1. Introduction
Periprosthetic joint infection (PJI) is a severe complication after total knee and hip arthroplasty, with an incidence of 1–2% in primary arthroplasties and up to 4–10% in prosthetic revision surgeries [1]. Despite multiple efforts, PJI is becoming increasingly important owing to the aging of the population, the higher prevalence of comorbidities, and increased life expectancy [2].
According to data from the National Inpatient Sample, a representative sample of all U.S. hospital discharges, the estimated annual incidence of primary total hip and knee arthroplasty per 100,000 persons increased, respectively, by 105% and 119%, between 2000 and 2014 [3]. According to the projection model of Sloan et al., by the year 2030, the volume of total hip arthroplasty is expected to increase by 71.2% to 635,000 procedures, and that of total knee arthroplasty will increase by 84.9% to 1.26 million procedures [3].
PJI not only affects patients, but also places a significant health and economic burden on the system, as many affected patients will undergo multiple surgical procedures and prolonged antibiotic therapy, with a higher cost per treatment [4,5].
PJI is unique, owing to the presence of an implant and its relationship with microorganisms and the immune system. The presence of material means that infection can occur with a much smaller inoculum, up to 100,000 times smaller in the case of Staphylococcus aureus, according to some studies, and may be caused by less virulent microorganisms, such as skin saprophytes [6,7]. In addition, bacterial adhesion and biofilm formation are associated with high resistance to antibiotic treatment. In vitro measurement of antibiotic sensitivity does not reflect in vivo sensitivity, as the required concentration cannot be achieved via systemic administration of the antibiotic [8].
The only two treatment options are implant replacement using a one or two-stage procedure, or in selected patients, debridement and antibiotic with implant retention (DAIR) followed by targeted antibiotic treatment [9]. In all these options, chemical debridement through tissue irrigation is a fundamental step, as it decreases bacterial load and rates of re-infection.
The numerous irrigation solutions currently available to orthopedic surgeons range from inexpensive established formulations to expensive commercial antibiofilm formulations. The most commonly used antiseptic solutions for irrigation are 0.35% or 10% povidone iodine, 0.05% chlorhexidine digluconate, 10% hydrogen peroxide, and 3% acetic acid, although none has proven to be superior to the others. While several studies compare the effectiveness of antiseptics on planktonic bacteria, they do not provide sufficient data on the ability of contemporary antiseptic solutions to eradicate biofilm in orthopedic materials [10,11,12]. Only Premkumar et al. [13] observed a clearly superior antibiofilm effect of 10% povidone-iodine after 3 min of exposure, with and without the addition of hydrogen peroxide, against the other solutions studied, such as Bactisure™ (Zimmer Biomet, Madrid, Spain), Irrisept (Irrimax, Orlando, FL, USA), polymyxin-bacitracin, and vancomycin, or Prontosan® diluted solution (B. Braun, Barcelona, Spain). Similarly, the optimal irrigation time and combination of antiseptics have not been addressed [14,15,16].
We attempted to answer these questions by performing an experimental in vitro study to determine which solution showed the best antibiofilm activity, how long it took to achieve its effect, and whether sequential combinations of solutions improved results.
2. Results
2.1. Single Antiseptics
Compared to the positive control, a statistically significant decrease in the median (IQR) log cfu/mL was observed for all antiseptics at all exposure times (1, 3, and 5 min) , Figure 1A). However, the decrease was less notable with AA3 at all exposure times, p < 0.001 , Figure 1A).
