1. Introduction
The developing brain is highly susceptible to adverse environmental conditions, leading to many potential and permanent structural alterations and adverse long-term effects on the behavioral and cognitive abilities of an individual. The early stages of life are marked by rapid brain growth and a dynamic process of synapse sculpting and pruning, making this time particularly prone to harmful disruptions [1]. Perinatal stressors like protein malnutrition, infections and neurotoxicant exposure have been implicated as risk factors for developing brain, leading to cognitive, behavioral and emotional impairments in both animals and humans, raising susceptibility to neuropsychiatric and neurodegenerative illnesses later in life [2,3,4]. Clinical investigations have revealed how exposure to environmental stressors early in life and throughout the developmental years of childhood and adolescence results in resilient or maladaptive behavior with a significant impact on cognition later in life [5]. Multiple types of stressors (multi-hit), such as exposure to prenatal and postnatal malnutrition [6,7], viral and bacterial infections, trauma, and social maltreatment and neurotoxins [8,9] act in variable combinations and profoundly disrupt brain development, thus may considerably increase the pathophysiology of neuropsychiatric disorders in affected individuals. Moreover, maternal inflammatory reactions during pregnancy [10], antenatal infection in preterm newborns [11] and neonatal infection [12] have also been linked to a lower intelligence quotient in offspring and increased chances of developing neuropsychiatric disorders such as schizophrenia and depression [13]. A combination of genetic, physical and environmental factors are known to interact to cause schizophrenia, resulting in the development, maintenance and evolution of the disease symptoms, but determining the exact origin of the disease is challenging [14,15].
Epidemiological data suggest that maternal malnutrition, a type of nutritional stress, has long been associated with changes in neurodevelopment, physical growth indices and brain structure [16,17]. Experimental malnutrition has been variously reported to affect the genesis, migration, plasticity and differentiation of neurons and glial cells during the critical periods of brain development [18,19,20,21,22,23], which are key factors in impaired neurocognitive development. Protein malnutrition has also been linked to an increased risk of neurodevelopmental and metabolic abnormalities in offspring, including autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD) and schizophrenia [24,25].
Experimental studies have also revealed behavioral and cognitive deficits, as well as physical development retardation and poor motor coordination in animal models following exposure to stressors such as maternal and early life infections, neurotoxicants, perinatal protein and protein-calorie malnutrition [22,26]. Perinatal protein malnutrition is also known to cause low anxiety in rodents, and such effects are directly proportional to the length of the period of malnutrition, and the longer exposure to a deficient diet results in long-lasting anxiolytic-like effects [18,27].
Moreover, the unhealthy lifestyles in which the children grow lead them to encounter viral and bacterial infections, which are common problems in already malnourished children, especially those from a low socio-economic background due to the compromised immune system [28]. Perinatal immune activation by viral and bacterial infections negatively impacts ongoing brain development and may enhance the risk of developing neuronal and other developmental disorders [4,29,30]. Lipopolysaccharide (LPS) is a Gram-negative bacterial cell wall component known to mimic bacterial infection in experimental conditions [31,32]. LPS-induced peripheral infection signals to the brain and causes microglial activation and extensive production of inflammatory cytokines, leading to behavioral pathology and cognitive impairment [33,34]. Prenatal exposure to LPS has been reported to alter developmental trajectories in neuron–microglia communication in the brains of young offspring, along with the imbalance in the offspring’s immune system. These prenatally exposed animals exhibit behavioral changes resembling schizophrenia-like phenotype in adulthood [35]. LPS-induced inflammation has also been associated with increased chances of anxiety disorders [36] and major depressive disorders [37].
Deltamethrin (DLT), a type II synthetic pyrethroid insecticide (cyano group at carboxyl α-position), has been largely used in agricultural, medical and domestic applications all over the world for more than 30 years [38,39]. Epidemiological data suggest a clear link between early life exposure to pyrethroid insecticides and neurodevelopmental disorders, viz., ADHD, ASD and developmental delay [39,40]. Pyrethroid exposure poses a public health concern, and the neurobehavioral performance deficiencies include decreased motor activity [41,42,43]. Occupational pesticide exposure has also been related to Parkinson’s disease (PD) [44], Alzheimer’s disease (AD) [45], amyotrophic lateral sclerosis (ALS) [46], dementia and cognitive impairments in several other diseases [47]. Other functions affected include delayed perceptual thinking and linguistic comprehension levels in children, as well as early growth and development impairments [48].
In this study, protein malnutrition (PMN) has been used as the prime stressor, and the F1 pups born to such nutritionally challenged dams were exposed to additive stressors, i.e., LPS and DLT, keeping in view their high susceptibility to infections and other environmental exposures. Thus, the concept of the multi-hit in the current context arises because of the susceptibility of early life development to numerous stressors, acting synergistically during perinatal life.
Thus, it is crucial to understand how the additive, synergistic or cumulative interplay of protein malnutrition, bacterial infection and a neurotoxicant pesticide affect behavioral development during adolescence and adulthood in terms of locomotor activity and anxiety status. Additionally, it was essential to establish whether there was any relationship between exposure to such stressors and the development of neurological disorders.
2. Materials and Methodology
Wistar albino rats used in the study were maintained under a standard animal house facility of the School of Studies in Neuroscience, Jiwaji University, Gwalior, in groups of 3 rats/cage (polypropylene cages; 52 cm × 28 cm × 22 cm) with clean and dust free husk bedding. The animals were maintained at a controlled temperature (25 ± 2 °C) and humidity (50–65%) with a fixed 12:12 h light–dark cycle and ad libitum food and water. The maintenance of the animal house was carried out as per the approved conditions and requirements of the Institutional Animal Ethics Committee of Jiwaji University, Gwalior (M.P), India.
Three-month-old virgin female rats (n = 32; body weight 140–150 gm) were selected from the breeding colony and shifted to control (20% protein; n = 16) and LP (8% protein; n = 16) diets for 15 days prior to mating to achieve a state of PMN before conception. Following acclimatization to the diet regimes, the females were placed for mating with healthy male rats in a ratio of 2:1. The pregnancy was confirmed through a vaginal smear test followed by increased body weights. Males were separated, and the pregnant females were maintained on their respective diets throughout gestation and lactation. The protein-malnourished mothers were equally fertile with no issues of pregnancies and mortality, except that the litter size was low. The day of birth was marked as postnatal day 0 for F1 pups. Both litter weight and size were recorded, and the pups were properly monitored for any abnormality and reared with their mothers in aseptic conditions. Litter size was adjusted to eight per dam to prevent dissimilarity among the groups due to different litter sizes. Both the control and LP diets were designed and procured from the National Institute of Nutrition (NIN) Hyderabad, India. The diet composition is tabulated as a .
2.1. Experimental Groups
The F1 pups obtained from both control and LP mothers were further grouped into eight groups (equal number of males and females), depending on the type of exposure (Figure 1).
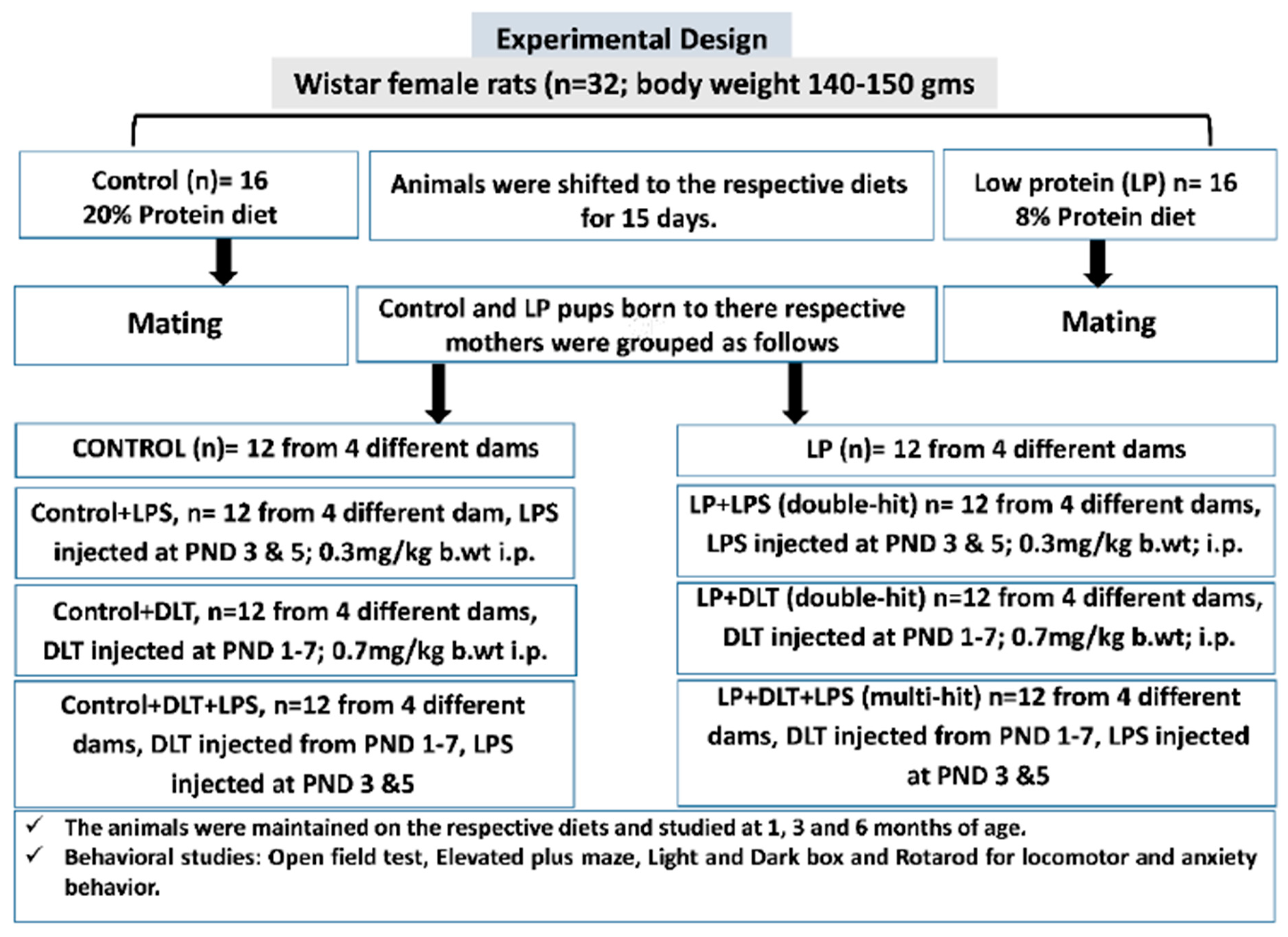
Figure 1. Overview of the experimental design and procedures followed.
