1. Introduction
Periodontitis is an inflammatory disease of all structures of the periodontium, maintained by microorganisms in the subgingival biofilm. Inflammatory changes can lead to loss of bone, collagen, and attachment. In advanced disease and without adequate treatment, the consequence may be loss of the affected teeth. Irreversible periodontal damage is usually preceded by reversible gingivitis due to dysbiotic microbial colonization of the tooth and/or gum.
In addition to this basic condition, systemic diseases represent cofactors for periodontitis, and some of these may in turn be influenced by the periodontitis itself. These systemic diseases include, for example, diabetes mellitus, cardiovascular disease (such as coronary artery disease), and prematurity [1,2,3].
In particular, the bidirectionality between diabetes mellitus and periodontitis has been well studied. Various clinical studies have shown that periodontitis is more common in diabetics than in non-diabetics [4,5,6]. Periodontitis, in turn, may also influence diabetes mellitus. For example, the risk of diabetics dying from coronary heart disease or nephropathy is significantly higher if they have advanced periodontitis [7].
In addition to diabetes, many studies have also shown an association between periodontitis and coronary heart disease [2,8,9].
Recently, in addition to the systemic diseases already mentioned, a link between periodontitis and Alzheimer’s disease (AD) has also been discussed [10,11,12,13]. A prospective clinical study showed that active, chronic periodontitis was associated with a decline in mental abilities in nursing home residents [14]. In particular, Porphyromonas gingivalis (P. gingivalis), one of the main pathobionts involved in periodontitis, appears to play a key role in this regard [11,15,16]. Thus, it is clear from these examples that periodontal health is closely related to systemic health.
Removal of the soft biofilm or mineralized deposits adhering to the tooth surface and thus the removal of periodontal pathogenic microorganisms (pathobionts) by scaling and root planing (SRP) is one of the main objectives of nonsurgical periodontal therapy and supportive periodontal therapy [17], regardless of the systemic factors that influence the event. Conventional methods such as the use of manual or ultrasound instruments are available for this purpose, and their effectiveness is considered to be comparable [18,19]. Another option is the use of air polishing systems or laser systems for cleaning affected tooth surfaces [20,21,22,23]. However, the benefit of root surface cleaning in periodontal therapy, e.g., with the Er:YAG laser, remains questionable due to the high heterogeneity of clinical studies [24]. Generally, it is possible to achieve a reduction in periodontal pathobionts using the various methods of surface instrumentation [25,26]. It cannot be assumed that complete removal of pathogens is achieved. Even the additional local application of an antibiotic—for example, using tetracycline-releasing sutures [27] or doxycycline-releasing gels [28,29]—cannot guarantee that all pathogens will be permanently removed. Furthermore, especially in advanced cases with high probing depths (≥6 mm), furcation involvement or, for example, root concavities, it becomes difficult or even impossible to perform this cleaning successfully through nonsurgical approaches alone; here, it then makes sense to consider surgical approaches (resective or regenerative) [30].
During such cleaning of the tooth or root surfaces, which is an invasive procedure, there is a risk of bacteremia [31,32,33]. In most cases, this bacteremia has no (or unnoticed) clinical relevance in the majority of patients. However, such bacteremia can cause dangerous infections in patients who have heart valve replacements or are immunosuppressed. Here, antibiotic prophylaxis and/or removal of the pathobionts makes all the more sense [34]. A direct effect of these bacteremia during nonsurgical periodontal therapy on other systemic diseases, as mentioned above, has not yet been proven. However, this topic itself should continue to be critically observed scientifically.
Another effective approach to the elimination of pathobionts could be the use of light energy, in the form of lasers. Diode lasers achieve particularly good results in adjunctive periodontal therapy [35,36]. They also appear to lead to the effective elimination of periodontal microorganisms. One study showed, in a split-mouth design, that the use of a diode laser (810 nm) before ultrasound scaling in patients with gingivitis resulted in a significant reduction in the prevalence of odontogenic bacteremia [37].
