1. Introduction
Diabetes is a devastating chronic disorder that is caused by a cascade of events manifested by the decrease in or the blockage of insulin release from the pancreas. Ineffective insulin production or use causes hyperglycemia, or high blood glucose, which leads to organ and tissue damage over time [1]. About 422 million people worldwide have diabetes, most in low- and middle-income countries, and 1.5 million die from it each year. The number and prevalence of people with diabetes have been rising for decades [2]. The global incidence of diabetes is on the rise, where 537 million persons in the age range of 20–79 years have been diagnosed, constituting 10.5% of the total adult population within this age bracket. The global prevalence of diabetes is expected to reach 643 million individuals by the year 2030 and is anticipated to rise further to 783 million by 2045 [3]. According to the International Diabetes Federation [4], there has been a consistent upward trend in the prevalence of diabetes in South-East Asian countries over the past two decades. The latest estimates have surpassed all previous projections.
The increased prevalence of diabetes necessitates the use of medicinal herbs for both treatment and prevention. An increasing number of people are looking for ways to incorporate natural bioactive substances in alternative medicine due to their low toxicity, low cost, fewer side effects, and ease of acquisition [5]. One promising herb is Psychotria malayana Jack, known in Malaysia as “salung”. It is widely distributed over the areas of the Philippines, Indonesia, Malaysia, and Papua New Guinea [6]. P. malayana is traditionally used in the treatment of diabetes, wounds, skin infections, and various other dermatological conditions [6,7]. This plant has been reported to possess α-glycosidase inhibitory activity towards carbohydrate hydrolysis and to slow glucose absorption [7]. α-Glucosidase inhibitors have been shown to be effective for people with type 2 diabetes mellitus [8]. According to a previous study by our research group, it was found that the methanol and water extracts of P. malayana leaf exhibited noteworthy α-glucosidase inhibitory activity, with IC50 values of 2.71 and 6.75 µg/mL, respectively. Palmitic acid, 1,3,5-benzenetriol, β-tocopherol, α-tocopherol, cholesta-7,9(11)-diene-3-ol, 24-epicampesterol, stigmast-5-ene, and monopalmitin were the active anti-diabetic metabolites identified in the extracts [7]. The anti-diabetic efficacy of the aqueous leaf extract was observed in an adult zebrafish model of type 1 diabetes. The extract demonstrated efficacy in reducing and normalizing the blood glucose levels of alloxan-induced zebrafish [9]. This prompts the further optimization of the P. malayana leaf extract to augment the inhibitory activity of α-glucosidase. In the pathogenesis of type 2 diabetes, the body’s insulin sensitivity diminishes, thereby inducing insulin resistance, which in turn triggers an inflammatory response. A reciprocal relationship can ensue, wherein heightened inflammation leads to increased insulin resistance, and conversely, increased insulin resistance exacerbates inflammation [10]. The overproduction of reactive species has the potential to exhaust the antioxidant defense system. Therefore, prolonged exposure to elevated levels of reactive oxygen species can result in enduring inflammation and insulin resistance [11,12]. Both the anti-inflammatory and antioxidant properties of OE were evaluated in this study to relate to its anti-diabetic property.
This study employed zebrafish for toxicity testing. The use of zebrafish embryos has gained significance in the fields of toxicology due to some advantages. The optical transparency exhibited by early-life stages of zebrafish enables the examination of visuo-neural circuitry functionality [13]. Zebrafish share 84% homology to gene-related diseases in mammals. Moreover, their use has as low cost, is time effective, and has easy maintenance [14].
Therefore, this study was designed to test the abilities of OE against diabetes, and inflammation and oxidative reactions, to evaluate its toxicity using a zebrafish embryo/larvae model, and to analyze its metabolites.
2. Results
2.1. Pharmacological Activity Study
The α-glucosidase inhibition (AGI), soybean lipoxygenase inhibitory (SLOXI), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, and ferric reducing antioxidant power (FRAP) activities of OE are outlined in . It shows that OE had high AGI activity, SLOXI activity, and antioxidant activities. The strength of the bioactivity is categorized as very strong, strong, moderate, and weak at the IC50 values of less than 50, 50–100, 101–150, and more than 150 ppm, respectively [15]. Therefore, OE has very strong AGI and antioxidant activities.
2.2. Toxicological Effect Analysis
2.3. Putative Metabolites of OE Detected by GC-MS and LC-MS
The compounds identified in the optimized extract of P. malayana leaves, after undergoing derivatization, were validated by cross-referencing the fragment m/z spectrum of each compound with the mass spectra database provided by the National Institute of Standards and Technology (NIST) 11. The chemical composition of the extracts was partially elucidated through GC-MS analysis following derivatization. displays the putative compounds, their retention time, peak area, similarity index (SI), and molecular formula. A variety of phytoconstituents belonging to different categories were identified, including organic acids (propanoic acid, butanedioic acid, pentanedioic acid, 1-cyclohexene-1-carboxylic acid, hexadecanoic acid, D-gluconic acid, and octadecanoic acid), sugar alcohols (arabinitol, ribitol, and myo-inositol), and simple sugars (D-(−)-tagatose, D-galactose, D-mannose, D-glucose, D-(+)-turanose, and D-turanose). The similarity index of all these compounds was equal to or greater than 90%. The putative compounds were identified and labelled at their corresponding peaks in the representative GC-MS chromatogram of the optimized extract (Figure 5).
