1. Introduction
Dengue fever (DF), a mosquito-borne tropical ailment caused by the Dengue virus (DENV), is characterized by flu-like symptoms and may sporadically progress to severe and life-threatening complications, such as dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) [1,2]. DF is the most widespread and rapidly increasing vector-borne disease (VBD) globally. It is now widespread in over 100 countries, affecting approximately 40% of the world’s population [3,4]. Each year, there are approximately 400 million infections, 500,000 severe cases of hospitalization, and a 2.5% mortality rate [5,6].
DENV, a member of the Flaviviridae family, is a single-stranded RNA (+ssRNA) virus [7]. Four serotypes of the virus (DENV-1, DENV-2, DENV-3, and DENV-4) can be distinguished [8,9,10], which differ in amino acid identity by 30–35% [11]. The treatment for dengue primarily relies on adjuvant therapy, as specific antiviral therapies are lacking [12]. There are also limitations to the use of a licensed dengue vaccine in naïve individuals not previously infected with DENV and in children under nine years of age [13,14]. An ideal dengue vaccine should possess cross-protective properties against all four serotypes, offer sustained efficacy, and ensure reliable safety. However, antibody-dependent enhancement (ADE) effects upon reinfection with other serotypes, and the absence of suitable animal models, make the development of an ideal dengue vaccine challenging [15,16]. Therefore, the development of safe and effective drugs for dengue treatment is urgently required.
The genome of DENV is approximately 10.7 kb in length and constitutes a single open reading frame (ORF) flanked by 5′ and 3′ untranslated regions (UTRs). The ORF of the genome encodes three structural proteins—capsid (C), premembrane/membrane (prM/M), and envelope (E)—along with seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [17,18,19]. Among these, NS5 acts as a methyltransferase and an RNA-dependent RNA polymerase (RdRp), both of which are essential for viral replication [20,21]. The DENV RdRp is responsible for both negative- and positive-stranded RNA synthesis during replication [22,23,24]. Since it lacks a mammalian counterpart and its sequence is conserved across all four serotypes with over 65% homology, it offers an attractive opportunity for the discovery of new antiviral drugs [25,26].
Interest in DENV NS5 has grown in recent years, and multiple RdRp inhibitors have been reported [27,28,29,30,31,32]. Notably, the Novartis Institute for Tropical Diseases has identified two promising allosteric inhibitors of DENV RdRp: 8-quinolyl sulfonamide (27) and 3-methoxybenzene ring (29) derivatives, demonstrating substantial activity, with IC50 values of 0.013–0.074 μM and 0.048–0.172 μM, respectively, against all clinically relevant dengue virus serotypes [9,33]. In the continuing pursuit of small molecular entities targeting DENV, we attempted to use compounds 27 and 29 as lead compounds to identify new anti-DENV compounds with higher activity. This article describes our efforts in this regard, shedding light on promising avenues for the development of future antiviral therapies and contributing to the fight against the dengue virus.
2. Results and Discussion
2.1. Compounds Design
2.2. Synthesis
Compound SW was obtained by coupling two key fragments: the benzenesulfonamide fragment (9) and the carboxylic acid fragment (25). The key intermediate, benzenesulfonamide (9), was synthesized in multiple steps according to the literature’s procedures (Scheme 1) [9,35,36]. 4-Bromo-3-methoxy-toluene (2) was obtained via nucleophilic displacement of 4-bromo-3-fluorotoluene (1) with sodium methoxide [37]. The 5-position of compound (2) was chlorosulfonated using chlorosulfonic acid, and then reacted with aqueous ammonia to yield the sulfonamide derivative 5-bromo-4-methoxy-2-methylbenzenesulfonamide (4) [9]. The amino group was protected using (Boc)2O in the presence of triethylamine and DMAP, resulting in tert-butyl ((5-bromo-4-methoxy-2-methylphenyl)sulfonyl)carbamate (5). The Suzuki– Miyaura cross-coupling reaction of compound 6 with thiophene boronic acid produced ((4-methoxy-2-methyl-5-(thiophene)yl)phenyl)sulfonyl) tert-butyl carbamate (6) [9]. The resulting compound 7 was subjected to halogenation of the thiophene ring using benzoyl peroxide and NBS, and the protecting group attached to the amino group was removed using trifluoroacetic acid to obtain 5-(5-bromothiophen-2-yl)-4-methoxy-2-methylbenzenesulfonamide (8) [9,38]. Under the catalysis of cuprous iodide, triethylamine, and the 1,1′-bis(diphenylphosphino)ferrocene-dichloropalladium(II) dichloromethane complex, compound 8 was cross-coupled with propargyloxytetrahydropyran to obtain the key intermediate 9 [39]. This intermediate was used directly in the subsequent step, with an overall yield of approximately 5%.
Carboxylic acids, such as 3-methoxybenzoic acid (25a), 5-methyl-2-thiophenecarboxylic acid (25g), and 4-pyrimidinecarboxylic acid (25h), are commercially available. Carboxylic acid (25b–f) and 25i were synthesized, as shown in Scheme 2. Briefly, commercially available resorcinol 10 and ethyl 2-oxocyclohexanecarboxylate 11 were cyclized in the presence of methanesulfonic acid to produce the corresponding coumarin analog 12, which was subsequently nucleophilized with bromide in DMF, followed by deprotection with sodium hydroxide to furnish 25b [40]. Compound 25c was synthesized by a Paal–Knorr reaction between 2,5-Hexanedione 13 and 4-aminobenzoic acid 14 formate in the presence of acetic acid [41]. The cyclization of methyl 2-cyclopentanonecarboxylate 15 and ethanimidamide 16 generated the pyrimidine derivative 17 in the presence of potassium tert-butoxide. Halogenation of the pyrimidine ring 17 in the presence of phosphorus oxychloride (POCl3) provided compound 18, which underwent nucleophilic reaction with lithium 3-amino-4-methylbenzoate, followed by deprotection with hydroxide·water (LiOH·H2O) to give 25d [42]. A mixture of itaconic acid 20 and 3,4-dichloroaniline 19 was melted by heating, and the product 25e was liberated with hydrochloric acid [43]. The reaction of trimellitic anhydride 21 with trimethylaniline 22 under acidic conditions yielded compound 25f [44]. Finally, condensation of 3,5-dimethylpyrazole 23 with ethyl bromoacetate 24, followed by hydrolysis with NaOH, yielded 25i [45].
