1. Introduction
As life expectancy has increased, it has become crucial to sustain and promote active aging [1]. Several factors contribute to active aging, and, among them, cognitive functioning plays a pivotal role in supporting everyday life duties and preserving autonomy and personal growth [2,3]. However, there is evidence that some cognitive abilities are more susceptible than others to life changes and to the consequent physiological decline [4]. For instance, cognitive functions that pertain to the crystallized intelligence domain are mainly preserved, while cognitive mechanisms associated with fluid intelligence and supporting cognitive control—like executive functions (EFs)—usually are more susceptible to decline [1,4,5,6,7,8]. Cognitive decline is characterized by structural and functional changes at a neural level involving a widespread network of brain regions and, in particular, fronto-parietal networks [9,10,11,12]. It follows that older adults (OAs) may present impairments in those abilities that mainly underlie cognitive control, which is fundamental in everyday life [13].
In recent decades, increasing evidence reported that creative thinking, and in particular divergent thinking (DT), may be preserved in aging (for a systematic review, see [14]) and may support cognitive functioning, with beneficial effects on both healthy and clinical populations [3,15,16,17,18,19]. Thinking creatively concerns the ability to generate a product or an idea that is both (i) unexpected and unusual and (ii) useful and appropriate to the context [20], breaking automatic responses for developing alternative behaviors, especially in situations that are new to the individual, and coping with possible everyday life difficulties [16,17]. In this vein, the ability to think divergently, that is, the ability to find more than one solution to open-ended demands [21], is a complex construct that involves both crystallized components of intelligence (i.e., semantic and autobiographical memories) to draw on for the development of new ideas and fluid-processing components (i.e., EFs) aimed at facilitating semantic associations (which are pivotal in the generation of creative ideas), inhibiting automatic thinking, and changing the attentional focus flexibly [22]. Therefore, DT is primarily a cognitive measure useful to investigate the individual’s creative potential [23,24].
DT is sustained by wide-spread neural networks and in particular by (i) the default mode network (DMN), (ii) the salience network (SN), and (iii) the executive control network (ECN). The DMN, which encompasses predominantly the medial prefrontal cortex (MPC), medial temporal lobes, posterior cingulate cortex (PCC), precuneus, and inferior parietal lobule (IPL), is supposed to be fundamental for imaginative thinking, generation of ideas, and retrieval and association of autobiographical and semantic knowledge [25,26,27]. The SN, which involves predominantly the anterior cingulate (ACC) and insular cortices, is fundamental for monitoring functions [28,29] and switching between DMN and ECN functions (e.g., [28,29]). Finally, the ECN, encompassing mainly lateral prefrontal and anterior inferior parietal regions, is crucial for goal-directed cognition and executive functioning, such as working memory and prepotent response inhibition [25,26].
A well-supported claim in the literature is that the DMN-ECN coupling, also modulated by the SN, reflects the dynamic interplay between spontaneous and controlled modes of thought, with DMN activated for idea generation but regulated by ECN in order to maintain specific task goals [26,29,30,31]. Interestingly, according to the Default–Executive Coupling Hypothesis of Aging (DECHA hypothesis; [25,32]), the functional coupling between DMN and ECN could be altered in aging, leading older adults to rely less on declining control processes (subtended predominantly by the ECN), and thereby rely more on crystalized intellectual capacities (mainly subtended by the DMN), to support goal-directed behaviors [13]. This potential shift in strategies is reflected in DT performance. Indeed, DT does not decline steadily during aging and it is assumed that OAs count more on crystallized components of intelligence (semantics) rather than fluid ones (cognitive control) [33], adopting a compensation mechanism. In this way, individual differences recorded in creative tasks between older and younger adults may be due to other cognitive abilities functioning, such as working memory or speed processing [3,34,35].
