1. Introduction
Sepsis is one of the leading causes of death in critically ill children, accounting for remarkably high mortality in all age groups regardless of geographic location and socioeconomic status and mainly affecting neonates and young children under 5 years of age [1]. Therefore, sepsis is a major driver of broad-spectrum antibiotic use and contributes to the emerging global threat of antimicrobial resistance. In turn, antimicrobial resistance negatively affects individuals with sepsis and contributes to the progression of infection to sepsis and septic shock by decreasing the effectiveness of available antimicrobial therapy. Currently, antimicrobial resistance in Gram-negative bacteria is a growing threat in Europe and worldwide [2,3]. The ability of these bacteria to acquire and modify new genetic material that confers on them new resistance mechanisms is a cause of great concern, especially in children, because of the more limited availability of antimicrobials in pediatric patients [4].
The choice of the empirical antimicrobial regimen in children with sepsis admitted to the Pediatric Intensive Care Unit (PICU) is a complex decision since prescribing physicians must weigh the need for broad-spectrum antibiotics with the concern about antimicrobial resistance. Although inadequate initial treatment in patients with septic shock decreases survival, inappropriate and unnecessarily prolonged use of broad-spectrum antimicrobials can also negatively influence morbidity and mortality in patients with sepsis [5,6,7]. This can negatively affect the course of sepsis since it facilitates the selection of resistant strains, increases C. difficile infections, and increases the need for long-term invasive devices, which can increase infectious complications and other adverse effects [8,9]. An update on MDR-sepsis epidemiology and on its associated risk factors among septic children would be helpful for physicians at the bedside.
