1. Introduction
Antimicrobial resistance is characterized as a “silent pandemic” by the global scientific community and is considered as one of the biggest threats to public health nowadays [1]. The World Health Organization (WHO) talks about a “post-antibiotic era” in which there will be no antibiotics suitable to deal with pan-resistant microbes [2]. In fact, it is estimated that by 2050 the problem of antimicrobial resistance will cause more deaths than cancer and diabetes combined [3].
Europe seems to share the same problem, as in 2015 the European Centre for Disease Prevention and Control (ECDC) recorded 33,110 deaths due to antibiotic-resistant microbes while predicting that by 2050 an economic crisis similar to that of 2008 will appear, due to the increased cost of hospitalizations and the decrease in productivity, as a result of antimicrobial resistance [4].
Regarding Greece, the prevalence of hospital infections is estimated to be about 10%, which means that 66,487 hospitalized patients suffer from some nosocomial pathogens annually [5]. The most frequently encountered pathogens are the following: Acinetobacter baumannii, Escherichia Coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecium and faecalis [6]. A major problem is the resistance of Klebsiella pneumoniae to third-generation cephalosporins and carbapenems (66.5% vs. 31.3% in the EU and 58.3% vs. 7.9% in the EU, respectively), the resistance of Pseudomonas aeruginosa to carbapenems and fluoroquinolones (48.9% vs. 16.5% in the EU and 46.8% vs. 18.9% in the EU, respectively), and the increasing multiresistance of Acinetobacter baumannii to cefepime, carbapenems, and aminoglycosides in both ICUs and clinical wards [5,6].
In Greece, there are only a few studies on the surveillance of antimicrobial resistance [5,6,7]. Our study aimed to estimate the antimicrobial susceptibility and multidrug resistance prevalence of certain clinical isolates in a regional hospital in Northern Greece during the last 6 years and to compare the difference between the 3-year time periods of 2018–2020 and 2021–2023. We expect that our study will lead to more effective antimicrobial treatment and better clinical outcomes.
2. Results
2.1. Six-Year Surveillance of Clinical Isolates’ Susceptibility to Certain Antibiotics
2.2. Epidemiology and Prevalence of Multidrug-Resistant (MDR) Clinical Isolates in 2018–2020 and 2021–2023
In total, 1767 patients were found to be infected by multidrug-resistant (MDR) bacterial strains during 2018–2023. Of that, 1094 (62%) were males aged 72 (3–101) years and 673 (38%) were females aged 77 (1–98) years. A significant increase (93%, almost 2 fold) in MDR bacteria was observed in males compared to females (19%) in the time period of 2021–2023 compared to the time period of 2018–2020 (p < 0.0001, r = −0.115, Figure 8A). Another difference was that cases with MDR isolates in men were observed in a younger age during the second time period, 2021–2023, compared to 2018–2020 (two-way ANOVA, p < 0.0001, r = 0.062, Figure 8B).
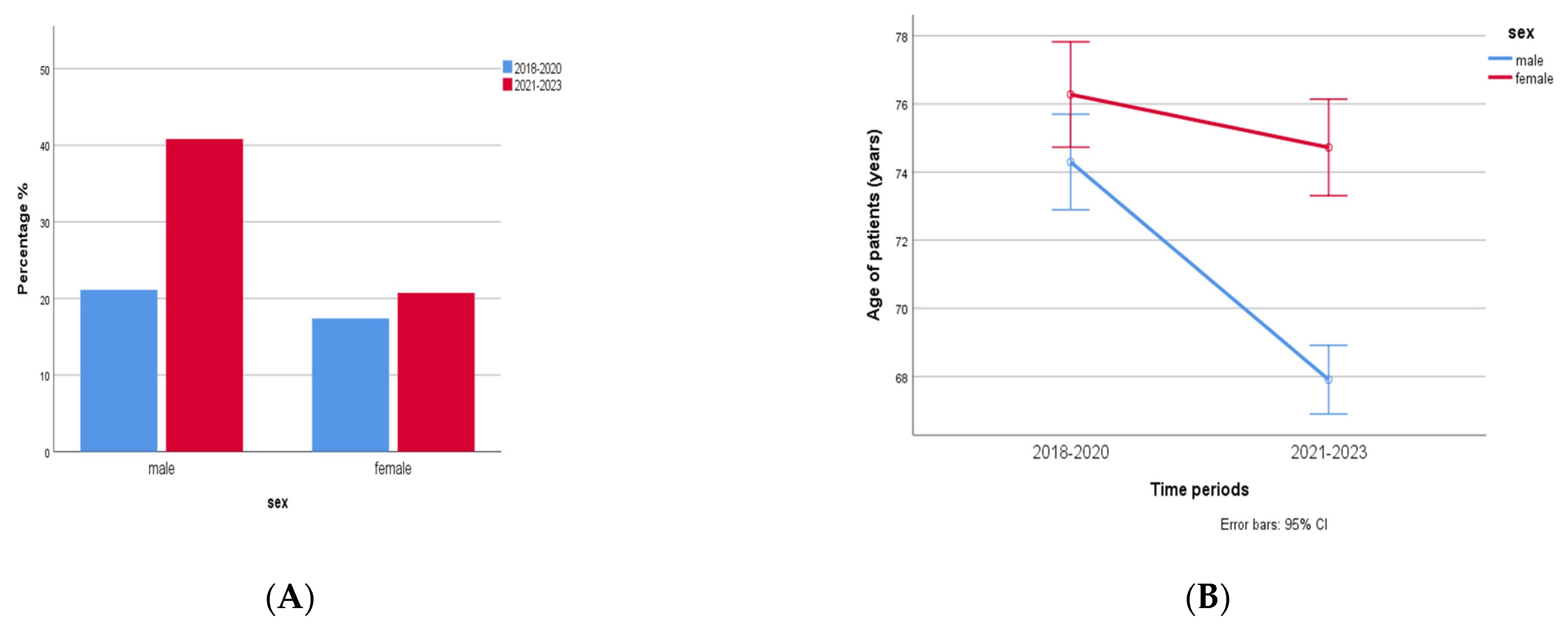
Figure 8. (A) MDR in males and females in 2018–2020 (n = 1094) and 2021–2023 (n = 673). A significant increase (2 fold) in MDR bacteria was observed in males compared to females in the time period 2021–2023 compared to the time period 2018–2020 (p < 0.0001, r = −0.115). (B) Cases with MDR isolates in men were observed in younger age during the second time period, 2021–2023, compared to 2018–2020 (two-way ANOVA, p < 0.0001, r = 0.062).