DT has also been linked to cognitive reserve (CR), which underlies the ability to perform a task or achieve an objective by recruiting alternative strategies and cognitive processes when the usual strategies are no longer possible, as can happen in the aging process [36,37,38]. CR can be increased throughout the lifespan by everyday life experiences and whether the individual is exposed to proper environmental stimuli, being a resource against age-related cognitive decline [38,39]. The educational level, occupational status, and commitment to cognitively stimulate leisure activities are possible factors that contribute to enhancing CR and are usually considered CR proxies [39]. Evidence showed the presence of a relationship between CR and DT in late adulthood [36,37,40,41], indicating the possibility that enhancing creative thinking may increase CR, thereby supporting cognitive functioning.
Nevertheless, to date, there are a few studies concerning such an issue, and also considering the key role of components related to cognitive functioning. Thus, due to the properties of DT and its relationships with crystallized and fluid-processing components and CR, the investigation of the relationships among these constructs in aging appears to be important.
Aims
According to the literature, the present study, firstly, aimed at investigating potential aging effects on DT abilities in both verbal and visual domains. Secondly, it explored the contribution of both crystallized and fluid (i.e., EFs) components of cognition in predicting performance in DT tasks. Finally, we investigated if crystalized components of intelligence mediate the relation between aging and DT, in line with the DECHA hypothesis, and therefore a potentially more “semanticized” over “controlled” cognitive processing in the elderly [13,42].
2. Materials and Methods
2.1. Participants
One hundred and thirty-three participants were recruited from two Italian regions, namely, Lombardy (70.6%) and Sardinia (28.6%).
All participants included in the sample had to meet the following inclusion criteria: age ≥ 60 years old; the absence of global cognitive impairments as defined by Mini-Mental State Examination (MMSE [43]) ≥ 24; psychological profile in the normal range; no history of neurologic impairments or neurosurgical interventions; and the absence of psychiatric disease or history of alcohol or drug addiction. From the initial sample of 133 participants, an additional screening procedure was carried out on the basis of neuropsychological tests administered during the experimental session (for further details, see Section 2.2. Procedure). The final sample consisted of 98 individuals (45 females; mean age = 72.44 years, SD = 6.35; mean education = 12.30 years, SD = 4.64).
2.2. Procedure
Data were collected from April 2021 to December 2022. All participants read and provided the informed consent. Two individual in-person sessions were scheduled, each of them lasting about 60 min, to evaluate DT, cognitive abilities, CR, and mood state. Participants who exhibited a pathological score in at least one neuropsychological test were excluded from the statistical analyses. All participants took part in the study on a voluntary basis. No incentive was provided.
The study was conducted according to the Declaration of Helsinki [44] and was approved by the Institutional Ethical Committee of the University of Bergamo and by the one of the Catholic University of the Sacred Heart in Milan.
2.3. Instruments
2.4. Statistical Analyses
The sample size for regression models was computed considering f2 as a measure of effect size. In detail, we assumed a medium effect size (f2 = 0.15), an α = 0.05, a power (1-β) of 0.80, number of total predictors = 8, and number of tested predictors = 3. Results yielded a sample size of 77 individuals.
All individual scores were transformed into standardized z-scores for statistical analyses. Four participants were excluded from the analyses because of missing data or abnormal z-scores values (± 3 SD). Therefore, the final sample entered into statistical analyses included 94 participants. Standardized scores of both visual and verbal DT subscales (i.e., fluency, flexibility, and originality) were averaged in order to obtain a composite score, respectively, for visual and verbal creativity. In correlation analyses, both parametric (Pearson’s r) and nonparametric (Spearman’s rho) coefficients were computed in order to explore the relations between sociodemographic variables (i.e., age and education), DASS scores, cognitive tests scores, and DT scores. Then, partial correlations between cognitive outcomes, CRIq total, and DT scores were calculated, controlling for both age and education. An additional EF composite score (mean of STROOP_I and TMT B-A) was computed by combining the Stroop test’s interference scores with TMT shifting index (TMT B-A) and was used in hierarchical regression models.
