1. Introduction
Developmental language disorder (DLD) is a heterogenous neurodevelopmental disorder that affects a child’s ability to comprehend and/or produce spoken and/or written language but cannot be attributed to hearing loss or overt neurological damage (coded in the ICD-11 §6A01.2). DLD affects around 7% of children in the US making it more prevalent than other neurodevelopmental disorders, such as autism spectrum disorders (ASD) and dyslexia [1,2,3,4]. Moreover, adults who were diagnosed with DLD as children often experience anxiety and depression and tend to struggle with social relationships, preferring environments and vocations that do not require strong language and literacy skills [5,6]. Despite the prevalence and profound life-long impact DLD can have on a person, little is understood about the neurological basis or etiology of the disorder or how observed language impairments arise.
DLD is typically diagnosed after the age of 4 (around the time a child enters into preschool), when it becomes clear that the child has fallen behind their same age peers in terms of receptive and expressive language skills [7]. Yet, it is likely that the neural substrates underlying the disorder are in place prior to receiving a diagnosis. Current research suggests that some combination of genetic and environmental factors influence neural development in this population, but it is unclear if aberrant brain pathology causes DLD or if DLD leads to altered brain structure and function [8]. Further, there is significant debate regarding theoretical accounts of language impairment patterns observed in children with DLD. To date, none of the neurological or theoretical explanations of DLD fully account for the range of symptoms across individuals or the differing results across research studies [9]. This disconnect has resulted in some researchers defining DLD as a heterogenous disorder that may actually be a spectrum disorder with different phenotypes, like ASD, or may even exist on the same continuum as ASD [10,11,12].
Core to this paper is the notion that the term heterogeneity is oftentimes misused to describe the differences found across studies that are better attributed to differences in research design (population identification, task demands, etc.). For example, if a study uses an assessment that poorly identifies children who have DLD (i.e., low sensitivity) compared to those who do not (i.e., low specificity), that could lead to the DLD group appearing to have more variability in measured behaviors [13,14]. Task demands may also influence how heterogeneous the DLD group appears, especially if the comparison group is poorly matched on age or other criteria. When studies accurately measure known areas of difficulty for children with DLD, such as morphosyntax, they in fact often perform similarly to one another (i.e., heterogeneity is reduced) [14]. In this overview, we broach the topic of heterogeneity briefly to suggest that children with DLD struggle with a range of linguistic and non-linguistic behaviors, but we do so with the knowledge that within specific domains of language they show more consistent impairment patterns than their typically developing peers. outlines language problems commonly reported in children with DLD.
Purpose
The careful characterization of heterogeneity in DLD is important to note because differing patterns of results for both behavioral and neuroimaging studies have obscured what may otherwise be true differences between children with DLD and TD children. As a result, findings have not been synthesized in a way that moves the field forward. As such, though we recognize that there are differences in outcomes across studies, here the aim of this overview is to shift the focus away from addressing outcome differences across studies to identifying converging evidence, so that we can begin to bridge the theoretical and imaging fields. While a few other overviews exist (see [16,17,18]), none that we are aware of link neuroimaging patterns with various theoretical approaches that attempt to capture the range of language impairment patterns found in DLD.
We approach this by first reviewing the underlying neuroimaging patterns to date in DLD (structural (Section 2) and functional (Section 3)). We then summarize a subset of common theoretical accounts of language impairment patterns (across production and comprehension) while attempting to bridge the gap between neuroimaging literature and theory to elucidate brain behavior connections in DLD (Section 5 and Section 6). As will become clear from this overview, the link between theoretical accounts of DLD and findings from neuroimaging research is based on limited evidence; therefore, we will conclude by proposing future directions for neuroimaging research to better understand how aberrant brain structure and function relates to observed language impairments in DLD (Section 7).
2. Structural Neuroimaging Findings in DLD
In this section and in Section 3 below, we describe the structural and functional outcomes of neuroimaging studies as a way to set up the integration of neuroimaging findings in support of theoretical models of language in DLD (Section 5, below).
The link between brain development and language outcomes in children with DLD is unclear, and this lack of connection is apparent when reviewing the DLD neuroimaging literature. Over the past 50 years, there have been fewer than 60 neuroimaging studies (excluding EEG studies) with children diagnosed with DLD. The majority of these studies have focused on structural brain differences when compared to language-unimpaired (neurotypical) children or children with other neurodevelopmental language disorders, such as children diagnosed with ASD and concomitant language impairment. Though there are some consistencies that will be discussed below, it is important to note that the picture portrayed here is tenuous at best due to the limited number of studies that confirm these consistent findings as compared to the larger number of studies that have contrasting results. In this paper, we determined that differences in participant selection and inclusion, diagnostic criteria, methodology, and analyses used underlie the disparate findings to date (see , Table A1). As such, comparing the results across studies and evaluating how structural and functional brain abnormalities contribute to language impairment in children with DLD is challenging. Nonetheless, in this section and in Section 3 below, we provide a general overview of structural and functional neuroimaging findings in DLD and highlight consistent patterns of results. Additionally, when appropriate, we link findings to patterns found with other language-impaired populations to provide credence to the structural and functional patterns found in DLD.
2.1. Structural Brain Differences
Across development, the human brain undergoes a wide variety of structural changes in order to support increasing cognitive demands and the acquisition of new skills [19]. The emergence of white matter pathways in the brain begins in utero following formation of the neural tube and production and migration of neurons [20,21]. The brain then continues to differentiate and refine following birth. Infancy and early childhood are periods of rapid brain development, where the myelination of axons and synaptic reorganization and pruning are abundant in order to strengthen neuronal populations that frequently fire together as well as support those neuronal networks associated with new skills [20,21]. As a result of these changes, the gross structure of the brain continues to visibly change within the first few years of life, with subtle decreases in gray matter volume and increases in white matter volume up until early adulthood [22]. Thus, changes in individual neurons as well as neural networks impact gross brain structure across development.
Any perturbations to the tightly orchestrated processes that contribute to brain development can contribute to a range of developmental disorders [23]. In fact, across different neurodevelopmental disorders, there is widespread evidence of volumetric brain differences compared to neurotypical peers [24,25,26,27,28,29]. For example, individuals with ASD have been shown to have whole and regional brain volume differences when compared to age-matched control children. Lange et al. (2015) found that young children with ASD had larger overall brain volumes than their typically developing peers but as they aged, they showed atypical regional volume decreases [30]. Findings such as these underscore the importance of investigating brain differences between typically and atypically developing populations, so that we can begin to uncover how structural (and functional) alterations contribute to language impairment. In the sections that follow, we provide an overview of structural brain differences in DLD starting with global brain volume, moving to a discussion of gray and white matter volume and integrity. It should be noted that the structure of this overview should not be viewed as an annotated summary of findings, but instead presents thematically related information based on the broader categories that were just described.
2.2. Regional Brain Differences
One of the most consistent findings in children with DLD is anomalous gray matter volume and symmetry within the perisylvian language zone. Though across the DLD literature a number of regions within this zone have been discussed [16,17], this section highlights three specific regions which have consistently been shown to have different characteristics in children with DLD as compared to TD children, namely the planum temporale and inferior frontal gyrus (Figure 1a). In addition to these standard language regions, the other region that will be discussed is the caudate nucleus, as it has been theorized to support speech and language processes (Figure 1b).
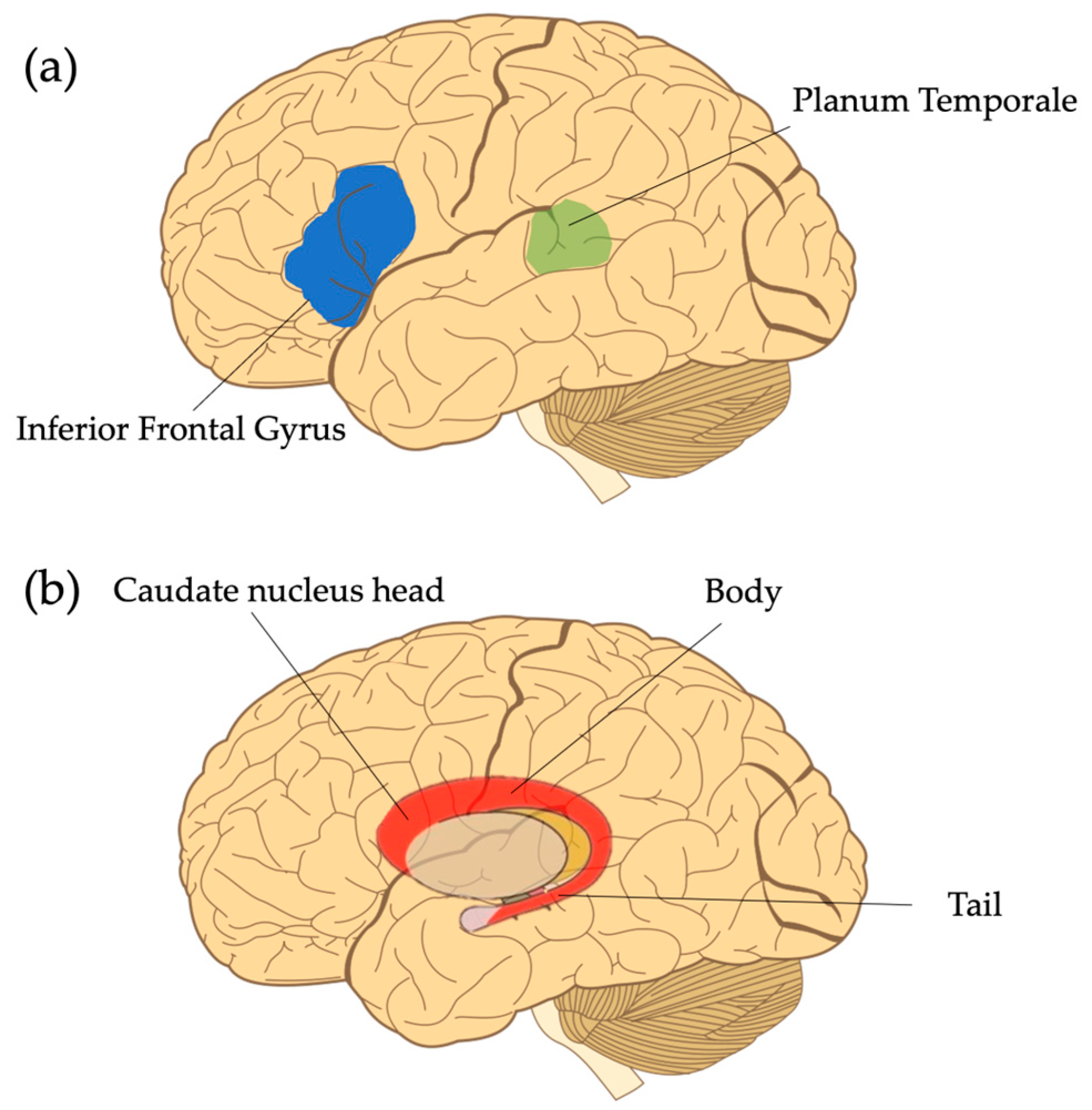
Figure 1. Commonly reported regions of interest in studies investigating structural gray matter differences in DLD. (a) The inferior frontal gyrus and the planum temporale. Note that the transparency of the planum temporal is meant to indicate that it is not visible on the lateral surface of the brain. (b) The caudate nucleus located subcortically. Note that it can be further subdivided into the head, body, and tail, but will be discussed as a whole in the text. Figure adapted from Hugh Guiney, CC BY-SA 3.0, via Wikimedia Commons https://en.m.wikipedia.org/wiki/File:Human-brain.SVG (accessed on 12 September 2023).
