1. Introduction
Adenoid cystic carcinoma (ACC) of the head and neck is an extremely rare type of cancer, accounting for about 1% of malignant head and neck tumors and 10% of malignant salivary gland tumors [1]. ACC is generally slow-growing but has a high potential for local recurrence and metastasis to distant organs; five-, ten-, and twenty-year survival rates for overall survival were 68%, 52%, and 28%, respectively [2,3]. Most patients with head and neck cancer (HNC) are treated with aggressive multidisciplinary approaches, including surgical resection, radiation therapy, and systemic chemotherapy, including molecular targeted agents or immune checkpoint inhibitors (ICIs). However, there is currently no standard systemic treatment for R/M ACC [4].
In recent years, ICI therapy has been developed and clinically applied to various cancers. Treatment with nivolumab and pembrolizumab, anti-programmed cell death-1 (PD-1) protein monoclonal antibodies, has been widely available for patients with recurrent or metastatic head and neck cancer (R/M HNC) [5]. The interaction between the PD-1 receptor and programmed cell death ligand-1 (PD-L1) inhibits T-cell immunological activities and is recognized as a representative mechanism of tumor immune evasion [6]. ICIs can enhance anti-tumor immune activity by blocking inhibitory signaling through the PD-1/PD-L1 pathway [7]. However, the response rate of ICI therapy has been shown to be 13–17% [8,9], and long-term response cases are limited. Therefore, the elucidation of biomarkers to predict ICI efficacy has been crucial for various types of solid tumors.
Recently, comprehensive genomic profiling (CGP) using next-generation sequencing (NGS) was developed in the clinical setting for the selection of promising molecularly targeted therapies. With the spread of CGP, cancer treatment has become increasingly personalized, and “precision medicine”, in which treatment is optimized for individuals according to their genetic mutations, is making progress [10]. Currently, biomarkers, such as microsatellite instability (MSI), the PD-L1 combined positive score (CPS), and tumor mutation burden (TMB), are used for selecting patients for clinical ICI therapy [11]. The population of MSI high is extremely low and could not be a universal marker for ICIs in patients with HNC [12]. It has been suggested that patients with TMB high had clinical benefits to ICIs, although PD-L1 CPS did not correlate with the efficacy of ICIs in real-world data in HNSCC [13]. There are cases in which ICIs are administered for ACC according to HNSCC. However, the efficacy of ICIs for ACC remains controversial [14] under the condition of the uninflamed and immunosuppressive tumor microenvironment in ACC [15,16].
This study examined the database of national genomic and clinical information established by the Center for Cancer Genomics and Advanced Therapy (C-CAT) based on the national policy to evaluate real-world data on genetic and clinical utility in R/M ACC of the head and neck. In addition, we investigated gene mutations using CGP in patients with R/M ACC who received ICIs according to HNSCC in our institution and evaluated the association between ICI response and predictive candidates, including TMB and genetic alterations in patients with ACC.
2. Materials and Methods
2.1. Patient Characterization on the C-CAT Database
We queried an anonymized database of genomic and clinical information on patients with cancer, collected from core hospitals using C-CAT. Cancer gene panel tests described in Section 2.3 were conducted at all designated hub hospitals in Japan. Gene profiling results were integrated into the C-CAT database at the National Cancer Center, Japan, and the data for each case from October 2021 could be utilized. The clinical data of C-CAT included age, sex, histology, type of cancer, treatment before and after the oncogene panel test, response to a drug, and type of CGP test. The study project in patients with R/M HNC was approved by the C-CAT Information Utilization Review Committee (proposal control number: CDU2022-021N).
2.2. Patient Population and Characterization at Tokyo Medical and Dental University (TMDU)
The clinical data, such as age, sex, performance status, and imaging evaluation before and after the systemic treatment for ACC, were collected. Computed tomography (CT) was performed at baseline and, thereafter, every 8–12 weeks until progression or treatment discontinuation. The 18-FDG-PET scan was performed once every year. We performed an oncogene panel test for R/M oral adenoid cystic carcinoma patients who were treated with immune checkpoint inhibitors as first-line therapy at our institution. In terms of informed consent, we obtained written consent from all patients for the use of genomic and clinical data for research purposes.
