1. Introduction
In the last years, CD73/adenosine has been prompted as a novel target in lung cancer research. CD73 is encoded by the gene NT5E and is crucial in numerous tumor cell-intrinsic and extrinsic functions [1]. Till now, CD73 is a dimeric ecto-5-nucleotidase (5′-NT) that is expressed on the exterior side of the plasma membrane. Each subunit has N- and C-terminal domains resembling bacterial 5′-NT enzymes. The metal ion binding site is in the N-terminal domain, while the substrate binding site and dimerization interface are in the C-terminal domain. The active enzyme site, formed by residues from both domains, is between them [2].
CD73 was studied in cancer for its role in immune suppression, but recent studies have elucidated far more functions related to this molecule [1,3,4]. CD73/adenosine signaling has been described to be involved in cardiac function [5,6], neural signaling [7,8], and renal function [9,10], among other defined functions [11].
CD73 is the primary manufacturer of extracellular adenosine. ATP is released from the cell, and CD39 at the cell surface dephosphorylates ATP to AMP. Subsequently, in a second step, CD73 converts AMP to adenosine, CD73 on the cell surface of cells is the rate-limiting step in the generation of extracellular adenosine [12]. A non-canonical pathway also leads to AMP production, but both paths eventually converge to CD73 activity [13]. Adenosine is a nucleoside necessary for cellular functions and for building RNA and DNA. In homeostatic conditions, the extracellular adenosine concentrations within tissues are in the low nM rank; however, cellular stress and cancer formation concentrations trigger the production of adenosine that can reach up to the 100 µM range [14,15,16].
CD73 is widely expressed in different tissues since adenosine is generated by hypoxia and chronic inflammation to limit tissue injury and host inflammatory damage [17]. CD73 transcription factors are principally regulated by signal transducers and activators of transcription 3 (STAT3)/hypoxia-inducible factor-1α (HIF-1α) cascade. Many cytokines, including transforming growth factor-β (TGFβ), interferons (IFNs), tumor necrosis factor (TNFα), interleukin-1β (IL-1β), and prostaglandin E2 (PGE2), induce CD73 expression.
CD73 is highly expressed in several cell types within the lung (Figure 1). The main expressing cells are the endothelial cells, fibroblasts, and different subsets of epithelial cells such as club cells, ciliated cells, and with less intensity, alveolar type II cells. Immune cells, including naïve B cells, naïve CD8-T cells, and memory B cells, are significant expressers of CD73, while CD4-T cells and lung resident macrophages express it to a lesser extent, according to immGen and Human Protein Atlas data. Lung tumors, mainly non-small cell lung cancer (NSCLC), showed robust CD73 expression signatures among all cancer types, particularly when associated with common oncogenic drivers of NSCLC, such as mutant epidermal growth factor receptor (EGFR) and KRAS [18,19]. CD73-derived adenosine binds to the surface of various immune cells such as the regulatory (Foxp3+) T cell (Tregs), effector T cell, natural killer (NK) cell, myeloid-derived suppressor cell (MDSC), macrophages, and B cell. It can regulate the tumor microenvironment and the tumor cell immunoevasion capacity.
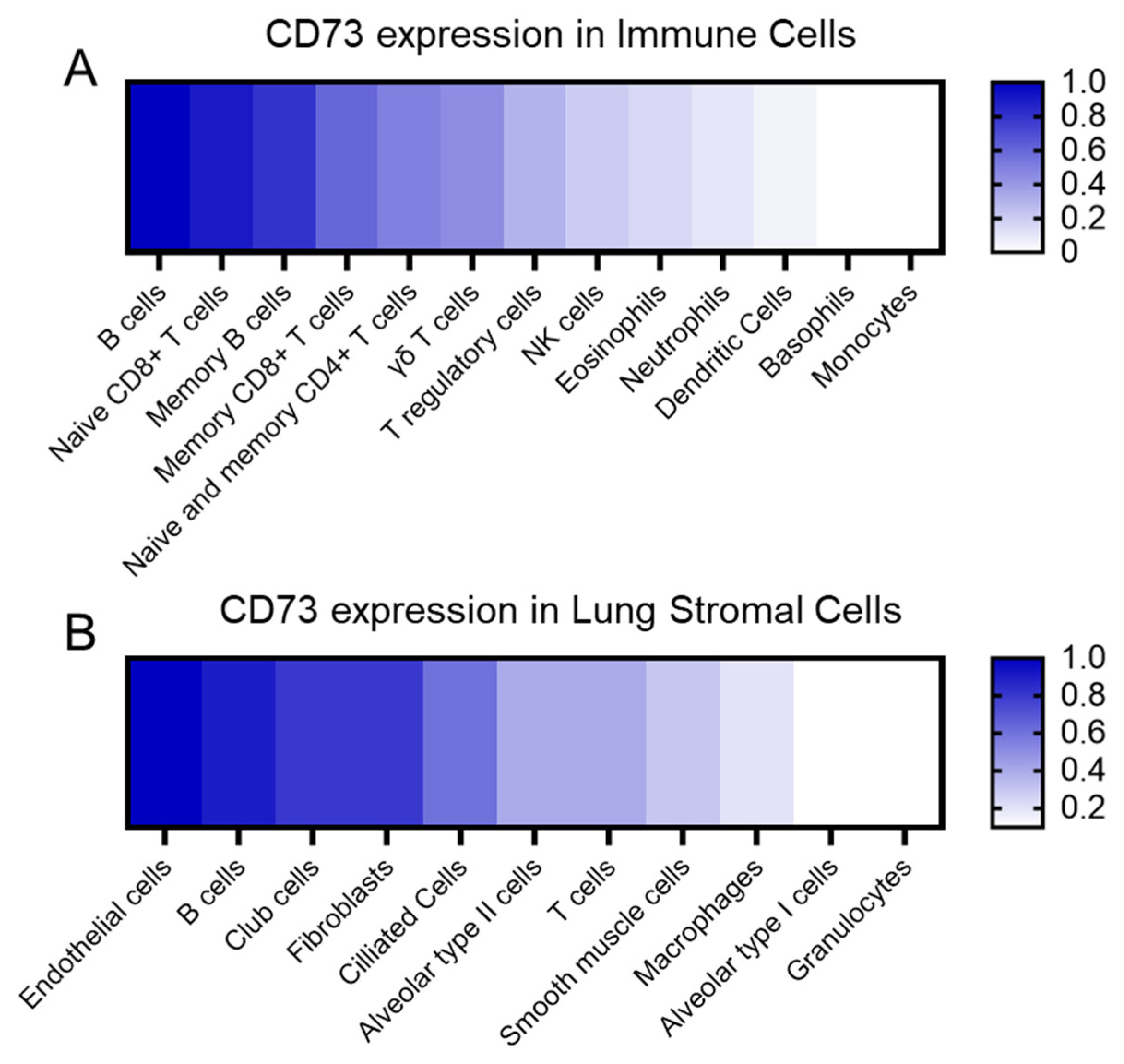
Figure 1. CD73 relative expression. (A) CD73 expression in the immune cell populations relative to the B cells, which are the main immune cells expressing CD73. (B) CD73 expression in the lung stromal cells relative to endothelial cells, which are the main expressing population. We summarized information from public transcriptomic and proteomic databases.
Nowadays, immune checkpoint inhibitor (ICI) therapies benefit only a subset of patients, challenging the scientific community to identify responders even with the existing predictive biomarkers like PD-L1. CD73 and the adenosine pathway are well known to disrupt the cytotoxic function of T cells, which is currently the main target of most clinical agents, and play a key role in tumor immunogenicity. Combining ICI drugs with adenosine pathway inhibitors holds promise for lung cancer therapy, despite the association of increased CD73 with therapy resistance [18,20,21].