Using 1-ethyl-3-(3-dimethylpropyl) carbodiimide (EDC)/1-hydroxybenzotriazole (HOBT) or 1-ethyl-3-(3-dimethylpropyl) base carbodiimide (EDC)/2-(7-azabenzotriazol-1-yl)-N, N, N’, N’-tetramethyluronium hexafluorophosphate (HATU) as a condensing agent, benzenesulfonamide (9) condensed with various carboxylic acids (25), followed by deprotection of tetrahydropyran with saturated ammonium chloride solution, afforded the title compounds SW-(a-i), as shown in Scheme 2, and the overall yield was around 50%. All compounds were characterized by 1H NMR, 13C NMR, and mass spectrometry. HPLC detection confirmed that the purity of each compound was greater than 95%.
2.3. Biological Evaluation
2.4. Molecular Dynamics (MD) Simulation and Analysis
To elucidate the binding modes of compounds SW-b and SW-d to DENV NS5, we compared them with those of compounds 27 and 29, bound to DENV NS5 using MD simulations conducted with Desmond [46]. Given the potential influence of chirality on small molecule–protein interactions, we conducted MD simulations considering two enantiomers of compound SW-b: S-SW-b and R-SW-b, even though compound SW-b was bioassayed as a racemate . To strike a balance between simulation accuracy and computational efficiency, the MD simulations had varying durations, ranging from 200–500 ns, with each run repeated three times to ensure consistent results. The duration depended on the structural stability of the protein–ligand complex.
An essential Indicator for assessing the simulated stability of protein–ligand complexes is the variation trend in the root-mean-square deviation (RMSD) values of proteins, and . illustrates the RMSD of the DENV NS5 protein Cα atoms and the ligand RMSD fit to protein (Lig fit Prot) during the MD simulations. The protein RMSD trajectory is shown in blue with values on the left Y-axis (Å), while the ligand RMSD trajectory is shown in red with values on the right Y-axis in (Å).
The results showed that the RMSD of DENV NS5 protein Cα atoms tended to stabilize during the simulation of several complexes. Notably, there were no instances in which the Lig fit Prot value significantly exceeded the corresponding Cα RMSD value. This confirms the reliability of the MD simulation results, indicating that the ligands did not significantly deviate from their initial binding sites. Starting from the initial frames of the crystal complexes of DENV NS5-27 and DENV NS5-29, the protein Cα atoms and ligands reached stability quickly after 25 ns, indicating highly stable crystal structures. Regarding the DENV NS5-S-SW-b docking complex, the RMSD values of protein Cα atoms and Lig fit Prot reached stability at 10 ns. Although the RMSD value of protein Cα atoms increased slightly from 100 ns to 200 ns, it subsequently regained restability after 200 ns. For the DENV NS5-R-SW-b docking complex, the RMSD values of the protein Cα atom and the Lig fit Prot changed simultaneously. After 220 ns, they stabilized and fluctuated at approximately 1.5 Å. For the DENV NS5-SW-d docking complex, the RMSD values of the protein Cα atoms reached stability at approximately 20 ns, while the RMSD values for Lig fit Prot were less stable. After simulating for 280 ns, both the RMSD values of the protein Cα atom and Lig fit Prot reached stability. Nevertheless, after conducting MD simulations lasting between 300 ns and 500 ns, both systems achieved a state of relative stability. This finding underscores their fundamental structural reliability.
The root-mean-square fluctuation (RMSF) results of the amino acid residues in the DENV NS5 complex with compounds 27, 29, SW-b and SW-d during the MD simulations are shown in Figure 4. The RMSF reflects the flexibility of amino acid residues; a greater fluctuation in RMSF indicates the higher flexibility of the corresponding amino acid residues [47]. Protein residues interacting with the ligand are marked with green vertical bars. The RMSF fluctuations are useful for determining the stability of protein binding to small molecules.
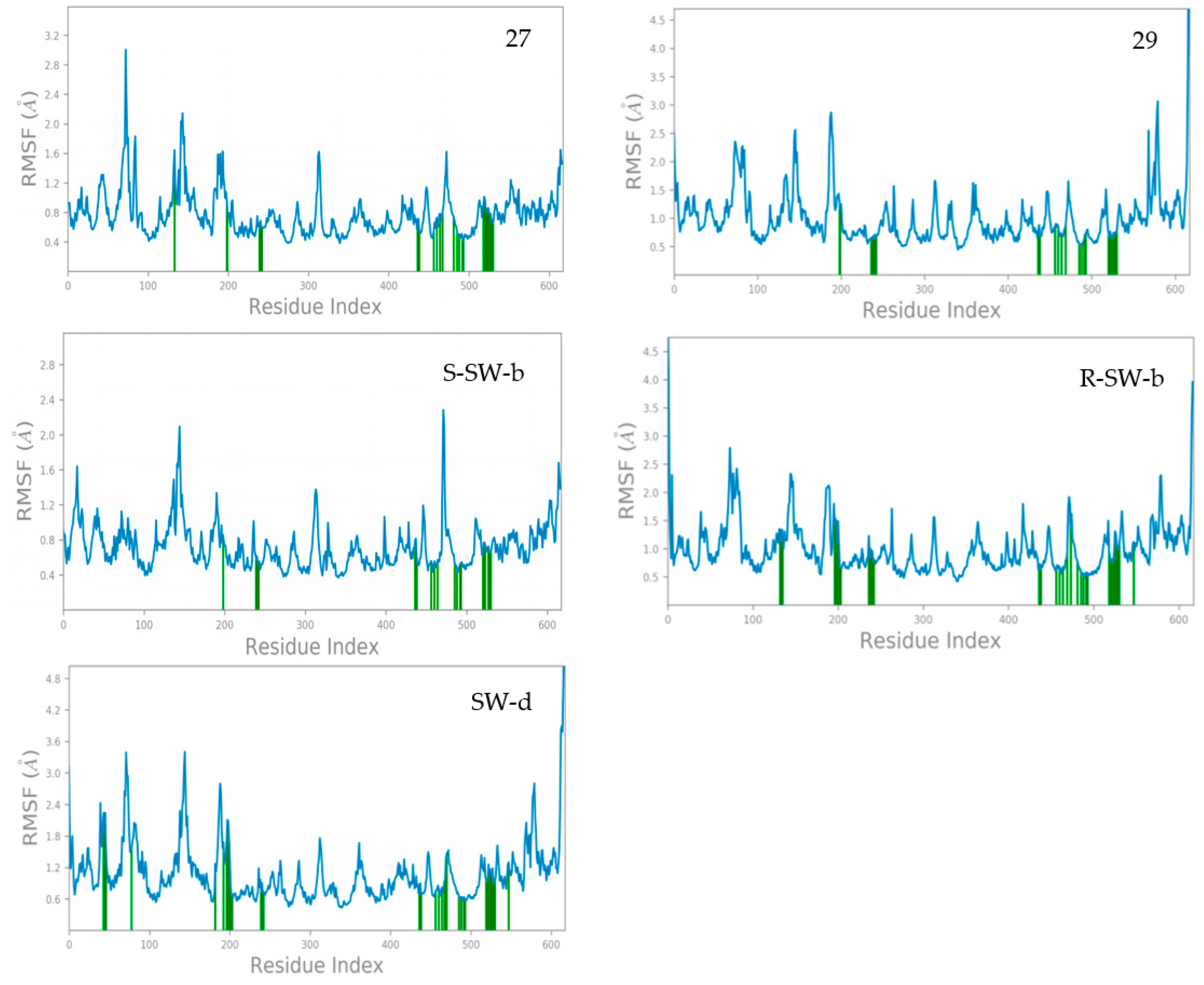
Figure 4. The RMSF of NS5 protein amino acid residues in MD simulations.
