1. Introduction
Adipose tissue constitutes a heterogenous organ, comprising distinct subtypes including WAT, beige adipose tissue, and BAT [1,2,3]. These adipose depots exhibit dissimilar morphological structures and physiological functions. WAT, being the largest lipid storage site within the body, chiefly constitutes white adipocytes [4]. Typical white adipocytes possess a singular large lipid droplet and a limited number of mitochondria, primarily involved in energy storage and provision. Impaired functionality of white adipocytes can result in disrupted lipid metabolism, lipodystrophy, and insulin resistance (IR) due to lipid spillage [5,6]. Moreover, excessive accumulation of WAT is associated with obesity, adversely impacting various physiological processes, and represents a prominent risk factor for the onset of type 2 diabetes and cardiovascular diseases [7]. Brown adipocytes exhibit a distinct phenotype characterized by the presence of multilocular small lipid droplets and a higher density of mitochondria [8]. These cells are prominently marked by elevated expression levels of mitochondrial uncoupling protein 1 (UCP1), which facilitates mitochondrial heat generation via the uncoupling of oxidative phosphorylation, thus ensuring the maintenance of basal body temperature in cold environments instead of relying on shivering [9]. Beige adipose tissue, displaying similar morphological and functional characteristics to BAT, is perceived as an intermediary state between WAT and BAT. The emergence of beige fat typically occurs after birth and is often juxtaposed with white adipocytes that transition between a browning or whitening state in response to environmental stimuli [4,8,10]. The induction of beige adipogenesis in WAT has been proposed as a potential therapeutic strategy for combating obesity and metabolic disorders associated with excessive weight gain.
Numerous investigations have established a close association between the concentration of vitamin D (VD) in systemic circulation and body adiposity [11]. Observational studies have consistently demonstrated a negative correlation between the serum levels of 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the bioactive form of VD, with measures of body mass index (BMI), subcutaneous fat mass, and visceral fat mass [12,13,14]. Multiple hypotheses have been posited to elucidate the underlying mechanisms connecting obesity with low serum 25(OH)D levels, encompassing volumetric dilution, adipose tissue sequestration, metabolic disturbances, and inadequate exposure to sunlight [15,16,17]. Mounting evidence lends support to the adipose tissue sequestration hypothesis, as research has consistently revealed that a high-fat diet augments the expression of Cyp2r1 in adipose tissue, facilitating the active uptake of vitamin D3 (cholecalciferol) and its subsequent conversion into 25(OH)D for storage within lipid droplets [18]. Furthermore, investigations have revealed that individuals with metabolically unhealthy obesity, which primarily involves the accrual of visceral fat, exhibit lower levels of 25(OH)D compared to those with metabolically healthy obesity, characterized by subcutaneous fat accumulation [19]. This observation implies that diverse loci of visceral fat deposition could exert varying effects on VD concentrations, thereby necessitating an exploration into the potential role of this factor in mediating metabolic dysregulation.
Moreover, the metabolic regulation of VD in adipose tissue is intricately linked to the VD receptor (VDR). Substantial evidence demonstrates widespread expression of VD-related metabolic enzymes and the VDR in human adipose tissue [20]. Interaction between the VDR and 1,25(OH)2D3 enables the direct or indirect modulation of the local VD metabolism as well as the regulation of adipocyte differentiation, inflammation, and other critical processes [20,21,22]. Interestingly, investigations have revealed higher expression levels of the VDR in the visceral adipose tissue (VAT) of obese individuals compared to lean subjects, while no significant differences were observed in the subcutaneous adipose tissue (SAT) [17]. Furthermore, in healthy non-obese individuals, elevated serum 25(OH)D levels were significantly associated with reduced VDR expression specifically in VAT, whereas this correlation was absent in SAT [23]. This reinforces the notion that VD may exert distinct effects on adipose tissue at different anatomical sites.
Therefore, this review focuses on the functional differences of VD in different types of adipocytes and provides new intervention ideas of micronutrients for the prevention and treatment of obesity.
2. Vitamin D Metabolism and Metabolic Health
Nature encompasses two primary forms of VD, namely vitamin D2 (ergocalciferol) and vitamin D3 [24]. Vitamin D2 predominantly originates from plant-based sources like mushrooms, while vitamin D3 primarily derives from cutaneous synthesis in response to ultraviolet (UV) light exposure and animal-based foods such as egg yolk, cod liver oil, sardines, and milk [25,26]. Both dietary vitamin D2 and vitamin D3 are absorbed into the bloodstream through chylomicron transport within the small intestine. Conversely, endogenous VD synthesis predominantly occurs through the conversion of 7-dehydrocholesterol in the skin, facilitated by exposure to UV light (290–315 nm). The process begins with the rapid conversion of a certain substance to vitamin D3, which is facilitated by heat. This newly formed vitamin D3 then binds to a specific protein known as vitamin D-binding protein (VDBP). Once bound, VDBP enters the circulatory system and is either transported to the liver or stored in fat along with the circulating blood [27]. Vitamin D3 may be further metabolized in the liver, enriched with hydroxylase enzymes like CYP2R1, resulting in the formation of 25(OH)D, which serves as the clinically utilized form for assessing VD status [28,29]. By the criteria established by the Endocrine Society, a serum concentration of 25 (OH) D ranging from 30 to 100 ng/mL is considered within the normal range. A concentration below 30 ng/mL (75 nmol/L) indicates insufficiency of VD, while a concentration below 20 ng/mL (50 nmol/L) indicates deficiency. Insufficient levels of VD can have detrimental effects on bone health, leading to fractures and bone loss [30,31]. Severe vitamin D deficiency (VDD) is defined by a serum 25 (OH) D concentration below 12 ng/mL (30 nmol/L), which significantly elevates the risk of mortality, immune system disorders, and other diseases [32].
The conversion of 25 (OH) D to its active form, 1,25 (OH) 2D3, occurs in the kidney through the enzymatic action of 1alpha hydroxylase (CYP27B1). This conversion facilitates the exertion of biological effects by 1,25 (OH) 2D3. These effects can be categorized into three main mechanisms:
(1) Genomic effect: The formed 1,25 (OH) 2D3-Vitamin D receptor (VDR)-Retinoid X Acid Receptor (RXR) complex translocates from the cytoplasm to the nucleus, where it binds to the VD response element (VDRE) on target genes. Consequently, the complex regulates the expression of numerous genes, totaling in the hundreds [33].
(2) Non-genomic effect: the membrane VDR activation initiates a rapid membrane priming reaction, triggering signaling pathways that contribute to biological responses [34,35].
(3) Epigenetic effects: these effects involve the regulation of miRNAs, DNA methylation, histone acetylation/deacetylation, and histone methylation/demethylation, which collectively impact gene expression regulation.
Ultimately, the elevated levels of 1,25 (OH) 2D3 induce the action of CYP24A1-mediated 24-hydroxylation. This process breaks down 1,25 (OH) 2D3 into biologically inactive calcific acid, which is ultimately excreted with bile, thereby completing the overall metabolic process [29].
VD exerts a crucial regulatory role in substance metabolism and displays significant potential in non-bone-related metabolic processes [36]. An increasing body of evidence has established a close association between circulating 25 (OH) D levels and the development and progression of cardiovascular disease, type 2 diabetes, IR, and obesity [14,37,38]. Investigations have revealed that in adipocytes, diet-induced VDD contributes to elevated levels of macrophage infiltration and inflammation within rat adipose tissue [39]. Furthermore, VD insufficiency reduces the activity of sirtuin1 (STRT1) and adenosine monophosphate-activated protein kinase (AMPK), which consequently influences energy metabolism and the occurrence of inflammatory responses [21].
Regarding the anti-inflammatory effects of VD on adipose tissue, consistent findings have been obtained from in vitro and in vivo studies. In both preadipocytes and adipocytes, 1,25 (OH) 2D3 inhibits the expression of IL-6, IL-1β, IL-8, MCP-1, and leptin while stimulating the expression of adiponectin [40,41,42,43]. Dietary VD supplementation also significantly attenuates the expression of chemokines and macrophage infiltration in mouse adipose tissue [43]. Cumulatively, the adverse effects of VD on skeletal muscle and adipose tissue contribute to the development and progression of IR and obesity.
