1. Introduction
The American College of Sports Medicine (ACSM) emphasizes the importance of selecting and consuming nutrients and supplements tailored to the type of exercise performed for optimal athlete performance [1,2]. In competitive sports, improvements ranging from 0.5% to 1.5% are considered critical for sporting performance [3]. The Australian Institute of Sport (AIS) categorizes supplements based on the level of evidence regarding their impacts on athlete performance, with beetroot juice (BJ) being categorized into Group A [4]. BJ contains a high concentration of inorganic nitrate (NO3−), a compound found in significant quantities in this food item and as a preservative in processed meats [5].
BJ has a NO3− concentration of up to 11.4 g/L [6]. After ingestion, it is reduced to nitrite (NO2−) through the action of nitrate reductase enzymes by anaerobic bacteria in the oral cavity [7]. Subsequently, NO2− is instantly converted into nitrous acid in the stomach and then nitrogen oxides (2HNO2 H2O + N2O3) [8]. Previous studies have demonstrated that NO2− enhances the bioavailability of nitric oxide (NO), a potent vasodilator, via the nitrate–nitrite–nitric oxide pathway, resulting in various physiological functions, such as improved skeletal muscle function and increased cardiorespiratory performance [9,10]. NO acts as a vasodilator in the muscle, where oxygen (O2) consumption is highest, allowing local blood flow to adapt to the O2 demand within skeletal muscle, thus providing a homogeneous distribution. Supporting this observation, prior research [11] has demonstrated that BJ supplementation, in gel form, prevents a decline in maximal voluntary contraction following three sets of handgrip isotonic exercises. Furthermore, it improves the saturation of muscle O2 during exercise recovery and reduces blood lactate levels. Nevertheless, when applying the same protocol for acute dose supplementation, the outcomes differed [12].
Additionally, it not only enhances vasodilation but also regulates cellular respiration and neurotransmission. As a consequence, it enhances other actions such as sprint capacity and cognitive performance [13,14,15,16,17]. In addition, it has been shown to improve performance in high-intensity intermittent efforts with short rest periods due to the faster resynthesis of phosphocreatine [18]. Furthermore, phytonutrients such as betalains and phenolic compounds are believed to be responsible for their antioxidant, anti-inflammatory, and analgesic effects [19,20], thus aiding in the recovery of muscle function [21,22]. Regarding betalains, which are considered among the primary plant inherent bioactive components of beets, the supplementation with 50 mg of betalain-rich concentrates, devoid of nitrate, resulted in a notable reduction in the 10 km race time for triathletes. Furthermore, it demonstrated a more significant decrease in the physiological increase in serum creatine kinase levels and fatigue following the supplementation [23]. The latter is considered key to the performance of athletes.
Therefore, NO3− is classified as a nutritional supplement that can directly enhance athletic performance, a stance supported by the International Olympic Committee (IOC) [24], as well as recent reviews [25,26]. Although the evidence in humans is less clear, previous studies in animals [27] observed that NO3− exerts a better result on type 2 muscle fibers compared to type 1 fibers. This effect could partially explain the benefits of muscle contraction, reducing the duration of the action potential of the motor unit and more rapidly improving conduction speed, allowing greater calcium release to maintain force production while promoting a decrease in fatigue.
In summary, BJ supplementation is associated with improvements in both aerobic and anaerobic exercises, albeit with limited insight into its potential synergistic impact. The evidence suggests that “it cannot be stated definitively whether the combination of BJ with other supplements has a positive or negative effect, but the effects of BJ supplementation may potentially be influenced by interactions with other supplements, such as caffeine” [9].
Although recent research has evaluated the benefits of certain ergogenic aids, including BJ, on athlete performance, these analyses have primarily focused on their isolated effects [28,29]. Our study aims to complement previous investigations by examining the potential synergistic effects of combining BJ with other permissible supplements available on the market.
Based on the foregoing, the objective of this study was to determine whether the combination of BJ with another nutritional supplement can enhance its beneficial effects and, therefore, improve physical performance in humans.
2. Material and Methods
2.1. Search Strategy
This systematic review adheres to the PRISMA guidelines [30]. Subsequently, the systematic review was registered in PROSPERO (CRD42023425965). A comprehensive literature search was conducted in two major databases—Web of Science and PubMed—considering articles published in the last 5 years (from 1 January 2018, to 29 January 2023). outlines the search strategy employed in the Web of Science and PubMed databases.
2.2. Selection Criteria
Firstly, the PICO strategy (Cochrane Handbook for Systematic Reviews of Interventions) was used to organize the systematic review with the seven required steps in https://training.cochrane.org (accessed on 2 March 2023). The selection criteria were as follows: (i) articles written in the English language; (ii) articles published in the Web of Science and PubMed databases; (iii) studies involving humans; (iv) original articles, randomized clinical trials, and quasi-experimental studies; (v) participants aged 18 to 65 years old; and (vi) articles published between January 2018 and January 2023. The exclusion criteria were as follows: (i) studies that included individuals with pathologies; (ii) studies that included animals; (iii) studies that included individuals under 18 years old and older adults (+65 years old); (iv) studies unrelated to physical activity and/or exercise; and (v) case studies, case reports, editorials, cross-sectional studies, longitudinal studies, systematic reviews, meta-analyses, and narrative reviews. After removing duplicates, eligibility was assessed by reviewing the title and abstract and, subsequently, reading the full text to evaluate eligibility.
