1. Introduction
Back pain is extremely common in the adult population, and it is one of the most frequent causes for patients to seek medical care [1,2,3]. The conservative medical treatments for addressing back pain consist of attempting oral medication and manual and exercise therapies. Interventional pain management modalities have been used for many years for the management of back pain [1,2,3]. The conservative medical treatments for addressing back pain consist of attempting oral medication and manual and exercise therapies. Commonly performed interventional procedures for back pain management include epidural steroid injections, caudal steroid injections, selective nerve root blocks, facet joints, and/or medial branch blocks. Such procedures provide a relatively long-term pain-alleviating effect, with the most advantageous outcome typically observed several weeks after the procedure [4]. Nevertheless, there may be differences in maximal efficacy and duration of action. Therefore, there are no uniform follow-up timings. The literature suggests that control visits are usually scheduled for the 1 week, 1 month, 2 months, and 3 months following the procedure. Sometimes, follow-ups occur on week 2, and in case of unsatisfactory anesthetic effect, the procedure may be repeated 3–4 weeks after the initial intervention. Although blind caudal epidural injections have a success rate of 74–90% [4], fluoroscopy-guided (FL-guided) techniques are more commonly used as they provide more accurate visualization and needle placement, resulting in subsequently accurate administration of the injectate and better outcomes [1,2,3,5].
The success rate of fluoroscopy-guided injections is generally higher compared to blind techniques and can vary depending on several factors, including individual patient factors, underlying cause and mechanism of pain, location of pain generators, specific injection technique, skill, and experience of specialists performing this procedure [5]. However, this technique has several negative effects, including radiation exposure, the contrast agent utilized to confirm appropriate drug deposition that has been associated with various side effects, including nausea and vomiting, hives, bronchospastic reaction, urticaria, hypotension, tachycardia, and anaphylactic reaction [2,3]. US-guided techniques have recently started gaining popularity among interventional pain physicians [6]. Ultrasound has proven to provide real-time visualizations of anatomical structures with less cost and reliable imaging for finding successful injection sites [7]. The success rate of US-guided low back pain interventions has been reported at 85–100% [8,9,10]. Previous studies compared the effects of caudal epidural steroid injections under the guidance of FL- and US-guided imaging by assessing post-procedural outcomes using the following scales: “Visual Analogue Scale” (VAS), “Visual Numeric Scale” (VNS), “Numeric Rating Scale” (NRS), “postoperative Oswestry Disability Index” (ODI), “Neck Disability Index” (NDI) functionality scores, and frequency of postoperative complications among patients [1,2,3,10]. Previous observational and randomized controlled trials reported mixed results regarding the efficacy and safety of these methods, but many of them did not find any significant difference. The objective of this SR&MA was to compare the US- and FL-guided interventions in reducing back pain intensity, functional outcomes, and complications.
2. Materials and Methods
2.1. Protocol
The current SR&MA was conducted following the PRISMA guidelines [11]. The protocol was publicly registered in the Open Science Framework at https://doi.org/10.17605/OSF.IO/CBQ82. We searched for RCTs, which studied the effects of the US-guided and FL-guided injections for back pain management, published before May 2023 in Scopus, PubMed, and the Cochrane Library (Figure 1). The following search terms and/or their combinations were used: “fluoroscopy”, “fluoroscopic guidance”, “fluoroscopy-guided”, “ultrasound”, “ultrasound-guided”, “sonography”, “diagnostic imaging”, “diagnostic”, “imaging”, “ultrasonography”, “ultrasonics”, “ultrasounds”, “back pain”, “low back pain”, “radicular pain”, and “epidural injections”.
