1. Introduction
Prediabetes (pre-DM) can lead to diabetes mellitus (DM) [1] and because, in most cases, there is a conglomeration of numerous cardiovascular risk factors (the so-called metabolic syndrome) [2], it remains in the attention of the medical community in the context of treatment and prevention [3,4,5]. There are two types of pre-DM [6]: impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) which can exist separately or together (if the patient reaches the criteria for both). Many complications, referred to as chronic diabetic (macro- and microvascular) complications, can be identified among patients with DM. As DM is generally an oligo- or asymptomatic disease (except for most serious cases with very high glucose levels), chronic complications may occur even just after a diagnosis of type 2 diabetes (T2DM). Interestingly, such complications can be found also within the group with pre-DM [7]. The reason for this is still unclear as from the general point of view diabetic complications derive from hyperglycaemia which, however, does not reach sufficient levels in pre-diabetic states. That is why not only glycaemia but also other parameters, which describe metabolic failure both in pre-DM and DM, are taken into account. It is known that even if glycaemia is well-controlled, patients with DM still express a residual risk of chronic complications [7]. This is probably due to risk factor accumulation accompanying carbohydrate disturbances or accumulation of previously introduced harmful functional and cell structure modifications as the effect of long-term metabolic disturbances. One of the main phenomena explaining the formation of those chronic complications is the creation of advanced glycation end-products (AGEs) [8]. Although the most known precursors of AGEs are glucose and fructose [9], an important role in the process is also played by the reactive dicarbonyls [8], with methylglyoxal (MGO) being the most reactive one [10]. The methylglyoxal-derived AGE, responsible for diabetic complications, is called methylglyoxal-derived hydroimidazolone 1 (MG-H1) [11]. The precursors of MGO are the intermediates of glycolysis (glyceraldehyde-3-phosphate and dihydroxyacetonephosphate) but those precursors can also be unrelated to the glucose metabolism pathway and may derive from the metabolism of fatty acid, amino acid, and ascorbic acid [12,13]. Because of those glucose-dependent and glucose-independent formations of AGEs and AGE precursors, with the possible correction of their levels [8] (which is still in the interest of scientific research), information about who could potentially benefit from the treatment is also desirable. It means that first it is required to identify group/s of people with abnormal levels of those substances. The patients at the initial stage of carbohydrate metabolism disorders, such as people with pre-DM, are the first line of research. Confirmation of abnormal levels of the metabolites for AGE formation in this group of patients may explain the potential mechanisms of the occurrence of chronic complications, so far referred to as hyperglycaemic, which possibly are highly glucose-independent.
In The Maastricht Study [14], Hanssen N. and colleagues concluded that MGO levels are associated with microvascular, but not macrovascular disorders. The authors also found higher fasting plasma MGO levels in subjects with prediabetes compared to healthy ones. In the study mentioned, 14.0% of the participants with pre-DM suffered from previously diagnosed cardiovascular disease (CVD). Due to discrepancies regarding the MGO concentration in patients with coronary artery disease in the literature [15,16], we conducted a preliminary analysis only on individuals without a previous history of confirmed coronary artery disease, stroke or intermittent claudication to exclude the potential impact of clinically important ischemia on the results.
The study aimed to assess the MGO concentration in individuals without previous CVD diagnosis who suffer from newly diagnosed prediabetes and to find if the difference between the levels of MGO in healthy individuals and patients with prediabetes exists. The confirmation of a high level of MGO in patients with prediabetes could potentially explain why early complications may start even before the diabetes is diagnosed.
2. Materials and Methods
2.1. Sample Origin
Frozen serum samples from patients with newly diagnosed IFG and/or IGT diagnosis based on European Association for the Study of Diabetes (EASD) criteria [17], and samples without such pathology were analysed by assessing the concentration of MGO. The material was obtained from Biobank where it had previously been anonymously deposited for another study, after the Bioethics Committee expressed consent for its use (No: KB385/2017).
The serum from both groups was obtained from patients with no previous vascular disease, cancer or any complications referred to as “chronic complications in diabetes” (people with pre-DM). In addition, to characterise the groups and assess the potential impact of other factors on the concentration of MGO, data from the interview, physical examination and the results of basic laboratory tests, which were previously collected in the study in which the subjects participated, were used [18]. The fatty liver was diagnosed only based on the ultrasound examination results obtained from patients’ records.
2.2. Sample Preparation and Analysis
The analysis of MGO concentration was carried out according to the method and process suggested in the literature [19,20,21,22], the course of which guarantees the most reliable results.
