1. Overview of MHC Class I and Class II Molecules
Tumor immunogenicity is largely dependent on the expression of Major Histocompatibility Complexes class I and II [MHC I, II, also known as Human Leukocyte Antigen complexes I, II (HLA I, II)]. These complexes are essential proteins capable of presenting foreign antigens or self-peptides to T lymphocytes for immunosurveillance, and also for tissue homeostasis in autoimmune and infectious diseases [1]. MHC I molecules are present in almost all nucleated cells in the human body, and are differently expressed in terms of the level of transcription, transduction, and epigenetic regulation [2]. In humans, the HLA locus is found in the short arm of chromosome 6, comprising three different loci named class I, class II, and class III [3,4]. Inherited in a Mendelian fashion, the HLA gene is the most complex and polymorphic system that exists in the human genome, being associated with more than 100 different diseases, particularly autoimmune disorders [1,5]. HLA class I molecules are composed of highly polymorphic and ubiquitously expressed classical HLA-A, -B and -C allotypes, and non-classical and less polymorphic HLA-E, -F, -G, -H, -J, -K, and -L allotypes (Figure 1). Other non-classical MHC class I related molecules include: Cluster of differentiation 1 (CD1), zinc-α2-glycoprotein (ZAG), neonatal Fc receptor (FcRn), MHC class I chain-related (MIC), endothelial Protein C Receptor (EPCR) and MHC class I-related molecule 1 (MR1), which can also bind to and present small molecules such as lipids, glycolipids, metabolites and modified peptides [6]. As discussed later, non-classic HLAs have a restricted expression pattern. To this day, there are approximately 25,228 different HLA class I alleles and 10,592 HLA class II alleles that have been sequenced and named [7].
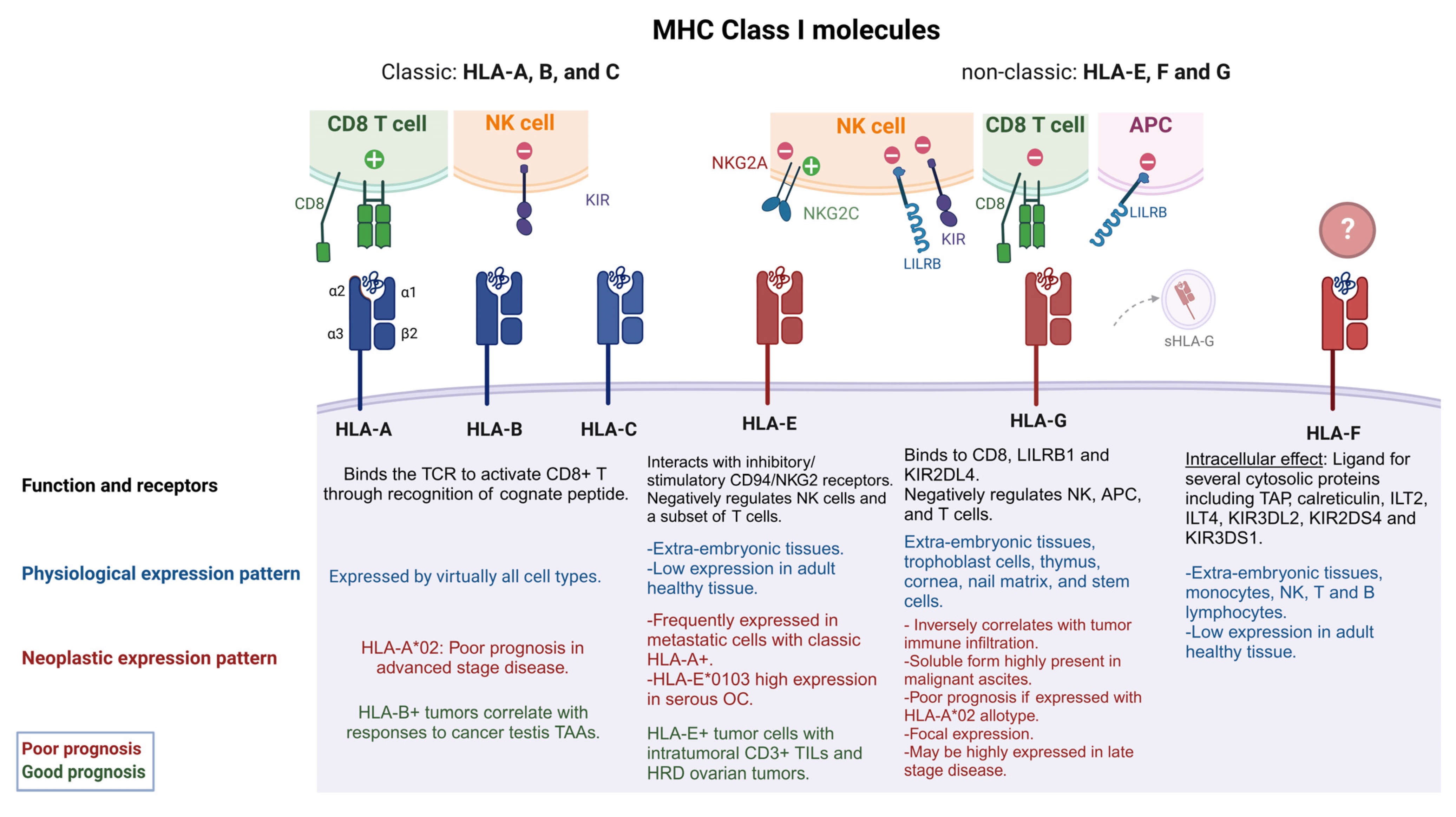
Figure 1. Classic and non-classic HLA I and their role in EOC tumorigenicity. HLA I molecules are formed from three α subunits (α1-3) and one B2M subunit (β2). Classic HLA I molecules include HLA-A, -B and –C while non-classic are known as HLA-E, -F, G. Classic HLA I molecules allow the presentation of endogenous peptides to CD8+ T cells, while non-classic HLA I molecules negatively regulate NK cell function. Some classic HLA Is (HLA-A*02) have been associated with poor EOC prognosis (red captions) when co-expressed with non- classic HLA-G and HLA-E [8]. HLA-E can interact with stimulatory or inhibitory receptors on NK cells and some T cells, while HLA-Gs have a broader immune-modulatory capability, having a negative effect on NKs, T cells and APCs. sHLA-G can also be found in extracellular vesicles and tumor derived exosomes. HLA-E and HLA-G expression levels have been associated with poor prognosis and late stage of disease. However, in some specific cases, HLA-E expression along T cell infiltration and HRD ovarian tumors have shown a good prognosis (green captions). HLA-F may have immune regulatory functions, but its binding partners are still unknown. See text for more details. HLA-A*02 refers to the allele group, TCR (T cell receptor), NK (natural killer cell), antigen presenting cell (APC), OC (ovarian cancer), HRD (homologous recombinant deficiency), TAP (transporter associated with antigen presentation), ILT2 [immunoglobulin (Ig)-like transcript 2], KIR3DS1 (killer cell Ig like receptor three Ig domains and short cytoplasmic tail 1), KIR3DL2 (KIR3D and long cytoplasmic tail 2), KIR2DS4 (KIR two Ig domains and short cytoplasmic tail 4), LILRB1 (Leukocyte Immunoglobulin-Like Receptor B1).
Classical and non-classical HLA I molecules form a heavy chain, presented in a glycosylated form on the cell surface, bound by non-covalent association to the invariant light chain β-2 microglobulin (β2M) which is coded in chromosome 15. The heavy chain makes three different domains (α1, -2, and -3) in the extracellular domain where α1 and α2 form a groove composed of hypervariable regions [9]. Peptides predominantly generated in the cytosol are transported to the endoplasmic reticulum through the transporter associated with antigen presentation (TAP) where other proteins such as tapasin mediate the binding of peptides within a range of 8–15 mer [10,11,12] to form an immunogenic peptide-MHC I complex (pMHCI). This complex is presented on the cell surface where it is potentially recognized by T cell receptors (TCR) on CD8+ T lymphocytes (CTLs). Some HLA I allotypes, such as a subgroup of the HLA-B locus, HLA Bw4, and HLA-Cw (HLA-C1 and HLA-C2) can engage with natural killer (NK) cells to produce an inhibitory signal [13,14,15] while HLA-E and -G allotypes can interact directly with CD94/NKG2 receptors on NK cells, inhibiting or inducing their activation [16,17,18] (Figure 1).
Similarly, HLA II complexes possess two polymorphic chains composed of five isotypes designated as HLA-DM, -DO, -DP, -DQ, -DR, with a more restrained expression. Peptides varying from 13–25 mer [19] derived from an extracellular origin can bind the MHC II groove to form a pMHCII complex in antigen presenting cells (APCs) including B lymphocytes, dendritic cells (DCs), macrophages, monocytes, Langerhans cells, endothelial cells, thymic epithelial cells, activated T lymphocytes, and some epithelial cells found in the cervical and colorectal regions, which can be recognized by TCR on CD4+ T lymphocytes [20,21,22]. Classical HLA class I, and to a greater extent HLA class II, can be detected in a soluble form (sHLA) in plasma, urine, and various other bodily fluids in healthy individuals [23,24].
The class III HLA region comprises more than 50 genes encoding for proteins not only involved in immunity (activation of complement, inflammation, immunoglobulin superfamily members, and cell stress) but also in hormonal synthesis, and extracellular matrix organization [25,26,27], the discussion of which is beyond the scope of this review article.
2. Ovarian Cancer Immunogenicity
As in most solid tumors, EOC cells downregulate MHC I expression as an immune evasion mechanism. Indeed, expression of MHC I genes is impaired in up to 60% of ovarian tumors [2,28,29]. Several EOC subtypes including serous, clear cell, endometrioid and mucinous, are immunogenic tumors capable of recruiting T cells into the tumor microenvironment (TME), resulting in positive prognoses [30,31,32,33]. Indeed, the presence of T cells specific to neoantigens expressed by EOC cells is strongly associated with increased survival [34,35] and the mechanisms related to immune cell infiltration are dependent on the antigen processing and presentation machinery (APM) components and MHC-I and -II status [36,37]. Nevertheless, the heterogeneity of HLA I allotype expression in a healthy cell is ultimately lost as tumors evolve to express fewer allotypes or completely lose HLA I expression [2,32,36].
The mechanisms by which MHC I expression is suppressed during tumor development has a major impact on the response to cancer immunotherapy [38]. Cancer cells lose or downregulate MHC I molecules because of the loss or decreased transcription of MHC I related genes or defects in APM components [39]. These defects can be classified as either “hard” or “soft”, depending on whether they are irreversible or reversible, respectively, by gene regulators or cytokines [39]. In healthy cells and cancer cells with soft defects, APM and MHC I genes can be induced by the IFN regulatory factor 1 (IRF-1), NF-κB, and the NOD-like receptor family caspase recruitment domain-containing 5 (NLRC5) in response to stimulatory cytokines such as TNF-α and IFN-γ [40,41,42].
EOC immunogenicity has been measured with humoral and cellular antitumor immune response markers detectable in peripheral blood, tumor sites, and ascites derived from EOC patients [43]. Goodell and colleagues were able to detect p53 antibodies in serum from 104 EOC patients, whose levels were positively correlated with overall survival [43]. Importantly, the presence of neoantigen-reactive T cells in patients with EOC can improve survival [34,35]. Brown et al. analyzed TCGA RNA-seq data from six EOC tumor sites in 515 patients, and identified mutational epitopes presented by the autologous HLA-A alleles that predicted tumor immunogenicity. These mutational epitopes triggered higher CTL content in the tumor niche and were associated with increased patient survival. However, tumors devoid of CTL infiltration lacked these mutational epitope signatures [34]. Wick and colleagues analyzed T cell reactivity towards 79 Tumor Associated Antigens (TAAs) originating from non-synonymous mutations identified by whole exome sequencing of autologous tumors, using T cells from the tumors of three EOC patients. A robust and specific CD8+ T-cell response to the mutated hydroxysteroid dehydrogenase-like protein 1 (HSDL1)L25V was detected in one patient at different levels over the course of disease recurrence, highlighting the evolving expression of neoantigens and the limit of naturally occurring antitumoral immunity recognition over EOC progression [35].
In fact, ovarian tumors generally possess intermediate or low mutational burdens as a consequence of a very low incidence of naturally processed and presented neoantigens that could generate a significant antitumoral response [44]. Nonetheless, TAA presentation is the pivotal factor enabling CTL-tumor cell recognition and killing [45]. The following section will elucidate the current understanding of the expression of HLA class I allotypes and their intricate association with tumor burden and survival outcomes.
2.1. Classic HLA Class I
Downregulation of classic MHC I is a prevalent immune evasion mechanism used by tumor cells to escape antitumor T-cell-mediated immune responses [46]. Under physiological conditions, classic HLA class I molecules are expressed by virtually all cell types, allowing for NK or T cell recognition to achieve immunosurveillance. A tissue microarray of 339 EOC samples stained for MHC I and β2M established a positive correlation between HLA I expression and increased patient survival independent of age, stage, level of cytoreduction, and exposure to chemotherapy [47]. Although specific allotypes, such as the HLA-A*02 subtype, correlate with poor prognosis in advanced-stage serous EOC [8], the HLA-B allotype is a positive predictor of the immune response to cancer testis’ TAAs [48]. While MHC I gene expression in EOC cells can be downregulated as a consequence of somatic mutations, these mutations are not common in EOC. Shukla et al. analyzed 7930 samples across 20 different tumor types and found that ovarian carcinoma, glioblastoma, and breast cancer largely lacked somatic mutations in HLA genes, being present in only 0–0.6% of the tumor samples [49].
