1. Introduction
Pediatrics is the field of medicine that centers on physical, social, and mental health from birth to the end of adolescence [1].
The pediatric population can be subcategorized, according to the “International Council for Harmonization” (ICH) topic E11 (CPMP/ICH/2711/99) and the ICH E11(R1), as preterm newborn infants (from the day of birth to the expected date of birth plus 27 days), term and post-term newborn infants (aged from 0 to 27 days), infants and toddlers (with 28 days to 23 months), children (aged between 2 and 11 years old), and adolescents (with age ranges from 12 to 16–18 years old, depending on region) (Figure 1).
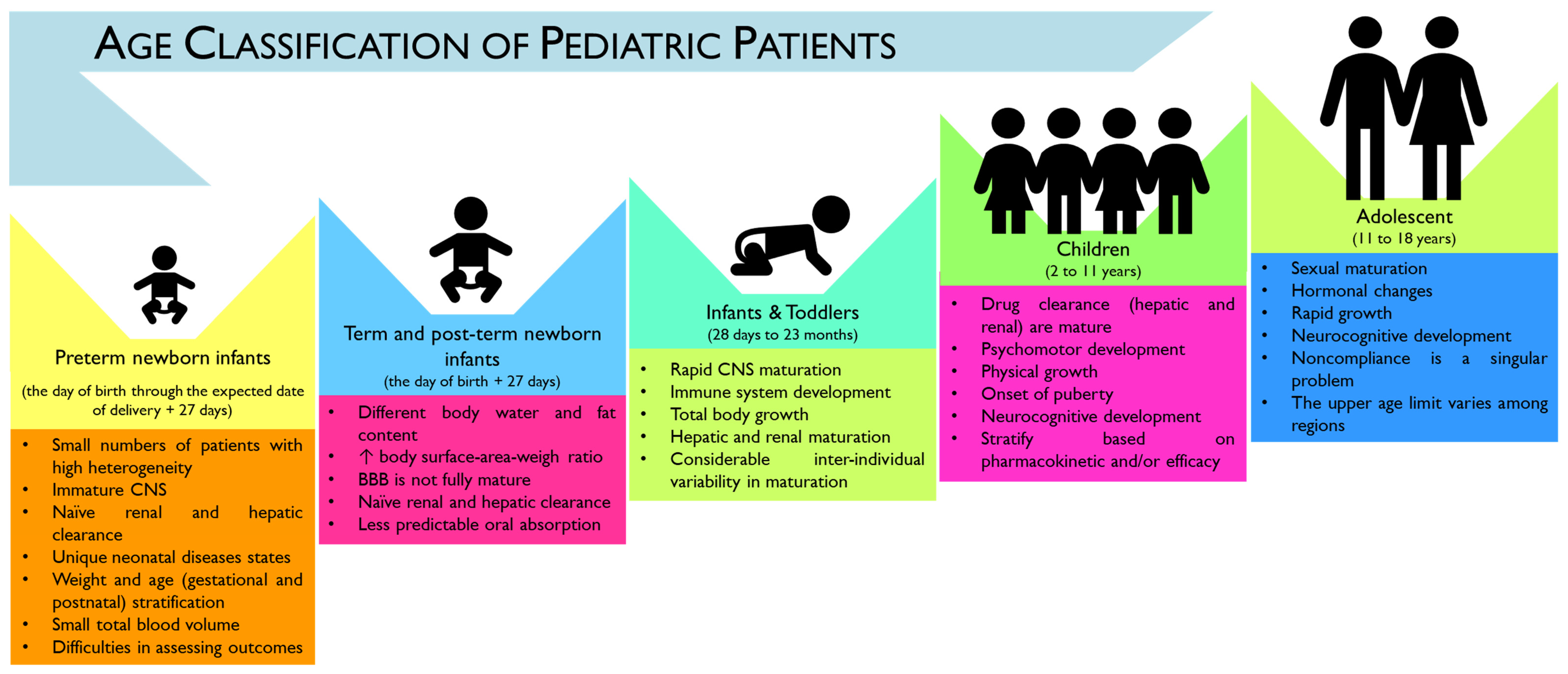
Figure 1. Infographic of the age categorization of pediatric patients according to the International Council for Harmonization (ICH) guideline E11(R1). As summarized, there is considerable heterogeneity in developmental categorization (e.g., physical, cognitive, and psychosocial) across pediatric ages [2]. Central nervous system (CNS), blood–brain barrier (BBB), increase (↑).
However, a considerable overlap can exist across the age subcategories, namely in physical, cognitive, and psychosocial development. Moreover, no consensus seems to exist on the upper age limit of pediatric patients, which may hamper the evaluation and development of age-appropriate treatment plans [3]. In particular, according to the American Academy of Pediatrics (AAP), the upper age limit of pediatrics is considered 21 years, with a proposed subcategorization of adolescence into three main groups: (1) early, represented by adolescents from 11 to 14 years old; (2) middle, for adolescents with ages between 15 and 17 years old; and (3) late adolescence ranging from 18 to 21 years old. However, this age limit has been questioned as increasing evidence has demonstrated that brain development only reaches adult levels of functioning by the third decade of life, which may contribute to the increase in complexity when addressing age-related pathologies and treatments [4].
Historically, the intrinsic heterogeneity in the pediatric population and the reduced number of individuals that can be included per each subcategory in clinical trials may have constituted fatal reasons to dub children as “therapeutic orphans” and for the “off-label” prescription of adult medication to pediatric patients. However, this paradigm has been shifting as it is well recognized that children cannot be considered mini-adults, since the developmental, physiological, and metabolic stages across these two age segments are critically different [5]. The impact on the pharmacokinetics (PK) and pharmacodynamics (PD) of the Active Pharmaceutical Ingredients (API) makes it unreasonable to translate dosage forms and dosage strengths straightforwardly from adults to children [6,7,8].
Therefore, a strategic workforce has been constructed to appropriately reply to disease burden across childhood, addressing the therapeutic deficit and developing age-appropriate formulations, in order to maximize efficacy and design quality, promote safety, minimize risks, and increase patient adherence to treatments [9,10].
Considering the route of administration, the most favored is the oral one. In contrast, the parenteral route remains reserved for more acute conditions, mainly when a quick onset is required [10]. Planning a pediatric oral formulation is challenging, and involves the choice of excipients, dosage form, and palatability [11]. For instance, the choice of dosage form for oral administration depends on the gut function and, thus, on both age and clinical condition [12]. Moreover, the choice of excipients for pediatric drug formulation has been questioned as certain excipients used in adult drug formulation are not adequate for pediatric use, with toxicological risks and safety issues in children [13]. Therefore, the collaboration of the European and the United States Pediatric Formulation Initiatives (PFIs) has resulted in the creation of the “Safety and Toxicity of Excipients for Pediatrics” (STEP) database that aims for the screening of excipients that can appropriately fit pediatric drug formulation [13,14,15]. Furthermore, a set of potentially inappropriate drugs for pediatric use has been released by the “Key Potentially Inappropriate Drugs in Pediatrics” tool, or “KIDs” List, with the primary goal of anticipating risks for adverse drug reactions (ADRs), decreasing severe ADRs, improving the quality of care, decreasing costs, and identifying subjects that need research in the pediatric population [16].
Despite the efforts made in the development of pediatric drug formulation, as well as in age-appropriate medical devices, clinical trials and approved drugs for the pediatric population remain constrained [17,18,19].
Nanotechnology has received enthusiasm among the scientific community, particularly in medicine and pharmaceutical fields, due to its potential to incorporate diagnostic and treatment tools in the same nanocarrier, enhance targetability to specific organs, decrease toxicity, and potentially reduce treatment schedules. At the same time, it provides a tool to increase patient compliance, which is an essential task concerning the pediatric population [20,21,22]. Together with nanomedicine, the advanced therapy medicinal products (ATMPs) have been considered by the European Parliament as the “therapies for the future” [23]. ATMPs are a heterogeneous group of biopharmaceuticals encompassing gene therapy, somatic cell therapy, tissue-engineering, and their combination. These nascent technologies have the potential to reduce or repair disease-causing cells, thereby introducing a curative approach to address the unmet medical needs and highlighting personalized precision medicine [24], with promising applications in the pediatric population [25].
Considering the timely subject and the undoubted shifting in the pediatric drug development paradigm, this literature review aims to outline the historical paradigm in pharmaceutical drug development for the pediatric age, delineating the pros and cons of using innovative therapies, such as nanomedicines and ATMPs, for treating pediatric pathologies, based on a fit-by-design approach, centering its reflection on the role of pharmaceutical technologists and developers in the conception of pediatric medicines.
2. Study Conception
A revision of the literature in different databases, such as Pubmed, Web of Sciences, and ScienceDirect, was carried out. Some of the following index terms were included: “advanced therapies medicinal products”, “cell therapy”, “child”, “children”, “gene therapy”, “nanomedicine”, “nanoparticles”, “nanotechnology”, “pediatrics”, “neoplasms”, “tissue engineering”, “drug formulation”, among others. In particular, the following MeSH terms were adopted: Pediatrics; Nanoparticles; Gene therapy; and Cell- and Tissue-Based Therapy. Other core databases were assessed, including https://www.ema.europa.eu/en, https://www.fda.gov/, https://clinicaltrials.gov/, https://www.clinicaltrialsregister.eu/ and https://www.nih.gov/, among others. When appropriate, the boolean operators “AND”, “OR” or “NOT” were applied. The inclusion criteria were the following: articles that contained one of the considered index terms in the title or in the abstract, and were presented preferentially in English. At least one author read the title and the abstract of the manuscripts to select the articles to be included as bibliographic support in this work.
3. Pediatric Drug Development: The Paradigm Is Shifting
The development of pediatric dosage forms and drug formulations has faced particular setbacks (Figure 2) [26,27,28] during the years, with widening repercussions in the off-label prescription of adult medications to pediatric patients [29].
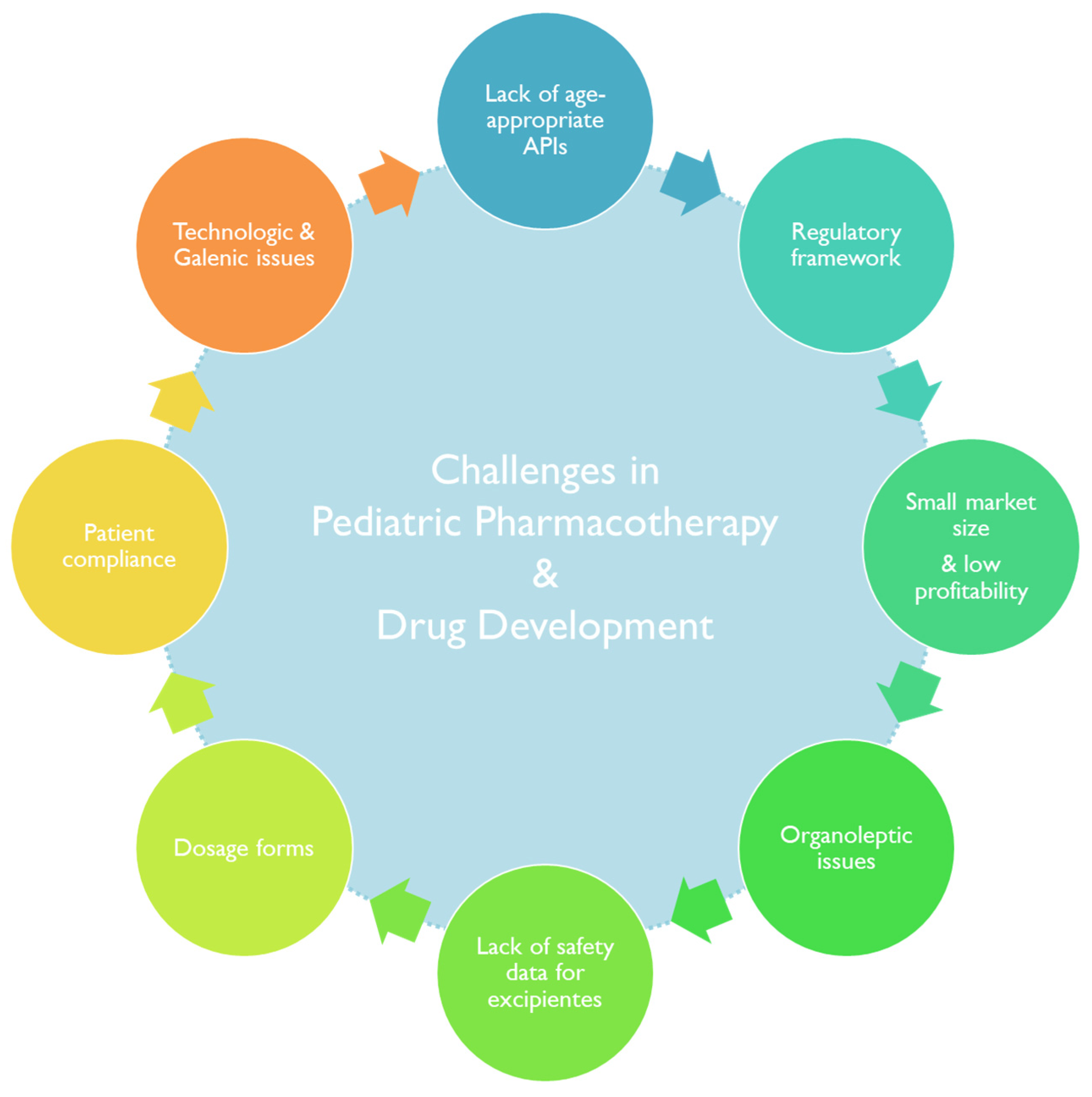
Figure 2. A summary of the factors impacting pharmacotherapy practice and the development of therapeutics aimed at pediatric patients.
However, the paradigm seems to be shifting, and overdue attention has been invested in overcoming the scarcity of pediatric age-appropriate medicines [30]. In the following section, a historical perspective of the regulatory landscape of pediatric medicines will be given. Next, some notes on the hit-or-miss game in research and financial investment in pediatric drug formulation, followed by an overview of some challenges in pediatric drug pharmacotherapy, will be provided.
3.1. Snapshot into the Pediatric Drug Development History
Implementing clinical trials as a new requirement for drug approval has rocked the pharmaceutical pipeline. The “Drug Efficacy Study Implementation Program”, conducted between 1938 and 1962, highlighted the need to reframe the clinical and pharmaceutical pipeline for drug approval. Since then, efforts have been raised to achieve the currently implemented step-by-step-based framework that encompasses drug discovery and development (D&D), pre-clinical studies, bridging first-in-human phase 0 studies, and clinical studies, phase I to IV (Figure 3) [31,32,33,34].

Figure 3. Infographic of the pharmaceutical pipeline and clinical trials timeline. In brief, a screening of potential drug candidates is performed during the drug discovery and development (D&D) step, which takes an average time of five years. The most promising compounds go further to pre-clinical trials under good laboratory practices (GLP), with a possible bridge to the clinical phase by taking advantage of the first-in-human trials (Phase 0), with interesting feedback on dosing and toxicity levels of the most promising candidate, which takes 18 months on average. As part of the investigational new drug (IND) portfolio, the clinical trials can go further, and if the treatment is effective and safe for human use the new drug application (NDA) obtains the approval of the regulatory agency (FDA). After, the pharmacovigilance post-marketing safety and efficacy studies are conducted over time [32,33]. High throughput screening (HTS).
The enrollment of the pediatric population in clinical studies has been steadily increasing [35]. In 1977, the American Academy of Pediatrics (AAP), together with the US Food and Drug Administration (FDA), delivered the “AAP guidelines on clinical studies in pediatric populations” [36]. Later, in 2007, Pediatric Regulation rose in the EU and the US, boosting pediatric drug development through marketing exclusivity incentives [37,38]. Moreover, since 2011, policies encouraging pediatric drug development and distribution have been launched in China [39]. Figure 4 outlines the regulatory background of pediatric drug approval in the US, the EU, and China [28,39,40,41,42].
