1. Introduction
Sarcopenia is characterized by a progressive decline in skeletal muscle mass and physical function, namely muscular strength and physical performance, that is commonly observed in older individuals. Multiple prospective studies have documented a decline in skeletal muscle mass of approximately 6% every decade following middle age [1]. This age-related condition has been shown to be on the rise in recent years [2]. Sarcopenia may lead to a variety of consequences, such as disability and increased mortality in the elderly, which has aroused widespread concern in society [3]. The risk of falling is increased by two–three times in the elderly when muscular strength declines as a result of muscle mass loss. Additionally, senior individuals with sarcopenia have a fall risk that is roughly 2.6 times greater than that of healthy elderly individuals, whereas elderly individuals with low muscle mass and muscular strength have mortality rates that are, respectively, 1.4 times and 2.34 times higher [4]. Consequently, the decline in skeletal muscle mass has resulted in major problems in the daily lives and overall well-being of older individuals [5]. The pathophysiology of sarcopenia is multifactorial. The process of aging disrupts the balance of skeletal muscle homeostasis, leading to an uneven distribution of anabolic and catabolic activities on the protein production pathway [6]. The development and progression of sarcopenia have been associated with many underlying risk factors. Physical fitness, inflammatory responses, antioxidants, mitochondrial function, hormones, the biochemistry of muscles, metabolic and cardiovascular disorders, neuromuscular junction dysfunction, insulin resistance, decreased number of satellite cells, and other clinical and physiological aspects of sarcopenia are discussed [7,8]. A previous study suggested that growth differentiation factor 8, p16 cyclin-dependent kinase 2A inhibitor, myogenic regulatory factors 4, and the stimulation of the Wnt pathway may lead to early sarcopenia development by changing satellite cell function, which would impair the restoring ability of skeletal muscle [9].
Presently, the Food and Drug Administration (FDA) has not approved any specific drugs to treat sarcopenia. Although several agents, such as appetite stimulants, growth hormone, myostatin inhibitors, activating II receptor drugs, β-receptor blockers, angiotensin-converting enzyme inhibitors, and troponin activators, are still recommended, the variable efficacy and side effects of these agents remain intractable [6].
Suitable protein intake and exercise are considered practical strategies to delay the progression of sarcopenia, given that there are no specific treatment options available for sarcopenia. However, a large number of patients suffering from sarcopenia are also combined with excess weight and osteoarthritis, which prevents them from maintaining regular and efficient levels of exercise. Due to their antioxidant, anti-inflammation, and anti-aging qualities with minimal toxicity, nutraceuticals and herbal medications have received a lot of interest recently for the prevention and treatment of sarcopenia [10].
Flavonoids are plant phenolic compounds with different medicinal properties such as antioxidant, anti-inflammation, and anti-cancer [11]. They are widely distributed in virtually every kind of plant, especially fruits and vegetables [12]. Also, flavonoids, which act as polyphenol antioxidants, are the main active components in many Chinese herbal medicines such as Alismatis rhizome and Achyranthis bidentate radix [13]. Flavonoids possess a fundamental chemical framework, including a 15-carbon skeleton, characterized by the presence of two phenyl rings (A and B) and a heterocyclic ring (C) that encompasses the oxygen atom [14]. Attributed to the C2=C3 functional double bond of the C ring in their chemical structure, flavonoids can be oxidized at different sites, leading to hydroxylation, methylation, and glycosylation [11]. Their variability functional group, degree of polymerization, degree of conjugation, and degree of substitution explain the broad and varied biological actions of flavonoids [11]. Depending on the chemical structure, flavonoids are divided into flavonols, flavan-3-ols, anthocyanidins, isoflavones, flavanones, and flavones [15]. Current evidence shows various flavonoids, such as rutin, nobiletin, luteolin, and quercetin have a favorable impact on skeletal muscle health by regulating protein homeostasis in vitro and in mouse models [16,17,18,19].
Although there was plenty of evidence showing the positive effect of flavonoids on muscle metabolism in animal. Not all clinical studies, nevertheless, have consistently shown a favorable effect [20,21,22,23]. Taking into account the aforementioned points, it should be indicated that an objective assessment of the effectiveness of flavonoids in the treatment of individuals with sarcopenia is needed. Therefore, we carried out a systematic review of randomized controlled trials (RCTs) to assess the effects of flavonoids on skeletal muscle mass, muscle function, and physical performance in sarcopenic adults.
