1. Introduction
Effective debridement and disinfection of the root canal space are crucial for the success of primary teeth endodontic treatment [1]. The main objective of root canal treatment is to eliminate microorganisms and their by-products from the root canal and prevent their re-entry [2]. Mechanical debridement can reduce bacterial counts but cannot completely eradicate them. To further decrease intra-radicular bacteria, various root canal irrigants and disinfection techniques are necessary. Moreover, there is a deficiency of evidence for complete microbial elimination [3].
The complex morphology of primary teeth root canals and the presence of bacteria in biofilms contribute to the incomplete eradication of intra-radicular bacteria, thereby hindering the antimicrobial activity of root canal irrigants [3]. The biofilms found in deciduous teeth with necrotic pulps mainly consist of anaerobic microorganisms, with aerobic microorganisms present in 60% of cases, streptococci in 85%, and Gram-negative bacilli in 15% of cases [4]. Gram-positive bacteria, for instance, Streptococcus and Enterococcus faecalis, are responsible for most endodontic failures due to their resistance to conventional treatment.
Enterococcus faecalis, a Gram-positive anaerobic coccus, can survive harsh conditions and withstand inadequate nutrition, with the capability of forming complex biofilms [3]. E. faecalis-dominant biofilms are highly resistant to conventional irrigants, which is attributed to the formation of extracellular polymeric matrix. Moreover, biofilms provide nutrition and protection to bacteria, increasing their resistance to antimicrobial agents [3].
Sodium hypochlorite is the preferred root canal irrigation material used in endodontic treatment due to its superior antibacterial properties, low surface tension, ease of use, and affordability [4]. However, its optimum used concentration, contact time, and temperature for clinical use are still debated. In paediatric dentistry, a concentration of 2.5% NaOCl is recommended to prevent complications related to pathological resorption areas in primary teeth roots. Although NaOCl effectively disinfects the root canal and dissolves soft tissue and pulpal residuals, its cytotoxicity can cause an acute injury to the periapical area if extruded beyond the apex [5]. The extrusion of NaOCl beyond the apex has been reported in about 42% of endodontic practitioners’ careers, with factors such as high syringe pressure, needle wedging, and large apex size playing a role, particularly in immature teeth [5]. The use of NaOCl in gel form has been introduced to minimise the potential side-effects associated with liquid NaOCl [6]. Studies have shown that the gel form reported significantly less extrusion in comparison with the solution form, especially when the apex diameter was less than 2.5 mm [4]. Sodium hypochlorite gel is suggested as a safer alternative with similar antimicrobial action as the NaOCl solution but with reduced apical extrusion.
Laser-photoactivated disinfection when combined with sodium hypochlorite offers several benefits, including fast penetration of the medication into the root canal, complete penetration of photosensitisers into biofilms and dentinal tubules, limited penetration and cytotoxicity in adjoining tissues, and absence of thermal side-effects [7]. The combination of a diode laser (940 nm) and NaOCl has a synergistic effect, enhancing the bactericidal action [8]. However, there is a lack of literature on the combination effects of sodium hypochlorite in gel form activated by an 810 nm diode laser for endodontic disinfection of deciduous root canals. Hence, an in vitro study was planned to test the null hypothesis, stating that there was no difference in the disinfecting efficacy of 2.5% sodium hypochlorite solution and 5.25% sodium hypochlorite gel against Enterococcus faecalis-contaminated primary teeth root canals, without laser activation and accompanied by 810 nm diode laser activation.
2. Materials and Methods
This in vitro study was granted ethical approval by the Institutional Review Board, Maratha Mandal’s, Nathajirao G. Halgekar Institute of Dental, Sciences & Research Centre, Belagavi, with number (2020-20/1391).
2.1. Study Design (Figure 1)
2.2. Baseline Microbial Analysis
At baseline, a microbial sample was obtained from each canal immediately before disinfection of root canal and inoculated on brain heart infusion media, and then incubated for 24–48 h to check for the growth of E. faecalis.
