1. Introduction
Oral medications are the preferred and widely accepted method of drug delivery due to their ease of administration, convenience for repeated and prolonged use, non-invasiveness, adaptability, scalability, and high patient compliance [1]. Nevertheless, certain patient groups, such as the elderly, children, individuals with Parkinson’s disease, and those recovering from anesthesia, often encounter challenges in swallowing or chewing solid dosage forms, particularly tablets and capsules [2]. In the United States, it is estimated that about 15 million people have dysphagia, and this represents about 4.6% of the population. Thus, extensive efforts have been undertaken to create innovative oral drug formulations that dissolve or disperse in the oral cavity, aiming to address the issue of swallowing difficulties [3]. Moreover, highly vascularized oral mucosa may increase permeability to many medications, thereby providing a rapid onset of action and increasing bioavailability, as reported elsewhere [4].
Oral disintegrating tablets (ODTs) are a type of solid oral dosage form designed to disintegrate rapidly within a matter of seconds when placed on the tongue without the necessity of water or chewing [5]. Oral thin films are a drug delivery system consisting of thin, flexible sheets that typically dissolve or disintegrate quickly, often within seconds, when placed in the mouth. They are intended to be placed either on the tongue or cheek and can be used to deliver a variety of medications, including over-the-counter (OTC) and prescription drugs [6,7].
While various names like thin strip, oral film, orally dissolving film, quick dissolve film, melt-away film, and wafer are employed to refer to the oral film dosage form, the European Medicines Agency officially designates it as an orodispersible film (ODF), or, as the United States Food and Drug Administration (U.S. FDA) commonly terms them, soluble films [8]. As per European Pharmacopeia (Ph. Eur.), ODFs are defined as sheets, either single or multilayered, composed of appropriate materials and are intended for rapid dispersion in the mouth. They rapidly disintegrate/dissolve in saliva to form a solution or suspension, thus enabling rapid absorption and delivery of the drug into the bloodstream or a rapid local effect. Moreover, ODFs offer rapid and consistent drug release, which can improve the bioavailability of some medications. The oral cavity is richly vascularized and has low enzymatic activity, which can potentially boost the bioavailability of drugs with low aqueous solubility. This route is advantageous for those drugs classified under the biopharmaceutical classification system (BCS) as Class II and Class IV. Rapid permeation across the mucosal lining of the oral cavity can circumvent acid hydrolysis in the stomach and initial hepatic metabolism. This pathway is particularly well-suited for potent medications, especially those designed for acute conditions, where they have an immediate therapeutic effect, mainly due to oromucosal and pregastric absorption, as well as direct access to the jugular vein [4]. Nevertheless, certain compounds are absorbed exclusively in the gastrointestinal tract after ingestion.
This review aims to comprehensively explore the current innovations and emerging trends of ODFs. It focuses on their design, formulation components, and manufacturing methods for potential applications in oral drug delivery. The objective is to provide a thorough understanding of ODF versatility, the recent advancements in polymers and plasticizers, and the integration of novel techniques like 3D printing. Additionally, the review seeks to emphasize the impact of incorporating nanoparticles in ODF formulations for enhanced oral drug delivery efficiency. This review adopts a systematic approach, conducting comprehensive literature searches on databases such as PubMed, Scopus, Web of Science, clinical trial databases, and patent databases. It focuses on keywords like ‘orodispersible film’, ‘polymers’, ‘manufacturing methods’, ‘3D printing’, ‘clinical trials’, ‘patents’, and ‘evaluation’. The inclusion and exclusion criteria ensure the selection of reliable studies and patents. Structured data extraction, critical appraisal, and synthesis of information are employed to present a cohesive narrative on the present advancements and evolving trends in orodispersible films while considering database-specific functionalities and intricacies.
The ODF, or strip, typically employs a hydrophilic polymer, preferably with mucoadhesive properties, and aims to achieve rapid disintegration within a short period. The primary driving factors responsible for the rapid drug release of ODFs, as anticipated, are the hydration and subsequent swelling of polymers due to water diffusion [9]. The primary objective of the majority of ODFs is to quickly dissolve or disintegrate in the oral cavity, forming a solution or suspension, which is then swallowed for absorption in the gastrointestinal tract [10].
The primary constraints frequently encountered with oral films pertain to their vulnerability in high-humidity environments and their limited capacity to accommodate a substantial drug dosage. illustrates a comparison between the advantages and drawbacks of orodispersible film drug delivery systems. The limited formulation size restricts the inclusion of additives for taste masking the drug, potentially impacting patient compliance.
2. Formulation Components
The components of oral films can differ depending on the particular formulation and intended purpose. However, there are common constituents typically found in oral film formulations, such as active pharmaceutical ingredients (APIs), polymers, plasticizers, sweetening agents, flavoring agents, coloring agents, salivary stimulating agents, stabilizers, surfactants, and solvents. In the context of pharmaceutical ODF dosage form development, critical quality attributes (CQAs) comprise the physical, chemical, biological, or microbiological properties and characteristics that must fall within specified limits to ensure the desired quality of the product. These attributes can be influenced by essential material qualities, including the quality of the API and the physicochemical properties of excipients, as well as critical process parameters such as the order of adding raw materials, the sequence of adding solutions, and the temperature of water [11]. illustrates a generic critical quality attribute for an orodispersible film.
2.1. APIs
APIs in ODFs can vary depending on the intended therapeutic purpose, and the selection depends on the drug’s solubility, stability, and desired pharmacokinetic profile. Currently, ODFs have been investigated with drugs belonging to different therapeutic categories, including anti-allergics and antihistamines, antibiotics, anti-inflammatories and analgesics, antidiarrheals, antiemetics, antidepressants, antipsychotics, anticonvulsants, cardiovascular medications, neurological medications, vitamins, and nutritional supplementation [12]. APIs are typically incorporated (5% w/w to 30% w/w) either as a solid dispersion, nanosuspension, micronized powder, or nanocrystals within the film matrix.
ODFs predominantly consist of BCS class I drugs, chosen primarily for their high solubility and permeability characteristics, although drugs from other classes are also integrated. The weight of the ODF, being less than 200 mg, limits the formulation space for the API, restricting its application to potent active ingredients. Notably, a higher molecular weight film former tends to yield a greater sample, dependent on its concentration. Due to the limited space within the oral cavity, ODFs with a size of 2 × 2 cm2 and a maximum thickness of 100 µm are considered acceptable [13]. During the polymer mixing process, several issues, such as the entrapment of air bubbles and the use of inappropriate casting solution viscosities, can affect the drug’s stability and dose uniformity. Moreover, the utilization of organic solvents during the process and the existence of residual solvents in the dried sample can potentially lead to toxicity issues and raise regulatory concerns [8].
2.2. Polymers
Oral films primarily comprise a polymer matrix, which provides film-forming properties with structural integrity. A range of polymers, including but not limited to starch, modified starches, hydroxypropyl methylcellulose (HPMC) (including hypromellose variants E3, E5, and E15), sodium carboxymethyl cellulose (NaCMC), gelatin, hydroxypropyl cellulose (HPC), hydroxyethyl cellulose (HEC), pectin, carboxymethyl cellulose (CMC), pullulan, locust bean gum, xanthan gum, guar gum, carrageenan, povidone polymers (polyvinylpyrrolidone, PVP), polyvinyl alcohol (PVA), polyethylene oxide (PEO), maltodextrins (MDXs), and various others have been studied as potential base materials for producing ODFs [8].
The film-forming polymer, which serves as the major component of the ODF, constitutes up to 65% of the weight based on the total dry weight of the film [14]. In some cases, a combination of polymers is employed to enhance the hydrophilicity, flexibility, mouthfeel attributes, and solubility of ODFs. These polymers should be non-toxic, non-irritating, free from leachable impurities, possess excellent wetting and spreadability, have a good shelf life, and should not promote secondary infections in the oral mucosa or dental regions. Additionally, the ODFs should have adequate peel, shear, and tensile strength. Among water-soluble polymers, gelatin, and hypromellose are the most commonly used to prepare oral strips. shows a comprehensive overview of the frequently used film-forming polymers, plasticizers, APIs, preparation methods, and the key highlights related to ODFs. The acceptability of ODFs depends upon film composition and the formation process, which affects disintegration, taste, texture, and mouthfeel attributes. The films produced should be transparent and free of air bubbles for aesthetic appeal and stability considerations.
2.3. Plasticizers
Plasticizers are low-molecular-weight additives added to a polymer solution to promote plasticity and flexibility. Plasticizers lower the glass-transition temperature of the polymers from a hard, glassy material to a soft, rubbery material. It is important to highlight that the moisture absorption of various plasticizers plays a crucial role in influencing various film characteristics [76]. Lipid plasticizers, such as fatty acids and their derivatives, lecithin, oils, and waxes, can be used to decrease the water vapor permeability of films due to their non-polar or hydrophobic nature [77]. However, the inclusion of lipids can introduce gloss and improve the visual appeal, yet it might impact the film’s cohesiveness and structural integrity, including a potential reduction in film strength.
Polysaccharide-based films possess a degree of rigidity, which necessitates the use of plasticizers to enhance their pliability for easier handling. Among the different plasticizers, glycerine can be considered an effective plasticizer, as seen with starch, methylcellulose, highly carboxymethylated starch, and HPMC films [78]. At equivalent concentration levels, hydrophilic plasticizers with smaller molecular weights, such as propylene glycol and glycerine, resulted in higher water vapor permeability compared to larger molecular weight plasticizers like PEG 400 in methylcellulose films. The effect of different plasticizers (glycerol, vitamin E TPGS, and triacetin) and their concentrations on the physicomechanical properties of pullulan-based oral films was disclosed [79]. Typically, the flexibility of protein-based films such as casein, gelatin, collagen, zein, and soy protein are commonly enhanced with plasticizers like glycerine, propylene glycol, sorbitol, PEG, sucrose, and oleic acid [80]. Apart from the cost, when choosing a plasticizer, it is essential to take into account three fundamental factors: compatibility, effectiveness, and durability.
The addition of plasticizers at a concentration ranging from 0–20% by weight of the dry polymer is employed to prevent undesirable physicomechanical and thermal properties of the polymeric films [79]. Hydrophilic cellulose-based polymers can be effectively combined with plasticizers containing polar hydroxyl groups, such as PEG, propylene glycol, glycerine, and polyols. In contrast, less hydrophilic cellulosic polymers can be plasticized with esters of citric acid and phthalic acid. Glycerine is frequently utilized to plasticize PVA due to its small molecular weight, low volatility, and compatibility with the polymer matrix [81]. Hydrophilic compounds, such as polyols (glycerine and sorbitol), are commonly used in starch films [82]. It is important to consider the characteristics of plasticizers to lower the glass-transition temperature of polymers to between 40 and 60 °C for non-aqueous solvent systems and below 75 °C for water-based solvent systems. Diethylene glycol can be used as a plasticizer for both HPMC- and PVA-based polymer films [83]. It was reported that the water absorption of Eudragit films is influenced by the specific Eudragit polymers and plasticizers employed. For instance, Eudragit E films, plasticized with diethyl phthalate, dibutyl phthalate, and tributyl citrate, exhibited a higher level of water absorption compared to those plasticized with triacetin [84]. A study examining the impact of varying PEG molecular weights and concentrations on the mechanical and thermomechanical attributes of free HPMC films revealed that the inclusion of a plasticizer led to a reduction in both of these characteristics [85].
The nature and quantity of plasticizers can indeed affect drug release by reducing the secondary bonding between polymer chains, thereby improving the mobility of the drug. Small amounts of plasticizer may make the polymer more rigid instead of making it softer, which is referred to as antiplasticization. This phenomenon can be leveraged as a formulation technique to control the permeability of drugs in the polymeric systems used in pharmaceuticals [86]. The release of the drug from a polymer system with plasticizers primarily depends on the physicochemical characteristics, specifically the solubility parameters of the plasticizer and the extent of plasticizer leaching. Plasticizers with lipophilic properties, such as dibutyl sebacate, typically remain within the polymeric system, resulting in the formation of robust and mechanically resilient coatings as the drug is released. Nonetheless, hydrophilic plasticizers have a tendency to wash out, which can result in reduced mechanical strength, potential cracking, or an increase in pore formation.
2.4. Sweetening and Flavoring Agents
Various techniques are utilized to mask the bitter or unpalatable taste of drugs in oral thin films. These methods include the utilization of sweetening agents, ion exchange resins, microparticles, inclusion complexes, and nanocarriers [87]. In recent times, lipids have garnered significant attention from researchers for their role in taste masking [88]. Various techniques, such as hot-melt extrusion (HMA), melt granulation, spray drying/congealing, and emulsification, can harness the taste-masking properties by utilizing lipids effectively. Sweeteners are generally added to mask the unpleasant taste and odors of APIs or excipients, improving palatability and patient acceptability. Sweeteners used in ODFs can be mainly classified into two types: natural sweeteners and artificial sweeteners. Glycyrrhizinic acid, derived from the root and rhizome extracts of licorice (Glycyrrhiza glabra), is a triterpenoid saponin widely utilized as a natural sweetener, which has been reported to be at least 30 times greater than sucrose [89]. Rebiana, a natural sweetener derived from the plant Stevia, possesses sweetness more than 200–300 times that of sucrose [90]. Despite artificial sweeteners being significantly sweeter than natural sugars, they are often associated with an aftertaste effect that can be alleviated by combining natural and artificial sweeteners. Typically, sweeteners are employed in concentrations of 3 to 6% w/w, either individually or in combination. Mannitol, a polyhydric alcohol, is extensively utilized as a sugar alcohol sweetener in ODFs due to its ability to enhance the physical integrity of the dosage form [91]. Additionally, mannitol offers exceptional stability and compatibility with APIs and has a non-hygroscopic nature, which is advantageous for ensuring the long-term integrity and stability of the dosage form. Sweeteners from significant sources, including sucrose, dextrose, fructose, glucose, liquid glucose, and maltose, are limited in their application within the diabetic community due to their substantial caloric content [92].Combining polyhydric alcohols like sorbitol, mannitol, isomalt, and maltitol can offer a pleasant mouthfeel and a refreshing cooling sensation. Aspartame is a dipeptide that provides a sugar-like taste at low concentrations and is nearly 200 times sweeter than sucrose. Aspartame has high stability, good compatibility with other excipients, and low caloric value, making it a preferred choice for low-calorie or sugar-free formulations [93]. Even though aspartame is used in the food and pharmaceutical sectors, a thorough understanding of its pros and cons is essential, particularly concerning potential health risks in children [94].