The aim of this study was to analyze the incidence rate of sepsis caused by multidrug-resistant (MDR) microorganisms and describe the factors associated with MDR bacteria among septic children admitted to the PICU in a Spanish cohort. Our secondary objective was to determine its influence on outcomes.
2. Results
2.1. Study Population
During this study period, a total of 12,295 children were admitted to the PICU, of whom 926 had at least one culture-proven infectious episode (7.3%). The presence of an inflammatory systemic response following sepsis criteria was reported in 432 (47%) of them. Patients’ demographic characteristics are summarized in . Overall, patients with sepsis had a median age of 11 months [4–55 months] and a PRISM III score 24 h after admission of 7 [3,4,5,6,7,8,9,10,11,12]. One hundred and forty-one (32.6%) patients had received antibiotic treatment in the 48 h prior to PICU admission. Forty-nine culture-proven sepsis (11.3%) was caused by a MDR bacteria and 383 culture-proven sepsis (88.7%) by a non-MDR bacteria (Figure 1, Flow chart), with a larger proportion of Gram-negative bacilli (GNB) in the MDR group (p < 0.001). Among the 383 children with non-MDR sepsis, there were 111 (29%) patients with prior MDR infection/colonization.
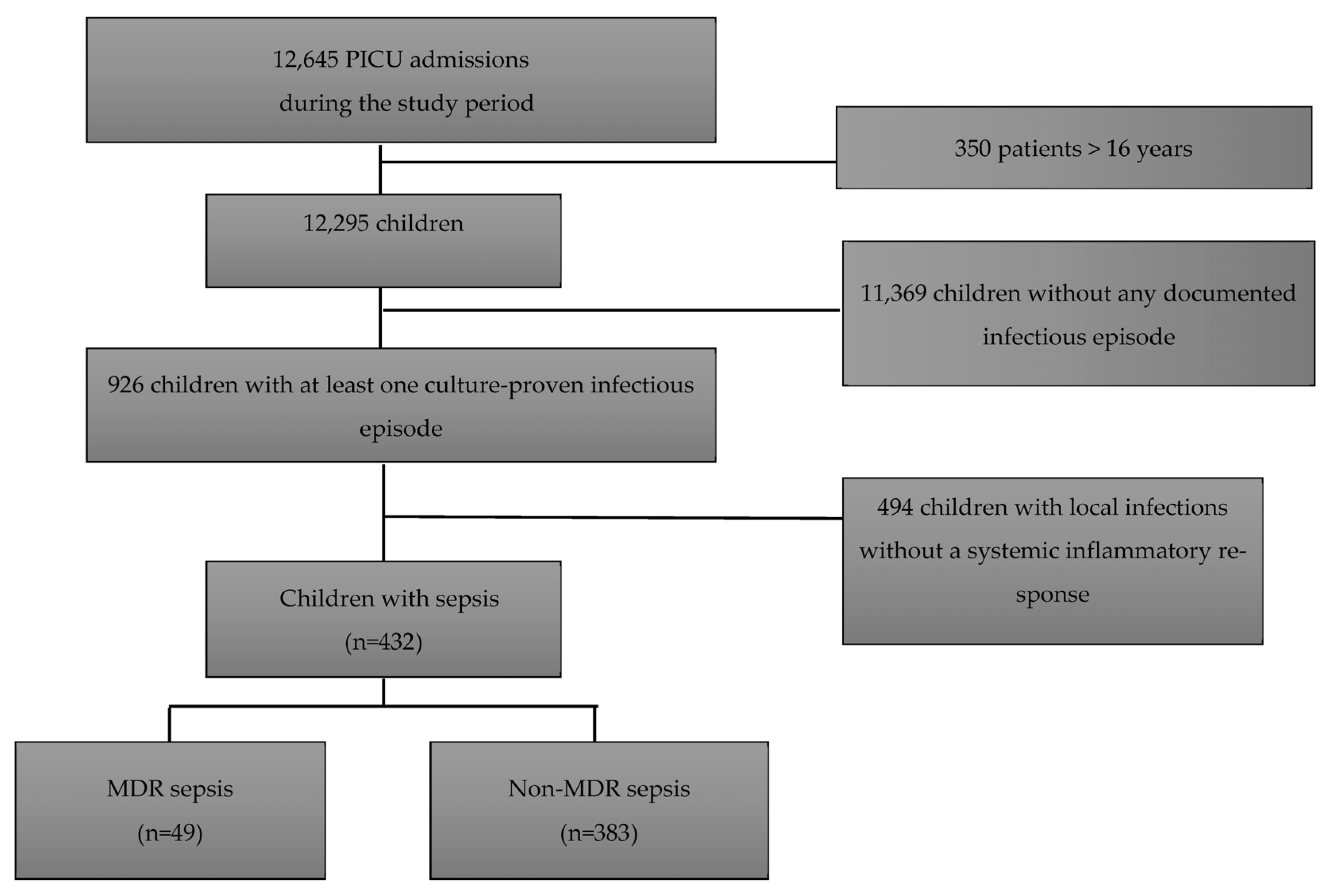
Figure 1. Study the flow chart.
2.2. Multidrug-Resistant Sepsis Rates, Overall Multidrug-Resistant Isolates, and Microorganism Profile over this Study Period
The rate of MDR bacteria among septic children ranged between 5.8 and 16.22% throughout this study period, with a significant increase since 2015 (p = 0.013) (Figure 2). ESBL-Enterobacterales were the most frequent causative microorganisms of MDR-sepsis (63.7% overall, Klebsiella spp. 36.7%), followed by P. aeruginosa spp. (16.3%), methicillin-resistant S. aureus (MRSA) (8%), S. maltophilia (6%), and other gram-negative bacteria (6%). Two Klebsiella spp. isolates produced metallobetalactamases, and one P. aeruginosa isolate was resistant to carbapenems.
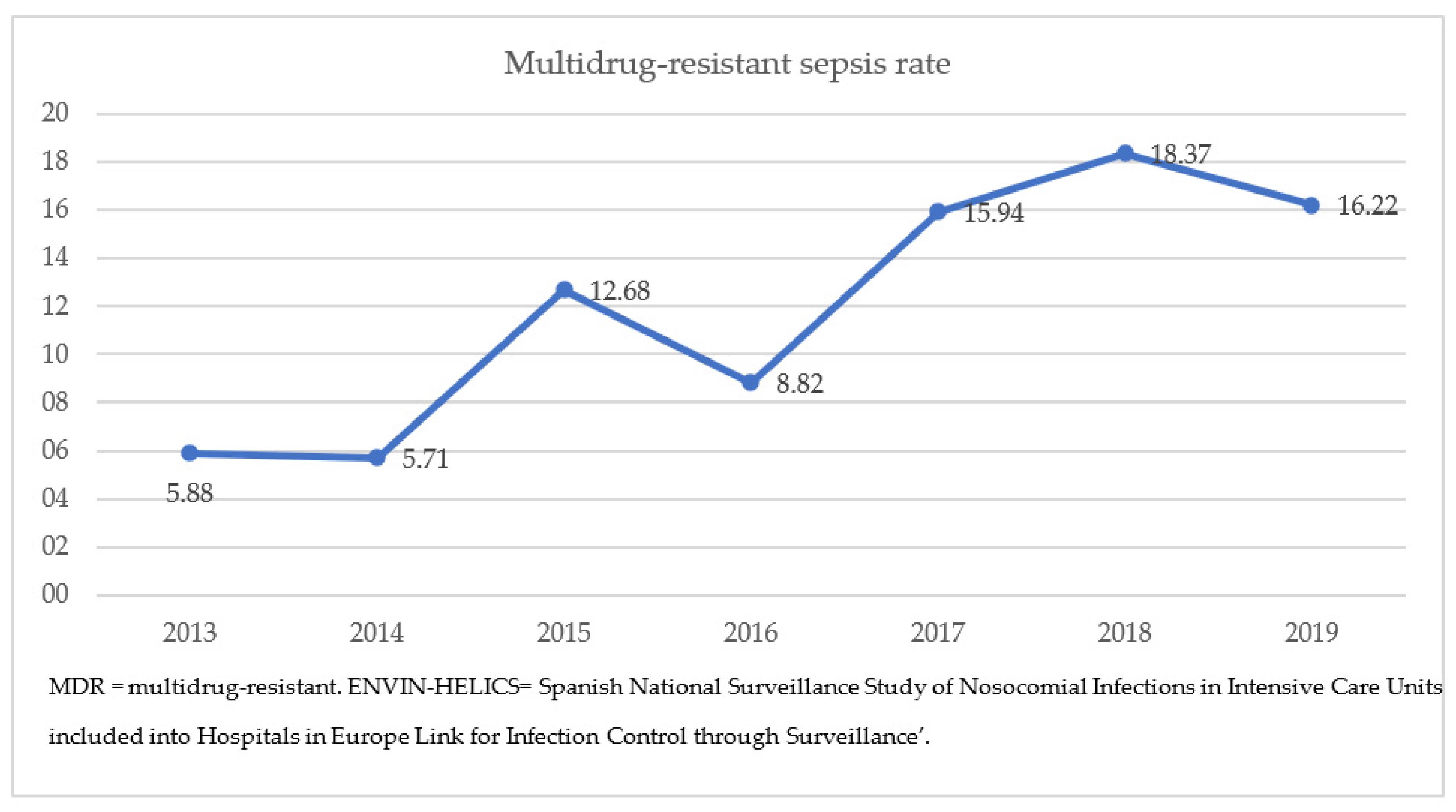
Figure 2. Evolution of the MDR sepsis rate in the pediatric ENVIN-HELICS registry, 2013–2019.
