1. Introduction
In recent years, various research has emerged investigating the role of empagliflozin in managing various aspects of diabetic complications. One of the studies that broadened the scope of empagliflozin usage was the EMPA-REG OUTCOME trial [1]. The trial demonstrated significant reductions in cardiovascular morbidity and mortality in patients with type 2 diabetes mellitus (T2DM) and established cardiovascular disease. This paved the way for a paradigm shift in the treatment strategy, with empagliflozin at the forefront of therapeutic regimens and now widely being recommended as a second-line diabetic medication after metformin [2,3]. In 2021, it was also approved for heart failure indications in patients without diabetes [4].
Chronic kidney disease (CKD) is prevalent in about 40% of individuals with type 2 diabetes, and patients with CKD are at a higher risk of adverse outcomes due to their compromised renal function [5]. Patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2 were not included, and over 70% of the patients in the trials had normal kidney function in the pivotal study. [1] To address these issues, several randomized controlled trials (RCTs) have been conducted to evaluate the various clinical efficacy and adverse effects of empagliflozin in patients with CKD. The EMPEROR-Reduced study demonstrated enhanced effectiveness in managing heart failure and deteriorating kidney function for the group with eGFRs of less than 60 mL/min/1.73 m2 [6]. The EMPA-REG OUTCOME study indicated there was no notable variance in side effects between the group with an eGFR less than 60 mL/min/1.73 m2 and those with standard kidney function [7]. Furthermore, the EMPA-KIDNEY study, which recently encompassed up to 30% of participants with an eGFR below 30 mL/min/1.73 m2, revealed that empagliflozin continues to be advantageous for declining kidney function and cardiovascular disease [8]. A pooled analysis of safety results from RCTs reported a beneficial effect on hyperkalemia and edema [9]. It seems the benefits of empagliflozin were generally consistent across a range of eGFR values [10].
Additionally, the real-world effectiveness of empagliflozin in patients with diabetes and CKD has recently been explored. Various studies investigated the long-term effects of empagliflozin on renal outcomes in a real-world setting and concluded that the drug did slow the progression of kidney disease in these patients [11,12]. Htoo et al. reported that empagliflozin significantly reduces the risk of MACEs (but not liraglutide) or HHF when compared to liraglutide or sitagliptin [13]. Furthermore, SGLT2 inhibitors have also been reported to reduce blood pressure, uric acid, and microalbuminuria or podocyturia, indicating they might have structural benefits for glomerular health [14].
However, most of the prior research has been conducted in the US or Europe. In fact, in the EMPA-REG OUTCOME trial, over 70% of participants were Caucasian, with merely 20% being Asian [1]. The effect or adverse drug reaction to a drug could vary based on genetic factors or medical practices across different racial groups. For instance, Asians are often reported to have a lower rate of cardiovascular disease risk compared to other ethnicities [15]. Differences in diabetes’ epidemiology and pathophysiology exist across various racial and ethnic groups [16,17,18]. Asian individuals with type 2 diabetes often receive their diagnosis relatively early, with about 20% being diagnosed before turning 40 years old [19]. This group with early onset diabetes also has an elevated risk for complications compared to those with a later onset [20]. Growing research indicates that Asians with type 2 diabetes have a heightened risk for CKD compared to other ethnicities [21,22].
A primary concern regarding adverse drug reactions to empagliflozin among CKD patients revolves around diabetic ketoacidosis (DKA) and dehydration [4]. So far, few studies have investigated the safety of these adverse effects specifically in Asians with CKD. While existing evidence does highlight beneficial renal outcomes and possible cardiovascular benefits, the risks associated with adverse reactions like diabetic ketoacidosis and dehydration need careful examination, especially for Asian populations. Although there have been numerous retrospective studies analyzing the effectiveness and safety of empagliflozin, none have compared results between CKD and non-CKD patients. Real-world evidence (RWE) studies hold value as they offer insights over longer durations and encompass a broader patient demographic [23,24]. They might be valuable in scenarios where RCT data may be lacking or unrepresentative of Asian population. Thus, our objective is to address these safety aspects through undertaking an RWE study focused on Korean CKD patients, utilizing insurance claim data.
2. Materials and Methods
2.1. Study Design and Sources
This was a retrospective cohort study evaluating the impact of empagliflozin compared to sitagliptin on safety outcomes in patients with CKD and T2DM. The analyzed health insurance data was officially provided by the Korean Health Insurance Review & Assessment Service (HIRA) [25]. In Korea, enrollment in the National Health Insurance (NHI) program is mandatory for 97% of the population. Healthcare facilities like clinics and hospitals file claims with the HIRA service to get reimbursement for both inpatient and outpatient care. These claims include details such as diagnoses coded according to the International Classification of Diseases, 10th revision (ICD10), procedures performed, prescription records, and demographic details.