2. Materials and Methods
Following the Cochrane approach, we conducted a systematic search of papers and published our findings in conformity with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24].
2.1. Search Strategy
In order to accomplish our objectives, the methodological approach for the current study was carried out utilizing the databases PubMed, Scopus, Embase, Cochrane, and Web of Science, as well as Google Scholar from inception up to June 2023. We performed the complete search in duplicate. We evaluated the eligibility of the title and abstract using predetermined inclusion and exclusion criteria. The full-text papers were checked for final inclusion if they were eligible. The following medical subject headings (MeSH) keywords were used: “Sarcopenia” AND (“2-Phenyl-Chromenes” OR “2 Phenyl Chromenes” OR “2-Phenyl-Benzopyran” OR “2 Phenyl Benzopyran” OR “2-Phenyl-Benzopyrans” OR “2 Phenyl Benzopyrans” OR “2-Phenyl-Chromene” OR “2 Phenyl Chromene” OR “Flavonoid” OR “Bioflavonoids” OR “Bioflavonoid” OR “Flavonoids” OR “Anthocyanins” OR “Benzoflavones” OR “beta-Naphthoflavone” OR “Biflavonoids” OR “Catechin” OR “Chalcones” OR “Flavanones” OR “Hesperidin” OR “Flavones” OR “Apigenin” OR “Diosmin” OR “Flavoxate” OR “Luteolin” OR “Flavonolignans” OR “Silymarin” OR “Silybin” OR “Flavonols” OR “Kaempferols” OR “Quercetin” OR “Rutin” OR “Hydroxyethylrutoside” OR “Isoflavones” OR “Coumestrol” OR “Genistein” OR ”Pterocarpans” OR “Rotenone” OR “Phloretin” OR “Polyphloretin Phosphate” OR “Proanthocyanidins”). The strategy of electronic searching is presented in detail in .
2.2. Study Selection
Studies included in this systematic review were RCTs that assessed the effects of flavonoids, flavonoids combined with other supplementation, or flavonoid-rich supplementations (compared with placebo or control or other intervention) on sarcopenia. Studies assessed the effects of flavonoids (used alone or in combination) and flavonoid-rich supplementations in sarcopenic adults were included. We assessed potential publications for inclusion after evaluating titles, abstracts, and methodologies. If studies were duplicate records, reviews, other article types (letters, perspectives, commentaries, editorials, case reports, cohort studies, and cross-sectional studies), or full-text inaccessible, they were eliminated. Studies were excluded if they were conducted in non-sarcopenia patients, in silico, in vitro, animal studies, used administration other than flavonoids or were written in other languages than English. The search yielded a total of 309 publications: Web of Science (n = 51), PubMed (n = 44), Scopus (n = 87), Google Scholar (n = 71), Embase (n = 47), and Cochrane (n = 9). After the screening, 6 references were included.
summarizes the patient, intervention/exposure, comparator, outcome, and study design (PICOS) criteria for studies to be included in and excluded from a systematic review.
2.3. Data Extraction
We evaluated the articles to determine if they should be included in the review and then extracted data into a standardized Microsoft Excel spreadsheet from the chosen articles. Relevant data were extracted, including year of publication, authorship, study location, participants’ gender, amount and condition, flavonoids type, type of study design, groups, dosage form, supplement dosage, intervention duration, outcome measurement, and main outcome.
2.4. Quality Assessment for Clinical Studies
With the following characteristics taken into account, we used the Cochrane Collaboration’s tool to evaluate the included RCTs for the risk of bias: (1) randomized sequence generation; (2) treatment allocation concealment; (3) participant blinding; (4) completeness of the outcome data; (5) selective outcome reporting; and (6) other sources of bias [25].
3. Results
There were 309 potentially eligible studies found after searching the Google Scholar, PubMed, Scopus, Embase, Cochrane, and Web of Science databases; 120 duplicate studies were eliminated. Based on title and methodology screening, and at least one of the following factors, a significant number of papers (183) were excluded: (1) reviews that were not filtered in the initial search; (2) in vitro, in silico, or in animal studies; (3) letters, commentaries, editorials, and case reports; (4) supplementation that does not contain any kind of flavonoids; or (5) full-text is not available. Figure 1 shows the study identification and selection procedures.