2.3. Data Extraction and Reliability
The search was conducted by (E.F.-C.). The titles and abstracts of all retrieved articles were read by (E.F.C., R.B.G., and F.R.-R.). A meeting was held to resolve disagreements regarding eligibility. For each included study, the following information was collected: first author, publication year, study type, main objective, number of subjects, gender, age, body weight, height, or body mass index (BMI) and type of exercise, exercise intervention type, supplementation protocols, evaluation methods, main results obtained, and conclusions. The selected articles were categorized based on the type of supplementation (BJ + citrulline malate, BJ + caffeine, BJ + carbohydrates, BJ + amino acids, BJ + potassium nitrate). Finally, the results of the studies that met the selection criteria were analyzed.
2.4. Quality Assessment and Level of Evidence
Bias risk was assessed using the critical appraisal tool for systematic reviews from the Joanna Briggs Institute. This tool includes various checklists specific to the study design. In this case, checklists for randomized clinical trials [31] and quasi-experimental studies [32] were used. Responses for each item had three possible categories: “yes” (criterion met), “no” (criterion not met) and “unclear”. The tools included thirteen items for randomized clinical trials and nine items for quasi-experimental studies. According to the above, studies were considered as “low-quality evidence” when ≤49% of the items were met, “medium-quality evidence” when 50–74% of the items were met, and “high-quality evidence” when ≥75% of the items were met. It should be noted that the responses “not applicable” and “unclear” were excluded from the evaluation, as they do not contribute to the quality of evidence. The three reviewers assessed the quality of the studies separately, and a consensus meeting was organized to resolve differences related to the classification of articles based on criteria fulfilment .
3. Results
Figure 1 shows the flowchart for the search strategy of the selected articles. A total of 700 studies were found in the two databases. After reading the titles and abstracts of each article, 340 studies were excluded as duplicates, and 126 studies were excluded because they were not relevant to the review topic. Subsequently, the eligibility of a total of 234 studies was assessed. After analyzing the exclusion criteria, finally, six studies were included in the analysis. Six studies had a randomized controlled trial design, and four studies had a quasi-experimental design.