The evolution of the overall MDR bacteria isolates (colonization/infection) in PICU patients over the six-year pre-pandemic period (2013–2019) is shown in Figure 3. The most prevalent MDR microorganisms were Gram-negative, and the most frequent MDR species were also ESBL-Enterobacterales, which increased significantly throughout this study period (p < 0.01), while other species remained somewhat stable. The proportion of previously infected/colonized patients who developed sepsis due to a MDR bacteria was significantly higher than that of patients without previous colonization (30.6% versus 6.6%, p < 0.001), and the prevalence of MDR bacteria increased significantly with the severity of the inflammatory response (25.3% vs. 35.7% vs. 48.9%; non-inflammatory response, sepsis, and septic shock, respectively, p < 0.001).
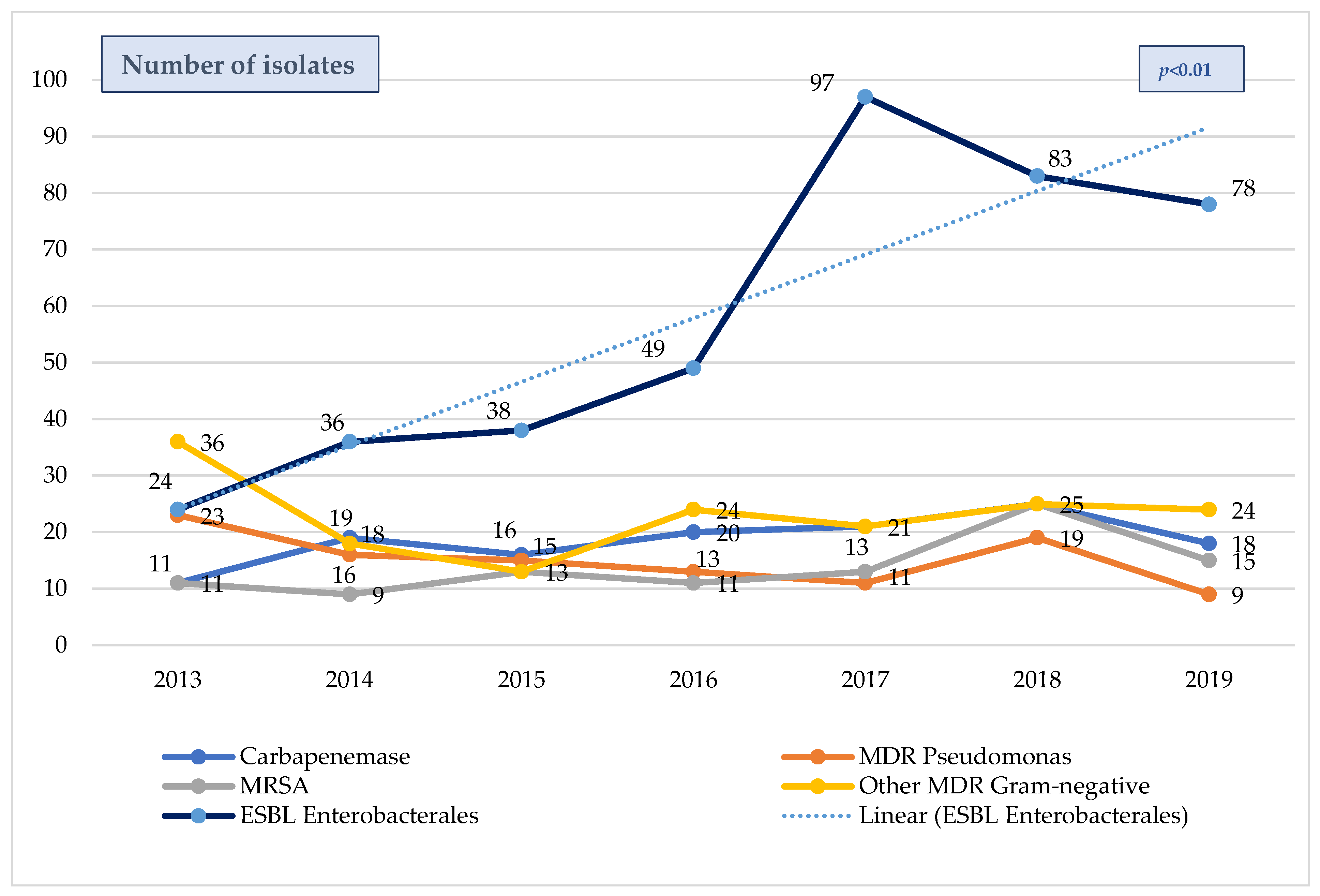
Figure 3. Evolution MDR bacteria isolates in septic patients from the pediatric ENVIN-HELICS registry 2013–2019. Includes colonization and infection. MDR = multi-drug-resistant. ESBL = extended spectrum β-lactamases. MRSA = methicillin-resistant Staphylococcus aureus. ENVIN-HELICS = Spanish National Surveillance Study of Nosocomial Infections in Intensive Care Units.
