1. Introduction
Combination immune checkpoint inhibition (cICI) with cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its ligand (PD-L1) inhibition is approved for use in patients with advanced melanoma and other malignancies including renal cell carcinoma and non-small cell lung cancer [1,2,3,4]. The improved survival benefit in these previously poor prognosis malignancies with combination therapy is offset by an increase in incidence and severity of collateral immune toxicity to healthy organs, collectively termed immune-related adverse events (irAEs) [2,5]. One of the most common irAEs after any ICI is thyroiditis [6,7]. In monotherapy clinical trials, thyroiditis was more common after blockade of the PD-1/PD-L1 pathway with an incidence of 8–38% compared to CTLA-4 inhibition with an incidence of 6–25% [7,8,9]. With the combination of ipilimumab and nivolumab in the landmark Checkmate 067 trial, hyper and/or hypothyroidism occurred in 28% [4]. Since then, observational studies have reported an incidence of thyroiditis after cICI of up to 56% [7,10].
In contrast to other forms of spontaneous thyroid autoimmunity, ICI therapy appears to induce a rapid onset, inflammatory thyroiditis resulting in a leak of pre-formed thyroid hormone and varying degrees of thyroid destruction [11]. Subclinical thyrotoxicosis, overt thyrotoxicosis and hypothyroidism are independently reported in clinical trial safety outcomes, but most probably reflect different time points of a common disease trajectory [12]. Hypothyroidism without a preceding thyrotoxic phase is less common [7].
At our center, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) is routinely utilized for tumor response assessment in patients receiving cICI for advanced melanoma [13,14]. A limited number of studies indicate that new increased metabolic activity in specific organs, including the thyroid gland, on routine FDG-PET/CT imaging performed in the first 3–6 months of treatment may be suggestive of irAEs [15,16,17,18]. While the finding of increased uptake of FDG in the thyroid after ICI is common [19], the temporal relationship between these findings and clinical and biochemical evidence of thyroiditis has not been systematically evaluated. We aimed to determine the association of FDG-PET/CT findings suggestive of thyroiditis developing in response to cICI as compared to the reference standard of biochemical assessment of thyroid function tests (TFTs). We further sought to evaluate interobserver agreement for FDG-PET/CT as a novel modality for the detection of ongoing thyroiditis and derive semiquantitative cut-offs values for this diagnosis.
2. Methods and Analysis
A pharmacy dispensing report was used to identify all patients who received at least one dose of cICI for metastatic melanoma between January 2016 and January 2019 at The Peter MacCallum Cancer Centre; a quaternary melanoma center in Melbourne, Victoria, Australia. Patients were eligible for inclusion if they had available FDG-PET/CT imaging at baseline and within six months of commencing cICI. Patients who had received prior first-line therapy with single-agent anti-PD-1, anti-CTLA-1 or BRAF/MEK inhibition were included. However, patients with a documented history of thyroiditis related to a prior line of immunotherapy were excluded. A chart review was undertaken to identify patients diagnosed with thyroiditis and the corresponding clinical presentation, TFT results and thyroid autoantibodies were recorded. Clinical events were followed for a minimum of 12 months.
2.1. Test Methods
2.2. Analysis
The reference standard for a diagnosis of thyroiditis was determined by TFTs. Clinical variables in patients with and without thyroiditis were compared using Fisher’s exact, χ2 and Wilcoxon rank sum tests. Time to diagnosis of thyroiditis and duration of the thyrotoxic phase were described as continuous variables with median and interquartile ranges (IQRs). The duration of the thyrotoxic phase was defined as the time in weeks from first TFT evidence of subclinical or overt thyrotoxicosis to normalization of TFTs or a TSH above the upper limit of normal (whichever occurred first). Patients diagnosed with thyroiditis who had indeterminate follow-up results were excluded from the analysis of the duration of the thyrotoxic phase.
The result of the index test was listed as a binary positive or negative. For the purpose of the analysis, equivocal results (one user only) were classified as negative. The diagnostic accuracy of FDG-PET/CT with respect to the reference standard was represented as sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) with 95% confidence intervals. The AUC for the overall performance of FDG-PET/CT was calculated as the mean of the estimated sensitivity and specificity by reported operating characteristics (ROC). Interobserver agreement for the diagnosis of thyroiditis by FDG-PET was assessed by the Cohen’s kappa statistics. The optimal cut-offs for thyroid SUVmax and its temporal changes suggestive of thyroiditis were selected based on a predictive model for optimal sensitivity and specificity. All statistical analyses were performed using STATA version 16.1 (STATA LP, College Station, TX, USA).
3. Results
3.1. Participants
Of 162 patients treated with combination ICIs from January 2016 to January 2019, 127 patients met the inclusion criteria for the study. Of the 35 patients excluded, 28 lacked post-treatment FDG-PET imaging and seven patients had a prior history of thyroiditis. The median age was 59 years (range 18–79) and 29% were female. Overall, 43/127 (34%) had a diagnosis of thyroiditis established with TFTs (Figure 1). There was no difference in patient demographics between those with and without thyroiditis . Prior exposure to anti-CTLA4 or anti-PD1 was not associated with new-onset thyroiditis after cICI. A higher proportion of patients with thyroiditis had previously received BRAF/MEK inhibition (58% versus 31%, p = 0.003).
