1. Introduction
Brain metastases (BMs) represent a central issue in the management of lung cancer, occurring in up to 50% of non-small-cell lung cancer (NSCLC) and over 70% of small-cell lung cancer (SCLC) patients during the course of their disease [1,2]. The incidence and prevalence of BMs are globally increasing due to the advances in diagnostic and therapeutic procedures that chronicize the natural history of lung cancer [3].
Considering the heterogeneity of presentations in this setting, an accurate prognostic definition is crucial to offer personalized therapies. The evolution of prognostic indices reflects the relevant changes that have occurred in the clinical practice of treating BMs. The original recursive partitioning analysis (RPA) was defined in the era of the “one size fits all approach”, when whole brain radiation therapy (WBRT) was considered the standard treatment for nearly all patients. At that time, only clinical factors such as age, Karnofsky performance status (KPS), status of the primary tumor and presence of extracranial metastases were included in the model [4]. The subsequent graded prognostic assessment (GPA) introduced the number of brain lesions, which underscored the importance of local treatments by surgery and focal radiation therapy (RT) in patients with a limited number of metastases [5]. Finally, the modern lung-cancer-specific GPAs (DS-GPA and Lung-molGPA) stratified the prognosis based on primary histology and molecular subtypes, highlighting the growing role of biomolecular diagnostic and systemic therapy (ST) in this scenario [6,7,8].
In the era of precision medicine, stereotactic radiosurgery (SRS) has gradually replaced the historical WBRT approach as a safer and more effective technique, and today represents a cornerstone of BM management [9].
Our review aims to offer an overview of the modern SRS strategies available to treat BMs from lung cancer, either alone or in combination with surgery and ST. In addition, we also describe in detail the molecular profile of lung cancer BMs and the emerging understanding of the role of the tumor microenvironment in influencing the response to standard therapies, revealing new potential prognostic and predictive biomarkers.
2. Stereotactic Radiosurgery (SRS)
SRS is a highly conformal technique, able to deliver a high dose of radiation in a single fraction to a well-defined target. This ablative and focal strategy mimics the effect of surgical resection through a less invasive approach, representing the ideal solution for small-sized BMs and a valid alternative when surgery is not feasible (generally for unresectable lesions critically located in deep or eloquent brain areas [10,11,12] or patients inoperable due to comorbidities).
2.1. Technical Solutions, Doses and Fractionations
Linear accelerators (LINACs), the Gamma Knife and Cyberknife are the platforms usually adopted for the radiosurgical treatment of BMs (Figure 1 shows a LINAC-based SRS approach).
Figure 1. 3D treatment planning reconstruction of a LINAC-based SRS treatment using conical-shaped collimators.
Over the decades, many technological advances have guaranteed a less invasive and more accurate delivery of SRS, and no clear advantages emerged in comparisons among these technical solutions in terms of efficacy or toxicity [13,14].
Different doses and fractionations have been evaluated and are adopted in clinical practice according to a risk-adaptive approach. A historical RTOG dose escalation trial settled on the maximum tolerated single-fraction doses as 24 Gray (Gy), 18 Gy and 15 Gy for lesions ≤ 20 mm, 21–30 mm and 31–40 mm in maximum diameter, respectively [15,16]. However, for larger lesions (>20 mm), 18–15 Gy or less could be detrimental with regard to local control (LC), and a higher dose results in an increased risk of radionecrosis (RN) [17].
RN is considered the main late complication of SRS, occurring from within a few months to years after radiation (up to 80% of cases have been reported within 3 years) [18]. The incidence rate is variable in the literature, usually ranging from 5 to 25% [19]. Possible risk factors are the volume of healthy brain tissue exposed to high doses of radiation, previous brain RT, concomitant ST and some specific histologies (such as ALK-translocated lung adenocarcinoma) [20]. The typical neuroradiological finding, a contrast-enhanced alteration with intralesional necrosis and perilesional edema, is not easily distinguishable from local tumor recurrence (Figure 2). Usually, gyriform lesions and edema with marginal or solid enhancement are more suggestive of tumor recurrence or a viable tumor. However, these patterns still have a low specificity and sensitivity [19].
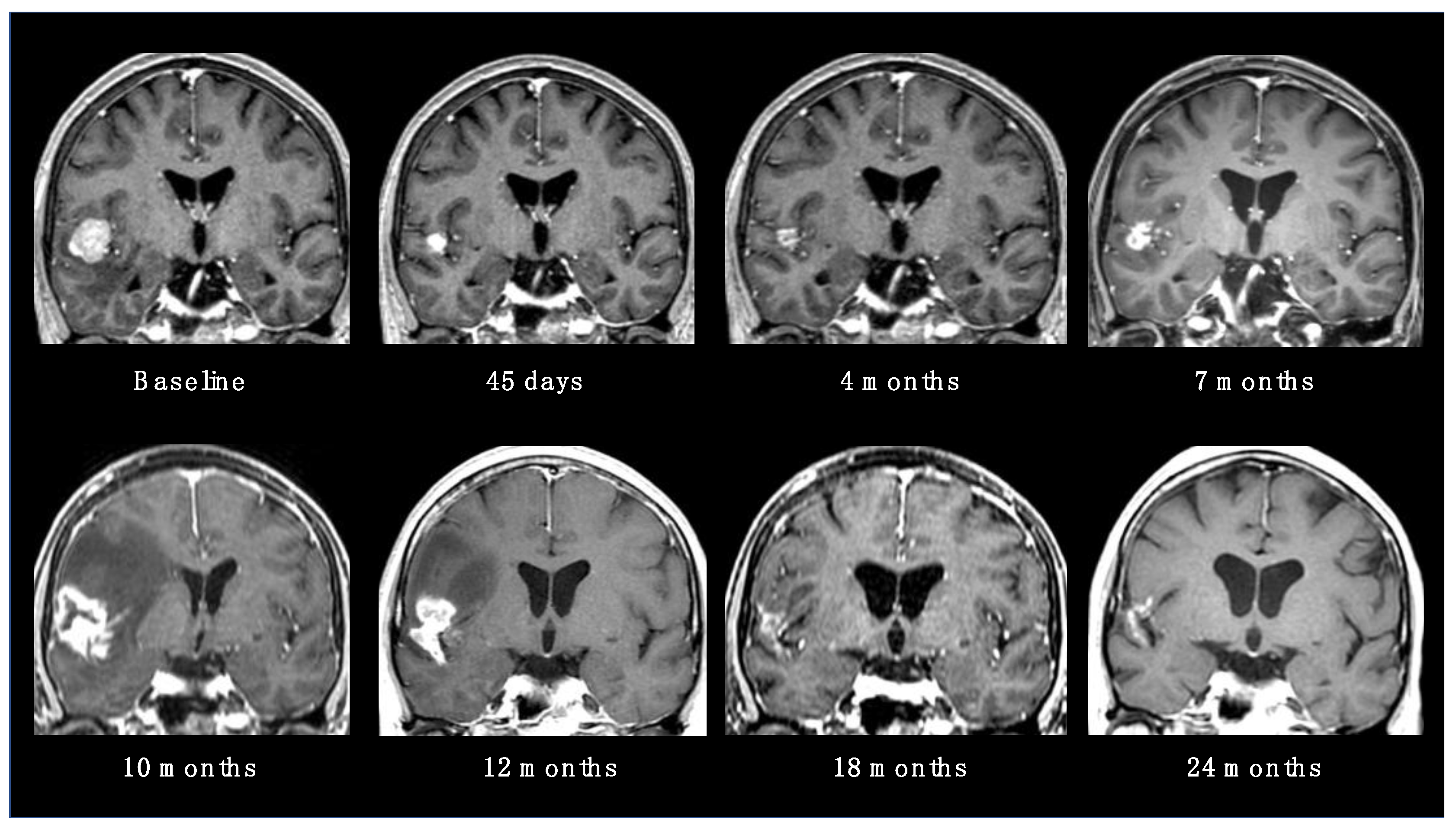
Figure 2. Radionecrotic evolution of a BM after SRS during a 24-month neuroradiological follow-up period.
For this reason, modern magnetic resonance (MR) sequences and functional imaging are essential for the differential diagnosis. In particular, a relative cerebral blood volume (rCBV) < 0.71 is highly suggestive of radionecrosis. Diffuse-weighted imaging is also highly efficient and even superior to rCBV in distinguishing recurrent tumors from RN. Moreover, positron emission tomography (PET) imaging with amino acid tracers may be extremely useful, because of the high amino acid uptake by tumor cells. Tracers, including Carbon-11 methionine, Fluoro-l-thymidine and Fluoroethyltyrosine, have been tested with promising results and very high sensitivity (up to 100%) and specificity (up to 93%) rates. [19]. Most RN presentations are asymptomatic and allow a conservative strategy, based on neuroradiological observation. When necrotic lesions cause symptoms, therapeutic options vary from corticosteroid administrations to the use of bevacizumab, up to the need for surgery in the case of large and edemigenous lesions [21].
Hypofractionated stereotactic RT (SRT) represents the ideal solution to improve LC without increasing the risk of RN related to focal RT in the management of large BMs. SRT delivers a lower dose per fraction in few (generally 3 or 5) fractions, thus allowing it to simultaneously deliver a higher biologically effective dose (BED) to the tumor and a lower radiobiological effect to the surrounding healthy brain tissue. Retrospective series of large brain lesions treated with SRT have accumulated over the years, and these have demonstrated better rates of LC and RN compared to SRS, as shown in two meta-analyses [22,23]. Ongoing randomized studies could definitively confirm these benefits (NCT03697343, NCT05222620, NCT05346367).
The international collaborative “HyTEC” (Hypofractionated Treatment Effects in the Clinic) project published data on tumor control probability (TCP, considered as the probability of LC) [24] and normal tissue complication probability (NTCP, considered as the risk of RN) [25] to guide dose and fractionation choices for SRS/SRT in BMs. The authors concluded that a 18–24 Gy single-fraction SRS is optimal for BMs ≤ 20 mm, while fractionated SRT should be preferred for lesions > 20 mm [24,25]. In clinical practice, the prescription dose for SRT treatment generally ranges from 24 to 30 Gy in three to five fractions. In addition to different RT dose fractionations, also the exposure of healthy brain tissue should be weighted in order to minimize the risk of RN. The recent UK consensus on normal tissue dose constraints suggests keeping the brain volume below 10–15 cc (including the target volume) for a maximum dose of 12 Gy (V12Gy) in a single fraction, whereas the dose received by 20 cc (D20cc) of the brain should not exceed 20 Gy and 24 Gy when SRT is delivered in three and five fractions, respectively [26]. shows some of the most commonly adopted SRS/SRT schedules for BMs, with the relative BED and equivalent dose in 2 Gy/fraction (EQD2) for tumor and brain parenchyma, alongside the dose constraints predictive for RN.
For further optimization of the therapeutic index in the management of large BMs, a novel approach named staged SRS (SSRS) has been investigated. It consists of delivering SRS in two or three fractions separated by an interval of a few weeks. Treatment replanning between each session is necessary to adapt RT volumes to the progressive shrinkage of the BM. Thus, a higher final BED on the tumor is achievable, while simultaneously maintaining a lower risk of RN. Despite the promising results of preliminary reports [27,28,29,30,31,32,33,34,35], further evidence is awaited before there is wide adoption of SSRS in clinical practice. Most studies included patients with BMs from different primary tumors, with NSCLC representing the most frequent (30–100%) histology. On the other hand, SCLC patients deserve specific consideration (as reported in the specific section below), as they were rarely included in studies investigating the role of SRS and SRT (≈5%).
2.2. SRS for Limited BMs
The management of oligometastatic brain disease, considered as a stage with a limited number (maximum 3–4) of BMs, indicating an intermediate status between the absence of brain lesions and a disseminated presentation, has evolved considerably during the past few decades. Historically, WBRT was considered the mainstay for BMs regardless of the number of lesions. Starting from the 1980s, several randomized clinical trials were conducted to investigate the role of focal treatments (either surgery or SRS) in this setting, enrolling a large number of NSCLC patients [36,37,38,39,40].
Firstly, the RTOG 9508 trial randomized patients with 1–3 BMs to receive WBRT + SRS versus WBRT alone. That study showed a better LC in the SRS group (1 y LC: 81% vs. 71%, p = 0.01), which resulted in an improved OS for patients with a single BM (median OS: 6.5 vs. 4.9 months, p = 0.039) [40]. The proven effectiveness of focal RT raised the question of the feasibility of omitting WBRT after SRS for oligometastatic BMs, possibly avoiding the neurocognitive dysfunctions related to extended radiation fields.
A second generation of randomized trials and a meta-analysis of individual patient data explored this scenario [41,42,43,44,45]. The results, despite the different primary endpoints considered, were univocal, showing similar OS rates despite a lower intracranial disease control in patients receiving SRS alone. At the same time, WBRT was confirmed to negatively impact both on neurocognitive function and quality of life (QoL) [42,43,44,46]. Furthermore, three secondary analyses of these trials, conducted using NSCLC patients, failed to prove a definitive advantage of additional WBRT over SRS after stratification for DS-GPA [47,48,49].
In conclusion, data from the aforementioned studies led to the progressive omission of WBRT for oligometastatic BMs in favor of focal therapies, which demonstrated a more favorable risk–benefit ratio (durable LC, lower toxicity and identical OS). Moreover, the feasibility of repeating SRS in case of further relapses (see the section dedicated to SRS retreatment below), with limited or no toxicity, encouraged this epochal transition in the treatment paradigm for oligometastatic BMs. Nowadays, according to current international guidelines, SRS alone is the central pillar for the management of a limited number of BMs [50,51,52,53].
2.3. SRS for Polymetastatic BMs
Although WBRT is still considered the standard RT approach for patients with multiple (≥5) BMs [54], the current trend is to propose the use of SRS also in this particular setting. Exploiting technological advances, we are now able to treat all the macroscopic metastatic deposits with focal RT (Figure 3), potentially improving LC and minimizing the exposure of uninvolved brain parenchyma [55].
Figure 3. Example of SRS for the treatment of multiple (≥5) BMs.
When SRS is offered with a LINAC-based approach, a single isocenter may be used to simultaneously treat multiple BMs, in order to reduce the delivery time and improve patients’ compliance [56,57,58,59].
Several studies investigated the role of SRS for polymetastatic BMs and provided the proof of principle for its feasibility, efficacy and safety. Yamamoto et al. led the first retrospective analyses, showing that carefully selected patients with up to 10 or more BMs are not unfavorable candidates to receive SRS alone [60,61,62,63,64]. Other multicenter retrospective [65] and prospective studies [66,67] confirmed these results, with LC, OS and toxicity rates comparable to those observed in oligometastatic patients. Despite the studies cited so far, robust data from phase III trials are still lacking to date. A Dutch trial, investigating WBRT versus SRS for patients with 4–10 BMs, prematurely interrupted the enrollment (mainly as a result of patients’ and doctors’ preference for SRS) and no definitive results were produced on the non-inferiority of SRS in terms of OS and brain-failure-free survival [68]. Another randomized controlled trial, presented at the American Society for Radiation Oncology (ASTRO) annual meeting in 2020, randomized patients with 4–15 non-melanoma BMs to receive WBRT or SRS. The radiosurgical treatment was associated with a reduced risk of neurocognitive deterioration without compromising OS (median OS: SRS 10.4 months vs. WBRT 8.4 months, p = 0.45) [69].
Ongoing phase III trials comparing hippocampal-avoiding WBRT (HA-WBRT) versus SRS (NCT03075072, NCT03550391, NCT04277403; see will provide further evidence for this scenario. Meanwhile, SRS should be offered to carefully selected patients with multiple BMs, while also taking into consideration impactful variables such as BM tumor volume [70,71] and BM velocity [72,73].
2.4. SRS Reirradiation
The use of upfront SRS with deferral of WBRT makes close MRI follow-up essential. Serial neuroimaging allows monitoring of the outcomes of treated lesions, in terms of LC and RN, and early detection of the eventual development of new intracranial metastases [74]. Literature data estimate a risk for local relapse of approximately 10–20% and for distant brain recurrence of up to 50% after SRS alone for BMs [41,42,43,44,45]. Although there is no consensus, SRS represents a valid salvage strategy for both local and distant intracranial recurrence, presenting considerable advantages over the other treatment modalities, such as WBRT, surgery and ST.
Multiple courses of SRS to treat new BMs appeared to be safe and effective for carefully selected patients, and it allowed them to avoid or delay WBRT [75,76]. Series conducted on NSCLC patients confirmed the feasibility of this approach [77,78]. The trial NRG-BN009 is an ongoing phase III trial investigating the impact of HA-WBRT in addition to SRS on patients with BM velocity ≥ 4 new brain metastases/year at the time of a first or second distant brain relapse after upfront SRS (NCT04588246; see .
The scenario of SRS reirradiation, in the strict sense, meaning the radiosurgical retreatment of a locally recurrent BM, is more controversial. Although surgery, when feasible, is generally considered the best salvage strategy after local relapse [79], repeated SRS is a viable alternative. Retrospective series [80,81] and two meta-analyses [82,83], including patients with BMs from different primary tumors, showed encouraging data in terms of LC, although retreatment carried a significant risk of RN. A recent large retrospective analysis validated these results for an NSCLC population [84]. A dose of 16–18 Gy in single fraction emerged as the most used, with the general belief that dose escalation may lead to an improvement in LC [81]. According to a recent study, fractionated SRT seemed to be a valuable option also for reirradiation, comparing favorably to single-fraction SRS with regard to LC and RN risk (1 y local failure: 27.7%; 1 y RN: 15.6%) [85]. REMASTEr (NCT05124912; see and Re-TREAT (NCT05126875; see are ongoing studies whose results are awaited to better define the role of SRS reirradiation.
To conclude, SRS may represent a valid solution in the spectrum of salvage therapies after prior SRS, allowing avoidance of the issues related to surgery or WBRT in this setting.