The severity of periodontal disease can also be exacerbated by specific genotypes that influence the immune response, with a well-known example being the interleukin-1 polymorphism. Likewise, periodontitis may start significantly earlier in such patients than in patients without this polymorphism [38]. In addition to the bactericidal effects of lasers, it is also plausible to consider photobiomodulatory effects, especially with blue and green laser light, which may have a positive influence on the immune response and post operative inflammation and wound healing [39]. A current approach in this context involves the use of a novel diode laser with a wavelength of 445 nm. In an in vitro study, this wavelength was shown to effectively reduce a broad spectrum of pathobionts, including Aggregatibacter actinomycetemcomitans, P. gingivalis, Staphylococcus aureus, and Candida albicans, with a relatively short application time and low total energy [40]. This approach needs to be investigated further. The aim of the present feasibility study was therefore to evaluate the bacteremia-preventive effect of disinfection with a diode laser before conventional nonsurgical periodontal therapy—testing the hypothesis that a bacteremia-preventive effect of laser irradiation prior to periodontal treatment can be demonstrated with the study design used.
2. Results
The present feasibility study demonstrated that the study design is capable of detecting pathobionts that had entered the bloodstream due to therapy-induced bacteremia. The laser parameters used also demonstrated a bacteremia-preventive effect.
2.1. Clinical Parameters
2.2. Microbiological and Immunological Parameters
3. Discussion
The present feasibility study demonstrated that the use of a diode laser with a wavelength of 445 nm has potential bacteremia-preventive effects when applied immediately before SRP. However, it must be clearly stated that this effect needs to be further investigated and proven in follow-up studies with larger numbers of patients. During periodontal treatment, transient bacteremia inevitably occurs. The incidence of bacteremia depends on the degree of periodontal inflammation [31]. Kinane et al. detected bacteremia in 13% of patients after ultrasound scaling and in 20% after periodontal probing [33]. In comparison with the present feasibility study, the blood samples in that study were only taken after the periodontal procedures, so not all bacteremia may have been detected due to phagocytosis of the bacteria by immune cells. In another study, bacteremia was detected in 73.8% of patients immediately after SRP, and 19% of the blood cultures were still positive 30 min after the completion of SRP [41]. Zhang et al. detected bacteremia in 33.3% of patients 5 min after the start of SRP [42]. These studies all detected bacteremia, but with marked differences in prevalence. This may be mainly due to the different methods used and different collection times [32]. To avoid missing bacteremia as much as possible, the collection times for blood cultures in the present study design were adjusted to follow the schedule described by Beutler et al. [43]. In contrast with most studies, the present feasibility study not only investigated the presence of bacteria in blood, but also took sulcus samples to demonstrate that the blood isolates actually originated from the periodontal pockets.
It has been shown that cleaning all quadrants of a dentition within 24 h resulted in an increased acute-phase response, in comparison to cleaning quadrants in individual sessions with a time interval of 1 week between each session [44]. However, periodontal microorganisms can enter the bloodstream not only during periodontal therapy, but also as a result of professional mechanical plaque removal, or through micro-injuries from mere chewing or tooth-brushing, in accordance with the rule that the more severe the periodontitis, the more severe the bacteremia [31,43,45]. In most cases, this is assumed to have no clinical consequences (or only unrecognized ones), but in vulnerable patient groups such as patients with heart valve replacements, there may be serious sequelae. One potential sequela is infective endocarditis, which should be avoided in such cases using antibiotic prophylaxis [46]. Clear disadvantages of systemic antibiotic prophylaxis are its effects on the whole body, including the commensal microbiome and increasing resistance rates. Employing a laser device, we do not assume that complete sterilization of the periodontal pocket is possible, but we hypothesize that at least such a temporary bacterial reduction in pockets is possible with laser disinfection through which bacteremia can be avoided.
In addition to the administration of systemic antibiotics to reduce or prevent transient bacteremia, local disinfection methods may also be an option. These include, for example, mouth rinses. However, a single mouth rinse containing chlorhexidine before scaling did not show any effect on bacteremia reduction in one study [47]. In addition to oral rinses, local laser administration may also be considered. In a clinical study, a diode laser with a wavelength of 810 nm was used before ultrasonic scaling in 22 patients with gingivitis, also with a split-mouth research design. Bacteremia was detected in 15 patients after ultrasound scaling alone, and after laser treatment in only eight patients (810 nm; flexible fiber optic 300 µm; repeated beam: 0.2 s on and 0.3 s off; output power 1.0 W; laser application duration for each tooth: 15 s) before ultrasound scaling [37].
