1. Introduction
Parkinson’s disease (PD) is a prevalent age-related and progressive neurodegenerative disorder and its primary cause is associated with the decline of dopaminergic neurons within the substantia nigra pars compacta [1,2,3,4,5]. The dopamine deficiency leads to a hypo-dopaminergic state in the basal ganglia, causing an imbalance in excitatory and inhibitory communication within the nigro-striatal and thalami-cortical circuits. As a consequence, it affects the network of brain structures responsible for planning, selecting, adapting, and executing motor functions [5,6,7,8]. After losing a significant portion of about 80% of dopamine-producing cells, clinical motor symptoms, including tremor, rigidity, bradykinesia, postural instability, and gait difficulties become noticeable [9,10,11]. Although treatments such as dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus (STN) can relieve axial motor symptoms, their effects on postural and gait control are still controversial [8,12,13,14,15].
While those motor symptoms can be very prominent and cause a significant loss in patient quality of life, PD can also be accompanied by numerous non-motor sensory symptoms [16,17,18,19]. Among those non-motor sensory symptoms, defective functionality of sensory systems such as visual [20,21,22,23], vestibular [8,24,25,26,27,28], and somatosensory systems [29,30,31,32,33,34,35,36,37,38] has been reported in PD patients. Since those sensory systems provide valuable information for controlling movements, impaired sensory system functionality might therefore contribute to PD patients’ motor symptoms. This might especially be true as those sensory systems gather information about the surrounding environment, the position of the body in space, and the relative position of each joint with respect to other parts of the body, as well as information about the condition of the ground on which the person is standing or walking [23,24,39,40]. Given that only the soles of the feet are in direct contact with the ground during standing or walking, particularly afferent information from plantar cutaneous mechanoreceptors appears to be crucial for motor control. Plantar cutaneous mechanoreceptors gather information about the pressure distribution and loading shifts underneath the foot during movements, and therefore are involved in adapting muscle contraction tone and contraction patterns [41,42,43,44,45,46,47,48,49,50,51,52]. The sensorimotor integration of plantar mechanoreceptors has already been investigated in several studies with individuals without neurological diseases. Those studies have shown that decreased plantar cutaneous sensation achieved by anesthesia of the foot sole led to impaired control of static and dynamic balance abilities, as well as gait performance [42,46,49,50,51,52,53,54,55,56]. Conversely, several studies have demonstrated that sensory stimulation of the foot sole using various types of shoe insoles improved balance and gait performance in PD patients [41,43,44,45]. Accordingly, those findings suggest that PD patients might suffer from impaired plantar tactile cutaneous perception, while mechanical stimulation facilitates the sensory signal and therefore enhances sensorimotor integration.
This makes sense from a pathophysiological point of view because the mechanisms of sensory symptoms in PD include findings of widespread deposits of α-synuclein, which is a fundamental pathological protein and a major component of Lewy bodies in PD [16,57,58,59,60,61,62]. Impaired plantar tactile cutaneous perception in PD patients is therefore quite conceivable, since it has been shown that α-synuclein affects numerous sensory-related structures of the central nervous system, as well as the peripheral nervous system, including the skin [57,63,64,65,66,67,68]. This is supported by the study from Nolano et al., who investigated skin biopsies and found peripheral denervation in PD patients. More specifically, the authors reported demyelination of epidermal nerve fibers of the glabrous and the hairy skin, as well as a significantly reduced number of mechanoreceptors in PD patients compared to those of healthy subjects. In particular, Meissner corpuscles, which detect mechanical vibration stimuli, may be affected, whereas the loss of those mechanoreceptors correlated with the disease severity of the patients. The authors concluded that peripheral deafferentation could play a major role in the pathogenesis of sensory dysfunction in PD [64]. Besides results showing impairments of the peripheral nervous system, there is also evidence for the defective central integration and processing of afferent information at a cerebral level in PD patients. Although the basal ganglia are considered well-established, primarily motor-related structures, they might also function as an active “sensory analyzer” for higher-level central somatosensory processing [69,70,71,72,73]. This is plausible, since the basal ganglia have projections into the thalamus and the cortex and receive input not only from motor areas, but also from cortical somatosensory areas, including the primary and the secondary somatosensory cortices [69,70,73,74]. Using 3D positron emission tomography, Boecker et al. showed that basal ganglia dysfunction in PD is characterized by abnormal sensory processing even for tasks devoid of any motor component [73]. Consequently, impaired tactile cutaneous perception in PD might be driven by both denervation of peripheral epidermal nerve fibers and mechanoreceptors and the defective central integration and processing of afferent information due to the diseased basal ganglia network [38,64,73,75,76,77,78,79,80,81,82,83,84,85,86].
Despite this clinical and experimental evidence of cutaneous denervation and impaired sensory processing in PD, investigations about tactile cutaneous perception are rare, especially for the foot sole, and the few existing studies show rather discordant results. While some studies found impaired plantar tactile cutaneous perception in PD patients [64,87,88], others failed to find PD affecting cutaneous thresholds for mechanical stimuli of the foot [48,89]. Lack of studies and contradicting results are also prominent for investigations about the effect of various therapies, such as anti-parkinsonian medication and STN-DBS for patients’ tactile cutaneous perception of the foot. According to the literature, anti-parkinsonian medication seems to have generally minor to no effects [48,64,87,89]. On the other hand, STN-DBS generally seems to be more promising for treating tactile cutaneous perception in PD [32,33,34,36,37,81,90,91,92]. However, existing studies have only focused on investigations of the upper body, including the torso, arms, hands, or fingers. To the best of our knowledge, no other studies have explored the effect of STN-DBS on tactile cutaneous perception in PD patients’ feet.
Specifically investigating plantar tactile cutaneous perception in PD patients could be beneficial for several reasons. It has been reported that sensory symptoms may occur prior to the presence of motor symptoms, so they could be used for early diagnosis of PD [16,93,94,95]. Moreover, this may help to distinguish between PD and various other neurodegenerative diseases, such as multiple system atrophy, which has been demonstrated with a high sensitivity and specificity of approx. 80% [87]. Consequently, testing plantar cutaneous sensation could serve as a potential surrogate marker. It may also add to our understanding of how PD affects the peripheral nervous system, as well as offer insight into sensorimotor integration and potentially help to better understand the cause of axial motor symptoms in PD. Thinking ahead, plantar tactile cutaneous perception measurements could help to develop and optimize low-budget therapy devices, such as textured insoles, to stimulate plantar cutaneous mechanoreceptors and consequently enhance motor performance in PD patients [41,43,44,45]. As this is the first study investigating the effects of STN-DBS on plantar tactile cutaneous perception in PD patients, it may provide valuable information for better understanding the neurophysiological processing of afferent sensory input. Furthermore, it could assist in counseling patients regarding suitable treatments and support clinicians in customizing and optimizing therapy strategies.
Therefore, we pursued two objectives with this study.
First, we investigated and characterized axial motor symptoms in PD patients, such as postural instability and gait difficulties. We examined whether PD patients treated with anti-parkinsonian medication and STN-DBS have superior motor control compared to patients treated with medication alone. We also tested healthy elderly subjects as a reference. We hypothesized that patients’ motor performance is worse compared to that of healthy subjects, and that the combination of medication and STN-DBS offers greater advantages in normalizing patients’ abnormal motor control compared to treatment with medication alone.
Second, we investigated somatosensory functionality by analyzing plantar cutaneous vibration perception thresholds (VPT) within the same study groups. Based on the pathophysiological mechanisms of PD and previous study findings, we hypothesized that plantar cutaneous vibration perception of patients would be impaired compared to that of healthy subjects. Furthermore, we assumed that medication in combination with STN-DBS would show superior effects on normalizing patients’ impaired plantar cutaneous vibration perception compared to treatment with medication alone.
2. Methods
2.1. Subjects
Three distinct study groups underwent evaluation: Patients with Parkinson’s disease (PD-MED), patients with Parkinson’s disease who previously underwent deep brain stimulation surgery (PD-MED–DBS), as well as healthy subjects. Both patient groups included subjects diagnosed with idiopathic Parkinson’s disease according to the diagnostic criteria of the Movement Disorders Society. They had to be at least 50 years old and have a disease severity between 2 and 3 on the Hoehn and Yahr scale [96]. All recruited PD patients primarily showed signs of postural instability and gait difficulty subtype of the disease. Subjects assigned to group PD-MED–DBS were required to have undergone bilateral high-frequency (≥130 Hz) deep brain stimulation surgery of the STN at least one year prior to guarantee optimized DBS settings and maximum efficiency [15,97]. The cut-off for the duration of DBS since surgery was defined as 5 years since studies have demonstrated a gradual decrease in stimulation efficacy over time [98,99,100]. All DBS patients included in this study showed a positive response to the surgery. Subjects of both patient groups underwent the examinations in the medication “on” state while under the influence of regular anti-parkinsonian medication, including levodopa. Patients in the PD-MED–DBS study group were additionally in the “on” stimulation state. The exclusion criteria for both patient groups comprised secondary pathologies affecting the motor and somatosensory systems, such as atypical parkinsonism, severe camptocormia, severe tremor, diabetes mellitus with polyneuropathy and normal pressure hydrocephalus. All patients had to be able to stand and walk without assistance. Patients with cognitive deficits (mini-mental state examination (MMSE) < 24/30), psychiatric issues, or severe depression were not considered for inclusion. All PD patients were recruited and underwent evaluations as part of the patient consultation at the Neurological Outpatient Clinic for Parkinson’s Disease and Deep Brain Stimulation in Gera, Germany, led by Christian Oehlwein. Relevant clinical data from the most recent neurological examination were provided for both patient groups . The control group, which comprised healthy elderly subjects, was examined in the laboratory of the Department of Human Locomotion (Chemnitz University of Technology, Germany). The healthy subjects had no injuries or diseases, and took no medication that could have affected cognition, postural performance, or cutaneous perception.
2.2. Equipment and Testing Procedures
Before data acquisition, all subjects were briefed about the purpose of this study and provided written informed consent. All procedures were conducted according to the recommendations of the Declaration of Helsinki and were approved by the ethics committee of the medical faculty of the University Leipzig (IRB number: 023/14-ff, 2 April 2014).
2.3. Data Processing
2.4. Statistical Analysis
Statistical analysis was performed using SPSS Statistics (IBM, version 29.0.0.0, Ehningen, Germany). The Shapiro–Wilk test (α = 0.05) was used to check for data distribution.
Differences between study groups were investigated using one-way analysis of variance for normally distributed data and the Kruskal–Wallis test for non-normally distributed data. To account for the study groups and testing conditions, the level of significance was Bonferroni-corrected individually. Effect sizes, r, were calculated as well. For the examination of the intra-group variability, an overall coefficient of variation for each test was computed.
For intra-group comparisons involving sex, body side, disease-dominant side, and AP vs. ML directions, the t-test for paired or independent samples was used for normally distributed data. In the case of non-normally distributed data, the Wilcoxon test was used.
3. Results
3.1. Demographic and Clinical Data
As shown in , there were considerably more male than female subjects in all study groups. On average, patient group PD-MED–DBS comprised younger subjects compared to both other study groups, with statistically significant differences compared to HS. Self-rated balance confidence and gait confidence were lower for both patient groups compared to the healthy subject group, HS. No differences were found for clinical data MMSE, UPDRS III, UPDRS total, or Hoehn and Yahr ratings between PD-MED and PD-MED–DBS. On average, PD-MED–DBS suffered from Parkinson’s disease for more than twice as long as PD-MED. The dominant disease side was evenly distributed among patients in group PD-MED. However, in group PD-MED–DBS, there were more patients with disease dominance on the right side of the body. In PD-MED, the time interval between the most recent clinical examination and the motor and vibration tests was longer and showed higher variability, in contrast to PD-MED–DBS. The mean duration of DBS since surgery was 33.1 ± 25.7 months, and patients’ self-rated satisfaction with DBS at the time of the tests was 80.1 ± 20.6%.
3.2. Motor Performance
3.3. Plantar Cutaneous Vibration Perception
No considerable differences between groups were found for contact forces or plantar temperatures. As shown in Figure 6, only the left metatarsal head of PD-MED showed statistically significant differences for the VPTs compared to HS. Nevertheless, patient VPTs seemed to be higher in general compared to VPTs from HS. Although there were no statistically significant differences between patient groups, VPTs from PD-MED tended to be higher compared to the VPTs from PD-MED–DBS. The overall coefficient of variation for VPTs showed slightly higher variability for HS (0.25 ± 0.05) compared to PD-MED (0.18 ± 0.02) and PD-MED–DBS (0.19 ± 0.03).
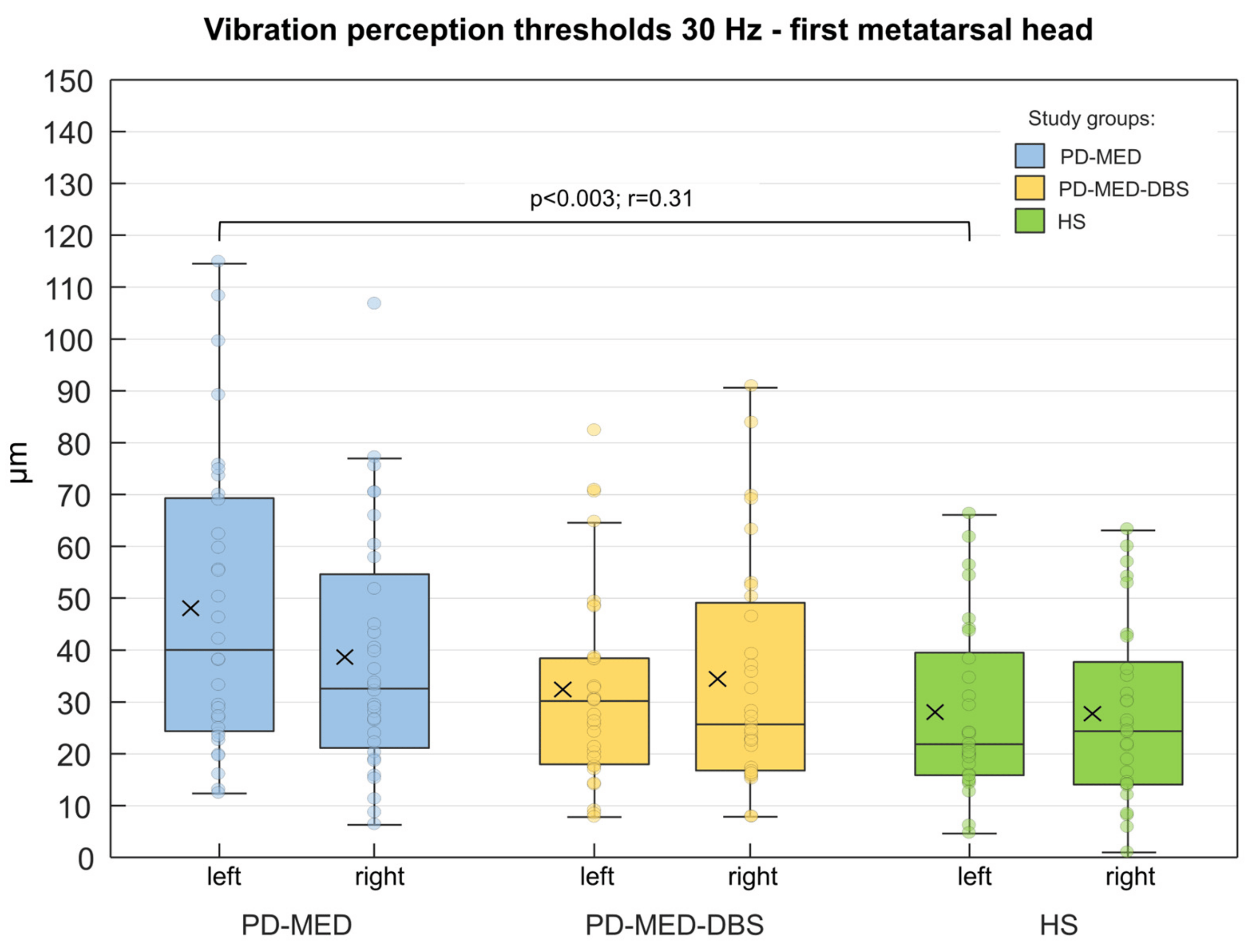
Figure 6. Group comparisons of the VPTs from the left and right first metatarsal head of the plantar foot. Displayed are the untransformed absolute VPTs. The cross within each box marks the mean value. Statistically significant differences (p < 0.0083) are shown as well as effect sizes, r.
