1. Introduction
Depression and obesity are highly prevalent conditions with relevant public health implications. In 2016, the World Health Organization (WHO) reported that approximately 2 billion adults were overweight, and 650 million of them were obese [1]. The WHO also reported that 5% of adults suffer from depression, and the alarming increase in this percentage during recent decades highlights the urgency of implementing more effective strategies against these devastating disorders [2]. Depression and obesity hold a bidirectional relationship with each other; that is, the presence of one increases the risk for developing the other, since they are, in many cases, comorbid conditions [3]. Therefore, it is mandatory to continue investigating the mechanisms responsible for the intricate metabolic and physiological pathways associated with both conditions. Particularly, both the KP and the ECS are known to be deregulated in some pathological conditions, including depression [4,5] and obesity, among others [6,7]. Depression and obesity are importantly influenced by physical exercise—defined as a planned, structured and repetitive activity that improves and maintains physical fitness and health—inducing changes in the mobilization of endocannabinoids (eCBs) and kynurenine metabolites (KYNs) [8,9]. eCB mobilization has been related to the activation of eCB receptor 1 (CB1) in the liver and adipose tissue, and a significant synthesis of eCB in skeletal muscle has been reported to regulate metabolism and restore energy following exercise [10]. On the other hand, the peripheric mobilization of KYNs has been related to suppression of KYN accumulation in the central nervous system (CNS) through an activation of KYN clearance in exercised skeletal muscle through the action of kynurenine aminotransferases enzymes (KATs) [11]. Thus, the effects of exercise training observed in obesity and depression models through modulation of these two pathways suggest an undescribed mechanism of crosstalk mediated by these metabolites, as reported in disease contexts like migraine [12]. Furthermore, the biological and biochemical signals responsible for modifying the rates of synthesis and degradation of both KYNs and eCBs, as well as the effects exerted by these molecules, which sometimes converge or overlap, will be described further. Therefore, the aim of this review is to analyze and discuss some studies performed recently to investigate the potential interactions between both systems, particularly those related to exercise-derived endocannabinoidome and kynurenine mechanisms, and to elucidate how the prescription of physical exercise could represent a new approach for the clinical management of these two conditions.
2. Modulation of the KP and the ECS through Physical Exercise
The benefits of regular practice of physical exercise for managing the symptoms of depression and obesity are widely accepted [13,14]. The American College of Sports Medicine recommends 150 min per week of moderate-intensity aerobic physical activity, 75 min per week of high-intensity aerobic exercise, or a combination of moderate and intense aerobic activity spread throughout the week, for weight loss and prevention of weight regain for adults [15]. The National Institute for Health and Care Excellence (NICE) guidelines recommend exercise interventions provided by a trained practitioner that include moderate-intensity aerobic exercise adapted to each individual’s needs [16]. Furthermore, exercise interventions have been shown to reduce depressive symptoms in adults and offer an additional treatment or adjuvant therapy [17]. Therefore, a sedentary lifestyle and physical inactivity are associated with high mortality rates and the development and progression of cancer, diabetes, hypertension, cardiovascular disease, depression, and obesity [18,19,20]. The mechanisms involved in the development of these diseases remain poorly understood [20,21]; however, exercise is involved in some metabolic pathways related to oxidative stress, immune responses, ECS, the conformation of intestinal microbiota, and the KP [22], which elicit adaptive changes which are observed, particularly, in myocytes. In turn, increasing mitochondrial biogenesis rates, anti-oxidant capacity, and protein synthesis, which generate a “pre-conditioned” state that protects cells from stressors that may occur during exercise, such as oxidative stress, increased temperature, and muscle atrophy, among others [23]. Particularly, some effects derived from physical exercise have been reported in the brain and the periphery, which positively influence the progression, clinical presentation, and prevention of various neurological, psychiatric, and metabolic diseases, including depression [24] and obesity [25].
2.1. The Kynurenine Pathway
Tryptophan (TRP) is an essential amino acid which is critical for protein synthesis; it is a precursor of the neurotransmitter serotonin, the hormone melatonin, and kynurenine catabolites, which are involved in the synthesis of the cellular cofactor nicotinamide adenine dinucleotide (NAD+). TRP is bound to albumin, either in plasma or serum, and only 5–10% is free to be immediately taken up by tissues and organs; thus, the availability of the KP relies on free TRP [11]. TRP is metabolized mainly through two metabolic pathways: the KP and the serotonin (5-hydroxytryptamine, 5-HT) pathway. A proportion of 95% of TRP comes from the diet; it is degraded through the KP to produce NAD, which is the preferred end product of the KP, and an essential cofactor which is necessary in maintaining energy metabolism [11] (Figure 1).

Figure 1. Main dysregulated enzymes and metabolites of the kynurenine pathway in obesity (orange) and both obesity and depression (pink). Created with BioRender.com.