2.3. Diode Laser Activation of Irrigants
In Group B (experimental group), after irrigation of the root canals, laser activation was performed in each subgroup with a special endodontic diode laser with a wavelength of 810 nm (Novolase Dual wave Endo Diode, Novolase Technologies, India) at an output power of 1 watt in continuous wave mode. Laser activation was carried out three times for 15 s each, with a 5 s interval between irradiations. The laser activation was delivered to the canal until it was 1 mm shorter than the working length using a special endodontic fibre tip, 200 µm in diameter (Novolase Technologies, Meerut, India). The hand piece was grasped at around an angle of 10 degrees between the fibre and the root canal wall. Irradiation was performed by circular movements starting at the apical part towards the coronal part (step-back technique) of the root canal. The procedure was carried out by a certified laser specialist who was blinded to the irrigants used.
2.4. Post-Intervention Microbial Analysis
Root canals were filled with sterile saline solution using a 30 G syringe, and dentin was scraped from inside the canals using a Hedstrom file #25. Then, a sterile paper point #25 was used to dry the canals for 60 s and then immersed in thioglycolates broth in 1.5 mL Eppendorf tubes, before being vortexed for 30 s and incubated for 24–48 h at 37 °C. Then, the inoculated broth suspension samples were inoculated in BHI agar and incubated for 48 h at 37 °C in a CO2 jar. After incubation, the colony-forming units of E. faecalis were assessed. All previous steps were performed under sterile aseptic conditions by a microbiologist who was blinded to interventional details.
2.5. Statistical Analysis
Statistical package for social sciences (SPSS) version 22 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA: IBM Corp) was used for statistical analysis. Data followed normal distribution. Mann–Whitney U Tests were used for group comparison and the Wilcoxon signed rank test was used for within-group comparisons between two groups. One-way analysis of variance (Kruskal–Wallis ANOVA) followed by the post hoc Mann–Whitney Test was used to compare subgroups. The significance level was set at p < 0.05.
3. Results
The pre-intervention bacterial colony-forming unit (CFU) values were in the range of 107, whereas post intervention, the values ranged from 0 to 106. The distribution was skewed; hence, a log10 transformation of data was performed and is presented.
In the current study, no statistically significant difference was reported in the E. faecalis colony-forming units (CFUs) between the subgroups both in the control and experimental groups pre-intervention. However, a statistically significant reduction in the E. faecalis CFUs post intervention in both groups was reported. The post hoc test revealed that the disinfection ability of sodium hypochlorite solution and gel was better than that of saline (p = 0.03, p = 0.004, respectively); however, there was no significant difference between sodium hypochlorite gel and solution. Similar results were observed in experimental groups. Sodium hypochlorite solution and gel were better than saline (p = 0.09, p = 0.02) .
A subgroup comparison between the control and experimental group revealed that there was no statistically significant difference except for saline (0.004) and sodium hypochlorite gel post intervention (0.02). There was a marked reduction in the E. faecalis counts post intervention in all the subgroups with a maximum reduction in the NaOCl gel group. In general, laser activation significantly improved the disinfection ability of the irrigant compared to non-activation , Figure 3).
