1. Introduction
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterized by social and behavioral deficits, impairment in verbal and nonverbal communication, and restricted, repetitive behaviors and interests [1]. According to the World Health Organization, the global prevalence of ASD in 2022 was 1 per 36 people [2]. The etiology of ASD remains elusive. A combination of multiple etiologies has been suggested, including genetic, epigenetic, immunologic, environmental [3,4], and metabolic abnormalities [5]. Mitochondrial dysfunction, neuroinflammation, and glutamate excitotoxicity are the most strongly implicated etiologies that correlate with the severity of ASD symptoms [6].
A growing body of evidence indicates that children with ASD experience motor function deficits not often seen in their typically developing (TD) counterparts [7]. Motor impairments, including deficits in gross and fine motor skills [8,9], may limit the participation of ASD children in sports and other physical activities (PAs) [10,11,12]. Low levels of physical activity, late motor skills, and fitness, especially in children and adolescents with ASD, may exacerbate social and emotional deficits and related comorbidities [13]. In addition, an association between the severity of motor and social impairments has been reported in children with ASD [14,15,16,17], suggesting that social impairments may also limit ASD children’s participation in PA.
PA, especially moderate and vigorous activities (MVPAs), is important for promoting health and overall quality of life among children, including children with ASD [18,19,20]. Recently, a growing interest in understanding the relationship between social and cognitive features of ASD and physical health outcomes has been widely noted [21]. Previous studies revealed that behavioral functioning in ASD children improved after performing some PA [22,23]. PA has been shown to effectively lower the frequency of stereotypical behavior episodes in children with ASD. It is believed that PA can cause significant changes in brain structure, function, and cognition in ASD children [24]. Long-term physical exercise modifies the structure of motor areas such as the cerebellum and motor cortex, as well as parts of the hippocampus, which is critical for learning, memory, and navigation [25].
Some studies reported that autistic people typically exhibit a reduction in physical activity due to social and behavioral abnormalities. Limited opportunity for exercise impacts the behavior of autistic children, leading to chronic illnesses, such as obesity, which is common in autistic patients [10,26,27]. Furthermore, it has been reported that age and gender may influence the outcome of PA reduction [10,28,29]. Specifically, ASD children’s physiological, cognitive, psychosocial, and behavioral functioning have been found to benefit from moderate to vigorous physical activity (MVPA) [30].
Since the effects of PA on cognition have important implications for improving performance in ASD children, accurate assessment of PA levels is important for studying PA patterns in ASD children. Previous studies have used accelerometer-based activity monitors and self-reported questionnaires to evaluate the level of PA. However, accelerometers have been widely accepted as a more reliable tool for the assessment of a range of PA types in a free-living environment [24,31,32].
One of the most widely used devices in PA research is the ActiGraph monitor (GT3X+), which captures children’s total body movements and free-play activities, including sitting, standing, walking, walking up the stairs, running, and cycling. It can accurately measure the orientation and immature motor movements of a child, making it suitable for the assessment of PA and sedentary activity in persons with disabilities [28,33]. For MVPA, moderate (3–5.99 METs) is defined as 2690–6166 counts/min and vigorous (6 METs) as 6167 counts/min. The total number of minutes of light-intensity physical activity is defined as the total number of minutes between 200 and 2690 counts/min [34].
To provide PA intervention programs aimed at enhancing health-related physical fitness on a daily, systematic, and individualized basis, it is essential to evaluate PA in people with ASD. The current study aimed to determine whether PA has a differential effect on children with ASD compared with their TD age-matched children. Our findings provide novel insights that should inform the development of effective interventional strategies.
2. Materials and Methods
2.1. Participants
Twenty-one children with autism aged 3–13 years (mean age 6.43 ± 2.29 years) were recruited from the Autism Research and Treatment Center, Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia. A diagnosis of autism was confirmed in all children using the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders. The exclusion criteria were comorbid ASD-related medical diseases or other neurological problems. The exclusion criteria were established by parent interviews and a review of the children’s medical records. The Childhood Autism Rating Scale (CARS), Social Responsiveness Scale (SRS), and sensory profile as measures of severity of the studied participants are presented in . It can be easily noticed that all are moderate–severe cases. For CARS, scores between 30 and 36.5 indicate mild to moderate autism, and scores from 37 to 60 indicate severe autism [34]. For SRS, 60–75 is considered mild to moderate, and a T-score >75 indicates severe impairment [35].
