1. Introduction
Cognitive impairment is a key phenotype in a range of disorders of the central nervous system (CNS). Currently, treatment strategies with robust efficacy and long-lasting effects on cognition are essentially lacking [1]. This lack of treatments is partially due to limited insights into the neurobiological processes involved in brain health and disease. Neuroplasticity refers to the capacity of the brain to generate new synapses, dendritic spines and, in general, to upgrade and change brain connectivity in response to learning [2]. These processes play a key role in adaptive CNS functioning and their disruption is associated with impaired cognitive and neurological functioning [3]. Uncovering the mechanisms involved in neuroplasticity is therefore crucial to improving CNS disorders.
The body’s ability to adapt to changes in oxygen levels is an evolutionary trait, but we are only beginning to understand the response of the CNS to lowered levels of ambient oxygen, also referred to as inspiratory hypoxia [4]. Hypoxia can consist of continuous (constant) or intermittent (cyclical, intervaled by brief periods of normoxia) stimuli with repeated (i.e., daily) or chronic exposure [5]. A common layperson’s perception has been that hypoxia is exclusively associated with negative effects on the CNS. In contrast, recent emerging evidence indicates that moderate doses of hypoxia (typically shorter intermittent or continuous repeated sessions of 10–16% O2 exposure over longer time periods (typically several weeks)) has possible therapeutic effects [6]. Specifically, a small pilot study exploiting intermittent hypoxia (eight cycles of five minutes of 10% O2 exposure with normoxia in between, repeated three times weekly for eight weeks) reported improved cognitive performance in seven elderly patients with amnestic mild cognitive impairment [7]. In line with this, another small study in 34 geriatric participants demonstrated improvements in cognitive and motor performance following 35–45 min of intermittent hypoxic (12% O2) and hyperoxic (30% O2) breathing repeated 2–3 times weekly for 5–6 weeks [8,9]. Remarkably, continuous hypoxia (11% O2) has also been associated with extending lifespan by 50% and delayed onset of neurological dysfunction in a mouse model of aging [10].
In contrast, studies investigating the effects of acute and severe doses of hypoxia (typically intensities of ≤9% O2) exposure consistently showed impaired cognitive performance in humans [11,12,13] and inhibited neuroplasticity, including neuronal apoptosis and increased oxidative stress, in animal models [14,15]. This apparent distinctive effect of hypoxia was also noted in a recent narrative review by Navarette-Opazo and Mitchell, where moderate intermittent hypoxia (9–16% O2) exposure with low cycle numbers produced mainly beneficial effects as opposed to severe intermittent hypoxia (3–8% O2 with more cycles), which produced mostly adverse effects across human and animal studies [6]. Taken together, this suggests that the balance between potential therapeutic vs. pathogenic effects of hypoxia depends on dose (i.e., frequency, duration, and intensity (level of hypoxia) per session as well as the length of the intervention) and exposure type (hypobaric vs. normobaric or intermittent vs. continuous). Nevertheless, there is a general lack of consensus regarding experimental procedures across hypoxia studies, resulting in many inconsistent findings due to variations in oxygen levels, intensity of exposure, and duration per session [6,16] as well as an inconsistent terminology, further complicating the comparability of efficacy across studies [5]. Given this, there is a need for systematic investigations of these moderate hypoxia interventions to determine the optimal intervention schedule.
The objective of this systematic review was to assess the effects of moderate intensities of hypoxia (i.e., 10–16% O2) exposure for longer periods (≥14 days) (i) on cognitive or neurological outcomes in humans and the methodological quality of these trials, and (ii) on measures of neuroplasticity and cognition in animal studies, to determine optimal treatment protocols as a guideline for future interventional studies in the field.
2. Methods
2.1. Data Source
This systematic review followed the procedures of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) 2020 statement [17]. A comprehensive systematic computerized search was conducted on the PubMED/MEDLINE, PsycInfo, and EMBASE and Cochrane Library databases from May to September 2023. The search profile involved the four elements, “Hypoxia”, “Cognitive, neurological, and neuroplasticity-related outcome”, “Intervention”, and “Exclusion criteria”, with each of their combinations and alternative key words in the respective databases (see for details on the search profile). A protocol of the review was registered on the online database PROSPERO (registration number: CRD42023469082).
2.2. Selection Criteria
The search criteria were defined in line with the population, intervention, comparison, and outcome (PICO) framework. The research question was: In both humans and animals (population), can moderate hypoxia exposure interventions (intervention), when compared to a normoxia control condition (comparison), affect cognitive and neurological function and markers of neuroplasticity (outcome)?
We included only original peer-reviewed empirical reports involving in vivo investigations of moderate hypoxia interventions on the brain. Eligible reports involved (a) both healthy individuals and individuals with clinical conditions, and animal subjects; (b) a longer intervention (≥14 days) of continuous or intermittent hypoxia of moderate intensity (as defined by O2 levels in the range of 10–16 ± 0.5% [6]), which could be normobaric (simulated altitude) or hypobaric (real altitude), which could also include combinations with other changes in O2 levels (i.e., hypoxia-hyperoxia treatment) as well as pharmacological or behavioral combined interventions to investigate potential synergistic effects; (c) parallel group studies or cross-over studies involving a normoxia control condition; (d) objective measure of cognitive and neurological function, including motor function, neuropsychiatric behavior, structural and functional neuroimaging, and objective neurobiological markers of brain plasticity, including neuronal morphology and myelination, oxidative stress, inflammation, erythropoietin, neurotrophins, and markers of Alzheimer’s pathology; and (e) articles published in English only. We excluded articles that investigated the effects of (I) acute hypoxia (less than 14 days); (II) severe or mild intensities of hypoxia interventions (≤9% or ≥17% O2); (III) had no normoxia control condition or did not include any hypoxic intervention (i.e., cross-sectional comparisons between high altitude vs. sea level populations); (IV) were written in languages other than English; and (V) were reviews, meeting abstracts, dissertations, and case reports.
2.3. Study Selection
Two authors (V.D. and J.M.L.) independently conducted a primary title/abstract screening for possible eligible studies and, following this, a secondary full-text screening using the Covidence systematic review software 2023, Veritas Health Innovation, Melbourne, Australia (www.covidence.org). Additional hand searches were further performed by tracking and screening citations in the included articles to ensure inclusion of all relevant reports. In all phases, articles were considered in accordance with the inclusion and exclusion criteria. Interrater reliability was measured as percentage agreement and was calculated as the number of agreements divided by the total number of screened references. Agreement between the two authors was high (primary screening: 97%; secondary screening: 96%). Disagreements were discussed and consensus was reached in all cases through discussions with a third author (KWM). Two authors (V.D. and J.M.) extracted the measures of interest and presented results from the eligible reports in and . The measures of interest were predefined in accordance with the objective of the review and included the following: author and year of publication, study design, population and sample characteristics, comparison groups, duration, and type of hypoxia intervention, O2 level, outcome measures, and main findings. If data were missing, they were defined as ‘no information’. The syntheses of the included reports were predefined according to human studies and animal studies , respectively.
2.4. Risk of Bias Assessment
The risk of bias within and across the included human reports was assessed by two authors (V.D. and J.M.). For interventional studies, risk of bias was evaluated in accordance with the revised Cochrane collaboration’s risk of bias 2 (RoB 2, 2021 version) tool (https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-crossover-trials, accessed on 3 October 2023). For case–control studies, risk of bias was assessed with the risk of bias in non-randomized studies—of interventions (ROBINS-I) tool [74]. Risk of bias evaluations were independently conducted by the two authors, and disagreements were discussed with a third author (KWM). If any information was missing in the included articles, additional searches for the registered studies on clinicaltrials.gov were performed, and a search for published study protocols were conducted on relevant search engines. and display the risk of bias evaluations for the included human studies. The PRISMA 2020 checklist was completed (see ).
3. Results
The systematic search, together with additional hand searches, identified 5582 articles (after removal of duplicates), and all these were included for the title/abstract screening (primary screening). Out of these, 315 articles were further evaluated for eligibility through a full-text reading (secondary screening). This resulted in 58 articles that met the inclusion criteria and were thus included in this review (Figure 1 depicts the PRISMA 2020 flowchart). Of these, ten reports were on humans (N = 274 individuals), and the remaining 48 studies were on animal models. and display the characteristics of the included studies investigating the effects of moderate hypoxia in human and animal subjects, respectively.
