1. Introduction
Skeletal muscle, comprising the largest tissue in the human body, plays a crucial role in maintaining physical functionality. Impairment of its proper function gives rise to musculoskeletal disorders, substantially diminishing overall quality of life. Moreover, this leads to an upsurge in morbidity and mortality rates, triggering significant socioeconomic repercussions for both families and communities. Consequently, there is an ongoing pursuit to elucidate the intricate molecular underpinnings of this issue and to devise innovative therapeutic approaches that can avert, or even reverse, the process of muscle atrophy [1,2].
MyoD functions as a key myogenic transcription factor in skeletal muscle regeneration [3,4]. In previous studies, primary myogenic cells derived from adult skeletal muscles of MyoD−/− mice were markedly divergent in their differentiation activity [5,6]. Interestingly, the restoration of MyoD expression from these cells was able to promote skeletal muscle differentiation. A series of genes are known to be involved in muscle differentiation: myogenic differentiation (MyoD), myogenin (MyoG), myosin heavy chain (MHC), and myomaker. These results can assess the therapeutic efficacy that affect MyoG and MHC expression by increasing MyoD transcriptional activity [7].
In the context of muscle atrophy, oxidative stress and inflammation stand out as pivotal molecular mechanisms [8,9,10]. Emerging evidence underscores the significance of escalated production of reactive oxygen species (ROS) within skeletal muscles. This phenomenon prominently triggers mitochondrial dysfunction, thereby elevating the presence of muscle atrophy-inducing factors, like the F-box protein Atrogin-1 and the muscle RING finger protein 1 (MuRF1) E3 ligases. Moreover, ROS have been associated with inactivity-induced muscle atrophy, as highlighted by several studies [11,12]. Notably, ROS have also been identified as a critical contributor to denervation-induced muscle atrophy [13]. Concurrently, inflammation emerges as a central pathological factor intricately linked to skeletal muscle dysfunction [14]. Its disruptive influence extends to impairing muscle homeostasis and myogenesis, while concurrently accentuating the activity of E3 ligases, ultimately amplifying the progression of skeletal muscle atrophy [15].
With the age-dependent imbalance between the synthesis and breakdown of muscle protein, accelerated muscle loss occurs and induces sarcopenia, a disease aroused from chronic muscle atrophy [16,17]. Sarcopenia is a relatively prevalent disease among the elderly, however, the gradual progress of muscle deterioration is a life-threatening, risk-provoking systemic dysfunction of skeletal muscle in the entire body [18,19]. When the diaphragm—the skeletal muscle which controls respiration through the continuous expansion and constriction of the muscle—starts to degrade, this malfunction of the lungs eventually gives rise to chronic pulmonary disease, COPD, which is one of the leading causes of death [20,21,22]. Also, sarcopenia-induced muscle wasting and weakness declines the strength of smooth muscle near blood vessels, increasing the possibility of causing hypertension and aortic stenosis directly related to mortality [23,24]. Thus, the early detection and diagnosis of sarcopenia and intensive care are critical for patients to evade related complications and severity increment [25,26]. In particular, drugs targeting muscle atrophy that can regulate muscle protein proliferation and reduction are urgently needed, since there is a scarcity of such FDA-approved drugs.
Herein, we validated the effects of RGX365, a protopanaxatriol-type rare ginsenoside fraction, on sarcopenia-related muscle atrophy. In our previous study, we revealed that RGX365 has various biological activities such as anti-diabetic, anti-septic, and anti-oxidant activity [27,28]. RGX365 also exhibits lung epithelial protection and vascular barrier integrity [29]. Based on its regenerative characteristics throughout systems, we presumed RGX365 might also participate in muscle regeneration and could be related to factors regulating the balance between skeletal muscle protein proliferation and degradation. In summary, we speculate that elevated ROS and activated inflammation may trigger downstream proteolysis pathways in skeletal muscle atrophy. Therefore, oxidative stress and inflammation might be a potential therapeutic target against denervation-induced skeletal muscle atrophy.
2. Materials and Methods
2.1. C2C12 Cell Culture and Differentiation
Mouse C2C12 myoblasts (CRL-1772™) obtained from ATCC were placed in cell culture dishes and nurtured in a growth medium, composed of DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (P/S), at 37 °C in an incubator containing 5% CO2. The differentiation protocol involved seeding 1 × 105 C2C12 myoblasts per well in 6-well plates, allowing them to adhere and grow for 24 h, followed by a transition to a differentiation medium with 2% horse serum and 1% penicillin/streptomycin. The medium was refreshed every alternate day for three days, during which we observed and recorded the morphological changes that signified successful myoblast differentiation into myotubes.
During the assessment of myogenesis activity, the experimental procedure involved the administration of RGX365 in conjunction with the differentiation medium. This was carried out over a period of three days, during which the culture medium containing the RGX365 was refreshed every alternate day. The differentiation medium, composed of DMEM supplemented with 2% horse serum and 1% penicillin/streptomycin (P/S), was refreshed daily to maintain optimal conditions for myoblast differentiation.
For the evaluation of the efficacy in reducing skeletal muscle atrophy, a distinct approach was taken. Following the successful differentiation of myoblasts into myotubes, an atrophy-inducing phase was initiated by exposing the myotubes to Dexamethasone, a known inducer of atrophy. Subsequently, the myotubes were subjected to treatment with a medium containing each compound. This medium was formulated to include RGX365 along with the necessary components to support myotube maintenance and growth.
2.2. Cell Viability Measurements
C2C12 cells were seeded at a density of 1 × 105 cells per well in a 96-well cell culture dish, and allowed to grow in proliferation media for 24 h. Extract powder of RGX365 was dissolved in DMSO with minimum volume providing complete solubility, and the solution was diluted with saline to varying concentrations (1.25, 2.5, 5, 10, 20 µg/mL). Subsequently, the cells were exposed to RGX365 for an additional 24 h. Following this treatment period, the culture medium was promptly replaced with fresh medium containing RGX365 every day. Next, the WST-1 assay kit obtained from Dojindo (Kumamoto, Japan) was introduced to the medium in the appropriate proportions. After a 40 min incubation, a microplate reader measured the optical density (OD) of the medium at a wavelength of 450 nm. This experiment was replicated six times, with each iteration consisting of three wells for replication.
2.3. MyHC Staining
At ambient room temperature, C2C12 myotube cells were fixed using a solution of 4% formaldehyde for a duration of 30 min. Following fixation, the cells underwent a triple wash with PBS supplemented with 0.1% Triton X-100. Subsequently, the cells were subjected to blocking using goat serum for a period of 1 h. Following the blocking step, the cells were exposed to mouse anti-MyHC (sc-32732, Santa Cruz Biotechnology, Dallas, TX, USA) at a temperature of 4 °C overnight. After a thorough washing process involving PBS for three cycles, the cells were incubated with a secondary antibody linked to Alexa Fluor 488. This incubation was carried out at a temperature of 37 °C for a duration of 1 h. Finally, the cells underwent a threefold wash utilizing PBS supplemented with 0.1% Triton X-100 once again. Subsequently, the myotubes were visualized and captured using a fluorescence microscope manufactured by Leica Microsystems (Mannheim, Germany).
2.4. Measurement of Myotube Diameter
The myotubes were imaged using a fluorescence microscope, and the diameter of each myotube was assessed using the Image J software (Image J 1.48V—Java 1.6.0_20(32-bit), National Institute of Health, Bethesda, MD, USA). The average diameter of the myotubes was determined by measuring the maximum diameter of individual myotubes. For every sample, ten random culture areas were chosen, and at least 100 myotubes were quantified for analysis.
