1. Introduction
Crimean–Congo haemorrhagic fever virus (CCHFV) is a tick-borne zoonotic virus in the species Crimean–Congo haemorrhagic fever orthonairovirus, genus Orthonairovirus, family Nairoviridae in the order Bunyavirales [1,2]. CCHFV has a tripartite, linear, single-stranded, negative-sense RNA genome. The three segments small (S), medium (M), and large (L) encode the nucleocapsid (N) and the NSs non-structural proteins, the two glycoproteins Gn and Gc and a non-structural protein NSm, and the RNA-dependent RNA-polymerase, respectively [3,4,5,6]. The virus causes haemorrhage, myalgia, and fever in humans and the disease was first described in Crimea in 1944–1945 [7]. It was later shown that the same virus was responsible for an illness identified in 1956 in the Democratic Republic of Congo and therefore was named the Crimean–Congo Haemorrhagic Fever virus [8,9,10]. The case fatality rate in CCHFV outbreaks is 10–40% according to the World Health Organisation (WHO) report [11]. CCHFV is classified into seven genetic lineages (Africa 1, 2, and 3, Asia 1 and 2, and Europe 1 and 2) that cause severe disease in humans, except the Europe 2 lineage [12].
Numerous wild and domestic animals, such as cattle, goats, sheep, ostriches, and hares, can become infected with CCHFV but do not show clinical symptoms of the disease [13,14,15,16,17]. These vertebrates act as amplification hosts. Although the viraemic phase is short in a majority of vertebrate hosts lasting 2–7 days [18,19], these hosts amplify the virus and support the virus spread from one tick to another [20,21,22]. Belobo et al. (2021) reported an overall worldwide CCHFV seroprevalence of 12.0% in animal species, whereas Spengler et al. (2016) stated seroprevalence of 19.3%, 21.7%, 28.1%, and 23.9% in cattle, donkey, goats, and sheep, respectively [23,24]. In Africa, much higher seroprevalence rates have been reported in livestock species, e.g., Uganda (36.5%), Zimbabwe (37%), Mauritania (67%), South Africa (74.2%) and Senegal (32.5%) [25,26,27,28]. However, few studies detected the CCHFV nucleic acids in vertebrates, most probably due to the short viraemic phase [29,30,31,32,33].
Rodents and shrews are widely distributed in peridomestic habitats and contact with human populations often occurs with a potential risk of disease transmission [34]. The rodent species commonly found in human settlements, e.g., Rattus rattus, Mastomys natalensis, Crocidura spp., and house mice (Mus spp.), are known to vector medically important hantaviruses and arenaviruses but also arboviruses like Wesselsbron (WSL) and Usutu (USUV) viruses [34,35,36]. However, the role of rodents in CCHFV transmission is not well understood, despite the fact that they can become infected through parasitized infected immature tick species, which in turn can infect/transmit the virus to humans who live in close proximity [37]. Whether CCHFV could be transmitted from rodent to human through other transmission pathways like urine, faces, rodent meat consumption, and other body fluids/secretions like rodent-borne viruses requires further investigations that will decipher their possible role as amplification/reservoir hosts in the CCHFV transmission cycle. Various studies have reported the presence of CCHFV antibodies in the mouse species Apodemus sylvaticus, brown rat Rattus norvegicus (Pakistan), Bushveld gerbil (Gerbilliscus leucogaster), Aethomys namaquensis, Rhabdomys pumilio, and Mastomys spp. (South Africa/Zimbabwe), Rattus rattus (Pakistan), Arvicanthis niloticus and Mastomys erythroleucus (Mauritania), and unidentified rodents in Iraq and Iran, among others [26,37,38,39,40]. Thus, rodents could play an important role in the transmission of CCHFV.
CCHFV is transmitted by ticks that serve as vectors and reservoirs and play an important role in CCHFV maintenance [41]. Hyalomma ticks of the family Ixodidae are the main vector and reservoir of the virus [9,10,26,42]. CCHFV has also been detected in other tick species of the genera Rhipicephalus, Amblyomma, and Dermacantor, although their role in virus maintenance and transmission is unclear [7,9,18]. Ticks are considered reservoirs as they support life-long and transstadial infections in contrast to short-term viremia observed in vertebrates [13,23,24,41]. Furthermore, non-systemic transmission of CCHFV can occur between ticks through co-feeding on a non-viraemic host as well as through venereal routes during mating [9,37,43,44,45,46].
Besides infection via the bite of a CCHFV-infected tick, humans can also become infected through contact with blood, secretions, organs, or other body fluids of infected persons and livestock during slaughter. Consequently, this makes people who are in close contact with animals like pastoralists, veterinarians, and abattoir workers more prone to CCHFV infections.
CCHFV is widely distributed and endemic in some parts of Africa, Europe, the Middle East, and Asia. The broad geographic spread is believed to be influenced by several factors including the wide distribution of the primary vector (Hyalomma ticks), the prolonged maintenance of the virus in ticks through horizontal and vertical transmission, the extended feeding times associated with ticks as well as the long-distance movement of livestock [9,10,47].
In Kenya, CCHFV was first detected in Rh. pulchellus in 1970 from dying sheep in the Kabete veterinary laboratories [7]. The first documented case of acute human infection with CCHFV was in the year 2000 from a farmer in western Kenya [47]. Subsequent studies have detected the virus in diverse tick species infesting livestock and antibodies against CCHFV were found in humans through serosurveys [15,16,30,31,48]. For instance, in Northeastern Kenya, the virus was detected in Hy. rufipes and Hy. truncatum infesting cattle and camels, and recently in Rh. decoloratus in western Kenya [30,31]. A recent study in Baringo and Kajiado counties confirmed livestock infestation with Rhipicephalus spp. and Hyalomma spp. ticks [49], which represent potential risk factors for CCHFV circulation. Furthermore, several serosurveys have revealed CCHFV seroprevalence rates of 14–35% in febrile patients from different geographical areas in Kenya [15,16,50]. Only a few studies have monitored CCHFV infection rates in livestock and wildlife in Kenya [29,32]. Much less investigated is the role of rodents in CCHFV transmission.
In this study, a one-health surveillance approach of CCHFV detection among a network of vertebrate hosts including humans was implemented. The study was conducted in two pastoralist-dominated areas, Kajiado and Baringo counties, in the Kenyan Rift Valley to help understand CCHFV transmission and circulation in these ecosystems. Specifically, evidence of CCHFV circulation and exposure in inapparent asymptomatic livestock, including cattle, goats, donkeys, sheep, and peridomestic rodents, was assessed. Additionally, syndromic surveillance of CCHFV in patients with febrile illness was conducted to determine the possible contribution of the virus to fevers of unknown origin.
2. Materials and Methods
2.1. Ethical Approval
The study was approved by the Kenya Medical Research Institute’s Scientific and Ethics Review Unit (SERU), (SERU No.3312) after gaining approval by the animal care and use committee. Additional approval for the study was accorded by the University of Pretoria, Faculty of Health Science’s Research Ethics Committee (Ethics Reference No. 568/2020).
