1. Introduction
Hematological cancers are a significant global health issue characterized by high mortality rates [1,2]. They negatively affect patients’ life expectancy and quality of life (QoL) and impose a substantial economic burden [3,4,5,6]. The clinical outcomes for hematological cancers have improved with the development of targeted therapies, such as small molecule inhibitors, monoclonal antibodies, and recombinant immunotoxins and, more recently, chimeric antigen receptor (CAR)-T cell therapies, antibody–drug conjugates, and bispecific T-cell engagers [7]. CAR-T therapies have emerged as a revolutionary treatment option, demonstrating remarkably effective and durable clinical responses for hematological cancers [8,9]. This therapy involves reprogramming the patient’s own T-cells to target the tumor cells wherein host T-cells are collected and are genetically modified ex vivo to express a CAR targeting a tumor-specific antigen [10]. To date, a total of six CAR-T therapies (tisagenlecleucel, axicabtagene ciloleucel, brexucabtagene autoleucel, lisocabtagene maraleucel, idecabtagene vicleucel, and ciltacabtagene autoleucel) have been approved by the United States Food and Drug Administration (FDA) for multiple hematological cancers [11,12,13] based on pivotal clinical trials demonstrating promising results of efficacy outcomes [14,15,16,17,18,19]. Notably, clinical trials focusing on CAR-T therapies have exhibited complete remission rates of 70–90% in relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) [14,15,16] and 40–58% in non-Hodgkin lymphoma (NHL) [17]. Additionally, an overall response rate of 97% was shown in relapsed or refractory multiple myeloma [19,20]. Furthermore, the complete response rate of 73.1% was shown in lenalidomide-refractory multiple myeloma in a phase 3 randomized open-label trial, CARTITUDE-4 [21].
CAR-T therapies have typically been administered in an inpatient setting followed by monitoring of patients closely for several weeks for serious side effects, such as cytokine release syndrome (CRS) and neurotoxicity [22]. However, the outpatient delivery of CAR-Ts is rapidly expanding for patients with a suitable benefit–risk clinical profile and based on overall greater predictability of the clinical course and patient preference. This has the potential to significantly reduce the treatment burden for patients and caregivers and the overall cost burden to the healthcare system associated with inpatient care [22]. Previous trials have demonstrated the feasibility of outpatient CAR-T administration and indicated that such outpatient infusion may be more convenient and preferred by patients and health systems [22,23,24,25]. Challenges in outpatient CAR-T administration, however, include the availability of trained multidisciplinary teams and the infrastructure required to identify and manage complications that need early intervention, suitable reimbursement policies, and caregiver education. Furthermore, patient-specific factors including disease characteristics, clinical status, predictability of adverse events (AEs), medical center proximity, and caregiver support impact the decision on the setting of CAR-T administration [22,26]. Although there are some standalone studies that have presented a case for outpatient delivery of CAR-T therapies, there is currently no published systematic literature review (SLR) comparing the clinical safety, efficacy, QoL, economic implication, and healthcare resource utilization (HCRU) of CAR-T administration in the two settings. This SLR aims to fill this gap by identifying and summarizing the existing clinical and economic evidence on CAR-T therapies and comparing the outcomes for inpatient versus outpatient CAR-T administration in patients with hematological cancer.
2. Methods
2.1. Study Design and Search Process
This SLR was carried out in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist [27] and the Cochrane handbook for systematic reviews of interventions, version 6.3 [28]. The protocol has not been registered. Records were retrieved from MEDLINE, Embase, and Cochrane electronic databases. Additionally, manual searching of conference proceedings, bibliographic sources, and other grey literature sources, such as Google, Google Scholar, and disease-specific websites/conferences [American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), American Society of Hematology (ASH), European Hematology Association (EHA), International Society for Pharmacoeconomics and Outcomes Research (ISPOR), and Academy of Managed Care Pharmacy (AMCP)] was carried out. The bibliographies of relevant SLRs on the research topic were also searched to identify any additional studies. The search strategies for each database are provided in . The literature search was limited to articles published from 1 January 2016 to 4 January 2023. The starting year was chosen as 2016 to ensure the coverage of all relevant studies that may have influenced the approvals of CAR-T therapy, the first of which occurred in 2017.
2.2. Eligibility Criteria
This SLR included studies reporting relevant outcomes of CAR-T therapy administration in both outpatient and inpatient settings or only the outpatient setting among patients with lymphoma, ALL, or multiple myeloma. Relevant outcomes included safety, efficacy, QoL, costs, and HCRU measures. Studies that did not report the setting of CAR-T administration were excluded. Clinical trials and observational (prospective and retrospective) studies were included whereas non-human studies were excluded. The screening of articles to evaluate conformance to eligibility criteria was performed independently by two reviewers and any disagreements were resolved in discussion with a third reviewer.
2.3. Data Extraction
Two reviewers independently extracted the following data from the included studies: study characteristics, patient characteristics, treatment-related information, and outcomes of interest. Any inconsistencies were resolved through discussion between the two reviewers. If necessary, a third reviewer was consulted to mediate and reach a consensus.