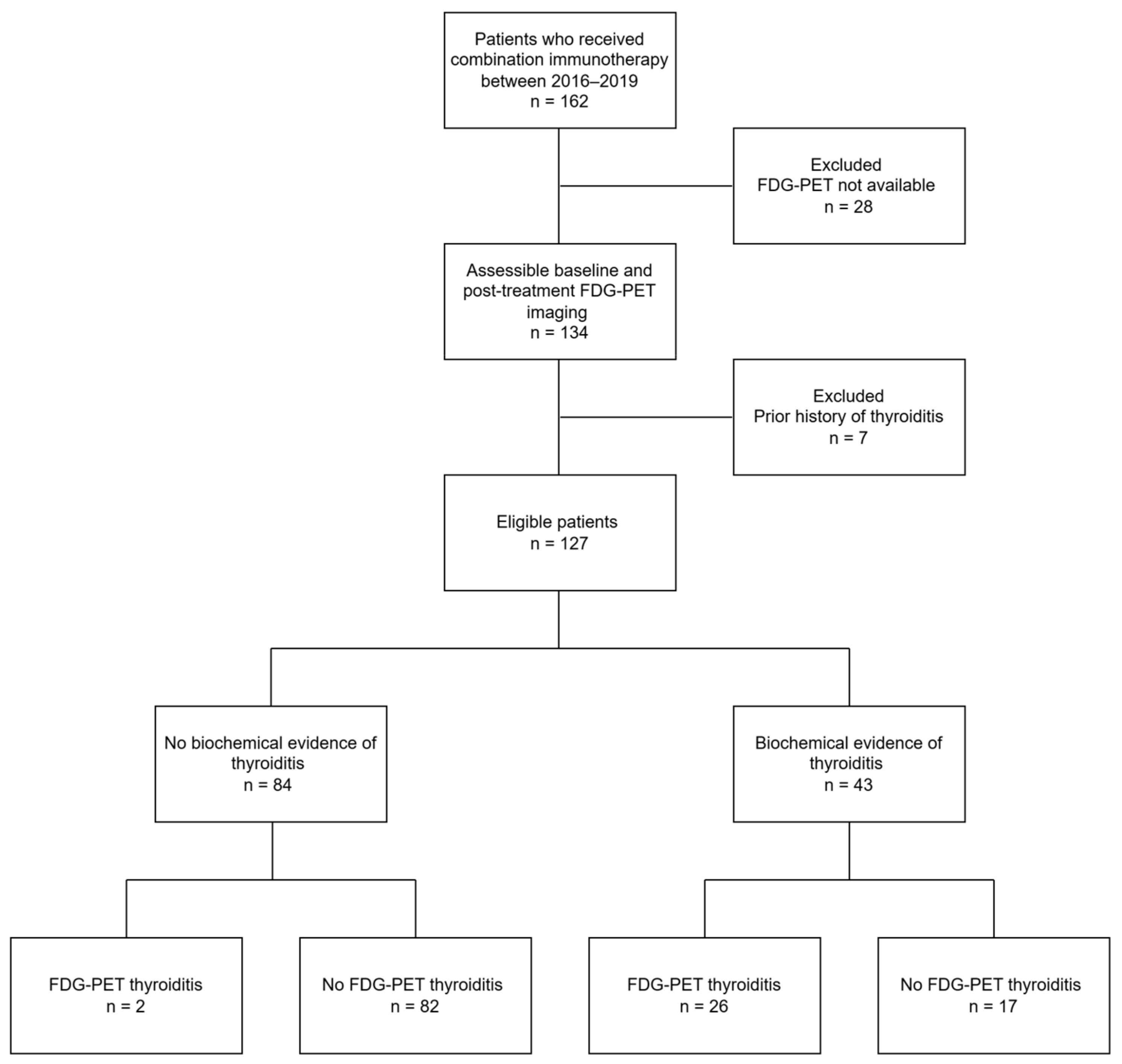
Figure 1. Consort diagram outlining patient eligibility and summary of FDG-PET/CT and clinical findings.
3.2. Established Thyroiditis
Thyroiditis was diagnosed biochemically at a median of 3 weeks from commencement of cICI (range 1–15). 37/43 (86%) patients had a documented thyrotoxic phase, which was overt in 34 and subclinical in three, and lasted a median duration of 4.5 weeks. The thyrotoxic phase was transient in all cases. Six patients first presented with hypothyroidism, and 19/37 (51%) patients with a transient thyrotoxic phase progressed to a hypothyroid phase. Overall, thyroxine replacement was commenced in 23/43 patients. Once commenced, thyroxine withdrawal was not attempted by the treating clinicians. Most patients (38/43) were managed in the outpatient setting with mild symptoms. Five patients required hospital admission during the thyrotoxic phase, although four were primarily receiving treatment for another contemporaneous irAE. Two patients complained of mild transient neck pain which did not require treatment with glucocorticoids. TPO and TG ab were measured in 17/43 patients and were elevated in six (35%) and 11 (65%) respectively. TSHrAb was measured in 13 people. Only one individual had a high titre of TSHrAb (>40 IU/L, reference range < 1.8 IU/L). Thyroid hormone levels in this individual followed a pattern of minimally symptomatic transient thyrotoxicosis followed by permanent hypothyroidism. In this case, the presence of TSHrAb did not herald prolonged thyroid hormone overproduction.
3.3. Index Test Results
FDG/PET-CT was performed in all patients at a median of 11 weeks from commencement of cICI (range 3–32). FDG/PET-CT was declared positive by both NMPs in 26/43 (60%) patients with thyroiditis but only 2/84 (2%) patients not diagnosed with thyroiditis , resulting in an AUC of 0.87 [95% CI 0.8, 0.94]. Recognizing the temporal discordance in biochemical testing and scanning in many patients, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 61% [95% confidence interval (CI) 44–75], 98% [95% CI 92–100%], 93% [95% CI 77–91] and 83% [95% CI 74–90], respectively.
Temporal evolution of the thyroid FDG uptake in a patient with thyroiditis is demonstrated in Figure 2. In 22/28 patients with PET-thyroiditis and available further follow-up FDG PET/CT, resolution of FDG uptake to baseline was noted in all cases by a median of 28.6 weeks (range 17.9–158) from the start of cICIs.