Sucralose, a chlorinated derivative of sucrose, exhibits excellent stability and compatibility with other ingredients over a wide pH range and is recognized for its absence of adverse effects on dental health. With approximately 200 times the sweetness of sucrose, acesulfame potassium provides a quick onset of sweet taste. Notably heat stable, pH stable, and non-reactive in Maillard reactions, acesulfame potassium proves suitable for diverse formulation and manufacturing processes. These sweetening agents, along with appropriate flavoring agents, play a critical role in improving the taste, palatability, and patient compliance of ODFs and ODTs. The selection of a sweetener depends on factors such as the taste profile, stability, compatibility, manufacturing process, and the target patient population.
The extensive contact between oral films and the oral mucosa underscores the significance of directing a part of the development efforts toward creating a pleasing and palatable formulation. Perception of the flavors depends on the ethnicity, age, and liking of the people [95]. In ODF formulations, it is common to add flavors at a concentration of up to 10% w/w. To enhance the flavor intensity and improve the mouthfeel attributes, cooling agents can also be introduced. Flavoring agents play a crucial role in enhancing the palatability and overall sensory experience of ODFs. The selection and incorporation of appropriate flavoring agents are essential to mask any unpleasant taste or medicinal bitterness that may be associated with the API or other excipients in the film. Flavoring agents not only improve the taste but also contribute to patient compliance, especially in pediatric and geriatric populations.
2.5. Saliva-Stimulating Agent
Saliva-stimulating agents, also known as sialagogues, are substances that can increase saliva production in the mouth. They can be beneficial for individuals with conditions such as dry mouth (xerostomia) or for those who may have difficulty producing adequate saliva [14]. The inclusion of these agents can expedite the disintegration process, leading to the rapid dissolution of ODF within a short time. Frequently employed agents for stimulating salivation in rapidly dissolving strip formulations encompass acids like citric acid, malic acid, lactic acid, ascorbic acid, and tartaric acid. These substances are usually included individually or in combination, with concentrations typically falling in the range of 3 to 5% by weight relative to the film. Furthermore, xylitol, a type of sweetener, has been proven to possess saliva-stimulating properties, as evidenced by studies conducted on chewing gum [96].
2.6. Coloring Agents
Coloring agents are often added to ODFs to enhance their appearance or differentiate between different formulations. Specific coloring agents can vary depending on the regulatory requirements, formulation considerations, and desired aesthetics. Commonly used coloring agents in ODFs include pigments such as titanium dioxide to achieve a lighter or opaque appearance in the film and iron oxides to provide shades of red, yellow, or brown in the film. Moreover, FD and C-approved coloring agents, such as indigo carmine and brilliant blue dyes, are used to provide a blue color, fast green FCF (for coloring food) to create green, and tartrazine dye is often used to achieve a yellow color in the film [58]. Agents should be incorporated into the ODF formulation at a maximum concentration of 1% by weight, specifically when certain ingredients or medications within the formulation are insoluble or in suspension [97]. It is important to note that the use of coloring agents in pharmaceutical products is subject to regulations and guidelines set by regulatory authorities, such as the U.S. FDA or the European Medicines Agency. These agencies provide specifications and limitations on the use of coloring agents to ensure safety and suitability for oral consumption.
2.7. Stabilizing Agents
These are essential components in ODFs to maintain their structural integrity, prevent degradation, and ensure the desired properties of the film [49]. These agents help in preserving the film’s texture, flexibility, and ability to dissolve quickly. Several stabilizing agents are commonly used in ODF formulations, including HPC, PVP, xanthan gum, sodium alginate, locust bean gum, carrageenan, gelatin, sorbitol, and surfactant [98]. Typically, these agents enhance the viscosity and uniformity of the dispersion, collaborating with other excipients and active ingredients to establish a unified and enduring ODF dosage form. In order to enhance the film properties, such as spreadability and dispersibility, generally, minor quantities of surfactants and emulsifying agents are included. The specific choice and combination of stabilizing agents depend on the desired characteristics of the film and the specific requirements of the formulation.
3. Manufacturing Methods
The primary methods utilized to prepare ODFs include the solvent casting technique, electrospinning, hot-melt extrusion (HME), and the emerging technique of 3D printing (3DP) for personalized dosing [99]. When developing a film formulation and its processes, it is beneficial to consider the drug product CQAs that align with the quality target profile attributes.
3.1. Solvent Casting
At present, solvent casting has emerged as the widely preferred technique for practical, feasible manufacturing and the scaling up of oral films [100]. On a small scale, for the preparation of ODFs in a laboratory setting, polymer(s) and other necessary ingredients are dissolved in a suitable solvent to form a homogeneous solution. The solution is then cast onto a substrate, and the solvent is allowed to evaporate, leaving behind a thin film. The manufacturing process of ODFs on an industrial scale using the solvent casting technique consists of multiple steps, starting with the precise dispensing of the drug, excipients, and non-toxic FDA-approved class III solvents, followed by their homogeneous dispersion in a low-shear or high-shear mixer under thermostatic control [4]. Nonetheless, it is not recommended to utilize high-shear mixers when dealing with encapsulated drug actives, as this method can lead to the removal of the encapsulating material. The slurry undergoes solvent evaporation within a hot-air oven set at a designated temperature. Subsequently, it is applied onto a meticulously chosen liner using a knife-over-roll coater equipped with a precise pin gauge. The resulting dried laminates are rolled up into master rolls and subsequently cut into individual dose units based on the desired dimensions of the ODF. These single-dose units are enclosed within pouches or sachets using packaging and sealing machinery. The solvent casting method used to prepare ODFs is similar to the manufacturing process of buccal film, as depicted in Figure 1. The child-resistant and tamper-proof packaging material is designed to protect against adverse environmental conditions and ensure ease of use. It is of utmost importance to determine the weight of each film unit being packaged, as the drug dosage in the ODF is directly dependent on its weight. One of the key advantages of this dosage form is its flexibility in generating multiple dose units simply by adjusting the size of the film. Various factors, such as the solvent evaporation rate, airflow velocity of the hot air, pin gauge dimensions, and conveyor belt speed, influence the formation of the cast film. Scaling up the production of ODFs using the solvent casting technique poses several challenges. Equipment adaptation is often necessary, and the transition to larger-scale equipment must preserve the desired film characteristics. Maintaining uniformity and homogeneity of the film on a larger scale is crucial, as variabilities in the mixing and casting processes can lead to inconsistencies. Ensuring the integrity of the film, and avoiding defects such as cracking or uneven thickness is a challenge that grows with the scale of production. Implementing effective quality control measures becomes more intricate, demanding rigorous batch-to-batch consistency and adherence to regulatory standards. The increased scale also introduces considerations of elevated costs related to equipment, materials, and energy consumption. Environmental factors like temperature and humidity become more pronounced in larger-scale production, affecting the drying process and the overall quality of the films. Addressing these challenges requires meticulous planning, optimization of processes, and a commitment to maintaining high-quality standards throughout the scaled-up production of ODFs.
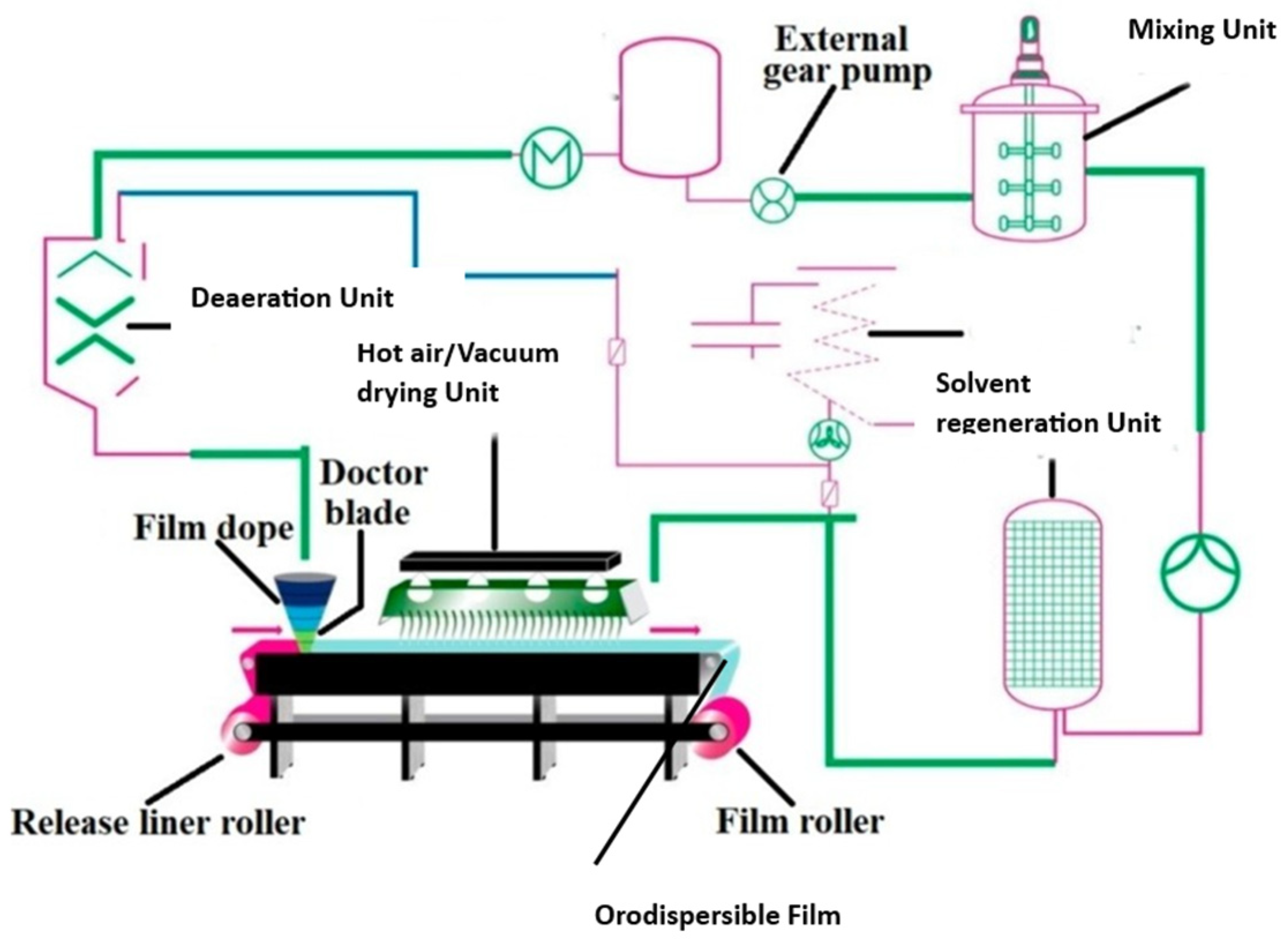
Figure 1. Schematic diagram displaying the typical solvent casting processes involved in orodispersible film manufacture (adapted from [4], published by MDPI, 2021).
Quetiapine fumarate ODF production, at a pilot scale, has been executed through the solvent casting method, following the principles of quality by design [101]. The optimized films were prepared by employing HPMC E5 as the film former and propylene glycol as the plasticizer at a temperature range of 65–70 °C.
The viscoelastic characteristics of the casting solution or dispersion significantly influence the film’s characteristics, such as content uniformity, thickness, morphology, and drug release. To overcome the typical problem of poor drug loading, investigators have developed a porous ODF using HPMC polymer via the solvent casting method [10]. A proposed solution for addressing the issue of content uniformity is the introduction of a new unit-dose plate that can be filled with the required volume of a constituent mixture [102]. An alternative method to ensure the content’s uniformity is by using doctor-blade film coaters, wherein a slurry is uniformly applied to a substrate through a metering blade to achieve the desired thickness [100]. To ensure the uniformity of the dispersion, the rheological properties and solids’ content are estimated, and in-process sterility testing is conducted to identify any potential bioburden. Various researchers have observed that the mechanical properties of the films, such as folding endurance, tensile strength, toughness, puncture strength, and film thickness, were significantly impacted by the formulation compositions [70,75]. For instance, different domperidone ODFs prepared using PVPK-90 were discovered to significantly influence the physical properties, mechanical properties, film thickness, and drug release rate of the resulting films [103]. The formation of the cast film on the selected liner is influenced by several variables, which encompass the rate of solvent evaporation, air flow velocity, positioning of the heat source, pin gauge dimensions, and conveyor belt speed [104]. The characteristics of the intermediate liner, such as the contact angles and surface tensions, can influence the film’s quality. Investigations conducted on hydrochlorothiazide ODFs prepared with HPC or HPMC as film-forming agents have demonstrated remarkable influences of the production process on the film’s attributes [105]. The film can be manufactured similarly as a transdermal patch—as a large sheet that can be cut into specific dimensions consisting of individual dosage units and packaged in pharmaceutically acceptable packaging materials. Such a formulation advancement would enable users to readily recognize their medication, thereby enhancing safety and adherence. Lately, researchers have demonstrated that the inclusion of nanocrystal dispersions or microparticles in ODFs can lead to improved drug dissolution rates [106] or enable extended drug delivery [107]. Nevertheless, it is important to consider that the inclusion of such particles may potentially impact the mechanical characteristics of the film.
3.2. Three-Dimensional Printing (3DP) Method
The utilization of additive manufacturing techniques such as 3DP has significantly reduced the preparation time while improving the mechanical characteristics of films. Numerous 3DP methods, including the fused deposition method (FDM), HME, and print-fill, have been extensively studied, with thermoplastic polymers serving as the primary components [108]. Among these methods, FDM is particularly well-suited for special populations requiring customized dosing or controlled release kinetics. The feasibility of using HME 3DP for preparing ODFs with MDX was investigated [108]. To determine the printable design space for individualized formulations, the impact of the critical formulation and process variables were evaluated. These personalized films have the potential to be employed in a clinical setting, maximizing therapeutic efficacy and minimizing adverse drug reactions. In 2015, the U.S. FDA approved SPRITAM® (levetiracetam) (Langhorne, PA, USA), marking a milestone as the first ODT formulation with a porous structure manufactured through 3D printing using the Zip®Dose technology. Because 3D printing can produce dosage form designs with performance characteristics difficult to create with conventional methods, personalized ODFs also have the potential to improve drug efficacy and/or reduce adverse events, which should lead to improved patient outcomes [109,110]. As part of a study, a thermal inkjet printer designed for commercial use was adapted to print personalized ODFs that incorporated varying doses (1.25 and 3 mg) of warfarin. The fabrication of the film involved the use of HPMC (20% w/w) and glycerol (3% w/w) as its constituent materials [111]. To address the challenge of taste masking in ODFs, the production of multilayered films with separate taste-masking layers using FDM 3DP has been outlined [111]. Filaments were created by blending PEO and PVA with ibuprofen and acetaminophen as model drugs, each at designated temperature ranges. In contrast to the 3DP methods previously documented, the oral adhesive films fabricated through FDM exhibited superior structural integrity and content uniformity. The FDM technique faces a significant limitation due to its elevated processing temperature, which greatly restricts its practical utility for thermolabile pharmaceuticals. It was proposed that a combination of FDM and inkjet printing can be efficiently utilized to incorporate thermolabile drugs [112]. The utilization of extrusion 3DP, which directly extrudes and prints semisolid materials like gels or pastes (Figure 2), offers a solution to overcome the limitation of the FDM process, specifically the need for material filaments [113].