2.3. White Matter Pathways
Historically, investigations into brain function have focused on the size and engagement of cortical and subcortical gray matter regions of the brain. However, a missing piece of those investigations are the connections that allow gray matter regions to coordinate activity. Since we know brain regions do not operate in silos, recent technological advances have provided scientists a way to measure the important connections that exist between gray matter regions, known as white matter, which comprises the structural wiring of the brain (Figure 3). Due to the early development of the brain, white matter pathways are already present by 30 weeks of gestation [77]. However, between birth and two years of age, children undergo a period of rapid brain development, highly influenced by genes and the environment, that helps further shape the neural architecture of the brain [20,78]. As children continue to develop, white matter volume continues to increase until around the fourth decade of life to support improvements in cognitive skills [19,79,80].
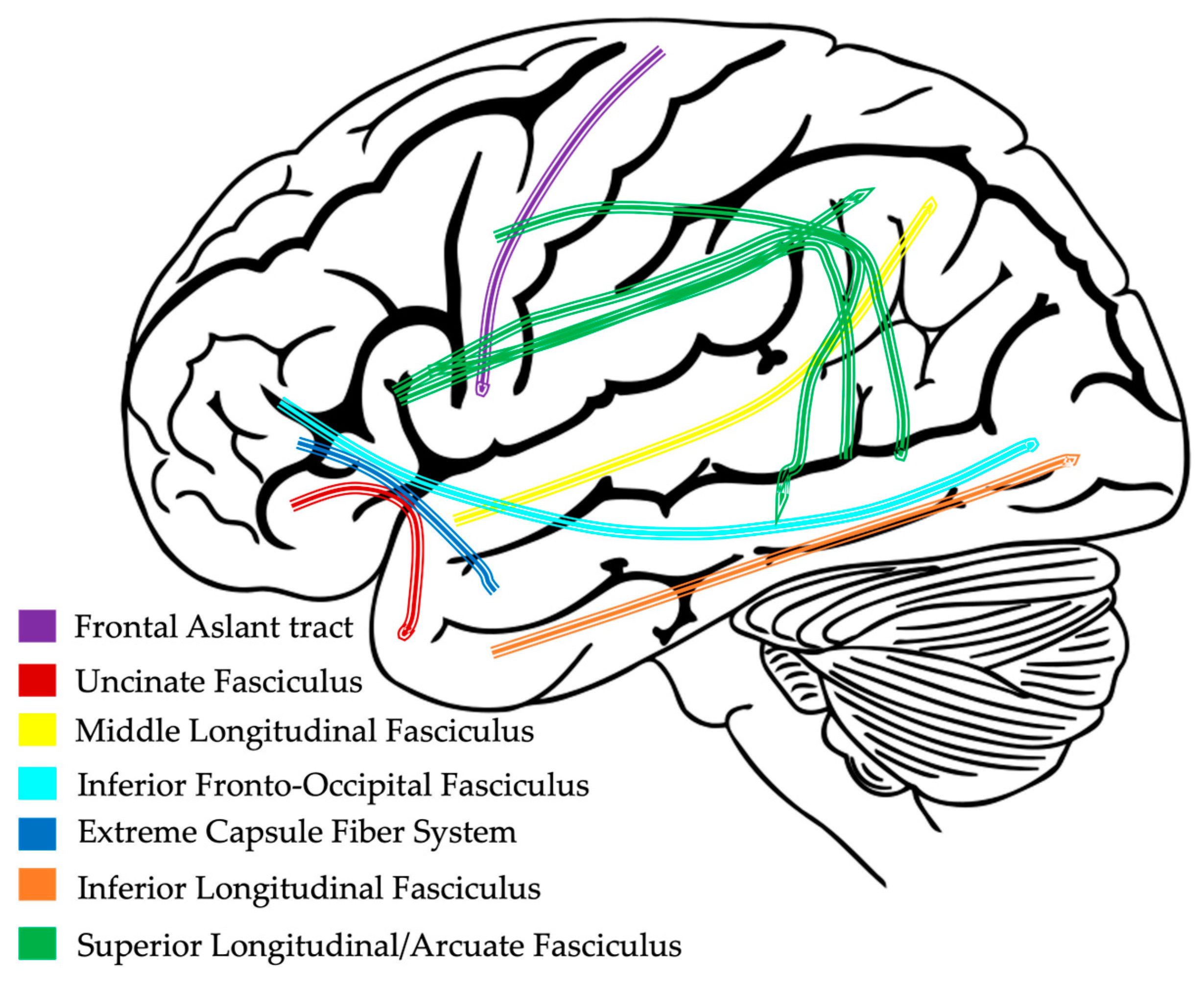
Figure 3. Example of white matter pathways connecting gray matter regions in the left hemisphere.
There is a strong relationship between neural activity associated with new skills and the formation of efficient, myelinated white matter pathways that connect gray matter regions throughout the brain [19]. As a result of this critical interaction, any deviation in the typical formation of white matter pathways will likely contribute to functional impairments [20]. Prior research has demonstrated a link between white matter alterations and language impairment in children with a range of neurodevelopmental disorders, including autism spectrum disorder, dyslexia and other reading disorders, and epilepsy [81,82,83,84]. While research on white matter connectivity in children with DLD is limited, like other neurodevelopmental populations, there seems to be a connection between language impairment and altered white matter volume and diffusivity (movement of water molecules along white matter pathways). Here, we provide an overview of these findings starting with white matter volume changes in DLD as compared to TD and then we turn attention to the diffusivity of white matter tracts involved in language processing within dorsal and ventral regions.
3. Functional Neuroimaging Findings in DLD
Functional magnetic resonance imaging (fMRI) provides researchers (and clinicians) a glimpse into the window of the active brain. The examination of brain activity during specific tasks can inform theories of behavior, in this case, language.
3.1. Functional Magnetic Resonance Imaging (fMRI) Studies
Studies investigating functional brain activity in children with DLD using fMRI vary significantly across the tasks that are used and the age ranges studied. However, the general picture suggests that children with DLD differ across activation levels (hypo- and hyper-activation), locations, and laterality patterns of brain activation when compared to typically developing children during language-related tasks. Since fMRI results are largely based on task demands, it is important to understand that different tasks will produce different patterns of activation. However, the goal here is to highlight consistent patterns of regional activation differences across the broader categories of expressive and receptive tasks (though see for a more detailed review of activation patterns across specific tasks). As will become clear from the discussion below, more studies are needed to not only verify results (particularly across a wider range of tasks) but to also explore whether differences in the level, location, or laterality of activation patterns are due to other factors such as maturational changes (as discussed in prior sections).
3.2. Cerebral Blood Flow Patterns
There is a tight coupling between neuronal activity and increased blood flow to active brain regions (e.g., neurovascular coupling). When neurons are active, they send chemical and electrical signals to blood vessels to dilate which in turn increases the flow of blood and allows an abundance of oxygen and glucose to be delivered to neurons as a fuel source. As neurons consume the influx of oxygen, the blood becomes deoxygenated. It is this change in oxygen content on which the fMRI or blood oxygen level dependent (BOLD) signal is based [105]. However, if the relationship between neural activity and the BOLD response is not accurately modeled via the hemodynamic response function (HRF), then activation patterns may be misinterpreted. Prior research has revealed that the HRF may deviate from the canonical pattern with clinical populations [106]. For example, following a stroke, the amount of time it takes for blood to perfuse neural tissue (i.e., transit delay time) may be longer than normal and the amount of blood that gets delivered to neural tissue may be reduced [99,107,108]. Thus, when conducting fMRI studies with suspected neurologically compromised populations, including neurodevelopmental groups, it is critical that the modeled HRF reflects the true nature of blood flow in the brain in order to extract accurate BOLD signal estimates.