The control group was comprised of 30 age-matched TD children (mean age 7.2 ± 3.14 years) who attended the pediatric clinic of King Khalid University Hospital, Riyadh, Saudi Arabia, for routine follow-up. They had no clinical signs or symptoms indicative of neuropsychiatric disorders. Children with any neurological, endocrine, cardiovascular, pulmonary, liver, or kidney disease were excluded from the study.
Informed consent for the study was obtained from parents or legal guardians of the investigated subjects as approved by the ethical guidelines of medicine of King Saud University number 13/3945//IRB.
2.2. Physical Activity Measurement
Physical activity was measured using the ActiGraph GT3X+ accelerometer (ActiGraph, Pensacola, FL, USA). Parents or caregivers were instructed on how to operate the device and attach it to the child’s waist. Participants were instructed to wear the monitor during all activities except for swimming, showering/bathing, and during their sleep. Parents or guardians were provided with a log to record times when the accelerometer was not worn. Previous studies showed that at least 6 days of recordings were needed for accurate evaluation of sedentary behavior [36,37]. Participants kept the accelerometer for at least 7 consecutive days. To be included in the study, participants had to have accelerometer recordings for a minimum of 6 days, including at least 1 weekend day, and at least 10 h of recordings each day. Participants who did not meet these criteria were excluded from the study. Activity levels were stratified based on accelerometer counts per minute, according to the protocol described by Freedson [38]. Time spent in sedentary behavior, light-intensity physical activity, and moderate–vigorous physical activity (MVPA) was quantified using cut-point thresholds established specifically for preschool children.
2.3. Anthropometric Measurements
Participants’ height was measured in centimeters to the nearest tenth of a millimeter. Weight was measured in kilograms to the nearest gram, using an electronic scale (SECA S-214, Basel, Switzerland stadiometer). Measurements were taken twice for each participant, and mean values were recorded. BMI reference values have been set by the World Health Organization (WHO), and their formula was used to assess the quantity of fat in controls and children with autism [39]. The metabolic equivalent of tasks (METs) was estimated using the ActiGraph regression equation developed by Freedson et al. [38], which utilizes counts and age to estimate METs. In the current study, we selected ActiGraph cut points in preschool-age children to estimate metabolic equivalent. To estimate fat percent, the skin folds at the triceps and subscapular area were measured by Lange Skinfold Caliper. Waist and hip circumferences were measured using a measuring tape, and the data were used to calculate the hip-to-waist ratio. Muscle strength was estimated by measuring hand grip strength using Takei Hand Grip Dynamometer. All measurements were taken twice, and averages were recorded.
2.4. Statistical Analysis
Data analysis was performed using Statistical Package for Social Studies (SPSS) version 22 (IBM Corp., New York, NY, USA) unless otherwise indicated. Continuous variables were expressed as mean ± standard deviation. Significance of observed differences was evaluated using the t-test with normally distributed variables and the Wilcoxon Mann–Whitney test with non-normally distributed variables. Differences associated with a p-value < 0.05 were considered significant. Statistical power was estimated using G*Power version 3.1.9.4 [18]. Power was calculated for the study’s sample sizes (21 autism and 30 control) for each of the predictor variables. Correlations were computed using Spearman correlations (r), and a p-value was calculated to indicate the significance of the correlation, with a p-value < 0.05 indicating significance. When comparing correlation coefficients between groups, multiple computations were performed to determine whether observed differences between group correlation coefficients met statistical significance. First, Fisher z transformation was applied to the pair of correlation coefficients to be compared, r1 and r2, converting them to the Fisher z scores z1 and z2, respectively. This was performed using the equation shown below (1). A Z-test was then calculated by dividing the difference between z1 and z2 by the standard error of that difference, as shown below (2). A p-value was calculated, with values < 0.05 indicating statistical significance. Fisher z transformation and the Z-test were performed using the syntax written by Weaver and Wuensch (Weaver 2013) for SPSS.