2.3. Comparison between MDR and Non-MDR Septic Groups
Baseline overall and differential features between MDR and non-MDR sepsis patients are listed in . Compared to children with non-MDR sepsis, children with MDR sepsis were younger and had more frequent previous chronic diseases and malnutrition. These patients also received more frequent antibiotics 48 h prior to admission and total parenteral nutrition. There were no statistically significant differences in the proportion of community-onset/intrahospital sepsis, malignancy, immunosuppression, or previous hospital days pre-PICU between both groups. shows the differences in the multivariate analysis of risk factors for mortality depending on the age group. Independent risk factors associated with MDR-sepsis were the use of antibiotics 48 h prior to PICU (OR 2.38 [1.06–5.36]; p = 0.037) and PICU sepsis debut (OR 2.58 [1.07–6.26]; p = 0.037) in >1 year-old children, and previous malnourishment (OR 4.99 [1.11–22.4]; p = 0.037) in <1 year-old children.
Baseline overall and differential risk factors for mortality between MDR and non-MDR sepsis patients are listed in .
The median PICU length of stay (overall sepsis) was 20 days [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], and their overall PICU mortality rate was 12.7%. PICU stay was longer in children with MDR-sepsis compared to children with non-MDR sepsis (25.5 vs. 19 days; p = 0.006), but there were not statistically significant differences in PICU mortality (16.3% vs. 12.2%; p = 0.423) between both groups. Septic shock (OR 6.03 [2.75–13.2]; p <0.001), immunosuppression (OR 3.69 [1.73–2.79]; p = 0.001), and PICU sepsis (OR 2.95 [1.16–7.47]; p = 0.022) were independent factors associated with mortality in overall sepsis. In septic MDR-colonized/Infected children (n = 160), immunosuppression (OR 3.4 [1.2–10.1]) and the presence of septic shock (OR 4.3 [1.3–14]) were independent risk factors for mortality. In those with MDR-sepsis, only septic shock (OR 50.7 [1.4–8]).
3. Discussion
This is the first epidemiologic study to assess MDR rates and variables potentially associated with MDR-sepsis among critically ill children with sepsis and septic shock in a large Spanish cohort. Despite its low rate, MDR-sepsis had a significant impact on PICU stays, although it showed no impact on mortality. In this cohort, the most frequent MDR species were ESBL-Enterobacterales, and this fact may have influenced our results due to a more favorable resistance profile and susceptibility to antimicrobials. Noteworthy, we found no difference in the proportion of intrahospital/community-onset MDR-sepsis compared to non-MDR-sepsis. However, we observed that > 48 h of antibiotics prior to PICU admission, PICU sepsis in children > 1 year-old, and malnourishment in children <1 year-old were associated with a higher risk for MDR microorganisms in septic critically ill children. These findings may have important clinical implications.
In our study, the median age of sepsis patients was 11 months, and almost half of the episodes occurred in children under 1 year old, indicating that, as described in the literature, a significant proportion of sepsis affects younger children aged below two years old [1,10]. Moreover, the median age of patients with sepsis caused by MDR bacteria was lower than sepsis caused by drug-susceptible microorganisms (8 vs. 12 months), even though only 22% of children were <1 year-old in the MDR-group vs. 49.3% in the non-MDR group. This may be explained by the great proportion of children affected by chronic conditions in the MDR-sepsis group.
