BEIJING, April 26, 2023 - CASI Pharmaceuticals, Inc. (Nasdaq: CASI), a Cayman incorporated biopharmaceutical company focused on developing and commercializing innovative therapeutics and pharmaceutical products, today reported financial results for the year ended December 31, 2022, and provided an update on key highlights for 2022.
Wei-Wu He, Ph.D., CASI’s Chairman and Chief Executive Officer, commented, “We are pleased to report $10.2 million in EVOMELA® revenues for the fourth quarter, $38 million for the full-year 2022. We have achieved our goal for our full-year 2022 revenue growth to exceed 2021 revenues, with a 26.7% increase in year on year sales revenue. Our team in China delivered strong performance despite the adverse impact of COVID-19 including of a Q3-4 rapid national spread of the pandemic and lockdowns in several major cities in China. Through the efforts of the CASI team and our commercial group of hematology sales and medical marketing specialists in China, we have built a strong foundation for our commercial franchise. We plan to continue building our commercial franchise throughout 2023 and beyond.”
Dr. He continued, “Advancement, development, and commercialization of the portfolio remains our strategic focus. 2022 marks a major milestone for CASI and our partner Juventas; the CNCT-19 New Drug Application (NDA) was accepted by National Medical Products Administration (NMPA) in December 2022. We are now diligently preparing for the anticipated CNCT-19 launch in China, as we continue the development of the regulatory framework for BI-1206 in China. We completed dosing the first patient in the BI-1206 phase I trial in China. CB-5339 also received Clinical Trial Application approval from the NMPA in January 2023. The Phase 1 dose escalation and expansion study of CID-103, in patients with previously treated, relapsed or refractory multiple myeloma is closed to further accrual in France and the UK. We plan to build on the momentum to drive our portfolio forward by executing on several milestones in the quarters ahead.”
Key Highlights for 2022
EVOMELA® (melphalan for injection)
Prior to EVOMELA’s entry into the Chinese market, an average of 800 stem cell transplants per year were conducted in the multiple myeloma (MM) treatment setting. Following EVOMELA’s launch in August of 2019, CASI worked closely with KOLs to drive market awareness and expedite adoption in the Chinese market. In 2022, nearly 10,000 patients were treated with EVOMELA. CASI continues to pursue a similar strategy with respect to marketing efforts and physician visits to further the adoption of stem cell transplantation as a standard of care in the MM treatment setting and will continue working to address the persistent high unmet need in this patient population.
CNCT19 (CD19 targeted CAR-T)
Our partner, Juventas Cell Therapy Ltd (“Juventas”), continues the development of CNCT19, an autologous CD19 CAR-T investigative product for which CASI has co-commercial and profit-sharing rights. CNCT19 is being developed as a potential treatment for patients with hematological malignancies which express CD19 including, B-cell acute lymphoblastic leukemia (“B-ALL”) and B-cell non-Hodgkin lymphoma (“B-NHL”). The Phase 1 studies in B-ALL and B-NHL in China have been completed by Juventas. The Phase 2 B-ALL and B-NHL registration studies are both currently enrolling. In December 2020, CNCT19 received Breakthrough Therapy Designation based on initial data from the ongoing single-arm, open-label, non-randomized, dose-escalation, Phase 1 study designed to determine the safety and efficacy of CNCT19 in B-ALL. Since then, the National Medical Products Administration (NMPA) granted CTA approval for CNCT19 in two indications (relapsed/refractory B-All and B-NHL) in Nov. 2019. Additionally, earlier this year, the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation (ODD) to Juventas, for CNCT19, for the treatment of patients with Acute Lymphoblastic Leukemia (ALL). Currently, there are no domestically developed CD-19 CAR-T therapy marketed in China. CASI intends for CNCT (CD19 CAR-T) to be locally developed and manufactured to be more affordable and widely accessible to patients. Dec. 15, 2022, China National Medical Products Administration (NMPA) has accepted the new drug application (NDA) from Juventas Cell Therapy, Ltd., (Juventas) for CNCT19.
CB-5339 (VCP/p97 inhibitor)
CB-5339 CTA application for the Multiple Myeloma indication is in preparation after receiving an acceptance letter for the CB-5339 IND package from the China Center of Drug Evaluation. Cleave Therapeutics is responsible for the ex-China development of CB-5339, an oral second-generation, small molecule VCP/p97 inhibitor, and is evaluating the molecule in a Phase 1 clinical trial in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).