2.3. Comprehensive Genomic Profiling (CGP) Analysis
The C-CAT database currently includes the genomic information on all types of tumors from the Foundation One® Companion Diagnostic (F1CDx; Foundation Medicine, Inc., Cambridge, MA, USA) test, Foundation One® Liquid Companion Diagnostic (F1LCDx; Foundation Medicine, Inc.) test, and National Cancer Center (NCC) Oncopanel test. F1CDx and F1LCDx comprehensively analyzed 324 gene alterations, including substitutions, insertions, deletions, copy number changes, selective gene rearrangements, and calculated genomic signatures, such as MSI and TMB [17]. The NCC Oncopanel test examines mutations, amplifications, and homozygous deletions of the entire coding region of 127 genes of clinical or preclinical relevance, along with rearrangements [18]. The F1CDx test was used to obtain the genomic profiling in ACC of head and neck at our institution. The F1CDx assay used formalin-fixed paraffin-embedded (FFPE) tumor tissue samples obtained via biopsy or surgical operation, and the suitable tumor specimens for testing were selected by pathologists.
2.4. Expert Panel Discussion
Each result in the CGP test report was discussed at a molecular tumor board meeting by a multidisciplinary team. The board consisted of experts, such as clinical oncologists, pathologists, clinical geneticists, bioinformaticians, genomic researchers, and genetic counselors, and discussed issues, such as genetically informed treatment options and interpretation of somatic/genetic mutations. When a gene alteration is the target of a molecular targeted therapy, it is considered an actionable mutation. A drug is available for human use either as an antibody or as a small molecule compound with an IC50 concentration in the nanomolar range [19]. Evidence-level classification of gene aberrations was decided according to the Clinical Practice Guidance for NGS in Cancer Diagnosis and Treatment Edition 2.1 issued by three associations: the Japanese Society of Medical Oncology (JSMO), Japan Society of Clinical Oncology (JSCO), and Japanese Cancer Association (JCA) [20].
2.5. Efficacy Evaluation of ICI Therapy
We used anti-PD-1 inhibitors, such as nivolumab or pembrolizumab, and evaluated tumor response according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria [21]. The objective response rate (ORR) was defined as the total number of patients who had complete response (CR) or partial response (PR). Disease control rate (DCR) was defined as the total number of patients with CR, PR, or stable disease (SD).
2.6. Biomarker Assessment
PD-L1 CPS score was evaluated in FFPE tumor samples using the PD-L1 immunohistochemistry (IHC) 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA, USA). TMB, defined as the total number of somatic mutations per million base pairs in the tumor genome, was measured using the CGP test [22]. TMB was divided into three groups: low (5 Muts/Mb or less), intermediate (6–9 Muts/Mb), and high scores (10 Muts/Mb or more), as described in a previous report [13].
3. Results
3.1. Comprehensive Patient Population and Genetic Characterization of CGP in C-CAT Data
Between June 2019 and June 2023, of the 53,906 patients enrolled in the C-CAT database, 1886 were classified with R/M HNC, of which 263 were categorized with ACC. Among them, 250 (F1 test) and 13 (NCC Oncopanel) patients were analyzed, respectively (Figure 1A). We found a total of 272 gene alterations and showed the top 10 most frequent genetic mutations detected in 263 ACC patients. The commonly mutated genes were NOTCH1 (34%), followed by SPEN (17%), KMT2D (16%), LTK (15%), and KDM6A (14%), which is mainly consistent with a previous large study [23]. MYB structure variants were about 14% (Figure 1B).

Figure 1. The scheme of total population and genetic information in ACC patients from CGP in the C-CAT database. (A) The scheme of the population from the C-CAT database using the F1(F1CDx or F1LCDx) test and NCC Oncopanel test. Information of 53,906 patients was registered in C-CAT, with a total of 263 patients diagnosed with R/M ACC of head and neck. (B) Percentage of the top 10 most frequent genes and variant types by histological type. Color coding indicates the variant type. (C) Percentage of TMB value in 59 patients who received ICI. TMB value is indicated as low (5 Muts/Mb or less), intermediate (6–9 Muts/Mb), and high (10 Muts/Mb or more).