In all three groups, women had lower VPTs than men: however, these were only statistically significant for PD-MED. In PD-MED, the VPTs of the left foot were significantly higher compared to the VPTs of the right foot. Moreover, an effect of the disease-dominant side was found but was not consistent throughout the two patient groups. For instance, patients in PD-MED with left-sided disease dominance showed higher VPTs for the left foot (46.7 µm ± 24.4 µm) compared to the right foot (34.9 µm ± 20.5 µm). Nevertheless, this effect was not found for patients in PD-MED with right-sided disease dominance or for patients in PD-MED–DBS.
4. Discussion
4.1. Motor Performance
Our first objective was to analyze patients’ axial motor symptoms, such as postural instability and gait difficulties, in comparison to healthy elderly subjects. We also analyzed whether patients treated with anti-parkinsonian medication in conjunction with STN-DBS had superior motor control compared to patients treated with medication alone. Therefore, we implemented different motor tests and used a pressure distribution platform to objectively assess subjects’ motor performance.
4.2. Plantar Cutaneous Vibration Perception
Our second objective was to investigate tactile somatosensory functionality of the plantar skin in PD patients in comparison to healthy subjects. We also analyzed the therapeutic effects of whether anti-parkinsonian medication alone or medication in conjunction with DBS show different effects on tactile somatosensory functionality of the plantar skin in PD patients. Therefore, we implemented a customized vibration exciter to analyze subjects’ plantar cutaneous vibration perception thresholds.
We hypothesized that PD patients have higher plantar cutaneous vibration perception thresholds than healthy subjects, which was confirmed by our results. Both of our patient groups, PD-MED and PD-MED–DBS, showed generally higher thresholds; however, statistical significance was only found between PD-MED and HS. Although investigations about tactile cutaneous perception of vibration or pressure in PD are rare, especially for the foot sole, there are a few studies which help to explain our results. Compliance with our study findings can be found in three other studies [64,87,88], while two other studies failed to find PD affecting cutaneous thresholds for mechanical stimuli of the foot [48,89]. Testing cutaneous sensory functionality and analyzing skin biopsies of the foot, Nolano et al. showed that PD patients have significantly increased tactile thresholds, which is strongly associated with patients’ significant loss of epidermal nerve fibers and mechanoreceptors, such as Meissner corpuscles [64]. In another study, Prätorius et al. found significantly higher thresholds in PD patients when analyzing five sites of the plantar foot using a vibration exciter at 30 Hz and Semmes-Weinstein Monofilaments for touch pressure perception. Their results showed that for each tested location (except the heel), the thresholds of PD patients were at least twice as high as those in the healthy control subjects [88]. Using electrical sinusoidal stimulation at 5, 250, and 2000 Hz at the external malleolus of the foot, Ikeda et al. also found significantly increased perception thresholds for PD patients compared to those of healthy controls [87]. However, McKeown et al. reported intact cutaneous functionality in PD. Using sophisticated methods, the authors investigated vibro-tactile thresholds at 30 and 250 Hz at the first metatarsal head of the foot and failed to find evidence of elevated plantar thresholds in PD patients [48]. Doty et al. also found no impaired tactile pressure sensitivity in PD patients at the medial sole of the foot and the plantar halluces using a forced-choice staircase threshold test paradigm [89]. The lack of agreement between different study results might primarily be attributed to varying methodological factors, such as the different severity of the disease between the study groups investigated. In this regard, Nolano et al. found that disease severity correlates with the loss of epidermal nerve fibers and Meissner corpuscles in PD. Hence, disease severity, which is associated with disease duration, might be a valuable factor influencing tactile perception [64]. However, when comparing the findings of different studies, there appears to be no consistent pattern for the influence of disease severity and duration on tactile cutaneous perception. In other words, even in studies in which patients had suffered from PD for a relatively long period or had a higher severity of the disease at the time of measurement, no differences in tactile perception were found between PD patients and healthy subjects [48,89]. As sensory perception underlies multifactorial influences, further factors, such as different measurement devices, testing other anatomical locations, and varying vibration frequencies, might also play a role. Furthermore, since only the current study and that by McKeown et al. controlled external factors, such as contact force, this could be another factor influencing tactile perception thresholds [48]. In addition, potential differences in patient skin temperature, which was only controlled in our study, might have had an unknown effect on tactile perception in other studies. Since our healthy subjects were slightly older compared to our patient groups, this could have biased their VPTs towards higher values as an effect of aging. Therefore, study groups with comparable age would have shown differences more dramatically. Finally, the lack of statistical power due to small sample sizes might have contributed to different results as well. However, in addition to studies that investigated tactile perception of the foot, there are a number of studies that analyzed tactile perception at other anatomical locations in PD, including the arms, hands, fingers, and the torso [32,33,34,36,37,38,81,90]. Most of these studies found that PD increases tactile perception thresholds, which confirms the results of our study.
Given that only the soles of the feet are in direct contact with the ground while standing and walking, afferent information from plantar cutaneous mechanoreceptors is crucial input for motor control. Plantar cutaneous mechanoreceptors gather information about the pressure distribution and load shifts underneath the foot during movements, and therefore are involved in adapting muscle contraction tone and contraction patterns. Sensorimotor integration of plantar mechanoreceptors has already been investigated in several studies with individuals without neurological disease. In summary, decreased plantar cutaneous sensation achieved by anesthesia of the foot sole leads to impaired control of static and dynamic balance abilities [42,46,49,50,51,52,53,54,55,56]. Conversely, sensory stimulation of the foot sole has been shown to improve balance and gait performance. This has been demonstrated in several studies with PD patients using various types of shoe insoles. For instance, Phuenpathom et al. analyzed the effect of mechanical pressure stimulation of the foot sole during the initiation of gait in PD patients. They used insoles with thickened silicon pads and found that the pressure stimulation of the plantar foot skin reduced the freezing of gait, which is a devastating motor symptom in PD [43]. In another study, Qiu et al. reported that textured insoles decreased postural sway and improved balance stability under challenging conditions in the PD group due to facilitating afferent information from mechanoreceptors of the foot sole [44]. Another study analyzed predicting factors of falls in PD. Investigating muscle activity and spatial–temporal parameters during walking, Jenkins et al. found an improvement in the overall stability and safety of gait when using stimulating ribbed insoles. More specifically, the authors reported increased single-limb support time, which implies improved overall stability and normalized timing of the peak activation of the tibialis anterior muscles [45]. Novak and Novak used an elastic vibrating insole that delivered 70 Hz suprathreshold vibration bursts to the heel and the forefoot during the stance phase of gait. Their findings indicate that step-synchronized vibration stimulation of the plantar foot improves gait steadiness in PD with predominant balance impairment. Suprathreshold vibration stimulation improved gait performance by normalizing stride variability, walking speed, stride length, and cadence through enhanced sensory feedback [41]. In another study, the authors stated that the difference in touch thresholds they found between PD patients with and without a history of falling might be an association between reduced peripheral sensation and increased postural instability in the fallers group [282]. In summary, since the stimulation of the plantar foot enhances motor performance in PD patients, the link between reduced plantar sensation and motor symptoms in PD seems plausible. Hence, the reduced plantar cutaneous vibration perception found in our patient groups might be another factor contributing to motor symptoms in PD, such as postural instability or gait difficulties.
The causes of sensory symptoms in PD are multifactorial; however, there is a strong association with widespread deposits of α-synuclein, a fundamental pathological protein, which is also a major component of malicious Lewy bodies in PD patients [16,57,58,59,60,61,62]. Based on neuroanatomical models, the progression of α-synuclein might already begin in the prodromal stage of the disease, initiating in the lower brainstem, autonomic nervous system, and olfactory bulb, advancing in a caudal to rostral direction affecting the diencephalon, basal forebrain, medial temporal lobe structures, and finally neocortical areas [16,65,66,83,283,284,285]. While the progression of sensory symptoms in PD might be related not only to the extension of α-synuclein in specific dopaminergic structures, it also can be present in neurons, presynaptic terminals, and glial cells in the autonomic nervous system, the retina, the central and peripheral nervous systems, and therefore in epidermal nerves of the skin [57,63,64,65,67,68]. Studies have reported that sensory deficits are related to cutaneous denervation in PD, predominantly by α-synuclein. Investigating skin and cutaneous nerves from the abdominal wall in PD patients, Ikemura et al. found extensive Lewy body accumulation in up to 70% of the investigated cases with Lewy stages II and III, which corresponds to preclinical and early stages of PD [63]. Using skin biopsy to assess peripheral denervation, Nolano et al. found a lower density of intrapapillary myelinated endings of the glabrous skin in PD patients compared to healthy subjects. In both the glabrous and the hairy skin, the authors also observed axonal swelling and myelin abnormalities, such as paranodal and distal demyelination, profile segmentation and occasional internodal demyelination of the epidermal nerve fibers. This also includes myelinated axons of the fiber type Aβ which are responsible for conducting afferent tactile information from mechanoreceptors, such as the Meissner corpuscles, to the central nervous system [286]. More specifically, the number of Meissner corpuscles that detect mechanical vibration stimuli was significantly reduced. Furthermore, Meissner corpuscles even presented a wide range of anomalies, which, according to the authors, suggests the coexistence of degenerative and regenerative processes. The loss of Meissner corpuscles also correlated with the disease severity of the patients. The authors concluded that peripheral deafferentation, including Meissner corpuscles in PD patients, could play a major role in the pathogenesis of sensory dysfunction and could account (at least partly) for the impairment in sensory function in PD [64]. Since we predominantly investigated the functionality of Meissner corpuscles, the findings from Nolano et al. might therefore be one reasonable explanation for why our PD patients also showed impaired plantar cutaneous vibration sensitivity. As those studies mainly show degeneration and deficits of the peripheral nervous system in PD patients, there is also evidence for defective central integration and processing of afferent information at a cerebral level in PD. Although the basal ganglia are considered well-established primarily motor-related structures, there is conjoining clinical and experimental evidence supporting basal ganglia as active “sensory analyzers” for higher-level central somatosensory processing [69,70,71,72,73]. This is plausible, since the basal ganglia are connected to the cortex and receive input from not only motor areas but also cortical somatosensory areas [69,70]. Particularly the STN, one of the main input structures of the basal ganglia, receives projections from multiple cortical, predominantly sensorimotor, areas, whereas its disease-related hyperactivity might cause the loss of functional specificity and ultimately alter somatosensory and sensorimotor integration processing of tactile afferent information [36,287,288,289]. Boecker et al. investigated altered activity of various brain structures, including regional cerebral blood flow and metabolism, using 3D positron emission tomography while applying vibration stimuli to the skin of the index finger. Their results showed that sensory-evoked brain activation in PD patients was reduced in subcortical (basal ganglia) and cortical (parietal and frontal) areas compared to that of healthy control subjects. More specifically, PD patients showed decreased activation of the contralateral sensorimotor and lateral premotor cortex, the contralateral secondary somatosensory cortex, the contralateral posterior cingulate, the bilateral prefrontal cortex (Brodmann area 10), and the contralateral basal ganglia. In contrast, there was a relative enhanced activation of ipsilateral sensory cortical areas, notably caudal primary and secondary somatosensory cortices and the insular cortex, in PD patients compared to healthy subjects. The authors interpreted their findings as an indication of either altered central focusing and gating of afferent sensory impulses or enhanced compensatory recruitment of associative sensory areas in the presence of patients’ basal ganglia dysfunction. Hence, with their findings, Boecker et al. showed that basal ganglia dysfunction in PD is characterized by abnormal sensory processing, even for tasks devoid of any motor component [73]. In this context, other studies have also suggested that altered tactile perception, including impaired shape discrimination and tactile acuity, reduced roughness detection at the fingertips, altered two-point tactile discrimination thresholds and abnormal weight perception thresholds, are the result of defective central processing attributed to the diseased basal ganglia in PD [38,75,76,77,78,79,80,81,82]. It is assumed that the so-called “neural noise” in the somatosensory loops of the basal ganglia may also contribute to the increase in tactile detection thresholds [84,85,86,90]. Disease-related changes in the receptive fields for tactile inputs to the basal ganglia may introduce “noise” into sensory perception, resulting in increased thresholds and reduced discriminative capacities for different sensory modalities [84,85]. This might be emphasized by excessive pathological synchronous neural activity in the beta frequency band (8–35 Hz) throughout the cortico-basal ganglia network in PD patients. Accordingly, cortical oscillations in the beta-range “contaminate” the oscillatory activity of the basal ganglia and prevent their desynchronization, which is essential for movement control, but can possibly also play a role in sensory processing [86,290,291]. Besides impaired basal ganglia functionality, the dysfunction of extranigral pathways, including the brainstem nuclei, diencephalic and cortical areas, as well as extra-encephalic structures, such as the spinal cord and the autonomic enteric plexus, might be associated with sensory deficits in PD [61,90,292]. In summary, impaired tactile cutaneous perception in PD might be driven by denervation of peripheral epidermal nerve fibers and mechanoreceptors, as well as by defective central integration and processing of afferent information at a cerebral level. Therefore, those pathophysiological mechanisms might help to explain the increased plantar cutaneous vibration thresholds found in our patient groups.