3. Results
Demographic and anthropometric data are presented in . Twenty-one children with ASD and 30 age-matched TD children were included in this study. We found that METs were higher in ASD children compared with TD children (p = 0.001). All other demographic and anthropometric characteristics did not significantly differ between the two groups of children.
We observed a modest but statistically significant decrease in total sedentary bouts in ASD children. We did not observe any differences in total activity counts or total time spent (p = 0.957) in all types of PA between the two groups.
Our results revealed that there was a highly significant difference in total time spent in sedentary activity (p < 0.001) and in the total sedentary activity counts (p < 0.001) in the control group compared with the ASD group. The results also indicated that there were no significant differences between groups for the total counts and time spent in LPA and MPA. However, ASD children spent more time than TD engaging in VPA (p = 0.017).
The total counts on different axes showed that PA varied substantially depending on the axis. PA assessed by axis 2 (horizontal or forward and backward motion) showed a highly significant difference in ASD compared to TD (p = 0.001), whereas axis 1 (vertical or upward and downward motion) and axis 3 (lateral or left and right motion) showed no significant difference. Total vector magnitude counts (VM counts) were also significantly higher in the ASD group compared with TD (p = 0.024). Furthermore, counts per minute (CPMs) were significantly higher in ASD over all axes compared with TD (p = 0.001), as well as for the vector magnitude CPM (p ≤ 0.001) (Figure 1 and .
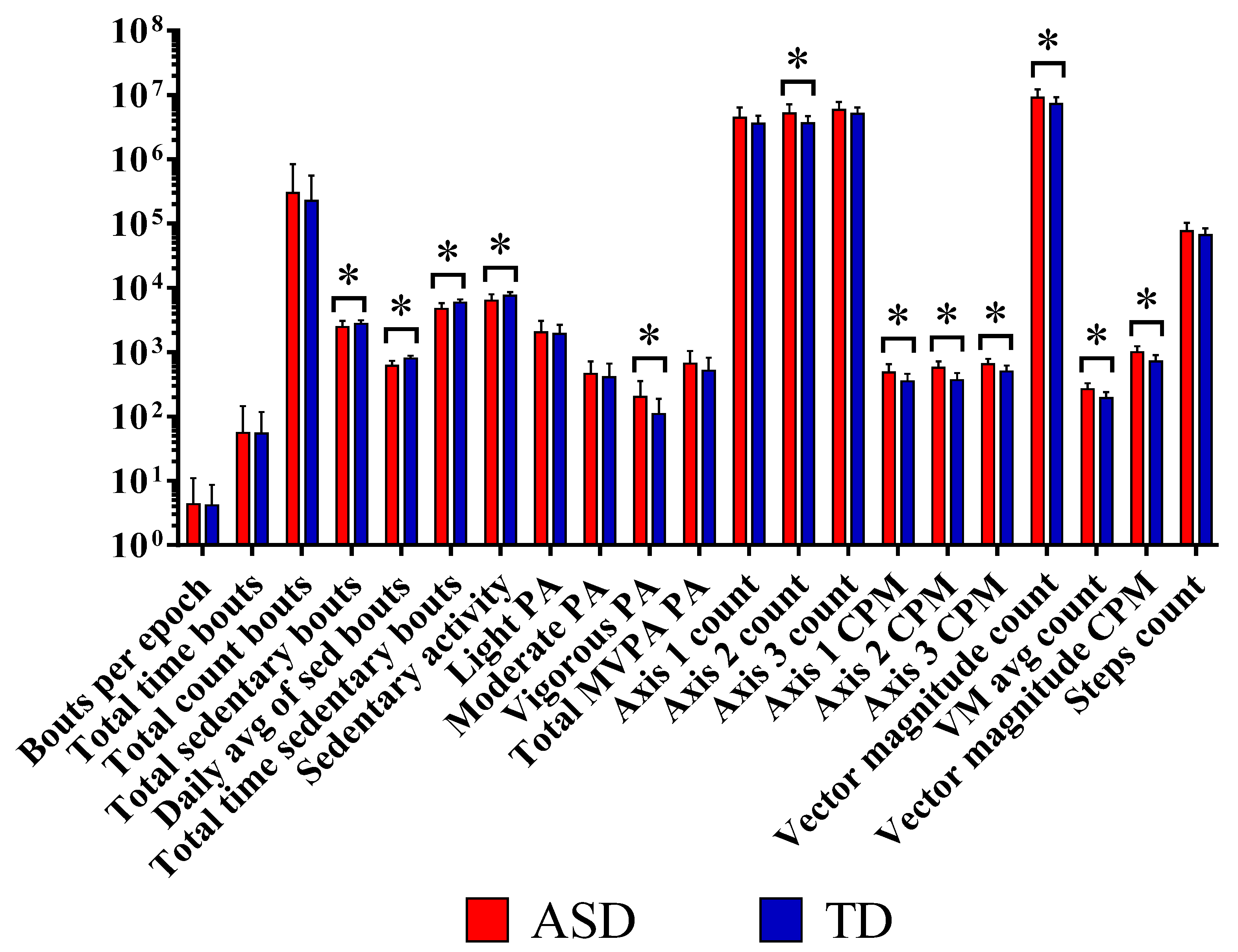
Figure 1. The step counts per day showed higher correlation with vigorous-intensity physical activity (r = 0.759; p = 0.001 and MVPA, r = 0.668; p = 0.001) than with light- (r = 0.552; p = 0.009) or moderate-intensity activity (r = 0.549; p = 0.01) among ASD participants. In the control group, the step counts per day were more highly correlated with moderate-intensity physical activity and MVPA (r = 0.633; p = 0.001, r = 0.664; p = 0.001, respectively) than with vigorous-intensity activity (r = 0.497; p = 0.005).
When the correlation between step counts and total energy expenditure in METs was calculated, the correlation was slightly higher in ASD than in the control group (r = 0.768, p = 0.001; 0.773, p = 0.001). * Significant correlations with p < 0.05.
4. Discussion
Research focusing on the association between ASD symptoms and PA has substantially increased over the last few years. Previous research demonstrated that PA has important implications for the improvement of some social, cognitive, and behavioral features [18,19,20,21]. To the best of our knowledge, the current study is the first to assess sedentary behavior and physical activity in Saudi ASD children.