2.5. Fluorescence Intensity of Cellular ROS
The Cellular ROS Assay Kit (ab113851) from Abcam (Cambridge, UK) was used to detect generated ROS in the myotubes. Dexamethasone-induced C2C12 myotube cells were treated with a solution of RGX365 and its components. The cells were washed with the buffer and stained with the DCFDA reagent diluted with DMEM medium for 45 min at 37 °C in an incubator containing 5% CO2. Images of the myotubes were taken after 24 h via fluorescence microscopy.
2.6. Western Blotting
The expressed levels of p-AKT, AKT, p-mTOR, and mTOR were evaluated in C2C12 myotubes to confirm upregulation of the Akt/mTOR pathway induced by RGX365 and its components, which indicates muscle hypertrophy. The expression of PGC-1α, NRF1, and Tfam was also investigated through immunoblotting to verify the efficacy of RGX365 and its components on muscle repair. Expressed proteins in C2C12 myotube cell lysate were quantified and loaded on the acrylamide gel. After gel electrophoresis, immunoblotting was conducted with antibodies of p-AKT (Cell signaling technology, Danvers, MA, USA, cat no #4060, dilution 1:1000), AKT (Cell signaling technology, Danvers, MA, USA, cat no #9272, dilution 1:1000), p-mTOR (Cell signaling technology, Danvers, MA, USA, cat no #5536, dilution 1:1000), and mTOR (Cell signaling technology, Danvers, MA, USA, cat no #2983, dilution 1:1000). PGC1-α ((Cell signaling technology, Danvers, MA, USA, cat no #2178, dilution 1:500), NRF1 (Cell signaling technology, Danvers, MA, USA, cat no #69432, dilution 1:1000), and Tfam (Cell signaling technology, Danvers, MA, USA, cat no #8076, dilution 1:1000) antibodies were also used to validate protein expression. α-Tublin was detected using α-Tublin antibody (1:10,000, Santa Cruz Biotechnology, Inc., sc-5286) and quantified as a reference protein.
2.7. Animal Experiment
Male C57BL/6 mice at two different age groups, specifically 2 months old and 23 months old, were procured from Orient Bio (Seongnam, Republic of Korea). These mice were accommodated in polycarbonate cages, situated in a controlled environment set at temperatures ranging from 20 to 25 °C with humidity levels of 40 to 45%. The lighting schedule was maintained on a 12 h light and 12 h dark cycle. The mice were provided with a standard rodent pellet diet and unrestricted access to water. Within each cage, a group of five animals was housed, following a period of 12 days for acclimatization. All procedures involving animals were conducted in compliance with the guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) of Sungkyunkwan University.
The old mouse group (23-month-olds) was additionally fed RGX365 and each component once a day for 30 days. 20 µg of the powdered RGX365 and each compound was completely dissolved in DMSO and diluted with 100 µL of saline. The solution was administered through oral gavage using a ball tip needle. Ginsenoside RGX365 was purchased from AREZ Co., Ltd. (Daejeon, Republic of Korea).
After 30 days of oral administration, grip strength was measured using the Grip Strength Test (Bioseb) before sacrifice. Grip strength was measured by standard protocols; pulling the tail of the mouse and hanging it onto the apparatus until it releases the grasping tool. After five measurements per mouse, the mean of each peak force was divided by body weight.
2.8. Histological Staining
After euthanasia, the tibialis anterior (TA) muscle of the hindlimb was exposed. The fascia was carefully peeled off with forceps and the TA muscle was separated. The TA muscle tissue was then embedded in OCT compound (Sakura Finetek Japan Co., Tokyo, Japan) and snap-frozen in liquid nitrogen. Samples were cross sectioned in 10 µm thickness at the mid belly region, placed on the slide, and fixed with 4% paraformaldehyde (PFA) solution. The slide was stained with hematoxylin (Sigma, St. Louis, MO, USA) and then immediately washed with distilled water. Eosin (Sigma, St. Louis, MO, USA) was used for counterstaining and the slide was dipped in ethanol with increasing concentration and xylene. After covering the slide with cover-glass, the slide was analyzed with an optical microscopy.
2.9. Immunohistochemistry and Morphometric Analysis
Other snap-frozen TA muscle sample slides (10 µm thickness) were used for immunohistochemistry analysis. The slides were fixed in 4% PFA or acetone and blocked in 2% bovine serum albumin (BSA) in PBS for 1 h following overnight incubation with mouse anti-MyHC (sc-32732, Santa Cruz Biotechnology, Dallas, TX, USA). After PBS washing, the slides were incubated with goat anti-mouse Alexa Fluor 488 secondary antibody for 1 h at room temperature. Then the slides were washed three times in PBS, mounted with fluorescence medium (Vector Laboratories, Burlingame, CA, USA), and analyzed using a fluorescence microscope (Leica microsystem, Mannheim, Germany).
Five non-overlapping images of each muscle cross-section were taken, and the minimum mouse fiber diameter or cross-sectional area (CSA) was determined by NIS Elements AR 3.2 software (Nikon, Melville, NY, USA) or Image J software. The minimum mouse diameter was defined as the minimum distance between the opposite boundary of the muscle fiber and parallel tangents, and it was found to be very insensitive to deviation from the optimal cross-sectional profile 1. The data was calculated as a percentage distribution. The average of the TA muscle diameter or fiber CSA threshold for the total 2000–3500 muscle fibers was preset in the muscle section using NIS Elements software, and remained constant throughout the quantification of all muscles.
2.10. Quantitative RT-PCR
C2C12 or TA muscle tissues were lysed and total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). Using reverse transcription, cDNA was produced by RNA with oligo dT primer and M-MLV reverse transcriptase (Enzynomics, Daejeon, Republic of Korea). cDNA was mixed with TOPreal™ qPCR 2X PreMIX (SYBR Green with high ROX, Enzynomics, Daejeon, Republic of Korea) and primers specific to target genes. qRT-PCR was performed using SYBR green on the ABI 7500 system. mRNA levels of atrogin-1, MuRF1, MyoD, MyoG, MyHC 1, and 36B4 as the reference gene were analyzed .
2.11. Blood Biochemistry
Collected murine blood samples from the sarcopenia mouse model treated with RGX365 were analyzed using the Fuji DRI-CHEM NX500i (Fujifilm Corp., Tokyo, Japan) at the Chiral Material Core Facility Center of Sungkyunkwan University. Serum levels of blood urea nitrogen (BUN), lactate dehydrogenase (LDH), creatinine, aminotransferase (AST), alanine aminotransferase (ALT), and albumin (ALB) were examined.
2.12. Cytokine ELISA
IL-1β ELISA kit (88-7013A-88), IL-6 ELISA kit (KMC0061), and TNF-α ELISA kit (BMS607-3) from Invitrogen (Waltham, MA, USA) were used to determine cytokine levels in sarcopenia-induced murine serum. A 96-well microplate was pre-coated with the biotin-conjugate anti-mouse antibody and incubated for 2 h on a microplate shaker at room temperature. Coated wells were washed with washing buffer three times to remove unbound antibodies, loaded with the serum samples, and then incubated at 4 °C overnight. Following the incubation, the plate was washed with the washing buffer three times and each well was loaded with 50 µL of Streptavin-HRP solution. After 2 h of incubation at room temperature, washing buffer was added and the wells were washed 3 times. Substrate solution was added to the wells and the absorbance was measured at 450 nm with the TECAN microplate reader when the reaction ended.