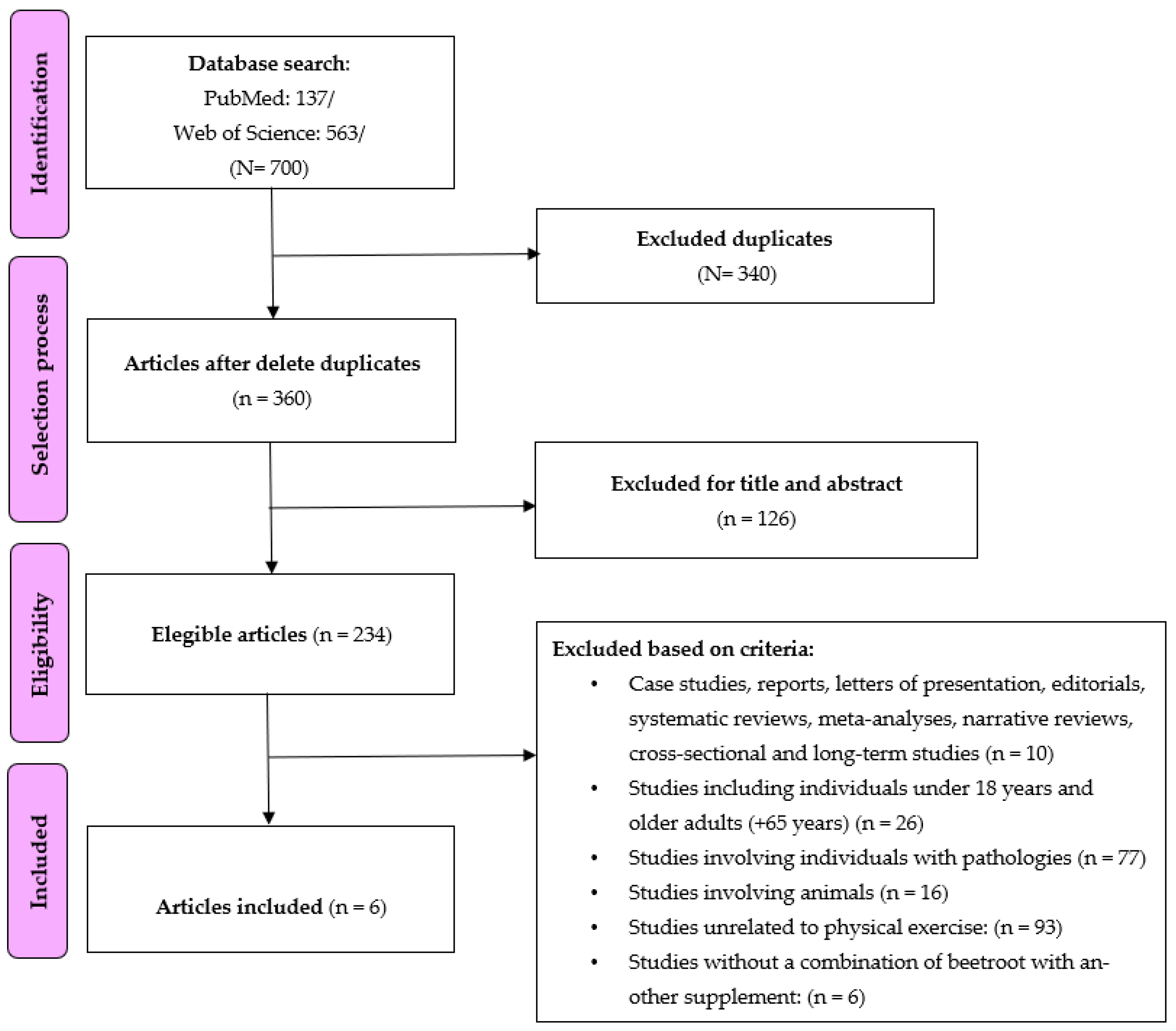
Figure 1. Flow chart used for searching studies.
provides a summary of the studies included in this review. This review covers data from 106 participants, with sample sizes ranging from 9 to 32 subjects. All studies reported the gender of the participants. None of the studies only included females; only one study included both males and females [34] and five studies only included males [35,36,37,38,39], and All studies reported the age of the participants [34,35,36,37]. The ages of the subjects ranged from 18 to 64 years. The samples were drawn from five different countries: two studies were conducted in Spain, one in Scotland, one in France, one in Iran, and one in Sweden.
Regarding the sample characteristics, four of the six studies focused on athletes such as soccer players [39], triathletes [38], and runners [34,36], while the remaining two studies included physically active individuals in their sample [35,37].
To analyze the effect of supplementation, four studies [36,37,38,39] applied various aerobic tests. Two studies considered strength exercises such as squatting [37] and leg extension [35].
The results revealed that three studies did not show significant effects with the intake of BJ in combination with other supplements like caffeine (CAF) [34,39] and nitrate (N), i.e., N + CIT and N + ARG [35]. In contrast, the other three studies observed positive effects on performance in different tests after the intake of BJ in combination with supplements such as caffeine [37], citrulline [38], and carbohydrates [36].
Additionally, an analysis was conducted to determine the quality of the studies included in this review. For this purpose, the Joanna Briggs Institute criteria checklist was used . Different criteria were applied depending on the study characteristics. In this regard, Figure 2 shows that three of the four randomized controlled trials (RCT) met 100% of the criteria [35,37,39], while one met 92.3% [38]. Of the remaining three quasi-experimental studies, two met 77% of the criteria [34,36]. All studies met ≥ 75% of the criteria, classifying them as high quality (HQ).
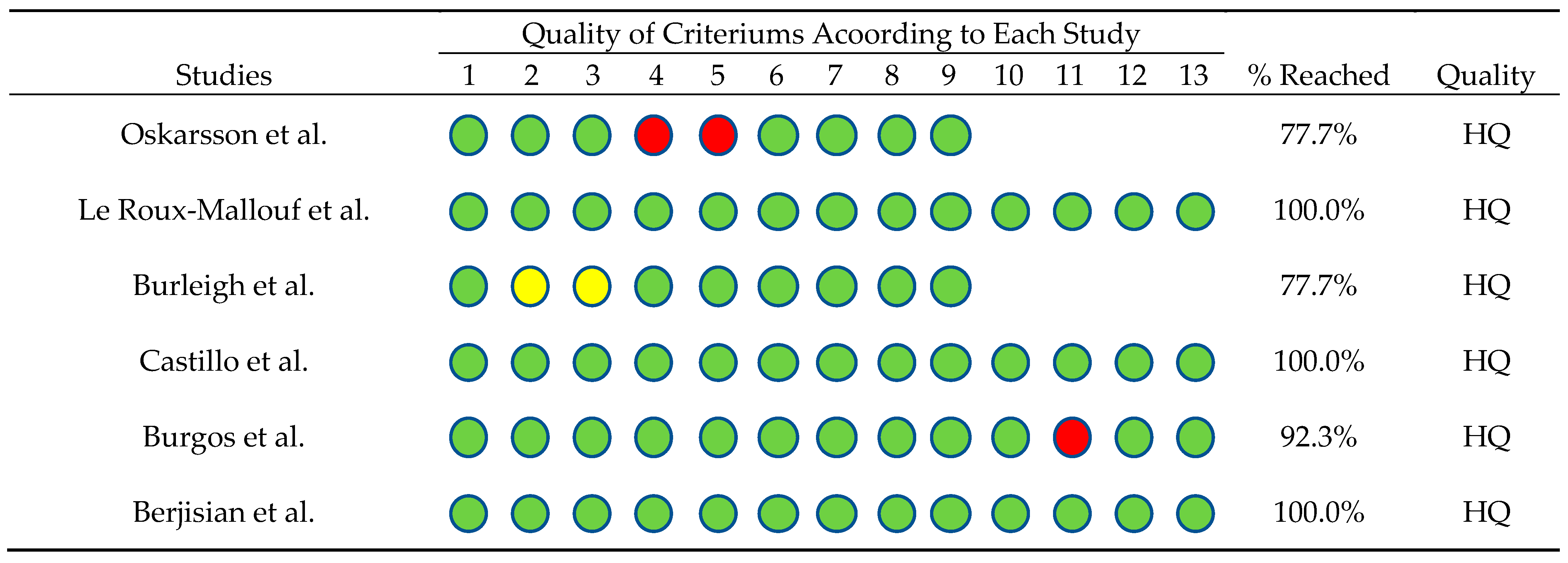
Figure 2. Checklist from the Joanna Briggs Institute’s criteria according to the kind of study, percentage of criteria reached, and quality level of evidence [34,35,36,37,38,39]. Green circles: criterion met, red circles: criterion not met and yellow circles: unclear information.