Sampling of livestock (cattle, goats, and sheep) was conducted by a team including a Kenya Veterinary Board (KVB)-registered veterinarian or/an animal health technician after obtaining written consent from the respective county governments and verbal consent from local farmers. Additionally, informed written consent was obtained from all febrile patients sampled.
2.2. Study Sites
The survey was conducted in different locations within Baringo (0.4695° N, 35.9833° E) and Kajiado (1.7617° S, 36.0255° E) counties from January 2020 to December 2021 as part of a larger project that aimed at understanding the transmission dynamics of arboviruses [51,52]. Both field sites are located in the Kenyan Rift Valley and are semi-arid ecologies mainly inhabited by nomadic pastoralist communities and a history of arbovirus circulation [16,49,50,53,54,55] (Figure 1).
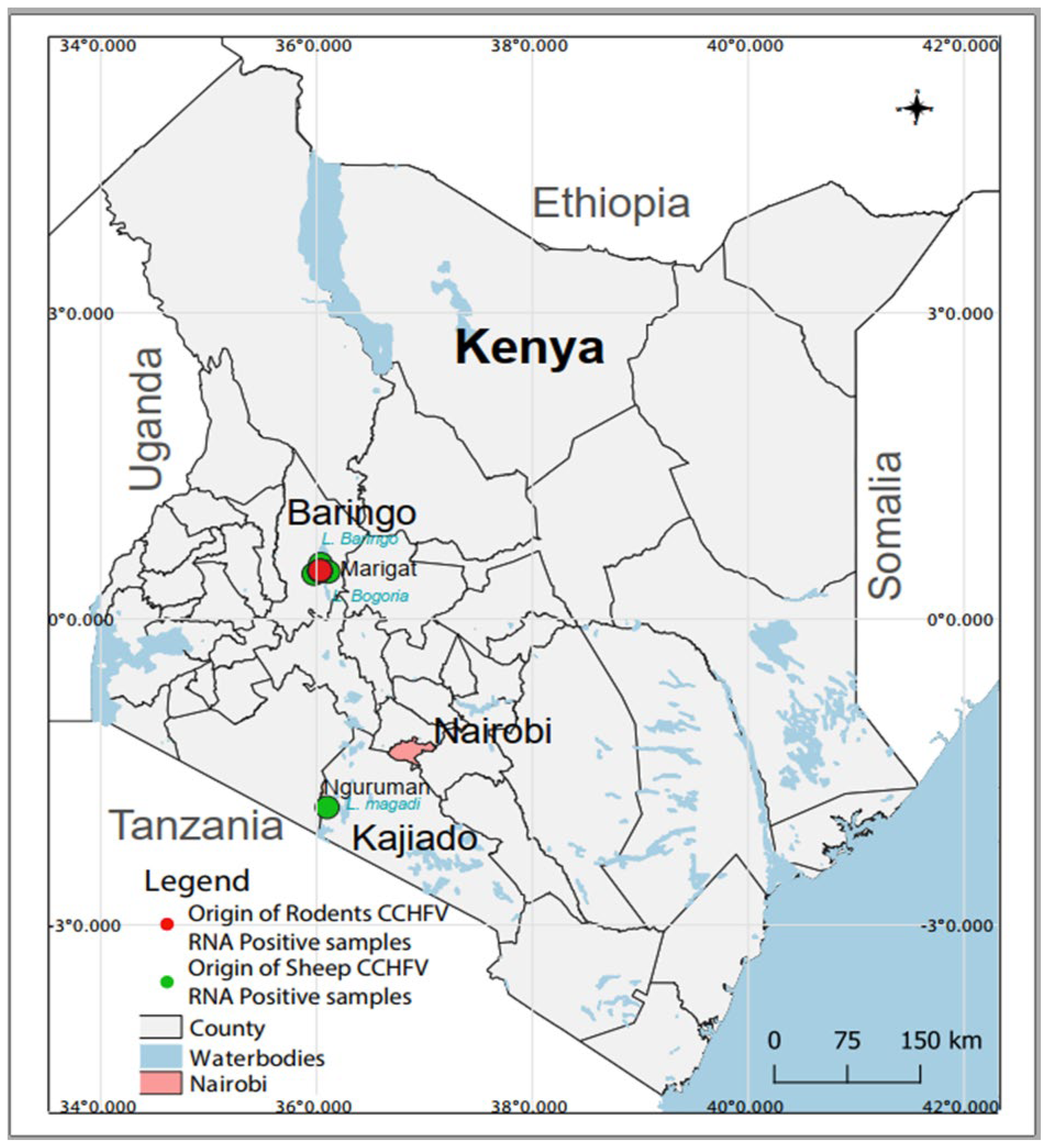
Figure 1. Map of Kenya showing sampling sites and the origin of CCHFV RNA-positive samples. The map was created in the open source GIS software, QGIS 3.22 using GPS co-ordinates and shape files derived from Natural Earth (http://www.naturalearthdata.com/, a free GIS data source accessed on 20 April 2023) and Africa Open data (https://africaopendata.org/dataset/kenya-counties-shapefile, license Creative Commons, accessed on 20 April 2023).
2.3. Human Sampling
Human sampling was conducted at Marigat Subcounty Hospital (Baringo County) and Entasopia Health Centre (Kajiado County). Patients (male and female) ≥5 years presenting with a clinical case definition of acute febrile illness characterized by fever (body temperature ≥ 38 °C) and at least one of the following clinical manifestations: cough, joint pain, headache, chills, general body malaise, and any signs of bleeding and neurological abnormalities were formally recruited for the study after obtaining their written consent and, in the case of children, consent from guardians. Clinical demographics and risk factor data were collected from the patients using a questionnaire and a data sheet.
A total of 5 mL of blood from each participant was collected into BD Vacutainer® serum tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with a clot activator by a trained and licensed phlebotomist. The serum was separated by centrifuging at 1500 rpm for 10 min using an Eppendorf™ 5702 Series Centrifuge. Samples were stored in liquid nitrogen at the health facilities until transportation on dry ice to the Emerging Infectious Disease (EID) Laboratory at icipe, Nairobi, where they were stored at −80 °C until further analysis.
2.4. Livestock Sampling
A random sampling of domesticated livestock (cattle, sheep, goats, and donkeys) aged 1–3 years was conducted twice a year after the short and long rains by a registered veterinarian and/or an animal health technician after obtaining verbal consent from the farmers. Animal characteristics like age and sex as well as information on vaccination history, clinical signs, and location of sampling were recorded in a data capture form. Animals were randomly selected and whole blood from the jugular vein was collected using a 10 mL BD Vacutainer® with EDTA and one pre-coated with serum activator for serum. Samples were transported on dry ice to the EID Laboratory at icipe, Nairobi, and stored at −80 °C until further analysis.
2.5. Sampling of Peridomestic Rodents
Rodents were trapped using the LFAHD Folding Live Capture Rodent/Rat/Mouse traps (https://www.shermantraps.com/animal-traps/). Two to four traps were placed inside houses and their surroundings within the study sites (Baringo and Kajiado counties) according to the National Museum of Kenya (NMK) guidelines (unpublished). The traps were baited with a mixture of locally available peanut butter and white oats, opened at dusk, checked every morning, and left closed during the day. Trapped specimens were placed in a handling bag, weighed, had their sex and age recorded in a data capture form, and identified to the genus or species based on morphological and geographical criteria according to the Kingdon Guide to African Mammals [56] and East African Mammals [57]. Species were further confirmed by molecular analyses [58,59].