BI-1206 (Anti-FcyRIIB antibody)
Along with our partner, BioInvent, we continue to progress the development and regulatory framework for BI-1206 in China. The National Medical Products Administration (NMPA) granted BI-1206 Clinical Trial Application (CTA) approval in December 2021. EC approval from a leading investigational site was granted in January 2022. BI-1206 is currently being investigated in two Phase 1/2 trials. One is evaluating the BI-1206 combination with rituximab for the treatment of non-Hodgkin lymphoma, which includes patients with FL, MCL and marginal zone lymphoma (MZL) who have relapsed or are refractory to rituximab. A second Phase 1/2 trial is investigating BI-1206 in combination with anti-PD1 therapy Keytruda® (pembrolizumab) in solid tumors. Earlier this year the U.S. FDA granted orphan drug designation, for BI-1206, for the treatment of follicular lymphoma (FL), the most common form of slow-growing non-Hodgkin lymphoma (NHL).
CID-103 (Anti-CD38 Mab)
CID-103 is a fully human IgG1 anti-CD38 monoclonal antibody recognizing a unique epitope that has demonstrated encouraging preclinical efficacy and safety profile compared to other anti-CD38 monoclonal antibodies. CASI maintains exclusive global rights and is developing CID-103 for the treatment of patients with multiple myeloma. The Phase 1 dose escalation and expansion study of CID-103 in patients with previously treated relapsed or refractory multiple myeloma is closed to further accrual in France and the UK. Future multiple myeloma development activities will be focused on China. In May 2022, CASI entered into a sublicense agreement with Precision Autoimmune Therapeutics, who will carry out the development activities for the autoimmune indications for CID-103.
Fourth Quarter & Full-Year 2022 Financial Results
Further information regarding the Company, including its Annual Report on Form 20-F for the year ended December 31, 2022, can be found at www.casipharmaceuticals.com.
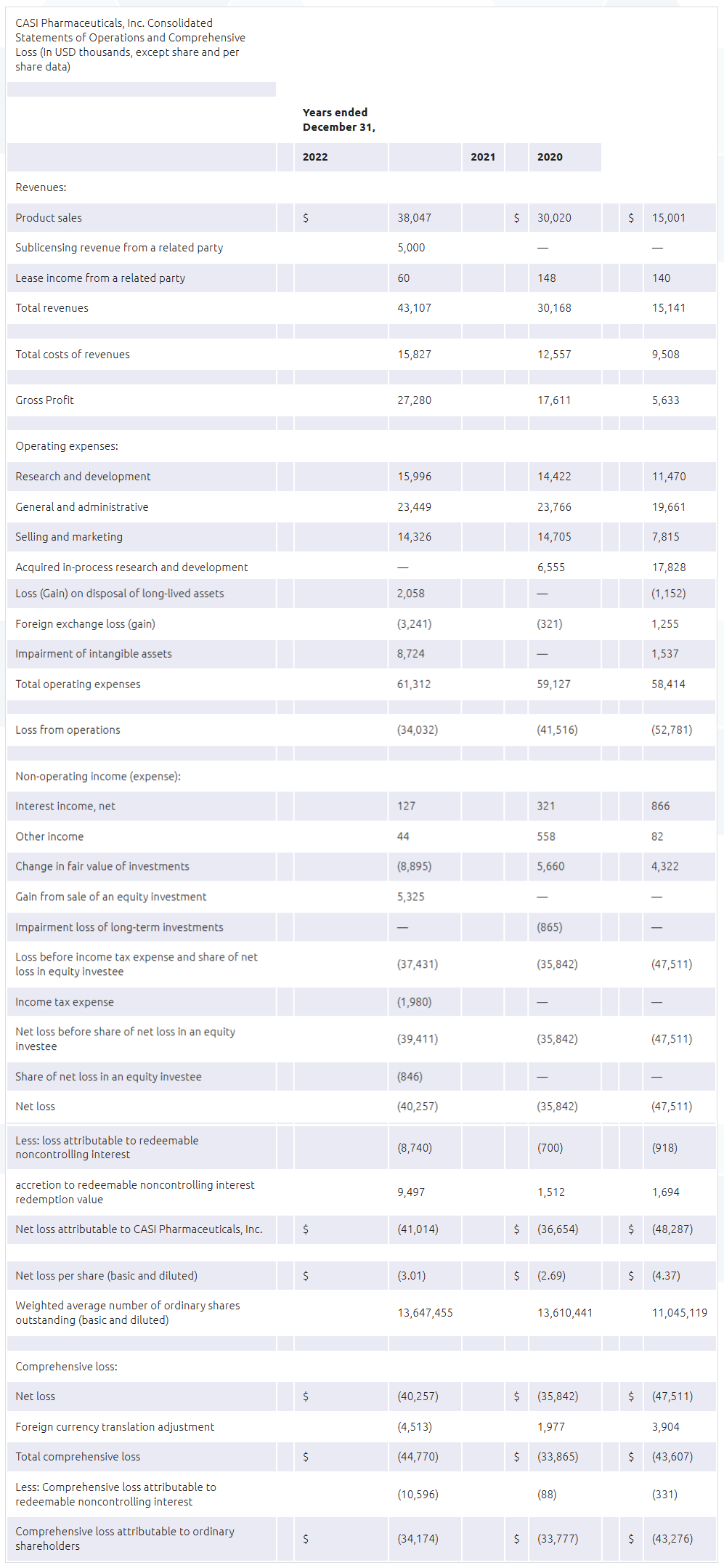
- Revenues mainly consist of product sales of EVOMELA. Total revenue was $43.1 million for the year ended December 31, 2022, including EVOMELA sales of $38.0 million, compared to $30 million for the year ended December 31, 2021.