2.13. Statistical Analysis
Each experiment was conducted with a minimum of three independent repetitions. Results are presented as means, along with their corresponding standard deviations (SD). The statistical analysis was executed using SPSS for Windows, version 16.0 (SPSS, Chicago, IL, USA). To assess the significance of differences among the test groups, a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was applied. Statistical significance was established for p-values below 0.05.
3. Results
3.1. RGX365 Increases Cell Viability and Induces High MyHC Expression in Muscle Cells
First of all, of the C2C12 cells, we validated that the treatment with RGX365 showed a noticeable increment of cell viability compared to the control group, which did not show any significant difference among treated doses (1.25 µg/mL, 2.5 µg/mL, 5 µg/mL, 10 µg/mL and 20 µg/mL) (Figure 1A). To further investigate whether increased cell viability was derived from the myogenesis effects of RGX365, we conducted MyHC staining of C2C12 cells treated with or without RGX365, and quantified the diameter of myotubes and MyHC expression following an experimental scheme (Figure 1B). The results showed that the treatment with RGX365 caused a considerable difference in myotube diameter, revealing that the sizes of myotubes whose diameter is over 20 µm are remarkably increased as the dose of RGX rises (Figure 1C,D). Furthermore, the transcription level of MyHC also increased following dose elevation, upward to an over twofold increase with C2C12 treated using 10 µg/mL of RGX365 (Figure 1E). Overall, the thickening of myotubes treated with RGX365 was induced by augmenting the magnitude of the MyHC expression, gradually increasing per se concentration.
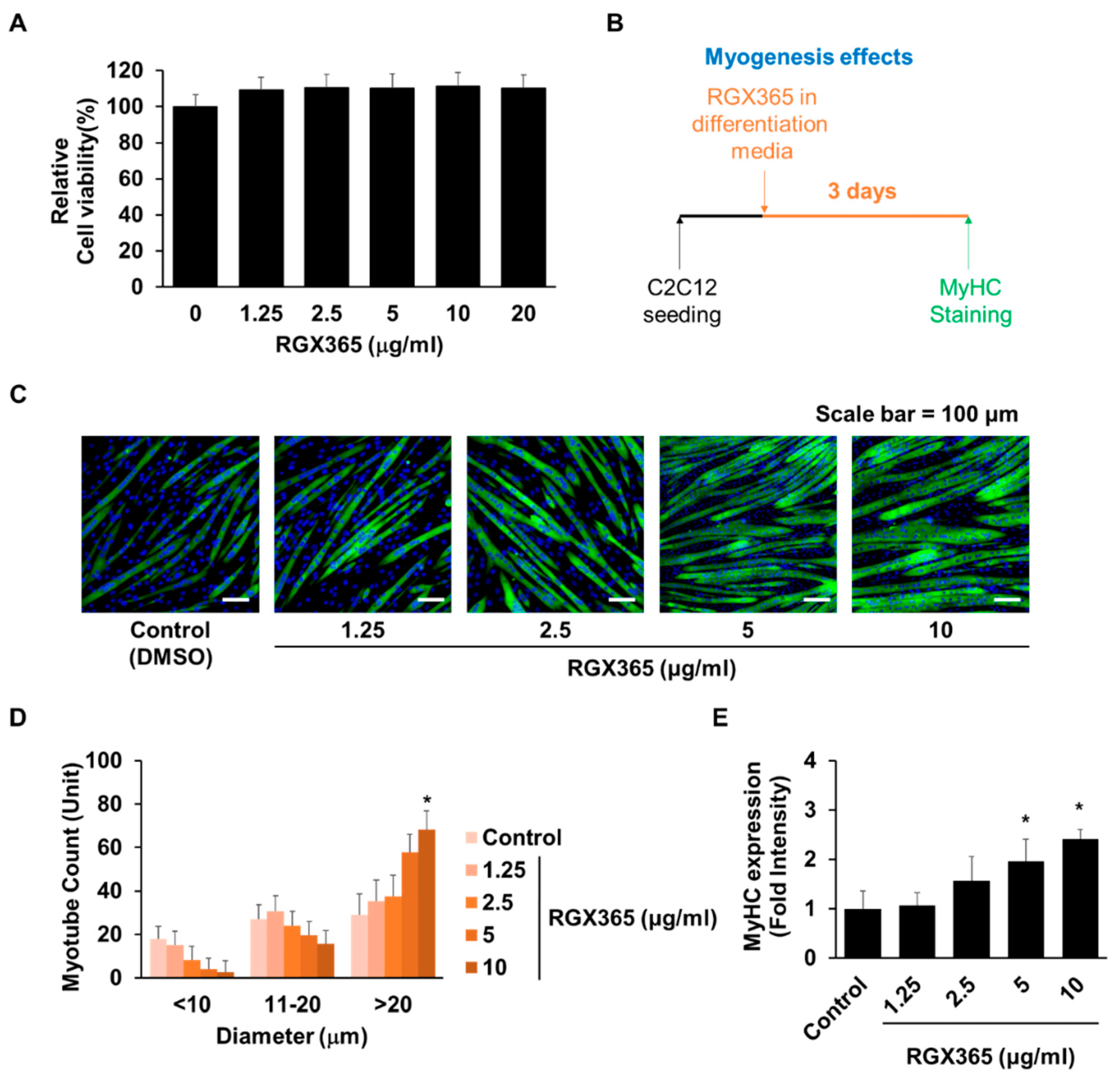
Figure 1. Myogenic effects of RGX365. (A) Cell viability of C2C12 cells in different concentrations of RGX365. * p < 0.05 vs. non-treated group. (p value was obtained by Student’s t test). (B) Experimental scheme for RGX365 treatment. (C,D) MyHC staining for morphological observation (scale bar = 100 µm) and quantification of myotubes. (E) Quantification of MyHC fluorescence intensity. * p < 0.05 vs. the control group. (One-way ANOVA with Tukey’s correction). Each experiment was triplicated, after which the results were recorded and calculated.
3.2. RGX365 Increases Cell Viability and Induces High MyHC Expression in Skeletal Muscle Cells
As previously proposed, RGX365 is a chemical compound composed of five constituents: Rg2, Rg4, Rg6, Rh1, and Rh4 (Figure 2A) [29]. Since we observed increased cell viability upon RGX365 treatment in vitro, we next examined the effect of each component with the efficacy of RGX365. Distinct from others, Rg6 and Rh4 remarkably elevated cell survival among the five composites (Figure 2B). Through MyHC staining of C2C12, we also found that myotubes of C2C12 treated with 10 µg/mL of Rg6 and Rh4 are thicker than those treated with other components, showing substantial amounts of myotubes whose diameter is larger than 20 µm, in comparison to the others (Figure 2C,D). Transcripts of MyHC also upregulated with the treatment of Rg6 and Rh4, indicating that though RGX365 showed the most efficacy, Rg6 and Rh4 are necessary and closely related to the function of RGX365 (Figure 2E).