MDR Klebsiella pneumoniae (700/1767, 39.6%) and Acinetobacter baumannii (643/1767, 36.4%) were the dominant MDR bacteria, followed by Pseudomonas aeruginosa (192/1767, 10.9%) and Enterococcus faecium (171/1767, 9.7%). The prevalence of bacteria with multidrug resistance is shown in Figure 9.
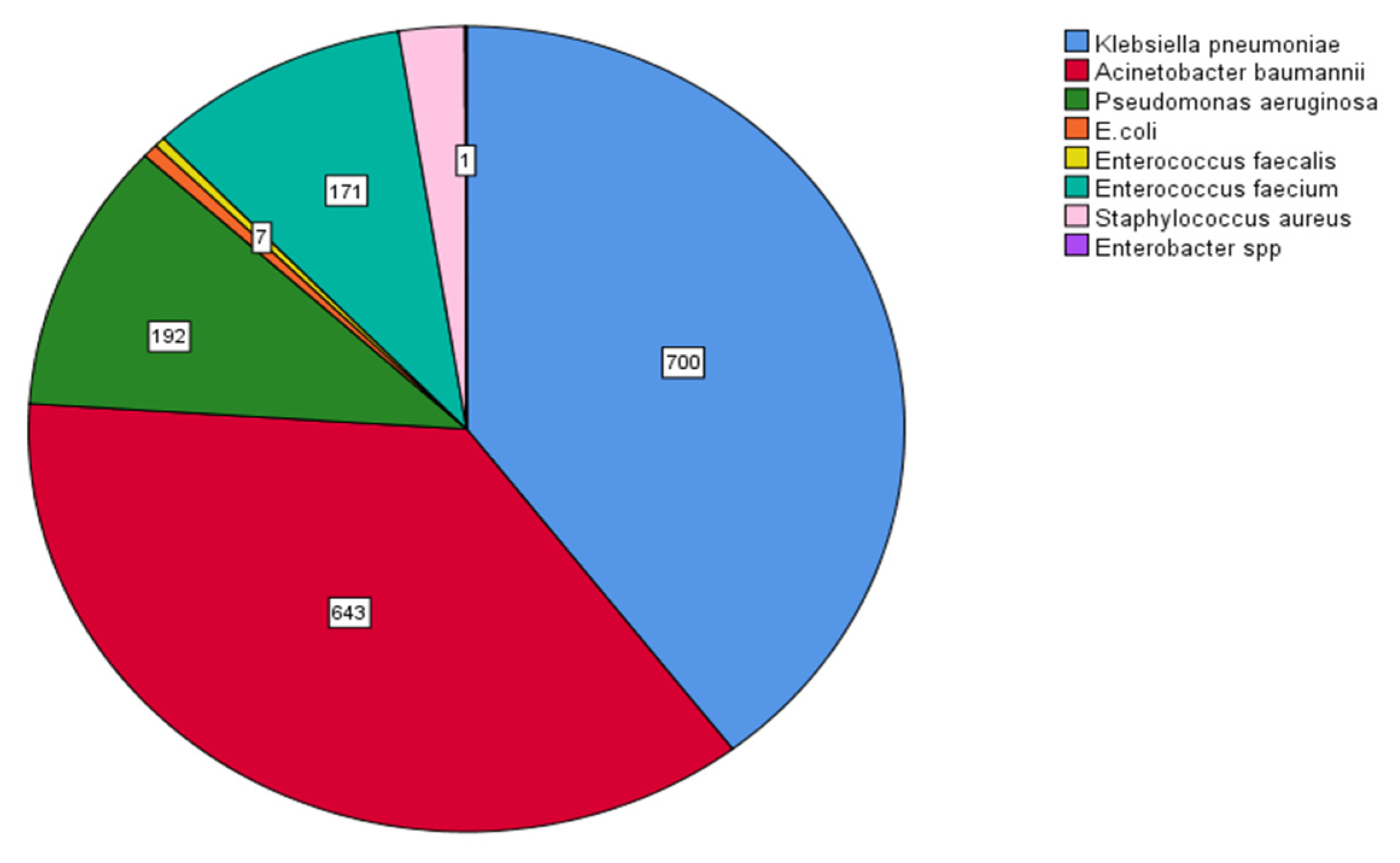
Figure 9. Prevalence of multidrug-resistant (MDR) bacteria. MDR Klebsiella pneumoniae (700/1767, 39.6%), Acinetobacter baumannii (643/1767, 36.4%), Pseudomonas aeruginosa (192/1767, 10.9%), and Enterococcus faecium (171/1767, 9.7%).
In particular, when bacterial strains were analyzed according to the kind of antimicrobial resistance (multidrug resistance, MDR; excessive drug resistance, XDR; pan drug resistance, PDR) and the presence of VRE and MRSA strains, XDR clinical isolates were the most prevalent ones (1015/1767, 57.4%), followed by MDR strains (570/1767, 32.3%) and PDR strains (159/1767, 9%). The prevalence of VRE and MRSA isolated bacterial strains was low (0.8% and 0.5%, respectively) (Figure 10).