2.3. Statistical Analysis
Statistical analyses were based on a database collected from 42 participants, divided into two independent main groups: a study group (N = 31), and a control group (N = 11).
The variables subjected to statistical analysis were on dichotomous and quotient scales. In the case of variables on quotient scales, their conformity to a normal distribution and homogeneity of variance were determined. The normality of the distribution was verified with the Shapiro–Wilk test, while the Levene and Brown–Forsyth tests were used to verify the homogeneity of variance. All analysed variables in the quotient scales met the normality of the distribution criterion.
To characterise the two groups compared, basic descriptive statistics were determined within each group: size, mean value, standard deviation, 95% significance interval for the mean value (±95% CI) and bifurcation in the case of quotient variables, and count tables with percentages in the case of dichotomous variables.
The statistical significance of the correlations between variables on the quotient scales was assessed by calculating Pearson’s r linear correlation matrices. The statistical significance of the calculated r-Pearson parameter values was determined by the t-test.
The significance of Pearson’s 〖chi〗^2 statistic was used to assess the degree of correlation between the dichotomous variables and the constructed bivariate (2 × 2) tables.
The statistical significance of the differences between the mean values of the quotient variables in the two compared groups—test and control—was assessed depending on the result of the test confirming the homogeneity of variance: the parametric Student’s t-test for independent samples or the Cochran-Cox Z-test with independent variance estimation.
In all statistical analyses performed, a significance level of α = 0.05 was assumed. Statistical analysis was performed using the Statistica 13.3 PL computer programme from StatSoft.
The funding source had no involvement in any part of the study. The corresponding author confirms that the authors had full access to all the data in the study and assumed final responsibility for the decision to submit it for publication.
3. Results
Samples from 31 patients with pre-DM and 11 healthy volunteers were evaluated.
There was no difference between the groups in the following basic parameters: age, creatinine, alanine aminotransferase (ALT), total cholesterol level (TCL), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL) , sex, use of nicotine, hypertension, history of diabetes in family, using of hypertensive drugs or fibrates .
Statistical significance was found when groups were compared for body weight, BMI, fasting glucose level , fatty liver and use of statins .