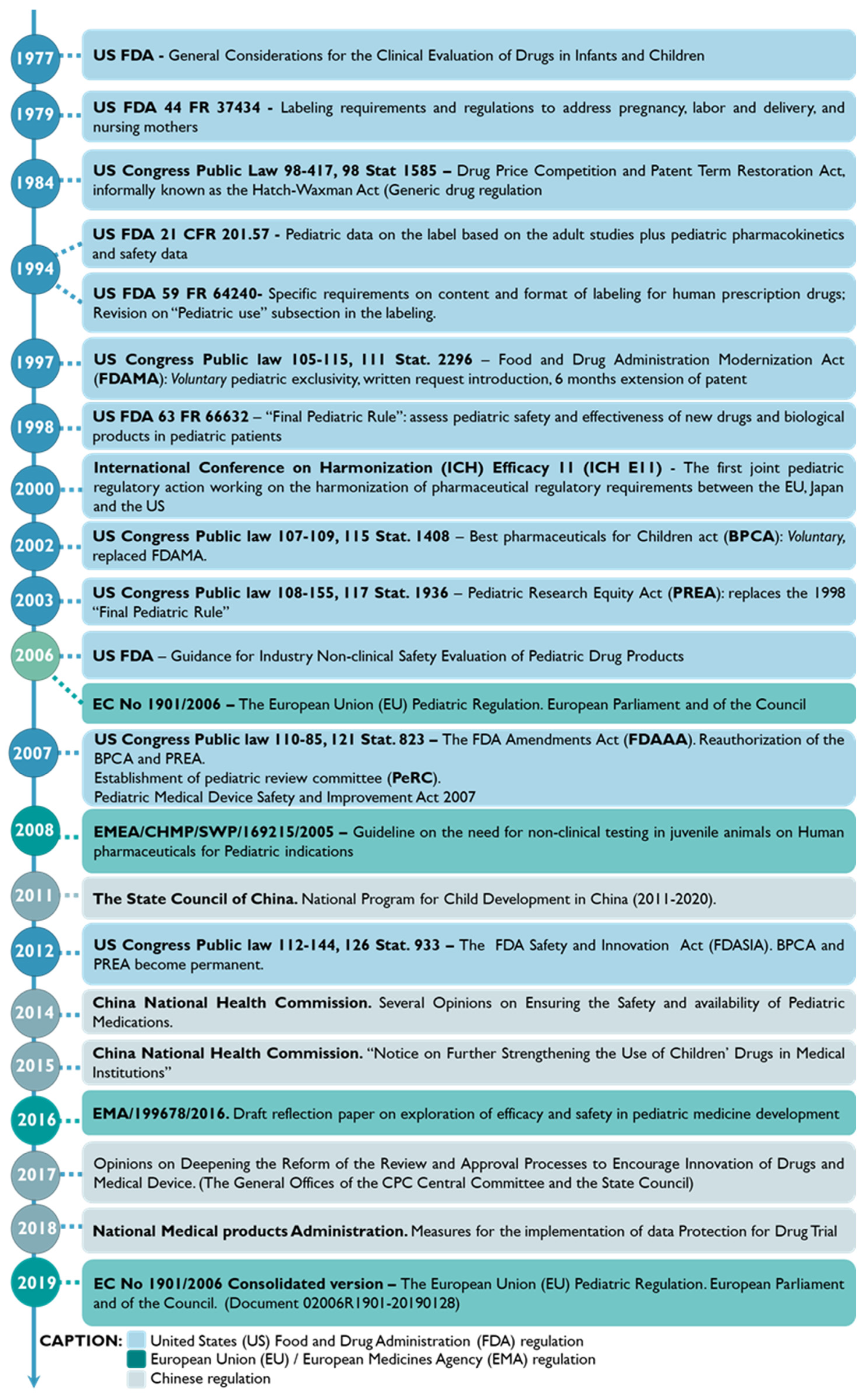
Figure 4. Historical roadmap of the regulation and guidelines developed for clinical studies related to the pediatric population in China, the European Union (EU), and the United States (US) [28,39,40,41,42].
The Orphan Drug Act seems to be a boon for pediatric medicine [43]. Moreover, supportive initiatives, such as the Pediatric Research Equity Act (PREA), the Best Pharmaceuticals for Children Act (BPCA), and/or the Pediatric Investigation Plan (PIP), have provided a carrot-and-stick approach to pediatric medicine advancements [43,44].
The implementation of EU directive no. 1901/2006 has promoted the accessibility of medicines for individuals under 18 years old, without compromising the access of adults to these products or the well-being of children, requiring the investigation of safety and efficacy and quality on an age-appropriate based approach. Notably, to promote investment in the development of new drug candidates and formulations when preparing a marketing authorization application (MAA), the pharmaceutical industry is requested to include a PIP to address the safety of the medicine for the pediatric population [10].
Despite these achievements, significant heterogeneity in funding sources, pediatric clinical conditions, and study characteristics still impact the participation of the pediatric population in clinical trials [40,45]. Based on a search performed on the https://clinicaltrials.gov/ database, it was possible to detect that among 462,303 registered clinical trials, only 90,920 were designed for children (from birth to 17 years old) (data collected on 14 August 2023). Moreover, in the EU Clinical Trials Register database, EudraCT, from the 43,644 clinical trials reported, only 7229 were conducted in the population less than 18 years old [46]. Moreover, some issues regarding age-appropriate equipment and medical techniques, a “child-friendly” environment, pediatric expert physicians and other health professionals, together with the management of caregivers, may also contribute to limiting the enrollment of the pediatric population in clinical trials [27].
3.2. Constrains in Drug Development for Pediatric Patients
4. Nanomedicine for Pediatric Healthcare
Nanomedicine has emerged through the conjugation of two main fields, namely nanotechnology and medicine. The European Technology Platform on Nanomedicine (ETPN) defined the term nanomedicine as the use of nanotechnology to achieve advances in healthcare by exploiting unique bio and physicochemical properties of materials at the nano scale [131]. On the other hand, the EMA refers to nanomedicine as the application of nanosized components with specific advantageous properties, such as better targeting and bioavailability of therapeutics, new modes of therapeutic action, and nanostructured surfaces/scaffolds for engineered tissues [132]. Among the most studied nanoparticles intended for the prophylaxis, diagnosis, and treatment of diseases are inorganic, lipid-based, and polymeric-based nanoparticles (Figure 7) [20].
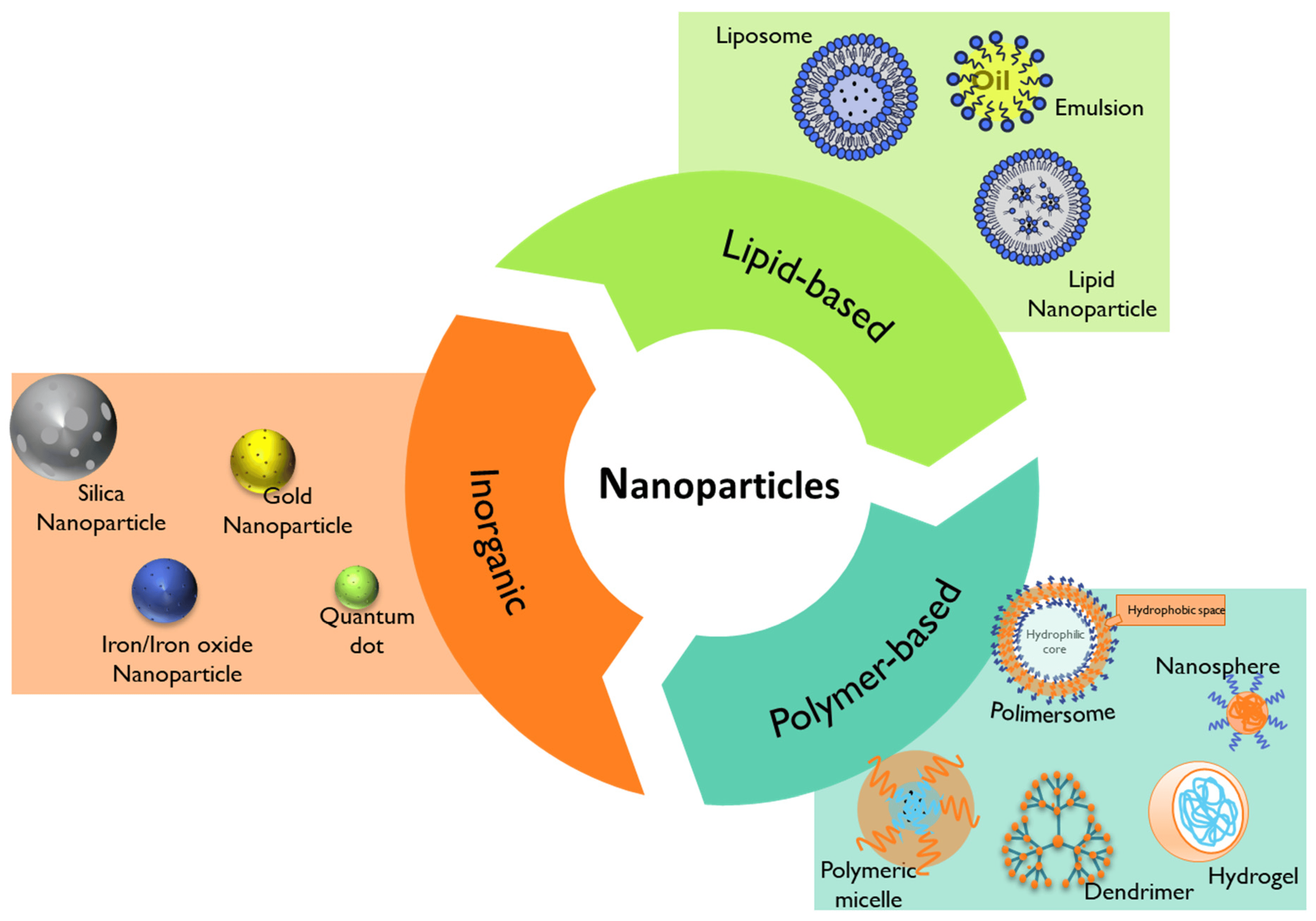
Figure 7. Summary of the different types of nanoparticles that can be used in nanomedicine.
In the field of pediatric medicine, the use of nanomedicine has offered innovative solutions for the diagnosis and treatment of various conditions, particularly in cancer [133,134,135], infection [136], dentistry [137], dermatology [138], and nutrition [139].
4.1. Lipid-Based Nanoparticles
Lipid-based nanoparticles comprise liposomes, lipid nanoparticles, and emulsions (Figure 7) [118]. Their advantageous properties, like biocompatibility, formulation simplicity, and payload flexibility, make them the most highly approved nanomedicines by the FDA [20,118].
Liposomes are typically composed of phospholipids, which can form unilamellar and multilamellar vesicular structures which allow the delivery of hydrophilic, hydrophobic, and lipophilic drugs in the same system. Liposomes can be modified to extend their circulation and enhance delivery, avoiding rapid detection from the reticuloendothelial system (RES) [118].
Nano-emulsions are heterogeneous oil-in-water or water-in-oil emulsions mainly formed by oil droplets containing the API, stabilized by surfactants and cosurfactants and dispersed in an aqueous external phase [20]. They are usually prepared using Generally Recognized as Safe (GRAS)-grade excipients approved by the FDA [140], and possess high loading capacity for lipophilic APIs with some thermodynamically reported instabilities [141].
The development of next-generation lipid nanoparticles, namely solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), has emerged to overcome some limitations of the conventional lipid-based nanosystems [20,142]. Lipid-based nanoparticles like SLN and NLCs can offer the targeted delivery of drugs, increase the bioavailability of hydrophobic drugs, and protect sensitive active compounds [20].
Lipid-based nanoparticles have been widely investigated for various applications, namely in cancer [143,144] and more recently in the formulations of the mRNA COVID-19 nano-vaccines [145], with some of them approved by the FDA for different therapeutic purposes .
Among these, liposomes are the most widely studied in pediatrics, and transversal variations in the PK parameters have been registered between the adult and the pediatric populations [161,162].
Furthermore, significant differences between the participation of children (birth–17 years) versus adults in clinical trials using liposomes (clinicalTrial.gov database, data collected by 7 August 2023) have been registered. In fact, of 285 clinical trials that are currently recruiting or not yet recruiting, only 31 include liposomes in pediatrics (birth–17 years), with the majority of them addressing cancer treatment .
Other types of lipid nanoparticles, such as in situ self-assembly nanoparticles (ISNPs), have been investigated. For example, child-friendly Lopinavir/Ritonavir pediatric granules utilizing ISNPs were developed. In vivo pre-clinical data demonstrated that the orally administered formulation improved lopinavir bioavailability and concentration in the brain and lymphoid tissues, the target sites of the HIV [163]. In another study, Rodríguez-Nogales et al. formulated nano-assemblies using squalenoyl-gemcitabine and alkyl-lysophospholipid edelfosine with a nanoprecipitation method. Their results revealed that the 50 nm nanoparticles presented a high uptake by human osteosarcoma cells, resulting in antitumoral activity and enhanced gemcitabine and edelfosine pharmacokinetic profiles [164].
4.2. Polymer-Based Nanoparticles
Polymer-based nanoparticles are colloidal systems made up of natural, semi-synthetic, or synthetic polymers (Figure 8), allowing for a wide variety of possible architectures and characteristics [20,162]. They include dendrimers, polymeric micelles, polymersomes, nanospheres, and nanogels (Figure 7) with diverse clinical applications [20]. Usually, natural polymers present fewer toxic effects than synthetic polymers [165].
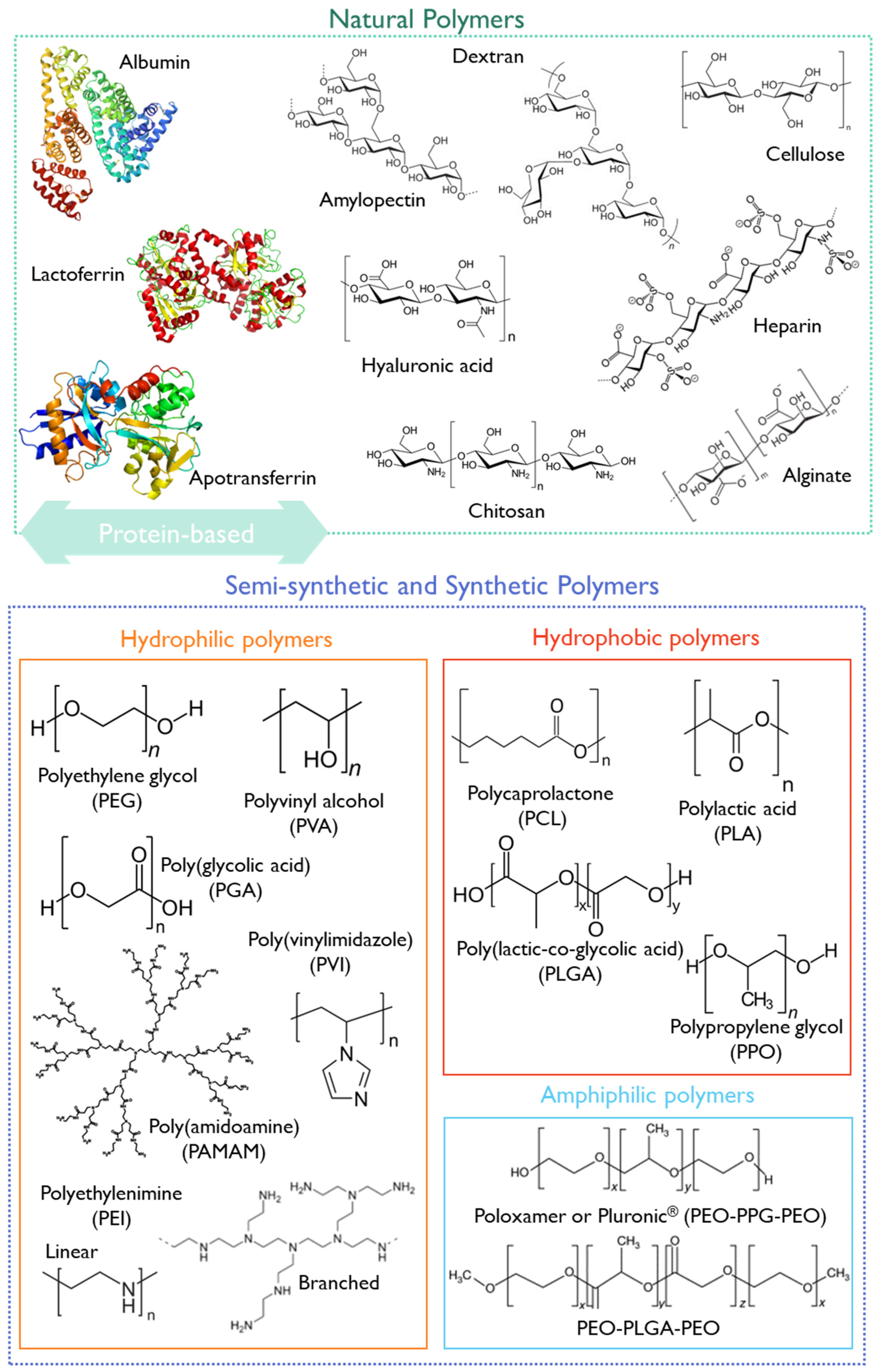
Figure 8. Structural representation of some natural, semi-synthetic, and synthetic polymers.