3.2. Outcomes of ICI Therapy in C-CAT Data
Of the 263 patients with R/M ACC, 59 (22%) received immune checkpoint inhibitors, of which 26 were treated with nivolumab and 33 with pembrolizumab. First, we examined the treatment efficacy of 59 ACC patients. Of these, seventeen (29%) cases were not evaluable (NE). Two (3%) patients experienced CR, three (5%) had PR, twenty-six (44%) had SD, and eleven (19%) had PD; the ORR was 8%, and the DCR was 53%. The patients were divided into three groups (low, intermediate, and high) according to TMB values to evaluate the differences in ICI treatment efficacy, as described in a previous report [13]. Of 59 ACC patients, 54 (92%) were in the low TMB, 4 (7%) were in the intermediate, and 1 (2%) was in the high group (Figure 1C). In the TMB-low group, the best response to ICIs was CR in 2/54 (4%), PR in 3/54 (6%), and SD in 23/54 (43%) patients, and, therefore, the ORR was 9% and the DCR was 52%. The TMB-intermediate group showed SD in 2/4 (50%) patients and DCR of 50%. One patient in the TMB-high group was SD A). The median TMB was 2 Muts/Mb (range, 0–11). The median age of the patients with ACC was 61 years (range: 34–77 years), and 54% were female. In terms of results by gender, the ORR was 7% and the DCR was 56% for males and 9% and 50% for females, respectively B).
Furthermore, we compared the patients who received ICIs as first-line therapy with second-line or later-line therapy to eliminate the ambiguous difference between therapy lines. Of the 59 patients, 28 received ICIs as the first-line therapy and 31 received ICIs as the second-line or later-line therapy. In the 28 patients who received ICIs as first-line therapy, 1 (4%) patient experienced CR, 1 (4%) had PR, 8 (29%) had SD, 7 (25%) had PD, and 11 (39%) were NE; the ORR was 7% and the DCR was 36%. Of 28 patients, 25 (89%) were in the low-TMB group, 3 (11%) in the intermediate group, and no patients were in the high group. The best response to ICIs in the TMB-low group was CR in 1/25 (4%), PR in 1/25 (4%), and SD in 6/25 (24%) patients, and, therefore, the ORR was 8% and the DCR was 32%. The TMB-intermediate group showed SD in 2/3 (66%) patients and a DCR of 67% A). In the 31 patients who received ICIs as the second-line or later-line therapy, 1 (3%) patient experienced CR, 2 (6%) had PR, 18 (58%) had SD, 4 (13%) had PD, and 6 (19%) were NE; the ORR was 10% and the DCR was 68%. Of 31 patients, 29 (94%) were in the low-TMB group, 1 (3%) in the intermediate group, and 1 (3%) was in the high group. The best response to ICIs in the TMB-low group was CR in 1/29 (3%), PR in 2/29 (7%), and SD in 17/29 (59%) patients, and, therefore, the ORR was 11% and the DCR was 69%. One patient in the TMB-intermediate group was PD, and one patient in the TMB-high group was SD B). Thus, the patients who received ICIs as first-line or second/later-line therapy had a similar ORR, and TMB could not be a predictive marker for ICI therapy due to the extremely low number of patients with ACC of the head and neck.
Next, we examined the treatment efficacy in ACC patients with NOTCH1, KDM6A, BRAF mutations, and MYB structural variants, as these genes were commonly observed and associated with poorer prognosis in ACC [23,24]. NOTCH1, BRAF mutations, and MYB structural variants were found in thirteen, four, five, and five patients, respectively. In NOTCH1 and BRAF mutation cases, the ORR was 0% and the DCR was about 50%. In KDM6A mutation cases, the ORR was 0% and the DCR was 20%. In the cases with MYB structure variants, one (20%) patient experienced CR, and one (20%) had PR; both ORR and DCR were 40% . The five cases that were CR or PR are shown in . The median TMB was 1.26 Muts/Mb for CR and PR cases that did not correlate with treatment response.