It was observed that the crystal structure of the DENV NS5-27 complex (PDB: 5K5M) exhibited high stability. With the exception of the N-terminal and C-terminal regions of the protein and some amino acid residues, most amino acid residues had RMSF values below 2.0 Å. Specifically, the RMSF values of amino acid residues directly associated with compound 27 were basically less than 1.2 Å. In contrast, the crystal structure of the DENV NS5-29 complex was less stable compared to the DENV NS5-27 complex. Most amino acid residues had RMSF values below 3.0 Å, except for those located at the N-terminal and C-terminal regions of the protein. Furthermore, the RMSF values of amino acid residues directly interacting with compound 29 were basically less than 1.0 Å. In general, the complex structure of DENV NS5 with these two small-molecule inhibitors was stable. Compared with compounds 27 and 29, compound S-SW-b demonstrated greater stability within the DENV NS5 protein. The RMSF values of the amino acid residues in the DENV NS5-S-SW-b complex basically did not exceed 2.0 Å, and the RMSF value of the amino acid residues directly interacting with compound S-SW-b was basically less than 0.8 Å. The stability of compound R-SW-b in the DENV NS5 protein was lower than that of compound S-SW-b but comparable to that of compound 29. The RMSF values of amino acid residues in the DENV NS5-R-SW-b complex reached up to 2.5 Å, whereas the RMSF values of amino acid residues directly interacting with compound R-SW-b was basically less than 1.5 Å. Compared with the DENV NS5-27, 29, and SW-b complexes, the stability of the DENV NS5-SW-d complex was slightly less pronounced. While a few amino acid residues exhibited RMSF values slightly exceeding 3.0 Å, the RMSF value of amino acid residues directly interacting with compound SW-d was approximately 1.8 Å. Overall, the complex formed between DENV NS5 and compound SW-d demonstrated a high degree of stability.
The mode of action of the NS5–ligand complex was further resolved by protein– ligand contact and protein–ligand interaction scores. Protein–ligand contacts (or ‘interactions’) are categorized into four types: hydrogen bonds (HB), hydrophobic contacts (HP), ionic bridges, and water bridges (WB). Each interaction type contains more specific subtypes; for example, HP falls into three subtypes: ion–pi interactions (ion–pi), pi–pi stacking (π–π), and non-specific hydrophobic interactions (ns HP). A 2D summary of the protein–ligand contact analysis results for NS5 binding with compounds 27, 29, S-SW-b, R-SW-b, and SW-d is displayed (Figure 5). This can be visualized more intuitively in stick mode (Figure 6). Protein–ligand interactions, such as HB, WB, ion-pi interactions, and π–π stacking, which occurred with a probability of over 30% during the simulation, are displayed. A standardized stacked bar graph presents the protein–ligand interaction fractions of the four possible types of bond interactions (HB, HP, ionic, and WB) for NS5 binding with compounds 27, 29, SW-b, and SW-d. The interaction fraction of the protein with the corresponding residues is represented by a standardized stacked bar graph; for example, a value of 0.7 means that a specific interaction is maintained for 70% of the simulation time. Values above 1.0 are possible because certain protein residues may form multiple contacts of the same subtype with the ligand. A timeline representation of the interactions and contacts (hydrogen bonds and hydrophobic, ionic, and water bridges) is shown (Figure 6). The top panel shows the total number of specific contacts between the protein and ligand throughout the trajectory. The bottom panel shows the residues that interacted with the ligand in each trajectory frame. Some residues made more than one specific contact with the ligand, which is represented by the darker orange shade, according to the scale to the right of the plot.