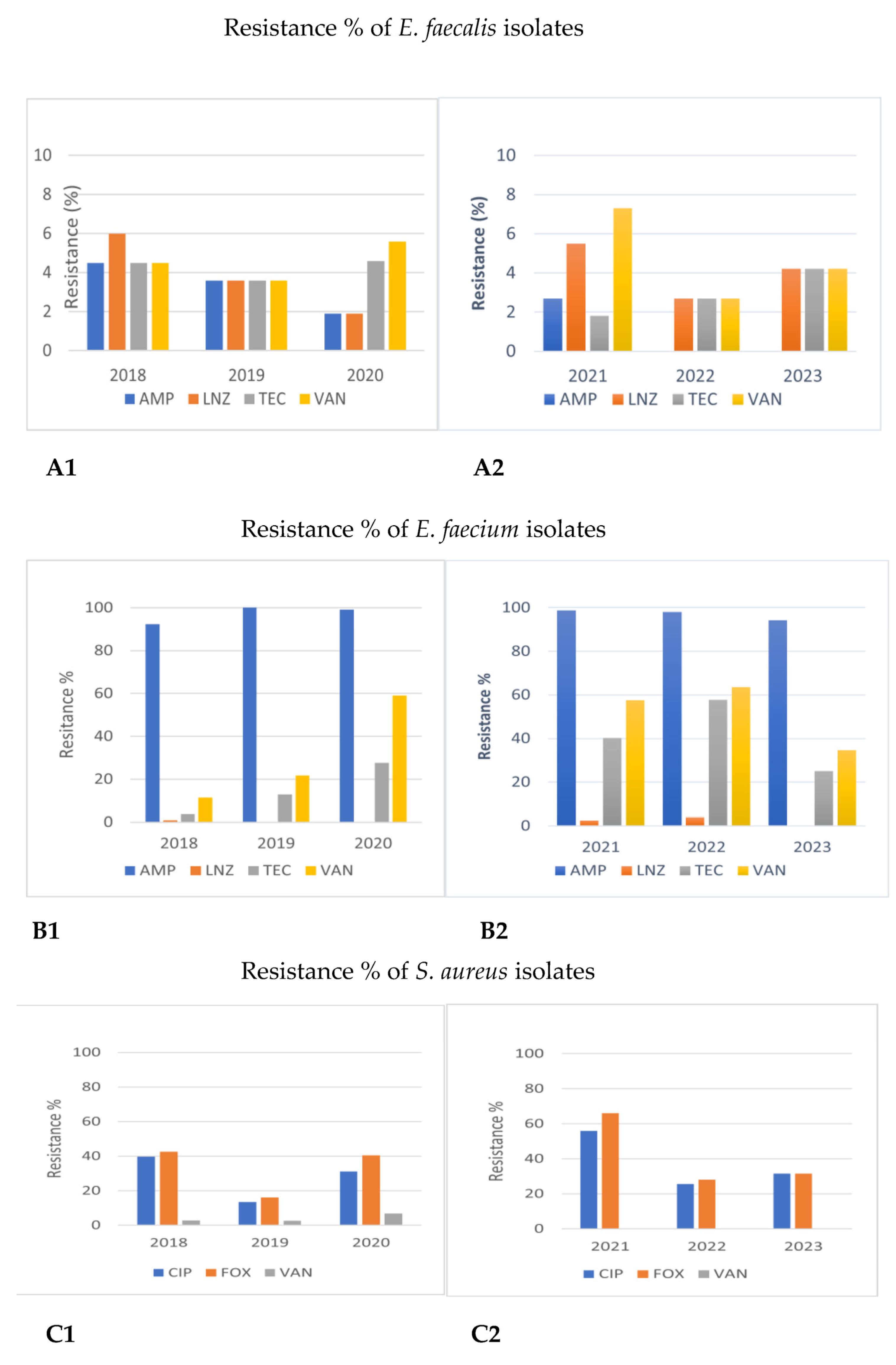
Figure 1. Comparison of antimicrobial resistance of Gram-positive cocci to certain antibiotics between 2018–2020 and 2021–2023. (A1,A2) Antimicrobial resistance of Enterococcus faecalis in 2018–2020 and 2021–2023. (B1,B2) Antimicrobial resistance of Enterococcus faecium in 2018–2020 and 2021–2023. (C1,C2) Antimicrobial resistance of Staphylococcus aureus in 2018–2020 and 2021–2023. AMP: ampicillin; LNZ: linezolid; TEC: teicoplanin; VAN: vancomycin; CIP: ciprofloxacin: FOΧ: cefoxitin.
The intensive care unit (ICU) harbored the majority of MDR isolates (1053/1760, 59.8%), followed by the department of internal medicine (377/1760, 21.4%). The prevalence of MDR in the community patients (hospital outpatient and emergency care units) was also high (211/1760, 11%) (Figure 11).
Most MDR isolates were obtained from urine cultures (673/1748, 38.5%) and from pharyngeal (355/1748, 20.3%) and rectal specimens (340/1748, 19.5%) and were collected in the ICU from blood- and catheter-related cultures (254/1748, 14.6%) and rarely from other biological products such as bronchial and injury specimens, etc. (Figure 12).
The comparison of MDR prevalence between 2018–2020 and 2021–2023 revealed a significant rise in Klebsiella pneumoniae (300/1767 in 2018–2020 vs. 400/1767 in 2021–2023, p = 0.002, r = 0.073), a non-significant rise in Acinetobacter baumannii (246/1767 in 2018–2020 vs. 397/1767 in 2021–2023, p > 0.05) and Pseudomonas aeruginosa (70/1767 in 2018–2020 vs. 122/1767 in 2021–2023, p > 0.05), respectively, and a significant rise in Enterococcus faecium (39/1767 in 2018–2020 vs. 132/1767 in 2021–2023) MDR isolates in the course of time (p < 0.0001, r = 0.105), as shown in Figure 13.