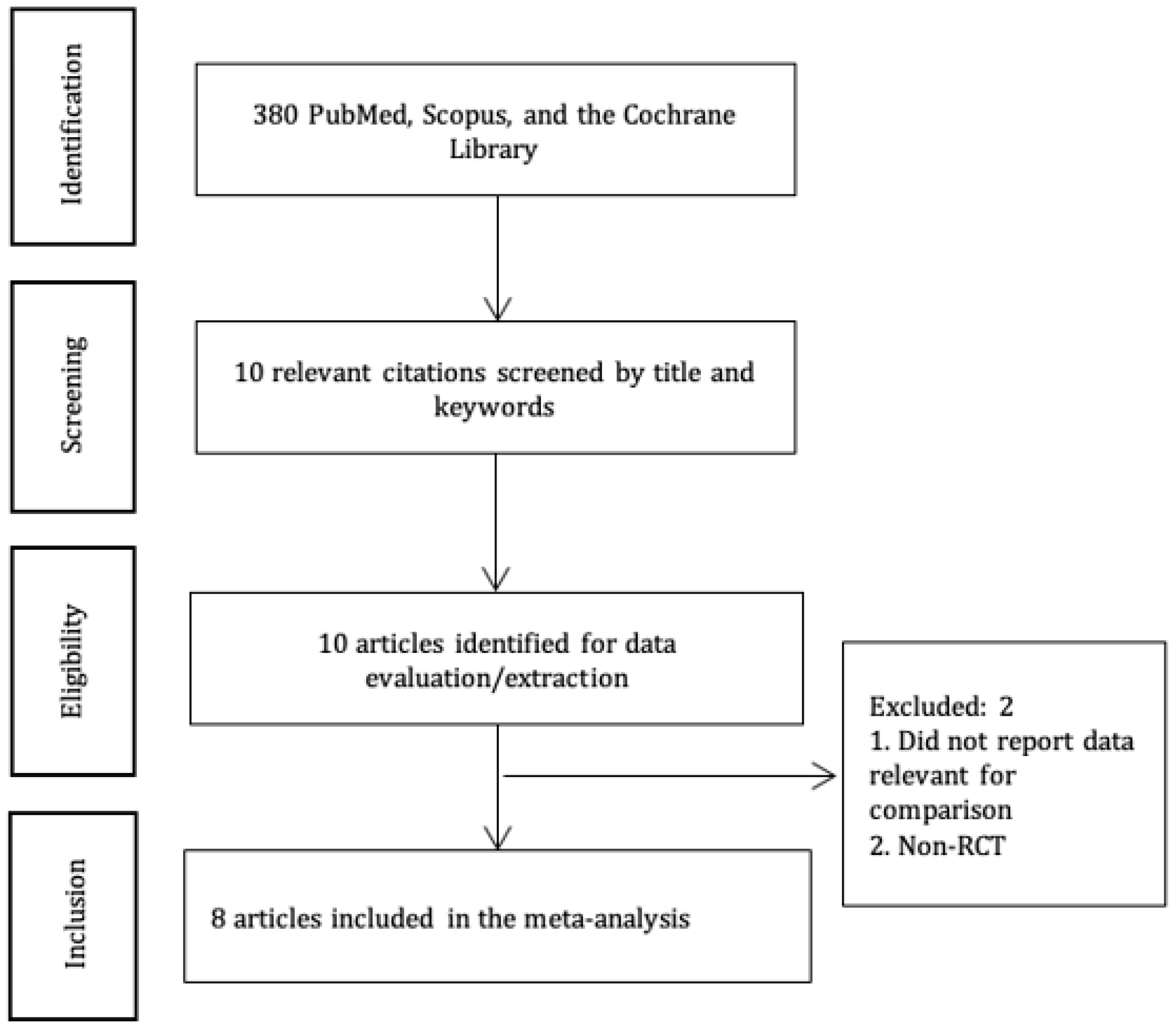
Figure 1. PRISMA diagram.
Two authors conducted the screening. First, the articles were screened based on titles. Then, abstracts were screened based on inclusion/exclusion criteria. Finally, full texts were screened, and those articles that reported outcomes of interest for this study were included in the meta-analysis. In case of disagreements, a third author was consulted.
We considered the following criteria for inclusion: (a) study design: “randomized controlled trials” (RCTs); (b) age: 18 years and older; and (c) procedures: interventions for back pain management performed either under US and/or fluoroscopic guidance. We considered studies published in the English language.
We excluded studies if they included (a) pediatric patients; (b) study designs other than RCT; or (c) poorly described methodology or inadequately reported findings.
2.2. Outcomes
The primary outcome of our meta-analysis is post-procedural pain intensity. The secondary outcomes were functional outcomes (ODI/NDI) and postprocedural complications.