- Costs of revenues were $15.8 million for the year ended December 31, 2022 compared to $12.6 million for the year ended December 31, 2021.
- General and administrative expenses for the year ended December 31, 2022 were $23.4 million, compared with $23.8 million for the year ended December 31, 2021.
- Selling and marketing expenses for the year ended December 31, 2022, were $14.3 million, compared with $14.7 million for the year ended December 31, 2021.
- Research and development expenses for the year ended December 31, 2022 were $16.0 million, compared with $14.4 million for the year ended December 31, 2021.
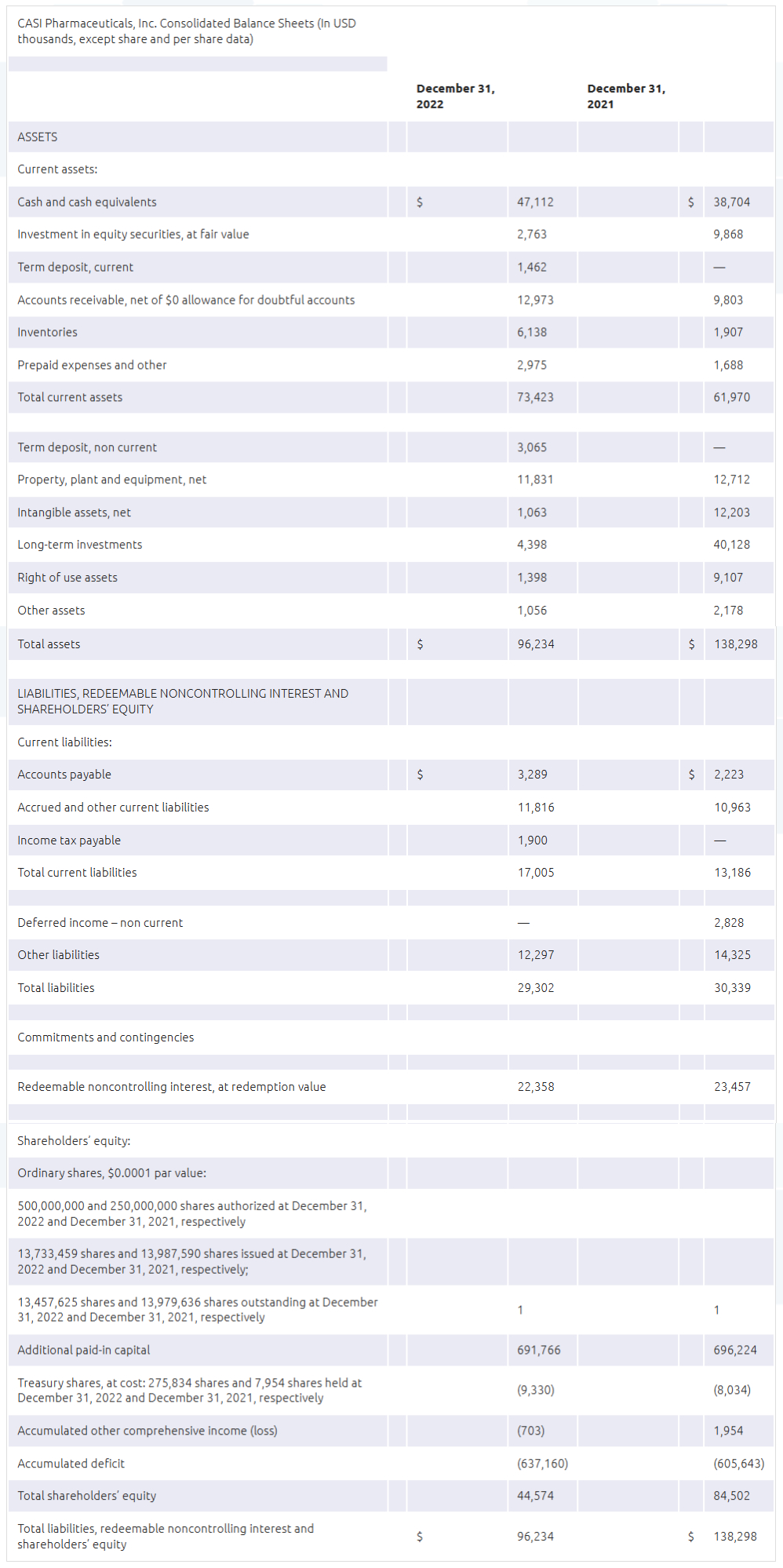
- Net loss for the year ended December 31, 2022 was $40.3 million compared to $35.8 million for the year ended December 31, 2021. As of December 31, 2022, CASI had cash and cash equivalents of $47.1 million compared to $38.7 million as of December 31, 2021.
About CASI Pharmaceuticals
CASI Pharmaceuticals, Inc. is a biopharmaceutical company focused on developing and commercializing innovative therapeutics and pharmaceutical products in China, the United States, and throughout the world. The Company is focused on acquiring, developing, and commercializing products that augment its hematology oncology therapeutic focus as well as other areas of unmet medical need. The Company intends to execute its plan to become a leader by launching medicines in the Greater China market, leveraging the Company’s China-based regulatory and commercial competencies and its global drug development expertise. The Company’s operations in China are conducted through its wholly owned subsidiary, CASI Pharmaceuticals (China) Co., Ltd., located in Beijing, China. More information on CASI is available at www.casipharmaceuticals.com.
Forward-Looking Statements
This announcement contains forward-looking statements. These statements are made under the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as “will,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “confident” and similar statements. Among other things, the business outlook and quotations from management in this announcement, as well as the Company’s strategic and operational plans, contain forward-looking statements. The Company may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the “SEC”), in in its annual report to shareholders, in press releases and other written materials and in oral statements made by its officers, directors or employees to third parties. Statements that are not historical facts, including statements about the Company’s beliefs and expectations, are forward-looking statements. Forward-looking statements involve inherent risks and uncertainties. A number of