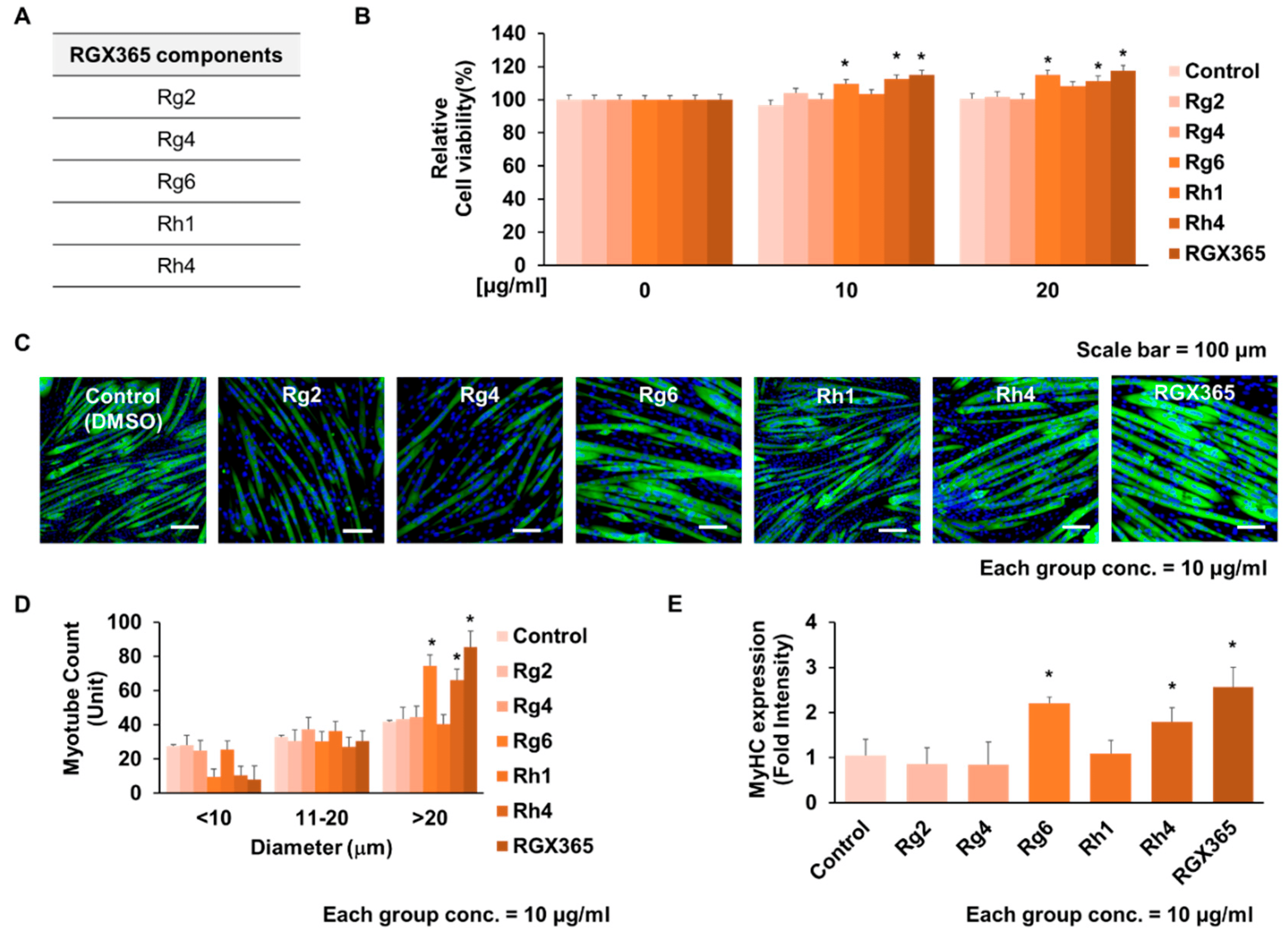
Figure 2. Effect of RGX365 constituents on activating C2C12 cell differentiation. (A) Component list of RGX365. (B) Cell viability of C2C12 cells in different concentrations of RGX365 components (ginsenoside Rg2, Rg4, Rg6, Rh1, and Rh4). (C,D) MyHC staining for morphological observation (scale bar = 100 µm) and quantification of myotubes. (E) Quantification of MyHC fluorescence intensity. * p < 0.05 vs. the control group. (One-way ANOVA with Tukey’s correction). Each experiment was triplicated, after which the results were recorded and calculated.
3.3. RGX365 Gives Therapeutic Effects to Muscle Atrophy with Increased MyHC Expression
By causing dexamethasone-induced skeletal muscle atrophy on C2C12, the muscle recovery effects of RGX365 and each of its components were assessed following the experimental scheme (Figure 3A). As a result, muscle thickening with enlarged myotube diameters was observed during morphological analysis of the RGX365-treated group (Figure 3B), while narrow myotubes with a diameter smaller than 10 µm were frequently detected in dexamethasone-treated myotubes (Figure 3C). This indicates efficacy of RGX365 on the regeneration of atrophic muscle and the protection of skeletal muscle against dexamethasone. Consistent with the previous results, Rg6 and Rg4 in particular showed better therapeutic effects than the other composites regarding skeletal muscle atrophy (Figure 3B,C). We next validated whether the recovery of myotubes originated from upregulated MyHC expression induced by RGX365. There was a clear elevation in the MyHC expression level induced by RGX365, and Rg6 and Rh4 showed a clear difference in MyHC expression levels compared to the other single compound treated groups, which is increased to certain amounts (Figure 3D). Oxidative stress and chronic inflammation are key contributors to the progression of sarcopenia [30]. Ginsenosides possess antioxidative properties that may counteract the detrimental effects of reactive oxygen species, thereby preserving muscle function and reducing the rate of muscle loss [31]. We estimated that treatment with RGX365, which is also a kind of ginsenoside fraction, would alleviate ROS generation and subsequently reduce skeletal muscle wasting. In this respect, the fluorescence intensity of ROS generated in the C2C12 myotube was evaluated. The increased ROS generation derived from Dexamethasone-induced skeletal muscle atrophy showed significant decline when treated with RGX365 (Figure 3E). Expression and post translational modification, especially the phosphorylation of proteins regulating the damage and repair of skeletal muscle, were examined to determine the therapeutic effects of RGX365 and its components on skeletal muscle atrophy. The Akt/mTOR signaling pathway is well documented as a critical skeletal muscle regulator, particularly of muscle weight loss prevention and size increment. Further characterized, the phosphorylation of Akt and mTOR indicates their activation, and this represents the hypertrophy of myotubes [32]. Given that, we investigated the expression and relative ratio of Akt, p-Akt, mTOR, and p-mTOR with western blot analysis. Compared to the Dexamethasone-treated group, myotubes treated with RGX365 or its components showed a notable increment of phosphorylated Akt and mTOR, while the level of non-phosphorylated forms remained analogous (Figure 3F–H). We next confirmed other key factors regulating skeletal muscle protection and regeneration, PGC-1α, NRF1, and Tfam. Phosphorylation of the transcriptional coactivator PGC-1α interacts with transcriptional factors including NRF-1, and NRF-1 upregulates the translation of Tfam. In cytosol, Tfam is imported into mitochondria and promotes mitochondrial biogenesis [33,34]. With dexamethasone, expression of PGC-1α, NRF1, and Tfam decreased against the control group. However, RGX365 rescued the expression of PGC-1α, NRF1, and Tfam, causing an even larger increase than in the control group, which indicates the skeletal muscle hypertrophy occurring after muscle damage. We also validated that components of RGX365 upregulate PGC-1α signaling pathway-mediated muscle regeneration to a certain extent (Figure 3G,H). This indicates that the administration of RGX365 downregulates the breakdown of myotubes by ameliorating ROS production. After all, with regard to muscle regeneration along with muscle protection, RGX365 has therapeutic effects to stimulate MyHC expression, and Rg6 and Rg4 account for a big part of it.