2.4. Quality Assessment and Risk of Bias
The Cochrane risk-of-bias tool version 2 (RoB 2) for randomized controlled trials (RCTs) [29], the Downs and Black (Downs 1998) checklist for non-RCTs [30], and the Newcastle and Ottawa scale (NOS) for observational studies [31] were utilized for quality assessment.
2.5. Data Analysis
Evidence identified from the systematic literature search was analyzed qualitatively. The compiled evidence was tabulated, summarized, and presented graphically for the following elements of the research: study details (trial design, tumor type, treatment setting, sample size, and follow-up duration) and outcomes presented (safety, efficacy, QoL, HCRU, and costs incurred). Safety outcomes included the cytokine release syndrome (CRS), neurologic toxicities, and other toxicities reported in individual publications while the collated efficacy outcomes included the complete response (CR), partial response (PR), overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). HCRU measures reported included the rate of, time to, and reasons for hospitalization, length of hospital stay, rate of ICU admissions and length of stay, and outpatient visits. Costs incurred in different follow-up periods post-infusion were compiled and categorized as available.
The data were reported separately for outpatient and inpatient cohorts and included evidence from comparative as well as single cohort studies.
3. Results
3.1. Literature Search Results
Database searches identified 7701 initial records. After deduplication, 5648 records remained for screening against inclusion and exclusion criteria. A total of 1125 records met the relevant criteria and an additional 83 records were obtained from supplementary sources including Google Scholar, conference proceedings, and a bibliography of identified studies and SLRs, resulting in a total of 1208 records. Ultimately, 38 records that reported outcomes for patients who underwent infusion/management in the outpatient setting or in both outpatient and inpatient settings were considered for qualitative synthesis (Figure 1).
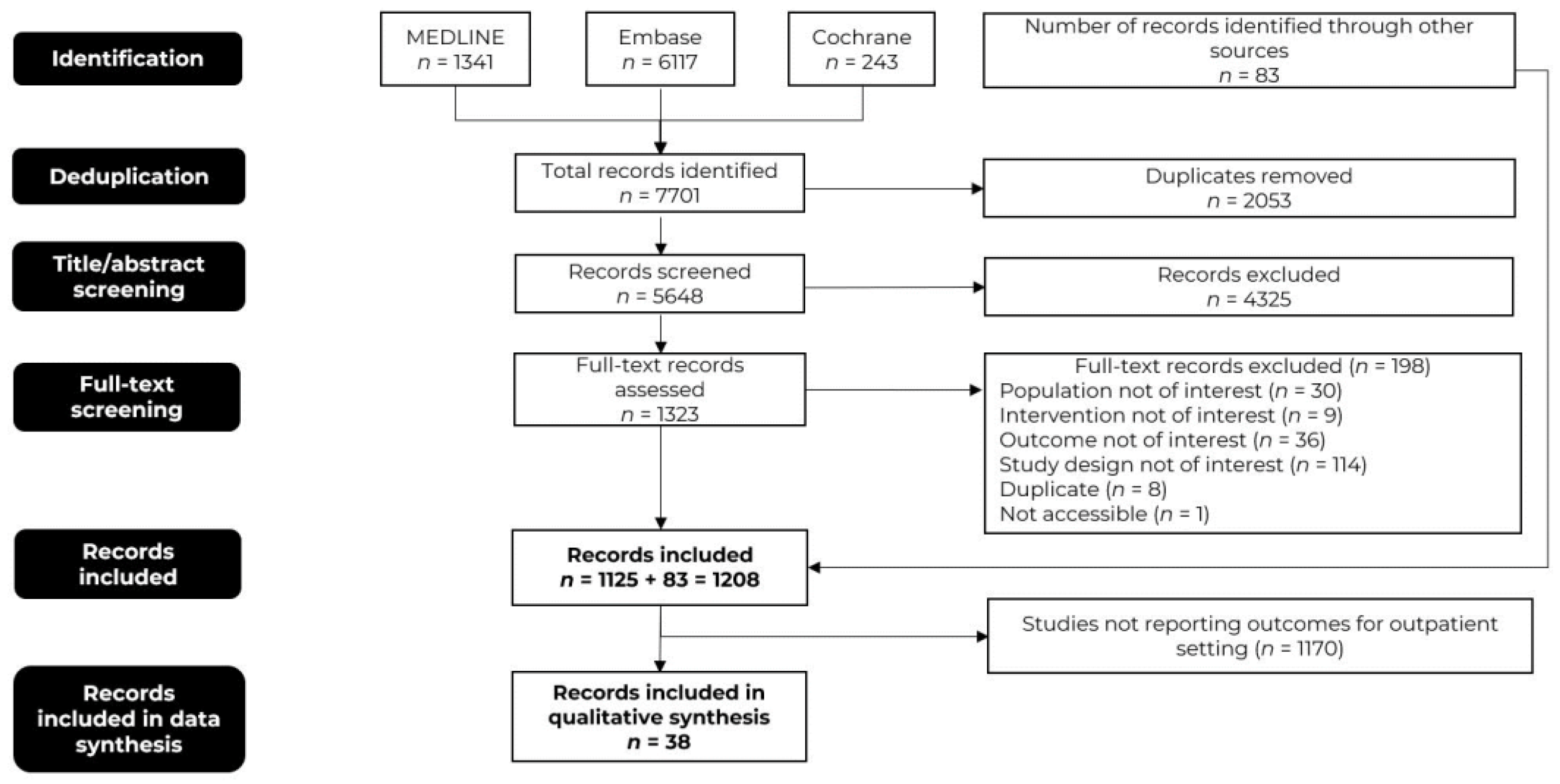
Figure 1. The preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram.
