1. Introduction
Ulcerative colitis is one of the main forms of inflammatory bowel disease (IBD) affecting the rectum and colon that is characterized by an imbalance in proinflammatory and anti-inflammatory reactivity [1,2]. The remarkable rise in IBD worldwide, including in most developing nations, affects millions of individuals and is a major public health issue that may raise the risk of colon cancer [3,4]. Several factors contribute to the etiology of UC including microbial, environmental, and genetic factors [5].
Although the exact pathogenesis of UC is still unknown [6], it is characterized by relapsing and remitting mucosal inflammation [7,8]. In particular, macrophages play a significant role in inflammatory disorders by engendering the cytokines interleukin (IL)-1β and tumor necrosis factor-α (TNF-α) and other inflammatory mediators such as nitric oxide (NO) and prostaglandins [9]. Chronic inflammation has been linked to a lower risk of colitis-associated colorectal cancer by increasing the production of pro-inflammatory cytokines such as IL-6, IL-1ß, IL-17, and TNF-α. Therefore, targeting NF-kB inflammation pathways together with Wnt/ß-catenin signaling may act to control colorectal carcinogenesis [10]. In fact, it has been reported that the negative regulation of the Wnt signaling pathway by the degradation of β-catenin, a transcriptional coactivator of the Wnt signaling pathway, allows its subsequent translocation to the nucleus and activation of Wnt target genes by associating with LEF-1/TCF proteins [11].
The standard treatment methods for UC use medications targeting inflammation and the immune system including mesalamine, sulfasalazine, and corticosteroids that, taken alone or in association, contribute to treating this disease [12]. Unfortunately, these drugs are linked with side effects and patients eventually become refractory or intolerant over time [13]. Therefore, the research for alternative and/or supplementary treatments among herbal and traditional medicines has been highly motivated [14,15]. Recent studies have focused on natural products and supplements obtained from plants with anti-inflammatory effects, low toxicity, and minimal side effects [16].
Urtica dioica, often known as common nettle, is one of the most commonly used medicinal plants in the world due to its biologically active compounds [17]. The leaves of this plant have been reported to show hypotensive, anti-inflammatory, hypoglycemic, analgesic, anti-ulcer, antioxidant, antimicrobial, cytoprotective, and anticancer activities [18,19]. Some of the chemicals in this plant include lignan, secolignan, norlignan, alkaloid, sesquiterpenoid, flavonoid, triterpenoid, sphingolipid, and sterol [20,21]. The trichomes of the nettle contain formic acid, acetyl choline, serotonin, and histamine [22].
According to various studies, the stinging nettle plant contains biologically active chemicals such as phenols and flavonoids that can help reduce free radical generation by diverse pharmacological properties such as antioxidative and anti-inflammatory properties and may play a role in the prevention of intestinal inflammation. The use of water as a solvent showed the highest total phenolic content values as well as producing a significant effect on the antioxidant capacity of the extracts [23].
The aim of this study was to evaluate the prophylactic coloprotective action of AEUD on DSS-induced ulcerative colitis via the regulation of inflammatory reactions and antioxidant properties in a colitis rat model.
2. Materials and Methods
2.1. Animals
Healthy adult male Wistar rats (weighing between 180 and 200 g) were purchased from the Society of Pharmaceutical Industries of Tunisia (SIPHAT, Ben-Arous, Tunisia) and acclimatized for 1 week before performing any experiment. All animals were housed under safe laboratory conditions in a temperature- and humidity-controlled room (22–24 °C, 70%) and kept on a 12 h light/dark cycle using hygrometer, thermometer, and timer settings with food and tap water available ad libitum. All animal procedures were performed in accordance with the Guidelines for Care and Use of Animals Laboratory and approved by the Bio-Medical Ethics Committee (CEBM) for the Care and Use of Animals for scientific purpose (JORT454002 (6 May 2021)). Furthermore, all experiments were performed at the same time of day (8 h).
2.2. AEUD Preparation
Leaves of Urtica dioica were collected from Beja, Tunisia, in March 2021 and were identified by Dr. Chokri Hafsi, a Professor at the University of Jendouba. The Voucher specimens (No. SO.325) have been deposited with the herbarium of the Higher Institute of Biotechnology of Beja, Tunisia. After drying in an oven at 50 °C for 48 h, the leaves were ground into fine powder using a blender. An amount of 10 g of the powder mixture was dissolved in 100 mL of bi-distilled water and incubated in a shaker for 24 h. Then, the extract solution obtained was filtered, concentrated in a water bath under vacuo, frozen, and lyophilized. AEUD was used for the phytochemical and mineral determination and in vivo experiments.
2.3. AEUD Phytochemical and Mineral Analysis
A phytochemical characterization of AEUD was made by determining the total phenolic compounds according to the colorimetric method of Folin–Ciocalteu. Briefly, 500 µL of the extract was added to 10 mL of water and 0.5 mL of Folin–Ciocalteu reagent. After 5 min, 8 mL of 7.5% sodium carbonate solution was added. The reaction was kept in the dark for 2 h and was measured at 765 nm using a UV-visible detector spectrophotometer. Gallic acid was applied as a standard, and the results were expressed in milligram gallic acid equivalent per gram dry matter (mg GAE/g DM) [24].
The extract solution (0.5 mL) was mixed with 500 μL of 50% Folin–Ciocalteu reagent. The mixture was then allowed to stand for a 2–5 min period followed by the addition of 1.0 mL of 20% sodium carbonate. After 10 min incubation at room temperature, the mixture was centrifuged for 5 min (1000× g), and the absorbance of the supernatant was measured at 730 nm. The total tannin content was expressed as mg of tannic acid equivalents/g DM [25].
The total flavonoid content was detected using the AlCl3 colorimetric method. In fact, 1 mL of the sample was mixed with 1 mL of 2% AlCl3 solution. After 15 min incubation at room temperature, the optical density of their action mixture was evaluated at 430 nm. Quercetin was used as a reference standard and the total flavonoid content was expressed as milligram quercetin equivalent per gram dry matter (mg QE/g DM) [26]. The total sugar level was determined using a previous procedure [27].
Atomic spectroscopy was used to detect the contents of magnesium (Mg), zinc (Zn), iron (Fe), manganese (Mn), molybdenum (Mo), and copper (Cu) in AEUD.
2.4. Experimental Procedure
The study was continued for 21 days and a total of 36 rats were divided into six groups, each consisting of six animals, including: Group 1: normal control given only saline solution with oral intake of NaCl (0.9%, 5 mL/kg, b.w.); Group 2: the colitis group receiving DSS (5%) in the drinking water; Group 3: the reference group, MESA was administered to the rats at 100 mg/kg by gavage from day 0 to 21; Groups 4, 5, and 6: AEUD given at 50, 100, and 200 mg/kg once a day by gavage route for 21 days.
Ulcerative colitis was induced in rats by administering 5% DSS in the drinking water from day 15 to 21, except for the control group. During DSS treatment, stool consistency, the presence of blood in the feces, body weight, and food intake were examined and documented daily. After 21 days of experiment, animals were anesthetized to avoid any kind of stress which could distort the results and sacrificed by decapitation. The entire colon was measured; then, a portion of the colon tissue was stored in 10% buffered formalin for histopathological analysis and the remaining colon tissue was stored at −80 °C for further biochemical analysis.
2.5. Evaluation of Clinical Colitis and Colonic Weight and Length Measurement
The assessment of clinical colitis included daily monitoring of disease activity score determined on the basis of stool consistency, blood in the stool, and weight loss during exposure to DSS. The relevant specific criteria that were used to calculate the DAI are presented in [28]. The samples of the large intestine were weighed and the colon lengths were measured.