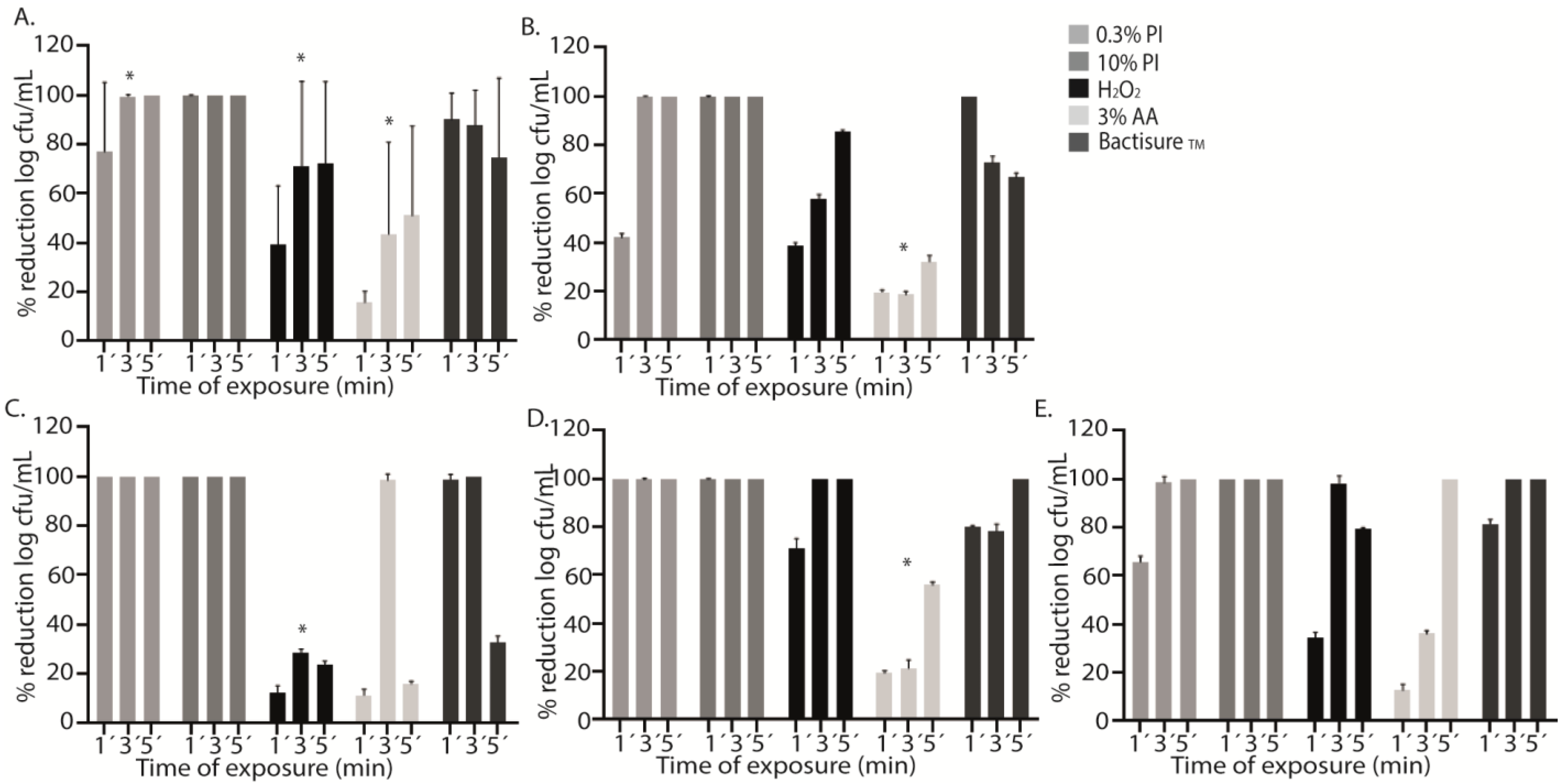
Figure 1. Graphical representation of the efficacy of different antiseptics against each microorganism. The graphs show the mean percentage reduction in log cfu/mL of each antiseptic (0.3% PI, 0.3% povidone iodine; 10% PI, 10% povidone iodine; H2O2, hydrogen peroxide; AA3, 3% acetic acid, and Bactisure™) in all microorganisms (A) and for each species studied separately: methicillin-susceptible Staphylococcus aureus (MSSA) (B), methicillin-resistant Staphylococcus aureus (MRSA) (C), Staphylococcus epidermidis (D), and Pseudomonas aeruginosa (E) at three different exposure times (1′, 3′, and 5′). The differences between log cfu/mL reduction at different exposure times for each antiseptic were statistically significant (* p < 0.001) for 0.3% PI, H2O2, and AA3 (A). AA showed a lower bacterial decrease at the three exposure times (* p < 0.001) (B). H2O2 showed a lower bacterial decrease than 0.3% PI, 10% PI, and Bactisure™ (* p < 0.001) (C). AA3 showed a lower bacterial decrease at the three exposure times (* p < 0.001) (D).
In PI treatment, we did not obtain any cfu from the sonicated discs after 3 and 5 min of exposure, whereas after 1 min of exposure, the mean (SD) percentage reduction reached only 77.1% (12.0%). As with PI, no bacteria were recovered from sonicated discs after PI10 treatment, regardless of exposure time , Figure 1A).
In contrast, treatment with H2O2, AA3, and Bactisure™ did not reach 100% reduction in cfu counts. The mean (SD) percentage reduction in log cfu/mL after 1 min, 3 min, and 5 min with H2O2 was, respectively, 39.0% (22.1%), 71.1% (32.1%), and 72.1% (29.7%). The mean (SD) percentage reduction in log cfu/mL after 1 min, 3 min, and 5 min with AA3 was, respectively, 15.7% (4.4%), 43.9% (34.7%), and 51.2% (33.0%). The mean (SD) percentage reduction in log cfu/mL after 1 min, 3 min, and 5 min with Bactisure™ was, respectively, 91.0% (9.5%), 87.8% (13.0%), and 74.5% (29.4%) , Figure 1A).
H2O2, AA3, and Bactisure™ reached a significantly lower bacterial decrease at all exposure times compared to PI and PI10 in terms of median log cfu/mL count .