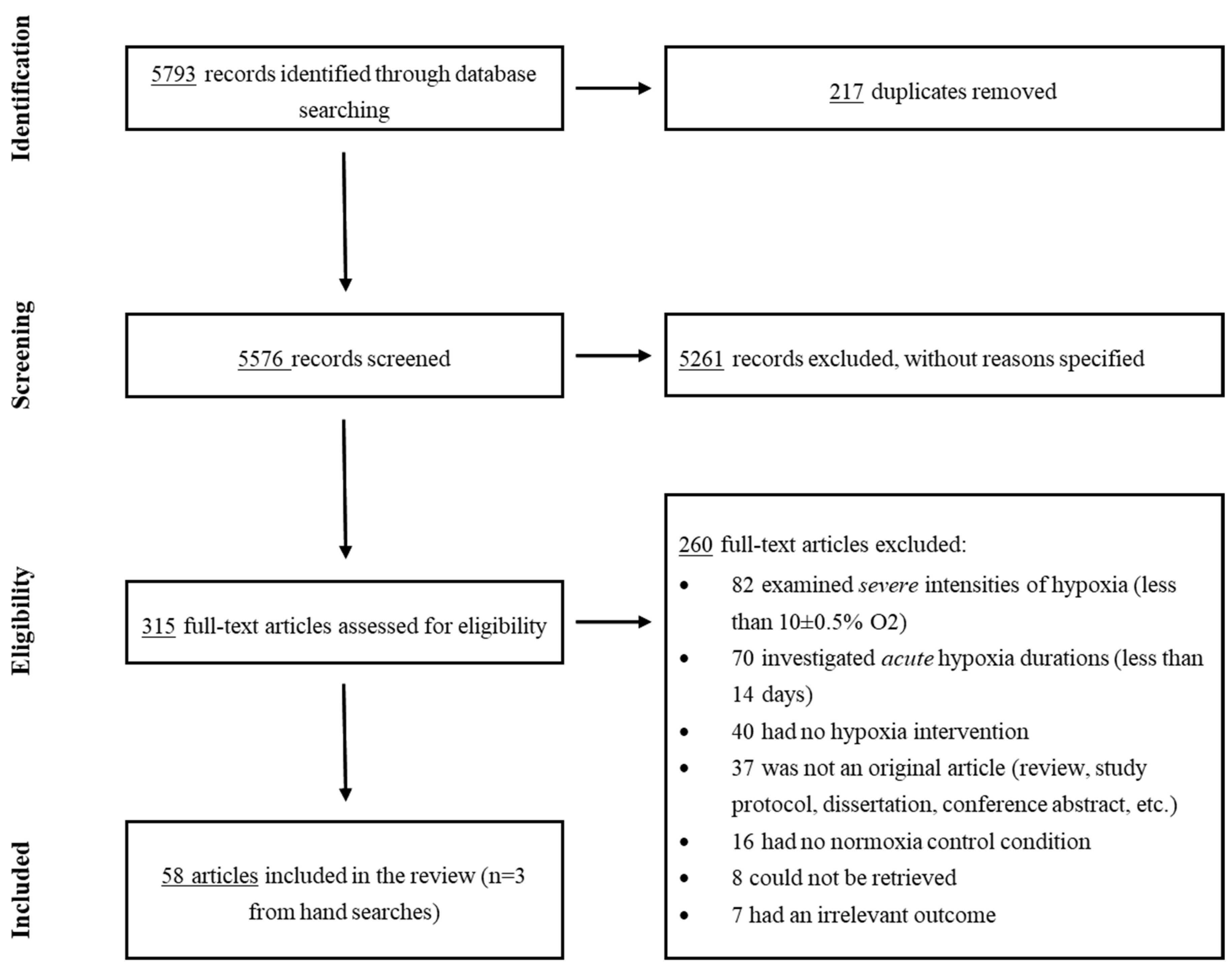
Figure 1. PRISMA 2020 Flowchart.
4. Effects of Moderate Hypoxia in Human Studies
Of the eight human studies, six (75%) observed largely beneficial effects of intermittent moderate hypoxia (i.e., 10–14% O2 or 80% SpO2) or hypoxia-hyperoxia repeated for 30–90 min sessions for 3–7 weeks on all or most cognitive and/or neurological outcomes in healthy participants, geriatric participants, and patients with mild cognitive impairment [8,9,19,22,23,24,25]. One study (12.5%) in patients with traumatic brain injury reported no changes in any outcome after continuous 10–14% O2 for 30 min to two hours repeated over 5–12 weeks [21]. Finally, one study (12.5%) in college students found negative effects after two years of chronic high-altitude living (3658 m corresponding to approx. 13% O2) [20].
4.1. Cognition
Eight studies investigated the effects of hypoxia exposure on cognition (three reports had 100% overlapping samples and are therefore counted as one study here) [8,9,18]. Of these, six studies examined global cognitive functioning assessed by either a comprehensive test battery [21] or a screening tool [9,19,22,24,25]. Two studies with N = 25–34 geriatric patients reported improved global cognition after relatively similar treatment protocols, involving normobaric, intermittent hypoxia-hyperoxia breathing (3–30 cycles of 10–14% and 30–40% O2 for 30–45 min repeated 2–3 times weekly for 5–6 weeks) combined with physical training compared to normoxic breathing and physical training [18,19]. Two studies of N = 28 mild cognitive impairment patients observed global cognitive improvement following normobaric intermittent hypoxia-hyperoxia (12% and 33% O2 for 15 min repeated five times per week for three weeks) compared to normoxia controls [24,25]. Two studies observed no effect of hypoxia on global cognition [21,24]. One of these was of N = 9 patients with traumatic brain injury, who underwent two hours of continuous approx. 12% O2 repeated for 12 weeks in addition to muscle electrostimulation [21]. The other study was of N = 34 older adults after normobaric intermittent hypoxia (80–90% SpO2) breathing for 15 min repeated five times per week over four weeks [22].
Three studies specifically investigated the effects of hypoxia on attention and processing speed [20,21,22]. One study of N = 34 older adults reported improved attention, but no group-by-time change in processing speed, after six weeks of combined normobaric, intermittent hypoxia (90–80% SpO2 for one hour repeated three times weekly for six weeks) with a full body strength–endurance training program [22]. One study with N = 9 patients with traumatic brain injury detected no difference in processing speed after two hours of hypobaric, continuous approx. 12% O2 repeated three times per week over six weeks combined with muscle electrostimulation [21]. Another case–control study comparing N = 49 college students moving to high altitude (hypobaric, chronic exposure to 3658 m corresponding to approx. 13% O2) with N = 49 college students living at sea level found no group-by-time difference in processing speed, but a within-group decline in students who lived for two years at high altitude [20]. Nevertheless, students (N = 98) were allocated to high altitude vs. sea level groups based on their a priori acceptance to a college located at either high altitude or sea level, yielding bias in the randomization process in this study (see for risk of bias evaluation).
Two studies further examined the effects of hypoxia on verbal memory [20,21]. One study observed no change after hypobaric continuous 12% O2 for two hours repeated three times weekly for six weeks in addition to muscle electrostimulation for N = 9 patients with traumatic brain injury [21]. Accordingly, the other study comparing college students living at high altitude (hypobaric approx. 13% O2 chronically for two years) vs. sea level showed no difference in verbal memory performance, although there was a within-group effect showing verbal memory decline in the chronic high-altitude group [20].
Finally, two studies investigated the effects of hypoxia interventions on executive functioning [21,23]. One study in older adults comparing hypoxia and aerobic training (N = 17) with normoxia and aerobic training (N = 16) found no significant difference in executive functioning, but a within-group improvement in the hypoxia and aerobics group (normobaric hypoxia consisted of four weeks of intermittent 90–80% SpO2 for 90 min repeated three times per week) [23]. The other study with N = 9 patients with traumatic brain injury found no change in executive functioning after two hours of continuous hypobaric 12% O2 for six weeks in addition to muscle electrostimulation compared to a normoxia control group [21].
Taken together, these findings suggest that smaller doses of normobaric intermittent hypoxia (with moderate intensities between 10 and 14% O2) applied for 30 to 90 min sessions enhance cognitive performance [18,19,22,23,24], whereas higher doses of hypoxia (e.g., 12% O2 continuously applied for two hours over 12 weeks or continuous high-altitude exposure chronically for two years) may not change cognition [21] or negatively impact cognition [20].
4.2. Motor Function
One study of N = 34 geriatric patients investigated the effect of normobaric intermittent hypoxic-hyperoxic (10–14%–30–40% O2) breathing for 3–9 cycles over 30–45 min combined with multimodal training repeated 2–3 times per week over 5–6 weeks on motor function in three reports (100% overlapping sample) [8,9,18]. While patients in the hypoxia-hyperoxia group exhibited improved performance on a walking test compared to normoxia, no change was detected in the remaining three tests of motor function and mobility [8,9,18]. The study thus suggests a positive effect of intermittent hypoxia exposure on some aspects of motor function.