2.6. Biochemical Assays
Blood samples were collected in lithium heparin tubes and then plasma was obtained by centrifugation (4000 t/min/4 °C for 15 min) and stored at −80 °C until analysis. Plasma levels of C-reactive protein (CRP), amylase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), blood sugar, urea, and creatinine were measured using automated enzymatic assays (ProXL). Potassium (K+) and sodium (Na+) were measured using a Cornley AFT-300 Electrolytes Analyzer (Precimed, China).
2.7. Determination of Hematological Parameters
Hematological parameters including hemoglobin (Hb), hematocrit (Hct), red blood cells (RBCs), white blood cells (WBCs) as well as hematological indices such as mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean cellular hemoglobin concentration (MCHC) were commonly analyzed using an electronic automate (HORIBA-ABX Pentra XL 80 (Bioplus, China)).
2.8. Assessment of DSS-Induced Oxidative Stress in the Colonic Tissues
Colonic lipid peroxidation was determined by measuring MDA using the double heating method. In brief, aliquots of colonic homogenates were mixed with a BHT–TCA solution that contained 1% BHT (w/v) dissolved in 20% TCA (w/v) and centrifuged at 1000× g for 5 min at 4 °C. The supernatant was mixed with 0.5 N HCl and 120 mM TBA in 26 mM Tris and heated at 80 °C for a duration of 10 min. The absorbance of the resulting chromophore was determined at 532 nm after cooling. The levels of MDA were determined using an extinction coefficient for the MDA–TBA complex of 1.56 × 105 M−1 cm−1 [29].
For the determination of glutathione peroxidase (GPx), 1 mL of reaction mixture containing 0.2 mL colonic homogenate supernatant, 0.2 mL (0.1 M) phosphate buffer pH 7.4, 0.2 mL GSH (4 mM), and 0.4 mL H2O2 (5 mM) was incubated at 37 °C for 1 min and the reaction was stopped by addition of 0.5 mL TCA (5%, w/v). After centrifugation at 1500× g for 5 min, an aliquot (0.2 mL) of the supernatant was combined with 0.5 mL of 0.1 M phosphate buffer pH 7.4 and 0.5 mL of DTNB (10 mM) and the absorbance was read at 412 nm. The GPx activity was expressed in nanomolar of GSH consumed per minute per milligram of protein.
The superoxide dismutase (SOD) activity level was obtained using modified epinephrine assays. At alkaline pH, the superoxide anion (O2−) causes the auto-oxidation of epinephrine to adrenochrome while competing with this reaction: SOD decreases the formation of adrenochrome. An SOD unit is the quantity of extract that inhibits the rate of adrenochrome formation by 50%. The enzyme extract was added to 2 mL reaction mixture containing 10 µL bovine catalase (0.4 U/µL), 20 µL epinephrine (5 mg/mL), and 62.5 mM sodium carbonate/ bicarbonate buffer pH 10.2. Absorbance changes were observed at 480 nm [30].
The catalase (CAT) activity was evaluated by measuring the initial rate of hydrogen peroxide (H2O2) disappearance at 240 nm. The reaction mixture contained 33 mM H2O2 in 50 mM phosphate buffer at pH 7.0 and the activity of CAT was calculated using the extinction coefficient of 40 mM−1 cm−1 for H2O2 [31,32].
The colonic mucosal H2O2 level was determined. Briefly, hydrogen peroxide reacts with p-hydroxybenzoic acid and 4-aminoantipyrine in the presence of peroxidase leading to the formation of quinoneimine that has a pink color detected at 505 nm [33].
2.9. Histopathological Analysis
Colonic tissue specimens from the distal portion were collected, washed with ice-cold saline solution, and fixed in phosphate-buffered formalin (10%). Then, specimens were embedded in blocks of paraffin, sliced into 3 to 5 μm sections, stained with hematoxylin and eosin (H&E), and assessed for mucosal damage, ulceration, erosions, hemorrhage, and necrosis by a pathologist in a blinded manner under light microscopy equipped with a color video camera for digital imaging [34].
2.10. Statistical Analysis
All values were evaluated as mean ± standard error of the mean. The statistical significance of differences between groups was measured using SPSS statistical program software version 20 using one-way analysis of variance with post hoc Tukey’s multiple comparison test. A value of p < 0.05 was considered significant.
3. Results
3.1. Bioactive Compound Profile and Mineral Composition
illustrates our obtained results; AEUD is an excellent source of functional compounds such as total phenolics (247.65 ± 2.69 mg EAG/g MS), total tannins (188.29 ± 2.94 mg ECat/g MS), flavonoids (34.08 ± 0.53 mg EQt/g MS), and sugars (32.3 ± 2.4 mg Eq Glucose/g MS).
Our outcomes showed that AEUD contained a high concentration of magnesium, iron, and zinc .
3.2. Effects of AEUD or MESA on Colitis Clinical Symptoms and Colonic Weight/Length
Rats in the negative control group exhibited variations in body weight compared to their body weight at day 15, and food and water intakes. The rats showed a decrease in the food and water intakes in the model group versus those in the negative control group, which clarifies the body weight variation. In this context, the body weight of DSS-induced colitis rats varied significantly between 5.60 ± 0.80 g and 2.4 ± 0.21 g between day 15 and day 21 when comparing the negative control to the DSS group, respectively. However, it notably recovered in both the 50 and 100 mg/kg AEUD-treated groups (4.57 ± 0.38 g and 4.62 ± 0.40 g, respectively) and the MESA group (4.52 ± 0.50 g). Pretreatment with AEUD or MESA also improved the accompanying symptoms, which returned to approach ordinary qualities, in comparison to the DSS group .
The DAI score is a common parameter used for evaluating the severity of colitis. As expected, in the rats receiving DSS for a week (from day 15 to day 21) and compared with the normal control group, there was a significant increase in the DAI score of the DSS group indicating that this group showed substantial variation in body weight as well as stool consistency and bloody stool.
On the other hand, the DAI scores were decreased in rats pretreated with AEUD with different extract doses and MESA (Figure 1).