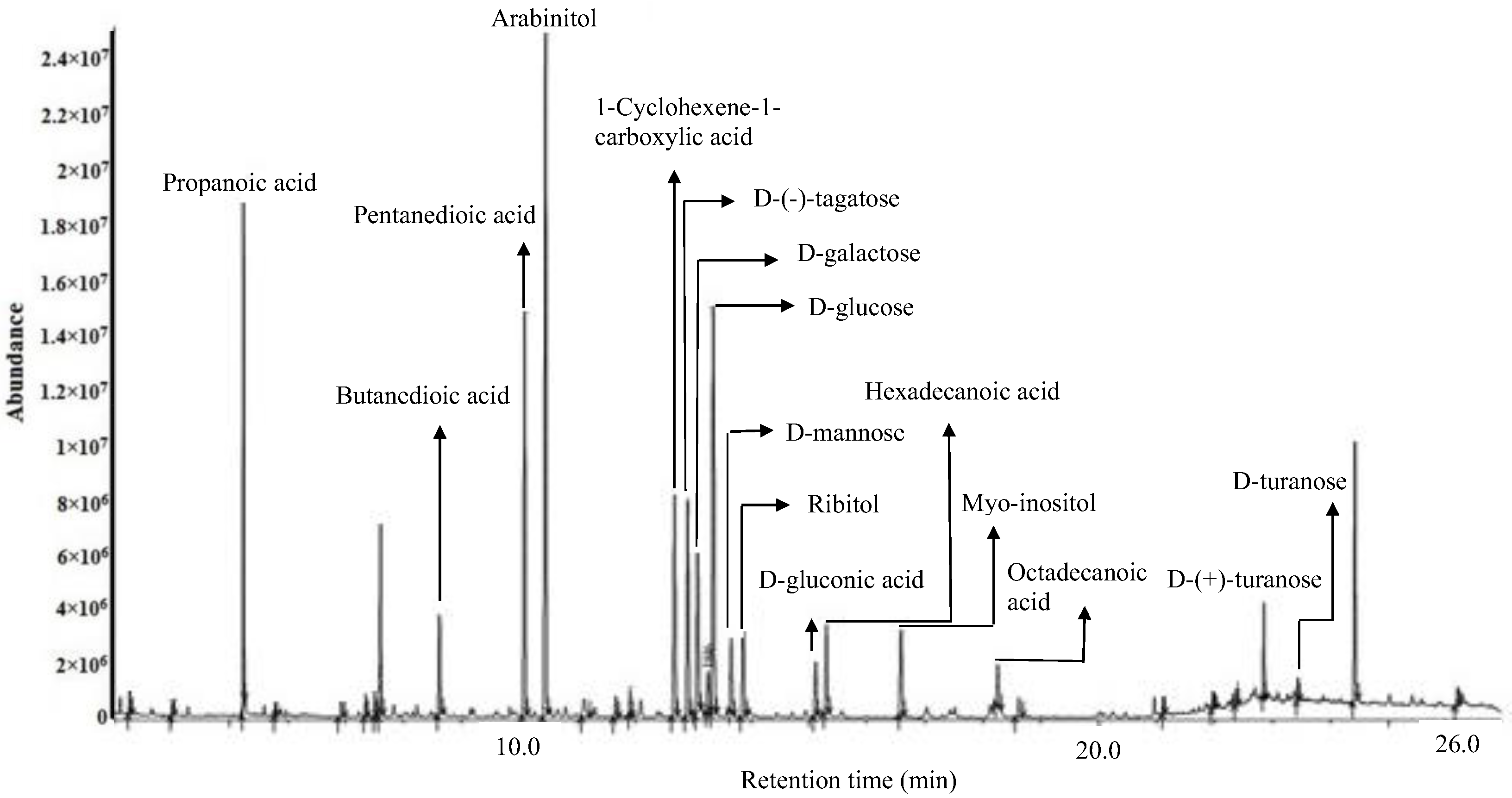
Figure 5. GC−MS chromatogram of the derivatized optimized P. malayana leaf extract.
Twelve putative compounds identified in OE of P. malayana utilizing the LC-MS spectra are depicted in Figure 6. Several classes of bioactive compounds, including flavonoids, flavonoid glycosides, chromones, tannins, phenylpropanoids, and sugar, were identified. The nomenclature, retention time, observed m/z, neutral mass, mass error, response, adducts, and molecular formula of the putative compounds are presented in . The compounds that were identified in this study include cnidimol F, procyanidin B2, stachyose, moracin M-3′-O-β-D-glucopyranoside, isoaloeresin A, procyanidin A2, kushenol O, leucopelargonidin, sennoside B, procyanidin B3, isorhamnetin, and tenuifoliside B. The compounds were identified and labeled based on their respective peaks in the OE chromatogram of the liquid chromatography–mass spectrometry (LC-MS) as in Figure 6.
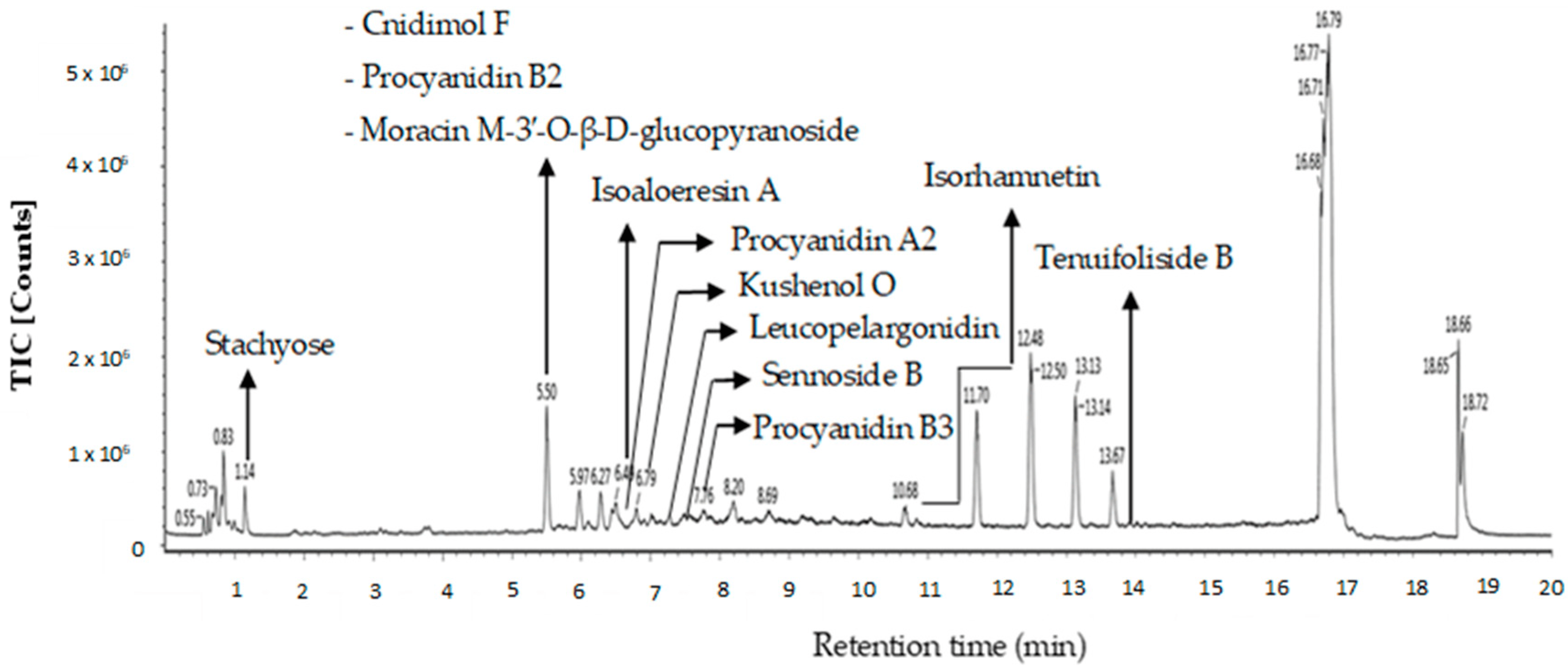
Figure 6. LC−MS chromatogram of OE.