3. SRS and Surgery
Surgery plays a central role in the treatment of BMs, remaining the gold standard for large and edemigenous lesions. It ensures a decompressive effect with immediate symptom relief and, when required, provides histological diagnosis and molecular determination [51,52,53]. Despite these advantages, the LC of resected BMs is not durable after surgery alone, with recurrence rates reaching 50% [86]. Furthermore, there is a risk of leptomeningeal dissemination (LMD) of tumor cells intrinsic to the surgical procedure [87]. In this scenario, RT may represent not only an alternative but also a complementary solution to surgery, in order to improve clinical outcomes. A paradigm shift has occurred also in the adjuvant setting, with the historic WBRT approach being abandoned due to the lack of survival benefits [43,86,88], in favor of postoperative focal treatments that maximize LC without an excessive neurotoxicity.
3.1. Adjuvant SRS
Focal RT of the surgical cavity after surgical resection of BMs represents the current standard of care in many centers and is recommended by the most recent guidelines [51,52,53]. Using one (SRS) or a few (SRT) sessions of ablative RT, it is possible to reduce the risk of local recurrence associated with exclusive surgery. In the last two decades, several retrospective series investigated the role of adjuvant SRS, and a recent meta-analysis, pooling 3458 patients from 50 studies, revealed high LC rates (1 y LC: 83.7%) and a low toxicity profile (1 y RN rate: 6.9%) [89]. Two randomized trials confirmed the efficacy and safety of this approach [90,91]. Mahajan and colleagues randomized patients to receive adjuvant SRS versus observation after gross total resection of 1–3 BMs. The results showed an excess of local recurrence in the observation group (1 y freedom from local recurrence: 43% vs. 72% in the SRS arm, p = 0.015) [90]. In the multicenter NCCTG N107C/CEC.3 study, adjuvant SRS was compared to adjuvant WBRT for patients with a single resected BM. WBRT resulted in better overall intracranial control at 1 year compared to the SRS group (81.5% vs. 40.7%, p = 0.003) [91,92]. In fact, no difference was observed in terms of LC between the two arms, according to a secondary analysis of the trial presented at the ASTRO 2022 meeting (1 y LC: 79.2% vs. 86.5%, p = 0.099) [93]. On the other hand, the preservation of neurocognitive functions and QoL were significantly better in the SRS group [91,92]. For completeness, the trial showed no differences with regard to OS between the study arms [90,91,92].
Based on this evidence, adjuvant SRS of the surgical cavity has progressively become the recommended treatment in patients with resected BMs, although many uncertainties still need to be resolved. First, the optimal dose and fractionation to obtain an ideal balance between LC and the risk of RN is still under investigation. Considering that surgery is generally proposed for large symptomatic lesions, it is very common to face large postoperative volumes, which may not be adequately treated with a single-fraction SRS. As described for intact BMs, fractionated SRT seems to be the ideal solution to this problem. Several series and meta-analyses have suggested an excellent benefit–risk ratio for adjuvant SRT [94,95,96,97,98], and the completion of ongoing randomized trials is awaited for stronger evidence (NCT04114981, NCT05160818; see . Other issues related to postoperative focal treatment depend on the dynamic adaptation of the surgical cavity, which complicates the identification of target volumes and the timing of adjuvant RT. Given this heterogeneous scenario, consensus contouring guidelines published in 2017 provided technical and practical recommendations with the aim of standardizing postoperative SRS for completely resected BMs [99]. Regarding the optimal timing, an interval from surgery of 4–6 weeks is suggested [100]. Finally, adjuvant SRS is thought to be inferior to WBRT in reducing the risk of LMD after surgery, with a reported incidence of up to 35% after postoperative focal RT [101]. LMD may even appear with a distinct nodular pattern, which is less likely to be symptomatic and has a better OS rate than the classical linear pattern when treated with adjuvant SRS [9].
To conclude, adjuvant SRS/SRT is considered a valuable treatment strategy after surgical resection of BMs, although further evidence from larger retrospective studies and randomized trials is eagerly awaited to clarify the role of focal treatments in this setting.
3.2. Neoadjuvant SRS
Neoadjuvant SRS represents the new frontier in the management of resectable BMs. All the critical issues related to adjuvant SRS led to the investigation of a preoperative strategy; the rationale is to sterilize any microscopic disease before the macroscopic resection of the brain lesion. A better definition of the irradiation target with shrinkage of the treatment volumes (Figure 4), a reduced risk of RN and LMD and a contraction of the overall treatment time are all potential advantages over postoperative focal RT.
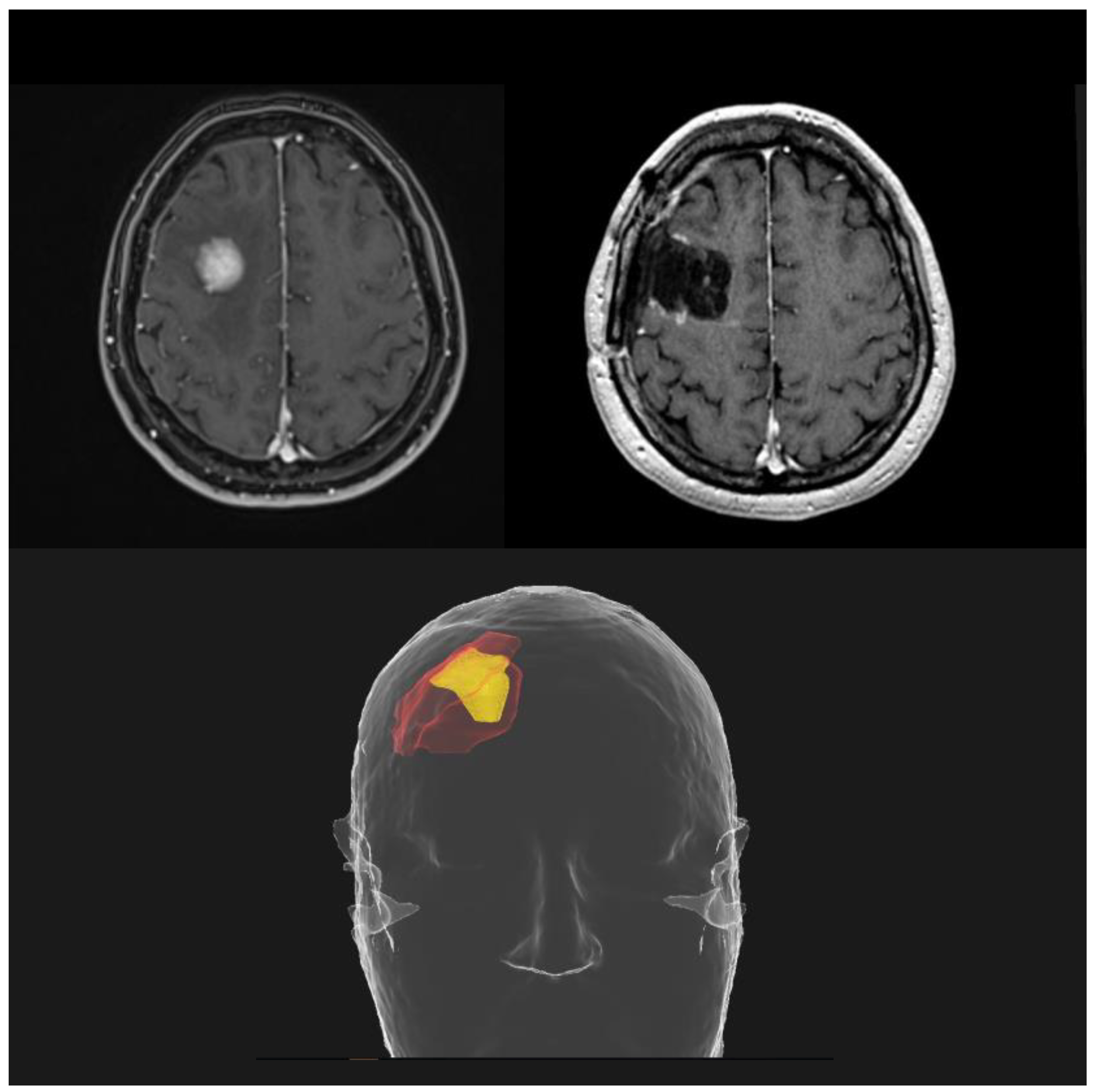
Figure 4. Potential reduction in treatment volumes using a preoperative SRS approach (yellow), compared to postoperative SRS (red).
On the other hand, the main limitations of a neoadjuvant approach are the risk of irradiating a lesion with an unproven histological confirmation and delaying surgery in those urgent cases that require rapid intervention to control a symptomatic lesion. Neoadjuvant RT is typically performed as a single fraction of 16 Gy, given a few days before surgery; more recently, fractionated SRT (median doses of 24–30 Gy in 3–5 fractions) was proposed with the intention to improve the therapeutic index [102].
Several single-arm studies collected retrospective and prospective data of patients treated with neoadjuvant SRS and showed favorable oncological outcomes and toxicity profiles (low rates of RN and LMD) [103,104,105,106,107,108,109,110,111]. The trial PROPS-BM, a multicenter cohort study at five American institutions, represents the largest published series in this setting. With a median time between SRS and surgery of 1 day (range 1–3 days), the median prescribed dose at the 80% isodose line was 15 Gy. The treatment was effective (1 y LC: 85%; 1 y distant control: 63.7%; 1 y OS: 57.7%) with a low rate of complications (LMD rate: 7.9%; RN rate: 7.1%) [112].
Ongoing studies, particularly randomized phase III trials comparing neoadjuvant versus adjuvant SRS (NCT03741673, NCT03750227, NCT04474925, NCT05438212, NCT05545007; see , will provide further information about this promising approach.
4. SRS and Systemic Therapy (ST)
Historically, ST played a secondary role in the management of BMs, due to the blood–brain barrier limiting the penetration to the CNS of the traditional chemotherapeutical agents. Except for highly chemosensitive histologies, such as SCLC, intracranial response rates to chemotherapy were considered unsatisfactory [50]. The modern targeted therapies, particularly immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs), markedly improved the prognosis for specific oncological settings and strengthened the role of ST in the treatment of BMs.
In NSCLC, new-generation targeted agents, such as osimertinib in the case of EGFR mutation other than exon 20 insertions [113,114], and alectinib, ceritinib, brigatinib, lorlatinib and ensartinib in the case of ALK translocation [115,116,117,118,119,120], have shown excellent results for patients with BMs, with intracranial overall response rates (ORRs) ranging between 78% and 91%, as well as encouraging tolerability results. Moreover, ROS1, RET and NTRK rearrangements, MET exon 14 skipping mutation and KRAS G12C mutations represent further molecular markers targetable by drugs, for which novel specific inhibitors have shown encouraging intracranial activity [121,122,123,124,125,126,127,128,129]. However, all these prospective data derive from small subsets of patients enrolled in clinical trials with treated and/or asymptomatic BMs, and this limitation should be considered when translating them to clinical practice. summarizes these findings.
At the same time, ICIs demonstrated a potential intracranial activity for NSCLC patients both when used alone (ORR range: 16.4–55.9%) [133,134,135,136,137,138] and in association with chemotherapy [139,140].
This led to the investigation of a multimodal approach, integrating SRS and modern ST to exploit both these highly effective treatment solutions in different BM scenarios. In addition to the potential synergistic effect achievable with the concurrent administration of SRS and ST [141,142], there is growing interest regarding the possibility of starting upfront ST and delaying or omitting focal treatments [143,144]. To date, no evidence from randomized trials is available to define the optimal combination in terms of timing, safety and efficacy for the new ST options and SRS, requiring a case-by-case evaluation within multidisciplinary discussions.
4.1. SRS and Immune Checkpoint Inhibitors (ICI)
The advent of ICIs improved the prognosis for NSCLC at different disease stages [145,146,147] and significantly reduced the incidence of BMs (incidence of BMs: 6.5% after durvalumab versus 11.8% after placebo, according to the long-term results of the PACIFIC trial) [148], thus drawing attention to the CNS activity of these drugs.
In recent years, the association of SRS and immunotherapy for the treatment of BMs was tested in several retrospective series. Three meta-analyses showed better LC and OS when the two treatment modalities are concurrently administered (1 y LC: 73.2–89.2%; 1 y OS: 64.6–68%) versus a sequential approach (1 y LC: 67.8–69.5%; 1 y OS: 42.7–58%) [149,150,151]. In addition to the increased LC, the synergy between RT and ICIs also seemed to improve distant intracranial control, reducing the risk of developing new brain lesions [152]. Moreover, the literature has reported cases of specific immune responses after SRS-ICI, such as abscopal effect, pseudoprogressions or immune-related adverse events, occasionally related to a better prognosis [153,154]. Although all these studies were predominantly conducted on melanoma patients, NSCLC series confirmed their results [155,156,157].
Regarding toxicities, some reports described an increased RN risk (reported RN rates: 16–37.5%) after concurrent SRS and ICIs [158,159,160]. Despite this, meaningful data have argued in favor of the feasibility of concurrent therapies maintaining acceptable RN rates (<10% at 1 year) [161,162].
Larger prospective trials, possibly limited to a specific histology, are needed to better characterize the efficacy and toxicity profiles of SRS-ICI strategies (NCT02696993, NCT04042220, NCT04047602, NCT04291092, NCT04427228, NCT04787185, NCT04889066, NCT05522660, NCT05703269; see . The evidence available to date is insufficient to strongly recommend the use of upfront ICIs alone, deferring RT when it would be indicated according to current practice.
4.2. SRS and Tirosine-Kinase Inhibitors (TKIs)
Oncogene-addicted NSCLC is characterized by a particularly high incidence of BMs (about 20% in the case of EGFR-mutated and up to 40% for ALK-rearranged tumors at the diagnosis) [163]. TKIs revolutionized the prognosis in this scenario and gained an important role in patients presenting with BMs. The excellent CNS efficacy of new-generation EGFR and ALK inhibitors allowed the consideration of modern ST as an effective single-modality treatment, reserving RT as a consolidation or salvage option in case of persistent or progressive disease [164,165,166]. In light of the aforementioned results of subgroup analyses from randomized trials [113,114,115,116,117,118,119], upfront TKIs are currently accepted by international guidelines for selected patients with multiple asymptomatic BMs from EGFR-mutated and ALK-translocated NSCLC [51,52,53].
Nevertheless, RT remains a highly effective focal approach and its omission in favor of ST alone may be detrimental, both in terms of intracranial control [167] and survival [168], even in the oncogene-addicted population. Notably, Magnuson et al. demonstrated that SRS followed by first-generation EGFR inhibitors significantly improves OS compared to EGFR inhibitors alone (median OS: 46 vs. 25 months, p < 0.001) in TKI-naïve patients with newly diagnosed BMs. Patient selection was extremely important, as SRS seemed to play a key role only in the oligometastatic setting (82% of the patients in the SRS + EGFR inhibitors group had less than 5 BMs) [168].
Therefore, another strand of interest aims to analyze the combination of SRS and TKIs, outlining the potential synergistic effect and the toxicity profile. The first studies investigated WBRT and old-generation TKIs, showing promising but inconclusive results [169,170,171]. The paradigm shift towards the new generation of TKIs with better CNS penetration and the progressive expansion of focal RT techniques have further modified the treatment landscape in this setting.
For EGFR-mutated patients, a few retrospective series investigated the association between osimertinib and SRS, showing a positive impact on clinical outcomes and a potential OS benefit in patients with upfront oligometastatic brain involvement [172,173,174]. In particular, a recent study by Zhao and colleagues showed that upfront cranial therapies like SRS or surgery in addition to osimertinib may provide extra survival benefits compared to osimertinib alone (median OS: 39 vs. 27 months, p = 0.041) [173].
ALK translocation, as well as the other molecular drivers targetable by drugs in NSCLC (ROS1, MET, BRAF, RET, HER2 and NTRK), are less common [175], making it difficult to accumulate robust data on combined SRS-TKI treatment in these specific settings [171,176]. Conversely, KRAS mutations are detected in 25–30% of patients with lung adenocarcinoma. However, the use of KRAS inhibitors has been expanding only in the last few years [128,129], and their combination with SRS represents an interesting frontier to explore in the future.
In the context of polymetastatic BMs, different strategies can be adopted for these patients. The efficacy of modern ST can be exploited with the administration of upfront TKIs in order to downstage CNS disease, avoiding upfront WBRT and converting some patients into SRS candidates [177]. On the other hand, upfront SRS remains feasible also for multiple BMs, with promising results according to a recent multicenter analysis on patients with EGFR-mutated and ALK-translocated NSCLC [178].
To conclude, SRS and TKIs are two effective treatment solutions for the management of BMs from oncogene-addicted NSCLC, allowing patients to avoid WBRT even in the case of multiple lesions. The results from ongoing studies investigating the combination of focal RT and modern target inhibitors (NCT03497767, NCT03769103, NCT04905550, NCT04908956, NCT05033691, NCT05236946, NCT05522660; see are awaited to better define the risks and benefits of the concurrent administration.
4.3. SRS and SCLC
The management of BMs from SCLC deserves specific considerations. SCLC is a poor prognosis disease, with a great tendency to brain metastatic dissemination and intracranial progression [179]. In this scenario, WBRT is still considered the standard of care, delivered alternatively with prophylactic intent [180,181] or to treat macroscopic BMs, even for patients with a low CNS involvement [182]. Historically, SCLC histology was considered an exclusion criterion from randomized trials investigating the role of SRS for BMs, resulting in a lack of prospective evidence [183]. Nevertheless, the development of advanced neuroimaging, with the possibility of omitting prophylactic cranial irradiation (PCI) in favor of MRI surveillance [184], and the introduction of ICIs in combination with standard chemotherapy regimens [185,186,187,188], paved the way for a paradigm shift potentially including focal RT techniques.
In the last few years, an increasing number of institutions published retrospective studies exploring the safety and the effectiveness of SRS for SCLC patients [189,190,191,192,193,194,195,196,197]. FIRE-SCLC is a cohort study representing the largest series available to date; over 700 patients with BMs from SCLC received first-line SRS, reporting favorable outcomes and no inferiority in terms of OS after propensity-score-matched comparison with first-line WBRT. In particular, patients developing oligometastatic brain involvement seemed to have a favorable prognosis when treated with SRS (median OS: 11 months for patients receiving SRS to a single BM) [198]. Recently, a systematic review and meta-analysis was conducted with the aim of strengthening these findings. This study showed longer OS following SRS compared with WBRT ± SRS boost (HR: 0.85, 95% CI 0.75–0.97) or WBRT alone (HR: 0.77; 95% CI 0.72–0.83) [199].