They can be biodegradable or non-biodegradable. As biodegradable polymers undergo biodegradation in vivo through enzymatic or non-enzymatic pathways producing biocompatible or harmless by-products, they have been preferred in nanomedicine, particularly for pediatrics [162]. The performance of polymeric biodegradable formulations can be improved by (1) using FDA-approved biodegradable polymers, (2) administering the formulations in situ, (3) using combined therapies, such as immunotherapy or radiotherapy, and (4) applying the on-demand delivery of molecularly targeted agents [166].
Some examples of biodegradable polymers are polysaccharides, such as hyaluronic acid, chitosan, dextrin, or alginate (Figure 8).
Chitosan is a natural biocompatible and biodegradable cationic polymer with low toxicity. It is based on deacetylated chitin [167] obtained from crustaceans, insects, squibs-centric diatoms, or fungi [168]. At an acidic pH, chitosan presents a high density of positive charges that deliver mucoadhesive properties, and a suitable environment for complexing anionic polymers or nucleic acids [169]. Moreover, it can entrap poorly water-soluble drugs, combining antimicrobial, anti-inflammatory, and wound-healing effects [170]. This polymer has been classified by the FDA as GRAS [171], and is approved as a biomaterial for use in tissue engineering and drug delivery applications [172]. Furthermore, chitosan has been applied in developing pediatric formulations , and some chitosan formulations underwent clinical trials, as summarized in .
However, concerns regarding the source, purity, and immunogenicity of chitosan have hampered its approval for pharmaceutical applications [172].
Hyaluronic acid (HA) is a mucopolysaccharide present in the extracellular matrix, synovial fluid, and connective tissues, consisting of D-glucuronic acid and (1-b-3) N-acetyl-D-glucosamine alternating units (Figure 8) [166]. HA is biocompatible, non-immunogenic, and biodegradable, and presents a viscoelastic nature, making it suitable for nanomedicine applications [166]. Cluster of differentiation-44 (CD44) is a main receptor of HA and is overexpressed in solid tumors, making it suitable for cancer-targeting purposes [178]. Due to its versatile properties, HA has been studied for pediatric drug formulations, aiming at increased patient compliance through the modification of the dosage form or by decreasing the dosing frequency [179,180,181]. Moreover, HA has already undergone clinical trials, with 91 registered entries addressing the pediatric population (birth to 17 years).
Another group of natural polymers is the protein-based biomaterials, such as albumin, lactoferrin, or apotransferrin (Figure 8).
Albumin is a water-soluble globular protein present in ca. 50% of the total plasma body mass. Due to its hemocompatibility, albumin has been applied for intravenous gene and drug delivery. Consequently, an albumin-based nanosystem for the delivery of paclitaxel (Abraxane®) received FDA approval in 2005. According to the information approved by the FDA in 2020 (Reference ID: 4661467), the safety and effectiveness of Abraxane® have not been established in pediatric patients so far. However, in 2013, a Phase 1/2 clinical trial (NCT01962103) was begun aiming to find the safe dose of nab-paclitaxel, Abraxane®, in children with solid tumors, and to see if it could constitute a treatment for children and young adults with solid tumors (1 ≤ 18 years old in Phase 1 and 2 ≤ 24 years old in Phase 2).
Lactoferrin (LF) is a natural cationic iron-binding glycoprotein present in milk, with antiviral, anti-inflammatory, antioxidant, anti-cancer, and immune-stimulating effects [182,183]. LF receptors are known to be overexpressed in cancer and endothelial brain cells, making them suitable for active tumor targeting or crossing the blood–brain barrier (BBB) via receptor-mediated transcytosis for brain delivery. In addition, LF-based nanocarriers were found to have a pH-dependent release profile. At an acidic pH, a faster drug release is observed, which could increase drug release in acidic sites such as the tumor tissue microenvironment and could enhance the therapeutic efficacy of the encapsulated hydrophobic active molecules [182,184]. Commercial preparations of bovine lactoferrin, recognized as GRAS by the FDA, are commonly used in in vitro and in vivo testing. Recently, recombinant human lactoferrin has also become available [185]. Ahmed et al. [186] developed LF-based nanoparticles containing carboplatin to address retinoblastoma in children. Apotransferrin-based nanoparticles were also prepared as they are also implicated in iron transport [186,187]. In another study, Narayana et al. developed carboplatin and etoposide-loaded LF nanoparticles to address retinoblastoma treatment in vitro [188].
Semi-synthetic or synthetic polymers have also been exploited for pediatric applications. The FDA-approved synthetic polymer PEG is widely used due to its biocompatibility and biodegradability [162,189]. It is often combined with other more hydrophobic polymers or other API nanocarriers since it provides stealth properties and improves the pharmacological properties of nanomedicines. However, some allergic reactions were reported when using PEG as an excipient in pediatric drug formulation, which may limit its use (as reported above, Section 3.2.5).
Polycaprolactone (PCL) is recognized as non-toxic and suitable for controlled/sustained drug and vaccine delivery owing to its high permeability in relation to drugs [166]. Conjugates of PLC with PEG have recently been reviewed [190]. Krishnan et al. produced PEG-PCL nanoparticles using the nanoprecipitation method, aiming at treating leukemia in the pediatric population. The in vivo results have demonstrated improved life quality and survival in mice in the dexamethasone-loaded nanoparticles group compared to the free drug group [191].
The FDA-approved polymer poly lactic-co-glycolic acid (PLGA) has shown suitable properties for drug delivery, with improved circulation time and permeability. PLGA is an aliphatic polyester polymer that comprises a synthetic copolymer of lactic acid (α-hydroxy propanoic acid) and glycolic acid (hydroxy acetic acid) with demonstrated potential for drug delivery and tissue engineering scaffolds [192]. The 50:50 ratio of lactic to glycolic acid monomers and molecular weight PLGA (3–9 kDa) have been associated with decreased half-time and fastest degradation [161]. PLGA-PEG nanoparticles have been synthesized and decorated with a CD133 aptamer to target salinomycin delivery to CD133+ pediatric osteosarcoma cancer stem cells [193].
Other synthetic polymers (Figure 8), such as polyethyleneimine (PEI), poly(vinylimidazole) (PVI), or poly(amidoamine) (PAMAM), will be discussed in more detail in Section 5.3 due to their unique properties for gene delivery.
Due to their versatility, the arrangement of different polymers can result in different nanoparticle architectures. The following sections will give a brief overview of the use of polymeric micelles and dendrimers in pediatric nanomedicine.
4.3. Inorganic Nanoparticles
Inorganic nanoparticles encompass metal nanoparticles (iron, gold, silver, and zinc) or rare-earth metal nanoparticles (lanthanum oxide, La2O3 or ytterbium oxide, Yb2O3) and silica nanoparticles, among others [20]. They have been widely used to diagnose and treat atherosclerosis or cancer [20]. The FDA has approved some inorganic nanoparticles intended for iron replacement therapies or for treating anemia and associated diseases . Among them, Venofer® and Ferrlecit® have been studied for pediatric interventions. Venofer® is an iron oxide nanoparticle coated with sucrose used for the slow dissolution of iron following intravenous injection, preventing a rapid and toxic increase in free iron in the blood. Ferrlecit® is a stable macromolecular complex of sodium ferric gluconate in sucrose [161].
Ongoing research in this field has highlighted the possible application of inorganic nanoparticles in diagnosing, treating, and monitoring pediatric brain tumors [206] and other pathologies [130]. Moreover, the application of hybrid nanoparticles has also revealed promising features [207]. For example, the use of Angiopep-2 (An)-PEG-doxorubicin (DOX)-gold nanoparticles (AuNPs) could penetrate the BBB and target glioma cells (Figure 9) [207].

Figure 9. (A) Schematic representation and (B) delivery procedure of the angiopep-2-PEG-doxorubicin-gold nanoparticles (An-PEG-DOX-AuNPs). Briefly, the LRP1 receptor could mediate An-PEG-DOX-AuNP penetration through the BBB and targeting to glioma cells, after which DOX would be released at the tumor site or in tumor cells and enter into nuclei to induce tumor cell apoptosis. Reprinted from [207], copyright (2014), with permission from Elsevier.
4.4. Challenges in Using Nanotherapy in Pediatrics
As reviewed by us previously, the bright side of the coin in the application of nanotechnology in medicine may obscure dark shadows and it should further evolve as an auxiliary to circumvent troubleshooting in nanomedicine [20]. These challenges may impact not only the adult population, but particularly the pediatric population, as limited information for this age group is available [20,68]. Moreover, most preclinical studies to assess the impact of the physicochemical properties of nanosystems are conducted in adult models after intravenous administration, while the preferential route of administration for pediatrics is p.o. [208]. Additionally, the evaluation of the PK parameters of the nanoformulations could also be hindered, as reviewed elsewhere [161]. Other issues regarding the application of nanotherapies in pediatrics are transversal to those present for different dosage forms. However, here it is more evident because the topic of nanomedicine is more recent, and there is a vast unknown to explore [209].
In Figure 10, a snapshot of the main issues that remain to be overcome in using nanotherapies in the pediatric age is presented.
When designing a nanomedicine intended for pediatric application, it would be beneficial to consider some of these points, particularly regarding the safety and efficacy that could contribute to long-term effects [210]. It would also be relevant to study how environmental exposure to nanoparticles could impact children’s health, development, and their treatment response [211].
Moreover, ethical concerns regarding informed consent in this age group for enrollment in clinical trials, the lack of public understanding of nanotechnology, and socioeconomic issues may also limit the studies using nanoparticles in children [130]. The pros and cons of nanomedicine should cross all stages during the nanomedicine design and development, focusing on the well-being and the best interest of children.
Taking into account potential benefits, nanomedicine has been dubbed, together with ATMPs, as the “therapies for the future” by the European Parliament [23]. The following section summarizes some advancements and issues of ATMPs, mainly focusing on the pediatric population.
5. Advanced Therapy Medicinal Products (ATMPs) for Pediatric Healthcare
ATMPs are medicinal products that encompass (1) gene therapy medicinal products (GTMPs), (2) somatic cell therapy medicinal products (sCTMPs), (3) tissue-engineered products (TEPs), and (4) combined ATMPs (e.g., tissue or cell-associated with a device) (Figure 11) [212,213]. The decision dendrogram regarding the different types of ATMPs can be found in the EMA reflection paper: EMA/CAT/600280/2010 rev.1.
5.1. ATMPs—Legal Framework in the European Union
The EMA is the agency that regulates the free movement of ATMPs within the EU, to facilitate market access to these medicines, foster the competitiveness of European pharmaceutical companies, and ensure health protection for patients. The EMA’s Innovation Task Force (ITF) arose to promote the development of effective, innovative medicines that could be available to patients promptly. Based on this, the EMA has encouraged the development of ATMPS by offering advisory services and incentives.
As for all medicinal products, to obtain marketing authorization, the development of ATMPs should follow the requirements of good manufacturing practice (GMP) stated in the Commission Directive 2003/94/EC, with a specific focus on the GMP guidelines that mainly address ATMPs, presented in the “Good Manufacturing Practice for Advanced Therapy Medicinal Products” (C(2017) 7694 final guideline, from Brussels 2017-11-22). Some important EU GMP guidelines could also be of interest for ATMPs manufacturing, such as Annex 2 of the “Manufacture of biological active substances and medicinal products for human use”, Annex 13 of the “Manufacture of investigational medicinal products”, and Annex 16 of the “Certification by a qualified person and batch release”. Moreover, good clinical practice (GCP) requirements should also be applied for ATMPs, which are described in the Commission Directive 2005/28/EC with complementary details specific for ATMPs in the EC guideline C(2019) 7140 final (Brussels, 2019-10-10). Aligned with these, good laboratory practice (GLP) procedures also need to be taken into consideration concerning ATMPs [214].
Furthermore, in February 2018, the EMA released a draft of the revised guidelines on safety and efficacy follow-up and risk management of ATMPs, EMEA/149995/2008 rev.1. The guideline describes specific aspects of pharmacovigilance, risk management planning, safety, and efficacy follow-up of authorized ATMPs, as well as some elements of clinical follow-up of patients treated with ATMPs.
The overall regulation of ATMPs is summarized in Regulation (EC) No 1394/2007, with a distinct reference to the Committee for Advanced Therapies (CAT) that ensures the trinomial of quality, safety, and efficacy of ATMPs, and provides an up-to-date overview of the scientific landscape in the field. The CAT is also responsible for providing recommendations and scientific advice on classifying ATMPs (Article 17 of Regulation (EC) No. 1394/2007). Micro-, small- and medium-sized companies can also submit requests for certification to the CAT under the Article 18 of Regulation (EC) No 1394/2007, corresponding to the ATMPs regulation.
The Marketing Authorization Application (MAA) procedure for ATMPs requires their evaluation based on the centralized procedure, described in the Regulation (EC) No 726/2004, with the preliminary assessment from the CAT, which deals with the classification of the ATMPs [215]. The centralized procedure can encompass three types of marketing authorization (MA): standard marketing authorization, conditional marketing authorization, and marketing authorization under exceptional circumstances (Figure 12) [216]. A typical MA is conferred when no additional information on quality, safety, and efficacy or in the benefit–risk balance of the medicinal product under evaluation is required regarding that presented in the MAA. A conditional MA may be applied when an unmet medical need supports the availability of medicine to patients before the comprehensive clinical data. An MA attributed in exceptional circumstances occurs only in extreme situations, like when a disease is rare or a clinical endpoint is difficult to measure, and the safety and efficacy data required for a standard MA are pretty challenging to obtain based on the limited data originated from the reduced number of patients [215].
The centralized procedure is characterized by a single application, evaluation, and authorization through all the EU member states, including the European Free Trade Association (EFTA) members, such as Iceland, Liechtenstein, and Norway. In the case of medicines derived from biotechnology processes, orphan medicinal products, and medicines aiming to treat diseases such as cancer or HIV, the centralized procedure is compulsory [215,216]. On the other hand, it could be optional in cases when the new active substances provide other indications than those stated previously, or for those that present scientific, therapeutic, and technical innovation, or whose authorization is considered of particular relevance for the public and animal health in the EU [215,216].
In the centralized procedure, after receiving the application from the developers of the ATMPs, the CAT prepares a draft opinion about the quality, safety, and efficacy of the ATMPs received [216]. Based on the CAT opinion report, the CHMP adopts an opinion recommending (or not) the authorization of the ATMP to the European Commission, which is responsible for the final decision.
Interestingly, the so-called “hospital scheme exemption application” under Regulation EC No 1394/2007 launched the opportunity for a national authorization of non-industrially manufactured ATMPs. Based on this, if an ATMP is designed and produced for an individual patient, it can be used on a non-routine basis in a hospital under the exclusive responsibility of a specific medical practitioner [216].