3. Adipose Tissue Function and Metabolic Health
Adipose tissue represents a heterogeneous organ characterized by substantial plasticity, encompassing diverse cell types including preadipocytes, immune cells, and mesenchymal stem cells [44]. Throughout its life cycle, adipose tissue undergoes a dynamic process of expansion and compression in response to shifting metabolic demands. A higher adipose tissue mass generally correlates with compromised metabolic health. However, adipose tissue expansion serves as a mechanism to safely store surplus nutrients and prevent their accumulation in other tissues. Nevertheless, excessive adipose tissue undermines metabolic health, whereas insufficient adipose tissue, as observed in lipodystrophy, can lead to metabolic disorders such as diabetes, hypertriglyceridemia, non-alcoholic fatty liver disease, and adipose tissue-related endocrine dysfunctions [45]. Hence, the proper functioning of adipose tissue is crucial for maintaining metabolic well-being.
Adipose tissue plays a critical role in the biosynthesis and breakdown of free fatty acids and other nutrients. It further modulates the metabolic functions of other organs through the secretion of adipokines, including adiponectin and leptin [46]. Hence, the preservation of a proper equilibrium among adipocyte hypertrophy, proliferation, fibrosis, and lipolysis, along with the normal synthesis and release of adipokines, holds paramount importance for metabolic well-being. However, it is noteworthy that distinct types of adipose tissue exhibit divergent contributions to metabolic health.
3.1. White Adipose Tissue
WAT is a prominent organ within the human body, exhibiting a significant capacity for storing and releasing energy in the form of triglycerides. Furthermore, WAT secretes adipokines in response to variations in systemic energy levels [47,48]. In normal circumstances, WAT adapts to increased lipid storage demands by undergoing adipocyte expansion, specifically hyperplasia, and hypertrophy, to maintain a consistent energy supply during periods of food deprivation. However, aberrations in one’s lifestyle and dietary composition have given rise to chronic overnutrition, leading to the surpassing of the tolerable threshold for stored triglycerides. Consequently, excess triglycerides are transferred to other metabolic organs, such as the muscle and liver, resulting in ectopic lipid accumulation. This accumulation plays a mediating role in the development of IR and complications related to obesity [3,49].
Based on anatomy, WAT can be subdivided into VAT and SAT [50]. The ratio between these two depots holds substantial implications for metabolic health. Metabolically unhealthy individuals with obesity exhibit a heightened visceral adiposity index (a specific index incorporating waist circumference, triglycerides, and HDL to indirectly represent VAT function) and a homeostatic model assessment of insulin resistance (HOMA-IR), ultimately resulting in poorer metabolic parameters when compared to their metabolically healthy obese counterparts [51,52]. Notably, metabolically healthy individuals with obesity possess lower amounts of visceral fat, whereas metabolically unhealthy individuals with normal weight exhibit higher levels of visceral fat [53]. This evidence strongly suggests that increased VAT significantly correlates with more severe IR and metabolic diseases associated with obesity, even in individuals of normal weight [54]. This correlation can be attributed to VAT’s propensity for lipolysis and immune cell infiltration, consequently resulting in the production of proinflammatory factors such as TNF-α, IL-6, and IL-1β, which contribute to IR. Nonetheless, these processes also serve as critical means of internal organ protection [55,56,57,58]. The distinction between VAT and SAT is further evident in the volume of adipocytes, which tends to be larger in VAT and smaller in SAT. As a consequence, hypertrophic adipocytes manifest IR [59,60]. This disparity is also responsible for explaining why obesity characterized by SAT accumulation is comparatively healthier than that dominated by VAT [61].
3.2. Brown Adipose Tissue and Beige Adipose Tissue
In the human body, BAT has relatively little quantity and is selectively distributed in specific anatomical parts, such as the neck and scapula [62]. BAT possesses distinct characteristics, including a darker appearance, multiloculated lipid droplets, and a greater mitochondrial density within individual brown adipocytes, which play a crucial role in maintaining lipolysis homeostasis by promoting fat burning [8,63]. During infancy, the thermogenic effect of BAT largely determines cold resistance. By employing uncoupled oxidative phosphorylation rather than shivering, BAT generates heat to sustain the basal body temperature of organisms in cold environments [9]. Recent investigations have revealed that the significance of BAT extends beyond thermogenesis and body temperature regulation, encompassing important implications in the fine-tuning of glucose and lipid metabolism as well as insulin sensitivity [64]. Activation of BAT can substantially enhance whole-body energy expenditure by more than 100% in mice and 40–80% in humans, concurrently reducing plasma triglyceride levels [65,66]. However, the content of BAT gradually diminishes with age in humans [67]. Consequently, the activation of adult BAT holds considerable importance in maintaining metabolic health. Additionally, researchers have identified a special type of “hypothermogenic” beige adipocytes, which possess larger lipid droplets, fewer mitochondria, and reduced thermogenic gene expression levels [68]. Beige adipose tissue represents an intermediary state between WAT and BAT [8,10]. Beige adipocytes can transform mature white adipocytes, a plastic process primarily occurring within SAT [69,70,71]. Cold exposure, catecholamines, exercise, adipokines, and other stimuli can induce the browning or beginning of WAT [72,73,74,75]. However, obesity may impede the thermogenic function of BAT, leading to a significant reduction in BAT activity among obese individuals, irrespective of their BMI. Notably, this reduction in BAT activity exhibits a strong negative correlation with VAT mass [76,77]. While similar results were not observed in the BAT of diet-induced obese mice, ob/ob mice exhibited a decrease in the mRNA expression levels of UCP1 in their BAT [78].
4. Vitamin D Plays an Important Role in Adipose Tissue
The relationship between VD and adipose tissue is intricate. Adipose tissue, particularly VAT, serves as a primary reservoir for VD, with approximately 65% of the whole-body vitamin D3 and 35% of 25(OH)D being stored in lipid droplets within adipose tissue [79,80,81]. Conversely, adipocytes express VD-related metabolic enzymes and VDR, which in turn regulate the local VD metabolism, adipogenesis, lipid metabolism, thermogenesis, inflammation, and apoptosis through direct or indirect interactions with 1,25(OH)2D3 [4,29].
4.1. Vitamin D and Visceral Adipose Tissue
VAT has been observed to contain approximately 20% higher levels of VD compared to SAT, although the precise reasons for this difference remain unclear [81]. Serum VD levels show a negative correlation with VAT content and serve as an important indicator for assessing the expression of the VDR within VAT [74,82,83]. While the precise relationship between the VDR in VAT and serum 1,25(OH)2D3 has yet to be fully elucidated, the current findings provide support for a dose–response relationship between the severity of VDD and low VDR expression [84]. Notably, VDR expression in VAT is significantly lower in individuals with a normal BMI compared to those who are obese (BMI = 30–40 kg/m2) or severely obese (BMI > 40 kg/m2). Several studies have found a positive correlation between waist circumference and VDR expression in VAT, as well as a negative correlation between 25(OH)D and VDR expression in VAT [23]. These results align with findings from animal studies involving VDR overexpression, suggesting that the VDR plays a role in the proliferation or hypertrophy of visceral adipocytes and is closely associated with the development of central obesity.