Skeletal muscle is the main reservoir of amino acids, which, under critical conditions, can be displaced for energy production by some organs [26] and contributes to KYN metabolism, and its composition and performance can be modified through exercise. In muscle fibers, there is a low expression of indoleamine 2,3-dioxygenase (IDO) and minimal tryptophan 2,3-dioxygenase (TDO) enzymatic activity, so the KYN produced in the liver or immune cells and circulating TRP are imported into muscle fibers through the large neutral amino acids transporters (LATs) following an open competition with other amino acids [27]. The most abundant enzymes within muscle fibers are KATs, KYNU, KMO, and 3-hydroxyanthranilate 3,4-dioxygenase (3HAO). KMO and 3HAO require molecular oxygen for their catalytic activity, whereas KATs and KYNU require α-keto acids. Therefore, during exercise, the increased O2 consumption required to sustain higher energy production from mitochondria could affect the KP by reducing the enzymatic activity of KMO and 3HAO and by inhibiting a class of prolyl-hydroxylases (PHDs); these utilize α-ketoglutarate as a substrate under hypoxia conditions, leading to the use of α-ketoglutarate availability by KATs [28]. Chronic endurance exercise increases skeletal muscle expression of all four KATs in humans through the PGC-1α1-PPARα/δ pathway to convert KYN to KYNA, elevating the serum concentration [29]. However, it remains unknown how KYNA leaves muscle fibers [30]. KYNA does not cross the brain–blood barrier (BBB), so decreasing KYN levels in the brain could reduce the stress-mediated effects underlying depressive symptoms [31]. PGC-1a co-activators and its variants PGC-1a1 and PGC-1a4 modulate processes related to energy metabolism and muscle mass maintenance by regulating physiological adaptations to exercise and promoting myokine secretion to regulate systemic energy expenditure [32]. Therefore, while PGC-1a1 regulates bioenergetic cellular processes, PGC-1a4 modulates muscle hypertrophy by repressing myostatin expression [27,33].
2.2. The Endocannabinoid System
The ECS is an endogenous signaling system that plays a central role in the development and function of the nervous system [70]. The ECS is formed by multifunctional components such as cannabinoid receptors (mainly CB1 and CB2, as these are the most frequently studied), their endogenous ligands, endocannabinoids (eCB), and several proteins involved in the transport, synthesis, and degradation of other neurotransmitters [70]. eCBs are derived from neural membrane lipids (i.e., phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol-4,5-bisphosphate) that are released and can diffuse to neurons, microglia, and astrocytes. The most studied eCBs to date are 2-arachidonoylglycerol (2-AG), N-arachidonoyl-ethanolamine, anandamide (AEA), and oleamide (OEA), but new molecules related to cannabinoids have been emerging in the literature [68]. eCBs bind to their receptors, mainly CB1 receptors in neurons and muscle cells and CB2 receptors in microglia and peripheral immune cells, where they exert their modulatory functions on diverse cellular processes [71] (Figure 2).
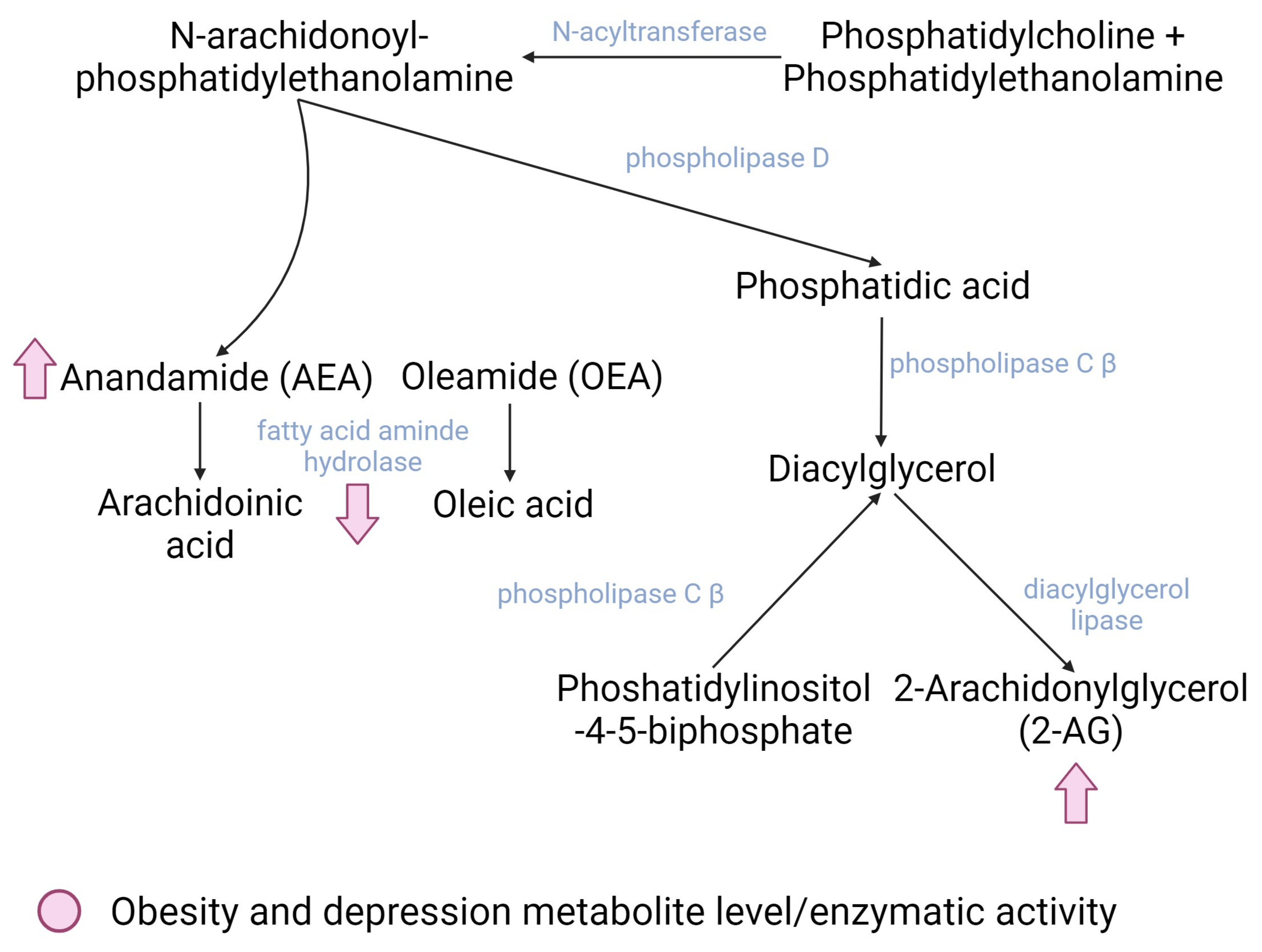
Figure 2. Main dysregulated enzymes and metabolites of the endocannabinoid system in both obesity and depression (pink). Created with BioRender.com.