This review provides insights into the latest literature on the adenosine pathway, particularly CD73, in lung cancer and other tumors. We will highlight the signaling of the CD73/adenosine pathway in tumoral cells and their interaction in lung stroma and immune system players. We will explain the current knowledge of this pathway in both pre-clinical models and clinical settings focusing on lung cancer. Finally, the review summarizes existing evidence of therapies and clinical trials involving the adenosine pathway, discussing prospects for treating lung cancer.
2. Materials and Methods
2.1. Search Strategy and Study Selection
The review was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. The literature search was independently conducted by three researchers (M.S., O.M-C., and R.G-P.), using the two major databases, PubMed and Scopus, before March 2023. The search used different combinations of terms such as cancer AND (CD73) or lung cancer AND (CD73). In addition, CD73 AND (tumor microenvironment OR lung stromal cells OR immune cells).
Articles were considered eligible for the review if they met the following inclusion criteria: (i) evaluation of the biological effect of the signaling through CD73/adenosine pathway in all kinds of cancer processes or lung cell populations, (ii) studies in vitro and in vivo with data of CD73 in tumoral cells or lung cell populations, (iii) data related to cell proliferation, wound healing, cell invasion, hypoxia, cellular pathways related to migration and invasion processes that CD73 and adenosine were mentioned. Exclusion criteria were (1) case reports, editorials, abstracts, unpublished studies, book chapters, and commentaries; (2) in silico analysis only; (3) full-length articles in non-English language; and (4) none of the above-mentioned assays.
Articles without free full text available were searched through the University Autonoma de Barcelona digital library, or via direct contact with the authors. The full-text versions of all potentially relevant studies were screened, and disagreements were resolved through discussions between the authors until consensus was achieved. A search of references of included studies and previous reviews on the topic was also performed by hand to include additional relevant studies according to our selection criteria.
2.2. Systematic Review Process and Data Extraction
Overall, around 2000 articles were preliminarily identified in the literature search. After excluding duplicates, three independent reviewers (M.S., O.M-C., and R.G-P.) screened titles and abstracts of 956 records. Investigators were blinded to each other’s decisions. Information about study design and methodology, cells or subjects evaluated, reported results, and effects were extracted and summarized by each author. In the end, 125 articles were eligible due to their scientific interest and used for this review.
3. CD73 in the Interaction between Tumor Cells and Immune and Lung Resident Cells
In this section, we describe the interaction between tumor cells and the immune cells and stromal cells in the lung tissue focusing on the CD73/adenosine axis. Stromal–immune cell crosstalk essentially modifies the lung microenvironment, which may promote a carcinogenic milieu and may impact tumoral cell proliferation.
3.1. Cell–Cell Interaction between Tumor Cells with Immune Cells
The interaction between tumor cells and immune cells is a heterogeneous and complex process that plays a fundamental role in the development and progression of cancer. While the immune system has mechanisms like NK cells, cytotoxic T cells, and antigen-presenting cells to eliminate cancer cells, their interaction can lead to factors promoting tumor growth. Tumor cells, in turn, develop mechanisms to inhibit immune responses. One of the molecules identified as an important regulator of this interaction is the CD73 receptor. CD73 overexpression is associated with cancer progression, suppression of the immune system, poorer prognosis, and increased metastasis risk in various cancers [23]. The CD39/CD73 complex and the extracellular adenosine play a crucial role in cancer cells evading the immune system. This chapter will explore how CD73/adenosine expression impacts various immune cells and influences the lung tumoral microenvironment; we have summarized this chapter in a diagram in Figure 2.
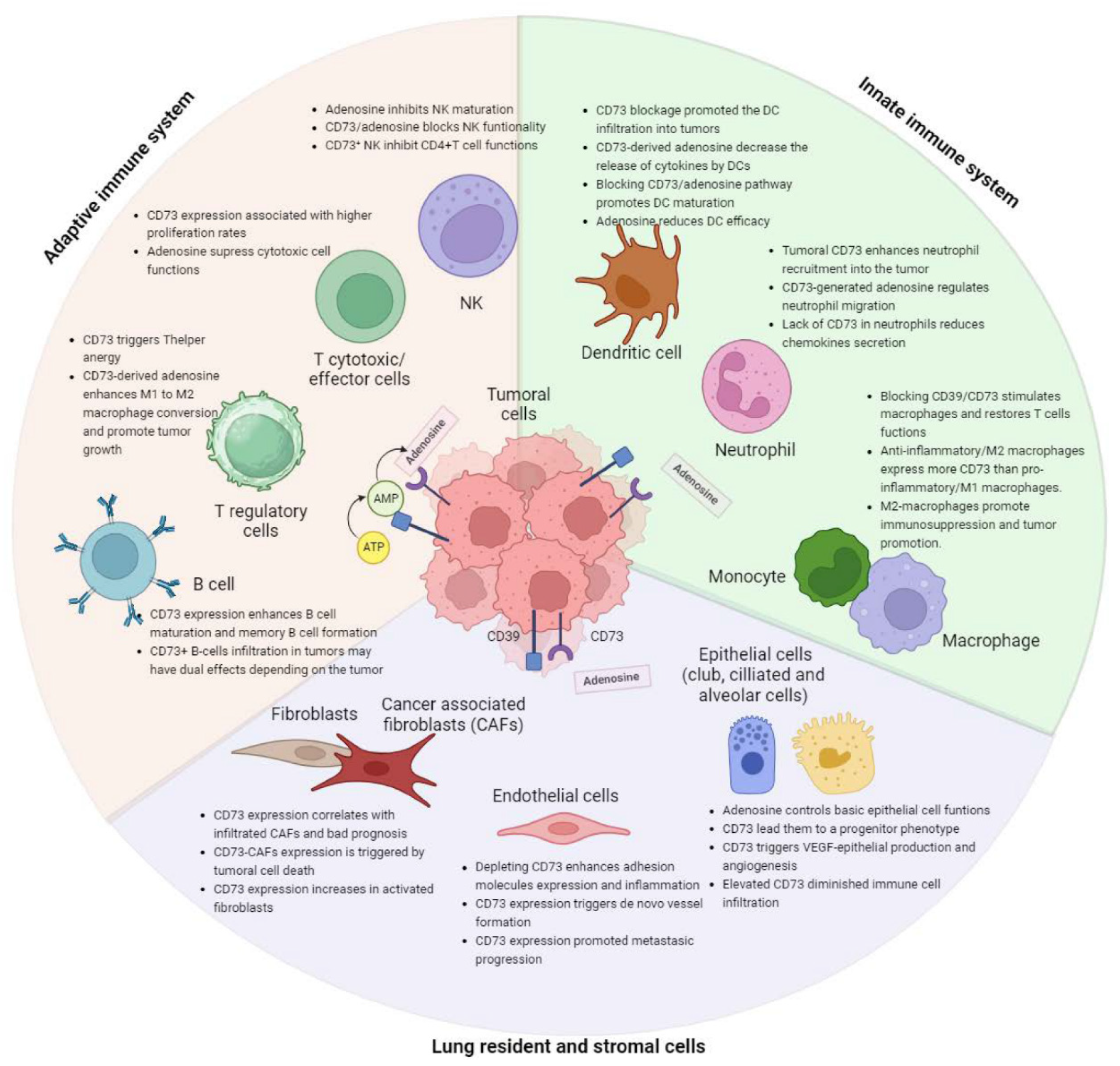
Figure 2. Summary of the effects of CD73/adenosine within all lung resident and immune cells related to carcinogenesis.
