1. Introduction
Constipation is the most frequently reported gastrointestinal (GI) disorder and is characterized by symptoms including infrequent bowel movements, hard consistency of stools, excessive straining, the sensation of incomplete evacuation, and abdominal bloating [1]. The occurrence of constipation is a detriment to the quality of life in multiple aspects, including restrictions on physical functioning, body pain, and also psychological distress. Long-term constipation may contribute to multiple serious maladies, including colorectal cancer, irritable bowel syndrome, and other GI diseases, or even death [2]. Based on epidemiological studies reported in the past three decades, the global prevalence of constipation is estimated to be 10.1% according to the Rome IV criteria [3], with female gender, older age, lower socioeconomic status, less physical activity, depressed psychological status, and physical abuse being identified as common risk factors [4,5]. Regardless of the etiologies, laxatives are the mainstay of pharmacological treatment for potential long-term therapy in constipation patients [6]. However, the quick alleviative effects of pharmacological treatments on constipation are also combined with undesirable side effects, such as diarrhea, nausea, dehydration, and drug dependence [7]. Thus, more effective and safer intervention strategies are needed.
In recent decades, dietary regulation with dietary fiber (DF) or polysaccharide products to treat constipation has received public attention. DF is defined as indigestible carbohydrate (CHO) polymers with a degree of polymerization ≥ 10, according to the Codex Alimentarius Commission, and mainly includes natural or synthetic polysaccharides (cellulose, hemicellulose, pectin, β-glucans, and other CHO polymers), oligosaccharides, resistant starch, and resistant dextrin [8]. For instance, psyllium is a typical laxative DF that is already approved by the Food and Drug Administration (https://www.fda.gov/, accessed on 1 July 2023) and the European Food Safety Authority (https://www.efsa.europa.eu/, accessed on 1 July 2023). In addition, current research shows that polysaccharides from bamboo shavings [9], Holothuria leucospilota [10], Spirulina platensis [11], Atractylodis macrocephala [12], and Platycodon grandiflorum [13] also have potential for alleviating constipation.
The main mechanism by which DF allays constipation is related to its good hydration properties, which help with intestinal peristalsis and increase stool moisture and volume [14]. However, the GI fermentation of DF is also considered an important factor in relieving constipation symptoms. Indigestible fibers can be completely or partially fermented by the gut microbiota in the large intestine, thereby improving intestinal microecology and prompting the production of beneficial metabolites such as short-chain fatty acids (SCFAs) [15]. These organic acids containing 1−6 carbon atoms (e.g., acetic acid, propionic acid, butyric acid, valeric acid) play an essential role in GI transit and defecation. Wang et al. [16] reported that constipated mice treated with resistant acylated or butylated starches presented an increased abundance of acetic acid- or butyric acid-producing bacteria and an enhanced production of acetic acid and butyric acid, which were associated with accelerated intestinal transit and increased stool moisture. Moreover, GI fermentation products of DF also prompt the secretion of GI hormones related to appetite, digestion, and GI motility [17,18]. Lan et al. [19] observed that the motilin (MTL, an excitatory GI hormone) level was reduced in diphenoxylate-induced constipated rats, while inulin and isomalto-oligosaccharide administration alleviated constipation and reestablished the MTL level, as well as increased the SCFA content within the colon. Notably, DF with different compositions and structures may differ greatly in physicochemical properties and GI fermentation performance, which results in divergent responses to constipation interventions. Therefore, it is important to explore the relationship between the structural and fermentative characteristics of DF and its laxative effects to pursue an effective and easy-to-implement therapy for alleviating constipation.
Sweet potato (Ipomoea batatas [L.] Lam.) is one of the most widely consumed crops globally and has higher DF content than typical staple foods, such as rice and wheat flour [20]. In general, sweet potato is regarded as a healthy food for GI disorders, e.g., constipation, diarrhea, and inflammatory bowel disease [21]. In our previous study, a soluble fiber composite (SDF-S) was extracted from steamed sweet potato, and it presented superior activity to inulin in promoting Lactobacillus spp. proliferation and fermentation in vitro [22]. In our recent preliminary experiment, it was also shown to alleviate loperamide-induced constipation in mice at a dose of 400 mg/(kg·bw·d) (as shown in ). In the present study, two polysaccharide components with different structures were isolated from SDF-S and were characterized in terms of their chemical composition, molecular weight (Mw), monosaccharide profile, and Fourier transform infrared spectroscopy (FT-IR) spectrum. Their effects on constipation were evaluated by determining the GI motility, stool parameters, intestinal histology, and GI hormone levels in constipated mice. Meanwhile, the fecal SCFA profile and gut microbiota were also analyzed for a better understanding of the fermentation characteristics of the two polysaccharide components. This study is important for understanding the relationship between the fermentation characteristics of polysaccharides and their therapeutic effects on constipation and may be helpful for the selection of polysaccharide laxatives.
2. Materials and Methods
2.1. Materials and Chemicals
The preparation of SDF-S was conducted using the method that we previously reported [22]. Briefly, 20 min steamed sweet potato was freeze-dried, powdered, and extracted with hot water (40 °C) with the assistance of ultrasonic treatment. After removing starch and protein components with Taka-diastase, amyloglucosidase, and pancreatin, the supernatant of the extract was collected by centrifugation, concentrated, and precipitated with ethanol. The sediment was collected, freeze-dried, and denoted as SDF-S. The lot of SDF-S used in this study was composed of 91.86% glucose, with an average molecular weight of 5.32 kDa. The detailed composition and structural information are presented in . SDF-S was further separated by using a DEAE Sepharose Fast Flow (DEAE-FF) ion-exchange column, which was purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China).
Monosaccharide standards (L-arabinose, L-fucose, L-rhamnose, D-galactose, D-glucose, D-mannose, D-ribose, D-glucuronic acid, and D-galacturonic acid) and SCFA standards (acetic acid, propionic acid, n-butyric acid, n-valeric acid, isobutyric acid, and isovaleric acid) were purchased from Sigma-Aldrich Co. LLC (Shanghai, China). Loperamide hydrochloride was purchased from Xi’an Janssen Pharmaceutical Ltd. (Xi’an, China). Phenolphthalein was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Gum arabic was purchased from Yonghua Chemical Co., Ltd. (Suzhou, China). Activated carbon was purchased from Macklin Inc (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits to quantify MTL, gastrin (GAS), substance P (SP), vasoactive intestinal peptide (VIP), and somatostatin (SS) levels in serum were purchased from Lengton Biotech Co., Ltd. (Shanghai, China). All other chemicals and reagents used in this study were of analytical grade.