Two separate hierarchical regression analyses were performed, considering both DT measures (i.e., visual and verbal) as dependent variables. In both regression analyses, age, education, and gender were entered as predictors in the first model, measures of EFs (i.e., executive composite score, Digit Span Backward, and SDMT) in the second model, measures of crystallized intelligence (WAIS_voc) in the third model, and cognitive reserve index (CRIq total) in the fourth model. Additional mediation models were then tested in order to investigate the effects of executive/fluid processing mechanisms (executive composite score), crystallized intelligence (WAIS_voc), and cognitive reserve index (CRIq_tot) as potential mediators of the relationship between age and visual DT.
3. Results
and reported the mean and SD of sociodemographic variables, neuropsychological tests, and creativity tests.
3.1. Correlations
As expected, age and education were significantly correlated to almost all cognitive outcomes, except for verbal episodic memory (see . Significant correlations were found between age, education, and visual DT composite score (r = −0.406, p < 0.001; r = 0.220, p = 0.033). Notably, considering visual and verbal DT subscales, we found that age correlated with all visual subscales and only with verbal originality subscale (only a trend: p = 0.06) (see .
Moreover, significant partial correlations were found between visual DT, CRIq total (r = 0.235, p = 0.027), and WAIS_voc (r = 0.278, p = 0.008) scores.
3.2. Regression Models
Only visual creative thinking models were found to be significant. Furthermore, Model 1, Model 3, and Model 4 were significant with age as the only significant predictor in Model 1. Increasing age corresponded to a diminished DT score, whereas higher WAIS_voc scores and CRIq total predicted better visual DT scores (see .
3.3. Mediation Models
Only the WAIS_voc was found to be a significant mediator in the relation between age and visual DT (see Figure 1 and .
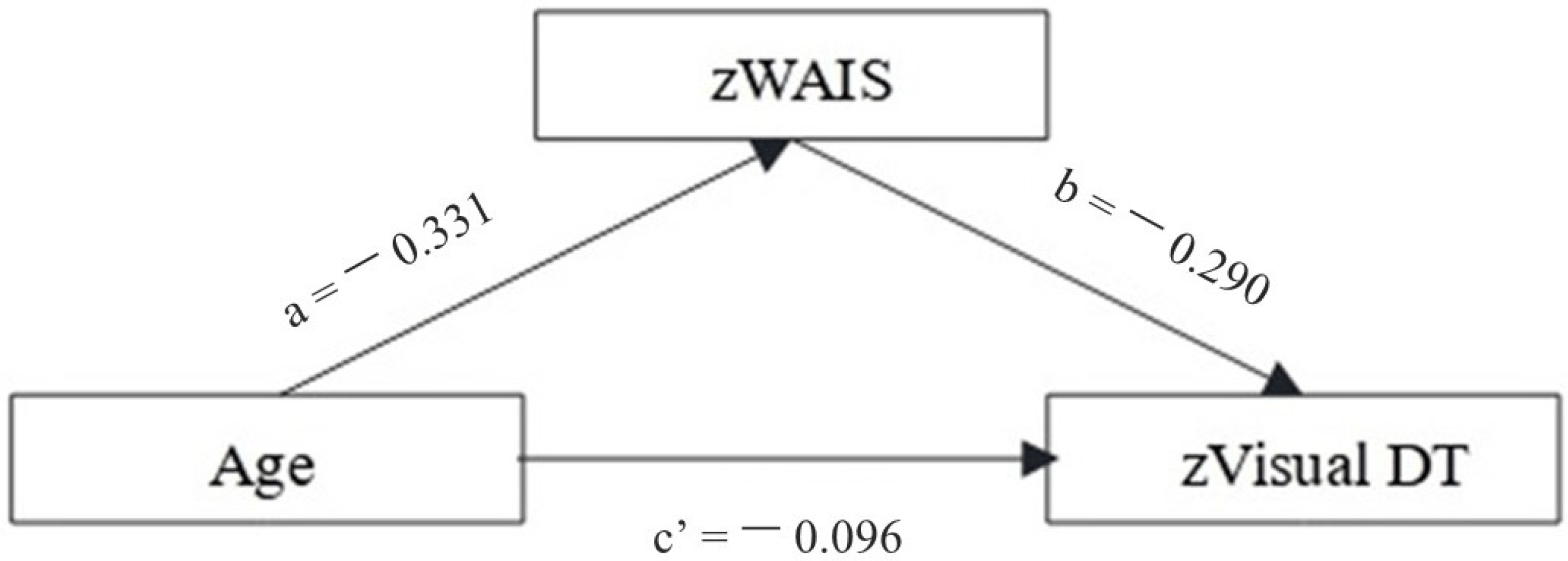
Figure 1. Mediation analysis investigating the role of crystallized intelligence in mediating the relationship between age and visual divergent thinking. Betas were reported for each component (see also .