When analyzed for the type of antimicrobial resistance, MDR and PDR strains increased significantly during 2021–2023 when compared to 2018–2020 (MDR, p < 0.0001, r = 0.136; PDR p < 0.0001, r = 0.147). A non-significant rise in XDR strains in 2021–2023 when compared to these in 2018–2020 was also observed (p > 0.05). MDR strains were 274/680 in 2018–2020 and 296/1087 in 2021–2023, XDR strains were 374/680 in 2018–2020 and 641/1087 in 2021–2023, and PDR strains were 25/680 in 2018–2020 and increased to 134/1087 in 2021–2023. VRE and MRSA strains had a similar prevalence between the two time periods (VRE: 6/680 in 2018–2020 vs. 9/1087 in 2021–2023; MRSA: 1/680 in 2018–2020 vs. 7/1087 in 2021–2023) (Figure 14).
A non-significant increase in MDR strains located in the ICU was observed (661/1083) in 2021–2023 when compared to the number of isolates that presented in the ICU from 2018 to 2020 (392/677), p > 0.05. Smaller but significant increases in 2021–2023 were observed in the department of internal medicine (164/677 in 2018–2020 vs. 213/1083 in 2021–2023, p = 0.023, r = 0.054) and in the other wards (12/677 in 2018–2020 vs. 58/1083 in 2021–2023, p < 0.0001, r = 0.089). A non-significant rise in MDR strains in outpatient and emergency care units (91/677 in 2018–2020 vs. 120/1083 in 2021–2023, p > 0/05) was also observed (Figure 15).
3. Discussion
The WHO and the US Centers for Disease Control and Prevention (CDC) have characterized antimicrobial-resistant bacteria as a universally accelerating serious health threat [8,9]. Although there is not yet an integral, systematic, international surveillance of antimicrobial resistance [8], various reports indicate that more than 2 million infections due to antibiotic-resistant pathogens with 29,000 deaths occur in the United States per year, with a health care cost of more than USD 4.7 billion [9]. In Europe, data from national surveillance networks report over 33,000 deaths and 874,000 disability-adjusted life years due to hospital- and community-acquired infections every year, accounting for USD 1.5 billion in annual health costs [4,10]. The problem seems to occur in developed rather than developing countries, in which communicable infectious diseases remain the main cause of mortality [11], while recent data report increasing rates of MDR resistance as well [12].
Although antimicrobial resistance (AMR) genes exist naturally in the environment, the systematic empirical administration of broad-spectrum antibiotics in hospital settings has increased their presence in this area [13]. Therefore, in 2017, the WHO published a list of pathogens requiring the urgent discovery and administration of new antibiotic agents.
Within this list, certain pathogens named the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) group were characterized as of ‘priority status’ [14,15,16]. Through genetic mutations and the acquisition of mobile genetic elements (plasmids) [17], ESKAPE pathogens have developed resistance mechanisms against many antibiotic classes including oxazolidinones, lipopeptides, macrolides, fluoroquinolones, tetracyclines, and b-lactams and their combinations with b-lactamase inhibitors as well as last-line antibiotic groups such as carbapenems, glycopeptides, and polymyxins [18,19,20,21,22,23]. Additionally, resistance to lipoglycopeptides, although rare, has been reported recently [24].
Among European Union (EU) countries, Greece reports consistently higher-than-EU-average rates of resistance in the following hospital-acquired pathogens: Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterococcus faecium [6]. Carbapenem resistance in Enterobacterales causing hospital-acquired infections is 43.7% in our country, whereas other EU countries (Estonia, Finland, Iceland) report 0%. Particularly, a major problem seems to be the resistance of Klebsiella pneumoniae to third-generation cephalosporins and carbapenems (66.5% for Greece vs. 31.3% for EU and 58.3% vs. 7.9%, respectively), the resistance of Pseudomonas aeruginosa to carbapenems and fluoroquinolones (48.9% for Greece vs. 16.5% for EU and 46.8% vs. 18.9%, respectively), and the increasing multiresistance of Acinetobacter baumannii to cefepime, carbapenems, and aminoglycosides in both ICUs and clinical wards [5,6]. For example, the proportion of carbapenem-resistant Acinetobacter baumannii isolates in blood stream infections changed for meropenem from 79.7% and 95.3% in 2010 to 94.4% and 98.1% in 2017 in clinical wards and ICUs, respectively [6].
Our study estimated the antimicrobial resistance of clinical isolates in a 227-bed general hospital in Katerini, Greece. Katerini is the capital city of Pieria Regional Unit in Northern Greece. The municipal unit of Katerini has a population of 80,700 (according to the 2021 census) [25] and it is the second most populous urban area in the region after the city of Thessaloniki (the biggest city in Northern Greece). The General Hospital of Katerini is the only hospital in this regional unit and has approximately 2500 annual admissions.
Our findings concerning the susceptibility of bacterial strains of the ESKAPE pathogens to certain agents reveal some very interesting points. Regarding MRSA, a similar prevalence seemed to occur in our study and other published national data [6]. Ciprofloxacin resistance, although not stable, remains quite high [6]. It is encouraging that the susceptibility to glycopeptide (VAN, vancomycin; TEC, teicoplanin) and lipopeptide antibiotics (DAP, daptomycin) and oxazolidinones (LNZ, linezolid) is constantly high. As for Enterococci, Enterococcus faecalis was more sensitive than Enterococcus faecium to all antimicrobial classes except linezolid. However, the observed increasing level of Enterococcus faecium non-susceptibility to vancomycin and teicoplanin in our study, especially during 2021–2023, is problematic and highlights the need for close monitoring, although high resistance levels were reported from other EU countries as well [26].