Until the results from the ongoing trials (NCT03297788, NCT04804644, NCT03391362, NCT04516070; see are available, SRS can be considered a therapeutic option for carefully selected patients with SCLC BMs.
5. Towards Novel Integrated Treatments: The Role of Molecular Profiling and Microenvironment Characterization
5.1. A—Molecular Profile of Lung Cancer BMs: Genomic Characterization
The molecular profile of lung cancer BMs has been investigated through comprehensive, multimodal “-omic” analyses to better characterize the driver alterations enabling BM establishment, progression and resistance to treatments [200]. Most studies have employed next-generation sequencing (NGS) assays to investigate genetic alterations including single-nucleotide variations (SNVs), small insertions and deletions (InDels) and gene rearrangements. Within a cohort of 552 NSCLCs, Wang et al. characterized 153 matched BM specimens, demonstrating for the first time that RET fusions increase the risk of BM development. Furthermore, the significance of EGFR exon 21 mutations and exon 19 frameshift deletions as predictive alterations of BM development was also confirmed [201,202]. The authors also assessed the incidence of ALK alterations [201], reporting the retention of ALK-EML4 translocations in BMs compared with primary tumors, and a rate of about 3–11% for ALK amplifications in these samples [203,204]. A whole exome sequencing (WES) approach on a series of 40 samples, comprising both primary lung adenocarcinoma (AC) and BMs, showed a discrepancy in the mutational landscape between primary and secondary lesions. BMs were found to be characterized by a higher level of genomic alterations both in terms of copy number alterations (CNAs) and single-nucleotide variants (SNVs), with a low rate (8.3%) of shared mutations between BM and primary lung cancer. A further recent comprehensive genomic characterization of lung AC BMs investigated new putative private driver alterations and, in accordance with other studies [205], confirmed the preservation of primary AC driver mutations involving TP53, KRAS, STK11, KEAP1 and EGFR. Moreover, a phylogenetic analysis revealed that the accumulation of somatic mutations, as well as the genetic divergence and heterogeneity between primary tumors and BMs, are the result of the concurrent evolution of primary subclones and metastatic ones which can be explained through a parallel progression model [204].
Concerning relevant CNAs, in addition to the well-established role of the loss of homozygosity of CDKN2A/B, BMs can harbor either focal amplifications of MYC (8q24.21) or YAP1, BIRC3 and TMEM123 (11q.22.2), or of a cluster of metalloproteinases including MMP13, EDNRA, ARHGAP10 and NR3C (4q31.23). Interestingly, the comparison between 58 BMs and their matched primary ACs demonstrated that the mentioned chromosomal alterations (8q24.21, 11q22.2, 4q31.23) are only present in the BMs, determining a clonal divergency during the metastatic process and potentially favoring a low response to RT, as previously reported for other tumors [206,207]. Finally, patient-derived xenograft (PDX) mouse models confirmed these results demonstrating the role of YAP1, MMP13 and MYC, whose overexpression confers a greater propensity to grow in brain microenvironments, probably due to MYC-mediated activation of stemness-like phenotypes and pathways [208,209].
Molecular characterization of a series of 79 stage IV lung squamous cell carcinomas (SQCCs) and matched BMs revealed that the latter retain the PTEN loss of function from their primitive tumors [210]. PTEN loss of function has been described as an important alteration in lung cancer BMs because it mediates the activation of anti-apoptotic pathways such as NF-kB, COX-2, MAPK and PI3K, causing a higher resistance to therapies which induce apoptosis like RT [211]. SQCC-BMs retain primitive tumors’ alterations, but also harbor novel private ones, such as the 5q31 gain where proto-cadherins reside [212].
The proto-oncogene MET is an important player involved in metastatic processes, given its key role in the epithelial–mesenchymal transition (EMT). Stella et al. investigated the mutational landscape of MET in a case series of 68 samples of lung cancer (NSCLCs and neuroendocrine tumors—NETs), showing a higher rate of mutations and amplifications in BMs, a finding associated with higher RT resistance [213]. These findings are especially relevant for NETs since fewer data are available regarding these tumors due to the low availability of specimens caused by the lower rate of surgical resections [214]. Despite these challenges, Dono et al. evaluated the genomic alterations of a series of BMs from different tumors, including 10 BMs from SCLC. Genetic analysis, also performed using data from the COSMIC and TGCA databases, demonstrated that SCLC-BMs are enriched in alterations of specific genes: the epigenetic regulator ARID1A, component of the SWI/SNF complex, and the proto-oncogene FGF10 [215]. Mouse models have also been developed to perform in vivo characterization. BM cannot be investigated in genetically engineered mouse models (GEMMs) since the timeframe for their development is not sufficient before death occurs because of primary tumor progression and/or other metastasis development. On the other hand, cell-derived xenograft (CDX) and orthotopic mice, generated by injecting patient-collected circulating tumoral cells (CTCs) [214,216], develop BMs, allowing the characterization of these entities [216,217,218]. Moreover, the development of SCLC-organoids is a novel tool which might improve the molecular characterization of this entity; Quaranta et al. developed a protocol to establish cerebral organoids of SCLC metastasis to recapitulate the dissemination process and to characterize BM profiles the interactions between SCLC cells and brain parenchyma cells, and the response to therapeutical regimens, including RT [219].
An overview of lung cancer BMs’ molecular profile investigated through “-omic” analyses is provided in Figure 5.
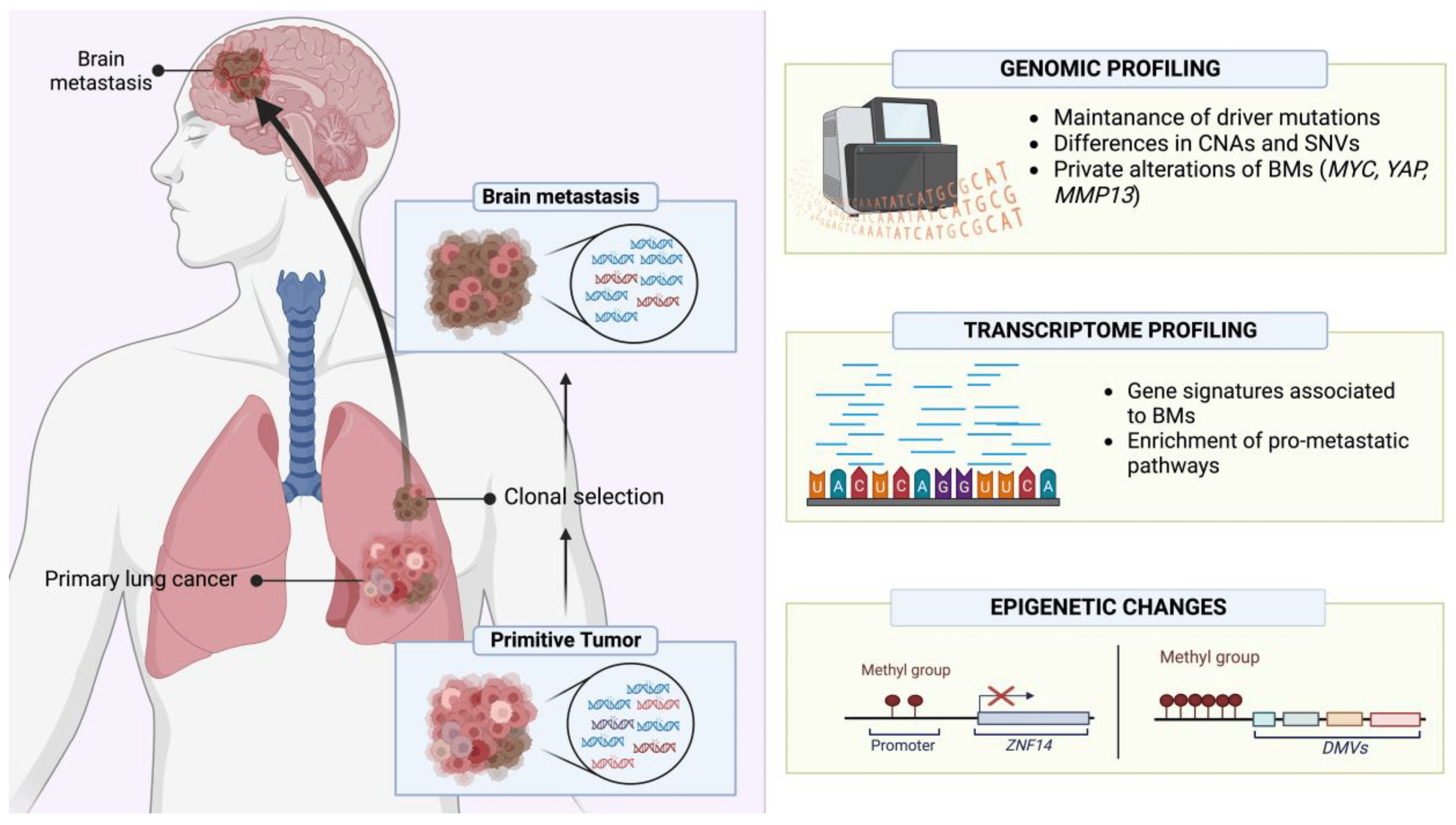
Figure 5. Schematic overview of the molecular characteristics of lung cancer BMs investigated through “-omic” analyses. Created with BioRender (https://www.biorender.com/, accessed on 13 September 2023).
5.2. B—Molecular Profile of Lung Cancer BMs: Transcriptomic and Epigenetic Features
Genomic characterization is just one type of tumor molecular profiling. Indeed, transcriptomic and epigenetic characterization are also critical to achieve a comprehensive molecular dissection of a tumor. Gene expression analysis demonstrated the activation of pro-invasion/metastatic signaling in BMs, especially of Rap1, whose role during invasion processes has been previously reported for primary brain tumors. Moreover, BMs showed an enrichment in pathways involved in RT resistance, such as PI3K/AKT [220,221,222,223]. Kramer et al. profiled 91 fresh-frozen samples from patients affected by NSCLC, among whom 32 had developed BMs, to assess the risk of patients to develop BMs and to find new tumoral biomarkers. The RNA-seq analysis demonstrated a signature of 22 transcripts which was highly associated with BM risk. Moreover, the pathway analysis found an enrichment of genes related to oxidative phosphorylation, in particular those associated with the respiratory chain complex I [224]. To elucidate the risk of BM development, Koh et al. analyzed 36 lung AC cases with BMs and 36 lung AC cases without BM, comparing the expression of a large series of immune-related genes. The analysis found a gene signature of 11 immune-related genes (DPP4, ICAM1, RARRES3, CD74, CSF2, HLA-DMB, ICAM5, MUC1, CCL18, RORC and ICAM4) associated with a higher risk of BM development, and this signature was further validated by immunohistochemical stainings to verify protein expression [225]. A further study, focused on BM cells retrieved from the cerebrospinal fluid (CSF) of lung adenocarcinoma patients, studied the gene signatures associated with BM development. This transcriptome analysis showed that CTCs in CSF harbor an enrichment in genes associated with cell adhesion, especially those necessary for the interaction with vessel cells and tissue colonization, as well as EMT, extracellular matrix remodeling and metabolic activities, highlighting how these pathways are essential for both the tumor spread and seeding in distant sites [226] (Figure 5).
DNA methylation profiling is now an important, clinically relevant tool, allowing the determination of tumor types according to their epigenetic signatures. Epigenetic analysis could also allow the identification of specific BM hallmarks. Therefore, Karlow et al. analyzed the methylation profile of BMs, comparing matched ‘trios’ of specimens retrieved from a series of 12 patients with lung adenocarcinoma and including lung normal tissue, lung primary cancer tissue and BMs. The profiling identified differentially methylated regions in metastatic lesions showing a gain of DNA methylation at specific sites. In particular, the promoter of ZNF154, a zinc finger related to the suppression of the metastatic process, was found to be hypermethylated, and thus silenced, in BM specimens. The analysis of DNA methylation valleys (DMVs), which are a hotspot of regulatory regions for several developmental genes [227], demonstrated that BMs are characterized by a gain of methylation, with the enrichment of heterochromatin markers such as H3K4me1, H3K9me3, H3K4me3, H3K27ac and H3K27me3, as well as a decrease in H3K36me3 methylation score, determining a suppression of polycomb repressor complex and boosting the metastatic process [228] (Figure 5).
These studies highlight that BMs are molecularly distinct entities compared to their primary tumors and harbor specific molecular traits associated with resistance to treatments, including RT. Further knowledge regarding these mechanisms will hopefully allow the current clinical gap to be filled and disclose novel effective targets for both BM prevention and treatment.
5.3. What’s Next? The Importance of Tumor Microenvironment and the Interplay with RT
Metastasis development is a strongly selective multistep process in which the role of the tumor microenvironment (TME) is crucial. Whether in primary or secondary sites, the interactions between tumoral cells and resident host cells shape the development and progression of neoplasms, also modulating their tropism to specific distant organs [229]. Regarding lung cancer, CNS is the predominant metastatic site and BMs occur frequently [230]. Cancer cells manage to cross the blood–brain barrier, hijacking its protective mechanisms and invading brain parenchyma to establish initial micrometastatic seeding [231]. Multiple cellular types within the TME are reprogrammed to promote cancer progression; astrocytes undergo several alterations in cellular morphology and gene expression through reactive gliosis, promoting the proliferation and chemoresistance of BMs [232,233]. Tumor-associated macrophages (TAMs) are driven towards M2 subtype differentiation, sustaining a more aggressive tumoral phenotype through the production of several cytokines capable of inducing angiogenesis, extracellular matrix remodeling and immunosuppression [234]. Dendritic cells (DCs) assume an immunosuppressive role in NSCLC, enhancing the ability of Treg cells to suppress T lymphocyte proliferation [235]. Even tumor-associated neutrophils (TANs) can differentiate in the pro-tumoral phenotype N2, which contributes to ECM remodeling, immune evasion, proliferation and angiogenesis [236].
In this setting, RT can induce changes in the TME that may modulate both tumor response and resistance through a subtle, highly complex balance between the activation and the suppression of the immune system [237]. Irradiation exerts an immunostimulating activity by increasing NK cell cytotoxicity, tumor infiltration by CD8+ cytotoxic T lymphocytes and M1 differentiation of macrophages, while reducing the level of infiltrating regulatory T cell (Treg) lymphocytes; these changes ultimately result in an inhibition of tumor growth [238]. In addition, irradiation increases the amount of major histocompatibility complex (MHC) molecules on the surface of the cells, and the secretion of several factors necessary for the activation of dendritic cells that initiate innate and adaptive immune responses. Unfortunately, pro-neoplastic changes are also induced, including the release of molecules, like the anti-inflammatory cytokine TGF-β, which promote tumor growth and invasion, inhibit cytotoxic cell functions and alter the autophagic and necrotic signaling, increasing the energetic supply of BM cells and suppressing death-inducing mechanisms [239,240]. On the other hand, the induced necrosis and necroptosis may activate the immune system against neoplastic cells through the establishment of a pro-inflammatory and pro-apoptotic microenvironment caused by TNF-α, INF-γ and IL-2 released by CD8+ T cells and NK cells [241,242]. These conflicting and intertwined relationships show the complex nature of the effects exerted by RT and highlight the therapeutic potential of targeting these interactions.
The main promoters of immunosuppression within the TME are Tregs, since they are resistant to RT and driven to proliferate by PD-1 [243]. Tregs are responsible for the production of TGF-β that suppresses the cytotoxic activity of CD8+ T cells, promotes the conversion of CD4+ T cells in Tregs and drives the differentiation of cancer-associated fibroblasts (CAFs), which contribute to the anti-apoptotic signaling by stimulating the PI3K pathway [244,245]. To counteract this mechanism, a high-dose regimen in hypo-fractionated stereotactic radiation therapy (HSRT) has been proposed to increase the number of CD8+ T cells, attenuate the release of TGF-β by CD4+ T cells and also reduce the number of Tregs [246].
Macrophages, especially the M2 subtype activated by inflammatory cytokines, are also involved in the establishment of an immunosuppressive TME. Even though the RT effect on macrophage differentiation needs to be fully elucidated, it has been reported that irradiation with either single or fractionated doses can activate both M1 and M2 macrophages, ultimately leading to tumor growth [247]. However, the RT-induced changes are still controversial, since an in vitro and in vivo study reported that irradiation of macrophages within the TME with 10 Gy, despite leading to an increase in the number of M1 cells and a reduction in M2 cells, nevertheless caused the upregulation of pro-survival pathways, such as NFκB and Bcl-2, increasing the invasive and metastatic phenotype [248]. Conversely, exposure to a low dose of radiation may cause reprograming of macrophages into M1 cells, which triggers infiltration of cytotoxic T lymphocytes (CTLs) into the TME [249].
Exposure of the TME to radiation causes secretion of IFN-γ, which induces the expression of MHC I and VCAM-1, resulting in lymphocyte trafficking and increasing anti-tumor immunity [250,251]. CTLs represent the most important anticancer cells in the TME, and highly radioresistant tumors may prevent the infiltration of lymphocytes [252]. Their activity directly depends on antigen presentation by DCs, whose antitumoral function can be enhanced by exposure of tumors to radiation and the subsequent upregulation of Toll-like receptors (TLRs) [253]. The higher presentation of damage-associated molecular patterns (DAMPs) to CTLs increases the release of anti-cancer cytokines, facilitating cytotoxicity against cancer cells [254]. Also, in this case, the high radiation doses administered in HSRT (at 10 Gy/fraction) may further amplify the release of immune-stimulating antigens and antigen presentation by DCs [255].
Given this evidence, several experimental studies have been conducted to modulate the TME and sensitize cancer cells to chemotherapy and/or RT [256]. The first pathway to be targeted was DNA repair, using inhibitors such as olaparib and veliparib, which are currently employed in clinical practice.
Since inflammation is a further important mechanism involved in tumor progression and resistance, strategies focused on its modulation have also been actively investigated. For instance, the enzyme COX-2 is known to be produced at higher levels in the TME and plays a key role in tumor growth and metastatization, so its targeting could be feasible for sensitizing cancer cells to RT [257]. It was demonstrated that inhibition of COX-2 with celecoxib increases apoptosis following irradiation in some types of cancer cells, and clinical studies confirmed the role of COX-2 targeting in tumor resistance, showing promising results in terms of relapse-free survival and OS in patients with breast and lung cancers, although these data were not confirmed in other clinical trials [258,259,260,261].