4. Discussion
The objective of this study was to determine whether the combination of beetroot juice (BJ) with another nutritional supplement can enhance its beneficial effects and, therefore, improve physical performance in humans.
Studies of BJ date back to the 1980s and were initially oriented towards clinical health [40,41,42,43,44,45], continuing in this direction until the 1990s [46,47,48,49]. From the year 2000, the first studies of the use of this supplement and its impact on physical exercise began to be published, focusing on its effects on reducing BP and oxygen consumption during exercise [46,47,48,49]. It was primarily determined that its consumption improves tolerance to high-intensity exercise in humans [48]. Subsequently, in 2013, the first systematic reviews on BJ and physical exercise began to appear [50,51].
A variety of studies confirm its utility through different research works [46,47,48,49,50,51], and its intake is even endorsed by the AIS [4] and the IOC [24]. However, it is essential to consider that supplement consumption is increasingly common and often, according to empirical evidence, tends to be combined with others. In this sense, this study aims to determine the effect of BJ intake and its potential enhancement when combined with other ergogenic aids applied to different sports and physical capabilities [37].
4.1. Combination of BJ in Aerobic Exercises
A total of four studies included in this review conducted aerobic tests to assess the effects of BJ supplementation in combination with another supplement [34,36,38,39]. Two of these studies combined BJ + CAF in soccer players [39] and runners [34]. In these studies, there were no differences in Yo-Yo test results, VO2max, running economy, heart rate, or lactate levels. A prior Australian study conducted in 2014 [52] found differences between the BJ + CAF combination and the placebo but did not find differences between the consumption of CAF alone and the BJ + CAF combination (Mean power 260 + 58 W and 258 + 59 W; p = 0.4, respectively). This Australian study suggests that a higher level of the athlete may require chronic BJ intake before a test to benefit performance, and it might work better in sports with higher intensity than just aerobic sports. These two aspects are relevant points to consider in these two studies since they did not achieve effects with the combination [34,39].
On the other hand, the study by Burgos et al. [38] investigated the effects of the combination of BJ + CIT in a group of triathletes. An improvement in aerobic power (p = 0.002) was found through chronic consumption of 3 g/day of CIT and 2.1 g/day of NO3- over 9 weeks. CIT’s ability to act as a buffer for muscular acidosis has previously been studied, which may help to reduce hyperammonemia and blood lactate accumulation in athletes involved in aerobic or strength activities [53]. In addition to the aforementioned effects of BJ, this would provide a performance boost in high-intensity exercise. Another systematic review [54] reported that CIT supplementation significantly reduced the rating of perceived exertion (7 studies, p = 0.03) and muscle soreness 24 h post-exercise (seven studies, p = 0.04). However, CIT supplementation did not significantly reduce muscle soreness 72 h post-exercise or lower blood lactate. This is attributed to the acute consumption of CIT (~8 g, 1 h before exercise), as opposed to the chronic administration registered in previous studies that yielded favorable outcomes. Therefore, the results should be considered with caution, as their effects may depend on the specific nature and duration of acute or chronic consumption.
Regarding the combination of BJ + CHO, the study by Burleigh et al. [36] examined the combination of enriched BJ + CHO (Powerade®, The Coca-Cola Company, Atlanta, GA, USA) in runners in an incremental test to exhaustion. An improvement in salivary pH was found in the BJ + CHO group compared to the depleted BJ + CHO group, CHO alone, and the control (water) group. This improvement is related to better intestinal substrate absorption efficiency and reduced dehydration, which could enhance physical performance. Most sports drinks have pH levels lower than the acceptable acidity of ~5.5 [55]. Additionally, the negative effect of an acidic and carbohydrate-rich diet could be exacerbated by reduced saliva flow during exercise [56]. In this case, BJ would contribute to preventing saliva acidification caused by the consumption of a sports drink during exercise (Figure 3).