Analysis of the effect of antiseptic solutions on specific microorganisms showed a mean (SD) percentage reduction in log cfu/mL for AA3 of only 23.3% (6.0%) (p < 0.001), with a lower bacterial decrease in MSSA and S. epidermidis at the three exposure times, reaching a mean (SD) percentage reduction of 31.2% (5.7%) and 56.6% (7.1%) after 5 min, respectively (p < 0.001) (Figure 1B,D). However, in P. aeruginosa and MRSA, complete bacterial eradication was reached at 5 min and 3 min, respectively (Figure 1C,E). H2O2 showed a lower bacterial decrease in MRSA than PI, PI10, and Bactisure™ (p < 0.001) , Figure 1C).
2.2. Sequential Combination of Antiseptics
Sequential washing for 9 min (3 min with each solution) with the sequential combinations 1, 2 and 3 (AA3 + H2O2 + PI10, AA3 + PI10 + H2O2, and H2O2 + AA3 + PI10) resulted in sterile cultures after sonication for all strains, whereas the sequential combination 4 (PI10 + H2O2 + AA3) only reached a log cfu/mL percentage reduction of 78.8%, p = 0.006, being less active for MRSA and P. aeruginosa (61.3% and 53.7%, respectively) , Figure 2A,C,E). However, in MSSA and S. epidermidis, complete bacterial eradication was achieved (Figure 2B,D).
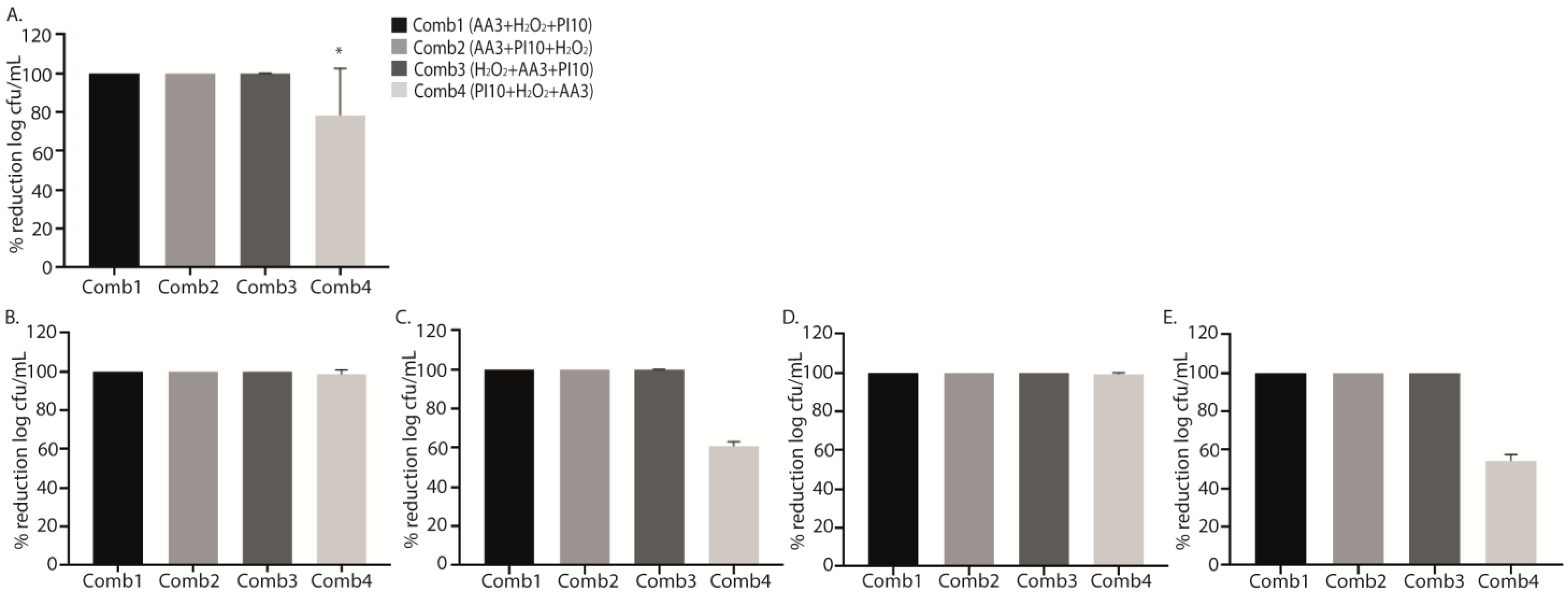
Figure 2. Graphical representation of the efficacy of the four sequential antiseptic combinations against each microorganism in terms of percentage reduction in log cfu/mL. Data show the mean percentage reduction in log cfu/mL for each sequential antiseptic combination applied against all microorganisms (A) and for each microorganism separately: methicillin-susceptible Staphylococcus aureus (MSSA) (B), methicillin-resistant Staphylococcus aureus (MRSA) (C), Staphylococcus epidermidis (D), and Pseudomonas aeruginosa (E) at a single exposure time of 3 min. The decrease in bacterial concentration for the sequential combination 4 (PI10 + H2O2 + AA3) was lower than for the other sequential combinations (* p = 0.006, in all comparisons) (A). No statistically significant differences were found according to species. Comb1, sequential combination 1; Comb2, sequential combination 2; Comb3, sequential combination 3; Comb4, sequential combination; PI, povidone iodine; H2O2, hydrogen peroxide; AA3, acetic acid.