2.2. Ethical Approval
Given that all participants were anonymized using a randomized identification number, there was no requirement for written informed consent. The study received approval from the Institutional Review Board of Seoul National University (IRB No. E2101/001-003) and adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [26].
2.3. Study Patients
This study included patients diagnosed with T2DM between 2016 and 2018. All included patients were adults (18 years or older) with established CKD (N18.4, N18.5, N18.6, E11.2, and E13.2) and T2DM (E11–E14) who were prescribed either empagliflozin or sitagliptin for the first time. We selected an active comparator (sitagliptin) as a proxy for the placebo. We chose an active comparator, sitagliptin, to stand in for the placebo due to its well-established use in observational studies. This decision was made because non-user comparator groups in such studies can show significant differences from actively treated patients, in contrast to RCTs [27]. Utilizing an active comparator can also aid in minimizing the risk of immortal time bias. Numerous other research has also used Dipeptidyl peptidase-4 (DPP4) inhibitors as comparators while evaluating the safety of SGLT-2, given the established safety records of DPP4 inhibitors [28,29,30,31,32]. The index date was determined to be the very first date each drug (empagliflozin or sitagliptin) was prescribed. The study period was before the new 2022 American Diabetes Association guidelines on type 2 diabetes were introduced [33], and empagliflozin and sitagliptin were commonly considered as second- or third-line options post metformin. In some cases, empagliflozin was prescribed after a DPP-4 inhibitor due to safety concerns associated with its use.
2.4. Key Variables
A total of 16 adverse effects including major adverse cardiovascular events (MACEs), all-cause mortality, myocardial infarction [MI], hospitalization for unstable angina, coronary revascularization, stroke, transient ischemic attack (TIA), hospitalization for heart failure (HHF), hypoglycemic events, urinary tract infections (UTIs), genital tract infections (GTIs), volume depletion, acute kidney injury (AKI), DKA, thromboembolic events, and bone fracture were analyzed. In each cohort, individuals with a history of these adverse effects were excluded, and separate cohorts were constructed. The operational definitions of outcomes were determined using the Korean Standard Classification of Diseases-7 codes or procedure codes . To minimize the influence of potential confounding variables, such as selection bias, we included a total of 71 covariates. These covered demographics, comorbidities, and disease/outcome-specific variables. All these covariates were evaluated within the year preceding the index date.
2.5. Statistical Analysis
Statistical analyses were performed for the intention-to-treat population. Patients were followed until the earliest occurrence of any of the following events: an outcome event, the date of the last follow-up, the date of switching diabetic medication to the other comparison group, or the end of the study period. The maximum follow-up period was set at 48 months. Empagliflozin users were matched 1:1 with sitagliptin users, and the distribution of the propensity score was inspected [34]. A standardized difference greater than 0.1 was considered indicative of an imbalance [35]. The Cox proportional hazard regression model was used to estimate the sex- and age-adjusted hazard ratio (aHR) of empagliflozin for adverse outcomes, with a 95% confidence interval (CI).
Sensitivity analyses were performed in two ways. First, patients who received at least 1 dose of each study drug were observed until ≤30 d after a patient’s last intake of medication. Additionally, we followed up patients who received the study drug for ≥30 d (cumulative) including events that only occurred ≤30 d after a patient’s last intake of medication (‘as-treated’ analysis). Analyses were performed with SAS Enterprise Guide version 7.1 (SAS Institute Inc., Cary, NC, USA).
2.6. Comparative Analysis
Using the same study design, patients in the normal kidney function (NKF) group were identified just like the CKD patients. The comparative analysis was conducted in a comparable fashion on patients with NKF and contrasted with the results obtained from patients with CKD. They were evaluated for the same set of 16 outcomes including MACEs, all-cause death, MI, and more. The statistical analysis, involving Cox proportional hazard regression and propensity score matching, was performed identically to maintain consistency. Thus, the outcomes in NKF patients were compared to the outcomes in CKD patients, thereby providing a comparison between the two distinct patient groups.