There are two main pathways for the biosynthesis of eCB. The first one involves the transfer of an arachidonic acid from phosphatidylcholine to phosphatidyl ethanolamine; this is mediated by the enzyme N-acyltransferase, which generates N-arachidonoyl-phosphatidyl ethanolamine (NAPE), an endocannabinoid precursor. NAPE is further degraded to AEA and phosphatidic acid. Phosphatidic acid is then degraded to diacylglycerol (DAG) by the phospholipase C β enzyme (PLCβ) that can also degrade phosphatidylinositol-4,5-bisphosphate (PIP2) to DAG, which constitutes the second route of endocannabinoid synthesis. Finally, DAG is hydrolyzed to 2-AG; this is mediated by the diacylglycerol lipase enzyme [70]. The degradation of AEA occurs primarily through the fatty acid amino hydrolase enzyme (FAAH), whereas 2-AG is degraded mainly through three enzymes: monoacylglycerol lipase (MGL) and alpha/beta domain hydrolases 6 and 12 (ABHD6 and 12) [70].
Among people with obesity, the key role of the ECS is the overproduction of eCBs by adipose tissue; this raises their central and peripheral circulating levels and is probably due to the higher availability of eCB precursors and eCB catabolism dysfunction. The attachment of eCb to CB1 increases hunger and willingness to intake food, decrease peristalsis, and delays stomach emptying [72]; however, the CB1 blockade precludes the attachment of AEA—the natural antidepressant endocannabinoid—resulting in depression symptoms [73].
Therefore, there is growing interest in determining the role of the ECS in health and disease processes because this system exerts several effects; in turn, it is influenced by many other signaling pathways involved in various physiological processes. The interest in generating more knowledge in the exercise-derived endocannabinoidome has been driven mainly because several of its participants have been considered as emerging targets for innovative therapeutic approaches [74].
3. Crosstalk between the KP and the ECS in Obesity and Depression during Exercise: Potential Functional Interactions
The ECS and the KP have been strongly implicated in obesity and depression, and the link between these two systems is via chronic low-grade systemic inflammation in obesity and the neuroinflammatory and dysregulated serotonergic component in depression [22]. Combined, these results constitute a novel challenge in the search of more and yet-unknown biologically relevant interactions between these systems; however, this evidence must be demonstrated in future clinical trials (Figure 3).
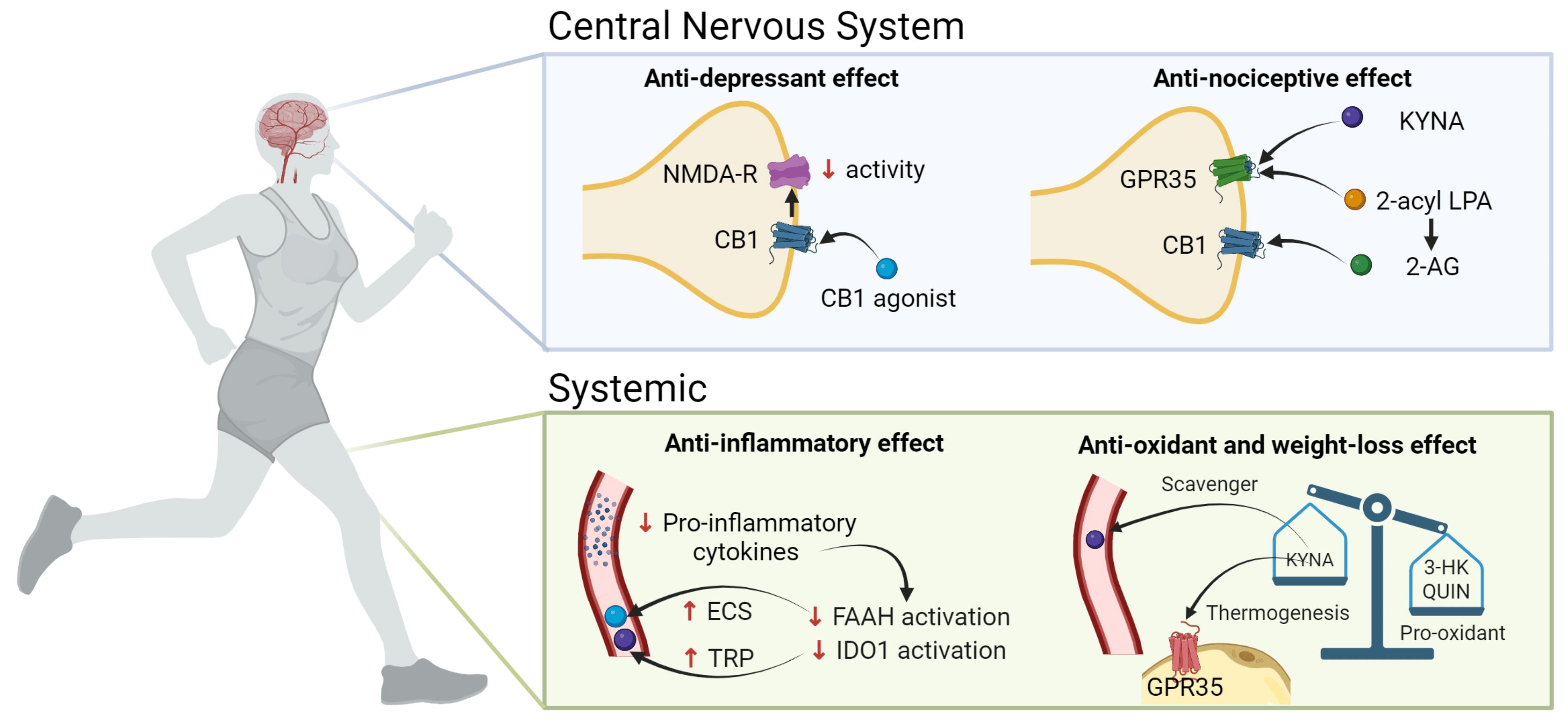
Figure 3. Exercise-induced modulation of the KP and the ECS capable of ameliorating both depressive symptoms and weight loss. 2-AG, 2-arachidonoyl glycerol; 3-HK, 3-hydroxykynurenine; CB1, cannabinoid receptor 1; KP, kynurenine pathway; ECS, endocannabinoid system; FAAH, fatty acid amino hydrolase; GPR35, G protein-coupled receptor 35; IDO,1 indoleamine 2,3-dioxygenase; KYNA, kynurenic acid; LPA, lysophosphatidic acid; NMDA-R, N-methyl-D-aspartate receptor; QUIN, quinolinic acid; TRP, Tryptophan. Created with BioRender.com.