Hypoxia is another important player in radioresistance and tumor progression. It has been suggested that HIF-1 is involved in both radioprotection and radiosensitization since it can activate p53 and apoptosis in irradiated cells, but also stimulates VEGF secretion to promote tumor vascularization [262]. Since the radioprotective effect of HIF-1 is highly dependent on p53 activity, in some conditions like the mutation and inactivation of p53, HIF-1 inhibition may be a promising strategy for the sensitization of tumors to RT [262,263]. However, the radioresistance induced by hypoxia could represent an issue in cases of treatment with anti-angiogenetic therapies against VEGF and its associated receptors, which may induce hypoxia. Actually, the concomitant irradiation of lung xenografts with the VEGF-receptor inhibitor cediranib induced tumor apoptosis and necrosis, as well as higher levels of hypoxia, but this was not associated with an acquired radioresistance since the maintenance of the inhibitor after RT treatment prevented the contribution of hypoxic cells to tumor regrowth and enhanced radiation response [264].
Considering the significant impact that the TME exerts on cancer progression, it is clear that novel strategies are warranted to modulate these interactions and counteract the pro-oncogenic mechanisms. As mentioned above, Tregs are more radioresistant than CTLs, and an increase in the Tregs to CTLs ratio is responsible for the establishment of an immunosuppressive environment in the TME. The main mechanism by which Tregs inhibit CTLs is the PD-1/PD-L1 axis, which is frequently reprogrammed by tumors as an escape mechanism from the immune system [265]. Thus, targeting this pathway in combination with RT may boost immune detection by cytotoxic cells and the exhaustion of Tregs within the TME, providing a biological rationale for the clinical efficacy of these therapeutic combinations [266,267]. Nowadays, several tumor types are being treated with immune checkpoint inhibitors (ICIs), and indeed immunotherapy with pembrolizumab and nivolumab has become a standard treatment option for many malignancies, including metastatic NSCLC [268,269]. Unfortunately, the BM response to anti-PD-L1 antibodies is poorer compared to that of primary lung lesions, probably due to a markedly decreased amount of PD-L1+ tumor infiltrating lymphocytes (TILs) in secondary lesions [270]. Moreover, exposure of cancer cells to radiation may cause upregulation of PD-L1, leading to resistance to subsequent doses of RT. Also, the genomic subset of NSCLC more prone to develop BMs could be associated with a diminished response to immunotherapy [271]; it has been reported that patients with EGFR mutations or ALK/ROS1 fusions present a characteristic low tumor mutational burden (TMB) and, despite the observed high expression of PD-L1 in tumoral cells, these patients present minimal benefits from ICIs and are associated with a short progression-free survival [272]. On the contrary, KRAS-mutated NSCLC patients present a high TMB and an increased infiltration of PD-L1-positive lymphocytes, resulting in an inflammatory TME which enables a better response to immunotherapy [273]. Multiple targeting of immune response modulators can also be envisaged; for instance, it was demonstrated that the simultaneous inhibition of PD-L1 and T cell immunoglobulin mucin-3 (TIM-3) in combination with RT may be more effective for tumor control [266].
Concerning other CNS cell types, Monteiro et al. demonstrated that BMs are promoted by high levels of S100A9 expressed by cancer cells following the inflammatory response caused by the interaction with reactive astrocytes. This molecule mediates resistance to RT through the NF-κB pathway, and its signaling could be impaired by the inhibition of its receptor RAGE [274]. S100A9 could thus be a relevant target for improving RT efficacy and also be exploited as a predictive biomarker since circulating levels of this protein before and immediately after WBRT treatment correlate with survival. Supporting this potential role, its inhibitor FPS-ZM1 showed a radiosensitizer effect independently of primary tumor origin and without side effects on healthy brain tissue, representing an innovative therapeutic option.
In conclusion, understanding the role exerted by the BM TME is critical to devise new strategies to lift the tumor-induced immunosuppression, improving the efficacy of both RT and ST, and ultimately extending disease control and survival in this challenging clinical setting.
An overview of RT influence on BM TME and the proposed modulation strategies is provided in Figure 6.
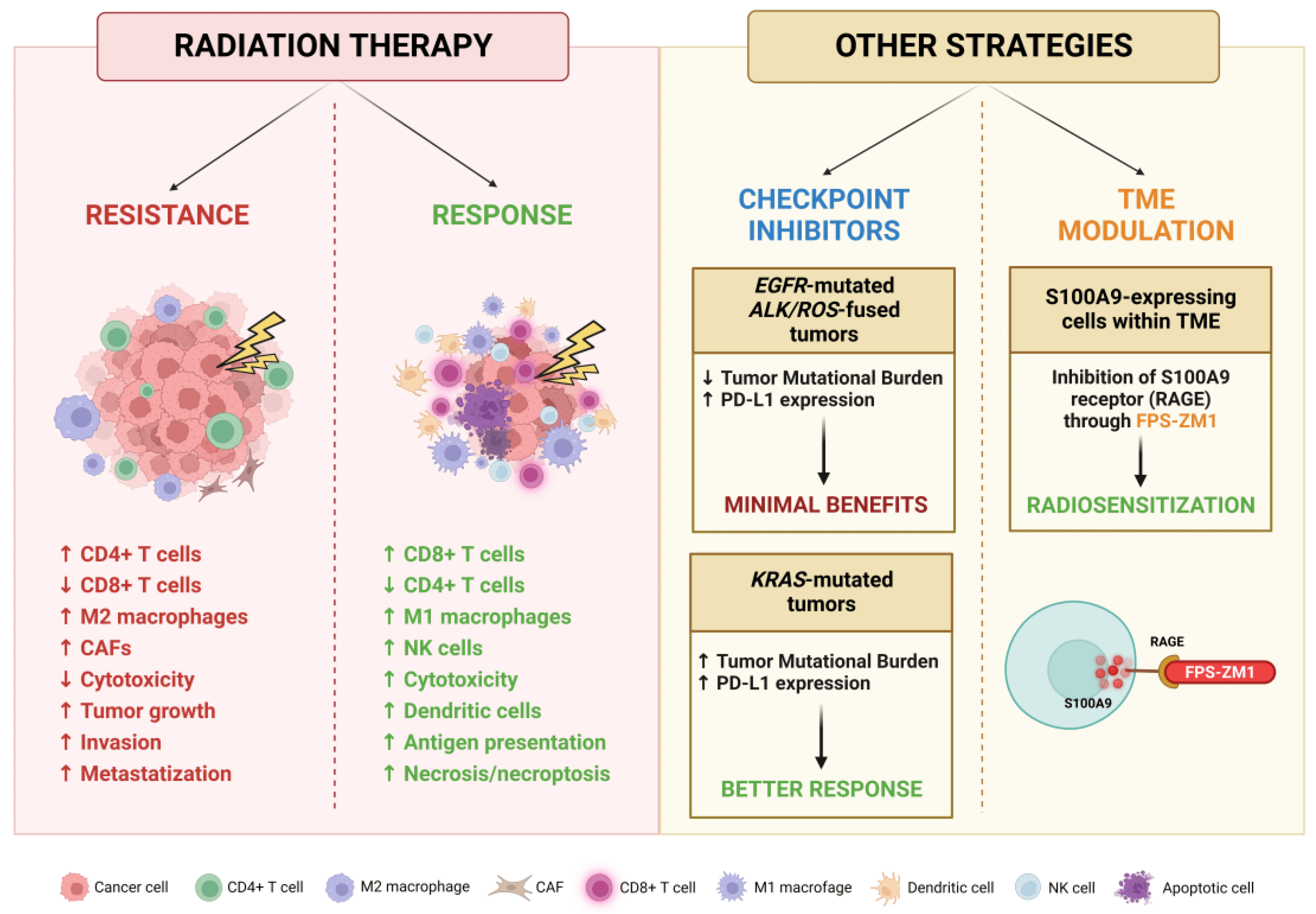
Figure 6. Schematic representation of effects exerted by RT on BM TME and potential strategies for its targeting. Created with BioRender.
6. Conclusions
SRS/SRT represents a cornerstone of lung cancer BM treatment, enabling LC in a high percentage of patients with lower rates of side effects compared to older modalities. SRS/SRT methods are also undergoing a fast-paced technical evolution, expanding their role in novel settings like patients with multiple BMs. The landscape of ST has also been completely reshaped in the last decade thanks to the deployment of ICIs and novel targeted therapies for oncogene-addicted tumors. Nevertheless, multiple open issues remain to be addressed, such as how to integrate these therapeutic tools most effectively for each patient. Finally, the revolution brought by ICIs has shown that neoplastic cells are just one side of the coin. Current translational research is highlighting the importance of both the BM-specific molecular profile of BMs and of TME interactions. These data will hopefully result in the identification of new vulnerabilities and potential targets, to improve RT efficacy and enable novel therapeutic strategies.
References
- Ulahannan, D.; Khalifa, J.; Faivre-Finn, C.; Lee, S.-M. Emerging Treatment Paradigms for Brain Metastasis in Non-Small-Cell Lung Cancer: An Overview of the Current Landscape and Challenges Ahead. Ann. Oncol. 2017, 28, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Nugent, J.L.; Bunn, P.A.; Matthews, M.J.; Ihde, D.C.; Cohen, M.H.; Gazdar, A.; Minna, J.D. CNS Metastases in Small Cell Bronchogenic Carcinoma: Increasing Frequency and Changing Pattern with Lengthening Survival. Cancer 1979, 44, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain Metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive Partitioning Analysis (RPA) of Prognostic Factors in Three Radiation Therapy Oncology Group (RTOG) Brain Metastases Trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A New Prognostic Index and Comparison to Three Other Indices for Patients With Brain Metastases: An Analysis of 1960 Patients in the RTOG Database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Effect of Tumor Subtype on Survival and the Graded Prognostic Assessment for Patients with Breast Cancer and Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2111–2117. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-MolGPA). JAMA Oncol. 2017, 3, 827. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; De, B.; Li, J.; Carpenter, D.; Kirkpatrick, J.; Milligan, M.; Shih, H.A.; Kutuk, T.; Kotecha, R.; Higaki, H.; et al. Graded Prognostic Assessment (GPA) for Patients With Lung Cancer and Brain Metastases: Initial Report of the Small Cell Lung Cancer GPA and Update of the Non-Small Cell Lung Cancer GPA Including the Effect of Programmed Death Ligand 1 and Other Prognostic Factors. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 60–74. [Google Scholar] [CrossRef]
- Mantovani, C.; Gastino, A.; Cerrato, M.; Badellino, S.; Ricardi, U.; Levis, M. Modern Radiation Therapy for the Management of Brain Metastases From Non-Small Cell Lung Cancer: Current Approaches and Future Directions. Front. Oncol. 2021, 11, 772789. [Google Scholar] [CrossRef]
- Chen, W.C.; Baal, U.H.; Baal, J.D.; Pai, J.S.; Boreta, L.; Braunstein, S.E.; Raleigh, D.R. Efficacy and Safety of Stereotactic Radiosurgery for Brainstem Metastases: A Systematic Review and Meta-Analysis. JAMA Oncol. 2021, 7, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Wu, H.-M.; Yang, H.-C.; Chen, C.-J.; Hu, Y.-S.; Lin, C.-J.; Guo, W.-Y.; Pan, D.H.-C.; Chung, W.-Y.; Lee, C.-C. Stereotactic Radiosurgery for Pituitary and Cavernous Sinus Metastases. J. Neurooncol. 2023, 161, 175–184. [Google Scholar] [CrossRef]
- Mantziaris, G.; Pikis, S.; Xu, Z.; Mullen, R.; Alzate, J.; Bernstein, K.; Kondziolka, D.; Wei, Z.; Niranjan, A.; Lunsford, L.D.; et al. Stereotactic Radiosurgery for Intraventricular Metastases: A Multicenter Study. Neurosurgery 2023, 92, 565–573. [Google Scholar] [CrossRef]
- Han, E.Y.; Wang, H.; Luo, D.; Li, J.; Wang, X. Dosimetric Comparison of Fractionated Radiosurgery Plans Using Frameless Gamma Knife ICON and CyberKnife Systems with Linear Accelerator-Based Radiosurgery Plans for Multiple Large Brain Metastases. J. Neurosurg. 2019, 132, 1473–1479. [Google Scholar] [CrossRef]
- Scorsetti, M.; Navarria, P.; Cozzi, L.; Clerici, E.; Bellu, L.; Franceschini, D.; Marzo, A.M.; Franzese, C.; Torri, V.; Reggiori, G.; et al. Radiosurgery of Limited Brain Metastases from Primary Solid Tumor: Results of the Randomized Phase III Trial (NCT02355613) Comparing Treatments Executed with a Specialized or a C-Arm Linac-Based Platform. Radiat. Oncol. 2023, 18, 28. [Google Scholar] [CrossRef]
- Shaw, E.; Scott, C.; Souhami, L.; Dinapoli, R.; Bahary, J.P.; Kline, R.; Wharam, M.; Schultz, C.; Davey, P.; Loeffler, J.; et al. Radiosurgery for the Treatment of Previously Irradiated Recurrent Primary Brain Tumors and Brain Metastases: Initial Report of Radiation Therapy Oncology Group Protocol (90-05). Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 647–654. [Google Scholar] [CrossRef]
- Shaw, E.; Scott, C.; Souhami, L.; Dinapoli, R.; Kline, R.; Loeffler, J.; Farnan, N. Single Dose Radiosurgical Treatment of Recurrent Previously Irradiated Primary Brain Tumors and Brain Metastases: Final Report of RTOG Protocol 90-05. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 291–298. [Google Scholar] [CrossRef]
- Thomsen, B.J.; Soliman, H. The Modern Management of Untreated Large (>2 cm) Brain Metastases: A Narrative Review. Chin. Clin. Oncol. 2022, 11, 16. [Google Scholar] [CrossRef]
- Walker, A.J.; Ruzevick, J.; Malayeri, A.A.; Rigamonti, D.; Lim, M.; Redmond, K.J.; Kleinberg, L. Postradiation Imaging Changes in the CNS: How Can We Differentiate between Treatment Effect and Disease Progression? Future Oncol. 2014, 10, 1277–1297. [Google Scholar] [CrossRef]
- Vellayappan, B.; Tan, C.L.; Yong, C.; Khor, L.K.; Koh, W.Y.; Yeo, T.T.; Detsky, J.; Lo, S.; Sahgal, A. Diagnosis and Management of Radiation Necrosis in Patients With Brain Metastases. Front. Oncol. 2018, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Bennett, E.E.; Xiao, R.; Kotecha, R.; Chao, S.T.; Vogelbaum, M.A.; Barnett, G.H.; Angelov, L.; Murphy, E.S.; Yu, J.S.; et al. Association Between Radiation Necrosis and Tumor Biology After Stereotactic Radiosurgery for Brain Metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 1060–1069. [Google Scholar] [CrossRef]
- Chung, C.; Bryant, A.; Brown, P.D. Interventions for the Treatment of Brain Radionecrosis after Radiotherapy or Radiosurgery. Cochrane Database Syst. Rev. 2018, 7, CD011492. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-Analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Choi, K.-S.; Park, E.S.; Cho, Y.H. Single- and Hypofractionated Stereotactic Radiosurgery for Large (> 2 cm) Brain Metastases: A Systematic Review. J. Neurooncol. 2021, 154, 25–34. [Google Scholar] [CrossRef]
- Redmond, K.J.; Gui, C.; Benedict, S.; Milano, M.T.; Grimm, J.; Vargo, J.A.; Soltys, S.G.; Yorke, E.; Jackson, A.; El Naqa, I.; et al. Tumor Control Probability of Radiosurgery and Fractionated Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Diez, P.; Hanna, G.G.; Aitken, K.L.; van As, N.; Carver, A.; Colaco, R.J.; Conibear, J.; Dunne, E.M.; Eaton, D.J.; Franks, K.N.; et al. UK 2022 Consensus on Normal Tissue Dose-Volume Constraints for Oligometastatic, Primary Lung and Hepatocellular Carcinoma Stereotactic Ablative Radiotherapy. Clin. Oncol. 2022, 34, 288–300. [Google Scholar] [CrossRef]
- Higuchi, Y.; Serizawa, T.; Nagano, O.; Matsuda, S.; Ono, J.; Sato, M.; Iwadate, Y.; Saeki, N. Three-Staged Stereotactic Radiotherapy without Whole Brain Irradiation for Large Metastatic Brain Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1543–1548. [Google Scholar] [CrossRef]
- Yomo, S.; Hayashi, M.; Nicholson, C. A Prospective Pilot Study of Two-Session Gamma Knife Surgery for Large Metastatic Brain Tumors. J. Neurooncol. 2012, 109, 159–165. [Google Scholar] [CrossRef]
- Yomo, S.; Hayashi, M. A Minimally Invasive Treatment Option for Large Metastatic Brain Tumors: Long-Term Results of Two-Session Gamma Knife Stereotactic Radiosurgery. Radiat. Oncol. 2014, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Angelov, L.; Mohammadi, A.M.; Bennett, E.E.; Abbassy, M.; Elson, P.; Chao, S.T.; Montgomery, J.S.; Habboub, G.; Vogelbaum, M.A.; Suh, J.H.; et al. Impact of 2-Staged Stereotactic Radiosurgery for Treatment of Brain Metastases ≥ 2 cm. J. Neurosurg. 2018, 129, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Dohm, A.; McTyre, E.R.; Okoukoni, C.; Henson, A.; Cramer, C.K.; LeCompte, M.C.; Ruiz, J.; Munley, M.T.; Qasem, S.; Lo, H.-W.; et al. Staged Stereotactic Radiosurgery for Large Brain Metastases: Local Control and Clinical Outcomes of a One-Two Punch Technique. Neurosurgery 2018, 83, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Higuchi, Y.; Serizawa, T.; Kawabe, T.; Nagano, O.; Sato, Y.; Koiso, T.; Watanabe, S.; Aiyama, H.; Kasuya, H. Three-Stage Gamma Knife Treatment for Metastatic Brain Tumors Larger than 10 cm3: A 2-Institute Study Including Re-Analyses of Earlier Results Using Competing Risk Analysis. J. Neurosurg. 2018, 129, 77–85. [Google Scholar] [CrossRef]
- Ginalis, E.E.; Cui, T.; Weiner, J.; Nie, K.; Danish, S. Two-Staged Stereotactic Radiosurgery for the Treatment of Large Brain Metastases: Single Institution Experience and Review of Literature. J. Radiosurg. SBRT 2020, 7, 105–114. [Google Scholar] [PubMed]
- Ito, D.; Aoyagi, K.; Nagano, O.; Serizawa, T.; Iwadate, Y.; Higuchi, Y. Comparison of Two-Stage Gamma Knife Radiosurgery Outcomes for Large Brain Metastases among Primary Cancers. J. Neurooncol. 2020, 147, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Damron, E.P.; Dono, A.; Chafi, H.; Martir, M.; Yu, T.-K.; Khwaja, S.; Amsbaugh, M.; Tandon, N.; Esquenazi, Y.; Blanco, A.I. Metastatic Neoplasm Volume Kinetics Following 2-Stage Stereotactic Radiosurgery. World Neurosurg. 2022, 161, e210–e219. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.C.; Hoekstra, F.H.; Tans, J.T.J.; Lambooij, N.; Metsaars, J.A.L.; Wattendorff, A.R.; et al. Treatment of Single Brain Metastasis: Radiotherapy Alone or Combined with Neurosurgery. Ann. Neurol. 1993, 33, 583–590. [Google Scholar] [CrossRef]