4. Interim Summary: Neuroimaging Patterns in DLD
Thus far, we have highlighted similarities as to the structural and functional differences in DLD as compared to TD across neuroimaging studies, while acknowledging that findings are based on limited evidence and often have contrasting results (likely due to methodological differences). We approached this portion of the overview by reporting converging evidence across structural (whole brain, gray matter, white matter, etc.) and functional measures (task-based activation and cerebral blood flow). The current state of the DLD neuroimaging literature suggests that structurally, when compared to typically developing children, children with DLD have smaller overall brain sizes, they show differences in whole brain and regional gray and white matter volume (potentially mediated by age), and they have altered white matter macro- and micro-structure of language-related tracts. Functionally, they demonstrate differences in the level of brain activation (i.e., hypo- and hyper-activation), the location of activation (i.e., regional differences), and laterality of activation (i.e., left vs. right hemisphere recruitment). They also show differences in the level and lateralization patterns of cerebral blood flow.
Importantly, while using a different approach, we identified similar regions of altered brain structure and function (planum temporale, inferior frontal gyrus, and caudate nucleus) to a prior systematic review of neuroimaging studies in DLD conducted by Mayes and colleagues (2015) [16]. While it is clear from both this overview and the review conducted by Mayes et al. (2015) that altered brain structure and function are important components of DLD pathology, the connection between neuroimaging findings and observed language deficits has been less apparent, as research findings from neuroimaging studies are often reported independently of theoretical research findings. Therefore, it is our goal in this paper to begin to make those connections so that moving forward as a field we can design more theoretically informed neuroimaging studies with more sophisticated linguistic material to better tease apart how aberrant brain structure and function relates to the range of reported language impairments in DLD.
In the next section, we briefly review selected theoretical accounts of DLD, and after each section, we comment on the potential link between the proposed theoretical account and the neuroimaging findings discussed above in an attempt to elucidate brain–behavior relationships.
5. Neuroimaging Evidence Supporting Theoretical Accounts of Language Impairment Patterns in DLD
In order to illustrate how neuroimaging evidence can better inform theoretical accounts of language impairment patterns in DLD, we now discuss some common theories that have been proposed to explain language impairment patterns in DLD.
Across the published studies, there are a range of theoretical accounts that attempt to describe and explain observable language error patterns in children with DLD. While errors are part of typical language development (overgeneralizations, pronoun resolution, etc.), here we refer to error patterns that do not resolve with time [122]. Theories outlining these error patterns can generally be divided into three well represented arguments in the literature that point to deficits specific to (1) linguistic knowledge (e.g., morphosyntax), (2) domain general language processing (e.g., phonological working memory, speech perception, etc.), or (3) non-linguistic cognitive processes associated with language (e.g., working memory, processing speed, etc.) [9]. In isolation, each of these theoretical approaches have merit; however, children with DLD can exhibit both linguistic and non-linguistic impairments, making it difficult to propose a single comprehensive and encompassing theory that can account for hallmark deficits, such as difficulty with grammar, while also explaining other less consistent findings, such as deficits in attention and speed of processing. It is our hope that by making connections between theoretical accounts of DLD and the more consistent neuroimaging findings, that we can point to potential areas to focus future research endeavors. To accomplish these goals, below we present three theoretical accounts of language impairment in DLD. After each account, we integrate the imaging findings described above so as to build a bridge between two seemingly disparate areas.
5.1. Theoretical Approach: Linguistic Knowledge
Deficits in morphology and syntax (i.e., morphosyntax) are ubiquitous in children diagnosed with DLD. These observations have led to proposals suggesting that language deficits stem from limitations in linguistic knowledge (e.g., tense marking rules, phrase structure rules, movement, etc.; . One of the earliest among the agreement, tense, and number marking accounts is the Extended Optional Infinitive (EOI) account [123]. According to Wexler (1994), around 4–5 years of age, typically developing children undergo a stage by which they optionally mark the tense and number on finite verbs in main clauses (e.g., she drinks coffee can optionally be *she drink coffee; the asterisk represents an ungrammatical utterance) [124]. In cases where tense/number is not marked, children tend to produce the infinitive form of the verb (i.e., drink). Building off of Wexler’s account, Rice et al. (1995) suggested that children with DLD remain in this optional infinitive stage for a period of time that extends beyond that of a typically developing child, before their grammatical usage catches up to that of an adult (if it does at all; see [125]).
The EOI account works well to characterize error patterns made by children diagnosed with DLD when speaking languages such as English and French, but it fails to account for the grammatical errors made by children who use other languages such as Italian and Spanish due to cross-linguistic differences in syntactic structure. Thus, a number of theoretical accounts followed which expanded on the EOI premise that children with DLD struggle with aspects of tense and agreement (see [125,126,127]). However, evidence has shown that children with DLD are not limited to errors in just tense and/or agreement as these accounts suggest, and thus, this theory underspecifies observed errors. To address this issue of limited scope, other accounts focused on structural complexity, such as the Representational Deficit for Dependent Relations (RDDR), suggesting that deficits stem from difficulty with performing these complex operations. One example would be the movement of a wh-question word (e.g., who) to the front of a sentence to form a question [122,128,129]. Though accounts such as the RDDR cover a wider range of deficits, especially across languages, they lack specificity, particularly concerning how and in which instances children with DLD struggle with complex operations. Further, the connection between deficits across different linguistic domains (i.e., syntax, morphology, and phonology) needs clarification since it is unclear how deficits in processing complex syntactic structures would also result in other deficits discussed in the sections below.
Other theories that focus on the application of rules, like the Narrow Rule Learning account [130], posit that children with DLD tend to stick to structures that have a high number of exemplars in the language, which may help bridge theoretical gaps, but no single linguistic theory can explain all of the inconsistencies in deficits across linguistic domains [122]. In addition, the majority of linguistic accounts have focused on observable production errors in individuals with DLD, which limits the ability to generalize to language deficits as a whole.
5.2. Theoretical Approach: Language Processing Accounts
Linguistic-based accounts of DLD, such as those described above, benefit from being able to explain specific error patterns in language use; however, they often fail to encompass the wide range of deficits across languages. Further, they may not accurately reflect how a child with DLD processes (as opposed to produces) language. Therefore, some theories posit that language impairment stems from aberrant processing in domain general language systems that impact language use . Among these theories are those that suggest that children with DLD have general auditory processing deficits. In a seminal series of studies in the 1970s, Tallal and Piercy [136,137], found that children with language impairment (those diagnosed with DLD and those with hearing impairments) performed worse than TD control children on a variety of tone and speech perception tasks that included categorization, discrimination, and temporal sequencing of auditory information. Children with DLD in particular seemed to struggle with auditory stimuli that were presented briefly or in rapid succession, which led the authors to suggest that the source of deficit stems from difficulties with temporal processing at the phoneme level.
In an attempt to expand on Tallal and colleague’s supposition of an underlying temporal processing deficit, Schwartz, Scheffler, and Lopez (2013) argued that children with DLD have deficits in perceptual processing, specifically, categorical perception and the use of perceptual speech cues, which in turn affects storage and access to lexical representations [138]. Though these perceptual accounts can be compelling, one point of weakness is in describing how they can lead to disruptions of grammatical processing as opposed to just lexical or phonological processing. Leonard, McGregor, and Allen (1992) attempted to address this limitation by proposing the Surface Account, which posited that the complex operations required to process grammatical markers taxes the system resulting in incomplete processing of grammatical morphemes [139]. It is argued that this difficulty is then further amplified by the low perceptual saliency of grammatical morphemes, which increases the amount of exposure needed to learn them. However, evidence in support of this theory is mixed (see [122] for a review); thus, as it stands, more work needs to be carried out to understand if and how these processing differences result in the range of deficits observed.
Phonological short-term memory (also referred to as phonological working memory) is another commonly cited source of language difficulty for children with DLD. Different models of how language is stored have been proposed, but the most prominent view of phonological short-term memory comes from Baddeley and colleagues [140,141,142]. Based on the model, Gathercole and Baddeley (1989) proposed that phonological short-term memory supports vocabulary development by helping to form stable phonological representations [143]. This proposal was supported by findings that demonstrated a relationship between poor nonword repetition skills and smaller vocabularies in children with DLD, indicating that they struggle to form and store stable phonological representations [144]. Thus, nonword repetition tasks have become a consistent and reliable measure for characterizing what many have suggested are phonological short-term memory impairments in children with DLD, but again, these theories are unable to account for observable impairments at the syntactic level during production and they tend to lack explanatory power for comprehension impairments [145]. Though there are processing theories that extend beyond the phoneme and word-level, the majority of studies have focused on this level. Thus, to better account for the full range of deficits observed in children with DLD, it is suggested that researchers look to other processing theories that have been proposed for other language-impaired populations as they may inform a larger range of observed deficits [146,147].
5.3. Theoretical Approach: Non-Linguistic Cognitive Processing
The notion that children with DLD have broader processing limitations beyond language has received considerable attention in the DLD literature . In looking at the literature from other well-studied language-impaired populations, namely individuals with aphasia, it has been proposed that linguistic knowledge remains intact in these individuals, but the ability to tap into the cognitive resources necessary to build the representations or perform complex operations with them are impaired (though see [151] for an opposing view) [152]. Studies investigating these limitations generally focus on aspects of working memory, such as resource allocation capacity and attention. Within the DLD literature, similar proposals have been investigated.