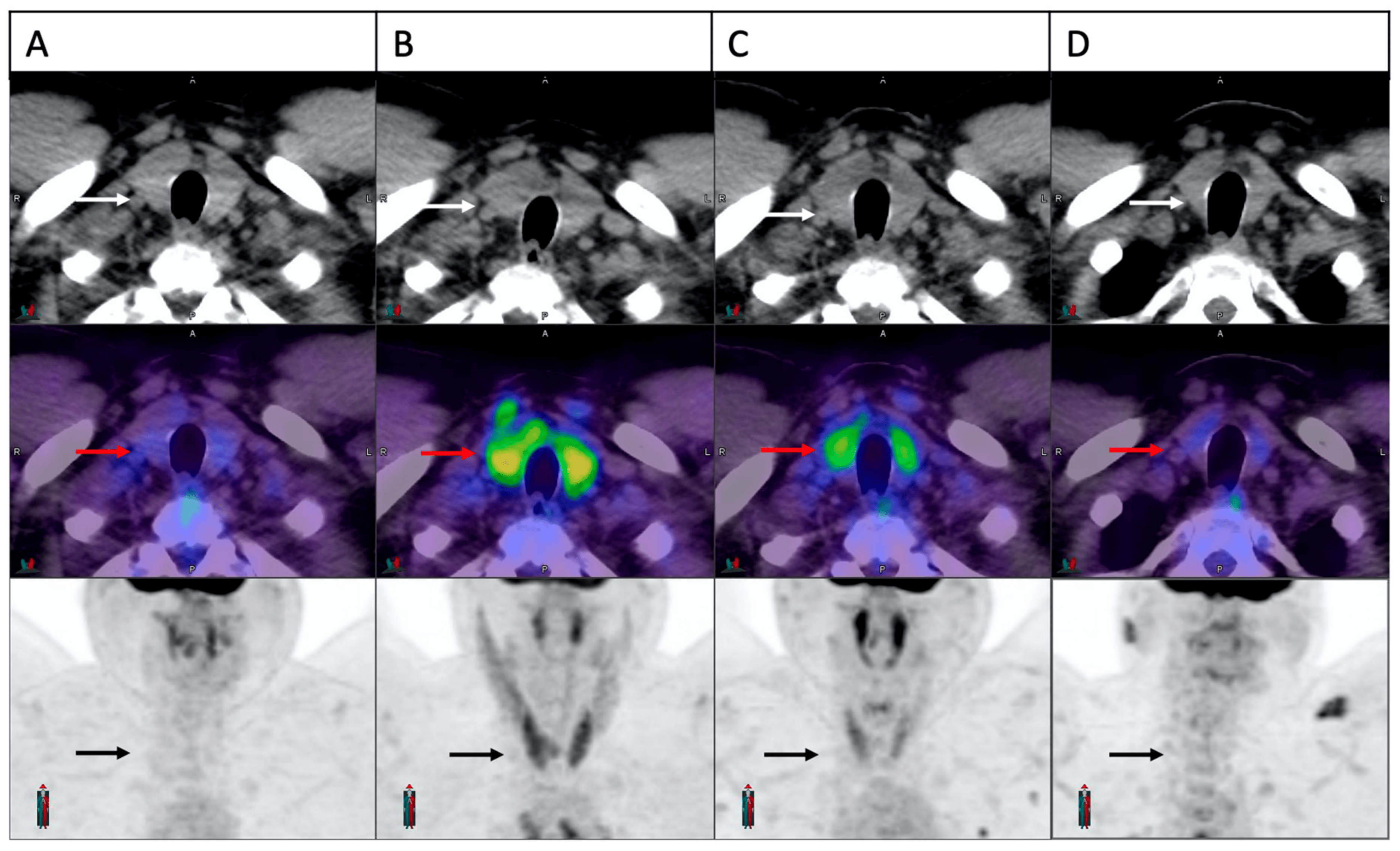
Figure 2. Temporal evolution of thyroid FDG uptake in a patient with cICI related thyroiditis. Trans-axial and maximum intensity projection images of the thyroid gland demonstrated in sequence on CT scan (white arrows), fusion image (red arrows) and PET scan (black arrows). Normal thyroid appearance is demonstrated at baseline (A). A significant increase in FDG uptake is noted on the first on-treatment scan at three weeks (B). Panel (C,D) demonstrate partial and complete resolution at 6 weeks and 24 weeks, respectively.
3.4. Clinical Correlation
The timing of FDG-PET/CT with respect to the biochemical diagnosis is illustrated in and Figure 5. In almost all cases, the biochemical diagnosis preceded the first on treatment FDG-PET/CT. There was no difference in the time interval between biochemical thyroiditis and the first FDG-PET/CT in patients with and without FDG-PET-detected thyroiditis.