In the present study, a laser wavelength that is effective against a broad spectrum of pathobionts—including A. actinomycetemcomitans, P. gingivalis, S. aureus, und C. albicans—was used for a bacteremia reduction approach. The wavelength used was 445 nm at a power of 0.5 W, and the laser was used in continuous-wave mode with a 320 µm fiber for 1 min for each tooth. In a pilot study, these parameters showed significant reductions in P. gingivalis in comparison with a control group in vitro [40]. This wavelength has a very high absorption level in the spectrum of hemoglobin and pigmented bacteria, which may explain the strong reduction in P. gingivalis. As this was a feasibility study and data were generated from only two patients, P. gingivalis was detected in sulcus samples in only one patient. The bacteremia-preventive properties of the laser treatment studied here might be of interest not only for vulnerable patient groups, but also for healthy patients. As mentioned above, periodontitis and Alzheimer’s disease have recently been associated. In particular, lipopolysaccharides of P. gingivalis have been detected in the brains of deceased patients with Alzheimer’s disease [48]. The laser treatment described here might be suitable for avoiding or at least reducing this type of bacterial migration as a result of periodontal therapy, thus potentially reducing the risk of systemic diseases.
As mentioned above, Graziani et al. demonstrated that the acute-phase response was greater in full-mouth nonsurgical therapy than in quadrant nonsurgical therapy [44]. Whether the same difference existed between the two experimental groups represented in the present study was also investigated. Unfortunately, due to the small number of patients, no clear trends can be demonstrated. In patient 1, IL-6 increased slightly 24 h after conventional treatment, but this was not detected 24 h after the appointment involving laser treatment; however, there was a transient increase in TNF-α 25 min after the start of SRP, which decreased again in the subsequent examinations. In patient 2, their TNF-α levels were already elevated at baseline and did not change during the course of the examinations on both dates. The other parameters also did not show any clear trends. Follow-up studies with larger numbers of patients may clarify whether laser disinfection with the corresponding reduction in, or prevention of, bacteremia is also associated with a lower acute-phase response.
Overall, despite the small number of patients and the limitations of this feasibility study, it can be concluded that the study protocol is capable of detecting bacteremia after periodontal therapy and that the laser disinfection method described could have potential bacteremia-reducing or even bacteremia-preventive properties. This needs to be confirmed in follow-up studies, which are currently underway.
4. Materials and Methods
4.1. Clinical Parameters
As a preparatory measure, situation models of both jaws in each patient were made in order to produce individual splints. These covered the occlusal surfaces and extended to the equator of all teeth. At six locations (mesio-vestibular, central vestibular, disto-vestibular, mesio-oral, central oral, and disto-oral), milled guiding grooves were used for defined insertion of a pressure-calibrated probe (Aesculap DB764R, Aesculap, Tuttlingen, Germany) into the gingival pocket. Using the individual splints produced in this way, the pocket depths, periodontal recession, clinical attachment level, and bleeding on probing were determined at the various examination time points by one investigator (P.J.). Bleeding points occurring within 30 s of probing were documented and reported as a percentage for the entire dentition. In addition, furcation involvement and the degree of looseness were recorded.
Pain sensation during treatment was assessed by the patients using a visual analog scale after the treatment session.
Body temperature was measured before and after treatment using an infrared thermometer in the ear (Thermoscan PRO 6000, Braun, Kronberg im Taunus, Germany).
4.2. Microbiological and Immunological Parameters
For local microbiological diagnosis, samples were taken using sterile paper points (ISO 45) in the two deepest pockets of each quadrant (eight in total) at baseline, 7 days after the first appointment/SRP, at the second appointment/SRP (14 days after the first SRP), and 7 days after the second appointment/SRP. These were then analyzed by culturing on various media (with and without the addition of sheep blood) at 37 °C in appropriate atmospheric conditions and identified by Maldi-TOF (mass spectrometry).