The rodents were euthanatized by cervical dislocation, and tissues (kidney, spleen, lung, heart, and liver samples) and blood were collected using cryovials and BD Vacutainer® tubes with serum activator, respectively. Serum was separated through centrifugation at 3000 rpm for 5 min. Samples were stored in liquid nitrogen until transportation to the EID Laboratory at icipe, Nairobi, where they were stored at −80˚C.
2.6. CCHFV IgG and IgM ELISA
The presence of CCHFV IgG antibodies in febrile patients was detected by ELISA according to the manufacturer’s instruction (VectoCrimean-CHF-IgG kit; Vector-Best, Russia, https://vector-best.ru). Positive samples were further tested for the presence of IgM antibodies by ELISA according to the manufacturer’s instruction (VectoCrimean-CHF-IgM ELISA kit; Vector-Best, Russia, https://vector-best.ru). Livestock and rodent serum samples were screened for CCHFV IgG antibodies by ELISA using the ID Screen® CCHF Double Antigen Multi-species ELISA kit (ID Vet, France, https://www.id-vet.com) according to the manufacturer’s instructions. Optical density (OD) values were determined using a BioTek ELX800 Microplate reader at 450 nm.
2.7. Statistical Data Analysis
Demographic data and the presence of CCHFV IgM and IgG antibodies expressed as positive or negative were analyzed using R version 4.2.0. Descriptive and univariable analyses were performed to understand how variables like age, gender, location as well as occupation and contact with animals could be linked to CCHFV exposure in humans. A comparison between the two study sites and different hosts was conducted using the chi-square test. The Agresti–Coull method was used to estimate the 95% confidence intervals (CIs). All tests were performed at a 5% significance level. Multiple logistic regression analysis based on likelihood ratio (LR) testing at a 5% level model reduction was performed to understand risk factors associated with CCHFV seropositivity.
2.8. RNA Extraction, PCR Screening, and Sequencing
Viral RNA was extracted from individual human serum samples, homogenized rodent tissues, and pooled livestock sera (consisting of 5–7 individual samples according to site and species) using the QIAamp Viral RNA Minikit (QIAGEN, Hilden Germany) according to the manufacturer’s protocol. Invitrogen SuperScript™ III Reverse Transcriptase (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used for cDNA synthesis and samples were screened by RT- PCR using the primers CCHFV F2 (5′-TGGACACCTTCACAAACTC-3′) and CCHFV R3 (5′-GACAAATTCCCTGCACCA-3′) amplifying a fragment of the nucleocapsid gene of 536 bp [60]. PCR products were analyzed using a 2% agarose gel stained with Invitrogen UltraPure™ Ethidium Bromide (Thermo Fisher Scientific Inc., USA). Amplicons were purified using the Applied Biosystems™ ExoSAP-IT™ PCR Product Cleanup Reagent (Thermo Fisher Scientific Inc., USA) according to the manufacturer’s instructions and sequenced in both directions at Macrogen, Europe B.V. Sequences were cleaned in Geneious Prime software (Version 2022.1.1) (https://www.geneious.com) and queried in the GenBank-NCBI database using the Basic Local Alignment Search Tool (BLAST) [61]. Sequencing of entire CCHFV genomes was attempted using the Illumina MiSeq platform as described previously [62].
2.9. Phylogenetic Analysis
CCHFV S segment sequences were downloaded from the GenBank NCBI database and a multiple sequence alignment with the Nairobi sheep disease virus (NSDV_KM464724) as an outgroup was performed using MAFFT [63]. A maximum likelihood phylogenetic analysis using PhyML [64] and the general time reversible (GTR) model and applying 1000 bootstrap replicates was inferred.
3. Results
3.1. CCHFV Serology
3.2. Detection of CCHFV in Livestock, Rodents and Humans
A total of 493 and 480 individual human and rodent samples, respectively, as well as 280 pooled livestock samples were screened for CCHFV by RT-PCR. Of these, four sheep pools (three originating from Marigat and one from Nguruman) and four rodent samples (originating from Marigat) contained CCHFV RNA. CCHFV was detected in one Rattus rattus and three Mus musculus (Figure 1). PCR amplicons were obtained for three human and five cattle samples, but sequencing was unsuccessful. Attempts to obtain more sequence information from the positive samples using NGS were not successful.
The partial CCHFV S segment sequences of 536 nucleotides generated both from sheep and rodents showed pairwise nucleotide identities of up to 99% among each other and of 96–98% to strains in the CCHFV Africa 3 lineage. Phylogenetic analyses showed that the sequences from this study formed a sub-clade within the Africa lineage 3 (Figure 4).
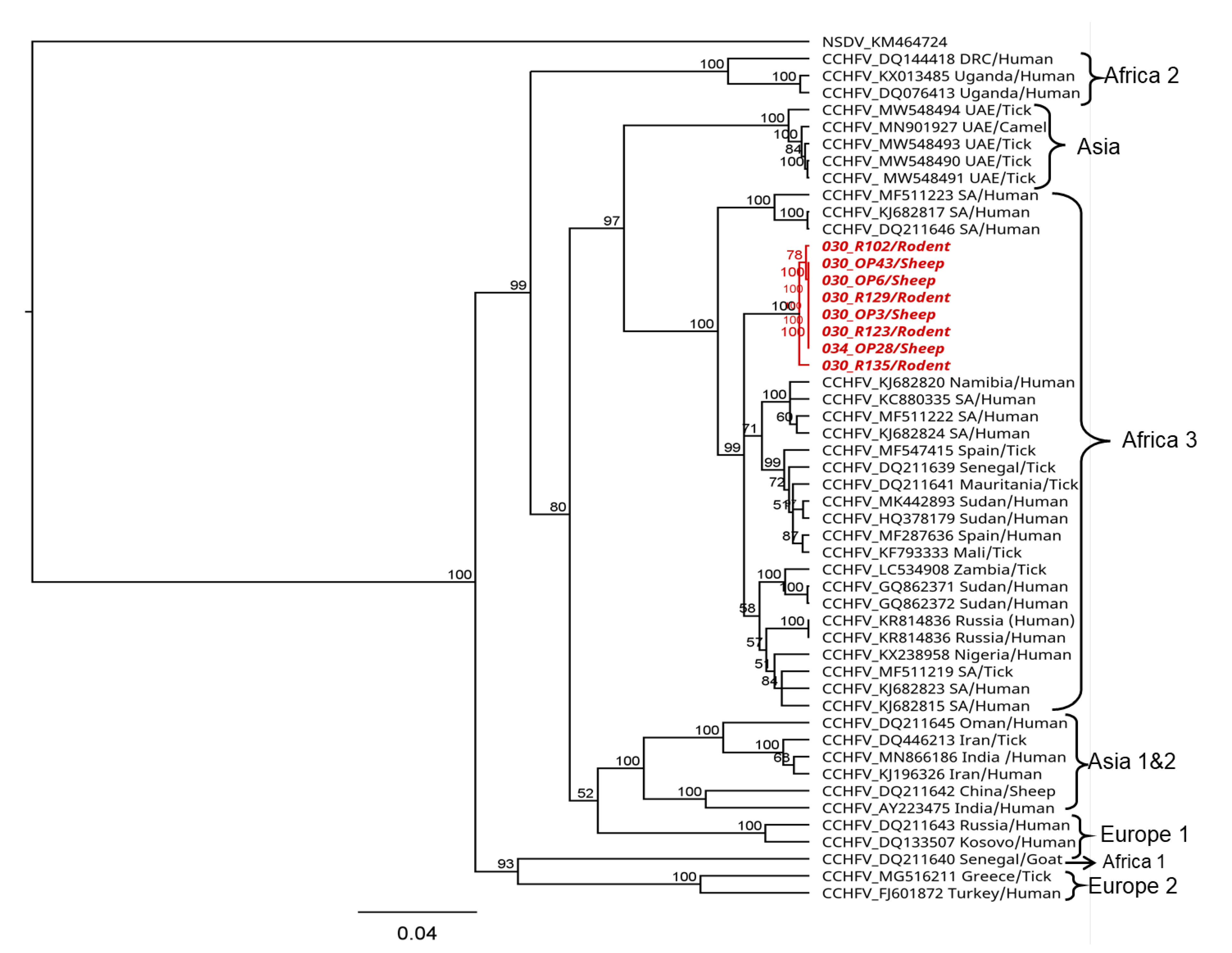
Figure 4. Phylogenetic relationship of CCHFV sequences detected in sheep and rodents from Kenya. Maximum likelihood (ML) phylogenetic analysis was based on a 536-nucleotide fragment of the nucleoprotein (S segment). Sequences were aligned using MAFFT and phylogenetic analyses and were inferred using PhyML v. 2.2.4 with the GTR substitution model employing 1000 bootstrap replicates. Sequences from this study are shown in red. Sequence accession number, country of origin, and host or vector species are indicated.