3. Discussion
This study builds upon our prior research that demonstrated the potential of the methanol extract of P. malayana leaf to exhibit strong anti-diabetic activity [7]. Then, in an effort to increase bioactivity and decrease toxicity, we optimized the extraction of this plant’s leaves. We currently have the trade secret number of TS 2023-01 and this extraction optimization has been registered for the patent filing process. In this study, the in vitro anti-diabetic, anti-inflammatory, and antioxidant properties, and the in vivo toxicity of OE were evaluated.
The AGI-IC50 of OE is 2.02 µg/mL. The optimization process successfully resulted in a more potent impact when compared to the previous study, which reported the AGI-IC50 of 2.71 µg/mL [7]. In addition, the present study demonstrates the anti-inflammatory impact of OE as per the lipoxygenase inhibition. The IC50 value of the extract for this enzyme was found to be 4.92 µg/mL. The present investigation represents the first report on the evaluation of the anti-inflammatory properties of P. malayana leaf extract.
α-Glucosidase inhibitors have the ability to slow down the release of D-glucose from oligosaccharides and disaccharides found in complex carbohydrates in our diet. This delay in glucose absorption leads to lower levels of glucose in the blood after a meal and helps to suppress postprandial hyperglycemia [16], while lipoxygenase (LOX) catalyzes the reaction between unsaturated fatty acids, resulting in the production of active lipid metabolites. These metabolites have been implicated in the pathogenesis of several disease conditions including diabetes [17,18,19,20].
OE demonstrated an excellent AGI activity that effectively prevented the absorption of disaccharides. This dual action may contribute to the enhanced anti-adipogenesis and anti-obesity efficacy of the extract, ultimately leading to a significant weight loss. In subsequent periods, it is imperative to ascertain the potential impact of optimized extract on glucose and lipid metabolism in pertinent tissues, including skeletal muscle and the liver [21].
Based on the results obtained from the FRAP and DPPH assays, OE exhibits antioxidant properties. The DPPH inhibitory activity of OE was found to be similar (IC50 = 13.08 µg/mL) compared to the results reported in a previous study (IC50 = 10.85 µg/mL) [7]. The FRAP assay of OE demonstrated the capability of an antioxidant inhibitor to react with a ferric tripyridyltriazine (Fe3+-TPTZ) complex, resulting in the formation of a colored ferrous tripyridyltriazine (Fe2+-TPTZ) [22]. Investigations were conducted to encompass a broad spectrum of compounds. The DPPH assay exclusively quantifies hydrophobic antioxidants, while the FRAP assay is designed to measure the antioxidant activity of hydrophilic compounds [23].
A hypothesis has been put forward suggesting an elevation in oxidative stress within individuals with diabetes. The activity of oxygen free radicals has the potential to initiate the process of lipid peroxidation. This, in turn, can stimulate the glycation of proteins, inactivating the enzymes, altering the collagen structure and function, and changing the basement membrane homeostasis. These events together can be followed by a series of complications, both short-term and long-term, ending with the occurrence of diabetes [24].
Several morphological defects were observed in the zebrafish exposed to the lower concentration than the one of LC50. It explains the cause of the zebrafish lethality. The present study revealed malformations of the embryo as early as 24 hpf, characterized by the occurrence of hatching defects, particularly at concentrations equal to or exceeding 100 µg/mL. Various degrees of morphological defects were observed in larval zebrafish, including yolk sac definitive, small eyes, and short body lengths. These manifestations can be collectively described as developmental retardation [25]. It can be caused by a lack of energy since energy is widely recognized as a fundamental requirement for living organisms to facilitate various physiological processes, including but not limited to growth, development, locomotion, and reproduction [26,27].
displays the embryos’ eye and yolk sizes as well as body length after the administration of various concentrations of OE. The decrease in the embryos’ eye and yolk sizes in a concentration-dependent manner was seen at concentrations of higher than and/or equal to 150 and 100 µg/mL, respectively. The alteration in yolk size has been linked to alterations in the functions of the glomerular filtration barrier [28]. The anomalous growth of the yolk size is suspected to lead to the compromised provision of nutrients to zebrafish embryos [29], while the occurrence of decreased eye size was attributed to the impairment of lipid metabolism and the obstruction of the water permeability barrier on the embryo surface [30].
The analysis of body length was limited to the group with a concentration of 50 µg/mL and negative control groups based on the embryos’ loss of the hatching ability when supplied with concentrations higher than or/and equal to 100 µg/mL of the plant extract. The potential for reduced hatchability of the embryos may be attributed to two factors that are the disruption of the hatching enzyme responsible for the digestion of the chorion and hypoxia [31]. Hypoxia refers to a condition wherein there is an insufficient supply of oxygen at the tissue level, leading to an inability to maintain proper homeostasis [32]. The observed reduction in zebrafish pigment development resulting from the exposure of optimized extract can be attributed to a decrease in tyrosinase activity and a downregulation of gene expression associated to melanogenesis [33,34].
shows that the administration of OE onto the embryo or larva resulted in the lack of a heartbeat. The reduction in heartbeats in zebrafish embryos has been observed to be linked to the presence of leaks in the endothelial vessels, often leading to cardiovascular dysfunctions [35,36].
The calculation of dosage is of utmost importance in the context of prescribing medication, as it is necessary to prevent the occurrence of adverse effects. This is particularly significant due to the requirement that the therapeutic dosage be maintained at a level below the LC50 value [7]. The toxicological impact of OE was assessed by determining the median lethal concentration (LC50) to be 224.29 µg/mL, which is higher than that of the previously reported value of 37.50 µg/mL for the methanol extract of this plant [7]. In accordance with the findings, a therapeutic index of 111.03 was attained, representing a significant increase of eightfold compared to the previously reported value of 13.84 for the methanol extract. This finding suggests that OE is safer and has the potential to be an anti-diabetic agent compared to our previous finding [7].