Control, Control+LPS, Control+DLT and Control+DLT+LPS groups:
Control group: F1 pups (n = 12; from four different dams) born to females fed with a 20% protein diet were considered as controls and used to assess various behavioral abilities at the ages of 1, 3 and 6 months.
Control rats exposed to bacterial mimetic LPS (Control+LPS group): A set of control F1 pups (n = 12; from four different dams) were exposed to LPS at the dose of 0.3 mg/kg body weight intraperitoneally on PND 3 followed by a booster dose on PND 5 and used to assess various behavioral abilities at the ages of 1, 3 and 6 months.
Control rat pups exposed to deltamethrin (Control+DLT group): Another set of control F1 pups (n = 12; from four different dams) were exposed to DLT at the daily dose of 0.7 mg/kg body weight, intraperitoneally from PND 1 to 7 and used to assess various behavioral abilities at the ages of 1, 3 and 6 months.
Control F1 rat pups exposed to both LPS and DLT (Control+DLT+LPS group): Another set of control F1 pups (n = 12; from four different dams) were exposed to both DLT and LPS at the above specified time points and doses and used to assess various behavioral abilities at the ages of 1, 3 and 6 months.
Protein-malnourished (PMN) groups:
PMN (LP) group: LP F1 pups (n = 12; from four different dams) born to females fed with an 8% protein diet and maintained on the same diet throughout the experimental period were considered as LP/PMN group. These LP F1 rats were maintained on an LP diet and assessed for various behavioral abilities at the ages of 1, 3 and 6 months.
LP F1 rat pups exposed to bacterial mimetic LPS (LP+LPS double-hit group): LP F1 pups (n = 12 from four different dams) were exposed to LPS at the dose of 0.3 mg/kg body weight, intraperitoneally on PND 3 followed by a booster dose on PND 5. These LP+LPS rats were maintained on an LP diet and assessed for various behavioral abilities at the ages of 1, 3 and 6 months.
LP F1 rat pups exposed to deltamethrin (LP+DLT double-hit group): Another set of LP F1 pups (n = 12; from four different dams) were exposed to DLT at a daily dose of 0.7 mg/kg body weight intraperitoneally from PND 1 to 7. These LP+DLT rats were maintained on an LP diet and assessed for various behavioral abilities at the ages of 1, 3 and 6 months.
LP F1 rat pups exposed to both LPS and DLT (LP+DLT+LPS multi-hit group): A third set of LP F1 pups (n = 12; from four different dams) were exposed to both DLT and LPS at the above specified time points and doses. These LP+DLT+LPS rats were maintained on an LP diet and assessed for various behavioral abilities at the ages of 1, 3 and 6 months.
Both LPS (E. coli, serotype O11:B4) and DLT (D9315-10MG) were procured from Sigma Aldrich. The LPS solution was prepared in sterilized phosphate-buffered saline (PBS), while DLT was dissolved in dimethyl sulfoxide (DMSO).
To perform low volume and error-free injection of DLT and LPS at a steady flow rate of 30 µL/min and to confirm absolute absorption, Stoelting Nanoinjector and Hamilton micro-syringe were used under hygienic settings. To counter any bias, control animals were injected with the vehicle alone. No anesthetic procedure was followed during the injection; pups were instead gently handled during the delivery of the injection and then quickly transferred to their respective dams to minimize the separation stress from the mother. To overcome sex-specific differences, an equal number of males and females were used in the study as post hoc analysis did not bring any sex-specific variations during data analysis.
2.2. Behavioral Studies
Specific behavioral abilities were assessed in F1 generation rats (n = 12/group/time point, consisting of 6 males and 6 females from different dams) of all the groups (Control, LP, Control+LPS, LP+LPS, Control+DLT, LP+DLT, Control+LPS+DLT and LP+DLT+LPS) at the ages of 1, 3 and 6 months.
3. Results
3.1. Physical Development
No statistically significant difference was noticed in the appearance of developmental or physical landmarks, including ear pinna detachment, eye opening, incisor eruption, vaginal opening and testes descent amongst all the eight groups. However, LP and LP exposed group animals (LP+LPS; LP+DLT; LP+DLT+LPS) displayed drastic fur loss with stunted body growth, whereas the control group and control-treated animals revealed healthy physical appearance in terms of both hair and body growth.
3.2. Double-Hit and Multi-Hit Exposure of Lipopolysaccharide and Deltamethrin Induced Hyperactivity and Low Anxious Behavior in Protein-Malnourished Rats
Open field analysis demonstrated that maternal protein malnourishment resulted in hyperactivity and low anxiety-like behavior, as revealed by significantly increased DT (Figure 3B), ST (Figure 3C), AT (Figure 3D), HC (Figure 3E), center zone entries (Figure 3F), center zone time (Figure 3G) and a significantly decreased resting time (Figure 3A) in LP rats at the age of 1, 3 and 6 months as compared to the age-matched control groups showing the effects of maternal protein malnutrition. Exposure with either LPS or DLT (LP+LPS and LP+DLT) or cumulative exposure of both LPS and DLT to LP rats (LP+LPS+DLT) resulted in further exaggeration in the hyperactivity and low-anxiety behavior with a highly significant increase in DT, AT, ST and HC at the age of 1, 3 and 6 months as compared to the age-matched control and LP alone animals. Such hyperactivity and low anxiety behavior were also observed in control animals exposed to either LPS or DLT or both LPS+DLT, but the mean values for DT, AT, ST and HC remained comparatively low as compared to their respective LP groups. Time in square analysis from open field tracks showed that LP multi-hit rats (LP+DLT+LPS) spent significantly more time in the center zone with increased center zone entries as compared to age-matched control and LP-alone animals. The locomotive hyperactivity behavior was highly pronounced at 3 months of age, and the animals remained hyperactive and anxious throughout their life, i.e., by 6 months of age studied in this investigation.

Figure 3. Open field test data showing hyperactivity/inattention and low anxiety-like symptoms in LP+DLT+LPS group rats in adolescence and adulthood: Bar graphs represent resting time (A), distance traveled (B), stereotypic time (C), ambulatory time (D), horizontal count (E), center zone entries (F) and center zone time (G) at 1, 3 and 6 months of age. All the treated group rats revealed significantly increased distance traveled, stereotypic time, ambulatory time, horizontal count, center zone entries and center zone time with low resting time when compared to age-matched control. However, LP+DLT+LPS group rats displayed highest level changes in all parameters as compared to control and LP alone rats. Representative open field activity track reports. *** p ≤ 0.001, for comparison between control group and treated animals; ### p ≤ 0.001, for comparison between LP group and treated group; $ p ≤ 0.05, $$ p ≤ 0.01, $$$ p ≤ 0.001, for comparison between Control+LPS and LP+LPS group; @ p ≤ 0.05, @@ p ≤ 0.01, @@@ p ≤ 0.001, for comparison between Control+DLT and LP+DLT group; & p ≤ 0.05, && p ≤ 0.01, &&& p ≤ 0.001, for comparison between Control+DLT+LPS and LP+DLT+LPS group.
Open field track reports also confirmed that the above data showed a low anxiety-like behavioral profile and locomotive hyperactivity in LP and LP-treated animals compared to age-matched controls, which showed normal exploratory behavior (Figure 4a,e,i). Combo exposure of LPS and DLT to both control and LP group animals further reduced the anxiety levels (Figure 4b–d,f–h,j–l) comparatively more in LP-treated animals (Figure 4n–p,r–t,v–x), with very frequent haphazard center zone arena exploration. Furthermore, multi-hit animals showed drastic behavioral impairments accompanied by irregular track activity with maximum periphery exploration/wall clinging called thigmotaxis and increased time spent in the center zone and corners, showing severe hyperactivity or low anxiety and fearless behavior. Additionally, the dense area in the center zone depicts stereotyped repetitive rearing and horizontal back-and-forth movements revealed by multi-hit animals, further suggesting their low-anxiety profile. The statistical mean and f-values, along with the level of significance, are given in and .