Despite the lack of HLA mutations, differences related to total HLA I and II expression in ovarian tumors occur. Using RNA-seq, immunohistochemistry, and flow cytometry analysis on 27 EOC samples, Schuster et al. revealed that most ovarian tumors display strong HLA I expression, and to some extent HLA II expression. However, only the EpCAM+ population was considered in the cancer cell subset which may not necessarily represent most EOC cells [50], and the degree of immune infiltration of the EOC samples was not included in the analysis, potentially resulting in an overestimated HLA expression in highly infiltrated tumors. In a more recent study, tissue sections from 30 untreated high-grade serous ovarian cancers (HGSC) were analyzed for MHC I staining and showed sub-clonal loss in 7/30 (23%), including areas of retained MHC class I expression immediately juxtaposed with areas of negative staining [51]. Neither of these studies classified the overall diversity of HLA class I allotypes being retained in the tumor tissue, which may turn out to be a notable weakness, as non-classic HLA class I expression may negatively impact patient survival as described in the following section.
2.2. Non-Classic HLA Class I
As cancer cells downregulate classic HLA I molecules to avoid CTL recognition, they can also circumvent detection and elimination by NK cells through alternative means. Non-classic HLA I molecules are less polymorphic and display distinct expression patterns in developing and adult tissues, exerting functions in both the innate and adaptive immune systems [52,53]. In many malignancies, non-classic HLA I allotypes are aberrantly expressed, perhaps as a consequence of the proximity of genes such as HLA-E, -F, and -G to the class I region on chromosome 6 [54]. Indeed, aberrant expression of non-classical HLA I molecules in tumors contributes to inhibition of NK cells, rendering tumor cells resistant to NK cell-mediated lysis [55]. The following sections summarize key studies underlining the potential effects of non-classic HLA molecules in EOC.
2.3. NLRC5, the Master Regulator of MHC Class I Expression
NLRC5 (also known as CITA) is a critical regulator of MHC I genes, as well as some related genes involved in MHC I-dependent APM via the formation of CITA enhanceosomes [41,93,94,95]. NLRC5 induces the expression of both classical and non-classical class I molecules, but also the main components of the APM pathway like β2M, immunoproteasome components (PSMB9, i.e., LMP2), and TAP1 [41]. The expression of MHC I and APM components strongly correlates with NLRC5 gene expression in multiple cancers such as lung, melanoma, thyroid, breast, prostate, uterine, and EOC. Defects in NLRC5 expression found in human tumors include genetic modifications such as copy number loss, somatic mutations, and promoter methylation, which strong downregulate MHC I expression [96,97]. An analysis of the NLRC5 gene in multiple cancer types revealed that EOC patients (n = 489) displayed the highest frequency of copy number loss at 72.2%. This loss was associated with the reduced expression of NLRC5 and MHC I and related genes, including HLA-A, HLA-B, HLA-C, B2M, LMP2, and LMP7 [96].
In summary, classic HLA I expression is associated with better survival for EOC patients while non-classic HLA I is more pronounced in aggressive and more advanced EOCs. Many unknown aspects related to non-classic HLA I, such as ligands, polymorphisms, and post-translational regulation, still need to be explored. Understanding these factors will help us to better grasp their influence on the EOC TME and, in particular, how they influence the response to treatment.
3. Other Tumor Microenvironment Factors Influencing Ovarian Cancer Immunogenicity
The EOC TME is highly immunosuppressive, frequently containing a tumor promoting network of cytokines, such as IL-10 and TGF-β, and also pro-inflammatory factors such as TNF-α [98]. In the following sections, these factors will be discussed in the context of the epithelial–mesenchymal transition (EMT) as increasing evidence suggests that these cytokines can influence HLA class I expression and overall tumor immunogenicity.
3.1. EMT Effects on HLA Expression in Cancer
Downregulation of HLA I expression has been linked to the EMT in melanoma, colorectal carcinoma, prostate adenocarcinoma, breast carcinoma, and EOC [99,100]. The EMT is a process through which epithelial cells shed cell–cell junctions, detach from the basement membrane, and undergo transcriptional and morphological changes to acquire mesenchymal characteristics and gain stem cell-like features such as the capacity for self-renewal [101,102]. Enhanced capacity for migration and invasion enables the cells to extravasate into the bloodstream and form metastatic colonies in peripheral tissues [103,104]. In prostate cancer cells, overexpression of the EMT transcriptional regulator Snail, or treatment with EMT-inducing Transforming Growth Factor Beta 1 (TGF β1), reduced the expression of HLA class I molecules [105]. Similar findings have been found in breast [106] and pulmonary cancers [107]. EpCAM+ EOC cells show high levels of the expression of MHC I classical haplotypes when compared to benign fallopian tube samples [108]. EpCAM is silenced in mesenchymal cancer cells [109]; this suggests that cancers that retain their epithelial features can also retain high levels of expression of the classical MHC I haplotypes.
In syngeneic tumors from mesenchymal cell lines established in the MMTV-PyMT mouse model of breast carcinoma, there was a remarkable decrease in MHC I expression alongside an increase in the checkpoint inhibitor PD-L1 compared to tumors arising from epithelial MMTV-PyMT cell lines expressing EpCAM and E-cadherin [106]. Evidence from this study and others points to the association of EMT with downregulation of MHC I components, whereby mesenchymal cells are able to avoid detection by immune cells by downregulating HLA and APM.
Strategies to bypass the negative effects of EMT on MHC I machinery have been proposed [110]. One possible strategy is to target EZH2, an epigenetic regulator whose activity is correlated with EMT in breast cancer and melanoma [111,112,113]. The combination of the EZH2 inhibitor GSK126 with anti-PD-1 in the anti-PD-1 resistant MOC1-esc1 mouse tumor model of head and neck squamous cell carcinoma reduced the growth of those tumors while also causing an increase in MHC I expression. This suggests that EZH2 expression synergizes with the immune checkpoint PD-L1 to reduce APM [114]. GSK126 treatment has also been found to upregulate classic MHC I allotypes in a human head and neck cancer cell line, suggesting similar effects to EZH2 in human cancers. Similarly, inhibition of the EMT in the highly metastatic 4T1 tumor model using an angiokinase inhibitor was found to upregulate MHC I expression and APM machinery [115]. It appears that the inverse relationship can also hold true, as the upregulation of the MHC I machinery protein B2M induced EMT in B2M-overexpressing clones of MCF7 (breast), H358 (lung), and SN12C (renal) cancer cells both in vivo and in vitro [116]. This suggests a complex relationship of bidirectional influences between MHC I, APM, and EMT in cancer cells, with EMT regulators such as EZH2 having a dampening impact on the machinery.
Despite the strong evidence for an association between EMT and the loss of MHC I/APM expression, this link is not universal. In a mesenchymal breast cancer cell line, a mesenchymal-to-epithelial reversion induced by upregulation of an EMT suppressor microRNA miR-200 [117] also suppressed PD-L1 expression and led to greater CD8+ T cell cytotoxicity. However, in a bioinformatic analysis of microRNAs affecting APM expression, high levels of the expression of miR-200a-5p suppressed TAP1 expression in melanoma [118]. Another analysis revealed that transgenic knock-in of miR-200c increased MHC I expression in murine mammary cancer cell line EO771 [119]. Thus, while the roles of these individual miR-200 molecules in suppressing EMT are well-established [120], they appear to have differing roles in the regulation of HLA and APM expression, which warrants further investigation.
Remarkably, a pan-cancer gene signature for epithelial–mesenchymal plasticity generated in our lab [121] includes genes for HLA-A, -C, and -E, TAP1, PSMB9, and B2M, suggesting that MHC I machinery is, at least in part, associated with the EMT in a variety of cancer cell lines and tumors from different origins. We leveraged this compiled single-cell dataset to compare HLA I gene expression against the average expression of a set of EMT genes, using either the cancer cell specific gene module [121] or the classical EMT hallmark module [122]. The results show a positive correlation for both classic and non-classic HLA I molecules (HLA-A-C/E-G) in eight different cancer types (Figure 2).
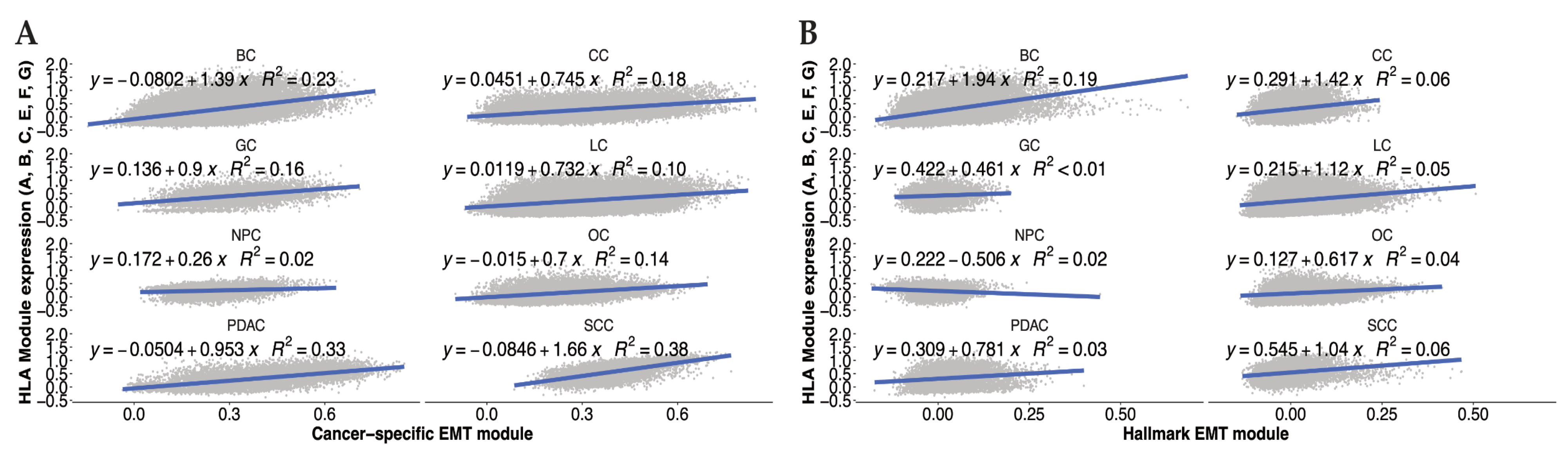
Figure 2. Average gene expression of MHC-I related HLA allotypes correlates with EMT in various cancer types. Expression of MHC-I related HLA allotypes (HLA A-G) scored in the cancer cell compartment of a variety of tumors (BC—Breast Cancer, CC—Colon Cancer, GC—Gastric Cancer, LC—Lung Cancer, NPC—Nasopharyngeal Cancer, OC—Ovarian Cancer, PDAC—Pancreatic Ductal Adenocarcinoma, SCC—Squamous Cell Carcinoma). HLA expression was correlated with EMT scores of those cells based on either (A) a cancer-specific EMT module published by Cook and Vanderhyden [120] or (B) the Hallmark EMT module from MSigDb.
For EOC, this correlation holds true for most of the individual HLA I allotypes . It is possible that this altered association between EMT and HLA expression is due to the fact that these are data from human tumors rather than from cell lines in vitro or mouse models, which suggests that the TME may well influence this relationship. Taken together, these findings suggest that the presence of HLA I molecules in cancer cells is associated with the presence of mesenchymal cells, and the EMT may promote the expression of HLA I molecules in certain cancer disease.
3.2. EMT Inducers and HLA I Expression
4. Therapeutic Strategies to Overcome the Lack of Immune Recognition in Ovarian Cancer
Over the past 25 years, there has been a tremendous effort to improve the response of cancers to immunotherapies to achieve tumor elimination. As the activity of CTLs and NK cells allows for successful antitumoral immunity, several approaches have been proposed to overcome immune evasion mechanisms established by the EOC TME. The following sections summarize the immune platforms under development that will potentially achieve better therapeutic outcomes for EOC. Figure 4 summarizes the therapeutic interventions aiming to increase EOC immunogenicity.