2.2. Preparation of Polysaccharide Fractions from SDF-S
DEAE-FF ion-exchange chromatography was employed to isolate polysaccharide fractions (named SSPs) from SDF-S based on the difference in charge. Firstly, SDF-S was dissolved at 20 mg/mL in distilled water and loaded onto a DEAE-FF column. Then, gradient elution was performed by sequentially using distilled water and NaCl solution with gradually increasing concentrations (0.1, 0.3, and 0.5 mol/L) as the eluent at a flow rate of 3.5 mL/min. Each 10.5 mL of the elution was collected, and its carbohydrate content was determined using the phenol–sulfuric acid method [23]. An elution curve was generated according to the number of the collection and its carbohydrate content. Elution fractions belonging to the same peak were combined, lyophilized, and re-dissolved at a concentration of 0.2 g/mL. Then, this solution was lyophilized again after dialysis at 4 °C for 24 h. As a result of these processes, two fractions were obtained from SDF-S, named SSP-1 and SSP-2, respectively.
2.3. Characterization of SSPs
2.4. Animals and Experimental Design
Eighty male Institute of Cancer Research (ICR) mice were purchased from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. (Jiaxing, China; animal license number: SCXK (ZHE) 2019-0001). After being conditioned for 7 d, the eighty male ICR mice were randomly divided into 5 groups, with sixteen mice in each group. These groups were treated as follows: (1) normal control group (NC), saline; (2) constipation model group (MC), saline + 10 mg/(kg·bw·d) loperamide; (3) positive control group (PC), 10 mg/(kg·bw·d) loperamide + 70 mg/(kg·bw·d) phenolphthalein; (4) SSP-1 group (SSP-1), 10 mg/(kg·bw·d) loperamide + 400 mg/(kg·bw·d) SSP-1; (5) SSP-2 group (SSP-2), 10 mg/(kg·bw·d) loperamide + 400 mg/(kg·bw·d) SSP-2. After a 14 d treatment, mice underwent a GI transit test and defecation function test according to the protocols described below.
During the experiments, all mice were housed in a specific pathogen-free laboratory at the Laboratory Animal Center of Zhejiang Academy of Agricultural Sciences (Hangzhou, China; facility license number: SYXK (ZHE) 2020-0022). Within the laboratory, the mice were maintained at a temperature of 22 ± 2 °C, a relative humidity of 55 ± 5%, and 12/12 h of automatic lighting (8:00 to 20:00 every day). Except for experimental practices, all of the mice were fed a commercial diet based on AIN-93 M and had free access to drinking water. Body weights and food intake were recorded daily.
2.5. Histological Observation
The fixed tissues underwent graded dehydration, paraffin embedding, sectioning, deparaffinization, clearing, and hematoxylin and eosin (H&E) staining according to Feldman et al.’s method [28], and then they were observed with a digital slide scanner (NanoZoomer S60, Hamamatsu Photonics K.K., Shizuoka, Japan).
2.6. Assessment of Serum GI Hormones
The levels of MTL, GAS, SP, VIP, and SS in serum were assayed according to the corresponding ELISA kit instructions. The absorbance values were determined at 450 nm using a microplate reader (SpectraMax 190, Molecular Devices LLC, San Jose, CA, USA).
2.7. Assessment of Fecal SCFA profile
The fecal SCFA profile was assayed according to the method reported by Zhao et al. [29], with slight modifications. Briefly, the mice feces were mixed with distilled water at a ratio of 1:9 and centrifuged at 10,000 rpm and 4 °C for 15 min. The supernatants were collected and acidified with crotonic acid–metaphosphoric acid solution (0.6464 g of crotonic acid in 100 mL of 2.5% (w/v) metaphosphoric acid solution) at −20 °C for 24 h. Then, the acidified supernatant was centrifuged at 12,000 rpm for 5 min, filtered, and loaded for SCFA quantification. A GC system (GC-2010 Plus, Shimadzu Corporation, Kyoto, Japan) was employed for quantification, along with a DB-FFAP column (30 m × 0.53 mm × 5 µm, Agilent Technologies Inc, Santa Clara, CA, USA) and a H2 flame ionization detector (FID). The injection volume was 1.0 µL, and N2 was used as the gas carrier at a flow rate of 12.0 mL/min. The initial column temperature was 70 °C, then increased to 180 °C at a rate of 15 °C/min, and finally increased to 240 °C at 40 °C/min. The temperature of the injection port and FID was kept at 250 °C. The flow rates of air, H2, and N2 in the detector were 400.0, 40.0, and 30.0 mL/min, respectively.
2.8. Bioinformatic Analysis Based on 16S rDNA Sequencing
All cecum contents were sent to LC-Bio Technology Co., Ltd., Hangzhou, Zhejiang Province, China, for bioinformatic analysis. DNA from different samples was extracted using a CTAB kit according to its instructions, and the purity was tested after concentration. The polymerase chain reaction (PCR) amplification of the V3 and V4 regions of bacterial 16S rDNA genes was carried out with the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′). The PCR products were purified with AMPure XT beads (Beckman Coulter Inc, Indianapolis, IN, USA) and quantified using Qubit (Thermo Fisher Scientific Inc, Waltham, MA, USA). All quantified amplicons were pooled into a library and paired-end-sequenced on a NovaSeq PE250 platform (Illumina Inc, San Diego, CA, USA).
The reads of each sample were merged using FLASH (version 1.2.7, https://ccb.jhu.edu/software/FLASH/index.shtml, accessed on 1 July 2023) and filtered using fqtrim (version 0.94, http://ccb.jhu.edu/software/fqtrim/, accessed on 1 July 2023) and Vsearch software (version 2.3.4, https://github.com/torognes/vsearch, accessed on 1 July 2023) to obtain high-quality clean tags. After dereplication using the Divisive Amplicon Denoising Algorithm (DADA2), the ASV table and sequences were obtained. Then, the ASV sequences were annotated with the SILVA database (release 138, https://www.arbsilva.de/documentation/release138/, accessed on 1 July 2023) for each representative sequence. Alpha diversity (Chao1, Simpson, Shannon, and Pielou’s evenness) and beta diversity (principal coordinate analysis (PCoA) and nonmetric multidimensional scaling (NMDS) based on weighted UniFrac distance) were calculated and normalized to the same sequences randomly using QIIME2 (version 2019.7, https://qiime2.org/, accessed on 1 July 2023). All statistical analyses were performed using R software (version 3.5.2). The Kruskal–Wallis and Wilcoxon rank sum tests were used for comparisons. All diagrams were generated using the Omicstudio platform (https://www.omicstudio.cn/index, accessed on 1 July 2023).