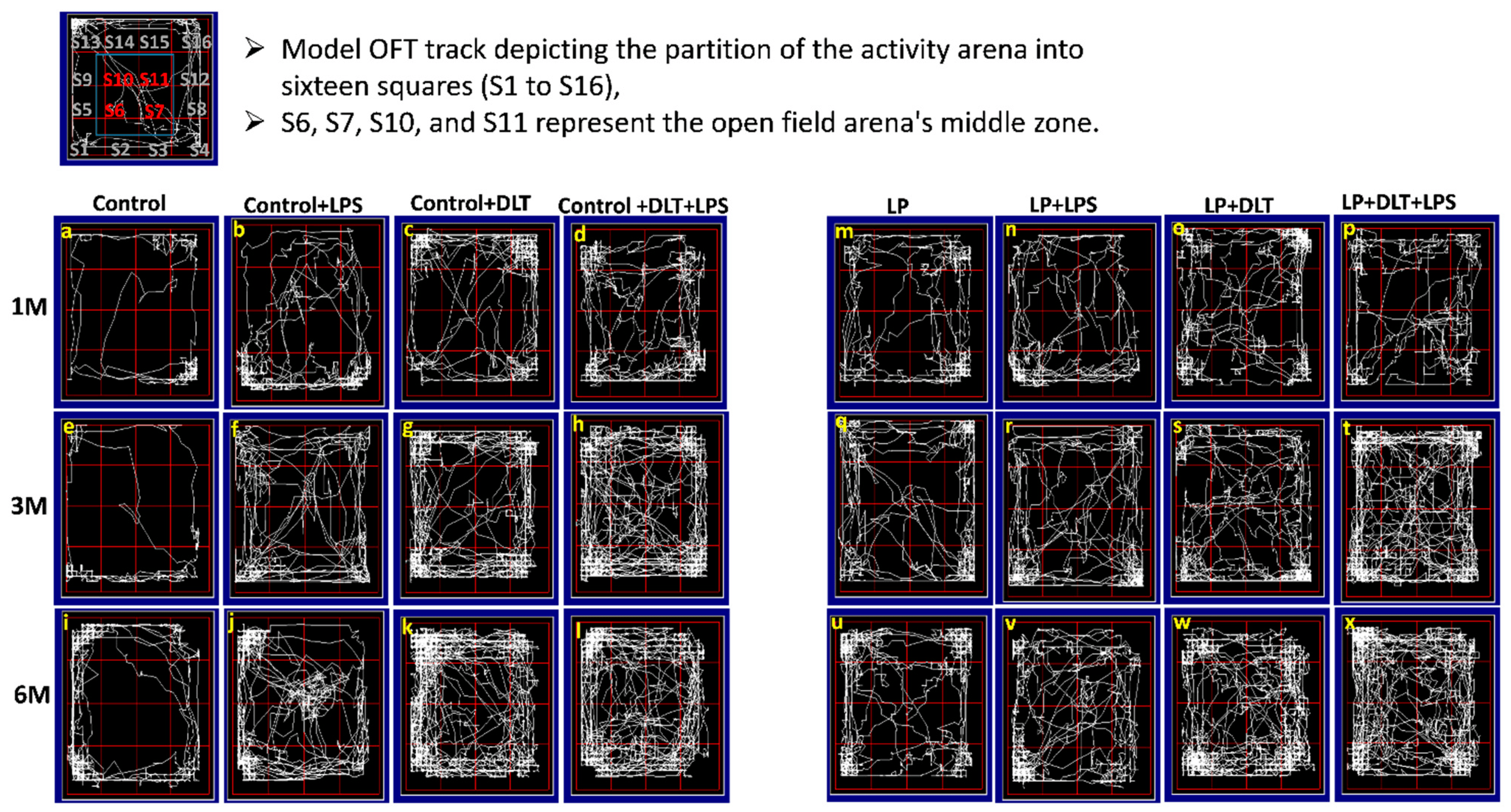
Figure 4. Revealed that control animals safely explore the open arena (a,e,i), while LP F1 rats showed increased exploration (m,q,u) and LP+LPS (n,r,v), LP+DLT (o,s,w) and LP+DLT+LPS (p,t,x) group rats exhibit further behavioral impairments as compared to control+LPS (b,f,j), control+DLT (c,g,k) and control+DLT+LPS (d,h,l). Moreover, LP+DLT+LPS group rats also present increased and haphazard center zone exploration (S6, S7, S10 and S11). Data were analyzed by one-way (comparison with control) and two-way (comparison between diet and infection) ANOVA and presented as mean ± SEM.
3.3. Elevated Plus Maze Test (EPM) Revealed Low Anxiety Phenotype in Protein-Malnourished Rats Treated with Lipopolysaccharide and Deltamethrin
The elevated plus maze (EPM) test also confirmed the low anxiety behavior following LPS and DLT exposure to LP rats. Data analysis from the EPM test revealed that LP animals spent significantly more time in open arms (% open arm time; Figure 5B) along with a significantly increased number of open arm entries (% open arm entries) (Figure 5A). In addition, both the time spent in the center zone and center zone entries (Figure 5C,D) were also significantly more in LP animals. However, upon single or combined treatment of LPS and DLT to LP animals, there was a further significant increase in both % open arm entries and % time spent in open arms, as well as the time spent in center zone and center zone entries with the maximum increase in the multi-hit treatment group (LP+LPS+DLT), demonstrating highly hyperactive and low anxiety-like phenotype as compared to age-matched control animals. The control animals also responded similarly to LPS or DLT or LPS+DLT treatments with a significant increase in % open arm entries and % open arm time as well as time in center zone and center zone entries at 1, 3 and 6 months, but to a lower degree as compared to their corresponding LP group rats. The number of open-arm entries increased gradually, with the complexity of exposure being highest in the LP multi-hit group (Figure 5E). Such behavior persisted consistently until the age of 6 months, as studied in this investigation. The data showing the mean and the f-values are tabulated in and .
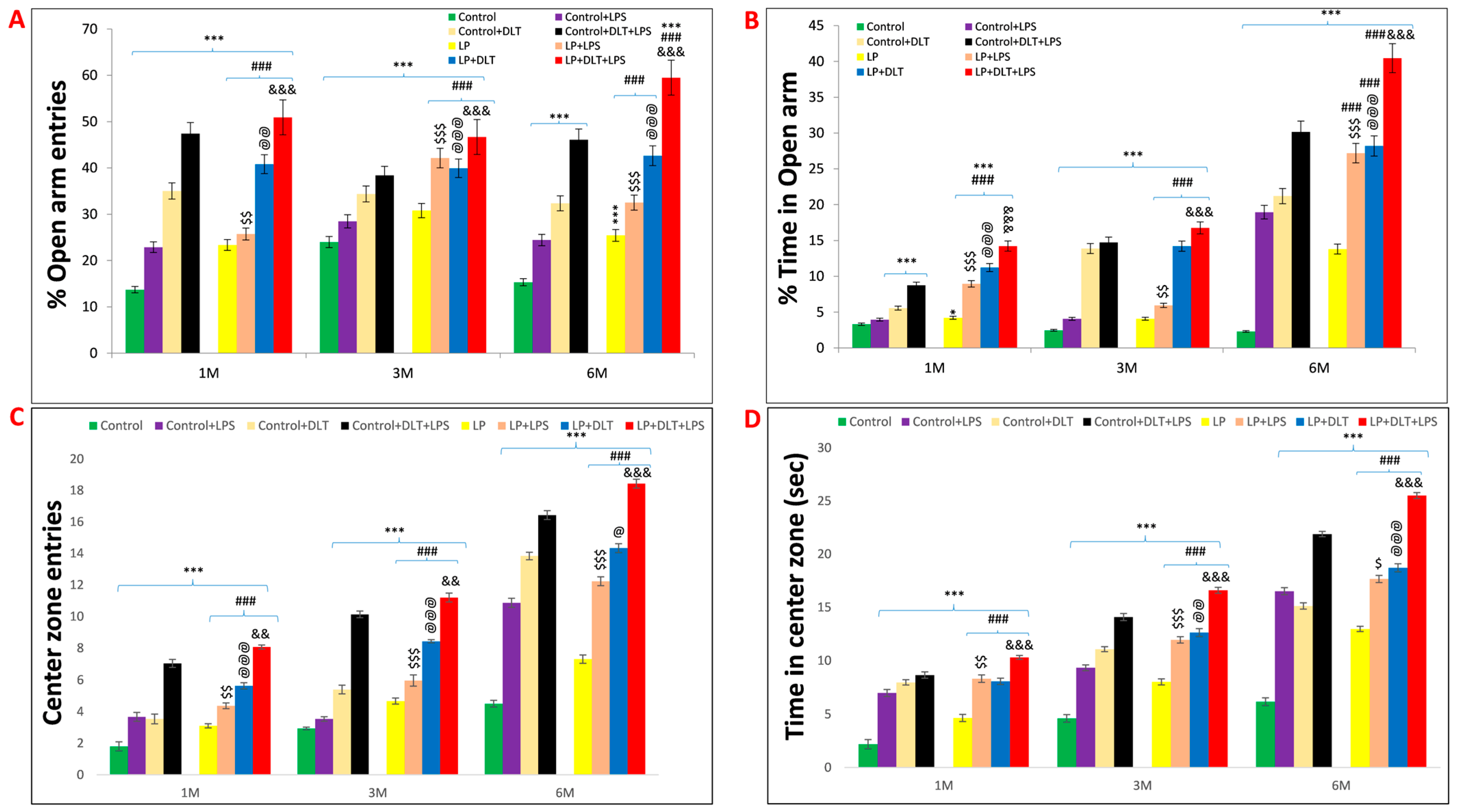
Figure 5. Elevated plus maze data also showed low anxiety, hyperactivity/inattention and low fear-like symptoms in LP+DLT+LPS group rats: Bar graphs representing the percent open arm entries (A), percent open arm time (B), center zone entries (C), center zone time (D) and total open arm entries (E) at 1, 3 and 6 months of postnatal age. From bar graphs, it was revealed that LP F1 rats prefer open arms and center zone exploration over closed arms, thus showing significantly increasing number of open arm entries and time (A,B), center zone entries and time (C,D), and total open arm entries (E) as compared to control group. However, LP+DLT+LPS group rats displayed further increase in all parameters as compared to control and LP alone animals. * p ≤ 0.05, *** p ≤ 0.001, for comparison between control group and treated animals; ### p ≤ 0.001, for comparison between LP group and treated group; $ p ≤ 0.05, $$ p ≤ 0.01, $$$ p ≤ 0.001, for comparison between Control+LPS and LP+LPS group; @ p ≤ 0.05, @@ p ≤ 0.01, @@@ p ≤ 0.001, for comparison between Control+DLT and LP+DLT group; && p ≤ 0.01, &&& p ≤ 0.001, for comparison between Control+DLT+LPS and LP+DLT+LPS group.
Elevated plus maze track reports also confirmed the above data showing the low anxiety-like behavioral profile in LP and LP-treated animals as compared to the respective age-matched control, which stays away from the open arms edges (Figure 6a,i,q). Combo exposure of LPS and DLT to both control (Control+LPS; Control+DLT; Control+DLT+LPS) and LP (LP+LPS; LP+DLT; LP+DLT+LPS) group animals further reduced the anxiety levels (Figure 6b–d,j–l,r–t), showing highly anxious profile in LP treated animals (Figure 6f–h,n–p,v–x), with very frequent open arm and center zone entries. Moreover, the multi-hit group rats frequently explored the open arms until the extreme distal ends, depicting hyperactivity or low-fear behavior (Figure 6d,l,t,h,p,x).