4. Discussion
The present study aimed at investigating aging effects on DT abilities and the possible relationships between fluid (i.e., EFs) and crystallized (i.e., WAIS_voc) components of cognition and CR in predicting DT performance in a sample of healthy OAs. We found an aging effect on visual DT performance but a beneficial effect of the crystallized component of cognition together with CR. Moreover, we observed a negative correlation between age and only the originality subscale of verbal DT.
In line with previous evidence (e.g., [33]), we found that age affected DT performance and visual DT, in particular, in later adulthood. No aging effect was reported instead for most of the verbal DT subscales, possibly indicating that the verbal domain is more stable in the elderly. Indeed, as reported in a previous study, verbal DT begins to decline after middle age and then remains globally stable, whereas visual DT abilities decline in later adulthood [33,54]. If we assume that visual and verbal DT rely on domain-specific processes [55], we should conclude that the verbal domain is less affected than the visual one by cognitive decline. Alternatively, we may also assume that the cognitive load required at different stages of visual and verbal DT, namely, the generation and evaluation of new ideas [36,56], is different in later adulthood and needs different compensation mechanisms. Executive functions/fluid components seem to be more involved in the evaluation stages of DT [31], whereas crystallized ones are more crucial in the generation stage. It follows that, in later adulthood, it could become easier to infer from verbal and autobiographical information (crystallized resources), which are generally stable during the lifespan, than relying on fluid/executive abilities, which are subjected to cognitive decline [6,7,8]. This could implicate differences in the generation and evaluation mechanisms in OAs. Given that verbal crystallized components of cognition are preserved and stable in later adulthood, we may hypothesize that verbal information could be more extended and accessible for the elderly, thus making idea generation less demanding, but evaluation more challenging. Indeed, aging was found to affect only the originality of verbal DT and globally DT performances did not correlate with any of attention and EF measures, indicating the implication of possible alternative cognitive strategies.
Accordingly, during the verbal DT task, individuals are specifically required to generate alternative uses for a given object, and in visual DT, they are required to draw as many figures as possible, without any context information or semantic constraint. This could implicate different cognitive demands and mechanisms involved. From a speculative point of view, assuming that OAs may create privilege for the crystallized component of cognition to complete the DT tasks, we can hypothesize that they rely on it even during visual idea generation. In order to draw figures from two parallel lines, individuals need to imagine the figure first, by accessing their semantic store, and then plan and execute the motor sequence of drawing. These additional cognitive steps implied in visual DT can potentially be more challenging for the elderly, being more cognitively demanding. Indeed, a physiological slowing of responses and a decline in vision together with difficulties in action planning and cognitive control are frequently observed in later adulthood. Consequently, visual DT being possibly more challenging than verbal DT, the amount of CR could play a role in guaranteeing adequate performances even in later adulthood, as shown in the present study, in line with previous evidence [36,37,40,41].