The precise assessment of physical activity levels is crucial for understanding the association between active lifestyle and health, especially when evaluating the effectiveness of intervention programs [13,22]. By assessing the level of PA for autistic children, it is possible to develop sports programs that support the health of this group. In the current study, the overall time spent in PA and the total activity level did not differ significantly between ASD and controls. This result agrees with previous studies. Sandt and Frey reported no differences between ASD children and controls in any physical activity setting [40].
4.1. Sedentary Activity in ASD Participants
In this study, ASD children spent less time in sedentary activity (SA) than TD (p = 0.001). This finding is in line with some previous studies comparing children with different disabilities, including ASD, with TD children in Europe and North America. For example, it has been reported that young children with ASD are more active and spend significantly less time in sedentary behavior compared with the control group [26,41], suggesting that the PA differential between ASD and TD may be age-related [11,14,28]. In contrast, other studies reported that ASD children were less physically active and had an increased sedentary lifestyle compared with TD, as sedentary activity ASD children may experience impairments in movement, communication, social skills, and behavior [10,11].
Our data indicated that the light and moderate activity counts were not significantly different between the two groups, but vigorous activity was significantly higher in ASD compared with TD. This finding contrasts with previous studies [11,42,43]. Researchers demonstrated that ASD children spent less time in PA compared with TD children. This finding could be attributed to their characteristic stereotypical and self-stimulating behaviors. They concentrate on negative habits, which prevent them from engaging in physical activity. It was noticed that the time spent in MVPA did not differ significantly between the studied groups (p = 0.132). It only accounts for a small amount of time in the day, and the majority of the time was spent in LPA and sedentary behavior.
shows a significantly stronger correlation between daily step counts and all physical activity intensities (time spent in light, moderate, and vigorous activity) in the ASD group than in the control group, despite the fact that there was no statistically significant difference between the two groups in terms of step counts. This result shows that the group with higher step counts spent a lot more time being physically active.