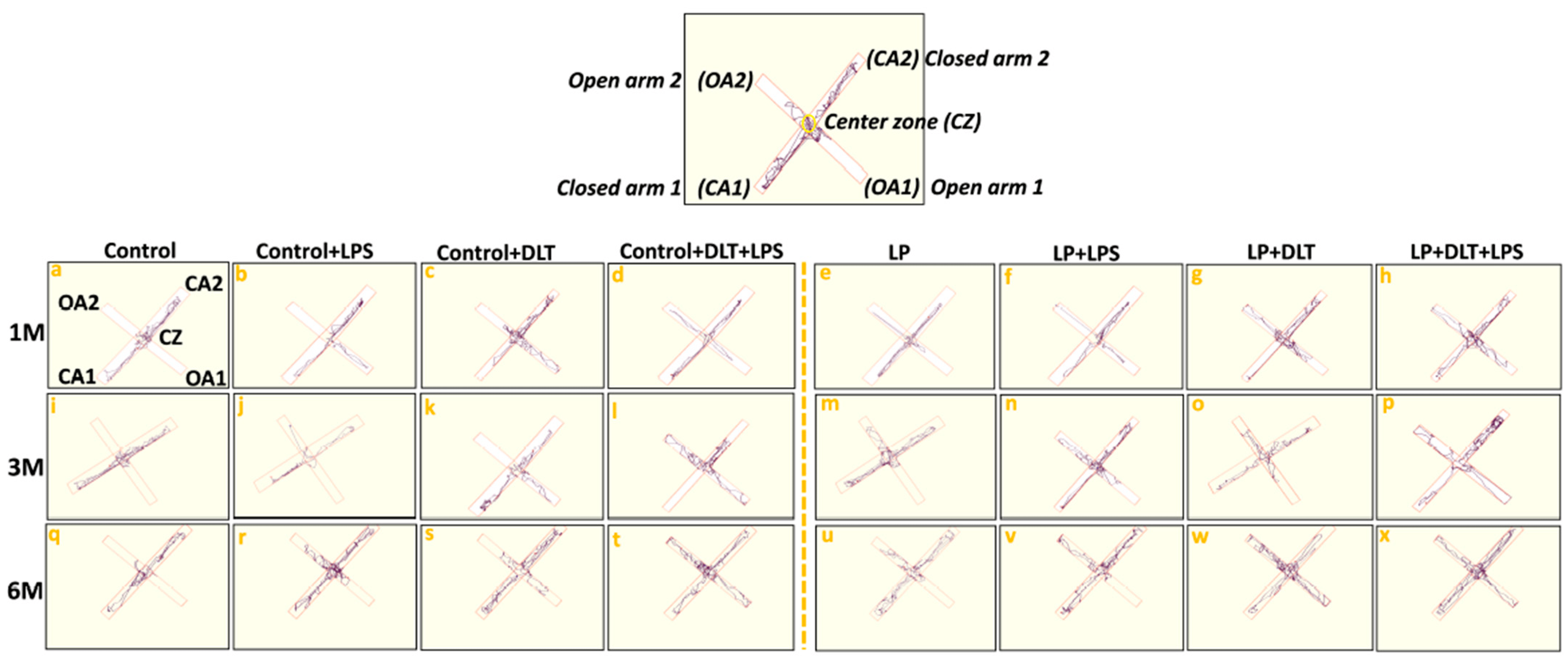
Figure 6. Track records indicate that control group rats behave normally while exploring the EPM arms with little percent open arm entries and time (a,i,q) as compared to LP group animals (e,m,u). However, exposed control+LPS (b,j,r), control+DLT (c,k,s), control+DLT+LPS (d,l,t), LP+LPS (f,n,v), LP+DLT (g,o,w) rats favored exploring open arms over closed ones and center zones. Such behavioral impairment was more severe in the LP+DLT+LPS group animals, showing low anxious profile with very frequent open arm and center zone entries (h,p,x). Moreover, the multi-hit group rats repeatedly explored the open arms to their most distal ends. Data were analyzed by one-way (comparison with control) and two-way (comparison between diet and infection) ANOVA and presented as mean ± SEM.
3.4. Light and Dark Box Test also Revealed Low Anxiety Behavior in Protein-Malnourished Rats Treated with LPS and DLT
OFT and EPM results were further supported by the light and dark box test. Data assessment from histograms of LD box revealed that LP animals showed significantly increased % light zone entries (Figure 7A) and % light zone time at 1, 3 and 6 months (Figure 7B), which indicates the low anxiety-like behavior with an increased tendency to explore the light zone in contrast to the biased normal nocturnal behavior of rats shown by age-matched control animals, revealing the impact of maternal protein malnutrition. Further exposure of LPS and DLT or both LPS+DLT to the control and LP F1 rats caused a sharp increase in both the % light zone entries and % light zone time, suggesting the impact of such double or multi-hit exposure in inducing hyperactivity and low anxiety. However, these changes were significantly higher in the LP multi-hit treated group, which indicates their higher susceptibility to developing such phenotypes.
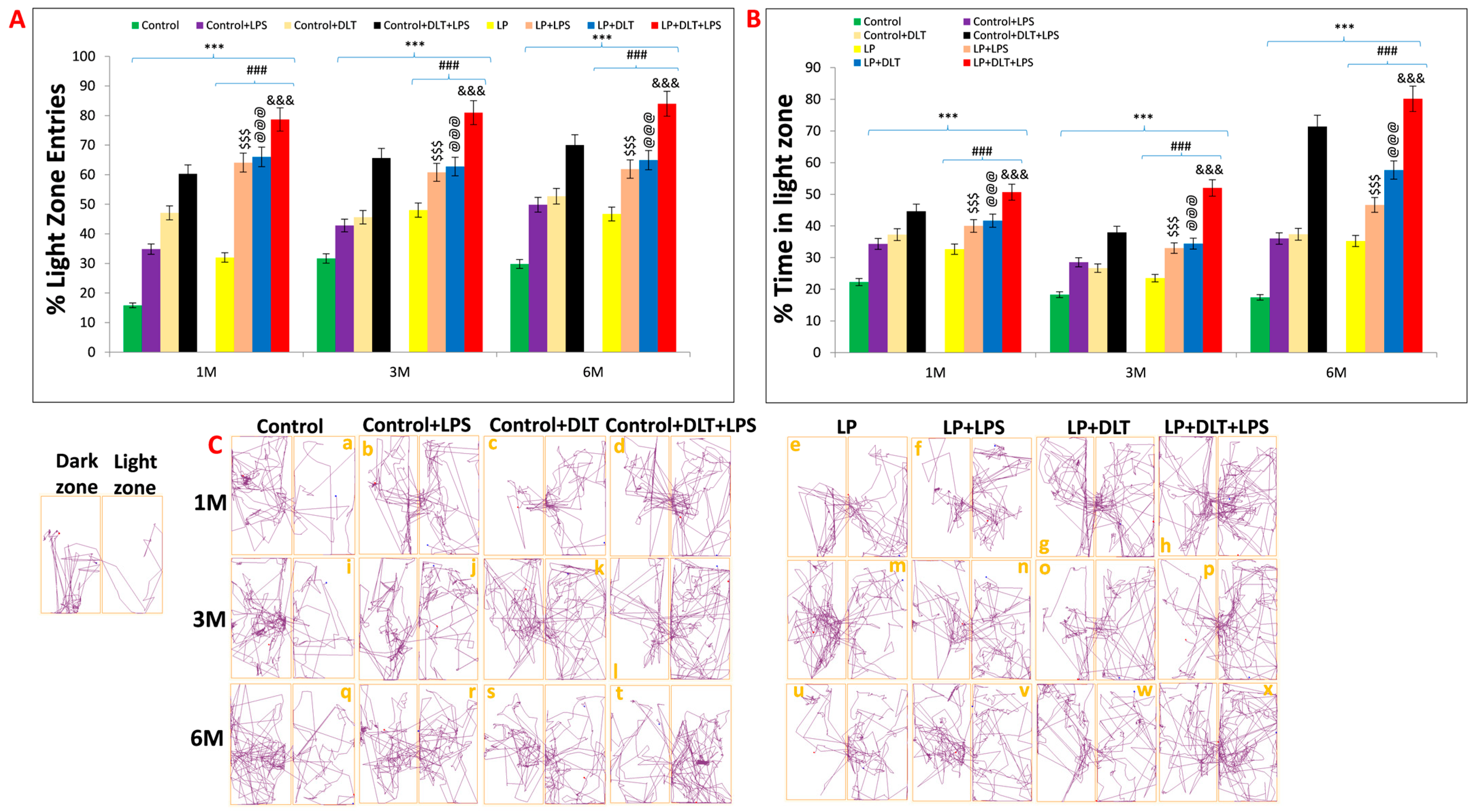
Figure 7. Light and dark box data also indicated low anxiety, hyperactivity/inattention and low fear-like symptoms in LP+DLT+LPS group: Bar graphs representing percent light zone entries (A) and percentage light zone time (B) at 1, 3 and 6 months of postnatal age: Data analysis showed that LP animals had significantly higher percent light zone entries (A) and percent light zone time (B) in contrast to age-matched control animals, which preferred dark zone. However, after exposure to either LPS or DLT or both LPS+DLT in control and LP F1 rats, a further significant rise in the percentage of light zone entries and light zone time was shown. Furthermore, such changes were significantly higher in the LP F1 stressed animals with maximum in LP+DLT+LPS group rats. Track records (C) also indicate that control group rats showed normal exploratory behavior upon exploring the light and dark zone with few percent light zone entries and time (a,i,q). However, cumulative exposure of LPS and DLT to control and LP F1 group rats revealed further increase in hyperactivity and low anxiety, which was evident by frequent entries into the light compartment. Such behavioral impairment was significantly maximum in LP+DLT+LPS group animals (h,p,x) as compared to age-matched control+LPS (b,j,r), control+DLT (c,k,s), control+DLT+LPS (d,l,t), LP (e,m,u), LP+LPS (f,n,v), LP+DLT (g,o,w) and other groups control Data were analyzed by one-way (comparion with control) and two-way (comparison between diet and infection) ANOVA and presented as mean ± SEM; *** p ≤ 0.001, for comparison between control group and treated animals; ### p ≤ 0.001, for comparison between LP group and treated group; $$$ p ≤ 0.001, for comparison between Control+LPS and LP+LPS group; @@@ p ≤ 0.001, for comparison between Control+DLT and LP+DLT group; &&& p ≤ 0.001, for comparison between Control+DLT+LPS and LP+DLT+LPS group.