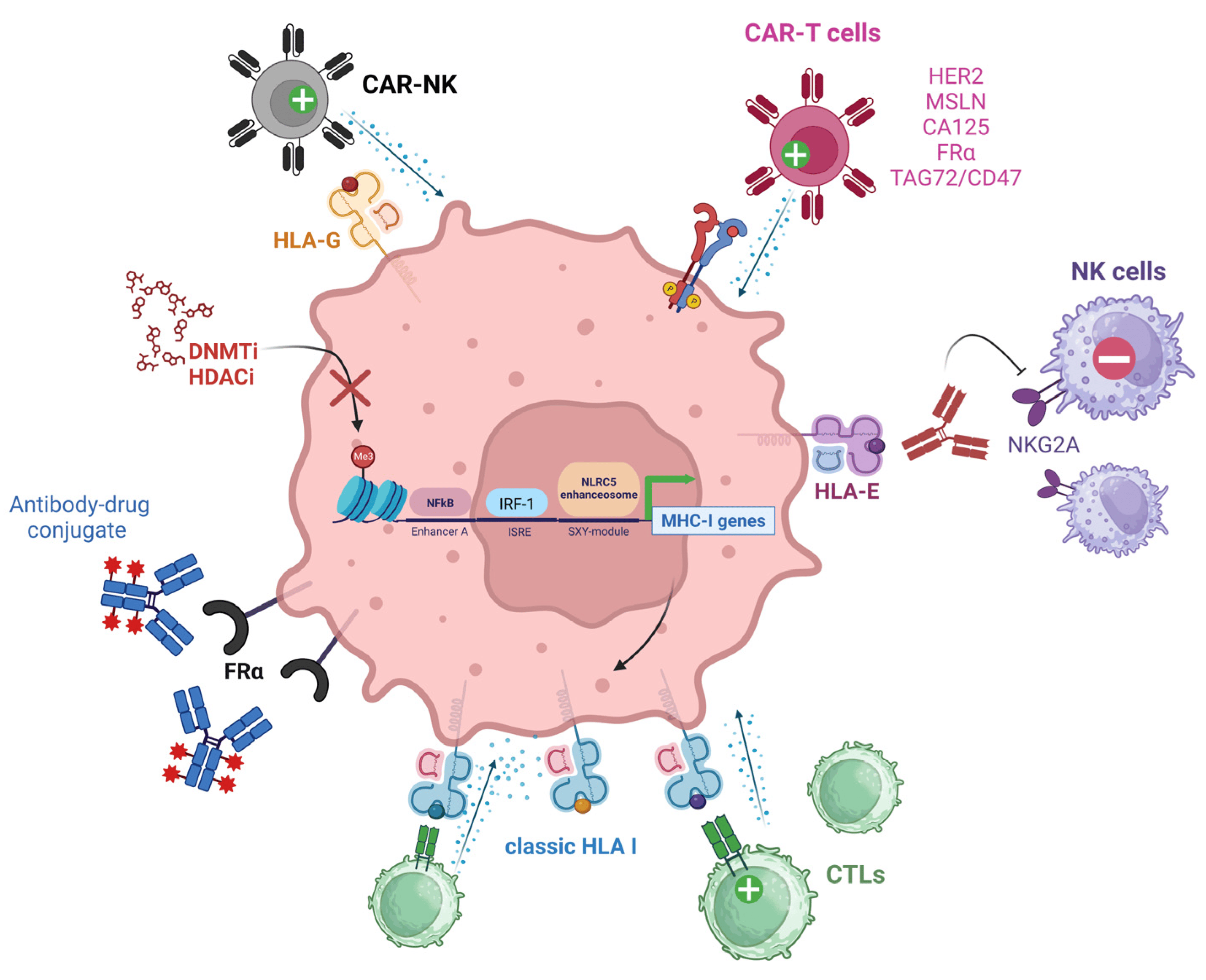
Figure 4. Therapeutic interventions aiming to increase EOC immunogenicity. TAAs highly expressed by EOC cells such as HER2, MSLN, CA125, FRα, and TAG42/CD47 can be targeted with CAR-T cells. FRα can be targeted by direct binding with antibody-drug conjugates. HLA-E+ ovarian tumors can be treated with antibodies blocking the HLA-E/NKG2A axis and impeding NK cell anti-tumoral activity. Poorly immunogenic ovarian tumors can be treated with DNMTi or HDACi agents aiming to decrease DNA hypermethylation or histone deacetylation (indicated by the red “X”). These epigenetic modifiers promote an increase in MHC I gene expression, type I IFN release, and TAA presentation, increasing CTL binding and antitumoral activity. HLA-G+ ovarian tumors can be treated with CAR-NK cells aiming to restore NK cytolytic functions. Green (+) symbols denote activation effect while red (−) symbols denote inhibitory signaling. NK (Natural Killer cell), CAR (Chimeric Antigen Receptor), HER2 (Human Epidermal Growth Factor Receptor 2), MSLN (Mesothelin), CA125 (Cancer Antigen 125), FRα (Alpha-Folate Receptor), TAG72 (Tumor-associated glycoprotein 72), NKG2A (NK group 2 member A receptor), CTLs (Cytotoxic T Lymphocytes), DNMTi (DNA methyltransferase inhibitor), HDACi (histone deacetylase inhibitor), IRF-1 (Interferon Regulatory Factor 1), ISRE (Interferon-Stimulated Response Element), NLRC5 (NLR family CARD domain containing 5).
4.1. Targeting TAAs and HLAs
4.2. Therapeutic Advances Targeting EMT in Cancer
Anti-EMT therapies possess the potential of reducing the invasion and spread of cancer cells, as well as being used in combination with other anti-tumor therapies to enhance patient response [192]. The quest to control the growth of the primary tumor and metastatic dissemination via attenuating EMT has received increasing attention recently [193,194]. Mesenchymal and partial-EMT cancer stem and stem-like cells have been characterized as resistant to drugs and other therapies mediated by increased drug efflux and the downregulation of apoptotic pathways [195]. Mesenchymal cells also upregulate HDACs as a means to stabilize EMT proteins [196,197]; blocking this activity with anti-EMT therapies might, therefore, sensitize mesenchymal cells to chemotherapy [198,199]. An inherent risk of using anti-EMT therapies is the potential to push cells towards the reverse mesenchymal-to-epithelial transition, which may encourage already circulating cancer cells to colonize their immediate surroundings. However, reversion to a more epithelial state could also restore MHC I re-expression resulting in a resurgence of anti-tumoral activity from immune cells. It is also important to remain cognizant that classical EMT-TFs, the putative targets for anti-EMT therapy, induce EMT in a context- and disease-dependent manner, therefore these therapies might not be effective across all cancers [193]. Figure 5 summarizes the EMT effect on the EOC TME.
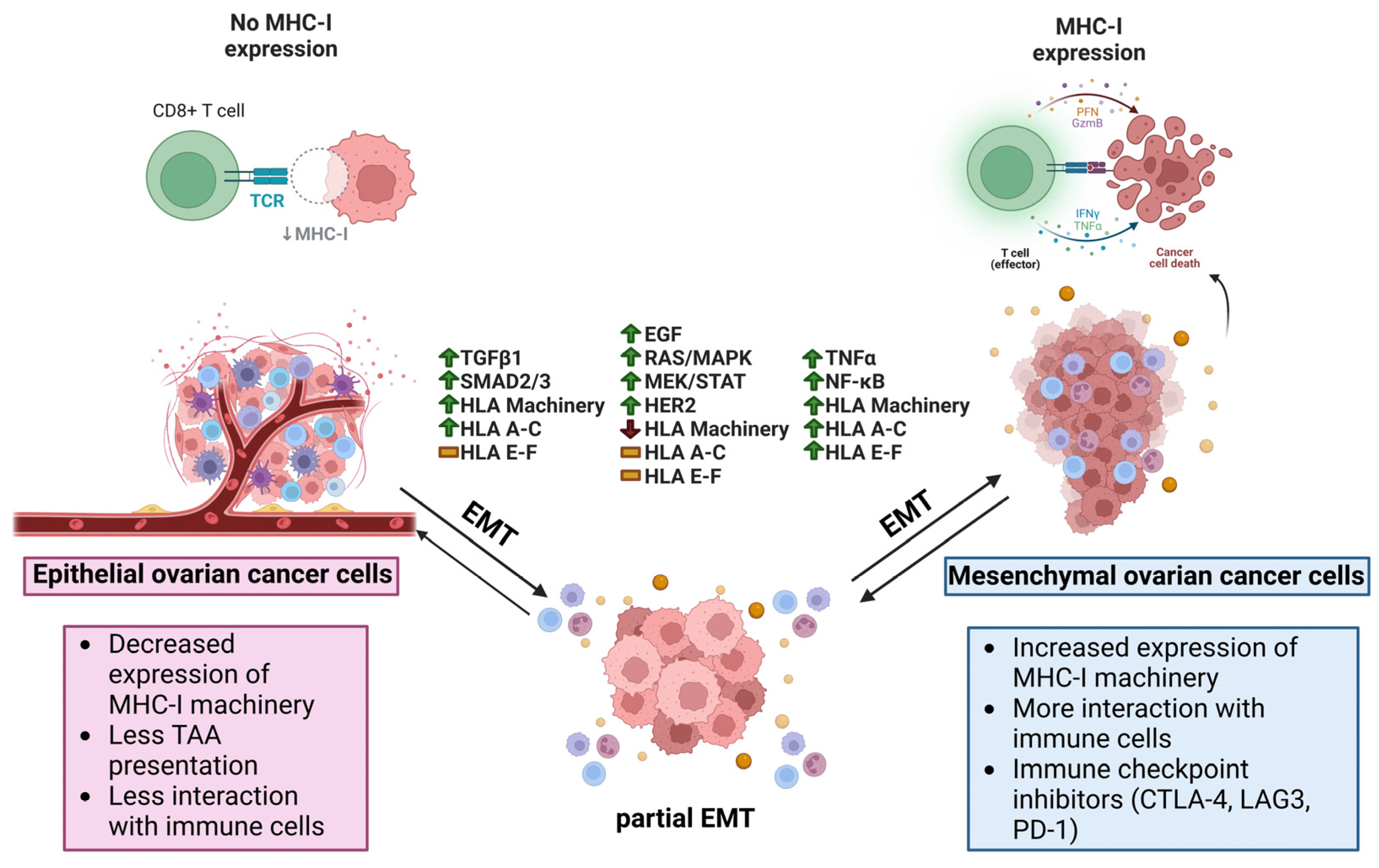
Figure 5. Potential relationship between antigen presentation and EMT in ovarian cancer. In ovarian cancer, epithelial cancer cells have decreased expression of classical HLA I related machinery, less interaction with immune cells, and less TAA presentation. Once stimulated with TGF-β1, EMT can begin increasing the expression of EMT-TFs such as SNAIL, SLUG, ZEB1, and TWIST1/2. At the same time, SMAD2/3 pathways are active, leading to the activation of APM and the re-expression of HLA-A, -B, and -C, and further leading to increased immunogenicity as the cells undergo EMT. If the EGF receptor (EGFR) is stimulated, the cells begin to undergo an EMT coinciding with activation of the RAS/MAPK, MEK/STAT, and HER2 pathways while seeing a reduction in APM and no changes to typical and atypical HLA I allotypes. EMT also beings if cancer cells are stimulated by TNF-α, including an increase in NF-κB that is upstream of APM and both typical and atypical HLA molecules. With increased immunogenicity in the mesenchymal phenotype as a result of more antigen presentation, immune cells like CD8+ T cells are able to effectuate their cytolytic functions on the cancer cells through PFN, GZMB, IFN-γ, and TNF-α. TAA (Tumor associated antigen), TGF-β1 (Transforming growth factor β1), EMT-TF (Epithelial-mesenchymal transition transcription factor), SNAIL (Zinc finger protein SNAI1), SLUG (Zinc finger protein SNAI2), ZEB1 (Zinc finger E-box binding homeobox1), TWIST1/2 (Twist-related protein 1/2), SMAD2/3 (Mothers against decapentaplegic homolog 2/3), APM (Antigen presenting machinery), HLA (Human leukocyte antigen), EGF (Epidermal growth factor), RAS/MAPK (Reticular activating system/Mitogen-activated protein kinase), MEK/STAT (Mitogen-activated protein kinase kinase/Signal transducer and activator of transcription), HER2 (Receptor tyrosine-protein kinase erbB-2), EMT (Epithelial-mesenchymal transition), NF-κB (Nuclear factor kappa-light-chain-enhancer of activated B cells), PFN (Perforin), GZMB (Granzyme B), IFN-γ (Interferon gamma).
5. Future Directions and Open Questions
This review focuses on the crucial role of MHC I molecules in ovarian tumor immunity. Despite the fact that MHC I molecules were discovered more than 60 years ago [212,213,214], and that MHC is among the most central fields in basic and clinical immunology [215], there are still many unknowns regarding the modulation of MHC I molecules, both classic and non-classic, in tumor immunity.
It is well established that the selective immune pressure on tumor cells allows for outgrowth of variants with low MHC I expression. However, it is still not clear if, under specific disease state(s), the EMT of the cancer cells could be directly linked to an increase in MHC I expression as shown in Figure 2. It remains unclear if these observations are based on gene expressions which do not necessarily represent protein expression, or whether these results reflect a relationship in human tumors that is not the same as in vitro or mouse model systems.
Since MHC I expression on the cell surface relies on the stability conferred by antigen binding, the more immunogenic the peptide, the longer the lifespan of the MHC I molecule on the cell surface [216,217]. Based on previous findings, we hypothesize that the mesenchymal state could render the cancer cells more immunogenic because the peptidome could be different from a more epithelial cancer cell, especially since more mesenchymal cells are associated with metastasis. However, the extent to which the proteome shapes the peptidome is still largely unknown during tumorigenesis. For instance, protein degradation was validated as an important factor in HLA I presentation [218], but the stability/instability of the proteome of more mesenchymal cancer cells has yet to be studied. Since EOC is known to generate neoantigens poorly, by becoming mesenchymal, cancer cells could perhaps produce more immunogenic peptides that could attract more CTLs to push immunoediting and CTL exhaustion further, as has been recently reported in breast cancer [119]. Therefore, immunopeptidome studies of EOC cells treated with EMT inducers such as TGFβ, compared with their more epithelial counterparts, may help elucidate how the TAAs generated under each context differ, and how they may interact with CTLs.