2.9. Data Analysis
For all the experiments, measurements were performed in triplicate. Data were collected from the experiments and were first subjected to the Shapiro–Wilk test to examine the normality. Data with a normal distribution are expressed as mean ± standard deviation (SD), and one-way analysis of variance (ANOVA) followed by the Duncan test was performed to compare the differences among groups. Data with a non-normal distribution are expressed as median ± SD, and the Kruskal–Wallis test was conducted to detect differences by comparing the data distribution between groups. Two-tailed Spearman’s correlation tests were conducted to assess the significance of the relationships among constipation-related parameters, fecal SCFAs, and GI microbiota. All of the analyses were conducted by using IBM SPSS program version 26.0 (IBM SPSS Inc, Chicago, IL, USA), with significance at p < 0.05. Figures were generated with OriginPro 2022 (OriginLab Co., Northampton, MA, USA).
3. Results and Discussion
3.1. Physicochemical Characterization of SSPs
According to our previous study [22], SDF-S is a low-Mw, resistant-dextrin-like DF and contains a variety of pectic substances . In the present study, two polysaccharide fractions, i.e., SSP-1 and SSP-2, were separated by using DEAE-FF ion-exchange chromatography, with water and 0.3 mol/L NaCl as the eluents, respectively (Figure 1), suggesting the neutral nature of SSP-1 and the acidic nature of SSP-2. SSP-1 was the main component of SDF-S, with a significantly higher yield of SSP-1 than SSP-2 (61.02 ± 4.62% vs. 12.88 ± 0.74 %) .
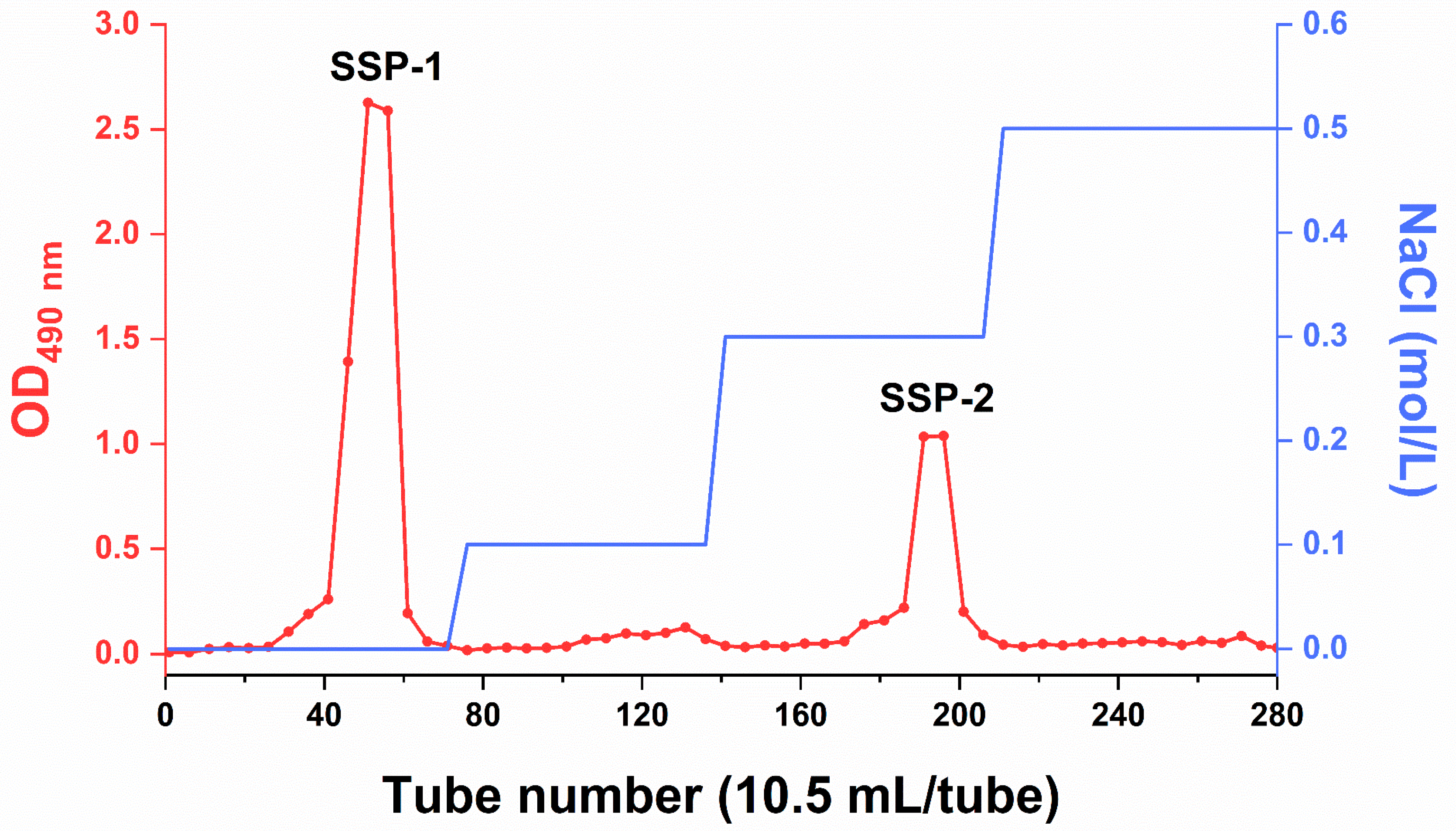
Figure 1. DEAE Sepharose Fast Flow chromatography of SDF-S. SSP-1 and SSP-2 signify, respectively, the neutral and acidic fractions isolated from soluble dietary fiber of steamed sweet potato; OD490 = optical density at 490 nm.
The FT-IR spectra of SSP-1 and SSP-2 (Figure 2) presented the typical characteristics of polysaccharides linked by β- and α-glycosidic bonds (900/919 cm−1 and 831/844 cm−1, respectively) [30]. Additionally, the spectrum of SSP-2 showed a unique, strong band at 1743 cm−1, which is attributed to the stretching of esterified C = O [31] and is in accordance with its much higher uronic acid content . The monosaccharide profiles suggest that SSP-1 is a neutral polysaccharide with glucose as the predominant constituent (99.49%), while SSP-2 is an acidic polysaccharide characterized by a 63.85% uronic acid content. Two typical acidic monosaccharides, galacturonic acid and glucuronic acid, were determined in SSP-2 with contents of 35.92% and 3.02%, respectively, but were not detected in SSP-1. SSP-1 showed a much lower Mw than SSP-2 (2.04 kDa vs. 41.66 kDa). Based on the above, it is suggested that SSP-1 may be a resistant-dextrin-like component of SDF-S, while SSP-2 may be degraded pectin [32].