3. Results
3.1. Demographics
A total of 932,465 patients diagnosed with type 2 diabetes and treated with either empagliflozin or sitagliptin were identified. From this group, 384,579 new users of these medications remained (Figure 1). Patients diagnosed with CKD were then selected, resulting in an eligible study cohort of 26,347 patients (6211 on empagliflozin, 20,136 on sitagliptin). The data revealed that sitagliptin users were older and had more frequent clinic visits (both inpatient and outpatient) compared to empagliflozin users and ). Moreover, a higher prevalence of coronary artery disease, including stroke, was observed among sitagliptin users relative to empagliflozin users.
Figure 1. Study flow chart.
A successful match was achieved between 6170 empagliflozin users and sitagliptin users. After matching, the differences in age, frequency of clinic visits, index date, cardiovascular risk factors, comedications, and comorbidities between the two groups were considerably diminished, resulting in a well-balanced cohort. All 71 covariates showed standardized differences well below 0.1. The median follow-up period was recorded as 0.9 years, with the median duration of prescription for anti-diabetic medications during the follow-up period being 0.9 years (interquartile range 0.2–2.3 years). The mean age of the patients was noted as 50.9 years, with men constituting 56.7% of the cohort (n = 6998). The baseline characteristics of patients with normal kidney function are presented in .
3.2. Risk of Safety Outcomes
The use of empagliflozin was associated with a notable reduction in the risk of MACEs, with an aHR of 0.74 (95% CI: 0.64–0.85) . The risk of all-cause mortality was also significantly reduced when treated with empagliflozin (aHR 0.47, 95% CI: 0.33–0.68). In the context of MI, empagliflozin usage resulted in a lower risk, as denoted by an aHR of 0.60 (95% CI: 0.45–0.81). However, for stroke, it did not show a significant difference in risk, with an aHR of 0.92 (95% CI: 0.74–1.13). Patients receiving empagliflozin demonstrated a decreased risk of hospitalization for unstable angina, presenting an aHR of 0.67 (95% CI: 0.57–0.79). Likewise, the risk of coronary revascularization was marginally reduced with an aHR of 0.79 (95% CI: 0.65–0.97). HHF showed a reduction in risk with an aHR of 0.66 (95% CI: 0.55–0.80). Hypoglycemic adverse events also had a slightly lower risk with empagliflozin treatment, as indicated by an aHR of 0.78 (95% CI: 0.62–0.97). Additionally, the risk of UTIs was slightly reduced, with an aHR of 0.90 (95% CI: 0.82–0.98). Notably, the risk of GTIs was increased with empagliflozin, with an aHR of 1.43 (95% CI: 1.27–1.61). There were no significant changes in the risk of transient ischemic attack, AKI, volume depletion, DKA, thromboembolic events, and fractures as indicated by the aHRs close to 1.
3.3. Sensitivity Analysis
For sensitivity analysis, after the follow-up of patients who received at least one dose of study drugs until ≤30 d after the last intake of medication, similar results were obtained in all 16 outcomes. Additional sensitivity analysis (including patients who received study drugs for ≥30 d including only events that occurred ≤30 d after a patient’s last intake of medications) did not produce meaningful changes in the study findings .
3.4. Comparative Analysis: CKD Group versus NKF Group
In a comparative analysis, we observed that CKD patients who used empagliflozin showed a protective effect against cardiovascular diseases. These included associations with MACEs, MI, coronary revascularization, angina, HHF, hypoglycemia, UTIs, GTIs, volume depletion, and thromboembolic events, as depicted in Figure 2. A notable observation was the risk of death in the CKD group with an aHR of 0.47 (95% CI: 0.33–0.68). Empagliflozin in the CKD group was not significantly associated with stroke, with an aHR of 0.92 (95% CI: 0.74–1.13); AKI, with an aHR of 0.94 (95% CI: 0.76–1.17); DKA, with an aHR of 0.96 (95% CI: 0.60–1.54); or fractures, with an aHR of 1.03 (95% CI: 0.92–1.14).