Although no significant differences were found, all data obtained from the treatments with the different sequential combinations showed a marked reduction in cell viability with respect to positive controls, with the mean (SD) percentage reduction for sequential combinations 1, 2, 3 and 4 (AA3 + H2O2 + PI10, AA3 + PI10 + H2O2, H2O2 + AA3 + PI10, and PI10 + H2O2 + AA3) being, respectively, 62.28 ± 33.26%, 77.63 ± 11.40%, 51.68 ± 32.99%, and 56.70 ± 36.43% (p = 0.103) , Figure 3).
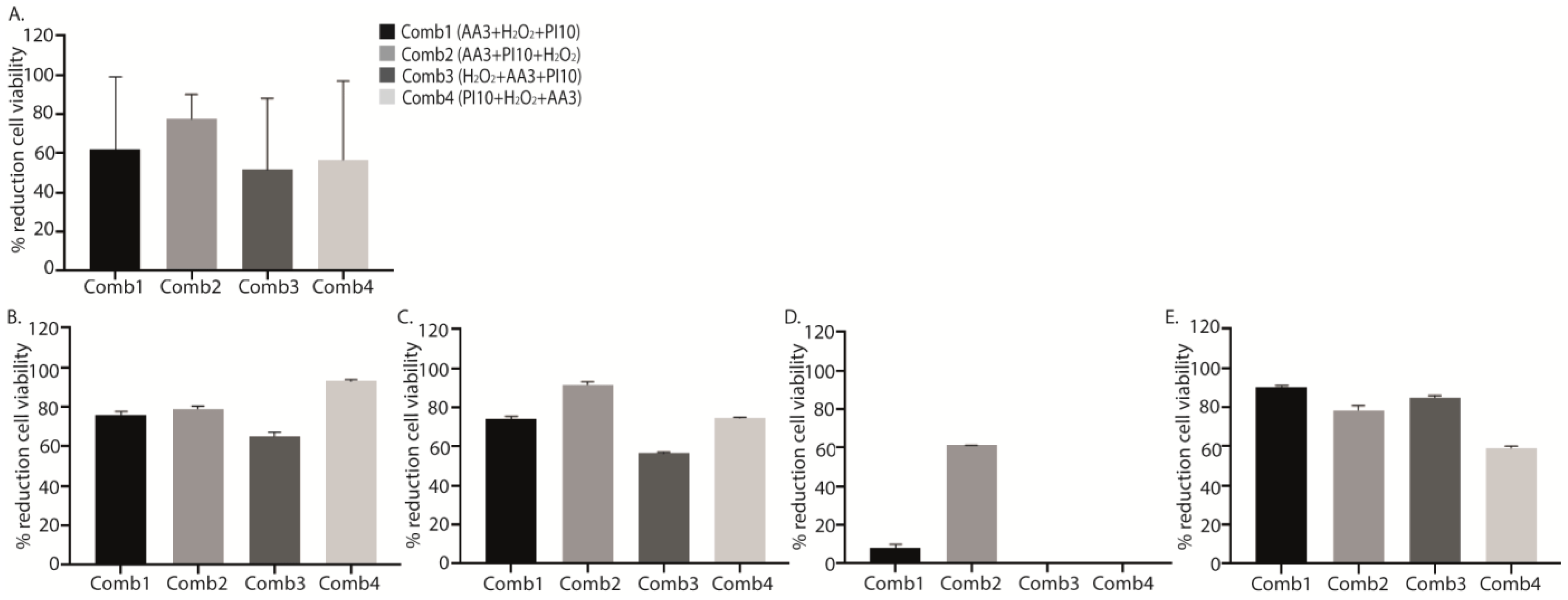
Figure 3. Graphical representation of the efficacy of the four sequential antiseptic combinations against each microorganism in terms of percentage reduction in log cfu/mL. Data show the mean percentage reduction in log cfu/mL for each sequential antiseptic combination applied against all microorganisms (A) and for each microorganism separately: methicillin-susceptible Staphylococcus aureus (MSSA) (B), methicillin-resistant Staphylococcus aureus (MRSA) (C), Staphylococcus epidermidis (D), and Pseudomonas aeruginosa (E) at a single exposure time of 3 min. No statistically significant differences were found in these analysis. Comb1, sequential combination 1; Comb2, sequential combination 2; Comb3, sequential combination 3; Comb4, sequential combination 4; PI, povidone iodine; H2O2, hydrogenated peroxide; AA3, acetic acid.