4.3. Neuroimaging
Three studies investigated the effects of hypoxia on the brain with neuroimaging methods [20,24,25]. Two similar studies with N = 28 patients with mild cognitive impairment and N = 13 healthy participants investigated the effect of normobaric, intermittent hypoxia-hyperoxia (15 min of cycles of 12% and 33% O2 five times per week repeated for three weeks) with electroencephalography (EEG) and found decreased P300 and N200 latencies, indicative of improved attention, working memory, and sensory processing, along with improved global cognition in the hypoxia group compared to normoxia controls [24,25]. One case–control study examined the effects of N = 49 students moving to study at a college at high altitude (3658 m corresponding to hypobaric continuous 13% O2 chronically for two years) vs. N = 49 students living at sea level on brain structure using structural magnetic resonance imaging (sMRI) [20]. Specifically, the chronic high-altitude group presented with decreased caudate grey matter volume compared to the control group, which correlated with poorer verbal memory performance [20]. Further, there was a within-group change in fractional anisotropy values in multiple white matter tracts in the high-altitude group, with both increases in thalamic and fronto-occipital regions and decreases in temporal regions [20]. While these neuroimaging findings are conflicting, it suggests that lower doses of, e.g., intermittent hypoxia interventions can improve brain functioning and associated cognitive performance, while higher doses, e.g., more chronic exposure at high altitude, may negatively affect structural brain measures.
4.4. Inflammation
One study investigated the effects of normobaric intermittent hypoxia-hyperoxia (four cycles of 12% and 33% O2 repeated five times weekly for three weeks) on markers of inflammation in N = 27 patients with mild cognitive impairment and healthy participants [25]. The study showed increased levels of inflammation following hypoxia compared to normoxia. This finding was interpreted as potential adaptive reprogramming, generating therapeutic effects against neuropatological changes, given the observed parallel cognitive improvement following hypoxia [25].
5. Risk of Bias Evaluations
presents the RoB 2 evaluations for the nine reports on controlled human trials included in the review. All studies were evaluated to be of ‘high risk’ in the overall assessment. The primary concerns of the methodology in these studies regarded inadequate information on (or a complete lack of) randomization (k = 5), suboptimal analyses without intention-to-treat statistical protocols (k = 9), and a lack of (information on) blinding in the outcome assessments (k = 6). displays the ROBINS-I evaluation for the single included observational study. This study was found to be of ‘serious risk of bias’ due to a lack of adjustment for important confounders, possible selection biases in the included participants, and a lack of information on assessor blinding [20]. Overall, the included human studies suffered from several methodological challenges according to the Cochrane guidelines, yielding a high risk of bias, which indicates that the study of hypoxia effects on cognition is still in its infancy and that the findings must be considered vague and very preliminary.
6. Effects of Moderate Hypoxia in Animal Studies
Of the 48 included animal studies, 28 (58.5%) observed adverse effects of hypoxia exposure [27,28,29,31,32,35,36,38,39,40,41,46,47,49,50,57,59,61,62,63,64,66,67,69,70,71,72], while 18 studies (37.5%) reported beneficial effects [26,33,34,37,42,43,44,45,48,51,53,55,56,58,60,65,68,73] and two studies (4%) found either no change in outcomes or mixed findings in both directions after hypoxia treatment [30,52].
6.1. Cognition and Neuropsychiatric Behavior
Thirty-eight studies investigated the effects of moderate hypoxia exposure on cognitive functioning and anxiety- and depression-like behavior in rodents (see . Thirty-three studies examined hypoxia-related changes in hippocampal-dependent learning and memory performance, mostly assessed with the Morris water maze paradigm (27 of 33 studies) [30,31,32,34,35,36,38,39,40,41,42,44,45,50,51,54,55,56,57,59,60,61,62,63,64,65,72]. Of these, 21 studies reported impaired learning and memory following various protocols with higher doses of hypoxia, including normobaric intermittent hypoxia (mainly 10% O2) chronically administered for +10 h daily over 2–4 weeks, or hypobaric continuous hypoxia of 11–13% O2 chronically administered over 4–12 weeks [31,32,35,36,38,39,40,41,50,54,57,59,61,62,63,64,66,67,69,71,72]. In contrast, nine studies, in which study designs predominantly included shorter, continuous hypobaric hypoxia exposures for four to six hours daily, found improved learning and memory (intensities between 10.8% and 16% O2 repeated for 14–28 days) [34,42,44,45,55,56,60,65,68]. Finally, two studies observed no difference in learning and memory following nomobaric intermittent 10% O2 for 21–30 days vs. normoxia controls [30,51].
Seven studies further investigated the effects of hypoxia on working memory, using working-memory versions of maze paradigms and object recognition paradigms [38,49,56,60,61,64,70]. Of these, four detected impaired working memory performance following both hypobaric or normobaric hypoxia, involving continuous exposures of 10.5–13.5% O2 chronically for 8–24 h daily over 2–4 weeks or intermittent 10% O2 exposure chronically for two weeks [38,49,63,70]. In contrast, two studies found improved performance following hypobaric continuous 14% O2 for 6 h repeated daily for 28 days [56] or normobaric continuous 11% O2 chronically for 28 days [60]. Finally, one study observed no difference in working memory after intermittent 10% O2, repeated for eight hours daily for 14 days [61].
Finally, 11 reports studied hypoxia-related effects on anxiety- and depression-like behavior, and locomotor functioning, which was mostly assessed with the open field test paradigm [27,39,44,45,50,57,61,67,68,71,73]. Of these, four studies found reduced anxious and depressive behavior (e.g., more time spent in open arms and physical state of fur) after 4 h daily continuous hypobaric 10.8–16% O2 repeated for 2–4 weeks [44,45,68,73]. However, four studies showed no difference in anxiety-related behavior following 2–4 weeks of 10%-16% O2, administered both repeated or chronically daily for 4–12 h [27,39,61,67]. Finally, three studies found increased anxiety-like behavior (higher locomotor activity and less time spent in open arms), mainly following intermittent hypoxia chronically for more than 8 h daily with intensities between 10% and 12.3% O2 over 14 days to 8 months [50,57,71].
Taken together, likely due to the very heterogeneous study designs and the sometimes questionable quality of the studies, the findings are highly inconsistent. Nevertheless, most studies report impaired cognitive functioning following larger doses of both normobaric and hypobaric hypoxia exposure, e.g., intermittent hypoxia with 10% O2 that was chronically administered over several weeks. However, studies exploiting lower doses of continuous and intermittent hypoxia (i.e., shorter durations of repeated frequency and higher O2 levels of 14–16%) find beneficiary effects on both learning and memory, working memory, anxiety- and depressive-like behavior.
6.2. Motor Function
Seven studies investigated the hypoxia-related effects on motor functioning in rodents [26,37,46,47,48,51,58]. Motor function was evaluated across many paradigms, but the most common was the Rotarod test assessing grip strength and motor coordination [75]. Four studies reported improved motor function following hypoxia exposure compared to normoxia [26,37,48,58]. Specifically, one study found enhanced motor–cognitive learning and enduring performance following three weeks of continuous 12% O2 exposure [58]. Two studies demonstrated preserved motor function after hypoxia, one involving intermittent 9.5–10% O2 with 5–8 daily cycles repeated for 20 days in models of ethanol withdrawal [37], and the other employing normobaric continuous 11% O2 eight hours daily for two weeks in a stroke model [48], when compared to normoxia-treated rodents. One study involving a spinal cord injury model found motor function improvement in one out of three tasks following 12 weeks of intermittent 11% O2, administered for 50 min sessions and repeated four days per week, with concurrent task-specific training [26]. In contrast, two studies observed impaired motor performance following 25–28 days of normobaric continuous 10–11% O2 exposure, either administered chronically for 12 [27] or 24 [46] hours daily, although this negative effect was restricted to one out of two motor tasks in one study [28]. The remaining study reported no change in motor function after six hours daily continuous 10% O2 repeated over 21 days [47]. Taken together, these findings suggest the possible beneficial effects of intermittent or continuous hypoxia with intensities of 11–12% O2 with repeated exposures over 14–21 days on motor performance and recovery in animal models.
6.3. In Vivo Neuroimaging
Four studies studied the effects of hypoxia on the brain using in vivo neuroimaging approaches in animals [29,64,70,71]. One EEG study in rats found reduced theta and delta activity following hypobaric, continuous 14% O2 chronically for 28 days [29]. This was hypothesized to be related to poorer cognitive performance, although this study did not include any direct measures of cognition. Another EEG study in mice found decreased P300 amplitude following hypobaric, continuous 12.5–13.5% O2 administered chronically for 14 days in addition to a reduction in working memory performance [70]. An fMRI study in rats found decreased resting-state activity after hypobaric continuous hypoxia administered chronically for 4 weeks (10% O2) alongside impaired memory [64]. Finally, a structural MRI study showed reduced hippocampal volume in rats subjected to continuous high altitude living (4250 m corresponding to approx. 12% O2) chronically for 8 months compared to rats living at sea level, which correlated with impaired memory performance [71]. These studies thus indicate the potentially negative effects of higher doses of hypobaric hypoxia or high-altitude exposure on cognition-related brain imaging measures in rodents.