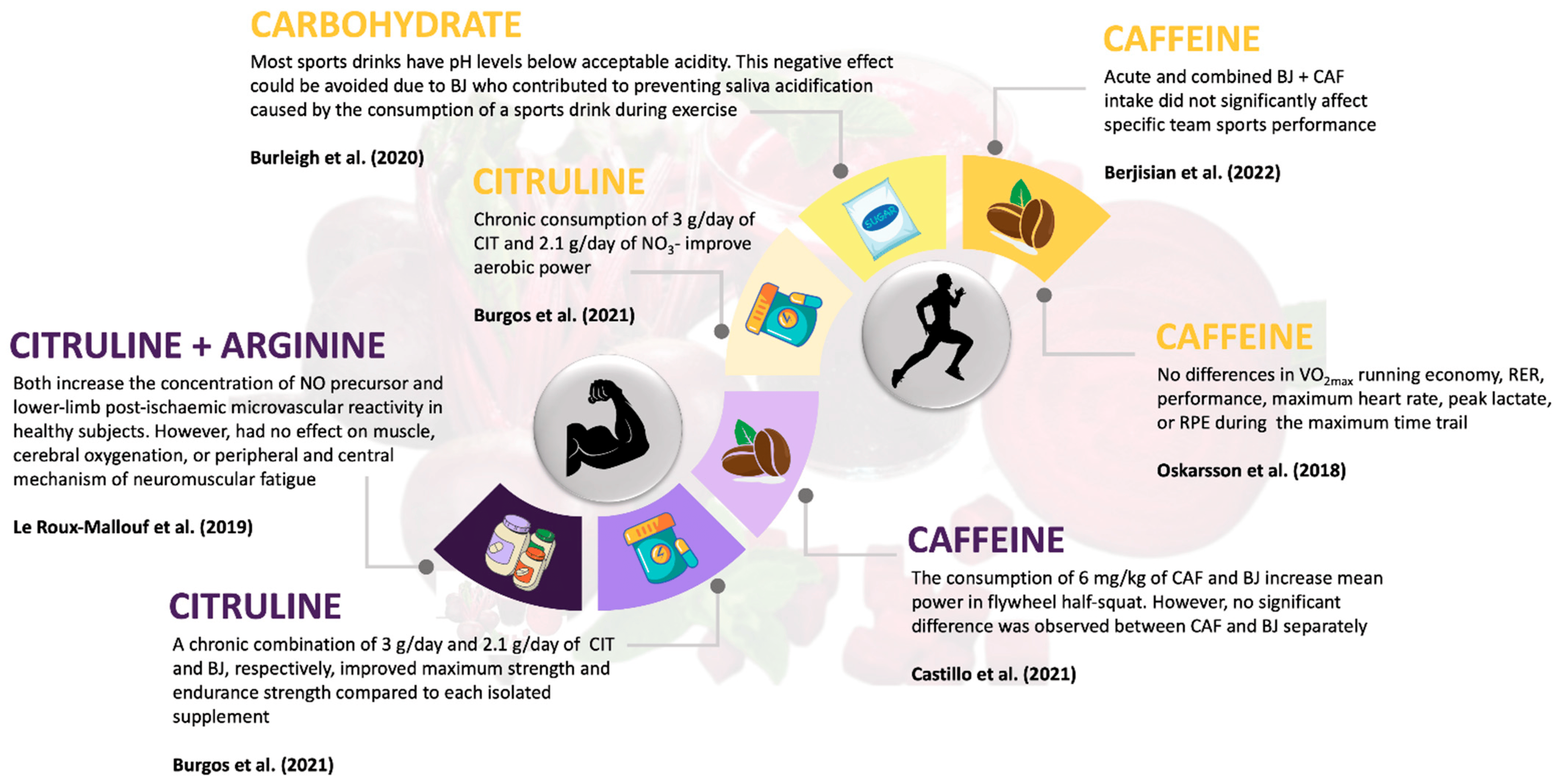
Figure 3. Interplay of BJ and its impact on strength and endurance performance in conjunction with other ergogenic aids [34,35,36,37,38,39].
4.2. Combination of BJ in Strength Exercises
Regarding the effects of BJ on muscle strength and power, it generally seems to be more effective. In the study by Castillo et al. [37], active men performed countermovement jump (CMJ) tests and squats after the acute consumption of BJ + CAF. The researchers concluded that prior intake of BJ + CAF seems to accelerate energy production capacity after a fatiguing protocol, maintaining CMJ performance in the post-test at 180 s compared to the pre-test. The effect of CAF on performance has been extensively studied. It has been defined that highly trained athletes are more sensitive to CAF and may experience even more improvement [57]. Furthermore, higher doses (6 mg·kg−1) may have greater positive effects [37]. However, despite the known distinct benefits of CAF and BJ, the study [37] could not establish a statistical difference between consuming only CAF or only BJ and the BJ + CAF combination. More specific studies are needed to address this research question.