There was no difference in MGO concentration between the groups. The mean value of MGO was 135.44 nM (±SD = 32.67) in the pre-DM group and 143.25 nM (±SD = 17.93), p = 0.46 (±95% CI) in the control group (Suppl. No 1). For the group size available in the study and the MGO mean values obtained, the power of the t-test was β = 0.1 and the analysis of the test’s power behaviour for larger groups (size simulation) did not show any power increase. For the calculation of approximately 120 study participants, the test reaches the power (β) of around 0.12, and the power of the test does not increase despite a further increase in the number of participants.
The positive linear correlation showed that the higher glycated haemoglobin (HbA1c), the higher MGO concentration (Figure 1) (p = 0.01). The power of the test was β = 0.74.
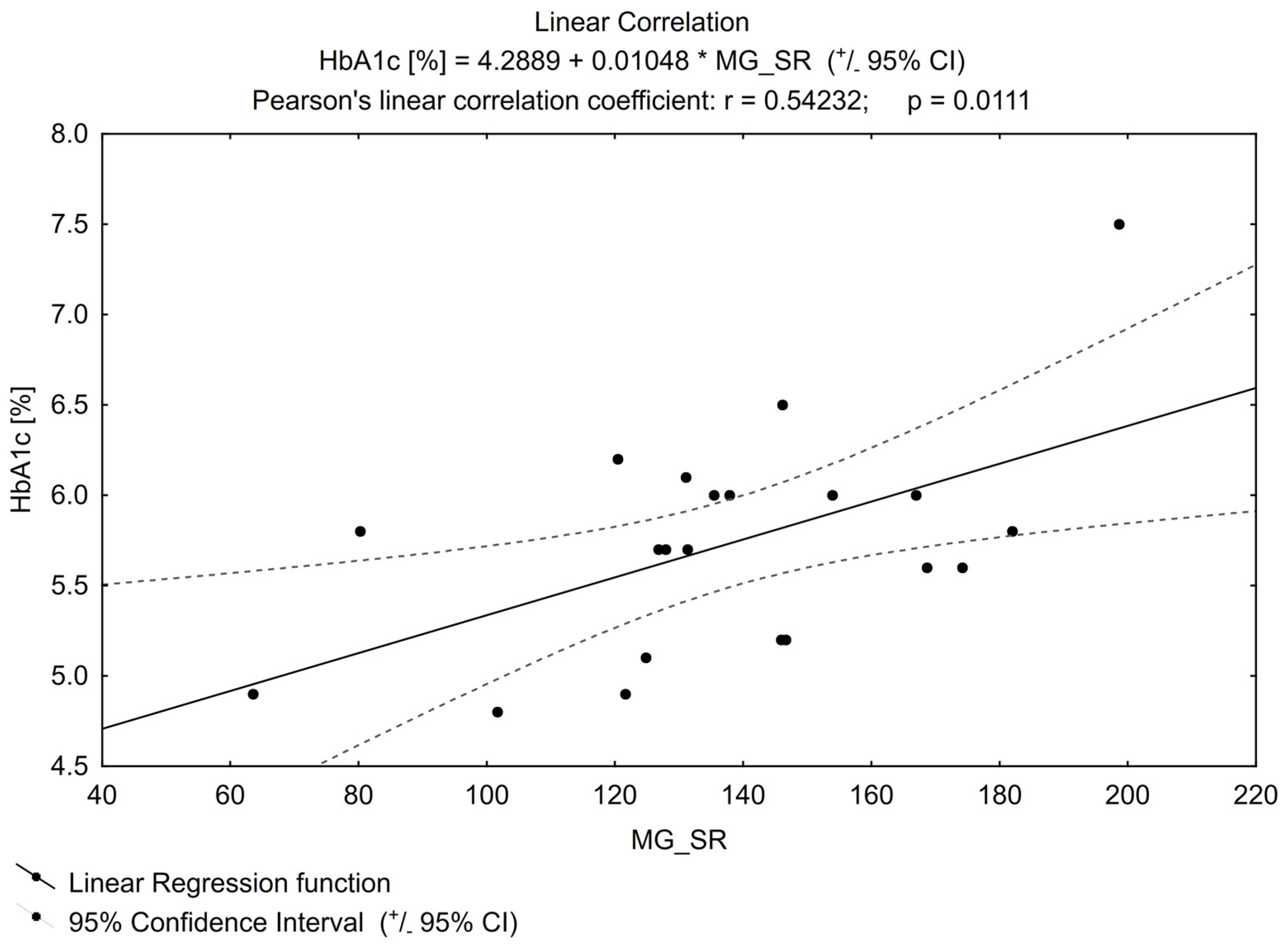
Figure 1. Linear correlation for HbA1c (glycated haemoglobin).
No other important correlation was found between MGO and the parameters that can impact its concentration (examples in Figure 2 and Figure 3), e.g., for TG or FPG.
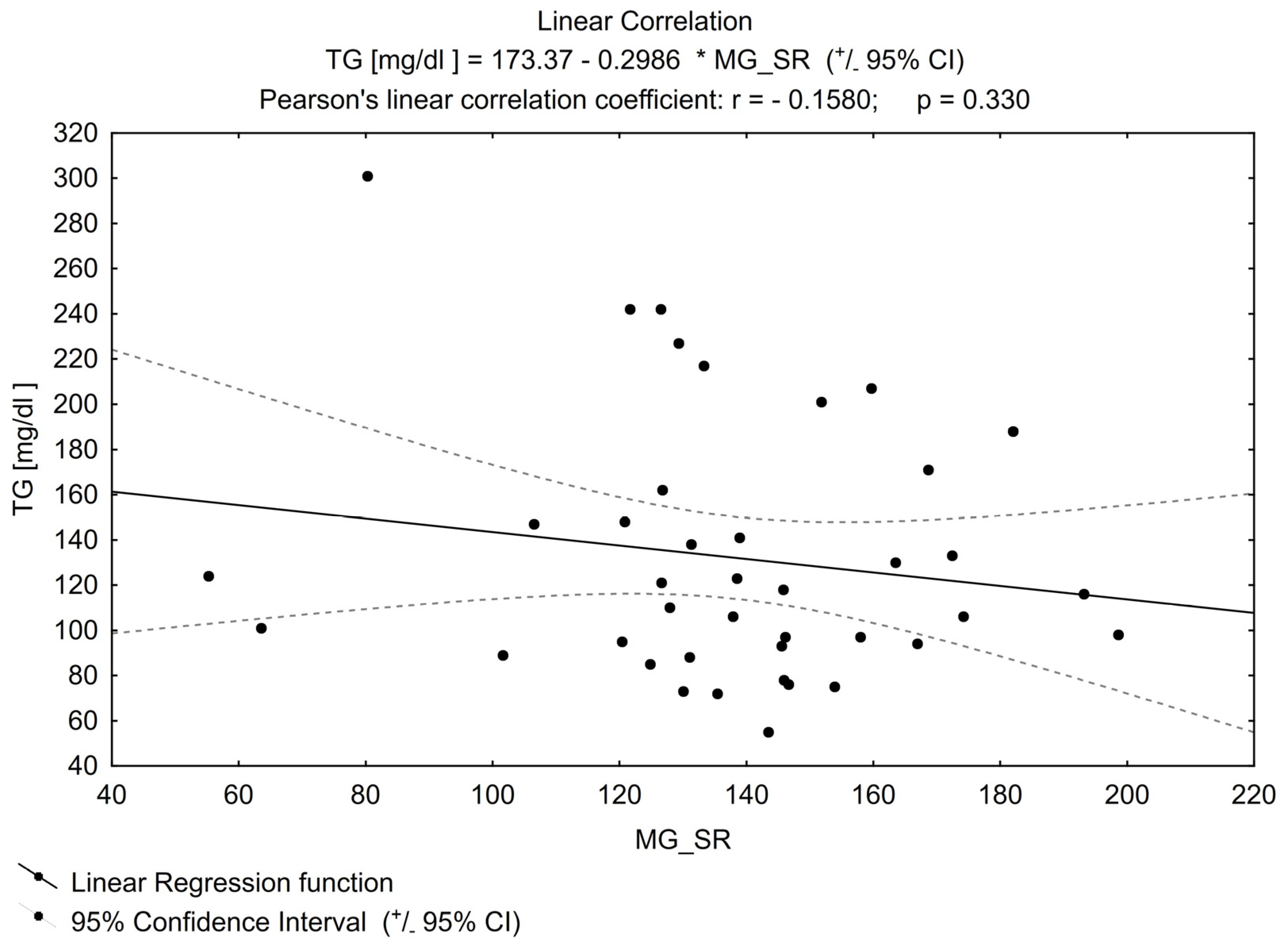
Figure 2. Linear correlation for TG (triglycerides).
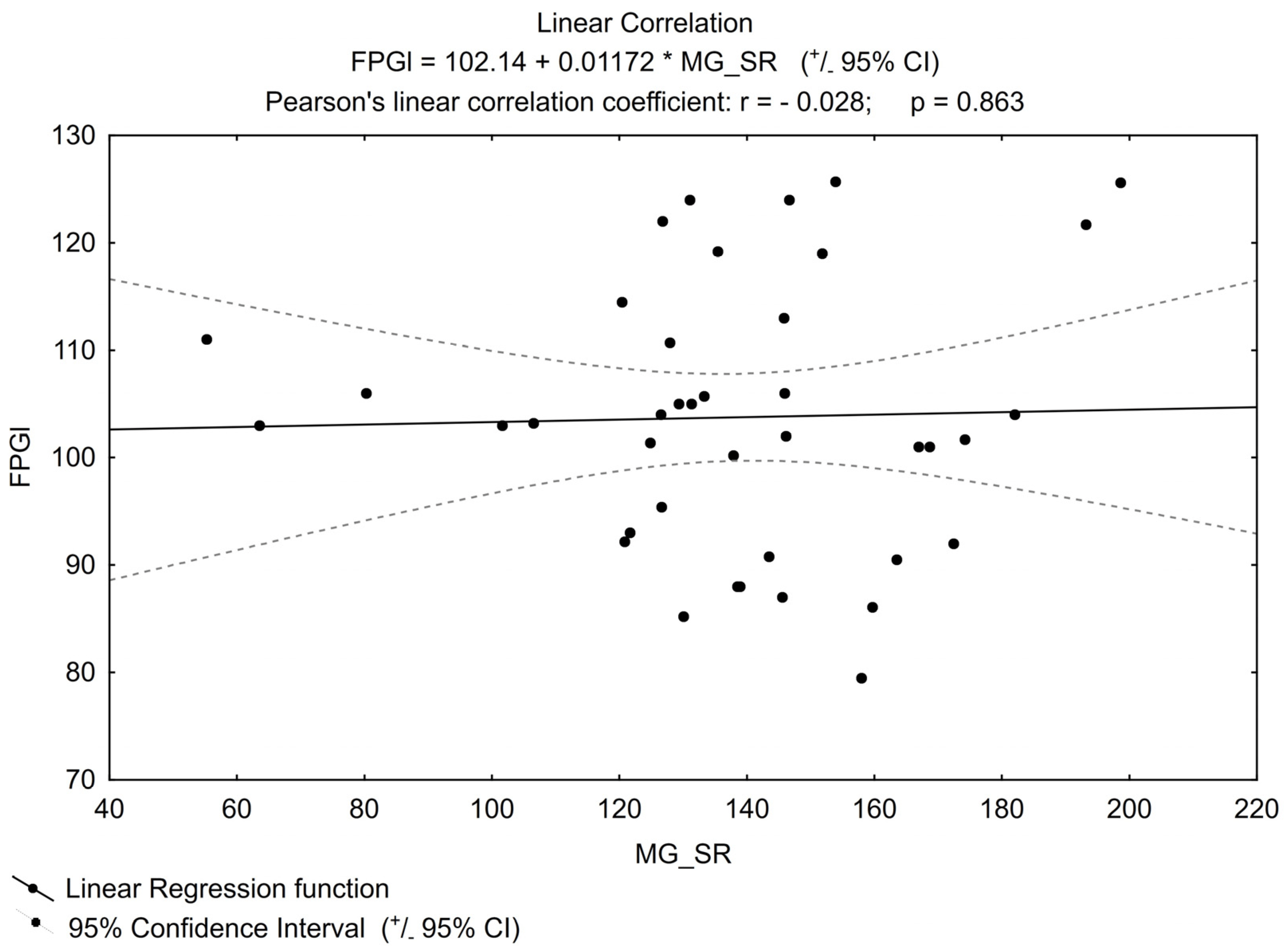
Figure 3. Linear correlation for FPGl (fasting plasma glucose level).