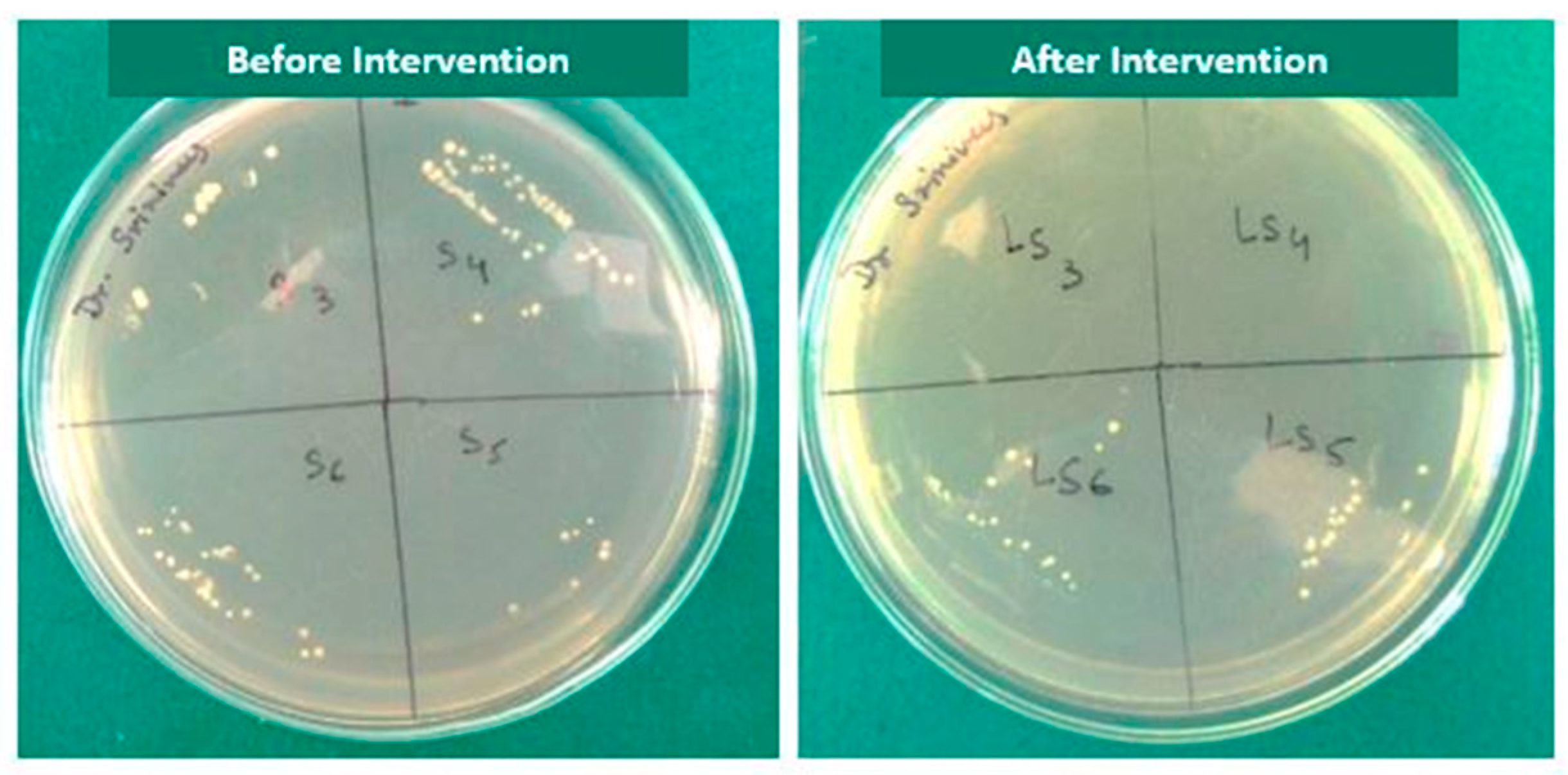
Figure 3. Culture media showing growth of E. faecalis before and after laser activated irrigation in all the subgroups.
4. Discussion
The study results favor acceptance of the null hypothesis where there was no significant difference between the disinfecting efficacy of 2.5% sodium hypochlorite solution and 5.25% sodium hypochlorite gel against Enterococcus faecalis-contaminated primary teeth root canals without laser activation. However, laser activation of sodium hypochlorite gel showed a superior disinfecting efficacy compared to non-activated gel.
A comparable result was reported in an in vivo study performed by Kotecha et al., where they treated multirooted primary teeth that had endodontic lesions using 5.25% sodium hypochlorite gel and solution, with both the interventions demonstrating comparable antimicrobial effectiveness when implemented as root canal disinfectants [10]. In addition, other in vitro studies have also demonstrated comparable efficacy of both sodium hypochlorite gel and solution [11,12,13].
Other studies by Hasna et al. and Luz et al. concluded that even though NaOCl solution reported a higher capacity for tissue dissolution than that of the gel form, both the sodium gel and solution forms were efficient in reducing the microbial load of E. faecalis [11,12].