Numerous enigmas persist regarding the intricate interplay between TMB, its impact on HLA expression, and the subsequent modulation of antitumoral immune responses. The TMB also plays a key role in both stemness and immunogenicity; BRCA1/2 genetic loss in EOC results in differential immunogenicity, with high immune infiltration being associated with PTEN-loss and BRCA1 promoter-methylated tumors, and low immune infiltration with a significantly shortened overall survival [219]. Nevertheless, EOCs with BRCA1/2 mutation and PTEN loss had significantly higher HRD scores, but displayed significantly fewer CD3+, CD8+, and FOXP3+ T cells [219]. In their recent study, Fumet et al. [61] showed that the HLA-E/CD94-NKG2A/2C axis influences antitumoral immunity in HRD ovarian tumors, revealing opportunities to explore and further investigate the interacting immune network in these specific tumor genotypes.
Many open questions remain regarding MHC I expression and tumorigenicity. How is a more “aggressive” genotype potentially more immunogenic and recognizable by CTLs? Is this a transient state of their stemness reprogramming? A loss of heterozygosity in HLA I represents a genetic barrier to effective immunotherapy, which would require alternative ways to harness the immune system to maximize clinical benefit [220].
What is the amplitude of HLA diversity/heterogeneity that would correlate with efficient immune responses in EOC? F. Garrido previously commented on the different HLA I expression profiles found in tumor clones maintained ex vivo, contrasting them with the homogeneous tumor cell lines used in laboratories, which are often derived from a single clone and therefore lacking HLA “diversity” [2]. Therefore, more relevant models need to be employed to better elucidate the nature of tumor immunogenicity.
What level of HLA expression influences tumor promotion and EMT? Does the grade and type of EOC affect HLA immune regulation in the TME differently? Ovarian cancer is not a single disease but comprises various subtypes with different molecular characteristics. The impact of MHC I expression can vary among these subtypes. It is essential to consider the specific subtype and its genetic constitution when evaluating the role of MHC I expression.
Standard treatment centered on neo-adjuvant chemotherapy using carboplatin/paclitaxel in EOC patients showed that HLA I expression in tumors decreases after treatment, hampering T cell recruitment and activation in the TME [221]. Therefore, strategies aiming to upregulate MHC-I expression during or after neoadjuvant chemotherapy could provide better treatment outcomes in these patients [221]. Epigenetic modulators (such as azacytidine) aiming to increase MHC I could be a good approach to increase immunogenicity.
In summary, the relationship between MHC I expression and ovarian cancer is multifaceted and context dependent. Generally, high MHC I expression can be favorable as it may enhance the immune response against the tumor. However, other factors, including the tumor’s genetic constitution, the EMT status of the cancer cells, and the treatment approach, need to be considered when assessing its significance in individual cases. Additionally, the field of cancer immunotherapy is continually evolving, and ongoing research may reveal new insights into the role of MHC I expression in the response of ovarian cancer to treatment.
References
- Shiina, T.; Hosomichi, K.; Inoko, H.; Kulski, J.K. The HLA Genomic Loci Map: Expression, Interaction, Diversity and Disease. J. Hum. Genet. 2009, 54, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F. HLA Class-I Expression and Cancer Immunotherapy. Adv. Exp. Med. Biol. 2019, 1151, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Sato, A. The HLA System. First of Two Parts. N. Engl. J. Med. 2000, 343, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Sato, A. The HLA System. Second of Two Parts. N. Engl. J. Med. 2000, 343, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Tapias, P.; Castiblanco, J.; Anaya, J.-M. HLA Association with Autoimmune Diseases. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- D’Souza, M.P.; Adams, E.; Altman, J.D.; Birnbaum, M.E.; Boggiano, C.; Casorati, G.; Chien, Y.; Conley, A.; Eckle, S.B.G.; Früh, K.; et al. Casting a Wider Net: Immunosurveillance by Nonclassical MHC Molecules. PLoS Pathog. 2019, 15, e1007567. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Barker, D.J.; Georgiou, X.; Cooper, M.A.; Flicek, P.; Marsh, S.G.E. IPD-IMGT/HLA Database. Nucleic Acids Res. 2020, 48, D948–D955. [Google Scholar] [CrossRef]
- Andersson, E.; Poschke, I.; Villabona, L.; Carlson, J.W.; Lundqvist, A.; Kiessling, R.; Seliger, B.; Masucci, G.V. Non-Classical HLA-Class I Expression in Serous Ovarian Carcinoma: Correlation with the HLA-Genotype, Tumor Infiltrating Immune Cells and Prognosis. Oncoimmunology 2015, 5, e1052213. [Google Scholar] [CrossRef]
- Stern, L.J.; Wiley, D.C. Antigenic Peptide Binding by Class I and Class II Histocompatibility Proteins. Structure 1994, 2, 245–251. [Google Scholar] [CrossRef]
- Chen, Y.; Sidney, J.; Southwood, S.; Cox, A.L.; Sakaguchi, K.; Henderson, R.A.; Appella, E.; Hunt, D.F.; Sette, A.; Engelhard, V.H. Naturally Processed Peptides Longer than Nine Amino Acid Residues Bind to the Class I MHC Molecule HLA-A2.1 with High Affinity and in Different Conformations. J. Immunol. 1994, 152, 2874–2881. [Google Scholar] [CrossRef]
- Rist, M.J.; Theodossis, A.; Croft, N.P.; Neller, M.A.; Welland, A.; Chen, Z.; Sullivan, L.C.; Burrows, J.M.; Miles, J.J.; Brennan, R.M.; et al. HLA Peptide Length Preferences Control CD8+ T Cell Responses. J. Immunol. 2013, 191, 561–571. [Google Scholar] [CrossRef]
- Trolle, T.; McMurtrey, C.P.; Sidney, J.; Bardet, W.; Osborn, S.C.; Kaever, T.; Sette, A.; Hildebrand, W.H.; Nielsen, M.; Peters, B. The Length Distribution of Class I Restricted T Cell Epitopes Is Determined by Both Peptide Supply and MHC Allele Specific Binding Preference. J. Immunol. 2016, 196, 1480–1487. [Google Scholar] [CrossRef]
- Lanier, L.L. NK Cell Recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef]
- Lanier, L.L.; Phillips, J.H. Inhibitory MHC Class I Receptors on NK Cells and T Cells. Immunol. Today 1996, 17, 86–91. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for Hla Class-I Molecules in Human Natural Killer Cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Söderström, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E Binds to Natural Killer Cell Receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- López-Botet, M.; Bellón, T. Natural Killer Cell Activation and Inhibition by Receptors for MHC Class I. Curr. Opin. Immunol. 1999, 11, 301–307. [Google Scholar] [CrossRef]
- Moretta, L.; Biassoni, R.; Bottino, C.; Mingari, M.C.; Moretta, A. Human NK-Cell Receptors. Immunol. Today 2000, 21, 420–422. [Google Scholar] [CrossRef]
- Chicz, R.M.; Urban, R.G.; Lane, W.S.; Gorga, J.C.; Stern, L.J.; Vignali, D.A.; Strominger, J.L. Predominant Naturally Processed Peptides Bound to HLA-DR1 Are Derived from MHC-Related Molecules and Are Heterogeneous in Size. Nature 1992, 358, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, T.; Ruiz-Cabello, F.; Garrido, F. Biological Implications of HLA-DR Expression in Tumours. Scand. J. Immunol. 1995, 41, 398–406. [Google Scholar] [CrossRef]
- Cruz-Tapias, P.; Castiblanco, J.; Anaya, J.-M. Major Histocompatibility Complex: Antigen Processing and Presentation; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Daar, A.S.; Fuggle, S.V.; Fabre, J.W.; Ting, A.; Morris, P.J. The Detailed Distribution of MHC Class II Antigens in Normal Human Organs. Transplantation 1984, 38, 293–298. [Google Scholar] [CrossRef]
- Aultman, D.; Adamashvili, I.; Yaturu, K.; Langford, M.; Gelder, F.; Gautreaux, M.; Ghali, G.E.; McDonald, J. Soluble HLA in Human Body Fluids. Hum. Immunol. 1999, 60, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Puppo, F.; Scudeletti, M.; Indiveri, F.; Ferrone, S. Serum HLA Class I Antigens: Markers and Modulators of an Immune Response? Immunol. Today 1995, 16, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, G.; Bahram, S. Genetics of the Central MHC. Curr. Opin. Immunol. 2004, 16, 668–672. [Google Scholar] [CrossRef]
- Horton, R.; Wilming, L.; Rand, V.; Lovering, R.C.; Bruford, E.A.; Khodiyar, V.K.; Lush, M.J.; Povey, S.; Talbot, C.C.; Wright, M.W.; et al. Gene Map of the Extended Human MHC. Nat. Rev. Genet. 2004, 5, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Schott, G.; Garcia-Blanco, M.A. MHC Class III RNA Binding Proteins and Immunity. RNA Biol. 2020, 18, 640–646. [Google Scholar] [CrossRef]
- Aust, S.; Felix, S.; Auer, K.; Bachmayr-Heyda, A.; Kenner, L.; Dekan, S.; Meier, S.M.; Gerner, C.; Grimm, C.; Pils, D. Absence of PD-L1 on Tumor Cells Is Associated with Reduced MHC I Expression and PD-L1 Expression Increases in Recurrent Serous Ovarian Cancer. Sci. Rep. 2017, 7, 42929. [Google Scholar] [CrossRef]
- Dholakia, J.; Scalise, C.B.; Katre, A.A.; Goldsberry, W.N.; Meza-Perez, S.; Randall, T.D.; Norian, L.A.; Novak, L.; Arend, R.C. Sequential Modulation of the Wnt/β-Catenin Signaling Pathway Enhances Tumor-Intrinsic MHC I Expression and Tumor Clearance. Gynecol. Oncol. 2022, 164, 170–180. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed Cell Death 1 Ligand 1 and Tumor-Infiltrating CD8+ T Lymphocytes Are Prognostic Factors of Human Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Vitale, M.; Pelusi, G.; Taroni, B.; Gobbi, G.; Micheloni, C.; Rezzani, R.; Donato, F.; Wang, X.; Ferrone, S. HLA Class I Antigen Down-Regulation in Primary Ovary Carcinoma Lesions: Association with Disease Stage. Clin. Cancer Res. 2005, 11, 67–72. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Warren, R.L.; Gibb, E.A.; Martin, S.D.; Spinelli, J.J.; Nelson, B.H.; Holt, R.A. Neo-Antigens Predicted by Tumor Genome Meta-Analysis Correlate with Increased Patient Survival. Genome Res. 2014, 24, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wick, D.A.; Webb, J.R.; Nielsen, J.S.; Martin, S.D.; Kroeger, D.R.; Milne, K.; Castellarin, M.; Twumasi-Boateng, K.; Watson, P.H.; Holt, R.A.; et al. Surveillance of the Tumor Mutanome by T Cells during Progression from Primary to Recurrent Ovarian Cancer. Clin. Cancer Res. 2014, 20, 1125–1134. [Google Scholar] [CrossRef]
- Han, L.Y.; Fletcher, M.S.; Urbauer, D.L.; Mueller, P.; Landen, C.N.; Kamat, A.A.; Lin, Y.G.; Merritt, W.M.; Spannuth, W.A.; Deavers, M.T.; et al. HLA Class I Antigen Processing Machinery Component Expression and Intratumoral T-Cell Infiltrate as Independent Prognostic Markers in Ovarian Carcinoma. Clin. Cancer Res. 2008, 14, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Santoiemma, P.P.; Reyes, C.; Wang, L.-P.; McLane, M.W.; Feldman, M.D.; Tanyi, J.L.; Powell, D.J. Systematic Evaluation of Multiple Immune Markers Reveals Prognostic Factors in Ovarian Cancer. Gynecol. Oncol. 2016, 143, 120–127. [Google Scholar] [CrossRef]
- Garrido, F.; Algarra, I. MHC Antigens and Tumor Escape from Immune Surveillance. Adv. Cancer Res. 2001, 83, 117–158. [Google Scholar] [CrossRef]
- Garrido, F.; Cabrera, T.; Aptsiauri, N. “Hard” and “Soft” Lesions Underlying the HLA Class I Alterations in Cancer Cells: Implications for Immunotherapy. Int. J. Cancer 2010, 127, 249–256. [Google Scholar] [CrossRef]