3.2. Cell–Cell Interaction between Tumor Cells with Lung Resident Cells
CD73 is widely expressed on the surface of numerous resident lung cell types, stomal and immune cells, including endothelial cells, and airway epithelial cells [74]. Understanding adenosine’s role in lung parenchyma and alveoli requires identifying its sources during homeostasis. Adenosine may originate from the interstitial compartment and penetrate the epithelial airway cells to reach the lumen. Transcriptomic and protein data indicated that lung endothelial cells are the main expressing cells in the lung, followed by club cells, fibroblasts, ciliated cells, alveolar cells, and smooth muscle cells (Figure 1). This chapter reviews the expression and function of CD73 in the key resident lung cells to elucidate its role in maintaining pulmonary homeostasis, and it is summarized in a schematic way in Figure 2.
4. Gene Signatures Regulating Immune Pathways in Tumor Microenvironment (TME)
The dynamic interplay between tumor and immune cells undergoes changes throughout carcinogenesis, endowing tumor cells with the capability to evade the host immune system [104]. Briefly, the prolonged selective pressure exerted by the immune system to eliminate cancer cells prompts the emergence of tumor clones adept at evading immune-mediated destruction, thereby promoting tumor growth [105]. These strategies hinge on the physiological mechanisms of immune response suppression and are the basis for the development and design of novel immunotherapeutics, as discussed below.
4.1. Gene Signatures Favoring Immune Response: IFNγ-Signature
IFNγ is a soluble cytokine primarily produced by the T-lymphocytes and natural killer cells in response to various inflammatory or immune stimuli. It binds to its receptors IFNGR1 and IFNGR2, activating Janus kinases (JAK1 and JAK2) and the signal transducer and activator of transcription 1 (STAT1) [106]. This activation prompts the expression of numerous immune-related genes, including those involved in immunorecognition, antigen presentation (TAP1, TAP2, B2M), and immune checkpoints like PD-L1 [107].
Genetic defects in IFNGR1/2 or JAK1 and JAK2 promote tumor refractoriness to IFNγ [108], particularly noted in melanoma where these alterations have been identified and associated with resistance to treatment with ICIs [109,110]. In lung cancer, limited research has revealed JAK1 and JAK2 loss-of-function mutations, with JAK2 mutant cells found to be unresponsive to IFNγ treatment [111]. Moreover, following antigen presentation to the T cell lymphocytes and IFNγ release, the expression of co-inhibitory immune checkpoints, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), the programmed cell death 1 (PD-1) or the PD-1 ligand (PD-L1) molecules occurs, a mechanism known as adaptive immune resistance. Most current immune checkpoint inhibitors in solid tumors target CTLA-4, PD-1, and PD-L1, serving as monoclonal antibodies that hinder their functions, while numerous other compounds are undergoing clinical investigation [112,113].
Thus, a proficient IFNγ signaling pathway and the presence of an inflammatory gene signature enriched by IFNγ response genes are emerging as a predictive biomarker for ICI with prognostic implications [114]. Indeed, Cristescu and colleagues proposed an 18-gene T-cell-inflamed gene expression profile, which was consistently associated with the response to immunotherapy in various types of cancer, including lung cancer [115].
While an association between CD73 and PD-L1 expression has been observed [17], it remains uncertain whether CD73 exerts an influence on the IFNγ pathway.
4.2. Gene Signatures Associated with an Immunosuppressive Milieu: The Adenosine Signature
The hypoxic tumor microenvironment induces hypoxia-inducible factor 1α (HIF-1 α), leading to the upregulation of adenosinergic molecules, including CD39, CD73, and the adenosine receptor A2BR [34,116]. These molecules are associated with metastatic disease and adverse clinical outcomes across various tumor types, including lung cancer [12,117].
Hypoxia induced the release of extracellular ATP within the tumor microenvironment, subsequently converted to adenosine, via CD39 and CD73. This process initiates a potent anti-inflammatory response, inhibiting the function of multiple host cells within TME, such as mast cells, endothelial cells, macrophages, neutrophils, NKs, DCs, and lymphocytes. Adenosine’s impact includes the stimulation of T cell anergy and the differentiation of CD4 cells into Foxp3+ Tregs, contributing to tumors’ immune escape and cancer growth [23,118,119,120].
Additionally, TGF-β enhances Treg cell function, suppressing T cytotoxic cells and NK cells [121]. It also drives differentiation of myeloid-derived suppressor cells into protumorgenic terminally differentiated MDSC characterized by high levels of cell surface CD39/CD73 expression [122].
Cristescu et al. identified a stroma/epithelial-to-mesenchymal transition/TGF-β signature, that was negatively associated with immunotherapy response and predictors of resistance, and may be potential targets for combination therapy strategies [115].
5. Novel Therapeutics Affecting Adenosine Pathway within TME
Efforts to counter adenosinergic molecules as a therapeutic approach have seen considerable progress. Preclinical studies using adenosine receptor inhibition in various tumor models have shown promising results, including restored immune cell function and tumor regression. For example, concurrent inhibition of A2AR and CD73 with monoclonal antibodies demonstrated synergistic effects compared with monotherapy in a mouse model with metastatic disease [123]. Another therapeutic strategy using MEDI9447 (MedImmune), a potent selective anti-CD73 human monoclonal antibody, either alone or in combination with the anti-PDL1 durvalumab, has been evaluated in phase 1 clinical trial with several tumor types, including lung cancer. Encouraging signs of antitumor activity were noted in tumor types typically resistant to immunotherapy [124]. CPI-444 (Corvus Pharmaceuticals), an orally selective A2AR antagonist, restored T cell signaling, and cytokine production suppressed by adenosine analogs in vitro. It showed promising outcomes in preclinical studies, including complete responses in more than half of the mice and induction of memory T cell responses, and with a synergistic activity in combination with anti-PD-L1 or anti-CTLA [125].
Given the broad activity of anti-PD(L)1 inhibitor but with some limitations, the blockage of the adenosine signaling pathway represents a novel therapeutic approach that has demonstrated an optimal safety profile and enhanced overall response rates in early phase trials, particularly using CD73 and A2AR inhibitors. In we show a comprehensive presentation of drugs targeting adenosinergic molecules that are at the moment used in clinical trials to improve the outcome for lung cancer patients. Beneficial outcomes for both monotherapy and combinations have been mostly lower than expected based on preclinical studies, emphasizing the need for more precise patient selection or biomarker integration to predict and optimize patient responses.
6. Conclusions
In summary, CD73’s multifaceted roles, from immune suppression to its pivotal role in generating extracellular adenosine, highlight its emerging significance in lung cancer and broader implications in cancer biology.
Abundantly expressed in various lung cell types, CD73 overexpression is strongly linked to non-small cell lung cancer (NSCLC) and common oncogenic drivers. Its interactions with immune cells, including Tregs, NK cells, and MDSCs, shape the tumor microenvironment, influencing immunoevasion. In the evolving landscape of cancer therapy, the combination of ICIs with adenosine pathway inhibitors presents a promising avenue. However, challenges such as therapy resistance associated with increased CD73 expression need to be addressed. This review provides a comprehensive overview of the current literature on the CD73/adenosine pathway in lung cancer emphasizing intricate signaling networks involving CD73 in tumoral cells, stroma, and the immune system. Additionally, it has explored the current state of therapies and clinical trials targeting the adenosine pathway, underscoring the need for more precise patient selection and biomarker integration to optimize outcomes.