In our study, E. coli, the most prevalent pathogen isolated from biological specimens, showed high susceptibility to many antibiotics, except amoxicillin/clavulanic acid and fluoroquinolones. Although resistance to the last-line group of antimicrobial agents (carbapenems, colistin) remained low during the whole 6-year period of testing, an increasing trend of non-susceptibility to third-generation cephalosporins and piperacillin/tazobactam is a serious problem since administration of an effective empirical antimicrobial treatment is essential for both hospitalized and community patients [6].
For Enterobacter species, because of the limited number of isolates during this 6-year surveillance study, more data on antimicrobial resistance need to be available in order to estimate certain conclusions. However, Enterobacter cloacae seems to have a much lower susceptibility to many antibiotic categories over time than Enterobacter aerogenes.
For Klebsiella pneumoniae, the major findings of this study are both the high resistance rates of isolates to all antibiotic classes, except amikacin (AMK) and colistin (COL), although a trend of increasing resistance to these agents is obvious, as well as a high proportion of multidrug resistance. Polemis et al. (2020) also reported high rates of carbapenem resistance [6].
Various Greek reports exist dealing with the phenomenon of high rates of carbapenem resistance among clinical isolates of K. pneumoniae since 2002. First, Verona integron-encoded Metallo-beta-lactamase (VIM) producers were the dominant ones during 2002–2007 [27] followed by the endemic presence of K. pneumoniae carbapenemase (KPC) producers since 2011 [28,29]. Afterwards, K. pneumoniae-producing New Delhi Metallo-beta-lactamase (NDM)-1 emerged in the country [30,31] and rapidly became the second most frequent carbapenemase encountered [32,33].
Regarding oxacillinase (OXA)-48-carbapenemases, since their entry in 2012, low rates of this carbapenemase class have been observed [33,34]. In our hospital, in the first study period (2018–2020), the dominant presence of KPC producers with a certain resistance phenotype (multiresistance with sensitivity only to amikacin and colistin) was the cause of the registered susceptibility to this agent. The increased amikacin resistance rate observed during the second study period (2021–2023) was due to the onset of NDM producers as well.
Regarding Acinetobacter baumannii, the extremely low susceptibility to all antibiotic categories, except colistin, and its predominance in hospital settings and especially in ICUs is an important finding of our study that has caused a great concern during the last years [5,6,35]. However, data on colistin-resistant/carbapenem-resistant A. baumannii isolates are more alarming and constitute a great challenge for both clinical practice and public health [36]. A. baumannii isolates in Greek hospitals produce almost exclusively the OXA-23 carbapenemase [37]. A limitation of this study is the determination of A. baumannii colistin susceptibility using automated system methods. Because strains resistant to colistin appear as false susceptible when tested automatically, both CLSI and EUCAST suggest the use of the broth microdilution method (BMD) as the gold-standard method [38].
A recent study that took place in our laboratory confirmed this finding, resulting in low accordance between these two methods (Vitek 2 and BMD), since 49.7% of A. baumannii strains were found sensitive to colistin using both Vitek 2 and BMD methods and 50.3% of strains were resistant to colistin when examined with the BMD method [39].
As for Pseudomonas aeruginosa, high rates of resistance to carbapenems and fluoroquinolones are in accordance with other national reports [5,6]. Additionally, similar resistance patterns to third- and fourth-generation cephalosporins (CAZ, ceftazidime; FEP, cefepime), piperacillin/tazobactam (PTZ), amikacin, and carbapenems exist between the two studied time periods. It seems that the decreased proportion of Pseudomonas aeruginosa VIM producers found during the last 20 years [40,41] combined with the increased presence of non-enzymatic mechanisms of carbapenem resistance [42,43] explain this phenomenon, as VIM producers exhibit multidrug-resistant phenotypes. Therefore, the low number of VIM producers could be responsible for the relatively low appearance of multidrug-resistant isolates observed in this study. Also very encouraging is the low resistance to colistin observed during these 6 years; this provides alternative therapeutic options to both hospital- and community-acquired infections caused by this microorganism.
Interesting findings about MDR resistance appeared during the pandemic years in our study. COVID-19 infection, with an estimated number of deaths higher than 6 million, has led to great morbidity and mortality worldwide [44]. Risk factors for developing COVID-19 in adults are considered as both demographic factors, such as older age, male sex, and ethnicity, and the presence of underlying diseases as well [44,45,46].
Additionally, reports demonstrate that COVID-19 patients are at greater risk of developing an MDR hospital-acquired infection due to their prolonged hospitalization, the increased rate of admissions to ICUs, and the need for mechanical ventilation [47,48]. Our data demonstrate that the significant increase in MDR pathogens in male patients (mainly in urine, blood, rectal, and pharyngeal specimens) observed in 2021–2023 when compared to 2018–2020 was caused mainly by admissions of patients with COVID-19 disease. In fact, older males (aged > 68 years) with mild and severe COVID-19 infection were the majority of hospital admissions during this time period, hospitalized in the ICU or either the internal medicine or COVID-19 clinical departments.
Another interesting finding of our study is the high rate of MDR pathogens in the community, as registered by the visits of outpatients to ambulatory and emergency care units of the hospital (11%). The explanation of this existing AMR reservoir, showing the spread of MDR pathogens from the hospital environment to the community, is probably the high antimicrobial consumption in our country. Greece has the highest percentage in consumption of antimicrobial agents among all European countries: in a 2020 ECDC report, antimicrobial consumption in defined daily doses (DDDs) per 100 inhabitants per day was 34.1 in Greece whereas the EU mean consumption was 19.4 DDDs per 100 inhabitants per day [5]. This overconsumption of antimicrobials may lead to the selection of resistant bacterial strains in Greece.