For example, Montgomery (2000) studied the ability of children with DLD to allocate resources during a word recall task [153]. Based on the assumptions of the working memory model proposed by Just and Carpenter (1992), the premise of their study was that people have a limited pool of resources by which they can process and store incoming information and children with DLD have even greater reductions in processing capacity [154]. They found that children with DLD could complete simple processing tasks, but as the task complexity increased (i.e., dual-load tasks where participants had to sort items by size and semantic category), performance worsened compared to controls, suggesting that children with DLD have reduced resources (e.g., processing capacities), which constrained the ability to successfully perform multiple, simultaneous cognitive operations. One big limitation of processing capacity theories is that it is not always clear what the “resource” being allocated is, though it is often conceived as an attentional one [141]. The problem with this is that children with DLD do not always demonstrate impairments in attention, or if they do, it tends to be situational. Further, it is challenging to design studies that can be falsified given that processing capacity is difficult to distinguish and measure.
Other non-linguistic cognitive theories have suggested that children with DLD are slower to perform a wide range of linguistic and non-linguistic tasks based on evidence from reaction time studies [122]. One early account for reduced processing speeds was the Generalized Slowing Hypothesis, which posits that children with DLD perform more slowly on both linguistic and non-linguistic tasks when compared to typically developing peers, and this difference is proportional to the complexity (i.e., the number of operations) required by the task [155]. Like with attentional studies, the problem with this hypothesis is that not all children with DLD show slowed reaction times on non-linguistic tasks, and those that do seem to do so selectively across different tasks [156]. Therefore, more work is needed to understand what causes some children to have slower processing speeds than others, as well as whether it affects broader, domain general neural systems or more specific ones.
One final group of domain-general cognitive theories discussed here is related to impaired learning systems [72]. The most comprehensive account of learning deficits in DLD is the Procedural Deficit Hypothesis (PDH) [73]. According to the PDH, children with DLD have a deficit in the procedural memory system. This system is comprised of frontal and subcortical brain regions that support the learning of linguistic, motor, and other non-linguistic sequences. The PDH posits that aberrant function of the procedural system can lead to impairments in any of the systems associated with it; thus, the PDH attempts to account for the wide range of linguistic and non-linguistic impairments reported in the DLD literature. It also makes a distinction between procedural and declarative systems. The procedural system is thought to underlie the acquisition and use of rule-governed grammatical computations, while the declarative system is thought to underlie storage of lexical knowledge, such as semantic features and the memorization of more arbitrary, word-specific information like irregular past-tense verb forms. Research investigating sequence-based procedural learning tasks has found that children with DLD demonstrate impairments in this realm but are less likely to show deficits in non-sequenced based declarative tasks [72]. However, Ullman and Pierpoint (2005) point out in their model that the procedural and declarative systems are not entirely independent of one another due to their connections with other systems, such as those involved in working memory, so it is possible for children with DLD to show deficits in processes supported by both systems [73]. This becomes particularly relevant when trying to account for smaller vocabularies in children with DLD, since the declarative system likely supports this process.
Though the PDH provides a nice framework for capturing one of the most striking impairments in DLD (grammatical errors), more work needs to be done to refine the hypothesis, particularly in terms of the functional boundaries between the procedural and declarative systems. Further, this model provides one of the most comprehensive accounts for the varied neuroimaging findings, but in an effort to account for such a broad range of deficits, the model posits that any deficit can be explained by the procedural system or connections to it, with limited evidence to support these claims.
5.4. Interim Summary: Linking Theory to Brain
There are a number of different accounts that have been proposed in an attempt to explain patterns of language impairment in children with DLD. We divided these theories into three well-represented categories, namely deficits in linguistic knowledge, language processing, and non-linguistic cognitive processing, and briefly discussed evidence supporting each. Each of these theories does well at capturing specific measured behaviors that differ in DLD compared to typically developing peers, but when zooming out more broadly to examine the range of behaviors reported (e.g., tense omission, poor phonological awareness, slower processing speed, etc.), there are a number of competing accounts that in isolation are not able to explain the broad scope of impairments in DLD. On one end of the theoretical continuum, the linguistic accounts lack explanatory power and are too narrow in scope, especially since most tend to focus on word-level, production errors; and on the other end, the more general non-linguistic processing accounts are too broad, as they cannot explain why language (and grammar) is affected above and beyond other cognitive systems in DLD if the deficit is in a domain-general system. One possibility is that DLD is a spectrum disorder (like autism) with different phenotypes [11]. If this is the case, neuroimaging evidence could help with the identification of different phenotypic groups by linking specific neural profiles to behavioral patterns. This would require much larger datasets than are typical in DLD research and would be most informative with longitudinal designs.
Alternatively, another approach could be to examine language abilities that are more commonly affected in children with DLD as a starting point to elucidate the relationship between altered brain structure/function and variable language profiles. For example, difficulty with the use of grammatical features of language and poor performance on nonword repetition tasks are common in DLD. Both of these abilities in typically developing individuals have been linked to the inferior frontal gyrus (IFG) [43,130,131]. In a recent study by Bahar and colleagues (2023) with children with DLD, the authors found that performance on a nonword repetition task was a significant predictor of surface area within the left IFG [41,46,133,134]. It may be the case that alterations within the left IFG are linked to difficulties with both of these language tasks. Further, given that the IFG is a densely connected hub within the language network, abnormal development of the region could impact other connected regions, such as the superior temporal sulcus. This would help explain empirical evidence indicating that language deficits exist across different language domains, as certain deficits could be linked back to a common neural substrate, and variations beyond those deficits could potentially be linked to alterations in structural and functional connections with other regions.
One other possibility is that the underlying neurobiological mechanisms supporting brain development are impacted in DLD. Recent work from Bahar et al. (2023) and Krishnan et al. (2022) is beginning to explore this area by investigating the underlying properties of brain tissue [41,74]. For example, as mentioned previously, Krishnan et al. (2022) measured gray matter myelin content in children and adolescence with DLD and found less myelin in the caudate nucleus and left inferior frontal gyrus. Together, these brain regions partially comprise the corticostriatal loop, which plays a role in sequential learning tasks such as learning the grammatical rules of a language. Based on their findings, the authors posited that children with DLD have difficulty learning the complex rules of language, as evinced by altered myelin content within this language learning circuitry (though they do not speculate on the causal direction of the relationship between reduced myelin and language abilities). Typical myelin development begins in utero and is subject to a number of carefully timed genetic processes that slowly give way to more environmental influences postnatally [76]. Thus, a reduction in myelin content could be related to specific genetic markers early on in DLD. Then, as a child continues to develop, experience could have a greater impact on the development of higher-order cognitive functions, like language learning. This idea is supported by the fact that myelination within the brain regions associated with these higher-order functions takes longer in comparison to sensory and motor regions to develop. This could help provide support for more domain-general theories, like the Procedural Deficit Hypothesis or the Generalized Slowing Hypothesis mentioned in Section 5.3 and Section 5.4. Unlike sensory and motor cortices, the brain regions involved with language processes take longer to “mature”; thus, early alterations to the underlying properties supporting brain function could have a detrimental impact on later-developing language network architecture, thus resulting in the range of language impairments that we observe in DLD.
6. Discussion: The Current State (and Limitations) in Linking Theory to Brain
It is clear that more work needs to be conducted to obtain a better sense of the neural organization and networks engaged in language processes in children diagnosed with DLD. Overall, the current state of the field suggests that children with DLD have atypical brain volume, laterality, and activation/connectivity patterns compared to their neurotypical peers. Behaviorally, one of the most striking impairments is in the production of grammatical morphemes, but research has demonstrated impairments in a range of other linguistic and non-linguistic tasks, such as vocabulary development, nonword repetition, and short-term memory (though it should be noted that it is difficult to create purely non-verbal short-term memory tasks). In terms of neuroimaging research, across both structural and functional brain studies, the planum temporale (located in the posterior superior temporal gyrus), the inferior frontal gyrus, and the caudate nucleus consistently show altered patterns in children with DLD when compared to typically developing children. Taken together, it is reasonable to speculate that atypical language development in DLD is not an environmental side effect but is in fact related to anomalous development of brain structures and/or function. However, few studies have attempted to link theoretical accounts of language impairment in DLD to neuroimaging findings, resulting in two disparate bodies of literature.
The goal of this overview was to synthesize the literature in a way that could help lay the groundwork for more theoretically motivated neuroimaging research. Though the connections made here are purely speculative based on the evidence presented, they do align well with the recent literature demonstrating structural and functional differences in the corticostriatal pathways that support language learning [41,72,74,159]. These recent studies utilize more consistent methodological approaches to make connections between altered brain structure and theoretical accounts of DLD, and they explore the underlying properties that support broader measures of structural brain development beyond brain volume (i.e., myelin, surface area, cortical thickness, etc.). This is important because prior neuroimaging research with children with DLD rarely overlaps in methodological approaches and often focuses on these gross brain measures, making it difficult to draw conclusions about the underlying contributors of altered brain structure or function in DLD. At this point, more research with larger sample sizes and carefully defined participant criteria is needed to replicate previous neuroimaging findings and build on our current knowledge of language impairment patterns in DLD. By laying a foundation for more theoretically motivated neuroimaging research, we may be able to better link neural differences to specific language profiles that align with current theoretical accounts of language deficits in DLD.