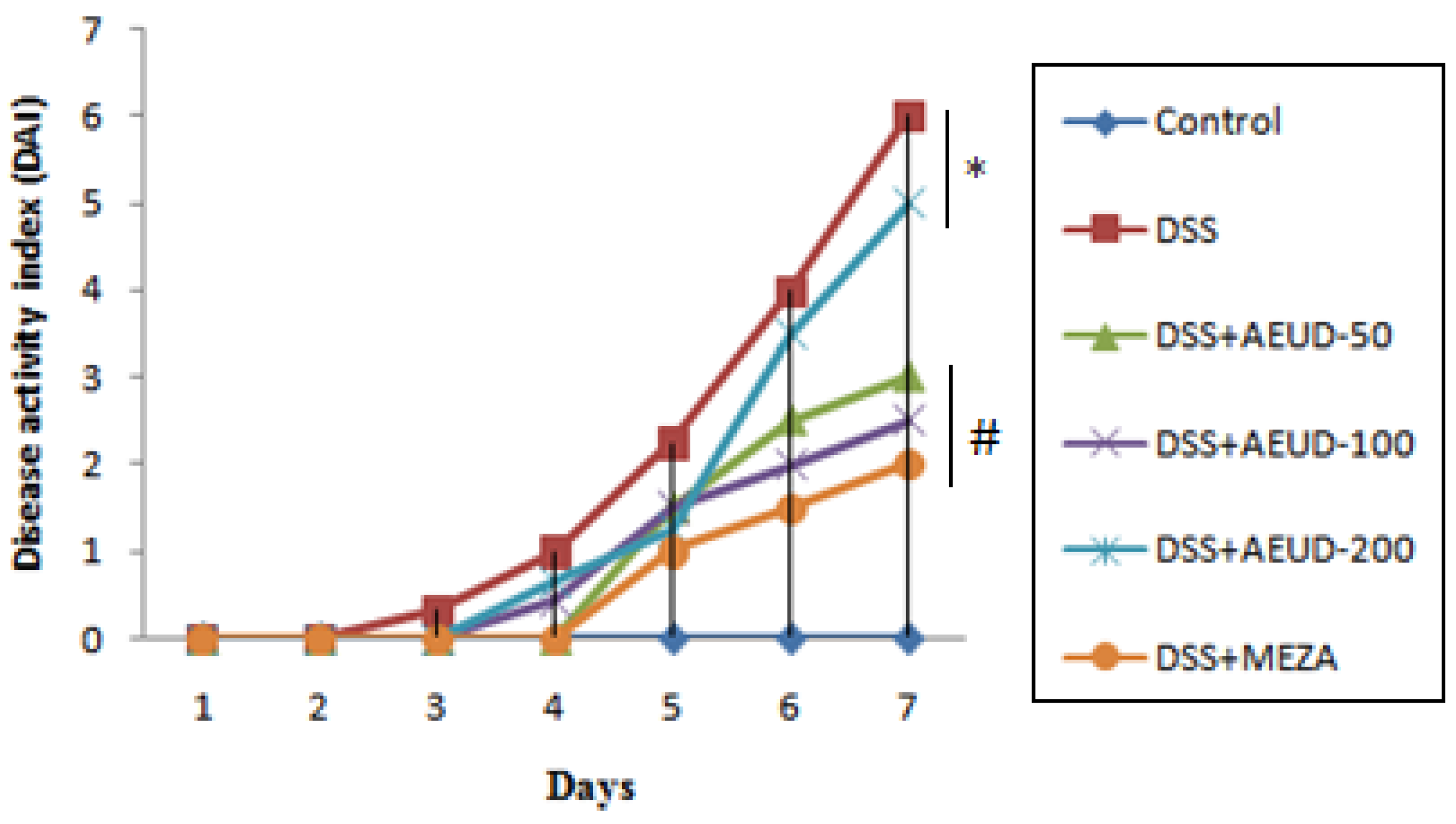
Figure 1. Effects of AEUD and MESA on disease activity index under colitis induced by DSS (from day 15 to day 21). Animals were pre-treated with three doses of AEUD (50, 100, and 200 mg/kg, b.w, p.o.), MESA (100 mg/kg, b.w, p.o.), or distilled water, and challenged with DSS (5%, w/v) in the drinking water or oral intake of NaCl (0.9%, 5 mL/kg, b.w.) during the last 7 days of treatment. Data are expressed as mean ± SEM (n = 6). * p < 0.05 compared to control group, # p < 0.05 compared to DSS group.
Shortening of the colon length and decreased weight are both indirect indicators of DSS-induced colitis severity. We assessed both colon length and weight in rats given DSS and/or AEUD/MESA. We found that the colon length/weight was dramatically decreased in the DSS-treated group contrasted to the control group. Rats given DSS + AEUD-50, DSS + AEUD-100, DSS + AEUD-200 (13.15 ± 0.58 cm, 12.83 ± 0.68 cm, 12.00 ± 1.09 cm), or DSS + MESA (14.87 ± 0.75 cm) showed a statistically significant decrease in the shortening of their colons in contrast with DSS (11.58 ± 1.29 cm) rats. Colon weight was higher in all groups given both DSS and AEUD or MESA when compared to rats given DSS alone .
3.3. Histological Observation and Evaluation
Micro-photographs of the colon sections are presented in Figure 2. The histopathological analysis of the colons of the control group and the pretreated-with-AEUD (50 mg/kg) group rats showed crypts with a normal histological structure containing normal mucosal epithelial cells along with submucosal glands with no observable ulceration or inflammation.
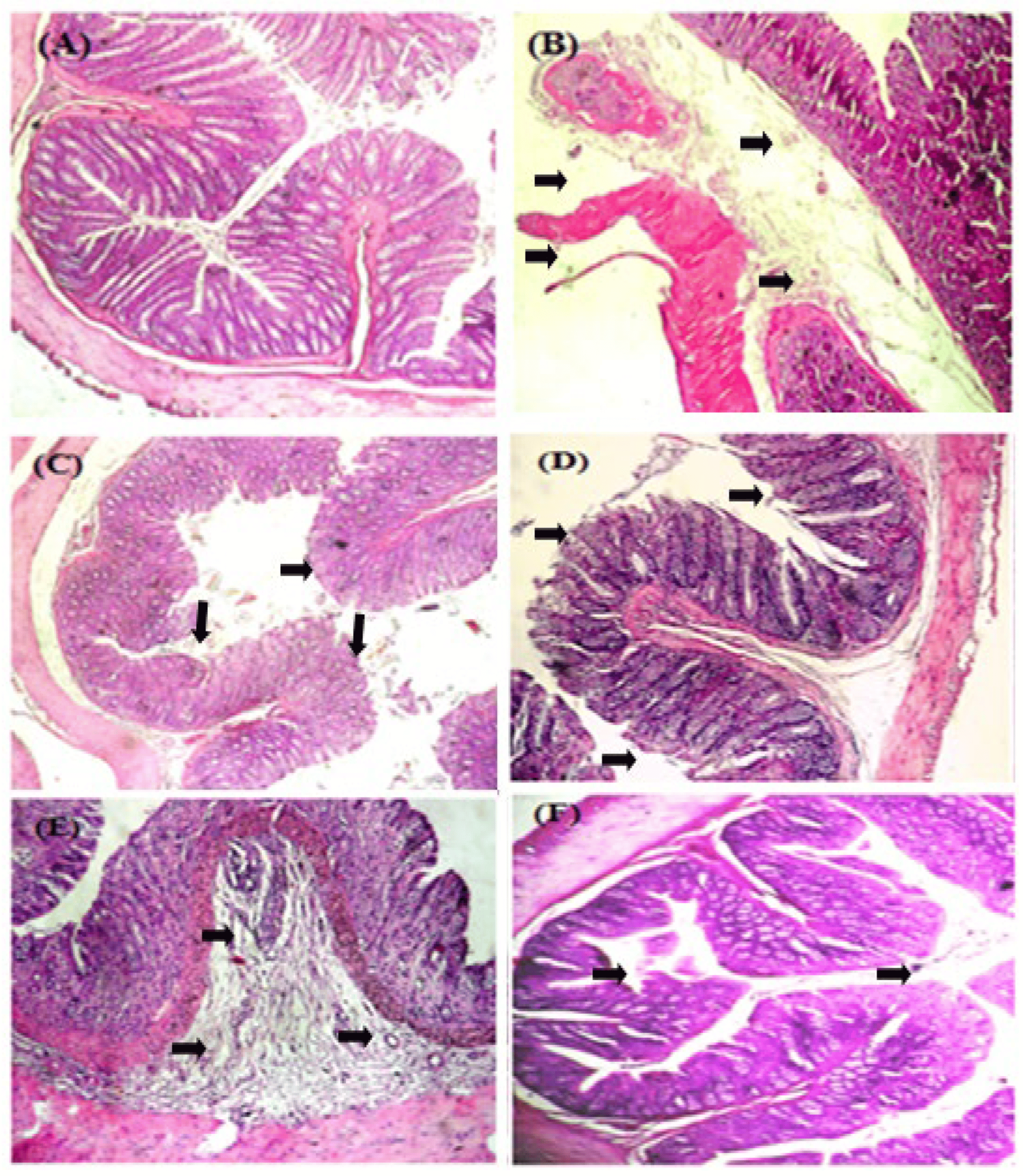
Figure 2. Effects of AEUD and MESA on the microscopic appearance of rat colons after DSS-induced colitis. Animals were pre-treated with various doses of AEUD (50, 100, and 200 mg/kg, b.w, p.o.), MESA (100 mg/kg, b.w, p.o.), or distilled water, and challenged with DSS (5%, w/v) or NaCl (0.9%, 5 mL/kg, b.w.) during the last 7 days of treatment. (A) H2O + NaCl, (B) DSS (5%, w/v) + H2O, (C) DSS + AEUD-50 mg/kg, (D) DSS + AEUD-100 mg/kg, (E) DSS + AEUD-200 mg/kg, (F) DSS + MESA (100 mg/kg, b.w., p.o.). The negative control group maintained normal colon morphology, whereas the DSS group showed multifocal areas of mucosal erosions with the loss of epithelial cells and inflammatory cell infiltration, edema, and ulceration (black arrows), (magnification ×40).