In the study by Burgos et al. [38], which supplemented with 3 g/day of CIT plus 2.1 g/day of BJ (300 mg/day of NIT) for 9 weeks, improved maximal strength and endurance-strength compared to separate CIT or BR supplementation. In this case, the chronic consumption of the BJ + CIT combination had significant positive effects.
On the other hand, the study by Le Roux-Mallouf et al. [35], conducted in 15 healthy men, examined the combination of NIT + CIT and NIT + ARG on isometric knee extension strength. The main results were an increased plasma concentration of NO precursors and enhanced post-ischemic vasodilation, but there was no significant effect on muscle and cerebral oxygenation or peripheral and central neuromuscular fatigue, and no improvement in exercise performance (Figure 3). The use of CIT as a nitric oxide promoter has previously been studied. Trexler et al. [58] studied the acute effects of CIT-Mal on lower limb strength but found its effect to be less pronounced than that of BJ. A recent systematic review and meta-analysis [59] concluded that CIT supplementation alone had a small ergogenic effect during strength training. Another study [60] conducted on trained strength athletes (a single 8 g dose of L-citrulline) did not enhance isometric force production, muscle endurance, or muscle oxygenation parameters. Apparently, acute doses of CIT supplementation are not sufficient to improve muscle oxygenation and strength parameters.
Finally, it is crucial to recognize that the various outcomes observed may have multiple causes, extending beyond the inherent efficacy of the supplement itself. Factors such as the diversity of the sample, encompassing both trained and untrained individuals, as well as the various disciplines under examination, can significantly influence the results. This context suggests that athletes who prefer using supplements might exhibit a higher likelihood of experiencing a placebo effect [61]. Furthermore, a prior study underscores that a combination of motivation, expectancy, and physiological factors can collectively determine whether the outcome is positive or negative [62]. This concept can clearly and eventually interfere as a confounding factor and reduce the gap between the ergogenic effect of a supplement and the placebo effect, diminishing the final statistical power found.
5. Conclusions
Few studies have been conducted regarding the combination of beetroot juice with other supplements in recent years. Our analysis indicates that there is evidence of its effectiveness, particularly when exercise intensity is higher. This applies to both aerobic and muscular strength activities. However, there appear to be greater benefits when the combination of beetroot juice with another supplement is consumed chronically. Studies involving the acute intake of two supplements in combination seem to have a lesser impact. Clearly, further research into the combination of beetroot juice with other supplements is needed to address unresolved questions, ultimately improving the health and athletic performance of individuals.
References
- Rodríguez, N.R.; Di Marco, N.M.; Langley, S. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New strategies in sport nutrition to increase exercise performance. Free Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.D.; Hopkins, W.G. Performance Enhancement at the Fifth World Congress on Sport Sciences; University of Otago: Dunedin, New Zealand, 1999; Volume 3. [Google Scholar]
- Australian Institute of Sport. ABCD Classification System. Available online: https://www.ais.gov.au/__data/assets/pdf_file/0006/1082517/AIS-Supplement-Framework-ABCD-System_v4.pdf (accessed on 17 December 2022).
- Murphy, M.; Eliot, K.; Heuertz, R.; Weiss, E. Whole beetroot consumption acutely improves running performance. J. Acad. Nutr. Diet 2012, 112, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Duncan, C.; Dougall, H.; Johnston, P.; Green, S.; Brogan, R.; Leifert, C.; Smith, L.; Golden, M.; Benjamin, N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995, 1, 546–551. [Google Scholar] [CrossRef]