4. Discussion
The data of this study show CCHFV circulation among humans, livestock, and peri-domestic rodents from two semi-arid ecologies within the Kenyan Rift Valley. Antibodies against CCHFV were detected in donkeys (31.4%), cattle (14.1%), sheep (9.8%), goats (8.1%), rodents (6.5%), and humans (5.9%). In addition, CCHFV RNA was detected in sheep (1.4%) and rodents (0.83%). The observations are consistent with previous detection of virus antibodies in febrile patients [15,16,50] and cattle [29,32] as well as wildlife [32]. Furthermore, the detection of CCHFV RNA in sheep, despite the short and low viremia, was consistent with CCHF infection in livestock [29,30,31,32,33]—an indication of active circulation at the sites. However, the detection of CCHFV RNA in peri-domestic rodents constitutes a novel finding, potentially implicating them in CCHFV epidemiology in the country. Taken together, these findings including the widespread detection of ticks [30,31] suggest that the virus is endemic in some regions of Kenya.
CCHFV infection in humans is lethal, with high fatality rates of up to 40% (WHO) during outbreaks depending on the infecting strain. Here, we detected IgM antibodies against CCHFV in nine cases of fevers of unknown origin (1.8%, 9/493), indicating evidence of recent or acute CCHFV infection, which may be the cause of febrile illness in these patients. However, the contribution of other aetiologies cannot be discounted, as fever is a common symptom of other local disease conditions, often with similarity in clinical presentation to those of arboviral pathogens [3,65]. The presence of antibodies against CCHFV in humans was not affected by gender, but by location and age, being higher in Marigat than Nguruman (8/9 88.9% (95% CI 54.3–100) and 1/9, 11.1% (95% CI (1–45.6), respectively), and in the age group ≥18 years (6/9, 66.7% (95% CI 35.1–88.3)). Geographic differences in risk profile could also be attributed to other factors, such as the abundance of competent tick species, exposure of humans to tick bites or degree of contact with animals. The higher presence of IgM and IgG antibodies in older patients was associated with the type of occupation and long-term involvement in activities that require frequent contact with livestock. Our data show that humans in contact with animals were more likely to be CCHFV-seropositive at both study sites. Interestingly, there was a strong positive association between CCHFV IgM-positive patients and retro-orbital pain, suggesting that this symptom could be a marker for CCHFV infection in the study area. Nevertheless, detailed socio-economic studies are needed to quantify the burden of CCHFV infection in humans.
Here, an overall CCHFV seropositivity of 14.19% was found in cattle, while a previous study found rates of 28% in cattle in Narok and Laikipia counties [29]. Seroprevalence was particularly higher in donkeys (31%), while the lowest rates were observed among small ruminants (sheep and goats). These data highlight the potential different virus exposure rates depending on the feeding preferences of tick species [29]. The likelihood of coming into contact with an infected tick and becoming infected with CCHFV was shown to increase with age [14,27,66] and is consistent with our findings of increased CCHFV seropositivity in older livestock, e.g., 10.2% in one-year-old animals, 11.1% in two-year-old animals, and 16.5% in three-year-old animals. This might also be true for the higher seropositivity rates detected in donkeys, which were older compared to other livestock species. CCHFV seropositivity in livestock may reach up to 80% in certain livestock species and regions [23]. For example, higher CCHFV seropositivity rates were found in Uganda (75.0%) [25], Senegal (57.1%) [27], Mali (13–95%) [67], Sudan (21% and 19.1%) [68], Mauritania (15–80%) [66,69], and South Africa (12.7%) [68,70], but lower seroprevalence rates of 3.13% were observed in Egypt [71]. The differences in CCHFV seroprevalence rates in livestock could be due to the implementation of tick control programs using acaricides and dips, or due to changes in tick species infesting different livestock species. For example, Ogola et al. (2022) found differential infestation of Hyalomma marginatum, the principal vector and reservoir of CCHFV, on sheep but not on other livestock species in the same study area [49].
This study presents the first known detection of CCHFV RNA in rodents in Kenya. CCHFV sequences were found in house rats (Rattus rattus) and house mice (Mus musculus), both species known to inhabit human dwellings. Rattus rattus (house rat) hosts a wide variety of harmful internal and external parasites, like fleas and tick larvae, that live on these rats and harbor disease-causing microorganisms that can infect humans, livestock, and other animals resulting in serious diseases [35,36]. Mus musculus lives as a human commensal in close association with humans, in houses and granaries [72,73,74]. The close interaction of these rodents with humans and livestock pastures may facilitate CCHFV transmission, e.g., by immature ticks that are mainly infesting rodents. However, in the absence of any evidence that CCHFV is a persistent infection and/or present in rodent excreta, it is unlikely that the virus can be transmitted via the same route as arenaviruses or hantaviruses. Further evidence of excretion will be required to justify such a form of transmission.