4.2. Vigorous PA in ASD Patients
It would be intriguing to investigate the association between increased vigorous PA in ASD patients compared with TD children and unbalanced excitatory/inhibitory neurotransmission, oxidative stress, and neuroinflammation as ASD etiological processes related to hyperactivity [44,45]. Individuals with autism have substantially greater glutamate, the primary excitatory neurotransmitter, and much lower gamma amino butyric acid (GABA), the primary inhibitory neurotransmitter [6,46]. Under normal physiological conditions, released glutamate is metabolized or taken up by neighboring astrocyte cells through glutamate transporters. When these pathways are disturbed, as in ASD, glutamate builds up and overexcites the N-methyl-D-aspartate (NMDA) receptors. These receptors, when triggered by excessive glutamate, function as a Ca2+ (calcium ion) channel. Because Mg2+ (magnesium) blocks the channel, these channels only operate when the cell membrane is depolarized. In ASD, the membrane is chronically depolarized, Mg2+ exits the channel, and Ca2+ influx is unrestricted for longer periods of time, leading to cell death via free radicals [47] or through mitochondrial overload, which results in free radical formation. Inflammatory mediators, increased oxidative stress, and decreased levels of brain-derived neurotrophic factor (BDNF) and other growth factors have more or less similar effects on glutamate microcircuits in ASD patients, regardless of whether the origin is centrally or peripherally derived. When mitochondria become damaged and electron leakage increases, ROS generation increases [48]. Theoretically, the higher PA in ASD patients could be due to the idea that the brain can use elevated glutamate as an energy source to dispose of the neurotransmitter’s excess levels. This explanation can find support by considering the fact that NMDA receptor antagonists reduce channel permeability and inhibit Ca2+ influx, providing neuroprotection, amending glutamate excitotoxicity, and perhaps ameliorating hyperactivity symptoms [44,49].
4.3. Vigorous PA and Sleep Disruptions in ASD Patients
Sleep disruptions are one of the most common comorbidities reported in ASD children, occurring in up to 80% of ASD children compared with 20–40% of typically developing children [50,51,52], and include insomnia, circadian rhythm disturbances, difficulty falling asleep, restless sleep, and frequent waking [53]. Most sleep comparison studies using objective (actigraphy) or subjective (questionnaires) assessments have found that children with ASD had lower sleep metrics than their typically developing counterparts [54]. In an attempt to find a link between the recorded increased aggressive PA and sleep problems in autistic individuals, it was interesting to consider the work of Wang et al. [54], who reported that some children with much higher PA have impaired sleep latency, bedtime resistance, and awakening latency. It was documented that minimal or excessive PA had a negative impact on sleep quality and quantity. Although sedentary living has frequently been suggested to explain this sleep disruption, it has been less frequently proven that an excess of PA may be deleterious. This can support the harmful effect of the increase in aggressive PA in our ASD participants compared with controls [55]. The suggested link between increased aggressive PA, glutamate excitotoxicity as a neurochemical characteristic of an autistic brain, and sleep disruption as a comorbidity in ASD patients could find support in the work of Bell et al. [56], in which they proved the relationship between glutamate excitotoxicity and sleep deficits in EcoHIV-infected mice.
4.4. Limitations
Our well-defined sample of children with a thorough diagnosis of ASD is one of our work’s strengths. However, our study has a number of limitations. First, it is descriptive; it does not investigate the impact of PA on selected comorbidities of ASD, such as anxiety, stress, and sleeping difficulties. Second is the small sample size, and our population was limited to ASD, which restricts the generalizability of the current findings. However, given the scarcity of studies in this field, our poor understanding of this group, the possibility for objective measurement practices, and potential correlations between PA and quality of life, the findings must be reported, replicated, and expanded.