In conclusion, CD73’s expanding role in cancer biology, particularly in lung cancer, offers a promising avenue for future research and therapeutic development. Understanding the nuances of the CD73/adenosine axis and its intricate interactions within the tumor microenvironment holds great potential for improving the treatment landscape for lung cancer patients.
References
- Resta, R.; Yamashita, Y.; Thompson, L.F. Ecto-Enzyme and Signaling Functions of Lymphocyte CD73. Immunol. Rev. 1998, 161, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Knapp, K.; Zebisch, M.; Pippel, J.; El-Tayeb, A.; Müller, C.E.; Sträter, N. Crystal Structure of the Human Ecto-5′-Nucleotidase (CD73): Insights into the Regulation of Purinergic Signaling. Structure 2012, 20, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Petruk, N.; Tuominen, S.; Åkerfelt, M.; Mattsson, J.; Sandholm, J.; Nees, M.; Yegutkin, G.G.; Jukkola, A.; Tuomela, J.; Selander, K.S. CD73 Facilitates EMT Progression and Promotes Lung Metastases in Triple-Negative Breast Cancer. Sci. Rep. 2021, 11, 6035. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Draisey, A.; Kazen, R.C.; Chung, C.; Phan, L.H.; Harvey, J.B.; Feng, J.; Xie, S.; Broaddus, R.R.; Bowser, J.L. Loss of CD73 Shifts Transforming Growth Factor-Β1 (TGF-Β1) from Tumor Suppressor to Promoter in Endometrial Cancer. Cancer Lett. 2021, 505, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Robin, E.; Marcillac, F.; Raddatz, E. A Hypoxic Episode during Cardiogenesis Downregulates the Adenosinergic System and Alters the Myocardial Anoxic Tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R614–R626. [Google Scholar] [CrossRef] [PubMed]
- Ndzie Noah, M.L.; Adzika, G.K.; Mprah, R.; Adekunle, A.O.; Koda, S.; Adu-Amankwaah, J.; Xu, Y.; Kanwore, K.; Wowui, P.I.; Sun, H. Estrogen Downregulates CD73/Adenosine Axis Hyperactivity via Adaptive Modulation PI3K/Akt Signaling to Prevent Myocarditis and Arrhythmias during Chronic Catecholamines Stress. Cell Commun. Signal 2023, 21, 41. [Google Scholar] [CrossRef]
- Smith, M.D.; Bhatt, D.P.; Geiger, J.D.; Rosenberger, T.A. Acetate Supplementation Modulates Brain Adenosine Metabolizing Enzymes and Adenosine A₂A Receptor Levels in Rats Subjected to Neuroinflammation. J. Neuroinflamm. 2014, 11, 99. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, J.; Wang, J.; Wang, X.; Gao, Q.; Tang, C.; Deng, J.; Xiong, Z.; Kong, X.; Guan, Y.; et al. Aberrant Adenosine Signaling in Patients with Focal Cortical Dysplasia. Mol. Neurobiol. 2023, 60, 4396–4417. [Google Scholar] [CrossRef]
- Tripathi, A.; Lin, E.; Xie, W.; Flaifel, A.; Steinharter, J.A.; Stern Gatof, E.N.; Bouchard, G.; Fleischer, J.H.; Martinez-Chanza, N.; Gray, C.; et al. Prognostic Significance and Immune Correlates of CD73 Expression in Renal Cell Carcinoma. J. Immunother. Cancer 2020, 8, e001467. [Google Scholar] [CrossRef]
- Takamatsu, D.; Kiyozawa, D.; Kohashi, K.; Kinoshita, F.; Toda, Y.; Ishihara, S.; Eto, M.; Oda, Y. Prognostic Impact of CD73/Adenosine 2A Receptor (A2AR) in Renal Cell Carcinoma and Immune Microenvironmental Status with Sarcomatoid Changes and Rhabdoid Features. Pathol. Res. Pract. 2023, 244, 154423. [Google Scholar] [CrossRef]
- Minor, M.; Alcedo, K.P.; Battaglia, R.A.; Snider, N.T. Cell Type- and Tissue-Specific Functions of Ecto-5′-Nucleotidase (CD73). Am. J. Physiol. Cell Physiol. 2019, 317, C1079–C1092. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The Ectonucleotidases CD39 and CD73: Novel Checkpoint Inhibitor Targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Allard, D.; Buisseret, L.; Stagg, J. The Adenosine Pathway in Immuno-Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Yegutkin, G.G. Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell 2019, 36, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Cousineau, I.; Allard, D.; Buisseret, L.; Pommey, S.; Chrobak, P.; Stagg, J. Adenosine A2a Receptor Promotes Lymphangiogenesis and Lymph Node Metastasis. Oncoimmunology 2019, 8, 1601481. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B. Adenosine--a Physiological or Pathophysiological Agent? J. Mol. Med. 2014, 92, 201–206. [Google Scholar] [CrossRef]
- Rocha, P.; Salazar, R.; Zhang, J.; Ledesma, D.; Solorzano, J.L.; Mino, B.; Villalobos, P.; Dejima, H.; Douse, D.Y.; Diao, L.; et al. CD73 Expression Defines Immune, Molecular, and Clinicopathological Subgroups of Lung Adenocarcinoma. Cancer Immunol. Immunother. 2021, 70, 1965–1976. [Google Scholar] [CrossRef]
- Chen, S.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Matei, D.E.; Zhang, Y.; Zhang, B. CD73: An Emerging Checkpoint for Cancer Immunotherapy. Immunotherapy 2019, 11, 983–997. [Google Scholar] [CrossRef]
- Yoshida, R.; Saigi, M.; Tani, T.; Springer, B.F.; Shibata, H.; Kitajima, S.; Mahadevan, N.R.; Campisi, M.; Kim, W.; Kobayashi, Y.; et al. MET-Induced CD73 Restrains STING-Mediated Immunogenicity of EGFR-Mutant Lung Cancer. Cancer Res. 2022, 82, 4079–4092. [Google Scholar] [CrossRef]
- Buisseret, L.; Pommey, S.; Allard, B.; Garaud, S.; Bergeron, M.; Cousineau, I.; Ameye, L.; Bareche, Y.; Paesmans, M.; Crown, J.P.A.; et al. Clinical Significance of CD73 in Triple-Negative Breast Cancer: Multiplex Analysis of a Phase III Clinical Trial. Ann. Oncol. 2018, 29, 1056–1062. [Google Scholar] [CrossRef]
- Roh, M.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Zhang, B. Targeting CD73 to Augment Cancer Immunotherapy. Curr. Opin. Pharmacol. 2020, 53, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res. 2010, 70, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Morandi, B.; Horenstein, A.L.; Chillemi, A.; Quarona, V.; Zaccarello, G.; Carrega, P.; Ferlazzo, G.; Mingari, M.C.; Moretta, L.; et al. A Non-Canonical Adenosinergic Pathway Led by CD38 in Human Melanoma Cells Induces Suppression of T Cell Proliferation. Oncotarget 2015, 6, 25602–25618. [Google Scholar] [CrossRef] [PubMed]