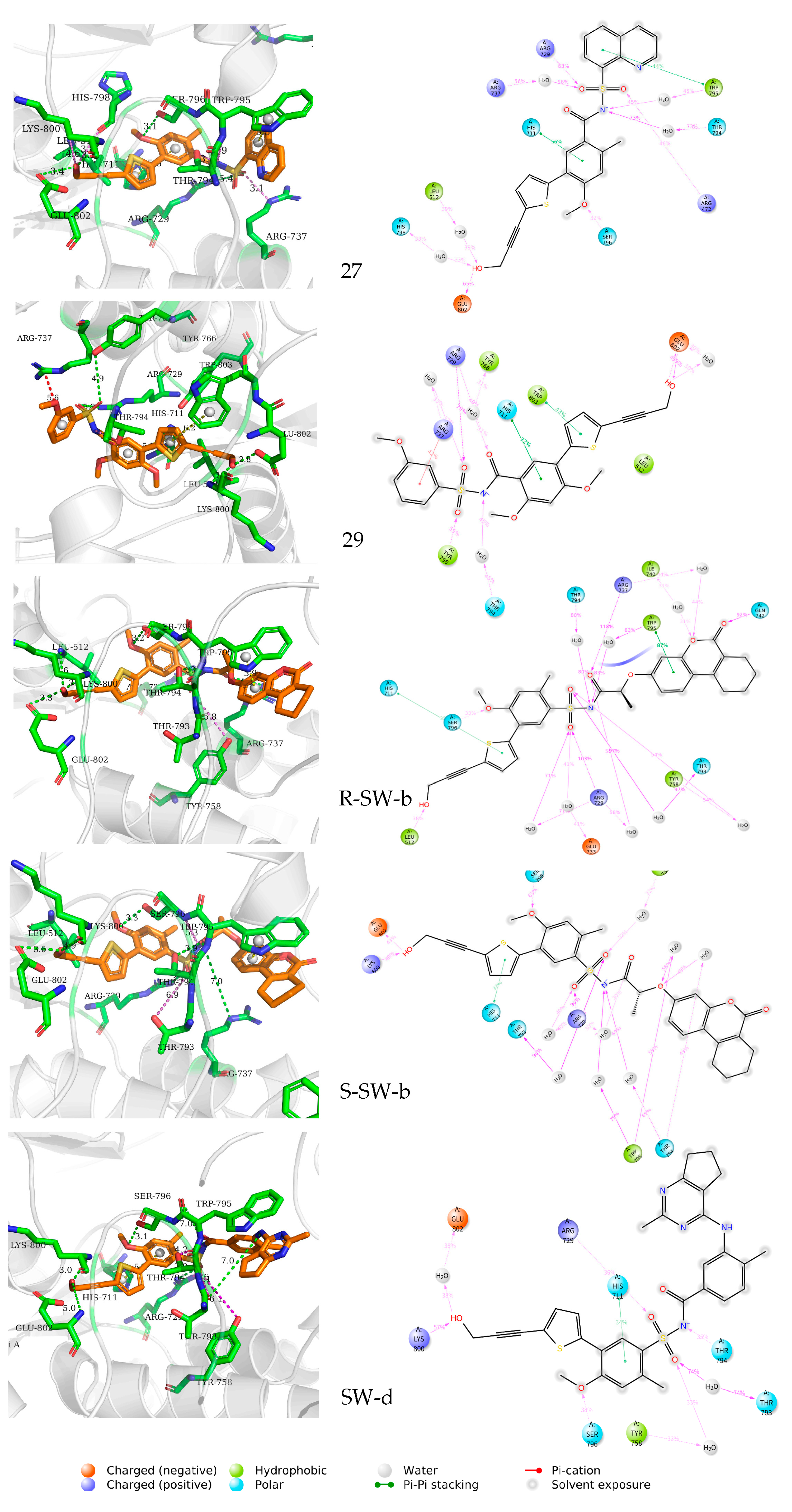
Figure 5. Two-dimensional representation and three-dimensional summary of the interaction analysis results of the NS5–ligand complexes (PDB: 5K5M). π–π stacking interactions are shown as yellow dashed lines, intermolecular hydrogen bonds as green dashed lines, and salt bridges as magenta dashed lines.
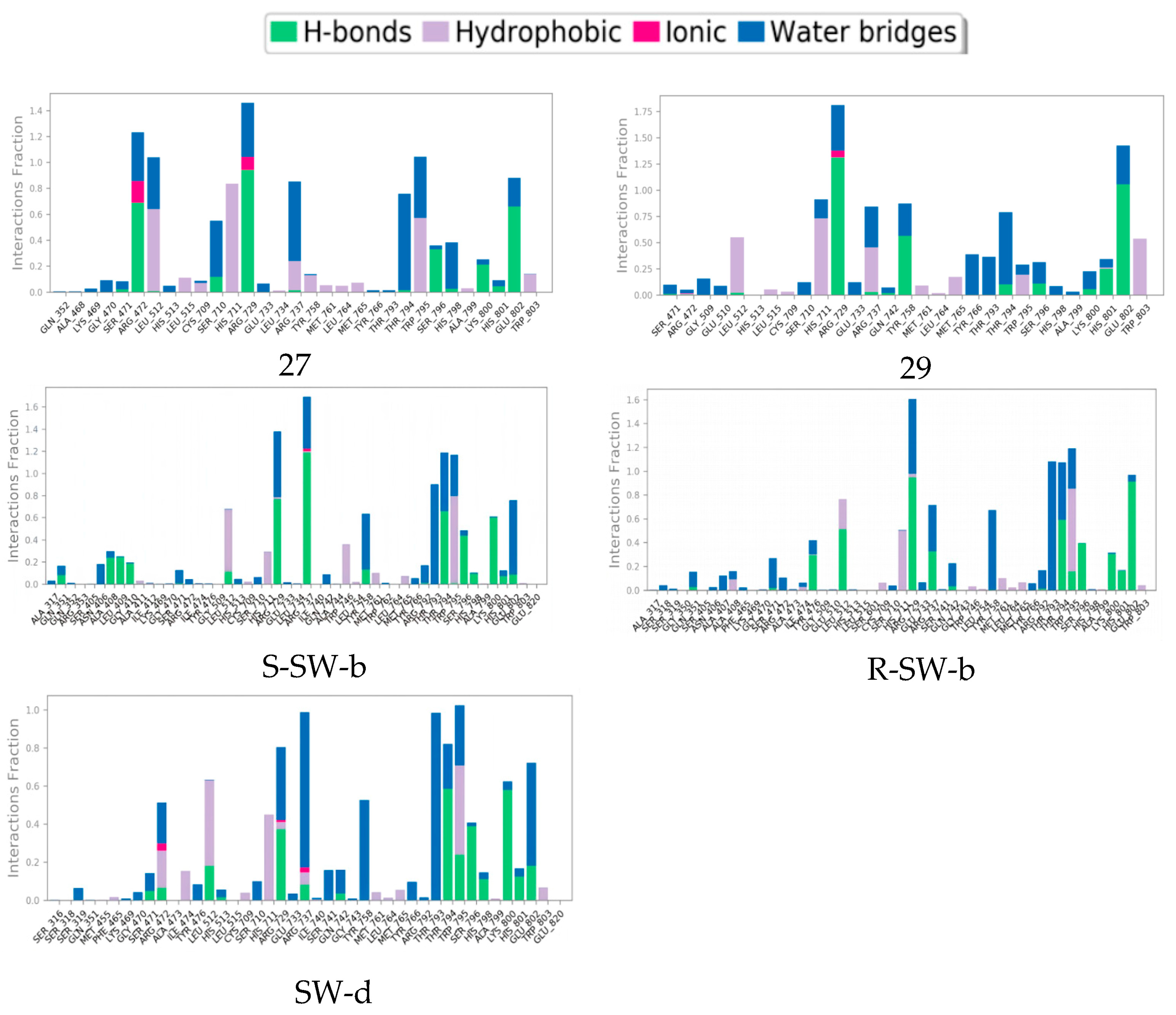
Figure 6. Interaction fraction summary of NS5–ligand contacts, normalized for total simulation time. Interaction fraction values over 1.0 indicate that a residue has multiple contact routes for interacting with the ligand.