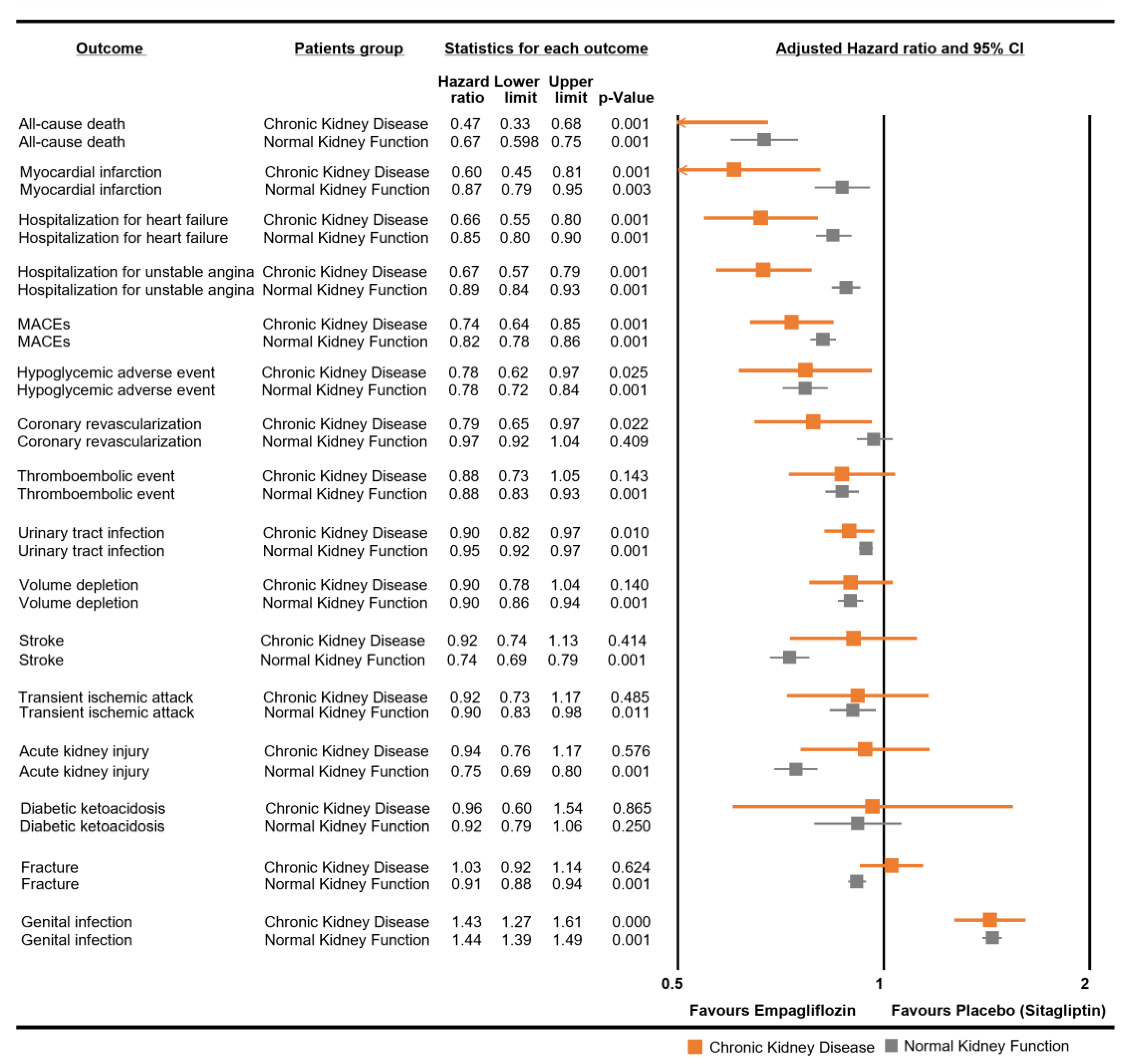
Figure 2. Comparative analysis of empagliflozin for each safety outcome in chronic kidney disease and normal kidney function patients. Normal kidney function patients refer to those not diagnosed with chronic kidney disease.
For NKF patients, the HRs for empagliflozin use were generally similar between both groups, except some outcomes. The risk of stroke was noted with an aHR of 0.74 (95% CI: 0.69–0.79). The association with the risk of AKI was marked by an aHR of 0.75 (95% CI: 0.69–0.80). Additionally, the risk of fractures was noted with an aHR of 0.91 (95% CI: 0.88–0.94) in the NKF group.
4. Discussion
This RWE study explored the safety outcomes of empagliflozin use in T2DM patients with CKD. To our knowledge, this is the first RWE study to examine a range of safety outcomes in Asian CKD patients. Despite concerns that these patients might not respond well to empagliflozin due to the reduced glucose reabsorption capabilities of their kidneys [4], the study found a substantial decrease in cardiovascular event risks in Asian CKD patients. These results are consistent with findings from the EMPA-REG OUTCOME, EMPEROR-REDUCED, and EMPA-KIDNEY studies [6,7,8].