An ATMP can also be classified as an orphan medical product by the Committee for Orphan Medicinal Products (COMP) of the EMA [216]. The orphan designation is attributed if the disease for which it is intended is a high-risk or chronically debilitating disease, it does not affect more than 5 in every 10,000 people, and there is no other satisfactory therapy. Therefore, it fills a gap and offers benefits to patients. The orphan designation procedure was implemented by the EMA in 2000 with Regulation (EC) No 141/2000 together with the amended Regulation (EC) No 847/2000 that provides definitions and rules for implementation [215]. More guidance could also be found in the 2016/C 424/03. More recently, the EMA released guidance on the designation of ultra-rare disease (Regulation (EU) No 536/2014). The EMA launched the PRIME initiative to accelerate the process of bringing medicines to market, which allows increasing support in developing treatments for unmet medical needs [217]. This scheme enforces communication between the applicant and the EMA from the earliest stages of drug development, which facilitates access to incentives to generate robust data on efficacy and safety for timely access evaluation at the time of application, culminating in the faster arrival of the new medicine to patients [215]. There could also be benefits from marketing exclusivity if designated as an orphan medicinal product. Additionally, even if a product is similar to the one approved, an MA can still be granted for the second product if it is safer, more effective, or otherwise clinically superior [215]. Due to the signs of progress in the development of innovative therapies, particularly in ATMPs, the definition of the concept of a similar medicinal product evolved and on 29 May 2018, the EC Regulation (EU) 2018/781 amended Regulation (EC) No. 847/2000 [215].
Regarding the MAA of ATMPs for the pediatric age, they faced the same routes of application as in adults [25], with crucial regulatory support provided in the Pediatric Regulation, Regulation (EC) No 1901/2006 and its amendment (EC) No 1902/2006. Recognizing the shortfall of pediatric treatments, the Pediatric Regulation offers incentives such as those stated in the pediatric-use marketing authorization (PUMA), access to the EMA-specific pediatric expert committee, and free advice to the industry. In line with this and to further promote the dissemination of pediatric trial results, clinical trials for pediatric interventions in the EU are entirely covered by the EU Clinical Trials Register [25].
Additionally, ATMPs’ post-authorization is guided by the EMA good pharmacovigilance practices (GVP), regarding the draft “Guideline on safety and efficacy follow-up and risk management of Advanced Therapy Medicinal Products” (EMEA/149995/2008 rev.1, 2018) that focuses on the single characteristics of ATMPs in line with Article 14 (4) of the Regulation (EC) No 1394/2007. It also offers a framework for the early mitigation of risks and their consequences to the patients, focusing on the post-authorization follow-up on the safety and efficacy of ATMPs.
The complexity and uniqueness of ATMPs, aligned with their intrinsic heterogeneity, brings some issues in the regulatory strategies, including the need for specialized and certified centers that could help in developing ATMPs, an active framework of follow-up, accessibility and financial and sustainable portfolios, access to robust clinical trials, and the development of animal models that better fit the human profile, all with the trinomial of quality, safety, and efficacy as the main pillars [218].
5.2. FDA and EMA-Approved ATMPs in Pediatrics
Most EMA-approved ATMPs are not indicated for pediatric patients (EMA/CAT/50775/2023). A similar profile has been registered in the US by the FDA [219]. summarizes the EMA and the FDA-approved ATMPs indicated for the pediatric age.
Most ATMPs currently on the market are GTMPs aimed at treating rare diseases. Interestingly, all the EMA-approved ATMPs listed in received orphan medicinal product status and were approved by the PRIority MEdicines (PRIME) scheme.
The following section shall propose key developments using ATMPs particularly targeting the pediatric population [25,220,221,222,223].
5.3. Gene Therapy
Gene therapy medicines relate to applying recombinant nucleic acids to treat, prevent, or cure a disease or medical disorder [224].
Gene therapy can be based on three main strategies: ex vivo, in vivo, or in situ [225]. Ex vivo gene therapy involves the genetic modification of cells outside the body to produce therapeutic factors and their subsequent transplantation into patients [226]. Unlike ex vivo therapy, in vivo gene therapy aims to modify the genetic repertory of target cells within living organisms [227].
The development of safer and more efficient viral vectors based on retro and lentiviruses, combined with improved technology for the scalable production of viral vectors, has enabled the successful therapy of rare genetic disorders [228]. In 2016, the first ex vivo gene therapy worldwide, StrimvelisTM, based on hematopoietic stem cells (HSC), was approved by the EMA for the treatment of severe combined immunodeficiency caused by adenosine deaminase deficiency (ADA-SCID) in pediatric patients that did not have an adequate cell donor [229]. A single infusion of autologous bone-marrow HSC, gene-corrected by γ-retrovirus-based technology, resulted in the long-term correction of T lymphocyte activity, immune reconstitution, and 100% survival during a 7-year follow-up. Additionally, the lack of leukemic transformation in transduced cell clones provides evidence of safety as well as hope for the successful approval of this type of gene therapy for other genetic diseases. Similar clinical benefits were observed in patients with Wiskott–Aldrich syndrome [230,231], X-linked adrenoleukodystrophy, metachromatic leukodystrophy [232,233], or transfusion dependent β-thalassemia [234,235,236] treated with lentiviral (LV) HSC therapy. In the case of X-linked severe combined immunodeficiency, LV therapy proved successful in both pediatric and adolescent patients suffering from secondary effects of previous allogeneic HSC transplant [237]. The use of such autologous therapy also circumvents the limitations of allogenic therapies by evading host–recipient immunologic differences and the need for severe immune suppression. Nonetheless, numerous factors can influence the therapeutic outcome in individual patients or different diseases, and they have been described in detail in a recent review by Naldini [228]. Additionally, the implementation of ex vivo therapy is limited by the requirements of highly specialized experts involved in all stages of the product production and performance, the short shelf life of genetically altered cells, and high costs. In order to fully exploit the therapeutic potential of ex vivo gene therapy, the long-term monitoring of risks related to insertional mutagenesis and oncogenesis, immunogenicity, and off-target effects is needed.
Alipogene tiparvovec (Glybera®) was the first approved gene therapy [222]. In 2022, the EMA approved UpstazaTM, a gene therapy medicine based on eladocagene exuparvovec, a functional gene within the adeno-associated viral vector, for use in children aged >18 months with severe aromatic L-amino acid decarboxylase (AADC) deficiency (EMA/365735/2022).
Nonetheless, despite the progress in viral vector development, some issues related to immunogenicity, low loading capacity, and difficulty in large-scale production are still limiting their translation into clinical practice and have inspired the investigation of alternative, potentially more successful and safer delivery vectors [238]. Therefore, to overcome challenges related to viral vectors, non-viral vectors based on cationic lipids or polymers have been pursued [238].
Examples of cationic lipids that are commercially available are 1,2-dioleoyl-3-trimethylammoniumpropane (DOTAP), N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethyl-ammonium chloride (DOTMA), 2,3-dioleyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium trifluoroacetate (DOSPA), and 1,2-dimyristyloxypropyl-3-dimethyl-hydroxyethyl ammonium bromide (DMRIE). Cationic liposomes have emerged as attractive gene vectors because they enhance pharmacokinetic properties and present relatively low immunogenicity [239]. However, the drawbacks of cationic lipid-based nanocarriers, such as poor stability, low transfection efficacy, and the generation of inflammatory responses, have limited their further application [239].
One of the most studied non-viral vectors for nucleic acid delivery is polyethyleneimine (PEI), which has been considered the gold standard since 1995. PEIs are a group of synthetic, water-soluble, linear, or branched polymers (Figure 8) composed of primary, secondary and tertiary amine groups that confer positive charge density at physiologic pH. Moreover, the “proton-sponge” effect makes them suitable for gene therapy, protecting the nucleic acid cargo from lysosomal degradation [238]. Currently, only one clinical trial is recruiting to test a PEI-based vaccine. The early phase I clinical trial, NCT04049864, aims to evaluate the safety and immunogenicity of a vaccine composed of DNA conjugated with a linear PEI (20 kDa) targeting relapsed neuroblastoma patients with ages between 1 and 20 years old (ClinicalTrials.gov database, accessed on 9 August 2023). The combined form of the vaccine includes an intramuscular injection of the DNA-PEI conjugate (polyplex) and oral administration using the attenuated Salmonella enterica as DNA vaccine carriers. The direct correlation between high molecular weight and high positive charge density may explain the scarcity of clinical data using PEI. While it is advantageous for high transfection efficiency, it may lead to undesirable, off-target toxicity [240]. Therefore, a balance between molecular weight and efficient transfection has been recommended, which has proved challenging. For this, PEI was grafted with hydrophobic moieties like lipoic acid, deoxycholic acid, cholesterol, or phospholipids to improve the transfection efficacy [238]. Furthermore, Wang et al. [241] developed hyperbranched-star PEG-g-PEI as a promising nonviral carrier for gene delivery in retinoblastoma, the most common malignant intraocular childhood tumor.
PEIs can also be conjugated with other polymers, such as Pluronics®, for gene and drug co-delivery [242]. This approach has also been tested for treating pediatric malignancies, such as osteosarcoma [243]. Despite being considered a high transfection non-viral vector, PEI is a non-biodegradable polymer that accumulates around the cell and triggers cytotoxicity, possibly hampering its translation to the clinic [244].
Poly(amidoamine) (PAMAM) are monodisperse and hyper-branched polymers (Figure 8), [245], that have been widely exploited for gene delivery. A major disadvantage of those common dendrimers is their toxicity, associated mainly with the chemistry of the surface amine groups. In a study performed by Wang et al. [246], the PAMAM dendrimer was modified with triazine-containing polymers as a strategy for efficient tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) gene therapy of osteosarcoma [246]. More recently, generation four of the PAMAM dendrimer has demonstrated potential for drug, peptide, and DNA delivery [247]. In spite of its high density of positive charges, which may contribute to its toxicity, in some cases, such as in cancer treatment, as the tumor cells present excessive intracellular negative charges, it could be selectively advantageous [247].
Poly(vinylimidazole) (PVI) (Figure 8) is a water-soluble polymer with a protonable imidazole group at acidic pHs [248]. PVI has additional biocompatibility properties, limited toxicity, and the ability to escape the endosome by activating the “proton sponge” mechanism [244]. The use of PVI for biomedical applications alone [249] or in combination with other polymers such as poly(acrylamide) [250] or chitosan [248] has already been exploited. Particularly, due to the presence of the imidazole group, PVI has been reported to present significant antibacterial activity [251,252].
Mumper et al. reported chitosan (Figure 8) as a potential gene carrier in the mid-1990s [253]. The pKa of amino groups on chitosan is around 6.5, so they tend to remain protonated at acidic and neutral pH [254]. Chitosan is positively charged and soluble in weakly acidic solutions, with a charge density dependent on the pH and the degree of deacetylation [255]. Ta et al. [256] formulated a chitosan hydrogel for pediatric osteosarcoma gene therapy using the pigment epithelium-derived factor (PEDF), with promising anti-tumor activity in vitro (SaOS-2 cells) and in vivo.
More recently, the Clustered Regularly Interspaced Short Palindromic Repeats)/CRISPR-associated protein 9 (CRISPR/Cas9) gene-editing technology has revolutionized gene therapy, as it can permanently correct deleterious base mutations or disrupt disease-causing genes with great precision and efficiency [257]. This technology can help reduce the risk of death in children under the age of five [258]. Therefore, its application to address infectious diseases in the pediatric population has been explored [258]. Malaria is a life-threatening infectious disease that children <5 years old are most vulnerable to, and it is transmitted through the bite of an infected Anopheles mosquito carrying the Plasmodium parasite. A CRISPR/Cas9-based gene editing approach based on the Fibrinogen-Related Protein 1 (FREP1) gene knockout, a fundamental protein for the survival of Plasmodium, was described by Dong et al. [259] in their search for malaria treatment. Moreover, CRISPR-Cas9 technology has been studied for the treatment of severe monogenic diseases, such as transfusion-dependent β-thalassemia (TDT) and sickle cell disease (SCD), by targeting the BCL11A erythroid-specific enhancer, which is responsible for the repression of γ-globin expression and fetal hemoglobin in erythroid cells [260]. Based on this approach, there are two clinical trials, NCT03655678 and NCT03745287, enrolling children (>12 years old) to assess the safety and efficacy of autologous CRISPR-Cas9 Modified CD34+ Human Hematopoietic Stem and Progenitor Cells (hHSPCs) in subjects with TDT and SDS, respectively [260]. In another study, Webber et al. developed a CRISPR/Cas9 system to correct COL7A1 gene [261], which causes recessive dystrophic epidermolysis bullosa (RDEB), a disease that affects the skin and other organs, in which children that are born with this condition are referred to as “butterfly child” [262].
5.4. Cell Therapy
Cell therapy spans multiple therapeutic areas, such as regenerative medicine, immunotherapy, and cancer therapy. It combines stem- and non-stem-cell-based unicellular or multicellular therapies, typically employing autologous or allogeneic cells, administered topically or as injectables, infusions, bioscaffolds, or scaffold-free systems [220]. The global cell therapy market size is estimated to achieve a CAGR of ca. 17% from 2023 to 2030 [263,264]. Although cell-based therapies present some safety concerns regarding potential tumorigenicity and high manufacturing costs, they have unique intrinsic features that offer the potential for enhanced efficacy against disease [221].
Stem cell therapies can be grouped into three categories: pluripotent stem cells (PSCs), adult stem cells (ASCs), and cancer stem cells (CSCs) [220]. Currently, the use of PSC- and ASC-derived organoids is considered a hot topic in translational stem cell research, as they can offer the three-dimensional (3D) structural and functional mimicry of organs in vitro [265]. Generally, the clinical use of CSCs has been motivated by their capacity to interfere with multiple signaling pathways, preventing cancer growth and relapse [266]. Across the world, stem and progenitor cell therapy is based on hematopoietic or mesenchymal cells, and is currently approved for various types of blood cancers, various blood disorders or tissue regeneration [267,268]. Cell-based therapies for humans primarily focused on bone marrow transplants for patients with blood-borne cancers in the middle of the XX century, and have resulted in a variety of currently FDA-approved products [221]. For example, allogeneic stem cell transplant therapy based on hematopoietic stem cells originating from umbilical cord blood (Omisirge®) was approved in 2023 by the FDA for use in patients above 12 years of age for the treatment of hematologic malignancies, while other therapies remain experimental in the USA.
A recent survey of ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) revealed 202 clinical studies related to the implementation of stem cell therapies for pediatric diseases [269]. Although the number of studies has tended to increase since 2007, the majority of the 112 completed studies) were short-term (<36 months), single-center clinical studies with a low number of recruited patients (<50) and without gender restrictions. Only about 30% of the studies with primary completion published results. While the studies were mostly based on HSC and mesenchymal stem cells, in the past 5 years the emphasis was mostly on allogeneic transplants. The low power of trials may obscure both clinically relevant results and adverse effects, stressing the need for larger multi-center studies in order to confirm clinical applicability and avoid experimental bias [269].
On the other hand, the management of non-stem-cell-based therapies has indicated the use of somatic cells that are isolated from the human body, propagated, expanded, selected, and subsequently administered to patients for curative, preventive, or diagnostic purposes [220]. Non-stem-cell-based cell therapies include fibroblasts, chondrocytes, keratinocytes, hepatocytes, pancreatic islet cells, and immune cells, such as T cells, dendritic cells (DCs), natural killer (NK) cells, or macrophages [220].
5.5. Tissue-Engineered Products
Tissue-engineered products contain or consist of engineered cells or tissues, and display properties when they are administered to human beings that allow them to regenerate, repair, or replace human tissue (EC No. 1394/2007). Cells or tissues are considered engineered if they fulfill at least one of the following conditions: (1) have been subjected to substantial manipulation or (2) are not intended to be used for the same essential function or functions in the recipient as in the donor [212].
Deguchi et al. [277] recently reviewed the use of tissue-engineered products with relevant applications to pediatric surgery.
5.6. Combined ATMPs
Combined ATMPs (cATMPs) are composed of a GTMP, sCTMP, or TEP in combination with one or more medical devices or one or more active implantable medical devices as an integral part of the product (EMA, EC No. 1394/2007), which may include devices such as biomaterial cell scaffolds or nanoparticles for gene therapy delivery [278].
cATMPs represent only 1% of the ATMPs that are under development in the EU [212]. Following these regulations (Directive 93/42/EEC and Directive 90/385/EEC) and the MEDical DEVices guidance document (MEDDEV), a medical device must be approved with the CE marking, an abbreviation in French of “Conformité Européenne” (European Conformity), prior to commercial availability in the EU. In this regard, any medical device that includes a cATMP must be previously approved with the CE marking by the notified bodies for its commercialization in the EU [212]. Moreover, when the final product includes a medical device, specific release tests may be required (EMA/CAT/80183/2014).