In summary, the positive relationships found between crystallized intelligence and visual DT confirms the pivotal role of semantic and prior knowledge in thinking creatively, allowing connections between weakly related concepts to form new ideas [57,58]. Accordingly, the mediation effect of WAIS_voc in the relation between age and visual DT performance supports the “semanticized” hypothesis of OAs cognition [13,42], where OAs are supposed to rely more on lifelong experiences and knowledge, rather than on fluid and controlled processes, to sustain goal-directed behaviors [13,42]. In this way, our results confirm that, although DT abilities may weaken during aging, if OAs rely on their previous experiences and knowledge as an alternative strategy, they can still perform adequately according to the contingent demands. Moreover, the observed mediative role of crystallized intelligence may underlie a shift toward autobiographical and semantic representations acquired during the lifespan to cope with high cognitive demands, according to the DECHA hypothesis [32].
Limitations
The study presents some limitations. Firstly, it adopted only one measure for each DT domain (i.e., verbal and visual). Therefore, future studies should apply at least two different measures per domain, to better understand the implication of mechanisms involved in creative cognition in later adulthood. Moreover, we cannot exclude that the individual’s attitude to prefer imagery or verbal strategies [59] in processing information may have influenced the results. Unfortunately, few studies have investigated the influence of cognitive styles on DT, mainly considering visual creativity and finding that a visual cognitive style, rather than a verbal one, can support the generation of original ideas [36]. Further studies should also consider such an individual difference and whether it contributes to sustaining DT in OAs.
5. Conclusions
The present study confirmed the strict relationship between DT and cognitive reserve, showing that, even if DT undergoes an age effect, OAs can sustain it by recruiting to a greater extent prior knowledge and crystallized abilities rather than declining control processes, possibly adopting compensatory mechanisms. Such results shed light on the potential role of DT, similar to CR, in counteracting possible cognitive impairments, allowing the recruitment of mostly spared cognitive abilities to functionally perform goal-oriented behaviors and satisfy cognitive demands, even when they are unusual, as it happens in DT tasks.
Implications
Considering that during aging OAs have to face new situations and difficulties, reporting higher probabilities of experiencing cognitive impairments [60], DT can contribute to coping with everyday life challenges by finding alternative and functional solutions, sustaining cognitive functions [16,17], and it appears crucial to delve into the potential effects that DT can have during later adulthood. In this way, the present findings support the design of effective interventions based on DT for promoting efficient cognitive functioning and sustaining prolonged autonomy. Moreover, these results might have significant practical implications such as the development of specific exercises for OAs aimed at differentially stimulating verbal and visual DT and the relative cognitive strategies. Verbal strategies could be useful in facing the eventual greater cognitive demand required by visual DT performances. On the other hand, stimulating visual DT could also promote more functional visual strategies. Notably, early interventions that specifically aim to expand and make people’s semantic store and networks more flexible could have a beneficial effect on both visual and verbal DT skills, preventing OAs’ decline. More studies are needed to address this issue.
Even if there is a lack of studies that investigated the cognitive mechanisms underlying DT in OAs, it appears important to maintain the focus on the issue, as life expectancy has been prolonged, and the prevention of cognitive impairments and dementia becomes an increasingly crucial issue.