- Chalmin, F.; Mignot, G.; Bruchard, M.; Chevriaux, A.; Végran, F.; Hichami, A.; Ladoire, S.; Derangère, V.; Vincent, J.; Masson, D.; et al. Stat3 and Gfi-1 Transcription Factors Control Th17 Cell Immunosuppressive Activity via the Regulation of Ectonucleotidase Expression. Immunity 2012, 36, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Mandapathil, M.; Szczepanski, M.J.; Szajnik, M.; Ren, J.; Lenzner, D.E.; Jackson, E.K.; Gorelik, E.; Lang, S.; Johnson, J.T.; Whiteside, T.L. Increased Ectonucleotidase Expression and Activity in Regulatory T Cells of Patients with Head and Neck Cancer. Clin. Cancer Res. 2009, 15, 6348–6357. [Google Scholar] [CrossRef] [PubMed]
- Zarek, P.E.; Huang, C.-T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A Receptor Signaling Promotes Peripheral Tolerance by Inducing T-Cell Anergy and the Generation of Adaptive Regulatory T Cells. Blood 2008, 111, 251–259. [Google Scholar] [CrossRef]
- Chatterjee, S.; Thyagarajan, K.; Kesarwani, P.; Song, J.H.; Soloshchenko, M.; Fu, J.; Bailey, S.R.; Vasu, C.; Kraft, A.S.; Paulos, C.M.; et al. Reducing CD73 Expression by IL1β-Programmed Th17 Cells Improves Immunotherapeutic Control of Tumors. Cancer Res. 2014, 74, 6048–6059. [Google Scholar] [CrossRef]
- Airas, L.; Hellman, J.; Salmi, M.; Bono, P.; Puurunen, T.; Smith, D.J.; Jalkanen, S. CD73 Is Involved in Lymphocyte Binding to the Endothelium: Characterization of Lymphocyte-Vascular Adhesion Protein 2 Identifies It as CD73. J. Exp. Med. 1995, 182, 1603–1608. [Google Scholar] [CrossRef]
- Airas, L.; Niemelä, J.; Salmi, M.; Puurunen, T.; Smith, D.J.; Jalkanen, S. Differential Regulation and Function of CD73, a Glycosyl-Phosphatidylinositol-Linked 70-kD Adhesion Molecule, on Lymphocytes and Endothelial Cells. J. Cell Biol. 1997, 136, 421–431. [Google Scholar] [CrossRef]
- Zhang, B. CD73 Promotes Tumor Growth and Metastasis. Oncoimmunology 2012, 1, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, L.; Song, Z.; Ren, M.; Yang, Y.; Li, J.; Shen, K.; Li, Y.; Ding, Y.; Yang, Y.; et al. Intratumoral CD73: An Immune Checkpoint Shaping an Inhibitory Tumor Microenvironment and Implicating Poor Prognosis in Chinese Melanoma Cohorts. Front. Immunol. 2022, 13, 954039. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Bazdar, D.A.; Albakri, M.; Ferrari, B.; Antonelli, C.J.; Freeman, M.L.; Dubyak, G.; Zender, C.; Sieg, S.F. CD8+ CD73+ T Cells in the Tumor Microenvironment of Head and Neck Cancer Patients Are Linked to Diminished T Cell Infiltration and Activation in Tumor Tissue. Eur. J. Immunol. 2020, 50, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Jia, B.; Zhao, C.; Claxton, D.F.; Sharma, A.; Annageldiyev, C.; Fotos, J.S.; Zeng, H.; Paulson, R.F.; Prabhu, K.S.; et al. Downregulation of CD73 Associates with T Cell Exhaustion in AML Patients. J. Hematol. Oncol. 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, L.B.; Rainbow, D.B.; Coppard, V.; Howlett, S.K.; Georgieva, Z.; Davies, J.L.; Mullay, H.K.; Hester, J.; Ashmore, T.; Van Den Bosch, A.; et al. Therapeutically Expanded Human Regulatory T-Cells Are Super-Suppressive Due to HIF1A Induced Expression of CD73. Commun. Biol. 2021, 4, 1186. [Google Scholar] [CrossRef] [PubMed]
- Gourdin, N.; Bossennec, M.; Rodriguez, C.; Vigano, S.; Machon, C.; Jandus, C.; Bauché, D.; Faget, J.; Durand, I.; Chopin, N.; et al. Autocrine Adenosine Regulates Tumor Polyfunctional CD73+CD4+ Effector T Cells Devoid of Immune Checkpoints. Cancer Res. 2018, 78, 3604–3618. [Google Scholar] [CrossRef] [PubMed]
- Conter, L.J.; Song, E.; Shlomchik, M.J.; Tomayko, M.M. CD73 Expression Is Dynamically Regulated in the Germinal Center and Bone Marrow Plasma Cells Are Diminished in Its Absence. PLoS ONE 2014, 9, e92009. [Google Scholar] [CrossRef]
- Bastian, J.F.; Ruedi, J.M.; MacPherson, G.A.; Golembesky, H.E.; O’Connor, R.D.; Thompson, L.F. Lymphocyte Ecto-5′-Nucleotidase Activity in Infancy: Increasing Activity in Peripheral Blood B Cells Precedes Their Ability to Synthesize IgG in Vitro. J. Immunol. 1984, 132, 1767–1772. [Google Scholar] [CrossRef]
- Yamashita, Y.; Hooker, S.W.; Jiang, H.; Laurent, A.B.; Resta, R.; Khare, K.; Coe, A.; Kincade, P.W.; Thompson, L.F. CD73 Expression and Fyn-Dependent Signaling on Murine Lymphocytes. Eur. J. Immunol. 1998, 28, 2981–2990. [Google Scholar] [CrossRef]
- Taylor, J.J.; Pape, K.A.; Jenkins, M.K. A Germinal Center-Independent Pathway Generates Unswitched Memory B Cells Early in the Primary Response. J. Exp. Med. 2012, 209, 597–606. [Google Scholar] [CrossRef]
- Kaji, T.; Ishige, A.; Hikida, M.; Taka, J.; Hijikata, A.; Kubo, M.; Nagashima, T.; Takahashi, Y.; Kurosaki, T.; Okada, M.; et al. Distinct Cellular Pathways Select Germline-Encoded and Somatically Mutated Antibodies into Immunological Memory. J. Exp. Med. 2012, 209, 2079–2097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-N.; Zhang, N.; Liu, H.-H.; Xia, P.; Zhang, C.; Song, J.-W.; Fan, X.; Shi, M.; Jin, L.; Zhang, J.-Y.; et al. Skewed CD39/CD73/Adenosine Pathway Contributes to B-Cell Hyperactivation and Disease Progression in Patients with Chronic Hepatitis B. Gastroenterol. Rep. 2021, 9, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Weitner, M.; Yang, A.; Akue, A.; Liu, X.; Schmidt, T.; Allman, W.R.; Akkoyunlu, M.; Derrick, S.C. Memory CD73+IgM+ B Cells Protect against Plasmodium Yoelii Infection and Express Granzyme B. PLoS ONE 2020, 15, e0238493. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.J.; Wu, Z.; David, P.; Mittelstädt, A.; Jacobsen, A.; Podolska, M.J.; Ubieta, K.; Brunner, M.; Kouhestani, D.; Swierzy, I.; et al. Tumor Infiltration with CD20+CD73+ B Cells Correlates with Better Outcome in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 5163. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Luke, J.J.; Hu, S.; Mahabhashyam, S.; Jones, W.B.; Marron, T.; Merchan, J.R.; Hughes, B.G.M.; Willingham, S.B. Anti-CD73 Antibody Activates Human B Cells, Enhances Humoral Responses and Induces Redistribution of B Cells in Patients with Cancer. J. Immunother. Cancer 2022, 10, e005802. [Google Scholar] [CrossRef] [PubMed]
- van de Veen, W.; Globinska, A.; Jansen, K.; Straumann, A.; Kubo, T.; Verschoor, D.; Wirz, O.F.; Castro-Giner, F.; Tan, G.; Rückert, B.; et al. A Novel Proangiogenic B Cell Subset Is Increased in Cancer and Chronic Inflammation. Sci. Adv. 2020, 6, eaaz3559. [Google Scholar] [CrossRef] [PubMed]