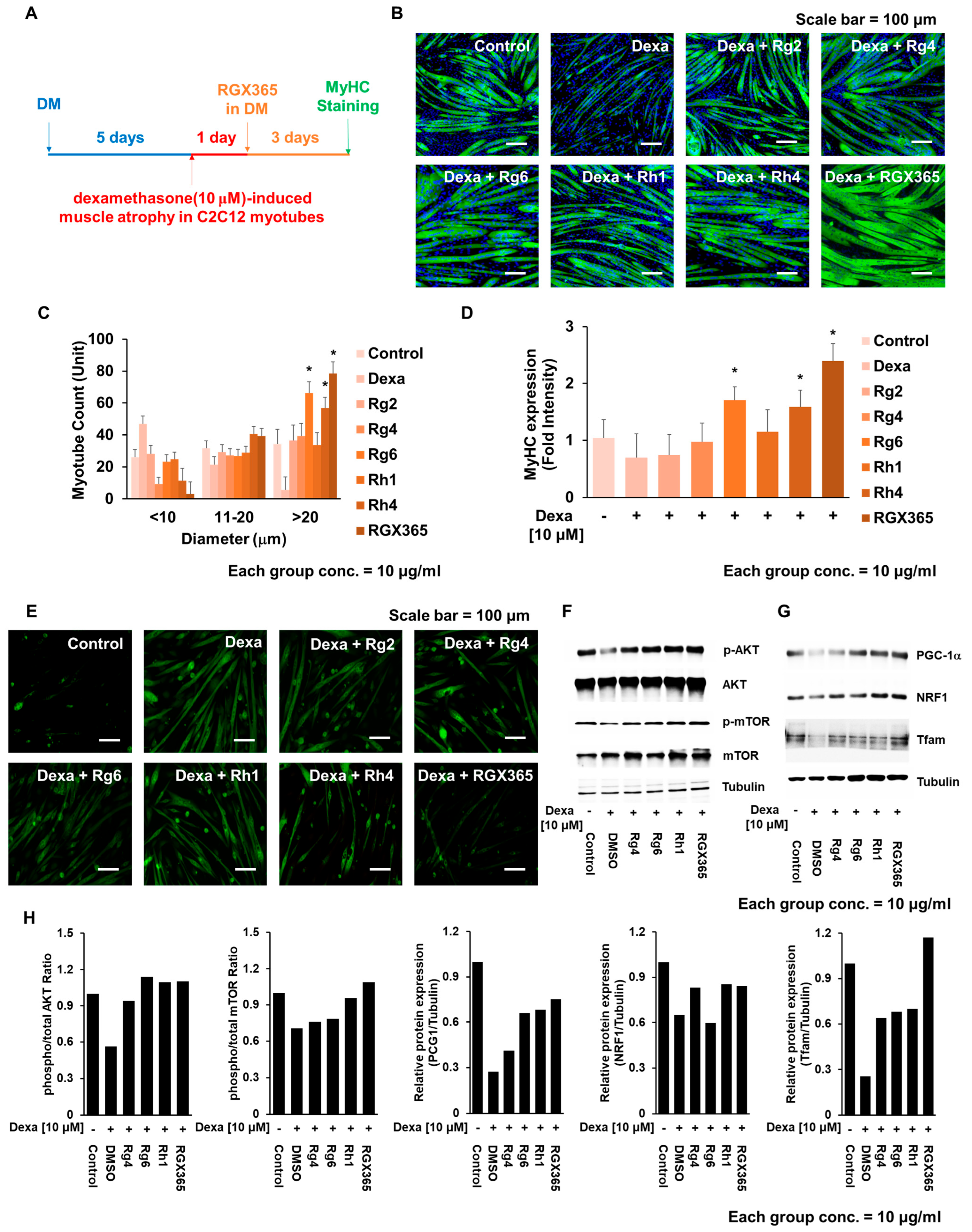
Figure 3. RGX365 protects C2C12 myotubes against dexamethasone-induced atrophy. (A) Experimental scheme for evaluating the effects of Dex on C2C12 myotubes with or without RGX365 treatment. (B,C) MyHC staining for morphological observation (scale bar = 50 µm) and quantification. Control = PBS, DEX = dexamethasone (10 µM) treatment group, DEX + each compound = dexamethasone (10 µM) combined with each molecule (10 µM) treatment group. (D) Quantification of MyHC fluorescence intensity. (E) Fluorescence imaging of ROS in dexamethasone-induced skeletal muscle atrophy with or without treatment with RGX365 or its components. (F) Western blot analysis of C2C12 myotube cell lysates on Akt/mTOR signaling pathway (G) Western blot analysis of C2C12 myotube cell lysates on mitochondria-mediated muscle repair. (H) Graphs represent the ratio between the phosphorylated protein ratio and the total amount of the targeted protein. Image Lab software 5.0 (Biorad Laboratories) was used to quantify the immunoreactive band density. Each experiment was triplicated, after which the results were recorded and calculated. * p < 0.05 vs. the dexamethasone treated group.
3.4. RGX365 Protects Muscle against Age-Dependent Atrophy and Regulates Related Gene Expression
To analyze the therapeutic effects of RGX365 on skeletal muscle regeneration in depth, we determined whether treatment with RGX365 and its components rescue muscle weight decline upon the loss of skeletal muscle due to age-dependent muscle atrophy in old mice. We orally administered RGX365 to 23-month-old mice daily for 30 days and sectioned skeletal muscle tissue to observe the density of the muscle. In consequence, mice treated with RGX365 recovered a high amount of muscle density, close to that of 2-month-old young mice. Within single component groups, mice treated with Rg6 and Rh4 showed a significant increase in muscle density (Figure 4A). Specifically, the increased area of cross-sectioned muscle and total muscle weight increment in the Rg6, Rh4, and RGX365-treated old mice group indicates high muscle differentiation induced by their efficacy (Figure 4B,C). Interestingly, we found that there was an outstanding augment of grip strength when RGX365 was administrated to old mice, and their grip strength was even greater than that of young mice (Figure 4D). We investigated this further at the transcriptional stage by analyzing mRNA expression. qRT-PCR was performed using 36B4 as the housekeeping gene. The primer sequences used for PCR are shown in . The mRNA expression of Atrogin-1 and MURF-1 was remarkably decreased in RGX365-treated old mice compared to normal old mice (Figure 4E). The age-dependent reduction in mRNA expression of MyoD and MyoG, which are transcription factors that regulate muscle development, differentiation, and repair, was rescued by RGX365, showing notable increment (Figure 4F). We concluded that RGX365 affects muscle regeneration-related mRNA expression, which is manifested by the enhancement of muscle quality and function.