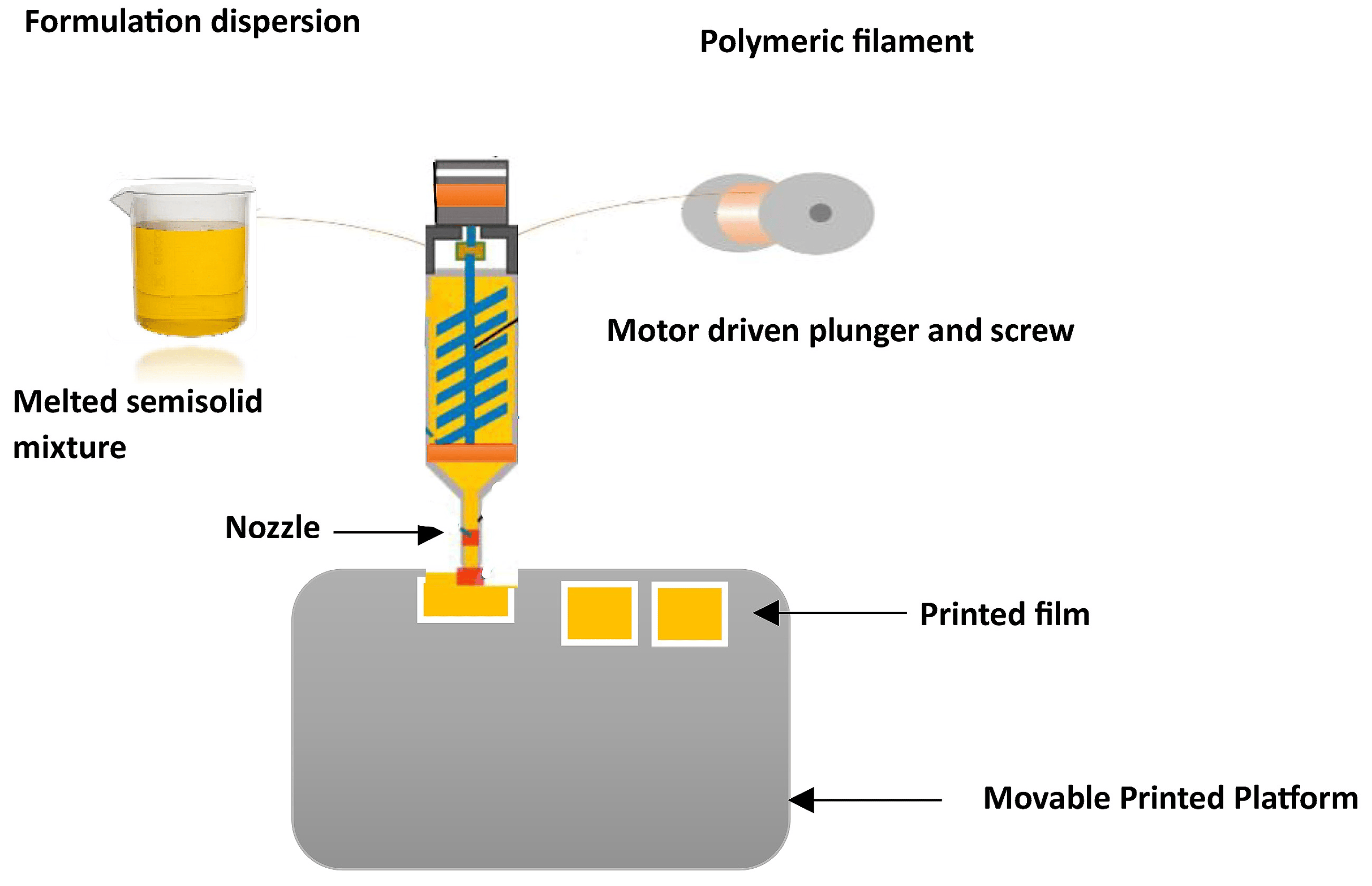
Figure 2. Illustration depicting ODF 3D printing based on the semisolid extrusion process.
3.3. Hot-Melt Extrusion (HME)
HME technology is a solvent-free, single-step continuous manufacturing process commonly employed to improve the water solubility of hydrophobic drugs through the formation of solid dispersions comprising both drugs and carrier materials (Figure 3). HME offers a promising and practical alternative to solvent casting, as it minimizes solvent usage and addresses issues associated with mixing and drying processes. An innovative approach combining the HME technique and solvent casting method has been utilized to develop ODFs incorporating micropellets, enabling extended drug release [107]. Manufacturing of a non-sticky, transparent, and uniform film using a hot-melt extrude equipped with a standard screw configuration has been described. The polymer matrix used for the melt extrusion process was comprised of modified starch with glycerol in addition to sweetening and saliva-simulating agents. The film formulations demonstrated fast disintegration (6 to 11 s) and demonstrated over 95% dissolution within 5 min [27]. In a recent investigation, HME was employed to achieve the continuous production of ODFs containing a substantial dosage of nimesulide, classified as a BCS II compound [114]. A mechanically robust film was successfully formulated, exhibiting an exceptional drug-loading percentage of up to 21.73% w/w and showcasing an optimal drug-release profile, further highlighting its effectiveness. The use of HME technology effectively improved the taste and enhanced the aqueous solubility of the drug in the development of mefenamic acid-loaded ODTs. This approach has the potential to be applied to the development of ODFs embedded with taste masking of bitter APIs, especially those belonging to BCS class II and class IV drugs [115].
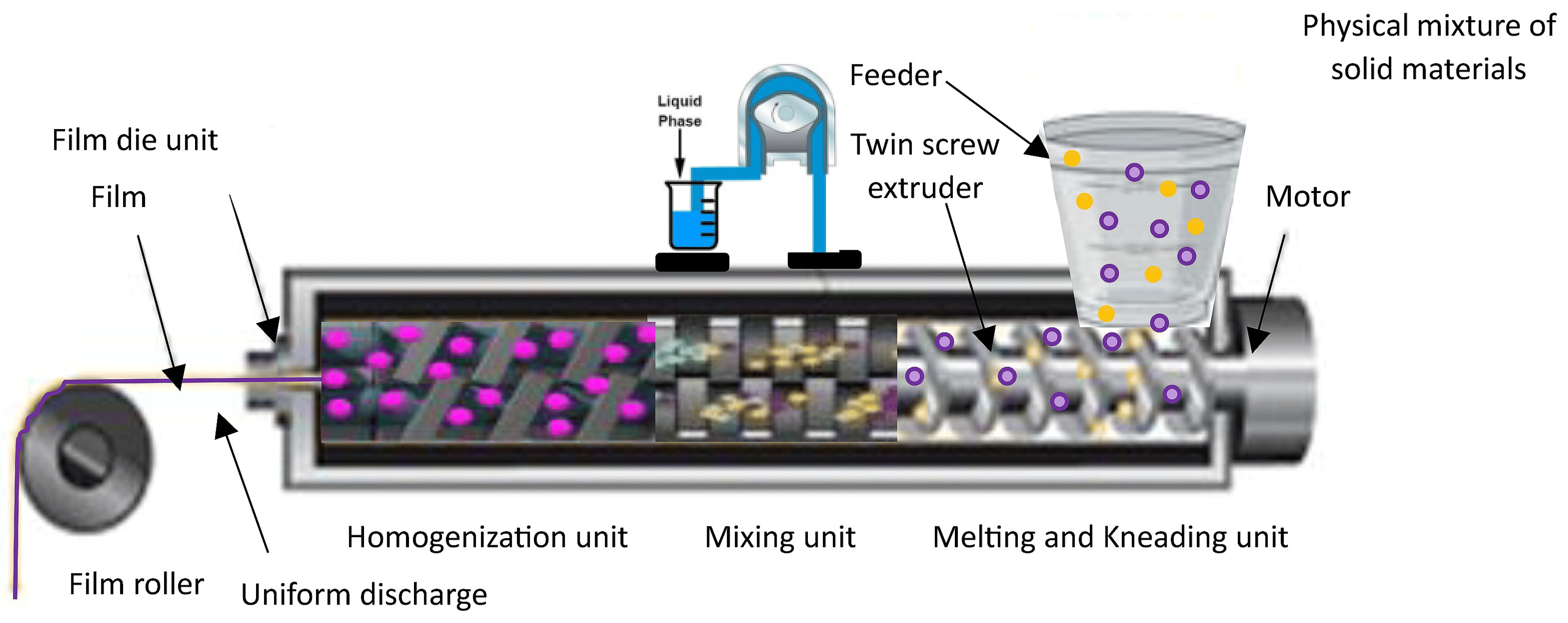
Figure 3. Manufacture of ODF utilizing hot-melt extrusion technique.
ODFs containing acetaminophen have been produced using Klucel™, HPC E5, and Soluplus® as carrier materials. The advanced physicochemical analyses, including Raman mapping, revealed the existence of drugs in an amorphous state in both the HME and 3D-printed films. Nevertheless, a relatively consistent distribution of amorphous drugs was observed in the 3D-printed films in comparison to the films fabricated using other techniques [116]. The findings indicate that combining the HME and 3DP methods holds significant promise for improving the physical attributes of formulations and producing ODFs with favorable features, such as rapid drug dissolution rates.
An extended drug-release film created by incorporating matrix drug-loaded particles has been reported [117]. These microparticles were fabricated by employing HME followed by milling, with diclofenac used as the model drug and Eudragit® RS (Essen, Germany) serving as the matrix former. The ODF was subsequently made by uniformly dispersing the microparticles in the ODF-forming polymer, which consisted of 15% HPMC and 6% glycerol as the plasticizer. Although the process faced challenges related to sedimentation and agglomeration, the incorporation of microparticles with a size of up to 500 µm resulted in ODFs with even drug distribution and satisfactory physical and mechanical properties.
3.4. Electrostatic Spray Deposition
The electrostatic spray process, also known as electrostatic spray deposition (Figure 4), applies a charged coating material, typically in the form of powders or liquid droplets or film, onto a charged surface using an electrostatically charged spray [2]. The electrostatic attraction between the charged particles and the surface causes them to adhere and form a uniform coating. This technique is commonly used in various industries for applications such as painting, powder coating, and surface treatment. In dry powder coating, the process of film formation occurs through the merging and smoothing out of individual powder particles, facilitated by the application of heat. It is advantageous to use thermoplastic polymers or pre-plasticized coating compositions with low glass-transition temperatures, ideally falling within a temperature range between 40 and 60 °C. This allows for film curing to take place at relatively low temperatures, which is beneficial when dealing with thermally sensitive drugs [118]. The extent of plasticization or reduction in glass-transition temperature significantly influences the particles’ ability to coalesce during the film formation process.
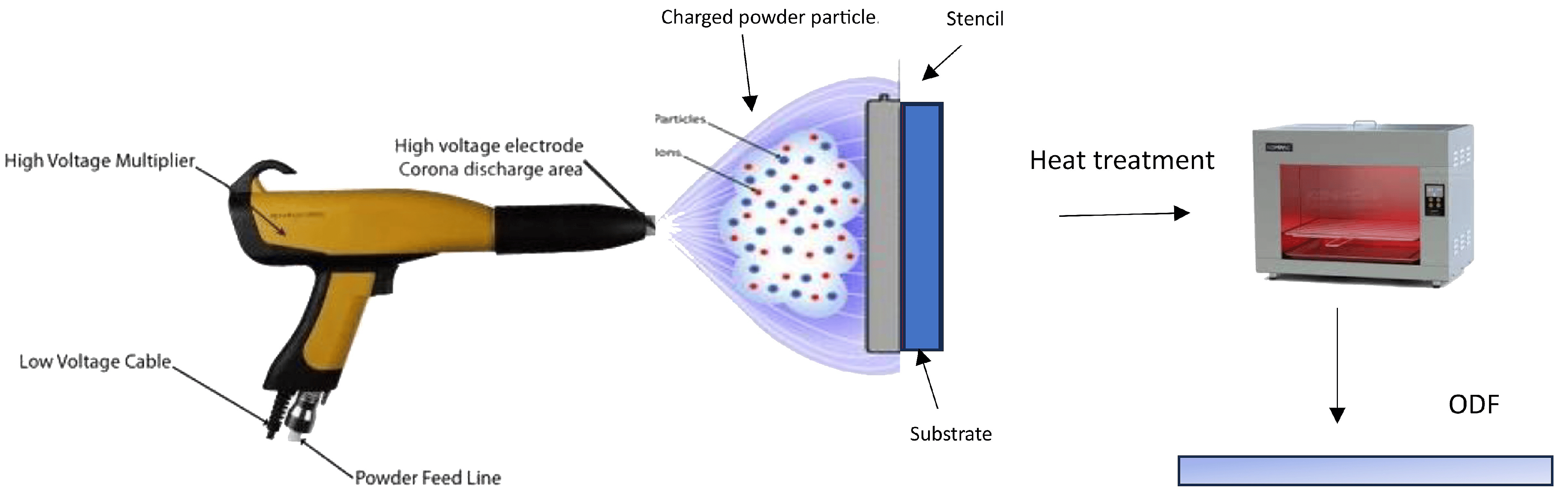
Figure 4. ODF fabrication based on electrospray powder deposition process.
A technique known as electrostatic powder deposition has been developed to create fast-dissolving PEO films for drug delivery, as detailed in the literature [119]. These films were prepared in various ways, including through a physical mixture of PEO and acetaminophen, as well as co-processed PEO and drug particles. The active films exhibited an average drug content of 97%, whereas the physical mixture of powders displayed more variability, with a relative standard deviation of 11.9%, compared to 1.8% in the films prepared using co-processed particles. Mechanical testing of the prepared films revealed that the active films could stretch up to 15%, in contrast to the Listerine® (New Brunswick, NJ, USA) strips at 1.6% and PEO films at 3.8%, mainly due to the plasticizing influence of the drug on PEO in the drug-containing films. Both active films achieved more than 85% drug release within a short time (2 min), indicating the potential of electrostatic powder deposition for creating free films for drug delivery. Moreover, the study highlighted that the efficiency of powder deposition through spraying was significantly influenced by various processing factors, such as the charging voltage, the distance between the spray gun tip and the substrate, and environmental humidity, as reported [120]. Additionally, for effective powder deposition, the sprayed powders must possess optimal electrical conductivity, which can be improved by increasing the environmental humidity. Overall, the full potential of this technique is not fully explored and needs further investigation.