To be able to detect systemic bacteremia after the treatments and compare it with the baseline findings, peripheral blood (25 mL) was drawn before the start of treatment, 25 min after the start, and 10 min after the end of the treatment in both the control group and the experimental group by one investigator (J.-S.W.). The patients fasted for this purpose to ensure standardization and the better accessibility of microbiological and immunological parameters. Peripheral blood cultures were analyzed using standard operational procedures by the laboratory diagnostic center at the University Hospital in Aachen, Germany. However, the blood cultures (aerobic and anaerobic) were kept for longer (maximum 21 days) and were plated several times to avoid overlooking any slow-growing species, with special emphasis on P. gingivalis and other black-pigmented bacteria. It should be noted that not every bacterial cell entering the bloodstream can be detected using this (or any) method, as most are very rapidly phagocytosed and thus killed and eliminated.
In addition, the samples were analyzed for immunological parameters in blood plasma to allow conclusions to be drawn concerning the acute-phase reaction. C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) were of particular interest. These parameters were also monitored 24 h after the respective treatment appointments.
The blood samples mentioned above were obtained from the median cubital veins. The areas of the puncture were carefully disinfected beforehand using a skin antiseptic (Octeniderm, Schülke, Norderstedt, Germany). For this purpose, the investigator responsible for blood sampling wore sterile gloves, which he kept on for the entire blood-sampling procedure. The puncture procedure was repeated at each blood collection time point. These precautions were implemented to minimize the possibility of contamination of the blood samples with skin commensals. The blood collection procedures themselves were performed with a Safety Multifly cannula (Sarstedt, Nürnbrecht, Germany). A blood culture adapter (Sarstedt, Nürnbrecht, Germany) was used for blood cultures; the aerobic blood culture bottle (Bactec Plus Aerobic/F Culture Vials 30 mL, Becton Dickinson, Franklin Lakes, NJ, USA) was inoculated first, followed by the anaerobic blood culture bottle (Bactec Lytic/10 Anaerobic/F Culture Vials 40 mL, Becton Dickinson, Franklin Lakes, NJ, USA). A blood collection system was then used for the immunological parameters (S-Monovette, Sarstedt, Nürnbrecht, Germany). The blood culture and immunological analyses were conducted in the laboratory diagnostic center at RWTH Aachen University Hospital, and the other microbiological analyses were conducted in the Division of Oral Microbiology and Immunology, Clinic for Operative Dentistry, RWTH Aachen University.
4.3. Treatment Procedure
Treatments in this study were performed using a single-blinded, randomized, split-mouth design. Which quadrants received the conventional treatment (control quadrants) and which quadrants received experimental laser disinfection before SRP were selected in advance using a randomized computer-generated random table. The patients did not know which quadrants were to receive the active laser disinfection, since the control quadrants received inactive laser disinfection—where the laser tip was guided into the sulcus without being switched on. To exclude potential bias, the physicians who performed SRP and the measurements were also not aware of which quadrants were to receive the actual laser disinfection. This was ensured by having a separate practitioner performing the laser disinfection procedure (A.B.).
Before conventional pocket cleaning, the periodontal pockets in the experimental study group were disinfected with a diode laser (SiroLaser Blue, Dentsply Sirona, Bensheim, Germany). The wavelength was 445 nm and the power was 0.5 W, with the laser in continuous-wave mode (laser class 4). A 320 µm fiber was used to disinfect/clean circularly around the diseased tooth for 1 min under constant movement. The laser light used was guided into the gingival pocket with an optical fiber. The device setting of 0.5 W corresponded to an effective power of 0.48 W. Assuming a Gaussian laser beam profile, the power density was 1210 W/cm2 (fiber tip diameter 0.32 mm; 0.48 W output power). Due to the low power density, this laser light is not harmful to the gums or other surrounding tissue. However, laser safety goggles must be worn during the irradiation procedure to protect the eyes.
All of the teeth with periodontal disease were then treated with manual instruments (Gracey curettes, Hu-Friedy, Leimen, Germany) and a piezoelectric scaler (Vector, Dürr Dental, Bietigheim-Bissingen, Germany) with a slimline scaler tip. The treatment end point was the clinically assessed absence of concrements on the root surfaces. This was assessed by a dentist who was experienced in periodontal treatment (F.K.), who was not aware of which teeth had received active or inactive laser disinfection.