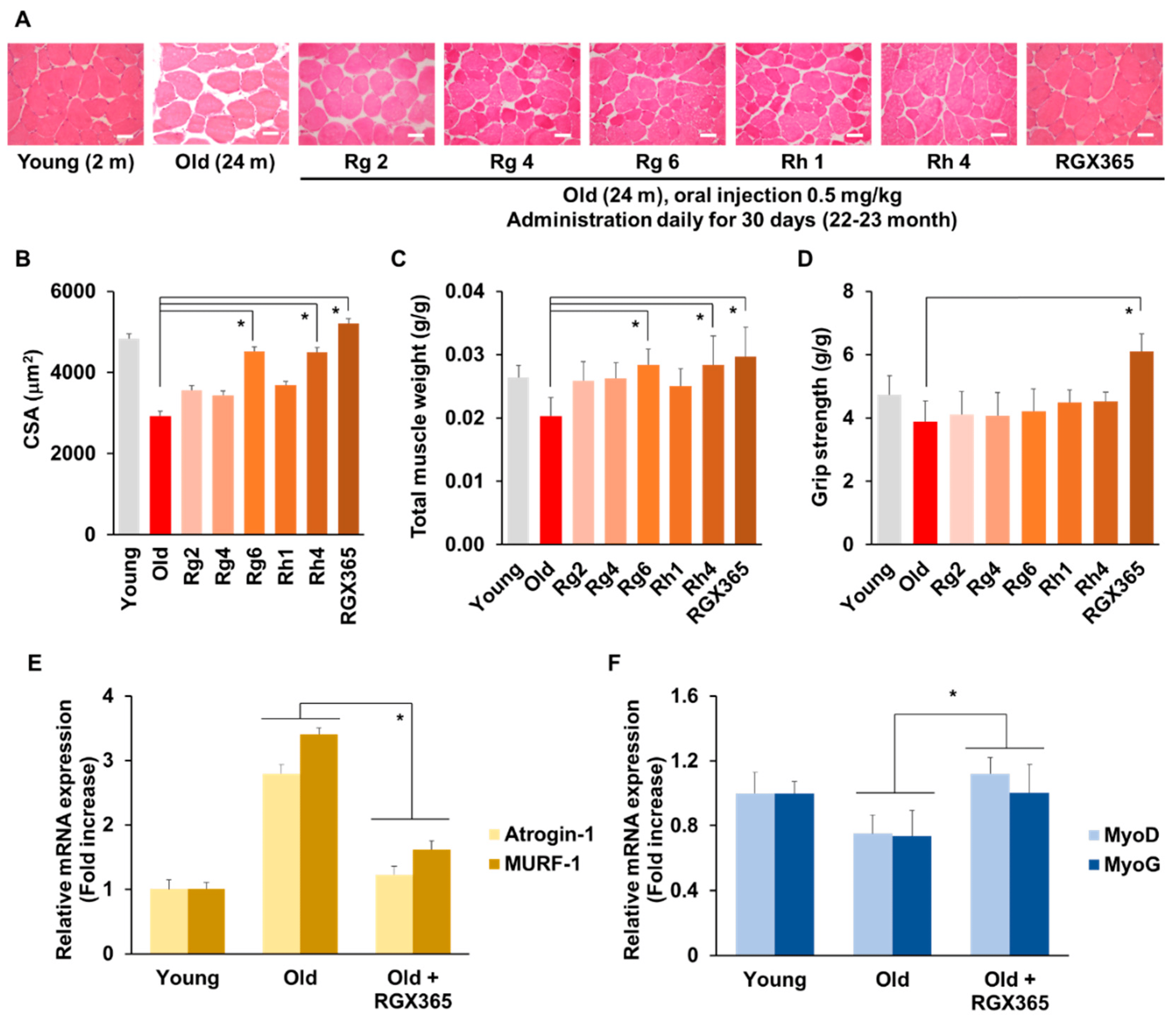
Figure 4. RGX365 enhances protective effect on senile sarcopenia. (A) Representative photomicrograph of H&E histological staining on TA muscle from PBS and ginsenosides Rg2, Rg4, Rg6, Rh1, Rh4, and RGX365. Scale bar: 75 µm. (B,C) Average values of the myofiber CSA (B) and TA wet weight (C) were measured from isolated muscles of each sample (n = 5). (D) Comparison of strength tests. The measurement values of the young (12-week-old) and old (2-year-old) mice with or without ginsenoside Rg2, Rg4, Rg6, Rh1, Rh4, and RGX365 treatment for 30 days. (E) Fold increase analysis of Atrogin-1 and MURF-1 mRNA expression. (F) Fold increase analysis of MyoD and MyoG mRNA expression. * p < 0.05 (One-way ANOVA with Tukey’s correction). Each experiment was triplicated, after which the results were recorded and calculated.
3.5. RGX365 Ameliorates Reactive Oxygen Species (ROS)-Mediated Progression of Sarcopenia
We validated ROS-induced tissue damage and its recovery after RGX365 administration through the quantification of tissue damage markers in the serum of the RGX365-treated sarcopenia mouse model. Blood urea nitrogen (BUN), lactate dehydrogenase (LDH), and creatinine, which are well documented as kidney damage markers, showed noticeable alleviation after treatment with RGX365 (Figure 5A). A relevant decrease of liver damage markers such as aminotransferase (AST), alanine aminotransferase (ALT), and albumin (ALB) was also indicated (Figure 5B). There was general decline in the production of proinflammatory cytokines IL-1β and TNF-α, though IL-6 did not show any significant difference (Figure 5C).
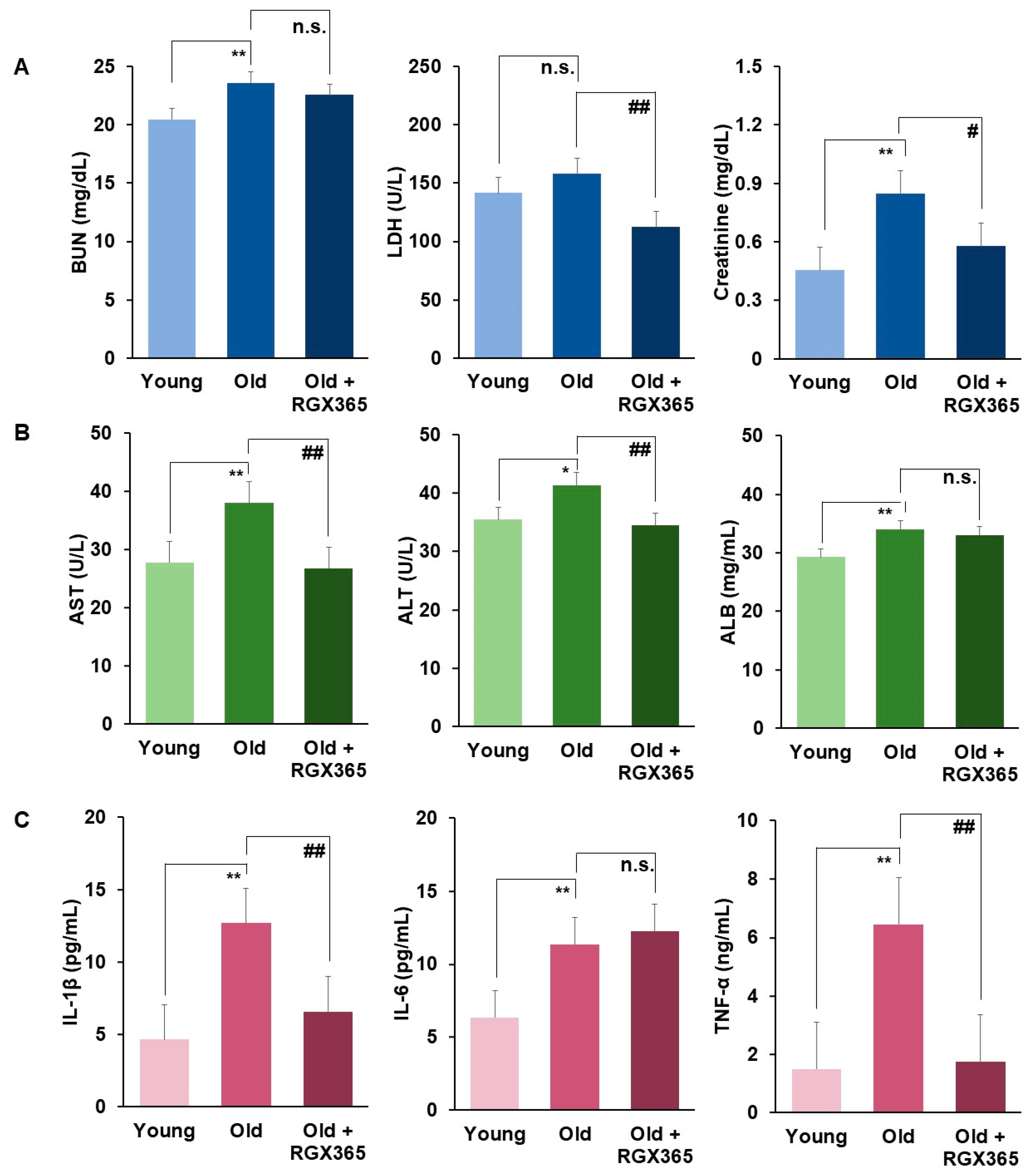
Figure 5. RGX365 alleviates tissue damage induced by ROS reduction. (A,B) Blood Chemistry analysis of sarcopenia mouse model. (A) Blood urea nitrogen (BUN), lactate dehydrogenase (LDH), and creatinine. (B) Aminotransferase (AST), alanine aminotransferase (ALT), and albumin (ALB). (C) Expression of proinflammatory cytokine (IL-1β, IL-6, and TNF-α) in sarcopenia mouse model. Data are mean ± SEM calculated for each group. * p < 0.05, ** p < 0.01 vs. the young group. # p < 0.05, ## p < 0.01 vs. the old group. p = n.s. (non-significant) vs. old group (One-way ANOVA with Tukey’s correction.) Each experiment was triplicated, after which the results were recorded and calculated.