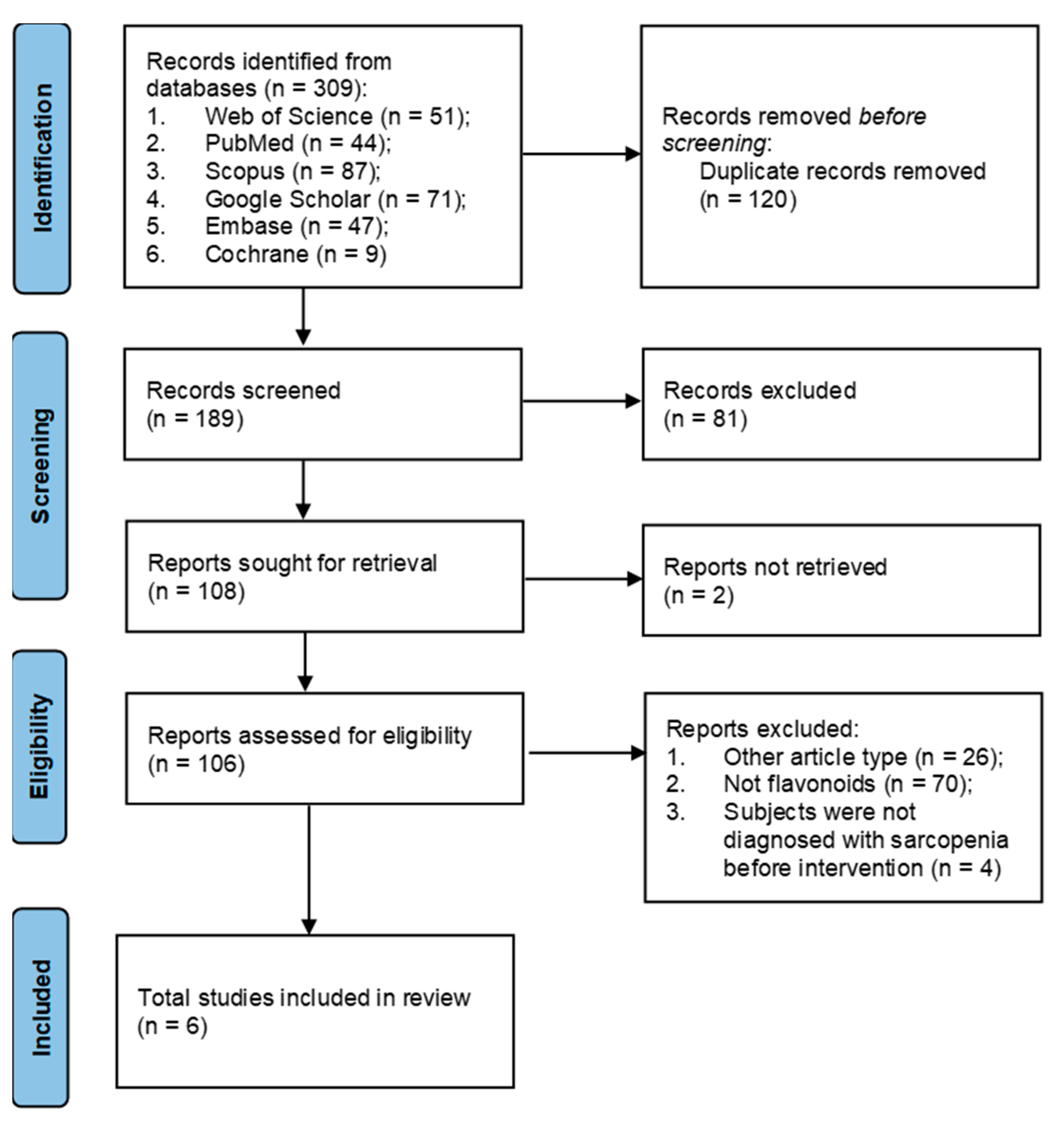
Figure 1. Flowchart of the selection process of the studies for systematic review.