3.2. Study Characteristics
The 38 included records were based on 21 unique studies, most of which were published in 2022 and 2023. The patient populations of these studies included individuals diagnosed with ALL, various types of lymphoma, such as B-cell lymphoma (BCL) and follicular lymphoma (FL), and multiple myeloma.
In total, 18 of these 21 studies were conducted in the United States [26,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. The TRANSFORM phase 3 clinical trial was conducted in the United States, Japan, and various European countries including Belgium, France, Germany, Italy, the Netherlands, Spain, Sweden, Switzerland, and the United Kingdom [64,65]. Additionally, the ELARA phase 2 clinical trial encompassed multinational locations, including centers in the United States and Australia [66,67]. Only one retrospective study did not report the specific location where it was conducted [68]. Eleven studies reported data on CAR-T administration in both outpatient and inpatient settings [26,32,33,34,35,36,37,38,39,40,41,42,43,44,52,53,54,58,61,62,64,65,66,67,68]. These publications were based on 5 clinical trials, namely OUTREACH [32,33,34,35,36,37,38], ELARA [66,67], PILOT [39,40,41], TRANSCEND NHL 001 [42,43,44], and TRANSFORM [64,65], where the choice of site of care for patients treated with CAR-T therapies was determined at the investigator’s discretion, taking into consideration the perspectives of multiple stakeholders including healthcare providers, patients, and caregivers.
Nine of the shortlisted studies reported efficacy outcomes including response rates and survival outcomes [36,37,39,42,47,55,57,60,63,67]. Only one clinical trial reported QoL in the patient groups of interest [38]. Five studies reported data on AEs, including CRS and neurologic toxicity [37,39,42,53,67]. Seven studies reported costs/reimbursement amounts associated with the administration of CAR-T in both settings [26,41,52,53,62,65,67]. Ten studies reported data on HCRU [26,37,39,41,52,53,54,62,67,68] in both settings while a further seven studies reported HCRU among patients who received CAR-T only in the outpatient setting [26,37,39,41,46,52,53,54,55,57,59,60,62,63,64,67,68]. The distribution of age, sex, performance status, number of prior treatment lines, and other patient characteristics varied across the studies. Details of the included studies and patient characteristics are shown in and .
3.3. Quality Assessment
All of the 21 studies included in the analysis underwent a quality assessment using relevant checklists based on their study design. The assessment aimed to evaluate the risk of bias and the overall quality of the studies. The ROB 2 checklist was used to assess the RCTs conducted by Kamdar in 2022 [64]. The findings from this assessment revealed a high risk of bias in the trial . The modified Downs and Black checklist with 27 items assessed eight non-randomized single-arm trials. highlights one study of good quality (score, 15–17), five of fair quality (score, 12–14), and two of poor quality (score < 11) [38,39,42,46,49,51,67]. The NOS criteria were used to assess the quality of double-arm and single-arm observational studies. Of the six double-arm studies, five were considered of good quality (score > 6) and one of fair quality (score 5) . Among the six single-arm studies, five were rated as good quality (score > 4) and one as fair quality (score 4) [26,41,45,53,54,55,57,59,60,62,63,68].
3.4. Clinical Outcomes
3.5. Economic Outcomes
4. Discussion
This comprehensive SLR on CAR-T therapies in patients with hematological cancer highlights the potential benefits of outpatient compared with inpatient administration. Safety outcomes, a key consideration in CAR-T treatments and a principal driver for traditionally treating patients in the inpatient setting, were comparable between those treated in the outpatient setting and those treated in the inpatient setting. Moreover, the analysis revealed comparable effectiveness outcomes between the two settings, including the response rates, duration of response, and survival outcomes where reported. Furthermore, both outpatient and inpatient cohorts experienced meaningful improvements in QoL measures. These findings collectively provide compelling evidence to clinicians and other decision-makers to actively consider administering CAR-T therapies in the outpatient setting for patients whose disease and clinical characteristics permit this. Treating institutions may upgrade their processes and protocols to encourage treatment in the outpatient setting, including preparing for and managing early complications.
In the reviewed studies, the choice of outpatient administration was at the investigator’s discretion, taking into account patient disease characteristics, clinical status, and logistical considerations, such as the availability of caregiver support and the ability to remain within a short distance from the treatment site for 30 days after infusion [32,34,35,36,37,38,39,42,43,44,64,65,66,67]. Typically, patients receiving CAR-T therapy in the outpatient setting are closely monitored by a multidisciplinary CAR-T therapy team and adhere to standard operating procedures for outpatient AE monitoring and management [32,34,35,36,37,38,66,67].
To further bolster the case for outpatient administration of CAR-T therapies, assessment of such administration in the real world with longer-term follow-up to allow for the evaluation of survival outcomes such as PFS and OS is warranted. These endpoints are crucial in evaluating the long-term benefits and potential risks associated with the two treatment settings [69].