- Noordijk, E.M.; Vecht, C.J.; Haaxma-Reiche, H.; Padberg, G.W.; Voormolen, J.H.C.; Hoekstra, F.H.; Tans, J.T.J.; Lambooij, N.; Metsaars, J.A.L.; Wattendorff, A.R.; et al. The Choice of Treatment of Single Brain Metastasis Should Be Based on Extracranial Tumor Activity and Age. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 711–717. [Google Scholar] [CrossRef]
- Mintz, A.H.; Kestle, J.; Rathbone, M.P.; Gaspar, L.; Hugenholtz, H.; Fisher, B.; Duncan, G.; Skingley, P.; Foster, G.; Levine, M. A Randomized Trial to Assess the Efficacy of Surgery in Addition to Radiotherapy in Patients with a Single Cerebral Metastasis. Cancer 1996, 78, 1470–1476. [Google Scholar] [CrossRef]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.-P.; et al. Whole Brain Radiation Therapy with or without Stereotactic Radiosurgery Boost for Patients with One to Three Brain Metastases: Phase III Results of the RTOG 9508 Randomised Trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs. Stereotactic Radiosurgery Alone for Treatment of Brain Metastases: A Randomized Controlled Trial. JAMA 2006, 295, 2483. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in Patients with Brain Metastases Treated with Radiosurgery or Radiosurgery plus Whole-Brain Irradiation: A Randomised Controlled Trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. Adjuvant Whole-Brain Radiotherapy Versus Observation After Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. JCO 2011, 29, 134–141. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs. Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, A.; Aoyama, H.; Kocher, M.; Neupane, B.; Collette, S.; Tago, M.; Shaw, P.; Beyene, J.; Chang, E.L. Phase 3 Trials of Stereotactic Radiosurgery With or Without Whole-Brain Radiation Therapy for 1 to 4 Brain Metastases: Individual Patient Data Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy Versus Observation in Patients With One to Three Brain Metastases From Solid Tumors After Surgical Resection or Radiosurgery: Quality-of-Life Results. JCO 2013, 31, 65–72. [Google Scholar] [CrossRef]
- Aoyama, H.; Tago, M.; Shirato, H. Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators Stereotactic Radiosurgery With or Without Whole-Brain Radiotherapy for Brain Metastases: Secondary Analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015, 1, 457–464. [Google Scholar] [CrossRef]
- Churilla, T.M.; Handorf, E.; Collette, S.; Collette, L.; Dong, Y.; Aizer, A.A.; Kocher, M.; Soffietti, R.; Alexander, B.M.; Weiss, S.E. Whole Brain Radiotherapy after Stereotactic Radiosurgery or Surgical Resection among Patients with One to Three Brain Metastases and Favorable Prognoses: A Secondary Analysis of EORTC 22952-26001. Ann. Oncol. 2017, 28, 2588–2594. [Google Scholar] [CrossRef]
- Churilla, T.M.; Ballman, K.V.; Brown, P.D.; Twohy, E.L.; Jaeckle, K.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Garces, Y.I.; et al. Stereotactic Radiosurgery With or Without Whole-Brain Radiation Therapy for Limited Brain Metastases: A Secondary Analysis of the North Central Cancer Treatment Group N0574 (Alliance) Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1173–1178. [Google Scholar] [CrossRef]
- Soffietti, R.; Abacioglu, U.; Baumert, B.; Combs, S.E.; Kinhult, S.; Kros, J.M.; Marosi, C.; Metellus, P.; Radbruch, A.; Villa Freixa, S.S.; et al. Diagnosis and Treatment of Brain Metastases from Solid Tumors: Guidelines from the European Association of Neuro-Oncology (EANO). Neuro-Oncology 2017, 19, 162–174. [Google Scholar] [CrossRef]
- Le Rhun, E.; Guckenberger, M.; Smits, M.; Dummer, R.; Bachelot, T.; Sahm, F.; Galldiks, N.; de Azambuja, E.; Berghoff, A.S.; Metellus, P.; et al. EANO-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up of Patients with Brain Metastasis from Solid Tumours. Ann. Oncol. 2021, 32, 1332–1347. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Ahluwalia, M.S.; Khan, O.H.; Asher, A.L.; Wefel, J.S.; Gondi, V. Whole-Brain Radiotherapy for Brain Metastases: Evolution or Revolution? J. Clin. Oncol. 2018, 36, 483–491. [Google Scholar] [CrossRef]
- Sahgal, A.; Ruschin, M.; Ma, L.; Verbakel, W.; Larson, D.; Brown, P.D. Stereotactic Radiosurgery Alone for Multiple Brain Metastases? A Review of Clinical and Technical Issues. Neuro-Oncology 2017, 19, ii2–ii15. [Google Scholar] [CrossRef]
- Amaya, D.; Shinde, A.; Wohlers, C.; Wong, K.C.C.; Novak, J.; Neylon, J.; Han, C.; Liu, A.; Dandapani, S.; Glaser, S. Dosimetric Comparison of Multiple vs Single Isocenter Technique for Linear Accelerator-Based Stereotactic Radiosurgery: The Importance of the Six Degree Couch. J. Appl. Clin. Med. Phys. 2021, 22, 45–49. [Google Scholar] [CrossRef]
- Bodensohn, R.; Kaempfel, A.-L.; Fleischmann, D.F.; Hadi, I.; Hofmaier, J.; Garny, S.; Reiner, M.; Forbrig, R.; Corradini, S.; Thon, N.; et al. Simultaneous Stereotactic Radiosurgery of Multiple Brain Metastases Using Single-Isocenter Dynamic Conformal Arc Therapy: A Prospective Monocentric Registry Trial. Strahlenther. Onkol. 2021, 197, 601–613. [Google Scholar] [CrossRef]
- Kim, G.J.; Buckley, E.D.; Herndon, J.E.; Allen, K.J.; Dale, T.S.; Adamson, J.D.; Lay, L.; Giles, W.M.; Rodrigues, A.E.; Wang, Z.; et al. Outcomes in Patients With 4 to 10 Brain Metastases Treated With Dose-Adapted Single-Isocenter Multitarget Stereotactic Radiosurgery: A Prospective Study. Adv. Radiat. Oncol. 2021, 6, 100760. [Google Scholar] [CrossRef]
- Kraft, J.; van Timmeren, J.E.; Mayinger, M.; Frei, S.; Borsky, K.; Stark, L.S.; Krayenbuehl, J.; Zamburlini, M.; Guckenberger, M.; Tanadini-Lang, S.; et al. Distance to Isocenter Is Not Associated with an Increased Risk for Local Failure in LINAC-Based Single-Isocenter SRS or SRT for Multiple Brain Metastases. Radiother. Oncol. 2021, 159, 168–175. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ide, M.; Jimbo, M.; Aiba, M.; Ito, M.; Hirai, T.; Usukura, M. Gamma Knife Radiosurgery with Numerous Target Points for Intracranially Disseminated Metastases. In Radiosurgery 1997; Karger Publishers: Basel, Switzerland, 1998; Volume 2, pp. 94–109. [Google Scholar]
- Yamamoto, M.; Kawabe, T.; Sato, Y.; Higuchi, Y.; Nariai, T.; Barfod, B.E.; Kasuya, H.; Urakawa, Y. A Case-Matched Study of Stereotactic Radiosurgery for Patients with Multiple Brain Metastases: Comparing Treatment Results for 1-4 vs. ≥ 5 Tumors: Clinical Article. J. Neurosurg. 2013, 118, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kawabe, T.; Sato, Y.; Higuchi, Y.; Nariai, T.; Watanabe, S.; Kasuya, H. Stereotactic Radiosurgery for Patients with Multiple Brain Metastases: A Case-Matched Study Comparing Treatment Results for Patients with 2–9 versus 10 or More Tumors. J. Neurosurg. 2014, 121, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, Y.; Higuchi, Y.; Kasuya, H.; Barfod, B.E. A Cohort Study of Stereotactic Radiosurgery Results for Patients With 5 to 15 Versus 2 to 4 Brain Metastatic Tumors. Adv. Radiat. Oncol. 2020, 5, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Sato, Y.; Higuchi, Y.; Kasuya, H. Stereotactic Radiosurgery Results for Patients with 5–10 versus 11-20 Brain Metastases: A Retrospective Cohort Study Combining 2 Databases Totaling 2319 Patients. World Neurosurg. 2021, 146, e479–e491. [Google Scholar] [CrossRef]
- Hughes, R.T.; McTyre, E.R.; LeCompte, M.; Cramer, C.K.; Munley, M.T.; Laxton, A.W.; Tatter, S.B.; Ruiz, J.; Pasche, B.; Watabe, K.; et al. Clinical Outcomes of Upfront Stereotactic Radiosurgery Alone for Patients With 5 to 15 Brain Metastases. Neurosurgery 2019, 85, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic Radiosurgery for Patients with Multiple Brain Metastases (JLGK0901): A Multi-Institutional Prospective Observational Study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Nichol, A.; Ma, R.; Hsu, F.; Gondara, L.; Carolan, H.; Olson, R.; Schellenberg, D.; Germain, F.; Cheung, A.; Peacock, M.; et al. Volumetric Radiosurgery for 1 to 10 Brain Metastases: A Multicenter, Single-Arm, Phase 2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 312–321. [Google Scholar] [CrossRef]
- Hartgerink, D.; Bruynzeel, A.; Eekers, D.; Swinnen, A.; Hurkmans, C.; Wiggenraad, R.; Swaak-Kragten, A.; Dieleman, E.; van der Toorn, P.-P.; Oei, B.; et al. A Dutch Phase III Randomized Multicenter Trial: Whole Brain Radiotherapy versus Stereotactic Radiotherapy for 4–10 Brain Metastases. Neurooncol. Adv. 2021, 3, vdab021. [Google Scholar] [CrossRef]
- Li, J.; Ludmir, E.B.; Wang, Y.; Guha-Thakurta, N.; McAleer, M.F.; Settle, S.H.; Yeboa, D.N.; Ghia, A.J.; McGovern, S.L.; Chung, C.; et al. Stereotactic Radiosurgery versus Whole-Brain Radiation Therapy for Patients with 4–15 Brain Metastases: A Phase III Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S21–S22. [Google Scholar] [CrossRef]
- Bhatnagar, A.K.; Flickinger, J.C.; Kondziolka, D.; Lunsford, L.D. Stereotactic Radiosurgery for Four or More Intracranial Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Banfill, K.E.; Bownes, P.J.; St Clair, S.E.; Loughrey, C.; Hatfield, P. Stereotactic Radiosurgery for the Treatment of Brain Metastases: Impact of Cerebral Disease Burden on Survival. Br. J. Neurosurg. 2012, 26, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Farris, M.; McTyre, E.R.; Cramer, C.K.; Hughes, R.; Randolph, D.M.; Ayala-Peacock, D.N.; Bourland, J.D.; Ruiz, J.; Watabe, K.; Laxton, A.W.; et al. Brain Metastasis Velocity: A Novel Prognostic Metric Predictive of Overall Survival and Freedom From Whole-Brain Radiation Therapy After Distant Brain Failure Following Upfront Radiosurgery Alone. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 131–141. [Google Scholar] [CrossRef]
- Yamamoto, M.; Aiyama, H.; Koiso, T.; Watanabe, S.; Kawabe, T.; Sato, Y.; Higuchi, Y.; Kasuya, H.; Barfod, B.E. Validity of a Recently Proposed Prognostic Grading Index, Brain Metastasis Velocity, for Patients With Brain Metastasis Undergoing Multiple Radiosurgical Procedures. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Hanssens, P.; Karlsson, B.; Yeo, T.T.; Chou, N.; Beute, G. Detection of Brain Micrometastases by High-Resolution Stereotactic Magnetic Resonance Imaging and Its Impact on the Timing of and Risk for Distant Recurrences. J. Neurosurg. 2011, 115, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Shultz, D.B.; Modlin, L.A.; Jayachandran, P.; Von Eyben, R.; Gibbs, I.C.; Choi, C.Y.H.; Chang, S.D.; Harsh, G.R.; Li, G.; Adler, J.R.; et al. Repeat Courses of Stereotactic Radiosurgery (SRS), Deferring Whole-Brain Irradiation, for New Brain Metastases After Initial SRS. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C.G.; Gurewitz, J.; Kavi, A.; Bernstein, K.; Silverman, J.; Mureb, M.; Donahue, B.; Kondziolka, D. Survival and Outcomes in Patients with ≥ 25 Cumulative Brain Metastases Treated with Stereotactic Radiosurgery. J. Neurosurg. 2021, 137, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Mariya, Y.; Sekizawa, G.; Matsuoka, Y.; Seki, H.; Sugawara, T. Outcome of Stereotactic Radiosurgery for Patients with Non-Small Cell Lung Cancer Metastatic to the Brain. J. Radiat. Res. 2010, 51, 333–342. [Google Scholar] [CrossRef]
- Lee, W.-J.; Choi, J.-W.; Kong, D.-S.; Seol, H.J.; Nam, D.-H.; Lee, J.-I. Clinical Outcomes of Patients with Multiple Courses of Radiosurgery for Brain Metastases from Non-Small Cell Lung Cancer. Sci. Rep. 2022, 12, 10712. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.A.; Brown, S.; Reiner, A.S.; Young, R.J.; Chen, J.; Bale, T.A.; Rosenblum, M.K.; Newman, W.C.; Brennan, C.W.; Tabar, V.; et al. Salvage Resection of Recurrent Previously Irradiated Brain Metastases: Tumor Control and Radiation Necrosis Dependency on Adjuvant Re-Irradiation. J. Neurooncol. 2021, 155, 277–286. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Jung, S.; Jung, T.-Y.; Moon, K.-S.; Jang, W.-Y.; Park, J.-Y.; Song, T.-W.; Lim, S.-H. Repeat Stereotactic Radiosurgery for Recurred Metastatic Brain Tumors. J. Korean Neurosurg. Soc. 2018, 61, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Sneed, P.K.; Chan, J.W.; Ma, L.; Braunstein, S.E.; Theodosopoulos, P.V.; Fogh, S.E.; Nakamura, J.L.; Boreta, L.; Raleigh, D.R.; Ziemer, B.P.; et al. Adverse Radiation Effect and Freedom from Progression Following Repeat Stereotactic Radiosurgery for Brain Metastases. J. Neurosurg. 2023, 138, 104–112. [Google Scholar] [CrossRef]
- Loi, M.; Caini, S.; Scoccianti, S.; Bonomo, P.; De Vries, K.; Francolini, G.; Simontacchi, G.; Greto, D.; Desideri, I.; Meattini, I.; et al. Stereotactic Reirradiation for Local Failure of Brain Metastases Following Previous Radiosurgery: Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020, 153, 103043. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Didwania, P.; Lehrer, E.J.; Palmer, J.D.; Trifiletti, D.M.; Sheehan, J.P. Repeat Stereotactic Radiosurgery for Locally Recurrent Brain Metastases Previously Treated with Stereotactic Radiosurgery: A Systematic Review and Meta-Analysis of Efficacy and Safety. J. Radiosurg. SBRT 2022, 8, 1–10. [Google Scholar]
- Kowalchuk, R.O.; Niranjan, A.; Lee, C.-C.; Yang, H.-C.; Liscak, R.; Guseynova, K.; Tripathi, M.; Kumar, N.; Peker, S.; Samanci, Y.; et al. Reirradiation With Stereotactic Radiosurgery After Local or Marginal Recurrence of Brain Metastases From Previous Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 726–734. [Google Scholar] [CrossRef]
- Yan, M.; Lee, M.; Myrehaug, S.; Tseng, C.-L.; Detsky, J.; Chen, H.; Das, S.; Yeboah, C.; Lipsman, N.; Costa, L.D.; et al. Hypofractionated Stereotactic Radiosurgery (HSRS) as a Salvage Treatment for Brain Metastases Failing Prior Stereotactic Radiosurgery (SRS). J. Neurooncol. 2023, 162, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative Radiotherapy in the Treatment of Single Metastases to the Brain: A Randomized Trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Tewarie, I.A.; Jessurun, C.A.C.; Hulsbergen, A.F.C.; Smith, T.R.; Mekary, R.A.; Broekman, M.L.D. Leptomeningeal Disease in Neurosurgical Brain Metastases Patients: A Systematic Review and Meta-Analysis. Neurooncol. Adv. 2021, 3, vdab162. [Google Scholar] [CrossRef]
- Kayama, T.; Sato, S.; Sakurada, K.; Mizusawa, J.; Nishikawa, R.; Narita, Y.; Sumi, M.; Miyakita, Y.; Kumabe, T.; Sonoda, Y.; et al. Effects of Surgery With Salvage Stereotactic Radiosurgery Versus Surgery With Whole-Brain Radiation Therapy in Patients With One to Four Brain Metastases (JCOG0504): A Phase III, Noninferiority, Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Akanda, Z.Z.; Hong, W.; Nahavandi, S.; Haghighi, N.; Phillips, C.; Kok, D.L. Post-Operative Stereotactic Radiosurgery Following Excision of Brain Metastases: A Systematic Review and Meta-Analysis. Radiother. Oncol. 2020, 142, 27–35. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-Operative Stereotactic Radiosurgery versus Observation for Completely Resected Brain Metastases: A Single-Centre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative Stereotactic Radiosurgery Compared with Whole Brain Radiotherapy for Resected Metastatic Brain Disease (NCCTG N107C/CEC·3): A Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Palmer, J.D.; Klamer, B.G.; Ballman, K.V.; Brown, P.D.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; et al. Association of Long-Term Outcomes With Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Breen, W.; Dooley, K.E.; Twohy, E.; Brown, P.D.; Ballman, K.; Cerhan, J.H.; Urbanic, J.J.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; et al. Patterns of Failure after Stereotactic Radiosurgery vs. Whole Brain Radiotherapy for Resected Brain Metastases: Central Imaging Review of the N107C/CEC.3 (Alliance) Phase III Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 1063–1064. [Google Scholar] [CrossRef]