- Grund, J.; Iben, K.; Reinke, S.; Bühnen, I.; Plütschow, A.; Müller-Meinhard, B.; Garcia Marquez, M.A.; Schlößer, H.A.; von Tresckow, B.; Kellermeier, F.; et al. Low B-Cell Content Is Associated with a CD73-Low Tumour Microenvironment and Unfavourable Prognosis in Classic Hodgkin Lymphoma. Br. J. Haematol. 2023, 201, 1097–1102. [Google Scholar] [CrossRef]
- Kicova, M.; Michalova, Z.; Coma, M.; Gabzdilova, J.; Dedinska, K.; Guman, T.; Bernatova, S.; Hajikova, M.; Giertlova, M.; Veselinyova, D.; et al. The Expression of CD73 on Pathological B-Cells Is Associated with Shorter Overall Survival of Patients with CLL. Neoplasma 2020, 67, 933–938. [Google Scholar] [CrossRef]
- Neo, S.Y.; Yang, Y.; Record, J.; Ma, R.; Chen, X.; Chen, Z.; Tobin, N.P.; Blake, E.; Seitz, C.; Thomas, R.; et al. CD73 Immune Checkpoint Defines Regulatory NK Cells within the Tumor Microenvironment. J. Clin. Investig. 2020, 130, 1185–1198. [Google Scholar] [CrossRef]
- Lee, Y.S.; Radford, K.J. The Role of Dendritic Cells in Cancer. Int. Rev. Cell Mol. Biol. 2019, 348, 123–178. [Google Scholar] [CrossRef]
- Canale, F.P.; Ramello, M.C.; Núñez, N.; Araujo Furlan, C.L.; Bossio, S.N.; Gorosito Serrán, M.; Tosello Boari, J.; Del Castillo, A.; Ledesma, M.; Sedlik, C.; et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8+ T Cells. Cancer Res. 2018, 78, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Arab, S.; Kheshtchin, N.; Ajami, M.; Ashurpoor, M.; Safvati, A.; Namdar, A.; Mirzaei, R.; Mousavi Niri, N.; Jadidi-Niaragh, F.; Ghahremani, M.H.; et al. Increased Efficacy of a Dendritic Cell-Based Therapeutic Cancer Vaccine with Adenosine Receptor Antagonist and CD73 Inhibitor. Tumour Biol. 2017, 39, 1010428317695021. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Chiang, S.-F.; Chen, C.-Y.; Hong, W.-Z.; Chen, T.-W.; Chen, W.T.-L.; Ke, T.-W.; Yang, P.-C.; Liang, J.-A.; Shiau, A.-C.; et al. Targeting CD73 Increases Therapeutic Response to Immunogenic Chemotherapy by Promoting Dendritic Cell Maturation. Cancer Immunol. Immunother. 2023, 72, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of Neutrophil Function by Adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- van Waeg, G.; Van den Berghe, G. Purine Catabolism in Polymorphonuclear Neutrophils. Phorbol Myristate Acetate-Induced Accumulation of Adenosine Owing to Inactivation of Extracellularly Released Adenosine Deaminase. J. Clin. Investig. 1991, 87, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in Immunity and Inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, J.; Cao, H.; Xiao, G.; Wang, Z.; Zhang, X.; Zhang, N.; Wu, W.; Zhang, H.; Wang, Q.; et al. Identification of CD73 as a Novel Biomarker Encompassing the Tumor Microenvironment, Prognosis, and Therapeutic Responses in Various Cancers. Cancers 2022, 14, 5663. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Thompson, L.F.; Karhausen, J.; Cotta, R.J.; Ibla, J.C.; Robson, S.C.; Colgan, S.P. Endogenous Adenosine Produced during Hypoxia Attenuates Neutrophil Accumulation: Coordination by Extracellular Nucleotide Metabolism. Blood 2004, 104, 3986–3992. [Google Scholar] [CrossRef]
- Junger, W.G. Immune Cell Regulation by Autocrine Purinergic Signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef]
- Reutershan, J.; Vollmer, I.; Stark, S.; Wagner, R.; Ngamsri, K.-C.; Eltzschig, H.K. Adenosine and Inflammation: CD39 and CD73 Are Critical Mediators in LPS-Induced PMN Trafficking into the Lungs. FASEB J. 2009, 23, 473–482. [Google Scholar] [CrossRef]
- Liu, T.-T.; Wang, Y.-L.; Zhang, Z.; Jia, L.-X.; Zhang, J.; Zheng, S.; Chen, Z.-H.; Shen, H.-H.; Piao, C.-M.; Du, J. Abnormal Adenosine Metabolism of Neutrophils Inhibits Airway Inflammation and Remodeling in Asthma Model Induced by Aspergillus Fumigatus. BMC Pulm. Med. 2023, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Pop, L.M.; Laine, A.; Iyengar, P.; Vitetta, E.S.; Hannan, R. Key Role for Neutrophils in Radiation-Induced Antitumor Immune Responses: Potentiation with G-CSF. Proc. Natl. Acad. Sci. USA 2016, 113, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-Associated Neutrophils (TAN) Develop pro-Tumorigenic Properties during Tumor Progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Zhu, X.; Zhang, H.; Yang, G.H.Y.; Friesen, T.J.; Shipley, M.; Maeda, D.Y.; Zebala, J.A.; McKay-Fleisch, J.; Meredith, G.; et al. Neutrophil Content Predicts Lymphocyte Depletion and Anti-PD1 Treatment Failure in NSCLC. JCI Insight 2019, 4, e130850. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed]
- Schaider, H.; Oka, M.; Bogenrieder, T.; Nesbit, M.; Satyamoorthy, K.; Berking, C.; Matsushima, K.; Herlyn, M. Differential Response of Primary and Metastatic Melanomas to Neutrophils Attracted by IL-8. Int. J. Cancer 2003, 103, 335–343. [Google Scholar] [CrossRef]
- Strell, C.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Neutrophil Granulocytes Promote the Migratory Activity of MDA-MB-468 Human Breast Carcinoma Cells via ICAM-1. Exp. Cell Res. 2010, 316, 138–148. [Google Scholar] [CrossRef]
- Hamidzadeh, K.; Mosser, D.M. Purinergic Signaling to Terminate TLR Responses in Macrophages. Front. Immunol. 2016, 7, 74. [Google Scholar] [CrossRef]
- Zanin, R.F.; Braganhol, E.; Bergamin, L.S.; Campesato, L.F.I.; Filho, A.Z.; Moreira, J.C.F.; Morrone, F.B.; Sévigny, J.; Schetinger, M.R.C.; de Souza Wyse, A.T.; et al. Differential Macrophage Activation Alters the Expression Profile of NTPDase and Ecto-5′-Nucleotidase. PLoS ONE 2012, 7, e31205. [Google Scholar] [CrossRef]