3.5. Electrospinning Method
The electrospinning method involves the transformation of polymer solution droplets from a spherical shape to a conical shape, resulting in the production of nanosized fiber filaments (Figure 5). Electrospun nanofibers have attracted considerable interest because of their distinctive characteristics and potential applications. These nanofibers feature a high surface area-to-volume ratio and impressive mechanical attributes and can be customized to exhibit specific functionalities, including controlled release, filtration, tissue engineering, and applications related to energy storage [121]. Optimizing the diameter and structure of the resulting fibers can be achieved by manipulating different factors, including the polymer concentration, solution viscosity, electric field intensity, and spinning distance. Electrospun oral films offer versatile applications, including tailored solutions for addressing specific oral conditions like periodontitis or mucositis, as well as serving as effective drug delivery platforms for systemic treatments. Electrospinning offers versatility in the design and fabrication of oral films, allowing for customization of their properties and characteristics [122]. This enables the development of personalized medicine approaches or targeted therapies tailored to specific patient needs. Furthermore, oral films composed of nanofibers demonstrate impressive mechanical robustness and flexibility, all the while enabling the precise and controlled release of medications at a predetermined rate [123]. According to the report, the hyperbranched nanofiber formulations displayed remarkable mechanical durability and effective drug delivery properties. These findings indicate that the hyperbranched cellulose nanofiber holds significant promise as a feasible alternative to commercially accessible dosage forms [124]. Sequential electrospinning was employed to successfully fabricate a multilayer film, with the exterior layers comprising ethyl cellulose nanofibers, while the inner layer contained gelatin nanofibers loaded with curcumin [125]. Such a strategy could be adopted to develop ODFs with incompatible drugs as a combination dosage form. A study compared electrospun orally disintegrating films with conventional casting films for the formulation of rizatriptan using PVP and PVA as polymers [126]. Electron microscopy imaging revealed that the electrospun-based films exhibited a nanoporous structure, whereas the casted films did not display any discernible pores. In both film types, the drug was evenly distributed, with no evident interactions between the drug and the polymers. Furthermore, the electrospun-based films demonstrated increased bioavailability and a faster Tmax in comparison with the marketed films and tablets. This investigation inferred that electrospun-based films outperformed the conventional casted films in terms of their in vivo pharmacological efficacy, potentially due to the nanostructure achieved via the electrospinning approach. Ensuring appropriate dosage forms for children is crucial for promoting treatment adherence and effective pharmacotherapy, particularly in low-resource settings where long-term treatment is required. A study has been conducted for the development of an isoniazid-loaded ODF based on an electrospinning technique utilizing a combination of natural and synthetic polymers [127]. The ODFs consisted of nanofibers with satisfactory thermal stability and potential drug amorphization. Rapid disintegration (<15 s) and rapid drug release of isoniazid in less than 60 s were noticed. The developed ODFs exhibited several features that are valuable for pediatric patients, including ease of administration, favorable drug loading, and rapid drug-release properties.
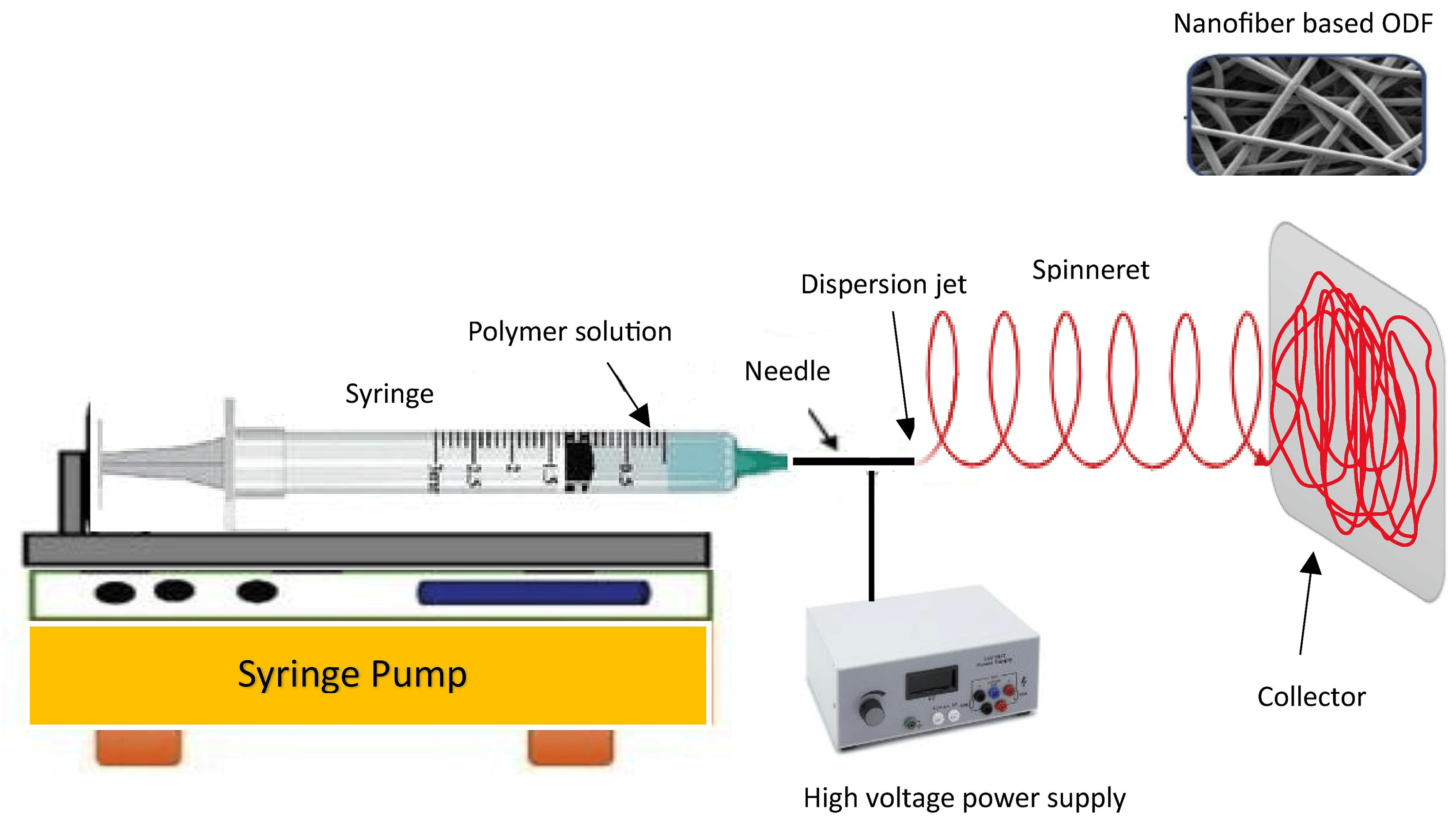
Figure 5. Steps involved in the electrospinning process for preparing ODFs.
A feasibility study explored the use of the electrospinning process to create nanofibers loaded with sildenafil, employing pullulan as the film-forming polymer and water as the sole solvent [128]. By optimizing the electrospinning parameters, such as the shear force, viscosity, and surface tension of the polymer solution, a continuous production of nanofiber-based ODFs was achieved. It was demonstrated that the disintegration of electrospun products proved to be exceptionally rapid and compliant with both Ph. Eur., and USP standards. The delivery of biopharmaceuticals to the oral mucosa presents various promising applications in the field of medicine, such as localized treatment, enhanced patient convenience, and improved patient compliance compared to traditional injection-based delivery methods. The delivery of biopharmaceuticals through the oral mucosa presents several challenges, including the mucosal membrane barrier, enzymatic degradation, and achieving controlled release, which needs to be addressed for successful implementation. To overcome this challenge, researchers explored the use of a dual-layer mucoadhesive patch produced through electrospinning using lysozyme as a model antimicrobial protein [129]. Nanofibers were made of PVA/Eudragit RS100 polymers using an ethanol/water mixture as the solvent. The resulting fibrous membranes exhibited a cumulative drug release of 90 ± 13% within 2 h. Confocal microscopy along with dual fluorescent fiber labeling, demonstrated the even distribution of lysozyme and polymers throughout the patch. The formulation achieved high encapsulation efficiency (93.4 ± 7.0%) and preserved enzyme activity (96.1 ± 3.3%). This study underscores the considerable potential of this drug delivery system for delivering therapeutic proteins to the oral mucosa. While the electrospinning process has proven to be a valuable technique for fabricating nanofibers with a wide range of applications, it does have some limitations. There are a few primary limitations of the electrospinning process, including process complexity, limited scalability, solvent compatibility, fiber morphology control, material compatibility, limited fiber orientation, and mechanical strength.
4. Nanoparticle-Embedded ODFs
The advantages of embedding either polymeric or lipid nanoparticles into ODFs are multifaceted, including enhanced drug solubility and permeability, controlled release, targeted drug delivery, taste masking, and improved stability during storage and transportation [130,131,132]. Embedding nanoparticles into ODFs usually requires specialized formulation methods like solvent casting, HME, and spray drying. These techniques offer the necessary control and compatibility for successfully embedding nanoparticles in ODFs while addressing the challenges associated with their integration into the film formulation. A schematic diagram depicting the typical nanoparticle types and methods employed for incorporating nanoparticles into ODFs is presented in Figure 6. These techniques are employed to achieve the even dispersion of nanoparticles while preserving the film’s mechanical characteristics and overall integrity. Despite the promising potential of nanoparticle-loaded ODFs, there are challenges to address, including concerns about nanoparticle toxicity, regulatory compliance, and quality control. It is essential to thoroughly evaluate these aspects to ensure the safety and efficacy of such formulations. Several studies have suggested that formulating APIs in lipid nanoparticles can enhance their oral bioavailability [133,134]. Additionally, lipid formulations have been shown to mitigate the impact of food on API absorption [135]. The main approach to improving the bioavailability of APIs in lipid dispersions revolves primarily around creating surface-active monoglycerides. These monoglycerides, in combination with bile salts, give rise to mixed micelles that can encapsulate drug molecules. These mixed micelles are believed to facilitate the direct absorption of the API in conjunction with the lipids.
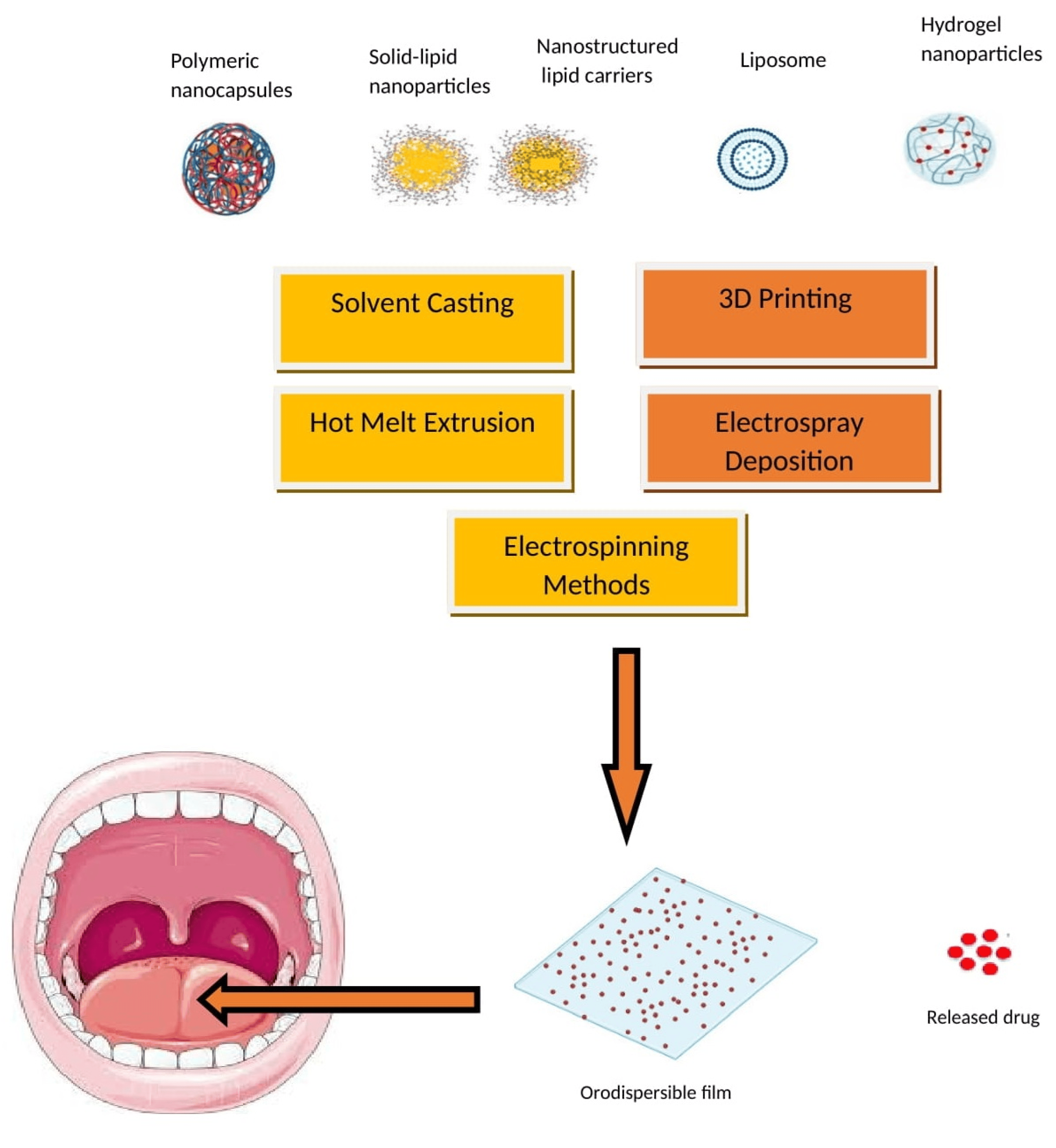
Figure 6. Nanoparticle types and incorporation methods in ODFs.