The role of rodents in CCHFV transmission is not well known, although various studies have confirmed the presence of antibodies in rodents from Africa (South Africa, Zimbabwe, Senegal, Mauritania, and Central Africa Republic), Asia (Pakistan, Iraq, and Iran) and Europe (Hungary) [7,17,23,26,38]. In this study, we report an overall seropositivity of 6.5% (6/93, 95% CI 2.7–13.6). Sera from five Mastomys natalensis from Nguruman and one Rattus rattus from Marigat contained antibodies against CCHFV. The presence of antibodies in multimammate mice (Mastomys spp.) has also been reported in Mauritania (27%) and South Africa/Zimbabwe (0.3%) [26,39]. Nonetheless, the small sample size for seropositivity may not present conclusive results, and further studies with larger sample sizes and robust optimized serological assays are recommended to ascertain the true CCHFV seroprevalence in rodents. Generally, the presented data are inconclusive in confirming rodents as reservoir hosts for CCHFV, although it does suggest that they could facilitate virus amplification. Further investigation is necessary to determine whether viremia could persist, thereby supporting virus spread.
The phylogenetic analysis of the partial CCHFV nucleoprotein sequences detected in this study revealed up to 99% nucleotide identity among the sequences and 96–98% nucleotide identity among strains in the African lineage 3 that have been reported to cause disease in humans [75,76,77,78,79]. This could confirm the potential risk of the virus in circulation to cause disease in the human population, although there is a need for generating whole genome sequences to allow for detailed characterization and fine-scale inferences on the genetic diversity of circulating strains in the area. In this study, attempts to generate more genome information from the CCHFV-positive samples were not successful but would be necessary for a comprehensive phylogenetic analysis and to shed light on the genetic diversity of circulating strains in the area.
Finally, the low human and livestock seropositivity reported in this study compared to what has been previously reported in other parts of Kenya, as well as in neighboring countries, can only be attributed to well-organized and managed tick control measures practiced in the areas. However, the findings confirm the possibility of CCHFV’s contribution to febrile illnesses. Although various seroprevalence studies have reported the detection of CCHFV IgM antibodies in febrile patients in different parts of Kenya [15,16,50], the true burden of CCHFV infection and its overall contribution to febrile cases cannot be ascertained. This is because of the lack of CCHFV surveillance systems in the country and routine diagnostics in the healthcare system. Considering our findings and previous reports, it is imperative to establish robust diagnostic techniques for routine diagnosis in healthcare facilities and to equip healthcare professionals with essential knowledge on the diagnosis and management of CCHFV. This will ensure early detection and prevention through the implementation of appropriate control measures. Furthermore, the scope of our study was limited to only two counties in Kenya. Consequently, the data only provide a basis for further studies and recommend nationwide one health surveillance to determine the true burden of CCHFV, which can comprehensively inform the healthcare system.
5. Conclusions
Overall, the data of our study provide evidence for active circulation of CCHFV in Kenya shown via the detection of viral RNA in sheep and rodents, the presence of IgM antibodies against CCHFV in febrile patients, and the presence of IgG antibodies against CCHFV in donkeys, cattle, sheep, and goats. The presence of IgM antibodies in febrile patients suggests the possibility of the contribution of CCHFV to undiagnosed febrile cases. Together, our findings emphasize the importance of one health active surveillance in vectors, livestock, humans, and rodents to understand the transmission and circulation of CCHFV. So far, rodents and shrews that live in close proximity to humans have been mostly neglected in disease surveillance, despite the potential risk of transmitting disease.
References
- Kuhn, J.H.; Adkins, S.; Agwanda, B.R.; Al Kubrusli, R.; Alkhovsky, S.V.; Amarasinghe, G.K.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2021 Taxonomic Update of Phylum Negarnaviricota (Riboviria: Orthornavirae), Including the Large Orders Bunyavirales and Mononegavirales. Arch. Virol. 2021, 166, 3513–3566. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; et al. Taxonomy of the Order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.; González-Scarano, F. Emerging Infectious Diseases: The Bunyaviridae. J. Neurovirol. 2005, 11, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Briese, T.; Bird, B.; Kapoor, V.; Nichol, S.T.; Lipkin, W.I. Batai and Ngari Viruses: M Segment Reassortment and Association with Severe Febrile Disease Outbreaks in East Africa. J. Virol. 2006, 80, 5627–5630. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.D.; Trappier, S.G.; Sanchez, A.J.; Meyer, R.F.; Goldsmith, C.S.; Zaki, S.R.; Dunster, L.M.; Peters, C.J.; Ksiazek, T.G.; Nichol, S.T. A Reassortant Bunyavirus Isolated from Acute Hemorrhagic Fever Cases in Kenya and Somalia. Virology 2001, 291, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, C.; Venter, M.; Lwande, O.; Swanepoel, R.; Sang, R. Phylogenetic Analysis of Bunyamwera and Ngari Viruses (Family Bunyaviridae, Genus Orthobunyavirus) Isolated in Kenya. Epidemiol. Infect. 2016, 144, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraal, H. The Epidemiology of Tick-Borne Crimean-Congo Hemorrhagic Fever in Asia, Europe, and Africa. J. Med. Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Maupin, G.O.; Ksiazek, T.G.; Rollin, P.E.; Khan, A.S.; Schwarz, T.F.; Lofts, R.S.; Smith, J.F.; Noor, A.M.; Peters, C.J.; et al. Molecular Investigation of a Multisource Outbreak of Crimean-Congo Hemorrhagic Fever in the United Arab Emirates. Am. J. Trop. Med. Hyg. 1997, 57, 512–518. [Google Scholar] [CrossRef]
- Kuehnert, P.A.; Stefan, C.P.; Badger, C.V.; Ricks, K.M. Crimean-Congo Hemorrhagic Fever Virus (CCHFV): A Silent but Widespread Threat. Curr. Trop. Med. Rep. 2021, 8, 141–147. [Google Scholar] [CrossRef]
- Shayan, S.; Bokaean, M.; Shahrivar, M.R.; Chinikar, S. Crimean-Congo Hemorrhagic Fever. Lab Med. 2015, 46, 180–189. [Google Scholar] [CrossRef]