- Benjamin, N.; O’Driscoll, F.; Dougall, H.; Duncan, C.; Smith, L.; Golden, M.; McKenzie, H. Stomach NO synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports. Med. 2014, 44, S35–S45. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; Nascimento, L.A.; Volino-Souza, M.; Mesquita, J.S.; Alvares, T.S. Beetroot-based gel supplementation improves handgrip strength and forearm muscle O2 saturation but not exercise tolerance and blood volume in jiu-jitsu athletes. Appl. Physiol. Nutr. Metab. 2018, 43, 920–927. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; do Nascimento, L.A.; Volino-Souza, M.; do Couto Vellozo, O.; Alvares, T.S. A single oral dose of beetroot-based gel does not improve muscle oxygenation parameters, but speeds up handgrip isometric strength recovery in recreational combat sports athletes. Biol. Sport 2020, 37, 93–99. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Clifford, T.; Allerton, D.M.; Brown, M.A.; Harper, L.; Horsburgh, S.; Keane, K.M.; Stevenson, E.J.; Howatson, G. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl. Physiol. Nutr. Metab. 2017, 42, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Jonvik, K.L.; Nyakayiru, J.; van Loon, L.J.; Verdijk, L.B. Can elite athletes benefit from dietary nitrate supplementation? J. Appl. Physiol. (1985) 2015, 119, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Wylie, L.J.; Fulford, J.; Kelly, J.; Black, M.I.; McDonagh, S.T.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Dietary nitrate improves sprint performance and cognitive function during prolonged intermittent exercise. Eur. J. Appl. Physiol. 2015, 115, 1825–1834. [Google Scholar] [CrossRef]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. Beetroot juice is more beneficial than sodium nitrate for attenuating muscle pain after strenuous eccentric-bias exercise. Appl. Physiol. Nutr. Metab. 2017, 42, 1185–1191. [Google Scholar] [CrossRef]
- Daab, W.; Bouzid, M.A.; Lajri, M.; Bouchiba, M.; Saafi, M.A.; Rebai, H. Chronic Beetroot Juice Supplementation Accelerates Recovery Kinetics following Simulated Match Play in Soccer Players. J. Am. Coll. Nutr. 2021, 40, 61–69. [Google Scholar] [CrossRef]
- Kozłowska, L.; Mizera, O.; Gromadzińska, J.; Janasik, B.; Mikołajewska, K.; Mróz, A.; Wąsowicz, W. Changes in Oxidative Stress, Inflammation, and Muscle Damage Markers Following Diet and Beetroot Juice Supplementation in Elite Fencers. Antioxidants 2020, 9, 571. [Google Scholar] [CrossRef]
- Montenegro, C.F.; Kwong, D.A.; Minow, Z.A.; Davis, B.A.; Lozada, C.F.; Casazza, G.A. Betalain-rich concentrate supplementation improves exercise performance and recovery in competitive triathletes. Appl. Physiol. Nutr. Metab. 2017, 42, 166–172. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef]
- Tanabe, Y.; Fujii, N.; Suzuki, K. Dietary Supplementation for Attenuating Exercise-Induced Muscle Damage and Delayed-Onset Muscle Soreness in Humans. Nutrients 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Cano, L.; Lago-Rodríguez, Á.; Domínguez, R. The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 762. [Google Scholar] [CrossRef] [PubMed]
- Esen, O.; Domínguez, R.; Karayigit, R. Acute Beetroot Juice Supplementation Enhances Intermittent Running Performance but Does Not Reduce Oxygen Cost of Exercise among Recreational Adults. Nutrients 2022, 14, 2839. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.A.; Bishop, D.J. Effects of Dietary Supplements on Adaptations to Endurance Training. Sports Med. 2020, 50, 25–53. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E239–E245. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic reviews of effectiveness. In BJI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; 2020; Available online: https://synthesismanual.jbi.global (accessed on 4 April 2023). [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.; Holly, C.; Kahlil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an Umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar]
- Castillo, D.; Rodríguez-Fernández, A.; Ramírez-Campillo, R.; Raya-González, J. Effects of caffeine, beetroot juice and its interaction consumption on exercise-related fatigue. Kinesiology 2021, 53, 185–192. [Google Scholar] [CrossRef]
- Berjisian, E.; McGawley, K.; Saunders, B. Acute effects of beetroot juice and caffeine co-ingestion during a team-sport-specific intermittent exercise test in semi-professional soccer players: A randomized, double-blind, placebo-controlled study. BMC Sports. Sci. Med. Rehabil. 2022, 14, 52. [Google Scholar] [CrossRef]
- Burgos, J.; Viribay, A.; Fernández-Lázaro, D.; Calleja-González, J.; González-Santos, J.; Mielgo-Ayuso, J. Combined Effects of Citrulline Plus Nitrate-Rich Beetroot Extract Co-Supplementation on Maximal and Endurance-Strength and Aerobic Power in Trained Male Triathletes: A Randomized Double-Blind, Placebo-Controlled Trial. Nutrients 2022, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, M.C.; Sculthorpe, N.; Henriquez, F.L.; Easton, C. Nitrate-rich beetroot juice offsets salivary acidity following carbohydrate ingestion before and after endurance exercise in healthy male runners. PLoS ONE 2020, 15, e0243755. [Google Scholar] [CrossRef] [PubMed]