References
- Oschwald, J.; Guye, S.; Liem, F.; Rast, P.; Willis, S.; Röcke, C.; Jäncke, L.; Martin, M.; Mérillat, S. Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Rev. Neurosci. 2019, 31, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T. Consequences of Age-Related Cognitive Declines. Annu. Rev. Psychol. 2012, 63, 201–226. [Google Scholar] [CrossRef]
- Fusi, G.; Palmiero, M.; Lavolpe, S.; Colautti, L.; Crepaldi, M.; Antonietti, A.; Di Domenico, A.; Colombo, B.; Di Crosta, A.; La Malva, P.; et al. Aging and Psychological Well-Being: The Possible Role of Inhibition Skills. Healthcare 2022, 10, 1477. [Google Scholar] [CrossRef]
- Mather, M. Aging and cognition. WIREs Cogn. Sci. 2010, 1, 346–362. [Google Scholar] [CrossRef]
- Li, H.; Hirano, S.; Furukawa, S.; Nakano, Y.; Kojima, K.; Ishikawa, A.; Tai, H.; Horikoshi, T.; Iimori, T.; Uno, T.; et al. The Relationship Between the Striatal Dopaminergic Neuronal and Cognitive Function with Aging. Front. Aging Neurosci. 2010, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Polk, T.A.; Mikels, J.A.; Taylor, S.F.; Marshuetz, C. Cerebral aging: Integration of brain and behavioral models of cognitive function. Dialogues Clin. Neurosci. 2001, 3, 151–165. [Google Scholar] [CrossRef]
- Verhaeghen, P.; Cerella, J. Aging, executive control, and attention: A review of meta-analyses. Neurosci. Biobehav. Rev. 2002, 26, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghen, P. Aging and vocabulary score: A meta-analysis. Psychol. Aging 2003, 18, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S.; Barch, D.M. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci. Biobehav. Rev. 2002, 26, 809–817. [Google Scholar] [CrossRef]
- Bäckman, L.; Nyberg, L.; Lindenberger, U.; Li, S.C.; Farde, L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef]
- Cropley, V.L.; Fujita, M.; Innis, R.B.; Nathan, P.J. Molecular Imaging of the Dopaminergic System and its Association with Human Cognitive Function. Biol. Psychiatry. 2006, 59, 898–907. [Google Scholar] [CrossRef]
- Damoiseaux, J.S. Effects of aging on functional and structural brain connectivity. Neuroimage 2017, 160, 32–40. [Google Scholar] [CrossRef]
- Spreng, R.N.; Lockrow, A.W.; DuPre, E.; Setton, R.; Spreng, K.A.P.; Turner, G.R. Semanticized autobiographical memory and the default—Executive coupling hypothesis of aging. Neuropsychologia 2018, 110, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fusi, G.; Lavolpe, S.; Crepaldi, M.; Rusconi, M.L. The Controversial Effect of Age on Divergent Thinking Abilities: A Systematic Review. J. Creat. Behav. 2021, 55, 374–395. [Google Scholar] [CrossRef]
- Flaherty, A.W. Brain Illness and Creativity: Mechanisms and Treatment Risks. Can. J. Psychiatry 2011, 56, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Colautti, L.; Baldassini, D.; Colombo, V.; Mottura, S.; Sacco, M.; Sozzi, M.; Corbo, M.; Rusconi, M.L.; Antonietti, A. CREC: The role of serious games in improving flexibility in thinking in neuropsychological rehabilitation: CREC. Br. J. Educ. Technol. 2018, 49, 717–727. [Google Scholar] [CrossRef]
- Fusi, G.; Ferrari, E.; Zanetti, M.; Crepaldi, M.; Bersanini, C.; Paladino, A.; Colautti, L.; Rozzini, L.; Antonietti, A.; Rusconi, M.L. A Comparison of Divergent Thinking Abilities Between Healthy Elderly Subjects and MCI Patients: Preliminary Findings and Implications. Front. Psychol. 2020, 11, 738. [Google Scholar] [CrossRef]
- Colautti, L.; Magenes, S.; Rago, S.; Zanaboni Dina, C.; Cancer, A.; Antonietti, A. Creative Thinking in Tourette’s Syndrome: An Uncharted Topic. Front. Psychol. 2021, 12, 649814. [Google Scholar] [CrossRef]