In addition, the release of bile salts can potentially improve the solubility of orally administered inadequately aqueous soluble APIs, thus facilitating their immediate absorption. While there have been limited studies on integrating solid lipid nanoparticles into mucoadhesive films, there has been even less focus on incorporating lipid microparticles into ODFs [136]. In a recent study [137], researchers explored the potential of utilizing HPMC film as a promising vehicle for solid lipid particles. Their findings demonstrated that the lipid content in the film matrix could reach as high as 54 wt.%, depending on the specific type of triglyceride nanoparticles integrated. To create lipid dispersions for inclusion in ODFs, a high-pressure homogenization process was employed, with formulations composed of 10% lipid, 5% stabilizer, and 0.5% sodium dodecyl sulfate (SDS). Some formulations solely relied on the surfactant SDS for stabilization, with a composition of 10% lipid and 0.5% SDS. During the evaluation of the stabilizers, various particle formulations were tested for three different triglycerides (tristearin, tripalmitin, and trimyristin). The most promising outcomes were observed with combinations of a polymer and the surfactant SDS, as well as formulations containing only SDS. Two specific particle formulations, HPMC/SDS and SDS, stood out as highly promising due to their ability to achieve substantial lipid contents and produce high-quality films. For tristearin, lipid contents of up to 0.50 were achievable in the ODFs with HPMC/SDS stabilization, and they were up to 0.54 for the SDS-based formulation. It is worth noting that the maximum lipid load was lower for the films loaded with tripalmitin (0.40) and trimyristin (0.29) compared to tristearin. A noteworthy aspect of this study was the successful redispersal of lipid nanoparticles from the film matrix without compromising their nanoparticulate properties. Additionally, the films exhibited commendable mechanical properties and disintegration times. Moreover, this study demonstrated the feasibility of substantially stabilizing lipid particles in the metastable α-polymorphic form for a duration of at least 6 months when utilizing HPMC/SDS to stabilize the triasterin suspension [137]. This breakthrough discovery opens up new possibilities for leveraging HPMC films to enhance the delivery of lipid-based drug formulations.
One innovative technique involves integrating a self-micro emulsifying system into the film, as previously explored [138]. However, this method demands a substantial quantity of oil, posing a challenge to the long-term integrity and stability of the film. To address this concern, another strategy was employed, i.e., reducing the drug to the nano-scale before its incorporation into ODFs. Research has shown a remarkable 11-fold increase in the in vitro drug release from polymeric films embedded with nanoparticles in comparison to the pure drug [139]. Other researchers have reported identical improvements in ex vivo permeation, emphasizing the substantial promise of using nanoparticle-loaded ODFs for the delivery of poorly soluble compounds [140,141]. In a recent study, scientists employed the Box–Behnken method to design and fabricate ODFs loaded with ketoprofen nanoparticles [142]. The amorphous state of drug-embedded nanoparticles within the ODFs was verified through the absence of distinct crystalline patterns and the absence of endothermic peaks of ketoprofen in X-ray diffraction and modulated differential scanning calorimetry analyses, respectively. The optimized formulation demonstrated a nearly fourfold increase in permeability compared to pure drugs. Furthermore, the dissolution rates of the drug and the optimized drug-containing ODF in a pH 1.2 solution reached approximately 30% and 95% at the 60 min mark, respectively. Pharmaceutical nanosuspensions are necessary when dealing with problematic drug molecules that cannot form salts, have high molecular weights, require a large dose, possess high log p-values, and have high melting points, as these factors make it difficult to create suitable drug formulations using other methods. A variety of techniques are employed to create nanosuspensions with diverse particle sizes. These methods include top-down approaches like dry and wet milling, co-grinding, and high-pressure homogenization, as well as bottom-up techniques such as antisolvent precipitation, liquid emulsion, and sonoprecipitation methods [143]. Nanosuspension-based oral thin films offer a promising approach to drug delivery, addressing the challenges associated with solubility, bioavailability, and patient compliance for a variety of pharmaceutical compounds. Fast-dissolving oral films of buspirone were created by employing the solvent evaporation method to transform a nanosuspension with the film-forming agents HPMC E5 and PVA. Buspirone oral films exhibited remarkable physical and mechanical characteristics and displayed good stability, and the in vitro assessments revealed an initial rapid drug release followed by a sustained release [21]. The inclusion of nanoparticles in oral films was anticipated to improve the dissolution and permeability properties of various poorly water-soluble drugs. A fast-dissolving oral film containing the poorly aqueous soluble and low bioavailable drug, lercanidipine, was fabricated as nanoparticles through the antisolvent evaporation technique. These formulations demonstrated a substantial enhancement in the in vitro dissolution rate and ex vivo permeation [21].
Pharmacokinetic investigations involving lutein nanocrystals in fast-dissolving oral films in rats demonstrated a significant reduction in the Tmax and a substantial increase in the Cmax compared to the oral solution. Additionally, the AUC0–24 h of nanocrystal fast-dissolving oral films was approximately two times larger than that of the oral solution, confirming a substantial enhancement in both the rate and extent of bioavailability [144].
A research study explores the application of the wet-milling technique to produce the nanosized hydrophobic drug loratadine, resulting in enhanced solubility and quicker dissolution [145]. These nanosized materials (<400 nm) were then employed in the formulation of ODFs that disintegrate rapidly, typically in less than 60 s. Although the drug’s crystalline structure remains unaltered, there is a significant improvement in its bioavailability. When administered to rats, the nanocrystal ODF leads to a 5.69-fold increase in the AUC0–24 h compared to the original drug, with a faster onset of action, typically within 30 min. The simplification of the formulation process and the enhancements in drug solubility and bioavailability have positioned the ODF as a promising drug delivery method for loratadine. In another interesting investigation, poorly aqueous soluble drugs such as fenofibrate and naproxen were formulated as nanoparticles using various strategies and were incorporated into ODFs [146]. The research findings revealed that the dose of API that can be administered in a single ODF significantly relies on the chosen formulation approach and the physicochemical characteristics of the API. The amorphous solid dispersion-ODFs and films incorporating API-loaded lipid nanoemulsions emerged as the most favorable film formulations. These formulations resulted in a decrease in the API dissolution time when compared to ODFs containing non-formulated API microparticles. Despite slightly compromised mechanical film properties compared to API-free film formulations, these ODFs achieved rapid disintegration times. For naproxen in the solid dispersion-ODFs, an API loading of up to 8 wt.% was attained without encountering recrystallization. In contrast, during the formulation of FENO in the films, the API content was limited to 2 wt.%, indicating unique intermolecular interactions between the APIs and the film-forming matrix.
5. In Vitro and In Vivo Evaluation Methods
The assessment of ODFs involves a range of essential tests to ensure their quality and performance. These tests encompass measurements of thickness, weight consistency, film durability, flexibility, water absorption, swelling, surface characteristics, and moisture content. Evaluating the taste perceptibility of an ODF is crucial, as it significantly influences patient acceptance and adherence to the medication. Additionally, mechanical properties such as tensile strength, puncture strength, elongation at break, elastic modulus, porosity, and folding endurance are evaluated, typically following the ASTM D882-01 standard [147]. The microstructural, compositional, and thermal characteristics of the films were examined by employing a range of analytical techniques, including scanning electron microscopy, X-ray diffraction, differential scanning calorimetry, and Fourier-transform infrared spectroscopy. Numerous investigations have underscored the impact of the disintegration evaluation method on the final results of gelatin-based ODFs. In the case of the slide frame method, where a solution drop is placed on the film’s surface, and the time for the solution to dissolve the film and form a hole is measured, it has demonstrated favorable disintegration times of around 30 s in multiple research studies [54]. In contrast, when using the Petri dish method, the sample is immersed in a solution, and the time taken for the film to fully disintegrate is documented. Research conducted with this approach has documented disintegration times exceeding 5 min [55]. offers a comprehensive compilation of frequently utilized in vitro techniques for the evaluation of ODFs. These techniques are invaluable in pharmaceutical formulation development for researchers to assess the quality, performance, and characteristics of ODFs in a controlled laboratory setting. The schematic representation of the texture analyzer, Franz diffusion cell, dissolution apparatus type II, and dissolution apparatus type V, utilized for the in vitro evaluation of ODFs, is presented in the Figure 7, Figure 8 and Figure 9.
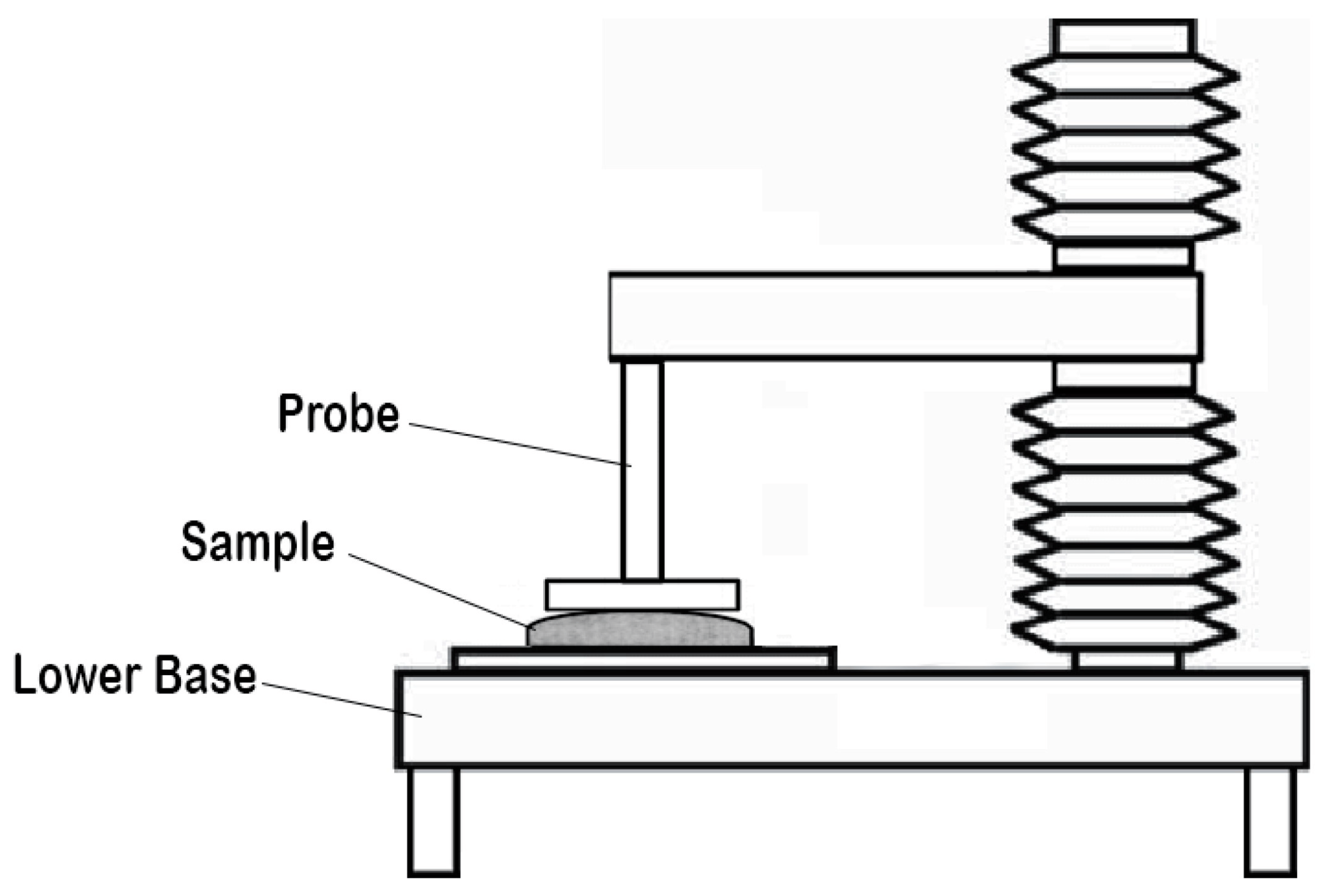
Figure 7. Schematic diagram of the texture analyzer (adapted from [167], published by MDPI, 2020).
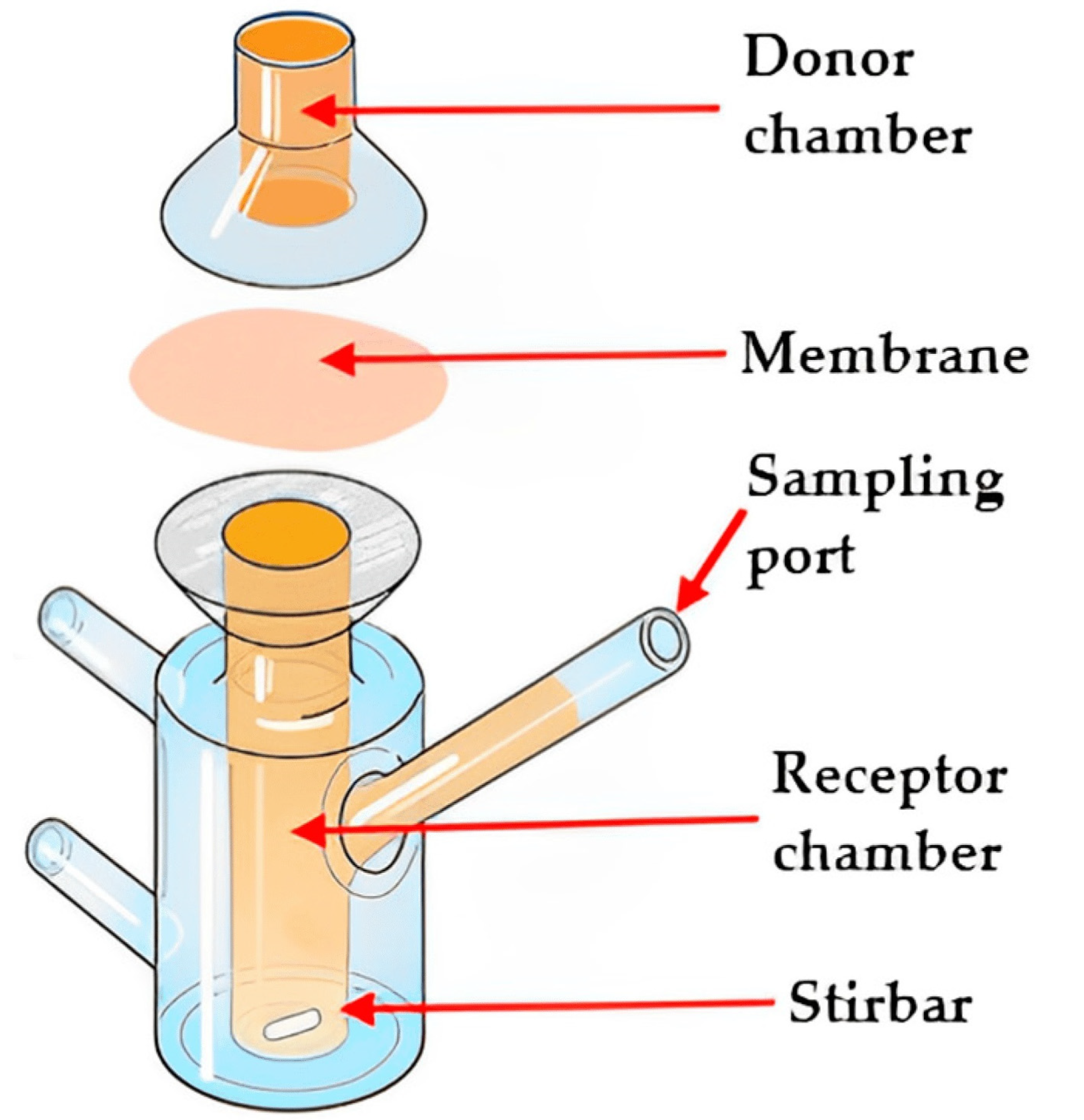
Figure 8. Illustration of the Franz diffusion cell (adapted from [168], published by MDPI, 2023).