3.3. Outcomes of ICI Therapy and Sequencing Results in TMDU
Between 26 February 2020 and 31 May 2023, four patients with R/M ACC who were treated with immune checkpoint inhibitors as first-line therapy underwent CGP with an F1 test at TMDU. We examined the treatment response of four ACC patients. All patients expressed more than 1% of PD-L1 CPS, which allowed for the administration of ICIs. All patients were treated with pembrolizumab; of these, three patients experienced SD, and one had PD. TMB values were ≤5 Muts/Mb in all patients A).
We evaluated the genetic mutations detected in four R/M ACC patients who were treated for ICI at our institution. The commonly mutated genes were NOTCH1, TP53, and TERT promoter. MYB rearrangement and MYB-NFIB fusion were found in the same case B). Of the four patients, one patient with MYB structure variants who had a long-term stable disease is shown below.
3.4. Case Presentation (Case No.3 in Table 5A,B)
A 63-year-old man was diagnosed with a submandibular gland tumor and underwent a submandibular adenectomy in December 2012. The intraoperative frozen section revealed ACC, so concurrent neck dissection (level I-IIA) was performed. Thereafter, he underwent adjuvant radiotherapy (60 Gy) in January 2013. In February 2020, a CT scan showed metastasis to the left lower lobe of the lung, and he underwent an anatomic segmentectomy. Histopathological examination identified a metastatic tumor consistent with ACC of the submandibular gland tumor. In addition, in October 2020, a CT scan confirmed metastasis to the right lower lobe of the lung, an anatomic segmentectomy was performed, and a histopathologic examination identified a metastatic tumor consistent with ACC of the primary tumor. Furthermore, the 18-FDG-PET scan showed new metastasis to both side lobes of the lung, and the NGS panel using F1CDx revealed that the TMB was 1 Muts/Mb and showed mutated genes, including BRAF, NOTCH1, and MYB structural variants B). Pembrolizumab was started in January 2022 (Figure 2A), and two months after administration, a CT scan showed a significant reduction in the target lesions. Seventeen months have passed since pembrolizumab’s first administration, and the target lesions were maintained without new recurrence and metastases (Figure 2B).
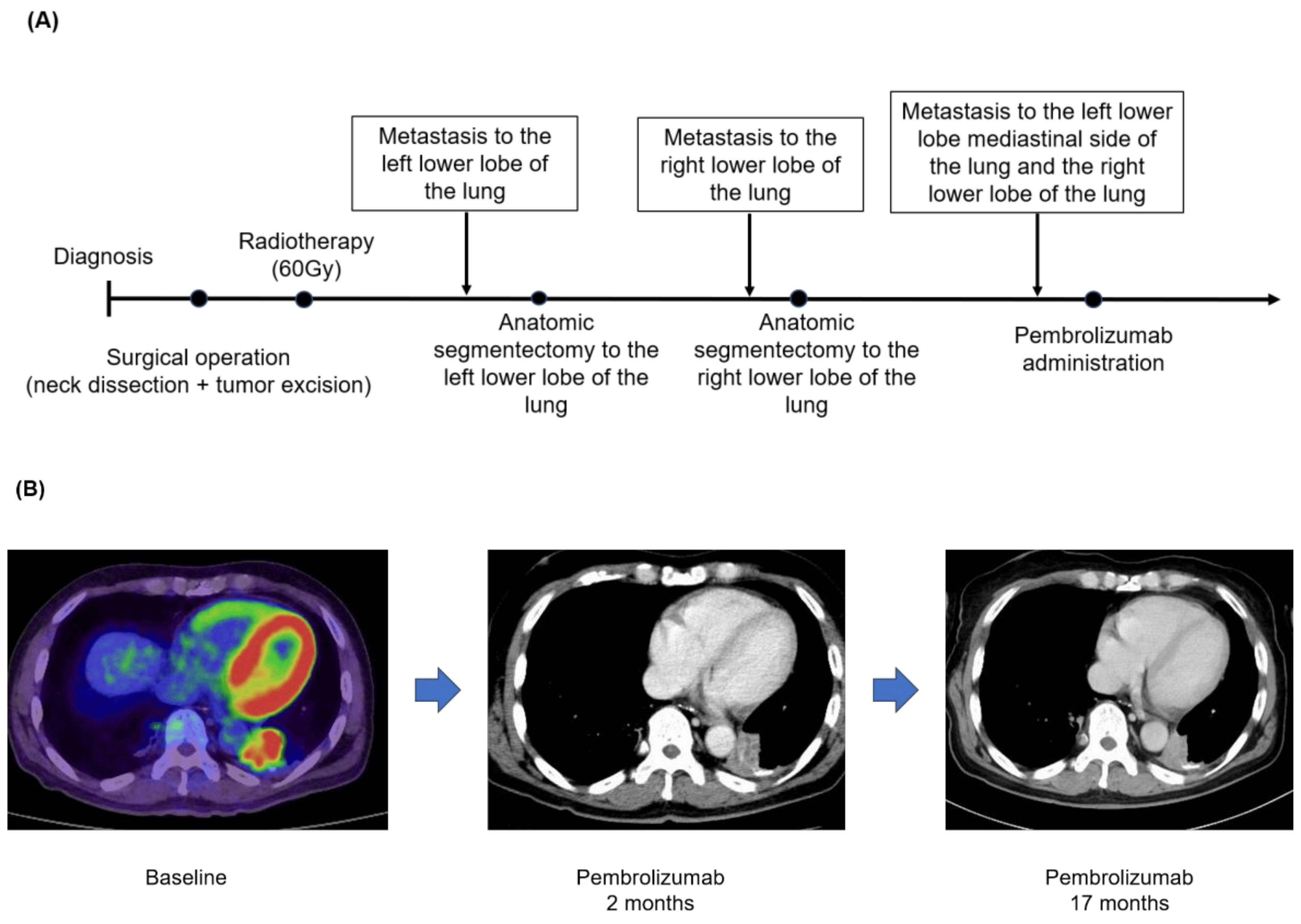
Figure 2. (A) Clinical course. (B) FDG-PET or CT scan before therapy with pembrolizumab, 2 months after treatment, and 17 months since he initiated the ICI therapy. The red color means FDG uptake.
4. Discussion