6.4. Neuronal Morphological Changes
Twenty-three studies examined hypoxia-related changes in neuronal morphology in rodents (see . Out of these, 13 studies investigated neurodegeneration, which involved apoptosis in the hippocampus [30,35,36,40,61,62,69,71,73], frontal and temporal cortex [43,70], and the basal forebrain [57]. Eight studies reported increased neuroapoptosis following higher hypoxic doses, involving 8+ hours of daily normobaric or hypobaric hypoxia (intensities between 10 and 13% O2) chronically for 2–8 weeks when compared to normoxia controls [35,36,40,57,62,69,71,72]. Two studies found no apoptosis, one after normobaric intermittent 10% O2 exposure chronically for one month [30], and one after hypobaric continuous 11–14% O2 repeated for four hours daily for two weeks [73]. One study found prevented neuroapoptosis in Alzheimer’s disease rats treated with hypobaric continuous hypoxia for 4 h daily (12.5% O2) repeated for two weeks compared to normoxia treatment [43].
Eight studies investigated neuroregenerative processes following hypoxia exposure in rodents, including hippocampal neurogenesis [28,33,45,55,56,73] and long-term potentiation [42,68]. Of these, seven found enhanced neurogenesis or long-term potentiation exploiting different protocols of low-dose hypoxia, primarily hypobaric continuous hypoxia of 11–16% O2 repeated for 4–6 h daily for 2–4 weeks [33,42,45,55,56,68,73]. The remaining study found decreased neurogenesis after normobaric, continuous 10% O2 chronically for two weeks [28].
Furthermore, five studies investigated hippocampal synaptic morphology [41,54,56,61,68]. Two studies found increased synaptic plasticity following 4–6 h repeated hypobaric continuous hypoxia (10.8–14.2% O2) for four weeks [56,68]. In contrast, two studies reported synaptic loss after intermittent or continuous hypoxia for 6–8 h repeated daily with intensities between 10 and 11.1% O2 [41,61]. The final study observed no change in synaptic morphology after 12 weeks of hypobaric, continuous 11% O2 chronic exposure [54].
Finally, two studies investigated changes in dendritic morphology [38,56]. One study found increased dendritic plasticity after continuous hypobaric hypoxia (6 h daily at 14.2% O2) repeated for one month [56], whereas one study reported decreased dendritic branching following intermittent 10% O2 exposure with 240 cycles for 12 h per day for 20 days, although this effect was only observed in male, and not female, rats [38].
Taken together, the findings from these animal models suggest that hypoxia exposure mostly has a positive impact on neuronal morphological changes in the brain, although findings vary with the direction of these changes seemingly being dependent on the dose and intensity of exposure, with lower doses producing most consistent beneficial effects.
6.5. Myelination
Four reports studied hypoxia-related changes in myelination, including myelinogenesis and degeneration and levels of mature myelin [27,28,46,54]. Three found reduced myelination after chronic exposure to normobaric continuous hypoxia (10–11% O2) for 2–4 weeks [27,28,46]. One study did not observe any change in myelination after hypobaric continuous hypoxia (chronic 11% O2) compared to normoxia [54]. Based on this, higher hypoxic doses involving lower levels of oxygen (<11%) and longer-duration chronic exposure might negatively affect myelination processes.
6.6. Neurotrophins
Six studies investigated if moderate hypoxia affects neurotrophin levels, which involve brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and neurotrophin-3 (NT-3) levels [45,48,51,52,53,73]. All studies reported increased neutrophin levels after varied types of moderate hypoxia protocols, mainly involving either intermittent hypoxia with 10 cycles per day (approx. one hour) repeated for 3–10 weeks, or continuous hypoxia with repeated exposure chronically up to eight weeks. Taken together, these results indicate that both intermittent and continuous moderate hypoxia increases neurotrophin levels.
6.7. Erythropoietin
Two studies examined the effects of hypoxia on erythropoietin (EPO) levels, a multifunctional growth factor known for its neuroplastic properties [51,73]. One study in rats reported increased EPO levels in the hippocampus after four hours daily repeated 11% O2 exposure (hypobaric continuous hypoxia) over two weeks compared to normoxia controls [73]. In line with this, a study in a mouse model of Alzheimer’s disease found increased cerebrocortical EPO following normobaric intermittent hypoxia with 10 cycles of 10% O2 and normoxia, administered for one hour daily repeated over 2–3 weeks, compared to normoxia-treated mice [51]. This suggests that moderate hypoxia exposure can heighten EPO expression.
6.8. Neuroinflammation
Five studies in rodents investigated the hypoxia-related changes in neuroinflammation markers, i.e., pro-inflammatory cytokines like interleukin-6 and tumor necrosis factor alpha [46,57,59,65,71]. Of these, four studies found increased levels of neuroinflammation following higher doses of both intermittent and continuous hypoxia (chronic exposure of 10–12% O2 for 25 days to eight months) compared to normoxia [46,57,59,65,71]. However, one study demonstrated reduced neuroinflammation following hypobaric continuous hypoxia (14% O2) repeated for 4 h daily for 14 days in a mouse model of Alzheimer’s disease [65]. These findings indicate that hypoxia can alter neuroinflammatory markers, with some evidence that higher doses (e.g., with lower intensity levels (10–12%) of O2 for longer durations) may increase inflammation, while more moderate doses (e.g., repeated 14% O2 for shorter durations) may alleviate inflammation.
6.9. Oxidative Stress
Eight studies investigated the effect of hypoxia on levels of oxidative stress in rodents, involving increased lipid peroxidation and decreased antioxidant levels in the brain [31,35,44,52,57,66,71,72]. Seven of these found increased levels of oxidative stress following various protocols, including intermittent (320–480 cycles of 10% O2) with chronic exposures for 14 days to 8 months [31,35,52,57,66,71,72]. One study found no significant effect on oxidative stress after hypobaric continuous hypoxia (12.5% O2) administered for 4 h daily, repeated for 14 days [44]. These findings indicate that longer-duration and chronic exposures of hypoxia can heighten levels of oxidative stress in the brain.
6.10. Markers of Alzheimer’s Disease
Six studies investigated how hypoxia affects Alzheimer’s disease markers, including tau phosphorylation [39,63,66], β-amyloid [41], amyloid plaque [65], and hippocampal vascular density [60]. Four studies reported increased levels of Alzheimer’s disease markers, indicating worsened pathology, following continuous hypoxia (6–8 h daily exposures of 10–11% O2) repeated for 2–8 weeks [39,41,63,66]. However, one study of hypobaric continuous hypoxia (14% O2 given in four hour sessions repeated for two weeks) demonstrated decreased levels of amyloid plaque in the hippocampus (but not in the cortex) in an Alzheimer’s disease model in mice [65]. Another study found increased hippocampal vascular density following normobaric continuous hypoxia with 11% O2 chronically for 28 days, which lasted for at least two months after hypoxia treatment [60]. This effect contrasts with decreased vascular density in neurodegenerative conditions such as Alzheimer’s disease. Overall, the conflicting findings indicate that hypoxia can affect Alzheimer’s disease markers, although the direction is controversial and possibly dose-dependent.
7. Discussion
This systematic review identified 58 articles investigating the effects of moderate hypoxia exposure on cognitive and neurological functions and markers of neuroplasticity across humans and animal subjects. Of these, eight studies were conducted in humans (three articles overlapping in sample) with various CNS conditions like traumatic brain injury and mild cognitive impairment or healthy individuals, whereas 48 studies were conducted in rodents, including models of spinal cord injury and Alzheimer’s disease. In the human reports, six studies (75%) found beneficial effects for hypoxia exposure, while the remaining studies observed either no efficacy (k = 1; 12.5%) or impairment (k = 1; 12.5%). The findings were more variable in the animal studies with around 28 studies (58.5%) demonstrating the negative effects of hypoxia exposure on measures of learning and memory, apoptosis, inflammation, and oxidative stress, while 18 studies (37.5%) reported positive effects, and two studies (4%) showed either no or mixed findings. In general, studies exploiting lower doses of normobaric intermittent or continuous hypoxia exposure with sessions ranging from 30 min to 4 h repeated over 2–12 weeks showed the most consistent beneficial effects, while studies applying higher doses of both normobaric or hypobaric, intermittent or continuous hypoxia with +6 h sessions, chronically administered over 2 weeks to 2 years, showed more consistent negative effects. Indeed, 84% of the ‘low dose’ hypoxia studies (k = 9 in humans; k = 11 in animals) observed beneficial effects on measures of global cognition, memory, attention, motor function, and neuroplasticity markers (i.e., increased levels of neurotrophins, EPO, neurogenesis, etc.) [9,18,19,22,23,24,26,42,43,44,45,53,65,69,73]. In contrast, only 18% of the studies with higher doses of hypoxia exposure (k = 1 in humans; k = 37 in animals) showed beneficial effects, while 79% reported negative effects on cognition, brain structure, oxidative stress, inflammation, and Alzheimer’s disease markers [20,27,28,31,32,35,36,38,39,40,41,47,49,50,54,57,59,61,62,63,64,66,69,70,71,72]. Importantly, the risk of bias was high for all human studies, indicating the very preliminary state of the current research on hypoxia and CNS disorders.