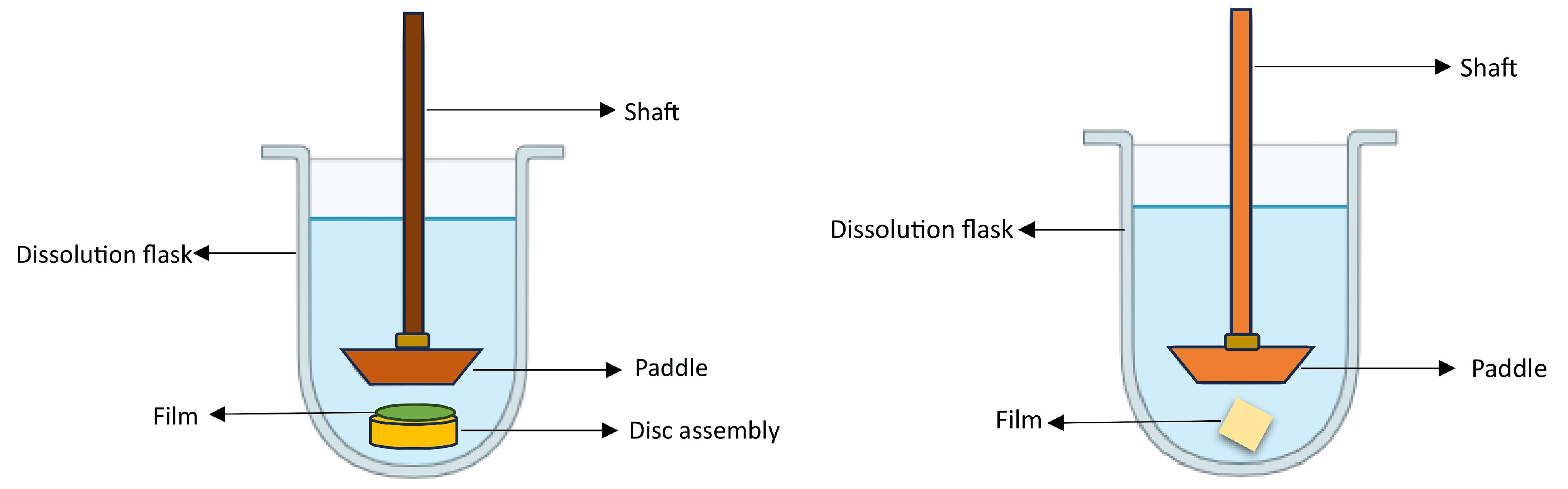
Figure 9. Schematic illustration of the USP dissolution apparatus type II (left) and dissolution apparatus type V (paddle-over-disk (right)).
The evaluation of ODFs extends beyond in vitro tests, with the use of animal models for predicting the in vivo absorption. Allometric equations facilitate the dose conversion between humans and animals [169]. In vivo studies often rely on pharmacokinetic parameters like AUC, Tmax, and Cmax to estimate the drug absorption of nanoparticles, which can be tracked and assessed in tissues using fluorescent labeling. Nevertheless, it is crucial to acknowledge that the plasma drug concentration cannot differentiate between absorption from the pregastric and intestinal regions [4]. Ex vivo mucosal permeation models, which closely mimic the histological features of the buccal membrane, offer more accurate predictions of the in vivo drug absorption compared to an evaluation employing artificial membranes [170]. These models provide valuable insights into the intricate processes governing nanoparticle transport, including their interactions with mucus barriers.
Patients are more likely to adhere to their treatment regimens when the taste of the ODF is palatable, making it a key factor in the overall therapeutic experience. Therefore, conducting sensory assessments to understand the taste characteristics and how they affect the patient’s willingness to use the product is essential in pharmaceutical and healthcare research. There are currently limited testing methods capable of accurately evaluating the sensory aspects that affect the acceptability of ODFs. In the reported study, four ODFs were fabricated using PVA and NaCMC as the primary components through the 3DP method [171]. The in vitro disintegration times were tested using the Petri dish, oral cavity model, and bio-tribology methods. The data showed that an increase in the molecular weight of the polymers had an exponential impact on the time it took for the films to disintegrate in the Petri dish and oral cavity. Moreover, polymeric films with higher molecular sizes exhibited a stronger adhesion to the upper palate in the oral cavity method. Bio-tribology analysis revealed that films with a higher molecular weight disintegrated more rapidly and had a lower coefficient of friction, suggesting potentially favorable oral perception but also a certain stickiness due to increased viscosity. These evaluation techniques have the potential to assist formulators in designing, testing, and reformulating ODFs that can achieve rapid disintegration and offer improved sensory attributes when consumed, thereby enhancing overall treatment efficacy and acceptability. In summary, the assessment of oral thin films involves a comprehensive battery of tests to ensure their physical and mechanical properties, as well as their suitability for drug delivery. While the in vitro evaluations are crucial, ex vivo and in vivo models using animal and mucosal tissue provide valuable insights into real-world drug absorption and biodistribution.
6. Clinical Translation and Future Prospects
ODF is applicable for local delivery in mouth conditions, such as a local anesthetic in dental procedures, bacterial or fungal infections, sore throats, oral ulcers, pharyngitis, or tonsillitis [7]. ODF offers numerous benefits, making it particularly advantageous in emergency scenarios and for patients with schizophrenia and dysphagia. ODFs prepared with small molecules were successfully developed for local as well as systemic effects [73,172]. offers a concise overview of the clinical trials, both ongoing and completed, pertaining to various drug-loaded ODFs designed for oral delivery. Back in 2010, the FDA authorized the first prescription ODF, known by the trade name Zuplenz, and contains ondansetron as its API. Since then, many prescription and non-prescription ODFs have been approved and have gained popularity worldwide. Nonetheless, numerous formulation hurdles still exist, including the challenging issues of unpalatable-tasting drugs and high dosages. The feasibility of ODFs for the delivery of nanoparticles has been extensively explored [173,174].
Many companies have secured patents for their ODFs, and lists some examples of these patented ODFs, along with a summary of their innovation. An in-depth examination of patent technologies employed in the production of ODFs has been recently reviewed [175]. highlights an overview of selected commercial examples of ODFs and their respective therapeutic categories. This information offers valuable insights into the diversity of ODFs in the market and the therapeutic areas they are primarily designed to address.
ODFs are typically categorized into three classes based on different criteria, such as dissolution properties, layering characteristics, and the nature of the API. Dissolution-based ODFs can be categorized as rapidly dissolving, with dissolution occurring in less than 30 s; moderately dissolving, taking between 1 to 30 min; or slowly dissolving, requiring more than 30 min to fully dissolve. Monolayer ODFs generally include an API, a film former, a plasticizer, and inactive ingredients. On the other hand, bilayer or oral films have an API layer along with an additional taste-masking or mucoadhesive layer, acting as a separator between the two. Multilayer films employ an API layer positioned between two layers, making this approach beneficial for effectively combining incompatible drugs [176]. The widely used approach for creating ODFs involves the solvent casting method, wherein active substances are dissolved or dispersed in biocompatible polymeric films, followed by evaporation. However, there is a growing interest in 3DP methods, including HME, FDM, and inkjet techniques. ODFs present certain drawbacks in drug delivery, including limitations in accommodating a substantial API load, challenges in taste masking, and potential variations in the uniformity of the content. Additionally, there may be limitations related to stability, shelf life, and susceptibility to environmental factors. These considerations underscore the importance of addressing multiple facets in ODF formulation to enhance overall efficacy and patient acceptance. The primary constraint linked to film dosage forms lies in the challenge of attaining a substantial payload within the restricted surface area. One possible approach to addressing this problem is to encapsulate a high payload of active substances in nanoparticles and then embed these nanoparticles in mucoadhesive polymeric materials. Presently, ongoing research is focused on nanoparticle-enabled films, investigating the diverse functionalization approaches aimed at improving diffusion through the oral mucosa and facilitating the systemic targeting of actives. The main issue resulting in the non-compliance of patients is the taste of drugs. However, the unpleasant taste of the drug in the film dosage form can be partially masked by using certain agents, such as a complexing agent (e.g., β-CD), a cooling agent (e.g., menthol), and sweetening agents like mannitol and aspartame sodium.
Numerous pharmaceutical companies are transitioning their product lines from ODTs to ODFs. Several APIs are being used for ODFs, targeting conditions like Parkinson’s disease, depression, schizophrenia, and Alzheimer’s disease. The identification of novel functional excipients with the capability to enhance penetration and the investigation of new approaches for oromucosal permeation, such as the ion-pair strategy, represent promising paths for advancing ODFs. The future of research and development in the field of ODFs may focus on 3DP techniques, which offer the potential to incorporate high drug loading, explore combinations of multiple drugs, and introduce compartmentalization for isolating incompatible drug components.
7. Conclusions
ODFs represent a versatile and patient-friendly dosage form that has gained significant attention in the pharmaceutical industry. ODFs offer numerous advantages, such as rapid drug delivery, improved patient compliance, and suitability for various drug types, including poorly soluble compounds and macromolecules. Recent advancements in the formulation of ODFs, including the incorporation of nanoparticles, have opened new avenues for enhancing drug delivery efficiency and expanding the range of drugs amenable to this administration route. However, further research and development are still needed to address the challenges related to taste masking, stability, and the incorporation of complex active ingredients. ODFs continue to be an exciting area of pharmaceutical innovation, with the potential to revolutionize drug delivery for a diverse spectrum of therapeutic uses. As the discipline progresses, ongoing efforts in formulation science, regulatory guidance, and clinical evaluation will be critical to fully realizing the possibilities of ODFs in improving patient outcomes and healthcare services.
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, K.; Kim, M.; Choi, D.H.; Jeong, S.H. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J. Pharm. Investig. 2017, 47, 183–201. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Orally Disintegrating Tablets; Food and Drug Administration: Silver Spring, MD, USA, 2008.
- Agency, E.M. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; European Medicines Agency: London, UK, 2013. [Google Scholar]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Oral films: Current status and future perspectives: I—Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Alaei, S.; Omidian, H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur. J. Pharm. Sci. 2021, 159, 105727. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. SOFTs—Structured orodispersible film templates. Eur. J. Pharm. Biopharm. 2019, 137, 209–217. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Quality by Design for Andas: An Example For Immediate-Release Dosage Forms; US Department of Health and Human Service—FDA: Rockville, MD, USA, 2012.