A remarkable finding was that the proportion of intrahospital sepsis was not significantly different between both groups, even with gram-negative rods accounting for 92% of MDR microorganisms. Thus, even taking into consideration the low statistical power due to the small sample, these close values deserve our attention. Classically, intrahospital and community infections have been known to have different causative agents, and antibiotic guidelines for empiric antibiotic therapy were therefore written accordingly [11]. In our cohort, a high proportion of children with MDR-sepsis (67.4%) came from the community, although MDR bacteria were responsible for only 10.4% of community-onset sepsis. In the era of the global dissemination of antimicrobial resistance, risk factors for the likelihood of drug-resistant infection should be cautiously evaluated, especially in septic shock [12,13]. In view of our results, other factors than the classical categorization between intrahospital/community onset, immunosuppression, or chronic conditions should be considered [14,15]. Analysis of our data in children > 1 year-old found that prior antibiotic prescription and PICU onset of sepsis were factors associated with higher risk for MDR-sepsis, as previously reported by other studies [14,16]. Noticeably, in our cohort, antibiotic prescription 48 h prior to admission increased by more than 200% the risk for MDR bacteria when assessing septic patients in the PICU, independent of previous hospitalization for more than 10 days and chronic conditions, which imply contact with the health care settings. So, while other factors are predictors for both MDR colonization and infection [17,18], among septic patients admitted to the PICU, prior >48 h antibiotic administration would be the only independent factor for considering a MDR bacteria etiology, based on our results.
Immunosuppression and malignancy have also been reported to be associated with a higher risk for MDR sepsis and sepsis mortality [18,19], along with other chronic comorbidities [1,10,16,20,21,22]. In our cohort, chronic conditions and malignancy seemed to be subrogates of worse outcomes, probably linked to a higher risk of intra-PICU sepsis and septic shock in that subgroup of patients. Despite the fact that MDR pathogens have been independently associated with an increased risk of death irrespective of age and the etiology of sepsis [2,13], no significant differences were observed in the mortality rate between patients with or without MDR bacterial sepsis in our cohort. An important reason for this is the small sample size of the MDR-sepsis group, which in turn might be due to the optional reporting of MDR cases. The worse outcomes in MDR-sepsis reported by other studies can be explained by greater virulence (i.e., MRSA) [14], but mainly by other factors involving the early and appropriate treatment of sepsis, such as the increased toxicity, inadequate dosage, the greater need for invasive or surgical procedures, and especially the delay in the start of the appropriate antibiotic therapy [23,24]. Accordingly, we found that immunosuppression was associated with higher mortality in overall and MDR-colonized/infected patients, but that the only independent and strongest factor associated with a higher risk of mortality among MDR septic critically ill children was the presence of septic shock (OR 50.7). These results are in line with Greenberg et al. [25], who found that the odds of death from sepsis in immunocompromised patients depended more on differences in care delivery than immunosuppressive medical conditions in a large multicenter study.
Another interesting result was that malnourishment was a strong risk factor for MDR-sepsis in infants, regardless of other known risk factors for sepsis such as total parenteral nutrition therapy or the presence of chronic disease. Classically, malnutrition has been reported as a risk factor for infections in low- and middle-income countries; however, it also affects children with comorbidities and increases healthcare exposure in high-income countries [2,26,27]. Apart from its known role on host defense and risk for infection [30], the pathophysiological mechanisms through which childhood malnutrition and intestinal microbiota impact MDR infections have recently been reviewed by Holowka et al. [29]. Curiously, the most prevalent microorganisms amongst most studies examining the burden of MDR infections/carriage in malnourished sub-Saharan Africans were ESBL-Enterobacterales [30,31,32].
Our results show an increasing rate of MDR bacterial sepsis from 2015 onwards, with the most frequent MDR species being ESBL-Enterobacterales in this cohort. These data are consistent with those published by other authors, in which the incidence of severe infections and sepsis caused by MDR bacteria has increased regardless of socioeconomic status, even tripling in the US [33], but increasing as well in Europe [34], Asia [35], and África [31]. This upward trend has also been observed in other neighboring countries, where the prevalence of ESBL-Enterobacterales in bloodstream isolates in 2022 from children from Portugal (26–41%), Italy (42–50%), and Greece (70%) has consistently increased over the last 10 years. These numbers are considerably higher than those reported in Spanish children (20–27%), according to the European Antimicrobial Resistance Surveillance Network (EARS-Net) [36] and then those reported by other Spanish studies (10.7% and 2.6% in healthy infants (8–16 months) and Spanish schoolchildren (3–13 years old), respectively) [37,38]. Despite the significant increase in infection/colonization by ESBL-Enterobacterales, the overall number of carbapenemase-producing Gram-negative bacteria in our Spanish cohort remained stable during this study period, as reported by EARS-Net in 2022 (0–8%), in contrast to Portugal (4.7–10%), Italy (3.6–18.7%), and Greece (>70%) [36]. These data correspond to global bloodstream infections, as there are no available data regarding PICU sepsis from these countries to compare with the Spanish cohort. However, our data confirm an alarming shift towards gram-negative predominance among MDR bacteria as well as its increase among septic children, raising concerns about a future increase in carbapenemase-producing Gram-negative bacteria following other countries’ patterns in the near future.