We herein conducted a retrospective study of the response to ICI therapy for patients with ACC of the head and neck based on CGP from the nationwide database and institutional cohort. Information on cancer gene panel tests conducted at all designated hospitals in Japan was, in principle, integrated into the C-CAT database. Thus, this database sheds light on the molecular biology or genetic findings in a rare type of cancer such as ACC.
First, we examined the clinical analysis of CGP in 263 ACCs of the head and neck in C-CAT data. The commonly mutated genes were NOTCH1 (34%), followed by SPEN (17%), KMT2D (16%), LTK (15%), KDM6A (14%), and MYB structure variants (14%). The NOTCH signaling pathway plays an important role in regulating cell proliferation and survival. NOTCH1 mutations in ACC occur in advanced-stage disease, distinct metastatic patterns, and poor prognosis [24]. KDM6A is important for the differentiation of embryonic stem cells and the development of various tissues and functions as a tumor suppressor [25]. NOTCH1 and KDM6A mutations have been reported to be associated with poorer prognosis compared to NOTCH1 and KDM6A wild-type patients, respectively [23].
MYB structure variants were called at a frequency of 14% from C-CAT data analysis, which was lower compared to the previous reports of 65–82% of ACC in the head and neck [26,27]. One reason is that MYB structure variants are not included in the list of NCC Oncopanel, and the other is that NGS panel tests (F1 and NCC Oncopanel) are exclusively performed using the DNA sequence, resulting in lower detection of structural variants, including rearrangement or fusion. MYB is a nuclear transcription factor and frequently occurs in the chromosome t(6;9) translocation with a transcription factor, NFIB, leading to the MYB-NFIB fusion that is considered to be a genetic hallmark of ACC [28]. MYB also increased metastasis by regulating ICAM1, VEGFA, MMP7, MMP9, and EMT-related markers, such as E-cadherin, vimentin, N-cadherin, and alpha-SMA. In addition, it was determined that MYB was closely associated with lung metastasis in patients with salivary adenoid cystic carcinoma through a xenograft mouse model [29] while it has been reported that no significant prognostic differences were observed between MYB-positive and MYB-negative ACC patients in the head and neck [30]. Importantly, the tyrosine kinase inhibitor (TKI) axitinib showed a trend toward superior progression-free survival (PFS) in ACC patients with MYB/NFIB rearrangements [31].