3.1. Study Characteristics
The characteristics of the studies are summarized in .
The study included publications that were published between 2007 and 2022. All subjects included in the six clinical trials were defined in sarcopenic conditions before intervention. A total of 440 participants were involved in these six RCTs and 81.6% of the subjects were female (359 female and 81 male). Four of the six included investigations [26,27,29,30] carried out in Asia, while two [28,31] were in the Americas. Two of the included RCTs [29,30] were conducted in a larger population of more than 100 participants, while the rest [26,27,28,31] were conducted in populations of less than 100 participants. This review included three RCTs [26,29,30] that assessed the effects of tea catechins (TCCs), two RCTs [27,28] that assessed the effects of epicatechin (EC), and one RCT [31] that assessed the effects of isoflavones. The duration of the trials ranged from 8 weeks to 6 months.
These RCTs included subjects with different diagnostic criteria. One study [26] referred to the Asia working group for sarcopenia (AWGS). One study [28] used the definition of the European Working Group on Sarcopenia in Older People (EWGSOP). One study [27] complied with Roubenoff’s view of sarcopenia [32]. The other three studies [29,30,31] did not describe their diagnostic criteria in detail, only that they used specific skeletal muscle index (SMI) values. shows the different sarcopenia diagnostic criteria used in all the included studies.
3.2. Study Outcomes
The main outcomes of the studies are summarized in . Some outcomes measured by the included studies were not assessed in the present review. Among the studies with outcomes of interest, all included studies measured skeletal muscle mass, and five of six studies [26,27,28,29,30] measured muscle strength and physical performance as the main parameters to evaluate sarcopenia status.
Four of the six studies [27,28,29,31] calculated SMI by dividing the estimated muscle mass by the square of the height to assess skeletal muscle mass. The other two studies [26,30] used estimated muscle mass measured by bioelectrical impedance analysis or dual-energy X-ray absorptiometry. Three of the five studies [26,29,30] assessed muscle strength by the measurement of maximal hand-grip strength and knee extension strength. One study [28] only measured maximal handgrip strength, and another study [27] used the measurement of maximal strength in leg press and chest press. For physical performance assessment, the included studies used the usual walking speed test, maximal walking speed test, Timed Up and Go test, six-minute walk test, step test, and sit-up test.
Based on the results presented by the authors of the RCTs included in the systematic review, three studies [26,27,31] showed flavonoids intervention improving skeletal muscle mass, two studies [28,29] showed flavonoids intervention improving muscle strength, and two studies [27,28] showed flavonoids intervention improving physical performance. Five of the involved six studies [26,27,28,29,31] found at least one of the outcomes is positively affected by flavonoids; it may be indicated that these studies presented effective flavonoids intervention for the sarcopenic individuals.
3.3. Quality Assessment
Figure 2 displays the bias concerns associated with the studies. They usually had a low risk of bias for the majority of domains, such as completeness of the outcome, selective reporting, an unclear risk of bias for the blinding of outcome assessment, and other bias. In three of six studies [26,29,30], the random sequence generation was at low risk, while in other studies [27,28,31] it was unclear. In three studies [27,28,31], there was a low risk for participant and staff blinding, while there was a high risk in the rest of the three studies [26,29,30]. The allocation concealment was at a low risk in one study [30], and unclear in five studies [26,27,28,29,31]. Overall, three of the included studies [27,28,31] were assessed as ones with a medium risk of bias, and the other three studies [26,29,30] were indicated with a high risk of bias.