The ARPEC (Antibiotic Resistance and Prescribing in European Children) study, carried out in 17 centers in 12 European countries over 3 years, collected 1441 isolates of resistant germs in blood cultures, among which 38.8% of K. pneumoniae, 29% of P. aeruginosa, and 23.9% of E. coli strains came from PICU [39]. Moreover, the presence of MDR bacteria in the community setting has also been noticed, to the point of considering them ubiquitous not only in the health environment but also in the community [40]. Najem et al. reported a 4.31% prevalence of MDR carriage in 3964 children screened prospectively for MDR at admission to a pediatric hospital in Germany, mainly gram-negative bacteria (3.64%), maybe reflecting the increase in children with multiple comorbidities, chronic diseases, and permanent invasive devices, but also other risk factors at home, including adults in contact with healthcare settings [41]. The impact of the COVID pandemic on our MDR Spanish PICU rates must be determined in the next few years.
Thus, in view of the above, standardization of MDR screening protocols and surveillance policies in the PICU is essential. Understanding local epidemiology and antimicrobial resistance patterns is key to adjusting the antibiotic regimen for patients with sepsis. This also raises an opportunity for the development of multidisciplinary antimicrobial stewardship programs to help improve the quality of antibiotic prescriptions and reduce the inappropriate use of antimicrobials in the PICU.
4. Strengths and Limitations
The main strength of our study is that it includes the largest and most complete registry regarding infections—including sepsis and septic—from patients admitted to 29 Spanish PICUs distributed throughout the national territory during the first 5 years of the ENVIN-HELICS study in children. Another advantage is that it includes different patient profiles and complexity, which makes it a varied and representative sample of the critically ill children in our country. Finally, this registry was prospectively defined and implemented with a risk-based approach.
Our study also has some limitations. First, our cohort was limited by the MDR microorganism incidence and by the 3-month per year full-reporting period, including risk factors. Given the low incidence of some of the events and risk factors, the power to demonstrate statistical differences was limited. Second, there is not a universal protocol requiring screening for MDR at PICU admission, and surveillance policies are not homogeneous among PICUs. Even so, our data also showed a significant increase in EBL colonization/infection rates. Third, this study was conducted in Spain, and the results may not be applicable to other settings due to the large variation in epidemiology, sepsis prevalence, case mix, and resource availability. Fourth, the usefulness of mortality as an outcome is limited since the impact of sepsis on survivors, who may present high morbidity and significant long-term complications and sequelae, cannot be evaluated. Finally, analysis of the pandemic and immediate post-pandemic period was not performed due to the high variability in PICU practices during the pandemics, including critically ill adult care, which could not be properly defined in the registry and may bias the results and data interpretation.
5. Materials and Methods
5.1. Study Design and Study Patients
This is an observational cohort study using data from the Spanish National Surveillance Study of Nosocomial Infections in Intensive Care Units included in the European registry ‘Hospitals in Europe Link for Infection Control through Surveillance’ (ENVIN-HELICS): an observational, prospective, multicentre, and nationwide study conducted in 29 PICUs and 219 adult ICUs in Spain during a 3-month period every year (from 1 April to 30 June). This registry provides relevant information about global infection rates (both community and HAI) in the PICU, including causative microorganisms and their antimicrobial resistance profiles. Data on all consecutively admitted patients are entered in a collaborative web database (http://hws.vhebron.net/envin-helics, accessed on 1 January 2023). Eligible subjects for this study were all children (≥1 month and ≤18-year-old patients) requiring PICU admission during this study period (2013–2019).
5.2. Data Collection
Variables collected for this analysis included demographic characteristics, Paediatric Risk of Mortality (PRISM III) score, comorbidities, diagnosis on admission, patient origin (community, hospital, other healthcare facilities), microbiological data, antibiotic susceptibility profile, and presence of inflammatory response and septic shock.
5.3. Study Definitions and Outcomes
Sepsis and septic shock were defined according to the Third International Consensus Definitions for Sepsis and Septic Shock [42]. Pediatric sepsis was defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection, and septic shock is a subset of sepsis patients with cardiovascular dysfunction that persists after adequate fluid resuscitation or needs vasoactive drugs. Patients were classified as having septic shock if vasopressors were initiated/increased within 24 h of the blood culture collection. Community-onset infections were those diagnosed prior to or during the first 48 h of hospital admission. Immunosuppression was defined by the presence of at least one of the following medical conditions: active hematologic malignancy, transplantation, immunosuppressive therapy, chemotherapy in the 30 days before PICU admission, chronic systemic steroid therapy, and autoimmune disease. Malignancy was defined as an active solid or hematologic cancer disease. Multidrug resistance (MDR) is defined in the registry according to the international expert proposal by Magiorakos et al. [43] as non-susceptibility to at least one agent in three or more antimicrobial categories (extended-spectrum penicillins, carbapenems, cephalosporins, aminoglycosides, and fluoroquinolones for Gram-negative bacteria; and ampicillin, vancomycin, fluoroquinolones, fosfomycin, and linezolid for Gram-positive bacteria). To assess the incidence rate of MDR among septic patients, we divided the number of patients with MDR-sepsis by the total number of septic patients. Colonized/infected children by MDR microorganisms were defined as patients with at least one MDR microorganism isolated in at least one clinical sample prior to or during PICU admission. The primary outcome was to study the incidence rate and factors associated with MDR-sepsis in the PICU. Secondary outcomes were PICU mortality and length of stay.