2.3. Data Extraction and Biostatistics Methods
Data extraction disputes were resolved via discussion among the authors. In case of further disagreements, an author not involved in data extraction was consulted. We extracted the following data from RCTs in the data table : 1st author, reference, country, study design and goals, patient age, study groups, number of patients, and interventions. If more than one study reported the outcomes of interest, we added them for data synthesis. Statistical methods were used for data conversions [12,13]. First, the data were extracted into an Excel file, and then a meta-analysis was conducted using the random effects model in RevMan 5.4 software, “the Cochrane Collaboration”. In data synthesis, the standardized mean difference was used for the outcomes of pain intensity and ODI/NDI, and the risk ratio was used for the incidence of postprocedural complications. The results were presented with forest plots, where green/blue squares with lines represent sample means with 95% CIs in a single study, whereas black diamonds represent the same for a group of studies (i.e. the overall result). I2 statistic was used to estimate the heterogeneity.
2.4. Methodological Assessment
We used the Cochrane Risk of Bias tool 2 [14] to check the methodological quality of studies. Each study was assessed in terms of bias in the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. For each of these domains, the risk of bias was graded as “high”, “low”, or “some concerns”. For example, for the randomization process, a “low” risk of bias implied appropriate allocation sequence generation and concealment that was well described and resulted in comparable groups at baseline. Based on the results for each domain, an overall assessment of bias for the study was drawn. If one to two domains were rated as having “some concerns”, the overall assessment for the study was “some concerns”. If three or more domains were rated as “some concerns” or at least one as “high risk”, the study was given an overall rate of “high” risk of bias. The main outcomes were assessed with GRADE [15] and presented in the Summary of Findings table. For each outcome, the certainty of evidence was evaluated based on the risk of bias, inconsistency, indirectness, and imprecision. Each of these criteria was rated as “not serious”, “serious”, or “very serious”. “Serious” or “very serious” concerns about the certainty of evidence downgraded the certainty of evidence from high to very low. Two authors conducted the quality assessment. Risk of bias assessed the randomization and blinding processes, incomplete accounting of patients or events, selective outcome reporting, and other potential sources of bias. Inconsistency took into account unexplained heterogeneity, the width of variance of the point estimates, and the overlapping of the confidence intervals. Indirectness checked whether the evidence was drawn from populations, interventions, outcome measures, or comparisons different from the ones of interest for a given outcome. Imprecision considered the number of patients and the width of the confidence interval. Upgrading was possible when the pooled effect size was large, when there was a dose–response effect, or when the effect could have been a result of residual confounding.
3. Results
We initially identified 380 articles that matched our search criteria (Figure 1). Eight articles [2,3,6,7,16,17,18,19] with 642 patients (US-guided group—321 and FL-guided group—321) were selected for a meta-analysis .
3.1. Qualitative Description of the Studies
The included studies were published between 2012 and 2018. The studies presented the results of pain measurements before the injection, at one and two weeks, and one, two, and three months post-operation, while ODI/NDI functionality scores were measured at baseline, two weeks, and one and three months. Immediate post-injection complications such as vasovagal reaction, transient headache, and facial flushing were the common reactions encountered by a minority of patients.
3.2. A Meta-Analysis of All Studies Combined
3.3. Assessment of Methodological Quality
Given the nature of the procedure, a certain degree of risk of bias was present due to the lack of double blinding. This has seriously affected the methodological quality of the studies. Based on the Cochrane risk of bias assessment, three studies had “some concerns” about the risk of bias, and four had a “high” risk of bias . Five outcomes were assessed for quality . Of them, one was of moderate quality, and four were of low quality. Detailed assessment of the outcomes is provided in the Evidence profile table in .
4. Discussion
In this SR&MA, we included eight RCTs, which compared ultrasound-guided and fluoroscopy-guided techniques for back pain management. Using ultrasound guidance alongside fluoroscopic confirmation could be a viable option for visual guidance for various interventions in back pain management [2,3,6,7,16,17,18,19].