5. Conclusions
The major finding of this study was that the ASD children were more physically active and less sedentary than TD. The outcomes of the current study should be interpreted with caution due to the small sample size. Therefore, larger studies with larger samples are needed to explore the potential role of physical activity engagement in the improvement of cognition and brain function in ASD children.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Shaw, K.A. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Rosenqvist, M.A.; Larsson, H.; Gillberg, C.; D’Onofrio, B.M.; Lichtenstein, P.; Lundström, S. Etiology of Autism Spectrum Disorders and Autistic Traits Over Time. JAMA Psychiatry 2020, 77, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Manoli, D.S.; State, M.W. Autism Spectrum Disorder Genetics and the Search for Pathological Mechanisms. Am. J. Psychiatry 2021, 178, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef]
- Pitzianti, M.; Fagioli, S.; Pontis, M.; Pasini, A. Attention Deficits Influence the Development of Motor Abnormalities in High Functioning Autism. Child Psychiatry Hum. Dev. 2021, 52, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Leech, K.A.; Tager-Flusberg, H.; Nelson, C.A. Development of fine motor skills is associated with expressive language outcomes in infants at high and low risk for autism spectrum disorder. J. Neurodev. Disord. 2018, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nordin, A.; Ismail, J.; Kamal Nor, N. Motor Development in Children With Autism Spectrum Disorder. Front. Pediatr. 2021, 9, 598276. [Google Scholar] [CrossRef]
- Bandini, L.G.; Gleason, J.; Curtin, C.; Lividini, K.; Anderson, S.E.; Cermak, S.A.; Maslin, M.; Must, A. Comparison of physical activity between children with autism spectrum disorders and typically developing children. Autism 2013, 17, 44–54. [Google Scholar] [CrossRef]
- Tyler, K.; MacDonald, M.; Menear, K. Physical Activity and Physical Fitness of School-Aged Children and Youth with Autism Spectrum Disorders. Autism Res. Treat. 2014, 2014, 312163. [Google Scholar] [CrossRef]
- Fang, Q.; Aiken, C.A.; Fang, C.; Pan, Z. Effects of Exergaming on Physical and Cognitive Functions in Individuals with Autism Spectrum Disorder: A Systematic Review. Games Health J. 2019, 8, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Toscano, C.V.A.; Ferreira, J.P.; Quinaud, R.T.; Silva, K.M.N.; Carvalho, H.M.; Gaspar, J.M. Exercise improves the social and behavioral skills of children and adolescent with autism spectrum disorders. Front. Psychiatry 2022, 13, 1027799. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.; Lord, C.; Ulrich, D.A. The Relationship of Motor Skills and Social Communicative Skills in School-Aged Children With Autism Spectrum Disorder. Adapt. Phys. Act. Q. 2013, 30, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Galloway, J.C.; Landa, R.J. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav. Dev. 2012, 35, 838–846. [Google Scholar] [CrossRef]
- Ohara, R.; Kanejima, Y.; Kitamura, M.; Izawa, K.P. Association between Social Skills and Motor Skills in Individuals with Autism Spectrum Disorder: A Systematic Review. Eur. J. Investig. Health Psychol. Educ. 2019, 10, 276–296. [Google Scholar] [CrossRef]
- West, K.L. Infant motor development in autism spectrum disorder: A synthesis and meta-analysis. Child Dev. 2019, 90, 2053–2070. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Huang, J.; Du, C.; Liu, J.; Tan, G. Meta-Analysis on Intervention Effects of Physical Activities on Children and Adolescents with Autism. Int. J. Environ. Res. Public Health 2020, 17, 1950. [Google Scholar] [CrossRef]
- Alhowikan, A. Benefits of physical activity for autism spectrum disorders: A systematic review. Saudi J. Sports Med. 2016, 16, 163. [Google Scholar] [CrossRef]
- Thomas, S.; Hinkley, T.; Barnett, L.M.; May, T.; Rinehart, N. Young Children with ASD Participate in the Same Level of Physical Activity as Children Without ASD: Implications for Early Intervention to Maintain Good Health. J. Autism Dev. Disord. 2019, 49, 3278–3289. [Google Scholar] [CrossRef]