- Tian, Y.; Lin, J.; Jing, H.; Wang, Q.; Wu, Z.; Duan, Y. Recent progress in orodispersible films-mediated therapeutic applications: A review. MedComm Biomater. Appl. 2023, 2, e34. [Google Scholar] [CrossRef]
- Nishigaki, M.; Kawahara, K.; Nawa, M.; Futamura, M.; Nishimura, M.; Matsuura, K.; Kitaichi, K.; Kawaguchi, Y.; Tsukioka, T.; Yoshida, K.; et al. Development of fast dissolving oral film containing dexamethasone as an antiemetic medication: Clinical usefulness. Int. J. Pharm. 2012, 424, 12–17. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Fonte, P.; Oliveira, A.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Optimization of two biopolymer-based oral films for the delivery of bioactive molecules. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.; Remedio, L.N.; Yoshida, C.M.; Carvalho, R.A. Carboxymethyl cellulose-based orally disintegrating films enriched with natural plant extract for oral iron delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102852. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Umemura, K.; Tahara, K.; Takeuchi, H. Formulation design of hydroxypropyl cellulose films for use as orally disintegrating dosage forms. J. Drug Deliv. Sci. Technol. 2018, 46, 93–100. [Google Scholar] [CrossRef]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B Biointerfaces 2018, 163, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.C.; Parfati, N.; Aryani, N.L.D.; Winantari, A.N.; Fitriani, E.W.; Pradana, A.T.; Nawatila, R.; Putranti, A.R.; Irine, F.; Angelica, F.; et al. Development, Evaluation, and Molecular Docking of Oral Dissolving Film of Atenolol. Pharmaceutics 2021, 13, 1727. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.; Bendas, E.R.; Ramadan, A.A.; Mostafa, D.A. Pharmaceutical and pharmacokinetic evaluation of a novel fast dissolving film formulation of flupentixol dihydrochloride. AAPS Pharmscitech 2014, 15, 1603–1610. [Google Scholar] [CrossRef]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef]
- Dahmash, E.Z.; Iyire, A.; Alyami, H.S. Development of orally dissolving films for pediatric-centric administration of anti-epileptic drug topiramate—A design of experiments (DoE) study. Saudi Pharm. J. 2021, 29, 635–647. [Google Scholar] [CrossRef]
- Al-Mogherah, A.I.; Ibrahim, M.A.; Hassan, M.A. Optimization and evaluation of venlafaxine hydrochloride fast dissolving oral films. Saudi Pharm. J. 2020, 28, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Lai, F.; Franceschini, I.; Corrias, F.; Sala, M.C.; Cilurzo, F.; Sinico, C.; Pini, E. Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study. Carbohydr. Polym. 2015, 121, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Quan, P.; Fang, L. Preparation of an oral thin film containing meclizine hydrochloride: In vitro and in vivo evaluation. Int. J. Pharm. 2015, 496, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Visser, J.C.; Klever, J.S.; Woerdenbag, H.J.; Frijlink, H.W.; Hinrichs, W.L.J. Orodispersible films based on blends of trehalose and pullulan for protein delivery. Eur. J. Pharm. Biopharm. 2018, 133, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid. Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Sizílio, R.H.; Galvão, J.G.; Trindade, G.G.G.; Pina, L.T.S.; Andrade, L.N.; Gonsalves, J.; Lira, A.A.M.; Chaud, M.V.; Alves, T.F.R.; Arguelho, M.; et al. Chitosan/pvp-based mucoadhesive membranes as a promising delivery system of betamethasone-17-valerate for aphthous stomatitis. Carbohydr. Polym. 2018, 190, 339–345. [Google Scholar] [CrossRef]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and evaluation of chitosan-based buccal bioadhesive films of zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- AnjiReddy, K.; Karpagam, S. In vitro and in vivo evaluation of oral disintegrating nanofiber and thin-film contains hyperbranched chitosan/donepezil for active drug delivery. J. Polym. Environ. 2021, 29, 922–936. [Google Scholar] [CrossRef]
- Dharmasthala, S.; Shabaraya, A.R.; Andrade, G.S.; Shriram, R.G.; Hebbar, S.; Dubey, A. Fast dissolving oral film of piroxicam: Solubility enhancement by forming an inclusion complex with β-cyclodextrin, formulation and evaluation. J. Young Pharm. 2019, 11, 1. [Google Scholar] [CrossRef]
- Timur, S.S.; Yüksel, S.; Akca, G.; Şenel, S. Localized drug delivery with mono and bilayered mucoadhesive films and wafers for oral mucosal infections. Int. J. Pharm. 2019, 559, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Gupta, R.; Vasanti, S. In vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm. Dev. Technol. 2007, 12, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singla, Y.P.; Narang, R.S.; Pandita, D.; Singh, S.; Narang, J.K. Frovatriptan loaded hydroxy propyl methyl cellulose/treated chitosan based composite fast dissolving sublingual films for management of migraine. J. Drug Deliv. Sci. Technol. 2018, 47, 230–239. [Google Scholar] [CrossRef]
- De Moraes, M.A.; Da Silva, C.F.; Vieira, R.S. Biopolymer Membranes and Films: Health, Food, Environment, and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Murthy, K.R. Formulation and evaluation of oral fast dissolving films of poorly soluble drug ezetimibe using transcutol Hp. Indian J. Pharm. Educ. Res. 2018, 52, 398–407. [Google Scholar] [CrossRef]
- Sharma, R.; Kamboj, S.; Singh, G.; Rana, V. Development of aprepitant loaded orally disintegrating films for enhanced pharmacokinetic performance. Eur. J. Pharm. Sci. 2016, 84, 55–69. [Google Scholar] [CrossRef]
- Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar]
- Panraksa, P.; Tipduangta, P.; Jantanasakulwong, K.; Jantrawut, P. Formulation of Orally Disintegrating Films as an Amorphous Solid Solution of a Poorly Water-Soluble Drug. Membranes 2020, 10, 376. [Google Scholar] [CrossRef]
- Shamma, R.; Elkasabgy, N. Design of freeze-dried Soluplus/polyvinyl alcohol-based film for the oral delivery of an insoluble drug for the pediatric use. Drug Deliv. 2016, 23, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Khames, A. Hexyl alginate derivative, an amphiphilic innovative buccal film-forming material of promising mechanical and release characteristics for the improvement of repaglinide bioavailability. Drug Des. Dev. Ther. 2019, 13, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, P.; Patil, J.; Reddy, M.V. Formulation and comparative pharmacokinetic evaluation of orodispersible tablets and films of nebivolol hydrochloride. J. Pharm. Investig. 2015, 45, 237–247. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- El-Bary, A.A.; Al Sharabi, I.; Haza’a, B.S. Effect of casting solvent, film-forming agent and solubilizer on orodispersible films of a polymorphic poorly soluble drug: An in vitro/in silico study. Drug Dev. Ind. Pharm. 2019, 45, 1751–1769. [Google Scholar] [CrossRef]
- Shi, L.L.; Xu, W.J.; Cao, Q.R.; Yang, M.; Cui, J.H. Preparation, characterization and in vitro evaluation of a polyvinyl alcohol/sodium alginate based orodispersible film containing sildenafil citrate. Pharmazie 2014, 69, 327–334. [Google Scholar] [PubMed]
- Dos Santos Garcia, V.A.; Borges, J.G.; Maciel, V.B.V.; Mazalli, M.R.; das Graças Lapa-Guimaraes, J.; Vanin, F.M.; de Carvalho, R.A. Gelatin/starch orally disintegrating films as a promising system for vitamin C delivery. Food Hydrocoll. 2018, 79, 127–135. [Google Scholar] [CrossRef]
- Borges, J.G.; Silva, A.G.; Cervi-Bitencourt, C.M.; Vanin, F.M.; Carvalho, R.A. Lecithin, gelatin and hydrolyzed collagen orally disintegrating films: Functional properties. Int. J. Biol. Macromol. 2016, 86, 907–916. [Google Scholar] [CrossRef]
- Dos Santos Garcia, V.A.; Gonçalves Borges, J.; Mazalli, M.R.; Lapa-Guimarães, J.d.G.; Vanin, F.M.; de Carvalho, R.A. Gelatin and pregelatinized starch orally disintegrating films: Properties and stability of vitamin C. J. Appl. Polym. Sci. 2017, 134, 44841. [Google Scholar] [CrossRef]
- Dos Santos Garcia, V.A.; Borges, J.G.; Osiro, D.; Vanin, F.M.; de Carvalho, R.A. Orally disintegrating films based on gelatin and pregelatinized starch: New carriers of active compounds from acerola. Food Hydrocoll. 2020, 101, 105518. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Vanin, F.M.; Carvalho, R.A.d.; Trindade, M.A.; Fávaro-Trindade, C.S. Characterization of low cost orally disintegrating film (ODF). Polímeros 2017, 27, 48–54. [Google Scholar] [CrossRef]
- Tedesco, M.P.; Monaco-Lourenço, C.A.; Carvalho, R.A. Gelatin/hydroxypropyl methylcellulose matrices—Polymer interactions approach for oral disintegrating films. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Woo, H.; Kim, I.-C.; Lee, K.H. Fish gelatin nanofibers prevent drug crystallization and enable ultrafast delivery. RSC Adv. 2017, 7, 40411–40417. [Google Scholar] [CrossRef]
- Pezik, E.; Gulsun, T.; Sahin, S.; Vural, İ. Development and characterization of pullulan-based orally disintegrating films containing amlodipine besylate. Eur. J. Pharm. Sci. 2021, 156, 105597. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.S.; Kumar, T.P.; Reddy, D.; Pathak, K.; Gowda, D.V.; Babu, A.V.N.; Aodah, A.H.; Khafagy, E.S.; Alotaibi, H.F.; Abu Lila, A.S.; et al. Development and Characterization of Pullulan-Based Orodispersible Films of Iron. Pharmaceutics 2023, 15, 1027. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bhide, Y.C.; Woerdenbag, H.J.; Huckriede, A.L.W.; Frijlink, H.W.; Hinrichs, W.L.J.; Visser, J.C. Development of an Orodispersible Film Containing Stabilized Influenza Vaccine. Pharmaceutics 2020, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Krull, S.M.; Ma, Z.; Li, M.; Davé, R.N.; Bilgili, E. Preparation and characterization of fast dissolving pullulan films containing BCS class II drug nanoparticles for bioavailability enhancement. Drug Dev. Ind. Pharm. 2016, 42, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Limpongsa, E.; Jaipakdee, N. Physical modification of Thai rice starch and its application as orodispersible film former. Carbohydr. Polym. 2020, 239, 116206. [Google Scholar] [CrossRef]
- Bodini, R.B.; Guimarães, J.d.G.L.; Monaco-Lourenço, C.A.; de Carvalho, R.A. Effect of starch and hydroxypropyl methylcellulose polymers on the properties of orally disintegrating films. J. Drug Deliv. Sci. Technol. 2019, 51, 403–410. [Google Scholar] [CrossRef]
- Liew, K.B.; Odeniyi, M.A.; Peh, K.K. Application of freeze-drying technology in manufacturing orally disintegrating films. Pharm. Dev. Technol. 2016, 21, 346–353. [Google Scholar] [CrossRef]
- Sha, H.; Yuan, C.; Cui, B.; Zhao, M.; Wang, J. Pre-gelatinized cassava starch orally disintegrating films: Influence of β-Cyclodextrin. Food Hydrocoll. 2022, 123, 107196. [Google Scholar] [CrossRef]
- Tamanini, F.; Moraes, B.S.; Amaral, C.S.T.; Carvalho, A.J.F.; Trovatti, E. Starch-based orodispersible film for diclofenac release. Braz. J. Pharm. Sci. 2023, 59, e211019. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.; Wang, B.; Luo, C.; Wang, Y.; Wang, H.; Chen, F.; Xiang, X. Development, In Vitro and In Vivo Evaluation of Racecadotril Orodispersible Films for Pediatric Use. AAPS PharmSciTech 2021, 22, 15. [Google Scholar] [CrossRef]
- Cupone, I.E.; Sansone, A.; Marra, F.; Giori, A.M.; Jannini, E.A. Orodispersible Film (ODF) Platform Based on Maltodextrin for Therapeutical Applications. Pharmaceutics 2022, 14, 2011. [Google Scholar] [CrossRef] [PubMed]
- Pechová, V.; Gajdziok, J.; Muselík, J.; Vetchý, D. Development of Orodispersible Films Containing Benzydamine Hydrochloride Using a Modified Solvent Casting Method. AAPS PharmSciTech 2018, 19, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Montanari, L.; Minghetti, P. Self Supporting Film for Pharmaceutical and Food. Use. Patent EP16893472004, 27 October 2004. [Google Scholar]
- Radicioni, M.; Castiglioni, C.; Giori, A.; Cupone, I.; Frangione, V.; Rovati, S. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des. Dev. Ther. 2017, 11, 1183–1192. [Google Scholar] [CrossRef]
- Radicioni, M.; Caverzasio, C.; Rovati, S.; Giori, A.M.; Cupone, I.; Marra, F.; Mautone, G. Comparative Bioavailability Study of a New Vitamin D3 Orodispersible Film Versus a Marketed Oral Solution in Healthy Volunteers. Clin. Drug Investig. 2022, 42, 151–161. [Google Scholar] [CrossRef]
- Cupone, I.E.; Roselli, G.; Marra, F.; Riva, M.; Angeletti, S.; Dugo, L.; Spoto, S.; Fogolari, M.; Giori, A.M. Orodispersible Film Based on Maltodextrin: A Convenient and Suitable Method for Iron Supplementation. Pharmaceutics 2023, 15, 1575. [Google Scholar] [CrossRef]
- Franceschini, I.; Selmin, F.; Pagani, S.; Minghetti, P.; Cilurzo, F. Nanofiller for the mechanical reinforcement of maltodextrins orodispersible films. Carbohydr. Polym. 2016, 136, 676–681. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N.d.F.F. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Aloui, H.; Baraket, K.; Sendon, R.; Silva, A.S.; Khwaldia, K. Development and characterization of novel composite glycerol-plasticized films based on sodium caseinate and lipid fraction of tomato pomace by-product. Int. J. Biol. Macromol. 2019, 139, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur. J. Pharm. Sci. 2017, 96, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Briddick, A.; Li, P.; Hughes, A.; Courchay, F.; Martinez, A.; Thompson, R.L. Surfactant and Plasticizer Segregation in Thin Poly(vinyl alcohol) Films. Langmuir 2016, 32, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.A.; Ilberg, V. Effect of different polyol-based plasticizers on thermal properties of polyvinyl alcohol:starch blends. Carbohydr. Polym. 2016, 136, 441–448. [Google Scholar] [CrossRef]
- Olechno, K.; Basa, A.; Winnicka, K. “Success Depends on Your Backbone”-About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 4872. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chen, K.S.; Run-Chu, L. Organic esters of plasticizers affecting the water absorption, adhesive property, glass transition temperature and plasticizer permanence of eudragit acrylic films. J. Control. Release 2000, 68, 343–350. [Google Scholar] [CrossRef]
- Honary, S.; Orafai, H. The effect of different plasticizer molecular weights and concentrations on mechanical and thermomechanical properties of free films. Drug Dev. Ind. Pharm. 2002, 28, 711–715. [Google Scholar] [CrossRef]
- Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties. Polymers 2020, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Vishvakarma, V.; Kaur, M.; Nagpal, M.; Arora, S. Role of Nanotechnology in Taste Masking: Recent Updates. Curr. Drug Res. Rev. 2023, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Joshi, U.; Singh, A.; Saharan, V.A. Lipids for Taste masking and Taste assessment in pharmaceutical formulations. Chem. Phys. Lipids 2021, 235, 105031. [Google Scholar] [CrossRef] [PubMed]
- Graebin, C.S. The pharmacological activities of glycyrrhizinic acid (“glycyrrhizin”) and glycyrrhetinic acid. Sweeteners 2018, 245–261. [Google Scholar] [CrossRef]