There were no significant differences found in the reduction in back pain at one week, one month, and three months following the interventions. Regarding postoperative functional outcomes assessed by the ODI and NDI, there was no difference between the two groups at one month, and three months after intervention. The rate of postoperative complications also did not differ significantly between US-guided and FL-guided techniques. Subgroup analysis that was performed for various outcomes (vasovagal reaction, transient headache, facial flushing) did not differ significantly between US-guided and FL-guided injections [2,3,6,7,16,17,18,19].
A recent meta-analysis compared US-guided injections (intervention group) with fluoroscopy- or computer tomography (CT)-guided injections (control group) in the spinal nerves [20]. Similar to our results, the study found no difference in pain scores between the US- and FL/CT-guided interventions for low back pain. Likewise, the authors concluded that there was “little to no difference” in ODI/NDI scores between the US- and FL-/CT-guided injections, similar to our results. The authors then conducted a sub-group analysis, separating the cervical group from the lumbar one. In this sub-group analysis, both pain and functional disability scores remained comparable between the US and the FL/CT groups. Regarding adverse events, the authors suggest that both major and minor complications seem to be less likely to occur in the US-guided intervention group. Moreover, US-guided injections were associated with a lower duration of the procedure [20].
Observational studies showed similar results. Thus, a study comparing “US-guided selective nerve root block”, “FL-guided interlaminar epidural block”, and “FL-guided transforaminal epidural block” for the management of radicular pain in the lower cervical spine demonstrated that although both the VNS and “neck disability index” improved within the groups at 1, 3, and 6 months in all groups, there were no differences between the groups. Moreover, the treatment success rate at all time points was not significantly different between the groups. US-guided interventions required a shorter duration and resulted in similar pain reduction and functional improvement. Thus, a US-guided technique might be considered for epidural steroid injection [21].
Similar results were yielded by another retrospective observational study of 54 patients with facet syndrome [22]. One month post-procedure, the pain scores improved within the group but did not differ between the US- and FL-guided groups. There were no major complications in either group. The researchers suggest using US guidance as a safe and equally effective substitute for FL-guided injections to avoid excessive irradiation.
Another observational study comparing US- and FL-guided caudal epidural steroid injection for the management of unilateral lower lumbar radicular pain also yielded similar results [23]. The study showed that the Oswestry Disability Index and verbal numeric scale scores improved at 3, 6, and 12 months in both groups. There were no statistical differences in verbal numeric scale scores and Oswestry Disability Index and the proportion of patients with successful treatment between the groups. Therefore, US-guided techniques might be considered in the management of lower lumbar radicular pain.
Ultrasound could be used with fluoroscopic confirmation for guiding sacroiliac joint injections in patients with SIJ arthritis. Both FL-guided and US-guided approaches for different methods of SIJ innervation are well described in a review article [24]. Even if fluoroscopic guidance is used for confirmation, the radiation exposure could be significantly reduced. There were no notable differences in accuracy, efficacy, or patient satisfaction between these two image-guided techniques for SIJ injections [19]. The rates of intra-articular injection during US-guided SIJ injections have shown significant variability in prior studies. Three previous studies have reported higher rates of intra-articular injection, ranging from 76.7% to 87.3% [25,26,27]. However, it is worth noting that the first two studies included relatively younger patients with inflammatory SIJ arthropathy and excluded patients with osteoarthritis of the SIJ. In contrast, our study included patients with an average age of 48.9 years who were more likely to have osteoarthritic changes. Accessing the intra-articular portion of the joint becomes more challenging in osteoarthritic conditions compared to inflammatory pathologies like rheumatoid arthritis or ankylosing spondylitis. Furthermore, a meta-analysis found that the risk difference of incorrect needle placement of US-guided injections was 13% for facet joint injections, as confirmed by CT, and 11% for lumbar medial branch blocks, as confirmed by FL guidance [28]. The quality of evidence, however, was from very low to low. Therefore, differences in intra-articular injection rates may be attributed to patient characteristics and pathologies studied.
Hartung et al. found that using ultrasound for sacroiliac joint (SIJ) arthropathy resulted in 40% of injections being administered intraarticularly [29]. Remarkably, both intra- and periarticular injections provided similar pain relief benefits. Other studies have also suggested that periarticular injections could have comparable outcomes to intraarticular injections. In Hartung’s study, the reduction in pain scores 1 month after the procedure (25% to 33%) was similar to that reported in the study by Soneji [17,27]. Overall, the success rate of intraarticular injections using the US for SIJ injections falls within the wide range reported in the literature, and the results may vary depending on the characteristics of the study populations.