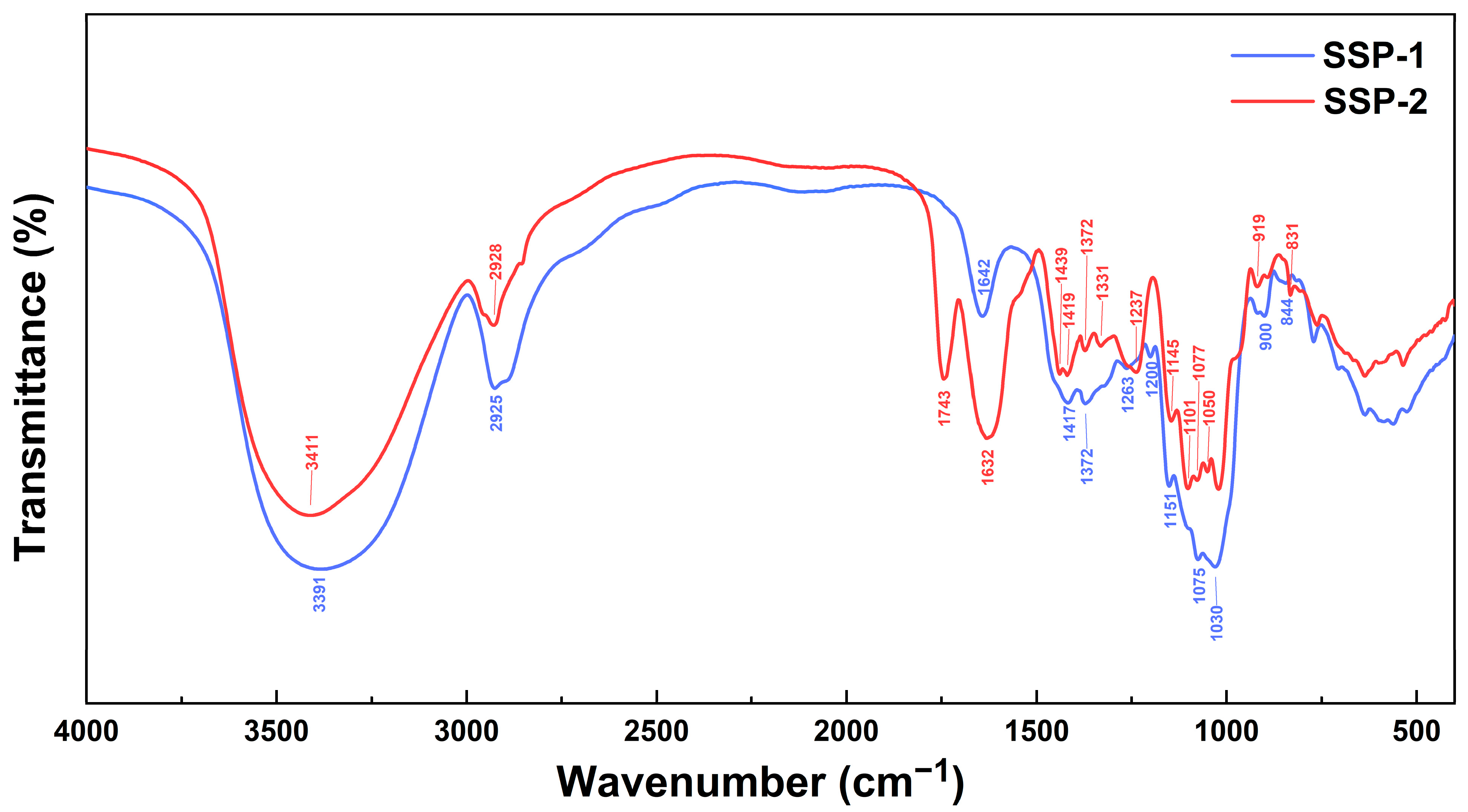
Figure 2. FT-IR spectra of SSPs. SSP-1 and SSP-2, respectively, signify the neutral and acidic fractions isolated from soluble dietary fiber of steamed sweet potato.
3.2. Effects of SSPs on GI Transit and Defecation Function
The results of GI transit and defecation function tests in constipated mice are presented in . Compared to the NC group, the MC group presented a significantly decreased GI transit rate (37.93 ± 4.67% vs. 80.19 ± 11.44%) and significantly longer defecation time of the first black stool (156.00 ± 10.15 min vs. 73.50 ± 3.11 min) (p < 0.05). In addition, the stool water content, pellet number, and weight in the MC group were also significantly lower (p < 0.05), which indicated that loperamide successfully induced constipation in mice. GI transit and defecation function were recovered in constipated mice administered phenolphthalein (the PC group), with no evidence of a significant difference in the GI transit rate or stool parameters being observed between the PC and NC groups, which is in accordance with the previous literature [33].
Both SSP-1 and SSP-2 displayed their potential for relieving constipation while exerting their effects in different aspects. Compared with the MC group, the defecation parameters, including the defecation time, stool water content, and pellet number, were all significantly improved in the SSP-1 and SSP-2 groups (p < 0.05). Additionally, the stool weight in the SSP-2 group was also significantly higher than in the MC and SSP-1 groups (p < 0.05). The GI transit rate also increased in the two SSP groups compared to the MC group, with a significant difference only being determined between the SSP-1 and NC groups (p < 0.05), as shown in . These results suggest that SSP-2 exerted its effect by improving defecation function, while SSP-1 presented superior effects to SSP-2 by promoting GI motility.
During the experiments, the mice in all groups showed similar weight gain and feeding behavior. No abnormal behavior, disease (other than constipation), or death was observed among mice in any of the groups, suggesting that the different administrations did not disturb the growth of the mice.