The results obtained were confirmed by the SEM images (Figure 4).
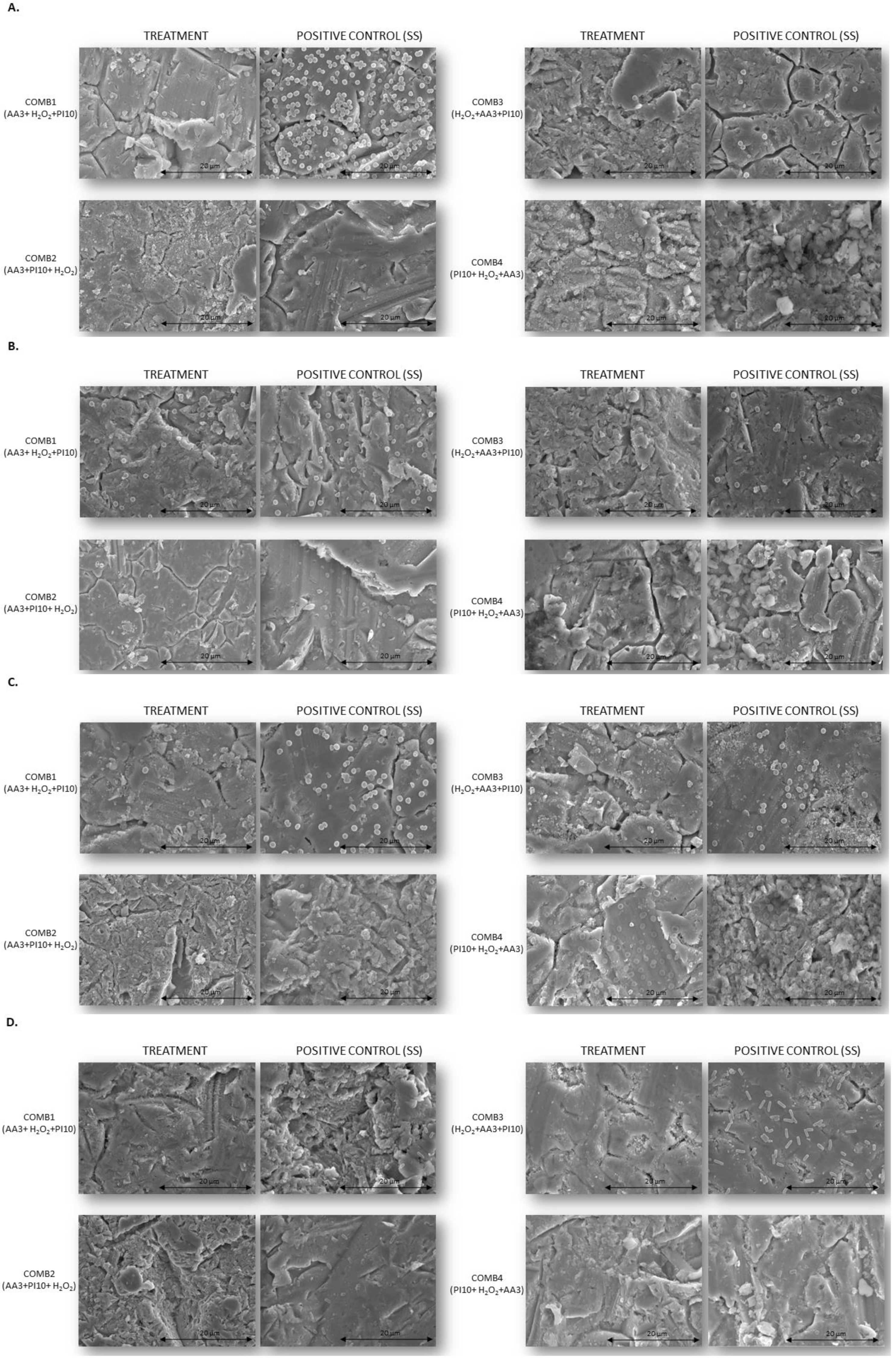
Figure 4. SEM images of biofilms treated with antiseptic combinations at a single exposure time of 3 min each vs. positive controls treated with sterile saline. All images were taken at 2500× magnification. (A). Methicillin-susceptible Staphylococcus aureus; (B). Methicillin-resistant Staphylococcus aureus; (C). Staphylococcus epidermidis; (D). Pseudomonas aeruginosa. Comb1, sequential combination 1; Comb2, sequential combination 2; Comb3, sequential combination 3; Comb4, sequential combination 4; PI, povidone iodine; H2O2, hydrogenated peroxide; AA3, acetic acid.
3. Discussion
Surgical and chemical debridement are key steps in the surgical treatment of PJI. They serve to decrease the bacterial load and rate of infection. However, there is no consensus on which antiseptic solution to use and for how long it should be applied. In fact, delegates at the 2018 International Consensus Meeting on Musculoskeletal Infection did not vote in favor of using antiseptics routinely in primary arthroplasty, although they did vote strongly in favor of using antiseptics during surgery for PJI, despite scarce evidence of their efficacy [17].