The colon tissues of the DSS (5%)-treated group were characterized by moderate to severe mucosal and submucosal inflammation along with infiltration of inflammatory cells, edema, and some ulcerations compared to normal tissue sections.
There was no significant difference between pretreated groups with MESA, AEUD (100 mg/kg), and AEUD (200 mg/kg); they showed mild erosion and mild focal mucosal and submucosal inflammatory cell infiltration, which were less severe findings compared to the colitis group and effectively reduced the signs of inflammation and retained the structural integrity with minimal pathological damage in colon tissue.
3.4. Biochemical Measures
As shown in , the plasma levels of inflammation marker (CRP), amylase, urea, creatinine, LDH, AST, ALT, and ALP were significantly higher in the DSS group than in the control group. In fact, compared with the control group, the CRP level in the DSS-treated group was significantly enhanced from 0.37 ± 0.08 mg/L to 2.05 ± 0.15 mg/L. With treatment with various doses of AEUD, the level of this inflammatory marker was reduced significantly to 0.42 ± 0.05 mg/L but not dose-dependently. The results further showed that the amylase activity in the DSS group was greatly increased (1047 ± 40.66 U/L) compared to the normal group (225 ± 14.86 U/L). Compared with that in the DSS group, the activity of amylase in the DSS + AEUD groups showed an attenuation of the increasing amylase activity. The same actions were noticed following the administration of MESA. Compared with those in the control group, AST, ALT, and ALP levels in the DSS-treated group were significantly elevated. In the AEUD treatment groups, these levels were lower than those in the DSS group and close to those in the control group. Added to that, we observed that treatment with AEUD greatly reduced the glycemic rise from 7.87 ± 0.42 mmol/L in the group of rats treated with DSS to 5.48 ± 0.29 mmol/L in the group treated with the higher dose of AEUD (200 mg/kg, BW, p.o.). Despite this, our outcomes indicated that the remaining parameters such as TC, TG, HDL, LDL, sodium, and potassium were not significantly changed in the DSS, MESA, and AEUD-treated groups versus the control group.
3.5. Hematological Parameters
The amount of platelets was lower in the DSS group of rats (557.33 ± 23.54 mEq/L) compared to the control group (903.5 ± 55.86 mEq/L). Similar to MESA, AEUD at various concentrations significantly increased the platelet amounts in the blood and were close to those in the control group. Furthermore, compared with the control treatment, DSS significantly upregulated the WBC concentration from 10.2 ± 0.28 to 14.96 ± 0.47 mg/L. While similar to MESA, AEUD at various concentrations significantly downregulated the WBC blood level . However, there were no improvements in Hb, Hct, erythrocytes, MCV, MCH, and MCHC.
3.6. Effects of DSS and AEUD on MDA and H2O2 Accumulation
DSS administration significantly (p < 0.05) increased the mean MDA level (54.06 ± 3.03 pmoles/mg protein) compared to that in normal rats (19.33 ± 2.90 pmoles/mg protein). Animals treated with MESA (100 mg/kg, BW, p.o.) and AEUD, especially at a dose of 100 mg/kg, exhibited a significant decrease in mean MDA level compared to colitis animals (26.04 ± 2.27 and 25.13 ± 2.96 pmoles/mg protein, respectively). Moreover, the hydrogen peroxide level was found to be raised significantly (p < 0.05) in colitis rats (1.02 ± 0.01 µmoles/mg protein) when compared with the negative control group (0.44 ± 0.04 µmoles/mg protein). Rats administered AEUD at the three doses of 50, 100, and 200 mg/kg and MESA exhibited significantly decreased levels of H2O2 compared to colitis rats (Figure 3A,B).
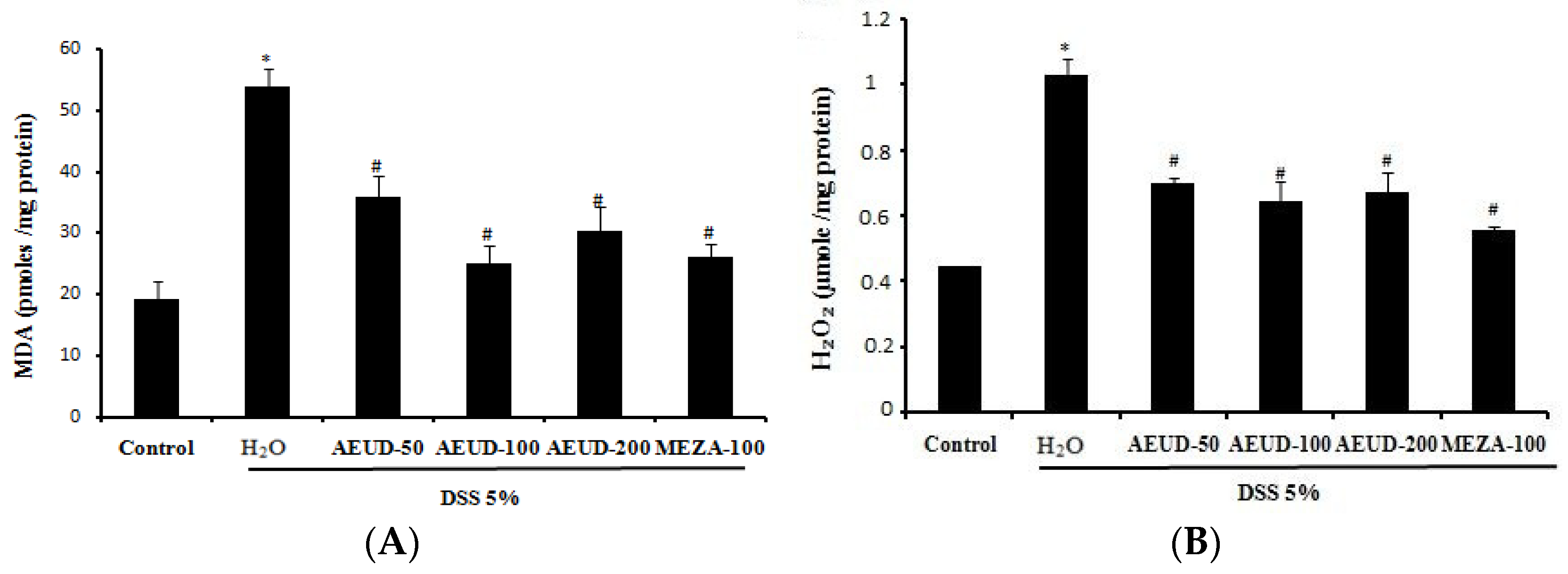
Figure 3. Effects of AEUD and MESA on dextran sulfate sodium (DSS)-induced changes in colonic mucosal MDA (A) and H2O2 (B) levels in rats. Animals were pre-treated with three doses of AEUD (50, 100, and 200 mg/kg, b.w, p.o.), MESA (100 mg/kg, b.w, p.o.), or distilled water, and challenged with DSS (5%, w/v) or NaCl (0.9%, 5 mL/kg, b.w.) during 7 days. The data are expressed as means ± S.E.M. (n = 6). *: p < 0.05 compared to control group. #: p < 0.05 compared to DSS group.