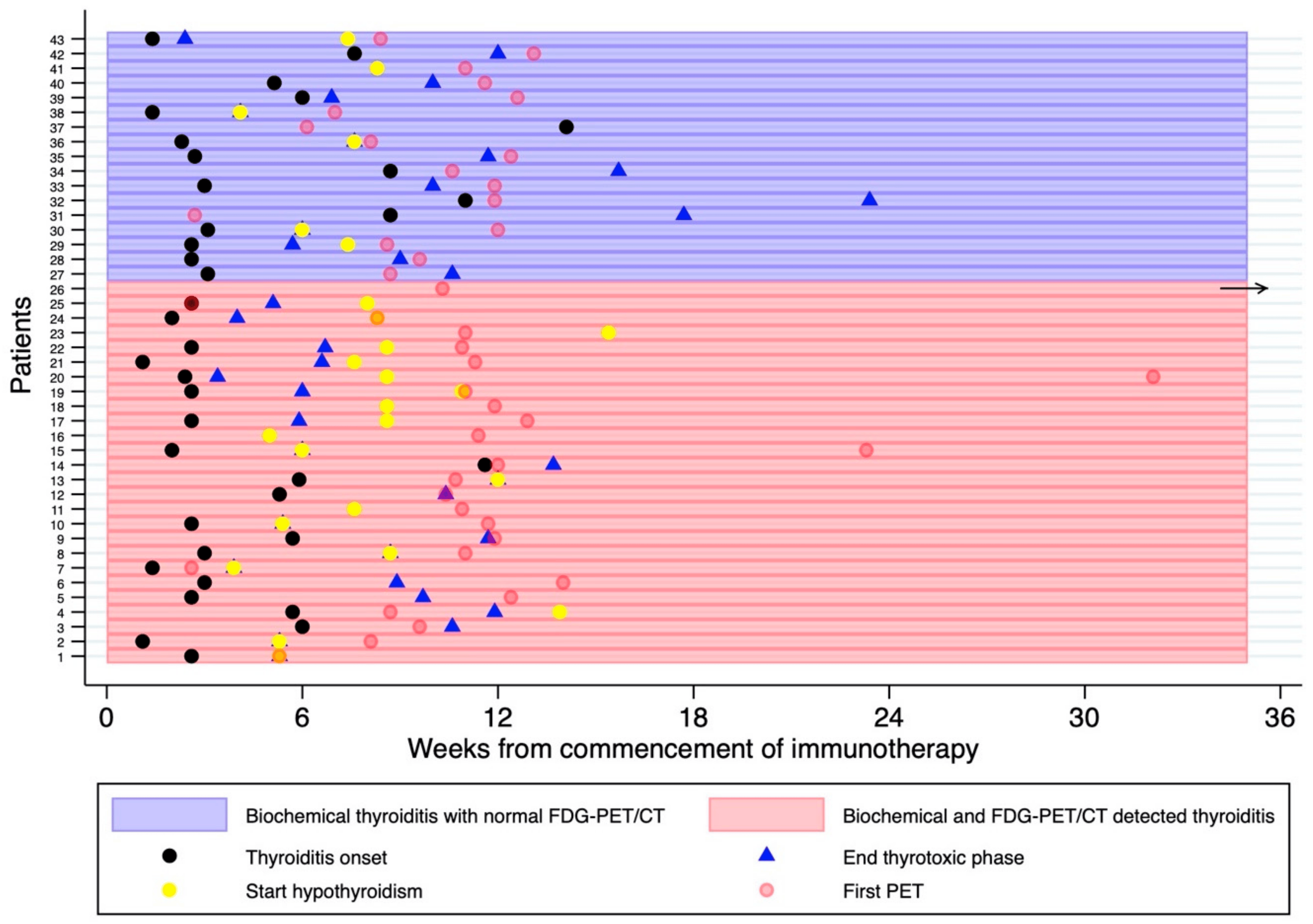
Figure 5. Swimmer Plot demonstrating the timing of the first on-treatment FDG-PET/CT with respect to the thyroiditis diagnosis, typically represented by an early thyrotoxic phase followed by euthyroid and/or hypothyroid phase. Thyroid function tests were performed every 4–6 weeks or more frequently if clinically indicated. The horizontal black arrow represents an outlier, whereby the thyrotoxicosis was diagnosed 97 months after the first FDG-PET/CT.
Overall, patients with FDG-PET-detected thyroiditis were more likely to develop overt hypothyroidism (71% versus 6%, p < 0.001). One patient had an abnormal diffuse thyroid uptake on FDG-PET at baseline (2 months prior to cICI), which resolved on the first follow up study (Figure 6). This patient had normal thyroid function at the time of the baseline FDG-PET but developed severe thyrotoxicosis (FT4 138 pmol/L, TSH < 0.01 mU/L) 10 days after commencing cICI, 2 months after the abnormal baseline FDG-PET/CT and 1.5 months before a normal follow up FDG-PET/CT. Autoantibodies were not measured. No prior anti-PD-1 or anti-CTLA-4 therapy had been administered in this case, indicating a probable pre-existing thyroid autoimmunity.
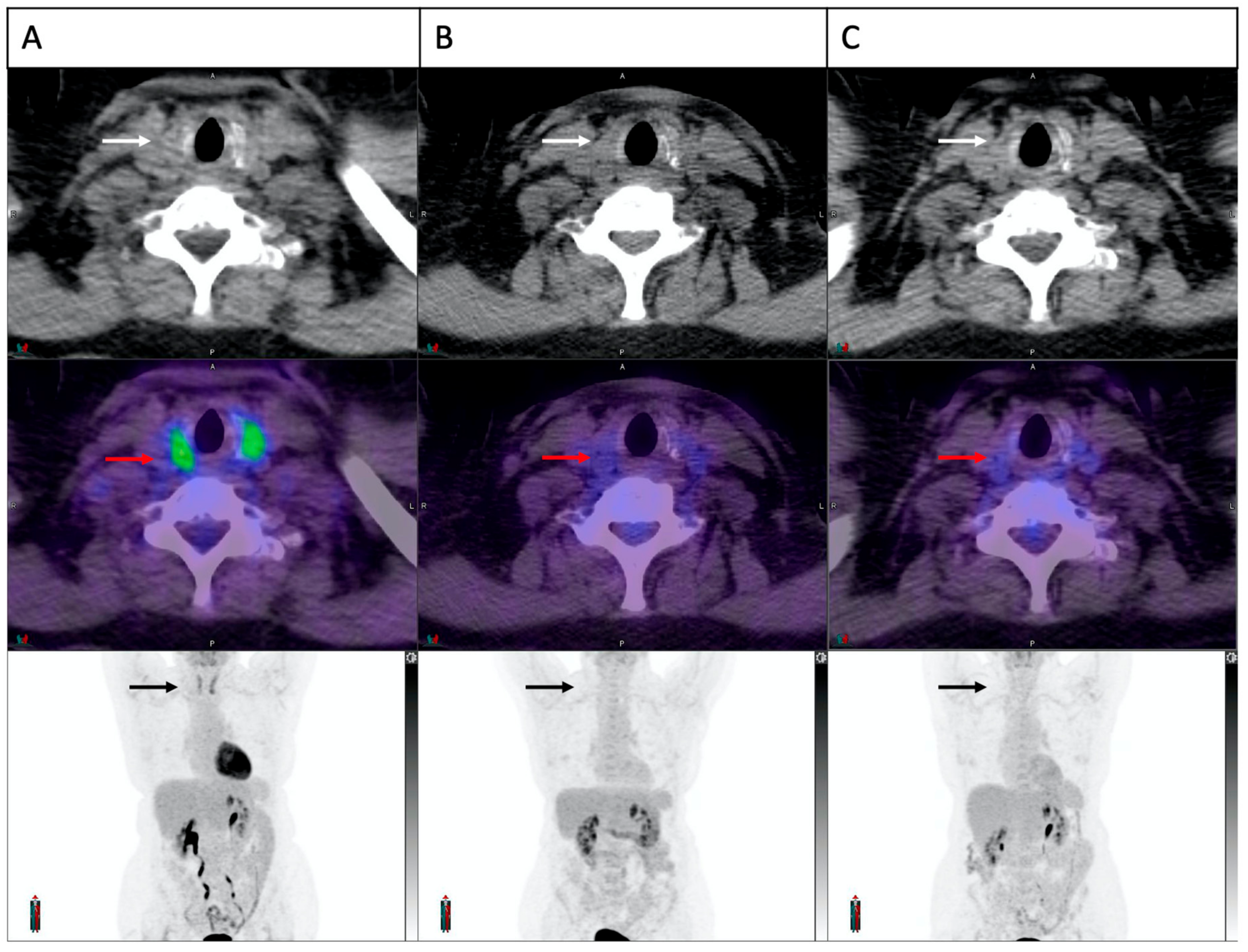
Figure 6. Increased thyroid FDG uptake at baseline in a patient who developed cICI related thyroiditis. Trans-axial and maximum intensity projection images of the thyroid gland demonstrated in sequence on CT scan (white arrows), fusion image (red arrows) and PET scan (black arrows). A clear increase in thyroid FDG uptake is demonstrated at baseline (A), which had resolved completely on both on-treatment scans at 6 and 14 weeks (B,C). The patient was euthyroid at baseline and developed biochemical thyroiditis 10 days after commencement of cICI.
There were two patients without clinical thyroiditis who had an abnormal FDG-PET/CT result. One patient was diagnosed with hypophysitis and secondary hypothyroidism. While there was no thyrotoxic phase, subsequent primary hypothyroidism could not be excluded. The second patient had normal TFTs two weeks before and one month after the abnormal FDG-PET and remained euthyroid.
3.5. Concomitant Muscle Uptake
In the 28 patients with FDG-PET/CT-detected thyroiditis, there were two patients with overt evidence of FDG-PET manifestations of myopathy. In both cases, transient diffuse skeletal muscle FDG uptake occurred concurrently with increased thyroid uptake and biochemical thyroiditis, suggesting the differential diagnoses of thyroid myopathy or concomitant inflammatory myositis (Figure 7) One patient remained asymptomatic while the other experienced significant proximal weakness for