4. Materials and Methods
4.1. Study Design
This retrospective study was performed by analyzing the annual reports of the Laboratory of Microbiology in General Hospital of Katerini, which is a city in Northern Greece. The duration of the study was almost 6 years, starting from 1 January 2018 and ending on 30 June 2023. It consisted of two parts: in the first part of the study, we investigated the antimicrobial susceptibility of certain bacteria during the whole 6-year time period using the WHONET Greek electronic surveillance system for monitoring AMR in hospitals based on routine data. This time period was chosen because, from 1 January 2018 and onwards, data from the newly established ICU were included in our reports, making data from older periods incomparable. On the other hand, the CLSI breakpoints were used as cutoff values for antimicrobial resistance until 30 June 2023, making data from newer periods incomparable. In the second part of the study, we analyzed and compared the epidemiology and prevalence of multidrug-resistant (MDR) bacterial isolates between the time periods of 2018–2020 and 2021–2023.
The analysis was focused on the most important bacteria that were monitored globally and regionally within the surveillance networks: Enterococcus faecalis and Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, Enterobacter cloacae, and Enterobacter aerogenes. The data were processed according to the institutional and national ethical standards and the World Medical Association (WMA) Declaration of Helsinki (1975), as revised in 2013. The protocol of the study was approved by the Ethics Committee of the General Hospital of Katerini (protocol code 8/30–8-2023).
The bacteria were isolated from biological products that were taken for diagnostic purposes from both hospitalized and community patients. Duplicate isolates were excluded from the study. Clinical isolates were identified by using classical bacteriology methods for microbial culture, on agar-based solid media, followed by biochemical testing of the obtained colonies performed by an automated Vitek 2 Compact system (Biomerieux, Craponne, France). Antibiotic sensibility was tested by using the minimal inhibiting concentration (MIC) method performed by the Vitek 2 Compact system. The interpretation of the antibiotic resistance was made according to CLSI (Clinical and Laboratory Standards Institute) guidelines (2022 edition).
MRSA (methicillin-resistant Staphylococcus aureus) strains were identified by using the Vitek 2 Compact Software based on an oxacillin non-susceptibility test for methicillin resistance. Multiple drug resistance (MDR) was defined as the antimicrobial resistance shown by a species of microorganism to at least one antimicrobial drug in three or more antimicrobial categories. Excessive drug resistance (XDR) was defined as the non-susceptibility of one bacteria species to all antimicrobial agents except in two or fewer antimicrobial categories. In conclusion, pan drug resistance (PDR) was defined as the non-susceptibility of bacteria to all antimicrobial agents in all antimicrobial categories [49].
The antibiotic classes that were used included penicillins (AMP, ampicillin; AMC, amoxicillin clavulanate), cephalosporins (FEP, cefepime; CTX, cefotaxime; CAZ, ceftazidime; TZP, piperacillin/tazobactam), carbapenems (IMP, imipenem; MEM, meropenem), fluoroquinolones (CIP, ciprofloxacin; LVX, levofloxacin), aminoglycosides (AMK, amikacin), sulfonamide (SXT, sulfamethoxazole/trimethoprim), and polymyxins (COL, colistin), and, furthermore, for the Gram-positive bacteria, glycopeptide antibiotics (VAN, vancomycin; TEC, teicoplanin), lipopeptide antibiotics (DAP, daptomycin), and oxazolidinones (LNZ, linezolid).
4.2. Statistical Analysis
Continuous variables are presented as a median and interquartile range, while categorical variables are described as counts and percentages. The comparison of the continuous values between the groups was performed with a nonparametric Mann–Whitney test while the comparison of the categorical variables was performed using Pearson chi-square or Fisher’s exact test. A univariate general linear (two-way ANOVA model) test was used to evaluate the association between age and sex in the two study periods. A Mann–Kendal test was performed for the trend analysis of the resistance to certain antibiotics regarding the ESKAPE pathogens during 2018–2023.
The SPSS 26.0 statistical package was used for the statistical analysis of the data, regarding the MDR rates, and p values < 0.05 were considered as statistically significant. The 5.6 software version of WHONET was used for the analysis of antimicrobial resistance rates of the isolates over time, making use of the CLSI 2021–2022 breakpoints.
5. Conclusions
An overall increasing antimicrobial resistance rate of the dominant pathogens that are responsible for hospital-acquired infections (Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterococcus faecalis) over time has been registered. Multidrug resistance is more common in Klebsiella pneumoniae and Acinetobacter baumannii isolates, especially in the ICU. The frequency of VRE and MRSA isolated bacterial strains remains relatively low. The profile of the hospitalized patient with an MDR infection, mostly from urine-, blood-, and catheter-related specimens, is male, over 68 years, having COVID-19 at the peak of the pandemic years and admitted to the ICU or the department of internal medicine. Furthermore, the presence of pathogens with excessive drug resistance and pan drug resistance during the last 6 years is alarming. It is urgent to establish hospital-based antimicrobial stewardship and programs for infection prevention and control as well as educational programs on rational antimicrobial use and infection control for health care workers.
References
- World Health Organization Ten Threats to Global Health in 2019. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 24 August 2023).