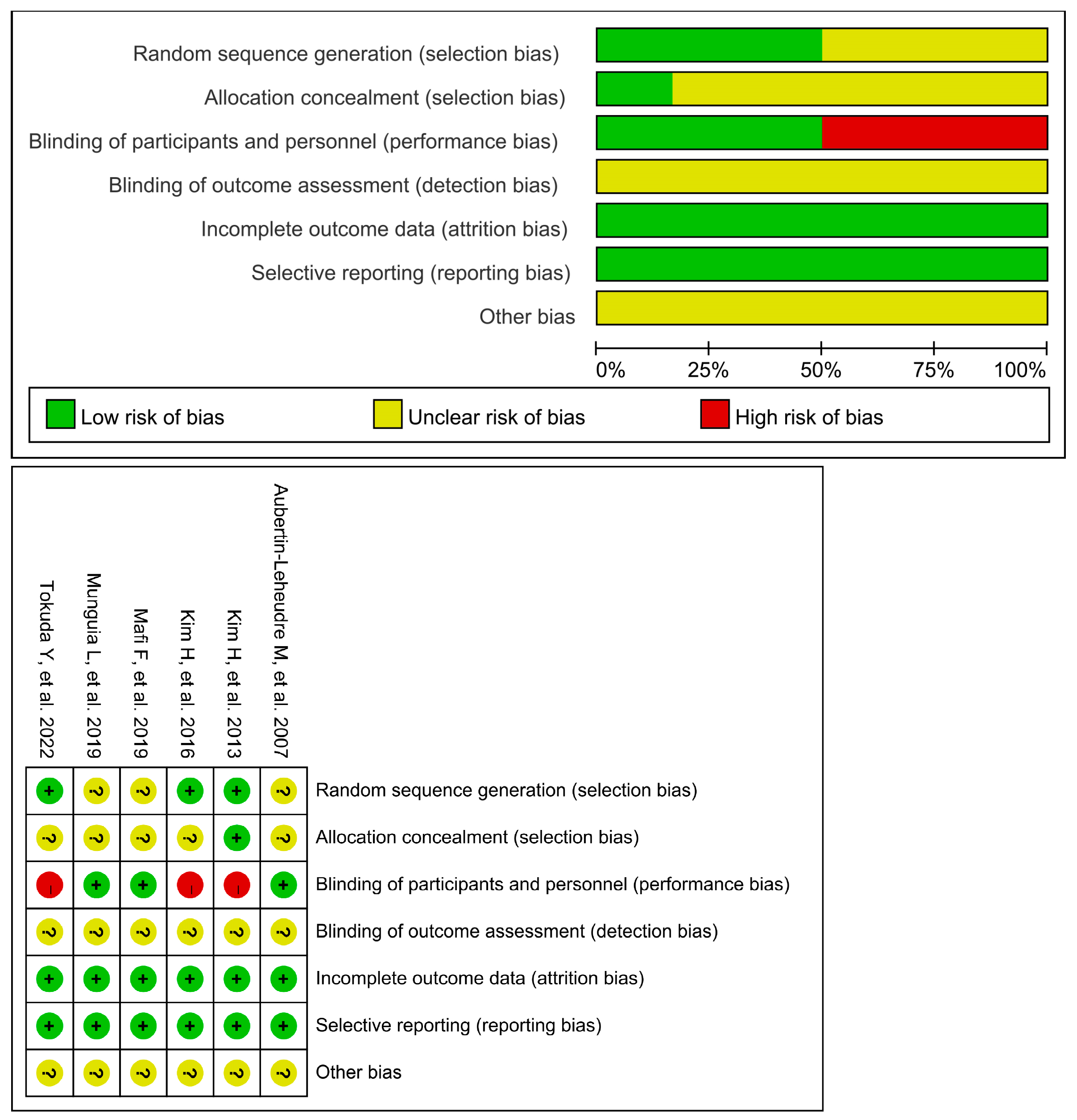
Figure 2. Risk of bias for all included studies [26,27,28,29,30,31].
3.4. Flavonoids
provides details on the impact of TCCs, EC, and isoflavones on skeletal muscle mass, muscle strength, and physical performance in sarcopenic adults.
4. Discussion
The purpose of this review was to assess the effect of flavonoids intervention on sarcopenic individuals. The included studies revealed that the majority of flavonoids interventions (five in six) turned out to be effective in improving skeletal muscle mass, muscle strength or physical performance in the group of sarcopenic individuals. In terms of risk of bias, three studies were indicated for a high risk of bias resulting from the participant blinding.
Numerous studies evaluated the effectiveness of flavonoids intervention in the prevention and management of sarcopenia among elderly adults not defined as sarcopenic before intervention. However, the outcomes are not as apparent as in the RCTs included in the present systematic review [22,23,33,34,35,36]. The interventions applied among sarcopenic individuals may be more effective than those applied in non-sarcopenic individuals.