3.3. Effects of SSPs on Intestinal Histological Morphology
Damage to the small intestinal villi can slow down the peristalsis of the intestine and the transit of excreta, thereby aggravating constipation. The morphology of the mouse small intestine is presented in Figure 3. After H&E staining, the NC group showed a complete intestinal structure with dense and neatly arranged villi, and the epithelial cells had well-separated crypts. However, in the MC group, the villi were disorganized, atrophied, or broken, and the crypts were short, distorted, or missing. Although the small intestinal villi in the PC and SSP-1 groups had some atrophy and rupturing, the villi and crypts in these groups were more intact than those in the CM group, whereas in the SSP-2 group, the villi were severely damaged like those in the MC group, and the crypts were also short, distorted, or missing. These results indicate that treatment with SSP-1 protected or promoted the recovery of the small intestine tissue in constipated mice.
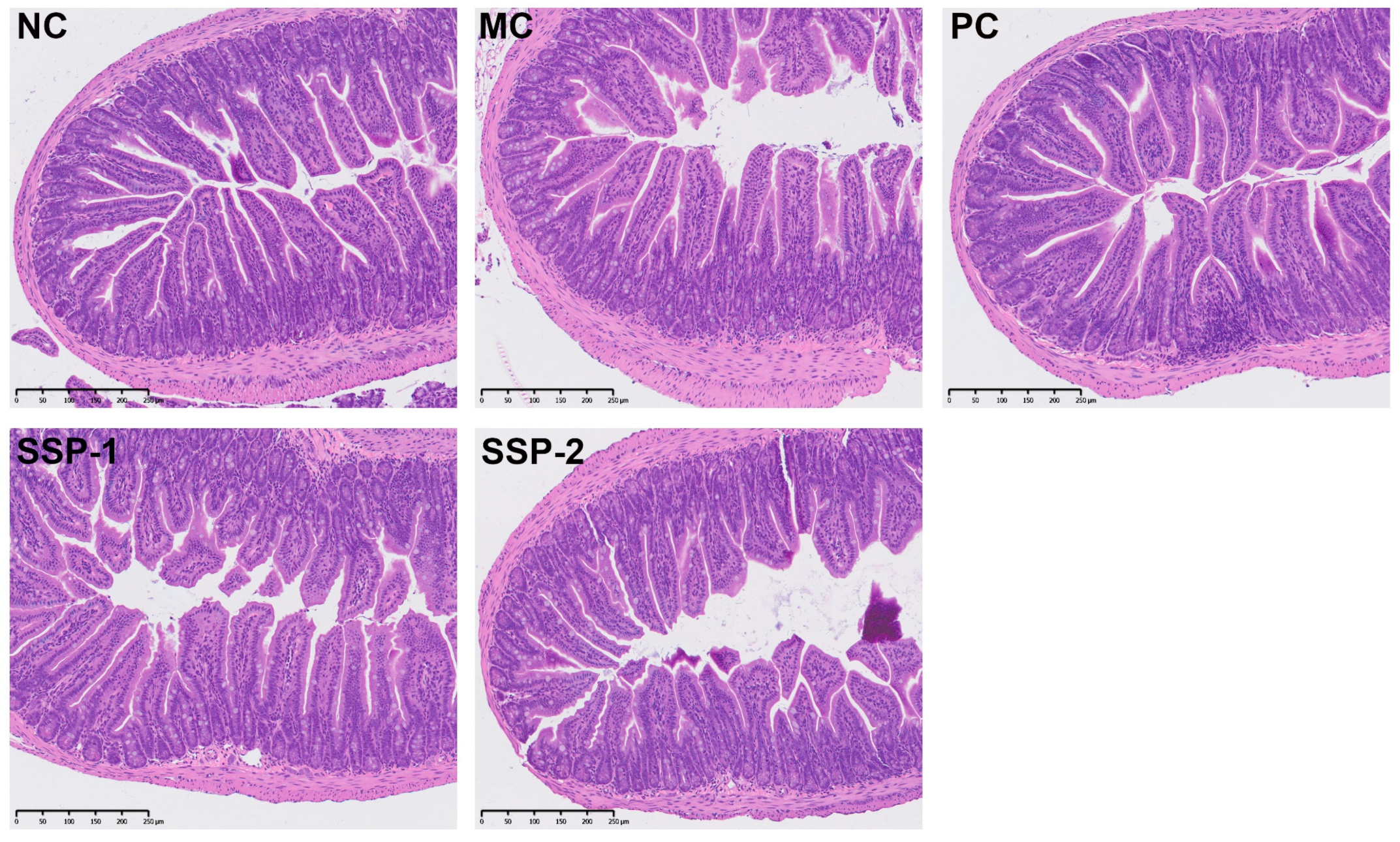
Figure 3. Morphology of mouse small intestine with H&E staining. NC = normal control group; MC = model control group; PC = positive control group; SSP-1 and SSP-2, respectively, signify the groups treated with the two fractions (SSP-1 and SSP-2) isolated from the soluble dietary fiber of steamed sweet potato.
3.4. Effects of SSPs on GI Hormone Levels in Serum
The levels of excitatory (GAS, MTL, and SP) and inhibitory GI hormones (VIP and SS) in serum were measured to further explore the mechanisms by which SSPs alleviate constipation. As presented in Figure 4, the GAS, MTL, and SP levels in the MC group were significantly lower than those in the NC group, while the VIP and SS levels were significantly upregulated (p < 0.05). The levels of GAS, SP, VIP, and SS in the PC group were not significantly different from those in the NC group (Figure 4 A,C,D,E), while the MTL level was significantly lower than that in the NC group but higher than that in the MC group (p < 0.05) (Figure 4B).

Figure 4. Serum levels of MTL (A), GAS (B), SP (C), VIP (D), and SS (E) in constipated mice. MTL = motilin; GAS = gastrin; SP = substance P; VIP = vasoactive intestinal peptide; SS = somatostatin. NC = normal control group; MC = model control group; PC = positive control group; SSP-1 and SSP-2, respectively, signify the groups treated with the two fractions (SSP-1 and SSP-2) isolated from the soluble dietary fiber of steamed sweet potato. Different letters between groups indicate significant differences (p < 0.05).
Compared to the MC group, the GAS and MTL levels in the SSP-1 group and SSP-2 group were both significantly higher (p < 0.05) (Figure 4A,B). In addition, the GAS level in these groups was even significantly higher than that in the NC group (p < 0.05) (Figure 4A). Administering SSP-1 and SSP-2 also increased the SP levels but decreased the VIP and SS levels in constipated mice to some extent, although no statistical difference was detected.