Given the absence of radiation exposure, lower cost, and convenience, US-guided techniques might be preferred for patients, doctors, and healthcare in general. The current guidelines for interventional management of chronic back pain recommend using fluoroscopic guidance. A qualitative review of studies on intradiscal injections for low back pain management revealed that almost 62% of the studies on the topic had performed the procedure under FL guidance (with or without comparators), and another 8.8% combine the FL and CT approaches [30]. Another narrative review described US, FL, and CT visualization in chronic pain interventions [31]. Despite the firm adherence to the guidelines on the use of fluoroscopy-guided procedures, adverse events have been reported. The complications may occur if the needle penetrates vitally important vessels, such as the anterior spinal artery [6,7,18]. Ultrasound guidance allows better visualization of vessels, nerves, soft tissue, and the spread of the anesthetic solution around the nerve. Therefore, it might provide an operator with better identification of vulnerable anatomical structures, while the value of fluoroscopy in identifying such anatomical structures is limited [2]. The advantages of the US are that it is radiation-free, easy to use, and it can offer continuous real-time needle guidance. US guidance also provides clear images of the sacral hiatus and detects the anatomic variations of the sacrum and sacral hiatus. In the previous studies, approximately 2% to 3% of the studied population had closed sacral canals, thus making “cervical epidural steroid injection” impossible for these subjects [17]. US-guided caudal epidural steroid injections” have been reported to achieve a 100% success rate in identifying the sacral hiatus in patients with radicular pain.
This systematic review has several major limitations. Only eight studies with relatively small sample sizes matched the inclusion criteria and were meta-analyzed. There was heterogeneity in the patient populations, the anatomical localization of back pain, and interventions included in the meta-analysis. Additionally, some studies employed an “ultrasound-assisted” approach, where ultrasound was used to locate the injection site, while others utilized dynamic guidance during the procedure. Given the nature of the intervention, there was a serious risk of bias in the included studies due to the lack of blinding. Finally, while there was no statistically significant difference observed between the two techniques, none of the included studies had a no-intervention arm to assess the effectiveness of the injections for low back pain. Despite these limitations, there was no evidence that US-guided techniques were overall inferior to FL-guided techniques. The advantages of the US include relative simplicity of performance, absence of radiological exposure, and continuous real-time needle guidance. However, while ultrasound guidance can decrease radiation exposure and enhance the visualization of soft tissues and vascular structures, there are a few disadvantages [29]. The quality of images can be impacted by patient morphology and injection location, and the technique is not effective for visualizing axial or spine structures where bone produces an acoustic shadow [32].
5. Conclusions
There were no significant differences between US-guided and FL-guided injections in reducing back pain intensity and complications at one month and three months after intervention. However, the model tends to favor the FL-guided injections over the US-guided injections in terms of functionality. Given the limitations of this meta-analysis, future RCTs are warranted to establish solid evidence on relevant outcomes for using these two techniques for back pain management.