Light and dark test track reports of animals also supported the above data, showing low anxiety profiles in LP and LP-treated animals as compared to age-matched control animals (Figure 7C(a,i,q)). Combo or multi-hit exposure of LPS and DLT in both control (Control+LPS; Control+DLT; Control+DLT+LPS) and LP (LP+LPS; LP+DLT; LP+DLT+LPS) group animals further showed reduced anxiety levels (Figure 7C(b–d,j–l,r–t)), comparatively more in LP treated animals (Figure 7C(f,g,n,o,v,w)) with irregular, random locomotor activity due to frequent entries into light compartment. Additionally, double or multi-hit animals depicted immobility at the doorway opening towards the light compartment, thus suggesting freezing behavior (Figure 7C(c,f,h,l,p,s,t,w,x). The statistical mean and f-values, along with the level of significance, are tabulated in and .
3.5. Cumulative Exposure of LPS and DLT to Protein-Malnourished (Multi-Hit) Rats Resulted in Hyperlocomotion and Motor Stereotypy Phenotype
The rotarod test was used to evaluate motor coordination. Interestingly, LP animals showed significantly increased latency to fall off from the accelerating rod of the rotarod at the age of 3 and 6 months as compared to age-matched control animals, indicating increased stereotyped behavior in LP animals, which tend them to keep moving purposelessly. Upon single or combined exposure of LPS and DLT to LP animals (LP+LPS, LP+DLT and LP+DLT+LPS), there was a further significant increase in latency to fall both at the age of 3 and 6 months. However, the multi-hit (LP+DLT+LPS) group rats were able to retain themselves for the maximum time on the accelerating rod, showing the highest latency to fall off both at 3 and 6 months of age, which reveals increased hyperactivity and motor stereotyped behavior when compared to the age-matched control group. Such behavioral deficits were also significantly observed in Control+LPS, Control+DLT and Control+DLT+LPS groups at 3 and 6 months of age when compared to age-matched normal control group. Such significantly higher retention time on rotating rotarod by the double and multi-hit group rats indicate increased hyperactivity and motor stereotyped behavior. The statistical mean and f-values, along with the level of significance, are mentioned in Figure 8 and and Table 10.
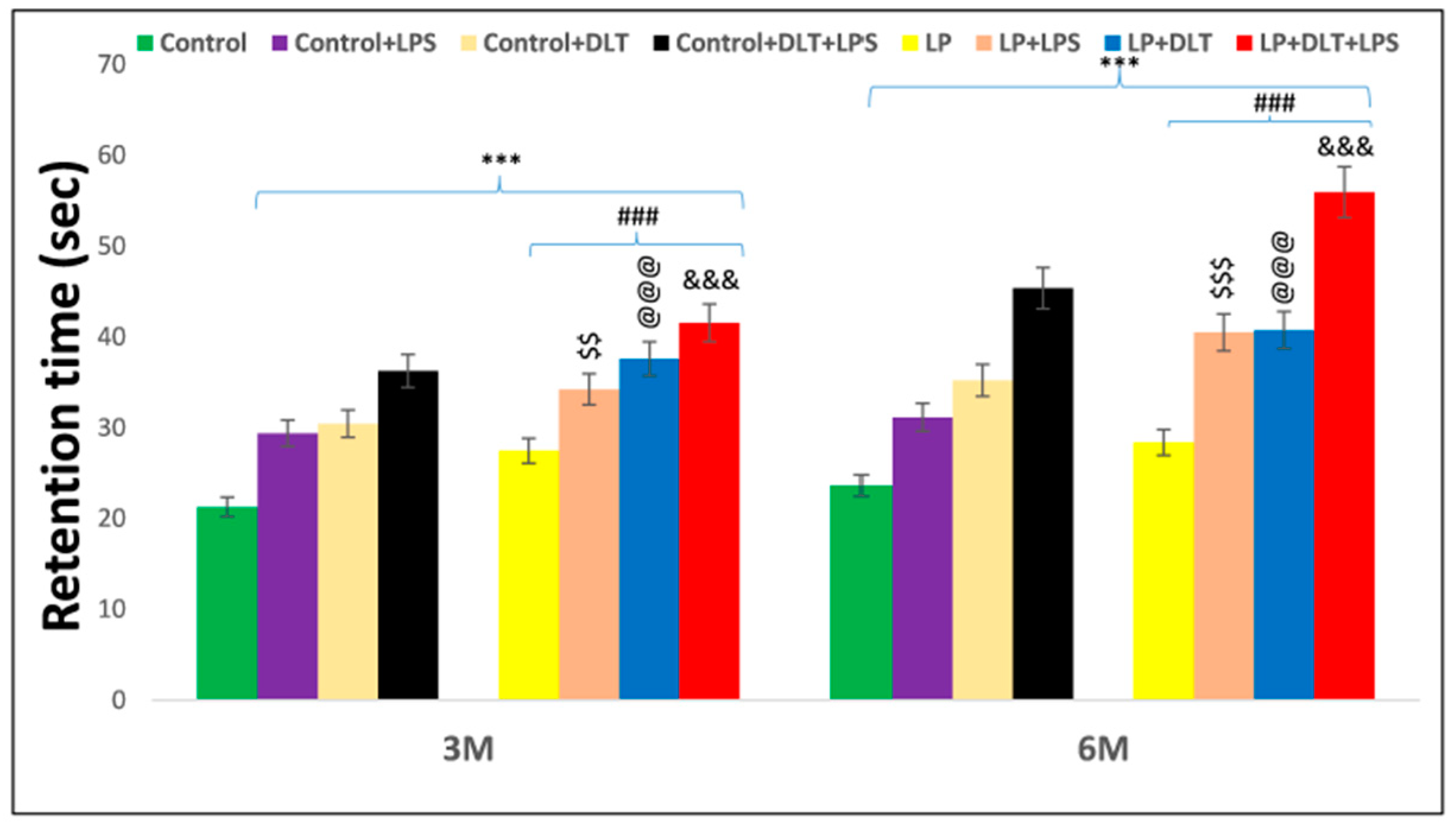
Figure 8. Rotarod performance displayed abnormal stereotypic behavior and inattention/hyperactive-like symptoms in LP+DLT+LPS group. Bar graph indicates the retention time on the accelerating rotating rod at 3 and 6 months of postnatal age. Control and LP F1 group rats exposed to either LPS or DLT or both LPS+DLT showed a significant increase in latency to fall off from the accelerating rotarod, indicating abnormal stereotyped motor behavior, which was more prominent in animals belonging to LP F1 treated groups and LP+LPS+DLT group rats indicated significantly maximum increased latency to fall off among all other groups showing a prominently abnormal stereotypic behavior than age-matched control rats. Data were analyzed by one-way (comparison with control) and two-way (comparison between diet and infection) ANOVA and presented as mean ± SEM; *** p ≤ 0.001; for comparison between control group and treated animals; ### p ≤ 0.001; for comparison between LP group and treated group; $$ p ≤ 0.01, $$$ p ≤ 0.001, for comparison between Control+LPS and LP+LPS group; @@@ p ≤ 0.001, for comparison between Control+DLT and LP+DLT group; &&& p ≤ 0.001, for comparison between Control+DLT+LPS and LP+DLT+LPS group.
4. Discussion
Perinatal stress has been widely documented as a contributing factor to several neurodevelopmental disorders, such as schizophrenia, ADHD, AD, PD and many others [50,51,52]. Many research groups have focused on the two-hit or dual-hit hypothesis, which postulates that an early genetic predisposition during critical developmental periods with a second hit, typically an environmental insult, such as an infection, nutritional deficiency or exposure to neurotoxicants, increases the likelihood of development of schizophrenia [30,53,54].
Even in the absence of genetic predisposition, perinatal life is inherently vulnerable to various environmental insults. Consequently, the presence of multiple stressors during early life, beyond the scope of the dual hit hypothesis, significantly increases the risk of schizophrenia and other neuropsychiatric disorders. In this study, we investigated the impact of multiple stressors during development and early life on the development of behavioral traits similar to schizophrenia and other psychotic disorders.
The severity of neurological problems is directly associated with the loss of body weight of an individual as it can increase the risk of systemic infections and may also raise the chances of morbidity and mortality in later life [55,56]. In this study, we recorded a persistent and significant loss of body weight in LP and LP-treated double-hit and multi-hit group rats as compared to the respective control rats. Such a significant loss of body weight may be due to the persistent overactivation of hypothalamic–pituitary–adrenal (HPA) axis as a response towards early life stressors [57], contributing to the development of metabolic dysregulation [58], as reported both in animal models [59] and human subjects [60]. In addition, the earlier reports from our lab also support that protein deprivation causes emaciation due to shrinkage of body size [22,23]. The available literature suggests that there is a direct link between the immune system and brain development. Both the brain and the immune system are not fully formed at birth but rather continue to mature in response to the postnatal environment, thus making them susceptible to early life stressors [61]. Studies in both humans [62] and animal models [63,64,65] suggest that a drop in body weight during early neonatal life following infection is an indication of sickness behavior. The population-based birth cohort studies have also found a link between low birth weight and many neuropsychological deficits, such as schizophrenia [66,67] and ADHD [68,69]. All these studies suggest that compromised physical growth and immune system following multi-hit exposure may predispose individuals to develop neuropsychiatric deficits.