Catechins are the main secondary metabolites of tea, belonging to the group of flavonoids [37]. Catechins’ potential health benefits for cardiovascular disease and other chronic conditions have been widely studied [38]. The group of catechins (flavan-3-ol) include: (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin-3-gallate, (−)-epigallocatechin, and EC [37]. These compounds exert anti-inflammatory effects by downregulating the expression of nuclear factor-κB and other proinflammatory cytokines [39]. According to the findings of Hung et al. [40], it was shown that EGCG exhibits stronger anti-inflammatory properties in comparison to other polyphenols found in green tea. Moreover, there were plenty of pre-clinical studies investigating the therapeutic effects of catechins on sarcopenia and the underlying mechanisms. Alway et al. [41] reported that catechins significantly attenuated the loss of hindlimb plantaris muscle mass, muscle fiber cross-sectional area, and tetanic force in the hindlimb suspension-induced sarcopenia rat model. The results demonstrated that catechins increased satellite cell proliferation and differentiation in skeletal muscles. Additionally, it was observed that catechins reduced oxidative stress and the abundance of the Bcl-2-associated X protein. Catechins have also been found to possess the ability to maintain muscle mass in aging rats. This effect is achieved, at least in part, by reducing protein degradation through the ubiquitin–proteasome pathway while simultaneously increasing the expression of anabolic factors [42].
In the present review, we include three studies [26,29,30] that used catechins (two studies [26,29] used catechins combined essential amino acid supplementation and one study [30] used catechins supplementation alone), and two studies [27,28] that used EC supplementation as an intervention, accounting for five out of six of the included studies. Catechins and the main compound appear to be the most promising flavonoids for treating sarcopenia.
All five studies evaluated skeletal muscle mass, muscle strength, and physical performance. The studies by Tokuda et al. [26] showed a significantly greater increase in muscle mass in the exercise combined with supplementation (catechins + essential amino acids) group compared with the RE group. The studies by Kim et al. [29] showed that essential amino acids combined with catechins also improved muscle strength. The study of Kim et al. [30] evaluated the effects of catechins alone but did not show a significant effect on skeletal muscle mass, muscle strength, or physical performance unless combined with exercise. While these studies did not show anti-sarcopenia effects from catechins alone, they nonetheless revealed the anti-sarcopenia effects of a combination form of catechins and essential amino acids.
EC is the main compound of catechins. Mafi F et al. [27] and Munguia et al. [28] investigated how EC significantly elevated the SMI, muscle strength, and physical performance in sarcopenic individuals. Mafi F et al. showed that EC also significantly increased the follistatin/myostatin ratio, which improves muscle hypertrophic responses and may be related to the increase in skeletal muscle mass after EC intervention. Moreover, Munguia et al. found a significant decrease in malondialdehyde (lipid peroxidation marker), protein carbonylation, and interleukin-6 levels after flavonoids intervention. This indicated that flavonoids treatment alleviated oxidative stress and inflammatory endpoints, with positive effects on muscle strength and physical performance.
Isoflavones, which are plentiful in soybeans, are virtually solely generated by plants in the Leguminosae family, and the majority of them have estrogenic properties [43]. It is well known that soy isoflavones have a strong lipid-lowering effect, which improves vasodilation and regulates fasting glucose and insulin levels [44]. Phytoestrogens may also have advantageous effects on muscle mass because of their anti-inflammatory properties or estrogen receptor affinities [45]. Furthermore, the cohort study conducted by Wu et al. [46] revealed a significant correlation between increased regular intake of soy food and handgrip strength among the general Chinese adult population, suggesting that the consumption of isoflavone-rich soy foods may potentially contribute to the enhancement of muscle health. In the present review, the study conducted by Aubertin-Leheudre et al. [31] evaluated the effects of isoflavone containing daidzein, glycitein, and genistein in 18 sarcopenic women. Although this study showed isoflavone significantly increased skeletal muscle mass, the subjects included in this study were limited (n = 18) and uneven (12 in the isoflavone group and 6 in the placebo group).