3.1. NMDAr and CB1
The co-localization of receptors is critical because it allows for protein coupling to occur between signaling cascades from different pathways. NMDA and CB1 receptors undergo pre- and post-synaptic co-localization [84], and the effects of this co-localization result in the opposite glutamatergic NMDA function through the reduction in glutamate release upon cannabinoid binding to CB1 (pre-synaptic) and the NMDA-elicited calcium increase which is secondary to endocannabinoid receptor antagonists (post-synaptic) [85]. Glutamate is the main ligand for NMDAr and the activation of this receptor is involved in synaptic processes. On the other hand, when CB1 is activated, mainly by 2-AG or AEA, the intracellular levels of Ca2+ increase, whereas the levels of K+ decrease in presynaptic neurons, inhibiting the release of glutamate to the synaptic space and affecting the functions of postsynaptic neurons [34,85]. The involvement of cannabinoids in decreasing the activity of NMDAr when binding to CB1 has been elegantly reviewed by some authors [12,85], but also it has been reported that ketamine, an antagonist of NMDAr, is effective as an antidepressant drug. However, this potential therapeutic effect has not been demonstrated for other NMDAr antagonists [86].
3.2. GPR35 and KYNA
In addition to CB1 and CB2, eCBs can act as the agonists to many G-protein-coupled receptors (GPRs) [87]. Particularly, GPR35 shares several structural and functional properties with cannabinoid receptors; it co-localizes and co-expresses with CB1 receptors [88] and can act as an endogenous receptor for KYNA, inducing anti-nociceptive effects in the nervous system [4,89]. This nociceptive function may represent a novel interaction between ECS and the nervous system, as modulated by KYNA levels [88]. Furthermore, the mediator 2-acyl lysophosphatidic acid can be enzymatically converted to 2-AG, which binds to CB1 and CB2; 2-AG is able also to bind to GPR35. KYNA activates GPR35 in mice, regulating adipose tissue energy homeostasis which stimulates the expression of lipid metabolism and thermogenic and anti-inflammatory genes in the adipose tissue. In human adipose tissue, GPR35 expression correlates with genes involved in transcriptional regulation of adipocyte browning, an event that can be explored as a therapeutic target for obesity [29]. The long-term systemic administration of KYNA to the brain of rats specifically elevates the abundance of functional CB1 receptors in them, which can be a compensatory mechanism on the ECS induced by the long-term KYNA exposure [90]. In this sense, it seems that GPR35, cannabinoid receptors, ECS, and the KP are linked by intrinsic ligand conversions [89].
3.3. Inflammation
Inflammation could explain some of the changes in the KP among people with obesity and depression [91]. The adipose tissue is the primary source of proinflammatory cytokines such as IL-6, tumor necrosis factor (TNF), and interferon gamma (IFN-γ) during inflammatory processes [92,93]. The adipose tissue is crucial in the study of obesity and is considered a risk factor for the development of other pathologies. Among patients with obesity, the KP is activated and the expression of the IDO1 gene is increased, eliciting the conversion of TRP to KYN [6] and the association of CRP (C-reactive protein) with high levels of QA and low KA/QA ratio; this reflects the proinflammatory and neurotoxic effects of QA and anti-inflammatory effects of KYNA [94]. Nevertheless, among people with depression, neither CRP nor BMI explain the differences when compared to healthy people [91]. The hypothesis that the interactions between ECS and KP metabolites could have clinical implications started with the finding of positive associations among some of these metabolites, particularly 2-AG, with IL-6 registered in patients with some psychiatric disorders compared to volunteers without these psychiatric disorders. The authors demonstrated relevant associations between IL-6 levels and the individual risk preference test and between PA levels and the test for neuroticism [95]. It has been suggested that inflammatory cytokines could be responsible for influencing eCB deposits among women with obesity, which, in turn, activate the ECS; in contrast, the expression of FAAH, which degrades the AEA in abdominal adipose tissue, is decreased, inhibiting the degradation of eCB [7]. FAAH and IDO1 enzymatic activities have been proposed to be able to promote depressive symptoms; therefore, these enzymes represent a potential therapeutic target in this disease [96]. Hence, the modulation of the proinflammatory condition, particularly in adipose tissue, can regulate both the ECS and KP and might improve depression-associated symptoms.