To further investigate how the cumulative interplay impact of all these stressors affects the behavior of multi-hit rats, a battery of behavioral tests was used. The open field test, frequently used to assess rodent locomotion, anxiety and stereotyped behavior, including grooming and rearing [70,71], demonstrated that maternal PMN results in hyperactivity and stereotyped behavior and a low anxiety-like behavioral phenotype, as assessed by a significant increase in DT, ST, AT, HC, center zone entries and center zone time. In addition, there was a significant decrease in RT, suggesting restless behavior. Such changes were further pronounced following exposure to LPS or DLT or additive exposure of both LPS+DLT to LP F1 rats, showing severe behavioral deficits with frequent central zone exploration as shown by an increase in center zone entries and time, depicting hyper-locomotion and spontaneous jumping against the wall of the open field arena or wall-hugging as a stereotyped behavior, which persisted through adolescence and adulthood. Increased center zone exploration is an indicator of low anxiety as the rodents have a natural tendency to avoid the open center [72], while the increased horizontal and vertical counts suggest increased exploratory activity [73,74]. OFT data also revealed that LP F1 rats exposed to both LPS and DLT spent more time in stereotypic activities like scratching; licking of the head, neck and trunk; and other grooming behaviors. Such behaviors were persistent throughout adolescence and adulthood. Stereotypies or repetitive behavior are the repetitions of certain motor patterns with no apparent goals or functions, which coincides with the theory of being unable to translate cognition into actions. Thus, LP multi-hit rats lose self-control mechanisms such as control of attention or emotions because they have lost the chain between knowledge and actions and lack the capacity to refrain from acting spontaneously [75,76]. In addition, the hyperactivity and thigmotaxis also indicate the stereotypic activity traversing the same locomotor trajectory repeatedly [77]. Although the equivalence of rodents and human repetitive behavior is variously argued, the probability of repetitive behavior and increased self-grooming in rodents is directly correlated with hyperactivity and stereotypy and may indicate spontaneous aggression, which has long been linked with obsessive–compulsive disorder (OCD). Grooming in rodents has become a useful strategy for modeling different mood and psychiatric disorders and understanding neural circuitries underlying complex motor patterns [78]. Thus, the increased self-grooming in rodents indicating complex repetitive, self-directed and sequentially patterned behaviors may correlate indirectly to human brain disorders, including chain of motor actions and complex patterning of motor activities [78]. The anxiety-like states also alter rodent self-grooming and its sequencing [79,80]. The increased self-grooming in rodents has been modeled with disease symptoms in OCD [81,82], ASD [83,84], anxiety and panic disorders, and schizophrenia [85,86]. Interestingly, the LP F1 rats, from early adolescence through late adulthood, exhibited increased central time duration in addition to overall increased locomotion, indicating hyperactivity, motor stereotypy and low anxiety symptoms that are typical of both ADHD [87] and schizophrenic [88,89] patients. This suggests that increased movement and stereotypic behavior in LP and LP multi-hit group rats is indicative of their inability to adapt to novel conditions or signs of inadequate habituation [90,91]. When tested with rotarod, these single and multi-hit animals maintained themselves on the accelerating rod of the rotarod for a longer time, showing significantly increased latency to fall off when compared with the control animals. This indicates their motor stereotypy behavior that makes them keep moving abnormally and purposelessly. The motor stereotypies have been described as complex, repetitive, rhythmic, often bilateral movements with a typical onset in early childhood [92]. Such movements in the multi-hit rats may be the resultant of hyperactivity, low anxiety and fearless behaviors as revealed by open field test, elevated plus maze, and light and dark test. Over time, the emotional responses may become dissipated and replaced by hyperactivity and stereotyped responses. In this study, motor stereotypy refers to an excessive repetition of one type of motor response [93], i.e., prolonged maintenance on the rotating rod of the rotarod and increased latency to fall off by multi-hit animals. Such exaggerated hyperactivity and repetitive and stereotyped behavior also indirectly indicate an inattention behavior, as rats are reluctant to engage in tasks requiring sustained attention. Such behavior shown by multi-hit rats is also seen in many neuropsychiatric conditions, such as schizophrenia [94,95], OCD [96], autism spectrum disorder (ASD) and ADHD [97,98]. Recently, exposure to DLT alone has been linked to long-term neurobehavioral deficits in rodents when exposed postnatally [40] or in offspring when exposed during pregnancy [99]. Locomotor hyperactivity, attention deficits and elevated dopamine transporter and receptor levels commonly reported following DLT exposure are also typically seen in children with ADHD [99]. Studies examined with exposure to maternal protein deficiency or newborn viral or bacterial infections have similarly demonstrated impairment in behavior as measured by the OFT paradigm [22,23,30,100,101] consistent with what was observed in the present study.
These OFT results were further supported by EPM data, another commonly used test to assess anxiety-like phenotype, as rodents show an innate fear of height, which conflicts with curiosity and a drive for spontaneous exploration [102,103]. A higher degree of anxiety is often indicated by more frequent entries and time spent in the closed arms of EPM, while the higher open-arms exploration suggests anxiolytic behavior [71,102,104]. Rodents naturally favor closed arms and, in a typical 10 min experiment, spend the majority of their time in the closed arms [104,105]. The multi-hit animals tested in the EPM spent the most time in open arms with significantly increased % time and % entries along with significantly more center zone exploration, indicating hyperactive, low anxiety and fearless phenotype. Such changes increase gradually with the complexity of exposure and age, revealing that perinatal stressors lead to prolonged and consistent behavioral changes through adolescence to adulthood, as also seen in most schizophrenic patients. Low anxiety phenotype was also reported following exposure to viral and bacterial infection in rats [30] and humans [106,107], mouse models of tauopathy [108] and exposure to bisphenol A [109]. Unconditional anxiety responses were tested in rats by light and dark zone exploration tests [110]. Similar to the EPM, the rats were placed in an open arena that included protected (dark compartment) and unprotected (bright compartment) areas with free access between the two [111]. Most rodents exhibit a natural affinity for protected, dark spaces [71,106,112]; thus, increased avoidance of the light chamber is interpreted as increased anxiety [113]. However, increased time spent in the light zone or shorter entry delays are interpreted as signs of anxiolytic behavior or low anxiety-like phenotype [110,114]. Light and dark box tests also revealed hyperactivity and low anxiety-like behavior as multi-hit LP rats showed a significant increase in light zone exploration with increased % light zone entries and % light zone time. The increased time spent in the light zone or shorter entry delays are interpreted as signs of anxiolytic behavior or low anxiety-like phenotype [110,114].
The present study thus suggests that perinatal additive/cumulative multi-hit exposure of PMN, LPS and deltamethrin during early life leads to long-term deficits in terms of decreased bodyweight, hyperactivity, motor stereotypy and low anxiety, typically seen in patients with neuropsychiatric disorders like schizophrenia and ADHD. Although the heritability of ADHD and schizophrenia is quite high but not absolute [115,116], environmental influences during prenatal and postnatal development might also be playing a substantial role in the pathogenesis of these diseases. The results obtained in this study thus suggest that the cumulative impact of early life stressors may act as crucial risk factors and may pre-dispose individuals to develop such psychopathologies during adolescence and adulthood.
5. Conclusions
The present results indicate that additive exposure to multiple perinatal stressors during early life may lead to severe behavioral abnormalities in terms of hyperactivity, motor stereotypy and low-anxiety phenotype in adulthood, which may mimic the pathophysiology of neuropsychiatric disorders like ADHD and schizophrenia and may enhance the risk of developing such psychopathologies in adulthood.