In this review, using consistent and specific criteria to define sarcopenia may be crucial in recruiting participants who had low levels of muscle mass and function to receive the expected benefit of flavonoids supplementation. However, only two of the included studies used AWGS and EWGSOP criteria. Although global diagnostic criteria for sarcopenia are now available, some included studies relied on expert scholarly views of sarcopenia at the time, which may have led to a risk of uncertain bias. Further RCTs that use specific operational criteria for sarcopenia are required.
The present review provided an overview of the effectiveness of flavonoids interventions in the treatment of individuals diagnosed with sarcopenia based on the RCTs. There were several limitations of this study that must be indicated. Firstly, the small number of studies, sample size, and unbalanced gender ratio of the participants. Although a comprehensive search of six electronic databases has been undertaken, it is possible that some RCTs meeting the criteria of this review were overlooked, which may be present in another language or unpublished articles. Then, the discrepancy in participants’ recruitment and methodology could also have influenced the outcome of this study. Half of the included studies indicated a high risk of bias, which reduces the possibility of being able to draw conclusions. Only two studies included in this review conducted sarcopenia-related biochemical analyses to investigate the underlying mechanism of flavonoids intervention. Hence, the effect of flavonoids supplementation on skeletal muscle mass, strength, and physical performance in individuals with sarcopenia could be underestimated.
5. Conclusions
The majority of interventions studied within RCTs included in the systematic review, based on flavonoids/flavonoids combined with other supplementation/flavonoid-rich supplementations, were effective in the treatment of sarcopenia.
In the future, more well-designed RCTs need to be conducted in this area to better identify the effectiveness and also reveal the underlying mechanism of the effect of flavonoids intervention in sarcopenic adults.
References
- Janssen, I. Evolution of sarcopenia research. Appl. Physiol. Nutr. Metab. 2010, 35, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.C.; Dennison, E.M.; Cooper, C. Epidemiology of Sarcopenia: Determinants Throughout the Lifecourse. Calcif. Tissue Int. 2017, 101, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.P.; Rejeski, W.J.; Espeland, M.A.; Miller, M.E.; Church, T.S.; Fielding, R.A.; Gill, T.M.; Guralnik, J.M.; Newman, A.B.; Pahor, M.; et al. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P). J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Kim, S.W.; Kim, J.W.; Park, H.Y. Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia-A Narrative Review. Life 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Borrás, C.; Viña, J. Genistein, a tool for geroscience. Mech. Ageing Dev. 2022, 204, 111665. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef] [PubMed]
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.S.; Lee, W.K.; Lee, S.J.; Lee, S.Y.; Seo, J.H.; Kim, E.J.; Choe, Y.I.; Kim, S.G.; Yoo, J.I. Rutin Prevents Dexamethasone-Induced Muscle Loss in C2C12 Myotube and Mouse Model by Controlling FOXO3-Dependent Signaling. Antioxidants 2023, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Zhang, Y.; Qu, T.Q.; Sang, X.Q.; Li, Y.X.; Ren, F.Z.; Wen, P.C.; Sun, Y.N. Nobiletin Improves D-Galactose-Induced Aging Mice Skeletal Muscle Atrophy by Regulating Protein Homeostasis. Nutrients 2023, 15, 1801. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Shin, S.K.; Kwon, E.Y. Luteolin Protects Against Obese Sarcopenia in Mice with High-Fat Diet-Induced Obesity by Ameliorating Inflammation and Protein Degradation in Muscles. Mol. Nutr. Food Res. 2023, 67, e2200729. [Google Scholar] [CrossRef] [PubMed]
- Le, N.H.; Kim, C.S.; Park, T.; Park, J.H.; Sung, M.K.; Lee, D.G.; Hong, S.M.; Choe, S.Y.; Goto, T.; Kawada, T.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef] [PubMed]