Also, these proinflammatory cytokines can cross the BBB [97] through specific cytokine transporters and circumventricular organs and reach the brain [98]. Additionally, under the physiological context of low-grade inflammation—as seen in obesity—there can be a loss of the BBB’s integrity that can increase the infiltration of these cytokines, promoting an inflammatory condition in the CNS that impacts the progression of neurologic and psychiatric diseases [99]. Thus, the modulation of the KP via CB2 activation has been suggested as a potential target to counteract chronic inflammation and its associated effects concerning some diseases in the CNS. The CB2 stimulation has been linked to decreased levels of various proinflammatory cytokines, such as IFN-γ, which typically increase IDO activity [22]. In addition, upon its activation, CB2 increases anti-inflammatory cytokine levels, including those of interleukin-10 (IL-10) [100], and these increments in IL-10 can effectively interfere with IDO activity [101].
3.4. Oxidative Stress
Some of the KP metabolites can exert cytotoxic functions that favor either the generation of oxidative stress or protect cells from these oxidant molecules. 3-HK inhibits complexes I, II, and IV of the electron transport chain [102] and promotes the generation of reactive oxygen species [103]. However, 3-HK has been described also as a free radical scavenger [104]. QUIN generates oxidative stress by excitotoxicity [105], while KYNA exhibits scavenger activity [106,107]. On the other hand, there is evidence that CB1 activation promotes oxidative stress [108]; however, its neurological effects are mainly positive, showing antidepressive, anti-inflammatory, enhanced memory, and neuroplasticity improvements [109].
Finally, there are two research areas that we would like to put forward for further investigation which will provide a better understanding of the topic of this review: the use of phytocannabinoids combined with physical exercise as an alternative treatment of obesity and depression; research to determine why physical exercise could not induce long-lasting changes in the KP metabolites in both health and illness.
4. Conclusions
The endocannabinoid system and the kynurenine pathway can interact with each other through their metabolites—endocannabinoids and kynurenines—which are released during acute exercise and which are capable of mediating exercise-derived benefits among people with obesity and depression [110,111]. Many of the benefits of physical exercise, especially in the context of diseases, can be attributed to the action of eCBs and kynurenines in their signaling tissues and the activation of downstream pathways, or by their metabolic transformation to other molecules [112]. Clear evidence of these benefits remains limited, because many possible outcomes (aerobic capacity, adverse effects, and quality of life) have not been included in the corresponding research [113]; however, the interaction of both systems can improve understanding of why exercise is helpful in these diseases. Finally, considering the failure of rimonabant as a treatment for obesity (the treatment led to significant weight loss but had severe adverse trends toward depressed mood and anxiety), we suggest that exercise-derived endocannabinoidomes and kynurenines offer potential therapeutic targets. In this recommendation, we place emphasis on the increment of KYNA, AEA, and 2-AG circulating levels, which have significant benefits in both obesity and depression: (a) KYNA induces an anti-inflammatory environment in the adipose tissue, preventing weight gain and rescuing adiposity in subcutaneous WAT through GPR35 receptors and KYN clearance in the periphery; this reduces depressive symptoms. (b) AEA and 2-AG, together with other myokines, such as BDNF, improve cognitive functions and mood.
References
- Obesity and Overweight; Health Report. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Depressive Disorder; Health Report. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 31 March 2023).
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M.; Berk, M. Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Adv. Protein Chem. Struct. Biol. 2012, 88, 27–48. [Google Scholar]
- Huang, W.J.; Chen, W.W.; Zhang, X. Endocannabinoid system: Role in depression, reward and pain control (Review). Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Dadvar, S.; Ferreira, D.M.S.; Cervenka, I.; Ruas, J.L. The weight of nutrients: Kynurenine metabolites in obesity and exercise. J. Intern. Med. 2018, 284, 519–533. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Disanzo, B.L.; Wang, X.; Yang, R.; Gong, D. Adipose tissue endocannabinoid system gene expression: Depot differences and effects of diet and exercise. Lipids Health Dis. 2011, 10, 194. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in human—Possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef]
- Joisten, N.; Schumann, M.; Schenk, A.; Walzik, D.; Freitag, N.; Knoop, A.; Thevis, M.; Bloch, W.; Zimmer, P. Acute hypertrophic but not maximal strength loading transiently enhances the kynurenine pathway towards kynurenic acid. Eur. J. Appl. Physiol. 2020, 120, 1429–1436. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Zádor, F.; Dvorácskó, S.; Bohár, Z.; Benyhe, S.; Tömböly, C.; Párdutz, Á.; Vécsei, L. Interactions between the Kynurenine and the Endocannabinoid System with Special Emphasis on Migraine. Int. J. Mol. Sci. 2017, 18, 1617. [Google Scholar] [CrossRef]