3.7. Effects of DSS and AEUD on Antioxidant Enzyme Activities
Figure 4 A–C represents the effects of AEUD at diverse doses (50, 100, and 200 mg/kg) and MESA on SOD, CAT, and GPx antioxidant activities in the colon tissues of all groups of animals. DSS-induced UC animals demonstrated significant depletions in antioxidative enzyme levels (0.53 ± 0.007 U/mg protein, 2.55 ± 0.19 µmoles H2O2/mg protein, and 0.28 ± 0.05 µmoles GSH/min/mg protein, respectively), revealing a depletion in free radical scavenging activity compared to the normal control group (1.11 ± 0.03 U/mg protein, 6.54 ± 0.13 µmoles H2O2/mg protein, and 0.48 ± 0.03 µmoles GSH/min/mg protein, respectively). Animals treated with AEUD at various doses and MESA at a single dose of 100 mg/kg demonstrated significant replenishment in mean SOD, CAT, and GPx levels compared to UC animals, indicating restoration of free radical scavenging activity. Dose-dependence was noticed only when observing the SOD and GPx activities.
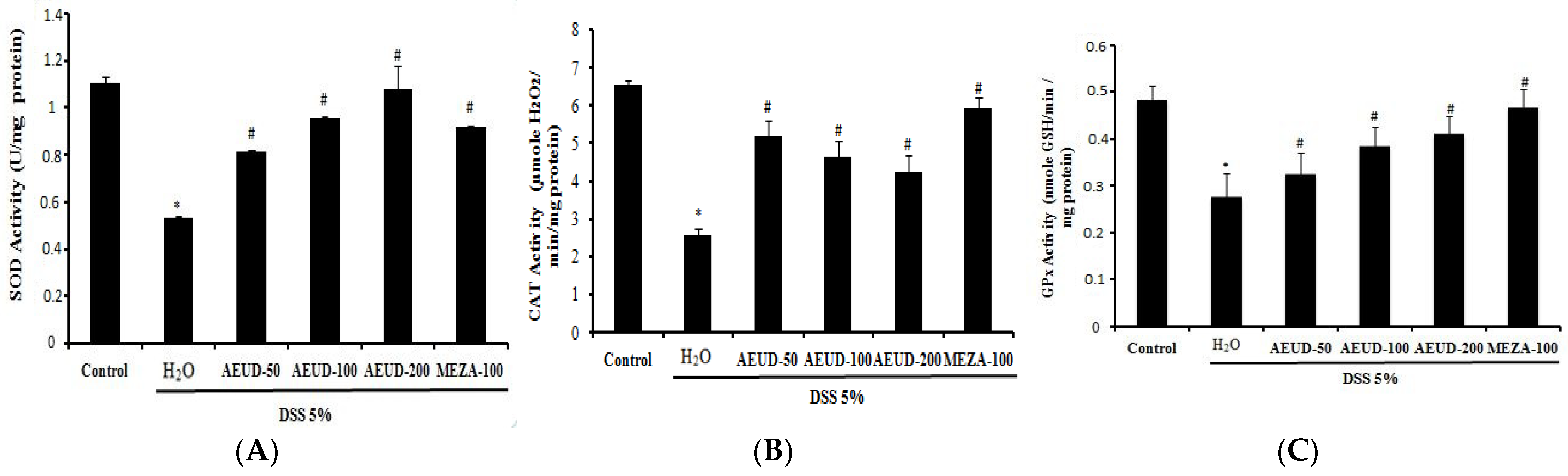
Figure 4. Effects of AEUD and MESA on colonic mucosal antioxidant enzyme activities: SOD (A), CAT (B), and GPx (C) during DSS–induced colitis in rats. Animals were pre-treated with three doses of AEUD (50, 100, and 200 mg/kg, b.w, p.o.), MESA (100 mg/kg, b.w, p.o.), or distilled water, and challenged with DSS (5%, w/v) or NaCl (0.9%, 5 mL/kg, b.w.) during 7 days. The data are expressed as means ± S.E.M. (n = 6). *: p < 0.05 compared to control group. #: p < 0.05 compared to DSS group.
4. Discussion
UC is an inflammatory disorder of the colon with a complicated etiology. 5-ASA, often known as mesalazine, is one of the most commonly used medicines in the treatment of UC. However, these treatments have the potential to have negative side effects. Consequently, there is growing interest in using the numerous natural components found in traditional herbs. In this respect, the prophylactic coloprotective action of the total extract of Urtica dioica leaves was investigated in DSS-induced UC in rats.
Firstly, we evaluated the phytochemical and mineral composition of AEUD which has been used as a natural remedy for ages [35]. The current results exhibit that AEUD is rich in bioactive compounds including phenolic compounds, tannins, flavonoids, and sugar. Furthermore, AEUD contains high mineral levels such as those of magnesium, iron, and zinc. These data are similar to those of previous studies which confirm that stinging nettle’s leaves are becoming more well-known because they contain a wide range of chemical components such as flavonoids, phenolic compounds, organic acids, minerals, and vitamins [36], as well as tannins, fatty acids, volatile compounds, polysaccharides, isolectins, sterols, terpenes, and proteins [37,38].
Secondly, the current study was designed to investigate the protective effects of AEUD in a DSS-induced ulcerative colitis model. The DSS–induced colitis model is one of the most widely used models. It can potentially cause damage to intestinal epithelial cells (IECs) [39]. The resulting injury has symptoms and characteristics similar to UC in humans [40]. In this regard, rats treated with DSS in their drinking water demonstrated a significant decrease in clinical parameters such as food intake, water consumption, body weight gain, and colonic length/weight due to severe tissue edema, necrosis, goblet cell hyperplasia, and inflammatory cell infiltration [41], but an increase in the DAI. MESA and AEUD treatment significantly improved these symptoms. Previously, many studies have shown the benefits of pure polyphenols or polyphenol-rich extracts in preventing colonic length/weight decreases in colitis rats, which is indicative of the therapeutic efficacy of prospective anti-ulcerative medicines [42,43].
DSS control rats had mild to severe mucosal and submucosal inflammation, inflammatory cell infiltration, edema, and some ulcerations as compared to normal tissue sections. On the other hand, the colonic tissues of the rats treated with AEUD (50 mg/kg) were determined to have normal histological structure.