4. Discussion
Basic and clinical data showed that the abnormal serum concentration of MGO was connected with the cardiovascular incidence in individuals with DM, which may at least partly explain a higher cardiovascular risk in this group of patients [16,23,24,25] if its contribution to the creation of AGEs, mentioned in the introduction, is considered. Higher plasma MGO levels positively correlated with the known cardiovascular risk markers like HbA1c value and albumin/creatinine ratio in the urine of patients with T2DM [26,27,28,29]. In our analysis, as in the studies mentioned above, the HbA1c value correlated positively with MGO concentration the higher the HbA1c value, the higher the MGO value. In our study, for obvious reasons, HbA1c was determined mainly in the pre-DM group (it was only incidental in the control group). According to local recommendations, glycated haemoglobin was not a parameter that allowed excluding diabetes in healthy people, but only a parameter to be used for monitoring already diagnosed disorders of carbohydrate metabolism [30]. Therefore, it was not collected for the previous study that the subjects participated in. Our study, in this regard, confirms the relationship between MGO and the aforementioned glycated blood protein for pre-DM, which was previously reported only in a group of patients with DM.
It is also emphasized in the literature that a higher concentration of MGO in plasma correlates with mortality and amputation risk in patients with critical ischemia of the lower extremities, regardless of the presence of diabetes [31]. In addition, urinary MG-H1 levels were positively correlated with cardiovascular parameters including blood pressure and lipid concentration in obese but healthy women. This has given rise to suggestions that the level of AGEs in urine, and not only the known risk factors, can serve as an independent and sensitive marker for cardiovascular risk assessment in obese individuals in the future [32]. The indication of the above correlations of MGO level with poor prognosis inspired scientists to search for new therapeutic strategies aimed at reducing the concentration of this molecule (via its detoxication or prevention of formation) which finally can help to reduce macro- and microvascular complications [33,34]. This strategy seems to have a future, because there are known substances, available on the market but used for another purpose, with MGO-binding properties, e.g., metformin (regardless of its hypoglycaemic effect) [35,36,37,38]. Several other compounds have also been found to be potentially useful in an anti-MGO strategy [39,40,41]. Therefore, there remains the question of selecting a group of individuals who will be able to benefit from such therapies, because they are characterised by an abnormal level of MGO. However, since determination of MGO concentration in serum or urine is not available in public laboratories, it may be useful to determine the phenotype of people for whom the use of MGO-lowering substances is likely to be a targeted therapy.
Taking into account the accumulation of pathological states that often accompany prediabetes and their relationship with the formation of AGEs [40], it seemed that the presence of elevated methylglyoxal levels (compared to healthy individuals) in this group was highly probable. However, the newly diagnosed, hypoglycaemic drug-naïve patients with pre-DM in our study did not have elevated MGO levels, compared to a healthy control group, although they presented features typical of the metabolic syndrome and : glucose levels, hepatic steatosis, body weight and BMI, which differentiated the two groups. In many countries, the aforementioned metformin, which is credited with reducing MGO levels, is used in the treatment of patients with pre-DM, although, certainly, the only intention of such recommendations is to lower glucose levels. However, in our study, the results obtained could not be a response to metformin use, because none of the participants had previously been prescribed such treatment. The fasting MGO results we found are opposite to the Hanssen N et al. study [14]. Although we are aware that sample processing can lead to an overestimation of MGO concentration, which may be released in the already collected sample during its storage or processing [20,21], it should be emphasized that the material used for our study was obtained, processed and stored under the same conditions for both groups. For this reason, we were able to compare the concentration of MGO between the study groups, and it is worth noting that the reported concentration values are within the ranges shown by other authors for the healthy population: 60–250 nM [20,22]. It should also be mentioned that, compared to our study, in the study by Hanssen N. et al., none of the analysed groups (even healthy subjects) had MGO levels within the suggested range for healthy. The other differences include the older analysed population in the Maastricht Study (almost 10 years older for each group) and statistical differences between the groups regarding the age in the Hanssen N et al. study [14]. For comparison of the MGO concentration in both groups, the analysis of the power for t-student test value in our study indicates that the power level of > 0.8 (β > 0.8) cannot be obtained regardless of sample size. This suggests no statistical significance between the groups for MGO concentration values.