- Minniti, G.; Esposito, V.; Clarke, E.; Scaringi, C.; Lanzetta, G.; Salvati, M.; Raco, A.; Bozzao, A.; Maurizi Enrici, R. Multidose Stereotactic Radiosurgery (9 Gy × 3) of the Postoperative Resection Cavity for Treatment of Large Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Doré, M.; Cebula, H.; Thillays, F.; Proust, F.; Darié, I.; Martin, S.-A.; Delpon, G.; Lefebvre, F.; Noël, G.; et al. Hypofractionated Stereotactic Radiation Therapy to the Resection Bed for Intracranial Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1179–1189. [Google Scholar] [CrossRef]
- Faruqi, S.; Ruschin, M.; Soliman, H.; Myrehaug, S.; Zeng, K.L.; Husain, Z.; Atenafu, E.; Tseng, C.-L.; Das, S.; Perry, J.; et al. Adverse Radiation Effect After Hypofractionated Stereotactic Radiosurgery in 5 Daily Fractions for Surgical Cavities and Intact Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Sandhu, N.; Jin, M.C.; Wang, E.; Jaoude, J.A.; Schofield, K.; Zhang, C.; Liu, E.; Gibbs, I.C.; Hancock, S.L.; et al. Stereotactic Radiosurgery for Resected Brain Metastases: Single-Institutional Experience of Over 500 Cavities. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Eitz, K.A.; Lo, S.S.; Soliman, H.; Sahgal, A.; Theriault, A.; Pinkham, M.B.; Foote, M.C.; Song, A.J.; Shi, W.; Redmond, K.J.; et al. Multi-Institutional Analysis of Prognostic Factors and Outcomes After Hypofractionated Stereotactic Radiotherapy to the Resection Cavity in Patients With Brain Metastases. JAMA Oncol. 2020, 6, 1901–1909. [Google Scholar] [CrossRef]
- Soliman, H.; Ruschin, M.; Angelov, L.; Brown, P.D.; Chiang, V.L.S.; Kirkpatrick, J.P.; Lo, S.S.; Mahajan, A.; Oh, K.S.; Sheehan, J.P.; et al. Consensus Contouring Guidelines for Postoperative Completely Resected Cavity Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 436–442. [Google Scholar] [CrossRef]
- Minniti, G.; Niyazi, M.; Andratschke, N.; Guckenberger, M.; Palmer, J.D.; Shih, H.A.; Lo, S.S.; Soltys, S.; Russo, I.; Brown, P.D.; et al. Current Status and Recent Advances in Resection Cavity Irradiation of Brain Metastases. Radiat. Oncol. 2021, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Foreman, P.M.; Jackson, B.E.; Singh, K.P.; Romeo, A.K.; Guthrie, B.L.; Fisher, W.S.; Riley, K.O.; Markert, J.M.; Willey, C.D.; Bredel, M.; et al. Postoperative Radiosurgery for the Treatment of Metastatic Brain Tumor: Evaluation of Local Failure and Leptomeningeal Disease. J. Clin. Neurosci. 2018, 49, 48–55. [Google Scholar] [CrossRef]
- Rajkumar, S.; Liang, Y.; Wegner, R.E.; Shepard, M.J. Utilization of Neoadjuvant Stereotactic Radiosurgery for the Treatment of Brain Metastases Requiring Surgical Resection: A Topic Review. J. Neurooncol. 2022, 160, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Asher, A.L.; Burri, S.H.; Wiggins, W.F.; Kelly, R.P.; Boltes, M.O.; Mehrlich, M.; Norton, H.J.; Fraser, R.W. A New Treatment Paradigm: Neoadjuvant Radiosurgery Before Surgical Resection of Brain Metastases With Analysis of Local Tumor Recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Burri, S.H.; Asher, A.L.; Crocker, I.R.; Fraser, R.W.; Zhang, C.; Chen, Z.; Kandula, S.; Zhong, J.; Press, R.H.; et al. Comparing Preoperative With Postoperative Stereotactic Radiosurgery for Resectable Brain Metastases: A Multi-Institutional Analysis. Neurosurgery 2016, 79, 279–285. [Google Scholar] [CrossRef]
- Patel, K.R.; Burri, S.H.; Boselli, D.; Symanowski, J.T.; Asher, A.L.; Sumrall, A.; Fraser, R.W.; Press, R.H.; Zhong, J.; Cassidy, R.J.; et al. Comparing Pre-Operative Stereotactic Radiosurgery (SRS) to Post-Operative Whole Brain Radiation Therapy (WBRT) for Resectable Brain Metastases: A Multi-Institutional Analysis. J. Neurooncol. 2017, 131, 611–618. [Google Scholar] [CrossRef]
- Patel, A.R.; Nedzi, L.; Lau, S.; Barnett, S.L.; Mickey, B.E.; Moore, W.; Bindal, S.; Wardak, Z.; Dan, T.; Timmerman, R.; et al. Neoadjuvant Stereotactic Radiosurgery Before Surgical Resection of Cerebral Metastases. World Neurosurg. 2018, 120, e480–e487. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Miller, K.R.; Asher, A.L.; Heinzerling, J.H.; Moeller, B.J.; Lankford, S.P.; McCammon, R.J.; Fasola, C.E.; Patel, K.R.; Press, R.H.; et al. Preoperative Stereotactic Radiosurgery before Planned Resection of Brain Metastases: Updated Analysis of Efficacy and Toxicity of a Novel Treatment Paradigm. J. Neurosurg. 2019, 131, 1387–1394. [Google Scholar] [CrossRef]
- Vetlova, E.; Golbin, D.A.; Golanov, A.V.; Potapov, A.A.; Banov, S.M.; Antipina, N.; Kostjuchenko, V.V.; Usachev, D.Y.; Belyaev, A.Y.; Goryaynov, S. Preoperative Stereotactic Radiosurgery of Brain Metastases: Preliminary Results. Cureus 2017, 9, e1987. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, S.; Mitsuya, K.; Yasui, K.; Kimura, K.; Onoe, T.; Ogawa, H.; Asakura, H.; Harada, H.; Hayashi, N. Neoadjuvant Fractionated Stereotactic Radiotherapy Followed by Piecemeal Resection of Brain Metastasis: A Case Series of 20 Patients. Int. J. Clin. Oncol. 2022, 27, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Perlow, H.K.; Matsui, J.K.; Ho, C.; Prasad, R.N.; Liu, K.; Upadhyay, R.; Klamer, B.; Wang, J.; Damante, M.; et al. Fractionated Pre-Operative Stereotactic Radiotherapy for Patients with Brain Metastases: A Multi-Institutional Analysis. J. Neurooncol. 2022, 159, 389–395. [Google Scholar] [CrossRef]
- Udovicich, C.; Ng, S.P.; Tange, D.; Bailey, N.; Haghighi, N. From Postoperative to Preoperative: A Case Series of Hypofractionated and Single-Fraction Neoadjuvant Stereotactic Radiosurgery for Brain Metastases. Oper. Neurosurg. 2022, 22, 208–214. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Dhakal, R.; Vaslow, Z.K.; Dan, T.; Mishra, M.V.; Murphy, E.S.; Patel, T.R.; Asher, A.L.; Yang, K.; Manning, M.A.; et al. Preoperative Radiosurgery for Resected Brain Metastases: The PROPS-BM Multicenter Cohort Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Han, J.-Y.; Katakami, N.; Kim, H.R.; Hodge, R.; Kaur, P.; Brown, A.P.; Ghiorghiu, D.; et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J. Clin. Oncol. 2018, 36, 2702–2709. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef]
- Gadgeel, S.; Peters, S.; Mok, T.; Shaw, A.T.; Kim, D.W.; Ou, S.I.; Pérol, M.; Wrona, A.; Novello, S.; Rosell, R.; et al. Alectinib versus Crizotinib in Treatment-Naive Anaplastic Lymphoma Kinase-Positive (ALK+) Non-Small-Cell Lung Cancer: CNS Efficacy Results from the ALEX Study. Ann. Oncol. 2018, 29, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Shaw, A.T.; Bearz, A.; Camidge, D.R.; Solomon, B.J.; Bauman, J.R.; Bauer, T.M.; Peters, S.; Toffalorio, F.; Abbattista, A.; et al. Intracranial and Extracranial Efficacy of Lorlatinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer Previously Treated with Second-Generation ALK TKIs. Ann. Oncol. 2021, 32, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M.; Barlesi, F.; Bertino, E.M.; van den Bent, M.J.; Wakelee, H.A.; Wen, P.Y.; Chiu, C.-H.; Orlov, S.; Chiari, R.; Majem, M.; et al. ASCEND-7: Efficacy and Safety of Ceritinib Treatment in Patients with ALK-Positive Non–Small Cell Lung Cancer Metastatic to the Brain and/or Leptomeninges. Clin. Cancer Res. 2022, 28, 2506–2516. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, D.-W.; Tiseo, M.; Langer, C.J.; Ahn, M.-J.; Shaw, A.T.; Huber, R.M.; Hochmair, M.J.; Lee, D.H.; Bazhenova, L.A.; et al. Exploratory Analysis of Brigatinib Activity in Patients With Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer and Brain Metastases in Two Clinical Trials. J. Clin. Oncol. 2018, 36, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Bauer, T.M.; Ou, S.-H.I.; Liu, G.; Hayashi, H.; Bearz, A.; Penkov, K.; Wu, Y.-L.; Arrieta, O.; Jassem, J.; et al. Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non-Small-Cell Lung Cancer From the Phase III CROWN Study. J. Clin. Oncol. 2022, 40, 3593. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs. Crizotinib for Patients With Anaplastic Lymphoma Kinase−Positive Non–Small Cell Lung Cancer. JAMA Oncol. 2021, 7, 1617–1625. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; Soo, R.A.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in Advanced ROS1-Positive Non-Small-Cell Lung Cancer: A Multicentre, Open-Label, Single-Arm, Phase 1-2 Trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Subbiah, V.; Gautschi, O.; Tomasini, P.; de Braud, F.; Solomon, B.J.; Shao-Weng Tan, D.; Alonso, G.; Wolf, J.; Park, K.; et al. Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J. Clin. Oncol. 2023, 41, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.S.; Tan, D.S.W.; Lee, D.H.; Misch, D.; Garralda, E.; Kim, D.-W.; et al. Safety and Efficacy of Pralsetinib in RET Fusion-Positive Non-Small-Cell Lung Cancer Including as First-Line Therapy: Update from the ARROW Trial. Ann. Oncol. 2022, 33, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14–Mutated or MET-Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients With MET Exon 14–Skipping Non-Small Cell Lung Cancer: Long-Term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023. online first. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1–2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.-H.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Zhou, C.; Kim, S.W.; Reungwetwattana, T.; Zhou, J.; Zhang, Y.; He, J.; Yang, J.J.; Cheng, Y.; Lee, S.H.; Bu, L.; et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A randomised phase 3 study. Lancet Respir Med. 2019, 7, 437–446. [Google Scholar] [CrossRef]
- Negrao, M.V.; Spira, A.I.; Heist, R.S.; Jänne, P.A.; Pacheco, J.M.; Weiss, J.; Gadgeel, S.M.; Velastegui, K.; Yang, W.; Der-Torossian, H.; et al. Intracranial Efficacy of Adagrasib in Patients From the KRYSTAL-1 Trial With KRASG12C-Mutated Non-Small-Cell Lung Cancer Who Have Untreated CNS Metastases. J. Clin. Oncol. 2023, JCO2300046. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for Patients with Melanoma or Non-Small-Cell Lung Cancer and Untreated Brain Metastases: Early Analysis of a Non-Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Schalper, K.A.; Gettinger, S.N.; Mahajan, A.; Herbst, R.S.; Chiang, A.C.; Lilenbaum, R.; Wilson, F.H.; Omay, S.B.; Yu, J.B.; et al. Pembrolizumab for Management of Patients with NSCLC and Brain Metastases: Long-Term Results and Biomarker Analysis from a Non-Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2020, 21, 655–663. [Google Scholar] [CrossRef]
- Reck, M.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Caro, R.B.; Linardou, H.; Burgers, J.; Gallardo, C.; Nishio, M.; Peters, S.; et al. 122MO Nivolumab (NIVO) + Ipilimumab (IPI) as First-Line (1L) Treatment (Tx) for Patients (Pts) with Advanced NSCLC (ANSCLC) and Baseline (BL) Brain Metastases (Mets): Intracranial and Systemic Outcomes from CheckMate 227 Part 1. Ann. Oncol. 2021, 32, S1430–S1431. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Herbst, R.S.; de Castro, G.; Hui, R.; Peled, N.; Kim, D.-W.; Novello, S.; Satouchi, M.; Wu, Y.-L.; Garon, E.B.; et al. Outcomes With Pembrolizumab Monotherapy in Patients With Programmed Death-Ligand 1-Positive NSCLC With Brain Metastases: Pooled Analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin. Res. Rep. 2021, 2, 100205. [Google Scholar] [CrossRef]
- Teixeira Loiola de Alencar, V.; Guedes Camandaroba, M.P.; Pirolli, R.; Fogassa, C.A.Z.; Cordeiro de Lima, V.C. Immunotherapy as Single Treatment for Patients With NSCLC With Brain Metastases: A Systematic Review and Meta-Analysis-the META-L-BRAIN Study. J. Thorac. Oncol. 2021, 16, 1379–1391. [Google Scholar] [CrossRef]
- Kilickap, S.; Özgüroğlu, M.; Sezer, A.; Gumus, M.; Bondarenko, I.; Gogishvili, M.; Türk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.A.; et al. 10MO EMPOWER-Lung 1: Cemiplimab (CEMI) Monotherapy as First-Line (1L) Treatment of Patients (Pts) with Brain Metastases from Advanced Non-Small Cell Lung Cancer (ANSCLC) with Programmed Cell Death-Ligand 1 (PD-L1) ≥50%—3-Year Update. J. Thorac. Oncol. 2023, 18, S42–S43. [Google Scholar] [CrossRef]
- Nadal, E.; Rodriguez-Abreu, D.; Massuti, B.; Juan-Vidal, O.; Huidobro Vence, G.; Lopez, R.; de Castro Carpeño, J.; Estival, A.; Campelo, R.G.; Sullivan, I.; et al. Updated Analysis from the ATEZO-BRAIN Trial: Atezolizumab plus Carboplatin and Pemetrexed in Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer with Untreated Brain Metastases. JCO 2022, 40, 9010. [Google Scholar] [CrossRef]
- Reck, M.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab with Two Cycles of Chemotherapy versus Chemotherapy Alone (Four Cycles) in Advanced Non-Small-Cell Lung Cancer: CheckMate 9LA 2-Year Update. ESMO Open 2021, 6, 100273. [Google Scholar] [CrossRef]
- Cho, A.; Untersteiner, H.; Hirschmann, D.; Shaltout, A.; Göbl, P.; Dorfer, C.; Rössler, K.; Marik, W.; Kirchbacher, K.; Kapfhammer, I.; et al. Gamma Knife Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy or Targeted Therapy. Cancers 2020, 12, 3668. [Google Scholar] [CrossRef]
- Koide, Y.; Nagai, N.; Miyauchi, R.; Kitagawa, T.; Aoyama, T.; Shimizu, H.; Tachibana, H.; Kodaira, T. Radiotherapy or Systemic Therapy versus Combined Therapy in Patients with Brain Metastases: A Propensity-Score Matched Study. J. Neurooncol. 2022, 160, 191–200. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Lou, E. The Past, Present, and Future Management of Brain Metastases in EGFR-Mutant Non-Small Cell Lung Cancer. Neuro-Oncology 2021, 23, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Franchi, M.; Zaffaroni, M.; Vincini, M.G.; de Marinis, F.; Spaggiari, L.; Orecchia, R.; Marvaso, G.; Jereczek-Fossa, B.A. Upfront Advanced Radiotherapy and New Drugs for NSCLC Patients with Synchronous Brain Metastases: Is the Juice Worth the Squeeze? A Real-World Analysis from Lombardy, Italy. Cancers 2023, 15, 1103. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.D.; Marin-Acevedo, J.A.; Pellini, B. Immunotherapy for Advanced Non-Small Cell Lung Cancer: A Decade of Progress. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e105–e127. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Peterson, J.; Brown, P.D.; Sheehan, J.P.; Quiñones-Hinojosa, A.; Zaorsky, N.G.; Trifiletti, D.M. Treatment of Brain Metastases with Stereotactic Radiosurgery and Immune Checkpoint Inhibitors: An International Meta-Analysis of Individual Patient Data. Radiother. Oncol. 2019, 130, 104–112. [Google Scholar] [CrossRef]
- Voronova, V.; Lebedeva, S.; Sekacheva, M.; Helmlinger, G.; Peskov, K. Quantification of Scheduling Impact on Safety and Efficacy Outcomes of Brain Metastasis Radio- and Immuno-Therapies: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 1609. [Google Scholar] [CrossRef]
- Badrigilan, S.; Meola, A.; Chang, S.D.; Rezaeian, S.; Nemati, H.; Almasi, T.; Rostampour, N. Stereotactic Radiosurgery with Immune Checkpoint Inhibitors for Brain Metastases: A Meta-Analysis Study. Br. J. Neurosurg. 2022, 1–11. [Google Scholar] [CrossRef]