4. Discussion
Age-related sarcopenia, the progressive loss of skeletal muscle mass and function that occurs with aging, has garnered increasing attention due to its escalating risk and prevalence in contemporary society [17]. As the global population continues to age, the implications of sarcopenia are becoming more pronounced, posing significant challenges to public health, quality of life, and healthcare systems [35]. The rise in the prevalence of age-related sarcopenia can be attributed to a multitude of factors [36]. Firstly, the aging population itself contributes to this phenomenon. Advancements in healthcare and improved living conditions have led to increased life expectancies, resulting in a larger elderly demographic susceptible to age-related physiological changes. Nutritional factors also play a pivotal role. Modern dietary habits, characterized by high-calorie, low-nutrient diets, often lack the essential nutrients required to maintain muscle mass and strength [37]. This imbalance can accelerate the loss of muscle tissue and hinder the body’s ability to regenerate muscle fibers [38].
The pursuit of effective interventions to ameliorate senile sarcopenia has gained momentum, with various approaches being explored, including the potential benefits of incorporating natural compounds like ginseng into dietary strategies [39]. Ginseng, a widely used herbal remedy derived from the root of Panax species, has garnered attention for its potential to combat age-related health challenges [40]. Ginseng’s anti-inflammatory effects could potentially mitigate the chronic low-grade inflammation often observed in sarcopenic patients, thereby promoting muscle regeneration and overall muscle health [41]. Furthermore, ginseng’s influence on metabolism is noteworthy. It has been suggested that ginsenosides might enhance energy metabolism, improve glucose regulation, and support mitochondrial function [42]. As impaired energy metabolism is implicated in the development of sarcopenia, ginseng’s potential to modulate these pathways could have positive implications for muscle preservation and strength maintenance.
Black ginseng (BG), which undergoes a unique process of being steamed and dried nine times, exhibits diverse pharmacological properties encompassing anti-diabetic, wound healing, immunostimulatory, antioxidant, anti-inflammatory, and antiseptic effects [43]. This includes the presence of protopanaxadiol (PPD) and protopanaxatriol (PPT) ginsenoside types. Notably, rare ginsenosides such as Rb1, Rc, Rd (major PPD-type), and Re, Rf, Rg1 (major PPT-type) are found in BG, characterized by their high polarity due to multiple high-molecular-weight sugars present within their structures [44]. In BG, the principal PPT-type ginsenosides comprise rare ginsenosides including Rg2, Rg4, Rg6, Rh1, and Rh4. Recently, these specific rare ginsenosides—Rg2, Rg4, Rg6, Rh1, and Rh4—have been noted for their potent anti-tumor, anti-inflammatory, and immunomodulatory effects [29,45,46]. However, owing to their scarcity and high cost, the utilization of these efficacious rare ginsenosides, particularly Rg2, Rg4, Rg6, Rh1, and Rh4 present in BG, has necessitated the development of a large-scale separation and purification technique for their potential application as therapeutic agents. In our prior research endeavors, we successfully devised an approach to obtain substantial quantities of PPT-type rare ginsenosides, a method that has been named RGX365. This innovative technique addresses the challenge of securing substantial amounts of these valuable compounds and holds significant potential for advancing their utilization in therapeutic applications.
Here, we determined the effects of a ginsenoside-derived molecule, RGX365, on skeletal muscle cell malfunction caused by dexamethasone-induced muscle atrophy. We found that treatment with RGX365 increased the cell viability and myogenic effects of C2C12 and induced higher expression of MyHC. In particular, two specific components of RGX365—Rg6 and Rh4—showed significant efficacy compared to the others. We also investigated whether RGX365 also improved muscle regeneration in old mice whose skeletal muscle atrophy was already thoroughly in progress. It turned out that RGX365 indubitably rescued skeletal muscle weight and volume, and increased muscle proliferation in old mice. Remarkably, regarding skeletal muscle function, the grip strength of the group of old mice treated with RGX365 was dramatically increased. These enhancements in skeletal muscle function were regulated by the expression of related markers (Atrogin-1, MuRF-1, MyoD, and MyoG), which was verified by the decrease or increase of mRNA expression. Additionally, in vivo treatment with RGX365 inhibits ROS scavenging and severe inflammatory responses, revealing its effects in modulating NETosis in infectious diseases such as COVID-19 [28]. Based on validation in previous studies, we hypothesized that the mechanism of action of RGX365 would also have beneficial effects on senile sarcopenia.
Our study revealed that administering RGX365 to senile sarcopenia mice for a duration of 30 days yielded favorable outcomes. The application of RGX365 exhibited positive effects, comparable to those seen in countering myogenic impacts and inhibiting muscular proteolytic enzymes. The effective utilization of RGX365 hinges upon four fundamental physiological aspects—absorption, distribution, metabolism, and excretion (ADME)—which collectively influence the compound’s disposition within an organism and its therapeutic efficacy.
These physiological and pharmacokinetic factors collectively influence the levels of RGX365 in the body, consequently affecting its performance and pharmacological activity. To reach skeletal muscles, RGX365 typically necessitates absorption into the bloodstream, followed by transportation to target cells. Elements like solubility, gastric emptying time, intestinal transit time, chemical stability in the stomach, and the ability to permeate the intestinal wall all contribute to the extent of RGX365 absorption post oral administration. The degree of absorption plays a pivotal role in determining RGX365’s bioavailability. Moreover, the role of reactive oxygen species (ROS) in inducing skeletal muscle atrophy is well established. Drawing from our current findings, RGX365 holds the potential to deliver advantageous effects within the skeletal muscle system.
5. Conclusions
To sum up, our study introduces a new perspective by unveiling the potential of rare ginsenoside RGX365 in addressing the complexities of sarcopenia. This marks a significant advancement as we, for the first time, shed light on RGX365’s ability to counteract oxidative stress and temper inflammatory responses. These actions orchestrate a cascade effect that not only curtails muscle proteolysis, but also serves as a robust defense mechanism against the progression of skeletal muscle atrophy. This study not only highlights RGX365’s promise as a therapeutic solution for sarcopenia, but also uncovers an innovative application for these rare ginsenosides. This newfound dimension opens avenues for further exploration, offering potential benefits across various facets of health and wellness. As we continue to seek innovative interventions for the challenges posed by sarcopenia, the discovery of RGX365’s potential brings renewed optimism for healthier aging and enhanced quality of life.