- Hobart, M.; Ramassar, V.; Goes, N.; Urmson, J.; Halloran, P.F. The Induction of Class I and II Major Histocompatibility Complex by Allogeneic Stimulation Is Dependent on the Transcription Factor Interferon Regulatory Factor 1 (IRF-1): Observations in IRF-1 Knockout Mice. Transplantation 1996, 62, 1895–1901. [Google Scholar] [CrossRef]
- Meissner, T.B.; Li, A.; Biswas, A.; Lee, K.-H.; Liu, Y.-J.; Bayir, E.; Iliopoulos, D.; van den Elsen, P.J.; Kobayashi, K.S. NLR Family Member NLRC5 Is a Transcriptional Regulator of MHC Class I Genes. Proc. Natl. Acad. Sci. USA 2010, 107, 13794–13799. [Google Scholar] [CrossRef]
- Naumann, M.; Scheidereit, C. Activation of NF-Kappa B in Vivo Is Regulated by Multiple Phosphorylations. EMBO J. 1994, 13, 4597–4607. [Google Scholar] [CrossRef]
- Goodell, V.; Salazar, L.G.; Urban, N.; Drescher, C.W.; Gray, H.; Swensen, R.E.; McIntosh, M.W.; Disis, M.L. Antibody Immunity to the P53 Oncogenic Protein Is a Prognostic Indicator in Ovarian Cancer. J. Clin. Oncol. 2006, 24, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.D.; Brown, S.D.; Wick, D.A.; Nielsen, J.S.; Kroeger, D.R.; Twumasi-Boateng, K.; Holt, R.A.; Nelson, B.H. Low Mutation Burden in Ovarian Cancer May Limit the Utility of Neoantigen-Targeted Vaccines. PLoS ONE 2016, 11, e0155189. [Google Scholar] [CrossRef]
- Luo, N.; Nixon, M.J.; Gonzalez-Ericsson, P.I.; Sanchez, V.; Opalenik, S.R.; Li, H.; Zahnow, C.A.; Nickels, M.L.; Liu, F.; Tantawy, M.N.; et al. DNA Methyltransferase Inhibition Upregulates MHC-I to Potentiate Cytotoxic T Lymphocyte Responses in Breast Cancer. Nat. Commun. 2018, 9, 248. [Google Scholar] [CrossRef]
- Taylor, B.C.; Balko, J.M. Mechanisms of MHC-I Downregulation and Role in Immunotherapy Response. Front. Immunol. 2022, 13, 844866. [Google Scholar] [CrossRef] [PubMed]
- Rolland, P.; Deen, S.; Scott, I.; Durrant, L.; Spendlove, I. Human Leukocyte Antigen Class I Antigen Expression Is an Independent Prognostic Factor in Ovarian Cancer. Clin. Cancer Res. 2007, 13, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Szender, J.B.; Eng, K.H.; Matsuzaki, J.; Miliotto, A.; Gnjatic, S.; Tsuji, T.; Odunsi, K. HLA Superfamily Assignment Is a Predictor of Immune Response to Cancer Testis Antigens and Survival in Ovarian Cancer. Gynecol. Oncol. 2016, 142, 158–162. [Google Scholar] [CrossRef]
- Shukla, S.A.; Rooney, M.S.; Rajasagi, M.; Tiao, G.; Dixon, P.M.; Lawrence, M.S.; Stevens, J.; Lane, W.J.; Dellagatta, J.L.; Steelman, S.; et al. Comprehensive Analysis of Cancer-Associated Somatic Mutations in Class I HLA Genes. Nat. Biotechnol. 2015, 33, 1152–1158. [Google Scholar] [CrossRef]
- Schuster, H.; Peper, J.K.; Bösmüller, H.-C.; Röhle, K.; Backert, L.; Bilich, T.; Ney, B.; Löffler, M.W.; Kowalewski, D.J.; Trautwein, N.; et al. The Immunopeptidomic Landscape of Ovarian Carcinomas. Proc. Natl. Acad. Sci. USA 2017, 114, E9942–E9951. [Google Scholar] [CrossRef]
- Griesinger, L.; Nyarko-Odoom, A.; Martinez, S.A.; Shen, N.W.; Ring, K.L.; Gaughan, E.M.; Mills, A.M. PD-L1 and MHC Class I Expression in High-Grade Ovarian Cancers, Including Platinum-Resistant Recurrences Treated with Checkpoint Inhibitor Therapy. Appl. Immunohistochem. Mol. Morphol. 2023, 31, 197–203. [Google Scholar] [CrossRef]
- Monos, D.S.; Winchester, R.J. The Major Histocompatibility Complex. In Clinical Immunology: Principles and Practice, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 79–92. [Google Scholar]
- Wyatt, R.C.; Lanzoni, G.; Russell, M.A.; Gerling, I.; Richardson, S.J. What the HLA-I!—Classical and Non-Classical HLA Class I and Their Potential Roles in Type 1 Diabetes. Curr. Diab. Rep. 2019, 19, 159. [Google Scholar] [CrossRef]
- Menier, C.; Saez, B.; Horejsi, V.; Martinozzi, S.; Krawice-Radanne, I.; Bruel, S.; Le Danff, C.; Reboul, M.; Hilgert, I.; Rabreau, M.; et al. Characterization of Monoclonal Antibodies Recognizing HLA-G or HLA-E: New Tools to Analyze the Expression of Nonclassical HLA Class I Molecules. Hum. Immunol. 2003, 64, 315–326. [Google Scholar] [CrossRef]
- Marín, R.; Ruiz-Cabello, F.; Pedrinaci, S.; Méndez, R.; Jiménez, P.; Geraghty, D.E.; Garrido, F. Analysis of HLA-E Expression in Human Tumors. Immunogenetics 2003, 54, 767–775. [Google Scholar] [CrossRef]
- Wei, X.; Orr, H.T. Differential Expression of HLA-E, HLA-F, and HLA-G Transcripts in Human Tissue. Hum. Immunol. 1990, 29, 131–142. [Google Scholar] [CrossRef]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; López-Botet, M.; Geraghty, D.E. HLA-E Is a Major Ligand for the Natural Killer Inhibitory Receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef]
- Speiser, D.E.; Valmori, D.; Rimoldi, D.; Pittet, M.J.; Liénard, D.; Cerundolo, V.; MacDonald, H.R.; Cerottini, J.C.; Romero, P. CD28-Negative Cytolytic Effector T Cells Frequently Express NK Receptors and Are Present at Variable Proportions in Circulating Lymphocytes from Healthy Donors and Melanoma Patients. Eur. J. Immunol. 1999, 29, 1990–1999. [Google Scholar] [CrossRef]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E Expression by Gynecological Cancers Restrains Tumor-Infiltrating CD8+ T Lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef]
- Eugène, J.; Jouand, N.; Ducoin, K.; Dansette, D.; Oger, R.; Deleine, C.; Leveque, E.; Meurette, G.; Podevin, J.; Matysiak, T.; et al. The Inhibitory Receptor CD94/NKG2A on CD8+ Tumor-Infiltrating Lymphocytes in Colorectal Cancer: A Promising New Druggable Immune Checkpoint in the Context of HLAE/Β2m Overexpression. Mod. Pathol. 2020, 33, 468–482. [Google Scholar] [CrossRef]
- Fumet, J.-D.; Lardenois, E.; Ray-Coquard, I.; Harter, P.; Joly, F.; Canzler, U.; Truntzer, C.; Tredan, O.; Liebrich, C.; Lortholary, A.; et al. Genomic Instability Is Defined by Specific Tumor Microenvironment in Ovarian Cancer: A Subgroup Analysis of AGO OVAR 12 Trial. Cancers 2022, 14, 1189. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.J. The CGAS–CGAMP–STING Pathway Connects DNA Damage to Inflammation, Senescence, and Cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Nguyen, S.; Beziat, V.; Dhedin, N.; Kuentz, M.; Vernant, J.P.; Debre, P.; Vieillard, V. HLA-E Upregulation on IFN-γ-Activated AML Blasts Impairs CD94/NKG2A-Dependent NK Cytolysis after Haplo-Mismatched Hematopoietic SCT. Bone Marrow. Transpl. 2009, 43, 693–699. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, R.; Xie, S.; Wen, X.; Wang, H.; Gao, X.; Guo, L. Human Leukocyte Antigen-E Alleles and Expression in Patients with Serous Ovarian Cancer. Cancer Sci. 2015, 106, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Ishitani, A.; Geraghty, D.E. HLA-F Is a Surface Marker on Activated Lymphocytes. Eur. J. Immunol. 2010, 40, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, S.D.; Biro, P.A.; Holmes, C.H. HLA-F Is a Predominantly Empty, Intracellular, TAP-Associated MHC Class Ib Protein with a Restricted Expression Pattern1. J. Immunol. 2000, 164, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Lepin, E.J.M.; Bastin, J.M.; Allan, D.S.J.; Roncador, G.; Braud, V.M.; Mason, D.Y.; van der Merwe, P.A.; McMichael, A.J.; Bell, J.I.; Powis, S.H.; et al. Functional Characterization of HLA-F and Binding of HLA-F Tetramers to ILT2 and ILT4 Receptors. Eur. J. Immunol. 2000, 30, 3552–3561. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F and MHC Class I Open Conformers Are Ligands for NK Cell Ig-like Receptors. J. Immunol. 2013, 191, 3553–3562. [Google Scholar] [CrossRef]
- Burian, A.; Wang, K.L.; Finton, K.A.K.; Lee, N.; Ishitani, A.; Strong, R.K.; Geraghty, D.E. HLA-F and MHC-I Open Conformers Bind Natural Killer Cell Ig-Like Receptor KIR3DS1. PLoS ONE 2016, 11, e0163297. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Lee, N.; Burian, A.; Pyo, C.-W.; Tykodi, S.S.; Warren, E.H.; Yee, C.; Riddell, S.R.; Geraghty, D.E. HLA-F and MHC-I Open Conformers Cooperate in a MHC-I Antigen Cross-Presentation Pathway. J. Immunol. 2013, 191, 1567–1577. [Google Scholar] [CrossRef]
- Hrbac, T.; Kopkova, A.; Siegl, F.; Vecera, M.; Ruckova, M.; Kazda, T.; Jancalek, R.; Hendrych, M.; Hermanova, M.; Vybihal, V.; et al. HLA-E and HLA-F Are Overexpressed in Glioblastoma and HLA-E Increased After Exposure to Ionizing Radiation. Cancer Genom. Proteom. 2022, 19, 151–162. [Google Scholar] [CrossRef]
- Fang, W.; Xia, Y. LncRNA HLA-F-AS1 Attenuates the Ovarian Cancer Development by Targeting MiR-21-3p/PEG3 Axis. Anti Cancer Drugs 2022, 33, 671. [Google Scholar] [CrossRef]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; DeMars, R. A Class I Antigen, HLA-G, Expressed in Human Trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Cirulli, V.; Zalatan, J.; McMaster, M.; Prinsen, R.; Salomon, D.R.; Ricordi, C.; Torbett, B.E.; Meda, P.; Crisa, L. The Class I HLA Repertoire of Pancreatic Islets Comprises the Nonclassical Class Ib Antigen HLA-G. Diabetes 2006, 55, 1214–1222. [Google Scholar] [CrossRef]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.-M.; Carosella, E.D. Expression of HLA-G in Human Cornea, an Immune-Privileged Tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef]
- Menier, C.; Rabreau, M.; Challier, J.-C.; Le Discorde, M.; Carosella, E.D.; Rouas-Freiss, N. Erythroblasts Secrete the Nonclassical HLA-G Molecule from Primitive to Definitive Hematopoiesis. Blood 2004, 104, 3153–3160. [Google Scholar] [CrossRef]
- Crisa, L.; McMaster, M.T.; Ishii, J.K.; Fisher, S.J.; Salomon, D.R. Identification of a Thymic Epithelial Cell Subset Sharing Expression of the Class Ib HLA-G Molecule with Fetal Trophoblasts. J. Exp. Med. 1997, 186, 289–298. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; Moreau, P.; LeMaoult, J.; Carosella, E.D. The Dual Role of HLA-G in Cancer. J. Immunol. Res. 2014, 2014, 359748. [Google Scholar] [CrossRef]
- Barbaro, G.; Inversetti, A.; Cristodoro, M.; Ticconi, C.; Scambia, G.; Di Simone, N. HLA-G and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2023, 24, 2557. [Google Scholar] [CrossRef] [PubMed]
- Contini, P.; Ghio, M.; Poggi, A.; Filaci, G.; Indiveri, F.; Ferrone, S.; Puppo, F. Soluble HLA-A,-B,-C and -G Molecules Induce Apoptosis in T and NK CD8+ Cells and Inhibit Cytotoxic T Cell Activity through CD8 Ligation. Eur. J. Immunol. 2003, 33, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, L.; Flørenes, V.A.; Skrede, M.; Dong, H.P.; Nielsen, S.; McMaster, M.T.; Nesland, J.M.; Shih, I.-M.; Davidson, B. Expression of HLA-G in Malignant Mesothelioma and Clinically Aggressive Breast Carcinoma. Virchows Arch. 2006, 449, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rouas-Freiss, N.; Moreau, P.; Ferrone, S.; Carosella, E.D. HLA-G Proteins in Cancer: Do They Provide Tumor Cells with an Escape Mechanism? Cancer Res. 2005, 65, 10139–10144. [Google Scholar] [CrossRef] [PubMed]
- Carosella, E.D.; Favier, B.; Rouas-Freiss, N.; Moreau, P.; Lemaoult, J. Beyond the Increasing Complexity of the Immunomodulatory HLA-G Molecule. Blood 2008, 111, 4862–4870. [Google Scholar] [CrossRef]
- Lin, A.; Zhang, X.; Xu, H.-H.; Xu, D.-P.; Ruan, Y.-Y.; Yan, W.-H. HLA-G Expression Is Associated with Metastasis and Poor Survival in the Balb/c Nu/Nu Murine Tumor Model with Ovarian Cancer. Int. J. Cancer 2012, 131, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Fujii, T.; Unno, N.; Yamashita, T.; Hyodo, H.; Miki, A.; Hamai, Y.; Kozuma, S.; Taketani, Y. Human Leukocyte Antigen-G-Expressing Cells Differently Modulate the Release of Cytokines from Mononuclear Cells Present in the Decidua versus Peripheral Blood. Am. J. Reprod. Immunol. 2001, 45, 94–99. [Google Scholar] [CrossRef]