Several recent experimental and clinical investigations have elucidated the anti-inflammatory properties of VD in the liver, muscle, and adipose tissue, in which the inhibition of monocyte chemoattractant protein 1 (MCP-1) and macrophage recruitment has been observed [4,85,86]. Hypomethylation of inflammation-related adipokines (BCL5, CXCL8, IL-12A) has been observed in the adipose tissue of individuals with VDD, particularly in obese subjects. This association is linked to an increase in total fat mass, visceral fat mass, and impaired insulin sensitivity [87,88]. In the study conducted by Imaduddin Mirza et al., it was found that macrophage infiltration in the VAT of obese individuals demonstrated a dose-dependent increase with the increasing VDD [88]. Conversely, restoring VD status has been shown to improve the inflammatory response observed in the VAT of obese individuals [89]. Animal studies have reported similar findings, observing that diet-induced VDD exaggerates adipocyte hypertrophy and recruits adipose tissue macrophages to the epididymal adipose tissue, resulting in elevated levels of IL-6 and TNF-α [21,39]. Furthermore, VDD has been associated with reduced STRT1 and AMPK activity, affecting energy metabolism and triggering inflammatory responses [21]. In mice subjected to 12 weeks of VD restriction, a significant increase in NF-kB levels was observed in the visceral fat [39]. Moreover, female mice with fat-specific knockdown of the VDR showed an increase in VAT mass, resembling the VDD phenotype [90]. Interestingly, Matthews et al. found that in the context of high-fat feeding, mice with fat-specific VDR knockout exhibited higher serum 25 (OH) D levels compared to control mice, with no significant differences observed in the visceral adipocyte area [90]. This finding may be attributed to the upregulation of thermogenic proteins like UCP1, which promote lipolysis in VAT. Furthermore, studies have indicated that VD supplementation restricts macrophage migration in the adipocytes of obese mice, thereby exerting an anti-inflammatory effect [43]. Collectively, these findings underscore the crucial involvement of VD in VAT accumulation and inflammation, suggesting that it may serve as a significant regulatory factor in the pathogenesis of central obesity. Moreover, the VD/VDR complex may play a role in modulating the NLRP3/caspase 1 pathway, though the underlying mechanisms in adipocytes remain unknown [91]. Further investigations are warranted to substantiate the suggested connections between the NLRP3 inflammasome, obesity, IR, and VD, which may shed light on the potential role of VD in attenuating IR and metabolic disorders.
4.2. Vitamin D and Subcutaneous Adipose Tissue
VDD has been observed to promote SAT accumulation in the forearm, arm, and thigh regions of the human body, similar to its effect on VAT [92]. Animal studies have consistently demonstrated an increase in SAT in high-fat diet-induced VDD animals [21]. However, some studies have reported a negative correlation between serum 25 (OH) D levels and body fat, but no significant association between serum 25 (OH) D and SAT [93]. In the absence of differences in food and energy intake, high-fat diet-fed VDD animals exhibited significantly higher epididymal fat deposition compared to high-fat diet-fed VD-sufficient animals, while the SAT did not differ between the two groups [94,95]. This finding suggests that VDD may preferentially facilitate lipid accumulation in VAT rather than SAT under high-fat diet conditions. Interestingly, BMI and HOMA-IR were found to be positive predictors of VDR expression in SAT [23]. The response of SAT to 1,25 (OH)2D3 treatment and the expression of the VDR vary depending on the degree of obesity [23,96]. Moreover, in obese male patients, it has been noted that isoproterenol-mediated lipolysis in abdominal SAT is attenuated, leading to a reduction in the release of 1,25(OH)2D3 [97]. However, a 12-week period of energy restriction and body fat loss did not result in significant changes in the 25 (OH) D level in the SAT or serum 25 (OH) D concentration [98]. This suggests that weight loss may trigger the release of 25 (OH) D in SAT, but the magnitude of release may not be sufficient to cause noticeable changes in serum 25 (OH) D. Alternatively, the released 25 (OH) D may undergo inactivation and subsequent excretion through the classical negative feedback regulation pathway. Notably, the expression of CYP2J2, CYP27A1 (25-hydroxylase), and CYP27B1 (1α-hydroxylase) in SAT is lower in obese individuals, whereas the expression level of CYP24A1 was significantly increased after weight loss [17]. This supports the notion that VD inactivation is heightened in SAT following weight loss.
VD also plays a crucial role in the inflammatory processes occurring in SAT. In a study, SAT samples obtained from obese subjects were manipulated in vitro with IL-1β intervention to induce an inflammatory model. Following incubation with 1,25 (OH)2D3, the mRNA levels of IL-6, IL-8, and MCP-1 in the cells were significantly decreased [99]. Although this result was not observed in SAT samples from subjects who orally consumed VD supplements, it is important to consider that obesity can impair the effectiveness of VD supplementation. Furthermore, the results from in vitro studies underscore the close association between the anti-inflammatory effect of VD and changes in the VDR in tissues. Notably, it has been demonstrated that upregulated VDR mRNA and downregulated CYP27B1 mRNA are positively correlated with the expression levels of IL-1β, IL-6, and IL-8 mRNA in SAT samples from obese subjects [100]. However, it should be noted that some studies have shown that VDR expression is higher in VAT but not in SAT in obese patients [17]. In non-obese subjects, a clear negative correlation was observed between serum VD levels and VDR expression in VAT, but this phenomenon was not observed in SAT [23]. These findings suggest that VD metabolism and VDR expression in SAT are relatively stable, likely due to SAT’s higher stability and anti-lipolytic effect. With the progression of obesity, metabolic changes associated with VD may initially occur in VAT rather than SAT. However, further research is needed to elucidate the underlying mechanisms.
4.3. VDR Regulates Adipose Tissue Browning and Alters Energy Expenditure
VDR expression levels have a significant impact on the quality of BAT. Studies have demonstrated that mice with fat-specific VDR overexpression exhibit a considerably higher BAT content compared to control mice, whereas fat-specific VDR knockout and whole-body VDR knockout mice have a lower BAT content [101,102]. The activity of uncoupling proteins (UCPs) is directly modulated by VD/VDR signaling, and the dose-dependent inhibition of brown adipocyte differentiation by 1,25 (OH)2D3 has been observed [78,103]. Primary BAT culture experiments have confirmed the ability of 1,25 (OH)2D3 to directly inhibit UCP expression [104]. VDR overexpression suppresses brown adipocyte differentiation and peroxisome proliferator-activated receptor gamma (PPAR-γ) activation by downregulating peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) and PR domain containing 16 (PRDM16), subsequently reducing the expression levels of UCP1, UCP2, and UCP3 in BAT. These effects are reversed upon VDR knockdown [78,102,105]. Both VDR knockout and 1α-hydroxylase (CYP27B1) knockout mice exhibit weight loss and increased UCP1 expression in BAT [104]. Similarly, the UCP1 gene expression was significantly upregulated in the VAT of mice with fat-specific VDR knockout, leading to increased energy expenditure and promotion of the browning process in WAT [90]. Furthermore, VD supplementation has been shown to inhibit the browning of WAT in mice with chronic kidney disease [106]. Collectively, these findings indicate that the VDR serves as a negative regulator of fat browning.
UCP2, a member of the UCPs family, occupies a pivotal position in the regulation of energy metabolism. Previous investigations have revealed that the administration of low doses of VD can suppress Ucp2 expression by bolstering mitochondrial localization and ATP generation, thus restraining the initiation of apoptosis [107]. Conversely, high doses of VD can trigger this process. In UCP2-transfected 3T3-L1 cells, 1,25(OH)2D3 displayed the dose-dependent stimulation of cytosolic Ca2+ levels, leading to a 25% increase in mitochondrial Ca2+ levels, thereby further propelling adipocyte apoptosis [108]. These results are consistent with the observation of lean phenotype and excessive energy expenditure in VDR knockout mice. It is postulated that VDR-null mice undergo adipocyte apoptosis, resulting in insufficient adipose tissue to maintain body temperature, prompting augmented UCPs expression in BAT and WAT to generate heat. Nevertheless, VD may mitigate this phenomenon by stimulating the differentiation of MSCs into preadipocytes, which in turn inhibits the expression of UCPs. It is imperative to further explore the role of the 1,25(OH)2D3/VDR-UCPs pathway system in the regulation of BAT development and function in obesity.