Wilkins et al. have recently presented a pipeline for ATMPs, demonstrating that combined ATMPs possess great interest in cardiovascular system diseases, along with ophthalmology/endocrine/nutritional/metabolic/genetic disorders and hematological malignancies [279]. Besides that, no clinical trial seems to be registered in the EU database using cATMP for neurological applications [278].
In 2013, the only cATMP approved in the EU was for the repair of knee cartilage defects. However, it was withdrawn in 2014 for commercial reasons due to the closure of the EU manufacturing site [212]. Later, in 2017, Spherox was recommended for marketing authorization to repair cartilage knee defects in adult populations (EMA/CHMP/315817/2017).
The current lack of combined ATMP approaches on the market and in clinical trials may represent an important research and investment opportunity [278].
6. Future Perspectives and Final Remarks
The pediatric drug development landscape has undergone significant changes in recent decades in moving towards more efficient and safer therapeutics, but some issues remain unaddressed. Factors that can impact the pediatric pharmacotherapy practice and drug development are (1) a lack of approved APIs for the pediatric population, (2) a deficiency in regulatory clarity, (3) low market size and profitability, (4) age-appropriate drug formulations, (5) a lack of safety data for excipients used in pediatric drug development, (6) the route of administration not being age-adjusted, (7) complete pharmacokinetic data not being available, and/or (8) difficulties in establishing in vivo models that can mimic different pediatric subgroups, leading to the need for novel technologic and galenic requirements. Using nanomedicine seems to provide a way to overcome some of the reported issues. Preclinical and clinical studies offer promising results in improving the solubility, organoleptic properties, therapeutic efficiency, and safety of a broad spectrum of APIs. However, a considerable rift needs to be crossed until most of the bench-formulated nanomedicines can be translated to the patient’s bedside, particularly in the case of pediatric nanomedicines. A workforce has been proposed that joins pharmaceutical developers and physicians in standardizing procedures for the development of pediatric formulations. The advent of ATMPs brings the possibility of curing pediatric pathologies with complete remission of the disease. However, challenging questions regarding their safety and immunogenic adverse effects persist. These innovative therapies also provide challenges for healthcare systems and drug developers, summarized in the “four As”: authorization, availability, assessment, and affordability.
Moreover, pharmacovigilance issues may also hamper the number of ATMPs currently available in clinical practice. Furthermore, economic problems due to the high costs necessary for the development of these technologies, as well as the limited revenue, may also impair investment in this area. Aligned with these, a call for action emitted by the Alliance for Regenerative Medicine (ARM) revealed that the EU is becoming stagnant compared to the U.S. and Asia in the number of therapeutic developers, clinical trials, and investments nurturing the development of ATMPs.
Therefore, some so-far-unanswered questions have arisen: (1) does pediatric medicine continue to be a “therapeutic orphan?” (2) Are the healthcare and the economic systems prepared for a personalized medicine perspective? (3) Are governments, the regulatory entities, and society prepared for the technophilic and technophobic demands proposed by the nanomedicine advancements, particularly employing intelligent nanomaterials, and those arising from ATMPs?
Pediatric medicine continues to be a hot and challenging topic to be investigated and the role of pharmaceutical developers is undoubtedly crucial in the pre-conception, design, and formulation, to the clinical phase of them, taking into consideration a common international framework established in the trinomial pillars of quality, efficacy, and safety in a fit-by-design perspective.
References
- Rimsza, M.E.; Hotaling, C.A.J.; Keown, M.E.; Marcin, J.P.; Moskowitz, W.B.; Sigrest, T.D.; Simon, H.K. Definition of a Pediatrician. Pediatrics 2015, 135, 780–781. [Google Scholar] [CrossRef]
- Appropriate ICH Expert Working Group. E11(R1) Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population; Adopted on 18 August 2017. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e11r1-addendum-clinical-investigation-medicinal-products-pediatric-population (accessed on 12 July 2023).
- Sawyer, S.M.; McNeil, R.; Francis, K.L.; Matskarofski, J.Z.; Patton, G.C.; Bhutta, Z.A.; Esangbedo, D.O.; Klein, J.D. The age of paediatrics. Lancet Child Adolesc. Health 2019, 3, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Hardin, A.P.; Hackell, J.M.; Simon, G.R.; Boudreau, A.D.A.; Baker, C.N.; Barden, G.A.; Meade, K.E.; Moore, S.B.; Richerson, J.; Brown, O.W.; et al. Age limit of pediatrics. Pediatrics 2017, 140, e20172151. [Google Scholar] [CrossRef]
- Maheshwari, M.; Sanwatsarkar, S.; Katakwar, M. Pharmacology related to paediatric anaesthesia. Indian J. Anaesth. 2019, 63, 698. [Google Scholar] [CrossRef]
- Ernest, T.B.; Elder, D.P.; Martini, L.G.; Roberts, M.; Ford, J.L. Developing paediatric medicines: Identifying the needs and recognizing the challenges. J. Pharm. Pharmacol. 2010, 59, 1043–1055. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef]
- O’Brien, F.; Clapham, D.; Krysiak, K.; Batchelor, H.; Field, P.; Caivano, G.; Pertile, M.; Nunn, A.; Tuleu, C. Making medicines baby size: The challenges in bridging the formulation gap in neonatal medicine. Int. J. Mol. Sci. 2019, 20, 2688. [Google Scholar] [CrossRef]
- Walsh, J.; Schaufelberger, D.; Iurian, S.; Klein, S.; Batchelor, H.; Turner, R.; Gizurarson, S.; Boltri, L.; Alessandrini, E.; Tuleu, C. Path towards Efficient Paediatric Formulation Development Based on Partnering with Clinical Pharmacologists and Clinicians, a Conect4children Expert Group white Paper; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021; p. bcp.14989. [Google Scholar]
- Vieira, I.; Sousa, J.J.; Vitorino, C. Paediatric Medicines—Regulatory Drivers, Restraints, Opportunities and Challenges. J. Pharm. Sci. 2021, 110, 1545–1556. [Google Scholar] [CrossRef]
- Ogbonna, J.D.N.; Cunha, E.; Attama, A.A.; Ofokansi, K.C.; Ferreira, H.; Pinto, S.; Gomes, J.; Marx, Í.M.G.; Peres, A.M.; Lobo, J.M.S.; et al. Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug. Pharmaceuticals 2022, 15, 1331. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Development of Paediatric Medicines: Points to Consider in Formulation. Available online: https://www.who.int/publications/m/item/trs970-annex-5-development-of-paediatric-medicines-points-to-consider-in-formulation (accessed on 12 July 2023).
- Salunke, S.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database. Part 1—A need assessment study. Int. J. Pharm. 2012, 435, 101–111. [Google Scholar] [CrossRef]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the paediatric population: A review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Salunke, S.; Brandys, B.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: Part 2—The pilot version. Int. J. Pharm. 2013, 457, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.S.; Thackray, J.; Matson, K.L.; McPherson, C.; Lubsch, L.; Hellinga, R.C.; Hoff, D.S. Key potentially inappropriate drugs in pediatrics: The KIDs list. J. Pediatr. Pharmacol. Ther. 2020, 25, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Grant-Kels, J.M. The Meanings of “Pediatric Drug Development”. Ther. Innov. Regul. Sci. 2019, 53, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, S.; Ando, Y. Gap between pediatric and adult approvals of molecular targeted drugs. Sci. Rep. 2020, 10, 17145. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.C. The Scarcity of Approved Pediatric High-Risk Medical Devices. JAMA Netw. Open 2021, 4, e2112760. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef]
- Marques, M.S.; Lima, L.A.; Poletto, F.; Contri, R.V.; Kulkamp Guerreiro, I.C. Nanotechnology for the treatment of paediatric diseases: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103628. [Google Scholar] [CrossRef]
- Pires, L.R.; Vinayakumar, K.B.; Turos, M.; Miguel, V.; Gaspar, J. A Perspective on Microneedle-Based Drug Delivery and Diagnostics in Paediatrics. J. Pers. Med. 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Scientific Foresight (STOA). Therapies for the Future—Advanced Therapies & Nanomedicine. Available online: https://epthinktank.eu/2017/11/22/therapies-for-the-future-advanced-therapies-nanomedicine/ (accessed on 17 July 2023).
- Pizevska, M.; Kaeda, J.; Fritsche, E.; Elazaly, H.; Reinke, P.; Amini, L. Advanced Therapy Medicinal Products’ Translation in Europe: A Developers’ Perspective. Front. Med. 2022, 9, 757647. [Google Scholar] [CrossRef]
- Lederer, C.W.; Koniali, L.; Buerki-Thurnherr, T.; Papasavva, P.L.; La Grutta, S.; Licari, A.; Staud, F.; Bonifazi, D.; Kleanthous, M. Catching Them Early: Framework Parameters and Progress for Prenatal and Childhood Application of Advanced Therapies. Pharmaceutics 2022, 14, 793. [Google Scholar] [CrossRef]
- Kimland, E.; Odlind, V. Off-label drug use in pediatric patients. Clin. Pharmacol. Ther. 2012, 91, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Bucci-Rechtweg, C. Enhancing the Pediatric Drug Development Framework to Deliver Better Pediatric Therapies Tomorrow. Clin. Ther. 2017, 39, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.A.; Catapano, M.; Hirschfeld, S.; Giaquinto, C. Paediatric drug development: The impact of evolving regulations. Adv. Drug Deliv. Rev. 2014, 73, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Coppes, M.J.; Jackson, C.; Connor, E.M. I-ACT for Children: Helping close the gap in drug approval for adults and children. Pediatr. Res. 2022, 93, 1786–1787. [Google Scholar] [CrossRef] [PubMed]
- Burckart, G.J.; Kim, C. The Revolution in Pediatric Drug Development and Drug Use: Therapeutic Orphans No More. J. Pediatr. Pharmacol. Ther. 2020, 25, 565. [Google Scholar] [CrossRef]
- Greene, J.A.; Podolsky, S.H. Reform, Regulation, and Pharmaceuticals—The Kefauver–Harris Amendments at 50. N. Engl. J. Med. 2012, 367, 1481–1483. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). The Drug Development Process. Available online: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process (accessed on 11 January 2022).
- Réda, C.; Kaufmann, E.; Delahaye-Duriez, A. Machine learning applications in drug development. Comput. Struct. Biotechnol. J. 2020, 18, 241–252. [Google Scholar] [CrossRef]
- Fernandez, E.; Perez, R.; Hernandez, A.; Tejada, P.; Arteta, M.; Ramos, J.T. Factors and Mechanisms for Pharmacokinetic Differences between Pediatric Population and Adults. Pharmaceutics 2011, 3, 53. [Google Scholar] [CrossRef]
- Subramanian, D.; Cruz, C.V.; Garcia-Bournissen, F. Systematic Review of Early Phase Pediatric Clinical Pharmacology Trials. J. Pediatr. Pharmacol. Ther. 2022, 27, 609. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Drugs. Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics 1977, 60, 91–101. [Google Scholar] [CrossRef]
- Severin, T.; Corriol-Rohou, S.; Bucci-Rechtweg, C.; an Haack, K.; Fuerst-Recktenwald, S.; Lepola, P.; Norjavaara, E.; Dehlinger-Kremer, M.; Haertter, S.; Cheung, S.Y.A. How is the Pharmaceutical Industry Structured to Optimize Pediatric Drug Development? Existing Pediatric Structure Models and Proposed Recommendations for Structural Enhancement. Ther. Innov. Regul. Sci. 2020, 54, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Rose, K. The Challenges of Pediatric Drug Development. Curr. Ther. Res. Clin. Exp. 2019, 90, 128–134. [Google Scholar] [CrossRef]
- Wu, W.; Tang, Z.; Chen, J.; Gao, Y. Pediatric drug development in China: Reforms and challenges. Pharmacol. Res. 2019, 148, 104412. [Google Scholar] [CrossRef]
- Joseph, P.D.; Craig, J.C.; Caldwell, P.H.Y. Clinical trials in children. Br. J. Clin. Pharmacol. 2015, 79, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, F.H.; Huang, S.Y.; Zhang, S.G.; Chen, H.W. The best pharmaceuticals for children—What can we do? Transl. Pediatr. 2020, 9, 86–92. [Google Scholar] [CrossRef]
- The European Parliament and the Council EUR-Lex-02006R1901-20190128-EN. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1901-20190128 (accessed on 11 January 2022).
- Grand View Research (GVR) Pharmaceutical Manufacturing Market Size Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/pharmaceutical-manufacturing-market (accessed on 29 June 2022).
- Milne, C.P. More Efficient Compliance with European Medicines Agency and Food and Drug Administration Regulations for Pediatric Oncology Drug Development: Problems and Solutions. Clin. Ther. 2017, 39, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, X.; Zhou, L.; Li, L.; Zhang, T. Updated analysis of pediatric clinical studies registered in ClinicalTrials.gov, 2008–2019. BMC Pediatr. 2021, 21, 212. [Google Scholar] [CrossRef]
- EU Clinical Trials Register. Clinical Trials Register, Age Range: “Under 18”. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search (accessed on 14 August 2021).
- Van der Gronde, T.; Uyl-de Groot, C.A.; Pieters, T. Addressing the challenge of high-priced prescription drugs in the era of precision medicine: A systematic review of drug life cycles, therapeutic drug markets and regulatory frameworks. PLoS ONE 2017, 12, e0182613. [Google Scholar] [CrossRef]
- PwC Health Research Institute. Medical Cost Trend: Behind the Numbers 2022: PwC. Available online: https://www.pwc.com/us/en/industries/health-industries/library/behind-the-numbers.html (accessed on 28 January 2022).
- Speer, E.M.; Lee, L.K.; Bourgeois, F.T.; Gitterman, D.; Hay, W.W.; Davis, J.M.; Javier, J.R. The state and future of pediatric research—An introductory overview. Pediatr. Res. 2023, 2023, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Intelligence, M. Pediatric Drugs Market Size & Share Analysis—Industry Research Report—Growth Trends. Available online: https://www.mordorintelligence.com/industry-reports/pediatric-drugs-market (accessed on 3 August 2023).
- Gitterman, D.P.; Langford, W.S.; Hay, W.W. The uncertain fate of the National Institutes of Health (NIH) pediatric research portfolio. Pediatr. Res. 2018, 84, 328–332. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). RePORT—RePORTER—Search Term “Pedriatic”. Available online: https://reporter.nih.gov/search/Uv2KFJNsBkKhF-a4LG7iOA/projects/charts?shared=true (accessed on 18 January 2022).