- Celik, O.; Yildiz, B.O. Obesity and physical exercise. Minerva Endocrinol. 2021, 46, 131–144. [Google Scholar] [CrossRef]
- Chalder, M.; Wiles, N.J.; Campbell, J.; Hollinghurst, S.P.; Haase, A.M.; Taylor, A.H.; Fox, K.R.; Costelloe, C.; Searle, A.; Baxter, H.; et al. Facilitated physical activity as a treatment for depressed adults: Randomised controlled trial. BMJ 2012, 344, e2758. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
- NICE. Depression in adults: Treatment and management. In NICE Guideline No. 222; NICE: Manchester, UK, 2022. [Google Scholar]
- Heissel, A.; Heinen, D.; Brokmeier, L.L.; Skarabis, N.; Kangas, M.; Vancampfort, D.; Stubbs, B.; Firth, J.; Ward, P.B.; Rosenbaum, S.; et al. Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression. Br. J. Sports Med. 2023, 57, 1049–1057. [Google Scholar] [CrossRef]
- Knight, J.A. Physical inactivity: Associated diseases and disorders. Ann. Clin. Lab. Sci. 2012, 42, 320–337. [Google Scholar]
- Metcalfe, A.J.; Koliamitra, C.; Javelle, F.; Bloch, W.; Zimmer, P. Acute and chronic effects of exercise on the kynurenine pathway in humans—A brief review and future perspectives. Physiol. Behav. 2018, 194, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Valente-Silva, P.; Ruas, J.L. Tryptophan-Kynurenine Metabolites in Exercise and Mental Health. In Hormones, Metabolism and the Benefits of Exercise; Spiegelman, B., Ed.; Springer: Cham, Switzerland, 2017; pp. 83–91. [Google Scholar]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef]
- Zádor, F.; Joca, S.; Nagy-Grócz, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Benyhe, S.; Vécsei, L. Pro-Inflammatory Cytokines: Potential Links between the Endocannabinoid System and the Kynurenine Pathway in Depression. Int. J. Mol. Sci. 2021, 22, 5903. [Google Scholar] [CrossRef]
- Powers, S.K. Exercise: Teaching myocytes new tricks. J. Appl. Physiol. 2017, 123, 460–472. [Google Scholar] [CrossRef]
- Rimer, J.; Dwan, K.; Lawlor, D.A.; Greig, C.A.; McMurdo, M.; Morley, W.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2012, 7, Cd004366. [Google Scholar]
- Hopps, E.; Caimi, G. Exercise in obesity management. J. Sports Med. Phys. Fitness 2011, 51, 275–282. [Google Scholar]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392.e5. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Ruas, J.L. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef] [PubMed]
- Harkin, A. Muscling in on depression. N. Engl. J. Med. 2014, 371, 2333–2334. [Google Scholar] [CrossRef]
- Correia, J.C.; Ferreira, D.M.; Ruas, J.L. Intercellular: Local and systemic actions of skeletal muscle PGC-1s. Trends Endocrinol. Metab. 2015, 26, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, Y.; Tadaishi, M.; Nagaike, Y.; Morita, A.; Ogawa, Y.; Ezaki, O.; Takai-Igarashi, T.; Kitaura, Y.; Shimomura, Y.; Kamei, Y.; et al. PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS ONE 2014, 9, e91006. [Google Scholar] [CrossRef]
- Liu, J.J.; Movassat, J.; Portha, B. Emerging role for kynurenines in metabolic pathologies. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 82–90. [Google Scholar] [CrossRef]
- Allison, D.B.; Fontaine, K.R.; Manson, J.E.; Stevens, J.; VanItallie, T.B. Annual deaths attributable to obesity in the United States. JAMA 1999, 282, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Hubmann, H.; Pilz, S.; Schauenstein, K.; Renner, W.; März, W. Beyond cholesterol--inflammatory cytokines, the key mediators in atherosclerosis. Clin. Chem. Lab. Med. 2004, 42, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, J.; Gao, J.; Cheng, J.; Xie, H.; Zhang, L.; Wang, Y.H.; Gao, Z.; Wang, Y.; Wang, X.; et al. Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat. Commun. 2022, 13, 3489. [Google Scholar] [CrossRef]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated with Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Polyzos, K.A.; Ovchinnikova, O.; Berg, M.; Baumgartner, R.; Agardh, H.; Pirault, J.; Gisterå, A.; Assinger, A.; Laguna-Fernandez, A.; Bäck, M.; et al. Inhibition of indoleamine 2, 3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe−/− mice. Cardiovasc. Res. 2015, 106, 295–302. [Google Scholar] [CrossRef]
- Wells, G.; Kennedy, P.T.; Dahal, L.N. Investigating the Role of Indoleamine 2, 3-Dioxygenase in Acute Myeloid Leukemia: A Systematic Review. Front. Immunol. 2021, 12, 651687. [Google Scholar] [CrossRef]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front. Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; et al. Erratum: The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2016, 24, 1821. [Google Scholar] [CrossRef]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Murakami, E. A possible diabetogenic role for tryptophan metabolites and effects of xanthurenic acid on insulin. Am. J. Clin. Nutr. 1971, 24, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 181. [Google Scholar] [CrossRef]
- Gómez-Galán, M.; De Bundel, D.; Van Eeckhaut, A.; Smolders, I.; Lindskog, M. Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol. Psychiatry 2013, 18, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C. Disorders of memory and plasticity in psychiatric disease. Dialogues Clin. Neurosci. 2013, 15, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012, 73, 962–977. [Google Scholar] [CrossRef]
- Gibney, S.M.; McGuinness, B.; Prendergast, C.; Harkin, A.; Connor, T.J. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav. Immun. 2013, 28, 170–181. [Google Scholar] [CrossRef]