The majority of the human studies found beneficial effects of moderate hypoxia exposure on measures of cognitive and neurological functioning. These positive studies were characterized by similar treatment schedules of low-dose normobaric, intermittent hypoxia involving fewer cycles (i.e., 3–30 cycles) and shorter (i.e., 30–90 min) durations with intensities between 10 and 14% O2, and most were in combination with concurrent physical training programs. This suggests that low-dose intermittent and repeated hypoxia training could be an efficacious intervention on the functioning of the CNS and may be particularly efficacious when combined with a motor-cognitive intervention. Indeed, the animal studies that showed beneficial effects of hypoxia involved relatively similar treatment procedures with session durations ranging from 50 min to 4 h, mainly continuous with repeated exposures over 2–12 weeks with moderate intensities between 10 and 16% O2, although only one of these studies involved concurrent physical training [26]. Accordingly, mechanistic findings from these animal studies suggest that markers of neuroplasticity (i.e., neuronal and synaptic growth, increased neurotrophins and EPO levels in the brain) are targeted by physiological manipulation of oxygen and may underlie the observed effects on cognitive and neurological functioning across animals and humans. Taken together, this indicates that lower doses of both intermittent and continuous hypoxia training with moderate O2 levels and repeated short sessions could be an effective intervention targeting CNS disease by stimulating neuroplasticity markers, with potentially synergistic effects arising from concurrent training as it exploits the neuroplastic potential generated by the hypoxia exposure. Nevertheless, these findings are still preliminary due to the high risk of bias and small sample sizes, and future large-scale randomized controlled trials are thus highly warranted to assess the potential neuroprotective effects of moderate hypoxia exposure.
Regardless of the most consistently positive effects of hypoxia interventions in the human studies, it is noted that most of the animal studies (56%) showed adverse effects. Possible mechanisms for these observed negative effects involve increased neuroapoptosis, heightened oxidative stress, increased Alzheimer’s disease markers, and inflammation in the brain as evident from the preclinical studies. There were some commonalities across these studies. Firstly, animal subjects often live in impoverished conditions, and it was not clear if animals from the included studies had access to enriched environments or were deprived of environmental stimulation during the studies [76]. This could have impeded the optimal physiological effects of hypoxia on neuroplasticity. Indeed, studies in which animals were living in environmentally enriched conditions, such as access to running wheels [58] or physical training [26] showed beneficial effects of hypoxia. The seeming lack of environmental enrichment on most animal hypoxia studies contrasts with the human studies in two ways: (i) firstly, human participants are not subjected to impoverished conditions but live their normal enriched lives during trial participation, and (ii) the human hypoxia interventions mostly (78% of studies) combined hypoxia exposure with other activities such as physical exercise [8,9,18,19,22,23,24]. Therefore, it is possible that physical activity and enriched environments are crucial for maximizing the neuroplastic potential generated by hypoxia exposure, leading to cognitive improvements [77]. More generally, this discrepancy between animal and human studies illustrates the limitations of using animal models to investigate the functioning of the human CNS [78], which may contribute to the sometimes poor predictive validity of treatment effects in animal models for efficacy on CNS disorders in humans [79].
Further, most studies observing negative effects, including a majority of the animal studies, employed higher doses of hypoxia (i.e., longer sessions of continuous or intermittent hypoxia repeated from +6 h daily to also chronic exposure, e.g., moving to high altitude or 12 h daily). This provides insights into the optimal dosing and timing of hypoxia interventions, pointing to more positive effects from normobaric hypoxia and lower doses (i.e., shorter, continuous or intermittent hypoxic sessions for less than 4 h, repeated 3–5 times weekly or daily) and multimodal interventions. This is in line with the concept of hormesis [80], which stipulates that the body should be exposed to small doses of stress (e.g., hypoxia) in order to compensate in a beneficial way, i.e., by triggering markers of neuroplasticity. In contrast, higher hypoxic doses (i.e., too severe O2 intensities, and too long or chronic exposure) may result in excessive stress for the system, potentially explaining the observed negative findings in studies employing hypoxia for longer durations. Indeed, studies have generally shown that severe intensities (less than 10% O2) of hypoxia have a negative impact on the brain [81,82,83]. Further, commonly employed ‘chronic intermittent hypoxia’ models of sleep apnea with intermittent 10% O2 exposure administered chronically over several weeks have also produced consistent neuropathogenic effects [84]. On the contrary, it is also relevant to speculate whether even milder forms of hypoxia may be sufficient, although underdosing at, e.g., 17% O2 is also unlikely to generate the desired effects [85]. Nonetheless, some studies exploiting even shorter periods of moderate hypoxia interventions with 10–16% O2 but for less than 14 days found beneficiary effects on executive functioning in healthy individuals [86], walking abilities in patients after spinal cord injury [87], and alleviated memory impairment in a rat model of stroke [88], although these were not included in the present review. Taken together, this suggests that the optimal intensity and dosing of hypoxia for exploiting neuroplastic benefits are likely to be moderate around 10–16% O2 with shorter intermittent or continuous types of exposures involving 30 min to 4 h sessions, with repeated frequency +3 days weekly over 2–6 weeks.
A limitation of this systematic review is that it did not include a quantification of the effects of the hypoxia interventions through meta-analysis. However, there were several issues with conducting a meta-analysis on the studies included in this review. Firstly, the research field is at a very early stage as reflected by the high risk of bias across the human studies. Secondly, there was high heterogeneity in the treatment schedules and outcome measures, which would complicate the comparison of the effects across studies. Another limitation was the predefined inclusion criteria of solely moderate intensities of hypoxia (oxygen levels in the range of 10–16%) and minimum duration of 14 days with no upper cut-off for duration. These criteria are somewhat broad and yield heterogeneous treatment protocols. Nevertheless, the aim of this review was to provide an umbrella perspective on this emerging field and provide insights into the potential optimal treatment schedules of hypoxia training that can guide future interventional studies. Finally, the translational approach comparing clinical and preclinical studies further allows for deeper mechanistic insights of moderate hypoxia interventions.
In conclusion, emerging translational evidence suggest that lower doses of moderate hypoxia exposure can improve aspects of cognitive and neurological functioning and markers of neuroplasticity, including learning and memory, motor abilities, neuronal and synaptic growth, BDNF, and EPO levels. Specifically, this review revealed most consistent benefits of normobaric hypoxia exposures with moderate intensities between 10 and 16% O2, administered either intermittently or continuously, but for relatively short durations (30 min to 4 h sessions), repeatedly over 2–12 weeks. Further, most cognitive and neurological benefits occurred when the hypoxia treatments were combined with hyperoxic breathing or concurrent motor–cognitive strategies such as physical exercise or rehabilitation, possibly due to synergistic effects on neuroplastic processes. However, no definite conclusions regarding efficacy can yet be drawn given the high risk of bias in all of the human hypoxia studies due to small sample sizes, and lack of randomization and assessor blinding. Larger, methodologically stronger randomized controlled studies are thus highly warranted. If such studies replicate cognitive and neurological improvements following moderate hypoxia, this can have the potential to advance treatments targeting neuroplasticity dysfunctions and cognitive decline across CNS disorders.