Previous in vitro studies reported that exposure times between 1 and 5 min were sufficient for the antiseptics to be effective and do not excessively increase operative time [13,18,19]. However, based on our results, 1 min did not lead to complete bacterial eradication in several experiments.
Regarding the efficacy of each antiseptic when used individually, 0.3% PI applied for at least 3 min may be the best choice, owing to its lower toxicity, 1 min application with PI10 would be also sufficient. Our data are consistent with those of other, previous studies that tested PI. Shohat et al. analyzed 31,331 cases of PJI, in which 0.3% PI was used for 3 min, and found the rate of PJI to be 2.34 times lower than for the control group (0.6% vs. 1.3%, p < 0.001) [20]. Oduwole et al. studied the effect of PI on S. aureus and S. epidermidis, reporting that in addition to its known bactericidal effect, it can also inhibit the development of S. epidermidis and S. aureus biofilm by repressing transcription of polysaccharide intercellular adhesin [18]. Ernest et al. studied the effect of PI10 on cobalt chrome, stainless steel, and titanium alloy discs with MSSA biofilm and found that irrigation for 5 min reduces biofilm growth by 95%, and that this option was superior to H2O2, ClO2, and Dakin’s solution [19]. Premkumar et al. analyzed the anti-biofilm activity of several antiseptic solutions on different surfaces of common implants such as PMMA and polyethylene, and found that PI10 and its combination with 4% H2O2 were the most effective, although the concentrations are considered cytotoxic [13]. One of the concerns associated with antiseptics in vivo is soft tissue toxicity at the concentration used, and the possibility of inhibiting tissue healing. Romano et al. studied the cytotoxicity of 0.3%PI, 0.5% PI, 0.5% H2O2, and 1.5% H2O2 after 1, 3, and 5 min of exposure, reporting a gradual increase in tissue toxicity according to exposure time, except for 0.3% PI, for which the lowest toxicity was recorded [21]. Therefore, we consider that PI0.3 could be used for prophylaxis, rather than PI10, whose toxicity in soft tissues is still a concern. However, all toxicity studies were performed in vitro and examined antiseptics used for prophylactic purposes (healthy joint). These results have not been validated in the context of PJI, and there is no evidence that PI0.3 3 min is more toxic than PI10 1 min. Our in vitro studies lead us to believe that it could be.
Bactisure™ is a commercialized irrigation solution that includes ethanol, acetic acid, sodium acetate, and benzalkonium chloride. Its anti-biofilm activity has previously been tested by Kia et al., who reported no differences in the reduction in bacterial load of S. aureus compared to PI [15]. We recorded better results for PI than for Bactisure™ after 3 and 5 min of irrigation and PI10 at all exposure times.
While combining solutions may have a synergistic effect, not all solutions can be combined [22], as 4% chlorhexidine gluconate precipitates with PI and H2O2, and there is no evidence of its anti-biofilm activity; therefore, it is not recommended in combinations. In addition, 0.5% sodium hypochlorite generates gas when combined with PI or H2O2, and the combination of AA and H2O2 can form peracetic acid. This last interaction does not affect our results, since, during the combinations, the 3 min sequential exposure with each of the different solutions is always followed by a saline wash to rinse the previous solution. Therefore, the only antiseptics that do not react with each other are PI and H2O2. In fact, in their in vitro study, Premkumar et al. showed that H2O2 had a synergistic effect with PI, their combination being the most effective [13].
In our in vitro study, the highest anti-biofilm activity was achieved when antiseptics were combined. In addition, the sequential order in which each antiseptic was applied affected the results, as the only sequential combination that did not reach complete bacterial eradication was that in which AA3 was not used in the first step. Therefore, since AA3 disrupts the biofilm, we believe that it should be used at the beginning, followed by PI and H2O2, which both have a bactericidal effect (sequential combination 2).
We believe that specific triple treatment is a key factor in chemical debridement during surgery for PJI. In some in vitro studies, an increase in the antibacterial effect of PI was observed after the application of H2O2; therefore, it can be stated that H2O2 has a synergistic effect with iodine. This effect was not observed when iodine was used in isolation. However, in highly mature biofilm, the sequential combination of H2O2 and PI might not be 100% effective. The use of acetic acid prior to this sequential combination is based on its ability to alter the polysaccharide matrix as an acidic liquid, not on its antiseptic effect, as it needs prolonged exposure to show this effect. In line with the mechanism of action of AA3, our in vitro study revealed this effect when we used AA3 at the beginning of the sequential combination, but not when it was used after other antiseptic solutions.