The kidneys play a crucial role in the underlying mechanisms of T2DM. Firstly, in healthy individuals, the kidneys account for about 20% to 25% of the body’s endogenous glucose production during fasting, due to the process of gluconeogenesis. Secondly, the kidneys are pivotal in managing blood glucose levels as they oversee both glucose filtration and reabsorption processes [36,37]. At this point, empagliflozin has received more attention for its glucosuria mechanism. Moreover, empagliflozin’s benefits in CKD patients appear to extend beyond just inducing glucosuria, positively impacting cardiovascular and renal dynamics, reducing inflammation, and providing protection to end-organs. Several mechanisms have been proposed for the effects of empagliflozin, including its role in natriuresis, ketogenesis, lipid metabolism, improvements in cellular and endothelial functions, and antiproliferative effects on certain types of cancer. One of the mechanisms involves aiding sodium excretion [38], which in turn activates transforming growth factor, offering hemodynamic protection to the kidneys [39,40,41]. Concerns have been primarily raised regarding AKI when empagliflozin is used in patients with CKD [42] due to vasoconstriction or volume depletion; however, such events are usually temporary. Our study did not identify any enhanced risks of AKI or volume depletion in Asian CKD patients. Additionally, empagliflozin boosts endogenous G production that promotes lipid oxidation and ketone body utilization. Studies have reported an increase in lipolysis and a reduction in visceral fat [43]. In CKD patients with decreased renal function, the development of DKA has been a concern due to decreased ketone excretion; however, this study found that empagliflozin did not increase the risk of DKA. Furthermore, empagliflozin has been associated with improved arterial vascular stiffness and reduced resistance [44,45]. It has shown a decline in both cardiac and vascular sympathetic nerve activities, and it exhibits anti-inflammatory properties [46,47] and mitigates fibrosis [45,48]. All these factors provide a strong rationale for its potential cardiovascular benefits, especially in CKD patients with deteriorated renal function. However, our study did not find significant changes in the risk for stroke, transient ischemic attack, thromboembolic events, and fractures.
Our research strength lies in its comprehensive analysis of various adverse effects. To date, only a few studies have investigated the effectiveness or safety of empagliflozin in Asian patients with CKD. Sugiyama et al. reported that SGLT2 inhibitor enhanced kidney protection, leading to a marked improvement in the reduction in eGFR and proteinuria in Japanese patients [12]. Another study also showed a reduction in risk for developing end-stage kidney disease in Singaporean patients [49]. However, a significant limitation of these studies is their focus solely on CKD’s progression, neglecting to consider cardiovascular events and side effects. We examined a range of safety outcomes in Asian CKD patients and concluded that the pattern of results closely aligns with those observed in Western populations. A few key points should be considered when interpreting these results. For cardiovascular outcomes, Asian individuals often exhibit a stronger reaction to antihypertensive medications that influence the renin–angiotensin–aldosterone system. [50]. Another study also indicates racial distinctions in plasma renin activity levels [51]. This suggests reducing overall sodium content as a potential therapeutic target, showing why Asian individuals might be also responsive to empagliflozin. It is reported that GTIs were considerably less frequent in Asians than in Western populations, and this could be attributed to better hygiene practices in Asia [52]. However, GTIs also seem to be an issue with the use of empagliflozin in the Asian population.
For those with significant renal impairment in CKD (<30 mL/min/1.73 m2), the area under the concentration–time curve of empagliflozin increased 1.7–2.7-fold, and its half-life was increased by 1.4-fold compared to NKF patients [53,54]. Indeed, our comparative analyses have shown that Asian CKD patients showed a higher occurrence of adverse reactions irrespective of being on sitagliptin or empagliflozin compared to NKF patients. Nevertheless, the HRs for empagliflozin use in relation to various outcomes were generally consistent between both groups, with a few exceptions. SGLT2 inhibitors are as effective to use in CKD patients as they are in NKF patients while preventing a variety of cardiovascular events. The cardiovascular safety benefits of empagliflozin appear to be further maximized in CKD patients. However, the comparative analysis did reveal no significant results for stroke, AKI, and fractures in CKD patients compared to NKF patients. Other RWE studies have also reported safety in other NKF patients with these results (stroke [55], AKI [56,57,58,59], and fractures [60]). This could be explained by the underlying disease state and co-morbidities in CKD patients and warrants further research to explore these risks in more detail. Even though empagliflozin did not reveal any new safety concerns, these findings underline the significance of tailored therapeutic strategies in CKD patients. This fact emphasizes the importance of patient education and close monitoring to prevent and manage such complications.