Contrasting results were observed in a study by Zand et al. where sodium hypochlorite in solution form exhibited better antibacterial efficacy than the gel form [14], while a clinical study by Karatas et al. using NaOCl gel during root canal preparation resulted in reduced post-operative pain on day 1 when compared to NaOCl solution [15].
However, an in vitro investigation by Faria et al. demonstrated that the 3% sodium hypochlorite solution reported better penetrability in dentinal tubules (p < 0.05) than the gel form, and these results indicated that the gel form penetration into dentinal tubules is limited due to the increase in viscosity. This can be considered a limitation of the NaOCl gel form, in addition to other problems like the dubious penetration into unreachable regions, the inability to remove debris from root canals, and needing to add saline solution in addition to the gel for passive ultrasonic irrigation, which could dilute and lose the effectiveness of NaOCl gel [16].
In the current study, root canal irrigation with 5.25% NaOCl gel activated with an 810 nm diode laser resulted in the complete eradication of Enterococcus faecalis. However, a few studies showed the bactericidal efficacy of a 940 nm diode-laser-activated 5% NaOCl solution used as an irrigant against E. faecalis [8,17,18,19,20]. Moreover, the bactericidal efficiency improved by using the erbium-laser-activated irrigation (LAI) of 0.5% NaOCl against E. faecalis biofilm in a few studies [21,22,23].
However, Cristo et al. showed that there is no significant improvement in the antibacterial efficacy of low-powered (0.5 W) Er, Cr:YSGG laser activation on low concentrations of NaOCl used as a root canal irrigant [24].
The photoactivated disinfection mechanism has a significant role in the antibacterial effect of the laser, as when it is combined with NaOCl, it resulted in several benefits; for instance, the acceleration of irrigant penetration inside the root canal, which leads to a rapid bactericidal effect; the complete removal of biofilms and, consequently, the efficient penetration of photosensitisers inside dentinal tubules; and the controlled penetration of the photosensitiser into the adjoining bone and periodontal ligament, which decreases the cytotoxicity and limits the thermal side-effects on the adjacent tissues [25].
As primary teeth exhibit bizarre internal geometry and other features such as furcal connections and horizontal anastomoses that are not common for permanent teeth, the root canal treatment of primary teeth is a more complicated procedure than for permanent teeth [25]. Recent radiographic imaging systems have revealed that remanent pulp tissues might persist due to using mechanical root canal instrumentation only. Hence, irrigation as well as instrumentation together ensure the complete debridement and disinfection of root canals [25].
Furthermore, it was reported that the largest number of microorganisms is present in the main root canal of primary teeth; yet, a significant portion of microorganisms is present in lateral canals, apical ramifications, as well as dentinal tubules. These place a burden on the clinician to accomplish all the aforementioned tasks in a duration that matches the attention span of our young paediatric patients [10].
A mixture of bacterial species is associated with root canal infections in primary teeth, including aerobic and anaerobic microorganisms as well as facultative microorganisms. It was found that Enterococcus faecalis, Porphyromonas gingivalis, and Treponema denticola are the most dominant bacterial species found in primary teeth root canals [26]. Also, E. faecalis had a reported prevalence of 63% in the necrotic pulp of primary root canals, in addition to being occasionally present in the initial infections of permanent teeth [15].
When NaOCl is added into water, it dissociates into Na+ and OCl− ions that form the predominant form of HOCl in acidic or neutral pH media, which are responsible for the antimicrobial effect of the most widely used root canal irrigant [27]. NaOCl is the only irrigant that can dissolve necrotic pulp tissues with a minimum remanent of dentinal collagen; yet, it could not dissolve the smear layer.
However, NaOCl reported cytotoxic activities that may cause acute injuries to the periapical tissues when it extends beyond the root apex, causing haemolysis, ulcerations, and the destruction of endothelial and fibroblast cells, and this resulted in emphysema, trismus, and sensory-motor defects [28]. The extruded irrigant amount might be interrelated to many factors, such as extra pressure from the syringes, needle wedging, and the width of the apical foramen that is noticed frequently in immature teeth [22]. The use of NaOCl in solution form increased the risk of its extrusion beyond the apex, posing a risk to paediatric patients [29].