In silico studies showed that the binding patterns of the S1 fragments of compounds SW-b and SW-d were different. In compound SW-b, the reverse acylsulfonamide amide moiety formed HB with the side chain of R737. Additionally, in compound R-SW-b, the propargyl alcohol hydroxyl moiety of S3 fragments formed HB with the side chain of L512. Compound SW-d mainly formed WB on the side chain R737 (Figure 4). During the MD simulations (Figure 5), S-SW-b formed hydrogen bonds with Q351, A408, L409, G410, S471, L512, R729, R737, Y758, R792, T794, W795, S796, H798, K800, H801, and E802. Of these, R729, R737, T794, and K800 had interaction fractions > 0.6. R-SW-b formed conventional hydrogen bonds with Q351, S471, Y476, L512, R729, R737, Q742, R792, T794, W795, S796, K800, H801, and E802. Of these, R729, T794, and E802 had interaction fractions > 0.6. SW-d formed conventional hydrogen bonds with S471, R472, L512, H513, R729, R737, Q742, T794, W795, S796, H798, K800, H801, and E802. Of these, T794 and K800 had interaction fractions > 0.6. Comparison with the protein–ligand contacts of compounds 27 and 29 shows that Compound 27 formed conventional hydrogen bonds with S471, R472, L512, S710, R729, R737, T794, S796, H798, K800, H801, and E802. Of these, R472, R729, and E802 had interaction fractions > 0.6. Compound 29 formed conventional hydrogen bonds with S471, R472, L512, R729, R737, Q742, Y758, T794, S796, K800, H801, and E802. Of these, R729 and E802 had interaction fractions > 0.6. Compounds SW-b and SW-d increased protein–ligand contact compared to compounds 27 and 29. In addition, the benzene ring of the S1 fragments of SW-b and SW-d showed π–π stacking with W795. Compared with compounds 27 and 29, the reverse acylsulfonamide fragment reduced the WB between T794 and NH but increased the WB between the sulfonyl group and R729 and T794. These findings suggest that compounds SW-b and SW-d possess similar or improved stabilities compared to compounds 27 and 29.
The binding free energy profiles of the DENV NS5 protein complexes were evaluated using the MM/PBSA method. The estimated binding free energy of the DENV NS5 protein with compounds 27, 29, R-SW-b, S-SW-b, and SW-d were −82.138 kcal/mol, −79.716 kcal/mol, −83.090 kcal/mol −95.966 kcal/mol, and −82.003 kcal/mol, respectively, while S-SW-b was the best one among these compounds, confirming that it had a strong affinity. Additionally, the free energy of the protein–ligand complexes was composed of multiple components of energy (van der Waals, Coulomb, Covalent and Hbond, solvation, and Packing); solvation Lipo and van der Waals force was the major contributor to the total binding free energy (Figure 7). It was noteworthy that, compared to other compounds, a substantial reduction in the number of solvation and Lipo energy in S-SW-b can be observed, which may be a reason why it has the lowest total binding free energy.
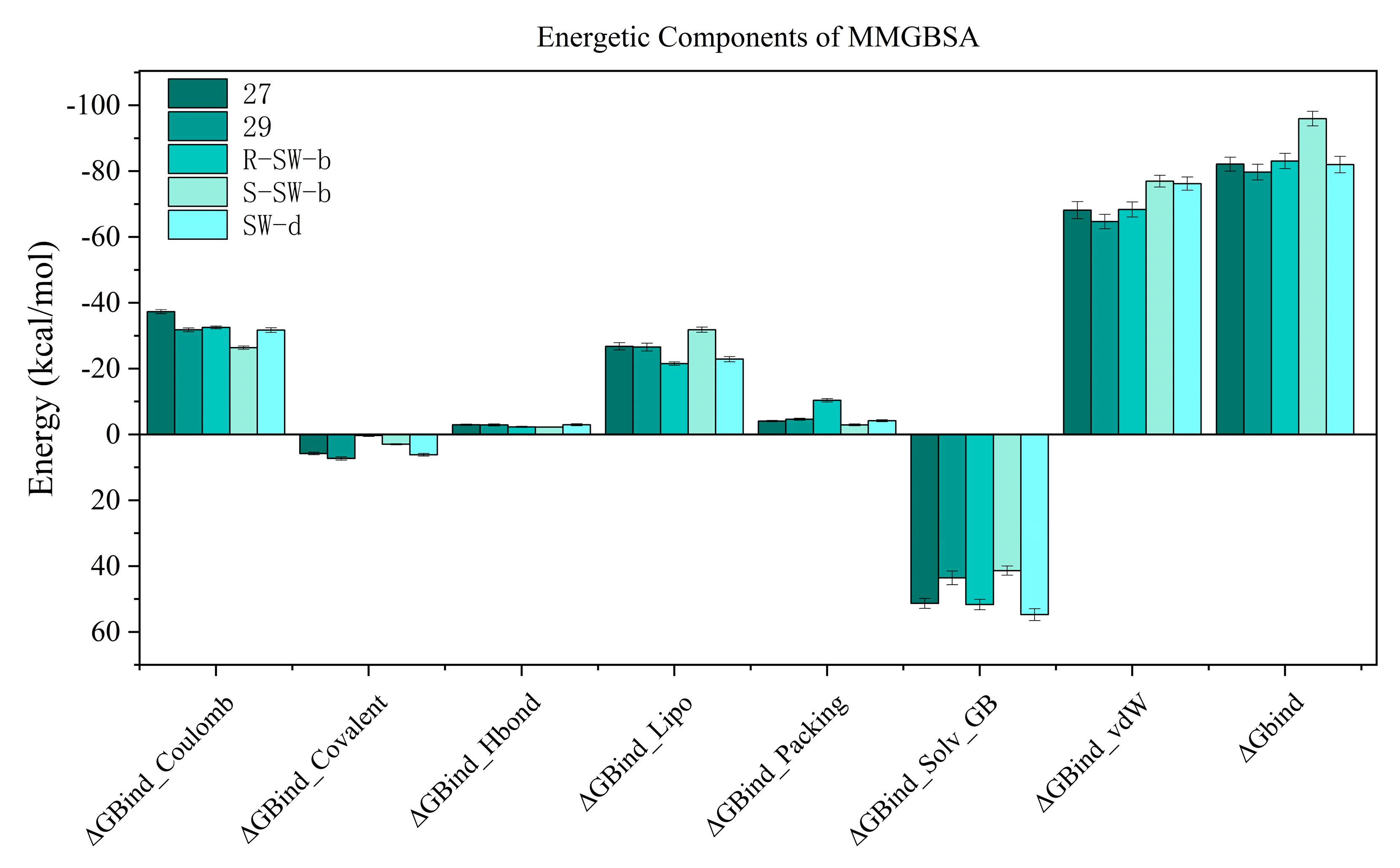
Figure 7. MM−GBSA energies of molecular simulation trajectory for compounds 27, 29, R−SW−b, S−SW−b, and SW−d.