Gas chromatography–mass spectrometry (GC-MS) was employed for the identification and the analysis of 16 OE putative compounds. These compounds include propanoic acid, butanedioic acid, pentanedioic acid, 1-cyclohexene-1-carboxylic acid, hexadecanoic acid, D-gluconic acid, octadecanoic acid, arabinitol, ribitol, myo-inositol, D-(−)-tagatose, D-galactose, D-mannose, D-glucose, D-(+)-turanose. D-mannose, hexadecanoic acid (also known as palmitic acid), and myo-inositol, which have previously been identified in this plant [7]. The major compounds detected were propanoic acid, butanedioic acid (succinic acid), hexadecanoic acid (palmitic acid), and D-(−)-tagatose. These compounds exhibited a similarity index of 95% or higher using National Institute of Standards and Technology (NIST) Standard Database 11.
The study conducted by Natrus et al. [37] demonstrated that propanoic acid exhibited advantageous properties in mitigating endoplasmic reticulum stress induced by diabetes in the ventromedial hypothalamus of rats. In the study conducted by Al-Lahham and Rezaee [38], it was observed that the application of propanoic acid to human subcutaneous adipose tissue led to a notable decrease in inflammatory indicators such as TNF-α and IP-10, as well as MMP-9 and CD163, the macrophage markers. Propanoic acid is hazardous when supplied to male western albino rats with an escalating risk of hepatic damage [39]. Propanoic acid exhibits significant antioxidant properties, effectively neutralizing reactive oxygen species, including hydroxyl radicals, thereby contributing to detoxification processes. Desmodium canum, a plant with recognized medicinal properties, contains succinic acid as its primary hypoglycemic agent. This organic acid demonstrates a hypoglycemic impact after a one-time intake as it effectively causes a blood glucose reduction in Sprague Dawley rats [40]. The study conducted by Chien et al. [41] reported the anti-inflammatory properties of succinic acid derivatives. These derivatives were found to exhibit potential immunomodulatory effects against RAW264.7 macrophage cells [42].
According to Hardy et al. [43], palmitic acid administration resulted in the inhibition of glucose uptake specifically in response to insulin stimulation while having no effect on basal glucose uptake. According to Wu et al. [44], it was observed that palmitic acid can induce the upregulation of inflammatory cytokines in macrophages. These cytokines are known to have a significant impact on the inflammatory mechanisms involved in the development of atherosclerosis. Additionally, it was observed that the alteration in microbiota indirectly led to the induction of endoplasmic reticulum stress and subsequent liver damage in zebrafish. The microbiota containing palmitic acid facilitated the uptake, resulting in increased palmitic acid accumulation in the liver and exacerbated hepatotoxicity in zebrafish [45]. According to previous research, there is evidence suggesting that palmitic acid could potentially serve as a significant risk factor for the development of coronary heart disease [46]. OE at concentrations of 100 µg/mL and higher, exhibited a reduced heartbeat compared to the negative control. This observation could potentially be attributed to the presence of palmitic acid.
The study conducted by Ensor et al. [47] demonstrated that D-tagatose exhibited significant efficacy in reducing HbA1c levels among individuals diagnosed with type 2 diabetes, as compared to the administration of a placebo. The study conducted by Kruger et al. [48] did not observe any evidence suggesting that D-tagatose has the potential to enhance the occurrence of lymphoma cancer cells in mouse L5178Y, regardless of the presence or absence of metabolic activation. Furthermore, the study found no alterations in the micronucleus formation of polychromatic erythrocytes in the bone marrow, leading to the conclusion that D-tagatose does not possess genotoxic properties [48]. Research involving the administration of D-tagatose in Sprague Dawley rats at three different doses (4, 12, and 20 g/kg body weight/day) via gastric intubation showed no toxicity or clinical effects in the rats [49,50]. Therefore, it is possible that D-(−)-tagatose does not contribute to the observed toxicity effect exhibited by OE but expresses a prominent effect on anti-diabetic and anti-inflammatory activities.
According to Mashayekh-Amiri et al. [51], myo-inositol is characterized as a second messenger and an insulin sensitizer, which enhances the maintenance of glucose homeostasis and assumes a significant function in the regulation of glucose. Additionally, it demonstrates anti-inflammatory properties by decreasing the expression of the mRNA of TNF-α [52]. Myo-inositol is a well-established antioxidant molecule [53].
The dystrophic pathology of limb muscles in fukutin-related protein (FKRP)-mutant mice was found to be improved when treated with ribitol, which was associated with focal inflammatory infiltrates [54]. Nevertheless, it was observed that ribitol facilitated a rise in the glutathione peroxidase, catalase, and superoxide dismutase activity. This effect is likely a result of an augmented generation of the superoxide radical [55].
LC-MS detected some putative compounds such as isorhamnetin, isoaloeresin A, moracin M-3′-O-β-D-glucopyranoside, procyanidin B3, and leucopelargonidin. Isorhamnetin is a bioactive molecule that undergoes O-methylation and is frequently present in medicinal plants including Ginkgo biloba, Hippophae rhamnoides, and Oenanthe javanica [56]. These flavonoids are beneficial in managing diabetes [57]. The therapeutic effects of oral isorhamnetin were examined on a streptozotocin-induced diabetes model. Experimental animals received 10 mg/kg or 20 mg/kg isorhamnetin for 10 days. Isorhamnetin reduced oxidative stress and hyperglycemia, suggesting an anti-diabetic impact [56]. The anti-diabetic properties of isorhamnetin extend beyond the evidential blood glucose levels’ decline. A similar effect was observed in the red blood cells, the sciatic nerve, and the lenses of the rat model where the sorbitol aggregation was stopped [58,59]. Several studies have shown that it is capable of scavenging DPPH and ABTS radicals, as well as inhibiting lipid peroxidation [60,61,62]. Grdović et al. [63] found that isorhamnetin from Castanea sativa may protect against streptozotocin-induced oxidative damage and β-cell apoptosis. The study also found a correlation between observed benefits and the antioxidant activity. Isorhamnetin reduced malondialdehyde (MDA) and intracellular reduced glutathione (GSH) levels while increasing superoxide dismutase (SOD) and catalase (CAT) activity, demonstrating this relationship. The study conducted by Yang et al. [64] provided evidence that isorhamnetin has the potential to mitigate acute lung injury generated by lipopolysaccharide (LPS) through the inhibition of COX-2 expression in male BALB/c mice. Nevertheless, Gong et al. [65] reported this compound’s toxic activity. Dong et al. [66] described isorhamnetin as having been seen to have cytotoxic effects on H9C2 cardiomyocytes. Similarly, Liang et al. [67] discovered that isorhamnetin also demonstrates cytotoxicity towards mouse primary hepatocytes. Furthermore, Zhang et al. [68] found that isorhamnetin promotes DNA damage in HepG2 cells.