Some studies found significantly less extrusion of NaOCl in gel form when compared to the solution form, when the apical foramen diameter was less than 2.5 mm. This occurred in spite of the high plunger pressure of the syringe when the gel form was used in comparison to the solution form at the same flow rate [29,30]. Hence, it was decided to test the photo-activated disinfection ability of sodium hypochlorite gel in the present study. Several research studies have found that laser activation leads to a clear modulation in the NaOCl reaction rate that significantly improves the production and consumption of available chlorine and oxygen ions compared to ultrasound activation [31].
The diode laser bactericidal effect could have resulted from its greater penetration depth (1000 μm) in comparison to NaOCl, which penetrates only to 100 μm of the dentinal tubules [21]. Also, diode laser thermal photodisruptive action at unreachable parts of dentin resulted in an improvement in its bactericidal action [32].
In the current study, a fine diameter of the fibre-optic tip of 200 µm was used to improve the delivery of laser light to the root canal walls efficiently, resulting in a reduced bacterial count. Jambagi et al. found that the small fibre-optic tip enhanced the power density of the tip as well as improved the accessibility to the apical third, resulting in effectively extending its use with curved canals [32].
The study’s strength lies in its preliminary attempt to evaluate the disinfection efficacy of laser-activated sodium hypochlorite gel with the traditional technique. However, further in vivo studies with a large sample size should be conducted to explore and validate the evidence related to the study findings. Disinfection efficacy could be tested on root canal biofilm models rather than on a single microorganism. Further studies testing different wavelengths and doses of lasers and the disinfection efficacy of photo activated therapy could give valuable insights into endodontic disinfection therapy.
5. Conclusions
Within the limitation of the current in vitro setting, the utilisation of 5.25% sodium hypochlorite gel as a root canal irrigant, activated with an 810 nm diode laser, led to the complete eradication of Enterococcus faecalis in primary teeth root canals. If clinical trials confirm the disinfection effectiveness of laser activation of sodium hypochlorite gel, this innovative approach, known for its safety and patient-friendliness, has the potential to significantly enhance the clinical outcomes of root canal therapy in primary teeth, promoting cutting-edge dental care for young patients.
References
- Walia, V.; Goswami, M.; Mishra, S.; Walia, N.; Sahay, D. Comparative Evaluation of the Efficacy of Chlorhexidine, Sodium Hypochlorite, the Diode Laser and Saline in Reducing the Microbial Count in Primary Teeth Root Canals–An In Vivo Study. J. Lasers Med. Sci. 2019, 10, 268. [Google Scholar] [CrossRef]
- Sohrabi, K.; Sooratgar, A.; Zolfagharnasab, K.; Kharazifard, M.J.; Afkhami, F. Antibacterial activity of diode laser and sodium hypochlorite in Enterococcus faecalis-contaminated root canals. Iran. Endod. J. 2016, 11, 8. [Google Scholar] [PubMed]