GAS, MTL, SP, VIP, and SS are all essential GI hormones involved in bowel movement. As excitatory hormones, GAS, MTL, and SP can stimulate the secretion of gastric acid and digestive enzymes, thereby promoting GI peristalsis and emptying [34,35,36]. They are usually found at lower levels in constipated patients [37]. In contrast, both VIP and SS inhibit smooth muscle contraction, and SS also exerts broad inhibitory effects on GI hormone secretion (e.g., GAS and SP) [38,39]. In agreement with previous studies [9], the loperamide-treated mice showed remarkable reductions in GAS, MTL, and SP levels, as well as detectable increases in VIP and SS levels, while the up/downregulation of these GI hormones was reversed after treatment with polysaccharides. Thus, the deficiency of excitatory GI hormones and the excessive production of inhibitory GI hormones exert a negative influence on GI motility in mice and lead to constipation. The results demonstrate that both SSP-1 and SSP-2 enhanced the levels of excitatory GI hormones, especially GAS and MTL, thereby improving GI motility in constipated mice.
3.5. Effects of SSPs on Fecal SCFA Profile
In this study, the contents of acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, valeric acid, and total SCFAs (the sum of six SCFAs) in mice feces in each group were measured to evaluate the effects of SSPs on gut microbiota metabolites. As shown in Figure 5, except for valeric acid, the contents of all single SCFAs and total SCFAs in the MC group were significantly lower than those in the NC group (p < 0.05). SSP treatments improved SCFA production in constipated mice. Compared to the MC group, both the SSP-1 and SSP-2 groups showed significantly higher fecal contents of acetic acid, isovaleric acid, and valeric acid (p < 0.05). Additionally, the acetic acid content in the SSP-1 group was significantly higher than in the SSP-2 group. Conversely, the isobutyric acid content in the SSP-2 group was significantly higher than that in the SSP-1 group (p < 0.05).
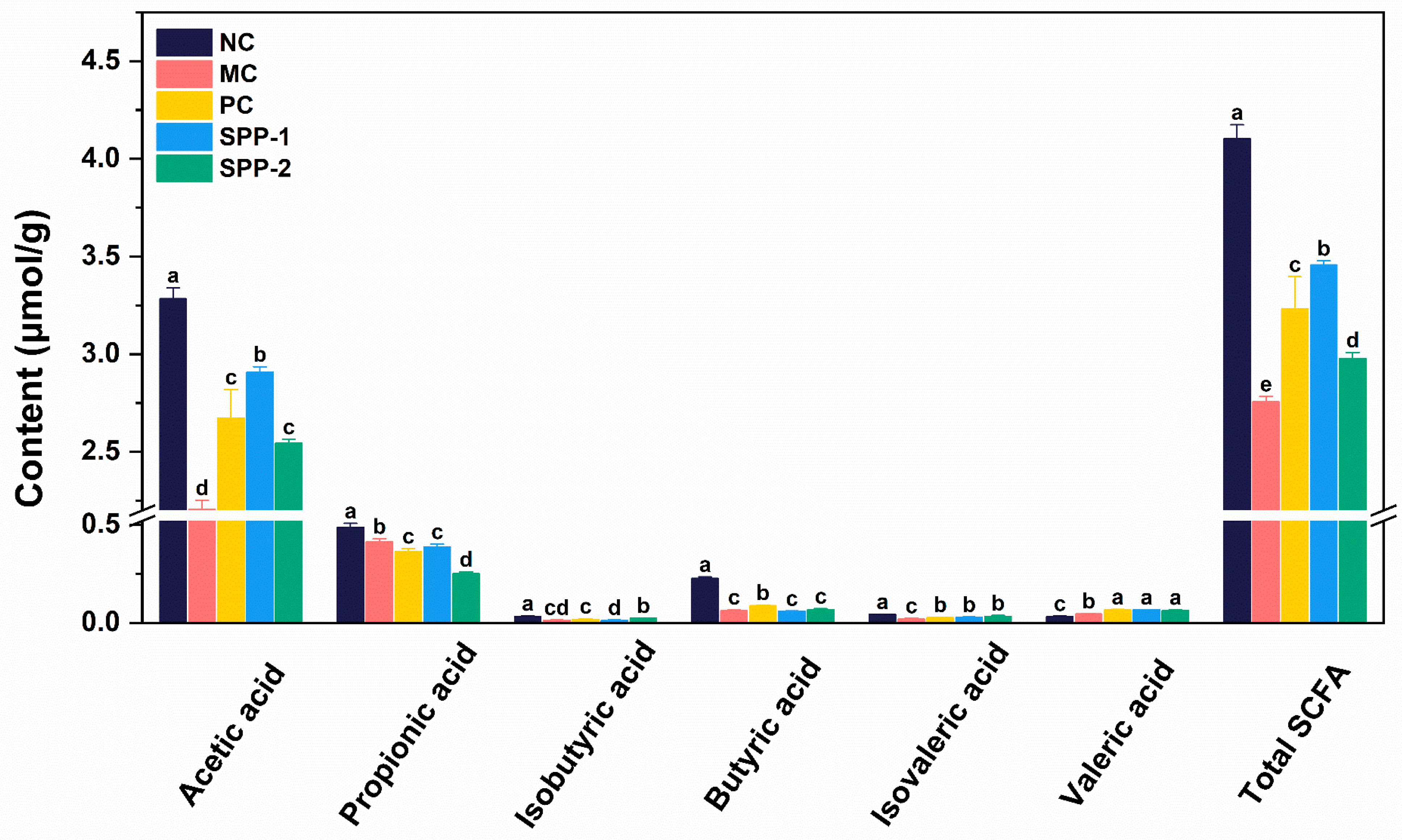
Figure 5. SCFA profile in feces of constipated mice. NC = normal control group; MC = model control group; PC = positive control group; SSP-1 and SSP-2, respectively, signify the groups treated with the two fractions (SSP-1 and SSP-2) isolated from the soluble dietary fiber of steamed sweet potato. Different letters between groups indicate significant differences (p < 0.05).
The function of SCFAs in the intestinal tract mainly includes stimulating peristalsis, promoting fluid secretion, increasing osmotic pressure, and protecting the mucosal barrier, thus performing a vital role in alleviating constipation [40,41]. Therefore, we further analyzed the correlation between SCFAs and other constipation-related parameters. As presented in , the acetic acid content was positively related to the GI transit rate, stool water content, and serum MTL level but was negatively related to the serum VIP level, defecation time, and SS level (p < 0.05). Butyric acid was found to be positively related to the MTL level and SP level but was negatively related to the VIP and SS levels (p < 0.05). Positive correlations were found between the fecal isobutyric acid content and the stool weight and MTL level, and a negative correlation was observed between the fecal isobutyric acid content and the serum VIP level (p < 0.05). Fecal isovaleric acid was also positively correlated with the stool pellet number, stool weight, stool water content, GI transit rate, and serum MTL level, while it was negatively correlated with the VIP level (p < 0.05). Additionally, a positive correlation between fecal valeric acid and GAS and a negative correlation between fecal propionic acid and GAS were detected (p < 0.05).