Isoaloeresin A is a prominent constituent that is present in Aloe ferox, a plant species known for its abundant anti-inflammatory compounds [69,70]. This plant inhibits the secretion of pro-inflammatory markers and cytokines, which are responsible for inducing significant inflammation that ultimately results in acute respiratory distress [71]. However, there is a lack of evidence pertaining to the potential anti-diabetic, antioxidant, anti-inflammatory capabilities as well as the toxicological effects of this compound.
Moracin M-3′-O-β-D-glucopyranoside was extracted from the leaves of Morus insignis and exhibited a significant hypoglycemic effect in streptozotocin-induced hyperglycemic rats [72]. The selection of moracin M-3′-O-β-D-glucopyranoside as a promising agent was based on its established anti-diabetic activities [73]. In addition, it demonstrates significant potential in the therapeutic treatment of inflammatory illnesses. This compound exhibited 100% inhibitory activity against soluble epoxide hydrolase, as evidenced by IC50 values of 7.7 μM [74]. However, there is a lack of research investigating the effects of antioxidants on moracin M-3′-O-β-D-glucopyranoside.
Procyanidin B3 demonstrates significant inhibitory activity against glucosidase and possesses antioxidant properties, indicating its potential effectiveness in the prevention and treatment of diabetes and in mitigating oxidative stress [75,76,77,78]. Procyanidin B3 demonstrates inherent antioxidant characteristics and displays significant inhibitory effects on lectin-like oxidized LDL receptor-1, a crucial factor associated with the progression of arteriosclerosis [79]. This compound has an IC50 value of 40.1 µg/mL, indicating the inhibition of α-glucosidase [80]. Procyanidin B3 on the other hand displayed an IC50 value of 7.9 µg/mL in the DPPH test, demonstrating an antioxidant effect, as well as 34.5 µg/mL in the H2O2 assay [80]. Procyanidin B3 effectively suppressed the generation of inflammation in human nucleus pulposus cells exposed to LPS by upregulating the inducible nitric oxide synthase and COX-2 expression [81]. The potency of procyanidin B3 in mitigating inflammation generated by 12-O-tetradecanoylphorbol-13-acetate in mouse ears surpassed that of indomethacin and glycyrrhetinic acid, which are commonly employed as anti-inflammatory drugs [82].
Leucopelargonidin extracted from Pseudarthria viscida roots increases insulin release in the serum of diabetic rats [83,84]. Cherian et al. [85] found that leucopelargonidin derivatives from Ficus bengalensis bark significantly increased β-cell insulin production in vitro. Leucopelargonidin-3-O-α-L rhamnoside dimethoxy ether showed antioxidant activity in hyperlipidemic rats [86]. It was not lethal even at 1.8 g/kg in experimental animals [87].
In summary, the presence of these putative compounds has been found to significantly contribute to the potent anti-diabetic, anti-inflammatory, and antioxidant properties of OE. Myo-inositol, isorhamnetin, and procyanidin B3 demonstrated all three bioactivities.
4. Materials and Methods
4.1. Materials
4.2. Methods
5. Conclusions
OE demonstrated a greater level of inhibitory activity against α-glucosidase compared to the prior state of the art. It also displayed high efficacy in the inhibition of soybean lipoxygenase-induced inflammation and antioxidant properties. The results of the toxicity assessment conducted on zebrafish embryos demonstrated that the toxicity of OE was less than the prior state of the art; thus, OE has a higher therapeutic index. This suggests that OE is more potent and is safer for pharmaceutical use. Upon careful examination of all relevant factors pertaining to this invention, it becomes evident that OE holds significant practical value. OE holds theoretical significance in the context of developing medicine and healthcare products for the future management of diabetes mellitus. This discovery establishes a robust foundation for conducting in vivo and clinical trials in the future. However, additional research is necessary to isolate, identify, and quantify the novel bioactive compounds responsible for the anti-diabetic and anti-inflammation activities.
6. Patents
The preparation of OE was classified as a trade secret (Reference number: TS 2023-01) registered at International Islamic University Malaysia dated 23 June 2023. The patent filing process was registered at International Islamic University Malaysia (application ID: 3070, dated 24 July 2023).
References
- International Diabetes Federation. Available online: https://idf.org/about-diabetes/what-is-diabetes/ (accessed on 2 September 2023).
- World Health Organization (WHO). Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 10 September 2023).
- Kumar, A.; Gangwar, R.; Ahmad Zargar, A.; Kumar, R.; Sharma, A. Prevalence of diabetes in India: A review of IDF Diabetes Atlas 10th edition. Curr. Diabetes Rev. 2023, 20, 2024. [Google Scholar] [CrossRef]
- International Diabetes Federation, IDF Diabetes Atlas 10th Edition. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 2 September 2023).