- Roshdy, N.N.; Kataia, E.M.; Helmy, N.A. Assessment of antibacterial activity of 2.5% NaOCl, Chitosan nano-particles against Enterococcus faecalis contaminating root canals with and without diode laser irradiation: An in vitro study. Acta Odontol. Scand. 2019, 77, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Öter, B.; Topcuoglu, N.; Tank, M.K.; Cehreli, S.B. Evaluation of antibacterial efficiency of different root canal disinfection techniques in primary teeth. Photomed. Laser Surg. 2018, 36, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, H.K.; Siren, E.K.; Waltimo, T.M.; Orstavik, D.; Haapasalo, M.P. Inactivation of local root canal medicaments by dentine- an in vitro study. Int. Endod. J. 2000, 33, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Nesser, S.F.A.; Bshara, N.G. Evaluation of the apical extrusion of sodium hypochlorite gel in immature permanent teeth: An in vitro study. Dent. Med. Probl. 2019, 56, 149–153. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.B.; Cai, S.; Simionato, M.R.; Lage-Marques, J.L. High-power diode laser in the disinfection in depth of the root canal dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, e68–e72. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Baz, P.; Martín-Biedma, B.; Ruíz-Pinon, M.; Rivas-Mundina, B.; Bahillo, J.; Seoane-Prado, R.; Perez-Estévez, A.; Gude, F.; De Moor, R.; Varela-Patiño, P. Combined sodium hypochlorite and 940 nm diode laser treatment against mature E. faecalis biofilms in-vitro. J. Lasers Med. Sci. 2012, 3, 116–121. [Google Scholar]
- Yavagal, C.M.; Patil, V.C.; Yavagal, P.C.; Kumar, N.K.; Hariharan, M.; Mangalekar, S.B. Efficacy of Laser Photoacoustic Streaming in Paediatric Root Canal Disinfection-An Ex-Vivo Study. Contemp. Clin. Dent. 2021, 12, 44–48. [Google Scholar] [CrossRef]
- Kotecha, N.; Shah, N.C.; Doshi, R.J.; Kishan, K.V.; Luke, A.M.; Shetty, K.P.; Mustafa, M.; Pawar, A.M. Microbiological Effectiveness of Sodium Hypochlorite Gel and Aqueous Solution When Implemented for Root Canal Disinfection in Multirooted Teeth: A Randomized Clinical Study. J. Funct. Biomater. 2023, 14, 240. [Google Scholar] [CrossRef]
- Shamsi, P.N.; Yeganeh, L.A.B.; Saberi, B.V.; Azadeh, K.F.P.; Kashani, T. Antibacterial effect of sodium hypochlorite gel and solution on Enterococcus faecalis. J. Dentomaxillofac. Radiol. Pathol. Surg. 2017, 6, 27–31. [Google Scholar]
- Hasna, A.A.; Da Silva, L.P.; Pelegrini, F.C.; Ferreira, C.L.R.; de Oliveira, L.D.; Carvalho, C.A.T. Effect of sodium hypochlorite solution and gel with/without passive ultrasonic irrigation on Enterococcus faecalis, Escherichia coli and their endotoxins. F1000Research 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Luz, L.B.; Santana, R.; Prates, A.W.; Froelich, J.; De Melo, T.A.; Montagner, F.; Luisi, S.B. Antimicrobial action, ph, and tissue dissolution capacity of 2.5% sodium hypochlorite gel and solution. J. Health Biol. Sci. 2019, 7, 121–125. [Google Scholar] [CrossRef]
- Zand, V.; Lotfi, M.; Soroush, M.H.; Abdollahi, A.A.; Sadeghi, M.; Mojadadi, A. Antibacterial Efficacy of Different Concentrations of Sodium Hypochlorite Gel and Solution on Enterococcus faecalis Biofilm. Iran. Endod. J. 2016, 11, 315–319. [Google Scholar]
- Karataş, E. Postoperative pain after the use of sodium hypochlorite gel and solution forms: A randomized clinical study. Eur. Endod. J. 2020, 6, 34–37. [Google Scholar] [CrossRef]
- Faria, G.; Viola, K.S.; Coaguila-Llerena, H.; Oliveira, L.R.A.; Leonardo, R.T.; Aranda-García, A.J.; Guerreiro-Tanomaru, J.M. Penetration of sodium hypochlorite into root canal dentine: Effect of surfactants, gel form and passive ultrasonic irrigation. Int. Endod. J. 2019, 52, 385–392. [Google Scholar] [CrossRef]
- Vaid, D.; Shah, N.; Kothari, D.; Bilgi, P. Additive effect of photoactivated disinfection on the antibacterial activity of QMix 2 in 1 against 6-week Enterococcus faecalis biofilms: An in vitro study. J. Conserv. Dent. 2017, 20, 41–45. [Google Scholar] [CrossRef]