4.4. Vitamin D Supplementation Regulates Adipose Tissue Metabolic Health
VDD plays a pivotal role in the pathogenesis and progression of metabolic disorders, such as IR. To maintain serum 25 (OH) D levels within the recommended range of 20 to 100 ng/mL, the Endocrine Society recommends a daily intake of 1500 to 2000 IU or higher of VD to mitigate the risk of non-skeletal diseases [25]. Nevertheless, the reproducibility of the effect of VD supplementation in improving serum 25 (OH) D levels is limited, particularly in the context of obesity [109,110]. It is noteworthy that systematic reviews and meta-analyses have reported a significant increase in serum leptin levels and a decrease in the levels of C-reactive protein in individuals with type 2 diabetes mellitus following VD supplementation. These findings are beneficial for ameliorating systemic inflammation [111,112]. Therefore, some studies have suggested that the efficacy of VD supplementation may be impaired in the presence of obesity, and the beneficial effects of supplementation may only be observable in individuals with inadequate VD levels or severe disorders of glucose and lipid metabolism [110]. Hence, changes in serum 25 (OH) D levels after VD supplementation should be carefully considered.
In a trial conducted on obese Wistar rats, supplementation with 800 IU of VD demonstrated a remarkable ability to attenuate weight gain and decrease the deposition of abdominal fat [113]. Consistent with these findings, obese Wistar rats receiving a higher dosage of 2400 IU of VD exhibited reduced levels of VAT leptin and MCP-1 mRNA, along with elevated levels of adiponectin, as compared to obese rats supplemented with either 800 IU or placebo [114]. However, the study did not ascertain any variations in the serum 25 (OH) D levels pre- and post-vitamin D supplementation, thereby making it inconclusive to determine whether the favorable effects of VD supplementation on obesity can be attributed to changes in VD levels.
The absence of any observable changes in the physiological morphology of adipocytes following VD supplementation has led to the suggestion that it may not possess beneficial effects on the development of obesity. Nonetheless, it should be noted that certain studies have demonstrated significant improvements in adipose tissue inflammation and reduction in liver tissue steatosis in obese C57BL/6J mice subjected to VD supplementation [115,116]. In diet-induced obese mice (an animal model of metabolic inflammation) and mice injected intraperitoneally with lipopolysaccharide (an animal model of acute inflammation), VD has been shown to decrease the levels of proinflammatory cytokines and chemokines in both adipocytes and VAT [43]. Furthermore, VD supplementation has been found to lower the mRNA expression levels of Cyp27A1, Cyp24A1, and cubilin in epidydimal white adipose tissue (eWAT), indicating a possible modulation of its metabolism through reducing the uptake and activation of 25 (OH) D [117]. Glucose transport in the adipose tissue of high-fat diet-fed mice also increased after VD supplementation, suggesting a potential improvement in obesity-induced glucose metabolism disorders [118]. Notably, VD in conjunction with high calcium intake can activate calcium-mediated apoptotic pathways in adipose tissue, activating calcium-dependent apoptotic proteases calpain and caspase-12, which presents a potential avenue for obesity prevention and treatment [119]. However, the reliability and controllability of this approach warrant further investigation, ensuring that healthy cells are not compelled to undergo apoptosis while promoting the apoptosis of dysfunctional adipocytes to curb inflammatory infiltration arising from hypertrophy.
5. VDR Plays a Key Role in the Regulation of Adipogenesis
VDR plays a crucial role in adipogenesis. Systemic VDR-null mice display diminished adipose tissue mass, augmented overall energy expenditure, and resistance to high-fat diet-induced obesity, which coincides with notable enhancements in glucose tolerance and insulin sensitivity [102,120]. Furthermore, these defects in cellular adipogenesis become more evident as the mice age [101]. Similarly, another study found no significant difference in VAT cell size between VDR knockout mice and wild-type mice at 21 days of age. However, as the mice reached 8 months of age, VDR knockout mice exhibited significantly smaller fat cell areas compared to wild-type mice, accompanied by alopecia and increased energy expenditure [121]. Importantly, no disparity in VAT cell size was observed between VDR knockout mice at 21 days and 8 months [121]. In alignment with the lean phenotype observed in VDR knockout mice, VDR-deficient stem cells exhibited impaired adipogenesis in the presence and absence of 1,25(OH)2D3, and they hindered 1,25(OH)2D3-mediated lipogenesis in human adipogenic progenitor cells when a VDR antagonist was present. These findings indicate the vital role of the VDR in adipogenesis [122]. In a separate in vitro investigation, VDR knockdown was observed to hinder adipogenesis in 3T3-L1 cells, while 1,25(OH)2D3 demonstrated the ability to enhance the differentiation of human and mouse adipose tissue-derived stem cells (ASCs) into adipocytes [34,123]. Mesenchymal cells sourced from 6-month-old VDR-null mice displayed impeded adipogenesis in vitro; however, their differentiation capabilities were restored upon the stable expression of the VDR [122].
However, deletion specific to the VDR in adipose tissue did not fully replicate the lean phenotype observed in mice with a complete knockout of the VDR gene throughout the entire body. Adipose tissue-specific VDR knockout mice showed an increase in VAT weight, while SAT accumulation remained resistant to a high-fat diet [90]. This indicates that the VDR responds differently to the physiological regulation of VAT and SAT. Interestingly, in the absence of significant differences in food intake, mice with a VDR knockout specific to mature adipocytes exhibited a higher fat mass and increased serum leptin levels when exposed to the same high-fat diet as mice with a complete knockout of the VDR gene throughout the entire body [90]. This suggests that the lean phenotype and resistance to a high-fat diet resulting from whole-body VDR knockdown are not solely due to the loss of VDR action in mature adipocytes. The authors of this study discovered that even with a specific VDR knockdown, adipose tissue still exhibited a weak VDR expression, highlighting the possibility that the role of the VDR in MSCs and preadipocytes may be the underlying factor responsible for this difference. However, it is important to note that the PPAR-γ gene expression in VAT was significantly increased in both the total VDR knockout and adipose tissue-specific VDR knockout mice, whereas other genes related to adipogenesis, such as Steap4, Esr, Dok1, and Acaca, did not show any changes due to genotype differences [90]. This could be attributed to the fact that similar to the VDR, PPAR-γ also requires binding to RXR-α, and in the absence of the VDR, more PPAR-γ may bind to RXR-α to exert its effects.
Generalized or adipose tissue-specific overexpression of the VDR in mice results in fat accumulation and elevated leptin levels [102,119,124]. Specifically, overexpression of the VDR in mouse adipose tissue leads to increased body weight and fat mass, disruption of glucose and lipid metabolism, development of IR, and impairment of thermoregulation [102,125,126]. Similarly, VDR overexpression in adipocytes increases adipose tissue mass and lipid storage [122]. However, contrary to the phenotype observed in VDR overexpressing mice, multiple in vitro studies demonstrate that 1,25(OH)2D3, an active form of VD, has an inhibitory effect on lipid accumulation in mature adipocytes. 1,25(OH)2D3 reduces triglyceride (TAG) accumulation by enhancing basal and adrenergic-stimulated lipolysis, while also decreasing de novo lipogenesis in 3T3-L1 adipocytes [22]. It also stimulates the mRNA expression and fatty acid (FA) oxidation rate of FA oxidation-related genes, including CPT1A, PGC-1α, PPAR-α, and UCP1 in 3T3-L1 adipocytes [22,127]. Moreover, 1,25(OH)2D3 enhances insulin-stimulated AKT phosphorylation, GLUT4 translocation, and glucose transport in 3T3-L1 adipocytes [40,118]. It is worth noting that in the presence of an adipogenic medium, 1,25(OH)2D3 promotes lipid accumulation and upregulates the expression of genes involved in lipid synthesis, such as FABP4, FASN, and PPAR-γ [122]. These findings indicate that 1,25(OH)2D3 exerts a significant influence on lipolysis and lipid accumulation in mature adipocytes.
The disparities between in vitro and in vivo outcomes can potentially be attributed to divergent VD/VDR functions during various phases of cell differentiation. In this context, the VDR plays a pivotal role in facilitating the maturation of MSCs into fully developed adipocytes, thus augmenting adipocyte proliferation to enable the efficient storage of nutrients in a comparatively healthful state. Conversely, insufficiency of the VDR impedes the emergence of new, mature white adipocytes, initiates the browning process, and amplifies energy expenditure. “Although this might prove advantageous for combating obesity by promoting fat loss, it is imperative to maintain a sufficient population of white adipocytes for the sake of optimal metabolic function”. Taking an evolutionary standpoint, such a scenario is disadvantageous in terms of securing the adequate energy reserves needed to withstand periods of food scarcity.