This study’s results need to be considered within the limitations of an observational study. Our research employs a retrospective cohort methodology, and the HIRA database does not incorporate all details, such as lab outcomes for blood glucose tests, urine culture tests, or body weight. Despite our effort to control all potential confounders, there still may be residual confounding factors present. It should also be noted that the control utilized in this research was sitagliptin, not a genuine placebo. This could potentially impact the study’s external validity. Furthermore, the follow-up period in this study was relatively short, potentially limiting the ability to detect the long-term effects of the medications.
5. Conclusions
In conclusion, our study results suggest reassuring the efficacy and safety profiles of empagliflozin in CKD patients, and further supports its use in Asian population. As the number of patients with CKD continues to grow globally, the real-world evidence in this study could serve as a key step in providing evidence for future clinical decision-making around the use of empagliflozin in Asian CKD patients.
References
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin. Diabetes 2018, 36, 14–37. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Cefalu, W.T.; Januzzi, J.L., Jr.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.L.; Morris, P.B.; et al. 2018 ACC Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2018, 72, 3200–3223. [Google Scholar] [CrossRef] [PubMed]
- DailyMed. Drug Label: Empagliflozin (JARDIANCE). Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=faf3dd6a-9cd0-39c2-0d2e-232cb3f67565 (accessed on 1 June 2023).
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; de Boer, I.H. Clinical Manifestations of Kidney Disease Among US Adults with Diabetes, 1988–2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1801–1802. [Google Scholar] [CrossRef] [PubMed]
- Collaborative-Group, E.-K.; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Levin, A.; Nangaku, M.; Kadowaki, T.; Agarwal, R.; Hauske, S.J.; Elsasser, A.; Ritter, I.; Steubl, D.; Wanner, C.; et al. Safety of Empagliflozin in Patients with Type 2 Diabetes and Chronic Kidney Disease: Pooled Analysis of Placebo-Controlled Clinical Trials. Diabetes Care 2022, 45, 1445–1452. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Wheeler, D.C.; James, J.; Patel, D.; Viljoen, A.; Ali, A.; Evans, M.; Fernando, K.; Hicks, D.; Milne, N.; Newland-Jones, P.; et al. SGLT2 Inhibitors: Slowing of Chronic Kidney Disease Progression in Type 2 Diabetes. Diabetes Ther. 2020, 11, 2757–2774. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Yoshida, A.; Hieshima, K.; Kurinami, N.; Jinnouchi, K.; Tanaka, M.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Renoprotective Effects of Additional SGLT2 inhibitor Therapy in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease Stages 3b-4: A Real World Report From A Japanese Specialized Diabetes Care Center. J. Clin. Med. Res. 2019, 11, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Htoo, P.T.; Tesfaye, H.; Schneeweiss, S.; Wexler, D.J.; Everett, B.M.; Glynn, R.J.; Kim, S.C.; Najafzadeh, M.; Koeneman, L.; Farsani, S.F.; et al. Comparative Effectiveness of Empagliflozin vs Liraglutide or Sitagliptin in Older Adults With Diverse Patient Characteristics. JAMA Netw. Open 2022, 5, e2237606. [Google Scholar] [CrossRef] [PubMed]
- Durcan, E.; Ozkan, S.; Saygi, H.I.; Dincer, M.T.; Korkmaz, O.P.; Sahin, S.; Karaca, C.; Sulu, C.; Bakir, A.; Ozkaya, H.M.; et al. Effects of SGLT2 inhibitors on patients with diabetic kidney disease: A preliminary study on the basis of podocyturia. J. Diabetes 2022, 14, 236–246. [Google Scholar] [CrossRef]
- Jung, K.J.; Jang, Y.; Oh, D.J.; Oh, B.H.; Lee, S.H.; Park, S.W.; Seung, K.B.; Kim, H.K.; Yun, Y.D.; Choi, S.H.; et al. The ACC/AHA 2013 pooled cohort equations compared to a Korean Risk Prediction Model for atherosclerotic cardiovascular disease. Atherosclerosis 2015, 242, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Bakker, L.E.; Sleddering, M.A.; Schoones, J.W.; Meinders, A.E.; Jazet, I.M. Pathogenesis of type 2 diabetes in South Asians. Eur. J. Endocrinol. 2013, 169, R99–R114. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Seino, Y.; Fukushima, M.; Seino, S. beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr. Diabetes Rep. 2015, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Yeung, R.O.; Zhang, Y.; Luk, A.; Yang, W.; Sobrepena, L.; Yoon, K.H.; Aravind, S.R.; Sheu, W.; Nguyen, T.K.; Ozaki, R.; et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): A cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014, 2, 935–943. [Google Scholar] [CrossRef]
- Chan, J.C.; So, W.Y.; Yeung, C.Y.; Ko, G.T.; Lau, I.T.; Tsang, M.W.; Lau, K.P.; Siu, S.C.; Li, J.K.; Yeung, V.T.; et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: The SURE study: A randomized multicenter translational study. Diabetes Care 2009, 32, 977–982. [Google Scholar] [CrossRef]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef]
- Shah, A.; Kanaya, A.M. Diabetes and associated complications in the South Asian population. Curr. Cardiol. Rep. 2014, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Food-and-Drug-Administration-(FDA). Framework for FDA’s Real-World Evidence Program; FDA: Silver Spring, MD, USA, 2018.