References
- Miskowiak, K.W.; Seeberg, I.; Jensen, M.B.; Balanzá-Martínez, V.; del Mar Bonnin, C.; Bowie, C.R.; Carvalho, A.F.; Dols, A.; Douglas, K.; Gallagher, P.; et al. Randomised Controlled Cognition Trials in Remitted Patients with Mood Disorders Published between 2015 and 2021: A Systematic Review by the International Society for Bipolar Disorders Targeting Cognition Task Force. Bipolar Disord. 2022, 24, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Kays, J.L.; Hurley, R.A.; Taber, K.H. The Dynamic Brain: Neuroplasticity and Mental Health. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sasmita, A.O.; Kuruvilla, J.; Ling, A.P.K. Harnessing Neuroplasticity: Modern Approaches and Clinical Future. Int. J. Neurosci. 2018, 128, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Ehrenreich, H.; Gassmann, M.; Poustka, L.; Burtscher, M.; Hammermann, P.; Sirén, A.; Nave, K.; Miskowiak, K. Exploiting Moderate Hypoxia to Benefit Patients with Brain Disease: Molecular Mechanisms and Translational Research in Progress. Neuroprotection 2023, 1, 55–65. [Google Scholar] [CrossRef]
- Panza, G.S.; Burtscher, J.; Zhao, F. Intermittent Hypoxia: A Call for Harmonization in Terminology. J. Appl. Physiol. 2023, 135, 886–890. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic Potential of Intermittent Hypoxia: A Matter of Dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Schenck, H.; Hall, J.R.; Ross, S.E.; Kline, G.P.; Chen, S.; Mallet, R.T.; Chen, P. Intermittent Hypoxia Training for Treating Mild Cognitive Impairment: A Pilot Study. Am. J. Alzheimer’s Dis. Other Demen. 2020, 35, 1533317519896725. [Google Scholar] [CrossRef]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Effects of Intermittent Hypoxia-Hyperoxia on Mobility and Perceived Health in Geriatric Patients Performing a Multimodal Training Intervention: A Randomized Controlled Trial. BMC Geriatr. 2019, 19, 167. [Google Scholar] [CrossRef]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Intermittent Hypoxic-Hyperoxic Training on Cognitive Performance in Geriatric Patients. Alzheimer’s Dement. 2017, 3, 114–122. [Google Scholar] [CrossRef]
- Rogers, R.S.; Wang, H.; Durham, T.J.; Stefely, J.A.; Owiti, N.A.; Markhard, A.L.; Sandler, L.; To, T.L.; Mootha, V.K. Hypoxia Extends Lifespan and Neurological Function in a Mouse Model of Aging. PLoS Biol. 2023, 21, e3002117. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Barker-Collo, S.L.; Connell, C.J.W.; Gant, N. Acute Hypoxic Gas Breathing Severely Impairs Cognition and Task Learning in Humans. Physiol. Behav. 2015, 142, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Asmaro, D.; Mayall, J.; Ferguson, S. Cognition at Altitude: Impairment in Executive and Memory Processes under Hypoxic Conditions. Aviat. Space Environ. Med. 2013, 84, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, S.M.; Yuan, C.; Tian, H.J.; Li, P.; Gao, Y.Q. The Effects of Short-Term and Long-Term Exposure to a High Altitude Hypoxic Environment on Neurobehavioral Function. High Alt. Med. Biol. 2013, 14, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative Stress and Apoptosis after Acute Respiratory Hypoxia and Reoxygenation in Rat Brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Singh, S.B.; Mallick, B.; Muthuraju, S.; Ilavazhagan, G. High Altitude Memory Impairment Is Due to Neuronal Apoptosis in Hippocampus, Cortex and Striatum. J. Chem. Neuroanat. 2008, 36, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Mallet, R.T.; Pialoux, V.; Millet, G.P.; Burtscher, M. Adaptive Responses to Hypoxia and/or Hyperoxia in Humans. Antioxid. Redox Signal. 2022, 37, 887–912. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Bayer, U.; Glazachev, O.S.; Likar, R.; Burtscher, M.; Kofler, W.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S. Adaptation to Intermittent Hypoxia-Hyperoxia Improves Cognitive Performance and Exercise Tolerance in the Elderly. Adv. Gerontol. 2017, 7, 214–220. [Google Scholar] [CrossRef]
- Behrendt, T.; Bielitzki, R.; Behrens, M.; Glazachev, O.S.; Schega, L. Effects of Intermittent Hypoxia-Hyperoxia Exposure Prior to Aerobic Cycling Exercise on Physical and Cognitive Performance in Geriatric Patients-A Randomized Controlled Trial. Front. Physiol. 2022, 13, 899096. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, Q.; Wang, J.; Zhang, W.; Liu, J.; Li, B.; Xin, Z.; Liu, J.; Yin, H.; et al. Combined Fractional Anisotropy and Subcortical Volumetric Abnormalities in Healthy Immigrants to High Altitude: A Longitudinal Study. Hum. Brain Mapp. 2019, 40, 4202–4212. [Google Scholar] [CrossRef]
- Corral, L.; Conde, L.; Guillamo, E.; Blasi, J.; Juncadella, M.; Javierre, C.; Viscor, G.; Ventura, J.L. Circulating Progenitor Cells during Exercise, Muscle Electro-Stimulation and Intermittent Hypobaric Hypoxia in Patients with Traumatic Brain Injury: A Pilot Study. NeuroRehabilitation 2014, 35, 763–769. [Google Scholar] [CrossRef]
- Schega, L.; Peter, B.; Torpel, A.; Mutschler, H.; Isermann, B.; Hamacher, D. Effects of Intermittent Hypoxia on Cognitive Performance and Quality of Life in Elderly Adults: A Pilot Study. Gerontology 2013, 59, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Schega, L.; Peter, B.; Brigadski, T.; Lessmann, V.; Isermann, B.; Hamacher, D.; Torpel, A. Effect of Intermittent Normobaric Hypoxia on Aerobic Capacity and Cognitive Function in Older People. J. Sci. Med. Sport 2016, 19, 941–945. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Serebrovska, T.V.; Kholin, V.A.; Tumanovska, L.V.; Shysh, A.M.; Pashevin, D.A.; Goncharov, S.V.; Stroy, D.; Grib, O.N.; Shatylo, V.B.; et al. Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study. Int. J. Mol. Sci. 2019, 20, 5405. [Google Scholar] [CrossRef] [PubMed]
- Serebrovska, Z.O.; Xi, L.; Tumanovska, L.V.; Shysh, A.M.; Goncharov, S.V.; Khetsuriani, M.; Kozak, T.O.; Pashevin, D.A.; Dosenko, V.E.; Virko, S.V.; et al. Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment. Life 2022, 12, 432. [Google Scholar] [CrossRef]
- Arnold, B.M.; Toosi, B.M.; Caine, S.; Mitchell, G.S.; Muir, G.D. Prolonged Acute Intermittent Hypoxia Improves Forelimb Reach-to-Grasp Function in a Rat Model of Chronic Cervical Spinal Cord Injury. Exp. Neurol. 2021, 340, 113672. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, S.Y.; Li, R.X.; Liu, K.; Chen, J.F.; Yang, Y.J.; Deng, Y.B.; Wang, H.Z.; Xiao, L.; Mei, F.; et al. Chronic Exposure to Hypoxia Inhibits Myelinogenesis and Causes Motor Coordination Deficits in Adult Mice. Neurosci. Bull. 2021, 37, 1397–1411. [Google Scholar] [CrossRef]
- Chung, E.; Kong, X.; Goldberg, M.P.; Stowe, A.M.; Raman, L. Erythropoietin-Mediated Neuroprotection in a Pediatric Mouse Model of Chronic Hypoxia. Neurosci. Lett. 2015, 597, 54–59. [Google Scholar] [CrossRef]
- Erken, H.A.; Erken, G.; Colak, R.; Genç, O. Exercise and DHA Prevent the Negative Effects of Hypoxia on EEG and Nerve Conduction Velocity. High. Alt. Med. Biol. 2013, 14, 360–366. [Google Scholar] [CrossRef]
- Goldbart, A.; Row, B.W.; Kheirandish, L.; Schurr, A.; Gozal, E.; Guo, S.Z.; Payne, R.S.; Cheng, Z.; Brittian, K.R.; Gozal, D. Intermittent Hypoxic Exposure during Light Phase Induces Changes in CAMP Response Element Binding Protein Activity in the Rat CA1 Hippocampal Region: Water Maze Performance Correlates. Neuroscience 2003, 122, 585–590. [Google Scholar] [CrossRef]