It is important to consider that recent epidemiological studies have provided substantial evidence highlighting the close correlation between maternal VD status and the development of obesity in offspring. Maternal serum 25 (OH) D levels during pregnancy exert an influence on various adulthood obesity-related parameters, such as BMI, body fat percentage, and waist circumference, consequently increasing the susceptibility to obesity in later life [128,129]. Neonates born to 25 (OH) D-deficient mothers exhibit larger superficial and deep SAT volumes, which bear metabolic similarities to adult VAT, despite having similar birth weights and VAT masses as neonates born to 25 (OH) D-sufficient mothers [130]. Similarly, animal studies have demonstrated that the offspring of mothers with VD deficiency display an elevated body weight, glucose intolerance, and an augmented risk of obesity in adulthood [131,132,133]. This could be attributed to VD deficiency during pregnancy causing epigenetic changes in the offspring, such as differential methylation in promoters and CpG islands, leading to the increased expression of VDR and PPAR-γ in adipose tissue. This, in turn, promotes the proliferation and differentiation of preadipocytes, ultimately contributing to the obese phenotype in the offspring [134,135]. Other potential influencing factors include adipocyte dysplasia, enhanced oxidation of adipose tissue, aberrant synthesis and secretion of adipokines, as well as inflammation [132].
In summary, the VDR plays a role in modulating adipogenesis and exhibits varying regulatory effects on adipocytes during diverse stages of differentiation (Figure 1). Such discrepancies between in vivo and in vitro studies might be partly explained by this, although further research is necessary to fully elucidate the underlying mechanisms involved.
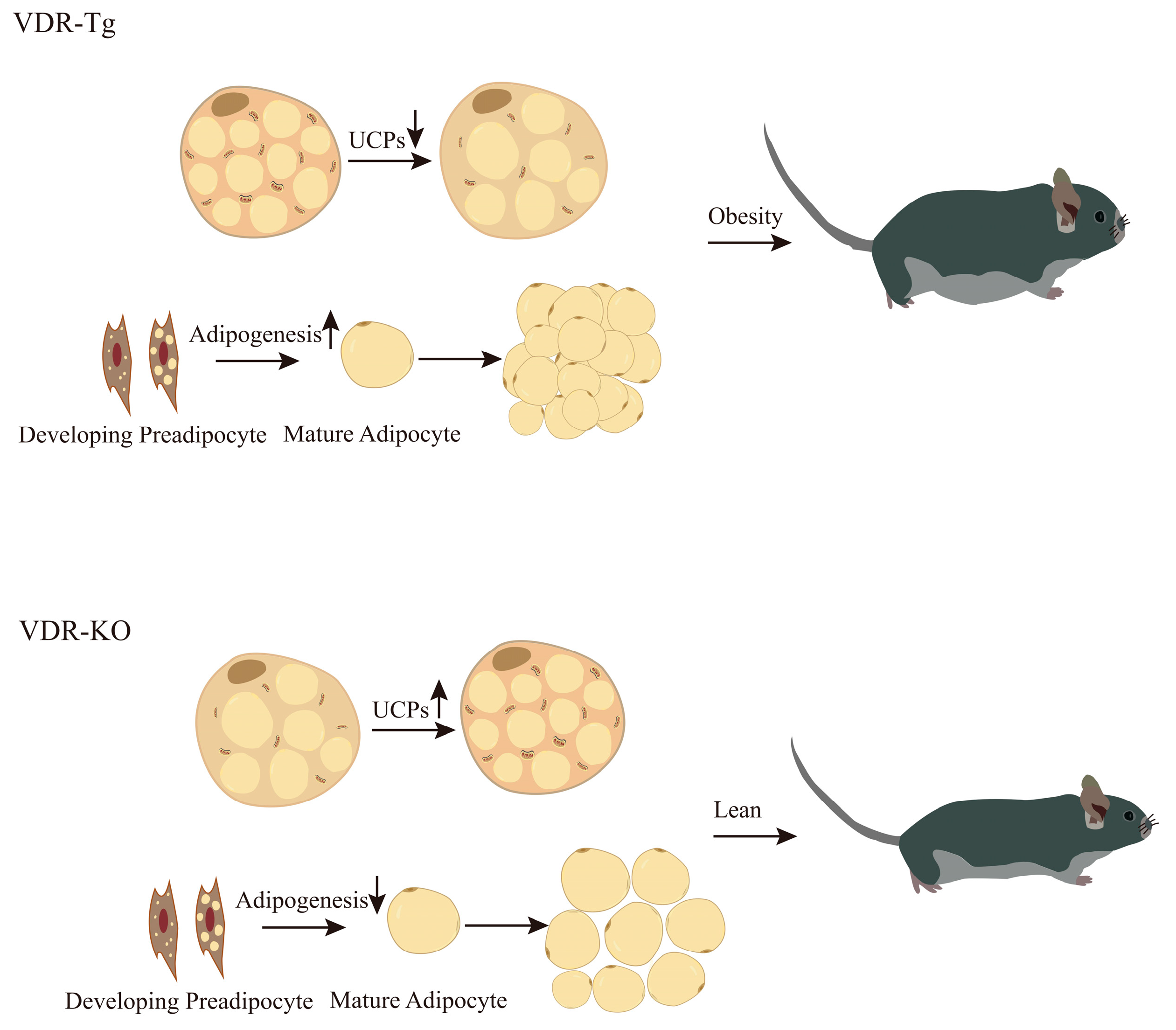
Figure 1. Role of VDR in the development of obesity. The presented figure depicts that the genetic manipulation of whole-body VDR, including knockout and overexpression, exerts notable effects on the browning phenomenon within white WAT as well as adipogenesis, consequently influencing the initiation and progression of obesity.
6. Summary
In summary, there exists a strong association between VD levels and the onset and progression of obesity, as well as adipose tissue metabolic capacity and lipogenesis. Adipose tissue serves as the primary reservoir for VD, where VD stored in lipid droplets can be released into the bloodstream during lipolysis. The 1,25(OH)2D3/VDR complex and its subsequent signaling pathways possess the ability to directly govern adipogenesis, thus playing a significant role in the modulation of insulin sensitivity and inflammation within adipose tissue. Distinct adipose tissue types demonstrate variations in cellular composition, metabolic characteristics, extracellular matrix composition, and susceptibility to alterations in the internal milieu, which might mediate the differential effects of VD in different adipose depots. The accumulation of VAT stands as the most sensitive and crucial indicative factor for identifying the presence of VDD high-risk individuals. While certain observational studies fail to support the favorable effects of VD supplementation in ameliorating obesity and its associated complications, the consolidated evidence from existing research supports the advantageous role of VD/VDR in regulating adipose tissue health and preventing obesity.