- WHO. Crimean-Congo Haemorrhagic Fever; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Hua, B.L.; Scholte, F.E.M.; Ohlendorf, V.; Kopp, A.; Marklewitz, M.; Drosten, C.; Nichol, S.T.; Spiropoulou, C.F.; Junglen, S.; Bergeron, É. A Single Mutation in Crimean-Congo Hemorrhagic Fever Virus Discovered in Ticks Impairs Infectivity in Human Cells. Elife 2020, 9, e50999. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.R.; Estrada-Peña, A.; Garrison, A.R.; Schmaljohn, C.; Spiropoulou, C.F.; Bergeron, É.; Bente, D.A. A Chronological Review of Experimental Infection Studies of the Role of Wild Animals and Livestock in the Maintenance and Transmission of Crimean-Congo Hemorrhagic Fever Virus. Antivir. Res. 2016, 135, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Zohaib, A.; Saqib, M.; Athar, M.A.; Hussain, M.H.; Sial, A.u.R.; Tayyab, M.H.; Batool, M.; Sadia, H.; Taj, Z.; Tahir, U.; et al. Crimean-Congo Hemorrhagic Fever Virus in Humans and Livestock, Pakistan, 2015–2017. Emerg. Infect. Dis. 2020, 26, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Irura, Z.; Tigoi, C.; Chepkorir, E.; Orindi, B.; Musila, L.; Venter, M.; Fischer, A.; Sang, R. Seroprevalence of Crimean Congo Hemorrhagic Fever Virus in Ijara District, Kenya. Vector-Borne Zoonotic Dis. 2012, 12, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Tigoi, C.; Lwande, O.; Orindi, B.; Irura, Z.; Ongus, J.; Sang, R. Seroepidemiology of Selected Arboviruses in Febrile Patients Visiting Selected Health Facilities in the Lake/River Basin Areas of Lake Baringo, Lake Naivasha, and Tana River, Kenya. Vector-Borne Zoonotic Dis. 2015, 15, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Földes, F.; Madai, M.; Németh, V.; Zana, B.; Papp, H.; Kemenesi, G.; Bock-Marquette, I.; Horváth, G.; Herczeg, R.; Jakab, F. Serologic Survey of the Crimean-Congo Haemorrhagic Fever Virus Infection among Wild Rodents in Hungary. Ticks Tick. Borne. Dis. 2019, 10, 101258. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Tsergouli, K.; Tsioka, K.; Mirazimi, A. Crimean-Congo Hemorrhagic Fever: Tick-Host-Virus Interactions. Front. Cell. Infect. Microbiol. 2017, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Bessell, P.R.; Nkongho, E.F.; Ngwa, V.N.; Tanya, V.N.; Sander, M.; Ndip, L.; Morgan, K.L.; Handel, I.G.; Mazeri, S.; et al. Seroepidemiology of Crimean-Congo Haemorrhagic Fever among Cattle in Cameroon: Implications from a One Health Perspective. PLoS Negl. Trop. Dis. 2022, 16, e0010217. [Google Scholar]
- Ergonul, O. Crimean-Congo Hemorrhagic Fever Virus. In Viral Hemorrhagic Fevers; CRC Press: Boca Raton, FL, USA, 2016; pp. 617–629. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean-Congo Haemorrhagic Fever. Antiviral Res. 2004, 64, 145–160. [Google Scholar] [CrossRef]
- Flick, R.; Whitehouse, C.A. Crimean-Congo Hemorrhagic Fever Virus. Curr. Mol. Med. 2005, 5, 754–758. [Google Scholar] [CrossRef]
- Spengler, J.R.; Bergeron, É.; Rollin, P.E. Seroepidemiological Studies of Crimean-Congo Hemorrhagic Fever Virus in Domestic and Wild Animals. PLoS Negl. Trop. Dis. 2016, 10, e0004210. [Google Scholar] [CrossRef] [PubMed]
- Belobo, J.T.E.; Kenmoe, S.; Kengne-Nde, C.; Emoh, C.P.D.; Bowo-Ngandji, A.; Tchatchouang, S.; Wobessi, J.N.S.; Mikangue, C.A.M.; Tazokong, H.R.; Bebey, S.R.K.; et al. Worldwide Epidemiology of Crimean-Congo Hemorrhagic Fever Virus in Humans, Ticks and Other Animal Species, a Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009299. [Google Scholar] [CrossRef] [PubMed]
- Balinandi, S.; von Brömssen, C.; Tumusiime, A.; Kyondo, J.; Kwon, H.; Monteil, V.M.; Mirazimi, A.; Lutwama, J.; Mugisha, L.; Malmberg, M. Serological and Molecular Study of Crimean-Congo Hemorrhagic Fever Virus in Cattle from Selected Districts in Uganda. J. Virol. Methods 2021, 290, 114075. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Swanepoel, R.; Shepherd, S.P.; McGillivray, G.M.; Searle, L.A. Antibody to Crimean-Congo Hemorrhagic Fever Virus in Wild Mammals from Southern Africa. Am. J. Trop. Med. Hyg. 1987, 36, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mangombi, J.B.; Roqueplo, C.; Sambou, M.; Dahmani, M.; Mediannikov, O.; Comtet, L.; Davoust, B. Seroprevalence of Crimean-Congo Hemorrhagic Fever in Domesticated Animals in Northwestern Senegal. Vector-Borne Zoonotic Dis. 2020, 20, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Msimang, V.; Weyer, J.; Le Roux, C.; Kemp, A.; Burt, F.J.; Tempia, S.; Grobbelaar, A.; Moolla, N.; Rostal, M.K.; Bagge, W.; et al. Risk Factors Associated with Exposure to Crimean-Congo Haemorrhagic Fever Virus in Animal Workers and Cattle, and Molecular Detection in Ticks, South Africa. PLoS Negl. Trop. Dis. 2021, 15, e0009384. [Google Scholar] [CrossRef]
- Blanco-Penedo, I.; Obanda, V.; Kingori, E.; Agwanda, B.; Ahlm, C.; Lwande, O.W. Seroepidemiology of Crimean-Congo Hemorrhagic Fever Virus (CCHFV) in Cattle across Three Livestock Pastoral Regions in Kenya. Dairy 2021, 2, 425–434. [Google Scholar] [CrossRef]
- Chiuya, T.; Masiga, D.K.; Falzon, L.C.; Bastos, A.D.S.; Fèvre, E.M.; Villinger, J. Tick-Borne Pathogens, Including Crimean-Congo Haemorrhagic Fever Virus, at Livestock Markets and Slaughterhouses in Western Kenya. Transbound. Emerg. Dis. 2021, 68, 2429–2445. [Google Scholar] [CrossRef]
- Sang, R.; Lutomiah, J.; Koka, H.; Makio, A.; Chepkorir, E.; Ochieng, C.; Yalwala, S.; Mutisya, J.; Musila, L.; Richardson, J.H.; et al. Crimean-Congo Hemorrhagic Fever Virus in Hyalommid Ticks, Northeastern Kenya. Emerg. Infect. Dis. 2011, 17, 1502–1505. [Google Scholar] [CrossRef]
- Obanda, V.; Agwanda, B.; Blanco-Penedo, I.; Mwangi, I.A.; King’ori, E.; Omondi, G.P.; Ahlm, C.; Evander, M.; Lwande, O.W. Livestock Presence Influences the Seroprevalence of Crimean Congo Hemorrhagic Fever Virus on Sympatric Wildlife in Kenya. Vector-Borne Zoonotic Dis. 2021, 21, 809–816. [Google Scholar] [CrossRef]
- Lutomiah, J.; Musila, L.; Makio, A.; Ochieng, C.; Koka, H.; Chepkorir, E.; Mutisya, J.; Mulwa, F.; Khamadi, S.; Miller, B.R.; et al. Ticks and Tick-Borne Viruses From Livestock Hosts in Arid and Semiarid Regions of the Eastern and Northeastern Parts of Kenya. J. Med. Entomol. 2014, 51, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Diagne, M.M.; Ndione, M.H.D.; Di Paola, N.; Fall, G.; Bedekelabou, A.P.; Sembène, P.M.; Faye, O.; Zanotto, P.M.d.A.; Sall, A.A. Usutu Virus Isolated from Rodents in Senegal. Viruses 2019, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Diagne, M.M.; Faye, M.; Faye, O.; Sow, A.; Balique, F.; Sembène, M.; Granjon, L.; Handschumacher, P.; Faye, O.; Diallo, M.; et al. Emergence of Wesselsbron Virus among Black Rat and Humans in Eastern Senegal in 2013. One Health 2017, 3, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, H. ADW: Rattus Rattus: Information. Available online: https://animaldiversity.org/accounts/Rattus_rattus/#5c07902a49ae8af145719eca77117bc9 (accessed on 13 November 2021).