- Mohamed Mahzir, K.A.; Abd Gani, S.S.; Hasanah Zaidan, U.; Halmi, M.I.E. Development of Phaleria macrocarpa (Scheff.) Boerl Fruits Using Response Surface Methodology Focused on Phenolics, Flavonoids and Antioxidant Properties. Molecules 2018, 23, 724. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.; Rahmawati, K.P.; Asnawati, D.; Ersalena, V.F.; Azwari, A. Characterization of Alkaloids from The Leaves of Psychotria malayana Jack of Lombok Island on The Basis of Gas Chromatography-Mass Spectroscopy. J. Pure Appl. Chem. Res. 2014, 3, 108–113. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ahmed, Q.U.; Nasir, M.H.M.; Supandi, F.; Taher, M.; Saiman, M.Z. Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack. Plants 2021, 10, 2688. [Google Scholar] [CrossRef]
- Akmal, M.; Wadhwa, R. Alpha Glucosidase Inhibitors; StatPearls Publishing: Rockville Pike, MD, USA, 2022. [Google Scholar]
- Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Che Mohamad, C.A.; Wan Sulaiman, W.M.A.; Abdul Wahab, R.; Ahmed, Q.U.; Abdul Ghaffar, M.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules 2019, 24, 1506. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Rudich, A.; Tirosh, A.; Potashnik, R.; Hemi, R.; Kanety, H.; Bashan, N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998, 47, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef]
- Benchoula, K.; Khatib, A.; Jaffar, A.; Ahmed, Q.U.; Sulaiman, W.M.A.W.; Wahab, R.A.; El-Seedi, H.R. The promise of zebrafish as a model of metabolic syndrome. Exp. Anim. 2019, 68, 407–416. [Google Scholar] [CrossRef]
- Irawan, C.; Putri, I.D.; Sukiman, M.; Utami, A.; Ismail; Putri, R.; Lisandi, A.; Pratama, A.N. Antioxidant activity of DPPH, CUPRAC, and FRAP methods, as well as activity of alpha-glucosidase inhibiting enzymes from Tinospora crispa (L.) stem ultrasonic extract. Pharmacogn. J. 2022, 14, 511–520. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.N.; Gao, B.; Li, P.; Inagaki, C.; Kawabata, J. Inhibitory effect on α-glucosidase by Adhatoda vasica Nees. Food Chem. 2008, 108, 965–972. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Ma, Q.; Lindsay, J.W.; Leone, K.A.; Ma, K.; Coben, J.; Galkina, E.V.; Nadler, J.L. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am. J. Physiol. 2011, 300, E410–E421. [Google Scholar] [CrossRef]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 2: Medicinal plants with inhibitory activity on arachidonate 12-lipoxygenase, 15-lipoxygenase and leukotriene receptor antagonists. Phytother. Res. 2005, 19, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, H.; Attaran, N.; Jafari, Z.; Saberi, M.R.; Seyedi, S.M.; Eshghi, H.; Pordel, M.; Riazi, M.M. Design and synthesis of 4-methoxyphenylacetic acid esters as 15-lipoxygenase inhibitors and SAR comparative studies of them. Bioorg. Med. Chem. 2009, 17, 2327–2335. [Google Scholar] [CrossRef]
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. α-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, T.Y.; Hong, J.Y.; Kim, G.J.; Oh, J.B.; Kim, M.J.; Apostolidis, E.; Lee, J.Y.; Kwon, Y.I. Anti-Obesity and Anti-Adipogenic Effects of Administration of Arginyl-Fructose-Enriched Jeju Barley (Hordeum vulgare L.) Extract in C57BL/6 Mice and in 3T3-L1 Preadipocytes Models. Molecules 2022, 27, 3248. [Google Scholar] [CrossRef] [PubMed]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]
- Capanoglu, E.; Kamiloglu, S.; Ozkan, G.; Apak, R. Evaluation of antioxidant activity/capacity measurement methods for food products. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 273–286. [Google Scholar]
- Boynes, J.W. Role of oxidative stress in development ofcomplication in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.Y.; Park, S.; Song, G.; Lim, W. Developmental toxicity of fipronil in early development of zebrafish (Danio rerio) larvae: Disrupted vascular formation with angiogenic failure and inhibited neurogenesis. J. Hazard. Mater. 2020, 385, 121531. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; He, L.; Zhang, T.; Li, C. Lentinan Impairs the Early Development of Zebrafish Embryos, Possibly by Disrupting Glucose and Lipid Metabolism. Processes 2022, 10, 120. [Google Scholar] [CrossRef]
- Hanke, N.; Staggs, L.; Schroder, P.; Litteral, J.; Fleig, S.; Kaufeld, J.; Pauli, C.; Haller, H.; Schiffer, M. “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. Biomed Res. Int. 2013, 2013, 658270. [Google Scholar] [CrossRef]
- Raldúa, D.; André, M.; Babin, P.J. Clofibrate and gemfibrozil induce an embryonic malabsorption syndrome in zebrafish. Toxicol. Appl. Pharmacol. 2008, 228, 301–314. [Google Scholar] [CrossRef] [PubMed]
- McCollum, C.W.; Ducharme, N.A.; Bondesson, M.; Gustafsson, J.A. Developmental toxicity screening in zebrafish. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 67–114. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanoparticle Res. 2009, 12, 1645–1654. [Google Scholar] [CrossRef]
- Bhutta, B.S.; Alghoula, F.; Berim, I. Hypoxia; StatPearls Publishing: Rockville Pike, MD, USA, 2022. [Google Scholar]
- Lajis, A.F.B. A Zebrafish Embryo as an Animal Model for the Treatment of Hyperpigmentation in Cosmetic Dermatology Medicine. Medicina 2018, 54, 35. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, S.H. Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Exp. Dermatol. 2015, 24, 761–766. [Google Scholar] [CrossRef]
- Yu, Y.L.; Li, H.L.; Wang, Y.C.; Qiao, X.G. The effect of thiram on heart development of zebrafish embryos. J. Inn. Mong. Univ. Natl. 2011, 3, 1–7. [Google Scholar]
- Hallare, A.V.; Schirling, M.; Luckenbach, T.; Kohler, H.R.; Triebskorn, R. Combined temperature and cadmium effects on developmental parameters and biomarker responses in zebrafish (Danio rerio) embryos. J. Therm. Biol. 2005, 30, 7–17. [Google Scholar] [CrossRef]
- Natrus, L.V.; Osadchuk, Y.S.; Lisakovska, O.O.; Labudzinskyi, D.O.; Klys, Y.G.; Chaikovsky, Y.B. Effect of Propionic Acid on Diabetes-Induced Impairment of Unfolded Protein Response Signaling and Astrocyte/Microglia Crosstalk in Rat Ventromedial Nucleus of the Hypothalamus. Neural Plast. 2022, 2022, 6404964. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S.; Rezaee, F. Propionic acid counteracts the inflammation of human subcutaneous adipose tissue: A new avenue for drug development. J. Fac. Pharm. 2019, 27, 645–652. [Google Scholar] [CrossRef]
