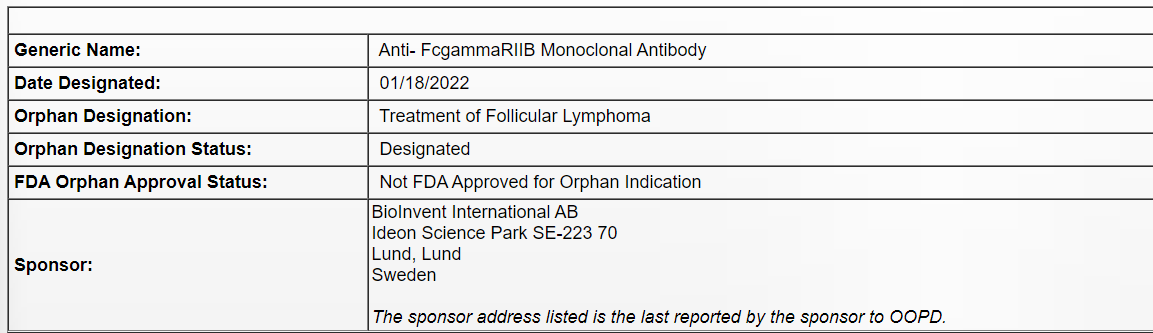
2022年1月20日,CASI宣布,其合作伙伴BioInvent International AB(以下简称BioInvent)旗下抗FcyRllB抗体BI-1206获得美国食品药品监督管理局(FDA)授予的孤儿药资格认定(Orphan Drug Designation, ODD),用于治疗滤泡性淋巴瘤(Follicular Lymphoma,FL)。滤泡性淋巴瘤是一种最常见的惰性非霍奇金淋巴瘤(Non-Hodgkin lymphoma,NHL)。
BI-1206是BioInvent在研的首要候选药物,正在进行两项I/II期临床研究:一项是与利妥昔单抗联用,用于治疗复发或难治性非霍奇金淋巴瘤(包括滤泡性淋巴瘤FL、套细胞淋巴瘤MCL及边缘区淋巴瘤MZL);另一项是与抗PD-1药物Keytruda®(帕博利珠单抗)联用,用于实体瘤的治疗。
CASI董事长及CEO何为无博士表示:“非常高兴BI-1206又取得了里程碑式的突破。FDA授予其‘孤儿药’称号,是对这款first-in-class产品的肯定和期待。目前,BI-1206已经在中国获得NHL临床试验批件。CASI拥有BI-1206大中华区独家商业权益,将与BioInvent密切合作,加速推进临床试验进展,早日将这一创新药带给更多患者。”