References
- Stolp, H.; Neuhaus, A.; Sundramoorthi, R.; Molnár, Z. The long and the short of it: Gene and environment interactions during early cortical development and consequences for long-term neurological disease. Front. Psychiatry 2012, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A., III; Gabard-Durnam, L.J. Early adversity and critical periods: Neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 2020, 43, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W. How do established developmental risk-factors for schizophrenia change the way the brain develops? Transl. Psychiatry 2021, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Milbocker, K.A.; Campbell, T.S.; Collins, N.; Kim, S.; Smith, I.F.; Roth, T.L.; Klintsova, A.Y. Glia-driven brain circuit refinement is altered by early-life adversity: Behavioral outcomes. Front. Behav. Neurosci. 2021, 2, 786234. [Google Scholar] [CrossRef]
- Sarkar, T.; Patro, N.; Patro, I.K. Neuronal changes and cognitive deficits in a multi-hit rat model following cumulative impact of early life stressors. Biol. Open 2020, 9, bio054130. [Google Scholar] [CrossRef]
- Onaolapo, O.J.; Onaolapo, A.Y. Nutrition, nutritional deficiencies, and schizophrenia: An association worthy of constant reassessment. World J. Clin. Cases. 2021, 9, 8295. [Google Scholar] [CrossRef]
- Sarkar, T.; Patro, N.; Patro, I.K. Cumulative multiple early life hits-a potent threat leading to neurological disorders. Brain Res. Bull. 2019, 147, 58–68. [Google Scholar] [CrossRef]
- Teissier, A.; Le Magueresse, C.; Olusakin, J.; da Costa, B.L.A.; De Stasi, A.M.; Bacci, A.; Kawasawa, Y.I.; Vaidya, V.A.; Gaspar, P. Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol. Psychiatry 2020, 25, 1159–1174. [Google Scholar] [CrossRef]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Dammann, O.; Kuban, K.C.; Leviton, A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Koponen, H.; Rantakallio, P.; Veijola, J.; Jones, P.; Jokelainen, J.; Isohanni, M. Childhood central nervous system infections and risk for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Culpepper, N.; Rapaport, M.H.; Buckley, P. Prenatal inflammation and neurodevelopment in schizophrenia: A review of human studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 92–100. [Google Scholar] [CrossRef]
- Akdeniz, C.; Tost, H.; Streit, F.; Haddad, L.; Wüst, S.; Schäfer, A.; Schneider, M.; Rietschel, M.; Kirsch, P.; Meyer-Lindenberg, A. Neuroimaging evidence for a role of neural social stress processing in ethnic minority–associated environmental risk. JAMA Psychiatry 2014, 71, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Uher, R. Gene–environment interactions in severe mental illness. Front. Psychiatry 2014, 5, 48. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal nutrition and neurodevelopment: A scoping review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Alamy, M.; Bengelloun, W.A. Malnutrition and brain development: An analysis of the effects of inadequate diet during different stages of life in rat. Neurosci. Biobehav. Rev. 2012, 36, 1463–1480. [Google Scholar] [CrossRef]
- Naik, A.A.; Patro, N.; Seth, P.; Patro, I.K. Intra-generational protein malnutrition impairs temporal astrogenesis in rat brain. Biol. Open 2017, 6, 931–942. [Google Scholar] [CrossRef]
- Patro, N.; Naik, A.A.; Patro, I.K. Developmental changes in oligodendrocyte genesis, myelination, and associated behavioral dysfunction in a rat model of intra-generational protein malnutrition. Mol. Neurobiol. 2019, 56, 595–610. [Google Scholar] [CrossRef]
- Reyes-Castro, L.A.; Rodriguez, J.S.; Charco, R.; Bautista, C.J.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. Int. J. Dev. Neurosci. 2012, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.A.; Patro, I.K.; Patro, N. Slow physical growth, delayed reflex ontogeny, and permanent behavioral as well as cognitive impairments in rats following intra-generational protein malnutrition. Front. Neurosci. 2015, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Patro, N.; Tiwari, P.K.; Patro, I.K. Maternal Spirulina supplementation during pregnancy and lactation partially prevents oxidative stress, glial activation and neuronal damage in protein malnourished F1 progeny. Neurochem. Int. 2020, 141, 104877. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Reyes, T.M. Offspring neuroimmune consequences of maternal malnutrition: Potential mechanism for behavioral impairments that underlie metabolic and neurodevelopmental disorders. Front. Neuroendocrinol. 2017, 47, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Dean, A.J. Maternal nutritional deficiencies and schizophrenia: Lessons from animal models with a focus on developmental vitamin D deficiency. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2016; Volume 23, pp. 243–264. [Google Scholar]
- Laus, M.F.; Vales, L.D.M.F.; Costa, T.M.B.; Almeida, S.S. Early postnatal protein-calorie malnutrition and cognition: A review of human and animal studies. Int. J. Environ. Res. Public Health 2011, 8, 590–612. [Google Scholar] [CrossRef]
- Françolin-Silva, A.L.; da Silva Hernandes, A.; Fukuda, M.T.H.; Valadares, C.T.; Almeida, S.S. Anxiolytic-like effects of short-term postnatal protein malnutrition in the elevated plus-maze test. Behav. Brain Res. 2006, 173, 310–314. [Google Scholar] [CrossRef]
- Walson, J.L.; Berkley, J.A. The impact of malnutrition on childhood infections. Curr. Opin. Infect. Dis. 2018, 31, 231. [Google Scholar] [CrossRef]
- Meyer, U. Prenatal poly (i: C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef]
- Sarkar, T.; Patro, N.; Patro, I.K. Perinatal exposure to synergistic multiple stressors leads to cellular and behavioral deficits mimicking Schizophrenia-like pathology. Biol. Open 2022, 11, bio058870. [Google Scholar] [CrossRef]
- Guan, Z.; Fang, J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav. Immun. 2006, 20, 64–71. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liang, J.; Chen, D.C.; Xiu, M.H.; De Yang, F.; Kosten, T.A.; Kosten, T.R. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology 2012, 222, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [PubMed]
- Chamera, K.; Trojan, E.; Szuster-Głuszczak, M.; Basta-Kaim, A. The potential role of dysfunctions in neuron-microglia communication in the pathogenesis of brain disorders. Curr. Neuropharmacol. 2020, 18, 408–430. [Google Scholar] [CrossRef]
- Vogelzangs, N.; De Jonge, P.; Smit, J.H.; Bahn, S.; Penninx, B.W. Cytokine production capacity in depression and anxiety. Transl. Psychiatry 2016, 6, e825. [Google Scholar] [CrossRef] [PubMed]
- Gaspersz, R.; Lamers, F.; Kent, J.M.; Beekman, A.T.; Smit, J.H.; van Hemert, A.M.; Schoevers, R.A.; Penninx, B.W. Anxious distress predicts subsequent treatment outcome and side effects in depressed patients starting antidepressant treatment. J. Psychiatry Res. 2017, 84, 41–48. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden age of insecticide research: Past, present, or future? Annu. Rev. Entomol. 1998, 43, 1–16. [Google Scholar] [CrossRef]
- Pitzer, E.M.; Sugimoto, C.; Gudelsky, G.A.; Adams, C.L.H.; Williams, M.T.; Vorhees, C.V. Deltamethrin exposure daily from postnatal day 3–20 in Sprague-Dawley rats causes long-term cognitive and behavioral deficits. Toxicol. Sci. 2019, 169, 511–523. [Google Scholar] [CrossRef]
- Pitzer, E.M.; Williams, M.T.; Vorhees, C.V. Effects of pyrethroids on brain development and behavior: Deltamethrin. Neurotoxicol. Teratol. 2021, 87, 106983. [Google Scholar] [CrossRef]
- Patro, N.; Shrivasta va, M.; Tripathi, S.; Patro, I.K. S100β upregulation: A possible mechanism of deltamethrin toxicity and motor coordination deficits. Neurotoxicol. Teratol. 2009, 31, 169–176. [Google Scholar] [CrossRef]
- Chen, N.N.; Luo, D.J.; Yao, X.Q.; Yu, C.; Wang, Y.; Wang, Q.; Wang, J.Z.; Liu, G.P. Pesticides induce spatial memory deficits with synaptic impairments and an imbalanced tau phosphorylation in rats. J. Alzheimer’s Dis. 2012, 30, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Belkadi, A.; Al-Haddad, S.; Richardson, J.R. Deltamethrin exposure inhibits adult hippocampal neurogenesis and causes deficits in learning and memory in mice. Toxicol. Sci. 2020, 178, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Azim, F.; Saju, H.; Zargaran, A.; Shirzad, M.; Kamal, M.; Fatema, K.; Rehman, S.; Azad, M.M.; Ebrahimi-Barough, S. Pesticides and Parkinson’s disease: Current and future perspective. J. Chem. Neuroanat. 2021, 115, 101966. [Google Scholar] [CrossRef]
- Li, Y.; Fang, R.; Liu, Z.; Jiang, L.; Zhang, J.; Li, H.; Liu, C.; Li, F. The association between toxic pesticide environmental exposure and Alzheimer’s disease: A scientometric and visualization analysis. Chemosphere 2021, 263, 128238. [Google Scholar] [CrossRef] [PubMed]
- Kamel, F.; Umbach, D.M.; Bedlack, R.S.; Richards, M.; Watson, M.; Alavanja, M.C.; Blair, A.; Hoppin, J.A.; Schmidt, S.; Sandler, D.P. Pesticide exposure and amyotrophic lateral sclerosis. Neurotoxicology 2012, 33, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 379–385. [Google Scholar] [CrossRef]
- Xue, Z.; Li, X.; Su, Q.; Xu, L.; Zhang, P.; Kong, Z.; Xu, J.; Teng, J. Effect of synthetic pyrethroid pesticide exposure during pregnancy on the growth and development of infants. Asia Pac. J. Public Health 2013, 25 (Suppl. 4), 72S–79S. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Patro, N.; Patro, I.K. Amelioration of neurobehavioral and cognitive abilities of F1 progeny following dietary supplementation with Spirulina to protein malnourished mothers. Brain Behav. Immun. 2020, 85, 69–87. [Google Scholar] [CrossRef]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- Hoeijmakers, L.; Lucassen, P.J.; Korosi, A. The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Front. Mol. Neurosci. 2015, 7, 103. [Google Scholar] [CrossRef]
- Monk, C.; Lugo-Candelas, C.; Trumpff, C. Prenatal developmental origins of future psychopathology: Mechanisms and pathways. Annu. Rev. Clin. Psychol. 2019, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.A.; Falkai, P.; Maier, W. Genetic and non-genetic vulnerability factors in schizophrenia: The basis of the two-hit hypothesis. J. Psychiatr. Res. 1999, 33, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Eyre, H.; Jacka, F.N.; Dodd, S.; Dean, O.; McEwen, S.; Debnath, M.; McGrath, J.; Maes, M.; Amminger, P.; et al. A review of vulnerability and risk for schizophrenia: Beyond the two-hit hypothesis. Neurosci. Biobehav. Rev. 2016, 65, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.L.; Herz, J.; Fernandes, A.; Rocha, J.; Sepodes, B.; Brito, M.A.; McGavern, D.B.; Brites, D. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J. Neuroinflammation 2015, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection-from underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef]