SCFAs are the major metabolites of indigestible carbohydrates fermented by the gut microbiota. Differences in fecal SCFA contents between the SSP-1 group and the SSP-2 group indicated their different fermentation characteristics, which also accounted for their different capacities for alleviating constipation in mice. The fermentation efficiency of neutral polysaccharides is usually higher than that of pectic polysaccharides, because few bacteria in the intestine can produce sufficient pectin hydrolases [42]. In agreement with existing evidence, the neutral polysaccharide, SSP-1, in this study produced more SCFAs in GI fermentation than the pectic fraction, SSP-2. As a result, the increased SCFAs, especially acetic acid, improved the osmotic pressure of the intestinal tract, promoted intestinal peristalsis, and increased the water content of intestinal contents [43], which explains the higher GI transit rate in the SSP-1 group compared to that in the SSP-2 group.
3.6. Effects of SSPs on the Gut Microbiota
4. Conclusions
This study compared the anti-constipation effects of two polysaccharide fractions, a neutral, resistant-dextrin-like polysaccharide and an acidic, pectin-derived polysaccharide, which were simultaneously isolated from steamed sweet potato extracts. The neutral fraction was superior to the acidic fraction in terms of the significant improvement in GI motility and better histological morphology of small intestinal villi, while the acidic fraction showed a better stool-bulking effect. The neutral fraction improved GI homeostasis by increasing the richness of beneficial bacteria such as Oscillibacter and producing more fecal acetic acid and total SCFAs. In contrast, the acidic fraction presented an inferior effect on regulating the GI microbiota. It could be concluded that the GI fermentation of polysaccharides played a more important role than its stool-bulking effect in alleviating constipation, and the production of acetic acid and total SCFAs accounted for the recovery of GI motility and defecation function in constipated mice. GI fermentability should be emphasized when a polysaccharide is considered as a potential therapeutic agent. While the gas production resulted from polysaccharide fermentation should be evaluated prudently, as far as the possible side effect is concerned.
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Talley, N.J.; Lasch, K.L.; Baum, C.L. A Gap in Our Understanding: Chronic Constipation and Its Comorbid Conditions. Clin. Gastroenterol. Hepatol. 2009, 7, 9–19. [Google Scholar] [CrossRef]
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global Prevalence of Functional Constipation According to the Rome Criteria: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef]
- Włodarczyk, J.; Waśniewska, A.; Fichna, J.; Dziki, A.; Dziki, Ł.; Włodarczyk, M. Current Overview on Clinical Management of Chronic Constipation. J. Clin. Med. 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Ford, A.C.; Mawe, G.M.; Dinning, P.G.; Rao, S.S.; Chey, W.D.; Simrén, M.; Lembo, A.; Young-Fadok, T.M.; Chang, L. Chronic Constipation. Nat. Rev. Dis. Primer 2017, 3, 17095. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, S. Constipation: Pathophysiology and Current Therapeutic Approaches. In Gastrointestinal Pharmacology; Greenwood-Van Meerveld, B., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2017; pp. 59–74. ISBN 978-3-319-56360-2. [Google Scholar]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of Functional Constipation in Children and Adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-Aligned Dietary Fiber Definitions Help to Bridge the ‘Fiber Gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef]
- Huang, J.; Lin, B.; Zhang, Y.; Xie, Z.; Zheng, Y.; Wang, Q.; Xiao, H. Bamboo Shavings Derived O-Acetylated Xylan Alleviates Loperamide-Induced Constipation in Mice. Carbohydr. Polym. 2022, 276, 118761. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Y.; Zeng, S.; Zheng, Y.; Wang, H.; Liao, H.; Song, J.; Zhang, X.; Cao, J.; Li, C. Polysaccharides from Holothuria Leucospilota Relieve Loperamide-Induced Constipation Symptoms in Mice. Int. J. Mol. Sci. 2023, 24, 2553. [Google Scholar] [CrossRef]
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina Platensis Ameliorates Diphenoxylate-Induced Constipation Symptoms in Mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101. [Google Scholar] [CrossRef]
- Yang, H.; Wu, C.; Chen, L.; Chang, X.; Luo, G.; Wu, K.; Tian, W.A. Macrocephala Polysaccharide Induces Alterations to Gut Microbiome and Serum Metabolome in Constipated Mice. Microb. Pathog. 2023, 178, 106084. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Song, J.; Zhai, X.; Cheng, N.; Xu, C.; Gui, S.; Chen, J. Improvement of Loperamide-Hydrochloride-Induced Intestinal Motility Disturbance by Platycodon Grandiflorum Polysaccharides through Effects on Gut Microbes and Colonic Serotonin. Front. Cell. Infect. Microbiol. 2023, 13, 1105272. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Yang, S.; Ma, Y.; Gong, Z.; Cui, W.; Wang, Y.; Wang, W. Extraction Optimization and Constipation-Relieving Activity of Dietary Fiber from Auricularia polytricha. Food Biosci. 2020, 33, 100506. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Acetic Acid and Butyric Acid Released in Large Intestine Play Different Roles in the Alleviation of Constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.; Zhong, F. The Effect of Viscous Soluble Dietary Fiber on Nutrient Digestion and Metabolic Responses II: In Vivo Digestion Process. Food Hydrocoll. 2020, 107, 105908. [Google Scholar] [CrossRef]
- Lan, J.; Wang, K.; Chen, G.; Cao, G.; Yang, C. Effects of Inulin and Isomalto-Oligosaccharide on Diphenoxylate-Induced Constipation, Gastrointestinal Motility-Related Hormones, Short-Chain Fatty Acids, and the Intestinal Flora in Rats. Food Funct. 2020, 11, 9216–9225. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 26 June 2023).