Figure 2 shows the sequence of individual steps in more detail.
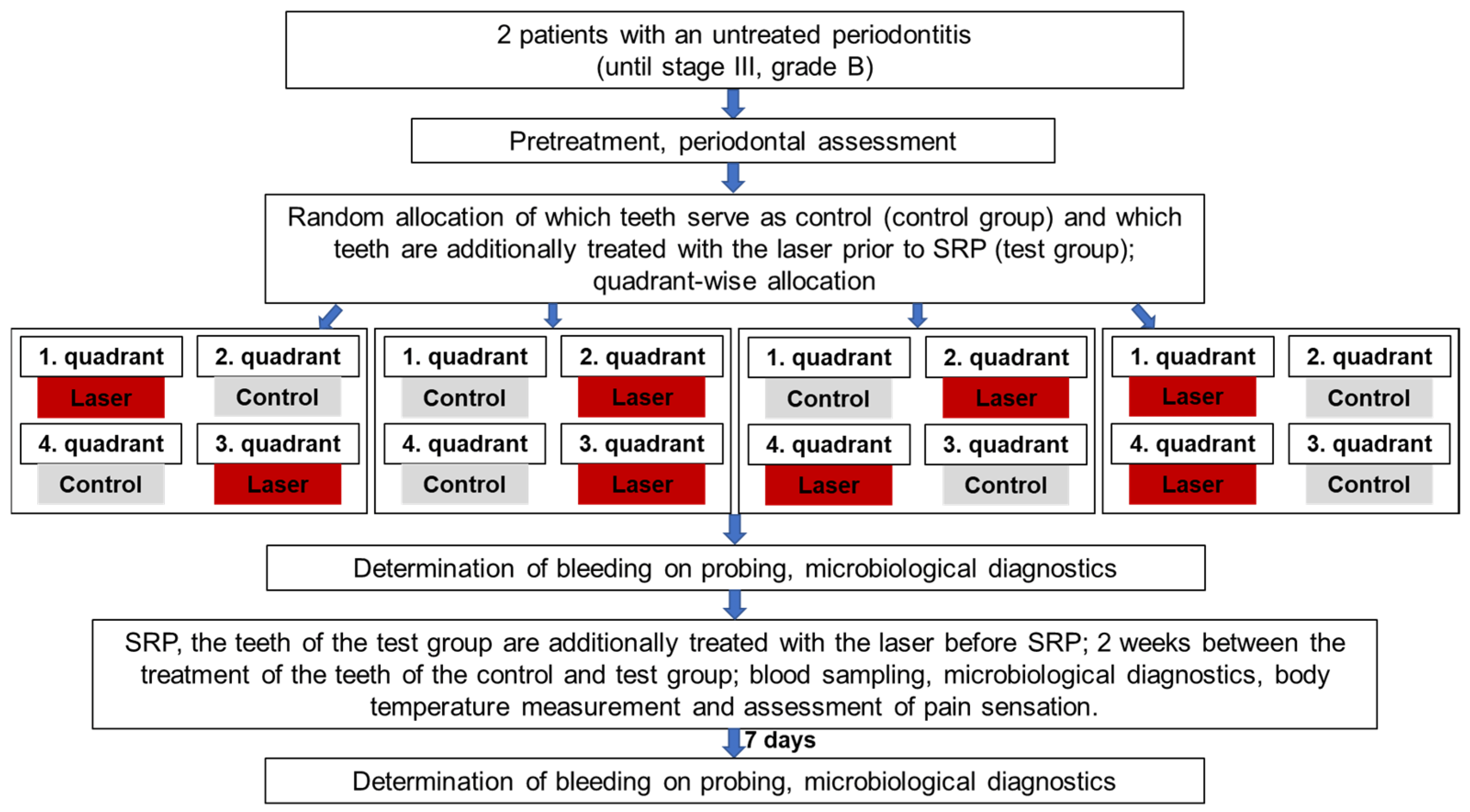
Figure 2. Flowchart for the study design.