the duration of the thyrotoxic phase. This patient developed biochemical thyrotoxicosis 9 days after commencement of cICI, while receiving high-dose glucocorticoid treatment for cerebral metastases. Over the following 3 weeks, progressive generalized muscle weakness was reported despite a decreasing dose schedule of glucocorticoids. Random cortisol and serum creatine kinase levels were normal. FDG-PET/CT performed two months after treatment commencement demonstrated interval development of thyroid and diffuse skeletal muscle uptake. The weakness resolved after transition to the hypothyroid phase.
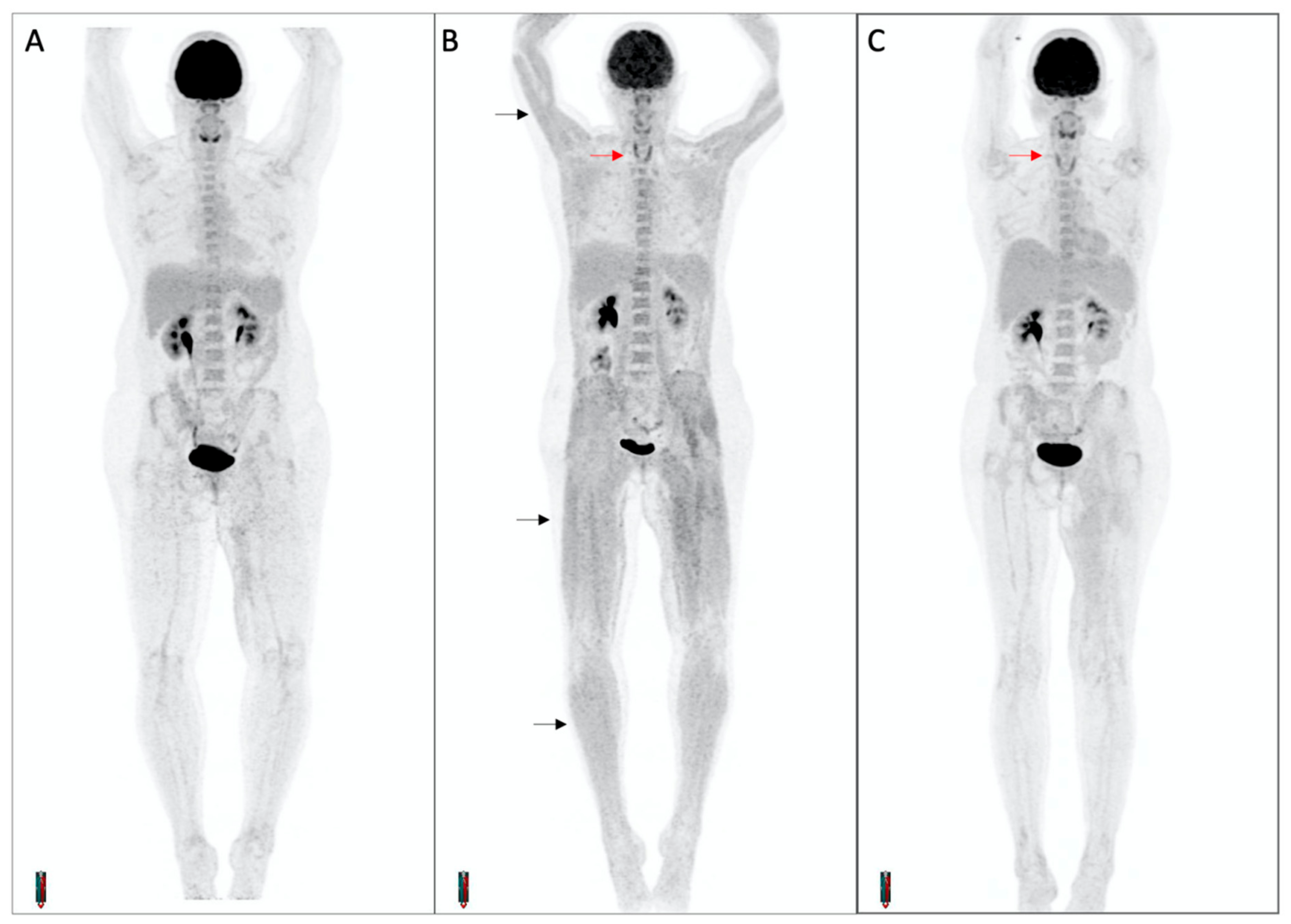
Figure 7. Diffuse skeletal muscle uptake of FDG in a patient with thyroiditis. Maximum intensity projection PET images at baseline (A) and on-treatment (B,C) demonstrate interval development of thyroiditis (red arrows) and diffuse skeletal muscle uptake (black arrows). The patient complained of progressive upper and lower limb weakness during the thyrotoxic phase, with a normal CK.
4. Discussion
Our data show that cICI-related thyroiditis was common, with a prevalence similar to the combination arm of the CheckMate 067 study (34% compared to 28%) [2]. FDG-PET/CT had a high specificity and positive predictive value for thyroiditis with respect to the reference standard biochemical test, although the temporal profiles of biochemical and scan findings were out of sync, primarily related to the discordant timing of routine biochemical testing and FDG PET/CT response assessment. Biochemical diagnosis of thyroiditis occurred early, at a median of 3 weeks from commencement of cICI. The lower sensitivity of FDG-PET/CT may relate to the timing of the imaging with respect to onset of the thyroiditis. With the median time of the first FDG-PET/CT scan 11 weeks, most patients had already developed biochemical abnormality and, in a subgroup of patients, the acute inflammatory phase (as represented by increased FDG uptake in the gland) may have resolved by the time of the first scan.
As changes in thyroidal uptake occur after the diagnosis is made by biochemical thyroid function tests, the incidental finding of diffusely increased FDG uptake on routine on-treatment FDG PET/CT is highly diagnostic of thyroiditis rather than thyroidal tumor metastases.
The initial thyrotoxic phase was generally detected prior to FDG-PET/CT evaluation and was largely resolved by the time FDG-PET/CT demonstrated evidence of ongoing increasing FDG uptake. This finding suggests that thyroid inflammation continues beyond the phase of initial thyroiditis with hormone release and may indicate an ongoing process of thyroid destruction.