When compared to the colitis group, the MESA or AEUD (100 mg/kg and 200 mg/kg) pretreated groups demonstrated mild erosion, mild focal mucosal inflammation, and mild submucosal inflammation. A previous investigation confirms that the administration of dextran alone does not result in any symptomatology in mice; it is actually the sulfate groups that are responsible for DSS toxicity [44]. In fact, DSS is responsible for altering the intestinal barrier integrity which disturbs the intestinal microbiome and homeostasis of intestinal immunity. The activation of the intestinal immune system and the migration of inflammatory cells into the intestine contribute to the maintenance of inflammation and intestinal lesions [45,46]. Many studies have demonstrated that the administration of several antioxidant agents reduces the severity of DSS-induced epithelial damage [47], mucosal inflammation, and erosion of surface epithelial cells [48,49].
AST and ALT levels were increased by DSS administration; however, AEUD reverted this rise in the DSS-treated rats. Cellular enzyme leakage into plasma is a well-known indicator of hepatic injury in conjunction with liver damage. Increased levels of these enzymes are reliable indicators of liver function because they show increased permeability, injury, and/or necrosis of hepatocytes [50].
CRP and WBCs are commonly used as markers of inflammation. We found an increase in these parameters in DSS-treated mice. In contrast, AEUD demonstrated great efficacy in preventing DSS-enhanced inflammatory mediators. In fact, CRP acts as an opsonin and activates complement, which causes the phagocytosis of bacterial and nuclear material. Therefore, CRP plays a crucial role in the innate immune system of the host and in the defense against autoimmunity [51]. High serum CRP levels in UC correlate well with disease activity and other inflammatory indicators such as WBCs. In a retrospective single-center cohort study, a higher WBC count at diagnosis was found to be associated with colectomy, underscoring the importance of WBCs in UC [52]. Many studies have found clinical cases of acute idiopathic pancreatitis and chronic pancreatitis related to IBD [53]. These findings demonstrated that pretreatment with AEUD or MESA reduced hyperamylasemia in DSS-treated rats. Increased permeability of the inflamed mucosa may be the cause of the pancreatic enzyme rise found in more severe or active disease; this is a mechanism previously suggested in persons with intestinal infarction who also have elevated serum amylase levels [54].
We found that AEUD significantly reduced the elevated glycemic level due to its ability to control blood sugar [55]. According to several studies, nettle enhances the release of insulin, which decreases blood sugar levels. This was demonstrated by examining diseased and healthy rats following intraperitoneal treatment with Urtica dioica aqueous extract [56].
The leaves of Urtica dioica have been reported to show anti-inflammatory properties and can be used to treat persistent inflammatory conditions. In this context, previous studies showed that biosynthesis of the arachidonic acid cascade enzymes, particularly the cyclooxygenases COX-1 and COX-2, was inhibited by leaf extracts, which reduced the formation of prostaglandins and thromboxane [57]. Furthermore, the PAF (platelet activating factor) system, inflammatory response, and antioxidant reaction are inhibited. The NF-κB system, which is implicated in immunity, is also affected [19,58]. In addition, a number of studies have demonstrated that leaf extracts block the release of interleukins IL-2 and IL-1, interferon (IFN), and the tumor necrosis factors (TNF) [59,60].
The ability of AEUD to combat oxidative damage and inflammation may be responsible for its protective action against liver injury related to oxidative damage [18]. Despite this, ROS are known to be beneficial species; however, excessive generation alters the redox balance and results in an oxidative stress state. In this context, it has been noted that an excessive amount of ROS is produced in subjects with UC. Hence, chronic oxidative stress has been demonstrated to have a significant impact on the persistence and etiology of ulcerative colitis [61]. In our investigation, exposure to DSS was accompanied by colonic oxidative damage that showed up as an increase in the H2O2 levels compared to the control group. Treatment with MESA and AEUD has proven to be useful in preventing colonic ROS excess caused by DSS intoxication. In fact, free radicals are molecules created by cellular metabolism, which can be destructive to biological tissues and cause injury to DNA, lipids, cell membranes, and proteins. It is commonly recognized that reactive oxygen species, especially the hydroxyl radical, contribute significantly to inflammation by causing membrane lipid peroxidation, which causes severe cellular damage [62]. Several studies have demonstrated that leaf extracts exhibit an antioxidant action by scavenging the DPPH radical (1,1-diphenyl-2-picrylhydrazyl). The majority of this antioxidant activity is caused by the presence of phenolic compounds [63,64].
One of the main causes of tissue lipid peroxidation is the excessive generation of ROS [65]. Our findings demonstrate that the colons of DSS control animals had higher MDA levels, whereas the administration of AEUD or MESA considerably decreased them. This shows that AEUD’s active ingredient has a protective impact that is remarkably similar to MESA’s protective effect in reducing oxidative damage. Our data are consistent with a number of previous reports that show that the antioxidant effect of AEUD is mostly due to the presence of phenolic compounds [63,64].
The activities of the main antioxidant enzymes SOD, CAT, and GPx were evaluated, since the occurrence of UC is significantly influenced by oxidative stress and inflammatory reactions [66]. SOD levels, GPx levels, and CAT activities dropped in the group treated with DSS, which could access mucosal cells via pinocytosis, causing cellular oxidation and disruption of the enzymatic antioxidant defense mechanism [67]. These levels increased and were comparable to those of the control group in the groups treated with AEUD and MESA. These outcomes are in accordance with the findings confirming that GPx, SOD, and CAT activities decreased in the colitis group under the action of the Urtica dioica antioxidant compounds [68,69,70,71].
5. Conclusions
The findings of this study demonstrate that AEUD improves colonic inflammation and oxidative stress in DSS-induced colitis by decreasing CRP levels, promoting the main antioxidant enzyme activities, and reducing colonic lipid peroxidation and H2O2 levels. Ultimately, AEUD alleviates DSS-induced colitis in rats and shows significant potential for bioactive compounds as a source of appropriate agents for the treatment of UC. However, further studies would help establish the local anti-inflammatory potential of the extract by evaluating the effect of AEUD on mediators of inflammation expressed in colonic tissues in colitis.