4.4. Patients
Two patients (two men, one 39 years of age and the other 63) with untreated generalized periodontitis (stage III, grade B) were recruited in the Clinic of Operative Dentistry, Periodontology, and Preventive Dentistry at Aachen University Hospital. The inclusion criteria were a minimum age of 18 years, no periodontal treatment in the previous 2 years, generalized periodontitis up to stage III, grade B, and the provision of written informed consent. The exclusion criteria were patients who had already received periodontal treatment within the previous 2 years, pregnancy, tobacco use, dental implants, metabolic diseases with possible influence on the healing process (especially diabetes), infectious diseases (such as human immunodeficiency virus or hepatitis B or C), patients undergoing chemotherapy, cystic fibrosis, and antibiotic treatment within the previous 3 months. All patients received detailed information regarding the conduction of the study and data protection. Each of the participants gave written informed consent to participate in the study. In the test plan of the present study submitted to the local ethics committee, particular attention was paid to benefit–risk assessment, the additional study-related measures (blood sampling and laser disinfection), and the use of the laser within its approval. The local ethics committee of the RWTH Aachen University Hospital saw/sees no concerns regarding the research project from an ethical and professional perspective. The study was conducted in full accordance with local and global ethical guidelines (World Medical Association Declaration of Helsinki, version X, 2013) and approved by the local ethics committee (reference number: EK 197/21; date of approval 17 August 2021).
4.5. Statistical Analysis
In this feasibility study, descriptive statistical analysis methods were mainly used for the two patients included. Null hypothesis significance testing is not appropriate unless the sample size is properly powered [49].
5. Conclusions
This feasibility study showed that the study protocol appears to be suitable for detecting bacteremia during periodontal therapy. Initial indications of a bacteremia-reducing or even bacteremia-preventive effect of the laser disinfection method described were observed. However, due to the small number of patients, this effect must continue to be investigated and proven in follow-up studies with larger numbers of patients.
References
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral. Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Blaizot, A.; Vergnes, J.N.; Nuwwareh, S.; Amar, J.; Sixou, M. Periodontal diseases and cardiovascular events: Meta-analysis of observational studies. Int. Dent. J. 2009, 59, 197–209. [Google Scholar] [PubMed]
- Manrique-Corredor, E.J.; Orozco-Beltran, D.; Lopez-Pineda, A.; Quesada, J.A.; Gil-Guillen, V.F.; Carratala-Munuera, C. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019, 47, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontol. 2000 2007, 44, 127–153. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Foster, N.; Taylor, J.J. Cross-susceptibility between periodontal disease and type 2 diabetes mellitus: An immunobiological perspective. Periodontol. 2000 2007, 45, 138–157. [Google Scholar] [CrossRef]
- Salvi, G.E.; Carollo-Bittel, B.; Lang, N.P. Effects of diabetes mellitus on periodontal and peri-implant conditions: Update on associations and risks. J. Clin. Periodontol. 2008, 35, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Nelson, R.G.; Tulloch-Reid, M.; Hanson, R.L.; Sievers, M.L.; Taylor, G.W.; Shlossman, M.; Bennett, P.H.; Genco, R.; Knowler, W.C. Periodontal disease and mortality in type 2 diabetes. Diabetes Care 2005, 28, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Mustapha, I.Z.; Debrey, S.; Oladubu, M.; Ugarte, R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: A systematic review and meta-analysis. J. Periodontol. 2007, 78, 2289–2302. [Google Scholar] [CrossRef]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A., 3rd; Dietrich, T.; Garcia, R.I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc. 2010, 58, 713–718. [Google Scholar] [CrossRef]