Second, we analyzed the efficacy of 59 ACC patients who were treated with ICIs. Due to its molecular and histological characteristics, such as lower immunogenicity, low TMB, fewer tumor-infiltrating lymphocytes, fewer dendritic cells, and lower levels of PD-1+ and CTLA4+ cells [15,32,33], the efficacy of ICI therapy has been limited, although promising data were also reported in R/M ACC [14,34]. In the C-CAT cohort, two (3%) patients experienced CR, three (5%) had PR, twenty-six (44%) had SD, and eleven (19%) had PD; the ORR was 8% and the DCR was 53%. It has been suggested that a higher value of TMB shows a positive association with higher response rates to ICI therapy in solid tumors. It was found that the median TMB was 2 Muts/Mb (range, 0–11) in ACC of the head and neck, while the TMB median was 5 Muts/Mb (range, 0–34) in our previous report in HNSCC [13]. There was only one case in the TMB-high group with SD. Therefore, there are only a few cases in patients with TMB high in R/M ACC, and TMB could not be a useful predictive marker.
Next, the efficacy of ICIs was analyzed with genetic mutations, such as NOTCH1, KDM6A, BRAF, and MYB. There are no response cases of ICIs with NOTCH1, KDM6A, or BRAF mutations, while MYB structural alteration showed 40% of ORR, suggesting MYB might be a candidate marker for a better prognosis for ICIs. Then, we examined the treatment response to ICIs and the clinical analysis of CGP in our institution. We found that the case with MYB structure variants had a long-term response, even with co-mutation of NOTCH1 and BRAF activation, suggesting that MYB alteration might have a strong effect on ICI efficacy. Intriguingly, a recent study showed that MYB is essential for the development of precursors of exhausted T cells, and the response to PD-1 checkpoint inhibition depends on MYB expression [35], which is consistent with our results. Furthermore, very recently, the addition of the PD-L1 inhibitor avelumab to axitinib has been shown to be beneficial in ACC of the head and neck [36], leading to its inclusion as a therapeutic option for ACC in the National Comprehensive Cancer Network guidelines.
To the best of our knowledge, this report could be the first in a real-world setting as there has been no report on the response of ICIs in ACC of the head and neck based on a comprehensive genomic profile. In C-CAT data, there were no ICI responses in patients with NOTCH1, KDM6A, or BRAF mutations, but there were response cases with MYB structure variants. In our institution, one patient with MYB structure variants had a long-term response, despite the presence of a NOTCH1 mutation. This result is expected to help predict the efficacy of ICI therapy in patients with R/M ACC of the head and neck.
There are a few limitations in this study. First, although the C-CAT database provided comprehensive results of genomic and clinical information regarding the response to therapies, such as the ORR or DCR, overall survival (OS) or progression-free survival (PFS) is not currently available. Therefore, we need to evaluate the survival rate in our institute; however, the sample size was very small so we could not perform any statistical analysis here. Second, this study was conducted domestically and retrospectively. In the future, further investigation should be prospectively conducted with an international multicentric cohort, including a larger sample size with MYB structural variants to confirm the results of this study.
5. Conclusions
In this study, we evaluated the clinical utility of CGP and responses to ICIs in head and neck ACCs using a nationwide database and institutional cohort. We found commonly mutated genes, such as NOTCH1 (34%), followed by SPEN (17%), KMT2D (16%), LTK (15%), KDM6A (14%), and MYB structure variants (14%). Interestingly, the population frequency of MYB structural variants was lower compared to previous reports. Notably, the C-CAT database revealed that ICI therapy did not respond in ACC patients with NOTCH1, KDM6A, or BRAF mutations, but there were response cases with MYB structure variants. Furthermore, we showed a case of long-term response to ICIs in our institution with MYB structural variants. ICI therapy could be a potential treatment option; however, the TMB value in ACC was relatively lower overall compared to HNSCC and could not be universally useful in predicting the efficacy of ICIs. The MYB structural variant might be a candidate for predictive biomarkers for immunotherapy in patients with R/M ACC.