- Food-and-Drug-Administration-(FDA). Submitting Documents Utilizing Real-World Data and Real-World Evidence to FDA for Drugs and Biologics. Available online: https://www.fda.gov/media/124795/download (accessed on 24 September 2021).
- Kim, J.A.; Yoon, S.; Kim, L.Y.; Kim, D.S. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Food-and-Drug-Administration-(FDA). Best Practices for Conducting and Reporting Pharmacoepidemiologic Safety Studies Using Electronic Healthcare Data; Guidance for Industry and FDA Staff; FDA: Silver Spring, MD, USA, 2013.
- Kim, Y.G.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Lee, K.W.; Kim, H.J. Sodium-glucose co-transporter-2 inhibitors and the risk of ketoacidosis in patients with type 2 diabetes mellitus: A nationwide population-based cohort study. Diabetes Obes. Metab. 2018, 20, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Lix, L.M.; Fralick, M.; Dell’Aniello, S.; Shah, B.R.; Ronksley, P.E.; Tremblay, E.; Hu, N.; Alessi-Severini, S.; Fisher, A.; et al. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis: A Multicenter Cohort Study. Ann. Intern. Med. 2020, 173, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.F.; Chen, S.W.; Liu, J.R.; Li, P.R.; Wu, L.S.; Chang, S.H.; Yeh, Y.H.; Kuo, C.T.; Chan, Y.H.; See, L.C. Major adverse cardiovascular and limb events in patients with diabetes and concomitant peripheral artery disease treated with sodium glucose cotransporter 2 inhibitor versus dipeptidyl peptidase-4 inhibitor. Cardiovasc. Diabetol. 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.M.; Kim, J.J.; Kim, H.J.; Sohn, H.S. Comparison of heart failure risk and medical costs between patients with type 2 diabetes mellitus treated with dapagliflozin and dipeptidyl peptidase-4 inhibitors: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2020, 19, 95. [Google Scholar] [CrossRef]
- Han, S.J.; Ha, K.H.; Lee, N.; Kim, D.J. Effectiveness and safety of sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in older adults with type 2 diabetes: A nationwide population-based study. Diabetes Obes. Metab. 2021, 23, 682–691. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice, C. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef]
- Parsons, L.S. Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. Available online: http://www2.sas.com/proceedings/sugi26/p214-26.pdf (accessed on 1 January 2017).
- NCSS-Statistical-Software. Data Matching—Optimal and Greedy. Available online: http://ncss.wpengine.netdna-cdn.com/wp-content/themes/ncss/pdf/Procedures/NCSS/Data_Matching-Optimal_and_Greedy.pdf (accessed on 1 July 2017).