In conclusion, our simulation results were consistent with the results of the biological activity detection. Among this group of compounds, the DENV NS5-SW-b complex demonstrated the highest stability, and compound SW-b exhibited the highest enzyme inhibitory activity. Conversely, the stability of the DENV NS5-SW-d complex was notably lower, and the corresponding compound, SW-d, displayed reduced enzyme inhibitory activity compared to compounds 27 and SW-b. This further validates the reliability of the simulation results. Moreover, our simulation results also indicated that the activity of compound S-SW-b should surpass that of its enantiomer R-SW-b.
3. Materials and Methods
3.1. Chemistry
3.2. Biological Evaluation
3.3. Virtual Screening Assay
Molecular docking was performed as described by Schrödinger (Maestro, 2020, Schrödinger Release 2020-1: Schrödinger, LLC, New York, NY, USA). Compounds 27 and 29, S-SW-b, R-SW-b, and SW-d were saved in *mol2 format.
3.4. Molecular Dynamics (MD) Simulation
For molecular dynamics (MD) simulation, Maestro’s Desmond module (Schrödinger, 2020) was used. A system builder panel using the OPLS3 force field was utilized to configure the biological system prior to MD simulation. To solvate the system, an SPC water model was employed, and an orthorhombic box with a 10 Å buffer distance was generated. In this study, MD simulations were conducted for a total duration of 200–500 ns on a GPU, maintaining a temperature of 300 K and a pressure of 1.01325 bar. The Simulation Interaction Diagram shows the ligand–protein interactions, including RMSD images, that determine the stability of the complex. The simulated complex was visualized using PyMOL software (version 2.0, Schrödinger) to observe the polar contacts between the ligand and receptor.
3.5. Thermodynamic Calculations: Binding Free Energy Calculation
Calculating the binding free energy can provide a thorough insight of the interaction of the protein–ligand complex. The binding free energy profiles of the DENV NS5 complex with compounds 27, 29, S-SW-b, R-SW-b and SW-d were assessed using thermal_mmgbsa.py (Schrödinger, 2020), which employs the Molecular Mechanics Poisson–Boltzmann Surface Area (MMPBSA) approach. A total of 50 snapshots from the final 50 ns of the MD production run were taken into account when estimating the binding free energy for each complex.
4. Conclusions
Starting with the potent non-nucleoside inhibitors 27 and 29, suitable replacement groups for the aryl sulfonamide fragment were identified through virtual compound construction, combined with docking-based virtual screening. Nine compounds were selected for the synthesis. The evaluation included multiple factors, including docking scores, free energy of binding to receptor proteins, predicted ADMET parameters (solubility, permeability, and absorbability), and considerations of structural diversity and feasibility of synthesis. Referring to the relevant literature, we developed a reasonable and feasible route to synthesize these compounds and assessed their anti-dengue virus activity. In the cytopathic effect assay on BHK-21 cells using the DENV2 NGC strain, both compounds SW-b and SW-d demonstrated comparable or superior activity against DENV2, with IC50 values of 3.58 ± 0.29 μM and 23.94 ± 1.00 μM, respectively, compared to that of compound 27 (IC50 = 19.67 ± 1.12 μM). Both SW-b and SW-d exhibited low toxicity levels, with CC50 values of 24.65 μM and 133.70 μM, respectively, resulting in selectivity indices of 6.89 and 5.58, respectively.
Structural analysis revealed that this increased inhibitory activity could be attributed to the strong π–π interaction between the benzene ring of their S1 fragments and W795, as well as enhancements in hydrogen bonding, hydrophobic interactions, electrostatic interactions, and van der Waals interactions. MD simulations confirmed the stable binding of SW-b and SW-d to NS5. These findings highlight the promising biological potential of SW-b and SW-d. However, further studies are required to evaluate their pharmacological and toxicity profiles in in vivo models. Our study provides valuable references and insights for the development of future drugs.