As the pathophysiological mechanisms mentioned above can develop inconsistently and therefore dominate either the left or the right cerebral hemispheres and body sides, this can cause laterality of symptoms. Since laterality is common for motor symptoms, such as tremor, it might also apply to sensory symptoms. This might at least be partly true for our findings, since we found an effect of a disease-dominant side in PD-MED. Patients with a disease-dominant left side showed higher vibration perception thresholds of the left foot compared to the right foot. This is supported by other studies, which also report laterality of sensory symptoms [36,64,83,293]. Nevertheless, we did not find this effect for patients in group PD-MED with a disease-dominant right side or for patients in group PD-MED–DBS, which is consistent with various other studies [34,37,81,87,89,95].
When analyzing whether anti-parkinsonian medication alone or medication in conjunction with DBS results in differences for tactile somatosensory functionality of the plantar skin in PD patients, we generally found higher vibration perception thresholds for group PD-MED compared to group PD-MED–DBS. Although the comparison between PD-MED and PD-MED–DBS had no statistical significance, our results showed a strong trend towards more impaired tactile perception for patients treated with anti-parkinsonian medication alone. This trend is also supported by the fact that the vibration thresholds only differed significantly between PD-MED and HS, while there was no difference between PD-MED–DBS and HS. Although the effect of anti-parkinsonian medication on sensory deficits, including noxious and innoxious tactile thresholds, and thermal perception in PD is controversial, reports about general insufficiency seem to dominate the literature [19,48,80,83,87,89,294]. For example, investigating plantar vibration perception thresholds in PD patients on and off medication, McKeown et al. found no acute effects of ceasing levodopa intake on plantar sensitivity [48]. Doty et al. also reported that plantar point pressure sensitivity thresholds were not affected by levodopa [89]. Moreover, Gierthmühlen et al. reported that levodopa did not influence detection thresholds or pain sensitivity [37]. Investigating pain perception as a sensory symptom in PD patients, insufficiency of medical treatment was also reported in another study with a large number of patients with early to moderate PD. In this epidemiological study, approx. 80% of PD patients reported no difference in pain between the on- and off-medication states [294]. It has even been reported that dopaminergic medication can worsen sensory symptoms in PD, such as proprioception, which might be related to medication-induced side effects due to heavy medication loads [19,89,265,295,296]. Although the processing of different sensory modalities, including proprioception, and noxious and innoxious tactile and thermal perception might not be the same, those studies show rather subtle effects of anti-parkinsonian medication on sensory symptoms and point towards little involvement of dopaminergic systems. Nevertheless, the contribution of dopaminergic systems to sensory symptoms in PD is still unclear.
On the other hand, several studies, including this current study, have shown that DBS of the STN is more promising than anti-parkinsonian medication alone for treating sensory symptoms in PD [19,32,33,34,36,37,83,90,91,92,95,297,298]. For example, Cury et al. stated that DBS has a clear effect on sensory thresholds and changes sensory abnormalities towards normal levels in PD patients [83]. Aman et al., who investigated haptic discrimination thresholds of the hand, also reported enhancements of more than 20% with DBS compared to cases without DBS [36]. The authors concluded that improved haptic precision might indicate improved somatosensory functionality by STN-DBS. Their results support the findings from Maschke et al., who also showed a 20% decrease in position sense threshold as a result of DBS [36,298]. In a more recent study, Sabourin et al. investigated specific settings of directional DBS electrodes on sensory symptoms using a quantitative sensory testing battery, including thermal, pressure, and vibration perception. Although the effects were subtle, their results demonstrated that DBS modulates thermal and mechanical cutaneous sensitivity. DBS pulse width modulated mechanical sensitivity, whereas the DBS total electrical energy modulated thermal sensitivity when using certain directional contacts of the electrodes [32]. Altering the stimulation frequency of DBS, Belasen et al. also analyzed its effects on sensory modalities, including cutaneous pressure and vibration perception. The authors reported that lower DBS frequencies resulted in changed detection thresholds for mechanical pressure and vibration to a greater extent than higher frequencies [33]. In another study, Cury et al. reported lower thermal and mechanical detection thresholds post DBS surgery compared to those detected pre surgery. According to the authors, their data confirmed the existence of sensory abnormalities in PD and suggested that DBS mainly influences detection thresholds rather than painful sensations. In particular, DBS had a significant effect on mechanical and thermal detection thresholds, which were modified toward normal values after DBS surgery. Accordingly, DBS modulated both large and small fiber-dependent sensory input [90]. In contrast, the results of Ciampi de Andrade et al. showed that the detection of large fiber-mediated sensations, including vibration sensations at 100 Hz, did not change in PD patients between on-stim and off-stim conditions. However, PD patients had lower sensitivity to mechanical and thermal pain in the on-stim condition [34]. Dogru Huzmeli et al. also reported reduced thresholds of cutaneous two-point discrimination in PD patients after DBS, suggesting improved somatosensory processing [92]. Using questionnaires such as the non-motor symptom scale, several other studies also found STN-DBS to improve sensory symptoms in PD patients [299,300,301]. Those study findings support our results, showing that DBS is more efficient in treating sensory symptoms and normalizing tactile cutaneous perception thresholds compared to anti-parkinsonian medication alone. This becomes even more interesting when we consider that our study group that received DBS was affected for twice as long as the group that received medication alone.
Since STN-DBS affects, first and foremost, the basal ganglia, changes in sensory perception are mainly associated with the modulation of somatosensory information at a cerebral level, while they probably have less effect on the peripheral nervous system per se [74,92,302,303,304,305,306]. The physiological mechanisms by which STN-DBS improves tactile cutaneous perception in PD patients remains unclear, but several hypotheses have been proposed. As STN-DBS acts on fibers and cells in close proximity to the implanted electrodes, an effect on specific somatosensory structures and pathways might be plausible, especially as the nearby thalamus plays a crucial role in processing sensory information [6,32,151,154,303]. In this context, it has been demonstrated that STN-DBS might modulate neural activity in the thalamus and other several cortical areas which are involved in processing tactile information [302,303,307]. Since the posterior parietal region receives information from prefrontal regions, the sensory cortex and multiple thalamic relay nuclei, STN-DBS may activate not only the frontal but also the parietal cortex, which suggests a contribution of the STN to sensory function [302,304]. The STN also has projections to the primary and secondary somatosensory cortices, which are responsible for processing tactile information, so that STN stimulation might affect sensory perception [32,74,302,304,305,308]. Using functional magnetic resonance imaging technology, DiMarzio et al. reported that the activity, especially of the primary somatosensory cortex, might be a promising indicator of whether sensory symptoms in PD patients respond to STN-DBS [308]. Another study has also mentioned that STN-DBS may alter the activity of the secondary somatosensory cortex, but this still has to be proven [74]. Three-dimensional positron emission tomography has also shown that DBS significantly increases the regional cerebral metabolic rate of glucose consumption in the frontal cortex, temporal cortex, parietal cortex, midbrain and basal ganglia, which may be associated with improved sensation [304,305]. Improved tactile perception through STN-DBS might therefore be the result of normalized inhibition–excitation communication of a comprehensive neuronal network, including numerous dopaminergic and nondopaminergic structures that are responsible for sensory processing [34,158,159,209,309]. Hence, DBS normalizes the disease-related hyperactivity of the STN, and consequently modulates the activation of the somatosensory cortex and enhances sensorimotor integration and processing of tactile afferent information [36,74,95,287,288,289,310]. It has been speculated that the high-frequency DBS signal overwrites the pathological activity of the STN, leading to dysfunction within the basal ganglia-thalamo-cortical connections [90,306,311]. Given the connectivity between the subthalamus, pallidus, and thalamus and the ascending projections into the somatosensory cortices, DBS-induced regulation of neuronal firing bursts that improve somatosensory processing seems plausible [36,90,265,266,311]. Thus, this mechanism is believed to restore the ability of thalamo-cortical relay cells to respond to depolarizing inputs involved in sensorimotor integration [312,313]. Moreover, it might reduce nigrostriatal “noise” and enhance the signal-to-noise ratio for a better signal discrimination, which is needed for tactile perception [36,90,311,314,315].
Since we found rather subtle effects of STN-DBS on tactile cutaneous perception thresholds compared to various other studies, we raised questions about the reasons for a lack of DBS efficacy. Hence, primarily methodological factors such as different surgical procedures and DBS settings must be considered when interpreting our results. In this context, Pötter-Nerger and Volkmann discussed the importance of distinguishing between a “primary” failure, attributed to suboptimal DBS settings, and a “secondary” failure, attributed to the diminishing benefits of stimulation due to disease progression [171]. Some of our STN-DBS patients were tested before their neurological consultation, while others were tested after. Consequently, those tested before the consultation did not benefit from potentially optimized DBS settings, introducing a potential bias toward lower efficacy in our results [180]. This could have been a potential issue in our study, considering that our STN-DBS patients had been suffering from the disease for more than twice as long as the patient group which received medication only. Furthermore, group PD-MED–DBS had relatively long STN stimulation intervals of approx. 3 years post surgery [98,99,100,171,179,180]. Further possible explanations include different study group compositions and the individual disease severity. Due to patients having high inter-individual symptom characteristics, a higher sample size might have been beneficial in detecting more robust group differences. Furthermore, conducting a longitudinal interventional study design to analyze tactile cutaneous perception before and after DBS surgery, with and without additional medication, instead of the cross-sectional design, could have provided a more accurate investigation of each therapy independently.
5. Conclusions
This study investigated axial motor symptoms and somatosensory functionality in PD patients and analyzed whether anti-parkinsonian medication in conjunction with STN-DBS shows different effects compared to medication alone. Healthy subjects were included as a reference. With respect to axial motor symptoms, we hypothesized that patients’ motor performance is worse compared to those of healthy subjects. Moreover, we assumed that medication in conjunction with additional STN-DBS is more advantageous in normalizing patients’ impaired motor performance compared to treatment with medication alone.
Regardless of the therapy, PD patients in both study groups showed higher postural sway in the AP and ML directions during quiescent bipedal stance compared to the healthy subject group HS. No differences could be found between treatment conditions. The functional limits of stability test revealed smaller base of support ranges, especially characterized by restricted limits of stability in the posterior direction for both patient groups, PD-MED and PD-MED–DBS, compared to the healthy subject group, HS. Moreover, patients in both groups needed longer times and moved slower during the test. Nevertheless, PD patients who received medication in conjunction with STN-DBS showed significantly larger limits of stability in the anterior direction compared to patients who received anti-parkinsonian medication alone. Patient gait was mainly characterized by slowness due to shorter strides and fewer steps per minute, which also reflected longer strides and double support times compared to HS. PD patients also showed broader stride widths compared to HS. Only minor differences pointing towards better gait performance were found for PD-MED–DBS compared to PD-MED. To summarize those results, we found that PD patients suffer from impaired postural stability during quasi-static and dynamic balance situations, as well as from impaired gait performance compared to the healthy subject group, which therefore confirms our hypothesis. Anti-parkinsonian medication in combination with STN-DBS tended to be superior for improving patients’ overall motor performance compared to medication alone. This was especially true for the limits of stability and gait. Nevertheless, as there were no significant improvements in the majority of the investigated motor parameters, we reject our hypothesis that anti-parkinsonian medication in combination with STN-DBS is superior for treating axial motor symptoms compared to medication alone. The differences between our results and those of studies demonstrating clear DBS-induced positive effects on motor performance can mainly be attributed to methodological factors, as described earlier. Therefore, our results suggest that testing patients’ postural and gait performance should be challenging and quantify various different aspects of motor performance in order to evaluate patients’ axial motor symptoms more comprehensively. As prominent clinical markers of patients’ motor disability, the limits of stability in the anterior and posterior directions and gait speed should be observed more carefully. Further studies examining the impact of DBS and anti-parkinsonian medication on axial motor symptoms should involve varying combinations of anti-parkinsonian medication and varying stimulation parameters. Besides STN-DBS, the effects of stimulating other target areas, such as the globus pallidus interna and the pedunculopontine nucleus, on motor performance should also be investigated. We also recommend additional investigations, including EMG analysis of various muscles controlling movements of the ankle, knee, and the hip joints, as well as 3D motion analysis for analyzing full body motion.
The second objective of this study was to investigate somatosensory functionality by analyzing plantar cutaneous VPTs within the same study groups. Based on the pathophysiological mechanisms of PD and previous study findings, we hypothesized that patients’ plantar cutaneous vibration perception is impaired compared to healthy subjects. Since our results showed that the plantar cutaneous vibration perception of PD patients who received only anti-parkinsonian medication was significantly higher compared to that of the healthy subject group, we can confirm our hypothesis. This suggests that the pathophysiological mechanisms of PD affect plantar tactile cutaneous perception. Moreover, our hypothesis is supported by the results of various other studies that found impaired tactile cutaneous perception in PD patients by analyzing numerous body regions, including the torso, arms, hands, and fingers, and the feet. Consistent with the argumentation above that plantar mechanoreceptor input contributes to motor control and the study findings that textured insoles improve motor performance in PD patients, our results also suggest that impaired plantar cutaneous vibration perception might therefore contribute to axial motor symptoms in PD patients. Hence, our results may be helpful for developing and implementing plantar tactile cutaneous perception-enhancing therapy strategies. Moreover, they can be used to design and optimize low-cost therapy devices, such as textured insoles, to stimulate plantar cutaneous mechanoreceptors and consequently enhance motor performance in PD patients. As this is the first study investigating the effects of STN-DBS on plantar tactile cutaneous perception in PD patients, we furthermore hypothesized that anti-parkinsonian medication in combination with STN-DBS might show superior effects on normalizing patients’ impaired plantar cutaneous vibration perception compared to medication alone. Therefore, based on our results that STN-DBS improves plantar cutaneous vibration perception and on other study findings that also showed that STN-DBS improves tactile cutaneous perception, we confirm our hypothesis. Our results suggest that PD patients’ impaired plantar tactile cutaneous perception improves through STN-DBS, presumably by normalizing the integration and processing of afferent input on a higher-order cerebral level. Further studies examining the impact of DBS and anti-parkinsonian medication on plantar tactile cutaneous perception should involve varying combinations of anti-parkinsonian medication and varying stimulation parameters. Besides STN-DBS, the effects of stimulating other target areas, such as the globus pallidus interna and the pedunculopontine nucleus, on tactile cutaneous perception should also be investigated. Moreover, subsequent studies should focus on examining the functionality of various cutaneous mechanoreceptors that are sensitive to vibration stimuli at different frequencies, and to noxious and innoxious pressure and temperature stimuli.