7. Future Directions: New Approaches in Linking Theory to Brain
There were a number of ways to craft this overview. We chose to categorize aspects of studies based on a variety of measures used to evaluate brain structure (i.e., whole brain volume, gray matter volume, white matter diffusivity, etc.) and brain function (i.e., task-based activation and cerebral blood flow). Our goal was to present converging evidence about neural regions implicated in DLD in order to link theory to brain. Importantly, using this approach, we found a similar pattern of results to a systematic review conducted by Mayes and colleagues (2015) in which they divided neuroimaging studies in DLD by the methodology employed (e.g., semi-automatic morphometry, voxel-based morphometry, etc.). Similarly to our findings, Mayes et al. (2015) reported that the posterior superior temporal gyrus, the inferior frontal gyrus, and the caudate are implicated in DLD pathology [16]. Given the consistencies across these reviews and more recent studies in DLD, future research will benefit from exploring the relationship between altered structure/function of these brain regions and performance on language tasks that are linked to activation within those regions.
A problem discussed throughout this overview, though, is the lack of consistency across studies in terms or the direction of volumetric differences (e.g., larger in the left hemisphere, smaller in the right, etc.) and the location and level of brain activation in the three brain regions frequently implicated in language impairment in DLD. One possibility is that broad measures of brain structure (i.e., volume) and brain function may overshadow important underlying properties that contribute to these gross measures. For example, as previously mentioned, Bahar et al. (2023) demonstrated that altered brain volume in DLD was largely driven by differences in surface area rather than cortical thickness, which is noteworthy as surface area and cortical thickness follow distinct developmental trajectories and are likely driven by distinct neurobiological mechanisms [41]. By exploring the underlying properties that index developmental changes, it can help to better account for inconsistent findings within the literature.
To date, one area of neuroimaging research that has received little attention is the processes underlying neuronal function and connectivity, particularly within the language network. Research from another language-impaired population, individuals with aphasia, has demonstrated that reduced cerebral blood flow to middle temporal regions correlates with auditory comprehension impairments, despite these regions appearing structurally uncompromised by the lesion [99]. It may be the case that important language regions, such as the left inferior frontal network, are hindered during development due to aberrant blood flow, underconnectivity, etc., resulting in those consistently observed morphosyntactic deficits in DLD. By examining distributed networks and the processes that underlie neuronal function (e.g., cerebral blood flow, glucose metabolism, etc.) in children with DLD (as compared to neurotypically developing children) it may help to further bridge theoretical accounts as well as aid in the identification of potential biomarkers of DLD.
References
- Tomblin, J.B.; Records, N.L.; Buckwalter, P.; Zhang, X.; Smith, E.; O’Brien, M. Prevalence of Specific Language Impairment in Kindergarten Children. J. Speech Lang. Hear. Res. 1997, 40, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.F.; Gooch, D.; Wray, C.; Baird, G.; Charman, T.; Simonoff, E.; Vamvakas, G.; Pickles, A. The impact of nonverbal ability on prevalence and clinical presentation of language disorder: Evidence from a population study. J. Child Psychol. Psychiatry 2016, 57, 1247–1257. [Google Scholar] [CrossRef]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.; Hollis, C.; Mawhood, L.; Rutter, M. Developmental language disorders—A follow-up in later adult life. Cognitive, language and psychosocial outcomes. J. Child Psychol. Psychiatry 2005, 46, 128–149. [Google Scholar] [CrossRef]
- Maggio, V.; Grañana, N.E.; Richaudeau, A.; Torres, S.; Giannotti, A.; Suburo, A.M. Behavior problems in children with specific language impairment. J. Child Neurol. 2014, 29, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Sansavini, A.; Favilla, M.E.; Guasti, M.T.; Marini, A.; Millepiedi, S.; Di Martino, M.V.; Vecchi, S.; Battajon, N.; Bertolo, L.; Capirci, O.; et al. Developmental Language Disorder: Early Predictors, Age for the Diagnosis, and Diagnostic Tools. A Scoping Review. Brain Sci. 2021, 11, 654. [Google Scholar] [CrossRef]
- Bishop, D.V. Cerebral asymmetry and language development: Cause, correlate, or consequence? Science 2013, 340, 1230531. [Google Scholar] [CrossRef]
- Schwartz, R.G. Handbook of Child Language Disorders, 2nd ed.; Psychology Press: London, UK, 2017. [Google Scholar] [CrossRef]
- Bishop, D.V.; Norbury, C.F. Exploring the borderlands of autistic disorder and specific language impairment: A study using standardised diagnostic instruments. J. Child Psychol. Psychiatry 2002, 43, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, H.S.; Camarata, S. Reconceptualizing developmental language disorder as a spectrum disorder: Issues and evidence. Int. J. Lang. Commun. Disord. 2019, 54, 79–94. [Google Scholar] [CrossRef]
- Tager-Flusberg, H. Do autism and specific language impairment represent overlapping language disorders? In Developmental Language Disorders; Psychology Press: London, UK, 2004; pp. 42–63. [Google Scholar]
- Pena, E.D.; Spaulding, T.J.; Plante, E. The composition of normative groups and diagnostic decision making: Shooting ourselves in the foot. Am. J. Speech Lang. Pathol. 2006, 15, 247–254. [Google Scholar] [CrossRef]
- Spaulding, T.J.; Plante, E.; Farinella, K.A. Eligibility criteria for language impairment. Lang. Speech Hear. Serv. Sch. 2006, 37, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.; Snowling, M.J.; Thompson, P.A.; Greenhalgh, T.; Consortium, C.; Adams, C.; Archibald, L.; Baird, G.; Bauer, A.; Bellair, J. Phase 2 of CATALISE: A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. J. Child Psychol. Psychiatry 2017, 58, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Mayes, A.K.; Reilly, S.; Morgan, A.T. Neural correlates of childhood language disorder: A systematic review. Dev. Med. Child Neurol. 2015, 57, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Brown, T.T. Specific language impairment. In Neurobiology of Language; Elsevier: Amsterdam, The Netherlands, 2016; pp. 899–912. [Google Scholar]
- Webster, R.I.; Shevell, M.I. Topical review: Neurobiology of specific language impairment. J. Child Neurol. 2004, 19, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Deoni, S. The development of brain white matter microstructure. Neuroimage 2018, 182, 207–218. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Yap, Q.J.; Teh, I.; Fusar-Poli, P.; Sum, M.Y.; Kuswanto, C.; Sim, K. Tracking cerebral white matter changes across the lifespan: Insights from diffusion tensor imaging studies. J. Neural Transm. 2013, 120, 1369–1395. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Gamst, A.C. Changes in volume with age—Consistency and interpretation of observed effects. Neurobiol. Aging 2005, 26, 1271–1274. [Google Scholar] [CrossRef]
- Pirozzi, F.; Nelson, B.; Mirzaa, G. From microcephaly to megalencephaly: Determinants of brain size. Dialogues Clin. Neurosci. 2018, 20, 267–282. [Google Scholar] [CrossRef]
- Bayard, F.; Nymberg Thunell, C.; Abé, C.; Almeida, R.; Banaschewski, T.; Barker, G.; Bokde, A.L.W.; Bromberg, U.; Büchel, C.; Quinlan, E.B.; et al. Distinct brain structure and behavior related to ADHD and conduct disorder traits. Mol. Psychiatry 2020, 25, 3020–3033. [Google Scholar] [CrossRef] [PubMed]
- Brieber, S.; Neufang, S.; Bruning, N.; Kamp-Becker, I.; Remschmidt, H.; Herpertz-Dahlmann, B.; Fink, G.R.; Konrad, K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 2007, 48, 1251–1258. [Google Scholar] [CrossRef]
- Hasan, K.M.; Molfese, D.L.; Walimuni, I.S.; Stuebing, K.K.; Papanicolaou, A.C.; Narayana, P.A.; Fletcher, J.M. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR Biomed. 2012, 25, 1263–1270. [Google Scholar] [CrossRef]
- Krain, A.L.; Castellanos, F.X. Brain development and ADHD. Clin. Psychol. Rev. 2006, 26, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Nickl-Jockschat, T.; Habel, U.; Maria Michel, T.; Manning, J.; Laird, A.R.; Fox, P.T.; Schneider, F.; Eickhoff, S.B. Brain structure anomalies in autism spectrum disorder-a meta-analysis of VBM studies using anatomic likelihood estimation. Hum. Brain Mapp. 2012, 33, 1470–1489. [Google Scholar] [CrossRef]
- Xia, Z.; Hoeft, F.; Zhang, L.; Shu, H. Neuroanatomical anomalies of dyslexia: Disambiguating the effects of disorder, performance, and maturation. Neuropsychologia 2016, 81, 68–78. [Google Scholar] [CrossRef]