Although we observed clear statistically significant differences according to the reduction in cfu counts, we were not able to confirm these results with respect to cell viability using flow cytometry, because samples had been refrigerated for several days, thus potentially affecting viability. In addition, the lack of correlation between percentage reduction in cfu counts and cell viability rate could be due to the presence of viable but nonculturable cells (VBNC), as we previously demonstrated with breast prostheses [23]. Therefore, it is important to analyze not only cfu counts, but also cell viability, as the presence of VBNC may have an important clinical impact.
One of the main limitations of the study was that it was based on a 24 h in vitro biofilm model, which may not mimic a real clinical scenario in which mature biofilms are formed [16]. In this regard, it is complicated to observe differences between high-efficacy solutions against early biofilm, as occurred in our study, given the high efficacy of PI10 and PI0.3. However, our conclusions remain unaffected: of the individual solutions, PI10 is the best, as it achieves 100% efficacy in less time (1 min). In addition, of the sequential combinations, AA3 + H2O2 + PI10 is the best; starting with AA3 enables biofilm disruption followed by the synergistic antiseptic action of H2O2 and PI.
Another limitation of the study is that the samples were refrigerated, potentially affecting cell viability, since higher cell viability values were expected in the positive controls. In addition, the mechanical effect of pulsed washing was not evaluated, since we performed the irrigation via immersion of the steel disc in the solutions tested. Moreover, since we only studied the antibiofilm activity of antiseptics on stainless steel implants, the results may differ depending on the material, as previously demonstrated [13]. It seems that the biofilm does not grow equally on all surfaces (plastic > titanium > PMMA), thus potentially influencing the antibiofilm capacity of the antiseptic solutions tested. Therefore, it would be interesting to consider this in future in vitro models. We did not test other irrigation solutions, such as surfactants, because they have no bactericidal effect and lead to regrowth, and in animal models, they failed to show better antibiophilic activity than saline [11]. Similarly, topical antibiotics were not evaluated, as there is evidence that their use does not improve the results after DAIR, with inadequate eradication rates reported for Staphylococcus spp. and E. coli [24]. Lastly, SEM studies should have been performed after each treatment step in the combinations to confirm the cumulative effect. However, this analysis is very time-consuming and expensive.
Therefore, our data should be validated with other materials, and further clinical data are necessary to determine whether these solutions can reduce PJI in in vivo models.
4. Materials and Methods
4.1. Setting
The study was performed in the microbiology laboratory of a tertiary institution in Madrid, Spain.
4.2. Laboratory Procedure
We designed an in vitro model with stainless steel disc implants based on a two-phase model with a contamination step (24 h biofilm), in which each strain was tested before being disinfected with antiseptics and their combinations (detailed below) or sterile saline (SS, for positive controls). Different exposure times were chosen to assess the impact on colony-forming unit (cfu) counts and, in combination assays, on cell viability and biofilm structure.
4.3. Availability of Data and Material
Datasets will be kept by the Microbiology and Infectious Diseases Department, and data collection will be registered in the repository of the Instituto de Salud Carlos III (ISCIII) under number C.0001228.
5. Conclusions
Based on our results, we propose a sequential combination of 9 min exposure of AA3 + PI10 + H2O2 (sequential combination 2). AA3 ensures chemical debridement and disruption of the biofilm, followed PI10, which has a bactericidal effect, then followed by H2O2, which has a bactericidal effect and increases the porosity of the cell wall. However, when sequential combinations are not available, PI may be a cost-effective alternative, as its anti-biofilm efficacy outperformed that of all the other solutions.
References
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Kitridis, D.; Tsikopoulos, K.; Givissis, P.; Chalidis, B. Mortality and complication rates in nonagenarians and octogenarians undergoing total hip and knee arthroplasty: A systematic review and meta-analysis. Eur. Geriatr. Med. 2022, 13, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Joint Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Morcos, M.W.; Kooner, P.; Marsh, J.; Howard, J.; Lanting, B.; Vasarhelyi, E. The economic impact of periprosthetic infection in total knee arthroplasty. Can. J. Surg. 2021, 64, E144–E148. [Google Scholar] [CrossRef] [PubMed]