References
- Weerasak, M.; Aju, M.; Hiroyuki, H.; Seidel, D. A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J. Med. Assoc. Thail. 2011, 94, 19–55. [Google Scholar]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived with Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- Berardelli, A. Neurophysiology of basal ganglia diseases. In Parkinson’s Disease and Related Disorders, Part I; Elsevier: Amsterdam, The Netherlands, 2007; pp. 67–75. ISBN 9780444519009. [Google Scholar]
- Agid, Y. Parkinson’s disease: Pathophysiology. Lancet 1991, 337, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S.C. Postural instability in patients with Parkinson’s disease. Epidemiology, pathophysiology and management. CNS Drugs 2013, 27, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. 1), 318–324. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Science, medicine, and the future: Parkinson’s disease. BMJ 1999, 318, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Shahed, J.; Jankovic, J. Motor symptoms in Parkinson’s disease. In Parkinson’s Disease and Related Disorders, Part I; Elsevier: Amsterdam, The Netherlands, 2007; pp. 329–342. ISBN 9780444519009. [Google Scholar]
- Benatru, I.; Vaugoyeau, M.; Azulay, J.-P. Postural disorders in Parkinson’s disease. Neurophysiol. Clin. 2008, 38, 459–465. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Coleman, S.; Galna, B.; Lord, S.; Rochester, L. Time-dependent changes in postural control in early Parkinson’s disease: What are we missing? Med. Biol. Eng. Comput. 2016, 54, 401–410. [Google Scholar] [CrossRef]
- Martin, W.R.W.; Wieler, M. Treatment of Parkinson’s disease. Can. J. Neurol. Sci. 2003, 30 (Suppl. 1), S27–S33. [Google Scholar] [CrossRef]
- May, D.S.; van Dillen, L.R.; Earhart, G.M.; Rawson, K.S.; Perlmutter, J.S.; Duncan, R.P. Effects of Subthalamic Nucleus Deep Brain Stimulation and Levodopa on Balance in People with Parkinson’s Disease: A Cross Sectional Study. Brain Sci. 2020, 10, 693. [Google Scholar] [CrossRef]
- Jellinger, K.A. Neuropathobiology of non-motor symptoms in Parkinson disease. J. Neural Transm. 2015, 122, 1429–1440. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F.; Bodis-Wollner, I. Parkinsons Disease and Nonmotor Dysfunction; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Hogg, E.; Wertheimer, J.; Graner, S.; Tagliati, M. Deep Brain Stimulation and Nonmotor Symptoms. Int. Rev. Neurobiol. 2017, 134, 1045–1089. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Maas, K.C.; Shah, V.V.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B.; Asaka, T.; Mancini, M. Functional limits of stability and standing balance in people with Parkinson’s disease with and without freezing of gait using wearable sensors. Gait Posture 2021, 87, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Fransson, P.-A. Strategic alterations of posture are delayed in Parkinson’s disease patients during deep brain stimulation. Sci. Rep. 2021, 11, 23550. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Fransson, P.-A. Effects of Deep Brain Stimulation on Postural Control in Parkinson’s Disease. Comput. Biol. Med. 2020, 122, 103828. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, B.; Burugupally, S.P. Postural Instability in Parkinson’s Disease: A Review. Brain Sci. 2019, 9, 239. [Google Scholar] [CrossRef]
- Vitale, C.; Marcelli, V.; Furia, T.; Santangelo, G.; Cozzolino, A.; Longo, K.; Allocca, R.; Amboni, M.; Marciano, E.; Barone, P. Vestibular impairment and adaptive postural imbalance in parkinsonian patients with lateral trunk flexion. Mov. Disord. 2011, 26, 1458–1463. [Google Scholar] [CrossRef]
- Bertolini, G.; Wicki, A.; Baumann, C.R.; Straumann, D.; Palla, A. Impaired tilt perception in Parkinson’s disease: A central vestibular integration failure. PLoS ONE 2015, 10, e0124253. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, Y.-J.; Horak, F.B. What Is Wrong with Balance in Parkinson’s Disease? J. Mov. Disord. 2015, 8, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Dimitrova, D.; Nutt, J.G. Direction-specific postural instability in subjects with Parkinson’s disease. Exp. Neurol. 2005, 193, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Termoz, N.; Halliday, S.E.; Winter, D.A.; Frank, J.S.; Patla, A.E.; Prince, F. The control of upright stance in young, elderly and persons with Parkinson’s disease. Gait Posture 2008, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.G.; Bloem, B.R. Postural control in Parkinson patients: A proprioceptive problem? Exp. Neurol. 2011, 227, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.; e Souza, T.M.; Bizarro, L.; Oliveira, A. Proprioceptive deficits in Parkinson’s disease: From clinical data to animal experimentation. Psychol. Neurosci. 2011, 4, 235–244. [Google Scholar] [CrossRef]
- Vaugoyeau, M.; Viel, S.; Amblard, B.; Azulay, J.P.; Assaiante, C. Proprioceptive contribution of postural control as assessed from very slow oscillations of the support in healthy humans. Gait Posture 2008, 27, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, S.; Khazen, O.; DiMarzio, M.; Staudt, M.D.; Williams, L.; Gillogly, M.; Durphy, J.; Hanspal, E.K.; Adam, O.R.; Pilitsis, J.G. Effect of Directional Deep Brain Stimulation on Sensory Thresholds in Parkinson’s Disease. Front. Hum. Neurosci. 2020, 14, 217. [Google Scholar] [CrossRef]
- Belasen, A.; Rizvi, K.; Gee, L.E.; Yeung, P.; Prusik, J.; Ramirez-Zamora, A.; Hanspal, E.; Paiva, P.; Durphy, J.; Argoff, C.E.; et al. Effect of low-frequency deep brain stimulation on sensory thresholds in Parkinson’s disease. J. Neurosurg. 2017, 126, 397–403. [Google Scholar] [CrossRef]
- de Andrade, D.C.; Lefaucheur, J.-P.; Galhardoni, R.; Ferreira, K.S.; Paiva, A.R.B.; Bor-Seng-Shu, E.; Alvarenga, L.; Myczkowski, M.L.; Marcolin, M.A.; de Siqueira, S.R.; et al. Subthalamic deep brain stimulation modulates small fiber-dependent sensory thresholds in Parkinson’s disease. Pain 2012, 153, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Cody, F.W.J.; O’Boyle, D.J. Identification of unilateral elbow-joint position is impaired by Parkinson’s disease. Clin. Anat. 2002, 15, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Aman, J.E.; Abosch, A.; Bebler, M.; Lu, C.-H.; Konczak, J. Subthalamic nucleus deep brain stimulation improves somatosensory function in Parkinson’s disease. Mov. Disord. 2014, 29, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Gierthmühlen, J.; Arning, P.; Binder, A.; Herzog, J.; Deuschl, G.; Wasner, G.; Baron, R. Influence of deep brain stimulation and levodopa on sensory signs in Parkinson’s disease. Mov. Disord. 2010, 25, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Maschke, M.; Tuite, P.J.; Krawczewski, K.; Pickett, K.; Konczak, J. Perception of heaviness in Parkinson’s disease. Mov. Disord. 2006, 21, 1013–1018. [Google Scholar] [CrossRef]
- Zia, S.; Cody, F.; O’Boyle, D. Joint position sense is impaired by Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2000, 47, 218–228. [Google Scholar] [CrossRef]
- Day, B.L.; Fitzpatrick, R.C. The vestibular system. Curr. Biol. 2005, 15, R583–R586. [Google Scholar] [CrossRef]
- Novak, P.; Novak, V. Effect of step-synchronized vibration stimulation of soles on gait in Parkinson’s disease: A pilot study. J. Neuroeng. Rehabil. 2006, 3, 9. [Google Scholar] [CrossRef]
- Viseux, F.J. The sensory role of the sole of the foot: Review and update on clinical perspectives. Neurophysiol. Clin. 2020, 50, 55–68. [Google Scholar] [CrossRef]
- Phuenpathom, W.; Panyakaew, P.; Vateekul, P.; Surangsrirat, D.; Hiransuthikul, A.; Bhidayasiri, R. Vibratory and plantar pressure stimulation: Steps to improve freezing of gait in Parkinson’s disease. Park. Relat. Disord. 2022, 105, 43–51. [Google Scholar] [CrossRef]
- Qiu, F.; Cole, M.H.; Davids, K.W.; Hennig, E.M.; Silburn, P.A.; Netscher, H.; Kerr, G.K. Effects of textured insoles on balance in people with Parkinson’s disease. PLoS ONE 2013, 8, e83309. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.E.; Almeida, Q.J.; Spaulding, S.J.; van Oostveen, R.B.; Holmes, J.D.; Johnson, A.M.; Perry, S.D. Plantar cutaneous sensory stimulation improves single-limb support time, and EMG activation patterns among individuals with Parkinson’s disease. Park. Relat. Disord. 2009, 15, 697–702. [Google Scholar] [CrossRef]
- Germano, A.M.C.; Heß, T.; Schmidt, D.; Milani, T.L. Effects of plantar hypothermia on quasi-static balance: Two different hypothermic procedures. Gait Posture 2018, 60, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Heß, T.; Milani, T.L.; Meixensberger, J.; Krause, M. Postural performance and plantar cutaneous vibration perception in patients with idiopathic normal pressure hydrocephalus. Heliyon 2021, 7, e05811. [Google Scholar] [CrossRef] [PubMed]
- McKeown, M.D.; Peters, R.M.; Pasman, E.P.; McKeown, M.J.; Carpenter, M.G.; Inglis, J.T. Plantar cutaneous function in Parkinson’s disease patients ON and OFF L-dopa. Neurosci. Lett. 2016, 629, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.F.; Oddsson, L.I.E.; de Luca, C.J. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 2004, 156, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Nurse, M.A.; Nigg, B.M. The effect of changes in foot sensation on plantar pressure and muscle activity. Clin. Biomech. 2001, 16, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.D.; Santos, L.C.; Patla, A.E. Contribution of vision and cutaneous sensation to the control of centre of mass (COM) during gait termination. Brain Res. 2001, 913, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.D.; McIlroy, W.E.; Maki, B.E. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 2000, 877, 401–406. [Google Scholar] [CrossRef]
- McKeon, P.O.; Hertel, J. Diminished Plantar Cutaneous Sensation and Postural Control. Percept Mot Ski. 2007, 104, 56–66. [Google Scholar] [CrossRef]
- McKeon, P.O.; Hertel, J. Plantar hypoesthesia alters time-to-boundary measures of postural control. Somatosens. Mot. Res. 2007, 24, 171–177. [Google Scholar] [CrossRef]
- Song, K.; Kang, T.K.; Wikstrom, E.A.; Jun, H.-p.; Lee, S.Y. Effects of reduced plantar cutaneous sensation on static postural control in individuals with and without chronic ankle instability. J. Sci. Med. Sport 2017, 20, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Germano, A.M.C.; Schmidt, D.; Milani, T.L. Effects of hypothermically reduced plantar skin inputs on anticipatory and compensatory balance responses. BMC Neurosci. 2016, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K. The role of α-synuclein in neurodegeneration—An update. Transl. Neurosci. 2012, 3, 1015. [Google Scholar] [CrossRef]
- Bennett, M.C. The role of α-synuclein in neurodegenerative diseases. Pharmacol. Ther. 2005, 105, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Adler, C.H.; Lue, L.; Sue, L.I.; Bachalakuri, J.; Henry-Watson, J.; Sasse, J.; Boyer, S.; Shirohi, S.; Brooks, R.; et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009, 117, 613–634. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Milenkovic, I.J.; Preusser, M.; Budka, H. Nigral burden of alpha-synuclein correlates with striatal dopamine deficit. Mov. Disord. 2008, 23, 1608–1612. [Google Scholar] [CrossRef]
- Gold, A.; Turkalp, Z.T.; Munoz, D.G. Enteric alpha-synuclein expression is increased in Parkinson’s disease but not Alzheimer’s disease. Mov. Disord. 2013, 28, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Zhang, Y.; Lim, K.L.; Tanaka, Y.; Huang, H.; Gao, J.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: Implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001, 7, 1144–1150. [Google Scholar] [CrossRef]
- Ikemura, M.; Saito, Y.; Sengoku, R.; Sakiyama, Y.; Hatsuta, H.; Kanemaru, K.; Sawabe, M.; Arai, T.; Ito, G.; Iwatsubo, T.; et al. Lewy body pathology involves cutaneous nerves. J. Neuropathol. Exp. Neurol. 2008, 67, 945–953. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Estraneo, A.; Selim, M.M.; Caporaso, G.; Stancanelli, A.; Saltalamacchia, A.M.; Lanzillo, B.; Santoro, L. Sensory deficit in Parkinson’s disease: Evidence of a cutaneous denervation. Brain 2008, 131, 1903–1911. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.D. Nervous system pathology in sporadic Parkinson disease. Neurology 2008, 70, 1916–1925. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Mori, F.; Tanji, K.; Orimo, S.; Takahashi, H. Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol. 2010, 120, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White Iii, C.L.; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Beudel, M.; Macerollo, A.; Brown, M.J.N.; Chen, R. Editorial: The Role of the Basal Ganglia in Somatosensory-Motor Interactions: Evidence from Neurophysiology and Behavior. Front. Hum. Neurosci. 2019, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Eördegh, G.; Paróczy, Z.; Márkus, Z.; Benedek, G. Multisensory integration in the basal ganglia. Eur. J. Neurosci. 2006, 24, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Colder, B. The basal ganglia select the expected sensory input used for predictive coding. Front. Comput. Neurosci. 2015, 9, 119. [Google Scholar] [CrossRef]
- Peller, M.; Zeuner, K.E.; Munchau, A.; Quartarone, A.; Weiss, M.; Knutzen, A.; Hallett, M.; Deuschl, G.; Siebner, H.R. The basal ganglia are hyperactive during the discrimination of tactile stimuli in writer’s cramp. Brain 2006, 129, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Boecker, H.; Ceballos-Baumann, A.; Bartenstein, P.; Weindl, A.; Siebner, H.R.; Fassbender, T.; Munz, F.; Schwaiger, M.; Conrad, B. Sensory processing in Parkinson’s and Huntington’s disease: Investigations with 3D H215O-PET. Brain 1999, 122 Pt 9, 1651–1665. [Google Scholar] [CrossRef]