The observed enhancement in QoL reported by Linhares et al. (2022) likely represents the comprehensive impact of CAR-T therapy on hematological cancer patients, regardless of the treatment setting [38]. Further research is needed to explore the underlying factors contributing to the improvement in QoL, paying particular attention to factors related to reduced hospitalization as this could enhance the overall patient experience and improve their QoL during treatment. It is also important to evaluate QoL at multiple time points after CAR-T therapy use and examine if the resolution of AEs reflects improved QoL outcomes. Furthermore, the utilization of other measures, such as the hospital anxiety and depression scale depression subscale (HADS-D), in patients with hematological cancer is important in understanding and addressing their mental health needs more specifically [70]. Additionally, a previous SLR emphasized the importance of identifying patients’ preferences for involvement in cancer treatment decisions. Establishing these preferences will encourage the healthcare system to become more responsive to individual patient needs and expectations and ultimately, contribute to improving their QoL [71].
The economic outcomes indicate that outpatient treatment may offer cost advantages over inpatient treatment, with patients treated in the latter incurring two to four times higher costs (USD 62,000–96,000) than by outpatient-treated patients (USD 16,000–38,000) [41,52,65]. The lower costs observed in the outpatient cohort were primarily driven by reduced hospitalization costs [41,62,65,67]. These findings align with an economic evaluation of CAR-T therapy based on the site of care, wherein outpatient CAR-T administration resulted in a substantial decrease in total costs (by 40.4%), with notable reductions observed in hospitalization, office visits, and procedural expenses [72]. However, a study by Yang (2022) found that the Medicare reimbursement in the outpatient cohort was slightly higher than that in the inpatient cohort during the first month post-infusion and comparable in subsequent months. The authors, however, attributed this to the inadequate reimbursement for CAR-T infusion in the inpatient setting whereas the outpatient setting reimbursement, which is covered under Medicare Part B, covers not only the CAR-T product cost more completely but also the handling, storage, and a portion of the physician’s service fees. Additional efforts are recommended to improve the reimbursement structure and care policies for CAR-T therapy in either infusion setting to suitably incentivize providers [26].
The outpatient cohort experienced more unplanned hospitalizations, with CRS being the main reason for hospitalization. However, the overall HCRU was lower in cases where data were available for both settings as the inpatient cohort exhibited longer stays and a higher HCRU [26,37,39,41,46,52,53,54,55,56,57,59,60,62,63,64,67,68]. An improved understanding of the predictive risk factors for CRS and neurotoxicity development, including patient disease characteristics and clinical status, can influence personalized decisions regarding outpatient administration as it may offer potential benefits in terms of overall resource utilization.
Outpatient CAR-T administration can potentially expand treatment access by freeing up inpatient capacity and addressing geographic obstacles. A prior economic model concluded that lower costs through outpatient administration could enable more patients to receive treatment with limited resources. [72].
This review provides a comprehensive analysis of both clinical and economic outcomes derived from clinical trials and observational studies concerning CAR-T therapies in a broad patient population with hematological cancer. The review was conducted in accordance with a predefined protocol, with clear inclusion and exclusion criteria, and adhered to the Cochrane guidelines for systematic review reporting. A comprehensive search strategy was employed to minimize reporting bias in the review process.
While this SLR adhered to rigorous selection criteria, it had some limitations. The included studies exhibited heterogeneity in terms of methodology and populations, which prevented direct comparisons. Additionally, in studies that reported outcomes related to the two settings, patients were not randomized between the settings, introducing the potential for bias and raising concerns about the comparability of reported outcomes. In line with the objective of this study, outcomes data were presented here only if reported by setting. The molecular aspect of the therapy has not been discussed as none of the identified publications referred to it as either the driver for the decision of inpatient vs. outpatient administration or as the cause of any difference in the outcomes. Furthermore, patient characteristics were not available for all the studies as the majority of the identified publications were conference abstracts with minimal information on patient characteristics.
5. Conclusions
Findings from our study showed comparable overall outcomes in safety, efficacy, and QoL between outpatient and inpatient CAR-T administration. While CAR-Ts are typically administered in an inpatient setting, outpatient administration of CAR-T can provide a reduced economic burden without negatively impacting clinical outcomes and should be actively considered where patient disease characteristics and logistical considerations permit this. Future research is needed to explore the impact of administration settings of new CAR-T therapies on patients with multiple myeloma and other hematological cancers.