- Chen, L.; Douglass, J.; Kleinberg, L.; Ye, X.; Marciscano, A.E.; Forde, P.M.; Brahmer, J.; Lipson, E.; Sharfman, W.; Hammers, H.; et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Trommer, M.; Adams, A.; Celik, E.; Fan, J.; Funken, D.; Herter, J.M.; Linde, P.; Morgenthaler, J.; Wegen, S.; Mauch, C.; et al. Oncologic Outcome and Immune Responses of Radiotherapy with Anti-PD-1 Treatment for Brain Metastases Regarding Timing and Benefiting Subgroups. Cancers 2022, 14, 1240. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Ahluwalia, M.S.; Gurewitz, J.; Bernstein, K.; Kondziolka, D.; Niranjan, A.; Wei, Z.; Lunsford, L.D.; Fakhoury, K.R.; Rusthoven, C.G.; et al. Imaging-Defined Necrosis after Treatment with Single-Fraction Stereotactic Radiosurgery and Immune Checkpoint Inhibitors and Its Potential Association with Improved Outcomes in Patients with Brain Metastases: An International Multicenter Study of 697 Patients. J. Neurosurg. 2022, 138, 1178–1187. [Google Scholar] [CrossRef]
- Singh, S.A.; McDermott, D.M.; Mattes, M.D. Impact of Systemic Therapy Type and Timing on Intracranial Tumor Control in Patients with Brain Metastasis from Non-Small-Cell Lung Cancer Treated With Stereotactic Radiosurgery. World Neurosurg. 2020, 144, e813–e823. [Google Scholar] [CrossRef]
- Scoccianti, S.; Olmetto, E.; Pinzi, V.; Osti, M.F.; Di Franco, R.; Caini, S.; Anselmo, P.; Matteucci, P.; Franceschini, D.; Mantovani, C.; et al. Immunotherapy in Association with Stereotactic Radiotherapy for Non-Small Cell Lung Cancer Brain Metastases: Results from a Multicentric Retrospective Study on Behalf of AIRO. Neuro-Oncology 2021, 23, 1750–1764. [Google Scholar] [CrossRef] [PubMed]
- Abdulhaleem, M.; Johnston, H.; D’Agostino, R.; Lanier, C.; LeCompte, M.; Cramer, C.K.; Ruiz, J.; Lycan, T.; Lo, H.-W.; Watabe, K.; et al. Local Control Outcomes for Combination of Stereotactic Radiosurgery and Immunotherapy for Non-Small Cell Lung Cancer Brain Metastases. J. Neurooncol. 2022, 157, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Colaco, R.J.; Martin, P.; Kluger, H.M.; Yu, J.B.; Chiang, V.L. Does Immunotherapy Increase the Rate of Radiation Necrosis after Radiosurgical Treatment of Brain Metastases? J. Neurosurg. 2016, 125, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Alexander, B.M.; Redig, A.J.; Schoenfeld, J.D.; Aizer, A.A. Immunotherapy and Symptomatic Radiation Necrosis in Patients With Brain Metastases Treated With Stereotactic Radiation. JAMA Oncol. 2018, 4, 1123–1124. [Google Scholar] [CrossRef]
- Kim, P.H.; Suh, C.H.; Kim, H.S.; Kim, K.W.; Kim, D.Y.; Aizer, A.A.; Rahman, R.; Guenette, J.P.; Huang, R.Y. Immune Checkpoint Inhibitor Therapy May Increase the Incidence of Treatment-Related Necrosis after Stereotactic Radiosurgery for Brain Metastases: A Systematic Review and Meta-Analysis. Eur. Radiol. 2021, 31, 4114–4129. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Kowalchuk, R.O.; Gurewitz, J.; Bernstein, K.; Kondziolka, D.; Niranjan, A.; Wei, Z.; Lunsford, L.D.; Fakhoury, K.R.; Rusthoven, C.G.; et al. Concurrent Administration of Immune Checkpoint Inhibitors and Single Fraction Stereotactic Radiosurgery in Patients With Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma Brain Metastases Is Not Associated With an Increased Risk of Radiation Necrosis Over Nonconcurrent Treatment: An International Multicenter Study of 657 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 858–868. [Google Scholar] [CrossRef]
- Wong, P.; Masucci, L.; Florescu, M.; Plourde, M.-E.; Panet-Raymond, V.; Pavic, M.; Owen, S.; Masson-Coté, L.; Ménard, C.; Routy, B.; et al. Phase II Multicenter Trial Combining Nivolumab and Radiosurgery for NSCLC and RCC Brain Metastases. Neurooncol. Adv. 2023, 5, vdad018. [Google Scholar] [CrossRef] [PubMed]
- Wrona, A.; Dziadziuszko, R.; Jassem, J. Management of Brain Metastases in Non-Small Cell Lung Cancer in the Era of Tyrosine Kinase Inhibitors. Cancer Treat. Rev. 2018, 71, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaekers, J.J.A.O.; Paats, M.S.; Dingemans, A.-M.C.; Hendriks, L.E.L. Central Nervous System Metastases and Oligoprogression during Treatment with Tyrosine Kinase Inhibitors in Oncogene-Addicted Non-Small Cell Lung Cancer: How to Treat and When? Transl. Lung Cancer Res. 2020, 9, 2599–2617. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, M.-J. Brain Metastases in Patients with Oncogenic-Driven Non-Small Cell Lung Cancer: Pros and Cons for Early Radiotherapy. Cancer Treat. Rev. 2021, 100, 102291. [Google Scholar] [CrossRef]
- Thomas, N.J.; Myall, N.J.; Sun, F.; Patil, T.; Mushtaq, R.; Yu, C.; Sinha, S.; Pollom, E.L.; Nagpal, S.; Camidge, D.R.; et al. Brain Metastases in EGFR- and ALK-Positive NSCLC: Outcomes of Central Nervous System-Penetrant Tyrosine Kinase Inhibitors Alone Versus in Combination With Radiation. J. Thorac. Oncol. 2022, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.-Y.; Chiang, C.-L.; Yang, H.-C.; Shen, C.-I.; Wu, H.-M.; Chen, Y.-W.; Chen, C.-J.; Luo, Y.-H.; Hu, Y.-S.; Lin, C.-J.; et al. Combined Stereotactic Radiosurgery and Tyrosine Kinase Inhibitor Therapy versus Tyrosine Kinase Inhibitor Therapy Alone for the Treatment of Non-Small Cell Lung Cancer Patients with Brain Metastases. J. Neurosurg. 2021, 137, 563–570. [Google Scholar] [CrossRef]
- Magnuson, W.J.; Lester-Coll, N.H.; Wu, A.J.; Yang, T.J.; Lockney, N.A.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J. Clin. Oncol. 2017, 35, 1070–1077. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Wang, M.; Robins, H.I.; Schell, M.C.; Werner-Wasik, M.; Komaki, R.; Souhami, L.; Buyyounouski, M.K.; Khuntia, D.; Demas, W.; et al. A Phase 3 Trial of Whole Brain Radiation Therapy and Stereotactic Radiosurgery Alone versus WBRT and SRS with Temozolomide or Erlotinib for Non-Small Cell Lung Cancer and 1 to 3 Brain Metastases: Radiation Therapy Oncology Group 0320. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1312–1318. [Google Scholar] [CrossRef]
- Welsh, J.W.; Komaki, R.; Amini, A.; Munsell, M.F.; Unger, W.; Allen, P.K.; Chang, J.Y.; Wefel, J.S.; McGovern, S.L.; Garland, L.L.; et al. Phase II Trial of Erlotinib plus Concurrent Whole-Brain Radiation Therapy for Patients with Brain Metastases from Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2013, 31, 895–902. [Google Scholar] [CrossRef]
- Johung, K.L.; Yeh, N.; Desai, N.B.; Williams, T.M.; Lautenschlaeger, T.; Arvold, N.D.; Ning, M.S.; Attia, A.; Lovly, C.M.; Goldberg, S.; et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J. Clin. Oncol. 2016, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ni, J.; Zeng, W.; Zhou, Y.; Guo, T.; Zeng, Y.; Zhao, Y.; Li, S.; Li, Y.; Yang, X.; et al. Clinical Value of Upfront Cranial Radiation Therapy in Osimertinib-Treated Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer With Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, S.; Yang, X.; Chu, L.; Wang, S.; Tong, T.; Chu, X.; Yu, F.; Zeng, Y.; Guo, T.; et al. Overall Survival Benefit of Osimertinib and Clinical Value of Upfront Cranial Local Therapy in Untreated EGFR-Mutant Nonsmall Cell Lung Cancer with Brain Metastasis. Int. J. Cancer 2022, 150, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-C.; Shen, Y.-C.; Chien, C.-R.; Liao, W.-C.; Chen, C.-H.; Hsia, T.-C.; Tu, C.-Y.; Chen, H.-J. The Optimal Therapy Strategy for Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer Patients with Brain Metastasis: A Real-World Study from Taiwan. Thorac. Cancer 2022, 13, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.J.; Kim, H.R.; Arcila, M.E.; Barron, D.; Chakravarty, D.; Gao, J.; Chang, M.T.; Ni, A.; Kundra, R.; Jonsson, P.; et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov. 2017, 7, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.; Eichholz, J.E.; Lebow, E.S.; Flynn, J.; Zhang, Z.; Walch, H.; Hubbeling, H.; Beal, K.; Moss, N.S.; Yu, K.K.; et al. Characterization of Central Nervous System Clinico-Genomic Outcomes in ALK-Positive Non-Small Cell Lung Cancer Patients with Brain Metastases Treated with Alectinib. Lung Cancer 2023, 178, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.; Patil, T.; Ross Camidge, D.; Bunn, P.A.; Schenk, E.L.; Pacheco, J.M.; Jurica, J.; Waxweiler, T.V.; Kavanagh, B.D.; Rusthoven, C.G. CNS Downstaging: An Emerging Treatment Paradigm for Extensive Brain Metastases in Oncogene-Addicted Lung Cancer. Lung Cancer 2023, 178, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Wandrey, N.E.; Gao, D.; Robin, T.P.; Contessa, J.N.; Singh, C.; Chiang, V.; Li, J.; Chen, A.; Wang, Y.; Sheehan, J.P.; et al. Multicenter Analysis of Stereotactic Radiosurgery for Multiple Brain Metastases from EGFR and ALK Driven Non-Small Cell Lung Cancer. Lung Cancer 2023, 176, 144–148. [Google Scholar] [CrossRef]
- Li, N.; Chu, Y.; Song, Q. Brain Metastasis in Patients with Small Cell Lung Cancer. Int. J. Gen. Med. 2021, 14, 10131–10139. [Google Scholar] [CrossRef]
- Aupérin, A.; Arriagada, R.; Pignon, J.P.; Le Péchoux, C.; Gregor, A.; Stephens, R.J.; Kristjansen, P.E.; Johnson, B.E.; Ueoka, H.; Wagner, H.; et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N. Engl. J. Med. 1999, 341, 476–484. [Google Scholar] [CrossRef]
- Slotman, B.; Faivre-Finn, C.; Kramer, G.; Rankin, E.; Snee, M.; Hatton, M.; Postmus, P.; Collette, L.; Musat, E.; Senan, S.; et al. Prophylactic Cranial Irradiation in Extensive Small-Cell Lung Cancer. N. Engl. J. Med. 2007, 357, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, C.G.; Camidge, D.R.; Robin, T.P.; Brown, P.D. Radiosurgery for Small-Cell Brain Metastases: Challenging the Last Bastion of Preferential Whole-Brain Radiotherapy Delivery. J. Clin. Oncol. 2020, 38, 3587–3591. [Google Scholar] [CrossRef]
- Robin, T.P.; Rusthoven, C.G. Radiosurgery for Small-Cell Lung Cancer Brain Metastases: A Review. J. Thorac. Dis. 2020, 12, 6234–6239. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamanaka, T.; Seto, T.; Harada, H.; Nokihara, H.; Saka, H.; Nishio, M.; Kaneda, H.; Takayama, K.; Ishimoto, O.; et al. Prophylactic Cranial Irradiation versus Observation in Patients with Extensive-Disease Small-Cell Lung Cancer: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 663–671. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum-Etoposide versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.-H.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.W.; Kong, D.S.; Lim, D.H.; Ahn, Y.C.; Nam, D.-H.; Lee, J.-I. The Role of Radiosurgery in Patients with Brain Metastasis from Small Cell Lung Carcinoma. J. Korean Neurosurg. Soc. 2011, 50, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Wegner, R.E.; Olson, A.C.; Kondziolka, D.; Niranjan, A.; Lundsford, L.D.; Flickinger, J.C. Stereotactic Radiosurgery for Patients with Brain Metastases from Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e21-27. [Google Scholar] [CrossRef]
- Yomo, S.; Hayashi, M. Upfront Stereotactic Radiosurgery in Patients with Brain Metastases from Small Cell Lung Cancer: Retrospective Analysis of 41 Patients. Radiat. Oncol. 2014, 9, 152. [Google Scholar] [CrossRef]
- Robin, T.P.; Jones, B.L.; Amini, A.; Koshy, M.; Gaspar, L.E.; Liu, A.K.; Nath, S.K.; Kavanagh, B.D.; Camidge, D.R.; Rusthoven, C.G. Radiosurgery Alone Is Associated with Favorable Outcomes for Brain Metastases from Small-Cell Lung Cancer. Lung Cancer 2018, 120, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Faramand, A.; Niranjan, A.; Kano, H.; Flickinger, J.; Lunsford, L.D. Primary or Salvage Stereotactic Radiosurgery for Brain Metastatic Small Cell Lung Cancer. J. Neurooncol. 2019, 144, 217–225. [Google Scholar] [CrossRef]
- Cordeiro, D.; Xu, Z.; Shepard, M.; Sheehan, D.; Li, C.; Sheehan, J. Gamma Knife Radiosurgery for Brain Metastases from Small-Cell Lung Cancer: Institutional Experience over More than a Decade and Review of the Literature. J. Radiosurg. SBRT 2019, 6, 35–43. [Google Scholar] [PubMed]
- Cifarelli, C.P.; Vargo, J.A.; Fang, W.; Liscak, R.; Guseynova, K.; Warnick, R.E.; Lee, C.-C.; Yang, H.-C.; Borghei-Razavi, H.; Maiti, T.; et al. Role of Gamma Knife Radiosurgery in Small Cell Lung Cancer: A Multi-Institutional Retrospective Study of the International Radiosurgery Research Foundation (IRRF). Neurosurgery 2020, 87, 664–671. [Google Scholar] [CrossRef]
- Jiang, W.; Haque, W.; Verma, V.; Butler, B.; Teh, B.S. Stereotactic Radiosurgery for Brain Metastases from Newly Diagnosed Small Cell Lung Cancer: Practice Patterns and Outcomes. Acta Oncol. 2019, 58, 491–498. [Google Scholar] [CrossRef]
- Dudnik, E.; Allen, A.M.; Michaeli, N.; Benouaich-Amiel, A.; Shochat, T.; Peled, N.; Finkel, I.; Zer, A.; Rotem, O.; Yust-Katz, S. Stereotactic Radiosurgery for Brain Metastases in Small Cell Lung Cancer: The Davidoff Cancer Center Experience. Isr. Med. Assoc. J. 2020, 22, 22–26. [Google Scholar] [PubMed]
- Rusthoven, C.G.; Yamamoto, M.; Bernhardt, D.; Smith, D.E.; Gao, D.; Serizawa, T.; Yomo, S.; Aiyama, H.; Higuchi, Y.; Shuto, T.; et al. Evaluation of First-Line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020, 6, 1028–1037. [Google Scholar] [CrossRef]
- Gaebe, K.; Li, A.Y.; Park, A.; Parmar, A.; Lok, B.H.; Sahgal, A.; Chan, K.K.W.; Erickson, A.W.; Das, S. Stereotactic Radiosurgery versus Whole Brain Radiotherapy in Patients with Intracranial Metastatic Disease and Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lancet Oncol. 2022, 23, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Y.; Xia, W.; Liu, B.; Ni, M.; Shen, J.; Bai, Y.; Weng, G.; Liu, W.; Yuan, S.; et al. Brain Metastases from Small Cell Lung Cancer and Non-Small Cell Lung Cancer: Comparison of Spatial Distribution and Identification of Metastatic Risk Regions. J. Neurooncol. 2023, 161, 97–105. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.; Fan, Y.; Jiang, Y. Multiple Treatment Modalities for Brain Metastasis in Patients with EGFR-Mutant Non-Small-Cell Lung Cancer. Onco Targets Ther. 2018, 11, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-Y.; Na, I.I.; Kim, C.H.; Park, S.; Baek, H.; Yang, S.H. EGFR Mutation and Brain Metastasis in Pulmonary Adenocarcinomas. J. Thorac. Oncol. 2014, 9, 195–199. [Google Scholar] [CrossRef]
- Preusser, M.; Berghoff, A.S.; Ilhan-Mutlu, A.; Magerle, M.; Dinhof, C.; Widhalm, G.; Dieckmann, K.; Marosi, C.; Wöhrer, A.; Hackl, M.; et al. ALK Gene Translocations and Amplifications in Brain Metastases of Non-Small Cell Lung Cancer. Lung Cancer 2013, 80, 278–283. [Google Scholar] [CrossRef]
- Jiang, T.; Yan, Y.; Zhou, K.; Su, C.; Ren, S.; Li, N.; Hou, L.; Guo, X.; Zhu, W.; Zhang, H.; et al. Characterization of Evolution Trajectory and Immune Profiling of Brain Metastasis in Lung Adenocarcinoma. Npj. Precis. Oncol. 2021, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Fang, Z.; Tang, S.; Cheng, R.; Li, Y.; Ren, S.; Su, C.; Min, W.; Guo, X.; Zhu, W.; et al. Mutational Landscape and Evolutionary Pattern of Liver and Brain Metastasis in Lung Adenocarcinoma. J. Thorac. Oncol. 2021, 16, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Mehta, M.; Griffith, J.; Panneerselvam, J.; Srivastava, A.; Kim, T.-D.; Janknecht, R.; Herman, T.; Ramesh, R.; Munshi, A. YAP1 Inhibition Radiosensitizes Triple Negative Breast Cancer Cells by Targeting the DNA Damage Response and Cell Survival Pathways. Oncotarget 2017, 8, 98495–98508. [Google Scholar] [CrossRef]