- Korsun, O.; Renvall, H.; Nurminen, J.; Mäkelä, J.P.; Pekkonen, E. Modulation of sensory cortical activity by deep brain stimulation in advanced Parkinson’s disease. Eur. J. Neurosci. 2022, 56, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Morse, J.R.; Lidsky, T.I. Somatosensory properties of globus pallidus neurons in awake cats. Exp. Brain Res. 1982, 46, 311–314. [Google Scholar] [CrossRef]
- Rothblat, D.S.; Schneider, J.S. Alterations in pallidal neuronal responses to peripheral sensory and striatal stimulation in symptomatic and recovered Parkinsonian cats. Brain Res. 1995, 705, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rothblat, D.S.; Schneider, J.S. Response of caudate neurons to stimulation of intrinsic and peripheral afferents in normal, symptomatic, and recovered MPTP-treated cats. J. Neurosci. 1993, 13, 4372–4378. [Google Scholar] [CrossRef]
- Seitz, R.J.; Roland, P.E. Vibratory stimulation increases and decreases the regional cerebral blood flow and oxidative metabolism: A positron emission tomography (PET) study. Acta Neurol. Scand. 1992, 86, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Diamond, S.G.; Markham, C.H. Parkinson’s disease: Sensory and motor problems in arms and hands. Neurology 1987, 37, 951–956. [Google Scholar] [CrossRef]
- Sathian, K.; Zangaladze, A.; Green, J.; Vitek, J.L.; DeLong, M.R. Tactile spatial acuity and roughness discrimination: Impairments due to aging and Parkinson’s disease. Neurology 1997, 49, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Cody, F.W.J.; O’Boyle, D.J. Discrimination of bilateral differences in the loci of tactile stimulation is impaired in subjects with Parkinson’s disease. Clin. Anat. 2003, 16, 241–247. [Google Scholar] [CrossRef]
- Weder, B.J.; Leenders, K.L.; Vontobel, P.; Nienhusmeier, M.; Keel, A.; Zaunbauer, W.; Vonesch, T.; Ludin, H.-P. Impaired somatosensory discrimination of shape in Parkinson’s disease: Association with caudate nucleus dopaminergic function. Hum. Brain Mapp. 1999, 8, 1–12. [Google Scholar] [CrossRef]
- Cury, R.G.; Galhardoni, R.; Fonoff, E.T.; Perez Lloret, S.; Dos Santos Ghilardi, M.G.; Barbosa, E.R.; Teixeira, M.J.; Ciampi de Andrade, D. Sensory abnormalities and pain in Parkinson disease and its modulation by treatment of motor symptoms. Eur. J. Pain 2016, 20, 151–165. [Google Scholar] [CrossRef]
- Conte, A.; Khan, N.; Defazio, G.; Rothwell, J.C.; Berardelli, A. Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat. Rev. Neurol. 2013, 9, 687–697. [Google Scholar] [CrossRef]
- Conte, A.; Modugno, N.; Lena, F.; Dispenza, S.; Gandolfi, B.; Iezzi, E.; Fabbrini, G.; Berardelli, A. Subthalamic nucleus stimulation and somatosensory temporal discrimination in Parkinson’s disease. Brain 2010, 133, 2656–2663. [Google Scholar] [CrossRef]
- Hammond, C.; Bergman, H.; Brown, P. Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci. 2007, 30, 357–364. [Google Scholar] [CrossRef]
- Ikeda, K.; Deguchi, K.; Kume, K.; Kamada, M.; Touge, T.; Masaki, T. Assessment of sensory perception and processing using current perception threshold in Parkinson’s disease. Neurol. Clin. Neurosci. 2013, 1, 209–213. [Google Scholar] [CrossRef]
- Prätorius, B.; Kimmeskamp, S.; Milani, T. The sensitivity of the sole of the foot in patients with Morbus Parkinson. Neurosci. Lett. 2003, 346, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Gandhi, S.S.; Osman, A.; Hurtig, H.I.; Pawasarat, I.; Beals, E.; Chung, I.; Dubroff, J.; Newberg, A.; Ying, G.-S.; et al. Point pressure sensitivity in early stage Parkinson’s disease. Physiol. Behav. 2015, 138, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.G.; Galhardoni, R.; Teixeira, M.J.; Dos Santos Ghilardi, M.G.; Silva, V.; Myczkowski, M.L.; Marcolin, M.A.; Barbosa, E.R.; Fonoff, E.T.; Ciampi de Andrade, D. Subthalamic deep brain stimulation modulates conscious perception of sensory function in Parkinson’s disease. Pain 2016, 157, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.G.; Galhardoni, R.; Fonoff, E.T.; Dos Santos Ghilardi, M.G.; Fonoff, F.; Arnaut, D.; Myczkowski, M.L.; Marcolin, M.A.; Bor-Seng-Shu, E.; Barbosa, E.R.; et al. Effects of deep brain stimulation on pain and other nonmotor symptoms in Parkinson disease. Neurology 2014, 83, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Dogru Huzmeli, E.; Yilmaz, A.; Okuyucu, E. Analysis of the effects of subthalamic nucleus deep brain stimulation on somatosensation in Parkinson’s disease patients. Neurol. Sci. 2020, 41, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Zhang, L.; Sperry, L.; Olichney, J.; Farias, S.T.; Shahlaie, K.; Chang, N.M.; Liu, Y.; Wang, S.-P.; Wang, C. Target Selection Recommendations Based on Impact of Deep Brain Stimulation Surgeries on Nonmotor Symptoms of Parkinson’s Disease. Chin. Med. J. 2015, 128, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Djaldetti, R.; Melamed, E. Sensory symptoms in Parkinson’s disease. In Parkinson’s Disease and Related Disorders, Part I; Elsevier: Amsterdam, The Netherlands, 2007; pp. 377–384. ISBN 9780444519009. [Google Scholar]
- Maruo, T.; Saitoh, Y.; Hosomi, K.; Kishima, H.; Shimokawa, T.; Hirata, M.; Goto, T.; Morris, S.; Harada, Y.; Yanagisawa, T.; et al. Deep brain stimulation of the subthalamic nucleus improves temperature sensation in patients with Parkinson’s disease. Pain 2011, 152, 860–865. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Beuter, A.; Modolo, J. Delayed and lasting effects of deep brain stimulation on locomotion in Parkinson’s disease. Chaos 2009, 19, 26114. [Google Scholar] [CrossRef]
- Castrioto, A.; Lozano, A.M.; Poon, Y.-Y.; Lang, A.E.; Fallis, M.; Moro, E. Ten-year outcome of subthalamic stimulation in Parkinson disease: A blinded evaluation. Arch. Neurol. 2011, 68, 1550–1556. [Google Scholar] [CrossRef]
- Zibetti, M.; Merola, A.; Rizzi, L.; Ricchi, V.; Angrisano, S.; Azzaro, C.; Artusi, C.A.; Arduino, N.; Marchisio, A.; Lanotte, M.; et al. Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov. Disord. 2011, 26, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Romito, L.M.; Daniele, A.; Piano, C.; Zinno, M.; Bentivoglio, A.R.; Albanese, A. Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 2010, 133, 2664–2676. [Google Scholar] [CrossRef] [PubMed]
- Germano, A.M.C.; Schlee, G.; Milani, T.L. Effect of cooling foot sole skin receptors on achilles tendon reflex. Muscle Nerve 2016, 53, 965–971. [Google Scholar] [CrossRef]
- Schlee, G.; Sterzing, T.; Milani, T.L. Foot sole skin temperature affects plantar foot sensitivity. Clin. Neurophysiol. 2009, 120, 1548–1551. [Google Scholar] [CrossRef]
- Nikaido, Y.; Akisue, T.; Kajimoto, Y.; Tucker, A.; Kawami, Y.; Urakami, H.; Iwai, Y.; Sato, H.; Nishiguchi, T.; Hinoshita, T.; et al. Postural instability differences between idiopathic normal pressure hydrocephalus and Parkinson’s disease. Clin. Neurol. Neurosurg. 2018, 165, 103–107. [Google Scholar] [CrossRef]
- Mancini, M.; Rocchi, L.; Horak, F.B.; Chiari, L. Effects of Parkinson’s disease and levodopa on functional limits of stability. Clin. Biomech. 2008, 23, 450–458. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Muthuraman, M.; Witt, K.; Weisser, B.; Fasano, A.; Deuschl, G. Postural control and freezing of gait in Parkinson’s disease. Park. Relat. Disord. 2016, 24, 107–112. [Google Scholar] [CrossRef]
- Ganesan, M.; Pal, P.K.; Gupta, A.; Sathyaprabha, T.N. Dynamic posturography in evaluation of balance in patients of Parkinson’s disease with normal pull test: Concept of a diagonal pull test. Park. Relat. Disord. 2010, 16, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Fröhlich, S.; Germano, A.M.C.; Kondragunta, J.; Agoitia Hurtado, M.F.D.C.; Rudisch, J.; Schmidt, D.; Hirtz, G.; Stollmann, P.; Voelcker-Rehage, C. Sensor-based systems for early detection of dementia (SENDA): A study protocol for a prospective cohort sequential study. BMC Neurol. 2020, 20, 84. [Google Scholar] [CrossRef]
- Schmidt, D.; Germano, A.M.; Milani, T.L.; Khaiyat, O. Subjective sensitivity data: Considerations to treat heteroscedasticity. Cogent Med. 2019, 6, 1673086. [Google Scholar] [CrossRef]
- Cobo, R.; García-Piqueras, J.; Cobo, J.; Vega, J.A. The Human Cutaneous Sensory Corpuscles: An Update. J. Clin. Med. 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- McGlone, F.; Reilly, D. The cutaneous sensory system. Neurosci. Biobehav. Rev. 2010, 34, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Hagander, L.G.; Midani, H.A.; Kuskowski, M.A.; Parry, G.J. Quantitative sensory testing: Effect of site and pressure on vibration thresholds. Clin. Neurophysiol. 2000, 111, 1066–1069. [Google Scholar] [CrossRef]
- Zippenfennig, C.; Wynands, B.; Milani, T.L. Vibration Perception Thresholds of Skin Mechanoreceptors Are Influenced by Different Contact Forces. J. Clin. Med. 2021, 10, 3083. [Google Scholar] [CrossRef]
- Mildren, R.L.; Strzalkowski, N.D.J.; Bent, L.R. Foot sole skin vibration perceptual thresholds are elevated in a standing posture compared to sitting. Gait Posture 2016, 43, 87–92. [Google Scholar] [CrossRef]
- Holowka, N.B.; Wynands, B.; Drechsel, T.J.; Yegian, A.K.; Tobolsky, V.A.; Okutoyi, P.; Mang’eni Ojiambo, R.; Haile, D.W.; Sigei, T.K.; Zippenfennig, C.; et al. Foot callus thickness does not trade off protection for tactile sensitivity during walking. Nature 2019, 571, 261–264. [Google Scholar] [CrossRef]
- Drechsel, T.J.; Monteiro, R.L.; Zippenfennig, C.; Ferreira, J.S.S.P.; Milani, T.L.; Sacco, I.C.N. Low and High Frequency Vibration Perception Thresholds Can Improve the Diagnosis of Diabetic Neuropathy. J. Clin. Med. 2021, 10, 3073. [Google Scholar] [CrossRef] [PubMed]
- Zippenfennig, C.; Drechsel, T.J.; Monteiro, R.L.; Sacco, I.C.; Milani, T.L. The Mechanoreceptor’s Role in Plantar Skin Changes in Individuals with Diabetes Mellitus. J. Clin. Med. 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Gomes Paiva, A.F.; Thoumie, P.; Missaoui, B. How far do stabilometric and clinical parameters correlate in peripheral neuropathies? Gait Posture 2017, 52, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.-A.; Scholtes, V.A.; Dallmeijer, A.J.; Twisk, J.W.; Harlaar, J. The importance of addressing heteroscedasticity in the reliability analysis of ratio-scaled variables: An example based on walking energy-cost measurements. Dev. Med. Child Neurol. 2012, 54, 267–273. [Google Scholar] [CrossRef]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Fransson, P.-A.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Patel, M. Deep brain stimulation in the subthalamic nuclei alters postural alignment and adaptation in Parkinson’s disease. PLoS ONE 2021, 16, e0259862. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Gavriliuc, O.; Boße, K.; Wolke, R.; Granert, O.; Deuschl, G.; Margraf, N.G. The Effect of Medication and Deep Brain Stimulation on Posture in Parkinson’s Disease. Front. Neurol. 2019, 10, 1254. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Horak, F.; Peterson, D. Anticipatory Postural Adjustment During Self-Initiated, Cued, and Compensatory Stepping in Healthy Older Adults and Patients with Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 1316–1324.e1. [Google Scholar] [CrossRef]
- Barbieri, F.A.; Polastri, P.F.; Baptista, A.M.; Lirani-Silva, E.; Simieli, L.; Orcioli-Silva, D.; Beretta, V.S.; Gobbi, L.T. Effects of disease severity and medication state on postural control asymmetry during challenging postural tasks in individuals with Parkinson’s disease. Hum. Mov. Sci. 2016, 46, 96–103. [Google Scholar] [CrossRef]
- Beretta, V.S.; Gobbi, L.T.B.; Lirani-Silva, E.; Simieli, L.; Orcioli-Silva, D.; Barbieri, F.A. Challenging Postural Tasks Increase Asymmetry in Patients with Parkinson’s Disease. PLoS ONE 2015, 10, e0137722. [Google Scholar] [CrossRef]
- Collomb-Clerc, A.; Welter, M.-L. Effects of deep brain stimulation on balance and gait in patients with Parkinson’s disease: A systematic neurophysiological review. Neurophysiol. Clin. 2015, 45, 371–388. [Google Scholar] [CrossRef]
- DiFrancisco-Donoghue, J.; Jung, M.-K.; Geisel, P.; Werner, W.G. Learning effects of the sensory organization test as a measure of postural control and balance in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 858–861. [Google Scholar] [CrossRef]
- Johnsen, E.L. Gait and postural instability in Parkinson’s disease treated with deep brain stimulation of the subthalamic nucleus. Dan. Med. Bull. 2011, 58, B4334. [Google Scholar] [PubMed]
- Rocchi, L. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.P.; McVey, M.A.; Lyons, K.E.; Pahwa, R.; Luchies, C.W. Postural sway in patients with mild to moderate Parkinson’s disease. Int. J. Neurosci. 2011, 121, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Scholz, E.; Guschlbauer, B.; Dichgans, J. Increased shortening reaction in Parkinson’s disease reflects a difficulty in modulating long loop reflexes. Mov. Disord. 1987, 2, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Schieppati, M.; Nardone, A. Free and supported stance in Parkinson’s disease. The effect of posture and ‘postural set’ on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain 1991, 114 Pt 3, 1227–1244. [Google Scholar] [CrossRef]