- Colautti, L.; Magenes, S.; Rago, S.; Camerin, S.; Zanaboni Dina, C.; Antonietti, A.; Cancer, A. Creative thinking in Tourette’s syndrome: A comparative study of patients and healthy controls. J. Clin. Exp. Neuropsychol. 2023, 1–16. [Google Scholar] [CrossRef]
- Dietrich, A. The cognitive neuroscience of creativity. Psychon. Bull. Rev. 2004, 11, 1011–1026. [Google Scholar] [CrossRef]
- Zhang, W.; Sjoerds, Z.; Hommel, B. Metacontrol of human creativity: The neurocognitive mechanisms of convergent and divergent thinking. Neuroimage 2020, 210, 116572. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, M.; Fusi, G.; Crepaldi, M.; Borsa, V.M.; Rusconi, M.L. Divergent thinking and the core executive functions: A state-of-the-art review. Cogn. Process. 2022, 23, 341–366. [Google Scholar] [CrossRef]
- Lubart, T.; Zenasni, F.; Barbot, B. Creative potential and its measurement. Int. J. Talent. Dev. Creat. 2013, 1, 41–51. [Google Scholar]
- Acar, S.; Tadik, H.; Myers, D.; Van Der Sman, C.; Uysal, R. Creativity and Well-being: A Meta-analysis. J. Creat. Behav. 2021, 55, 738–751. [Google Scholar] [CrossRef]
- Turner, G.R.; Spreng, R.N. Prefrontal Engagement and Reduced Default Network Suppression Co-occur and Are Dynamically Coupled in Older Adults: The Default–Executive Coupling Hypothesis of Aging. J. Cogn. Neurosci. 2015, 27, 2462–2476. [Google Scholar] [CrossRef]
- Beaty, R.E.; Christensen, A.P.; Benedek, M.; Silvia, P.J.; Schacter, D.L. Creative constraints: Brain activity and network dynamics underlying semantic interference during idea production. Neuroimage 2017, 148, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Zabelina, D.L.; Andrews-Hanna, J.R. Dynamic network interactions supporting internally-oriented cognition. Curr. Opin. Neurobiol. 2016, 40, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Snyder, A.Z.; Vincent, J.L.; Lustig, C.; Head, D.; Raichle, M.E.; Buckner, R.L. Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron 2017, 56, 924–935. [Google Scholar] [CrossRef]
- Goldberg, E. Creativity: The Human Brain in the Age of Innovation; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Beaty, R.E.; Kenett, Y.N.; Christensen, A.P.; Rosenberg, M.D.; Benedek, M.; Chen, Q.; Fink, A.; Qiu, J.; Kwapil, T.R.; Kane, M.J. Robust prediction of individual creative ability from brain functional connectivity. Proc. Natl. Acad. Sci. USA 2018, 115, 1087–1092. [Google Scholar] [CrossRef]
- Beaty, R.E.; Benedek, M.; Silvia, P.J.; Schacter, D.L. Creative Cognition and Brain Network Dynamics. Trends Cogn. Sci. 2016, 20, 87–95. [Google Scholar] [CrossRef]
- Beaty, R.E.; Chen, Q.; Christensen, A.P.; Kenett, Y.N.; Silvia, P.J.; Benedek, M.; Schacter, D.L. Default network contributions to episodic and semantic processing during divergent creative thinking: A representational similarity analysis. Neuroimage 2020, 209, 116499. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, M.; Nori, R.; Piccardi, L. Verbal and visual divergent thinking in aging. Exp. Brain Res. 2017, 235, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Foos, P.W.; Boone, D. Adult Age Differences in Divergent Thinking: It’s Just a Matter of Time. Educ. Gerontol. 2008, 34, 587–594. [Google Scholar] [CrossRef]
- Roskos-Ewoldsen, B.; Black, S.R.; Mccown, S.M. Age-related Changes in Creative Thinking. J. Creat. Behav. 2008, 42, 33–59. [Google Scholar] [CrossRef]
- Palmiero, M.; Di Giacomo, D.; Passafiume, D. Can Creativity Predict Cognitive Reserve? J. Creat. Behav. 2016, 50, 7–23. [Google Scholar] [CrossRef]
- Colombo, B.; Antonietti, A.; Daneau, B. The Relationships Between Cognitive Reserve and Creativity. A Study on American Aging Population. Front. Psychol. 2018, 9, 764. [Google Scholar] [CrossRef]