- Gozal, D.; Nair, D.; Goldbart, A.D. Physical Activity Attenuates Intermittent Hypoxia-Induced Spatial Learning Deficits and Oxidative Stress. Am. J. Respir. Crit. Care Med. 2010, 182, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Daniel, J.M.; Dohanich, G.P. Behavioral and Anatomical Correlates of Chronic Episodic Hypoxia during Sleep in the Rat. J. Neurosci. 2001, 21, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Row, B.W.; Gozal, E.; Kheirandish, L.; Neville, J.J.; Brittian, K.R.; Sachleben, L.R.; Guo, S.Z. Temporal Aspects of Spatial Task Performance during Intermittent Hypoxia in the Rat: Evidence for Neurogenesis. Eur. J. Neurosci. 2003, 18, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Narbona, R.; Delgado-García, J.M.; López-Ramos, J.C. Altitude Acclimatization Improves Submaximal Cognitive Performance in Mice and Involves an Imbalance of the Cholinergic System. J. Appl. Physiol. 2013, 114, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Hui-guo, L.; Kui, L.; Yan-ning, Z.; Yong-jian, X. Apocynin Attenuate Spatial Learning Deficits and Oxidative Responses to Intermittent Hypoxia. Sleep Med. 2010, 11, 205–212. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, Y.; Luo, J.; Wan, Y.; Liu, J.; Ge, R.L. Memantine Ameliorates Cognitive Impairment Induced by Exposure to Chronic Hypoxia Environment at High Altitude by Inhibiting Excitotoxicity. Life Sci. 2021, 270, 119012. [Google Scholar] [CrossRef]
- Ju, X.; Mallet, R.T.; Downey, H.F.; Metzger, D.B.; Jung, M.E. Intermittent Hypoxia Conditioning Protects Mitochondrial Cytochrome c Oxidase of Rat Cerebellum from Ethanol Withdrawal Stress. J. Appl. Physiol. 2012, 112, 1706–1714. [Google Scholar] [CrossRef]
- Kheirandish, L.; Gozal, D.; Pequignot, J.M.; Pequignot, J.; Row, B.W. Intermittent Hypoxia during Development Induces Long-Term Alterations in Spatial Working Memory, Monoamines, and Dendritic Branching in Rat Frontal Cortex. Pediatr. Res. 2005, 58, 594–599. [Google Scholar] [CrossRef]
- Lei, L.; Feng, J.; Wu, G.; Wei, Z.; Wang, J.Z.; Zhang, B.; Liu, R.; Liu, F.; Wang, X.; Li, H.L. HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. Int. J. Mol. Sci. 2022, 23, 16140. [Google Scholar] [CrossRef]
- Li, G.; Liu, J.; Guo, M.; Gu, Y.; Guan, Y.; Shao, Q.; Ma, W.; Ji, X. Chronic Hypoxia Leads to Cognitive Impairment by Promoting HIF-2α-Mediated Ceramide Catabolism and Alpha-Synuclein Hyperphosphorylation. Cell Death Discov. 2022, 8, 473. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, H.; Yang, J.; Ni, J.; Le, W. Chronic Hypoxia Facilitates Alzheimer’s Disease through Demethylation of γ-Secretase by Downregulating DNA Methyltransferase 3b. Alzheimer’s Dement. 2016, 12, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.J.; Chen, X.Q.; Weng, J.; Zhang, H.Y.; Pak, D.T.; Luo, J.H.; Du, J.Z. Hippocampal Spine-Associated Rap-Specific GTPase-Activating Protein Induces Enhancement of Learning and Memory in Postnatally Hypoxia-Exposed Mice. Neuroscience 2009, 162, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Manukhina, E.B.; Goryacheva, A.V.; Barskov, I.V.; Viktorov, I.V.; Guseva, A.A.; Pshennikova, M.G.; Khomenko, I.P.; Mashina, S.Y.; Pokidyshev, D.A.; Malyshev, I.Y. Prevention of Neurodegenerative Damage to the Brain in Rats in Experimental Alzheimer’s Disease by Adaptation to Hypoxia. Neurosci. Behav. Physiol. 2010, 40, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Manukhina, E.B.; Tseilikman, V.E.; Tseilikman, O.B.; Komelkova, M.V.; Kondashevskaya, M.V.; Goryacheva, A.V.; Lapshin, M.S.; Platkovskii, P.O.; Alliluev, A.V.; Downey, H.F. Intermittent Hypoxia Improves Behavioral and Adrenal Gland Dysfunction Induced by Posttraumatic Stress Disorder in Rats. J. Appl. Physiol. 2018, 125, 931–937. [Google Scholar] [CrossRef]
- Meng, S.X.; Wang, B.; Li, W.T. Intermittent Hypoxia Improves Cognition and Reduces Anxiety-Related Behavior in APP/PS1 Mice. Brain Behav. 2020, 10, e01513. [Google Scholar] [CrossRef]
- Ortega, S.B.; Kong, X.; Venkataraman, R.; Savedra, A.M.; Kernie, S.G.; Stowe, A.M.; Raman, L. Perinatal Chronic Hypoxia Induces Cortical Inflammation, Hypomyelination, and Peripheral Myelin-Specific T Cell Autoreactivity. J. Leukoc. Biol. 2016, 99, 21–29. [Google Scholar] [CrossRef]
- Perry, J.C.; D’Almeida, V.; Lima, M.M.S.; Godoi, F.R.L.; Vital, M.A.B.F.; Oliveira, M.G.M.; Tufik, S. Intermittent Hypoxia and Sleep Restriction: Motor, Cognitive and Neurochemical Alterations in Rats. Behav. Brain Res. 2008, 189, 373–380. [Google Scholar] [CrossRef]
- Pietrogrande, G.; Zalewska, K.; Zhao, Z.; Johnson, S.J.; Nilsson, M.; Walker, F.R. Low Oxygen Post Conditioning as an Efficient Non-Pharmacological Strategy to Promote Motor Function After Stroke. Transl. Stroke Res. 2019, 10, 402–412. [Google Scholar] [CrossRef]
- Row, B.W.; Kheirandish, L.; Cheng, Y.; Rowell, P.P.; Gozal, D. Impaired Spatial Working Memory and Altered Choline Acetyltransferase (CHAT) Immunoreactivity and Nicotinic Receptor Binding in Rats Exposed to Intermittent Hypoxia during Sleep. Behav. Brain Res. 2007, 177, 308–314. [Google Scholar] [CrossRef]
- Row, B.W.; Kheirandish, L.; Neville, J.J.; Gozal, D. Impaired Spatial Learning and Hyperactivity in Developing Rats Exposed to Intermittent Hypoxia. Pediatr. Res. 2002, 52, 449–453. [Google Scholar] [CrossRef]
- Ryou, M.-G.; Chen, X.; Cai, M.; Wang, H.; Jung, M.E.; Metzger, D.B.; Mallet, R.T.; Shi, X. Intermittent Hypoxia Training Prevents Deficient Learning-Memory Behavior in Mice Modeling Alzheimer’s Disease: A Pilot Study. Front. Aging Neurosci. 2021, 13, 674688. [Google Scholar] [CrossRef]
- Sakr, H.F.; Abbas, A.M.; El Samanoudy, A.Z. Effect of Vitamin E on Cerebral Cortical Oxidative Stress and Brain-Derived Neurotrophic Factor Gene Expression Induced by Hypoxia and Exercise in Rats. J. Physiol. Pharmacol. 2015, 66, 191–202. [Google Scholar]
- Satriotomo, I.; Nichols, N.L.; Dale, E.A.; Emery, A.T.; Dahlberg, J.M.; Mitchell, G.S. Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non- respiratory motor neurons. Neuroscience 2016, 13, 479–488. [Google Scholar] [CrossRef]
- Sharma, R.; Cramer, N.P.; Perry, B.; Adahman, Z.; Murphy, E.K.; Xu, X.; Dardzinski, B.J.; Galdzicki, Z.; Perl, D.P.; Dickstein, D.L.; et al. Chronic Exposure to High Altitude: Synaptic, Astroglial and Memory Changes. Sci. Rep. 2019, 9, 16406. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.; Qu, Z.; Li, D.; Si, P.; Qiao, Q.; Zhang, W.; Xue, Y.; Zhen, J.; Wang, W. Chronic Mild Hypoxia Promotes Hippocampal Neurogenesis Involving Notch1 Signaling in Epileptic Rats. Brain Res. 2019, 1714, 88–98. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.; Qu, Z.; Jia, L.; Li, D.; Zhen, J.; Wang, W. Chronic Intermittent Hypobaric Hypoxia Restores Hippocampus Function and Rescues Cognitive Impairments in Chronic Epileptic Rats via Wnt/Beta-Catenin Signaling. Front. Mol. Neurosci. 2020, 13, 617143. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, J.; Zhao, D.; Mo, H.; Zeng, Z.; Xiong, M.; Dong, M.; Hu, K. Effects of the Excitation or Inhibition of Basal Forebrain Cholinergic Neurons on Cognitive Ability in Mice Exposed to Chronic Intermittent Hypoxia. Brain Res. Bull. 2020, 164, 235–248. [Google Scholar] [CrossRef]