References
- Du, M.; Chen, W.; Liu, K.; Wang, L.; Hu, Y.; Mao, Y.; Sun, X.; Luo, Y.; Shi, J.; Shao, K.; et al. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J. Oncol. 2022, 2022, 1612702. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Allart-Vorelli, P.; Porro, B.; Baguet, F.; Michel, A.; Cousson-Gélie, F. Haematological cancer and quality of life: A systematic literature review. Blood Cancer J. 2015, 5, e305. [Google Scholar] [CrossRef]
- La Nasa, G.; Caocci, G.; Morelli, E.; Massa, E.; Farci, A.; Deiana, L.; Pintus, E.; Scartozzi, M.; Sancassiani, F. Health Related Quality of Life in Patients with Onco-hematological Diseases. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.; Leal, J.; Sullivan, R.; Luengo-Fernandez, R. Economic burden of malignant blood disorders across Europe: A population-based cost analysis. Lancet Haematol. 2016, 3, e362–e370. [Google Scholar] [CrossRef] [PubMed]
- Yucel, E.; Zhang, S.; Panjabi, S. Health-Related and Economic Burden Among Family Caregivers of Patients with Acute Myeloid Leukemia or Hematological Malignancies. Adv. Ther. 2021, 38, 5002–5024. [Google Scholar] [CrossRef]
- Sochacka-Ćwikła, A.; Mączyński, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies-The Last Decade Review. Cancers 2021, 14, 87. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Lei, W.; Xie, M.; Jiang, Q.; Xu, N.; Li, P.; Liang, A.; Young, K.H.; Qian, W. Treatment-Related Adverse Events of Chimeric Antigen Receptor T-Cell (CAR T) in Clinical Trials: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 3912. [Google Scholar] [CrossRef]
- Parikh, R.H.; Lonial, S. Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice. CA Cancer J. Clin. 2023, 73, 275–285. [Google Scholar] [CrossRef]
- Chimeric Antigen Receptor CAR T Cell Therapy Cancer Treatment Market Size Report. Available online: https://www.globenewswire.com/news-release/2022/06/25/2469076/0/en/Chimeric-Antigen-Receptor-CAR-T-Cell-Therapy-Cancer-Treatment-Market-Size-Report.html (accessed on 18 July 2023).
- Facts about Chimeric Antigen Receptor (CAR) T-Cell Therapy. Available online: https://www.lls.org/sites/default/files/2021-05/FSHP1_CART_Factsheet_Sept2020_Rev.pdf (accessed on 18 July 2023).
- Barros, L.R.C.; Couto, S.C.F.; da Silva Santurio, D.; Paixão, E.A.; Cardoso, F.; da Silva, V.J.; Klinger, P.; Ribeiro, P.; Rós, F.A.; Oliveira, T.G.M.; et al. Systematic Review of Available CAR-T Cell Trials around the World. Cancers 2022, 14, 2667. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.; Barrett, D.M. Current status of chimeric antigen receptor therapy for haematological malignancies. Br. J. Haematol. 2016, 172, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224ra225. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Quintás-Cardama, A. CD19 directed CAR T cell therapy in diffuse large B-cell lymphoma. Oncotarget 2018, 9, 29843–29844. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; An, G.; Sui, W.; Wang, T.; Zhang, X.; Yang, J.; Zhang, Y.; Zhang, L.; Zhu, D.; Huang, J.; et al. Phase 1 study of C-CAR088, a novel humanized anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. J. Immunother. Cancer 2022, 10, e005145. [Google Scholar] [CrossRef]
- Gagelmann, N.; Riecken, K.; Wolschke, C.; Berger, C.; Ayuk, F.A.; Fehse, B.; Kröger, N. Development of CAR-T cell therapies for multiple myeloma. Leukemia 2020, 34, 2317–2332. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.V.; Fernández de Larrea, C.; Martínez-López, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef]
- Myers, G.D.; Verneris, M.R.; Goy, A.; Maziarz, R.T. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J. Immunother. Cancer 2021, 9, e002056. [Google Scholar] [CrossRef]
- Bachier, C.; Palomba, M.; Abramson, J.; Andreadis, C.; Sehgal, A.; Godwin, J.; Hildebrandt, G.; Siddiqi, T.; Stevens, D.; Farazi, T.; et al. Outpatient Treatment with Lisocabtagene Maraleucel (liso-cel) in 3 Ongoing Clinical Studies in Relapsed/Refractory (R/R) Large B Cell Non-Hodgkin Lymphoma (NHL), Including Second-Line Transplant Noneligible (TNE) Patients: Transcend NHL 001, Outreach, and PILOT. Biol. Blood Marrow Transplant. 2020, 26, S25–S26. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Schuster, S.; Tam, C.; Borchmann, P.; Jaeger, U.; Waller, E.; Holte, H.; McGuirk, J.; Jaglowski, S.; Tobinai, K.; Andreadis, C.; et al. Sustained Disease Control for Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma: An Updated Analysis of Juliet, a Global Pivotal Phase 2 Trial of Tisagenlecleucel. Blood 2018, 132, 1684. [Google Scholar] [CrossRef]
- Yang, H.; Bollu, V.; Lim, S.; Tesfaye, M.; Dalal, A.A.; Lax, A.; Sethi, S.; Zhao, J. Healthcare resource use and reimbursement amount by site of care in patients with diffuse large B-cell lymphoma receiving chimeric antigen receptor T-cell (CAR-T) therapy–a retrospective cohort study using CMS 100% Medicare claims database. Leuk. Lymphoma 2022, 64, 339–348. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 18 July 2023).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Newcastle-Ottawa Quality Assessment form for Cohort and Case-Control Studies. Available online: https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed on 18 July 2023).