- Gitterman, D.P.; Hay, W.W.; Langford, W.S. Making the case for pediatric research: A life-cycle approach and the return on investment. Pediatr. Res. 2022 934 2022, 93, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.D.J.; Weiner, S.L.; Adamson, P.C.; Karres, D.; Reaman, G.; Rousseau, R.; Blanc, P.; Norga, K.; Skolnik, J.; Kearns, P.; et al. ACCELERATE—Five years accelerating cancer drug development for children and adolescents. Eur. J. Cancer 2022, 166, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, A.V.; Modi, N.; de Wildt, S.N.; Aurich, B.; Bakhtadze, S.; Sirvent, F.J.B.; Cabañas, F.; Campbell, L.; Casanova, M.; Charlton, P.; et al. Improving clinical paediatric research and learning from COVID-19: Recommendations by the Conect4Children expert advice group. Pediatr. Res. 2021, 91, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Vinci, R.J. The pediatric workforce: Recent data trends, questions, and challenges for the future. Pediatrics 2021, 147, e2020013292. [Google Scholar] [CrossRef]
- Beleck, A.; Nachman, S. Understanding Pediatric Drug Lag Time: Review of Selected Drug Package Inserts. J. Pediatr. Infect. Dis. Soc. 2021, 10, 509–513. [Google Scholar] [CrossRef]
- Malkawi, W.A.; Alrafayah, E.; Alhazabreh, M.; Abulaila, S.; Al-Ghananeem, A.M. Formulation Challenges and Strategies to Develop Pediatric Dosage Forms. Children 2022, 9, 488. [Google Scholar] [CrossRef]
- Tanaudommongkon, I.; John Miyagi, S.; Green, D.J.; Burnham, J.M.; van den Anker, J.N.; Park, K.; Wu, J.; McCune, S.K.; Yao, L.; Burckart, G.J. Combined Pediatric and Adult Trials Submitted to the US Food and Drug Administration 2012–2018. Clin. Pharmacol. Ther. 2020, 108, 1018. [Google Scholar] [CrossRef]
- Meng, M.; Zhou, Q.; Lei, W.; Tian, M.; Wang, P.; Liu, Y.; Sun, Y.; Chen, Y.; Li, Q. Recommendations on Off-Label Drug Use in Pediatric Guidelines. Front. Pharmacol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Allen, H.C.; Garbe, M.C.; Lees, J.; Aziz, N.; Chaaban, H.; Miller, J.L.; Johnson, P.; DeLeon, S. Off-Label Medication use in Children, More Common than We Think: ASystematic Review of the Literature. J. Okla. State Med. Assoc. 2018, 111, 776. [Google Scholar] [PubMed]
- Noel, G.J.; Nelson, R.M.; Bucci-Rechtweg, C.; Portman, R.; Miller, T.; Green, D.J.; Snyder, D.; Moreno, C.; Hovinga, C.; Connor, E. Inclusion of Adolescents in Adult Clinical Trials: Report of the Institute for Advanced Clinical Trials for Children’s Pediatric Innovation Research Forum. Ther. Innov. Regul. Sci. 2021, 55, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Rosenbaum, S.; Island, R. Developmental Pharmacokinetics in Pediatric Populations. J. Pediatr. Pharmacol. Ther. 2014, 19, 262–276. [Google Scholar] [CrossRef]
- Kelly, L.E.; Sinha, Y.; Barker, C.I.S.; Standing, J.F.; Offringa, M. Useful pharmacodynamic endpoints in children: Selection, measurement, and next steps. Pediatr. Res. 2018, 83, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Reflection Paper: Formulation of Choice for the Paediatric Population (EMEA/CHMP/PEG/194810/2005); European Medicines Agency: Amsterdam, The Netherlands, 2006; pp. 1–45. [Google Scholar]
- Food and Drug Administration (FDA). General Clinical Pharmacology Considerations for Pediatric Studies of Drugs, Including Biological Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-clinical-pharmacology-considerations-pediatric-studies-drugs-including-biological-products (accessed on 3 August 2023).
- Conklin, L.S.; Hoffman, E.P.; van den Anker, J. Developmental Pharmacodynamics and Modeling in Pediatric Drug Development. J. Clin. Pharmacol. 2019, 59, S87. [Google Scholar] [CrossRef]
- Sosnik, A.; Carcaboso, A.M. Nanomedicines in the future of pediatric therapy. Adv. Drug Deliv. Rev. 2014, 73, 140–161. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Paediatric pharmacokinetics: Key considerations. Br. J. Clin. Pharmacol. 2015, 79, 395–404. [Google Scholar] [CrossRef]
- Barker, C.I.S.; Standing, J.F.; Kelly, L.E.; Hanly Faught, L.; Needham, A.C.; Rieder, M.J.; de Wildt, S.N.; Offringa, M. Pharmacokinetic studies in children: Recommendations for practice and research. Arch. Dis. Child. 2018, 103, 695. [Google Scholar] [CrossRef]
- Siafaka, P.; Ipekci, E.; Caglar, E.Ş.; Ustundag Okur, N.; Buyukkayhan, D. Current Status of Pediatric Formulations for Chronic and Acute Children’ Diseases: Applications and Future Perspectives. Medeni. Med. J. 2021, 36, 152. [Google Scholar] [CrossRef]
- Leeder, J.S. Translating pharmacogenetics and pharmacogenomics into drug development for clinical pediatrics and beyond. Drug Discov. Today 2004, 9, 567–573. [Google Scholar] [CrossRef]
- Naji-Talakar, S.; Sharma, S.; Martin, L.A.; Barnhart, D.; Prasad, B. Potential implications of DMET ontogeny on the disposition of commonly prescribed drugs in neonatal and pediatric intensive care units. Expert Opin. Drug Metab. Toxicol. 2021, 17, 273. [Google Scholar] [CrossRef] [PubMed]
- Van Groen, B.D.; Pilla Reddy, V.; Badée, J.; Olivares-Morales, A.; Johnson, T.N.; Nicolaï, J.; Annaert, P.; Smits, A.; de Wildt, S.N.; Knibbe, C.A.J.; et al. Pediatric Pharmacokinetics and Dose Predictions: A Report of a Satellite Meeting to the 10th Juvenile Toxicity Symposium. Clin. Transl. Sci. 2021, 14, 29–35. [Google Scholar] [CrossRef]
- Jian, C.; Carpén, N.; Helve, O.; de Vos, W.M.; Korpela, K.; Salonen, A. Early-life gut microbiota and its connection to metabolic health in children: Perspective on ecological drivers and need for quantitative approach. eBioMedicine 2021, 69, 103475. [Google Scholar] [CrossRef] [PubMed]
- Leardini, D.; Venturelli, F.; Baccelli, F.; Cerasi, S.; Muratore, E.; Brigidi, P.; Pession, A.; Prete, A.; Masetti, R. Pharmacomicrobiomics in Pediatric Oncology: The Complex Interplay between Commonly Used Drugs and Gut Microbiome. Int. J. Mol. Sci. 2022, 23, 15387. [Google Scholar] [CrossRef]
- Walsh, J.; Griffin, B.T.; Clarke, G.; Hyland, N.P. Drug–gut microbiota interactions: Implications for neuropharmacology. Br. J. Pharmacol. 2018, 175, 4415. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, J.; McNamara, P.J. Using ontogeny information to build predictive models for drug elimination. Drug Discov. Today 2008, 13, 507–512. [Google Scholar] [CrossRef]
- Anderson, G.D. Children Versus Adults: Pharmacokinetic and Adverse-Effect Differences. Epilepsia 2002, 43, 53–59. [Google Scholar] [CrossRef]
- Johnson, T.N.; Jamei, M.; Rowland-Yeo, K. How Does In Vivo Biliary Elimination of Drugs Change with Age? Evidence from In Vitro and Clinical Data Using a Systems Pharmacology Approach. Drug Metab. Dispos. 2016, 44, 1090–1098. [Google Scholar] [CrossRef]
- Wollmer, E.; Ungell, A.L.; Nicolas, J.M.; Klein, S. Review of paediatric gastrointestinal physiology relevant to the absorption of orally administered medicines. Adv. Drug Deliv. Rev. 2022, 181, 114084. [Google Scholar] [CrossRef]
- Germovsek, E.; Barker, C.I.S.; Sharland, M.; Standing, J.F. Pharmacokinetic–Pharmacodynamic Modeling in Pediatric Drug Development, and the Importance of Standardized Scaling of Clearance. Clin. Pharmacokinet. 2019, 58, 39. [Google Scholar] [CrossRef]
- Barker, C.I.S.; Germovsek, E.; Hoare, R.L.; Lestner, J.M.; Lewis, J.; Standing, J.F. Pharmacokinetic/pharmacodynamic modelling approaches in paediatric infectious diseases and immunology. Adv. Drug Deliv. Rev. 2014, 73, 127–139. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, D.; Shu, Z.; Duan, Z.; Liu, Y.; Du, Q.; Zhang, Y.; Dong, Y.; Wang, T.; Hu, S.; et al. Population Pharmacokinetics and Model-Based Dosing Optimization of Teicoplanin in Pediatric Patients. Front. Pharmacol. 2020, 11, 594562. [Google Scholar] [CrossRef]
- Johnson, T.N.; Small, B.G.; Berglund, E.G.; Rowland Yeo, K. A best practice framework for applying physiologically-based pharmacokinetic modeling to pediatric drug development. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 967–972. [Google Scholar] [CrossRef]
- Khalid, S.; Rasool, M.F.; Masood, I.; Imran, I.; Saeed, H.; Ahmad, T.; Alqahtani, N.S.; Alshammari, F.A.; Alqahtani, F. Application of a physiologically based pharmacokinetic model in predicting captopril disposition in children with chronic kidney disease. Sci. Reports 2023, 13, 2697. [Google Scholar] [CrossRef] [PubMed]
- Ince, I.; Dallmann, A.; Frechen, S.; Coboeken, K.; Niederalt, C.; Wendl, T.; Block, M.; Meyer, M.; Eissing, T.; Burghaus, R.; et al. Predictive Performance of Physiology-Based Pharmacokinetic Dose Estimates for Pediatric Trials: Evaluation with 10 Bayer Small-Molecule Compounds in Children. J. Clin. Pharmacol. 2021, 61, S70–S82. [Google Scholar] [CrossRef]
- Ince, I.; Solodenko, J.; Frechen, S.; Dallmann, A.; Niederalt, C.; Schlender, J.; Burghaus, R.; Lippert, J.; Willmann, S. Predictive Pediatric Modeling and Simulation Using Ontogeny Information. J. Clin. Pharmacol. 2019, 59, S95–S103. [Google Scholar] [CrossRef]
- Liu, X.I.; Dallmann, A.; Wang, Y.M.; Green, D.J.; Burnham, J.M.; Chiang, B.; Wu, P.; Sheng, M.; Lu, K.; van den Anker, J.N.; et al. Monoclonal Antibodies and Fc-Fusion Proteins for Pediatric Use: Dosing, Immunogenicity, and Modeling and Simulation in Data Submitted to the US Food and Drug Administration. J. Clin. Pharmacol. 2019, 59, 1130–1143. [Google Scholar] [CrossRef]
- Lin, W.; Yan, J.H.; Heimbach, T.; He, H. Pediatric Physiologically Based Pharmacokinetic Model Development: Current Status and Challenges. Curr. Pharmacol. Rep. 2018, 4, 491–501. [Google Scholar] [CrossRef]
- Smith, L.; Leggett, C.; Borg, C. Administration of medicines to children: A practical guide. Aust. Prescr. 2022, 45, 188. [Google Scholar] [CrossRef] [PubMed]
- Galande, A.D.; Khurana, N.A.; Mutalik, S. Pediatric dosage forms-challenges and recent developments: A critical review. J. Appl. Pharm. Sci. 2020, 10, 155–166. [Google Scholar] [CrossRef]
- Nadeshkumar, A.; Sathiadas, G.; Sri Ranganathan, S. Administration of oral dosage forms of medicines to children in a resource limited setting. PLoS ONE 2022, 17, e0276379. [Google Scholar] [CrossRef] [PubMed]
- Rautamo, M.; Kvarnström, K.; Sivén, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. A Focus Group Study about Oral Drug Administration Practices at Hospital Wards—Aspects to Consider in Drug Development of Age-Appropriate Formulations for Children. Pharm. 2020, 12, 109. [Google Scholar] [CrossRef]
- Delgado-Charro, M.B.; Guy, R.H. Effective use of transdermal drug delivery in children. Adv. Drug Deliv. Rev. 2014, 73, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Hanning, S.M.; Walker, E.; Sutcliffe, E.; Tuleu, C. The rectal route of medicine administration for children: Let’s get to the bottom of it! Eur. J. Pharm. Biopharm. 2020, 157, 25–27. [Google Scholar] [CrossRef]
- Clinical Skills Content Medication Administration: Intramuscular Injections (Pediatric). Available online: https://elsevier.health/en-US/preview/intramuscular-injection-pediatrics (accessed on 4 August 2023).