Our finding that 53.5% of patients developed permanent hypothyroidism is similar to the published literature. In one study of 1246 patients treated with checkpoint inhibitors, permanent hypothyroidism requiring levothyroxine replacement occurred in 43% of patients who initially presented with overt thyrotoxicosis and only 3% of those whose initial presentation was subclinical thyrotoxicosis [22].
Indeed, patients who had FDG-PET/CT evidence of thyroiditis had a higher likelihood of developing overt hypothyroidism requiring ongoing thyroid hormone replacement. Hence, we postulate that the presence of increased FDG activity may indicate a more persistent and robust autoimmune response with destruction of thyrocytes by the adoptive immune system. Whether thyroid hormone replacement could be withdrawn when FDG uptake in the gland had resolved was not tested in this population, with clinicians continuing treatment once initiated. This practice is in line with the literature, which suggests most cases of overt hypothyroidism after ICI are permanent [7].
4.1. Pathophysiology of Thyroiditis
Currently, there are very little data as to the pathophysiology of ICI-related thyroiditis. As a glucose analogue radiotracer, FDG uptake in thyroiditis may reflect the increase glycolytic activity of activated CD8+ T cells infiltrating the gland [23]. Cytological and molecular analyses of thyroid tissue would be of benefit in understanding the underlying mechanisms. However, thyroid biopsy in otherwise recovering patients is difficult to justify [6]. In our cohort, TPOAb and TgAb were elevated in 35% and 65%, respectively, compared to the expected 95% and 80% reported in spontaneous autoimmune thyroid disease [24]. One observational study has shown that TgAb but not TPOAb positivity is associated with an increased likelihood of progression to permanent hypothyroidism following the hyperthyroid phase [25]. In our study, baseline thyroid antibody levels were not measured. Muir et al. have demonstrated across several studies that baseline TPOAb and TGAb positivity were more common in patients who developed thyroid irAEs than in those who did not, and patients where there was a greater than 50% increase in baseline antibody titre or new seroconversion were similarly more likely to experience thyroiditis than those who did not [22,25]. In thyroid irAE, antibody seroconversion may be a late or bystander response to a rapid pathogenic T cell autoimmunity, or antibody specificity in thyroid irAE may be different to that of spontaneous thyroiditis. The heterogeneity of the antibody response after treatment likely negates them as a clinically useful biomarker, however baseline antibody levels may be useful for risk prediction.