- Godwin, J.; Freytes, C.O.; Maris, M.; Stevens, D.A.; Hoda, D.; Mattar, B.; Varela, J.C.; Cherry, M.; Essell, J.; Courtright, J.; et al. Outcomes of Treatment with the Chimeric Antigen Receptor (CAR) T Cell Therapy Lisocabtagene Maraleucel (liso-cel) at Nonuniversity Medical Centers (NMCs): Initial Results from the Outreach Study in Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL). Transplant. Cell Ther. 2021, 27, S203–S204. [Google Scholar]
- Godwin, J.E.; Freytes, C.O.; Maris, M.; Stevens, D.A.; Hoda, D.; Mattar, B.; Varela, J.C.; Cherry, M.; Essell, J.; Courtright, J.; et al. Outcomes of Treatment with the Chimeric Antigen Receptor (CAR) T Cell Therapy Lisocabtagene Maraleucel (liso-cel) in the Nonuniversity Setting: Initial Results from the Outreach Study. Blood 2020, 136, 50–52. [Google Scholar] [CrossRef]
- Godwin, J.E.; Mattar, B.; Maris, M.; Bachier, C.; Stevens, D.A.; Hoda, D.; Varela, J.C.; Cherry, M.; Fanning, S.; Essell, J.; et al. Outreach: Preliminary safety & efficacy results from a phase 2 study of lisocabtagene maraleucel (LISO-CEL) in the nonuniversity setting. Hematol. Oncol. 2021, 39, 368–370. [Google Scholar] [CrossRef]
- Godwin, J.E.; Mattar, B.I.; Maris, M.B.; Bachier, C.R.; Stevens, D.A.; Hoda, D.; Varela, J.C.; Cherry, M.; Fanning, S.R.; Essell, J.H.; et al. Outreach: Preliminary safety and efficacy results from a phase 2 study of lisocabtagene maraleucel (liso-cel) in the non university setting. J. Clin. Oncol. 2021, 39, e19513. [Google Scholar] [CrossRef]
- Godwin, J.E.; Mattar, B.; Maris, M.; Bachier, C.; Stevens, D.; Hoda, D.; Varela, J.C.; Cherry, M.; Fanning, S.; Essell, J.; et al. Outreach: Results from a Phase 2 Study of Lisocabtagene Maraleucel (liso-cel) Administered As Inpatient (Inpt) or Outpatient (Outpt) Treatment in the Nonuniversity Setting in Patients (Pts) with R/R Large B-Cell Lymphoma (LBCL). Blood 2021, 138, 1762. [Google Scholar] [CrossRef]
- Linhares, Y.; Freytes, C.; Cherry, M.; Bachier, C.; Maris, M.; Hoda, D.; Varela, J.C.; Bellomo, C.; Cross, S.; Essell, J. Results from Outreach: A Phase 2 Study of Lisocabtagene Maraleucel (Liso-cel) Administered As Outpatient (Outpt) or Inpatient (Inpt) Treatment in the Community/Nonuniversity Setting in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL). Blood 2022, 140, 10416–10418. [Google Scholar] [CrossRef]
- Linhares, Y.; Liu, F.F.; Freytes, C.; Cherry, M.; Shi, L.; Liao, W.; Braverman, J.; Espinola, R.; Vedal, M.; Mattar, B. Lisocabtagene Maraleucel (Liso-cel) in Patients with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL) Treated As Outpatients or Inpatients in the Community/Nonuniversity Setting: Patient-Reported Outcomes/Health-Related Quality of Life from the Outreach Study. Blood 2022, 140, 10930–10931. [Google Scholar] [CrossRef]
- Sehgal, A.; Hoda, D.; Riedell, P.A.; Ghosh, N.; Hamadani, M.; Hildebrandt, G.C.; Godwin, J.E.; Reagan, P.M.; Wagner-Johnston, N.; Essell, J. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): An open-label, phase 2 study. Lancet Oncol. 2022, 23, 1066–1077. [Google Scholar] [CrossRef]
- Sehgal, A.R.; Godwin, J.; Pribble, J.; Wang, L.; Thorpe, J.; Hildebrandt, G.C. Lisocabtagene maraleucel (liso-cel) for treatment of second-line transplant noneligible (TNE) relapsed/refractory (R/R) aggressive non-hodgkin lymphoma (NHL): Initial results from the PILOT Study. Blood 2019, 134, 2882. [Google Scholar] [CrossRef]
- McGarvey, N.; Gitlin, M.; Lee, A.; Keating, S. Post-infusion monitoring costs by site of care among patients with relapsed or refractory large B-cell lymphoma who received second-line treatment with lisocabtagene maraleucel in the PILOT study. J. Manag. Care Spec. Pharm. 2022, 28, S32–S33. [Google Scholar]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Maloney, D.G.; Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Arnason, J.E.; Wang, M.; Forero, A.; Albertson, T.; Garcia, J.; et al. Preliminary Safety Profile of the CD19-Directed Defined Composition CAR T Cell Product JCAR017 in Relapsed/Refractory Aggressive B-NHL Patients: Potential for Outpatient Administration. Blood 2017, 130, 1552. [Google Scholar] [CrossRef]
- Palomba, M.L.; Garcia, J.; Wang, L.; Dehner, C.; Chung, K.C.; Maloney, D.G. TRANSCEND: Lisocabtagene Maraleucel (liso-cel; JCAR017) Healthcare Resource Utilization in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2018, 132, 3545. [Google Scholar] [CrossRef]