- One Health Global Leaders Group on Antimicrobial Resistance. World leaders join forces to fight the accelerating crisis of an antimicrobial resistance. Neurosciences 2021, 26, 112–113. [Google Scholar]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Kopsidas, I.; Theodosiadis, D.; Triantafyllou, C.; Koupidis, S.; Fanou, A.; Hatzianastasiou, S. Preventing Antimicrobial Resistance and Promoting Appropriate Antimicrobial Use in Inpatient Health Care in Greece 2022 (No. WHO/EURO: 2022-5837-45602-65411); World Health Organization; Regional Office for Europe: København, Denmark, 2022. [Google Scholar]
- Polemis, M.; Tryfinopoulou, K.; Giakkoupi, P.; Vatopoulos, A. WHONET-Greece Study Group. Eight-year trends in the relative isolation frequency and antimicrobial susceptibility among bloodstream isolates from Greek hospitals: Data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance–WHONET-Greece, 2010 to 2017. EuroSurveil 2020, 25, 1900516. [Google Scholar]
- Polemis, M.; Mandilara, G.; Pappa, O.; Argyropoulou, A.; Perivolioti, E.; Koudoumnakis, N.; Pournaras, S.; Vasilakopoulou, A.; Vourli, S.; Katsifa, H.; et al. COVID-19 and Antimicrobial Resistance: Data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance—WHONET-Greece (January 2018–March 2021). Life 2021, 11, 996. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: https://www.who.int/antimicrobial-resistance/publications/surveillancereport/en/ (accessed on 23 August 2023).
- Centers for Disease Control and Prevention. Antibiotic Resistant Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 20 August 2023).
- Weist, K.; Hogberg, L.D. ECDC publishes 2015 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Euro Surveill. 2016, 21, 30401. [Google Scholar] [CrossRef]
- Kayange, N.; Kamugisha, E.; Mwizamholya, D.L.; Jeremiah, S.; Mshana, S.E. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. 2010, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Seijas-Pereda, L.; Rescalvo-Casas, C.; Hernando-Gozalo, M.; Angmorkie-Eshun, V.; Agyei, E.; Adu-Gyamfi, V.; Sarsah, I.; Alfonso-Romero, M.; Cuadros-González, J.; Soliveri-de Carranza, J.; et al. The Antimicrobial Resistance (AMR) Rates of Enterobacterales in a Rural Hospital from the Eastern Region, Ghana: A Retrospective Study, 2022. Antibiotics 2023, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Akova, M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 2016, 7, 252–266. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Beatson, S.A.; Walker, M.J. Microbiology. Tracking antibiotic resistance. Science 2014, 345, 1454–1455. [Google Scholar] [CrossRef]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, S.; Mizutani, T.; Hiramatsu, K.; Kikuchi, K. Rapid acquisition of linezolid resistance in methicillin-resistant Staphylococcus aureus: Role of hypermutation and homologous recombination. PLoS ONE 2016, 11, e0155512. [Google Scholar] [CrossRef] [PubMed]
- Giddins, M.J.; Macesic, N.; Annavajhala, M.K.; Stump, S.; Khan, S.; McConville, T.H.; Mehta, M.; Gomez-Simmonds, A.; Uhleman, A.-C. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in bla (KPC-2)-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob. Agents Chemother. 2017, 62, e02101-17. [Google Scholar] [CrossRef]
- Herc, E.S.; Kauffman, C.A.; Marini, B.L.; Perissinotti, A.J.; Miceli, M.H. Daptomycin nonsusceptible vancomycin resistant Enterococcus bloodstream infections in patients with hematological malignancies: Risk factors and outcomes. Leuk. Lymphoma 2017, 58, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Kussmann, M.; Karer, M.; Obermueller, M.; Schmidt, K.; Barousch, W.; Moser, D.; Nehr, M.; Ramharter, M.; Poeppl, W.; Makristathis, A.; et al. Emergence of a dalbavancin induced glycopeptide/lipoglycopeptide non-susceptible Staphylococcus aureus during treatment of a cardiac device-related endocarditis. Emerg. Microbes Infect. 2018, 7, 202. [Google Scholar] [CrossRef]
- Census Results of the Population-Residents for the Year 2021 Concerning the Legal Population (Citizens) of Greece. Government Gazette 6951/Β/30 December 2022. Archived from the Original on 21 April 2023—Via Hellenic Statistical Authority (ELSTAT). Available online: https://olympiobima.gr/apografi-2021-se-123-245-atoma-o-plithysmos-tis-pierias-pinakas/ (accessed on 18 August 2023).
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe—Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017; ECDC: Stockholm, Sweden, 2018.
- Vatopoulos, A. High rates of metallo-beta-lactamase-producing Klebsiella pneumoniae in Greece—A review of the current evidence. Euro Surveill. 2008, 13, 8023. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Giakkoupi, P.; Maragos, A.; Bolikas, M.; Raftopoulos, V.; Papahatzaki, H.; Vrouhos, G.; Liakou, V.; Vatopoulos, A.C. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J. Infect. 2009, 58, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Giakkoupi, P.; Maltezou, H.; Polemis, M.; Pappa, O.; Saroglou, G.; Vatopoulos, A. Greek System for the Surveillance of Antimicrobial Resistance KPC-2-producing Klebsiella pneumoniae infections in Greek hospitals are mainly due to a hyperepidemic clone. Euro Surveill. 2009, 14, 19218. [Google Scholar] [CrossRef]