- Darwish, M.A.; Hoogstraal, H.; Roberts, T.J.; Ghazi, R.; Amer, T. A Sero-Epidemiological Survey for Bunyaviridae and Certain Other Arboviruses in Pakistan. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 446–450. [Google Scholar] [CrossRef]
- Gonzalez, J.; Mccormick, J.B.; Saluzzo, J.; Georges, A. Les Fièvres Hémorragiques d ’ Origine à Leur Africaines Étude Contribution En République Centrafricaine. Cah.—ORSTOM. Entomol. Médicale Et Parasitol. 1983, 21, 119–130. [Google Scholar]
- Saluzzo, J.F.; Digoutte, J.P.; Camicas, J.L.; Chauvancy, G. Crimean-Congo Haemorrhagic Fever And Rift Valley Fever In South-Eastern Mauritania. Lancet 1985, 325, 116. [Google Scholar] [CrossRef] [PubMed]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The Role of Ticks in the Maintenance and Transmission of Crimean-Congo Hemorrhagic Fever Virus: A Review of Published Field and Laboratory Studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef]
- Spengler, J.R.; Estrada-Peña, A. Host Preferences Support the Prominent Role of Hyalomma Ticks in the Ecology of Crimean-Congo Hemorrhagic Fever. PLoS Negl. Trop. Dis. 2018, 12, e0006248. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Camicas, J.L.; Cornet, J.P.; Faye, O.; Wilson, M.L. Sexual and Transovarian Transmission of Crimean-Congo Haemorrhagic Fever Virus in Hyalomma Truncatum Ticks. Res. Virol. 1992, 143, 23–28. [Google Scholar] [CrossRef]
- Bhowmick, S.; Kasi, K.K.; Gethmann, J.; Fischer, S.; Conraths, F.J.; Sokolov, I.M.; Lentz, H.H.K. Ticks on the Run: A Mathematical Model of Crimean-Congo Haemorrhagic Fever (CCHF)—Key Factors for Transmission. Epidemiologia 2022, 3, 116–134. [Google Scholar] [CrossRef]
- Bendary, H.A.; Rasslan, F.; Wainwright, M.; Alfarraj, S.; Zaki, A.M.; Abdulall, A.K. Crimean-Congo Hemorrhagic Fever Virus in Ticks Collected from Imported Camels in Egypt. Saudi J. Biol. Sci. 2022, 29, 2597–2603. [Google Scholar] [CrossRef]
- Burt, F.J.; Swanepoel, R. Molecular Epidemiology of African and Asian Crimean-Congo Haemorrhagic Fever Isolates. Epidemiol. Infect. 2005, 133, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Volynkina, A.; Lisitskaya, Y.; Kolosov, A.; Shaposhnikova, L.; Pisarenko, S.; Dedkov, V.; Dolgova, A.; Platonov, A.; Kulichenko, A. Molecular Epidemiology of Crimean-Congo Hemorrhagic Fever Virus in Russia. PLoS ONE 2022, 17, e0266177. [Google Scholar] [CrossRef]
- Dunster, L.; Dunster, M.; Ofula, V.; Beti, D.; Kazooba-Voskamp, F.; Burt, F.; Swanepoel, R.; DeCock, K.M. First Documentation of Human Crimean-Congo Hemorrhagic Fever, Kenya. Emerg. Infect. Dis. 2002, 8, 1005. [Google Scholar] [CrossRef]
- Gakuya, F.; Obanda, V.; Lwande, O.W.; Lutomiah, J.; Chepkorir, E.; Sang, R.; Mutisya, J.; Mulwa, F.; Michuki, G.; Venter, M.; et al. Isolation of Tick and Mosquito-Borne Arboviruses from Ticks Sampled from Livestock and Wild Animal Hosts in Ijara District, Kenya. Vector-Borne Zoonotic Dis. 2013, 13, 637–642. [Google Scholar] [CrossRef]
- Ogola, E.O.; Kopp, A.; Bastos, A.D.S.; Slothouwer, I.; Marklewitz, M.; Omoga, D.; Rotich, G.; Getugi, C.; Sang, R.; Torto, B.; et al. Jingmen Tick Virus in Ticks from Kenya. Viruses 2022, 14, 1041. [Google Scholar] [CrossRef] [PubMed]
- Nyataya, J.; Maraka, M.; Lemtudo, A.; Masakhwe, C.; Mutai, B.; Njaanake, K.; Estambale, B.B.; Nyakoe, N.; Siangla, J.; Waitumbi, J.N. Serological Evidence of Yersiniosis, Tick-Borne Encephalitis, West Nile, Hepatitis E, Crimean-Congo Hemorrhagic Fever, Lyme Borreliosis, and Brucellosis in Febrile Patients Presenting at Diverse Hospitals in Kenya. Vector-Borne Zoonotic Dis. 2020, 20, 348–357. [Google Scholar] [CrossRef]
- Omoga, D.C.A.; Tchouassi, D.P.; Venter, M.; Ogola, E.O.; Langat, S.; Getugi, C.; Eibner, G.; Kopp, A.; Slothouwer, I.; Torto, B.; et al. Characterization of a Novel Orbivirus from Cattle Reveals Active Circulation of a Previously Unknown and Pathogenic Orbivirus in Ruminants in Kenya. mSphere 2023, 8, e00488-22. [Google Scholar] [CrossRef]
- Omoga, D.C.A.; Tchouassi, D.P.; Venter, M.; Ogola, E.O.; Eibner, G.J.; Kopp, A.; Slothouwer, I.; Torto, B.; Junglen, S.; Sang, R. Circulation of Ngari Virus in Livestock, Kenya. mSphere 2022, 7, e00416-22. [Google Scholar] [CrossRef]
- Tchouassi, D.P.; Marklewitz, M.; Chepkorir, E.; Zirkel, F.; Agha, S.B.; Tigoi, C.C.; Koskei, E.; Drosten, C.; Borgemeister, C.; Torto, B.; et al. Sand Fly–Associated Phlebovirus with Evidence of Neutralizing Antibodies in Humans, Kenya. Emerg. Infect. Dis. 2019, 25, 681–690. [Google Scholar] [CrossRef]
- Marklewitz, M.; Tchouassi, D.P.; Hieke, C.; Heyde, V.; Torto, B.; Sang, R.; Junglen, S. Insights into the Evolutionary Origin of Mediterranean Sandfly Fever Viruses. mSphere 2020, 5, e00598-20. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Marfin, A.A.; Tukei, P.M.; Kuria, G.; Ademba, G.; Agata, N.N.; Ouma, J.O.; Cropp, C.B.; Karabatsos, N.; Reiter, P.; et al. First Recorded Outbreak of Yellow Fever in Kenya, 1992-1993. I. Epidemiologic Investigations. Am. J. Trop. Med. Hyg. 1998, 59, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Kingdon, J. Field Guide to African Mammals. Acad. Press 2015, 212–302, 314–319. [Google Scholar]