References
- Cinti, S. Adipose Organ Development and Remodeling. Compr. Physiol. 2018, 8, 1357–1431. [Google Scholar]
- Cypess, A.M. Reassessing Human Adipose Tissue. New Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Śliwińska, A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? Int. J. Mol. Sci. 2022, 23, 956. [Google Scholar] [CrossRef]
- Patni, N.; Garg, A. Lipodystrophy for the Diabetologist—What to Look For. Curr. Diabetes Rep. 2022, 22, 461–470. [Google Scholar] [CrossRef]
- Le Lay, S.; Magré, J.; Prieur, X. Not Enough Fat: Mouse Models of Inherited Lipodystrophy. Front. Endocrinol. 2022, 13, 785819. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue—A Translational Perspective. Endocr. Rev. 2023, 44, 143–192. [Google Scholar] [CrossRef]
- Xue, B.; Rim, J.-S.; Hogan, J.C.; Coulter, A.A.; Koza, R.A.; Kozak, L.P. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J. Lipid Res. 2007, 48, 41–51. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.-F. Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. [Google Scholar] [CrossRef]
- McGill, A.-T.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008, 7, 4. [Google Scholar] [CrossRef]
- Walsh, J.S.; Evans, A.L.; Bowles, S.; E Naylor, K.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016, 103, 1465–1471. [Google Scholar] [CrossRef]
- Konradsen, S.; Ag, H.; Lindberg, F.; Hexeberg, S.; Jorde, R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur. J. Nutr. 2008, 47, 87–91. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Drincic, A.T.; Armas, L.A.; van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. 2013, 37, 651–657. [Google Scholar] [CrossRef]
- Bonnet, L.; Hachemi, M.A.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Martin, J.-C.; Tourniaire, F.; Landrier, J.-F. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J. Steroid Biochem. Mol. Biol. 2019, 185, 39–46. [Google Scholar] [CrossRef]
- Esteghamati, A.; Aryan, Z.; Nakhjavani, M. Differences in vitamin D concentration between metabolically healthy and unhealthy obese adults: Associations with inflammatory and cardiometabolic markers in 4391 subjects. Diabetes Metab. 2014, 40, 347–355. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M.B. 1α, 25-Dihydroxyvitamin D and corticosteroid regulate adipocyte nuclear vitamin D receptor. Int. J. Obes. 2008, 32, 1305–1311. [Google Scholar] [CrossRef]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef]
- Chang, E.; Kim, Y. Vitamin D decreases adipocyte lipid storage and increases NAD-SIRT1 pathway in 3T3-L1 adipocytes. Nutrition 2016, 32, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Yuzbashian, E.; Asghari, G.; Hedayati, M.; Zarkesh, M.; Mirmiran, P.; Khalaj, A. Determinants of vitamin D receptor gene expression in visceral and subcutaneous adipose tissue in non-obese, obese, and morbidly obese subjects. J. Steroid Biochem. Mol. Biol. 2019, 187, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hengist, A.; Perkin, O.; Gonzalez, J.T.; Betts, J.A.; Hewison, M.; Manolopoulos, K.N.; Jones, K.S.; Koulman, A.; Thompson, D. Mobilising vitamin D from adipose tissue: The potential impact of exercise. Nutr. Bull. 2019, 44, 25–35. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Schoenmakers, I.; Bluck, L.J.C.; Ding, S.; Prentice, A. Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. Br. J. Nutr. 2011, 107, 1128–1137. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes. Rev. 2022, 23, e13453. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, R.; Delanghe, J.R.; Speeckaert, M.M. Vitamin D Deficiency: An Underestimated Factor in Sepsis? Int. J. Mol. Sci. 2023, 24, 2924. [Google Scholar] [CrossRef]
- Kauser, H.; Palakeel, J.J.; Ali, M.; Chaduvula, P.; Chhabra, S.; Lamichhane, S.L.; Ramesh, V.; O Opara, C.; Khan, F.Y.; Kabiraj, G.; et al. Factors Showing the Growing Relation Between Vitamin D, Metabolic Syndrome, and Obesity in the Adult Population: A Systematic Review. Cureus 2022, 14, e27335. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Research. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Park, E.; Lee, M.-J. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr. Res. Pract. 2020, 14, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Pescarmona, G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: Some preliminary emerging issues. Mol. Cell. Endocrinol. 2017, 450, 24–31. [Google Scholar] [CrossRef]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes: A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef]
- Zhang, R.; Li, B.; Gao, X.; Tian, R.; Pan, Y.; Jiang, Y.; Gu, H.; Wang, Y.; Wang, Y.; Liu, G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: Dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 810–819. [Google Scholar] [CrossRef]
- Hajhashemy, Z.; Shahdadian, F.; Ziaei, R.; Saneei, P. Serum vitamin D levels in relation to abdominal obesity: A systematic review and dose–response meta-analysis of epidemiologic studies. Obes. Rev. 2021, 22, e13134. [Google Scholar] [CrossRef]
- Borges, C.C.; Bringhenti, I.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Vitamin D restriction enhances periovarian adipose tissue inflammation in a model of menopause. Climacteric 2020, 23, 99–104. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Gouranton, E.; Romier, B.; Tourniaire, F.; Astier, J.; Malezet, C.; Amiot, M.; Landrier, J. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol. Nutr. Food Res. 2012, 56, 1771–1782. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 2012, 287, 42324–42332. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Trayhurn, P.; Bing, C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int. J. Obes. 2013, 37, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Peiretti, F.; Darmon, P.; Landrier, J.-F. Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice. Endocrinology 2015, 156, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Behl, T.; Kaur, I.; Singh, S.; Sharma, N.; Aleya, L. Targeting NLRP3 inflammasome as a chief instigator of obesity, contributing to local adipose tissue inflammation and insulin resistance. Environ. Sci. Pollut. Res. 2021, 28, 43102–43113. [Google Scholar] [CrossRef]
- Zammouri, J.; Vatier, C.; Capel, E.; Auclair, M.; Storey-London, C.; Bismuth, E.; Mosbah, H.; Donadille, B.; Janmaat, S.; Fève, B.; et al. Molecular and Cellular Bases of Lipodystrophy Syndromes. Front. Endocrinol. 2022, 12, 803189. [Google Scholar] [CrossRef]
- Funcke, J.-B.; Scherer, P.E. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. J. Lipid Res. 2019, 60, 1648–1697. [Google Scholar] [CrossRef]
- Heinonen, S.; Jokinen, R.; Rissanen, A.; Pietiläinen, K.H. White adipose tissue mitochondrial metabolism in health and in obesity. Obes. Rev. 2020, 21, e12958. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-Do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; Santos, I.D.O.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Metabolically Healthy Obesity (MHO) vs. Metabolically Unhealthy Obesity (MUO) Phenotypes in PCOS: Association with Endocrine-Metabolic Profile, Adherence to the Mediterranean Diet, and Body Composition. Nutrients 2021, 13, 3925. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Obstet. Gynecol. Surv. 2008, 63, 783–784. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proc. Natl. Acad. Sci. USA 2019, 116, 723–731. [Google Scholar]
- Item, F.; Konrad, D. Visceral fat and metabolic inflammation: The portal theory revisited. Obes. Rev. 2012, 13 (Suppl. 2), 30–39. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2017, 102, 3097–3110. [Google Scholar] [CrossRef]
- Björntorp, P. Metabolic difference between visceral fat and subcutaneous abdominal fat. Diabetes Metab. 2000, 26 (Suppl. 3), 10–12. [Google Scholar]
- Misra, A.; Vikram, N.K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [PubMed]