- Le Roux-Mallouf, T.; Laurent, J.; Besset, D. Effects of acute nitric oxide precursor intake on peripheral and central fatigue during knee extensions in healthy men. Exp. Physiol. 2019, 104, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, J.; McGawley, K. No individual or combined effects of caffeine and beetroot-juice supplementation during submaximal or maximal running. Appl. Physiol. Nutr. Metab. 2018, 43, 697–703. [Google Scholar] [CrossRef]
- Forrai, G.; Bánkövi, G.; Vágújfalvi, D. Betaninuria: A genetic trait? Acta Physiol. Acad. Sci. Hung. 1982, 59, 265–282. [Google Scholar]
- Ladd, K.F.; Archer, M.C.; Newmark, H.L. Increased endogenous nitrosation in smokers. IARC Sci. Publ. 1984, 57, 811–817. [Google Scholar]
- Hall, C.N.; Kirkham, J.S.; Northfield, T.C. Urinary N-nitrosoproline excretion: A further evaluation of the nitrosamine hypothesis of gastric carcinogenesis in precancerous conditions. Gut 1987, 28, 216–220. [Google Scholar] [CrossRef]
- Bos, P.M.; Van den Brandt, P.A.; Wedel, M.; Ockhuizen, T. The reproducibility of the conversion of nitrate to nitrite in human saliva after a nitrate load. Food Chem. Toxicol. 1988, 26, 93–97. [Google Scholar] [CrossRef]
- Obrist, R.; von Meiss, M.; Obrecht, J.P. Verwendung paramedizinischer Behandlungsmethoden durch Tumorpatienten. Eine Erhebung an 101 ambulanten Patienten. Dtsch. Med. Wochenschr. 1986, 111, 283–287. [Google Scholar] [CrossRef]
- Crespi, M.; Ohshima, H.; Ramazzotti, V.; Muñoz, N.; Grassi, A.; Casale, V.; Leclerc, H.; Calmels, S.; Cattoen, C.; Kaldor, J. Intragastric nitrosation and precancerous lesions of the gastrointestinal tract: Testing of an etiological hypothesis. IARC Sci. Publ. 1987, 84, 511–517. [Google Scholar]
- Pátkai, G.; Barta, J.; Varsányi, I. Decomposition of anticarcinogen factors of the beetroot during juice and nectar production. Cancer Lett. 1997, 114, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, M.A.; Nyhlin, H. Beeturia and colonic oxalic acid. QJM-Int. J. Med. 1995, 88, 711–717. [Google Scholar]
- Morant, R.; Jungi, W.F.; Koehli, C.; Senn, H.J. Warum benützen Tumorpatienten Alternativmedizin? [Why do cancer patients use alternative medicine?]. Schweiz. Med. Wochenschr. 1991, 121, 1029–1034. [Google Scholar] [PubMed]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; Dimenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The effect of nitrate supplementation on exercise performance in healthy individuals: A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab 2013, 23, 522–532. [Google Scholar] [CrossRef]
- Lane, S.C.; Hawley, J.A.; Desbrow, B.; Jones, A.M.; Blackwell, J.R.; Ross, M.L.; Zemski, A.J.; Burke, L.M. Single and combined effects of beetroot juice and caffeine supplementation on cycling time trial performance. Appl. Physiol. Nutr. Metab. 2014, 39, 1050–1057. [Google Scholar] [CrossRef]
- Stanelle, S.T.; McLaughlin, K.L.; Crouse, S.F. One Week of L-Citrulline Supplementation Improves Performance in Trained Cyclists. J. Strength Cond. Res. 2020, 34, 647–652. [Google Scholar] [CrossRef]
- Rhim, H.C.; Kim, S.J.; Park, J.; Jang, K.M. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Health Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef]
- Lussi, A.; Jaeggi, T.; Zero, D. The role of diet in the aetiology of dental erosion. Caries Res. 2004, 38 (Suppl. 1), 34–44. [Google Scholar] [CrossRef]
- Frese, C.; Frese, F.; Kuhlmann, S.; Saure, D.; Reljic, D.; Staehle, H.J.; Wolff, D. Effect of endurance training on dental erosion, caries, and saliva. Scand. J. Med. Sci. Sports 2015, 25, e319–e326. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Trexler, E.T.; Lazinica, B.; Pedisic, Z. Effects of caffeine intake on muscle strength and power: A systematic review and meta-analysis. J. Inter. Soc. Sports Nutr. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Keith, D.S.; Schwartz, T.A.; Ryan, E.D.; Stoner, L.; Persky, A.M.; Smith-Ryan, A.E. Effects of Citrulline Malate and Beetroot Juice Supplementation on Blood Flow, Energy Metabolism, and Performance During Maximum Effort Leg Extension Exercise. J. Strength Cond. Res. 2019, 33, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Vårvik, F.T.; Bjørnsen, T.; Gonzalez, A.M. Acute Effect of Citrulline Malate on Repetition Performance During Strength Training: A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Yang, Y.; Mangine, G.T.; Pinzone, A.G.; Ghigiarelli, J.J.; Sell, K.M. Acute Effect of L-Citrulline Supplementation on Resistance Exercise Performance and Muscle Oxygenation in Recreationally Resistance Trained Men and Women. J. Funct. Morphol. Kinesiol. 2023, 8, 88. [Google Scholar] [CrossRef]
- Hurst, P.; Foad, A.; Coleman, D.; Beedie, C. Athletes Intending to Use Sports Supplements Are More Likely to Respond to a Placebo. Med. Sci. Sports Exerc. 2017, 49, 1877–1883. [Google Scholar] [CrossRef]
- Beedie, C.J.; Foad, A.J. The placebo effect in sports performance: A brief review. Sports Med. 2009, 39, 313–329. [Google Scholar] [CrossRef]