5.4. Statistical Analysis
The statistical analysis was performed using IBM SPSS 25.0 Statistics®, IBM Corp. Released 2017, IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY, USA: IBM Corp. Categorical variables are shown as frequency (n) and percentage (%), whereas quantitative variables are summarized as median and interquartile range [IQR] because they are not normally distributed. The comparison of qualitative variables was performed using the χ2–test or Fisher’s exact test. Continuous variables were compared using the Mann–Whitney U-test and Kruskal–Wallis-test when not normally distributed. Univariate logistic regression analyses were performed for MDR-sepsis and mortality. All clinically relevant variables and variables with p < 0.2 from the univariate analysis were included in the multivariate analysis using the stepwise method. Results were presented as p-values and odds ratios/regression coefficients with a 95% confidence interval. Probability values (P) of less than 0.05 were considered statistically significant.
6. Conclusions
MDR bacteria increased significantly among septic children throughout this study period. ESBL-Enterobacterales were the most frequently isolated, with more than two-thirds of MDR-sepsis corresponding to children admitted from the community. In clinical practice, antibiotic use >48 h prior to PICU admission and malnourishment in infants should be considered when assessing the risk for MDR-sepsis, especially with septic shock, regardless of the location of the patient at the sepsis onset. As MDR bacteria are a global emerging threat, more multicenter international studies are needed to assess their impact on pediatric critically ill patients and implement global surveillance bundles.
References
- Rudd, K.E.; Johnson, S.C. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.; Buetti, N. EUROBACT-2 Study Group, ESICM, ESCMID ESGCIP and the OUTCOMEREA Network. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: The EUROBACT-2 international cohort study. Intensive Care Med. 2023, 49, 178–190. [Google Scholar] [CrossRef]
- Dettori, S.; Portunato, F. Severe infections caused by difficult-to-treat Gram-negative bacteria. Curr. Opin. Crit. Care 2023, 29, 438–445. [Google Scholar] [CrossRef]
- Bassetti, M.; Rodríguez-Baño, J. Should we take into account ESBLs in empirical antibiotic treatment? Intensive Care Med. 2016, 42, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Bassetti, M. Rationalizing antimicrobial therapy in the ICU: A narrative review. Intensive Care Med. 2019, 45, 172–189. [Google Scholar] [CrossRef]
- Fresán-Ruiz, E.; Izurieta-Pacheco, A.C. Antimicrobial Stewardship Improvement in Pediatric Intensive Care Units in Spain—What Have We Learned? Children 2022, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.L.; Plata-Menchaca, E.P. An approach to antibiotic treatment in patients with sepsis. J. Thorac. Dis. 2020, 12, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Bergl, P.A. COUNTERPOINT: Should Broad-Spectrum Antibiotics Be Routinely Administered to All Patients with Sepsis as Soon as Possible? No. Chest 2019, 156, 647–649. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Goldfarb, D.M. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Fresán-Ruiz, E.; Pons-Tomás, G. Device Exposure and Patient Risk Factors’ Impact on the Healthcare-Associated Infection Rates in PICUs. Children 2022, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Strich, J.R.; Heil, E.L. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. J. Infect. Dis. 2020, 222 (Suppl. 2), S119–S131. [Google Scholar] [CrossRef]
- Capsoni, N.; Bellone, P. Prevalence, risk factors and outcomes of patients coming from the community with sepsis due to multidrug resistant bacteria. Multidiscip. Respir. Med. 2019, 14, 23. [Google Scholar] [CrossRef]
- Folgori, L.; Bielicki, J. Future Challenges in Pediatric and Neonatal Sepsis: Emerging Pathogens and Antimicrobial Resistance. J. Pediatr. Intensive Care 2019, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sick-Samuels, A.C.; Goodman, K.E. A Decision Tree Using Patient Characteristics to Predict Resistance to Commonly Used Broad-Spectrum Antibiotics in Children With Gram-Negative Bloodstream Infections. J. Pediatr. Infect. Dis. Soc. 2020, 9, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Jones, T.M. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef]
- Chen, G.; Xu, K. Risk Factors of Multidrug-Resistant Bacteria in Lower Respiratory Tract Infections: A Systematic Review and Meta-Analysis. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 7268519. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Costa, P.; Atta, E.H. Infection with multidrug-resistant gram-negative bacteria in a pediatric oncology intensive care unit: Risk factors and outcomes. J. Pediatr. 2015, 91, 435–441. [Google Scholar] [CrossRef]
- Boeddha, N.P.; Schlapbach, L.J. Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: A prospective cohort study from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS). Crit. Care 2018, 22, 143. [Google Scholar] [CrossRef]