References
- Wang, W.H.; Urbina, A.N.; Chang, M.R.; Assavalapsakul, W.; Lu, P.L.; Chen, Y.H.; Wang, S.F. Dengue hemorrhagic fever—A systemic literature review of current perspectives on pathogenesis, prevention and control. J. Microbiol. Immunol. Infect. 2020, 53, 963–978. [Google Scholar] [CrossRef]
- Kuo, H.J.; Lee, I.K.; Liu, J.W. Analyses of clinical and laboratory characteristics of dengue adults at their hospital presentations based on the World Health Organization clinical-phase framework: Emphasizing risk of severe dengue in the elderly. J. Microbiol. Immunol. Infect. 2018, 51, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, J.; Simmons, C.P. The pathogenesis of dengue. Vaccine 2011, 29, 7221–7228. [Google Scholar] [CrossRef] [PubMed]
- Mousson, L.; Dauga, C.; Garrigues, T.; Schaffner, F.; Vazeille, M.; Failloux, A.B. Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) based on mitochondrial DNA variations. Genet. Res. 2005, 86, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, M.A.; Elnahary, E.K.; Elaiw, A.M. Stability of a secondary dengue viral infection model with multi-target cells. Alex. Eng. J. 2022, 61, 7075–7087. [Google Scholar] [CrossRef]
- Caillet-Saguy, C.; Lim, S.P.; Shi, P.Y. Polymerases of hepatitis C viruses and flaviviruses: Structural and mechanistic insights and drug development. Antivir. Res. 2014, 105, 8–16. [Google Scholar] [CrossRef]
- Koonin, E.V. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J. Gen. Virol. 1991, 72, 2197–2206. [Google Scholar] [CrossRef]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Yokokawa, F.; Nilar, S.; Noble, C.G.; Lim, S.P.; Rao, R.; Tania, S.; Wang, G.; Lee, G.; Hunziker, J.; Karuna, R.; et al. Discovery of potent non-nucleoside inhibitors of dengue viral RNA-dependent RNA polymerase from a fragment hit using structure-based drug design. J. Med. Chem. 2016, 59, 3935–3952. [Google Scholar] [CrossRef]
- Anoop, M.; Mathew, A.J.; Jayakumar, B.; Issac, A.; Nair, S.; Abraham, R.; Anupriya, M.G.; Sreekumar, E. Complete genome sequencing and evolutionary analysis of dengue virus serotype 1 isolates from an outbreak in Kerala, South India. Virus Genes 2012, 45, 1–13. [Google Scholar] [CrossRef]
- Qian, W.; Xue, J.X.; Xu, J.; Li, F.; Zhou, G.F.; Wang, F.; Luo, R.H.; Liu, J.; Zheng, Y.T.; Zhou, G.C. Design, synthesis, discovery and SAR of the fused tricyclic derivatives of indoline and imidazolidinone against DENV replication and infection. Bioorg. Chem. 2022, 120, 105639. [Google Scholar] [CrossRef] [PubMed]
- Songprakhon, P.; Thaingtamtanha, T.; Limjindaporn, T. Peptides targeting dengue viral nonstructural protein 1 inhibit dengue virus production. Sci. Rep. 2020, 10, 12933. [Google Scholar] [CrossRef]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Hj Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Tsai, Y.T.; Wang, S.F.; Wang, W.H.; Chen, Y.H. Dengue vaccine: An update. Expert Rev. Anti. Infect. Ther. 2021, 19, 1495–1502. [Google Scholar] [CrossRef]
- Wilder-Smith, A. Dengue vaccine development by the year 2020: Challenges and prospects. Curr. Opin. Virol. 2020, 43, 71–78. [Google Scholar] [CrossRef]
- Siriphanitchakorn, T.; Kini, R.M.; Ooi, E.E.; Choy, M.M. Revisiting dengue virus-mosquito interactions: Molecular insights into viral fitness. J. Gen. Virol. 2021, 102, 001693. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.K.; Garcia-Blanco, M.A.; Bradrick, S.S. Roles of pro-viral host factors in mosquito-borne flavivirus infections. Curr. Top. Microbiol. Immunol. 2018, 419, 43–67. [Google Scholar]
- Krishnan, M.N.; Garcia-Blanco, M.A. Targeting host factors to treat West Nile and dengue viral infections. Viruses 2014, 6, 683–708. [Google Scholar] [CrossRef]
- Egloff, M.P.; Decroly, E.; Malet, H.; Selisko, B.; Benarroch, D.; Ferron, F.; Canard, B. Structural and functional analysis of methylation and 5’-RNA sequence requirements of short capped RNAs by the methyltransferase domain of dengue virus NS5. J. Mol. Biol. 2007, 372, 723–736. [Google Scholar] [CrossRef]
- Issur, M.; Geiss, B.J.; Bougie, I.; Picard-Jean, F.; Despins, S.; Mayette, J.; Hobdey, S.E.; Bisaillon, M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA 2009, 15, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Acosta, E.G.; Kumar, A.; Bartenschlager, R. Revisiting dengue virus-host cell interaction: New insights into molecular and cellular virology. Adv. Virus Res. 2014, 88, 1–109. [Google Scholar] [PubMed]
- Selisko, B.; Wang, C.; Harris, E.; Canard, B. Regulation of Flavivirus RNA synthesis and replication. Curr. Opin. Virol. 2014, 9, 74–83. [Google Scholar] [CrossRef]
- Bollati, M.; Milani, M.; Mastrangelo, E.; Ricagno, S.; Tedeschi, G.; Nonnis, S.; Decroly, E.; Selisko, B.; De Lamballerie, X.; Coutard, B.; et al. Recognition of RNA Cap in the wesselsbron virus NS5 methyltransferase domain: Implications for RNA-capping mechanisms in flavivirus. J. Mol. Biol. 2009, 385, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Noble, C.G.; Shi, P.Y. The dengue virus NS5 protein as a target for drug discovery. Antivir. Res. 2015, 119, 57–67. [Google Scholar] [CrossRef]
- Koonin, E.V. Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and λ2 protein of reovirus. J. Gen. Virol. 1993, 74, 733–740. [Google Scholar] [CrossRef]
- Xu, H.T.; Colby-Germinario, S.P.; Hassounah, S.; Quashie, P.K.; Han, Y.; Oliveira, M.; Stranix, B.R.; Wain-berg, M.A. Identification of a pyridoxine-derived small-molecule inhibitor targeting dengue virus RNA-dependent RNA polymerase. Antimicrob Agents Chemother. 2015, 60, 600–608. [Google Scholar] [CrossRef]
- Cannalire, R.; Tarantino, D.; Astolfi, A.; Barreca, M.L.; Sabatini, S.; Massari, S.; Tabarrini, O.; Milani, M.; Querat, G.; Mastrangelo, E.; et al. Functionalized 2,1-benzothiazine 2,2-dioxides as new inhibitors of Dengue NS5 RNA-dependent RNA polymerase. Eur. J. Med. Chem. 2018, 143, 1667–1676. [Google Scholar] [CrossRef]