References
- Atallah, S.; Casiraghi, O.; Fakhry, N.; Wassef, M.; Uro-Coste, E.; Espitalier, F.; Sudaka, A.; Kaminsky, M.C.; Dakpe, S.; Digue, L.; et al. A prospective multicentre REFCOR study of 470 cases of head and neck Adenoid cystic carcinoma: Epidemiology and prognostic factors. Eur. J. Cancer 2020, 130, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.S.; Roh, J.L.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for survival and distant metastasis in 125 patients with head and neck adenoid cystic carcinoma undergoing primary surgery. J. Cancer Res. Clin. Oncol. 2020, 146, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Van Weert, S.; Bloemena, E.; van der Waal, I.; de Bree, R.; Rietveld, D.H.; Kuik, J.D.; Leemans, C.R. Adenoid cystic carcinoma of the head and neck: A single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013, 49, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Lorini, L.; Ardighieri, L.; Bozzola, A.; Romani, C.; Bignotti, E.; Buglione, M.; Guerini, A.; Lombardi, D.; Deganello, A.; Tomasoni, M.; et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021, 115, 105213. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.; Gillison, M.L.; Pfister, D.G.; Spencer, S.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Haendel, M.A.; Chute, C.G.; Robinson, P.N. Classification, Ontology, and Precision Medicine. N. Engl. J. Med. 2018, 379, 1452–1462. [Google Scholar] [CrossRef]
- Schlauch, D.; Fu, X.; Jones, S.F.; Burris, H.A., 3rd; Spigel, D.R.; Reeves, J.; McKenzie, A.J. Tumor-Specific and Tumor-Agnostic Molecular Signatures Associated With Response to Immune Checkpoint Inhibitors. JCO Precis. Oncol. 2021, 5, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Blons, H.; Cabelguenne, A.; Carnot, F.; Laccourreye, O.; de Waziers, I.; Hamelin, R.; Brasnu, D.; Beaune, P.; Laurent-Puig, P. Microsatellite analysis and response to chemotherapy in head-and-neck squamous-cell carcinoma. Int. J. Cancer 1999, 84, 410–415. [Google Scholar] [CrossRef]
- Noji, R.; Tohyama, K.; Kugimoto, T.; Kuroshima, T.; Hirai, H.; Tomioka, H.; Michi, Y.; Tasaki, A.; Ohno, K.; Ariizumi, Y.; et al. Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer. Cancers 2022, 14, 3476. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Fetten, J.V.; Michel, L.S.; Kriplani, A.; Morris, L.; Ostrovnaya, I.; Katabi, N.; Haque, S.; et al. A phase II trial cohort of nivolumab plus ipilimumab in patients (Pts) with recurrent/metastatic adenoid cystic carcinoma (R/M ACC). J. Clin. Oncol. 2019, 37, 6084. [Google Scholar] [CrossRef]
- Mosconi, C.; de Arruda, J.A.A.; de Farias, A.C.R.; Oliveira, G.A.Q.; de Paula, H.M.; Fonseca, F.P.; Mesquita, R.A.; Silva, T.A.; Mendonça, E.F.; Batista, A.C. Immune microenvironment and evasion mechanisms in adenoid cystic carcinomas of salivary glands. Oral Oncol. 2019, 88, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Guazzo, E.; Cooper, C.; Wilkinson, L.; Feng, S.; King, B.; Simpson, F.; Porceddu, S.; Panizza, B.; Coward, J.I.G. Therapeutic implications of immune-profiling and EGFR expression in salivary gland carcinoma. Head Neck 2021, 43, 768–777. [Google Scholar] [CrossRef]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef]
- Sunami, K.; Ichikawa, H.; Kubo, T.; Kato, M.; Fujiwara, Y.; Shimomura, A.; Koyama, T.; Kakishima, H.; Kitami, M.; Matsushita, H.; et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019, 110, 1480–1490. [Google Scholar] [CrossRef]