- Liu, W.; Sheng, H.; Xu, Y.; Liu, Y.; Lu, J.; Ni, X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: Relevant to proinflammatory cytokines and IDO activation. Behav. Brain Res. 2013, 242, 110–116. [Google Scholar] [CrossRef]
- Gong, X.; Chang, R.; Zou, J.; Tan, S.; Huang, Z. The role and mechanism of tryptophan—Kynurenine metabolic pathway in depression. Rev. Neurosci. 2023, 34, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, S.J.; Cunningham, M.J. The effect of a modified physical training program in reducing injury and medical discharge rates in Australian Army recruits. Mil. Med. 1999, 164, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; Ford, B.N.; Kuplicki, R.; Khalsa, S.; Teague, T.K.; Paulus, M.P. Acute administration of ibuprofen increases serum concentration of the neuroprotective kynurenine pathway metabolite, kynurenic acid: A pilot randomized, placebo-controlled, crossover study. Psychopharmacology 2022, 239, 3919–3927. [Google Scholar] [CrossRef]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, T.I.M.; Instanes, J.T.; Posserud, M.R.; Ulvik, A.; Kessler, U.; Haavik, J. Changes in Tryptophan-Kynurenine Metabolism in Patients with Depression Undergoing ECT-A Systematic Review. Pharmaceuticals 2022, 15, 1439. [Google Scholar] [CrossRef]
- Tateishi, H.; Setoyama, D.; Kang, D.; Matsushima, J.; Kojima, R.; Fujii, Y.; Mawatari, S.; Kikuchi, J.; Sakemura, Y.; Fukuchi, J.; et al. The changes in kynurenine metabolites induced by rTMS in treatment-resistant depression: A pilot study. J. Psychiatr. Res. 2021, 138, 194–199. [Google Scholar] [CrossRef]
- Bartoli, F.; Misiak, B.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Crocamo, C.; Savitz, J.B.; Carrà, G. The kynurenine pathway in bipolar disorder: A meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol. Psychiatry 2021, 26, 3419–3429. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; et al. Serum Kynurenines Correlate With Depressive Symptoms and Disability in Poststroke Patients: A Cross-sectional Study. Neurorehabil. Neural. Repair 2020, 34, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Dias, I.C.; Carabelli, B.; Ishii, D.K.; de Morais, H.; de Carvalho, M.C.; de Souza, L.E.R.; Zanata, S.M.; Brandão, M.L.; Cunha, T.M.; Ferraz, A.C.; et al. Indoleamine-2, 3-Dioxygenase/Kynurenine Pathway as a Potential Pharmacological Target to Treat Depression Associated with Diabetes. Mol. Neurobiol. 2016, 53, 6997–7009. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Olds, T.; Curtis, R.; Dumuid, D.; Virgara, R.; Watson, A.; Szeto, K.; O’Connor, E.; Ferguson, T.; Eglitis, E.; et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: An overview of systematic reviews. Br. J. Sports Med. 2023, 18, 1203–1209. [Google Scholar] [CrossRef]
- Isung, J.; Granqvist, M.; Trepci, A.; Huang, J.; Schwieler, L.; Kierkegaard, M.; Erhardt, S.; Jokinen, J.; Piehl, F. Differential effects on blood and cerebrospinal fluid immune protein markers and kynurenine pathway metabolites from aerobic physical exercise in healthy subjects. Sci. Rep. 2021, 11, 1669. [Google Scholar] [CrossRef]
- Paul, E.R.; Schwieler, L.; Erhardt, S.; Boda, S.; Trepci, A.; Kämpe, R.; Asratian, A.; Holm, L.; Yngve, A.; Dantzer, R.; et al. Peripheral and central kynurenine pathway abnormalities in major depression. Brain Behav. Immun. 2022, 101, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Millischer, V.; Erhardt, S.; Ekblom, Ö.; Forsell, Y.; Lavebratt, C. Twelve-week physical exercise does not have a long-lasting effect on kynurenines in plasma of depressed patients. Neuropsychiatr. Dis. Treat. 2017, 13, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Saran, T.; Mazur, A.; Łukasiewicz, J. The significance of physical activity in the prevention of depressive disorders. Psychiatr. Pol. 2021, 55, 1025–1046. [Google Scholar] [CrossRef]
- Sánchez Chapul, L.; Pérez de la Cruz, G.; Ramos Chávez, L.A.; Valencia León, J.F.; Torres Beltrán, J.; Estrada Camarena, E.; Carillo Mora, P.; Ramírez Ortega, D.; Baños Vázquez, J.U.; Martínez Nava, G.; et al. Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers’ and Swimmers’ Serum Samples. Antioxidants 2022, 11, 1223. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Colangeli, R.; Teskey, G.C.; Di Giovanni, G. Endocannabinoid-serotonin systems interaction in health and disease. Prog. Brain Res. 2021, 259, 83–134. [Google Scholar]
- Zdanowicz, A.; Kaźmierczak, W.; Wierzbiński, P. The endocannabinoid system role in the pathogenesis of obesity and depression. Pol. Merkur Lekarski 2015, 39, 61–66. [Google Scholar]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of Cannabinoids in Obesity. Int. J. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef]
- Schulz, P.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity? Nutrients 2021, 13, 373. [Google Scholar] [CrossRef]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Epstein, D.H.; Preston, K.L. Psychostimulant addiction treatment. Neuropharmacology 2014, 87, 150–160. [Google Scholar] [CrossRef]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D. Synthetic cannabinoids, organic cannabinoids, the endocannabinoid system, and their relationship to obesity, diabetes, and depression. Mol. Biol. 2018, 7, 2–4. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Gerdeman, G.L.; Seillier, A.; Giuffrida, A. Wired to run: Exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J. Exp. Biol. 2012, 215, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Garzón, J. The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: Implications in psychosis and schizophrenia. Front. Pharmacol. 2014, 4, 169. [Google Scholar] [CrossRef]