- Matsushita, K.; Yamada-Furukawa, M.; Kurosawa, M.; Shikama, Y. Periodontal Disease and Periodontal Disease-Related Bacteria Involved in the Pathogenesis of Alzheimer’s Disease. J. Inflamm. Res. 2020, 13, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.M.; Borrell, L.N.; Papapanou, P.N.; Elkind, M.S.; Scarmeas, N.; Wright, C.B. Periodontitis is associated with cognitive impairment among older adults: Analysis of NHANES-III. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.S.; Desrosiers, M.; Donegan, S.J.; Yepes, J.F.; Kryscio, R.J. Tooth loss, dementia and neuropathology in the Nun study. J. Am. Dent. Assoc. 2007, 138, 1314–1382. [Google Scholar] [CrossRef]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [PubMed]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Singhrao, S.K.; Harding, A.; Poole, S.; Kesavalu, L.; Crean, S. Porphyromonas gingivalis Periodontal Infection and Its Putative Links with Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 137357. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Arguello, E.I.; Ximenez-Fyvie, L.A.; Socransky, S.S. Controlling the plaque biofilm. Int. Dent. J. 2003, 53 (Suppl. 3), 191–199. [Google Scholar] [CrossRef]
- Drisko, C.L.; Cochran, D.L.; Blieden, T.; Bouwsma, O.J.; Cohen, R.E.; Damoulis, P.; Fine, J.B.; Greenstein, G.; Hinrichs, J.; Somerman, M.J.; et al. Position paper: Sonic and ultrasonic scalers in periodontics. Research, Science and Therapy Committee of the American Academy of Periodontology. J. Periodontol. 2000, 71, 1792–1801. [Google Scholar] [CrossRef]
- Tunkel, J.; Heinecke, A.; Flemmig, T.F. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 72–91. [Google Scholar] [CrossRef]
- Petersilka, G.J.; Tunkel, J.; Barakos, K.; Heinecke, A.; Haberlein, I.; Flemmig, T.F. Subgingival plaque removal at interdental sites using a low-abrasive air polishing powder. J. Periodontol. 2003, 74, 307–311. [Google Scholar] [CrossRef]
- Schwarz, F.; Bieling, K.; Venghaus, S.; Sculean, A.; Jepsen, S.; Becker, J. Influence of fluorescence-controlled Er:YAG laser radiation, the Vector system and hand instruments on periodontally diseased root surfaces in vivo. J. Clin. Periodontol. 2006, 33, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.S.; Krause, F.; Boecher, S.; Falk, W.; Birkenmaier, A.; Conrads, G.; Braun, A. Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model. Antibiotics 2021, 10, 1464. [Google Scholar] [CrossRef]
- Coluzzi, D.J.; Mizutani, K.; Yukna, R.; Al-Falaki, R.; Lin, T.; Aoki, A. Surgical laser therapy for periodontal and peri-implant disease. Clin. Dent. Rev. 2022, 6, 7. [Google Scholar] [CrossRef]
- Cobb, C.M. Lasers and the treatment of periodontitis: The essence and the noise. Periodontol. 2000 2017, 75, 205–295. [Google Scholar] [CrossRef]
- Braun, A.; Krause, F.; Hartschen, V.; Falk, W.; Jepsen, S. Efficiency of the Vector -system compared with conventional subgingival debridement in vitro and in vivo. J. Clin. Periodontol. 2006, 33, 568–574. [Google Scholar] [CrossRef]
- Eberhard, J.; Ehlers, H.; Falk, W.; Acil, Y.; Albers, H.K.; Jepsen, S. Efficacy of subgingival calculus removal with Er:YAG laser compared to mechanical debridement: An in situ study. J. Clin. Periodontol. 2003, 30, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Schmid, B.; Rutar, A.; Lang, N.P. Local antibiotic therapy guided by microbiological diagnosis. J. Clin. Periodontol. 2002, 29, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Burklin, T.; Schacher, B.; Ratka-Krueger, P.; Schaecken, M.T.; Renggli, H.H.; Fiehn, W.; Eickholz, P. Pharmacokinetic profile of a locally administered doxycycline gel in crevicular fluid, blood, and saliva. J. Periodontol. 2002, 73, 1285–1291. [Google Scholar] [CrossRef]