- Koscsó, B.; Csóka, B.; Kókai, E.; Németh, Z.H.; Pacher, P.; Virág, L.; Leibovich, S.J.; Haskó, G. Adenosine Augments IL-10-Induced STAT3 Signaling in M2c Macrophages. J. Leukoc. Biol. 2013, 94, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Montalbán Del Barrio, I.; Penski, C.; Schlahsa, L.; Stein, R.G.; Diessner, J.; Wöckel, A.; Dietl, J.; Lutz, M.B.; Mittelbronn, M.; Wischhusen, J.; et al. Adenosine-Generating Ovarian Cancer Cells Attract Myeloid Cells Which Differentiate into Adenosine-Generating Tumor Associated Macrophages—A Self-Amplifying, CD39- and CD73-Dependent Mechanism for Tumor Immune Escape. J. Immunother. Cancer 2016, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Perrot, I.; Michaud, H.-A.; Giraudon-Paoli, M.; Augier, S.; Docquier, A.; Gros, L.; Courtois, R.; Déjou, C.; Jecko, D.; Becquart, O.; et al. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep. 2019, 27, 2411–2425.e9. [Google Scholar] [CrossRef] [PubMed]
- Picher, M.; Burch, L.H.; Hirsh, A.J.; Spychala, J.; Boucher, R.C. Ecto 5′-Nucleotidase and Nonspecific Alkaline Phosphatase. Two AMP-Hydrolyzing Ectoenzymes with Distinct Roles in Human Airways. J. Biol. Chem. 2003, 278, 13468–13479. [Google Scholar] [CrossRef] [PubMed]
- Flocke, K.; Lesch, G.; Elsässer, H.P.; Bosslet, K.; Mannherz, H.G. Monoclonal Antibodies against 5′-Nucleotidase from a Human Pancreatic Tumor Cell Line: Their Characterization and Inhibitory Capacity on Tumor Cell Adhesion to Fibronectin Substratum. Eur. J. Cell Biol. 1992, 58, 62–70. [Google Scholar] [PubMed]
- Krüger, K.H.; Thompson, L.F.; Kaufmann, M.; Möller, P. Expression of Ecto-5′-Nucleotidase (CD73) in Normal Mammary Gland and in Breast Carcinoma. Br. J. Cancer 1991, 63, 114–118. [Google Scholar] [CrossRef]
- Strohmeier, G.R.; Lencer, W.I.; Patapoff, T.W.; Thompson, L.F.; Carlson, S.L.; Moe, S.J.; Carnes, D.K.; Mrsny, R.J.; Madara, J.L. Surface Expression, Polarization, and Functional Significance of CD73 in Human Intestinal Epithelia. J. Clin. Investig. 1997, 99, 2588–2601. [Google Scholar] [CrossRef]
- Nouwen, E.J.; Pollet, D.E.; Eerdekens, M.W.; Hendrix, P.G.; Briers, T.W.; De Broe, M.E. Immunohistochemical Localization of Placental Alkaline Phosphatase, Carcinoembryonic Antigen, and Cancer Antigen 125 in Normal and Neoplastic Human Lung. Cancer Res. 1986, 46, 866–876. [Google Scholar]
- Wang, L.; Dorn, P.; Simillion, C.; Froment, L.; Berezowska, S.; Tschanz, S.A.; Haenni, B.; Blank, F.; Wotzkow, C.; Peng, R.-W.; et al. EpCAM+CD73+ Mark Epithelial Progenitor Cells in Postnatal Human Lung and Are Associated with Pathogenesis of Pulmonary Disease Including Lung Adenocarcinoma. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L794–L809. [Google Scholar] [CrossRef]
- Bowser, J.L.; Blackburn, M.R.; Shipley, G.L.; Molina, J.G.; Dunner, K.; Broaddus, R.R. Loss of CD73-Mediated Actin Polymerization Promotes Endometrial Tumor Progression. J. Clin. Investig. 2016, 126, 220–238. [Google Scholar] [CrossRef]
- Antonioli, L.; Yegutkin, G.G.; Pacher, P.; Blandizzi, C.; Haskó, G. Anti-CD73 in Cancer Immunotherapy: Awakening New Opportunities. Trends Cancer 2016, 2, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, M.K.; Tervahartiala, M.; Kenessey, I.; Jalkanen, S.; Boström, P.J.; Salmi, M. Cell-Type-Specific CD73 Expression Is an Independent Prognostic Factor in Bladder Cancer. Carcinogenesis 2019, 40, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, B.G.; Charlebois, R.; Chouinard, G.; Allard, B.; Pommey, S.; Saad, F.; Stagg, J. CD73 Expression Is an Independent Prognostic Factor in Prostate Cancer. Clin. Cancer Res. 2016, 22, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Song, Z.; Li, L.; Zhang, X.; Liu, J.; Liu, X.; Qiu, L.; Qian, Z.; Zhou, S.; et al. Tumor CD73/A2aR Adenosine Immunosuppressive Axis and Tumor-Infiltrating Lymphocytes in Diffuse Large B-Cell Lymphoma: Correlations with Clinicopathological Characteristics and Clinical Outcome. Int. J. Cancer 2019, 145, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, S.; Salmi, M. VAP-1 and CD73, Endothelial Cell Surface Enzymes in Leukocyte Extravasation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, J.K.; Ridley, A.J. CD73 Represses Pro-Inflammatory Responses in Human Endothelial Cells. J. Inflamm. 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Minguet, S.; Huber, M.; Rosenkranz, L.; Schamel, W.W.A.; Reth, M.; Brummer, T. Adenosine and cAMP Are Potent Inhibitors of the NF-Kappa B Pathway Downstream of Immunoreceptors. Eur. J. Immunol. 2005, 35, 31–41. [Google Scholar] [CrossRef]
- Walker, G.; Langheinrich, A.C.; Dennhauser, E.; Bohle, R.M.; Dreyer, T.; Kreuzer, J.; Tillmanns, H.; Braun-Dullaeus, R.C.; Haberbosch, W. 3-Deazaadenosine Prevents Adhesion Molecule Expression and Atherosclerotic Lesion Formation in the Aortas of C57BL/6J Mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2673–2679. [Google Scholar] [CrossRef]
- Kalsi, K.; Lawson, C.; Dominguez, M.; Taylor, P.; Yacoub, M.H.; Smolenski, R.T. Regulation of Ecto-5′-Nucleotidase by TNF-Alpha in Human Endothelial Cells. Mol. Cell Biochem. 2002, 232, 113–119. [Google Scholar] [CrossRef]
- Bellingan, G.; Maksimow, M.; Howell, D.C.; Stotz, M.; Beale, R.; Beatty, M.; Walsh, T.; Binning, A.; Davidson, A.; Kuper, M.; et al. The Effect of Intravenous Interferon-Beta-1a (FP-1201) on Lung CD73 Expression and on Acute Respiratory Distress Syndrome Mortality: An Open-Label Study. Lancet Respir. Med. 2014, 2, 98–107. [Google Scholar] [CrossRef]
- Koszalka, P.; Ozüyaman, B.; Huo, Y.; Zernecke, A.; Flögel, U.; Braun, N.; Buchheiser, A.; Decking, U.K.M.; Smith, M.L.; Sévigny, J.; et al. Targeted Disruption of Cd73/Ecto-5′-Nucleotidase Alters Thromboregulation and Augments Vascular Inflammatory Response. Circ. Res. 2004, 95, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.F.; Eltzschig, H.K.; Ibla, J.C.; Van De Wiele, C.J.; Resta, R.; Morote-Garcia, J.C.; Colgan, S.P. Crucial Role for Ecto-5′-Nucleotidase (CD73) in Vascular Leakage during Hypoxia. J. Exp. Med. 2004, 200, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Chadjichristos, C.E.; Scheckenbach, K.E.L.; van Veen, T.A.B.; Richani Sarieddine, M.Z.; de Wit, C.; Yang, Z.; Roth, I.; Bacchetta, M.; Viswambharan, H.; Foglia, B.; et al. Endothelial-Specific Deletion of Connexin40 Promotes Atherosclerosis by Increasing CD73-Dependent Leukocyte Adhesion. Circulation 2010, 121, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Takedachi, M.; Qu, D.; Ebisuno, Y.; Oohara, H.; Joachims, M.L.; McGee, S.T.; Maeda, E.; McEver, R.P.; Tanaka, T.; Miyasaka, M.; et al. CD73-Generated Adenosine Restricts Lymphocyte Migration into Draining Lymph Nodes. J. Immunol. 2008, 180, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Henttinen, T.; Jalkanen, S.; Yegutkin, G.G. Adherent Leukocytes Prevent Adenosine Formation and Impair Endothelial Barrier Function by Ecto-5′-Nucleotidase/CD73-Dependent Mechanism. J. Biol. Chem. 2003, 278, 24888–24895. [Google Scholar] [CrossRef]