- Al-Daihan, S.; Shafi Bhat, R. Impact of Propionic Acid on Liver Damage in Rats. Int. J. Mol. Cell. Med. 2015, 4, 188–195. [Google Scholar] [PubMed]
- Lattibeaudiere, K.G.; Alexander-Lindo, R.L. Oleic Acid and Succinic Acid Synergistically Mitigate Symptoms of Type 2 Diabetes in Streptozotocin-Induced Diabetic Rats. Int. J. Endocrinol. 2022, 2022, 8744964. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, M.L.; Kuo, H.T.; Tsai, Y.C.; Lin, B.F.; Kuo, Y.H. Anti-inflammatory activities of new succinic and maleic derivatives from the fruiting body of Antrodia camphorata. J. Agric. Food Chem. 2008, 56, 7017–7022. [Google Scholar] [CrossRef] [PubMed]
- Razafindrakoto, Z.R.; Donno, D.; Tombozara, N.; Andriamaniraka, H.; Andrianjara, C.; Ramanitrahasimbola, D.; Beccaro, G.L. Antioxidant, Anti-Inflammatory, and Antidiabetic Activities of Leaves and Stems of Uapaca bojeri Bail. (EUPHORBIACEAE), an Endemic Plant of Madagascar. Pharmaceuticals 2020, 13, 71. [Google Scholar] [CrossRef]
- Hardy, R.W.; Ladenson, J.H.; Henriksen, E.J.; Holloszy, J.O.; McDonald, J.M. Palmitate stimulates glucose transport in rat adipocytes by a mechanism involving translocation of the insulin sensitive glucose transporter (GLUT4). Biochem. Biophys. Res. Commun. 1991, 177, 343–349. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Pang, X.; Wang, S.; Zhao, J.; Zhang, X.; Feng, L. Palmitic acid exerts pro-inflammatory effects on vascular smooth muscle cells by inducing the expression of C-reactive protein, inducible nitric oxide synthase and tumor necrosis factor-α. Int. J. Mol. Med. 2014, 34, 1706–1712. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Ran, C.; He, S.; Yang, Y.; Du, Z.; Zhang, J.; Zhou, Z. The Hepatotoxicity of Palmitic Acid in Zebrafish Involves the Intestinal Microbiota. J. Nutr. 2018, 148, 1217–1228. [Google Scholar] [CrossRef]
- Fattore, E.; Fanelli, R. Palm oil and palmitic acid: A review on cardiovascular effects and carcinogenicity. Int. J. Food Sci. Nutr. 2013, 64, 648–659. [Google Scholar] [CrossRef]
- Ensor, M.; Banfield, A.B.; Smith, R.R.; Williams, J.; Lodder, R.A. Safety and Efficacy of D-Tagatose in Glycemic Control in Subjects with Type 2 Diabetes. J. Endocrinol. Diabetes Obes. 2015, 3, 1065. [Google Scholar]
- Kruger, C.L.; Whittaker, M.H.; Frankos, V.H. Genotoxicity tests on D-tagatose. Regul. Toxicol. Pharmacol. 1999, 29, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.L.; Whittaker, M.H.; Frankos, V.H.; Schroeder, R.E. Developmental toxicity study of D-tagatose in rats. Regul. Toxicol. Pharmacol. 1999, 29, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Wyss, M.; Durán Agüero, S.; Angarita Dávila, L. D-Tagatose Is a Promising Sweetener to Control Glycaemia: A New Functional Food. Biomed Res. Int. 2018, 2018, 8718053. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh-Amiri, S.; Mohammad-Alizadeh-Charandabi, S.; Abdolalipour, S.; Mirghafourvand, M. Myo-inositol supplementation for prevention of gestational diabetes mellitus in overweight and obese pregnant women: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2022, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Arefhosseini, S.; Roshanravan, N.; Asghari, S.; Tutunchi, H.; Ebrahimi-Mameghani, M. Expression of inflammatory genes, WBC-derived inflammatory biomarkers, and liver function indices: Effects of myo-inositol supplementation in obese patients with NAFLD. J. Funct. Foods 2023, 104, 105524. [Google Scholar] [CrossRef]
- De Luca, M.N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, M.P.; Lu, P.; Blaeser, A.; Lu, Q.L. Ribitol restores functionally glycosylated α-dystroglycan and improves muscle function in dystrophic FKRP-mutant mice. Nat. Commun. 2018, 9, 3448. [Google Scholar] [CrossRef]
- Stone, V.; Kudo, K.Y.; August, P.M.; Marcelino, T.B.; Matté, C. Polyols accumulated in ribose-5-phosphate isomerase deficiency increase mitochondrial superoxide production and improve antioxidant defenses in rats’ prefrontal cortex. Int. J. Dev. Neurosci. 2014, 37, 21–25. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Choi, J.S.; Chung, H.Y. Antioxidant effects of isorhamnetin 3,7-di-O-beta-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, S.; Lee, H.S.; Kim, B.K.; Ohuchi, K.; Shin, K.H. Inhibitory effects of isorhamnetin-3-O-beta-D-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol. Pharm. Bull. 2005, 28, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Seo, K.; Ki, S.H.; Shin, S.M. Isorhamnetin Inhibits Reactive Oxygen Species-Dependent Hypoxia Inducible Factor (HIF)-1α Accumulation. Biol. Pharm. Bull. 2016, 39, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Pengfei, L.; Tiansheng, D.; Xianglin, H.; Jianguo, W. Antioxidant properties of isolated isorhamnetin from the sea buckthorn marc. Plant Foods Hum. Nutr. 2009, 64, 141–145. [Google Scholar] [CrossRef]
- Zuo, A.; Yu, Y.; Li, J.; Xu, B.; Yu, X.; Qiu, Y.; Cao, S. Study on the relation of structure and antioxidant activity of isorhamnetin, quercetin, phloretin, silybin and phloretin isonicotinyl hydrazone. Free Radic. Antioxid. 2011, 1, 39–47. [Google Scholar] [CrossRef]
- Grdović, N.; Dinić, S.; Arambašić, J.; Mihailović, M.; Uskoković, A.; Marković, J.; Poznanović, G.; Vidović, S.; Zeković, Z.; Mujić, A.; et al. The protective effect of a mix of Lactarius deterrimus and Castanea sativa extracts on streptozotocin-induced oxidative stress and pancreatic β-cell death. Br. J. Nutr. 2012, 108, 1163–1176. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.P.; Ni, Y.F.; Du, H.Y.; Wang, R.; Li, M.J.; Wang, W.C.; Li, M.M.; Wang, X.H.; Li, L.; et al. Protective Effect of Isorhamnetin on Lipopolysaccharide-Induced Acute Lung Injury in Mice. Inflammation 2016, 39, 129–137. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Dong, X.; Sun, G.; Luo, Y. Protective effect of isorhamnetin on oxidative stress induced by H2O2 in H9C2 cells. China Pharmacol. Bull. 2015, 31, 853–860. [Google Scholar]