- Lange, N.; Travers, B.G.; Bigler, E.D.; Prigge, M.B.D.; Froehlich, A.L.; Nielsen, J.A.; Cariello, A.N.; Zielinski, B.A.; Anderson, J.S.; Fletcher, P.T.; et al. Longitudinal Volumetric Brain Changes in Autism Spectrum Disorder Ages 6–35 Years. Autism Res. 2015, 8, 82–93. [Google Scholar] [CrossRef]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain charts for the human lifespan. Nature 2022, 604, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Gauger, L.M.; Lombardino, L.J.; Leonard, C.M. Brain morphology in children with specific language impairment. J. Speech Lang. Hear. Res. 1997, 40, 1272–1284. [Google Scholar] [CrossRef]
- Girbau-Massana, D.; Garcia-Marti, G.; Marti-Bonmati, L.; Schwartz, R.G. Gray–white matter and cerebrospinal fluid volume differences in children with specific language impairment and/or reading disability. Neuropsychologia 2014, 56, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Filipek, P.A.; Kemper, T.L.; Normandin, J.J.; Sanders, H.A.; Kennedy, D.N.; Caviness, V.S., Jr. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004, 55, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Nopoulos, P.C.; Tomblin, J.B. Abnormal subcortical components of the corticostriatal system in young adults with DLI: A combined structural MRI and DTI study. Neuropsychologia 2013, 51, 2154–2161. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Bakardjiev, A.; Hodgson, J.; Adrien, K.T.; Kennedy, D.N.; Filipek, P.A.; Caviness, V.S., Jr. Larger brain and white matter volumes in children with developmental language disorder. Dev. Sci. 2003, 6, F11–F22. [Google Scholar] [CrossRef]
- Soriano-Mas, C.; Pujol, J.; Ortiz, H.; Deus, J.; López-Sala, A.; Sans, A. Age-related brain structural alterations in children with specific language impairment. Hum. Brain Mapp. 2009, 30, 1626–1636. [Google Scholar] [CrossRef]
- Badcock, N.A.; Bishop, D.V.; Hardiman, M.J.; Barry, J.G.; Watkins, K.E. Co-localisation of abnormal brain structure and function in specific language impairment. Brain Lang. 2012, 120, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Carper, R.A.; Moses, P.; Tigue, Z.D.; Courchesne, E. Cerebral Lobes in Autism: Early Hyperplasia and Abnormal Age Effects. NeuroImage 2002, 16, 1038–1051. [Google Scholar] [CrossRef]
- Wierenga, L.M.; Langen, M.; Oranje, B.; Durston, S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage 2014, 87, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bahar, N.; Cler, G.J.; Krishnan, S.; Asaridou, S.S.; Smith, H.J.; Willis, H.E.; Healy, M.P.; Watkins, K.E. Differences in cortical surface area in developmental language disorder. bioRxiv 2023. [Google Scholar] [CrossRef]
- White, T.; Su, S.; Schmidt, M.; Kao, C.-Y.; Sapiro, G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010, 72, 36–45. [Google Scholar] [CrossRef]
- Fedorenko, E.; Thompson-Schill, S.L. Reworking the language network. Trends Cogn. Sci. 2014, 18, 120–126. [Google Scholar] [CrossRef]
- Friederici, A.D.; Chomsky, N.; Berwick, R.C.; Moro, A.; Bolhuis, J.J. Language, mind and brain. Nat. Hum. Behav. 2017, 1, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Hertrich, I.; Dietrich, S.; Ackermann, H. The margins of the language network in the brain. Front. Commun. 2020, 5, 519955. [Google Scholar] [CrossRef]
- Price, C.J. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010, 1191, 62–88. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Friedrich, P.; Fraenz, C.; Schlüter, C.; Beste, C.; Güntürkün, O.; Genç, E. Neurite architecture of the planum temporale predicts neurophysiological processing of auditory speech. Sci. Adv. 2018, 4, eaar6830. [Google Scholar] [CrossRef] [PubMed]
- Galuske, R.A.; Schlote, W.; Bratzke, H.; Singer, W. Interhemispheric asymmetries of the modular structure in human temporal cortex. Science 2000, 289, 1946–1949. [Google Scholar] [CrossRef]
- Dorsaint-Pierre, R.; Penhune, V.B.; Watkins, K.E.; Neelin, P.; Lerch, J.P.; Bouffard, M.; Zatorre, R.J. Asymmetries of the planum temporale and Heschl’s gyrus: Relationship to language lateralization. Brain 2006, 129, 1164–1176. [Google Scholar] [CrossRef]
- Foundas, A.L.; Leonard, C.M.; Gilmore, R.; Fennell, E.; Heilman, K.M. Planum temporale asymmetry and language dominance. Neuropsychologia 1994, 32, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N.; Levitsky, W. Human brain: Left-right asymmetries in temporal speech region. Science 1968, 161, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C.; Camou, S.L.; Reite, M.L.; Rogers, S.J. Planum temporale volume in children and adolescents with autism. J. Autism Dev. Disord. 2005, 35, 479–486. [Google Scholar] [CrossRef]
- Eckert, M.A.; Leonard, C.M. Structural imaging in dyslexia: The planum temporale. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Plante, E.; Swisher, L.; Vance, R.; Rapcsak, S. MRI findings in boys with specific language impairment. Brain Lang. 1991, 41, 52–66. [Google Scholar] [CrossRef]
- Cohen, M.; Campbell, R.; Yaghmai, F. Neuropathological abnormalities in developmental dysphasia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1989, 25, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Galaburda, A.M.; Sherman, G.F.; Rosen, G.D.; Aboitiz, F.; Geschwind, N. Developmental dyslexia: Four consecutive patients with cortical anomalies. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1985, 18, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, T.L.; Hesselink, J.R.; Sowell, E.; Tallal, P.A. Cerebral structure on magnetic resonance imaging in language-and learning-impaired children. Arch. Neurol. 1991, 48, 539–545. [Google Scholar] [CrossRef]
- Preis, S.; Jäncke, L.; Schittler, P.; Huang, Y.; Steinmetz, H. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia 1998, 36, 849–855. [Google Scholar] [CrossRef]
- De Fossé, L.; Hodge, S.M.; Makris, N.; Kennedy, D.N.; Caviness, V.S.; McGrath, L.; Steele, S.; Ziegler, D.A.; Herbert, M.R.; Frazier, J.A.; et al. Language-association cortex asymmetry in autism and specific language impairment. Ann. Neurol. 2004, 56, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Rogalsky, C.; Matchin, W.; Hickok, G. Broca’s area, sentence comprehension, and working memory: An fMRI study. Front. Hum. Neurosci. 2008, 2, 237. [Google Scholar] [CrossRef]
- Grodzinsky, Y. The neurology of syntax: Language use without Broca’s area. Behav. Brain Sci. 2000, 23, 1–21. [Google Scholar] [CrossRef]
- Martin, R.C. Language processing: Functional organization and neuroanatomical basis. Annu. Rev. Psychol. 2003, 54, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.M.; Prejawa, S.; Parker Jones, Ō.; Oberhuber, M.; Seghier, M.L.; Green, D.W.; Price, C.J. Dissecting the functional anatomy of auditory word repetition. Front. Hum. Neurosci. 2014, 8, 246. [Google Scholar] [CrossRef]
- Lee, J.C.; Dick, A.S.; Tomblin, J.B. Altered brain structures in the dorsal and ventral language pathways in individuals with and without developmental language disorder (DLD). Brain Imaging Behav. 2020, 14, 2569–2586. [Google Scholar] [CrossRef]
- Watkins, K.E.; Vargha-Khadem, F.; Ashburner, J.; Passingham, R.E.; Connelly, A.; Friston, K.J.; Frackowiak, R.S.; Mishkin, M.; Gadian, D.G. MRI analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain 2002, 125, 465–478. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.; O’Brien, L.M.; Kennedy, D.N.; Filipek, P.; Bakardjiev, A.; Hodgson, J.; Takeoka, M.; Makris, N. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain 2005, 128, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.R.; Wood, L.; Lu, D.; Houk, J.C.; Bitan, T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007, 1133, 136–144. [Google Scholar] [CrossRef]
- Crosson, B.; Benefield, H.; Cato, M.A.; Sadek, J.R.; Moore, A.B.; Wierenga, C.E.; Gopinath, K.; Soltysik, D.; Bauer, R.M.; Auerbach, E.J.; et al. Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. J. Int. Neuropsychol. Soc. 2003, 9, 1061–1077. [Google Scholar] [CrossRef]
- Tan, A.P.; Ngoh, Z.M.; Yeo, S.S.P.; Koh, D.X.P.; Gluckman, P.; Chong, Y.S.; Daniel, L.M.; Rifkin-Graboi, A.; Fortier, M.V.; Qiu, A.; et al. Left lateralization of neonatal caudate microstructure affects emerging language development at 24 months. Eur. J. Neurosci. 2021, 54, 4621–4637. [Google Scholar] [CrossRef]
- Thibault, S.; Py, R.; Gervasi, A.M.; Salemme, R.; Koun, E.; Lövden, M.; Boulenger, V.; Roy, A.C.; Brozzoli, C. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science 2021, 374, eabe0874. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Marzinzik, F.; Friederici, A.D.; Hahne, A.; Kupsch, A.; Schneider, G.-H.; Saddy, D.; Curio, G.; Klostermann, F. The Human Thalamus Processes Syntactic and Semantic Language Violations. Neuron 2008, 59, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Watkins, K.E.; Bishop, D.V.M. Neurobiological Basis of Language Learning Difficulties. Trends Cogn. Sci. 2016, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Ullman, M.T.; Pierpont, E.I. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex 2005, 41, 399–433. [Google Scholar] [CrossRef]