- Qin, Y.; Naumovski, N.; Ranadheera, C.S.; D’Cunha, N.M. Nutrition-Related Health Outcomes of Sweet Potato (Ipomoea batatas) Consumption: A Systematic Review. Food Biosci. 2022, 50, 102208. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Liu, X.; Hu, W.; Zhang, C.; Guo, Y.; Wu, W. Effects of Steaming on Sweet Potato Soluble Dietary Fiber: Content, Structure, and Lactobacillus Proliferation In Vitro. Foods 2023, 12, 1620. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, M.C.C.; Carpita, C. Measurement of Uranic Acids without Interference from Neutral Sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Qu, C.; Lin, G.; Zhang, Z.; Xie, J.; Chen, H.; Liang, J.; Li, C.; Wang, H.; Su, Z. Character and Laxative Activity of Polysaccharides Isolated from Dendrobium Officinale. J. Funct. Foods 2017, 34, 106–117. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Xu, Q.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacteria Exert Species-Specific Effects on Constipation in BALB/c Mice. Food Funct. 2017, 8, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sui, D.; Fu, W.; Yu, X.; Li, Y.; Wu, X.; Hou, Y.; Guo, M.; Xu, H. Laxative Effects of Yangyin Tongmi Capsule on a Model of Diphenoxylate-Induced Constipation in Mice. Evid. Based Complement. Alternat. Med. 2020, 2020, 1471824. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology; Day, C.E., Ed.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2014; Volume 1180, pp. 31–43. ISBN 978-1-4939-1049-6. [Google Scholar]
- Zhao, Z.; Liu, W.; Pi, X. In Vitro Effects of Stachyose on the Human Gut Microbiota. Starch-Stärke 2021, 73, 2100029. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Chen, M.; Gu, J.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant Activities of Sagittaria sagittifolia L. Polysaccharides with Subcritical Water Extraction. Int. J. Biol. Macromol. 2019, 134, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-J.; Hong, T.; Shi, H.-F.; Yin, J.-Y.; Koev, T.; Nie, S.-P.; Gilbert, R.G.; Xie, M.-Y. Probiotic Fermentation Modifies the Structures of Pectic Polysaccharides from Carrot Pulp. Carbohydr. Polym. 2021, 251, 117116. [Google Scholar] [CrossRef]
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. (Eds.) Chapter 4—Sweet Potato Pectin. In Sweet Potato Processing Technology; Academic Press: Cambridge, MA, USA, 2017; pp. 183–261. ISBN 978-0-12-812871-8. [Google Scholar]
- He, Y.; Liu, G.; Xia, C.; Chen, J.; Zhao, J.; Li, X.; Deng, J.; Wang, X.; Xiang, Z.; Zeng, P. Laxative Effect of Mulberry Ferment on Two Models of Constipated Mice. J. Funct. Foods 2022, 90, 104971. [Google Scholar] [CrossRef]
- Deloose, E.; Verbeure, W.; Depoortere, I.; Tack, J. Motilin: From Gastric Motility Stimulation to Hunger Signalling. Nat. Rev. Endocrinol. 2019, 15, 238–250. [Google Scholar] [CrossRef]
- Guilloteau, P.; Le Meuth-Metzinger, V.; Morisset, J.; Zabielski, R. Gastrin, Cholecystokinin and Gastrointestinal Tract Functions in Mammals. Nutr. Res. Rev. 2006, 19, 254–283. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Yik, Y.I.; Farmer, P.J.; King, S.K.; Chow, C.W.; Hutson, J.M.; Southwell, B.R. Gender Differences in Reduced Substance P (SP) in Children with Slow-Transit Constipation. Pediatr. Surg. Int. 2011, 27, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Weckbecker, G.; Lewis, I.; Albert, R.; Schmid, H.A.; Hoyer, D.; Bruns, C. Opportunities in Somatostatin Research: Biological, Chemical and Therapeutic Aspects. Nat. Rev. Drug Discov. 2003, 2, 999–1017. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent Advances in Vasoactive Intestinal Peptide Physiology and Pathophysiology: Focus on the Gastrointestinal System. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, 1801187. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Kim, C.C.; Kelly, W.J.; Patchett, M.L.; Tannock, G.W.; Jordens, Z.; Stoklosinski, H.M.; Taylor, J.W.; Sims, I.M.; Bell, T.J.; Rosendale, D.I. Monoglobus pectinilyticus Gen. Nov., sp. Nov., a Pectinolytic Bacterium Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2017, 67, 4992–4998. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, F.; Wang, Z.; Cao, J.; Li, C. Effect and Mechanism of Functional Compound Fruit Drink on Gut Microbiota in Constipation Mice. Food Chem. 2023, 401, 134210. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and Related Bacteria—From Metagenomic Species to Metabolic Features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, X.; Liu, X.; Yang, Y.; Li, Z. Chapter Seven—Autoimmune Diseases in China. In Advances in Immunology; Dong, C., Jiang, Z., Eds.; Advances in Immunology in China—Part A; Academic Press: Cambridge, MA, USA, 2019; Volume 144, pp. 173–216. [Google Scholar]
- Lu, W.; Li, J.; Gong, L.; Xu, X.; Han, T.; Ye, Y.; Che, T.; Luo, Y.; Li, J.; Zhan, R.; et al. H2S Modulates Duodenal Motility in Male Rats via Activating TRPV1 and KATP Channels. Br. J. Pharmacol. 2014, 171, 1534–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, X.; Deng, N.; Peng, X.; Tan, Z. Diarrhea with Deficiency Kidney-Yang Syndrome Caused by Adenine Combined with Folium senna Was Associated with Gut Mucosal Microbiota. Front. Microbiol. 2022, 13, 1007609. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, M.; Brown, S.; Chiarioni, G.; Dimidi, E.; Dudding, T.; Emmanuel, A.; Fox, M.; Ford, A.C.; Giordano, P.; Grossi, U.; et al. Chronic Constipation in Adults: Contemporary Perspectives and Clinical Challenges. 2: Conservative, Behavioural, Medical and Surgical Treatment. Neurogastroenterol. Motil. 2021, 33, e14070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan Oligosaccharides Attenuate Loperamide-Induced Constipation through Regulation of Gut Microbiota in Mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wei, X.; Deng, J.; Peng, C.; Ren, R.; Luo, Y.; Zhang, J.; Wei, X.; Hardiman, G.; Sun, Y.; et al. Integrating Omics and Network Pharmacology Reveals the Anti-Constipation Role of Chitosan with Different Molecular Weights in Constipated Mice. Int. J. Biol. Macromol. 2023, 235, 123930. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and Diet-Responsive Groups of Bacteria within the Human Colonic Microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zhu, H.; Qian, X.; Chen, Y.; Wang, Z.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Consumption of Butylated Starch Alleviates the Chronic Restraint Stress-Induced Neurobehavioral and Gut Barrier Deficits Through Reshaping the Gut Microbiota. Front. Immunol. 2021, 12, 755481. [Google Scholar] [CrossRef]