- Kingdon, J. East African Mammals, an Atlas of Evolution in Africa; Part b (Hares and Rodents); Academic Press: London, UK, 1974; Volume 2, ISBN 0124083420/9780124083424. [Google Scholar]
- Musila, S.; Monadjem, A.; Webala, P.W.; Patterson, B.D.; Hutterer, R.; De Jong, Y.A.; Butynski, T.M.; Mwangi, G.; Chen, Z.-Z.; Jiang, X.-L. An Annotated Checklist of Mammals of Kenya. Zool. Res. 2019, 40, 3–52. [Google Scholar] [CrossRef]
- Vences, M.; Nagy, Z.T.; Sonet, G.; Verheyen, E. DNA Barcodes Amphibians and Reptiles. In DNA Barcodes; Humana Press: Totowa, NJ, USA, 2012; p. 858. [Google Scholar] [CrossRef]
- Schwarz, T.F.; Nsanze, H.; Longson, M.; Nitschko, H.; Gilch, S.; Shurie, H.; Ameen, A.; Zahir, A.R.M.; Acharya, U.G.; Jager, G. Polymerase Chain Reaction for Diagnosis and Identification of Distinct Variants of Crimean-Congo Hemorrhagic Fever Virus in the United Arab Emirates. Am. J. Trop. Med. Hyg. 1996, 55, 190–196. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Langat, S.K.; Eyase, F.; Bulimo, W.; Lutomiah, J.; Oyola, S.O.; Imbuga, M.; Sang, R. Profiling of RNA Viruses in Biting Midges (Ceratopogonidae) and Related Diptera from Kenya Using Metagenomics and Metabarcoding Analysis. mSphere 2021, 6, e00551-21. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Gudo, E.S.; Ali, S.; António, V.S.; Chelene, I.R.; Chongo, I.; Demanou, M.; Falk, K.; Guiliche, O.C.; Heinrich, N.; Monteiro, V.; et al. Seroepidemiological Studies of Arboviruses in Africa. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1062, pp. 361–371. ISSN 0065-2598. [Google Scholar]
- Schulz, A.; Barry, Y.; Stoek, F.; Ba, A.; Schulz, J.; Haki, M.L.; Sas, M.A.; Doumbia, B.A.; Kirkland, P.; Bah, M.Y.; et al. Crimean-Congo Hemorrhagic Fever Virus Antibody Prevalence in Mauritanian Livestock (Cattle, Goats, Sheep and Camels) Is Stratified by the Animal’s Age. PLoS Negl. Trop. Dis. 2021, 15, e0009228. [Google Scholar] [CrossRef]
- Maiga, O.; Sas, M.A.; Rosenke, K.; Kamissoko, B.; Mertens, M.; Sogoba, N.; Traore, A.; Sangare, M.; Niang, M.; Schwan, T.G.; et al. Serosurvey of Crimean-Congo Hemorrhagic Fever Virus in Cattle, Mali, West Africa. Am. J. Trop. Med. Hyg. 2017, 96, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.A.; Mahmoud, M.A.M.; Aradaib, I.E. A Seroepidemiological Survey of Crimean Congo Hemorrhagic Fever among Cattle in North Kordufan State, Sudan. Virol. J. 2013, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Sas, M.A.; Mertens, M.; Isselmou, E.; Reimer, N.; El Mamy, B.O.; Doumbia, B.; Groschup, M.H. Crimean-Congo Hemorrhagic Fever Virus-Specific Antibody Detection in Cattle in Mauritania. Vector-Borne Zoonotic Dis. 2017, 17, 582–587. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; McCormick, J.B.; Swanepoel, R.; Van Middelkoop, A.; Harvey, S.; Kustner, H.G.V. Risk of Human Infections with Crimean-Congo Hemorrhagic Fever Virus in a South African Rural Community. Am. J. Trop. Med. Hyg. 1992, 47, 337–345. [Google Scholar] [CrossRef]
- Mohamed, M.; Said, A.-R.; Murad, A.; Graham, R. A Serological Survey of Crimean-Congo Haemorrhagic Fever in Animals in the Sharkia Governorate of Egypt. Vet. Ital. 2008, 44, 513–517. [Google Scholar] [PubMed]
- Holy, T.E.; Guo, Z. Ultrasonic Songs of Male Mice. PLoS Biol. 2005, 3, e386. [Google Scholar] [CrossRef] [PubMed]
- Cigarroa-Toledo, N.; Talavera-Aguilar, L.G.; Baak-Baak, C.M.; García-Rejón, J.E.; Hernandez-Betancourt, S.; Blitvich, B.J.; Machain-Williams, C. Serologic Evidence of Flavivirus Infections in Peridomestic Rodents in Merida, Mexico. J. Wildl. Dis. 2016, 52, 168–172. [Google Scholar] [CrossRef]
- Ballenger, L. ADW: Mus Musculus: Information. Available online: https://animaldiversity.org/accounts/Mus_musculus/ (accessed on 13 November 2021).
- Aradaib, I.E.; Erickson, B.R.; Karsany, M.S.; Khristova, M.L.; Elageb, R.M.; Mohamed, M.E.H.; Nichol, S.T. Multiple Crimean-Congo Hemorrhagic Fever Virus Strains Are Associated with Disease Outbreaks in Sudan, 2008–2009. PLoS Negl. Trop. Dis. 2011, 5, 2008–2009. [Google Scholar] [CrossRef]
- Bower, B.; Fletcher, T.E.; Mohamed, R.; Alzain, M.; Elhalawi, A.; Osman, A.; Semper, A.; Brooks, T.; Osborne, J.; Furneaux, J.; et al. Severe Undifferentiated Febrile Illness Outbreaks in the Federal Republic of Sudan—A Retrospective Epidemiological and Diagnostic Study. Int. J. Infect. Dis. 2016, 79 (Suppl. S1), 123–124. [Google Scholar] [CrossRef]
- Bukbuk, D.N.; Dowall, S.D.; Lewandowski, K.; Bosworth, A.; Baba, S.S.; Varghese, A.; Watson, R.J.; Bell, A.; Atkinson, B.; Hewson, R. Serological and Virological Evidence of Crimean-Congo Haemorrhagic Fever Virus Circulation in the Human Population of Borno State, Northeastern Nigeria. PLoS Negl. Trop. Dis. 2016, 10, e0005126. [Google Scholar] [CrossRef]
- Lukashev, A.N.; Klimentov, A.S.; Smirnova, S.E.; Dzagurova, T.K.; Drexler, J.F.; Gmyl, A.P. Phylogeography of Crimean Congo Hemorrhagic Fever Virus. PLoS ONE 2016, 11, e0166744. [Google Scholar] [CrossRef]
- Goedhals, D.; Bester, P.A.; Paweska, J.T.; Swanepoel, R.; Burt, F.J. Next-Generation Sequencing of Southern African Crimean-Congo Haemorrhagic Fever Virus Isolates Reveals a High Frequency of M Segment Reassortment. Epidemiol. Infect. 2014, 142, 1952–1962. [Google Scholar] [CrossRef]