- Attiguppe, P.R.; Tewani, K.K.; Naik, S.V.; Yavagal, C.M.; Nadig, B. Comparative Evaluation of Different Modes of Laser Assisted Endodontics in Primary Teeth: An In vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC124–ZC127. [Google Scholar] [CrossRef]
- Dai, S.; Xiao, G.; Dong, N.; Liu, F.; He, S.; Guo, Q. Bactericidal effect of a diode laser on Enterococcus faecalis in human primary teeth—an in vitro study. BMC Oral. Health 2018, 18, 154. [Google Scholar] [CrossRef]
- Mehrvarzfar, P.; Akhavan, H.; Rastgarian, H.; Akhlagi, N.M.; Soleymanpour, R.; Ahmadi, A. An in vitro comparative study on the antimicrobial effects of bioglass 45S5 vs. calcium hydroxide on Enterococcus faecalis. Iran. Endod. J. 2011, 6, 29. [Google Scholar]
- Neelakantan, P.; Cheng, C.Q.; Mohanraj, R.; Sriraman, P.; Subbarao, C.; Sharma, S. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er: YAG laser in vitro. Int. Endod. J. 2015, 48, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, P.; Merlos, A.; Sierra, J.M.; Camps-Font, O.; Arnabat-Dominguez, J.; Viñas, M. Effectiveness of low concentration of sodium hypochlorite activated by Er,Cr:YSGG laser against Enterococcus faecalis biofilm. Lasers Med. Sci. 2019, 34, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.A.; Taşdemir, T.; Buruk, C.K.; Çelik, D. Efficacy of Erbium, Chromium-doped Yttrium, Scandium, Gallium and Garnet Laser-activated Irrigation Compared with Passive Ultrasonic Irrigation, Conventional Irrigation, and Photodynamic Therapy against Enterococcus faecalis. J. Contemp. Dent. Pract. 2020, 21, 11–16. [Google Scholar] [CrossRef]
- Christo, J.E.; Zilm, P.S.; Sullivan, T.; Cathro, P.R. Efficacy of low concentrations of sodium hypochlorite and low-powered Er,Cr:YSGG laser activated irrigation against an Enterococcus faecalis biofilm. Int. Endod. J. 2016, 49, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Raducka, M.; Piszko, A.; Piszko, P.J.; Jawor, N.; Dobrzyński, M.; Grzebieluch, W.; Mikulewicz, M.; Skośkiewicz-Malinowska, K. Narrative Review on Methods of Activating Irrigation Liquids for Root Canal Treatment. Appl. Sci. 2023, 13, 7733. [Google Scholar] [CrossRef]
- Ahmed, H.M. Anatomical challenges, electronic working length determination and current developments in root canal preparation of primary molar teeth. Int. Endod. J. 2013, 46, 1011–1022. [Google Scholar] [CrossRef]
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological Aspects of Root Canal Infections and Disinfection Strategies: An Update Review on the Current Knowledge and Challenges. Front. Oral. Health 2021, 2, 672887. [Google Scholar] [CrossRef]
- Dioguardi, M.; Gioia, G.D.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef]
- Ozlek, E.; Neelakantan, P.; Khan, K.; Cheung, G.S.; Rossi-Fedele, G. Debris extrusion during root canal preparation with nickel-titanium instruments using liquid and gel formulations of sodium hypochlorite in vitro. Aust. Endod. J. 2021, 47, 130–136. [Google Scholar] [CrossRef]
- Naik, R.G.; Raviraj, G.A.; Yavagal, C.M.; Mandroli, P. Diode Lasers for Pediatric Endodontics: State-of-the-Art. J. Dent. Lasers 2017, 11, 7–13. [Google Scholar] [CrossRef]
- Mishra, A.; Koul, M.; Abdullah, A.; Khan, N.; Dhawan, P.; Bhat, A. Comparative Evaluation of Antimicrobial Efficacy of Diode Laser (Continuous Mode), Diode Laser (Pulse Mode), and 5.25% of Sodium Hypochlorite in Disinfection of Root Canal: A Short Study. Int. J. Clin. Pediatr. Dent. 2022, 15, 579–583. [Google Scholar] [PubMed]
- Jambagi, N.; Kore, P.; Dhaded, N.S. Comparison of Antimicrobial Efficacy of Diode Laser, Ultrasonic Activated and Conventional Irrigation with 2.5% NaOCl during RCT: An Interventional Study. J. Contemp. Dent. Pract. 2021, 22, 669–673. [Google Scholar] [PubMed]