- Lepper, C. and C.-M. Fan, Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 2010, 48, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Worthmann, A.; Schlein, C.; Berbée, J.F.P.; Rensen, P.C.N.; Heeren, J.; Bartelt, A. Effects of Pharmacological Thermogenic Adipocyte Activation on Metabolism and Atherosclerotic Plaque Regression. Nutrients 2019, 11, 463. [Google Scholar] [CrossRef]
- Angueira, A.R.; Shapira, S.N.; Ishibashi, J.; Sampat, S.; Sostre-Colón, J.; Emmett, M.J.; Titchenell, P.M.; Lazar, M.A.; Lim, H.-W.; Seale, P. Early B Cell Factor Activity Controls Developmental and Adaptive Thermogenic Gene Programming in Adipocytes. Cell Rep. 2020, 30, 2869–2878.e4. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Ikeda, K.; Chang, H.-Y.; Chang, C.-H.; Yoneshiro, T.; Oguri, Y.; Jun, H.; Wu, J.; Ishihama, Y.; Kajimura, S. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat. Metab. 2019, 1, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Dai, W.; Jang, M.J.; Medrano, L.; Li, Z.; Zhao, H.; Shao, M.; Tan, J.; Li, A.; Ning, T.; et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Investig. 2019, 130, 247–257. [Google Scholar] [CrossRef]
- Park, J.; Shin, S.; Liu, L.; Jahan, I.; Ong, S.-G.; Xu, P.; Berry, D.C.; Jiang, Y. Progenitor-like characteristics in a subgroup of UCP1+ cells within white adipose tissue. Dev. Cell 2021, 56, 985–999.e4. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kulterer, O.C.; Herz, C.T.; Prager, M.; Schmöltzer, C.; Langer, F.B.; Prager, G.; Marculescu, R.; Kautzky-Willer, A.; Hacker, M.; Haug, A.R.; et al. Brown Adipose Tissue Prevalence Is Lower in Obesity but Its Metabolic Activity Is Intact. Front. Endocrinol. 2022, 13, 858417. [Google Scholar] [CrossRef] [PubMed]
- Herz, C.T.; Kulterer, O.C.; Prager, M.; Schmöltzer, C.; Langer, F.B.; Prager, G.; Marculescu, R.; Kautzky-Willer, A.; Hacker, M.; Haug, A.R.; et al. Active Brown Adipose Tissue is Associated with a Healthier Metabolic Phenotype in Obesity. Diabetes 2021, 71, 93–103. [Google Scholar]
- Ricciardi, C.J.; Bae, J.; Esposito, D.; Komarnytsky, S.; Hu, P.; Chen, J.; Zhao, L. 1,25-Dihydroxyvitamin D3/vitamin D receptor suppresses brown adipocyte differentiation and mitochondrial respiration. Eur. J. Nutr. 2015, 54, 1001–1012. [Google Scholar] [CrossRef]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A. Vitamin D3 distribution and status in the body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef]
- Malmberg, P.; Karlsson, T.; Svensson, H.; Lönn, M.; Carlsson, N.-G.; Sandberg, A.-S.; Jennische, E.; Osmancevic, A.; Holmäng, A. A new approach to measuring vitamin D in human adipose tissue using time-of-flight secondary ion mass spectrometry: A pilot study. J. Photochem. Photobiol. B Biol. 2014, 138, 295–301. [Google Scholar] [CrossRef]
- Beckman, L.M.; Earthman, C.P.; Thomas, W.; Compher, C.W.; Muniz, J.; Horst, R.L.; Ikramuddin, S.; Kellogg, T.A.; Sibley, S.D. Serum 25(OH) Vitamin D concentration changes after roux-en-y gastric bypass surgery. Obesity 2013, 21, E599–E606. [Google Scholar] [CrossRef]
- Li, Y.-F.; Zheng, X.; Gao, W.-L.; Tao, F.; Chen, Y. Association between serum vitamin D levels and visceral adipose tissue among adolescents: A cross-sectional observational study in NHANES 2011–2015. BMC Pediatr. 2022, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, cardiometabolic risk, and Vitamin D status: The Framingham Heart Study. Diabetes 2009, 59, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Bunch, B.L.; Ma, Y.; Attwood, K.; Amable, L.; Luo, W.; Morrison, C.; Guru, K.A.; Woloszynska-Read, A.; Hershberger, P.A.; Trump, D.L.; et al. Vitamin D3 enhances the response to cisplatin in bladder cancer through VDR and TAp73 signaling crosstalk. Cancer Med. 2019, 8, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Hsieh, C.-C.; Kuo, H.-F.; Tsai, M.-K.; Yang, S.-N.; Kuo, C.-H.; Lee, M.-S.; Hung, C.-H. Effect of Vitamin D3 on Monocyte Chemoattractant Protein 1 Production in Monocytes and Macrophages. Acta Cardiol. Sin. 2014, 30, 144–150. [Google Scholar] [PubMed]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Hajiluian, G.; Nameni, G.; Shahabi, P. Adipose Tissue Inflammation and Oxidative Stress: The Ameliorative Effects of Vitamin D. Inflammation 2017, 40, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.C.; Salles, A.F.; Bringhenti, I.; Souza-Mello, V.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Adverse effects of vitamin D deficiency on the Pi3k/Akt pathway and pancreatic islet morphology in diet-induced obese mice. Mol. Nutr. Food Res. 2015, 60, 346–357. [Google Scholar] [CrossRef]
- Mirza, I.; Mohamed, A.; Deen, H.; Balaji, S.; Elsabbahi, D.; Munasser, A.; Naquiallah, D.; Abdulbaseer, U.; Hassan, C.; Masrur, M.; et al. Obesity-Associated Vitamin D Deficiency Correlates with Adipose Tissue DNA Hypomethylation, Inflammation, and Vascular Dysfunction. Int. J. Mol. Sci. 2022, 23, 14377. [Google Scholar] [CrossRef]
- Nimitphong, H.; Guo, W.; Holick, M.F.; Fried, S.K.; Lee, M. Vitamin D Inhibits Adipokine Production and Inflammatory Signaling Through the Vitamin D Receptor in Human Adipocytes. Obesity 2021, 29, 562–568. [Google Scholar] [CrossRef]
- Matthews, D.G.; D’angelo, J.; Drelich, J.; Welsh, J. Adipose-specific Vdr deletion alters body fat and enhances mammary epithelial density. J. Steroid Biochem. Mol. Biol. 2016, 164, 299–308. [Google Scholar] [CrossRef]
- Wu, M.; Lu, L.; Guo, K.; Lu, J.; Chen, H. Vitamin D protects against high glucose-induced pancreatic β-cell dysfunction via AMPK-NLRP3 inflammasome pathway. Mol. Cell. Endocrinol. 2022, 547, 111596. [Google Scholar] [CrossRef] [PubMed]
- Doğan, Y.; Kara, M.; Culha, M.A.; Özçakar, L.; Kaymak, B. The relationship between vitamin D deficiency, body composition, and physical/cognitive functions. Arch. Osteoporos. 2022, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, R.; Walschot, F.; Lips, P.; Lamb, H.J.; de Roos, A.; Rosendaal, F.R.; den Heijer, M.; de Jongh, R.T.; de Mutsert, R. Associations of different body fat deposits with serum 25-hydroxyvitamin D concentrations. Clin. Nutr. 2019, 38, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Futawaka, K.; Koyama, R.; Fukuda, Y.; Hayashi, M.; Imamoto, M.; Miyawaki, M.; Kasahara, M.; Tagami, T. Vitamin D3/VDR resists diet-induced obesity by modulating UCP3 expression in muscles. J. Biomed. Sci. 2016, 23, 56. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.-J.; Bendahan, D.; Bernard, M.; Martin, J.-C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Muñoz-Garach, A.; Serrano, M.; Garrido-Sánchez, L.; Bernal-López, M.R.; Fernández-García, D.; Moreno-Santos, I.; Garriga, N.; Castellano-Castillo, D.; Camargo, A.; et al. Serum 25-hydroxyvitamin D and adipose tissue vitamin D receptor gene expression: Relationship with obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 2015, 100, E591–E595. [Google Scholar] [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Goossens, G.H.; Blaak, E.E. Vitamin D release across abdominal adipose tissue in lean and obese men: The effect of ß-adrenergic stimulation. Physiol. Rep. 2019, 7, e14308. [Google Scholar] [CrossRef]
- Piccolo, B.D.; Dolnikowski, G.; Seyoum, E.; Thomas, A.P.; Gertz, E.R.; Souza, E.C.; Woodhouse, L.R.; Newman, J.W.; Keim, N.L.; Adams, S.H.; et al. Association between subcutaneous white adipose tissue and serum 25-hydroxyvitamin D in overweight and obese adults. Nutrients 2013, 5, 3352–3366. [Google Scholar] [CrossRef]
- Wamberg, L.; Cullberg, K.B.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Investigations of the Anti-inflammatory Effects of Vitamin D in adipose tissue: Results from an in vitro study and a randomized controlled trial. Horm. Metab. Res. 2013, 45, 456–462. [Google Scholar] [CrossRef]