- Krishnan, S.; Cler, G.J.; Smith, H.J.; Willis, H.E.; Asaridou, S.S.; Healy, M.P.; Papp, D.; Watkins, K.E. Quantitative MRI reveals differences in striatal myelin in children with DLD. eLife 2022, 11, e74242. [Google Scholar] [CrossRef]
- Corrigan, N.M.; Yarnykh, V.L.; Hippe, D.S.; Owen, J.P.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Myelin development in cerebral gray and white matter during adolescence and late childhood. Neuroimage 2021, 227, 117678. [Google Scholar] [CrossRef]
- Timmler, S.; Simons, M. Grey matter myelination. Glia 2019, 67, 2063–2070. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, Y.; He, Y. Graph theoretical modeling of baby brain networks. NeuroImage 2019, 185, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Dehaene-Lambertz, G.; Kulikova, S.; Poupon, C.; Hüppi, P.S.; Hertz-Pannier, L. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 2014, 276, 48–71. [Google Scholar] [CrossRef]
- Lebel, C.; Gee, M.; Camicioli, R.; Wieler, M.; Martin, W.; Beaulieu, C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage 2012, 60, 340. [Google Scholar] [CrossRef]
- Lebel, C.; Treit, S.; Beaulieu, C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019, 32, e3778. [Google Scholar] [CrossRef]
- Kaestner, E.; Balachandra, A.R.; Bahrami, N.; Reyes, A.; Lalani, S.J.; Macari, A.C.; Voets, N.L.; Drane, D.L.; Paul, B.M.; Bonilha, L. The white matter connectome as an individualized biomarker of language impairment in temporal lobe epilepsy. NeuroImage Clin. 2020, 25, 102125. [Google Scholar] [CrossRef]
- Langer, N.; Peysakhovich, B.; Zuk, J.; Drottar, M.; Sliva, D.D.; Smith, S.; Becker, B.L.; Grant, P.E.; Gaab, N. White matter alterations in infants at risk for developmental dyslexia. Cereb. Cortex 2017, 27, 1027–1036. [Google Scholar] [CrossRef]
- Olivé, G.; Slušná, D.; Vaquero, L.; Muchart-López, J.; Rodríguez-Fornells, A.; Hinzen, W. Structural connectivity in ventral language pathways characterizes non-verbal autism. Brain Struct. Funct. 2022, 227, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, J.; Wouters, J.; Vandermosten, M.; Ghesquière, P. Early dynamics of white matter deficits in children developing dyslexia. Dev. Cogn. Neurosci. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Fletcher, P.T.; Whitaker, R.T.; Tao, R.; Dubray, M.B.; Froehlich, A.; Ravichandran, C.; Alexander, A.L.; Bigler, E.D.; Lange, N.; Lainhart, J.E. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage 2010, 51, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Tachibana, M.; Rahman, S.; Kagitani-Shimono, K. Atypical structural connectivity of language networks in autism spectrum disorder: A meta-analysis of diffusion tensor imaging studies. Autism Res. 2022, 15, 1585–1602. [Google Scholar] [CrossRef]
- Dick, A.S.; Bernal, B.; Tremblay, P. The language connectome: New pathways, new concepts. Neuroscientist 2014, 20, 453–467. [Google Scholar] [CrossRef]
- Hickok, G.; Poeppel, D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 2004, 92, 67–99. [Google Scholar] [CrossRef]
- Saur, D.; Kreher, B.W.; Schnell, S.; Kümmerer, D.; Kellmeyer, P.; Vry, M.-S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040. [Google Scholar] [CrossRef]
- Roberts, T.; Heiken, K.; Zarnow, D.; Dell, J.; Nagae, L.; Blaskey, L.; Solot, C.; Levy, S.; Berman, J.; Edgar, J. Left hemisphere diffusivity of the arcuate fasciculus: Influences of autism spectrum disorder and language impairment. Am. J. Neuroradiol. 2014, 35, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Vydrova, R.; Komarek, V.; Sanda, J.; Sterbova, K.; Jahodova, A.; Maulisova, A.; Zackova, J.; Reissigova, J.; Krsek, P.; Kyncl, M. Structural alterations of the language connectome in children with specific language impairment. Brain Lang. 2015, 151, 35–41. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Hallquist, M.N.; Asato, M.; Luna, B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage 2014, 92, 356–368. [Google Scholar] [CrossRef]
- Verly, M.; Gerrits, R.; Sleurs, C.; Lagae, L.; Sunaert, S.; Zink, I.; Rommel, N. The mis-wired language network in children with developmental language disorder: Insights from DTI tractography. Brain Imaging Behav. 2019, 13, 973–984. [Google Scholar] [CrossRef]
- Liu, J.; Tsang, T.; Jackson, L.; Ponting, C.; Jeste, S.S.; Bookheimer, S.Y.; Dapretto, M. Altered lateralization of dorsal language tracts in 6-week-old infants at risk for autism. Dev. Sci. 2019, 22, e12768. [Google Scholar] [CrossRef]
- Baum, G.L.; Cui, Z.; Roalf, D.R.; Ciric, R.; Betzel, R.F.; Larsen, B.; Cieslak, M.; Cook, P.A.; Xia, C.H.; Moore, T.M.; et al. Development of structure–function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. USA 2020, 117, 771–778. [Google Scholar] [CrossRef]
- Liégeois, F.; Baldeweg, T.; Connelly, A.; Gadian, D.G.; Mishkin, M.; Vargha-Khadem, F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat. Neurosci. 2003, 6, 1230–1237. [Google Scholar] [CrossRef]
- De Guibert, C.; Maumet, C.; Jannin, P.; Ferré, J.-C.; Tréguier, C.; Barillot, C.; Le Rumeur, E.; Allaire, C.; Biraben, A. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia). Brain 2011, 134, 3044–3058. [Google Scholar] [CrossRef]
- Pigdon, L.; Willmott, C.; Reilly, S.; Conti-Ramsden, G.; Liegeois, F.; Connelly, A.; Morgan, A.T. The neural basis of nonword repetition in children with developmental speech or language disorder: An fMRI study. Neuropsychologia 2020, 138, 107312. [Google Scholar] [CrossRef]
- Abbott, N.T.; Baker, C.J.; Chen, C.; Liu, T.T.; Love, T.E. Defining Hypoperfusion in Chronic Aphasia: An Individualized Thresholding Approach. Brain Sci. 2021, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Thompson, C.K. Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front. Neurol. 2019, 10, 295. [Google Scholar] [CrossRef]
- Hugdahl, K.; Gundersen, H.; Brekke, C.; Thomsen, T.; Rimol, L.M.; Ersland, L.; Niemi, J. fMRI brain activation in a Finnish family with specific language impairment compared with a normal control group. J. Speech Lang. Hear. Res. 2004, 47, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Plante, E.; Patterson, D.; Sandoval, M.; Vance, C.J.; Asbjørnsen, A.E. An fMRI study of implicit language learning in developmental language impairment. NeuroImage Clin. 2017, 14, 277–285. [Google Scholar] [CrossRef]
- Dibbets, P.; Bakker, K.; Jolles, J. Functional MRI of task switching in children with specific language impairment (SLI). Neurocase 2006, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst, V.J.; Vannest, J.; Lee, G.; Hernandez-Garcia, L.; Plante, E.; Rajagopal, A.; Holland, S.K.; Consortium, C.A. Evidence that neurovascular coupling underlying the BOLD effect increases with age during childhood. Hum. Brain Mapp. 2015, 36, 1–15. [Google Scholar] [CrossRef]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 2021, 12, 3190. [Google Scholar] [CrossRef] [PubMed]
- Bonakdarpour, B.; Parrish, T.B.; Thompson, C.K. Hemodynamic response function in patients with stroke-induced aphasia: Implications for fMRI data analysis. NeuroImage 2007, 36, 322–331. [Google Scholar] [CrossRef]
- Brumm, K.P.; Perthen, J.E.; Liu, T.T.; Haist, F.; Ayalon, L.; Love, T. An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. Neuroimage 2010, 51, 995–1005. [Google Scholar] [CrossRef]
- Love, T.; Swinney, D.; Wong, E.; Buxton, R. Perfusion imaging and stroke: A more sensitive measure of the brain bases of cognitive deficits. Aphasiology 2002, 16, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Chiron, C.; Pinton, F.; Masure, M.; Duvelleroy-Hommet, C.; Leon, F.; Billard, C. Hemispheric specialization using SPECT and stimulation tasks in children with dysphasia and dystrophia. Dev. Med. Child Neurol. 1999, 41, 512–520. [Google Scholar] [CrossRef]
- Denays, R.; Tondeur, M.; Foulon, M.; Verstraeten, F.; Ham, H.; Piepsz, A.; Noël, P. Regional brain blood flow in congenital dysphasia: Studies with technetium-99m HM-PAO SPECT. J. Nucl. Med. 1989, 30, 1825–1829. [Google Scholar]
- Lou, H.; Henriksen, L.; Bruhn, P. Focal cerebral dysfunction in developmental learning disabilities. Lancet 1990, 335, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Ors, M.; Ryding, E.; Lindgren, M.; Gustafsson, P.; Blennow, G.; Rosén, I. SPECT findings in children with specific language impairment. Cortex 2005, 41, 316–326. [Google Scholar] [CrossRef]
- Tzourio, N.; Heim, A.; Zilbovicius, M.; Gerard, C.; Mazoyer, B.M. Abnormal regional CBF response in left hemisphere of dysphasic children during a language task. Pediatr. Neurol. 1994, 10, 20–26. [Google Scholar] [CrossRef]
- Hwang, J.W.; Lee, J.-B.; Kim, B.-N.; Lee, H.-Y.; Lee, D.-S.; Shin, M.-S.; Cho, S.-C. Regional cerebral perfusion abnormalities in developmental language disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-H.; Park, E.S.; Kim, D.Y.; Song, D.H.; Lee, J.D. The neuroradiological findings of children with developmental language disorder. Yonsei Med. J. 2007, 48, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.J.O.; Bishop, D.V.M. Cerebral dominance for language function in adults with specific language impairment or autism. Brain 2008, 131, 3193–3200. [Google Scholar] [CrossRef]