- Kanai, T.; Fujii, T.; Kozuma, S.; Yamashita, T.; Miki, A.; Kikuchi, A.; Taketani, Y. Soluble HLA-G Influences the Release of Cytokines from Allogeneic Peripheral Blood Mononuclear Cells in Culture. Mol. Hum. Reprod. 2001, 7, 195–200. [Google Scholar] [CrossRef]
- Babay, W.; Ben Yahia, H.; Boujelbene, N.; Zidi, N.; Laaribi, A.B.; Kacem, D.; Ben Ghorbel, R.; Boudabous, A.; Ouzari, H.-I.; Rizzo, R.; et al. Clinicopathologic Significance of HLA-G and HLA-E Molecules in Tunisian Patients with Ovarian Carcinoma. Hum. Immunol. 2018, 79, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Rebmann, V.; Chen, Y.-C.; Liu, H.-T.; Ali, S.Z.; Reinsberg, J.; McMaster, M.T.; Pfeiffer, K.; Chan, D.W.; Wardelmann, E.; et al. HLA-G Is a Potential Tumor Marker in Malignant Ascites. Clin. Cancer Res. 2003, 9, 4460–4464. [Google Scholar] [PubMed]
- Babay, W.; Boujelbene, N.; Ben Yahia, H.; Bortolotti, D.; Zemni, I.; Ouzari, H.-I.; Chelbi, H.; Mezlini, A.; Rizzo, R.; Zidi, I. Prognostic Significance of High Circulating SHLA-G in Ovarian Carcinoma. HLA 2021, 98, 357–365. [Google Scholar] [CrossRef]
- McCormick, J.; Whitley, G.S.J.; Le Bouteiller, P.; Cartwright, J.E. Soluble HLA-G Regulates Motility and Invasion of the Trophoblast-Derived Cell Line SGHPL-4. Hum. Reprod. 2009, 24, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.J.; Dijk, F.; Savci-Heijink, C.D.; Buist, M.R.; Kenter, G.G.; van de Vijver, M.J.; Jordanova, E.S. HLA-G Expression Is an Independent Predictor for Improved Survival in High Grade Ovarian Carcinomas. J. Immunol. Res. 2014, 2014, 274584. [Google Scholar] [CrossRef]
- Menier, C.; Prevot, S.; Carosella, E.D.; Rouas-Freiss, N. Human Leukocyte Antigen-G Is Expressed in Advanced-Stage Ovarian Carcinoma of High-Grade Histology. Hum. Immunol. 2009, 70, 1006–1009. [Google Scholar] [CrossRef]
- Downs, I.; Vijayan, S.; Sidiq, T.; Kobayashi, K.S. CITA/NLRC5: A Critical Transcriptional Regulator of MHC Class I Gene Expression. Biofactors 2016, 42, 349–357. [Google Scholar] [CrossRef]
- Staehli, F.; Ludigs, K.; Heinz, L.X.; Seguín-Estévez, Q.; Ferrero, I.; Braun, M.; Schroder, K.; Rebsamen, M.; Tardivel, A.; Mattmann, C.; et al. NLRC5 Deficiency Selectively Impairs MHC Class I- Dependent Lymphocyte Killing by Cytotoxic T Cells. J. Immunol. 2012, 188, 3820–3828. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y.; Chen, F.; Huang, Y.; Zhu, S.; Leng, Q.; Wang, H.; Shi, Y.; Qian, Y. NLRC5 Regulates MHC Class I Antigen Presentation in Host Defense against Intracellular Pathogens. Cell Res. 2012, 22, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Yoshihama, S.; Roszik, J.; Downs, I.; Meissner, T.B.; Vijayan, S.; Chapuy, B.; Sidiq, T.; Shipp, M.A.; Lizee, G.A.; Kobayashi, K.S. NLRC5/MHC Class I Transactivator Is a Target for Immune Evasion in Cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 5999–6004. [Google Scholar] [CrossRef] [PubMed]
- Yoshihama, S.; Vijayan, S.; Sidiq, T.; Kobayashi, K.S. NLRC5/CITA: A Key Player in Cancer Immune Surveillance. Trends Cancer 2017, 3, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers 2018, 10, E242. [Google Scholar] [CrossRef]
- Bubeník, J. Tumour MHC Class I Downregulation and Immunotherapy (Review). Oncol. Rep. 2003, 10, 2005–2008. [Google Scholar] [CrossRef]
- Norell, H.; Carlsten, M.; Ohlum, T.; Malmberg, K.-J.; Masucci, G.; Schedvins, K.; Altermann, W.; Handke, D.; Atkins, D.; Seliger, B.; et al. Frequent Loss of HLA-A2 Expression in Metastasizing Ovarian Carcinomas Associated with Genomic Haplotype Loss and HLA-A2-Restricted HER-2/Neu-Specific Immunity. Cancer Res. 2006, 66, 6387–6394. [Google Scholar] [CrossRef]
- Shimono, Y.; Ugalde, M.Z.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Down-Regulation of MiRNA-200c Links Breast Cancer Stem Cells with Normal Stem Cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef]
- Yang, M.-H.; Hsu, D.S.-S.; Wang, H.-W.; Wang, H.-J.; Lan, H.-Y.; Yang, W.-H.; Huang, C.-H.; Kao, S.-Y.; Tzeng, C.-H.; Tai, S.-K.; et al. Bmi1 Is Essential in Twist1-Induced Epithelial–Mesenchymal Transition. Nat. Cell. Biol. 2010, 12, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell. Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Weinberg, R.A. EMT and Cancer: More Than Meets the Eye. Dev. Cell 2019, 49, 313–316. [Google Scholar] [CrossRef]
- Chen, X.-H.; Liu, Z.-C.; Zhang, G.; Wei, W.; Wang, X.-X.; Wang, H.; Ke, H.-P.; Zhang, F.; Wang, H.-S.; Cai, S.-H.; et al. TGF-β and EGF Induced HLA-I Downregulation Is Associated with Epithelial-Mesenchymal Transition (EMT) through Upregulation of Snail in Prostate Cancer Cells. Mol. Immunol. 2015, 65, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Rashidian, M.; Reinhardt, F.; Bagnato, A.; Keckesova, Z.; Ploegh, H.L.; Weinberg, R.A. Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Res. 2017, 77, 3982–3989. [Google Scholar] [CrossRef]
- Pires, P.R.L.; Xavier, P.L.P.; Fukumasu, H. Abstract B180: Effects of EMT Process under MHC Class I and TAP1 Gene Expression Related to Antigen Presentation. Cancer Immunol. Res. 2019, 7, B180. [Google Scholar] [CrossRef]
- Porter, R.L.; Sun, S.; Flores, M.N.; Berzolla, E.; You, E.; Phillips, I.E.; Kc, N.; Desai, N.; Tai, E.C.; Szabolcs, A.; et al. Satellite Repeat RNA Expression in Epithelial Ovarian Cancer Associates with a Tumor-Immunosuppressive Phenotype. J. Clin. Invest. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.C.; Sankpal, N.V.; Gillanders, W.E. Functional Implications of the Dynamic Regulation of EpCAM during Epithelial-to-Mesenchymal Transition. Biomolecules 2021, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, Z.; ten Dijke, P. Harnessing Epithelial-Mesenchymal Plasticity to Boost Cancer Immunotherapy. Cell Mol. Immunol. 2023, 20, 318–340. [Google Scholar] [CrossRef]
- Zingg, D.; Debbache, J.; Schaefer, S.M.; Tuncer, E.; Frommel, S.C.; Cheng, P.; Arenas-Ramirez, N.; Haeusel, J.; Zhang, Y.; Bonalli, M.; et al. The Epigenetic Modifier EZH2 Controls Melanoma Growth and Metastasis through Silencing of Distinct Tumour Suppressors. Nat. Commun. 2015, 6, 6051. [Google Scholar] [CrossRef]
- Kleer, C.G.; Cao, Q.; Varambally, S.; Shen, R.; Ota, I.; Tomlins, S.A.; Ghosh, D.; Sewalt, R.G.A.B.; Otte, A.P.; Hayes, D.F.; et al. EZH2 Is a Marker of Aggressive Breast Cancer and Promotes Neoplastic Transformation of Breast Epithelial Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11606–11611. [Google Scholar] [CrossRef]
- Collett, K.; Eide, G.E.; Arnes, J.; Stefansson, I.M.; Eide, J.; Braaten, A.; Aas, T.; Otte, A.P.; Akslen, L.A. Expression of Enhancer of Zeste Homologue 2 Is Significantly Associated with Increased Tumor Cell Proliferation and Is a Marker of Aggressive Breast Cancer. Clin. Cancer Res. 2006, 12, 1168–1174. [Google Scholar] [CrossRef]
- Zhou, L.; Mudianto, T.; Ma, X.; Riley, R.; Uppaluri, R. Targeting EZH2 Enhances Antigen Presentation, Antitumor Immunity and Circumvents Anti-PD-1 Resistance in Head and Neck Cancer. Clin. Cancer Res. 2020, 26, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Xu, H.; Ma, L.; Li, C.; Qin, W.; Chen, X.; Yi, M.; Sun, L.; Liu, B.; Yuan, X. Nintedanib Enhances the Efficacy of PD-L1 Blockade by Upregulating MHC-I and PD-L1 Expression in Tumor Cells. Theranostics 2022, 12, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Josson, S.; Nomura, T.; Lin, J.-T.; Huang, W.-C.; Wu, D.; Zhau, H.E.; Zayzafoon, M.; Weizmann, M.N.; Gururajan, M.; Chung, L.W.K. Β2-Microglobulin Induces Epithelial to Mesenchymal Transition and Confers Cancer Lethality and Bone Metastasis in Human Cancer Cells. Cancer Res. 2011, 71, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Chockley, P.J.; Keshamouni, V.G. Immunological Consequences of Epithelial-Mesenchymal Transition in Tumor Progression. J. Immunol. 2016, 197, 691–698. [Google Scholar] [CrossRef]
- Lazaridou, M.-F.; Gonschorek, E.; Massa, C.; Friedrich, M.; Handke, D.; Mueller, A.; Jasinski-Bergner, S.; Dummer, R.; Koelblinger, P.; Seliger, B. Identification of MiR-200a-5p Targeting the Peptide Transporter TAP1 and Its Association with the Clinical Outcome of Melanoma Patients. OncoImmunology 2020, 9, 1774323. [Google Scholar] [CrossRef] [PubMed]
- Camp, F.A.; Brunetti, T.M.; Williams, M.M.; Christenson, J.L.; Sreekanth, V.; Costello, J.C.; Hay, Z.L.Z.; Kedl, R.M.; Richer, J.K.; Slansky, J.E. Antigens Expressed by Breast Cancer Cells Undergoing EMT Stimulate Cytotoxic CD8+ T Cell Immunity. Cancers 2022, 14, 4397. [Google Scholar] [CrossRef]
- Cavallari, I.; Ciccarese, F.; Sharova, E.; Urso, L.; Raimondi, V.; Silic-Benussi, M.; D’Agostino, D.M.; Ciminale, V. The MiR-200 Family of MicroRNAs: Fine Tuners of Epithelial-Mesenchymal Transition and Circulating Cancer Biomarkers. Cancers 2021, 13, 5874. [Google Scholar] [CrossRef]
- Cook, D.P.; Vanderhyden, B.C. Transcriptional Census of Epithelial-Mesenchymal Plasticity in Cancer. Sci. Adv. 2022, 8, eabi7640. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell. Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Scheel, C.; Eaton, E.N.; Li, S.H.-J.; Chaffer, C.L.; Reinhardt, F.; Kah, K.-J.; Bell, G.; Guo, W.; Rubin, J.; Richardson, A.L.; et al. Paracrine and Autocrine Signals Induce and Maintain Mesenchymal and Stem Cell States in the Breast. Cell 2011, 145, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Larocca, C.; Cohen, J.R.; Fernando, R.I.; Huang, B.; Hamilton, D.H.; Palena, C. An Autocrine Loop between TGF-Β1 and the Transcription Factor Brachyury Controls the Transition of Human Carcinoma Cells into a Mesenchymal Phenotype. Mol. Cancer Ther. 2013, 12, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, U.; Kowanetz, M.; Niimi, H.; Heldin, C.-H.; Moustakas, A. TGF-β and the Smad Signaling Pathway Support Transcriptomic Reprogramming during Epithelial-Mesenchymal Cell Transition. Mol. Biol. Cell 2005, 16, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. α-PD-1 Therapy Elevates Treg/Th Balance and Increases Tumor Cell PSmad3 That Are Both Targeted by α-TGFβ Antibody to Promote Durable Rejection and Immunity in Squamous Cell Carcinomas. J. Immunother. Cancer. 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Fradette, J.J.; Konen, J.M.; Moulder, S.; Zhang, X.; Gibbons, D.L.; Varadarajan, N.; Wistuba, I.I.; Tripathy, D.; Bernatchez, C.; et al. Targeting the Interplay between Epithelial-to-Mesenchymal-Transition and the Immune System for Effective Immunotherapy. Cancers 2019, 11, 714. [Google Scholar] [CrossRef]
- erglund, A.K.; Hinson, A.L.; Schnabel, L.V. TGF-β downregulates antigen processing and presentation genes and MHC I surface expression through a Smad3-dependent mechanism. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cook, D.P.; Vanderhyden, B.C. Context Specificity of the EMT Transcriptional Response. Nat. Commun. 2020, 11, 2142. [Google Scholar] [CrossRef]
- Machado, C.D.; Telles, P.D.; Nascimento, I.L. Immunological Characteristics of Mesenchymal Stem Cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef]