- Noble, C.G.; Lim, S.P.; Chen, Y.L.; Liew, C.W.; Yap, L.; Lescar, J.; Shi, P.Y. Conformational flexibility of the dengue virus RNA-dependent RNA polymerase revealed by a complex with an inhibitor. J. Virol. 2013, 87, 5291–5295. [Google Scholar] [CrossRef]
- Qadir, A.; Riaz, M.; Saeed, M.; Shahzad-Ul-Hussan, S. Potential targets for therapeutic intervention and structure based vaccine design against Zika virus. Eur. J. Med. Chem. 2018, 156, 444–460. [Google Scholar] [CrossRef]
- Manvar, D.; Kucukguzel, I.; Erensoy, G.; Tatar, E.; Deryabasogullari, G.; Reddy, H.; Talele, T.T.; Cevik, O.; Kaushik-Basu, N. Discovery of conjugated thiazolidinone-thiadiazole scaffold as anti-dengue virus polymerase inhibitors. Biochem. Biophys. Res. Commun. 2016, 469, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, S.; Velmurugan, D.; Gromiha, M.M. Identification of dengue viral RNA-dependent RNA polymerase inhibitor using computational fragment-based approaches and molecular dynamics study. J. Biomol. Struct. Dyn. 2016, 34, 1512–1532. [Google Scholar] [CrossRef]
- Lim, S.P.; Noble, C.G.; Seh, C.C.; Soh, T.S.; El Sahili, A.; Chan, G.K.; Lescar, J.; Arora, R.; Benson, T.; Nilar, S.; et al. Potent allosteric dengue virus NS5 polymerase inhibitors: Mechanism of action and resistance profiling. PLoS Pathog. 2016, 12, 1005737. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.L.; Xu, T.; Chen, Y.L.; Malet, H.; Egloff, M.P.; Canard, B.; Vasudevan, S.G.; Lescar, J. Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J. Virol. 2007, 81, 4753–4765. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Kalnmals, C.A. Stereoselective synthesis of exocyclic tetrasubstituted vinyl halides via ru-catalyzed halotropic cycloisomerization of 1,6-haloenynes. Org. Lett. 2017, 19, 2346–2349. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.; Tugny, C.; Salanouve, E.; Clavier, H.; Giordano, L.; Moraleda, D.; Gimbert, Y.; Mouriès-Mansuy, V.; Goddard, J.-P.; Fensterbank, L. Secondary phosphine oxide–gold(i) complexes and their first application in catalysis. Organometallics 2014, 33, 4051–4056. [Google Scholar] [CrossRef]
- Wucher, P.; Goldbach, V.; Mecking, S. Electronic influences in phosphinesulfonato palladium(II) polymerization catalysts. Organometallics 2013, 32, 4516–4522. [Google Scholar] [CrossRef]
- Alexandre, F.-R.; Brandt, G.; Caillet, C.; Chaves, D.; Convard, T.; Derock, M.; Gloux, D.; Griffon, Y.; Lallos, L.; Leroy, F.; et al. Synthesis and antiviral evaluation of a novel series of homoserine-based inhibitors of the hepatitis C virus NS3/4A serine protease. Bioorg. Med. Chem. Lett. 2015, 25, 3984–3991. [Google Scholar] [CrossRef]
- Nguyen, S.S.; Ferreira, A.J.; Long, Z.G.; Heiss, T.K.; Dorn, R.S.; Row, R.D.; Prescher, J.A. Butenolide synthesis from functionalized cyclopropenones. Org. Lett. 2019, 21, 8695–8699. [Google Scholar] [CrossRef]
- Xie, L.; Takeuchi, Y.; Cosentino, L.M.; Mcphail, A.T.; Lee, K.-H. Anti-AIDS agents. 42. synthesis and anti-HIV activity of disubstituted (3′R,4′R)-3′,4′-di-O-(S)-camphanoyl-(+)-cis-khellactone analogues. J. Med. Chem. 2001, 44, 664–671. [Google Scholar] [CrossRef]
- Mohsenzadeh, F.; Darabi, H.R.; Alivand, M.; Aghapoor, K.; Balavar, Y. Naturally occurring organic acids for organocatalytic synthesis of pyrroles via Paal–Knorr reaction. Res Chem. Intermed. 2020, 46, 5255–5262. [Google Scholar] [CrossRef]
- Gangjee, A.; Zhao, Y.; Raghavan, S.; Rohena, C.C.; Mooberry, S.L.; Hamel, E. Structure–activity relationship and in vitro and in vivo evaluation of the potent cytotoxic anti-microtubule agent N-(4-methoxyphenyl)-N,2,6-trimethyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-aminium chloride and its analogues as antitumor agents. J. Med. Chem. 2013, 56, 6829–6844. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Shi, Y.; Chen, J.; He, Z.; Xu, Z.; Zhao, Z.; Zhu, W.; Li, H.; Xu, Y.; Li, B.; et al. The discovery of new plant activators and scaffolds with potential induced systemic resistance: From jasmonic acid to pyrrolidone. MedChemComm 2016, 7, 1849–1857. [Google Scholar] [CrossRef]
- Mansoori, Y.; Atghia, S.V.; Sanaei, S.S.; Zamanloo, M.R.; Imanzadeh, G.; Eskandari, H. New, organo-soluble, thermally stable aromatic polyimides and poly(amide-imide) based on 2-[5-(3,5-dinitrophenyl)-1,3, 4-oxadiazole-2-yl]pyridine. Polym. Int. 2012, 61, 1213–1220. [Google Scholar] [CrossRef]
- Tang, L.; Ma, M.; Zhang, Q.; Luo, H.; Wang, T.; Chai, Y. Metal-free synthesis of pyrazoles from 1,3-diarylpropenes and hydrazines via multiple inter-/intramolecular C–H aminations. Adv. Synth. Catal. 2017, 359, 2610–2620. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Arunkumar, M.; Aravind, M.K.; Gayathri, S.; Rajkeerthana, S.; Mohankumar, V.; Ashok-kumar, B.; Varalakshmi, P. Probing marine brown macroalgal phlorotannins as antiviral candidate against SARS-CoV-2: Molecular docking and dynamics simulation approach. Mol. Divers. 2022, 26, 3205–3224. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, R.; Liu, F.; Zheng, T.; Wu, W. Molecular mechanism of interactions between inhibitory tripeptide GEF and angiotensin-converting enzyme in aqueous solutions by molecular dynamic simulations. J. Mol. Liq. 2018, 249, 389–396. [Google Scholar] [CrossRef]
- Medina, F.; Medina, J.F.; Colon, C.; Vergne, E.; Santiago, G.A.; Munoz-Jordan, J.L. Dengue virus: Isolation, propagation, quantification, and storage. Curr. Protoc. Microbiol. 2012, 27, 15D.2.1–15D.2.24. [Google Scholar] [CrossRef]
- Gong, E.Y.; Clynhens, M.; Ivens, T.; Lory, P.; Simmen, K.; Kraus, G. Cell-based antiviral assays for screening and profiling inhibitors against dengue virus. Methods Mol. Biol. 2013, 1030, 185–194. [Google Scholar]
- Farias, K.J.S.; Machado, P.R.L.; Da Fonseca, B.A.L. Chloroquine inhibits dengue virus type 2 replication in vero cells but not in C6/36 cells. Sci. World J. 2013, 2013, 282734. [Google Scholar] [CrossRef]