- Mathur, S.; Bulchandani, N.; Parihar, S.; Shekhawat, G.S. Critical review on steviol glycosides: Pharmacological, toxicological and therapeutic aspects of high potency zero caloric sweetener. Int. J. Pharmacol. 2017, 13, 916–928. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kearsley, M. Sweeteners and Sugar Alternatives in Food Technology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- DuBois, G.E.; Prakash, I. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers. Annu. Rev. Food Sci. Technol. 2012, 3, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Homler, B.E. Aspartame: Implications for the food scientist. In Aspartame; CRC Press: Boca Raton, FL, USA, 2020; pp. 247–262. [Google Scholar]
- Czarnecka, K.; Pilarz, A.; Rogut, A.; Maj, P.; Szymańska, J.; Olejnik, Ł.; Szymański, P. Aspartame-True or False? Narrative Review of Safety Analysis of General Use in Products. Nutrients 2021, 13, 1957. [Google Scholar] [CrossRef]
- Borges, J.G.; De Carvalho, R.A. Orally disintegrating films containing propolis: Properties and release profile. J. Pharm. Sci. 2015, 104, 1431–1439. [Google Scholar] [CrossRef]
- Cocco, F.; Cagetti, M.G.; Majdub, O.; Campus, G. Concentration in saliva and antibacterial effect of xylitol chewing gum: In vivo and in vitro study. Appl. Sci. 2020, 10, 2900. [Google Scholar] [CrossRef]
- Nagaraju, T.; Gowthami, R.; Rajashekar, M.; Sandeep, S.; Mallesham, M.; Sathish, D.; Kumar, Y.S. Comprehensive review on oral disintegrating films. Curr. Drug Deliv. 2013, 10, 96–108. [Google Scholar] [CrossRef]
- Manda, P.; Popescu, C.; Juluri, A.; Janga, K.; Kakulamarri, P.R.; Narishetty, S.; Narasimha Murthy, S.; Repka, M.A. Micronized Zaleplon Delivery via Orodispersible Film and Orodispersible Tablets. AAPS PharmSciTech 2018, 19, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Turković, E.; Vasiljević, I.; Drašković, M.; Parojčić, J. Orodispersible films—Pharmaceutical development for improved performance: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103708. [Google Scholar] [CrossRef]
- Allen, L.V., Jr. Basics of Compounding: Compounding Films. Int. J. Pharm. Compd. 2016, 20, 298–305. [Google Scholar] [PubMed]
- Bülbül, E.Ö.; Mesut, B.; Cevher, E.; Öztaş, E.; Özsoy, Y. Product transfer from lab-scale to pilot-scale of quetiapine fumarate orodispersible films using quality by design approach. J. Drug Deliv. Sci. Technol. 2019, 54, 101358. [Google Scholar] [CrossRef]
- Foo, W.C.; Khong, Y.M.; Gokhale, R.; Chan, S.Y. A novel unit-dose approach for the pharmaceutical compounding of an orodispersible film. Int. J. Pharm. 2018, 539, 165–174. [Google Scholar] [CrossRef]
- Zayed, G.M.; Rasoul, S.A.; Ibrahim, M.A.; Saddik, M.S.; Alshora, D.H. In vitro and in vivo characterization of domperidone-loaded fast dissolving buccal films. Saudi Pharm. J. 2020, 28, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Barhart, S.; Rathborne, N.; Hadgraft, J.; Roberts, M. Thin film oral dosage form in modified release drug delivery technology. Drug Pharm. Sci. 2008, 2, 209–216. [Google Scholar]
- Thabet, Y.; Breitkreutz, J. Orodispersible films: Product transfer from lab-scale to continuous manufacturing. Int. J. Pharm. 2018, 535, 285–292. [Google Scholar] [CrossRef]
- Alsofany, J.M.; Hamza, M.Y.; Abdelbary, A.A. Fabrication of Nanosuspension Directly Loaded Fast-Dissolving Films for Enhanced Oral Bioavailability of Olmesartan Medoxomil: In Vitro Characterization and Pharmacokinetic Evaluation in Healthy Human Volunteers. AAPS PharmSciTech 2018, 19, 2118–2132. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the new asset for the production of tailored medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.; Wu, L.; Liu, X.; Cheng, S.; Li, X. 3D printing: Innovative solutions for patients and pharmaceutical industry. Int. J. Pharm. 2023, 631, 122480. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of warfarin therapy using thermal ink-jet printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.; Amin, P. Orally Disintegrating Film of High-Dose BCS II Drug by Hot Melt Extrusion through Design of Experiment. J. Pharm. Innov. 2023, 18, 247–261. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Park, J.-B.; Alsulays, B.B.; Tiwari, R.V.; Almutairy, B.; Alshetaili, A.S.; Morott, J.; Shah, S.; Kulkarni, V.; Majumdar, S. Mefenamic acid taste-masked oral disintegrating tablets with enhanced solubility via molecular interaction produced by hot melt extrusion technology. J. Drug Deliv. Sci. Technol. 2015, 27, 18–27. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, A.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics 2021, 13, 1613. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef]
- Sauer, D.; Cerea, M.; DiNunzio, J.; McGinity, J. Dry powder coating of pharmaceuticals: A review. Int. J. Pharm. 2013, 457, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Prasad, L.K.; Keen, J.M.; LaFountaine, J.S.; Maincent, J.; Williams, R.O., III; McGinity, J.W. Electrostatic powder deposition to prepare films for drug delivery. J. Drug Deliv. Sci. Technol. 2015, 30, 501–510. [Google Scholar] [CrossRef]
- Prasad, L.K.; LaFountaine, J.S.; Keen, J.M.; Williams, R.O., 3rd; McGinity, J.W. Influence of process parameters on the preparation of pharmaceutical films by electrostatic powder deposition. Int. J. Pharm. 2016, 515, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nangare, S.; Jadhav, N.; Ghagare, P.; Muthane, T. Pharmaceutical Applications of Electrospinning. In Annales Pharmaceutiques Francaises; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–11. [Google Scholar]
- Yu, D.G.; Zhou, J. How can Electrospinning Further Service Well for Pharmaceutical Researches? J. Pharm. Sci. 2023, 112, 2719–2723. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Abdal-Hay, A.; Ivanovski, S.; Zhang, Y.S.; Sheikh, F.A. Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: Current status and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110756. [Google Scholar] [CrossRef] [PubMed]
- AnjiReddy, K.; Karpagam, S. Hyperbranched cellulose polyester of oral thin film and nanofiber for rapid release of donepezil; preparation and in vivo evaluation. Int. J. Biol. Macromol. 2019, 124, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef]
- Guo, X.; Cun, D.; Wan, F.; Bera, H.; Song, Q.; Tian, X.; Chen, Y.; Rantanen, J.; Yang, M. Comparative assessment of in vitro/in vivo performances of orodispersible electrospun and casting films containing rizatriptan benzoate. Eur. J. Pharm. Biopharm. 2020, 154, 283–289. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef]
- Ravasi, E.; Melocchi, A.; Arrigoni, A.; Chiappa, A.; Gennari, C.G.M.; Uboldi, M.; Bertarelli, C.; Zema, L.; Briatico Vangosa, F. Electrospinning of pullulan-based orodispersible films containing sildenafil. Int. J. Pharm. 2023, 643, 123258. [Google Scholar] [CrossRef]
- Edmans, J.G.; Murdoch, C.; Santocildes-Romero, M.E.; Hatton, P.V.; Colley, H.E.; Spain, S.G. Incorporation of lysozyme into a mucoadhesive electrospun patch for rapid protein delivery to the oral mucosa. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110917. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy-An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P. Development of asialoglycoprotein receptor-targeted nanoparticles for selective delivery of gemcitabine to hepatocellular carcinoma. Molecules 2019, 24, 4566. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Yt, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.M.; Hagesaether, E.; Tho, I. Solid lipid nanoparticle-loaded mucoadhesive buccal films—Critical quality attributes and in vitro safety & efficacy. Int. J. Pharm. 2021, 592, 120100. [Google Scholar] [CrossRef]
- Steiner, D.; Emmendörffer, J.F.; Bunjes, H. Orodispersible Films: A Delivery Platform for Solid Lipid Nanoparticles? Pharmaceutics 2021, 13, 2162. [Google Scholar] [CrossRef]
- Talekar, S.D.; Haware, R.V.; Dave, R.H. Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur. J. Pharm. Sci. 2019, 130, 215–224. [Google Scholar] [CrossRef]
- Shankar Raman, S.; Narayanan, V.H.B.; Durai, R. Lamotrigine Nanoparticle Laden Polymer Composite Oral Dissolving Films for Improving Therapeutic Potential of the Hydrophobic Antiepileptic Molecule. Assay Drug Dev. Technol. 2021, 19, 2–16. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Model-based description of disintegration time and dissolution rate of nanoparticle-loaded orodispersible films. Eur. J. Pharm. Sci. 2019, 132, 18–26. [Google Scholar] [CrossRef]
- Sinha, S.; Sonali; Garg, V.; Thapa, S.; Singh, S.; Chauhan, M.; Dutt, R.; Singh, R.P. Empagliflozin containing chitosan-alginate nanoparticles in orodispersible film: Preparation, characterization, pharmacokinetic evaluation and its in-vitro anticancer activity. Drug Dev. Ind. Pharm. 2022, 48, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.G.; Rathod, V.; Basim, P.; Gajera, B.; Dave, R.H. Understanding the Impact of Multi-factorial Composition on Efficient Loading of the Stable Ketoprofen Nanoparticles on Orodispersible Films Using Box-Behnken Design. J. Pharm. Sci. 2022, 111, 1451–1462. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chang, D.; Zhang, X.; Sui, H.; Kong, Y.; Zhu, R.; Wang, W. Oral fast-dissolving films containing lutein nanocrystals for improved bioavailability: Formulation development, in vitro and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, K.; Nguyen, H.T.; Nghiem, L.H.T.; Van Can, M.; Tran, T.H. Nanosized-Loratadine Embedded Orodispersible Films for Enhanced Bioavailability: Scalable Preparations and Characterizations. AAPS PharmSciTech 2022, 23, 78. [Google Scholar] [CrossRef]
- Steiner, D.; Tidau, M.; Finke, J.H. Embedding of Poorly Water-Soluble Drugs in Orodispersible Films-Comparison of Five Formulation Strategies. Pharmaceutics 2022, 15, 17. [Google Scholar] [CrossRef]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.M.; Minghetti, P.; Cilurzo, F. Trends in the Characterization Methods of Orodispersible Films. Curr. Drug Deliv. 2021, 18, 935–946. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Outlining critical quality attributes (CQAs) as guidance for the development of orodispersible films. Pharm. Dev. Technol. 2017, 22, 237–245. [Google Scholar] [CrossRef]
- Tsai, W.; Tsai, H.; Wong, Y.; Hong, J.; Chang, S.; Lee, M. Preparation and characterization of gellan gum/glucosamine/clioquinol film as oral cancer treatment patch. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Steiner, D.; Thabet, Y.; Breitkreutz, J.; Kwade, A. Comparative study on disintegration methods for oral film preparations. Eur. J. Pharm. Biopharm. 2018, 132, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. Int. J. Pharm. 2020, 589, 119876. [Google Scholar] [CrossRef] [PubMed]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Wu, J.; Shi, Z.; Zhao, P.; Su, H.; Wang, Q.; Jin, L. Nanofiber orodispersible films based on carboxymethyl curdlan and PEO: New delivery system for amlodipine besylate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128096. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-ghannam, A.A.; Al-Dhubiab, B.E.; Hasan, A.A. Mucoadhesive Film Embedded with Acyclovir Loaded Biopolymeric Nanoparticles: In vitro Studies. J. Young Pharm. 2017, 9, 100. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.; Song, I.O.; Lee, B.J.; Kang, C.Y.; Park, J.B. Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole. Pharmaceuticals 2022, 15, 895. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Saraiya, V.; Attimarad, M.; SreeHarsha, N.; Akrawi, S.H.; Shehata, T.M. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm. J. 2020, 28, 201–209. [Google Scholar] [CrossRef]
- Park, H.-R.; Seok, S.H.; Hwang, K.-M.; Kim, J.-Y.; Park, C.-W.; Park, E.-S. Formulation of sustained-release orodispersible film containing drug–resin complexes of donepezil hydrochloride. J. Pharm. Investig. 2022, 52, 259–272. [Google Scholar] [CrossRef]
- Ouda, G.I.; Dahmash, E.Z.; Alyami, H.; Iyire, A. A Novel Technique to Improve Drug Loading Capacity of Fast/Extended Release Orally Dissolving Films with Potential for Paediatric and Geriatric Drug Delivery. AAPS PharmSciTech 2020, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Hafner, A.; Lovrić, J.; Kregar, M.L.; Pepić, I.; Vanić, Ž.; Cetina-Čižmek, B.; Filipović-Grčić, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Pamlényi, K.; Regdon, G., Jr.; Nemes, D.; Fenyvesi, F.; Bácskay, I.; Kristó, K. Stability, permeability and cytotoxicity of buccal films in allergy treatment. Pharmaceutics 2022, 14, 1633. [Google Scholar] [CrossRef] [PubMed]
- Samaras, G.; Bikos, D.; Vieira, J.; Hartmann, C.; Charalambides, M.; Hardalupas, Y.; Masen, M.; Cann, P. Measurement of molten chocolate friction under simulated tongue-palate kinematics: Effect of cocoa solids content and aeration. Curr. Res. Food Sci. 2020, 3, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Ranmal, S.R.; Nhouchi, Z.; Keeley, A.; Adler, L.; Lavarde, M.; Pensé-Lhéritier, A.M.; Tuleu, C. Taste assessment for paediatric drug Development: A comparison of bitterness taste aversion in children versus Naïve and expert young adult assessors. Int. J. Pharm. 2023, 647, 123494. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Usui, R.; Ikezaki, H.; Tahara, K.; Takeuchi, H. An advanced technique using an electronic taste-sensing system to evaluate the bitterness of orally disintegrating films and the evaluation of model films. Int. J. Pharm. 2017, 531, 179–190. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mohd Jais, N.; Sulaiman, A.; Mohd Nor, M.Z.; Abdul Karim Shah, N.N.; Ariffin, S.H. Optimizing the processing factor and formulation of oat-based cookie dough for enhancement in stickiness and moisture content using response surface methodology and superimposition. Processes 2020, 8, 797. [Google Scholar] [CrossRef]
- Hossain, M.L.; Nguyen, M.; Benington, L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Application of a Customised Franz-Type Cell Coupled with HPTLC to Monitor the Timed Release of Bioactive Components in Complex Honey Matrices. Methods Protoc. 2023, 6, 70. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Morsy, M.A. Dose conversion between animals and humans: A practical solution. Indian J. Pharm. Educ. Res. 2022, 56, 600–607. [Google Scholar] [CrossRef]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Masen, M.; Cann, P.; Hanson, B.; Tuleu, C.; Orlu, M. Modernising Orodispersible Film Characterisation to Improve Palatability and Acceptability Using a Toolbox of Techniques. Pharmaceutics 2022, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Shen, B.; Xu, H.; Bai, J.; Dai, L.; Lv, Q.; Han, J.; Yuan, H. Formulation and optimization of a novel oral fast dissolving film containing drug nanoparticles by Box-Behnken design-response surface methodology. Drug Dev. Ind. Pharm. 2014, 40, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Dave, R.H. Formulation and characterization of acetaminophen nanoparticles in orally disintegrating films. Drug Deliv. 2016, 23, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.S.; Gowda, D.V.; Kumar, T.P.; Rosenholm, J.M. A Comprehensive Review of Patented Technologies to Fabricate Orodispersible Films: Proof of Patent Analysis (2000–2020). Pharmaceutics 2022, 14, 820. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V. Orodispersible Thin Film: A new patient-centered innovation. J. Drug Deliv. Sci. Technol. 2020, 59, 101843. [Google Scholar] [CrossRef]