- Stern, Y.; Barulli, D. Cognitive reserve. Handb. Clin. Neurol. 2019, 167, 181–190. [Google Scholar] [CrossRef]
- Opdebeeck, C.; Martyr, A.; Clare, L. Cognitive reserve and cognitive function in healthy older people: A meta-analysis. Aging Neuropsychol. Cogn. 2016, 23, 40–60. [Google Scholar] [CrossRef]
- Meléndez, J.C.; Alfonso-Benlliure, V.; Mayordomo, T.; Sales, A. Is Age Just a Number? Cognitive Reserve as a Predictor of Divergent Thinking in Late Adulthood. Creat. Res. J. 2016, 28, 435–441. [Google Scholar] [CrossRef]
- Câmara, J.; Mendes, A.; Caires, A.L.; Garcês, S.; Pocinho, M. Creativity and cognitive reserve in old age: An exploratory study in the Portuguese population. Psicologia 2020, 34, 229–235. [Google Scholar]
- Adnan, A.; Beaty, R.; Silvia, P.; Spreng, R.N.; Turner, G.R. Creative aging: Functional brain networks associated with divergent thinking in older and younger adults. Neurobiol. Aging 2019, 75, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Measso, G.; Cavarzeran, F.; Zappalà, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari, D.; Grigoletto, F. The mini-mental state examination: Normative study of an Italian random sample. Dev. Neuropsychol. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail making test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. A short version of the Stroop test: Normative data in an Italian population sample. Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Nocentini, U.; Giordano, A.; Vincenzo, S.D.; Panella, M.; Pasqualetti, P. The Symbol Digit Modalities Test—Oral version: Italian normative data. Funct. Neurol. 2006, 21, 93–96. [Google Scholar]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M. Tre test clinici di memoria verbale a lungo termine: Taratura su soggetti normali. Arch. Psicol. Neurol. Psichiatr. 1986, 47, 278–296. [Google Scholar]
- Orsini, A.; Pezzuti, L. WAIS-IV. Contributo Alla Taratura Italiana (70–90 Anni); Giunti OS: Firenze, Italy, 2015; Volume 1, pp. 1–70. [Google Scholar]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [CrossRef]
- Bottesi, G.; Ghisi, M.; Altoè, G.; Conforti, E.; Melli, G.; Sica, C. The Italian version of the Depression Anxiety Stress Scales-21: Factor structure and psychometric properties on community and clinical samples. Compr. Psychiatry 2015, 60, 170–181. [Google Scholar] [CrossRef]
- Sprini, G.; Tomasello, S. Torrance Tests of Creative Thinking (Test di Pensiero Creativo); Giunti OS Organizzazioni Speciali: Firenze, Italy, 1989. [Google Scholar]
- Palmiero, M. The effects of age on divergent thinking and creative objects production: A cross-sectional study. High. Abil. Stud. 2015, 26, 93–104. [Google Scholar] [CrossRef]
- Palmiero, M.; Nakatani, C.; Raver, D.; Belardinelli, M.O.; Van Leeuwen, C. Abilities within and Across Visual and Verbal Domains: How Specific Is Their Influence on Creativity? Creat. Res. J. 2010, 22, 369–377. [Google Scholar] [CrossRef]
- Finke, R.A. Imagery, Creativity, and Emergent Structure. Conscious. Cogn. 1996, 5, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Mednick, S. The associative basis of the creative process. Psychol. Rev. 1962, 69, 220–232. [Google Scholar] [CrossRef]
- Kenett, Y.N.; Faust, M. A Semantic Network Cartography of the Creative Mind. Trends Cogn. Sci. 2019, 23, 271–274. [Google Scholar] [CrossRef]
- Richardson, A. Verbalizer–visualizer: A cognitive style dimension. J. Ment. Imag. 1997, 1, 109–126. [Google Scholar]
- Naaldenberg, J.; Vaandrager, L.; Koelen, M.; Leeuwis, C. Aging Populations’ Everyday Life Perspectives on Healthy Aging: New Insights for Policy and Strategies at the Local Level. J. Appl. Gerontol. 2012, 31, 711–733. [Google Scholar] [CrossRef]