factors could cause actual results to differ materially from those contained in any forward-looking statement, including but not limited to the following: the risk that we may be unable to continue as a going concern as a result of our inability to raise sufficient capital for our operational needs; the possibility that we may be delisted from trading on The Nasdaq Capital Market if we fail to satisfy applicable continued listing standards; the volatility in the market price of our ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that adversely affect our ability to operate in China, our lack of experience in manufacturing products and uncertainty about our resources and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict when or if our product candidates will be approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency, PRC National Medical Products Administration, or other regulatory authorities; our inability to enter into strategic partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or future candidates; the risks relating to the need for additional capital and the uncertainty of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our other early-stage products under development; the risk that result in preclinical and clinical models are not necessarily indicative of clinical results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials; our ability to protect our intellectual property rights; the lack of success in the clinical development of any of our products; and our dependence on third parties; the risks related to our dependence on Juventas to conduct the clinical development of CNCT19 and to partner with us to co-market CNCT19; risks related to our dependence on Juventas to ensure the patent protection and prosecution for CNCT19; risks relating to the commercialization, if any, of our proposed products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); risks relating to interests of our largest shareholder and our Chairman and CEO that differ from our other shareholders; and risks related to the development of a new manufacturing facility by CASI Wuxi. Further information regarding these and other risks is included in the Company’s filings with the SEC. All information provided herein is as of the date of this announcement, and the Company undertakes no obligation to update any forward-looking statement, except as required under applicable law.
EVOMELA® is proprietary to Acrotech Biopharma LLC and its affiliates.
COMPANY CONTACT:
Rui Zhang
CASI Pharmaceuticals, Inc.
240.864.2643
ir@casipharmaceuticals.com
(Financial Table Follows)
COMPANY CONTACT:
CASI Pharmaceuticals, Inc.
Rui Zhang
240-864-2643
ir@casipharmaceuticals.com