- Akindolire, J.; Morcos, M.W.; Marsh, J.D.; Howard, J.L.; Lanting, B.A.; Vasarhelyi, E.M. The economic impact of periprosthetic infection in total hip arthroplasty. Can. J. Surg. 2020, 63, E52–E56. [Google Scholar] [CrossRef] [PubMed]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Staats, A.; Li, D.; Sullivan, A.C.; Stoodley, P. Biofilm formation in periprosthetic joint infections. Ann. Jt. 2021, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Barros, L.H.; Barbosa, T.A.; Esteves, J.; Abreu, M.; Soares, D.; Sousa, R. Early Debridement, antibiotics and implant retention (DAIR) in patients with suspected acute infection after hip or knee arthroplasty—Safe, effective and without negative functional impact. J. Bone Jt. Infect. 2019, 4, 300–305. [Google Scholar] [CrossRef]
- Goswami, K.; Cho, J.; Foltz, C.; Manrique, J.; Tan, T.L.; Fillingham, Y.; Higuera, C.; Della Valle, C.; Parvizi, J. Polymyxin and Bacitracin in the Irrigation Solution Provide No Benefit for Bacterial Killing in Vitro. J. Bone Joint Surg. Am. 2019, 101, 1689–1697. [Google Scholar] [CrossRef]
- Owens, B.D.; White, D.W.; Wenke, J.C. Comparison of Irrigation Solutions and Devices in a Contaminated Musculoskeletal Wound Survival Model. J. Bone Jt. Surg. 2009, 91, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kruckenhauser, E.-M.; Nogler, M.; Coraça-Huber, D. Use of Lavage Fluids in Arthroplasty to Prevent Postoperative Infections. Drug Res. 2013, 64, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Nishtala, S.N.; Nguyen, J.T.; Bostrom, M.P.G.; Carli, A.V. The AAHKS Best Podium Presentation Research Award: Comparing the Efficacy of Irrigation Solutions on Staphylococcal Biofilm Formed on Arthroplasty Surfaces. J. Arthroplast. 2021, 36, S26–S32. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Abdo, Z.E.; Rossman, S.R.; Kelly, M.A.; Piuzzi, N.S.; Higuera, C.A.; Schwarzkopf, R.; Springer, B.D.; Chen, A.F.; Parvizi, J. What Is the Optimal Irrigation Solution in the Management of Periprosthetic Hip and Knee Joint Infections? J. Arthroplast. 2021, 36, 3570–3583. [Google Scholar] [CrossRef] [PubMed]
- Kia, C.; Cusano, A.; Messina, J.; Muench, L.N.; Chadayammuri, V.; McCarthy, M.B.; Umejiego, E.; Mazzocca, A.D. Effectiveness of topical adjuvants in reducing biofilm formation on orthopedic implants: An in vitro analysis. J. Shoulder Elbow Surg. 2021, 30, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.A.; Wu, M.; Cochrane, N.H.; Belay, E.; Myntti, M.F.; James, G.A.; Ryan, S.P.; Seyler, T.M. Efficacy of common antiseptic solutions against clinically relevant microorganisms in biofilm. Bone Jt. J. 2021, 103-B, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.; Cho, J.; Fleischman, A.; Goswami, K.; Ketonis, C.; Kunutsor, S.K.; Makar, G.; Meeker, D.G.; Morgan-Jones, R.; Ortega-Peña, S.; et al. General Assembly, Prevention, Antiseptic Irrigation Solution: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, K.O.; Glynn, A.A.; Molony, D.C.; Murray, D.; Rowe, S.; Holland, L.M.; McCormack, D.J.; O’Gara, J.P. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J. Orthop. Res. 2010, 28, 1252–1256. [Google Scholar] [CrossRef]
- Ernest, E.P.; Machi, A.S.; Karolcik, B.A.; LaSala, P.R.; Dietz, M.J. Topical adjuvants incompletely remove adherent Staphylococcus aureus from implant materials: Topical Adjuvants and Biofilm Reduction. J. Orthop. Res. 2018, 36, 1599–1604. [Google Scholar] [CrossRef]
- Shohat, N.; Goh, G.S.; Harrer, S.L.; Brown, S. Dilute Povidone-Iodine Irrigation Reduces the Rate of Periprosthetic Joint Infection Following Hip and Knee Arthroplasty: An Analysis of 31,331 Cases. J. Arthroplast. 2022, 37, 226–231.e1. [Google Scholar] [CrossRef]
- Romano, V.; Di Gennaro, D.; Sacco, A.M.; Festa, E.; Roscetto, E.; Basso, M.A.; Ascione, T.; Balato, G. Cell Toxicity Study of Antiseptic Solutions Containing Povidone–Iodine and Hydrogen Peroxide. Diagnostics 2022, 12, 2021. [Google Scholar] [CrossRef]
- Siddiqi, A.; Abdo, Z.E.; Springer, B.D.; Chen, A.F. Pursuit of the ideal antiseptic irrigation solution in the management of periprosthetic joint infections. J. Bone Jt. Infect. 2021, 6, 189–198. [Google Scholar] [CrossRef]
- Fernández-Ibarburu, B.; Díaz-Navarro, M.; Ibarra, G.; Rivera, A.; Hafian, R.; Irigoyen, Ã.; Carrillo, R.; Pérez-Cano, R.; Muñoz, P.; García-Ruano, Á.; et al. Efficacy of Povidone Iodine Against Microbial Biofilms in Breast Implants with Different Textures: Results From an in vitro Study. Front. Microbiol. 2022, 13, 868347. [Google Scholar] [CrossRef]
- Azzam, K.A.; Seeley, M.; Ghanem, E.; Austin, M.S.; Purtill, J.J.; Parvizi, J. Irrigation and debridement in the management of prosthetic joint infection: Traditional indications revisited. J. Arthroplast. 2010, 25, 1022–1027. [Google Scholar] [CrossRef]