- Horak, F.B.; Nutt, J.G.; Nashner, L.M. Postural inflexibility in parkinsonian subjects. J. Neurol. Sci. 1992, 111, 46–58. [Google Scholar] [CrossRef]
- Dimitrova, D.; Horak, F.B.; Nutt, J.G. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J. Neurophysiol. 2004, 91, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Beretta, V.S.; Carpenter, M.G.; Barbieri, F.A.; Santos, P.C.R.; Orcioli-Silva, D.; Pereira, M.P.; Gobbi, L.T.B. Does the impaired postural control in Parkinson’s disease affect the habituation to non-sequential external perturbation trials? Clin. Biomech. 2021, 85, 105363. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Allum, J.H.J.; Honegger, F.; Adkin, A.L.; Bloem, B.R. Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Beretta, V.S.; Vitório, R.; Santos, P.C.R.D.; Orcioli-Silva, D.; Gobbi, L.T.B. Postural control after unexpected external perturbation: Effects of Parkinson’s disease subtype. Hum. Mov. Sci. 2019, 64, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness control of balance in quiet standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef]
- Néstor, G.-J. Parkinson’s Disease. Neurobiol. Dis. 2007, 51–67. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Mancini, M.; Carlson-Kuhta, P.; Gross, A.; Horak, F.B. Step initiation in Parkinson’s disease: Influence of initial stance conditions. Neurosci. Lett. 2006, 406, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sigward, S.; Fisher, B.; Salem, G.J. Altered Dynamic Postural Control during Step Turning in Persons with Early-Stage Parkinson’s Disease. Parkinsons. Dis. 2012, 2012, 386962. [Google Scholar] [CrossRef]
- Heß, T.; Oehlwein, C.; Milani, T.L. Anticipatory Postural Adjustments and Compensatory Postural Responses to Multidirectional Perturbations—Effects of Medication and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Brain Sci. 2023, 13, 454. [Google Scholar] [CrossRef]
- Błaszczyk, J.W.; Orawiec, R.; Duda-Kłodowska, D.; Opala, G. Assessment of postural instability in patients with Parkinson’s disease. Exp. Brain Res. 2007, 183, 107–114. [Google Scholar] [CrossRef] [PubMed]
- de La Casa-Fages, B.; Alonso-Frech, F.; Grandas, F. Effect of subthalamic nucleus deep brain stimulation on balance in Parkinson’s disease: A static posturographic analysis. Gait Posture 2017, 52, 374–380. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Collins, J.J.; de Luca, C.J.; Burrows, A.; Lipsitz, L.A. Open-loop and closed-loop postural control mechanisms in Parkinson’s disease: Increased mediolateral activity during quiet standing. Neurosci. Lett. 1995, 197, 133–136. [Google Scholar] [CrossRef]
- Visser, J.E.; Oude Nijhuis, L.B.; Janssen, L.; Bastiaanse, C.M.; Borm, G.F.; Duysens, J.; Bloem, B.R. Dynamic posturography in Parkinson’s disease: Diagnostic utility of the “first trial effect”. Neuroscience 2010, 168, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Blenkinsop, G.M.; Pain, M.T.G.; Hiley, M.J. Balance control strategies during perturbed and unperturbed balance in standing and handstand. R. Soc. Open Sci. 2017, 4, 161018. [Google Scholar] [CrossRef]
- Horak, F.B.; Nashner, L.M.; Diener, H.C. Postural strategies associated with somatosensory and vestibular loss. Exp. Brain Res. 1990, 82, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Doná, F.; Aquino, C.C.; Gazzola, J.M.; Borges, V.; Silva, S.; Ganança, F.F.; Caovilla, H.H.; Ferraz, H.B. Changes in postural control in patients with Parkinson’s disease: A posturographic study. Physiotherapy 2015, 102, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Am Grimbergen, Y.; Munneke, M.; Bloem, B.R. Falls in Parkinson’s disease. Curr. Opin. Neurol. 2004, 17, 405–415. [Google Scholar] [CrossRef]
- Bosek, M.; Grzegorzewski, B.; Kowalczyk, A.; Lubiński, I. Degradation of postural control system as a consequence of Parkinson’s disease and ageing. Neurosci. Lett. 2005, 376, 215–220. [Google Scholar] [CrossRef]
- Crenna, P.; Carpinella, I.; Lopiano, L.; Marzegan, A.; Rabuffetti, M.; Rizzone, M.; Lanotte, M.; Ferrarin, M. Influence of basal ganglia on upper limb locomotor synergies. Evidence from deep brain stimulation and L-DOPA treatment in Parkinson’s disease. Brain 2008, 131, 3410–3420. [Google Scholar] [CrossRef]
- Shohamy, D.; Myers, C.E.; Onlaor, S.; Gluck, M.A. Role of the basal ganglia in category learning: How do patients with Parkinson’s disease learn? Behav. Neurosci. 2004, 118, 676–686. [Google Scholar] [CrossRef]
- Maschke, M.; Gomez, C.M.; Tuite, P.J.; Konczak, J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 2003, 126, 2312–2322. [Google Scholar] [CrossRef]
- Samadi, P.; Rouillard, C.; Bédard, P.J.; Di Paolo, T. Functional neurochemistry of the basal ganglia. In Parkinson’s Disease and Related Disorders, Part I; Elsevier: Amsterdam, The Netherlands, 2007; pp. 19–66. ISBN 9780444519009. [Google Scholar]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Frey, K.A.; Studenski, S.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.H.; Albin, R.L.; Müller, M.L.T.M. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013, 81, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Barrantes, F.J. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Park. Dis. 2016, 2, 16001. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Ma, S.Y.; Lee, M.S.; Collan, Y.; Röyttä, M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Park. Relat. Disord. 2008, 14, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Karachi, C.; Grabli, D.; Bernard, F.A.; Tandé, D.; Wattiez, N.; Belaid, H.; Bardinet, E.; Prigent, A.; Nothacker, H.-P.; Hunot, S.; et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Investig. 2010, 120, 2745–2754. [Google Scholar] [CrossRef]
- Bonnet, A.-M.; Loria, Y.; Saint-Hilaire, M.-H.; Lhermitte, F.; Agid, Y. Does long-term aggravation of Parkinson’s disease result from nondopaminergic lesions? Neurology 1987, 37, 1539. [Google Scholar] [CrossRef] [PubMed]
- Pahapill, P.A.; Lozano, A.M. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000, 123 Pt 9, 1767–1783. [Google Scholar] [CrossRef] [PubMed]
- Zweig, R.M.; Jankel, W.R.; Hedreen, J.C.; Mayeux, R.; Price, D.L. The pedunculopontine nucleus in Parkinson’s disease. Ann. Neurol. 1989, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Graybiel, A.M.; Duyckaerts, C.; Javoy-Agid, F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc. Natl. Acad. Sci. USA 1987, 84, 5976–5980. [Google Scholar] [CrossRef]
- Rochester, L.; Yarnall, A.J.; Baker, M.R.; David, R.V.; Lord, S.; Galna, B.; Burn, D.J. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain 2012, 135, 2779–2788. [Google Scholar] [CrossRef]
- Oz, F.; Yucekeya, B.; Huzmeli, I.; Yilmaz, A. Does subthalamic nucleus deep brain stimulation affect the static balance at different frequencies? Neurocirugía 2023, 34, 60–66. [Google Scholar] [CrossRef]
- Sato, K.; Hokari, Y.; Kitahara, E.; Izawa, N.; Hatori, K.; Honaga, K.; Oyama, G.; Hatano, T.; Iwamuro, H.; Umemura, A.; et al. Short-Term Motor Outcomes in Parkinson’s Disease after Subthalamic Nucleus Deep Brain Stimulation Combined with Post-Operative Rehabilitation: A Pre-Post Comparison Study. Park. Dis. 2022, 2022, 8448638. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Bai, Y.; Zou, L.; Zhang, X.; Wang, H.; Gao, D.; Qin, G.; Ma, R.; Zhang, K.; Meng, F.; et al. Balance response to levodopa predicts balance improvement after bilateral subthalamic nucleus deep brain stimulation in Parkinson’s disease. NPJ Park. Dis. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, S.; Yu, Y.; Wang, Y.; Cheng, Y.; Yang, H.; Tong, X. Effect of Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) on balance performance in Parkinson’s disease. PLoS ONE 2020, 15, e0238936. [Google Scholar] [CrossRef] [PubMed]
- Szlufik, S.; Kloda, M.; Friedman, A.; Potrzebowska, I.; Gregier, K.; Mandat, T.; Przybyszewski, A.; Dutkiewicz, J.; Figura, M.; Habela, P.; et al. The Neuromodulatory Impact of Subthalamic Nucleus Deep Brain Stimulation on Gait and Postural Instability in Parkinson’s Disease Patients: A Prospective Case Controlled Study. Front. Neurol. 2018, 9, 906. [Google Scholar] [CrossRef]
- Pötter-Nerger, M.; Volkmann, J. Deep brain stimulation for gait and postural symptoms in Parkinson’s disease. Mov. Disord. 2013, 28, 1609–1615. [Google Scholar] [CrossRef]
- Colnat-Coulbois, S. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 780–787. [Google Scholar] [CrossRef]
- St George, R.J.; Carlson-Kuhta, P.; Burchiel, K.J.; Hogarth, P.; Frank, N.; Horak, F.B. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson disease. J. Neurosurg. 2012, 116, 1347–1356. [Google Scholar] [CrossRef]
- Benabid, A.-L.; Koudsié, A.; Benazzouz, A.; Fraix, V.; Ashraf, A.; Le Bas, J.F.; Chabardes, S.; Pollak, P. Subthalamic Stimulation for Parkinson’s Disease. Arch. Med. Res. 2000, 31, 282–289. [Google Scholar] [CrossRef]
- Maurer, C.; Mergner, T.; Xie, J.; Faist, M.; Pollak, P.; Lucking, C.H. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson’s disease. Brain 2003, 126, 1146–1163. [Google Scholar] [CrossRef]
- McNeely, M.E.; Earhart, G.M. Medication and subthalamic nucleus deep brain stimulation similarly improve balance and complex gait in Parkinson disease. Park. Relat. Disord. 2013, 19, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, L.; Carlson-Kuhta, P.; Chiari, L.; Burchiel, K.J.; Hogarth, P.; Horak, F.B. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease: Laboratory investigation. J. Neurosurg. 2012, 117, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; De Weerdt, W.; Dom, R.; Nuttin, B.; Peeraer, L.; Pattyn, A. Walking ability after implantation of a pallidal stimulator:analysis of plantar force distribution in patients with Parkinson’s disease. Park. Relat. Disord. 1998, 4, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Nantel, J.; McDonald, J.C.; Bronte-Stewart, H. Effect of medication and STN-DBS on postural control in subjects with Parkinson’s disease. Park. Relat. Disord. 2012, 18, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, N.; Mulligan, S.; Mahant, P.; Samanta, J.; Abbas, J.J. Deep brain stimulation amplitude alters posture shift velocity in Parkinson’s disease. Cogn. Neurodyn. 2012, 6, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Stack, E.; Ashburn, A.; Jupp, K. Postural instability during reaching tasks in Parkinson’s disease. Physiother. Res. Int. 2005, 10, 146–153. [Google Scholar] [CrossRef]
- Schieppati, M.; Hugon, M.; Grasso, M.; Nardone, A.; Galante, M. The limits of equilibrium in young and elderly normal subjects and in parkinsonians. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1994, 93, 286–298. [Google Scholar] [CrossRef]
- Behrman, A.L.; Light, K.E.; Flynn, S.M.; Thigpen, M.T. Is the functional reach test useful for identifying falls risk among individuals with Parkinson’s disease? Arch. Phys. Med. Rehabil. 2002, 83, 538–542. [Google Scholar] [CrossRef]
- Kelly, V.E.; Hyngstrom, A.S.; Rundle, M.M.; Bastian, A.J. Interaction of levodopa and cues on voluntary reaching in Parkinson’s disease. Mov. Disord. 2002, 17, 38–44. [Google Scholar] [CrossRef]
- Li, H.; Liang, S.; Yu, Y.; Wang, Y.; Cheng, Y.; Yang, H.; Tong, X. Clinical experience of comprehensive treatment on the balance function of Parkinson’s disease. Medicine 2020, 99, e20154. [Google Scholar] [CrossRef] [PubMed]
- St George, R.J.; Carlson-Kuhta, P.; Nutt, J.G.; Hogarth, P.; Burchiel, K.J.; Horak, F.B. The effect of deep brain stimulation randomized by site on balance in Parkinson’s disease. Mov. Disord. 2014, 29, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Schaeffer, W.J.; Margraf, N.G.; Munser, S.; Wrede, A.; Buhmann, C.; Deuschl, G.; Oehlwein, C. Effect of neurostimulation on camptocormia in Parkinson’s disease depends on symptom duration. Mov. Disord. 2015, 30, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, M.; Hvid, L.G.; Thrue, C.; Johansson, S.; Franzén, E.; Dalgas, U.; Langeskov-Christensen, M. Muscle Strength and Power in People with Parkinson Disease: A Systematic Review and Meta-analysis. J. Neurol. Phys. Ther. 2023, 47, 3–15. [Google Scholar] [CrossRef]
- Wolke, R.; Kuhtz-Buschbeck, J.P.; Deuschl, G.; Margraf, N.G. Insufficiency of trunk extension and impaired control of muscle force in Parkinson’s disease with camptocormia. Clin. Neurophysiol. 2020, 131, 2621–2629. [Google Scholar] [CrossRef]
- Santos, M.J.; Kanekar, N.; Aruin, A.S. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J. Electromyogr. Kinesiol. 2010, 20, 388–397. [Google Scholar] [CrossRef]
- Rougier, P.; Burdet, C.; Farenc, I.; Berger, L. Backward and forward leaning postures modelled by an fBm framework. Neurosci. Res. 2001, 41, 41–50. [Google Scholar] [CrossRef]
- Artieda, J.; Pastor, M.A.; Lacruz, F.; Obeso, J.A. Temporal discrimination is abnormal in Parkinson’s disease. Brain 1992, 115 Pt 1, 199–210. [Google Scholar] [CrossRef]
- Bernardinis, M.; Atashzar, S.F.; Jog, M.S.; Patel, R.V. Differential Temporal Perception Abilities in Parkinson’s Disease Patients Based on Timing Magnitude. Sci. Rep. 2019, 9, 19638. [Google Scholar] [CrossRef]
- Vaugoyeau, M.; Hakam, H.; Azulay, J.-P. Proprioceptive impairment and postural orientation control in Parkinson’s disease. Hum. Mov. Sci. 2011, 30, 405–414. [Google Scholar] [CrossRef]