- Wakhloo, D.; Scharkowski, F.; Curto, Y.; Javed Butt, U.; Bansal, V.; Steixner-Kumar, A.A.; Wüstefeld, L.; Rajput, A.; Arinrad, S.; Zillmann, M.R.; et al. Functional Hypoxia Drives Neuroplasticity and Neurogenesis via Brain Erythropoietin. Nat. Commun. 2020, 11, 1313. [Google Scholar] [CrossRef]
- Wang, H.; Yang, T.; Sun, J.; Zhang, S.; Liu, S. SENP1 Modulates Microglia-Mediated Neuroinflammation toward Intermittent Hypoxia-Induced Cognitive Decline through the de-SUMOylation of NEMO. J. Cell Mol. Med. 2021, 25, 6841–6854. [Google Scholar] [CrossRef]
- Warrington, J.P.; Csiszar, A.; Mitschelen, M.; Lee, Y.W.; Sonntag, W.E. Whole Brain Radiation-Induced Impairments in Learning and Memory Are Time-Sensitive and Reversible by Systemic Hypoxia. PLoS ONE 2012, 7, e30444. [Google Scholar] [CrossRef]
- Xu, L.H.; Xie, H.; Shi, Z.H.; Du, L.D.; Wing, Y.K.; Li, A.M.; Ke, Y.; Yung, W.H. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid. Redox Signal. 2015, 23, 695–710. [Google Scholar] [CrossRef]
- Yang, X.; Liu Dr, H.; Liu, X.; Chen, J. Thioredoxin and Impaired Spatial Learning and Memory in the Rats Exposed to Intermittent Hypoxia. Chin. Med. J. 2012, 125, 3074–3080. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Wang, W.; Xiao, Z.; Hong, Y. Multi-Vitamin B Supplementation Reverses Hypoxia-Induced Tau Hyperphosphorylation and Improves Memory Function in Adult Mice. J. Alzheimer’s Dis. 2016, 54, 297–306. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Liu, P.F.; Yue, Y.L.; Guo, J.S.; Wang, Z.C. Abnormal Brain Activity in Rats with Sustained Hypobaric Hypoxia Exposure: A Resting-State Functional Magnetic Resonance Imaging Study. Chin. Med. J. 2019, 132, 2621–2627. [Google Scholar] [CrossRef]
- Yue, X.; Zhou, Y.; Qiao, M.; Zhao, X.; Huang, X.; Zhao, T.; Cheng, X.; Fan, M.; Zhao, Y.; Chen, R.; et al. Intermittent Hypoxia Treatment Alleviates Memory Impairment in the 6-Month-Old APPswe/PS1dE9 Mice and Reduces Amyloid Beta Accumulation and Inflammation in the Brain. Alzheimer’s Res. Ther. 2021, 13, 194. [Google Scholar] [CrossRef]
- Zhang, C.E.; Yang, X.; Li, L.; Sui, X.; Tian, Q.; Wei, W.; Wang, J.; Liu, G. Hypoxia-Induced Tau Phosphorylation and Memory Deficit in Rats. Neurodegener. Dis. 2014, 14, 107–116. [Google Scholar] [CrossRef]
- Zhang, J.X.; Lu, X.J.; Wang, X.C.; Li, W.; Du, J.Z. Intermittent Hypoxia Impairs Performance of Adult Mice in the Two-Way Shuttle Box but Not in the Morris Water Maze. J. Neurosci. Res. 2006, 84, 228–235. [Google Scholar] [CrossRef]
- Zhang, J.X.; Chen, X.Q.; Du, J.Z.; Chen, Q.M.; Zhu, C.Y. Neonatal Exposure to Intermittent Hypoxia Enhances Mice Performance in Water Maze and 8-Arm Radial Maze Tasks. J. Neurobiol. 2005, 65, 72–84. [Google Scholar] [CrossRef]
- Zhang, N.; Shentu, Y.; Zhu, M.; Wang, H.; Yin, X.; Du, C.; Xue, F.; Fan, J.; Gong, Y.; Fan, X. Role of Ero1α in Cognitive Impairment Induced by Chronic Hypoxia. Brain Res. 2022, 1797, 148117. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, D.; Wu, G.; Ren, H.; Zhu, X.; Xie, W.; Zhang, Y.; Yang, L.; Peng, W.; Lai, C.; et al. Disrupted Gut Microbiota Aggravates Working Memory Dysfunction Induced by High-Altitude Exposure in Mice. Front. Microbiol. 2022, 13, 1054504. [Google Scholar] [CrossRef]
- Zhu, D.; He, B.; Zhang, M.; Wan, Y.; Liu, R.; Wang, L.; Zhang, Y.; Li, Y.; Gao, F. A Multimodal MR Imaging Study of the Effect of Hippocampal Damage on Affective and Cognitive Functions in a Rat Model of Chronic Exposure to a Plateau Environment. Neurochem. Res. 2022, 47, 979–1000. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, S.; Zhao, D.; Zeng, Z.; Mo, H.; Hu, K. Orexin A Improves the Cognitive Impairment Induced by Chronic Intermittent Hypoxia in Mice. Brain Res. Bull. 2021, 173, 203–210. [Google Scholar] [CrossRef]
- Zhu, X.H.; Yan, H.C.; Zhang, J.; Qu, H.D.; Qiu, X.S.; Chen, L.; Li, S.J.; Cao, X.; Bean, J.C.; Chen, L.H.; et al. Intermittent Hypoxia Promotes Hippocampal Neurogenesis and Produces Antidepressant-like Effects in Adult Rats. J. Neurosci. 2010, 30, 12653–12663. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Hamm, R.J.; Pike, B.R.; O’dell, D.M.; Lyeth, B.G.; Jenkins, L.W. The Rotarod Test: An Evaluation of Its Effectiveness in Assessing Motor Deficits Following Traumatic Brain Injury. J. Neurotrauma 1994, 11, 187–196. [Google Scholar] [CrossRef]
- Burrows, E.L.; Hannan, A.J. Towards Environmental Construct Validity in Animal Models of CNS Disorders: Optimizing Translation of Preclinical Studies. CNS Neurol. Disord. Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2013, 12, 587–592. [Google Scholar] [CrossRef]
- Mishra, A.; Patni, P.; Hegde, S.; Aleya, L.; Tewari, D. Neuroplasticity and Environment: A Pharmacotherapeutic Approach toward Preclinical and Clinical Understanding. Curr. Opin. Environ. Sci. Health 2021, 19, 100210. [Google Scholar] [CrossRef]
- Davies, C.; Hamilton, O.K.L.; Hooley, M.; Ritakari, T.E.; Stevenson, A.J.; Wheater, E.N.W. Translational Neuroscience: The State of the Nation (a PhD Student Perspective). Brain Commun. 2020, 2, fcaa038. [Google Scholar] [CrossRef]
- McGonigle, P. Animal Models of CNS Disorders. Biochem. Pharmacol. 2014, 87, 140–149. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis and Disease Resistance: Activation of Cellular Stress Response Pathways. Hum. Exp. Toxicol. 2008, 27, 155–162. [Google Scholar] [CrossRef]
- Titus, A.D.J.; Shankaranarayana Rao, B.S.; Harsha, H.N.; Ramkumar, K.; Srikumar, B.N.; Singh, S.B.; Chattarji, S.; Raju, T.R. Hypobaric Hypoxia-Induced Dendritic Atrophy of Hippocampal Neurons Is Associated with Cognitive Impairment in Adult Rats. Neuroscience 2007, 145, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, H.; Lee, J.S.; Park, K.S.; Jeon, G.S.; Shon, J.; Ahn, S.W.; Kim, S.H.; Lee, K.M.; Sung, J.J.; et al. Intermittent Hypoxia Can Aggravate Motor Neuronal Loss and Cognitive Dysfunction in ALS Mice. PLoS ONE 2013, 8, e81808. [Google Scholar] [CrossRef] [PubMed]
- Šimonová, Z.; Štěrbová, K.; Brožek, G.; Komárek, V.; Syková, E. Postnatal Hypobaric Hypoxia in Rats Impairs Water Maze Learning and the Morphology of Neurones and Macroglia in Cortex and Hippocampus. Behav. Brain Res. 2003, 141, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Sforza, E.; Roche, F. Chronic Intermittent Hypoxia and Obstructive Sleep Apnea: An Experimental and Clinical Approach. Hypoxia 2016, 2016, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Jain, I.H.; Goldberger, O.; Rezoagli, E.; Thoonen, R.; Chen, K.H.; Sosnovik, D.E.; Scherrer-Crosbie, M.; Mootha, V.K.; Zapol, W.M. Hypoxia Treatment Reverses Neurodegenerative Disease in a Mouse Model of Leigh Syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E4241–E4250. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive Function during Exercise under Severe Hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef] [PubMed]
- Hayes, H.B.; Jayaraman, A.; Herrmann, M.; Mitchell, G.S.; Rymer, W.Z.; Trumbower, R.D. Daily Intermittent Hypoxia Enhances Walking after Chronic Spinal Cord Injury A Randomized Trial. Neurology 2014, 82, 104–113. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Yang, Y.R.; Sun, S.H.; Liang, K.C.; Wang, R.Y. Post Ischemia Intermittent Hypoxia Induces Hippocampal Neurogenesis and Synaptic Alterations and Alleviates Long-Term Memory Impairment. J. Cereb. Blood Flow. Metab. 2013, 33, 764–773. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