- Malik, S.; Spencer, S.J. Early life stress and metabolism. Curr. Opin. Behav. Sci. 2019, 28, 25–30. [Google Scholar] [CrossRef]
- Spencer, S.J. Perinatal programming of neuroendocrine mechanisms connecting feeding behavior and stress. Front. Neurosci. 2013, 7, 109. [Google Scholar] [CrossRef]
- Peña, C.J.; Smith, M.; Ramakrishnan, A.; Cates, H.M.; Bagot, R.C.; Kronman, H.G.; Patel, B.; Chang, A.B.; Purushothaman, I.; Dudley, J.; et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat. Commun. 2019, 10, 5098. [Google Scholar] [CrossRef]
- Surkan, P.J.; Ettinger, A.K.; Hock, R.S.; Ahmed, S.; Strobino, D.M.; Minkovitz, C.S. Early maternal depressive symptoms and child growth trajectories: A longitudinal analysis of a nationally representative US birth cohort. BMC Pediatr. 2014, 14, 185. Available online: http://www.biomedcentral.com/1471-2431/14/185 (accessed on 21 July 2014). [CrossRef]
- Danese, A.; Lewis, S.J. Psychoneuroimmunology of early-life stress: The hidden wounds of childhood trauma? Neuropsychopharmacology 2017, 42, 99–114. [Google Scholar] [CrossRef]
- Shattuck, E.C.; Muehlenbein, M.P. Human sickness behavior: Ultimate and proximate explanations. Am. J. Phys. Anthropol. 2015, 157, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nemzek, J.A.; Hugunin, K.; Opp, M.R. Modeling sepsis in the laboratory: Merging sound science with animal well-being. Comp. Med. 2008, 58, 120–128. [Google Scholar] [PubMed]
- Bay-Richter, C.; Janelidze, S.; Hallberg, L.; Brundin, L. Changes in behaviour and cytokine expression upon a peripheral immune challenge. Behav. Brain Res. 2011, 222, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I. Sickness behavior, its mechanisms and significance. Anim. Health Res. Rev. 2008, 9, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.B.; Rantakallio, P.; Hartikainen, A.L.; Isohanni, M.; Sipila, P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: A 28-year follow-up of the 1966 north Finland general population birth cohort. Am. J. Psychiatry 1998, 155, 355–364. [Google Scholar] [CrossRef]
- Wahlbeck, K.; Forsén, T.; Osmond, C.; Barker, D.J.; Eriksson, J.G. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch. Gen. Psychiatry 2001, 58, 48–52. [Google Scholar] [CrossRef]
- Hatch, B.; Healey, D.M.; Halperin, J.M. Associations between birth weight and attention-deficit/hyperactivity disorder symptom severity: Indirect effects via primary neuropsychological functions. J. Child Psychol. Psychiatry 2014, 55, 384–392. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.Y.; Lee, J.; Jeong, G.H.; Lee, E.; Lee, S.; Lee, K.H.; Kronbichler, A.; Stubbs, B.; Solmi, M.; et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: An umbrella review. Lancet Psychiatry 2020, 7, 955–970. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2017, 19, 181–191. [Google Scholar] [CrossRef]
- La-Vu, M.; Tobias, B.C.; Schuette, P.J.; Adhikari, A. To approach or avoid: An introductory overview of the study of anxiety using rodent assays. Front. Behav. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A.; Racz, I.; Michel, K.; Zimmer, A. Diminished anxiety-and depression-related behaviors in mice with selective deletion of the Tac1 gene. J. Neurosci. 2002, 22, 10046–10052. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, C.R.; Ho, Y.J.; Schwarting, R.K. Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci. Biobehav. Rev. 2008, 32, 1544–1568. [Google Scholar] [CrossRef]
- Monterosso, J.; Ainslie, G. Beyond discounting: Possible experimental models of impulse control. J. Psychopharmacol. 1999, 146, 339–347. [Google Scholar] [CrossRef]
- Arce, E.; Santisteban, C. Impulsivity: A review. Psicothema 2006, 18, 213–220. [Google Scholar]
- Ralph, R.J.; Paulus, M.P.; Fumagalli, F.; Caron, M.G.; Geyer, M.A. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: Differential effects of D1 and D2 receptor antagonists. J. Neurosci. 2001, 21, 305–313. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Tuohimaa, P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: Potential utility for neurobehavioural stress research. J. Neurosci. Methods 2005, 143, 169–177. [Google Scholar] [CrossRef]
- Denmark, A.; Tien, D.; Wong, K.; Chung, A.; Cachat, J.; Goodspeed, J.; Grimes, C.; Elegante, M.; Suciu, C.; Elkhayat, S.; et al. The effects of chronic social defeat stress on mouse self-grooming behavior and its patterning. Behav. Brain Res. 2010, 208, 553–559. [Google Scholar] [CrossRef]
- Joel, D. Current animal models of obsessive compulsive disorder: A critical review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 374–388. [Google Scholar] [CrossRef]
- Feusner, J.D.; Hembacher, E.; Phillips, K.A. The mouse who couldn’t stop washing: Pathologic grooming in animals and humans. CNS Spectr. 2009, 14, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Sungur, A.Ö.; Vörckel, K.J.; Schwarting, R.K.; Wöhr, M. Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. J. Neurosci. Methods 2014, 234, 92–100. [Google Scholar] [CrossRef]
- Wolff, S.; Chess, S. A behavioural study of schizophrenic children. Acta Psychiatry Scand. 1964, 40, 438–466. [Google Scholar] [CrossRef] [PubMed]
- Roehr, B. American psychiatric association explains DSM-5. BMJ 2013, 346, f3591. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W. Micronutrients and diets in the treatment of attention-deficit/hyperactivity disorder: Chances and pitfalls. Front. Psychiatry 2020, 11, 102. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Teasdale, S.B.; Müller-Stierlin, A.S.; Ruusunen, A.; Eaton, M.; Marx, W.; Firth, J. Prevalence of food insecurity in people with major depression, bipolar disorder, and schizophrenia and related psychoses: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 63, 4485–4502. [Google Scholar] [CrossRef]
- Almeida, S.S.; Garcia, R.A.; Cibien, M.M.; De Araujo, M.; Moreira, G.; De Oliveira, L.M. The ontogeny of exploratory behaviors in early-protein-malnourished rats exposed to the elevated plus-maze test. Psychobiology 1994, 22, 283–288. [Google Scholar] [CrossRef]
- Bobyn, P.J.; Corbett, D.; Saucier, D.M.; Noyan-Ashraf, M.H.; Juurlink, B.H.; Paterson, P.G. Protein-energy malnutrition impairs functional outcome in global ischemia. Exp. Neurol. 2005, 196, 308–315. [Google Scholar] [CrossRef]
- Péter, Z.; Oliphant, M.E.; Fernandez, T.V. Motor stereotypies: A pathophysiological review. Front. Neurosci. 2017, 11, 171. [Google Scholar] [CrossRef]
- Ridley, R.M. The psychology of perseverative and stereotyped behaviour. Prog. Neurobiol. 1994, 44, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hoptman, M.J.; Ahmed, A.O. Neural foundations of mood-induced impulsivity and impulsive aggression in schizophrenia. Curr. Behav. Neurosci. Rep. 2016, 3, 248–255. [Google Scholar] [CrossRef]
- Van Erp, T.G.; Baker, R.A.; Cox, K.; Okame, T.; Kojima, Y.; Eramo, A.; Potkin, S.G. Effect of brexpiprazole on control of impulsivity in schizophrenia: A randomized functional magnetic resonance imaging study. Psychiatry Res. Neuroimaging 2020, 301, 111085. [Google Scholar] [CrossRef]
- Grassi, G.; Pallanti, S.; Righi, L.; Figee, M.; Mantione, M.; Denys, D.; Piccagliani, D.; Rossi, A.; Stratta, P. Think twice: Impulsivity and decision making in obsessive–compulsive disorder. J. Behav. Addict. 2015, 4, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, A.D.; Cost, K.T.; Anagnostou, E.; Lai, M.C.; Crosbie, J.; Schachar, R.; Georgiades, S.; Duku, E.; Szatmari, P. Inattention and hyperactive/impulsive component scores do not differentiate between autism spectrum disorder and attention-deficit/hyperactivity disorder in a clinical sample. Mol. Autism 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Grimm, O.; Kranz, T.M.; Reif, A. Genetics of ADHD: What should the clinician know? Curr. Psychiatry Rep. 2020, 22, 18. [Google Scholar] [CrossRef]
- Richardson, J.R.; Taylor, M.M.; Shalat, S.L.; Guillot, T.S., III; Caudle, W.M.; Hossain, M.M.; Mathews, T.A.S.R.; Cory-Slechta, D.A.; Miller, G.W. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J. 2015, 29, 1960–1972. [Google Scholar] [CrossRef]
- Singh, K.; Patro, N.; Pradeepa, M.; Patro, I. Neonatal lipopolysaccharide infection causes demyelination and behavioral deficits in adult and senile rat brain. Ann. Neurosci. 2017, 24, 146–154. [Google Scholar] [CrossRef]
- Baghel, M.S.; Singh, B.; Dhuriya, Y.K.; Shukla, R.K.; Patro, N.; Khanna, V.K.; Patro, I.K.; Thakur, M.K. Postnatal exposure to poly (I: C) impairs learning and memory through changes in synaptic plasticity gene expression in developing rat brain. Neurobiol. Learn. Mem. 2018, 155, 379–389. [Google Scholar] [CrossRef]
- Handley, S.L.; Mithani, S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch. Pharmacol. 1984, 327, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, F.; Alkadhi, K.A.; Salim, S. Understanding stress: Insights from rodent models. Curr. Res. Neurobiol. 2021, 2, 100013. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. 2008, 22, e1088. [Google Scholar] [CrossRef]
- Li, J.; Olsen, J.; Vestergaard, M.; Obel, C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. Eur. Child Adolesc. Psychiatry 2010, 19, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Menet, J.S.; Rosbash, M. When brain clocks lose track of time: Cause or consequence of neuropsychiatric disorders. Curr. Opin. Neurobiol. 2011, 21, 849–857. [Google Scholar] [CrossRef]
- Takeuchi, H.; Iba, M.; Inoue, H.; Higuchi, M.; Takao, K.; Tsukita, K.; Karatsu, Y.; Iwamoto, Y.; Suhara, T.; Miyakawa, T.; et al. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS ONE 2011, 6, e21050. [Google Scholar] [CrossRef]
- Tian, Y.H.; Baek, J.H.; Lee, S.Y.; Jang, C.G. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse 2010, 64, 432–439. [Google Scholar] [CrossRef]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef]
- Birkett, M.A.; Shinday, N.M.; Kessler, E.J.; Meyer, J.S.; Ritchie, S.; Rowlett, J.K. Acute anxiogenic-like effects of selective serotonin reuptake inhibitors are attenuated by the benzodiazepine diazepam in BALB/c mice. Pharmacol. Biochem. Behav. 2011, 98, 544–551. [Google Scholar] [CrossRef]
- Bailey, K.R.; Crawley, J.N. Anxiety-Related Behaviors in Mice. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2011; Chapter 5. [Google Scholar]
- Kulesskaya, N.; Voikar, V. Assessment of mouse anxiety-like behavior in the light–dark box and open-field arena: Role of equipment and procedure. Physiol. Behav. 2014, 133, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Trifu, S.C.; Kohn, B.; Vlasie, A.; Patrichi, B.-E. Genetics of schizophrenia (review). Exp. Ther. Med. 2020, 20, 3462–3468. [Google Scholar] [CrossRef] [PubMed]