References
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative Colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M. Intestinal Epithelium in Inflammatory Bowel Disease. Front. Med. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42, quiz e30. [Google Scholar] [CrossRef]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Genes and Environment: How Will Our Concepts on the Pathophysiology of IBD Develop in the Future? Dig. Dis. 2010, 28, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of Disease: Pathogenesis of Crohn’s Disease and Ulcerative Colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kang, J.H.; Song, K.H.; Park, M.S.; Kim, D.K.; Park, Y.J.; Choi, C.; Kim, H.M.; Kim, M.K.; Oh, S.H. Inonotus Obliquus Suppresses Proliferation of Colorectal Cancer Cells and Tumor Growth in Mice Models by Downregulation of β-Catenin/NF-κB-Signaling Pathways. Eur. J. Inflamm. 2013, 11, 615–629. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kang, J.-H.; Kim, D.-K.; Oh, S.H.; Kim, M.K. Orally Administered Aqueous Extract of Inonotus Obliquus Ameliorates Acute Inflammation in Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. J. Ethnopharmacol. 2012, 143, 524–532. [Google Scholar] [CrossRef]
- Kang, J.-H.; Jang, J.-E.; Mishra, S.K.; Lee, H.-J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol Peroxide from Chaga Mushroom (Inonotus obliquus) Exhibits Anti-Cancer Activity by down-Regulation of the β-Catenin Pathway in Colorectal Cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Luo, H.; Wang, N.; Li, J.; Xu, S.; Chen, K.; Feng, J.; Wu, L.; Li, S.; Liu, T.; et al. Portulaca Extract Attenuates Development of Dextran Sulfate Sodium Induced Colitis in Mice through Activation of PPARγ. PPAR Res. 2018, 2018, 6079101. [Google Scholar] [CrossRef] [PubMed]
- Duijvestein, M.; Battat, R.; Vande Casteele, N.; D’Haens, G.R.; Sandborn, W.J.; Khanna, R.; Jairath, V.; Feagan, B.G. Novel Therapies and Treatment Strategies for Patients with Inflammatory Bowel Disease. Curr. Treat. Options Gastroenterol. 2018, 16, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Im, J.P.; Ye, B.D.; Kim, Y.S.; Kim, J.S. Changing Treatment Paradigms for the Management of Inflammatory Bowel Disease. Korean J. Intern. Med. 2018, 33, 28–35. [Google Scholar] [CrossRef]
- Ko, J.K.; Auyeung, K.K. Inflammatory Bowel Disease: Etiology, Pathogenesis and Current Therapy. Curr. Pharm. Des. 2014, 20, 1082–1096. [Google Scholar] [CrossRef]
- Sakthivel, K.M.; Guruvayoorappan, C. Amentoflavone Inhibits iNOS, COX-2 Expression and Modulates Cytokine Profile, NF-κB Signal Transduction Pathways in Rats with Ulcerative Colitis. Int. Immunopharmacol. 2013, 17, 907–916. [Google Scholar] [CrossRef]
- Said, A.A.H.; Otmani, I.S.E.; Derfoufi, S.; Benmoussa, A. Highlights on Nutritional and Therapeutic Value of Stinging Nettle (Urtica dioica). Int. J. Pharm. Pharm. Sci. 2015, 7, 8–14. [Google Scholar]
- Devkota, H.P.; Paudel, K.R.; Khanal, H.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Abu-Reidah, I.M.; Altamimi, A.; Jaradat, N. Hydroethanolic Extract of Urtica dioica L. (Stinging Nettle) Leaves as Disaccharidase Inhibitor and Glucose Transport in Caco-2 Hinderer. Molecules 2022, 27, 8872. [Google Scholar] [CrossRef]
- Abdeltawab, A.A.; Ullah, Z.; Al-Othman, A.M.; Ullah, R.; Hussain, I.; Ahmad, S.; Talha, M. Evaluation of the Chemical Composition and Element Analysis of Urtica dioca. Afr. J. Pharm. Pharmacol. 2012, 6, 1555–1558. [Google Scholar] [CrossRef]
- Wang, J.; Pantopoulos, K. Regulation of Cellular Iron Metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kali, G. Study on Morpho-Anatomical and Histo-Chemical Charaterisation of Stinging Nettle, Urtica dioica L. in Uttarakhand, India. J. Pharmacogn. Phytochem. 2019, 8, 4325–4331. [Google Scholar]
- Flórez, M.; Cazón, P.; Vázquez, M. Antioxidant Extracts of Nettle (Urtica dioica) Leaves: Evaluation of Extraction Techniques and Solvents. Molecules 2022, 27, 6015. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-López, I.; Pérez, M.; Lamuela-Raventós, R.M. Total (poly)phenol analysis by the Folin-Ciocalteu assay as an anti-inflammatory biomarker in biological samples. Crit. Rev. Food Sci. Nutr. 2023, 7, 1–7. [Google Scholar] [CrossRef]
- Medini, M.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Parc, A.L.; Lee, H.; Chen, K.; Barile, D. Rapid Quantification of Functional Carbohydrates in Food. Prod. Food Nutr. Sci. 2014, 5, 71–78. [Google Scholar] [CrossRef]
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Sebai, H.; Marie, J.C.; Amri, M.; Marzouki, L.; El-Bennad, J. Preventive effect of carob (Ceratonia siliqua L.) in dextran sulfate sodium-induced ulcerative colitis in rat. RSC Adv. 2016, 6, 19992–20000. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Barnese, K.; Gralla, E.B.; Cabelli, D.E.; dan Valentine, J.S. The Role of Superoxide Anion in The Autoxidation of Epinephrine and A Simple Assay for Superoxide Dismutase. J. Am. Chem. Soc. 2008, 130, 4604–4606. [Google Scholar] [CrossRef]
- Selmi, S.; El-Fazaa, S.; Gharbi, N. Oxidative stress and alteration of biochemical markers in liver and kidney by malathion in rat pups. Toxicol. Ind. Health 2015, 31, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Abidi, A.; Nahdi, A.; Abdennabi, R.; Mlika, M.; Ben Ali, R.; Jameleddine, S. Olea europaea L. Leaf Extract Alleviates Fibrosis Progression and Oxidative Stress Induced by Bleomycin on a Murine Model of Lung Fibrosis. Dose Response 2023, 21, 15593258231200972. [Google Scholar] [CrossRef]
- Dingeon, B.; Ferry, J.P.; Roullet, A. Automatic assay of blood sugar by Trinder’s method. Ann. Biol. Clin. 1975, 33, 3–13. [Google Scholar]
- Stucchi, A.F.; Shofer, S.; Leeman, S.; Materne, O.; Beer, E.; McClung, J.; Shebani, K.; Moore, F.; O’Brien, M.; Becker, J.M. NK-1 Antagonist Reduces Colonic Inflammation and Oxidative Stress in Dextran Sulfate-Induced Colitis in Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1298–G1306. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of Pharmacological Uses of Urtica dioica and Others Benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef]
- Maietti, A.; Tedeschi, P.; Catani, M.; Stevanin, C.; Pasti, L.; Cavazzini, A.; Marchetti, N. Nutrient Composition and Antioxidant Performances of Bread-Making Products Enriched with Stinging Nettle (Urtica dioica) Leaves. Foods 2021, 10, 938. [Google Scholar] [CrossRef]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica dioica L.): A Review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.K. DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef]