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am. J. Physiol. Renal Physiol. 2015, 309, F889–F900. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Laffel, L.; Tamborlane, W.V.; Simons, G.; Hantel, S.; von Eynatten, M.; George, J.; Marquard, J.; Cherney, D.Z.I. Acute Effect of Empagliflozin on Fractional Excretion of Sodium and eGFR in Youth with Type 2 Diabetes. Diabetes Care 2018, 41, e129–e130. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Richter, K.; Blantz, R.C.; Thomson, S.; Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J. Am. Soc. Nephrol. 1999, 10, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, G.; Stattin, E.L.; Rudberg, S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol. Dial. Transplant. 2001, 16, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Inhibition of renal glucose reabsorption: A novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr. Pract. 2008, 14, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; McGuire, D.K.; Chilton, R.; Crowe, S.; Lund, S.S.; Woerle, H.J.; Broedl, U.C.; Johansen, O.E. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc. Dis. Res. 2016, 13, 119–126. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef]
- Aroor, A.R.; Das, N.A.; Carpenter, A.J.; Habibi, J.; Jia, G.; Ramirez-Perez, F.I.; Martinez-Lemus, L.; Manrique-Acevedo, C.M.; Hayden, M.R.; Duta, C.; et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc. Diabetol. 2018, 17, 108. [Google Scholar] [CrossRef]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Renal Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef]
- Lee, W.C.; Chau, Y.Y.; Ng, H.Y.; Chen, C.H.; Wang, P.W.; Liou, C.W.; Lin, T.K.; Chen, J.B. Empagliflozin Protects HK-2 Cells from High Glucose-Mediated Injuries via a Mitochondrial Mechanism. Cells 2019, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Ndibalema, A.R.; Kabuye, D.; Wen, S.; Li, L.; Li, X.; Fan, Q. Empagliflozin Protects Against Proximal Renal Tubular Cell Injury Induced by High Glucose via Regulation of Hypoxia-Inducible Factor 1-Alpha. Diabetes Metab. Syndr. Obes. 2020, 13, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.L.; Low, S.; Yeoh, E.; Lim, E.K.; Renaud, C.J.; Teoh, S.T.Y.; Tan, G.F.L.; Chai, C.C.; Liu, B.; Subramaniam, T.; et al. A real-world study on SGLT2 inhibitors and diabetic kidney disease progression. Clin. Kidney J. 2022, 15, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Wat, N.M.; So, W.Y.; Lam, K.S.; Chua, C.T.; Wong, K.S.; Morad, Z.; Dickson, T.Z.; Hille, D.; Zhang, Z.; et al. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care 2004, 27, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Eckert, G.J.; Hannon, T.S.; Liu, H.; Pratt, L.M.; Wagner, M.A.; Dimeglio, L.A.; Jung, J.; Pratt, J.H. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension 2014, 63, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Nangaku, M.; Hantel, S.; Okamura, T.; von Eynatten, M.; Wanner, C.; Koitka-Weber, A. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: Results from the EMPA-REG OUTCOME((R)) trial. J. Diabetes Investig. 2019, 10, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Sarashina, A.; Ueki, K.; Sasaki, T.; Tanaka, Y.; Koiwai, K.; Sakamoto, W.; Woerle, H.J.; Salsali, A.; Broedl, U.C.; Macha, S. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in Japanese patients with type 2 diabetes mellitus. Clin. Ther. 2014, 36, 1606–1615. [Google Scholar] [CrossRef]
- Macha, S.; Mattheus, M.; Halabi, A.; Pinnetti, S.; Woerle, H.J.; Broedl, U.C. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes. Metab. 2014, 16, 215–222. [Google Scholar] [CrossRef]
- Jang, H.Y.; Kim, I.W.; Oh, J.M. Using real-world data for supporting regulatory decision making: Comparison of cardiovascular and safety outcomes of an empagliflozin randomized clinical trial versus real-world data. Front. Pharmacol. 2022, 13, 928121. [Google Scholar] [CrossRef]
- Cahn, A.; Melzer-Cohen, C.; Pollack, R.; Chodick, G.; Shalev, V. Acute renal outcomes with sodium-glucose co-transporter-2 inhibitors: Real-world data analysis. Diabetes Obes. Metab. 2019, 21, 340–348. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Karasik, A.; Thuresson, M.; Melzer-Cohen, C.; Chodick, G.; Khunti, K.; Wilding, J.P.H.; Garcia Rodriguez, L.A.; Cea-Soriano, L.; Kohsaka, S.; et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): A multinational observational cohort study. Lancet Diabetes Endocrinol. 2020, 8, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.S.; Han, K.; Nam, Y.S.; Wittbrodt, E.T.; Fenici, P.; Kosiborod, M.N.; Heerspink, H.J.L.; Yoo, S.J.; Kwon, H.S. Renal outcomes and all-cause death associated with sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL 3 Korea). Diabetes Obes. Metab. 2021, 23, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, G.N.; Ferrandino, R.; Chang, A.; Surapaneni, A.; Chauhan, K.; Poojary, P.; Saha, A.; Ferket, B.; Grams, M.E.; Coca, S.G. Acute Kidney Injury in Patients on SGLT2 Inhibitors: A Propensity-Matched Analysis. Diabetes Care 2017, 40, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Bilezikian, J.P.; Thomas, G.N.; Hanif, W.; Kotsa, K.; Thayakaran, R.; Keerthy, D.; Tahrani, A.A.; Nirantharakumar, K. Initiation of dapagliflozin and treatment-emergent fractures. Diabetes Obes. Metab. 2018, 20, 1070–1074. [Google Scholar] [CrossRef]