- Gofshteyn, J.S.; Shaw, P.A.; Teachey, D.T.; Grupp, S.A.; Maude, S.; Banwell, B.; Chen, F.; Lacey, S.F.; Melenhorst, J.J.; Edmonson, M.J. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann. Neurol. 2018, 84, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.M.; Fitzgerald, J.C.; DiNofia, A.; Wray, L.; Leahy, A.B.; Li, Y.; Smith, L.T.; Burrows, E.K.; Ramos, M.; Motley, L.S. Inpatient and intensive care unit resource utilization after CD19-targeted chimeric antigen receptor T-cell therapy (CART19) for pediatric acute lymphoblastic leukemia (ALL). Biol. Blood Marrow Transplant. 2020, 26, S202–S203. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.; Redman, M.; Lee, S.Y.; Lee, D.H.; Ra, S.; Ujjani, C.S.; Dezube, B.J.; Poh, C.; Warren, E.H.; et al. Safety and Efficacy of Third Generation CD20 Targeted CAR-T (MB-106) for Treatment of Relapsed/Refractory B-NHL and CLL. Blood 2021, 138, 3872. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.; Redman, M.W.; Lee, S.Y.; Lee, D.H.; Ramachandran, A.; Ra, S.; Marzbani, E.A.; Graf, S.A.; Warren, E.H.; et al. Third Generation CD20 Targeted CAR T-Cell Therapy (MB-106) for Treatment of Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Blood 2020, 136, 38–39. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.; Redman, M.; Lee, S.; Lee, D.; Ra, S.; Ramachandran, A.; Lynch, R.; Smith, S.; Poh, C.; et al. Immunotherapy Using a 3rd Generation CD20 Targeted CAR T-Cell (MB-106) for Treatment of B-Cell Non-Hodgkin Lymphoma (B-NHL) and Chronic Lymphocytic Leukemia (CLL). HemaSphere 2021, 5, 335. [Google Scholar]
- Shadman, M.; Yeung, C.; Redman, M.; Lee, S.Y.; Lee, D.H.; Ra, S.; Qian, D.; Ujjani, C.; Dezube, B.; Poh, C.; et al. Efficacy and safety of a third generation CD20 CART (MB-106) for treatment of Relapsed/Refractory Follicular Lymphoma (FL). HemaSphere 2022, 6, 108–109. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hay, K.A.; Gust, J.; Hanaff, L.A.; Li, D.; Liles, W.C.; Wurfel, M.; Harju-Baker, S.; Myerson, D.; Gonzalez-Cuyar, L.; et al. Cytokine release syndrome (CRS) and neurotoxicity (NT) after CD19-specific chimeric antigen receptor-(CAR-) modified T cells. J. Clin. Oncol. 2017, 35, 3020. [Google Scholar] [CrossRef]
- Palomba, M.L.; Jun, M.P.; Garcia, J.; Lymp, J.; McGarvey, N.; Gitlin, M.; Pelletier, C.; Nguyen, A. Costs of Postinfusion Monitoring By Site of Care for Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL) Who Received Third-Line or Later Treatment with Lisocabtagene Maraleucel (liso-cel) in the Transcend NHL 001 and Outreach Trials. Blood 2020, 136, 16–17. [Google Scholar] [CrossRef]
- Denlinger, N.; Huang, Y.; Braunstein, Z.; Sigmund, A.; Bajwa, A.; Kapoor, N.; Agyeman, A.; Fisher, S.; Purdin, Z.; Neal, A.; et al. ABCL-509 Healthcare Utilization and Costs in Chimeric Antigen Receptor T-Cell Therapy for B-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2022, 22, S381–S382. [Google Scholar] [CrossRef]
- Chihara, D.; Liao, L.; Tkacz, J.; Lewing, B.; Franco, A.; Kilgore, K.M.; Nastoupil, L.J.; Chen, L. Real-World Effectiveness and Economic Impact Associated with Chimeric Antigen Receptor T-Cell Therapy Among Older Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma in US. Blood 2022, 140, 2421–2423. [Google Scholar] [CrossRef]
- Borogovac, A.; Keruakous, A.; Bycko, M.; Chakrabarty, J.H.; Ibrahimi, S.; Khawandanah, M.; Selby, G.B.; Yuen, C.; Schmidt, S.; Autry, M.T.; et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: Experience from a tertiary care center. Bone Marrow Transplant. 2022, 57, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Borogovac, A.; Keruakous, A.R.; Bycko, M.; Chakrabarty, J.H.; Ibrahimi, S.; Khawandanah, M.O.; Selby, G.B.; Yuen, C.; Schmidt, S.A.; Al-Juhaishi, T.; et al. Successful Development of an Outpatient Chimeric Antigen Receptor (CAR) T Cell Therapy Program. Blood 2021, 138, 4821. [Google Scholar] [CrossRef]
- Shao, Y.F.; Modi, D.; Kin, A.; Alavi, A.; Ayash, L.; Ratanatharathorn, V.; Uberti, J.P.; Deol, A. Feasibility of Outpatient CAR T Cell Therapy: Experience of a Single Institution. Blood 2021, 138, 4828. [Google Scholar] [CrossRef]