- El-Abhar, H.S.; Hammad, L.N.A.; Gawad, H.S.A. Modulating Effect of Ginger Extract on Rats with Ulcerative Colitis. J. Ethnopharmacol. 2008, 118, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Romier, B.; Schneider, Y.-J.; Larondelle, Y.; During, A. Dietary Polyphenols Can Modulate the Intestinal Inflammatory Response. Nutr. Rev. 2009, 67, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.H.; Murakami, A.; Tanaka, T.; Ohigashi, H. Dietary Rutin, but Not Its Aglycone Quercetin, Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice: Attenuation of pro-Inflammatory Gene Expression. Biochem. Pharmacol. 2005, 69, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran Sodium Sulfate Colitis Murine Model: An Indispensable Tool for Advancing Our Understanding of Inflammatory Bowel Diseases Pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.-E.; Conklin, L.S.; Centola, M.; Li, X. Distinct Cytokine Patterns Identified from Multiplex Profiles of Murine DSS and TNBS-Induced Colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Wéra, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef]
- Rajendiran, V.; Natarajan, V.; Devaraj, S.N. Anti-Inflammatory Activity of Alpinia Officinarum Hance on Rat Colon Inflammation and Tissue Damage in DSS Induced Acute and Chronic Colitis Models. Food Sci. Human. Wellness 2018, 7, 273–281. [Google Scholar] [CrossRef]
- Mazzon, E.; Esposito, E.; Di Paola, R.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Effects of Verbascoside Biotechnologically Produced by Syringa Vulgaris Plant Cell Cultures in a Rodent Model of Colitis. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 79–94. [Google Scholar] [CrossRef]
- Huang, T.-C.; Tsai, S.-S.; Liu, L.-F.; Liu, Y.L.; Liu, H.-J.; Chuang, K.P. Effect of Arctium Lappa L. in the Dextran Sulfate Sodium Colitis Mouse Model. World J. Gastroenterol. 2010, 16, 4193–4199. [Google Scholar] [CrossRef]
- Fernández, G.; Mena, M.P.; Arnau, A.; Sánchez, O.; Soley, M.; Ramírez, I. Immobilization Stress Induces C-Fos Accumulation in Liver. Cell Stress. Chaperones 2000, 5, 306–312. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.H.; Wright, T.T.; Shen, Z.Y.; Li, H.Y.; Zhu, W.; Potempa, L.A.; Ji, S.R.; Szalai, A.J.; Wu, Y. C-Reactive Protein Directly Suppresses Th1 Cell Differentiation and Alleviates Experimental Autoimmune Encephalomyelitis. J. Immunol. 2015, 194, 5243–5252. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; Thompson, K.; Lafleur, B.; Book, L.S.; Jackson, W.D.; O’Gorman, M.A.; Black, R.E.; Downey, E.; Johnson, D.G.; Matlak, M.E.; et al. Clinical Variables as Prognostic Tools in Pediatric-Onset Ulcerative Colitis: A Retrospective Cohort Study. Inflamm. Bowel Dis. 2011, 17, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.T.; Hoque, A.; Pasam, R.T.; Farhoud, A.; Abdelfattah, A.; Ramadorai, V.; Chaudrey, K.; Pellish, R. Acute idiopathic pancreatitis is associated with more aggressive disease course in Crohn’s disease but not in ulcerative colitis. BMC Gastroenterol. 2023, 23, 171. [Google Scholar] [CrossRef]
- Bellocchi, M.C.C.; Crinò, S.F.; De Marchi, G.; De Pretis, N.; Ofosu, A.; Caldart, F.; Ciccocioppo, R.; Frulloni, L. A Clinical and Pathophysiological Overview of Intestinal and Systemic Diseases Associated with Pancreatic Disorders: Causality or Casualty? Biomedicines 2023, 11, 1393. [Google Scholar] [CrossRef]
- Ranjbari, A.; Azarbayjani, M.A.; Yusof, A.; Halim Mokhtar, A.; Akbarzadeh, S.; Ibrahim, M.Y.; Tarverdizadeh, B.; Farzadinia, P.; Hajiaghaee, R.; Dehghan, F. In vivo and in vitro Evaluation of the Effects of Urtica dioica and Swimming Activity on Diabetic Factors and Pancreatic Beta Cells. BMC Complement. Altern. Med. 2016, 16, 101. [Google Scholar] [CrossRef]
- Farzami, B.; Ahmadvand, D.; Vardasbi, S.; Majin, F.J.; Khaghani, S. Induction of Insulin Secretion by a Component of Urtica dioica Leave Extract in Perifused Islets of Langerhans and Its in vivo Effects in Normal and Streptozotocin Diabetic Rats. J. Ethnopharmacol. 2003, 89, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Roschek, B.; Fink, R.C.; McMichael, M.; Alberte, R.S. Nettle Extract (Urtica dioica) Affects Key Receptors and Enzymes Associated with Allergic Rhinitis. Phytother. Res. 2009, 23, 920–926. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Khoshgozaran, L. Antinociceptive and Anti-Inflammatory Activities of Hydroethanolic Extract of Urtica dioica. Int. J. Biol. Pharm. Allied Sci. 2015, 1, 160–170. [Google Scholar]
- Konrad, L.; Müller, H.H.; Lenz, C.; Laubinger, H.; Aumüller, G.; Lichius, J.J. Antiproliferative Effect on Human Prostate Cancer Cells by a Stinging Nettle Root (Urtica dioica) Extract. Planta Med. 2000, 66, 44–47. [Google Scholar] [CrossRef]
- Yilmaz, B.; Basar, O.; Aktas, B.; Altinbas, A.; Ekiz, F.; Büyükcam, F.; Albayrak, A.; Ginis, Z.; Oztürk, G.; Coban, S.; et al. Effects of Urtica dioica Extract on Experimental Acute Pancreatitis Model in Rats. Int. J. Clin. Exp. Med. 2014, 7, 1313–1318. [Google Scholar]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Jabri, M.-A.; Aissani, N.; Tounsi, H.; Sakly, M.; Marzouki, L.; Sebai, H. Protective Effect of Chamomile (Matricaria recutita L.) Decoction Extract against Alcohol-Induced Injury in Rat Gastric Mucosa. Pathophysiology 2017, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Majedi, S.; Abdulsattar Faraj, T.; Jalal Ahmed, H.; HS Hussain, F. A Review of Biochemical Structures of Urtica dioica Metabolites and Their Pharmaceutical Effects. Chem. Rev. Lett. 2021, 4, 206–212. [Google Scholar]
- Kanter, M.; Coskun, O.; Budancamanak, M. Hepatoprotective Effects of Nigella sativa L and Urtica dioica L on Lipid Peroxidation, Antioxidant Enzyme Systems and Liver Enzymes in Carbon Tetrachloride-Treated Rats. World J. Gastroenterol. 2005, 11, 6684–6688. [Google Scholar] [CrossRef] [PubMed]
- Rtibi, K.; Grami, D.; Wannes, D.; Selmi, S.; Amri, M.; Sebai, H.; Marzouki, L. Ficus Carica Aqueous Extract Alleviates Delayed Gastric Emptying and Recovers Ulcerative Colitis-Enhanced Acute Functional Gastrointestinal Disorders in Rats. J. Ethnopharmacol. 2018, 224, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Mahmoud, S.M.; Amin, H.K.; Abdel Moneim, A.E. Ziziphus Spina-Christi Fruit Extract Suppresses Oxidative Stress and P38 MAPK Expression in Ulcerative Colitis in Rats via Induction of Nrf2 and HO-1 Expression. Food Chem. Toxicol. 2018, 115, 49–62. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Samman, F.S.; Elaidy, S.M.; Essawy, S.S.; Hassan, M.S. New Insights on the Modulatory Roles of Metformin or Alpha-Lipoic Acid versus Their Combination in Dextran Sulfate Sodium-Induced Chronic Colitis in Rats. Pharmacol. Rep. 2018, 70, 488–496. [Google Scholar] [CrossRef]
- Petronilho, F.; Michels, M.; Danielski, L.G.; Goldim, M.P.; Florentino, D.; Vieira, A.; Mendonça, M.G.; Tournier, M.; Piacentini, B.; Giustina, A.D.; et al. Diphenyl Diselenide Attenuates Oxidative Stress and Inflammatory Parameters in Ulcerative Colitis: A Comparison with Ebselen. Pathol. Res. Pract. 2016, 212, 755–760. [Google Scholar] [CrossRef]
- Ghasemi, S.; Moradzadeh, M.; Mousavi, S.H.; Sadeghnia, H.R. Cytotoxic Effects of Urtica dioica Radix on Human Colon (HT29) and Gastric (MKN45) Cancer Cells Mediated through Oxidative and Apoptotic Mechanisms. Cell. Mol. Biol. 2016, 62, 90–96. [Google Scholar]
- Gülçin, I.; Küfrevioglu, O.I.; Oktay, M.; Büyükokuroglu, M.E. Antioxidant, Antimicrobial, Antiulcer and Analgesic Activities of Nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]