- von Bueren, A.O.; Shalaby, T.; Oehler-Jänne, C.; Arnold, L.; Stearns, D.; Eberhart, C.G.; Arcaro, A.; Pruschy, M.; Grotzer, M.A. RNA Interference-Mediated c-MYC Inhibition Prevents Cell Growth and Decreases Sensitivity to Radio- and Chemotherapy in Childhood Medulloblastoma Cells. BMC Cancer 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.J.H.; Nayyar, N.; Bihun, I.; Dagogo-Jack, I.; Gill, C.M.; Aquilanti, E.; Bertalan, M.; Kaplan, A.; D’Andrea, M.R.; Chukwueke, U.; et al. Genomic Characterization of Human Brain Metastases Identifies Drivers of Metastatic Lung Adenocarcinoma. Nat. Genet. 2020, 52, 371–377. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, C.; Tang, L.; Chen, Q.; Guan, N.; Xu, K.; Guan, X. MYC Dysfunction Modulates Stemness and Tumorigenesis in Breast Cancer. Int. J. Biol. Sci. 2021, 17, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.J.; Zheng, S.; Aldape, K.; Hinds, P.W.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. PTEN Expression in Non-Small-Cell Lung Cancer: Evaluating Its Relation to Tumor Characteristics, Allelic Loss, and Epigenetic Alteration. Hum. Pathol. 2005, 36, 768–776. [Google Scholar] [CrossRef]
- Mortezaee, K.; Parwaie, W.; Motevaseli, E.; Mirtavoos-Mahyari, H.; Musa, A.E.; Shabeeb, D.; Esmaely, F.; Najafi, M.; Farhood, B. Targets for Improving Tumor Response to Radiotherapy. Int. Immunopharmacol. 2019, 76, 105847. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Shen, R.; Won, H.; Rekhtman, N.; Wang, L.; Sima, C.S.; Arora, A.; Seshan, V.; Ladanyi, M.; Berger, M.F.; et al. Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discov. 2015, 5, 610–621. [Google Scholar] [CrossRef]
- Stella, G.M.; Senetta, R.; Inghilleri, S.; Verdun di Cantogno, L.; Mantovani, C.; Piloni, D.; Scudeller, L.; Meloni, F.; Papotti, M.; Ricardi, U.; et al. MET Mutations Are Associated with Aggressive and Radioresistant Brain Metastatic Non-Small-Cell Lung Cancer. Neuro-Oncology 2016, 18, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-Cell Lung Cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Dono, A.; Takayasu, T.; Yan, Y.; Bundrant, B.E.; Arevalo, O.; Lopez-Garcia, C.A.; Esquenazi, Y.; Ballester, L.Y. Differences in Genomic Alterations Between Brain Metastases and Primary Tumors. Neurosurgery 2021, 88, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Winslow, M.M.; Sage, J. Mechanisms of Small Cell Lung Cancer Metastasis. EMBO Mol. Med. 2021, 13, e13122. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Gay, C.M.; Xi, Y.; Sivajothi, S.; Sivakamasundari, V.; Fujimoto, J.; Bolisetty, M.; Hartsfield, P.M.; Balasubramaniyan, V.; Chalishazar, M.D.; et al. Single-Cell Analyses Reveal Increased Intratumoral Heterogeneity after the Onset of Therapy Resistance in Small-Cell Lung Cancer. Nat. Cancer 2020, 1, 423–436. [Google Scholar] [CrossRef]
- Simpson, K.L.; Stoney, R.; Frese, K.K.; Simms, N.; Rowe, W.; Pearce, S.P.; Humphrey, S.; Booth, L.; Morgan, D.; Dynowski, M.; et al. A Biobank of Small Cell Lung Cancer CDX Models Elucidates Inter- and Intratumoral Phenotypic Heterogeneity. Nat. Cancer 2020, 1, 437–451. [Google Scholar] [CrossRef]
- Quaranta, V.; Linkous, A. Organoids as a Systems Platform for SCLC Brain Metastasis. Front. Oncol. 2022, 12, 881989. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, J.; Yu, Q.; Wu, H.; Liu, B.; Xiong, H.; Hu, G.; Zhao, J.; Yuan, X.; Liao, Z. Associations between Single-Nucleotide Polymorphisms in the PI3K-PTEN-AKT-MTOR Pathway and Increased Risk of Brain Metastasis in Patients with Non-Small Cell Lung Cancer. Clin. Cancer Res. 2013, 19, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, D.H.; Saporito-Irwin, S.; DeClue, J.E.; Wienecke, R.; Guha, A. Alterations in the Rap1 Signaling Pathway Are Common in Human Gliomas. Oncogene 1997, 15, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Evans, I.M.; Frolov, A.; Britton, G.; Pellet-Many, C.; Yamaji, M.; Mehta, V.; Bandopadhyay, R.; Li, N.; Brandner, S.; et al. A Crucial Role for DOK1 in PDGF-BB-Stimulated Glioma Cell Invasion through P130Cas and Rap1 Signalling. J. Cell Sci. 2014, 127, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-F.; Kim, J.-S.; Waldman, T. Radiation-Induced Akt Activation Modulates Radioresistance in Human Glioblastoma Cells. Radiat. Oncol. 2009, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Kamer, I.; Steuerman, Y.; Daniel-Meshulam, I.; Perry, G.; Izraeli, S.; Perelman, M.; Golan, N.; Simansky, D.; Barshack, I.; Ben Nun, A.; et al. Predicting Brain Metastasis in Early Stage Non-Small Cell Lung Cancer Patients by Gene Expression Profiling. Transl. Lung Cancer Res. 2020, 9, 682–692. [Google Scholar] [CrossRef]
- Koh, Y.W.; Han, J.-H.; Haam, S.; Lee, H.W. An Immune-Related Gene Expression Signature Predicts Brain Metastasis in Lung Adenocarcinoma Patients after Surgery: Gene Expression Profile and Immunohistochemical Analyses. Transl. Lung Cancer Res. 2021, 10, 802–814. [Google Scholar] [CrossRef]
- Ruan, H.; Zhou, Y.; Shen, J.; Zhai, Y.; Xu, Y.; Pi, L.; Huang, R.; Chen, K.; Li, X.; Ma, W.; et al. Circulating Tumor Cell Characterization of Lung Cancer Brain Metastases in the Cerebrospinal Fluid through Single-Cell Transcriptome Analysis. Clin. Transl. Med. 2020, 10, e246. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, H.; Wang, Q.; Zhou, C.; Wei, L.; Liu, X.; Zhang, W.; Zhang, Y.; Du, Z.; Wang, X.; et al. Genome-Wide Analyses Reveal a Role of Polycomb in Promoting Hypomethylation of DNA Methylation Valleys. Genome Biol. 2018, 19, 18. [Google Scholar] [CrossRef]
- Karlow, J.A.; Devarakonda, S.; Xing, X.; Jang, H.S.; Govindan, R.; Watson, M.; Wang, T. Developmental Pathways Are Epigenetically Reprogrammed during Lung Cancer Brain Metastasis. Cancer Res. 2022, 82, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar]
- Liu, W.; Powell, C.A.; Wang, Q. Tumor Microenvironment in Lung Cancer-Derived Brain Metastasis. Chin. Med. J. 2022, 135, 1781–1791. [Google Scholar] [CrossRef]
- García-Gómez, P.; Valiente, M. Vascular Co-Option in Brain Metastasis. Angiogenesis 2020, 23, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Jacob, L.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma-Astrocyte Gap Junctions Promote Brain Metastasis by CGAMP Transfer. Nature 2016, 533, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, N.; Zhu, W.; Wu, J.; Yang, X.; Teng, W.; Tian, J.; Fang, Z.; Luo, Y.; Chen, M.; et al. Modulation the Crosstalk between Tumor-Associated Macrophages and Non-Small Cell Lung Cancer to Inhibit Tumor Migration and Invasion by Ginsenoside Rh2. BMC Cancer 2018, 18, 579. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Dunbar, D.R.; Howie, S.E.; Sethi, T.; Gregory, C.D. Human Dendritic Cells Produce TGF-Beta 1 under the Influence of Lung Carcinoma Cells and Prime the Differentiation of CD4+CD25+Foxp3+ Regulatory T Cells. J. Immunol. 2009, 182, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Aloe, C.; Wang, H.; Vlahos, R.; Irving, L.; Steinfort, D.; Bozinovski, S. Emerging and Multifaceted Role of Neutrophils in Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 2806–2818. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Gandhi, S.; Chandna, S. Radiation-Induced Inflammatory Cascade and Its Reverberating Crosstalks as Potential Cause of Post-Radiotherapy Second Malignancies. Cancer Metastasis Rev. 2017, 36, 375–393. [Google Scholar] [CrossRef]
- Chen, Y.S.; Song, H.X.; Lu, Y.; Li, X.; Chen, T.; Zhang, Y.; Xue, J.X.; Liu, H.; Kan, B.; Yang, G.; et al. Autophagy Inhibition Contributes to Radiation Sensitization of Esophageal Squamous Carcinoma Cells. Dis. Esophagus 2011, 24, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Syed, V. TGF-β Signaling in Cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef]
- Rapoport, B.L.; Anderson, R. Realizing the Clinical Potential of Immunogenic Cell Death in Cancer Chemotherapy and Radiotherapy. Int. J. Mol. Sci. 2019, 20, 959. [Google Scholar] [CrossRef]
- Najafi, M.; Goradel, N.H.; Farhood, B.; Salehi, E.; Solhjoo, S.; Toolee, H.; Kharazinejad, E.; Mortezaee, K. Tumor Microenvironment: Interactions and Therapy. J. Cell. Physiol. 2019, 234, 5700–5721. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Kong, L.; Meng, X.; Yang, J.; Yu, J. Radiotherapy Combined with Immune Checkpoint Blockade Immunotherapy: Achievements and Challenges. Cancer Lett. 2015, 365, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cordes, N.; Seidler, J.; Durzok, R.; Geinitz, H.; Brakebusch, C. Beta1-Integrin-Mediated Signaling Essentially Contributes to Cell Survival after Radiation-Induced Genotoxic Injury. Oncogene 2006, 25, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.M.; Eneman, J.; Crocker, A.; White, J.; Tessitore, J.; Stanley, M.; Harlow, S.; Bunn, J.Y.; Weaver, D.; Muss, H.; et al. Cancer Associated Fibroblasts Stimulated by Transforming Growth Factor Beta1 (TGF-Beta 1) Increase Invasion Rate of Tumor Cells: A Population Study. Breast Cancer Res. Treat. 2008, 110, 39–49. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, H.; Ni, C.; Zhang, T.; Liu, L.; Lv, Q.; Zhang, Z.; Wang, Z.; Wu, D.; Wu, P.; et al. Hypofractionated Stereotactic Radiation Therapy Activates the Peripheral Immune Response in Operable Stage I Non-Small-Cell Lung Cancer. Sci. Rep. 2017, 7, 4866. [Google Scholar] [CrossRef]
- Tsai, C.-S.; Chen, F.-H.; Wang, C.-C.; Huang, H.-L.; Jung, S.-M.; Wu, C.-J.; Lee, C.-C.; McBride, W.H.; Chiang, C.-S.; Hong, J.-H. Macrophages from Irradiated Tumors Express Higher Levels of INOS, Arginase-I and COX-2, and Promote Tumor Growth. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing Radiation Modulates Human Macrophages towards a Pro-Inflammatory Phenotype Preserving Their pro-Invasive and pro-Angiogenic Capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an INOS+/M1 Phenotype That Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Sorensen, E.W.; Gerber, S.A.; Moran, J.P.; Frelinger, J.G.; Lord, E.M. Radiation-Induced IFN-Gamma Production within the Tumor Microenvironment Influences Antitumor Immunity. J. Immunol. 2008, 180, 3132–3139. [Google Scholar] [CrossRef]
- Lugade, A.A.; Moran, J.P.; Gerber, S.A.; Rose, R.C.; Frelinger, J.G.; Lord, E.M. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J. Immunol. 2005, 174, 7516–7523. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Xu, L.; Li, L.-F.; Liu, X.-X.; Gao, J.-X.; Bai, Y.-R. Inhibiting the CD8+ T Cell Infiltration in the Tumor Microenvironment after Radiotherapy Is an Important Mechanism of Radioresistance. Sci. Rep. 2018, 8, 11934. [Google Scholar] [CrossRef]
- Roses, R.E.; Datta, J.; Czerniecki, B.J. Radiation as Immunomodulator: Implications for Dendritic Cell-Based Immunotherapy. Radiat. Res. 2014, 182, 211–218. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The Tumour Microenvironment after Radiotherapy: Mechanisms of Resistance and Recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Grdina, D.J.; Murley, J.S.; Miller, R.C.; Mauceri, H.J.; Sutton, H.G.; Li, J.J.; Woloschak, G.E.; Weichselbaum, R.R. A Survivin-Associated Adaptive Response in Radiation Therapy. Cancer Res. 2013, 73, 4418–4428. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Sharma, K. COX-2 Signaling and Cancer: New Players in Old Arena. Curr. Drug Targets 2014, 15, 347–359. [Google Scholar] [CrossRef]
- Milas, L. Cyclooxygenase-2 (COX-2) Enzyme Inhibitors as Potential Enhancers of Tumor Radioresponse. Semin. Radiat. Oncol. 2001, 11, 290–299. [Google Scholar] [CrossRef]
- Han, Z.-Q.; Liao, H.; Shi, F.; Chen, X.-P.; Hu, H.-C.; Tian, M.-Q.; Wang, L.-H.; Ying, S. Inhibition of Cyclooxygenase-2 Sensitizes Lung Cancer Cells to Radiation-Induced Apoptosis. Oncol. Lett. 2017, 14, 5959–5965. [Google Scholar] [CrossRef]
- Shin, Y.K.; Park, J.S.; Kim, H.S.; Jun, H.J.; Kim, G.E.; Suh, C.O.; Yun, Y.S.; Pyo, H. Radiosensitivity Enhancement by Celecoxib, a Cyclooxygenase (COX)-2 Selective Inhibitor, via COX-2-Dependent Cell Cycle Regulation on Human Cancer Cells Expressing Differential COX-2 Levels. Cancer Res. 2005, 65, 9501–9509. [Google Scholar] [CrossRef]
- Bundred, N.J.; Cramer, A.; Morris, J.; Renshaw, L.; Cheung, K.-L.; Flint, P.; Johnson, R.; Young, O.; Landberg, G.; Grassby, S.; et al. Cyclooxygenase-2 Inhibition Does Not Improve the Reduction in Ductal Carcinoma in Situ Proliferation with Aromatase Inhibitor Therapy: Results of the ERISAC Randomized Placebo-Controlled Trial. Clin. Cancer Res. 2010, 16, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Dewhirst, M.W. HIF-1 and Tumour Radiosensitivity. Br. J. Cancer 2006, 95, 1–5. [Google Scholar] [CrossRef]
- Bruick, R.K. Expression of the Gene Encoding the Proapoptotic Nip3 Protein Is Induced by Hypoxia. Proc. Natl. Acad. Sci. USA 2000, 97, 9082–9087. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Telfer, B.A.; Shannon, A.M.; Babur, M.; Stratford, I.J.; Wedge, S.R. Inhibition of Vascular Endothelial Growth Factor Signalling Using Cediranib (RECENTIN.; AZD2171) Enhances Radiation Response and Causes Substantial Physiological Changes in Lung Tumour Xenografts. Br. J. Radiol. 2008, 81, S21–S27. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef] [PubMed]
- Oweida, A.; Hararah, M.K.; Phan, A.; Binder, D.; Bhatia, S.; Lennon, S.; Bukkapatnam, S.; Van Court, B.; Uyanga, N.; Darragh, L.; et al. Resistance to Radiotherapy and PD-L1 Blockade Is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin. Cancer Res. 2018, 24, 5368–5380. [Google Scholar] [CrossRef]
- Shitara, K.; Nishikawa, H. Regulatory T Cells: A Potential Target in Cancer Immunotherapy. Ann. N. Y. Acad. Sci. 2018, 1417, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Kim, R.; Keam, B.; Kim, S.; Kim, M.; Kim, S.H.; Kim, J.W.; Kim, Y.J.; Kim, T.M.; Jeon, Y.K.; Kim, D.-W.; et al. Differences in Tumor Microenvironments between Primary Lung Tumors and Brain Metastases in Lung Cancer Patients: Therapeutic Implications for Immune Checkpoint Inhibitors. BMC Cancer 2019, 19, 19. [Google Scholar] [CrossRef]
- Souza, V.G.P.; de Araújo, R.P.; Santesso, M.R.; Seneda, A.L.; Minutentag, I.W.; Felix, T.F.; Hamamoto Filho, P.T.; Pewarchuk, M.E.; Brockley, L.J.; Marchi, F.A.; et al. Advances in the Molecular Landscape of Lung Cancer Brain Metastasis. Cancers 2023, 15, 722. [Google Scholar] [CrossRef]
- Negrao, M.V.; Skoulidis, F.; Montesion, M.; Schulze, K.; Bara, I.; Shen, V.; Xu, H.; Hu, S.; Sui, D.; Elamin, Y.Y.; et al. Oncogene-Specific Differences in Tumor Mutational Burden, PD-L1 Expression, and Outcomes from Immunotherapy in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2021, 9, e002891. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, S.; Jin, R.; Wang, X.; Wang, F.; Zang, R.; Xu, H.; Lu, Z.; Huang, J.; Lei, Y.; et al. The Superior Efficacy of Anti-PD-1/PD-L1 Immunotherapy in KRAS-Mutant Non-Small Cell Lung Cancer That Correlates with an Inflammatory Phenotype and Increased Immunogenicity. Cancer Lett. 2020, 470, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Miarka, L.; Perea-García, M.; Priego, N.; García-Gómez, P.; Álvaro-Espinosa, L.; de Pablos-Aragoneses, A.; Yebra, N.; Retana, D.; Baena, P.; et al. Stratification of Radiosensitive Brain Metastases Based on an Actionable S100A9/RAGE Resistance Mechanism. Nat. Med. 2022, 28, 752–765. [Google Scholar] [CrossRef] [PubMed]