- Zhao, J.; Bollu, V.; Yang, H.; Dalal, A.; Tesfaye, M.; Ma, Q.; Lax, A.; Lim, S. Healthcare resource use (HRU) by infusion setting of chimeric antigen receptor T-cell (CAR-T) in patients with relapsed and refractory (r/r) diffuse large B-cell lymphoma (DLBCL): A retrospective cohort study using CMS 100% Medicare database. J. Clin. Oncol. 2021, 39, e19550. [Google Scholar] [CrossRef]
- Farooqui, N.; Sy-Go, J.P.T.; Miao, J.; Mehta, R.; Vaughan, L.E.; Bennani, N.N.; Wang, Y.; Bansal, R.; Hathcock, M.A.; Hayman, S.R. Incidence and Risk Factors for Acute Kidney Injury After Chimeric Antigen Receptor T-Cell Therapy. Mayo Clin. Proc. 2022, 97, 1294–1304. [Google Scholar] [CrossRef]
- Nasta, S.D.; Hughes, M.E.; Namoglu, E.C.; Garfall, A.; DiFilippo, H.; Ballard, H.J.; Barta, S.K.; Chong, E.A.; Frey, N.V.; Gerson, J.N. Outcomes of Tisagenlecleucel in Lymphoma Patients With Predominant Management in an Ambulatory Setting. Clin. Lymphoma Myeloma Leuk. 2022, 22, e730–e737. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, R.; Yang, H.; Liu, Q.; Zhao, J.; Lee, S.; Dalal, A.; Lim, S.; Bollu, V. Real-world healthcare resource utilization and costs associated with tisagenlecleucel and axicabtagene ciloleucel among patients with diffuse large B-cell lymphoma: An analysis of hospital data. J. Manag. Care Spec. Pharm. 2021, 27, S37–S38. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Yang, H.; Liu, Q.; Wang, T.; Zhao, J.; Lim, S.; Lee, S.; Dalal, A.; Bollu, V. Real-world healthcare resource utilization and costs associated with tisagenlecleucel and axicabtagene ciloleucel among patients with diffuse large B-cell lymphoma: An analysis of hospital data in the United States. Leuk. Lymphoma 2022, 63, 2052–2062. [Google Scholar] [CrossRef]
- Kirby, S.; Hoda, D.; Hunter, B. Successful Outpatient Treatment and Monitoring Following Administration of Various Anti-CD19 Chimeric Antigen Receptor Therapies in B-Cell Lymphomas. Blood 2022, 140, 10812–10813. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- McGarvey, N.; Gitlin, M.; Lee, A.; Keating, S. Post-infusion monitoring costs by site of care among patients with relapsed or refractory large B-cell lymphoma who received second-line treatment with lisocabtagene maraleucel in the TRANSFORM study. J. Manag. Care Spec. Pharm. 2022, 28, S33. [Google Scholar]
- Fowler, N.H.; Dickinson, M.; Ghosh, M.; Chen, A.; Andreadis, C.; Tiwari, R.; Masood, A.; Ramos, R.; Bollu, V.; Jousseaume, E.; et al. Assessment of Healthcare Resource Utilization and Costs in Patients with Relapsed or Refractory Follicular Lymphoma Undergoing CAR-T Cell Therapy with Tisagenlecleucel: Results from the Elara Study. Blood 2021, 138, 3533. [Google Scholar] [CrossRef]
- Fowler, N.H.; Dickinson, M.; Ghosh, M.; Chen, A.I.; Andreadis, C.; Tiwari, R.; Masood, A.; Ramos, R.; Jousseaume, E.; Thieblemont, C. Assessment of Healthcare Resource Utilization and Hospitalization Costs in Patients with Relapsed or Refractory Follicular Lymphoma Undergoing CAR-T Cell Therapy With Tisagenlecleucel: Results From the ELARA Study. Transplant. Cell Ther. 2023, 29, 60.e61–60.e64. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Anstadt, E.; Baron, J.; LaRiviere, M.; LaRose, M.; Landsburg, D.; Svoboda, J.; Nasta, S.; Gerson, J.; Barta, S.; et al. Impact of Radiotherapy on Hospitalization Burden Surrounding Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, E51–E52. [Google Scholar] [CrossRef]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology—A primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar]
- Senf, B.; Grabowski, K.; Spielmann, N.; Fettel, J. Quality of life and distress assessed with self and external assessment screening tools in patients with hematologic malignancies attending treatment in an acute hospital. Qual. Life Res. 2020, 29, 3375–3385. [Google Scholar] [CrossRef]
- Hubbard, G.; Kidd, L.; Donaghy, E. Preferences for involvement in treatment decision making of patients with cancer: A review of the literature. Eur. J. Oncol. Nurs. 2008, 12, 299–318. [Google Scholar] [CrossRef]
- Lyman, G.H.; Nguyen, A.; Snyder, S.; Gitlin, M.; Chung, K.C. Economic Evaluation of Chimeric Antigen Receptor T-Cell Therapy by Site of Care Among Patients with Relapsed or Refractory Large B-Cell Lymphoma. JAMA Netw. Open 2020, 3, e202072. [Google Scholar] [CrossRef]