References
- Dutt, V.; Gupta, S.; Dabur, R.; Injeti, E.; Mittal, A. Skeletal muscle atrophy: Potential therapeutic agents and their mechanisms of action. Pharmacol. Res. 2015, 99, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, S.S.; Singh, S.; Dabur, R. Natural products: Potential therapeutic agents to prevent skeletal muscle atrophy. Eur. J. Pharmacol. 2022, 925, 174995. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Kim, J.A.; Shon, Y.H.; Lim, J.O.; Yoo, J.J.; Shin, H.I.; Park, E.K. MYOD mediates skeletal myogenic differentiation of human amniotic fluid stem cells and regeneration of muscle injury. Stem Cell Res. Ther. 2013, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Legendre, N.P.; Biswas, A.A.; Lawton, A.; Yamamoto, S.; Tajbakhsh, S.; Kardon, G.; Goldhamer, D.J. Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration. Stem Cell Rep. 2018, 10, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Nelson, B.R.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 4109–4114. [Google Scholar] [CrossRef]
- Qiu, J.; Fang, Q.; Xu, T.; Wu, C.; Xu, L.; Wang, L.; Yang, X.; Yu, S.; Zhang, Q.; Ding, F.; et al. Mechanistic Role of Reactive Oxygen Species and Therapeutic Potential of Antioxidants in Denervation- or Fasting-Induced Skeletal Muscle Atrophy. Front. Physiol. 2018, 9, 215. [Google Scholar] [CrossRef]
- Kim, D.S.; Cha, H.N.; Jo, H.J.; Song, I.H.; Baek, S.H.; Dan, J.M.; Kim, Y.W.; Kim, J.Y.; Lee, I.K.; Seo, J.S.; et al. TLR2 deficiency attenuates skeletal muscle atrophy in mice. Biochem. Biophys. Res. Commun. 2015, 459, 534–540. [Google Scholar] [CrossRef]
- van Helvoort, H.A.; Heijdra, Y.F.; de Boer, R.C.; Swinkels, A.; Thijs, H.M.; Dekhuijzen, P.N. Six-minute walking-induced systemic inflammation and oxidative stress in muscle-wasted COPD patients. Chest 2007, 131, 439–445. [Google Scholar] [CrossRef]
- McClung, J.M.; Kavazis, A.N.; Whidden, M.A.; DeRuisseau, K.C.; Falk, D.J.; Criswell, D.S.; Powers, S.K. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J. Physiol. 2007, 585, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Smuder, A.J.; Judge, A.R. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y. Pathological state or cause of sarcopenia. Clin. Calcium 2017, 27, 45–52. [Google Scholar] [PubMed]
- Lee, W.; Lee, J.Y.; Lee, H.S.; Yoo, Y.; Shin, H.; Kim, H.; Min, D.S.; Bae, J.S.; Seo, Y.K. Thermosensitive Hydrogel Harboring CD146/IGF-1 Nanoparticles for Skeletal-Muscle Regeneration. ACS Appl. Bio. Mater. 2021, 4, 7070–7080. [Google Scholar] [CrossRef] [PubMed]
- Peris-Moreno, D.; Cussonneau, L.; Combaret, L.; Polge, C.; Taillandier, D. Ubiquitin Ligases at the Heart of Skeletal Muscle Atrophy Control. Molecules 2021, 26, 407. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef]
- Gea, J.; Agusti, A.; Roca, J. Pathophysiology of muscle dysfunction in COPD. J. Appl. Physiol. 2013, 114, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Ottenheijm, C.A.; Heunks, L.M.; Dekhuijzen, R.P. Diaphragm adaptations in patients with COPD. Respir. Res. 2008, 9, 12. [Google Scholar] [CrossRef]
- Pizzimenti, M.; Meyer, A.; Charles, A.L.; Giannini, M.; Chakfe, N.; Lejay, A.; Geny, B. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 2020, 11, 866–886. [Google Scholar] [CrossRef]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, N.; Koike, T.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Matsunaga, A.; Kuroiwa, M.; Arai, M. Assessment of Sarcopenia in the Intensive Care Unit and 1-Year Mortality in Survivors of Critical Illness. Nutrients 2021, 13, 2726. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.L.; Quinlan, J.I.; Dhaliwal, A.; Armstrong, M.J.; Elsharkawy, A.M.; Greig, C.A.; Lord, J.M.; Lavery, G.G.; Breen, L. Sarcopenia in chronic liver disease: Mechanisms and countermeasures. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G241–G257. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, J.E.; Song, G.Y.; Bae, J.S. Rgx365, a Rare Protopanaxatriol-Type Ginsenoside Fraction from Black Ginseng, Suppresses Inflammatory Gene iNOS via the Iinhibition of p-STAT-1 and NF-κB. Am. J. Chin. Med. 2020, 48, 1091–1102. [Google Scholar] [CrossRef]
- Park, H.H.; Kim, H.; Lee, H.S.; Seo, E.U.; Kim, J.E.; Lee, J.H.; Mun, Y.H.; Yoo, S.Y.; An, J.; Yun, M.Y.; et al. PEGylated nanoparticle albumin-bound steroidal ginsenoside derivatives ameliorate SARS-CoV-2-mediated hyper-inflammatory responses. Biomaterials 2021, 273, 120827. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of protopanaxatriol type ginsenoside fraction (Rgx365) on particulate matter-induced pulmonary injury. J. Toxicol. Environ. Health A 2019, 82, 338–350. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Hyatt, H.W.; Powers, S.K. Mitochondrial Dysfunction Is a Common Denominator Linking Skeletal Muscle Wasting Due to Disease, Aging, and Prolonged Inactivity. Antioxidants 2021, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Li Ji, L. Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J. Cell. Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef]
- Dhillon, R.J.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Kim, H.; Hirano, H.; Edahiro, A.; Ohara, Y.; Watanabe, Y.; Kojima, N.; Kim, M.; Hosoi, E.; Yoshida, Y.; Yoshida, H.; et al. Sarcopenia: Prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr. Gerontol. Int. 2016, 16 (Suppl. S1), 110–122. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhadra, R.; Schols, A.; van Helvoort, A.; Sambashivaiah, S. Nutrition in the prevention and management of sarcopenia—A special focus on Asian Indians. Osteoporos Sarcopenia 2022, 8, 135–144. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Bosaeus, I.; Rothenberg, E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc. Nutr. Soc. 2016, 75, 174–180. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng. Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Han, M.J.; Park, S.J.; Lee, S.J.; Choung, S.Y. The Panax ginseng Berry Extract and Soluble Whey Protein Hydrolysate Mixture Ameliorates Sarcopenia-Related Muscular Deterioration in Aged Mice. Nutrients 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Gao, S.; Zhao, D.; Li, X. Review of ginsenosides targeting mitochondrial function to treat multiple disorders: Current status and perspectives. J. Ginseng. Res. 2021, 45, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef]
- Jin, S.; Jeon, J.H.; Lee, S.; Kang, W.Y.; Seong, S.J.; Yoon, Y.R.; Choi, M.K.; Song, I.S. Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract. Molecules 2019, 24, 2618. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Gonzalez, J.A.; Sanchez, M.; Moratilla-Rivera, I.; Iglesias, I.; Gomez-Serranillos, M.P. Immunomodulatory, Anti-Inflammatory, and Anti-Cancer Properties of Ginseng: A Pharmacological Update. Molecules 2023, 28, 3863. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory Effects of Black Ginseng on Particulate Matter-Induced Pulmonary Injury. Am. J. Chin. Med. 2019, 47, 1237–1251. [Google Scholar] [CrossRef]