- Bremer, E.; Crozier, M.; Lloyd, M. A systematic review of the behavioural outcomes following exercise interventions for children and youth with autism spectrum disorder. Autism 2016, 20, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-T.; Luo, H.-J. Effect of physical activity interventions on children and adolescents with autism spectrum disorder: A systematic review and meta-analysis. Physiotherapy 2015, 101, e1685–e1686. [Google Scholar]
- Liang, X.; Li, R.; Wong, S.H.S.; Sum, R.K.W.; Sit, C.H.P. Accelerometer-measured physical activity levels in children and adolescents with autism spectrum disorder: A systematic review. Prev. Med. Rep. 2020, 19, 101147. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Amonkar, N.; Cleffi, C.; Srinivasan, S.; Bhat, A. Neural Effects of Physical Activity and Movement Interventions in Individuals With Developmental Disabilities-A Systematic Review. Front. Psychiatry 2022, 13, 794652. [Google Scholar] [CrossRef] [PubMed]
- Mccoy, S.M.; Jakicic, J.M.; Gibbs, B.B. Comparison of Obesity, Physical Activity, and Sedentary Behaviors Between Adolescents With Autism Spectrum Disorders and Without. J. Autism Dev. Disord. 2016, 46, 2317–2726. [Google Scholar] [CrossRef] [PubMed]
- Guner, U.U.C.; İrem, B. The Relationship between Nutrition-Physical Activity Behaviors of Autistic Children with Their Families and Children’s Obesity Levels During Covid Pandemic. J. Autism Dev. Disord. 2022. [Google Scholar] [CrossRef] [PubMed]
- Memari, A.H.; Ghaheri, B.; Ziaee, V.; Kordi, R.; Hafizi, S.; Moshayedi, P. Physical activity in children and adolescents with autism assessed by triaxial accelerometry. Pediatr. Obes. 2013, 8, 150–158. [Google Scholar] [CrossRef]
- Gehricke, J.G.; Chan, J.; Farmer, J.G.; Fenning, R.M.; Steinberg-Epstein, R.; Misra, M.; Parker, R.A.; Neumeyer, A.M. Physical activity rates in children and adolescents with autism spectrum disorder compared to the general population. Res. Autism Spectr. Disord. 2020, 70, 101490. [Google Scholar] [CrossRef]
- Li, R.; Liang, X.; Zhou, Y.; Ren, Z. A Systematic Review and Meta-Analysis of Moderate-to-Vigorous Physical Activity Levels in Children and Adolescents With and Without ASD in Inclusive Schools. Front. Pediatr. 2021, 9, 726942. [Google Scholar] [CrossRef]
- Hauck, J.L.; Ketcheson, L.R. Ulrich DA. Methodology to Promote Physical Activity Monitoring Adherence in Youth with Autism Spectrum Disorder. Front. Public Health 2016, 4, 206. [Google Scholar] [CrossRef]
- Haegele, J.; Zhu, X.; Bennett, H. Accelerometer Measured Physical Activity among Youth with Autism and Age, Sex, and Body Mass Index Matched Peers: A Preliminary Study. Disabil. Health J. 2021, 14, 101102. [Google Scholar] [CrossRef]
- Sirard, J.; Trost, S.; Pfeiffer, K.; Dowda, M.; Pate, R. Calibration and Evaluation of an Objective Measure of Physical Activity in Preschool Children. J. Phys. Act. Health 2011, 3. [Google Scholar] [CrossRef]
- Moulton, E.; Bradbury, K.; Barton, M.; Fein, D. Factor Analysis of the Childhood Autism Rating Scale in a Sample of Two Year Olds with an Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 49, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Schanding, G.T.; Nowell, K.P.; Goin-Kochel, R.P. Utility of the Social Communication Questionnaire-Current and Social Responsiveness Scale as Teacher-Report Screening Tools for Autism Spectrum Disorders. J. Autism Dev. Disord. 2011, 42, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport/Sports Med. Aust. 2011, 14, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Malow, B.; Fawkes, D.; Weiss, S.; Reynolds, A.; Loh, A.; Adkins, K.; Wofford, D.; Wyatt, A.; Goldman, S. Actigraphy in Children with Autism Spectrum Disorders: Strategies for Success. In Proceedings of the 2014 International Meeting for Autism Research, Atlanta, GA, USA, 14–17 May 2014; Available online: https://www.researchgate.net/publication/268130972 (accessed on 8 August 2022).