An important goal of preventing chronic complications resulting from metabolic disorders is to maintain normal lipid values, with LDL being the most important point of reference. Methylglyoxal also affects this molecule, as it modifies arginine residues of the protein component of LDL, and glycation of LDL by MGO results in the formation of VLDL, the most atherogenic molecule [42,43,44]. The pre-DM group and the control group differed, as mentioned above, in terms of some characteristics typical of the metabolic syndrome that adversely affect the formation of AGEs . In general, however, looking at the results of our work, the concentration of MGO in both groups is practically identical (even slightly lower in people with pre-DM, but without statistical significance) and comparable values of lipid metabolism parameters were shown. Looking for the cause of this surprising phenomenon and the lack of statistical significance, one can emphasize the small number of participants as the study limitation. Nevertheless, that number is typical of a preliminary study, the purpose of which is to outline the direction of further research. The only factor for which statistical significance was shown, and which could potentially “mitigate” the impact of adverse factors in the pre-DM group, was the use of statins . This medicine, which has a known pleiotropic effect [45,46,47], was prescribed to pre-DM patients by general practitioners (not researchers) due to abnormalities in the parameters of lipid metabolism at various, unspecified times before the blood samples were collected for the study. The aim of this decision was only to correct the lipid profile (patients did not need secondary prevention) and as we found, this profile did not differentiate the groups in our study, except for the fact that healthy individuals were not treated pharmacologically with statins. This draws our attention to the potential relationship between MGO concentration and statin intake (only the pre-DM group in the study). It cannot be ruled out that such pharmacotherapy, by lowering LDL levels or independently, contributed to the regulation of AGE formation by MGO in the studied group. In the above-mentioned big study [14], in all three groups (healthy, pre-DM, and DM) lipid-lowering treatment, which could impact the results, was noted. Despite the controversies sometimes raised in the literature on this group of drugs [48] (statins inhibit cholesterol biosynthesis but at the same time they have been advocated to suppress the synthesis of the most important natural antioxidants), statins’ pleiotropic effects, including anti-inflammatory ones, and thus the influence of the lipid-lowering therapy on the concentration of MGO cannot be excluded [49].
Knowing the relationships that link hyperglycaemia and other features of the metabolic syndrome with the formation of MGO, the aim of our study was to assess whether pre-DM patients would have higher levels of this substance than healthy subjects. The obtained results, showing no differences in MGO concentration between the groups, suggest the need to analyse the effects of drugs used to correct lipid disorders on AGEs formation, as it seems impossible that people with pre-DM and healthy individuals present the same cardiovascular risk expressed by MGO value.
The limitation of our study is the number of participants which results from the nature of the study (preliminary research).
5. Conclusions
The higher the HbA1c, the higher the methylglyoxal value confirmed in people with pre-diabetes. Nevertheless, the concentration of methylglyoxal in studied patients with prediabetes (mean value: 135.44 nM) was within the range previously found for the healthy population (60–250 nM) by other authors [20,22]. This was also confirmed in our study when the results from individuals with pre-diabetes were compared with those from the control group. Those unexpected results could be related to the introduction of statins. This observation requires confirmation in a further study, which should be directly dedicated to this issue.
References
- Available online: https://www.idf.org/metabolic_syndrome (accessed on 1 July 2023).