- Allard, B.; Turcotte, M.; Spring, K.; Pommey, S.; Royal, I.; Stagg, J. Anti-CD73 Therapy Impairs Tumor Angiogenesis. Int. J. Cancer 2014, 134, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Beavis, P.A.; Divisekera, U.; Liu, M.C.P.; Möller, A.; Darcy, P.K.; Smyth, M.J. CD73-Deficient Mice Are Resistant to Carcinogenesis. Cancer Res. 2012, 72, 2190–2196. [Google Scholar] [CrossRef]
- Xue, X.-M.; Liu, Y.-Y.; Chen, X.-M.; Tao, B.-Y.; Liu, P.; Zhou, H.-W.; Zhang, C.; Wang, L.; Jiang, Y.-K.; Ding, Z.-W.; et al. Pan-Cancer Analysis Identifies NT5E as a Novel Prognostic Biomarker on Cancer-Associated Fibroblasts Associated with Unique Tumor Microenvironment. Front. Pharmacol. 2022, 13, 1064032. [Google Scholar] [CrossRef]
- Zhou, J.; Schwenk-Zieger, S.; Kranz, G.; Walz, C.; Klauschen, F.; Dhawan, S.; Canis, M.; Gires, O.; Haubner, F.; Baumeister, P.; et al. Isolation and Characterization of Head and Neck Cancer-Derived Peritumoral and Cancer-Associated Fibroblasts. Front. Oncol. 2022, 12, 984138. [Google Scholar] [CrossRef]
- Yu, M.; Guo, G.; Huang, L.; Deng, L.; Chang, C.-S.; Achyut, B.R.; Canning, M.; Xu, N.; Arbab, A.S.; Bollag, R.J.; et al. CD73 on Cancer-Associated Fibroblasts Enhanced by the A2B-Mediated Feedforward Circuit Enforces an Immune Checkpoint. Nat. Commun. 2020, 11, 515. [Google Scholar] [CrossRef]
- Xing, F.; Saidou, J.; Watabe, K. Cancer Associated Fibroblasts (CAFs) in Tumor Microenvironment. Front. Biosci. 2010, 15, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Asif, P.J.; Longobardi, C.; Hahne, M.; Medema, J.P. The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis. Cancers 2021, 13, 4720. [Google Scholar] [CrossRef] [PubMed]
- Klatsky, A.L.; Morton, C.; Udaltsova, N.; Friedman, G.D. Coffee, Cirrhosis, and Transaminase Enzymes. Arch. Intern. Med. 2006, 166, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, Immunity and Cancer Immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [CrossRef]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The Roles of IFN Gamma in Protection against Tumor Development and Cancer Immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Shankaran, V.; Dighe, A.S.; Stockert, E.; Aguet, M.; Old, L.J.; Schreiber, R.D. Demonstration of an Interferon Gamma-Dependent Tumor Surveillance System in Immunocompetent Mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7556–7561. [Google Scholar] [CrossRef]
- Ribas, A. Releasing the Brakes on Cancer Immunotherapy. N. Engl. J. Med. 2015, 373, 1490–1492. [Google Scholar] [CrossRef]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Saigi, M.; Alburquerque-Bejar, J.J.; Mc Leer-Florin, A.; Pereira, C.; Pros, E.; Romero, O.A.; Baixeras, N.; Esteve-Codina, A.; Nadal, E.; Brambilla, E.; et al. MET-Oncogenic and JAK2-Inactivating Alterations Are Independent Factors That Affect Regulation of PD-L1 Expression in Lung Cancer. Clin. Cancer Res. 2018, 24, 4579–4587. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404.e9. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-Related mRNA Profile Predicts Clinical Response to PD-1 Blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Nebozhyn, M.; Zhang, C.; Albright, A.; Kobie, J.; Huang, L.; Zhao, Q.; Wang, A.; Ma, H.; Alexander Cao, Z.; et al. Transcriptomic Determinants of Response to Pembrolizumab Monotherapy across Solid Tumor Types. Clin. Cancer Res. 2022, 28, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Tak, E.; Jung, D.-H.; Kim, S.-H.; Park, G.-C.; Jun, D.Y.; Lee, J.; Jung, B.-H.; Kirchner, V.A.; Hwang, S.; Song, G.-W.; et al. Protective Role of Hypoxia-Inducible Factor-1α-Dependent CD39 and CD73 in Fulminant Acute Liver Failure. Toxicol. Appl. Pharmacol. 2017, 314, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Sinha, D.; Barkauskas, D.; Young, A.; Kalimutho, M.; Stannard, K.; Caramia, F.; Haibe-Kains, B.; Stagg, J.; Khanna, K.K.; et al. Adenosine 2B Receptor Expression on Cancer Cells Promotes Metastasis. Cancer Res. 2016, 76, 4372–4382. [Google Scholar] [CrossRef]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; Wong, M.K.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A Adenosine Receptor Protects Tumors from Antitumor T Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef]
- Stagg, J.; Smyth, M.J. Extracellular Adenosine Triphosphate and Adenosine in Cancer. Oncogene 2010, 29, 5346–5358. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine Generation Catalyzed by CD39 and CD73 Expressed on Regulatory T Cells Mediates Immune Suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Johnston, C.J.C.; Smyth, D.J.; Dresser, D.W.; Maizels, R.M. TGF-β in Tolerance, Development and Regulation of Immunity. Cell Immunol. 2016, 299, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Chen, X.; Li, L.; Li, Y.; Ping, Y.; Huang, L.; Yue, D.; Zhang, Z.; Wang, F.; et al. CD39/CD73 Upregulation on Myeloid-Derived Suppressor Cells via TGF-β-mTOR-HIF-1 Signaling in Patients with Non-Small Cell Lung Cancer. Oncoimmunology 2017, 6, e1320011. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Barkauskas, D.S.; Sult, E.; Hay, C.; Blake, S.J.; Huang, Q.; Liu, J.; Takeda, K.; Teng, M.W.L.; et al. Co-Inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-Tumor Immune Responses. Cancer Cell 2016, 30, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.; LoRusso, P.; Overman, M.; Noonan, A.M.; Kim, D.W.; Strickler, J.H.; Kim, S.-W.; Clarke, S.; George, T.J.; Grimison, P.S.; et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol. Immunother. 2023, 72, 2443–2458. [Google Scholar] [CrossRef]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.M.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef]