New onset Graves’ disease after ICI is rare but has been described in case reports, and guidelines do recommend measuring TSHrAb in any case of severe or symptomatic thyrotoxicosis to guide initiation of antithyroid drugs [26,27,28]. The one patient with a high titre of TSHrAb in our cohort experienced a brief thyrotoxic phase followed by permanent hypothyroidism. The absence of evidence of thyroid hormone overproduction in ICI-related thyroiditis prompts the recommendation that anti-thyroid drugs are unlikely to be useful in this setting. If commenced due to clinical concern or an elevated TSHrAb, TFTs should be monitored within 2-weekly intervals with a low threshold to cease treatment once the spontaneous thyrotoxic phase resolves.
4.2. FDG-PET/CT in ICI Related Thyroiditis
Incidental diffuse thyroid uptake on FDG-PET/CT is known to be associated with a higher incidence of spontaneous thyroid dysfunction in adults free of malignancy [29]. A limited number of studies have reviewed the role of FDG-PET/CT in ICI-related thyroiditis. At our center, we have previously demonstrated the accuracy of FDG-PET/CT in detecting both tumoral and systemic immune response. In our prior study, increased uptake in the thyroid following treatment was found in six patients, all of whom had biochemical correlation [15]. Yamauchi and colleagues reviewed results from baseline FDG-PET/CT imaging performed prior to ICI administration in 111 patients. A significant correlation between increased thyroid uptake before treatment and development of thyroiditis after treatment was observed (46.7% versus 4.2% p < 0.001) [16]. In our current study, all patients had TFTs prior to cICI and we excluded patients with a prior history of thyroid dysfunction. However, we did find evidence of pre-treatment FDG-PET/CT-detected thyroiditis in one patient who went on to develop new thyrotoxicosis soon after the first dose of cICI. In a small study of 18 lung cancer patients treated with nivolumab, baseline imaging was not associated with thyroiditis, though increased uptake in the thyroid 10–16 weeks after treatment commencement was predictive of hypothyroidism before the TSH became elevated [19]. In that study, only six patients had thyroiditis. The finding that the presence of thyroid metabolic activity on FDG-PET/CT is predictive of overt hypothyroidism was replicated in our study with a larger number of patients.
Because FDG-PET/CT imaging is commonly performed at an interval of 12 weeks from the onset of cICI largely to assess tumoral response and biochemical thyroiditis occurs within three weeks of commencing cICI, FDG-PET/CT findings suggestive of thyroiditis have limited diagnostic clinical utility. Nonetheless, an incidental observation of increased thyroid uptake should prompt careful monitoring of thyroid function if not already known to be abnormal. Whether thyroid hormone replacement can be safely withdrawn after resolution of active thyroid uptake of FDG on PET remains unknown.
4.3. Increased Incidence of Thyroiditis in Patients Previously Exposed to BRAF/MEK Inhibition
Our finding of a higher proportion of thyroiditis in patients who previously received BRAF/MEK inhibition (58% versus 31%, p = 0.003) has to our knowledge not been previously reported. Mitogen-activated protein kinase (MAPK) pathway signaling is important to thyroid biology as demonstrated in thyroid cancer. In papillary thyroid carcinoma driven by BRAF pathway mutations, there is decreased expression of sodium iodide symporter (NIS), TSH receptors and tumor cell specific MHC II. MHC class II expression in tumor cells increases antigen presentation to prime infiltrating CD4+ T cells, and decreased tumor cell MHC class II expression may result in immune escape. These proteins are restored by BRAF pathway inhibitors and thus enhancing immune response to tumor cells. It is not known if thyroid cell MHC class II expression is upregulated in normal thyroid cells to explain the increased immune response against thyroid cells following treatment with BRAF inhibitors seen in this cohort [30,31]. Perhaps inhibition of this pathway may pre-sensitize the thyroid to expressing alterative cell membrane autoantigens. Of note, prior treatment with CTLA-4 inhibitors or PD-1 inhibitors did not appear to prime the thyroid for thyroiditis events after cICI.
5. Conclusions
The primary role of FDG-PET/CT is monitoring of tumoral response and not for the evaluation of thyroiditis. While we admit the limitations in the utility of FDG PET/CT in the initial diagnosis of thyroiditis, the study has followed the recommendations for novel test performance characteristics in the analysis of the sensitivity/specificity/NPV/PPV of FDG PET/CT with respect to the reference standard test. With an increasing incidence of thyroid abnormalities detected on routine FDG-PET/CT imaging it is of great importance to understand the implications of these findings when interpreting the FDG PET/CT. To our knowledge, this is the first study to systematically evaluate the temporal profile of FDG-PET/CT findings of thyroiditis in relation to the biochemical and clinical course of thyroid dysfunction after cICI.
We demonstrate that biochemical thyrotoxicosis and hypothyroidism usually represent different time-points along the same disease trajectory, but a minority present with hypothyroidism not preceded by a thyrotoxic phase. Although routine FDG-PET/CT is commonly performed after the biochemical thyroid abnormality was detected, our study demonstrates that FDG-PET/CT has a high specificity and positive predictive value for ICI-related thyroiditis and correlates with progression to permanent hypothyroidism. We demonstrate high interobserver agreement for the detection of thyroiditis with FDG-PET and describe semiquantitative cut-off values using SUVmax and % change with respect to baseline.
References
- U.S. Food and Drug Administration. FDA Approves Nivolumab plus Ipilimumab and Chemotherapy for First-Line Treatment of Metastatic NSCLC. Drug Approvals and Databases 2020. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-plus-ipilimumab-and-chemotherapy-first-line-treatment-metastatic-nsclc (accessed on 4 July 2020).