- Liang, R.; Chen, J.; Zhi, D.; Fan, Y.; Liu, W.; He, X. Effects of isorhamnetin on human liver microsomes CYPs and rat primary hepatocytes. Drug Eval. Res. 2017, 40, 627–632. [Google Scholar]
- Zhang, Z.; Chen, S.; Mei, H.; Xuan, J.; Guo, X.; Couch, L.; Dobrovolsky, V.N.; Guo, L.; Mei, N. Ginkgo biloba leaf extract induces DNA damage by inhibiting topoisomerase II activity in human hepatic cells. Sci. Rep. 2015, 5, 14633. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kim, K. Anti-Propionibacterium acnes and the anti-inflammatory effect of Aloe ferox miller components. J. Herb. Med. 2017, 9, 53–59. [Google Scholar] [CrossRef]
- Mwale, M.; Masika, P.J. Analgesic and anti-inflammatory activities of Aloe ferox Mill. aqueous extract. Afr. J. Pharm. Pharmacol. 2010, 4, 291–297. [Google Scholar]
- Nalimu, F.; Oloro, J.; Kahwa, I.; Ogwang, P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe Ferox. Future J. Pharm. Sci. 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.S.; Alves, A.P.L.; Alves, G.B.C.; Cardoso, J.N.; Lopes, R.S.C.; Lopes, C.C. A new synthesis of alpha-bromo-acetophenones and its application in obtaining 2-benzoyl-benzofurans. Chem. Nova 2006, 29, 1259–1265. [Google Scholar]
- Kim, J.H.; Kim, T.J.; Kim, H.J.; Cho, C.W.; Kim, S.J.; Cho, H.S.; Kim, K.T.; Kand, J.S. A new high-performance liquid chromatographic method for the quality control of bio converted Mori Folium extracts with appropriate marker compounds related to antidiabetic. J. Anal. Sci. Technol. 2021, 12, 2. [Google Scholar] [CrossRef]
- Li, H.X.; Heo, M.; Go, Y.; Kim, Y.S.; Kim, Y.H.; Yang, S.Y.; Li, W. Coumarin and Moracin Derivatives from Mulberry Leaves (Morus alba L.) with Soluble Epoxide Hydrolase Inhibitory Activity. Molecules 2020, 25, 3967. [Google Scholar] [CrossRef]
- Schäfer, A.; Högger, P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol) effectively inhibit alpha-glucosidase. Diabetes Res. Clin. Pract. 2007, 77, 41–46. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Song, L.; Huang, D. Profiles and α-amylase inhibition activity of proanthocyanidins in unripe Manilkara zapota (chiku). J. Agric. Food Chem. 2012, 60, 3098–3104. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Lai, S.; Liu, C.; Huang, S.; Yang, H. Structure, antioxidant and α-amylase inhibitory activities of longan pericarp proanthocyanidins. J. Funct. Foods 2015, 14, 23–32. [Google Scholar] [CrossRef]
- Lu, Y.; Demleitner, M.F.; Song, L.; Rychlik, M.; Huang, D. Oligomeric proanthocyanidins are the active compounds in Abelmoschus esculentus Moench for its α-amylase and α-glucosidase inhibition activity. J. Funct. Foods 2016, 20, 463–471. [Google Scholar] [CrossRef]
- Mizuno, M.; Nakanishi, I.; Matsubayashi, S.; Imai, K.; Arai, T.; Matsumoto, K.I.; Fukuhara, K. Synthesis and antioxidant activity of a procyanidin B3 analogue. Bioorg. Med. Chem. Lett. 2017, 27, 1041–1044. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Prihantini, A.I.; Tachibana, S. α-Glucosidase inhibitor and antioxidant activity of procyanidin, an isolated compound from Quercus gilva Blume leaves. J. Appl. Pharm. Sci. 2022, 12, 213–218. [Google Scholar] [CrossRef]
- Shang, P.; Tang, Q.; Hu, Z.; Huang, S.; Hu, Y.; Zhu, J.; Liu, H. Procyanidin B3 alleviates intervertebral disc degeneration via interaction with the TLR4/MD-2 complex. J. Cell. Mol. Med. 2020, 24, 3701–3711. [Google Scholar] [CrossRef]
- Oizumi, Y.; Mohri, Y.; Hirota, M.; Makabe, H. Synthesis of procyanidin B3 and its anti-inflammatory activity. the effect of 4-alkoxy group of catechin electrophile in the Yb (OTf)(3)-catalyzed condensation with catechin nucleophile. J. Org. Chem. 2010, 75, 4884–4886. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.B.R.; Nambiasan, P.N.K. Phytochemical studies on Pseudarthria viscida. J. Res. Indian Med. Yoga 1976, 11, 104–106. [Google Scholar]
- Kuppusamy, R.; Shirwaikar, A.; Sam, K.G.; Kaitheri, S.K. Antidiabetic activity of Pseudarthria viscida aqueous root extract in neonatal streptozotocin induced NIDDM rats. Braz. J. Pharmacogn. 2012, 22, 1079–1084. [Google Scholar] [CrossRef]
- Cherian, S.; Kumar, R.V.; Augusti, K.T.; Kidwai, J.R. Antidiabetic effect of a glycoside of pelargonidin isolated from the bark of Ficus bengalensis Linn. Indian J. Biochem. Biophys. 1992, 29, 380–382. [Google Scholar]
- Daniel, R.S.; Mathew, B.C.; Devi, K.S.; Augusti, K.T. Antioxidant effect of two flavonoids from the bark of Ficus bengalensis Linn in hyperlipidemic rats. Indian J. Exp. Biol. 1998, 36, 902–906. [Google Scholar]
- Augusti, K.T.; Daniel, R.S.; Cherian, S.; Sheela, C.G.; Nair, C.R. Effect of leucopelargonin derivative from Ficus bengalensis Linn. on diabetic dogs. Indian J. Med. Res. 1994, 99, 82–86. [Google Scholar] [PubMed]
- Javadi, N.; Abas, F.; Abd Hamid, A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-based metabolite profiling of Cosmos caudatus leaves possessing alpha-glucosidase inhibitory activity. J. Food Sci. 2014, 79, C1130–C1136. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Mohamad, K.; Ooi, A.J.; Imiyabir, Z.; Chung, L.Y. Bioactivity-guided fractionation of the lipoxygenase and cyclooxygenase inhibiting constituents from Chisocheton polyandrus Merr. Fitoterapia 2012, 83, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M. Procedure: Preparation of DPPH Radical, and Antioxidant Scavenging Assay. DPPH Microplate Protoc. 2012, 7–9. [Google Scholar]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2014, 95, 204–209. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2013; pp. 1–22. [Google Scholar]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio—A general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar] [PubMed]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.U.; Uzir, B.F.; Nik Yusoff, N.I.; Perumal, V.; Abas, F.; Shaari, K.; Khatib, A. Identification of α-glucosidase inhibitors from Clinacanthus nutans leaf extract using liquid chromatography-mass spectrometry-based metabolomics and protein-ligand interaction with molecular docking. J. Pharm. Anal. 2019, 9, 91–99. [Google Scholar] [CrossRef]