- Ainscough, L.P.; Ford, J.L.; Morecroft, C.W.; Peak, M.; Turner, M.A.; Nunn, A.J.; Roberts, M. Accuracy of intravenous and enteral preparations involving small volumes for paediatric use: A review. Eur. J. Hosp. Pharm. 2018, 25, 66–71. [Google Scholar] [CrossRef]
- Linakis, M.W.; Roberts, J.K.; Lala, A.C.; Spigarelli, M.G.; Medlicott, N.J.; Reith, D.M.; Ward, R.M.; Sherwin, C.M.T. Challenges Associated with Route of Administration in Neonatal Drug Delivery. Clin. Pharmacokinet. 2015, 55, 185–196. [Google Scholar] [CrossRef]
- Fonceca, A.M.; Fox Ditcham, W.G.; Everard, M.L.; Devadason, S. Drug Administration by Inhalation in Children. In Kendig’s Disorder of the Respiratory Tract in Children; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–271.e3. [Google Scholar] [CrossRef]
- Volerman, A.; Kan, K.; Carpenter, D.; Press, V.G. Strategies for Improving Inhalation Technique in Children: A Narrative Review. Patient Prefer. Adherence 2021, 15, 665. [Google Scholar] [CrossRef]
- Khan, D.; Kirby, D.; Bryson, S.; Shah, M.; Rahman Mohammed, A. Paediatric specific dosage forms: Patient and formulation considerations. Int. J. Pharm. 2022, 616, 121501. [Google Scholar] [CrossRef]
- Lajoinie, A.; Henin, E.; Nguyen, K.A.; Malik, S.; Mimouni, Y.; Sapori, J.M.; Bréant, V.; Cochat, P.; Kassai, B. Oral drug dosage forms administered to hospitalized children: Analysis of 117,665 oral administrations in a French paediatric hospital over a 1-year period. Int. J. Pharm. 2016, 500, 336–344. [Google Scholar] [CrossRef]
- Montero-Padilla, S.; Velaga, S.; Morales, J.O. Buccal Dosage Forms: General Considerations for Pediatric Patients. AAPS PharmSciTech 2017, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Xu, Y.; Worsley, A.; Wong, I.C.K. Oral transmucosal drug delivery for pediatric use. Adv. Drug Deliv. Rev. 2014, 73, 50–62. [Google Scholar] [CrossRef]
- Sibum, I.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W.; Grasmeijer, F. Challenges for pulmonary delivery of high powder doses. Int. J. Pharm. 2018, 548, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Lobo, J.M.S.; et al. Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence. Pharmaceuticals 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Klingmann, V.; Reidemeister, S.; Breitkreutz, J. Patient-centric drug product development: Acceptability across patient populations—Science and evidence. Eur. J. Pharm. Biopharm. 2023, 188, 1–5. [Google Scholar] [CrossRef]
- Reker, D.; Blum, S.M.; Steiger, C.; Anger, K.E.; Sommer, J.M.; Fanikos, J.; Traverso, G. ‘Inactive’ ingredients in oral medications. Sci. Transl. Med. 2019, 11, eaau6753. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, G. Safety of Excipients in Pediatric Formulations—A Call for Toxicity Studies in Juvenile Animals? Children 2015, 2, 191. [Google Scholar] [CrossRef]
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef]
- Salunke, S.; Clapham, D.; Agrawal, A.; Hughes, K.; Nunn, T. Best practices for selection of excipients for paediatrics—Workshop reflection. Eur. J. Pharm. Biopharm. 2021, 160, 77–81. [Google Scholar] [CrossRef]
- Salunke, S.; Tuleu, C. The STEP database through the end-users eyes—Usability study. Int. J. Pharm. 2015, 492, 316–331. [Google Scholar] [CrossRef]
- Van Riet-Nales, D.A.; Schobben, A.F.A.M.; Vromans, H.; Egberts, T.C.G.; Rademaker, C.M.A. Safe and effective pharmacotherapy in infants and preschool children: Importance of formulation aspects. Arch. Dis. Child. 2016, 101, 662–669. [Google Scholar] [CrossRef]
- Bianchi, A.; Bottau, P.; Calamelli, E.; Caimmi, S.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Mori, F.; Paglialunga, C.; Saretta, F.; et al. Hypersensitivity to polyethylene glycol in adults and children: An emerging challenge. Acta Bio Medica Atenei Parm. 2021, 92, 2021519. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861. [Google Scholar] [CrossRef]
- Doyle, R.; Melo, M.; Teoh, C.; Wen, S.; Willcocks, S. Safety of mRNA vaccination for COVID-19 in children with polyethylene glycol (PEG)-asparaginase allergy. Pediatr. Allergy Immunol. 2023, 34, e13939. [Google Scholar] [CrossRef] [PubMed]
- Bigini, P.; Gobbi, M.; Bonati, M.; Clavenna, A.; Zucchetti, M.; Garattini, S.; Pasut, G. The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines. Nat. Nanotechnol. 2021, 16, 1169–1171. [Google Scholar] [CrossRef]
- Karaaslan, B.G.; Burtecene, N.; Mustu, U.; Ocak, S.; Kasapcopur, O.; Kıykım, A.; Cokugras, H. Evaluation of pediatric patients with suspected polyethylene glycol and polysorbate allergy before mRNA SARS-CoV2 vaccination. Allergol. Immunopathol. 2023, 51, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kardas, P.; Dabrowa, M.; Witkowski, K. Adherence to treatment in paediatric patients—Results of the nationwide survey in Poland. BMC Pediatr. 2021, 21, 16. [Google Scholar] [CrossRef]
- El-Rachidi, S.; LaRochelle, J.M.; Morgan, J.A. Pharmacists and Pediatric Medication Adherence: Bridging the Gap. Hosp. Pharm. 2017, 52, 124. [Google Scholar] [CrossRef] [PubMed]
- Turner-Bowker, D.M.; An Haack, K.; Krohe, M.; Yaworsky, A.; Vivas, N.; Kelly, M.; Chatterjee, G.; Chaston, E.; Mann, E.; Reaney, M. Development and content validation of the Pediatric Oral Medicines Acceptability Questionnaires (P-OMAQ): Patient-reported and caregiver-reported outcome measures. J. Patient-Rep. Outcomes 2020, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-centred pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Zamora, J.; Legaz, I.; Osuna, E.; Pérez-Cárceles, M.D. Age and education as factors associated with medication literacy: A community pharmacy perspective. BMC Geriatr. 2020, 20, 501. [Google Scholar] [CrossRef]
- Matson, P.A.; Bakhai, N.; Solomon, B.S.; Flessa, S.; Ramos, J.; Hammond, C.J.; Adger, H. Understanding caregiver acceptance of screening for family substance use in pediatric clinics serving economically disadvantaged children. Subst. Abus. 2022, 43, 282. [Google Scholar] [CrossRef]
- Mfoafo, K.; Tuleu, C.; Hanning, S.; Omidian, H.; Mfoafo, K. Exploring the Potential of Nanotechnology in Pediatric Healthcare: Advances, Challenges, and Future Directions. Pharmaceutics 2023, 15, 1583. [Google Scholar] [CrossRef]
- Fornaguera, C.; García-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef]
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Pérez De La Ossa, D.H.; Pita, R.; Vidal, J.M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine 2013, 8, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhai, B.; Yang, F.; Chen, Z.; Zhou, Q.; Paiva-Santos, A.C.; Yuan, Z.; Zhou, Y. Nanotechnology-Based Diagnostic and Therapeutic Strategies for Neuroblastoma. Front. Pharmacol. 2022, 13, 908713. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, C.; González-Fernández, Y.; Aldaz, A.; Couvreur, P.; Blanco-Prieto, M.J. Nanomedicines for pediatric cancers. ACS Nano 2018, 12, 7482–7496. [Google Scholar] [CrossRef]
- Yang, S.; Wallach, M.; Krishna, A.; Kurmasheva, R.; Sridhar, S. Recent Developments in Nanomedicine for Pediatric Cancer. J. Clin. Med. 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Rubey, K.M.; Brenner, J.S. Nanomedicine to fight infectious disease. Adv. Drug Deliv. Rev. 2021, 179, 113996. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Godhi, B.S.; Saha, S.; Singh, B.; Dinsa, K.; Bhagchandani, J.; Gautam, A. Use of nanoparticles in pediatric dentistry: A narrative review. J. Int. Oral Health 2022, 14, 357. [Google Scholar]
- Delouise, L.A. Applications of Nanotechnology in Dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef]
- Trandafir, L.M.; Dodi, G.; Frasinariu, O.; Luca, A.C.; Butnariu, L.I.; Tarca, E.; Moisa, S.M. Tackling Dyslipidemia in Obesity from a Nanotechnology Perspective. Nutrients 2022, 14, 3774. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in Translational Research—Opportunities and Challenges in Targeted Cancer Therapy. AAPS PharmSciTech 2014, 15, 694. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef]
- Basso, J.; Mendes, M.; Cova, T.; Sousa, J.; Pais, A.; Fortuna, A.; Vitorino, R.; Vitorino, C. A Stepwise Framework for the Systematic Development of Lipid Nanoparticles. Biomolecules 2022, 12, 223. [Google Scholar] [CrossRef]
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating Nanotechnology into Cancer Care. ACS Nano 2019, 13, 7370–7376. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zannikou, M.; Lofchy, L.; Li, Y.; Gaikwad, H.; Balyasnikova, I.V.; Simberg, D. Liposomal Extravasation and Accumulation in Tumors as Studied by Fluorescence Microscopy and Imaging Depend on the Fluorescent Label. ACS Nano 2021, 15, 11880–11890. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Praça, F.S.G.; Marinho, H.S.; Martins, M.B.F.; Gaspar, R.; Corvo, M.L.; Medina, W.S.G. Current aspects of breast cancer therapy and diagnosis based on a nanocarrier approach. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 749–774. [Google Scholar] [CrossRef]
- Forssen, E.A. The design and development of DaunoXome® for solid tumor targeting in vivo. Adv. Drug Deliv. Rev. 1997, 24, 133–150. [Google Scholar] [CrossRef]
- Gu, W.; Andrews, G.P.; Tian, Y. Recent Clinical Successes in Liposomal Nanomedicines. Int. J. Drug Discov. Pharmacol. 2023, 2, 52–59. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Shah, B.D.; Rozario, N.; Turba, E.P.; Bello, C.; Chavez, J.C.; Sokol, L.; Brayer, J.; Lancet, J.E. A Single-Arm, Open-Label Phase 2 Pilot Study of Vyxeos (CPX-351) in Adults with Relapsed or Refractory Acute Lymphoblastic Leukemia. Blood 2021, 138, 4399. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.P.; Leong, E.W.X.; Ge, R. Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines 2022, 10, 2179. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Arikayce Liposomal. Available online: https://www.ema.europa.eu/en/documents/assessment-report/arikayce-liposomal-epar-public-assessment-report_en.pdf (accessed on 9 August 2023).
- Meyerhoff, A.U.S. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin. Infect. Dis. 1999, 28, 42–48. [Google Scholar] [CrossRef]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal formulations of photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar] [CrossRef] [PubMed]
- Rouge, J. RNA and nanocarriers: Next generation drug and delivery platform take center stage. Trends Biotechnol. 2023, 41, 281–282. [Google Scholar] [CrossRef]
- Weng, Y.; Xiao, H.; Zhang, J.; Liang, X.J.; Huang, Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 2019, 37, 801–825. [Google Scholar] [CrossRef]
- Williams, R.M.; Kapadia, C.; Jaimes, E.A.; Heller, D.A. Nanotargeting to the kidney. In Regenerative Nephrology; Academic Press: Cambridge, MA, USA, 2022; pp. 439–449. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S.; Paulson, J.A. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Joseph, A.; Nance, E. Pharmacokinetics of nanotechnology-based formulations in pediatric populations. Adv. Drug Deliv. Rev. 2019, 151–152, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Nieto González, N.; Obinu, A.; Rassu, G.; Giunchedi, P.; Gavini, E. Polymeric and Lipid Nanoparticles: Which Applications in Pediatrics? Pharmaceutics 2021, 13, 670. [Google Scholar] [CrossRef]
- Pham, K.; Li, D.; Guo, S.; Penzak, S.; Dong, X. Development and in vivo evaluation of child-friendly lopinavir/ritonavir pediatric granules utilizing novel in situ self-assembly nanoparticles. J. Control Release 2016, 226, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, C.; Mura, S.; Couvreur, P.; Blanco-Prieto, M.J. Squalenoyl-gemcitabine/edelfosine nanoassemblies: Anticancer activity in pediatric cancer cells and pharmacokinetic profile in mice. Int. J. Pharm. 2020, 582, 119345. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–225. [Google Scholar]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, A.; Puri, V.; Aggarwal, G.; Maman, P.; Huanbutta, K.; Nagpal, M.; Sangnim, T. Chitosan-Based Polymer Blends for Drug Delivery Systems. Polymers 2023, 15, 2028. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, G.; Jain, A.; Tiwari, A.; Saraf, S.; Panda, P.K.; Katare, O.P.; Jain, S.K. Systematic optimization of cationic surface engineered mucoadhesive vesicles employing Design of Experiment (DoE): A preclinical investigation. Int. J. Biol. Macromol. 2019, 133, 1142–1155. [Google Scholar] [CrossRef]
- Nerli, G.; Gonçalves, L.M.D.; Cirri, M.; Almeida, A.J.; Maestrelli, F.; Mennini, N.; Mura, P.A. Design, Evaluation and Comparison of Nanostructured Lipid Carriers and Chitosan Nanoparticles as Carriers of Poorly Soluble Drugs to Develop Oral Liquid Formulations Suitable for Pediatric Use. Pharmaceutics 2023, 15, 1305. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Kantak, M.N.; Bharate, S.S. Analysis of clinical trials on biomaterial and therapeutic applications of chitosan: A review. Carbohydr. Polym. 2022, 278, 118999. [Google Scholar] [CrossRef] [PubMed]
- Korelc, K.; Larsen, B.S.; Gašperlin, M.; Tho, I. Water-soluble chitosan eases development of mucoadhesive buccal films and wafers for children. Int. J. Pharm. 2023, 631, 122544. [Google Scholar] [CrossRef]
- Priya Dharshini, K.; Fang, H.; Ramya Devi, D.; Yang, J.X.; Luo, R.H.; Zheng, Y.T.; Brzeziński, M.; Vedha Hari, B.N. pH-sensitive chitosan nanoparticles loaded with dolutegravir as milk and food admixture for paediatric anti-HIV therapy. Carbohydr. Polym. 2021, 256, 117440. [Google Scholar] [CrossRef]
- Dalvi, A.; Ravi, P.R.; Uppuluri, C.T. Rufinamide-Loaded Chitosan Nanoparticles in Xyloglucan-Based Thermoresponsive In Situ Gel for Direct Nose to Brain Delivery. Front. Pharmacol. 2021, 12, 691936. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Meshali, M.; Abdelghani, G. Optimization and Formulation of Cinnarizine-Loaded Chitosan Microspheres in Liquid Dosage Form for Pediatric Therapy. Drug Deliv. Lett. 2014, 4, 128–141. [Google Scholar] [CrossRef]
- Severino, P.; Silva, C.; Costa, T.; Silva, H.; Chaud, M.; Santana, M.H.; Souto, E.B. In Vivo Absorption of Didanosine Formulated in Pellets Composed of Chitosan Microspheres. Vivo 2014, 28, 1045–1050. [Google Scholar]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Pereira, M.; Silva, F.C.; Simões, S.; Ribeiro, H.M.; Almeida, A.J.; Marto, J. Innovative, Sugar-Free Oral Hydrogel as a Co-administrative Vehicle for Pediatrics: A Strategy to Enhance Patient Compliance. AAPS PharmSciTech 2022, 23, 691936. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F. Novel adhesive hyaluronic acid based solid dosage form for pediatric application. J. Drug Deliv. Sci. Technol. 2018, 44, 213–219. [Google Scholar] [CrossRef]
- Di Cicco, M.; Peroni, D.; Sepich, M.; Tozzi, M.G.; Comberiati, P.; Cutrera, R. Hyaluronic acid for the treatment of airway diseases in children: Little evidence for few indications. Pediatr. Pulmonol. 2020, 55, 2156–2169. [Google Scholar] [CrossRef]
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020, 164, 1046. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355. [Google Scholar] [CrossRef]
- Guzmán-Mejía, F.; Godínez-Victoria, M.; Molotla-Torres, D.E.; Drago-Serrano, M.E. Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus. Pharmaceuticals 2023, 16, 214. [Google Scholar] [CrossRef]
- Janicka, M.; Tomaszewska, M.; Ranoszek-Soliwoda, E.; Celichowski, K.; Grobelny, G.; Szymanski, J.; Conte, M.P.; Rosa, L.; Krzyzowska, M.; Janicka, M.; et al. Lactoferrin-Conjugated Nanoparticles as New Antivirals. Pharmaceutics 2022, 14, 1862. [Google Scholar] [CrossRef]
- Ahmed, F.; Ali, M.J.; Kondapi, A.K. Carboplatin loaded protein nanoparticles exhibit improve anti-proliferative activity in retinoblastoma cells. Int. J. Biol. Macromol. 2014, 70, 572–582. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology for Pediatric Retinoblastoma Therapy. Pharmaceuticals 2022, 15, 1087. [Google Scholar] [CrossRef] [PubMed]
- Narayana, R.V.L.; Jana, P.; Tomar, N.; Prabhu, V.; Nair, R.M.; Manukonda, R.; Kaliki, S.; Coupland, S.E.; Alexander, J.; Kalirai, H.; et al. Carboplatin- and Etoposide-Loaded Lactoferrin Protein Nanoparticles for Targeting Cancer Stem Cells in Retinoblastoma In Vitro. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Xu, X.; Barwe, S.P.; Yang, X.; Czymmek, K.; Waldman, S.A.; Mason, R.W.; Jia, X.; Rajasekaran, A.K. Dexamethasone-loaded Block Copolymer Nanoparticles Induce Leukemia Cell Deathand Enhances Therapeutic Efficacy: A Novel Application in PediatricNanomedicine. Mol. Pharm. 2013, 10, 2199. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377. [Google Scholar] [CrossRef]
- Ni, M.Z.; Xiong, M.; Zhang, X.C.; Cai, G.P.; Chen, H.W.; Zeng, Q.M.; Yu, Z.C. Poly(lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. Int. J. Nanomed. 2015, 10, 2537–2554. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef]
- De Castro, K.C.; Coco, J.C.; dos Santos, É.M.; Ataide, J.A.; Martinez, R.M.; do Nascimento, M.H.M.; Prata, J.; da Fonte, P.R.M.L.; Severino, P.; Mazzola, P.G.; et al. Pluronic® triblock copolymer-based nanoformulations for cancer therapy: A 10-year overview. J. Control Release 2023, 353, 802–822. [Google Scholar] [CrossRef]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.; Alvarez-Lorenzo, C.; Concheiro, A.; Veiga, F.; Figueiras, A. Nanotheranostic Pluronic-Like Polymeric Micelles: Shedding Light into the Dark Shadows of Tumors. Mol. Pharm. 2019, 16, 4757–4774. [Google Scholar] [CrossRef]
- Khodaei, A.; Jahanmard, F.; Madaah Hosseini, H.R.; Bagheri, R.; Dabbagh, A.; Weinans, H.; Amin Yavari, S. Controlled temperature-mediated curcumin release from magneto-thermal nanocarriers to kill bone tumors. Bioact. Mater. 2022, 11, 107–117. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved Nanomedicine against Diseases. Pharmaceutics 2023, 15, 774. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Werner, M.E.; Cummings, N.D.; Sethi, M.; Wang, E.C.; Sukumar, R.; Moore, D.T.; Wang, A.Z. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 463. [Google Scholar] [CrossRef]
- Ranade, A.A.; Joshi, D.A.; Phadke, G.K.; Patil, P.P.; Kasbekar, R.B.; Apte, T.G.; Dasare, R.R.; Mengde, S.D.; Parikh, P.M.; Bhattacharyya, G.S.; et al. Clinical and economic implications of the use of nanoparticle paclitaxel (Nanoxel) in India. Ann. Oncol. 2013, 24, v6–v12. [Google Scholar] [CrossRef]
- Huang, D.; Wu, D. Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 90, 713–727. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Szwed, A.; Rogalska, A.; Del Olmo, N.S.; Ortega, P.; Denel, M.; Jacenik, D.; Shcharbin, D.; de la Mata, F.J.; et al. Ruthenium Dendrimers against Human Lymphoblastic Leukemia 1301 Cells. Int. J. Mol. Sci. 2020, 21, 4119. [Google Scholar] [CrossRef]
- Chittasupho, C.; Aonsri, C.; Imaram, W. Targeted dendrimers for antagonizing the migration and viability of NALM-6 lymphoblastic leukemia cells. Bioorg. Chem. 2021, 107, 104601. [Google Scholar] [CrossRef] [PubMed]
- Guido, C.; Baldari, C.; Maiorano, G.; Mastronuzzi, A.; Carai, A.; Quintarelli, C.; De Angelis, B.; Cortese, B.; Gigli, G.; Palamà, I.E. Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers. Diagnostics 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef]
- Morford, L.L.; Bowman, C.J.; Blanset, D.L.; Bøgh, I.B.; Chellman, G.J.; Halpern, W.G.; Weinbauer, G.F.; Coogan, T.P. Preclinical safety evaluations supporting pediatric drug development with biopharmaceuticals: Strategy, challenges, current practices. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New challenges in the use of nanomedicine in cancer therapy. Bioengineered 2022, 13, 759. [Google Scholar] [CrossRef] [PubMed]
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Safety and Toxicity Implications of Multifunctional Drug Delivery Nanocarriers on Reproductive Systems In Vitro and In Vivo. Front. Toxicol. 2022, 4, 895667. [Google Scholar] [CrossRef] [PubMed]
- López-Paniagua, M.; de la Mata, A.; Galindo, S.; Blázquez, F.; Calonge, M.; Nieto-Miguel, T. Advanced Therapy Medicinal Products for the Eye: Definitions and Regulatory Framework. Pharmaceutics 2021, 13, 347. [Google Scholar] [CrossRef]
- European Union Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No 726/2004 (Text with EEA relevance). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:0121:0137:en:PDF (accessed on 17 July 2023).