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39 (Suppl. 15), 9506. [Google Scholar] [CrossRef]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Muir, C.A.; Tsang, V.H.M.; Menzies, A.M.; Clifton-Bligh, R.J. Immune Related Adverse Events of the Thyroid—A Narrative Review. Front. Endocrinol. 2022, 13, 886930. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Angell, T.E. Thyroiditis While Receiving Programmed Death Ligand 1 (PD-L1) Inhibitor Therapy for Nonthyroid Cancers Is Associated with Improved Overall Survival. Clin. Thyroid. 2020, 32, 65–68. [Google Scholar] [CrossRef]
- Muir, C.A.; Menzies, A.M.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Tsang, V. Phenotypic Differences in Thyroid Immune Related Adverse Events Following Treatment with Immune Checkpoint Inhibitors. J. Endocr. Soc. 2021, 5, A876–A877. [Google Scholar] [CrossRef]
- Yamauchi, I.; Sakane, Y.; Fukuda, Y.; Fujii, T.; Taura, D.; Hirata, M.; Hirota, K.; Ueda, Y.; Kanai, Y.; Yamashita, Y.; et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid 2017, 27, 894–901. [Google Scholar] [CrossRef]
- Iyer, P.C.; Cabanillas, M.E.; Waguespack, S.G.; Hu, M.I.; Thosani, S.; Lavis, V.R.; Busaidy, N.L.; Subudhi, S.K.; Diab, A.; Dadu, R. Immune-Related Thyroiditis with Immune Checkpoint Inhibitors. Thyroid 2018, 28, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Lewin, J.; Sayers, L.; Kee, D.; Walpole, I.; Sanelli, A.; Marvelde, L.T.; Herschtal, A.; Spillane, J.; Gyorki, D.; Speakman, D.; et al. Surveillance imaging with FDG-PET/CT in the post-operative follow-up of stage 3 melanoma. Ann. Oncol. 2018, 29, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Wallace, R.; Lo, S.N.; Galligan, A.; Weppler, A.M.; Hicks, R.J.; Sandhu, S. FDG PET/CT Prognostic Markers in Patients with Advanced Melanoma Treated with Ipilimumab and Nivolumab. Radiology 2023, 307, e221180. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Osman, M.M.; Weppler, A.M.; Wallace, R.; Galligan, A.; Lasocki, A.; Hunter, M.O.; Akhurst, T.; Hofman, M.S.; Lau, P.K.H.; et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2776–2786. [Google Scholar] [CrossRef]
- Yamauchi, I.; Yasoda, A.; Matsumoto, S.; Sakamori, Y.; Kim, Y.H.; Nomura, M.; Otsuka, A.; Yamasaki, T.; Saito, R.; Kitamura, M.; et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE 2019, 14, e0216954. [Google Scholar] [CrossRef] [PubMed]
- Cherk, M.H.; Nadebaum, D.P.; Barber, T.W.; Beech, P.; Haydon, A.; Yap, K.S. 18F-FDG PET/CT features of immune-related adverse events and pitfalls following immunotherapy. J. Med. Imaging Radiat. Oncol. 2022, 66, 483–494. [Google Scholar] [CrossRef]
- Lasocki, A.; Iravani, A.; Galligan, A. The imaging of immunotherapy-related hypophysitis and other pituitary lesions in oncology patients. Clin. Radiol. 2021, 76, 325–332. [Google Scholar] [CrossRef]
- Eshghi, N.; Garland, L.L.; Nia, E.; Betancourt, R.; Krupinski, E.; Kuo, P.H. 18F-FDG PET/CT Can Predict Development of Thyroiditis Due to Immunotherapy for Lung Cancer. J. Nucl. Med. Technol. 2018, 46, 260–264. [Google Scholar] [CrossRef]
- Kaalep, A.; Sera, T.; Oyen, W.; Krause, B.J.; Chiti, A.; Liu, Y.; Boellaard, R. EANM/EARL FDG-PET/CT accreditation—Summary results from the first 200 accredited imaging systems. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 412–422. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Muir, C.A.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Lo, S.N.; Carlino, M.S.; Tsang, V.H.M.; Menzies, A.M. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e3704–e3713. [Google Scholar] [CrossRef]
- Wachsmann, J.W.; Ganti, R.; Peng, F. Immune-mediated Disease in Ipilimumab Immunotherapy of Melanoma with FDG PET-CT. Acad. Radiol. 2017, 24, 111–115. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Muir, C.A.; Wood, C.C.G.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Carlino, M.S.; Menzies, A.M.; Tsang, V.H.M. Association of Antithyroid Antibodies in Checkpoint Inhibitor-Associated Thyroid Immune-Related Adverse Events. J. Clin. Endocrinol. Metab. 2022, 107, e1843–e1849. [Google Scholar] [CrossRef]
- Brancatella, A.; Viola, N.; Brogioni, S.; Montanelli, L.; Sardella, C.; Vitti, P.; Marcocci, C.; Lupi, I.; Latrofa, F. Graves’ Disease Induced by Immune Checkpoint Inhibitors: A Case Report and Review of the Literature. Eur. Thyroid J. 2019, 8, 192–195. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Rees Smith, B.; McLachlan, S.M.; Furmaniak, J. Autoantibodies to the Thyrotropin Receptor. Endocr. Rev. 1988, 9, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Chang, Y.; Kim, Y.; Kim, S.J.; Rhee, E.J.; Kwon, H.; Ahn, J.; Ryu, S. Diffusely Increased 18F-FDG Uptake in the Thyroid Gland and Risk of Thyroid Dysfunction: A Cohort Study. J. Clin. Med. 2019, 8, 443. [Google Scholar] [CrossRef]
- Schubert, L.; Mariko, M.L.; Clerc, J.; Huillard, O.; Groussin, L. MAPK Pathway Inhibitors in Thyroid Cancer: Preclinical and Clinical Data. Cancers 2023, 15, 710. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Zhang, P.; Zhang, W.; Ruan, X.; Tian, M.; Guo, S.; Zhang, W.; Zheng, X.; Zhao, L.; Gao, M. Inhibition of BRAF Sensitizes Thyroid Carcinoma to Immunotherapy by Enhancing tsMHCII-mediated Immune Recognition. J. Clin. Endocrinol. Metab. 2021, 106, 91–107. [Google Scholar] [CrossRef]