- Agyeman, P.K.A.; Schlapbach, L.J. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: A population-based cohort study. Lancet Child Adolesc. Health 2017, 1, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Klos, M.; Wojkowsja-Mach, J. Hospital-acquired Enterobacteriaceae bloodstream infections in children. Dev. Period Med. 2019, 23, 131–136. [Google Scholar] [PubMed]
- Folgori, L.; Bernaschi, P. Infection Control & Hospital Epidemiology Healthcare-Associated Infections in Pediatric and Neonatal Intensive Care Units: Impact of Underlying Risk Factors and Antimicrobial Resistane on 30-day case-fatality in Italy and Brazil. Infect. Control Hosp. Epidemiol. 2016, 37, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay and health care costs. Clin. Infect. Dis. 2006, 42 (Suppl. 2), 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Shorr, A.F. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Hohmann, S.F. Hospital Volume of Immunosuppressed Patients with Sepsis and Sepsis Mortality. Ann. Am. Thorac. Soc. 2018, 15, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Salemi, J.; Phillips, W. Malnutrition among Hospitalized Children in the United States: A 2012-2019 Update of Annual Trends. J. Acad. Nutr. Diet. 2023, 123, 109–116. [Google Scholar] [CrossRef]
- Khlevner, J.; Naranjo, K. Healthcare Burden Associated with Malnutrition Diagnoses in Hospitalized Children with Critical Illnesses. Nutrients 2023, 15, 3011. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Zambruni, M. Impact of Childhood Malnutrition on Host Defense and Infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef] [PubMed]
- Holowka, T.; Van Duin, D. Impact of childhood malnutrition and intestinal microbiota on MDR infections. JAC Antimicrob. Resist. 2023, 5, dlad051. [Google Scholar] [CrossRef]
- Ndir, A.; Diop, A. Epidemiology and Burden of Bloodstream Infections Caused by Extended-Spectrum Beta-Lactamase Producing Enterobacteriaceae in a Pediatric Hospital in Senegal. Forestier C, editor. PLoS ONE 2016, 11, e0143729. [Google Scholar] [CrossRef]
- Andersen, C.T.; Langendorf, C. Risk of community- and hospital-acquired bacteremia and profile of antibiotic resistance in children hospitalized with severe acute malnutrition in Niger. Int. J. Infect. Dis. 2022, 119, 163–171. [Google Scholar] [CrossRef]
- Maataoui, N.; Langendorf, C. Increased risk of acquisition and transmission of ESBL-producing Enterobacteriaceae in malnourished children exposed to amoxicillin. J. Antimicrob. Chemother. 2020, 75, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Braykov, N.P. Extended-Spectrum β-Lactamase–Producing and Third-Generation Cephalosporin-Resistant Enterobacteriaceae in Children: Trends in the United States, 1999–2011. J. Pediatr. Infect. Dis. Soc. 2014, 3, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Caselli, D.; Cesaro, S. Multidrug resistant Pseudomonas aeruginosa infection in children undergoing chemotherapy and hematopoietic stem cell transplantation. Haematologica 2010, 95, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Mehta, Y.; Paul, R. Sepsis Management in Southeast Asia: A Review and Clinical Experience. J. Clin. Med. 2022, 11, 3635. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Atlas of Infectious Diseases. European Antimicrobial Resistance Surveillance Network (EARS—Net). European Center of Diseases Control and Prevention. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 10 November 2023).
- Fernández-Reyes, M.; Vicente, D. High Rate of Fecal Carriage of Extended-Spectrum-β-Lactamase-Producing Escherichia coli in Healthy Children in Gipuzkoa, Northern Spain. Antimicrob. Agents Chemother. 2014, 58, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- López-Siles, M.; Moure, Z. Fecal carriage of extended-spectrum beta-lactamase-producing Enterobacterales in healthy Spanish schoolchildren. Front. Microbiol. 2023, 14, 1035291. [Google Scholar] [CrossRef]
- Bielicki, J.A.; Lundin, R. Antibiotic Resistance Prevalence in Routine Bloodstream Isolates from Children’s Hospitals Varies Substantially from Adult Surveillance Data in Europe. Pediatr. Infect. Dis. J. 2015, 34, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Ogyu, A. Available evidence of antibiotic resistance from extended-spectrum β-lactamase-producing Enterobacteriaceae in paediatric patients in 20 countries: A systematic review and meta-analysis. Bull. World Health Organ. 2019, 97, 486B–501B. [Google Scholar] [CrossRef]
- Najem, S.; Eick, D. High prevalence of multidrug-resistant Gram-negative bacteria carriage in children screened prospectively for multidrug resistant organisms at admission to a paediatric hospital, Hamburg, Germany, September 2018 to May 2019. Eurosurveillance 2022, 27, 2001567. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A. Multidrug-resistant, extensively drug-resistant, and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]