- Paniukov, D.; Lebel, R.; Giesbrecht, G.; Lebel, C. Cerebral Blood Flow Increases Across Early Childhood. Neuroimage 2020, 204, 116224. [Google Scholar] [CrossRef]
- Avants, B.B.; Duda, J.T.; Kilroy, E.; Krasileva, K.; Jann, K.; Kandel, B.T.; Tustison, N.J.; Yan, L.; Jog, M.; Smith, R. The pediatric template of brain perfusion. Sci. Data 2015, 2, 150003. [Google Scholar] [CrossRef]
- Moses, P.; Hernandez, L.M.; Orient, E. Age-related differences in cerebral blood flow underlie the BOLD fMRI signal in childhood. Front. Psychol. 2014, 5, 300. [Google Scholar] [CrossRef]
- Wu, C.; Honarmand, A.R.; Schnell, S.; Kuhn, R.; Schoeneman, S.E.; Ansari, S.A.; Carr, J.; Markl, M.; Shaibani, A. Age-related changes of normal cerebral and cardiac blood flow in children and adults aged 7 months to 61 years. J. Am. Heart Assoc. 2016, 5, e002657. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Sorond, F. Transcranial Doppler ultrasound: Technique and application. Semin. Neurol. 2012, 32, 411–420. [Google Scholar] [CrossRef]
- Leonard, L.B. Children with specific language impairment and their contribution to the study of language development. J. Child Lang. 2014, 41, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.L.; Wexler, K.; Cleave, P.L. Specific language impairment as a period of extended optional infinitive. J. Speech Lang. Hear. Res. 1995, 38, 850–863. [Google Scholar] [CrossRef]
- Wexler, K.; Lightfoot, D.; Homstein, N. Verb Movement; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Rice, M.L.; Wexler, K.; Hershberger, S. Tense over time: The longitudinal course of tense acquisition in children with specific language impairment. J. Speech Lang. Hear. Res. 1998, 41, 1412–1431. [Google Scholar] [CrossRef]
- Wexler, K.; Schütze, C.T.; Rice, M. Subject case in children with SLI and unaffected controls: Evidence for the Agr/Tns omission model. Lang. Acquis. 1998, 7, 317–344. [Google Scholar] [CrossRef]
- Wexler, K. Very early parameter setting and the unique checking constraint: A new explanation of the optional infinitive stage. Lingua 1998, 106, 23–79. [Google Scholar] [CrossRef]
- Van der Lely, H.K. SLI in children: Movement, economy, and deficits in the computational-syntactic system. Lang. Acquis. 1998, 7, 161–192. [Google Scholar] [CrossRef]
- Marshall, C.R.; Van Der Lely, H.K. The impact of phonological complexity on past tense inflection in children with Grammatical-SLI. Adv. Speech Lang. Pathol. 2007, 9, 191–203. [Google Scholar] [CrossRef]
- Ingram, D.; Carr, L. When morphology ability exceeds syntactic ability: A case study. In Proceedings of the Convention of the American Speech-Language-Hearing Association, New Orleans, LA, USA, 17–21 November 1994. [Google Scholar]
- Marshall, C.R.; van der Lely, H.K. A challenge to current models of past tense inflection: The impact of phonotactics. Cognition 2006, 100, 302–320. [Google Scholar] [CrossRef]
- Leonard, L.B.; Kueser, J.B. Five overarching factors central to grammatical learning and treatment in children with developmental language disorder. Int. J. Lang. Commun. Disord. 2019, 54, 347–361. [Google Scholar] [CrossRef]
- Tagarelli, K.M.; Shattuck, K.F.; Turkeltaub, P.E.; Ullman, M.T. Language learning in the adult brain: A neuroanatomical meta-analysis of lexical and grammatical learning. NeuroImage 2019, 193, 178–200. [Google Scholar] [CrossRef]
- Kuhnke, P.; Meyer, L.; Friederici, A.D.; Hartwigsen, G. Left posterior inferior frontal gyrus is causally involved in reordering during sentence processing. Neuroimage 2017, 148, 254–263. [Google Scholar] [CrossRef]
- Copland, D.A.; Brownsett, S.; Iyer, K.; Angwin, A.J. Corticostriatal regulation of language functions. Neuropsychol. Rev. 2021, 31, 472–494. [Google Scholar] [CrossRef]
- Tallal, P.; Piercy, M. Defects of Non-Verbal Auditory Perception in Children with Developmental Aphasia. Nature 1973, 241, 468–469. [Google Scholar] [CrossRef]
- Tallal, P.; Piercy, M. Developmental aphasia: Rate of auditory processing and selective impairment of consonant perception. Neuropsychologia 1974, 12, 83–93. [Google Scholar] [CrossRef]
- Schwartz, R.G.; Scheffler, F.L.V.; Lopez, K. Speech perception and lexical effects in specific language impairment. Clin. Linguist. Phon. 2013, 27, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Leonard, L.B.; McGregor, K.K.; Allen, G.D. Grammatical morphology and speech perception in children with specific language impairment. J. Speech Lang. Hear. Res. 1992, 35, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory and language: An overview. J. Commun. Disord. 2003, 36, 189–208. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working memory. In Psychology of Learning and Motivation; Elsevier: Amsterdam, The Netherlands, 1974; Volume 8, pp. 47–89. [Google Scholar]
- Gathercole, S.E.; Baddeley, A.D. Evaluation of the role of phonological STM in the development of vocabulary in children: A longitudinal study. J. Mem. Lang. 1989, 28, 200–213. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Baddeley, A.D. Phonological memory deficits in language disordered children: Is there a causal connection? J. Mem. Lang. 1990, 29, 336–360. [Google Scholar] [CrossRef]
- Estes, K.G.; Evans, J.L.; Else-Quest, N.M. Differences in the nonword repetition performance of children with and without specific language impairment: A meta-analysis. J. Speech Lang. Hear. Res. 2007, 50, 177–195. [Google Scholar] [CrossRef]
- Ferrill, M.; Love, T.; Walenski, M.; Shapiro, L.P. The time-course of lexical activation during sentence comprehension in people with aphasia. Am. J. Speech-Lang. Pathol. 2012, 21, S179–S189. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.K.; Gallant, J.E. Eye tracking reveals subtle spoken sentence comprehension problems in children with dyslexia. Lingua 2019, 228, 102708. [Google Scholar] [CrossRef]
- Brauer, J.; Anwander, A.; Perani, D.; Friederici, A.D. Dorsal and ventral pathways in language development. Brain Lang. 2013, 127, 289–295. [Google Scholar] [CrossRef]
- Perani, D.; Saccuman, M.C.; Scifo, P.; Anwander, A.; Spada, D.; Baldoli, C.; Poloniato, A.; Lohmann, G.; Friederici, A.D. Neural language networks at birth. Proc. Natl. Acad. Sci. USA 2011, 108, 16056–16061. [Google Scholar] [CrossRef]
- Labache, L.; Joliot, M.; Saracco, J.; Jobard, G.; Hesling, I.; Zago, L.; Mellet, E.; Petit, L.; Crivello, F.; Mazoyer, B.; et al. A SENtence Supramodal Areas AtlaS (SENSAAS) based on multiple task-induced activation mapping and graph analysis of intrinsic connectivity in 144 healthy right-handers. Brain Struct. Funct. 2019, 224, 859–882. [Google Scholar] [CrossRef] [PubMed]
- Love, T.; Swinney, D.; Walenski, M.; Zurif, E. How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain Lang. 2008, 107, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Hula, W.D.; McNeil, M.R. Models of attention and dual-task performance as explanatory constructs in aphasia. Semin. Speech Lang. 2008, 29, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.W. Relation of working memory to off-line and real-time sentence processing in children with specific language impairment. Appl. Psycholinguist. 2000, 21, 117–148. [Google Scholar] [CrossRef]
- Just, M.A.; Carpenter, P.A. A capacity theory of comprehension: Individual differences in working memory. Psychol. Rev. 1992, 99, 122. [Google Scholar] [CrossRef]
- Kail, R. A method for studying the generalized slowing hypothesis in children with specific language impairment. J. Speech Lang. Hear. Res. 1994, 37, 418–421. [Google Scholar] [CrossRef]
- Windsor, J.; Kohnert, K. The Search for Common Ground. J. SpeechLang. Hear. Res. 2004, 47, 877–890. [Google Scholar] [CrossRef]
- Jednoróg, K.; Marchewka, A.; Altarelli, I.; Monzalvo Lopez, A.K.; Van Ermingen-Marbach, M.; Grande, M.; Grabowska, A.; Heim, S.; Ramus, F. How reliable are gray matter disruptions in specific reading disability across multiple countries and languages? insights from a large-scale voxel-based morphometry study. Hum. Brain Mapp. 2015, 36, 1741–1754. [Google Scholar] [CrossRef]
- Friedrich, P.; Fraenz, C.; Schlüter, C.; Ocklenburg, S.; Mädler, B.; Güntürkün, O.; Genç, E. The relationship between axon density, myelination, and fractional anisotropy in the human corpus callosum. Cereb. Cortex 2020, 30, 2042–2056. [Google Scholar] [CrossRef]
- Krishnan, S.; Asaridou, S.S.; Cler, G.J.; Smith, H.J.; Willis, H.E.; Healy, M.P.; Thompson, P.A.; Bishop, D.V.M.; Watkins, K.E. Functional organisation for verb generation in children with developmental language disorder. NeuroImage 2021, 226, 117599. [Google Scholar] [CrossRef]
- Plante, E. MRI findings in the parents and siblings of specifically language-impaired boys. Brain Lang. 1991, 41, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Weismer, S.E.; Plante, E.; Jones, M.; Tomblin, J.B. A functional magnetic resonance imaging investigation of verbal working memory in adolescents with specific language impairment. J. Speech Lang. Hear. Res. 2005, 48, 405–425. [Google Scholar] [CrossRef]