- Apavaloaei, A.; Hesnard, L.; Hardy, M.-P.; Benabdallah, B.; Ehx, G.; Thériault, C.; Laverdure, J.-P.; Durette, C.; Lanoix, J.; Courcelles, M.; et al. Induced Pluripotent Stem Cells Display a Distinct Set of MHC I-Associated Peptides Shared by Human Cancers. Cell Rep. 2022, 40. [Google Scholar] [CrossRef]
- Bertone, S.; Schiavetti, F.; Bellomo, R.; Vitale, C.; Ponte, M.; Moretta, L.; Mingari, M.C. Transforming Growth Factor-Beta-Induced Expression of CD94/NKG2A Inhibitory Receptors in Human T Lymphocytes. Eur. J. Immunol. 1999, 29, 23–29. [Google Scholar] [CrossRef]
- Gunturi, A.; Berg, R.E.; Crossley, E.; Murray, S.; Forman, J. The Role of TCR Stimulation and TGF-Beta in Controlling the Expression of CD94/NKG2A Receptors on CD8 T Cells. Eur. J. Immunol. 2005, 35, 766–775. [Google Scholar] [CrossRef]
- Gooden, M.J.M.; van Hall, T. Infiltrating CTLs Are Bothered by HLA-E on Tumors. Oncoimmunology 2012, 1, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, Y.; Ge, Z.; Zhang, X.; Yan, Y.; Xie, Y. NLRC5 Deficiency Ameliorates Cardiac Fibrosis in Diabetic Cardiomyopathy by Regulating EndMT through Smad2/3 Signaling Pathway. Biochem. Biophys. Res. Commun. 2020, 528, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ni, M.; Li, X.; Meng, X.; Huang, C.; Li, J. NLRC5 Regulates TGF-Β1-Induced Proliferation and Activation of Hepatic Stellate Cells during Hepatic Fibrosis. Int. J. Biochem. Cell Biol. 2016, 70, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Romieu-Mourez, R.; François, M.; Boivin, M.-N.; Stagg, J.; Galipeau, J. Regulation of MHC Class II Expression and Antigen Processing in Murine and Human Mesenchymal Stromal Cells by IFN-γ, TGF-β, and Cell Density1. J. Immunol. 2007, 179, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tang, L.; Letterio, J.J.; Benveniste, E.N. The Smad3 Protein Is Involved in TGF-β Inhibition of Class II Transactivator and Class II MHC Expression1. J. Immunol. 2001, 167, 311–319. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef] [PubMed]
- Brea, E.J.; Oh, C.Y.; Manchado, E.; Budhu, S.; Gejman, R.S.; Mo, G.; Mondello, P.; Han, J.E.; Jarvis, C.A.; Ulmert, D.; et al. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer Immunol. Res. 2016, 4, 936–947. [Google Scholar] [CrossRef]
- Loi, S.; Dushyanthen, S.; Beavis, P.A.; Salgado, R.; Denkert, C.; Savas, P.; Combs, S.; Rimm, D.L.; Giltnane, J.M.; Estrada, M.V.; et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2016, 22, 1499–1509. [Google Scholar] [CrossRef]
- Franklin, D.A.; James, J.L.; Axelrod, M.L.; Balko, J.M. MEK Inhibition Activates STAT Signaling to Increase Breast Cancer Immunogenicity via MHC-I Expression. Cancer Drug Resist. 2020, 3, 603–612. [Google Scholar] [CrossRef]
- Inoue, M.; Mimura, K.; Izawa, S.; Shiraishi, K.; Inoue, A.; Shiba, S.; Watanabe, M.; Maruyama, T.; Kawaguchi, Y.; Inoue, S.; et al. Expression of MHC Class I on Breast Cancer Cells Correlates Inversely with HER2 Expression. Oncoimmunology 2012, 1, 1104–1110. [Google Scholar] [CrossRef]
- Velásquez, L.N.; Milillo, M.A.; Delpino, M.V.; Trotta, A.; Mercogliano, M.F.; Pozner, R.G.; Schillaci, R.; Elizalde, P.V.; Giambartolomei, G.H.; Barrionuevo, P. Inhibition of MHC-I by Brucella Abortus Is an Early Event during Infection and Involves EGFR Pathway. Immunol. Cell Biol. 2017, 95, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Pollack, B.P.; Sapkota, B.; Cartee, T.V. Epidermal Growth Factor Receptor Inhibition Augments the Expression of MHC Class I and II Genes. Clin. Cancer Res. 2011, 17, 4400–4413. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Moasser, M.M. The Epidermal Growth Factor Receptor Family: Biology Driving Targeted Therapeutics. Cell Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The Role of Epidermal Growth Factor Receptor in Cancer Metastasis and Microenvironment. Biomed. Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kerner, Z.J.; Hong, H.; Sun, J. Targeted Cancer Therapy with Tumor Necrosis Factor-Alpha. Biochem. Insights 2008, 2008, 15–21. [Google Scholar] [CrossRef]
- Lorenzi, S.; Forloni, M.; Cifaldi, L.; Antonucci, C.; Citti, A.; Boldrini, R.; Pezzullo, M.; Castellano, A.; Russo, V.; van der Bruggen, P.; et al. IRF1 and NF-KB Restore MHC Class I-Restricted Tumor Antigen Processing and Presentation to Cytotoxic T Cells in Aggressive Neuroblastoma. PLoS ONE 2012, 7, e46928. [Google Scholar] [CrossRef]
- Forloni, M.; Albini, S.; Limongi, M.Z.; Cifaldi, L.; Boldrini, R.; Nicotra, M.R.; Giannini, G.; Natali, P.G.; Giacomini, P.; Fruci, D. NF-ΚB, and Not MYCN, Regulates MHC Class I and Endoplasmic Reticulum Aminopeptidases in Human Neuroblastoma Cells. Cancer Res. 2010, 70, 916–924. [Google Scholar] [CrossRef]
- Nishio, H.; Yaguchi, T.; Sugiyama, J.; Sumimoto, H.; Umezawa, K.; Iwata, T.; Susumu, N.; Fujii, T.; Kawamura, N.; Kobayashi, A.; et al. Immunosuppression through Constitutively Activated NF-ΚB Signalling in Human Ovarian Cancer and Its Reversal by an NF-ΚB Inhibitor. Br. J. Cancer 2014, 110, 2965–2974. [Google Scholar] [CrossRef]
- Sadelain, M.; Rivière, I.; Riddell, S. Therapeutic T Cell Engineering. Nature 2017, 545, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 3 November 2023).
- Zhang, X.-W.; Wu, Y.-S.; Xu, T.-M.; Cui, M.-H. CAR-T Cells in the Treatment of Ovarian Cancer: A Promising Cell Therapy. Biomolecules 2023, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Bang, Y.-J. HER2-Targeted Therapies — a Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Olvera, A.; Dueñas-González, A.; Gallardo-Rincón, D.; Candelaria, M.; De la Garza-Salazar, J. Prognostic, Predictive and Therapeutic Implications of HER2 in Invasive Epithelial Ovarian Cancer. Cancer Treat Rev. 2006, 32, 180–190. [Google Scholar] [CrossRef]
- Sun, M.; Shi, H.; Liu, C.; Liu, J.; Liu, X.; Sun, Y. Construction and Evaluation of a Novel Humanized HER2-Specific Chimeric Receptor. Breast Cancer Res. 2014, 16, R61. [Google Scholar] [CrossRef]
- Chang, K.; Pastan, I. Molecular Cloning of Mesothelin, a Differentiation Antigen Present on Mesothelium, Mesotheliomas, and Ovarian Cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef]
- Ho, M.; Hassan, R.; Zhang, J.; Wang, Q.-C.; Onda, M.; Bera, T.; Pastan, I. Humoral Immune Response to Mesothelin in Mesothelioma and Ovarian Cancer Patients. Clin. Cancer Res. 2005, 11, 3814–3820. [Google Scholar] [CrossRef]
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, D.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-Specific Chimeric Antigen Receptor MRNA-Engineered T Cells Induce Anti-Tumor Activity in Solid Malignancies. Cancer Immunol. Res. 2014, 2, 112–120. [Google Scholar] [CrossRef]
- Rao, T.D.; Tian, H.; Ma, X.; Yan, X.; Thapi, S.; Schultz, N.; Rosales, N.; Monette, S.; Wang, A.; Hyman, D.M.; et al. Expression of the Carboxy-Terminal Portion of MUC16/CA125 Induces Transformation and Tumor Invasion. PLoS ONE 2015, 10, e0126633. [Google Scholar] [CrossRef]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor Biomarker to Cancer Therapy, a Work in Progress. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Chekmasova, A.A.; Rao, T.D.; Nikhamin, Y.; Park, K.J.; Levine, D.A.; Spriggs, D.R.; Brentjens, R.J. Successful Eradication of Established Peritoneal Ovarian Tumors in SCID-Beige Mice Following Adoptive Transfer of T Cells Genetically Targeted to the MUC16 Antigen. Clin. Cancer Res. 2010, 16, 3594–3606. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate Receptor Expression in Carcinomas and Normal Tissues Determined by a Quantitative Radioligand Binding Assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.H.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate Receptor Alpha as a Tumor Target in Epithelial Ovarian Cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Krco, C.J.; Erskine, C.L.; Goodman, K.; Kelemen, L.E.; Wettstein, P.J.; Low, P.S.; Hartmann, L.C.; Kalli, K.R. T-Cell Immunity to the Folate Receptor Alpha Is Prevalent in Women with Breast or Ovarian Cancer. J. Clin. Oncol. 2006, 24, 4254–4261. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A Phase I Study on Adoptive Immunotherapy Using Gene-Modified T Cells for Ovarian Cancer. Clin. Cancer Res. 2006, 12, 6106–6115. [Google Scholar] [CrossRef] [PubMed]
- Ebel, W.; Routhier, E.L.; Foley, B.; Jacob, S.; McDonough, J.M.; Patel, R.K.; Turchin, H.A.; Chao, Q.; Kline, J.B.; Old, L.J.; et al. Preclinical Evaluation of MORAb-003, a Humanized Monoclonal Antibody Antagonizing Folate Receptor-Alpha. Cancer Immun. 2007, 7, 6. [Google Scholar] [PubMed]
- Shimizu, T.; Fujiwara, Y.; Yonemori, K.; Koyama, T.; Sato, J.; Tamura, K.; Shimomura, A.; Ikezawa, H.; Nomoto, M.; Furuuchi, K.; et al. First-in-Human Phase 1 Study of MORAb-202, an Antibody–Drug Conjugate Comprising Farletuzumab Linked to Eribulin Mesylate, in Patients with Folate Receptor-α–Positive Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients with Platinum-Resistant Ovarian Cancer with High Folate Receptor Alpha Expression: Results from the SORAYA Study. JCO 2023, 41, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Martin, L.P.; O’Malley, D.M.; Matulonis, U.A.; Konner, J.A.; Perez, R.P.; Bauer, T.M.; Ruiz-Soto, R.; Birrer, M.J. Safety and Activity of Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha-Targeting Antibody-Drug Conjugate, in Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer: A Phase I Expansion Study. J. Clin. Oncol. 2017, 35, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Matulonis, U.A.; Birrer, M.J.; Castro, C.M.; Gilbert, L.; Vergote, I.; Martin, L.P.; Mantia-Smaldone, G.M.; Martin, A.G.; Bratos, R.; et al. Phase Ib Study of Mirvetuximab Soravtansine, a Folate Receptor Alpha (FRα)-Targeting Antibody-Drug Conjugate (ADC), in Combination with Bevacizumab in Patients with Platinum-Resistant Ovarian Cancer. Gynecol. Oncol. 2020, 157, 379–385. [Google Scholar] [CrossRef]
- Vergote, I.; Armstrong, D.; Scambia, G.; Teneriello, M.; Sehouli, J.; Schweizer, C.; Weil, S.C.; Bamias, A.; Fujiwara, K.; Ochiai, K.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study to Assess Efficacy and Safety of Weekly Farletuzumab in Combination with Carboplatin and Taxane in Patients with Ovarian Cancer in First Platinum-Sensitive Relapse. J. Clin. Oncol. 2016, 34, 2271–2278. [Google Scholar] [CrossRef]
- Shu, R.; Evtimov, V.J.; Hammett, M.V.; Nguyen, N.-Y.N.; Zhuang, J.; Hudson, P.J.; Howard, M.C.; Pupovac, A.; Trounson, A.O.; Boyd, R.L. Engineered CAR-T Cells Targeting TAG-72 and CD47 in Ovarian Cancer. Mol. Ther. Oncolytics. 2021, 20, 325–341. [Google Scholar] [CrossRef]
- Johnson, V.G.; Schlom, J.; Paterson, A.J.; Bennett, J.; Magnani, J.L.; Colcher, D. Analysis of a Human Tumor-Associated Glycoprotein (TAG-72) Identified by Monoclonal Antibody B72.3. Cancer Res. 1986, 46, 850–857. [Google Scholar]