- Boutry-Regard, C.; Vinyes-Parés, G.; Breuillé, D.; Moritani, T. Supplementation with Whey Protein, Omega-3 Fatty Acids and Polyphenols Combined with Electrical Muscle Stimulation Increases Muscle Strength in Elderly Adults with Limited Mobility: A Randomized Controlled Trial. Nutrients 2020, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Orsatti, F.L.; Maestá, N.; de Oliveira, E.P.; Nahas Neto, J.; Burini, R.C.; Nunes, P.R.P.; Souza, A.P.; Martins, F.M.; Nahas, E.P. Adding Soy Protein to Milk Enhances the Effect of Resistance Training on Muscle Strength in Postmenopausal Women. J. Diet. Suppl. 2018, 15, 140–152. [Google Scholar] [CrossRef]
- Choquette, S.; Dion, T.; Brochu, M.; Dionne, I.J. Soy isoflavones and exercise to improve physical capacity in postmenopausal women. Climacteric 2013, 16, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ 2009, 399, b2700. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Tokuda, Y.; Mori, H. Essential Amino Acid and Tea Catechin Supplementation after Resistance Exercise Improves Skeletal Muscle Mass in Older Adults with Sarcopenia: An Open-Label, Pilot, Randomized Controlled Trial. J. Am. Nutr. Assoc. 2023, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef]
- Munguia, L.; Ramirez-Sanchez, I.; Meaney, E.; Villarreal, F.; Ceballos, G.; Najera, N. Flavonoids from dark chocolate and (−)-epicatechin ameliorate high-fat diet-induced decreases in mobility and muscle damage in aging mice. Food Biosci. 2020, 37, 100710. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Suzuki, T.; Saito, K.; Yoshida, H.; Kojima, N.; Kim, M.; Sudo, M.; Yamashiro, Y.; Tokimitsu, I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 458–465. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Six months of isoflavone supplement increases fat-free mass in obese-sarcopenic postmenopausal women: A randomized double-blind controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Maesta, N.; Nahas, E.A.; Nahas-Neto, J.; Orsatti, F.L.; Fernandes, C.E.; Traiman, P.; Burini, R.C. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas 2007, 56, 350–358. [Google Scholar] [CrossRef]
- Thomson, R.L.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D. Muscle strength gains during resistance exercise training are attenuated with soy compared with dairy or usual protein intake in older adults: A randomized controlled trial. Clin. Nutr. 2016, 35, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Maruyama, K.; Yamamoto, N.; Saito, I. The effects of dietary licorice flavonoid oil supplementation on body balance control in healthy middle-aged and older Japanese women undergoing a physical exercise intervention: A randomized, double-blind, placebo-controlled trial. Aging Clin. Exp. Res. 2021, 33, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Mori, H. Effect of ingestion of essential amino acids and tea catechins after resistance exercise on the muscle mass, physical performance, and quality of life of healthy older people: A randomized controlled trial. Asia Pac. J. Clin. Nutr. 2021, 30, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, Q.; Li, Q.; Lin, H.; Li, J.; Zhu, M.; Liu, Z.; Wang, K. Integrative analysis of transcriptome and metab-olome reveals the mechanism of foliar application of Bacillus amyloliquefaciens to improve summer tea quality (Camellia sinensis). Plant Physiol. Biochem. 2022, 185, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef]
- Luk, H.Y.; Appell, C.; Chyu, M.C.; Chen, C.H.; Wang, C.Y.; Yang, R.S.; Shen, C.L. Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition. Antioxidants 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Miyazaki, H.; Fang, S.H.; Li, C.Y.; Suzuki, K. The Structural Characteristics of Green Tea Polyphenols on Lipopolysaccharide-Stimulated RAW Cells. J. Nutr. Biol. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Alway, S.E.; Bennett, B.T.; Wilson, J.C.; Sperringer, J.; Mohamed, J.S.; Edens, N.K.; Pereira, S.L. Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats. J. Appl. Physiol. 2015, 118, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, S.; Ma, S.; Suzuki, K. Effect of Genistein Supplementation on Exercise-Induced Inflammation and Oxidative Stress in Mice Liver and Skeletal Muscle. Medicina 2021, 57, 1028. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Petroni, M.L.; Caletti, M.T.; Dalle Grave, R.; Bazzocchi, A.; Aparisi Gómez, M.P.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Quan, J.; Wang, X.; Gu, Y.; Zhang, S.; Meng, G.; Zhang, Q.; Liu, L.; Wang, X.; Sun, S.; et al. Soy Food Consumption Is Inversely Associated with Handgrip Strength: Results from the TCLSIH Cohort Study. Nutrients 2023, 15, 391. [Google Scholar] [CrossRef] [PubMed]