- Pearson, K.G. Proprioceptive regulation of locomotion. Curr. Opin. Neurobiol. 1995, 5, 786–791. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience 2006, 141, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Konczak, J.; Corcos, D.M.; Horak, F.; Poizner, H.; Shapiro, M.; Tuite, P.; Volkmann, J.; Maschke, M. Proprioception and Motor Control in Parkinson’s Disease. J. Mot. Behav. 2009, 41, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Watson, S.; Halliday, G.M.; Heinemann, T.; Gerlach, M. Relationships between various behavioural abnormalities and nigrostriatal dopamine depletion in the unilateral 6-OHDA-lesioned rat. Behav. Brain Res. 2003, 139, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Martignoni, E.; Ferriero, G.; Pasetti, C. Balance and fear of falling in Parkinson’s disease. Park. Relat. Disord. 2005, 11, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Hariz, M.; Blomstedt, P. Deep brain stimulation for Parkinson’s disease. J. Intern. Med. 2022, 292, 764–778. [Google Scholar] [CrossRef]
- Agnesi, F.; Johnson, M.D.; Vitek, J.L. Deep brain stimulation: How does it work? Handb. Clin. Neurol. 2013, 116, 39–54. [Google Scholar] [CrossRef]
- Groiss, S.J.; Wojtecki, L.; Südmeyer, M.; Schnitzler, A. Deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2009, 2, 20–28. [Google Scholar] [CrossRef]
- Perlmutter, J.S.; Mink, J.W. Deep brain stimulation. Annu. Rev. Neurosci. 2006, 29, 229–257. [Google Scholar] [CrossRef]
- Bloem, B.R.; Beckley, D.J.; van Dijk, J.G.; Zwinderman, A.H.; Remler, M.P.; Roos, R.A. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson’s disease. Mov. Disord. 1996, 11, 509–521. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C. Evidence-based medical review update: Pharmacological and surgical treatments of Parkinson’s disease: 2001 to 2004. Mov. Disord. 2005, 20, 523–539. [Google Scholar] [CrossRef]
- Bejjani, B.P.; Gervais, D.; Arnulf, I.; Papadopoulos, S.; Demeret, S.; Bonnet, A.M.; Cornu, P.; Damier, P.; Agid, Y. Axial parkinsonian symptoms can be improved: The role of levodopa and bilateral subthalamic stimulation. J. Neurol. Neurosurg. Psychiatry 2000, 68, 595–600. [Google Scholar] [CrossRef]
- Lubik, S.; Fogel, W.; Tronnier, V.; Krause, M.; König, J.; Jost, W.H. Gait analysis in patients with advanced Parkinson disease: Different or additive effects on gait induced by levodopa and chronic STN stimulation. J. Neural Transm. 2006, 113, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Goto, S.; Hamasaki, T.; Kuratsu, J.-I. Effect of bilateral subthalamic nucleus stimulation on levodopa-unresponsive axial symptoms in Parkinson’s disease. Acta Neurochir. 2008, 150, discussion 22. [Google Scholar] [CrossRef]
- Benabid, A.L. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 2003, 13, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Krack, P.; Batir, A.; van Blercom, N.; Chabardes, S.; Fraix, V.; Ardouin, C.; Koudsie, A.; Limousin, P.D.; Benazzouz, A.; LeBas, J.F.; et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 2003, 349, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Faist, M. Effect of bilateral subthalamic nucleus stimulation on gait in Parkinson’s disease. Brain 2001, 124, 1590–1600. [Google Scholar] [CrossRef]
- Zanardi, A.P.J.; da Silva, E.S.; Costa, R.R.; Passos-Monteiro, E.; Dos Santos, I.O.; Kruel, L.F.M.; Peyré-Tartaruga, L.A. Gait parameters of Parkinson’s disease compared with healthy controls: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 752. [Google Scholar] [CrossRef]
- Roper, J.A.; Kang, N.; Ben, J.; Cauraugh, J.H.; Okun, M.S.; Hass, C.J. Deep brain stimulation improves gait velocity in Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2016, 263, 1195–1203. [Google Scholar] [CrossRef]
- Grabli, D.; Karachi, C.; Welter, M.-L.; Lau, B.; Hirsch, E.C.; Vidailhet, M.; François, C. Normal and pathological gait: What we learn from Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 979–985. [Google Scholar] [CrossRef]
- Stolze, H.; Klebe, S.; Poepping, M.; Lorenz, D.; Herzog, J.; Hamel, W.; Schrader, B.; Raethjen, J.; Wenzelburger, R.; Mehdorn, H.M.; et al. Effects of bilateral subthalamic nucleus stimulation on parkinsonian gait. Neurology 2001, 57, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Kuhtz-Buschbeck, J.P.; Drücke, H.; Jöhnk, K.; Illert, M.; Deuschl, G. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Charlett, A.; Weller, C.; Purkiss, A.G.; Dobbs, S.M.; Dobbs, R.J. Breadth of base whilst walking: Effect of ageing and parkinsonism. Age Ageing 1998, 27, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kirollos, C.; Charlett, A.; O’Neill, C.J.; Kosik, R.; Mozol, K.; Purkiss, A.G.; Bowes, S.G.; Nicholson, P.W.; Hunt, W.B.; Weller, C.; et al. Objective measurement of activation of rigidity: Diagnostic, pathogenetic and therapeutic implications in parkinsonism. Br. J. Clin. Pharmacol. 1996, 41, 557–564. [Google Scholar] [CrossRef]
- Bovonsunthonchai, S.; Vachalathiti, R.; Pisarnpong, A.; Khobhun, F.; Hiengkaew, V. Spatiotemporal gait parameters for patients with Parkinson’s disease compared with normal individuals. Physiother. Res. Int. 2014, 19, 158–165. [Google Scholar] [CrossRef]
- Brinkerhoff, S.A.; Murrah, W.M.; Roper, J.A. All Known Cemeteries of Clayton County, Georgia; Ancestors Unlimited: Jonesboro, Georgia, 1986; ISBN 0123456789. [Google Scholar]
- Bugalho, P.; Alves, L.; Miguel, R. Gait dysfunction in Parkinson’s disease and normal pressure hydrocephalus: A comparative study. J. Neural Transm. 2013, 120, 1201–1207. [Google Scholar] [CrossRef]
- Weller, C.; Humphrey, S.J.; Kirollos, C.; Bowes, S.G.; Charlett, A.; Dobbs, S.M.; Dobbs, R.J. Gait on a shoestring: Falls and foot separation in parkinsonism. Age Ageing 1992, 21, 242–244. [Google Scholar] [CrossRef]
- Johnsen, E.L.; Sunde, N.; Mogensen, P.H.; Ostergaard, K. MRI verified STN stimulation site–gait improvement and clinical outcome. Eur. J. Neurol. 2010, 17, 746–753. [Google Scholar] [CrossRef]
- Navratilova, D.; Krobot, A.; Otruba, P.; Nevrly, M.; Krahulik, D.; Kolar, P.; Kolarova, B.; Kaiserova, M.; Mensikova, K.; Vastik, M.; et al. Deep Brain Stimulation Effects on Gait Pattern in Advanced Parkinson’s Disease Patients. Front. Neurosci. 2020, 14, 814. [Google Scholar] [CrossRef]
- Johnsen, E.L.; Mogensen, P.H.; Sunde, N.A.; Ostergaard, K. Improved asymmetry of gait in Parkinson’s disease with DBS: Gait and postural instability in Parkinson’s disease treated with bilateral deep brain stimulation in the subthalamic nucleus. Mov. Disord. 2009, 24, 588–595. [Google Scholar] [CrossRef]
- Peppe, A.; Chiavalon, C.; Pasqualetti, P.; Crovato, D.; Caltagirone, C. Does gait analysis quantify motor rehabilitation efficacy in Parkinson’s disease patients? Gait Posture 2007, 26, 452–462. [Google Scholar] [CrossRef]
- Allen, N.E.; Canning, C.G.; Sherrington, C.; Fung, V.S.C. Bradykinesia, muscle weakness and reduced muscle power in Parkinson’s disease. Mov. Disord. 2009, 24, 1344–1351. [Google Scholar] [CrossRef]
- Bloem, B.R.; Grimbergen, Y.A.M.; Cramer, M.; Willemsen, M.; Zwinderman, A.H. Prospective assessment of falls in Parkinson’s disease. J. Neurol. 2001, 248, 950–958. [Google Scholar] [CrossRef]
- St George, R.J.; Nutt, J.G.; Burchiel, K.J.; Horak, F.B. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology 2010, 75, 1292–1299. [Google Scholar] [CrossRef]
- van Nuenen, B.F.L.; Esselink, R.A.J.; Munneke, M.; Speelman, J.D.; van Laar, T.; Bloem, B.R. Postoperative gait deterioration after bilateral subthalamic nucleus stimulation in Parkinson’s disease. Mov. Disord. 2008, 23, 2404–2406. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Poole, R.; Johnson, J.; Halawa, O.; Huang, X. Arm swing magnitude and asymmetry during gait in the early stages of Parkinson’s disease. Gait Posture 2010, 31, 256–260. [Google Scholar] [CrossRef]
- Adkin, A.L.; Frank, J.S.; Jog, M.S. Fear of falling and postural control in Parkinson’s disease. Mov. Disord. 2003, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Cakit, B.D.; Saracoglu, M.; Genc, H.; Erdem, H.R.; Inan, L. The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson’s disease. Clin. Rehabil. 2007, 21, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Müller, M.L.; Kotagal, V.; Koeppe, R.A.; Kilbourn, M.R.; Gilman, S.; Albin, R.L.; Frey, K.A. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J. Cereb. Blood Flow Metab. 2012, 32, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Malouin, F.; Richards, C.L.; Jackson, P.L.; Dumas, F.; Doyon, J. Brain activations during motor imagery of locomotor-related tasks: A PET study. Hum. Brain Mapp. 2003, 19, 47–62. [Google Scholar] [CrossRef]
- Jahn, K.; Deutschländer, A.; Stephan, T.; Strupp, M.; Wiesmann, M.; Brandt, T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage 2004, 22, 1722–1731. [Google Scholar] [CrossRef]
- Weinberger, M.; Hamani, C.; Hutchison, W.D.; Moro, E.; Lozano, A.M.; Dostrovsky, J.O. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp. Brain Res. 2008, 188, 165–174. [Google Scholar] [CrossRef]
- Lozano, A.M.; Snyder, B.J. Deep brain stimulation for parkinsonian gait disorders. J. Neurol. 2008, 255 (Suppl. 4), 30–31. [Google Scholar] [CrossRef]
- Cossu, G.; Pau, M. Subthalamic nucleus stimulation and gait in Parkinson’s Disease: A not always fruitful relationship. Gait Posture 2017, 52, 205–210. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain 1994, 117 Pt 5, 1169–1181. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Ability to modulate walking cadence remains intact in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1532–1534. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 2005, 128, 2250–2259. [Google Scholar] [CrossRef]
- Bloem, B.R.; Grimbergen, Y.A.M.; van Dijk, J.G.; Munneke, M. The “posture second” strategy: A review of wrong priorities in Parkinson’s disease. J. Neurol. Sci. 2006, 248, 196–204. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain 1996, 119 Pt 2, 551–568. [Google Scholar] [CrossRef]
- Aziz, T.Z.; Davies, L.; Stein, J.; France, S. The role of descending basal ganglia connections to the brain stem in parkinsonian akinesia. Br. J. Neurosurg. 1998, 12, 245–249. [Google Scholar] [CrossRef]
- Brozova, H.; Barnaure, I.; Ruzicka, E.; Stochl, J.; Alterman, R.; Tagliati, M. Short- and Long-Term Effects of DBS on Gait in Parkinson’s Disease. Front. Neurol. 2021, 12, 688760. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Haq, I.U.; Hwynn, N.; Oyama, G.; Okun, M.; Tillman, M.D.; Hass, C.J. Low-frequency Versus High-frequency Subthalamic Nucleus Deep Brain Stimulation on Postural Control and Gait in Parkinson’s Disease: A Quantitative Study. Brain Stimul. 2015, 8, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Mera, T.O.; Filipkowski, D.E.; Riley, D.E.; Whitney, C.M.; Walter, B.L.; Gunzler, S.A.; Giuffrida, J.P. Quantitative analysis of gait and balance response to deep brain stimulation in Parkinson’s disease. Gait Posture 2013, 38, 109–114. [Google Scholar] [CrossRef]
- Brozova, H.; Barnaure, I.; Alterman, R.L.; Tagliati, M. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2009, 72, 770, author reply 770-1. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Gruendlinger, L.; Scollins, L.; O’Herron, S.; Tarsy, D. Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov. Disord. 2009, 24, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Rizzone, M.; Lopiano, L.; Lanotte, M.; Ferrarin, M. Impact of subthalamic nucleus stimulation on the initiation of gait in Parkinson’s disease. Exp. Brain Res. 2006, 172, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.T.; Dilda, V.; Hakim, B.; MacDougall, H.G. Validation of 24-hour ambulatory gait assessment in Parkinson’s disease with simultaneous video observation. Biomed. Eng. Online 2011, 10, 82. [Google Scholar] [CrossRef]
- Peppe, A.; Pierantozzi, M.; Chiavalon, C.; Marchetti, F.; Caltagirone, C.; Musicco, M.; Stanzione, P.; Stefani, A. Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: Effects on gait in Parkinson’s disease. Gait Posture 2010, 32, 512–518. [Google Scholar] [CrossRef]
- Xie, J.; Krack, P.; Benabid, A.L.; Pollak, P. Effect of bilateral subthalamic nucleus stimulation on parkinsonian gait. J. Neurol. 2001, 248, 1068–1072. [Google Scholar] [CrossRef]
- Ferrarin, M.; Rizzone, M.; Lopiano, L.; Recalcati, M.; Pedotti, A. Effects of subthalamic nucleus stimulation and l-dopa in trunk kinematics of patients with Parkinson’s disease. Gait Posture 2004, 19, 164–171. [Google Scholar] [CrossRef]
- Ferrarin, M.; Rizzone, M.; Bergamasco, B.; Lanotte, M.; Recalcati, M.; Pedotti, A.; Lopiano, L. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson?: S disease. Exp. Brain Res. 2005, 160, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Bleuse, S.; Delval, A.; Blatt, J.L.; Derambure, P.; Destée, A.; Defebvre, L. Effect of bilateral subthalamic nucleus deep brain stimulation on postural adjustments during arm movement. Clin. Neurophysiol. 2011, 122, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Crenna, P.; Marzegan, A.; Rabuffetti, M.; Rizzone, M.; Lopiano, L.; Ferrarin, M. Effect of L-dopa and subthalamic nucleus stimulation on arm and leg swing during gait in Parkinson’s Disease. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Volume 2007, pp. 6665–6668. [Google Scholar] [CrossRef]