- European Medicines Agency (EMA). Support for Advanced-Therapy Developers | GLP Requirements. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/advanced-therapies/support-advanced-therapy-developers (accessed on 10 September 2023).
- Detela, G.; Lodge, A. EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation. Mol. Ther. Methods Clin. Dev. 2019, 13, 205–232. [Google Scholar] [CrossRef]
- Iglesias-Lopez, C.; Agustí, A.; Vallano, A.; Obach, M. Current landscape of clinical development and approval of advanced therapies. Mol. Ther. Methods Clin. Dev. 2021, 23, 606–618. [Google Scholar] [CrossRef]
- Iglesias-Lopez, C.; Agustí, A.; Obach, M.; Vallano, A. Regulatory Framework for Advanced Therapy Medicinal Products in Europe and United States. Front. Pharmacol. 2019, 10, 921. [Google Scholar] [CrossRef]
- Smith, M.D.; Brune, J.C.; Wildemann, B.; Pruss, A. Whither Advanced Therapy Medicinal Products? Transfus. Med. Hemotherapy 2013, 40, 449. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 9 August 2023).
- El-Kadiry, A.E.H.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef]
- Bashor, C.J.; Hilton, I.B.; Bandukwala, H.; Smith, D.M.; Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 2022, 21, 655–675. [Google Scholar] [CrossRef]
- Buckland, K.F.; Bobby Gaspar, H. Gene and cell therapy for children—New medicines, new challenges? Adv. Drug Deliv. Rev. 2014, 73, 162–169. [Google Scholar] [CrossRef]
- Ligon, J.A.; Wessel, K.M.; Shah, N.N.; Glod, J. Adoptive Cell Therapy in Pediatric and Young Adult Solid Tumors: Current Status and Future Directions. Front. Immunol. 2022, 13, 846346. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Advanced Therapy Medicinal Products: Overview. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview (accessed on 18 July 2023).
- Papanikolaou, E.; Bosio, A. The Promise and the Hope of Gene Therapy. Front. Genome Ed. 2021, 3, 618346. [Google Scholar] [CrossRef] [PubMed]
- Gowing, G.; Svendsen, S.; Svendsen, C.N. Ex vivo gene therapy for the treatment of neurological disorders. Prog. Brain Res. 2017, 230, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Soofiyani, S.R.; Baradaran, B.; Lotfipour, F.; Kazemi, T.; Mohammadnejad, L. Gene Therapy, Early Promises, Subsequent Problems, and RecentBreakthroughs. Adv. Pharm. Bull. 2013, 3, 249. [Google Scholar] [CrossRef]
- Naldini, L. Genetic engineering of hematopoiesis: Current stage of clinical translation and future perspectives. EMBO Mol. Med. 2019, 11, e9958. [Google Scholar] [CrossRef]
- Aiuti, A.; Roncarolo, M.G.; Naldini, L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: Paving the road for the next generation of advanced therapy medicinal products. EMBO Mol. Med. 2017, 9, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Aiuti, A.; Biasco, L.; Scaramuzza, S.; Ferrua, F.; Cicalese, M.P.; Baricordi, C.; Dionisio, F.; Calabria, A.; Giannelli, S.; Castiello, M.C.; et al. Lentiviral hematopoietic stem cell gene therapy in patients with wiskott-aldrich syndrome. Science 2013, 341, 1233151. [Google Scholar] [CrossRef]
- Hacein-Bey Abina, S.; Gaspar, H.B.; Blondeau, J.; Caccavelli, L.; Charrier, S.; Buckland, K.; Picard, C.; Six, E.; Himoudi, N.; Gilmour, K.; et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA 2015, 313, 1550–1563. [Google Scholar] [CrossRef]
- Cartier, N.; Hacein-Bey-Abina, S.; Bartholomae, C.C.; Veres, G.; Schmidt, M.; Kutschera, I.; Vidaud, M.; Abel, U.; Dal-Cortivo, L.; Caccavelli, L.; et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009, 326, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Eichler, F.; Duncan, C.; Musolino, P.L.; Orchard, P.J.; De Oliveira, S.; Thrasher, A.J.; Armant, M.; Dansereau, C.; Lund, T.C.; Miller, W.P.; et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med. 2017, 377, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Montini, E.; Lorioli, L.; Cesani, M.; Fumagalli, F.; Plati, T.; Baldoli, C.; Martino, S.; Calabria, A.; Canale, S.; et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013, 341, 1233158. [Google Scholar] [CrossRef]
- Sessa, M.; Lorioli, L.; Fumagalli, F.; Acquati, S.; Redaelli, D.; Baldoli, C.; Canale, S.; Lopez, I.D.; Morena, F.; Calabria, A.; et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: An ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet 2016, 388, 476–487. [Google Scholar] [CrossRef]
- Marktel, S.; Scaramuzza, S.; Cicalese, M.P.; Giglio, F.; Galimberti, S.; Lidonnici, M.R.; Calbi, V.; Assanelli, A.; Bernardo, M.E.; Rossi, C.; et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ß-thalassemia. Nat. Med. 2019, 25, 234–241. [Google Scholar] [CrossRef]
- De Ravin, S.S.; Wu, X.; Moir, S.; Anaya-O’Brien, S.; Kwatemaa, N.; Littel, P.; Theobald, N.; Choi, U.; Su, L.; Marquesen, M.; et al. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016, 8, 335ra57. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Kim, B.K.; Ke, X.; Yeo, Y. In-vitro and in-vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate. Biomaterials 2019, 217, 119296. [Google Scholar] [CrossRef]
- Li, L.; He, Z.Y.; Wei, X.W.; Gao, G.P.; Wei, Y.Q. Challenges in CRISPR/CAS9 Delivery: Potential Roles of Nonviral Vectors. Hum. Gene Ther. 2015, 26, 452–462. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999, 45, 268–275. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhao, P.; Chen, Z.; Lin, Q. Hyperbranched-star PEI-g-PEG as a nonviral vector with efficient uptake and hypotoxicity for retinoblastoma gene therapy application. Colloid Interface Sci. Commun. 2022, 50, 100647. [Google Scholar] [CrossRef]
- Domingues, C.S.D.C.; Serambeque, B.P.; Laranjo Cândido, M.S.; Marto, C.M.M.; Veiga, F.J.B.; Sarmento Antunes Cruz Ribeiro, A.B.; Figueiras, A.R.R.; Botelho, M.F.R.; Dourado, M.A.R.F. Epithelial-mesenchymal transition and microRNAs: Challenges and future perspectives in oral cancer. Head Neck 2018, 40, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Domingues, C.; Jarak, I.; Veiga, F.; Figueiras, A. Osteosarcoma from the unknown to the use of exosomes as a versatile and dynamic therapeutic approach. Eur. J. Pharm. Biopharm. 2022, 170, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, E.B.; Sezgintürk, M.K. Poly(amidoamine) (PAMAM): An emerging material for electrochemical bio(sensing) applications. Talanta 2016, 148, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Shao, N.; Hu, Z.; Chen, H.; Xu, L.; Wang, C.; Cheng, Y.; Xiao, J. Triazine-modified dendrimer for efficient TRAIL gene therapy in osteosarcoma. Acta Biomater. 2015, 17, 115–124. [Google Scholar] [CrossRef]
- Flores-Mejía, R.; Fragoso-Vázquez, M.J.; Pérez-Blas, L.G.; Parra-Barrera, A.; Hernández-Castro, S.S.; Estrada-Pérez, A.R.; Rodrígues, J.; Lara-Padilla, E.; Ortiz-Morales, A.; Correa-Basurto, J. Chemical characterization (LC–MS–ESI), cytotoxic activity and intracellular localization of PAMAM G4 in leukemia cells. Sci. Rep. 2021, 11, 8210. [Google Scholar] [CrossRef]
- Kandasamy, G.; Danilovtseva, E.N.; Annenkov, V.V.; Krishnan, U.M. Poly(1-vinylimidazole) polyplexes as novel therapeutic gene carriers for lung cancer therapy. Beilstein J. Nanotechnol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Pack, D.W.; Putnam, D.; Langer, R. Design of Imidazole-Containing Endosomolytic Biopolymers for Gene Delivery. Biotechnol. Bioeng. 2000, 67, 217–223. [Google Scholar] [CrossRef]
- Jeon, W.Y.; Choi, Y.B.; Kim, H.H. Ultrasonic synthesis and characterization of poly(acrylamide)-co-poly(vinylimidazole)@MWCNTs composite for use as an electrochemical material. Ultrason. Sonochem. 2018, 43, 73–79. [Google Scholar] [CrossRef]
- Abdellatif Soliman, S.M.; Sanad, M.F.; Shalan, A.E. Synthesis, characterization and antimicrobial activity applications of grafted copolymer alginate-g-poly(N-vinyl imidazole). RSC Adv. 2021, 11, 11541. [Google Scholar] [CrossRef]
- Massoudi, S.; Bagheri, M.; Beygi Khosrowshahi, Y.; Hosseini, M. Antibacterial and cytotoxicity assessment of poly (N-vinyl imidazole)/nitrogen-doped graphene quantum dot nanocomposite hydrogels. Polym. Bull. 2023, 80, 6471–6494. [Google Scholar] [CrossRef]
- MacLaughlin, F.C.; Mumper, R.J.; Wang, J.; Tagliaferri, J.M.; Gill, I.; Hinchcliffe, M.; Rolland, A.P. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J. Control Release 1998, 56, 259–272. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Revuri, V.; Huh, K.M.; Lee, Y.K. Polysaccharide based nano/microformulation: An effective and versatile oral drug delivery system. In Nanostructures for Oral Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 409–433. [Google Scholar] [CrossRef]
- Ritthidej, G.C. Nasal Delivery of Peptides and Proteins with Chitosan and Related Mucoadhesive Polymers. In Peptide and Protein Delivery; Academic Press: Cambridge, MA, USA, 2011; pp. 47–68. [Google Scholar] [CrossRef]
- Ta, H.T.; Dass, C.R.; Larson, I.; Choong, P.F.M.; Dunstan, D.E. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials 2009, 30, 4815–4823. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Vigliotti, V.S.; Martinez, I. Public health applications of CRISPR: How children’s health can benefit. Semin. Perinatol. 2018, 42, 531. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Simões, M.L.; Marois, E.; Dimopoulos, G. CRISPR/Cas9 -mediated gene knockout of Anopheles gambiae FREP1 suppresses malaria parasite infection. PLOS Pathog. 2018, 14, e1006898. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Webber, B.R.; Osborn, M.J.; McElroy, A.N.; Twaroski, K.; Lonetree, C.; DeFeo, A.P.; Xia, L.; Eide, C.; Lees, C.J.; McElmurry, R.T.; et al. CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. NPJ Regen. Med. 2016, 1, 16014. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, J.P. Butterfly Children/Epidermolysis Bullosa. Pondicherry J. Nurs. 2021, 14, 66–68. [Google Scholar] [CrossRef]
- Grand View Research (GVR). Cell Therapy Market Size, Share And Growth Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/cell-therapy-market (accessed on 9 August 2023).
- Mordor Intelligence Cell Therapy Market—Size, Trends, Industry Analysis & Growth. Available online: https://www.mordorintelligence.com/industry-reports/cell-therapy-market (accessed on 9 August 2023).
- Azar, J.; Bahmad, H.F.; Daher, D.; Moubarak, M.M.; Hadadeh, O.; Monzer, A.; Bitar, S.A.; Jamal, M.; Al-Sayegh, M.; Abou-Kheir, W. The Use of Stem Cell-Derived Organoids in Disease Modeling: An Update. Int. J. Mol. Sci. 2021, 22, 7667. [Google Scholar] [CrossRef]
- Clarke, M.F. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019, 380, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Hao, J.; Wang, L.; Tan, Y.; Tian, Y.; Li, S.; Ma, A.; Fu, B.; Dai, J.; Zhai, P.; et al. Developing Standards to Support the Clinical Translation of Stem Cells. Stem Cells Transl. Med. 2021, 10, S85–S95. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, K. Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 2023, 1, 608–609. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, C.; Chen, Z.; Li, Y.; Dai, J.; Zhang, J.; Shu, Q. Clinical trials of stem cell-based therapies for pediatric diseases: A comprehensive analysis of trials registered on ClinicalTrials.gov and the ICTRP portal site. Stem Cell Res. Ther. 2022, 13, 307. [Google Scholar] [CrossRef]
- Yáñez-Muñoz, R.J.; Grupp, S.A. CAR-T in the clinic: Drive with care. Gene Ther. 2018, 25, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Joechner, A.; Li, Z.; Yang, S.F.; Schlegel, P. Development of CAR T Cell Therapy in Children—A Comprehensive Overview. J. Clin. Med. 2022, 11, 2158. [Google Scholar] [CrossRef] [PubMed]
- Blache, U.; Popp, G.; Dünkel, A.; Koehl, U.; Fricke, S. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat. Commun. 2022, 13, 5225. [Google Scholar] [CrossRef]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Hucks, G.; Rheingold, S.R. The journey to CAR T cell therapy: The pediatric and young adult experience with relapsed or refractory B-ALL. Blood Cancer J. 2019, 9, 10. [Google Scholar] [CrossRef]
- Moretti, A.; Ponzo, M.; Nicolette, C.A.; Tcherepanova, I.Y.; Biondi, A.; Magnani, C.F. The Past, Present, and Future of Non-Viral CAR T Cells. Front. Immunol. 2022, 13, 867013. [Google Scholar] [CrossRef]
- Zhao, N.; Song, Y.; Xie, X.; Zhu, Z.; Duan, C.; Nong, C.; Wang, H.; Bao, R. Synthetic biology-inspired cell engineering in diagnosis, treatment, and drug development. Signal Transduct. Target. Ther. 2023, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Zambaiti, E.; De Coppi, P. Regenerative medicine: Current research and perspective in pediatric surgery. Pediatr. Surg. Int. 2023, 39, 167. [Google Scholar] [CrossRef] [PubMed]
- Lally, C.; Joyce, K.; Pandit, A. Biomaterials enhancing performance of cell and nucleic-acid therapies: An opportunity in the brain. Biomater. Biosyst. 2022, 5, 100036. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, G.C.; Lanyi, K.; Inskip, A.; Ogunbayo, O.J.; Brhlikova, P.; Craig, D. A pipeline analysis of advanced therapy medicinal products. Drug Discov. Today 2023, 28, 103549. [Google Scholar] [CrossRef] [PubMed]