- Liu, Q.; Bhat, M.; Bowen, W.D.; Cheng, J. Signaling pathways from cannabinoid receptor-1 activation to inhibition of N-methyl-D-aspartic acid mediated calcium influx and neurotoxicity in dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2009, 331, 1062–1070. [Google Scholar] [CrossRef]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Reggio, P.H. An Update on Non-CB(1), Non-CB(2) Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017, 2, 265–273. [Google Scholar] [CrossRef]
- Zádor, F.; Nagy-Grócz, G.; Kekesi, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Horvath, G.; Benyhe, S.; Vécsei, L. Kynurenines and the Endocannabinoid System in Schizophrenia: Common Points and Potential Interactions. Molecules 2019, 24, 3709. [Google Scholar] [CrossRef]
- Zhao, P.; Abood, M.E. GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors. Life Sci. 2013, 92, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zádor, F.; Nagy-Grócz, G.; Dvorácskó, S.; Bohár, Z.; Cseh, E.K.; Zádori, D.; Párdutz, Á.; Szűcs, E.; Tömböly, C.; Borsodi, A.; et al. Long-term systemic administration of kynurenic acid brain region specifically elevates the abundance of functional CB(1) receptors in rats. Neurochem. Int. 2020, 138, 104752. [Google Scholar] [CrossRef]
- Farup, P.G.; Hamarsland, H.; Mølmen, K.S.; Ellefsen, S.; Hestad, K. The Kynurenine Pathway in Healthy Subjects and Subjects with Obesity, Depression and Chronic Obstructive Pulmonary Disease. Pharmaceuticals 2023, 16, 351. [Google Scholar] [CrossRef]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Heilman, P.; Hill, M.N.; Coussons-Read, M.; Brundin, L.; Coccaro, E.F. Role of the kynurenine pathway and the endocannabinoid system as modulators of inflammation and personality traits. Psychoneuroendocrinology 2019, 110, 104434. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Martínez, A.R.; Viveros-Paredes, J.M.; Hidalgo-Franco, G.V.; Pardo-González, E.; Chaparro-Huerta, V.; González-Castañeda, R.E.; Flores-Soto, M.E. Chronic Inhibition of FAAH Reduces Depressive-Like Behavior and Improves Dentate Gyrus Proliferation after Chronic Unpredictable Stress Exposure. Behav. Neurol. 2021, 2021, 6651492. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Skorobogatov, K.; De Picker, L.; Verkerk, R.; Coppens, V.; Leboyer, M.; Müller, N.; Morrens, M. Brain Versus Blood: A Systematic Review on the Concordance Between Peripheral and Central Kynurenine Pathway Measures in Psychiatric Disorders. Front. Immunol. 2021, 12, 716980. [Google Scholar] [CrossRef]
- Małkiewicz, M.A.; Szarmach, A.; Sabisz, A.; Cubała, W.J.; Szurowska, E.; Winklewski, P.J. Blood-brain barrier permeability and physical exercise. J. Neuroinflamm. 2019, 16, 15. [Google Scholar]
- Parastouei, K.; Aarabi, M.H.; Hamidi, G.A.; Nasehi, Z.; Arani, S.-K.; Jozi, F.; Shahaboddin, M.E. A CB2 Receptor Agonist Reduces the Production of Inflammatory Mediators and Improves Locomotor Activity in Experimental Autoimmune Encephalomyelitis. Rep. Biochem. Mol. Biol. 2022, 11, 1–9. [Google Scholar] [CrossRef]
- Quintana, F.J.; Murugaiyan, G.; Farez, M.F.; Mitsdoerffer, M.; Tukpah, A.M.; Burns, E.J.; Weiner, H.L. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2010, 107, 20768–20773. [Google Scholar] [CrossRef]
- O’Farrell, K.; Harkin, A. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 2017, 112, 307–323. [Google Scholar] [CrossRef]
- Lee, K.J.; Jung, K.H.; Cho, J.Y.; Lee, S.T.; Kim, H.S.; Shim, J.H.; Lee, S.K.; Kim, M.; Chu, K. High-Fat Diet and Voluntary Chronic Aerobic Exercise Recover Altered Levels of Aging-Related Tryptophan Metabolites along the Kynurenine Pathway. Exp. Neurobiol. 2017, 26, 132–140. [Google Scholar] [CrossRef]
- Sathyasaikumar, K.V.; de la Cruz, V.P.; Pineda, B.; Cervantes, G.I.V.; Ortega, D.R.; Donley, D.W.; Severson, P.L.; West, B.L.; Giorgini, F.; Fox, J.H.; et al. Cellular Localization of Kynurenine 3-Monooxygenase in the Brain: Challenging the Dogma. Antioxidants 2022, 11, 315. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Patel, V.; Kashiwaya, Y.; Liaudet, L.; Evgenov, O.V.; Mackie, K.; Haskó, G.; Pacher, P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc. Res. 2010, 85, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? Int. J. Mol. Sci. 2020, 21, 4221. [Google Scholar] [CrossRef]
- Danielsson, L.; Papoulias, I.; Petersson, E.L.; Carlsson, J.; Waern, M. Exercise or basic body awareness therapy as add-on treatment for major depression: A controlled study. J. Affect. Disord. 2014, 168, 98–106. [Google Scholar] [CrossRef]
- Moraes, H.S.; Silveira, H.S.; Oliveira, N.A.; Portugal, E.M.M.; Araújo, N.B.; Vasques, P.E.; Bergland, A.; Santos, T.M.; Engedal, K.; Coutinho, E.S.; et al. Is Strength Training as Effective as Aerobic Training for Depression in Older Adults? A Randomized Controlled Trial. Neuropsychobiology 2020, 79, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fuller, O.K.; Whitham, M.; Mathivanan, S.; Febbraio, M.A. The Protective Effect of Exercise in Neurodegenerative Diseases: The Potential Role of Extracellular Vesicles. Cells 2020, 9, 2182. [Google Scholar] [CrossRef]
- Pastén, C.S.; Caneo, C. Addition of aerobic exercise to antidepressant drug monotherapy for major depressive disorder in adults. Medwave 2022, 22, e8670. [Google Scholar]