- Voulgari, E.; Gartzonika, C.; Vrioni, G.; Politi, L.; Priavali, E.; Levidiotou-Stefanou, S.; Tsakris, A. The Balkan region: NDM-1-producing Klebsiella pneumoniae ST11 clonal strain causing outbreaks in Greece. J. Antimicrob. Chemother. 2014, 69, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Giakkoupi, P.; Tryfinopoulou, K.; Kontopidou, F.; Tsonou, P.; Golegou, T.; Souki, H.; Tzouvelekis, L.; Miriagou, V.; Vatopoulos, A. Emergence of NDM-producing Klebsiella pneumoniae in Greece. Diagn. Microbiol. Infect. Dis. 2013, 77, 382–384. [Google Scholar] [CrossRef]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasević, A.T.; Tzouvelekis, L.; Miriagou, V.; Vatopoulos, A. European Survey of Carbapenemase-Producing Enterobacterales (EuSCAPE) Working Group Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacterales (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar]
- Galani, I.; Karaiskos, I.; Karantani, I.; Papoutsaki, V.; Maraki, S.; Papaioannou, V.; Tsorlini, H.; Charalampaki, N.; Toutouza, M.; Vagiakou, H.; et al. On Behalf of The Study Collaborators Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Euro Surveill. 2018, 23, 1700775. [Google Scholar] [CrossRef]
- Voulgari, E.; Zarkotou, O.; Ranellou, K.; Karageorgopoulos, D.E.; Vrioni, G.; Mamali, V.; Themeli-Digalaki, K.; Tsakris, A. Outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in Greece involving an ST11 clone. J. Antimicrob. Chemother. 2013, 68, 84–88. [Google Scholar] [CrossRef]
- Dey, D.K.; Park, J.; Kang, S.C. Genotypic, phenotypic, and pathogenic characterization of the soil isolated Acinetobacter courvalinii. Microb. Pathog. 2020, 149, 104287. [Google Scholar] [CrossRef]
- Oikonomou, O.; Sarrou, S.; Papagiannitsis, C.C.; Georgiadou, S.; Mantzarlis, K.; Zakynthinos, E.; Dalekos, G.N.; Petinaki, E. Rapid dissemination of colistin and carbapenem resistant Acinetobacter baumannii in Central Greece: Mechanisms of resistance, molecular identification and epidemiological data. BMC Infect. Dis. 2015, 15, 559. [Google Scholar] [CrossRef]
- Pournaras, S.; Dafopoulou, K.; Del Franco, M.; Zarkotou, O.; Dimitroulia, E.; Protonotariou, E.; Poulou, A.; Zarrilli, R.; Tsakris, A. Greek Study Group on Acinetobacter Antimicrobial Resistance Predominance of international clone 2 OXA-23-producing-Acinetobacter baumannii clinical isolates in Greece, 2015: Results of a nationwide study. Int. J. Antimicrob. Agents 2017, 49, 749–753. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Recommendations for MIC Determination of Colistin (Polymyxin E) as Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. EUCAST. 2016. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf (accessed on 5 March 2021).
- Tsalidou, M.; Bostanitis, I.; Samaras, K.; Skoumpa, K.; Gara, S.; Varsou, E.; Goutsiou, R.; Bostanitis, C.; Papaioannidou, P. Colistin susceptibility testing methods of Acinetobacter baumannii isolates: Verifying accuracy and reliability among two methods. EMJ Microbiol. Infect. Dis. 2021, 2, 31–32. [Google Scholar]
- Tsakris, A.; Pournaras, S.; Woodford, N.; Palepou MF, I.; Babini, G.S.; Douboyas, J.; Livermore, D.M. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J. Clin. Microbiol. 2000, 38, 1290–1292. [Google Scholar] [CrossRef]
- Giakkoupi, P.; Petrikkos, G.; Tzouvelekis, L.S.; Tsonas, S.; Legakis, N.J.; Vatopoulos, A.C. WHONET Greece Study Group Spread of integron-associated VIM-type metallo-beta-lactamase genes among imipenem-nonsusceptible Pseudomonas aeruginosa strains in Greek hospitals. J. Clin. Microbiol. 2003, 41, 822–825. [Google Scholar] [CrossRef]
- Meletis, G.; Vavatsi, N.; Exindari, M.; Protonotariou, E.; Sianou, E.; Haitoglou, C.; Sofianou, D.; Pournaras, S.; Diza, E. Accumulation of carbapenem resistance mechanisms in VIM-2-producing Pseudomonas aeruginosa under selective pressure. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 253–258. [Google Scholar] [CrossRef]
- Liakopoulos, A.; Mavroidi, A.; Katsifas, E.A.; Theodosiou, A.; Karagouni, A.D.; Miriagou, V.; Petinaki, E. Carbapenemase-producing Pseudomonas aeruginosa from central Greece: Molecular epidemiology and genetic analysis of class I integrons. BMC Infect. Dis. 2013, 13, 505. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Papaioannidou, P.; Skoumpa, K.; Bostanitis, C.; Michailidou, M.; Stergiopoulou, T.; Bostanitis, I.; Tsalidou, M. Age, Sex and BMI Relations with Anti-SARS-CoV-2-Spike IgG Antibodies after BNT162b2 COVID-19 Vaccine in Health Care Workers in Northern Greece. Microorganisms 2023, 13, 1279. [Google Scholar] [CrossRef]
- Vassilaki, N.; Gargalionis, A.N.; Bletsa, A.; Papamichalopoulos, N.; Kontou, E.; Gkika, M.; Patas, K.; Theodoridis, D.; Manolis, I.; Ioannidis, A.; et al. Impact of Age and Sex on Antibody Response Following the Second Dose of COVID-19 BNT162b2 mRNA Vaccine in Greek Healthcare Workers. Microorganisms 2021, 13, 1725. [Google Scholar] [CrossRef] [PubMed]
- La, Y.; Hong, J.Y.; Lee, H.S.; Lee, E.H.; Lee, K.H.; Song, Y.G.; Kim, S.B.; Han, S.H. Increase of multidrug-resistant bacteria after the COVID-19 pandemic in South Korea: Time-series analyses of a long-term multicenter cohort. J. Infect. 2022, 85, 702–769. [Google Scholar] [CrossRef]
- Hu, S.; You, Y.; Zhang, S.; Tang, J.; Chen, C.; Wen, W.; Wang, C.; Cheng, Y.; Zhou, M.; Feng, Z. Multidrug-resistant infection in COVID-19 patients: A meta-analysis. J. Infect. 2023, 86, 66–117. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria. Clin. Microbiol. Infect. 2012, 1, 268–281. [Google Scholar] [CrossRef] [PubMed]