References
- Chen, C.P.C.; Tang, S.F.T.; Hsu, T.-C.; Tsai, W.-C.; Liu, H.-P.; Chen, M.J.L.; Date, E.; Lew, H.L. Ultrasound Guidance in Caudal Epidural Needle Placement. Anesthesiology 2004, 101, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.; Lee, J.H.; Kim, J.; Park, K.D.; Lee, W.Y.; Park, Y. Ultrasound-Guided Selective Nerve Root Block versus Fluoroscopy-Guided Transforaminal Block for the Treatment of Radicular Pain in the Lower Cervical Spine: A Randomized, Blinded, Controlled Study. Skeletal Radiol. 2013, 42, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Evansa, I.; Logina, I.; Vanags, I.; Borgeat, A. Ultrasound versus Fluoroscopic-Guided Epidural Steroid Injections in Patients with Degenerative Spinal Diseases: A Randomised Study. Eur. J. Anaesthesiol. 2015, 32, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Stitz, M.Y.; Sommer, H.M. Accuracy of Blind versus Fluoroscopically Guided Caudal Epidural Injection. Spine 1999, 24, 1371–1376. [Google Scholar] [CrossRef]
- Manchikanti, L.; Cash, K.A.; Pampati, V.; McManus, C.D.; Damron, K.S. Evaluation of Fluoroscopically Guided Caudal Epidural Injections. Pain Physician 2004, 7, 81–92. [Google Scholar] [CrossRef]
- Akkaya, T.; Ozkan, D.; Kertmen, H.; Sekerci, Z. Caudal Epidural Steroid Injections in Postlaminectomy Patients: Comparison of Ultrasonography and Flouroscopy. Turk. Neurosurg. 2017, 27, 420–425. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Ma, L.; Cai, Z.; Meng, C.; Qi, S.; Zhou, H. Ultrasound-Guided Versus Fluoroscopy-Controlled Lumbar Transforaminal Epidural Injections: A Prospective Randomized Clinical Trial. Clin. J. Pain 2016, 32, 103–108. [Google Scholar] [CrossRef]
- Loizides, A.; Gruber, H.; Peer, S.; Galiano, K.; Bale, R.; Obernauer, J. Ultrasound Guided versus CT-Controlled Pararadicular Injections in the Lumbar Spine: A Prospective Randomized Clinical Trial. AJNR Am. J. Neuroradiol. 2013, 34, 466–470. [Google Scholar] [CrossRef]
- Hashemi, M.; Dadkhah, P.; Taheri, M.; Haji Seyed Abootorabi, S.M.; Naderi-Nabi, B. Ultrasound-Guided Lumbar Transforaminal Epidural Injections; A Single Center Fluoroscopic Validation Study. Bull. Emerg. Trauma 2019, 7, 251–255. [Google Scholar] [CrossRef]
- Blanchais, A.; Le Goff, B.; Guillot, P.; Berthelot, J.-M.; Glemarec, J.; Maugars, Y. Feasibility and Safety of Ultrasound-Guided Caudal Epidural Glucocorticoid Injections. Joint Bone Spine 2010, 77, 440–444. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE Guidelines: A New Series of Articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Hazra, A.K.; Bhattacharya, D.; Mukherjee, S.; Ghosh, S.; Mitra, M.; Mandal, M. Ultrasound versus Fluoroscopy-Guided Caudal Epidural Steroid Injection for the Treatment of Chronic Low Back Pain with Radiculopathy: A Randomised, Controlled Clinical Trial. Indian. J. Anaesth. 2016, 60, 388–392. [Google Scholar] [CrossRef]
- Soneji, N.; Bhatia, A.; Seib, R.; Tumber, P.; Dissanayake, M.; Peng, P.W.H. Comparison of Fluoroscopy and Ultrasound Guidance for Sacroiliac Joint Injection in Patients with Chronic Low Back Pain. Pain Pract. 2016, 16, 537–544. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.-H.; Park, K.D.; Ahn, J.K.; Park, J.; Jee, H. Ultrasound-Guided vs. Fluoroscopy-Guided Caudal Epidural Steroid Injection for the Treatment of Unilateral Lower Lumbar Radicular Pain: A Prospective, Randomized, Single-Blind Clinical Study. Am. J. Phys. Med. Rehabil. 2013, 92, 575–586. [Google Scholar] [CrossRef]
- Jee, H.; Lee, J.-H.; Park, K.D.; Ahn, J.; Park, Y. Ultrasound-Guided versus Fluoroscopy-Guided Sacroiliac Joint Intra-Articular Injections in the Noninflammatory Sacroiliac Joint Dysfunction: A Prospective, Randomized, Single-Blinded Study. Arch. Phys. Med. Rehabil. 2014, 95, 330–337. [Google Scholar] [CrossRef]