- Jonas, M.I.; Kuryłowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Kozniewski, K.; Puzianowska-Kuznicka, M. Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level. Int. J. Mol. Sci. 2019, 20, 5272. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean Phenotype and resistance to diet-induced obesity in vitamin d receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, Y.; Kong, J. VDR regulates energy metabolism by modulating remodeling in adipose tissue. Eur. J. Pharmacol. 2019, 865, 172761. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Kusudo, T. Bidirectional effect of vitamin D on brown adipogenesis of C3H10T1/2 fibroblast-like cells. PeerJ 2023, 11, e14785. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Szeto, F.L.; Zhang, W.; Ye, H.; Kong, J.; Zhang, Z.; Sun, X.J.; Li, Y.C. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am. J. Physiol. Metab. 2009, 296, E820–E828. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.W.; Ding, W.; Hoffman, H.M.; Wang, Z.; Hao, S.; Zheng, R.; Gonzalez, A.; Zhan, J.-Y.; Zhou, P.; Li, S.; et al. Vitamin D ameliorates adipose browning in chronic kidney disease cachexia. Sci. Rep. 2020, 10, 14175. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Sun, X. Calcitriol and energy metabolism. Nutr. Rev. 2008, 66, S139–S146. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M.B. Role of uncoupling protein 2 (UCP2) expression and 1alpha, 25-dihydroxyvitamin D3 in modulating adipocyte apoptosis. FASEB J. 2004, 18, 1430–1432. [Google Scholar] [CrossRef]
- Bassatne, A.; Chakhtoura, M.; Saad, R.; Fuleihan, G.E.-H. Vitamin D supplementation in obesity and during weight loss: A review of randomized controlled trials. Metabolism 2019, 92, 193–205. [Google Scholar] [CrossRef]
- Seida, J.C.; Mitri, J.; Colmers, I.N.; Majumdar, S.R.; Davidson, M.B.; Edwards, A.L.; Hanley, D.A.; Pittas, A.G.; Tjosvold, L.; Johnson, J. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3551–3560. [Google Scholar] [CrossRef]
- Hajimohammadi, M.; Shab-Bidar, S.; Neyestani, T.R. Vitamin D and serum leptin: A systematic review and meta-analysis of observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2016, 71, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tian, L.; Xiao, Y.; Huang, G.; Zhang, M. Effect of Vitamin D Supplementation on Some Inflammatory Biomarkers in Type 2 Diabetes Mellitus Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2018, 73, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.M.; Biscaia, P.B.; Brunoski, J.; Ribeiro, R.A.; Franco, G.C.N.; Scomparin, D.X. Vitamin D supplementation decreases visceral adiposity and normalizes leptinemia and circulating TNF-α levels in western diet-fed obese rats. Life Sci. 2021, 278, 119550. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Gregorio, B.; Sampaio, F.; Sanchez, R.; Risopatrón, J. Role of Vitamin D in the Development of Obesity Current Research in Nutrition and Food Science. Int. J. Morphol. 2017, 35, 1568–1575. [Google Scholar] [CrossRef]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.-F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef]
- Jahn, D.; Dorbath, D.; Kircher, S.; Nier, A.; Bergheim, I.; Lenaerts, K.; Hermanns, H.M.; Geier, A. Beneficial Effects of Vitamin D Treatment in an Obese Mouse Model of Non-Alcoholic Steatohepatitis. Nutrients 2019, 11, 77. [Google Scholar] [CrossRef]
- Bonnet, L.; Karkeni, E.; Couturier, C.; Astier, J.; Dalifard, J.; Defoort, C.; Svilar, L.; Martin, J.-C.; Tourniaire, F.; Landrier, J.-F. Gene Expression Pattern in Response to Cholecalciferol Supplementation Highlights Cubilin as a Major Protein of 25(OH)D Uptake in Adipocytes and Male Mice White Adipose Tissue. Endocrinology 2017, 159, 957–966. [Google Scholar] [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef]
- Weber, K.; Erben, R.G. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J. Anim. Physiol. Anim. Nutr. 2013, 97, 675–683. [Google Scholar] [CrossRef]
- Schutkowski, A.; Max, D.; Bönn, M.; Brandsch, C.; Grundmann, S.M.; Hirche, F.; Staege, M.S.; Stangl, G.I. Vitamin D Does Not Play a Functional Role in Adipose Tissue Development in Rodent Models. Mol. Nutr. Food Res. 2018, 62, 1700726. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Simmons, K.M.; Brunton, J.; Salinero, A.; Chittur, S.V.; Welsh, J.E. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J. Cell. Physiol. 2013, 228, 2024–2036. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.M.; Tzameli, I.; Astapova, I.; Lam, F.S.; Flier, J.S.; Hollenberg, A.N. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J. Biol. Chem. 2006, 281, 11205–11213. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Lieben, L.; Watanabe, M.; Perino, A.; Auwerx, J.; Schoonjans, K.; Verstuyf, A. Vitamin D and energy homeostasis—Of mice and men. Nat. Rev. Endocrinol. 2014, 10, 79–87. [Google Scholar] [CrossRef]
- Tao, T.; Kobelski, M.M.; Saini, V.; Demay, M.B. Adipose-specific VDR Deletion Leads to Hepatic Steatosis in Female Mice Fed a Low-Fat Diet. Endocrinology 2022, 163, bqab249. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Kang, S.; Goyal, A.; Zhang, K.; You, J.Y.; Carey, M.; Jain, S.; Bhansali, S.; Kehlenbrink, S.; Guo, P.; et al. Insulin-sensitizing effects of vitamin D repletion mediated by adipocyte vitamin D receptor: Studies in humans and mice. Mol. Metab. 2020, 42, 101095. [Google Scholar] [CrossRef]
- Larrick, B.M.; Kim, K.-H.; Donkin, S.S.; Teegarden, D. 1,25-Dihydroxyvitamin D regulates lipid metabolism and glucose utilization in differentiated 3T3-L1 adipocytes. Nutr. Res. 2018, 58, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Boyle, V.T.; Thorstensen, E.B.; Thompson, J.M.D.; E McCowan, L.M.; A Mitchell, E.; Godfrey, K.M.; Poston, L.; Wall, C.R.; Murphy, R.; Cutfield, W.; et al. The relationship between maternal 25-hydroxyvitamin D status in pregnancy and childhood adiposity and allergy: An observational study. Int. J. Obes. 2017, 41, 1755–1760. [Google Scholar] [CrossRef]
- Miliku, K.; Felix, J.F.; Voortman, T.; Tiemeier, H.; Eyles, D.W.; Burne, T.H.; McGrath, J.J.; Jaddoe, V.W. Associations of maternal and fetal vitamin D status with childhood body composition and cardiovascular risk factors. Matern. Child Nutr. 2018, 15, e12672. [Google Scholar] [CrossRef] [PubMed]
- Tint, M.T.; Chong, M.F.; Aris, I.M.; Godfrey, K.M.; Quah, P.L.; Kapur, J.; Saw, S.M.; Gluckman, P.D.; Rajadurai, V.S.; Yap, F.; et al. Association between maternal mid-gestation vitamin D status and neonatal abdominal adiposity. Int. J. Obes. 2018, 42, 1296–1305. [Google Scholar] [CrossRef]
- Reichetzeder, C.; Chen, H.; Föller, M.; Slowinski, T.; Li, J.; Chen, Y.-P.; Lang, F.; Hocher, B. Maternal vitamin D deficiency and fetal programming—Lessons learned from humans and mice. Kidney Blood Press. Res. 2014, 39, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, Y.; Zhang, Q.; Xiao, X. The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review. Nutrients 2023, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Seipelt, E.M.; Tourniaire, F.; Couturier, C.; Astier, J.; Loriod, B.; Vachon, H.; Pucéat, M.; Mounien, L.; Landrier, J. Prenatal maternal vitamin D deficiency sex-dependently programs adipose tissue metabolism and energy homeostasis in offspring. FASEB J. 2020, 34, 14905–14919. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hong, Q.; Wang, X.; Zhu, L.; Wu, T.; Xu, P.; Fu, Z.; You, L.; Wang, X.; Ji, C.; et al. The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in rat offspring. Sci. Rep. 2018, 8, 365. [Google Scholar] [CrossRef]
- Belenchia, A.M.; Jones, K.L.; Will, M.; Beversdorf, D.Q.; Vieira-Potter, V.; Rosenfeld, C.S.; Peterson, C.A. Maternal vitamin D deficiency during pregnancy affects expression of adipogenic-regulating genes peroxisome proliferator-activated receptor gamma (PPARγ) and vitamin D receptor (VDR) in lean male mice offspring. Eur. J. Nutr. 2018, 57, 723–730. [Google Scholar] [CrossRef]