- Matsudera, S.; Kano, Y.; Aoyagi, Y.; Tohyama, K.; Takahashi, K.; Kumaki, Y.; Mitsumura, T.; Kimura, K.; Onishi, I.; Takemoto, A.; et al. A Pilot Study Analyzing the Clinical Utility of Comprehensive Genomic Profiling Using Plasma Cell-Free DNA for Solid Tumor Patients in Japan (PROFILE Study). Ann. Surg. Oncol. 2021, 28, 8497–8505. [Google Scholar] [CrossRef]
- Naito, Y.; Aburatani, H.; Amano, T.; Baba, E.; Furukawa, T.; Hayashida, T.; Hiyama, E.; Ikeda, S.; Kanai, M.; Kato, M.; et al. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int. J. Clin. Oncol. 2021, 26, 233–283. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Valero Mayor, C.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N., Jr.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Broun, A.; Ge, K. Lysine Demethylase KDM6A in Differentiation, Development, and Cancer. Mol. Cell Biol. 2020, 40, e00341-20. [Google Scholar] [CrossRef]
- Brill, L.B., 2nd; Kanner, W.A.; Fehr, A.; Andrén, Y.; Moskaluk, C.A.; Löning, T.; Stenman, G.; Frierson, H.F., Jr. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod. Pathol. 2011, 24, 1169–1176. [Google Scholar] [CrossRef]
- Rettig, E.M.; Tan, M.; Ling, S.; Yonescu, R.; Bishop, J.A.; Fakhry, C.; Ha, P.K. MYB rearrangement and clinicopathologic characteristics in head and neck adenoid cystic carcinoma. Laryngoscope 2015, 125, E292–E299. [Google Scholar] [CrossRef]
- Humtsoe, J.O.; Kim, H.S.; Jones, L.; Cevallos, J.; Boileau, P.; Kuo, F.; Morris, L.G.T.; Ha, P. Development and Characterization of MYB-NFIB Fusion Expression in Adenoid Cystic Carcinoma. Cancers 2022, 14, 2263. [Google Scholar] [CrossRef]
- Xu, L.H.; Zhao, F.; Yang, W.W.; Chen, C.W.; Du, Z.H.; Fu, M.; Ge, X.Y.; Li, S.L. MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int. J. Oncol. 2019, 54, 1579–1590. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Lao, X.; Liang, Y. The value of MYB as a prognostic marker for adenoid cystic carcinoma: Meta-analysis. Head Neck 2019, 41, 1517–1524. [Google Scholar] [CrossRef]
- Ho, A.L.; Dunn, L.; Sherman, E.J.; Fury, M.G.; Baxi, S.S.; Chandramohan, R.; Dogan, S.; Morris, L.G.; Cullen, G.D.; Haque, S.; et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann. Oncol. 2016, 27, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Gay, L.M.; Wang, K.; Vergilio, J.A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.M.; Russell, J.; Ali, S.; et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann. Oncol. 2017, 28, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Witte, H.M.; Gebauer, N.; Lappöhn, D.; Umathum, V.G.; Riecke, A.; Arndt, A.; Steinestel, K. Prognostic Impact of PD-L1 Expression in Malignant Salivary Gland Tumors as Assessed by Established Scoring Criteria: Tumor Proportion Score (TPS), Combined Positivity Score (CPS), and Immune Cell (IC) Infiltrate. Cancers 2020, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.L.; Le Tourneau, C.; et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am. J. Clin. Oncol. 2018, 41, 1083–1088. [Google Scholar] [CrossRef]
- Tsui, C.; Kretschmer, L.; Rapelius, S.; Gabriel, S.S.; Chisanga, D.; Knöpper, K.; Utzschneider, D.T.; Nüssing, S.; Liao, Y.; Mason, T.; et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 2022, 609, 354–360. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Sousa, L.G.; Feng, L.; Mott, F.; Blumenschein, G.; Altan, M.; Bell, D.; Bonini, F.; Li, K.; Marques-Piubelli, M.L.; et al. Phase II Clinical Trial of Axitinib and Avelumab in Patients With Recurrent/Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2023, 41, 2843–2851. [Google Scholar] [CrossRef]