- Patianna, G.; Valente, N.A.; D’Addona, A.; Andreana, S. In vitro evaluation of controlled-release 14% doxycycline gel for decontamination of machined and sandblasted acid-etched implants. J. Periodontol. 2018, 89, 325–330. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Participants, E.F.P.W.; Methodological, C. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- Forner, L.; Larsen, T.; Kilian, M.; Holmstrup, P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 2006, 33, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Horliana, A.C.; Chambrone, L.; Foz, A.M.; Artese, H.P.; Rabelo Mde, S.; Pannuti, C.M.; Romito, G.A. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: A systematic review. PLoS ONE 2014, 9, e98271. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Riggio, M.P.; Walker, K.F.; MacKenzie, D.; Shearer, B. Bacteraemia following periodontal procedures. J. Clin. Periodontol. 2005, 32, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, C.M.; Brizuela, M. Antibiotic Prophylaxis in Dental and Oral Surgery Practice. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Caruso, U.; Nastri, L.; Piccolomini, R.; d’Ercole, S.; Mazza, C.; Guida, L. Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol. 2008, 31, 513–518. [Google Scholar] [PubMed]
- Zhao, P.; Song, X.; Wang, Q.; Zhang, P.; Nie, L.; Ding, Y.; Wang, Q. Effect of adjunctive diode laser in the non-surgical periodontal treatment in patients with diabetes mellitus: A systematic review and meta-analysis. Lasers Med. Sci. 2021, 36, 939–950. [Google Scholar] [CrossRef]
- Assaf, M.; Yilmaz, S.; Kuru, B.; Ipci, S.D.; Noyun, U.; Kadir, T. Effect of the diode laser on bacteremia associated with dental ultrasonic scaling: A clinical and microbiological study. Photomed. Laser Surg. 2007, 25, 250–256. [Google Scholar] [CrossRef]
- Kornman, K.S.; Crane, A.; Wang, H.Y.; di Giovine, F.S.; Newman, M.G.; Pirk, F.W.; Wilson, T.G., Jr.; Higginbottom, F.L.; Duff, G.W. The interleukin-1 genotype as a severity factor in adult periodontal disease. J. Clin. Periodontol. 1997, 24, 72–77. [Google Scholar] [CrossRef]
- Serrage, H.; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M.R. Under the spotlight: Mechanisms of photobiomodulation concentrating on blue and green light. Photochem. Photobiol. Sci. 2019, 18, 1877–1909. [Google Scholar] [CrossRef]
- Schaefer, B. The Effect of Dental Laser Systems on Periodontal Pathogenic Microorganisms In Vitro. Bachelor’s Thesis, Christian-Albrechts-Universität zu Kiel, Kiel, Germany, 2015. [Google Scholar]
- Lafaurie, G.I.; Mayorga-Fayad, I.; Torres, M.F.; Castillo, D.M.; Aya, M.R.; Baron, A.; Hurtado, P.A. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J. Clin. Periodontol. 2007, 34, 873–879. [Google Scholar] [CrossRef]
- Zhang, W.; Daly, C.G.; Mitchell, D.; Curtis, B. Incidence and magnitude of bacteraemia caused by flossing and by scaling and root planing. J. Clin. Periodontol. 2013, 40, 41–52. [Google Scholar] [CrossRef]
- Beutler, J.; Jentsch, H.F.R.; Rodloff, A.C.; Stingu, C.S. Bacteremia after professional mechanical plaque removal in patients with chronic periodontitis. Oral Dis. 2019, 25, 1185–1194. [Google Scholar] [CrossRef]
- Graziani, F.; Cei, S.; Orlandi, M.; Gennai, S.; Gabriele, M.; Filice, N.; Nisi, M.; D’Aiuto, F. Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2015, 42, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.O.; Nys, M.; De, M.P.; Charpentier, J.; Albert, A.; Legrand, V.; Rompen, E.H. Systemic release of endotoxins induced by gentle mastication: Association with periodontitis severity. J. Periodontol. 2002, 73, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Dayer, M.; Lockhart, P.B.; Prendergast, B. Antibiotic Prophylaxis of Infective Endocarditis. Curr. Infect. Dis. Rep. 2017, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Balejo, R.D.P.; Cortelli, J.R.; Costa, F.O.; Cyrino, R.M.; Aquino, D.R.; Cogo-Muller, K.; Miranda, T.B.; Moura, S.P.; Cortelli, S.C. Effects of chlorhexidine preprocedural rinse on bacteremia in periodontal patients: A randomized clinical trial. J. Appl. Oral Sci. 2017, 25, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Tickle-Degnen, L. Nuts and bolts of conducting feasibility studies. Am. J. Occup. Ther. 2013, 67, 171–176. [Google Scholar] [CrossRef]