- Chauhan, S.C.; Vinayek, N.; Maher, D.M.; Bell, M.C.; Dunham, K.A.; Koch, M.D.; Lio, Y.; Jaggi, M. Combined Staining of TAG-72, MUC1, and CA125 Improves Labeling Sensitivity in Ovarian Cancer: Antigens for Multi-Targeted Antibody-Guided Therapy. J. Histochem. Cytochem. 2007, 55, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, M.P.; Venkatraman, G.; Singh, A.P.; Chauhan, S.C.; Johansson, S.L.; Jain, M.; Smith, L.; Davis, J.S.; Remmenga, S.W.; Batra, S.K. Expression of TAG-72 in Ovarian Cancer and Its Correlation with Tumor Stage and Patient Prognosis. Cancer Lett. 2007, 251, 247–257. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.M.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- Tinker, A.V.; Hirte, H.W.; Provencher, D.; Butler, M.; Ritter, H.; Tu, D.; Azim, H.A.; Paralejas, P.; Grenier, N.; Hahn, S.-A.; et al. Dose-Ranging and Cohort-Expansion Study of Monalizumab (IPH2201) in Patients with Advanced Gynecologic Malignancies: A Trial of the Canadian Cancer Trials Group (CCTG): IND221. Clin. Cancer Res. 2019, 25, 6052–6060. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Oaknin, A.; Sanchez-Simon, I.; Salgado, A.C.; Patel, S.P.; Oza, A.; Das, M.; Kourtesis, P.; Ascierto, M.L.; Diamond, J.R. 518 Phase 1B Trial of Monalizumab (NKG2A Inhibitor) plus Durvalumab: Safety and Efficacy in Patients with Metastatic Ovarian, Cervical, and Microsatellite-Stable Endometrial Cancers. Int. J. Gynecol. Cancer 2020, 30. [Google Scholar] [CrossRef]
- Jan, C.-I.; Huang, S.-W.; Canoll, P.; Bruce, J.N.; Lin, Y.-C.; Pan, C.-M.; Lu, H.-M.; Chiu, S.-C.; Cho, D.-Y. Targeting Human Leukocyte Antigen G with Chimeric Antigen Receptors of Natural Killer Cells Convert Immunosuppression to Ablate Solid Tumors. J. Immunother. Cancer 2021, 9, e003050. [Google Scholar] [CrossRef]
- Stone, M.L.; Chiappinelli, K.B.; Li, H.; Murphy, L.M.; Travers, M.E.; Topper, M.J.; Mathios, D.; Lim, M.; Shih, I.-M.; Wang, T.-L.; et al. Epigenetic Therapy Activates Type I Interferon Signaling in Murine Ovarian Cancer to Reduce Immunosuppression and Tumor Burden. Proc. Natl. Acad. Sci. USA 2017, 114, E10981–E10990. [Google Scholar] [CrossRef] [PubMed]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via DsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.L.; Chiappinelli, K.B.; Li, H.; Murphy, L.M.; Travers, M.E.; Topper, M.J.; Mathios, D.; Lim, M.; Shih, I.-M.; Wang, T.-L.; et al. Reply to Haffner et al.: DNA Hypomethylation Renders Tumors More Immunogenic. Proc. Natl. Acad. Sci. USA 2018, 115, E8583–E8584. [Google Scholar] [CrossRef] [PubMed]
- Moufarrij, S.; Srivastava, A.; Gomez, S.; Hadley, M.; Palmer, E.; Austin, P.T.; Chisholm, S.; Diab, N.; Roche, K.; Yu, A.; et al. Combining DNMT and HDAC6 Inhibitors Increases Anti-Tumor Immune Signaling and Decreases Tumor Burden in Ovarian Cancer. Sci. Rep. 2020, 10, 3470. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.B.; Meza-Perez, S.; Londoño, A.; Katre, A.; Peabody, J.E.; Smith, H.J.; Forero, A.; Norian, L.A.; Straughn, J.M.; Buchsbaum, D.J.; et al. Epigenetic Modifiers Upregulate MHC II and Impede Ovarian Cancer Tumor Growth. Oncotarget 2017, 8, 44159–44170. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Rasco, D.W.; Heath, E.I.; Munster, P.N.; Schellens, J.H.M.; Isambert, N.; Tourneau, C.L.; O’Neil, B.; Mathijssen, R.H.J.; Lopez-Martin, J.A.; et al. Phase I Study of CC-486 Alone and in Combination with Carboplatin or Nab-Paclitaxel in Patients with Relapsed or Refractory Solid Tumors. Clin. Cancer Res. 2018, 24, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Loo Yau, H.; Chakravarthy, A.; Wang, B.; Shen, S.Y.; Ettayebi, I.; Ishak, C.A.; Bedard, P.L.; Abdul Razak, A.; R Hansen, A.; et al. An Open-Label, Phase II Multicohort Study of an Oral Hypomethylating Agent CC-486 and Durvalumab in Advanced Solid Tumors. J. Immunother. Cancer 2020, 8, e000883. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Stewart, T.A.; Thompson, E.W.; Monteith, G.R. Targeting EMT in Cancer: Opportunities for Pharmacological Intervention. Trends Pharmacol. Sci. 2014, 35, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs. 2021, 211, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Sun, T. Editorial: Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target in Cancer. Front. Oncol. 2023, 13. [Google Scholar]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of Epithelial-Mesenchymal Transition through Epigenetic and Post-Translational Modifications. Mol. Cancer 2016, 15. [Google Scholar] [CrossRef]
- Wu, S.-M.; Jan, Y.-J.; Tsai, S.-C.; Pan, H.-C.; Shen, C.-C.; Yang, C.-N.; Lee, S.-H.; Liu, S.-H.; Shen, L.-W.; Chiu, C.-S.; et al. Targeting Histone Deacetylase-3 Blocked Epithelial-Mesenchymal Plasticity and Metastatic Dissemination in Gastric Cancer. Cell Biol. Toxicol. 2023, 39, 1873–1896. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial–Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, Cancer Stem Cells and Drug Resistance: An Emerging Axis of Evil in the War on Cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hernando-Calvo, A.; Carvajal, R.D.; Morris, V.K.; Paik, P.K.; Zandberg, D.P.; Kaczmar, J.M.; Niculescu, L.; Bohr, D.; Reiners, R.; et al. A Phase 1 Trial of the Bifunctional EGFR/TGFβ Fusion Protein BCA101 Alone and in Combination with Pembrolizumab in Patients with Advanced Solid Tumors. JCO 2022, 40, 2513. [Google Scholar] [CrossRef]
- Kim, C.; Liu, S.V.; Crawford, J.; Torres, T.; Chen, V.; Thompson, J.; Tan, M.; Esposito, G.; Subramaniam, D.S.; Giaccone, G. A Phase I Trial of Dasatinib and Osimertinib in TKI Naïve Patients with Advanced EGFR-Mutant Non-Small-Cell Lung Cancer. Front. Oncol. 2021, 11, 728155. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Thoma, C.; Goodall, R.J.; Lyons, T.J.; Gaitskell, K.; Wiggans, A.J.; Bryant, A. Epidermal Growth Factor Receptor Blockers for the Treatment of Ovarian Cancer. Cochrane Database Syst. Rev. 2018, 2018, CD007927. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Dumbrava, E.I.; Jiang, Y.; Thein, K.Z.; Naing, A.; Hong, D.S.; Fu, S.; Piha-Paul, S.A.; Tsimberidou, A.M.; Janku, F.; et al. Dual EGFR Blockade with Cetuximab and Erlotinib Combined with Anti-VEGF Antibody Bevacizumab in Advanced Solid Tumors: A Phase 1 Dose Escalation Triplet Combination Trial. Exp. Hematol. Oncol. 2020, 9, 7. [Google Scholar] [CrossRef]
- Seo, J.; Ha, J.; Kang, E.; Cho, S. The Role of Epithelial–Mesenchymal Transition-Regulating Transcription Factors in Anti-Cancer Drug Resistance. Arch. Pharm. Res. 2021, 44, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Kalafut, J.; Okon, E.; Czapinski, J.; Halasa, M.; Przybyszewska, A.; Miziak, P.; Okla, K.; Rivero-Muller, A.; Stepulak, A. Histone Deacetylase Inhibitors and Phenotypical Transformation of Cancer Cells. Cancers 2019, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Boulding, T.; McCuaig, R.D.; Tan, A.; Hardy, K.; Wu, F.; Dunn, J.; Kalimutho, M.; Sutton, C.R.; Forwood, J.K.; Bert, A.G.; et al. LSD1 Activation Promotes Inducible EMT Programs and Modulates the Tumour Microenvironment in Breast Cancer. Sci. Rep. 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Martín-Otal, C.; Lasarte-Cia, A.; Serrano, D.; Casares, N.; Conde, E.; Navarro, F.; Sánchez-Moreno, I.; Gorraiz, M.; Sarrión, P.; Calvo, A.; et al. Targeting the Extra Domain A of Fibronectin for Cancer Therapy with CAR-T Cells. J. Immunother. Cancer 2022, 10, e004479. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Lu, Z.-R. Targeting Fibronectin for Cancer Imaging and Therapy. J. Mater. Chem. B Mater. Biol. Med. 2017, 5, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, Q.; Liu, Y.; Gao, Y.; Qu, Z.; Mo, L.; Xu, Y.; Chen, R.; Shi, L. A Small Vimentin-Binding Molecule Blocks Cancer Exosome Release and Reduces Cancer Cell Mobility. Front. Pharmacol. 2021, 12, 627394. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ye, M.; Zhou, J.; Wang, Z.; Zhu, X. Targeting E-Cadherin Expression with Small Molecules for Digestive Cancer Treatment. Am. J. Transl. Res. 2019, 11, 3932–3944. [Google Scholar] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-Cadherin in Cancer Metastasis, Its Emerging Role in Haematological Malignancies and Potential as a Therapeutic Target in Cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [PubMed]
- Dausset, J. Iso-leuco-anticorps. Acta Haematol. 1958, 20, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.; Rolfs, M.R. Fetomaternal Leukocyte Incompatibility. J. Clin. Invest. 1958, 37, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Van Rood, J.J.; Eernisse, J.G.; Van Leeuwen, A. Leucocyte Antibodies in Sera from Pregnant Women. Nature 1958, 181, 1735–1736. [Google Scholar] [CrossRef] [PubMed]
- Thorsby, E. A Short History of HLA. Tissue Antigens. 2009, 74, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Harndahl, M.; Rasmussen, M.; Roder, G.; Dalgaard Pedersen, I.; Sørensen, M.; Nielsen, M.; Buus, S. Peptide-MHC Class I Stability Is a Better Predictor than Peptide Affinity of CTL Immunogenicity. Eur. J. Immunol. 2012, 42, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, F.; Bazzaro, M.; Canella, A.; Marastoni, M.; Traniello, S.; Gavioli, R. The lifespan of major histocompatibility complex class I/peptide complexes determines the efficiency of cytotoxic T-lymphocyte responses. Immunology 1999, 96, 411–415. [Google Scholar] [CrossRef]
- Bassani-Sternberg, M.; Pletscher-Frankild, S.; Jensen, L.J.; Mann, M. Mass Spectrometry of Human Leukocyte Antigen Class I Peptidomes Reveals Strong Effects of Protein Abundance and Turnover on Antigen Presentation*[S]. Mol. Cell. Proteom. 2015, 14, 658–673. [Google Scholar] [CrossRef]
- Kraya, A.A.; Maxwell, K.N.; Eiva, M.A.; Wubbenhorst, B.; Pluta, J.; Feldman, M.; Nayak, A.; Powell, D.J.; Domchek, S.M.; Vonderheide, R.H.; et al. PTEN Loss and BRCA1 Promoter Hypermethylation Negatively Predict for Immunogenicity in BRCA-Deficient Ovarian Cancer. JCO Precis. Oncol. 2022, 6, e2100159. [Google Scholar] [CrossRef] [PubMed]
- Chowell, D.; Morris, L.G.T.; Grigg, C.M.; Weber, J.K.; Samstein, R.M.; Makarov, V.; Kuo, F.; Kendall, S.M.; Requena, D.; Riaz, N.; et al. Patient HLA Class I Genotype Influences Cancer Response to Checkpoint Blockade Immunotherapy. Science 2018, 359, 582–587. [Google Scholar] [CrossRef]
- Brunekreeft, K.L.; Paijens, S.T.; Wouters, M.C.A.; Komdeur, F.L.; Eggink, F.A.; Lubbers, J.M.; Workel, H.H.; Van Der Slikke, E.C.; Pröpper, N.E.J.; Leffers, N.; et al. Deep Immune Profiling of Ovarian Tumors Identifies Minimal MHC-I Expression after Neoadjuvant Chemotherapy as Negatively Associated with T-Cell-Dependent Outcome. Oncoimmunology 2020, 9, 1760705. [Google Scholar] [CrossRef]
- Stevenson, J.P.; Kindler, H.L.; Papasavvas, E.; Sun, J.; Jacobs-Small, M.; Hull, J.; Schwed, D.; Ranganathan, A.; Newick, K.; Heitjan, D.F.; et al. Immunological Effects of the TGFβ-Blocking Antibody GC1008 in Malignant Pleural Mesothelioma Patients. Oncoimmunology 2013, 2, e26218. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Golan, T.; Ikeda, M.; Milella, M.; Taieb, J.; Wainberg, Z.A.; Wang, L.-W.; Gyambibi, N.; Lopez-Martin, E.M.; Xu, K.; et al. Phase III Study (DaNIS-2) of the Anti–TGF-β Monoclonal Antibody (MAb) NIS793 with Nab-Paclitaxel/Gemcitabine (NG) versus NG Alone in Patients (Pts) with First-Line Metastatic Pancreatic Ductal Adenocarcinoma (MPDAC). JCO 2022, 40, TPS4193. [Google Scholar] [CrossRef]
- Pesch, A.M.; Pierce, L.J.; Speers, C.W. Modulating the Radiation Response for Improved Outcomes in Breast Cancer. JCO Precis. Oncol. 2021. [Google Scholar] [CrossRef]
- Kaczmar, J.M.; Zandberg, D.P.; Wong, D.J.L.; Yilmaz, E.; Sherman, E.J.; Hernando-Calvo, A.; Sacco, A.G.; Chung, C.H.; Bohr, D.; Reiners, R.; et al. Dose Expansion Results of the Bifunctional EGFR/TGFβ Inhibitor BCA101 with Pembrolizumab in Patients with Recurrent, Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2023. [Google Scholar] [CrossRef]