- Tuomilehto, J.; Wolf, E. Primary prevention of diabetes mellitus. Diabetes Care 1987, 10, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Barry, E.; Craig, D.; Airoldi, M.; Bevan, G.; Greenhalgh, T. Preventing type 2 diabetes: Systematic review of studies of cost-effectiveness of lifestyle programmes and metformin, with and without screening, for pre-diabetes. BMJ Open 2017, 7, e017184. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Knowler, W.C.; Crandall, J.P.; Perreault, L.; Edelstein, S.L.; Jeffries, S.L.; Molitch, M.E.; Pi-Sunyer, X.; Darwin, C.; Heckman-Stoddard, B.M.; et al. Metformin for diabetes prevention: Insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017, 60, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Siegel, K.R.; Ng, B.P.; Jawanda, S.; Proia, K.K.; Zhang, X.; Albright, A.L.; Zhang, P. Cost-effectiveness of diabetes prevention interventions targeting high-risk individuals and whole populations: A systematic review. Diabetes Care 2020, 43, 1593–1616. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Nikolaos, P.; Ziegler, D. Prediabetic neuropathy: Does it exist? Curr. Diab. Rep. 2012, 12, 376–383. [Google Scholar]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef] [PubMed]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. (Landmark Ed.) 2019, 24, 186–211. [Google Scholar]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and mechanism of the reaction of aminoguanidine with the oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 2000, 60, 55–65. [Google Scholar] [CrossRef]
- LO, T.W.; Westwood, M.E.; Mclellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N -acetylarginine, N-acetylcysteine, and N-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Maillard degradation pathways of vitamin C. Angew. Chem. Int. Ed. Engl. 2013, 52, 4887–4891. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Where does plasma methylglyoxal originate from? Diabetes Res. Clin. Pract. 2013, 99, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.; Scheijen, J.; Houben, A.; van de Waarenburg, M.; Berendschot, T.; Webers, C.; Reesink, K.; van Greevenbroek, M.; van der Kallen, C.; Schaper, N.; et al. Fasting and post-oral-glucose-load levels of methylglyoxal are associated with microvascular, but not macrovascular, disease in individuals with and without (pre)diabetes: The Maastricht Study. Diabetes Metab. 2021, 47, 101148. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Engelen, L.; Ferreira, I.; Scheijen, J.L.J.M.; Huijberts, M.S.; van Greevenbroek, M.M.J.; van der Kallen, C.J.H.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D.A.; et al. Plasma levels of advanced glycation endproducts Nε-(carboxymethyl)lysine. Nε-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: The Hoorn and CODAM studies. J. Clin. Endocrinol. Metab. 2013, 98, E1369–E1373. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Beulens, J.W.; van Dieren, S.; Scheijen, J.L.; van der A, D.L.; Spijkerman, A.M.; van der Schouw, Y.T.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: A case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes 2015, 64, 257–265. [Google Scholar] [CrossRef] [PubMed]
- The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC); The European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Sutkowska, E.; Fortuna, P.; Kałuża, B.; Sutkowska, K.; Wiśniewski, J.; Gamian, A. Metformin has no impact on nitric oxide production in patients with pre-diabetes. Biomed. Pharmacother. 2021, 140, 111773. [Google Scholar] [CrossRef]
- Gensberger-Reigl, S.; Huppert, J.; Pischetsrieder, M. Quantification of reactive carbonyl compounds in icodextrin-based peritoneal dialysis fluids by combined UHPLC DAD and -MS/MS detection. J. Pharm. Biomed. Anal. 2016, 118, 132–138. [Google Scholar] [CrossRef]
- Henning, C.; Liehr, K.; Girndt, M.; Ulrich, C.; Glomb, M.A. Extending the spectrum of alpha-dicarbonyl compounds in vivo. J. Biol. Chem. 2014, 289, 28676–28688. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Schalkwijk, C.G. Quantification of glyoxal, methylglyoxal and 3- deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: Evaluation of blood specimen. Clin. Chem. Lab. Med. 2014, 52, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Scheijen, J.L.; Jorsal, A.; Parving, H.-H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D.; Schalkwijk, C.G. Higher plasma methylglyoxal levels are associated with incident cardiovascular disease in individuals with type 1 diabetes: A 12-year follow-up study. Diabetes 2017, 66, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Westerink, J.; Scheijen, J.L.; van der Graaf, Y.; Stehouwer, C.D.; Schalkwijk, C.G.; Algra, A.; Grobbee, R.D.; Rutten, G.E.; Visseren, F.L.; et al. Higher plasma methylglyoxal levels are associated with incident cardiovascular disease and mortality in individuals with type 2 diabetes. Diabetes Care 2018, 41, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Nin, J.W.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.H.; Tarnow, J.; Rossing, P.; Stehouwer, C.D. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes Care 2011, 34, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Randell, E.; Han, Y.; Adeli, K.; Krahn, J.; Meng, Q.H. Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin. Biochem. 2011, 44, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, Q.H.; Gordon, J.R.; Khandwala, H.; Wu, L. Proinflammatory and proapoptotic effects of methylglyoxal on neutrophils from patients with type 2 diabetes mellitus. Clin. Biochem. 2007, 40, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Castiglione, S.; Macrì, F.; Giuliani, A.; Ramini, D.; Vinci, M.C.; Tortato, E.; Bonfigli, A.R.; Olivieri, F.; Raucci, A. Circulating levels of AGEs and soluble RAGE isoforms are associated with all-cause mortality and development of cardiovascular complications in type 2 diabetes: A retrospective cohort study. Cardiovasc. Diabetol. 2022, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.M.; Vistisen, D.; Fleming, T.; Nawroth, P.P.; Rossing, P.; Jørgensen, M.E.; Lauritzen, T.; Sandbaek, A.; Witte, D.R. Methylglyoxal is associated with changes in kidney function among individuals with screen-detected Type 2 diabetes mellitus. Diabet. Med. 2016, 33, 1625–1631. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. Guidelines on the management of patients with diabetes. A position of Diabetes Poland. Clin. Diab. 2021, 10, 1–113. [Google Scholar]
- Hanssen, N.M.; Teraa, M.; Scheijen, J.L.; Van de Waarenburg, M.; Gremmels, H.; Stehouwer, C.D.; Verhaar, M.C.; Schalkwijk, C.G. Plasma methylglyoxal levels are associated with amputations and mortality in severe limb ischemia patients with and without diabetes. Diabetes Care 2021, 44, 157–163. [Google Scholar] [CrossRef]
- Baye, E.; Mark, A.B.; Poulsen, M.W.; Andersen, J.M.; O Dragsted, L.; Bügel, S.G.; de Courten, B. Associations between urinary advanced glycation end products and cardiometabolic parameters in metabolically healthy obese women. J. Clin. Med. 2019, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Barski, O.A.; Ahmed, Y.; O’Toole, T.E.; Conklin, D.J.; Bhatnagar, A.; Srivastava, S. Reductive metabolism of AGE precursors: A metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes 2009, 58, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero-Lopez, D.; Lecomte, M.; Moinet, G.; Patereau, G.; Lagarde, M.; Wiernsperger, N. Reaction of metformin with dicarbonyl compounds. Possible implication in the inhibition of advanced glycation end product formation. Biochem. Pharmacol. 1999, 58, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Kender, Z.; Fleming, T.; Kopf, S.; Torzsa, P.; Grolmusz, V.; Herzig, S.; Schleicher, E.; Rácz, K.; Reismann, P.; Nawroth, P.P. Effect of metformin on methylglyoxal metabolism in patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2014, 122, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.M.; Sullivan, D.L. Metformin for prevention of type 2 diabetes. Ann. Pharmacother. 2004, 38, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Yee, L.T.L.; Thallas, V.; Lassila, M.; Candido, R.; Jandeleit-Dahm, K.A.; Thomas, M.C.; Burns, W.C.; Deemer, E.K.; Thorpe, S.R.; et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes 2004, 53, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.M.D.; Soro-Paavonen, A.; Sheehy, K.; Li, J.; Calkin, A.C.; Koitka, A.; Rajan, S.N.; Brasacchio, D.; Allen, T.J.; Cooper, M.E.; et al. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia 2011, 54, 681–689. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other AGE-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Rabbani, N.; Chittari, M.V.; Bodmer, C.W.; Zehnder, D.; Ceriello, A.; Thornalley, P.J. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010, 59, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Godfrey, L.; Xue, M.; Shaheen, F.; Geoffrion, M.; Milne, R.; Thornalley, P.J. Glycation of LDL by methylglyoxal increases arterial atherogenicity: A possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes 2011, 60, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Vermeer, M.A.; Stehouwer, C.D.; Koppele, J.T.; Princen, H.M.; van Hinsbergh, V.W. Effect of methylglyoxal on the physico-chemical and biological properties of low-density lipoprotein. Biochem. Biophys. Acta 1998, 1394, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Deplazes, E.; Cranfield, C.G.; Garcia, A. The role of structure and biophysical properties in the pleiotropic effects of statins. Int. J. Mol. Sci. 2020, 21, 8745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends. Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Tikhaze, A.K.; Kapel’ko, V.I.; Shepel’kova, G.S.; Shumaev, K.B.; Panasenko, O.M.; Konovalova, G.G.; Belenkov, Y.N. Mechanisms of oxidative modification of low density lipoproteins under conditions of oxidative and carbonyl stress. Biochemistry 2007, 72, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of advanced glycation end product (AGE) formation and accumulation. Handb. Exp. Pharmacol. 2021, 264, 395–423. [Google Scholar]