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Recommendations on Physical Activity For health; World Health Organization (WHO): Geneva, Switzerland, 2010. [Google Scholar]
- Sandt, D.D.R.; Frey, G.C. Comparison of Physical Activity Levels between Children with and Without Autistic Spectrum Disorders. Adapt. Phys. Act. Q. 1998, 22, 146–159. [Google Scholar] [CrossRef]
- Ketcheson, L.; Hauck, J.L.; Ulrich, D. The levels of physical activity and motor skills in young children with and without autism spectrum disorder, aged 2–5 years. Autism 2017, 22, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N. A Functional Analysis of Physical Activity in Children with Autism Spectrum Disorder. Thesis Projects. 28. 2021. Available online: https://scholarship.rollins.edu/mabacs_thesis/28 (accessed on 8 August 2022).
- Pan, C.-Y. Objectively Measured Physical Activity Between Children With Autism Spectrum Disorders and Children Without Disabilities During Inclusive Recess Settings in Taiwan. J. Autism Dev. Disord. 2008, 38, 1292–1301. [Google Scholar] [CrossRef]
- Elia, J.; Izaki, Y.; Ambrosini, A.; Hakonarson, H. Glutamatergic Neurotransmission in ADHD: Neurodevelopment and Pharmacological Implications. J. Pediatr. Neonatol. 2020, 1, 1006. [Google Scholar]
- Baskerville, R.; McGrath, T.; Castell, L. The effects of physical activity on glutamate neurotransmission in neuropsychiatric disorders. Front. Sports Act. Living 2023, 5, 1147384. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A. GABA and Glutamate Imbalance in Autism and Their Reversal as Novel Hypothesis for Effective Treatment Strategy. Autism Dev. Disord. 2020, 18, 46–63. [Google Scholar] [CrossRef]
- Lipton, S.A.; Nicotera, P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium 1998, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat. Rev. Drug Discov. 2006, 5, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.; Fadeuilhe, C.; Gisbert, L.; Setien, I.; Delgado, M.; Corrales, M.; Richarte, V.; Ramos-Quiroga, J.A. Sleep in adults with autism spectrum disorder and attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2020, 38, 1–24. [Google Scholar] [CrossRef]
- Gunes, S.; Ekinci, O.; Feyzioglu, A.; Ekinci, N.; Kalinli, M. Sleep problems in children with autism spectrum disorder: Clinical correlates and the impact of attention deficit hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2019, 15, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Stutz, J.; Eiholzer, R.; Spengler, C. Effects of evening exercise on sleep in healthy participants: A systematic review and metaanalysis. Sports Med. 2019, 49, 269–287. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goodlin-Jones, B.; Hertz-Picciotto, I. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population based study. J. Sleep Res. 2008, 17, 197–206. [Google Scholar] [CrossRef]
- Wang, F.; Boros, S. The effect of physical activity on sleep quality: A systematic review. Eur. J. Physiother. 2019, 23, 1–8. [Google Scholar] [CrossRef]
- Bricout, V.A.; Pace, M.; Guinot, M. Sleep and Physical Activity in Children with Autism Spectrum: About 3 Clinical Cases. Austin J. Autism Relat. Disabil. 2018, 4, 1049–1052. [Google Scholar]
- Bell, B.J.; Hollinger, K.R.; Deme, P.; Sakamoto, S.; Hasegawa, Y.; Volsky, D.; Kamiya, A.; Haughey, N.; Zhu, X.; Slusher, B.S. Glutamine antagonist JHU083 improves psychosocial behavior and sleep deficits in EcoHIV-infected mice. Brain Behav. Immun. Health 2022, 9, 100478. [Google Scholar] [CrossRef]