- St George, R.J.; Carlson-Kuhta, P.; King, L.A.; Burchiel, K.J.; Horak, F.B. Compensatory stepping in Parkinson’s disease is still a problem after deep brain stimulation randomized to STN or GPi. J. Neurophysiol. 2015, 114, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Weaver, F.M.; Follett, K.A.; Stern, M.; Luo, P.; Harris, C.L.; Hur, K.; Marks, W.J.; Rothlind, J.; Sagher, O.; Moy, C.; et al. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology 2012, 79, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Rizzone, M.; Ferrarin, M.; Pedotti, A.; Bergamasco, B.; Bosticco, E.; Lanotte, M.; Perozzo, P.; Tavella, A.; Torre, E.; Recalcati, M.; et al. High-frequency electrical stimulation of the subthalamic nucleus in Parkinson’s disease: Kinetic and kinematic gait analysis. Neurol. Sci. 2002, 23 (Suppl. 2), S103–S104. [Google Scholar] [CrossRef]
- Krystkowiak, P.; Blatt, J.-L.; Bourriez, J.-L.; Duhamel, A.; Perina, M.; Blond, S.; Guieu, J.-D.; Destée, A.; Defebvre, L. Effects of Subthalamic Nucleus Stimulation and Levodopa Treatment on Gait Abnormalities in Parkinson Disease. Arch. Neurol. 2003, 60, 80–84. [Google Scholar] [CrossRef]
- Moreau, C.; Defebvre, L.; Destée, A.; Bleuse, S.; Clement, F.; Blatt, J.L.; Krystkowiak, P.; Devos, D. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2008, 71, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Wagle Shukla, A.; Moro, E.; Gunraj, C.; Lozano, A.; Hodaie, M.; Lang, A.; Chen, R. Long-term subthalamic nucleus stimulation improves sensorimotor integration and proprioception. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1020–1028. [Google Scholar] [CrossRef]
- Sailer, A.; Molnar, G.F.; Paradiso, G.; Gunraj, C.A.; Lang, A.E.; Chen, R. Short and long latency afferent inhibition in Parkinson’s disease. Brain 2003, 126, 1883–1894. [Google Scholar] [CrossRef]
- Knight, E.J.; Testini, P.; Min, H.-K.; Gibson, W.S.; Gorny, K.R.; Favazza, C.P.; Felmlee, J.P.; Kim, I.; Welker, K.M.; Clayton, D.A.; et al. Motor and Nonmotor Circuitry Activation Induced by Subthalamic Nucleus Deep Brain Stimulation in Patients with Parkinson Disease: Intraoperative Functional Magnetic Resonance Imaging for Deep Brain Stimulation. Mayo Clin. Proc. 2015, 90, 773–785. [Google Scholar] [CrossRef]
- Vitek, J.L. Deep brain stimulation: How does it work? Cleve. Clin. J. Med. 2008, 75 (Suppl. 2), S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Hamel, W.; Fietzek, U.; Morsnowski, A.; Schrader, B.; Herzog, J.; Weinert, D.; Pfister, G.; Müller, D.; Volkmann, J.; Deuschl, G.; et al. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: Evaluation of active electrode contacts. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1036–1046. [Google Scholar] [CrossRef]
- Vitek, J.L. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord. 2002, 17, S69–S72. [Google Scholar] [CrossRef]
- Santaniello, S.; McCarthy, M.M.; Montgomery, E.B.; Gale, J.T.; Kopell, N.; Sarma, S.V. Therapeutic mechanisms of high-frequency stimulation in Parkinson’s disease and neural restoration via loop-based reinforcement. Proc. Natl. Acad. Sci. USA 2015, 112, E586–E595. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Matar, E.; Ward, P.B.; Bolitho, S.J.; Gilat, M.; Pearson, M.; Naismith, S.L.; Lewis, S.J.G. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain 2013, 136, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Deniau, J.-M.; Degos, B.; Bosch, C.; Maurice, N. Deep brain stimulation mechanisms: Beyond the concept of local functional inhibition. Eur. J. Neurosci. 2010, 32, 1080–1091. [Google Scholar] [CrossRef]
- Arai, N.; Yokochi, F.; Ohnishi, T.; Momose, T.; Okiyama, R.; Taniguchi, M.; Takahashi, H.; Matsuda, H.; Ugawa, Y. Mechanisms of unilateral STN-DBS in patients with Parkinson’s disease: A PET study. J. Neurol. 2008, 255, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Geday, J.; Østergaard, K.; Johnsen, E.; Gjedde, A. STN-stimulation in Parkinson’s disease restores striatal inhibition of thalamocortical projection. Hum. Brain Mapp. 2009, 30, 112–121. [Google Scholar] [CrossRef]
- Stefani, A.; Lozano, A.M.; Peppe, A.; Stanzione, P.; Galati, S.; Tropepi, D.; Pierantozzi, M.; Brusa, L.; Scarnati, E.; Mazzone, P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 2007, 130, 1596–1607. [Google Scholar] [CrossRef]
- Florio, T.; Scarnati, E.; Confalone, G.; Minchella, D.; Galati, S.; Stanzione, P.; Stefani, A.; Mazzone, P. High-frequency stimulation of the subthalamic nucleus modulates the activity of pedunculopontine neurons through direct activation of excitatory fibres as well as through indirect activation of inhibitory pallidal fibres in the rat. Eur. J. Neurosci. 2007, 25, 1174–1186. [Google Scholar] [CrossRef]
- Hanakawa, T.; Katsumi, Y.; Fukuyama, H.; Honda, M.; Hayashi, T.; Kimura, J.; Shibasaki, H. Mechanisms underlying gait disturbance in Parkinson’s disease: A single photon emission computed tomography study. Brain 1999, 122 Pt 7, 1271–1282. [Google Scholar] [CrossRef]
- Limousin, P.; Greene, J.; Pollak, P.; Rothwell, J.; Benabid, A.L.; Frackowiak, R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann. Neurol. 1997, 42, 283–291. [Google Scholar] [CrossRef]
- Di Biase, L.; Pecoraro, P.M.; Carbone, S.P.; Caminiti, M.L.; Di Lazzaro, V. Levodopa-Induced Dyskinesias in Parkinson’s Disease: An Overview on Pathophysiology, Clinical Manifestations, Therapy Management Strategies and Future Directions. J. Clin. Med. 2023, 12, 4427. [Google Scholar] [CrossRef]
- Rizzone, M.G.; Fasano, A.; Daniele, A.; Zibetti, M.; Merola, A.; Rizzi, L.; Piano, C.; Piccininni, C.; Romito, L.M.; Lopiano, L.; et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: From the advanced phase towards the late stage of the disease? Park. Relat. Disord. 2014, 20, 376–381. [Google Scholar] [CrossRef]
- Kerr, G.K.; Worringham, C.J.; Cole, M.H.; Lacherez, P.F.; Wood, J.M.; Silburn, P.A. Predictors of future falls in Parkinson disease. Neurology 2010, 75, 116–124. [Google Scholar] [CrossRef]
- Dickson, D.W.; Uchikado, H.; Fujishiro, H.; Tsuboi, Y. Evidence in favor of Braak staging of Parkinson’s disease. Mov. Disord. 2010, 25 (Suppl. 1), S78–S82. [Google Scholar] [CrossRef]
- Kingsbury, A.E.; Bandopadhyay, R.; Silveira-Moriyama, L.; Ayling, H.; Kallis, C.; Sterlacci, W.; Maeir, H.; Poewe, W.; Lees, A.J. Brain stem pathology in Parkinson’s disease: An evaluation of the Braak staging model. Mov. Disord. 2010, 25, 2508–2515. [Google Scholar] [CrossRef]
- Wolters, E.C.; Braak, H. Parkinson’s disease: Premotor clinico-pathological correlations. In Parkinson’s Disease and Related Disorders; Springer: Berlin/Heidelberg, Germany, 2006; pp. 309–319. [Google Scholar] [CrossRef]
- Nakanishi, T.; Tamaki, M.; Mizusawa, H.; Akatsuka, T.; Kinoshita, T. An experimental study for analyzing nerve conduction velocity. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 484–487. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Rodriguez, M.; Guridi, J.; Mewes, K.; Chockkman, V.; Vitek, J.; DeLong, M.R.; Obeso, J.A. The subthalamic nucleus in Parkinson’s disease: Somatotopic organization and physiological characteristics. Brain 2001, 124, 1777–1790. [Google Scholar] [CrossRef]
- Romanelli, P.; Bronte-Stewart, H.; Heit, G.; Schaal, D.W.; Esposito, V. The functional organization of the sensorimotor region of the subthalamic nucleus. Stereotact. Funct. Neurosurg. 2004, 82, 222–229. [Google Scholar] [CrossRef]
- Calvert, G.A.; Thesen, T. Multisensory integration: Methodological approaches and emerging principles in the human brain. J. Physiol. Paris 2004, 98, 191–205. [Google Scholar] [CrossRef]
- Kühn, A.A.; Kupsch, A.; Schneider, G.-H.; Brown, P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci. 2006, 23, 1956–1960. [Google Scholar] [CrossRef]
- Kühn, A.A.; Williams, D.; Kupsch, A.; Limousin, P.; Hariz, M.; Schneider, G.-H.; Yarrow, K.; Brown, P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain 2004, 127, 735–746. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Bratzke, H.; Hamm-Clement, J.; Sandmann-Keil, D.; Rüb, U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J. Neurol. 2002, 249 (Suppl. 3), iii1–iii5. [Google Scholar] [CrossRef]
- Riederer, P.; Sian-Hülsmann, J. The significance of neuronal lateralisation in Parkinson’s disease. J. Neural Transm. 2012, 119, 953–962. [Google Scholar] [CrossRef]
- Silverdale, M.A.; Kobylecki, C.; Kass-Iliyya, L.; Martinez-Martin, P.; Lawton, M.; Cotterill, S.; Chaudhuri, K.R.; Morris, H.; Baig, F.; Williams, N.; et al. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Park. Relat. Disord. 2018, 56, 27–32. [Google Scholar] [CrossRef]
- O’Suilleabhain, P.; Bullard, J.; Dewey, R.B. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J. Neurol. Neurosurg. Psychiatry 2001, 71, 607–610. [Google Scholar] [CrossRef]
- Mancini, F.; Comi, C.; Oggioni, G.D.; Pacchetti, C.; Calandrella, D.; Moja, M.C.; Riboldazzi, G.; Tunesi, S.; Fante, M.D.; Manfredi, L.; et al. Prevalence and features of peripheral neuropathy in Parkinson’s disease patients under different therapeutic regimens. Park. Relat. Disord. 2014, 20, 27–31. [Google Scholar] [CrossRef]
- Dellapina, E.; Ory-Magne, F.; Regragui, W.; Thalamas, C.; Lazorthes, Y.; Rascol, O.; Payoux, P.; Brefel-Courbon, C. Effect of subthalamic deep brain stimulation on pain in Parkinson’s disease. Pain 2012, 153, 2267–2273. [Google Scholar] [CrossRef]
- Maschke, M.; Tuite, P.J.; Pickett, K.; Wächter, T.; Konczak, J. The effect of subthalamic nucleus stimulation on kinaesthesia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 569–571. [Google Scholar] [CrossRef]
- Ortega-Cubero, S.; Clavero, P.; Irurzun, C.; Gonzalez-Redondo, R.; Guridi, J.; Obeso, J.A.; Rodriguez-Oroz, M.C. Effect of deep brain stimulation of the subthalamic nucleus on non-motor fluctuations in Parkinson’s disease: Two-year’ follow-up. Park. Relat. Disord. 2013, 19, 543–547. [Google Scholar] [CrossRef]
- Reich, M.M.; Ray Chaudhuri, K.; Ashkan, K.; Hulse, N.; Costello, A.; Moriarty, J.; Samuel, M. Changes in the non-motor symptom scale in Parkinson’s disease after deep brain stimulation. Basal Ganglia 2011, 1, 131–133. [Google Scholar] [CrossRef]
- Witjas, T.; Kaphan, E.; Régis, J.; Jouve, E.; Chérif, A.A.; Péragut, J.-C.; Azulay, J.P. Effects of chronic subthalamic stimulation on nonmotor fluctuations in Parkinson’s disease. Mov. Disord. 2007, 22, 1729–1734. [Google Scholar] [CrossRef]
- Taktakishvili, O.; Sivan-Loukianova, E.; Kultas-Ilinsky, K.; Ilinsky, I.A. Posterior parietal cortex projections to the ventral lateral and some association thalamic nuclei in Macaca mulatta. Brain Res. Bull. 2002, 59, 135–150. [Google Scholar] [CrossRef]
- Davis, K.D.; Kwan, C.L.; Crawley, A.P.; Mikulis, D.J. Functional MRI Study of Thalamic and Cortical Activations Evoked by Cutaneous Heat, Cold, and Tactile Stimuli. J. Neurophysiol. 1998, 80, 1533–1546. [Google Scholar] [CrossRef]
- Hilker, R.; Voges, J.; Weisenbach, S.; Kalbe, E.; Burghaus, L.; Ghaemi, M.; Lehrke, R.; Koulousakis, A.; Herholz, K.; Sturm, V.; et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: Evidence from a FDG-PET study in advanced Parkinson’s disease. J. Cereb. Blood Flow Metab. 2004, 24, 7–16. [Google Scholar] [CrossRef]
- Trost, M.; Su, S.; Su, P.; Yen, R.-F.; Tseng, H.-M.; Barnes, A.; Ma, Y.; Eidelberg, D. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage 2006, 31, 301–307. [Google Scholar] [CrossRef]
- Oswal, A.; Beudel, M.; Zrinzo, L.; Limousin, P.; Hariz, M.; Foltynie, T.; Litvak, V.; Brown, P. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain 2016, 139, 1482–1496. [Google Scholar] [CrossRef] [PubMed]
- Herzog, J.; Weiss, P.H.; Assmus, A.; Wefer, B.; Seif, C.; Braun, P.M.; Pinsker, M.O.; Herzog, H.; Volkmann, J.; Deuschl, G.; et al. Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain 2008, 131, 132–145. [Google Scholar] [CrossRef]
- DiMarzio, M.; Rashid, T.; Hancu, I.; Fiveland, E.; Prusik, J.; Gillogly, M.; Madhavan, R.; Joel, S.; Durphy, J.; Molho, E.; et al. Functional MRI Signature of Chronic Pain Relief from Deep Brain Stimulation in Parkinson Disease Patients. Neurosurgery 2019, 85, E1043–E1049. [Google Scholar] [CrossRef]
- Di Giulio, I.; St George, R.J.; Kalliolia, E.; Peters, A.L.; Limousin, P.; Day, B.L. Maintaining balance against force perturbations: Impaired mechanisms unresponsive to levodopa in Parkinson’s disease. J. Neurophysiol. 2016, 116, 493–502. [Google Scholar] [CrossRef]
- Hartmann, C.J.; Hirschmann, J.; Vesper, J.; Wojtecki, L.; Butz, M.; Schnitzler, A. Distinct cortical responses evoked by electrical stimulation of the thalamic ventral intermediate nucleus and of the subthalamic nucleus. Neuroimage Clin. 2018, 20, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; D’Alessandro, G.; Bioulac, B.; Hammond, C. High-frequency stimulation in Parkinson’s disease: More or less? Trends Neurosci. 2005, 28, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.E.; Terman, D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J. Comput. Neurosci. 2004, 16, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Oliviero, A.; Mazzone, P.; Insola, A.; Tonali, P.; Di Lazzaro, V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J. Neurosci. 2001, 21, 1033–1038. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodríguez-Oroz, M.C.; Benitez-Temino, B.; Blesa, F.J.; Guridi, J.; Marin, C.; Rodriguez, M. Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov. Disord. 2008, 23 (Suppl. 3), S548–S559. [Google Scholar] [CrossRef]
- Sudhyadhom, A.; McGregor, K.; Okun, M.S.; Foote, K.D.; Trinastic, J.; Crosson, B.; Bova, F.J. Delineation of motor and somatosensory thalamic subregions utilizing probabilistic diffusion tractography and electrophysiology. J. Magn. Reson. Imaging 2013, 37, 600–609. [Google Scholar] [CrossRef]