- Kimura, R.; Yamamoto, N.; Watanabe, J.; Ono, Y.; Hongo, M.; Miyakoshi, N. Comparative Efficacy of Ultrasound Guidance and Fluoroscopy or Computed Tomography Guidance in Spinal Nerve Injections: A Systematic Review and Meta-Analysis. Eur. Spine J. 2023. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, W.Y.; Kim, J.W.; Cho, K.R.; Nam, S.H.; Park, Y. Ultrasound-Guided Selective Nerve Root Block versus Fluoroscopy-Guided Interlaminar Epidural Block versus Fluoroscopy-Guided Transforaminal Epidural Block for the Treatment of Radicular Pain in the Lower Cervical Spine: A Retrospective Comparative Study. Pain Res. Manag. 2020, 2020, 9103421. [Google Scholar] [CrossRef] [PubMed]
- Touboul, E.; Salomon-Goëb, S.; Boistelle, M.; Sobhy Danial, J.; Deprez, V.; Goëb, V. Lumbar Zygapophyseal Joints Injections under Ultrasound Guidance an Alternative to Fluoroscopy Guidance in the Management of Low Back Pain. Sci. Rep. 2022, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Kim, T.K.; Lee, W.Y.; Ahn, J.; Koh, S.H.; Park, Y. Ultrasound-Guided Versus Fluoroscopy-Guided Caudal Epidural Steroid Injection for the Treatment of Unilateral Lower Lumbar Radicular Pain: Case-Controlled, Retrospective, Comparative Study. Medicine 2015, 94, e2261. [Google Scholar] [CrossRef] [PubMed]
- Soto Quijano, D.A.; Otero Loperena, E. Sacroiliac Joint Interventions. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 171–183. [Google Scholar] [CrossRef]
- Pekkafahli, M.Z.; Kiralp, M.Z.; Başekim, C.C.; Silit, E.; Mutlu, H.; Oztürk, E.; Kizilkaya, E.; Dursun, H. Sacroiliac Joint Injections Performed with Sonographic Guidance. J. Ultrasound Med. 2003, 22, 553–559. [Google Scholar] [CrossRef]
- Klauser, A.; De Zordo, T.; Feuchtner, G.; Sögner, P.; Schirmer, M.; Gruber, J.; Sepp, N.; Moriggl, B. Feasibility of Ultrasound-Guided Sacroiliac Joint Injection Considering Sonoanatomic Landmarks at Two Different Levels in Cadavers and Patients. Arthritis Rheum. 2008, 59, 1618–1624. [Google Scholar] [CrossRef]
- Hartung, W.; Ross, C.J.; Straub, R.; Feuerbach, S.; Schölmerich, J.; Fleck, M.; Herold, T. Ultrasound-Guided Sacroiliac Joint Injection in Patients with Established Sacroiliitis: Precise IA Injection Verified by MRI Scanning Does Not Predict Clinical Outcome. Rheumatology 2010, 49, 1479–1482. [Google Scholar] [CrossRef]
- Ashmore, Z.M.; Bies, M.M.; Meiling, J.B.; Moman, R.N.; Hassett, L.C.; Hunt, C.L.; Cohen, S.P.; Hooten, W.M. Ultrasound-Guided Lumbar Medial Branch Blocks and Intra-Articular Facet Joint Injections: A Systematic Review and Meta-Analysis. Pain Rep. 2022, 7, e1008. [Google Scholar] [CrossRef]
- Hofmeister, M.; Dowsett, L.E.; Lorenzetti, D.L.; Clement, F. Ultrasound- versus Fluoroscopy-Guided Injections in the Lower Back for the Management of Pain: A Systematic Review. Eur. Radiol. 2019, 29, 3401–3409. [Google Scholar] [CrossRef]
- Migliore, A.; Sorbino, A.; Bacciu, S.; Bellelli, A.; Frediani, B.; Tormenta, S.; Pirri, C.; Foti, C. The Technique of Intradiscal Injection: A Narrative Review. Ther. Clin. Risk Manag. 2020, 16, 953–968. [Google Scholar] [CrossRef]
- Wang, D. Image Guidance Technologies for Interventional Pain Procedures: Ultrasound, Fluoroscopy, and CT. Curr. Pain Headache Rep. 2018, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.W.H.; Narouze, S. Ultrasound-Guided Interventional Procedures in Pain Medicine: A Review of Anatomy, Sonoanatomy, and Procedures: Part I: Nonaxial Structures. Reg. Anesth. Pain Med. 2009, 34, 458–474. [Google Scholar] [CrossRef] [PubMed]

