1. Introduction
According to the International Coordination Committee for the Registration of Pharmaceutical Products for Human Use (ICH) guidelines and the M9 Bioequivalence Exemptions Based on the Biopharmaceutical Classification System [1,2,3,4] permeability is closely related to the rate and extent of drug absorption in the body and is one of the key factors in the transport of drugs in the body through expanded membranes and is relevant to drug development. Pharmaceutical excipients are non-physiologically active substances in pharmaceuticals that affect their absorption and bioavailability in the body [5,6,7]. For poorly permeable drugs, permeation enhancers are commonly used [8,9,10,11], for example, fatty alcohols, which increase drug permeability by altering the structure and properties of biofilms. Co-solvents could provide a way to address the issue of insoluble drugs, but they also lead to change in permeability [12].
SBE-β-CD [13,14,15,16,17,18], a new pharmaceutical excipient often used as a co-solvent, has been included in USP44-NF39 under the name Betadex Sulfobutyl Ether Sodium, and is currently available as Captisol® [19,20] and Dexolve® [21]. It is widely used in biologics and insoluble drugs [22,23,24]. With an average degree of substitution of 6.5, sulfobutyl can be substituted at the 2-, 3- and 6-hydroxyl groups of the β-glucose unit, ameliorating the nephrotoxicity problems of β-cyclodextrins [25], which is a better co-solvent for solubility. However, usually, the increase in drug solubility with co-solvents is followed by a corresponding change in drug permeability, and there is a delicate balance between the two effects [12,26,27,28]. Under certain pH conditions, when the osmotic effect of increased solubility exceeds the inhibition of permeability by the co-solvent, an increase in drug permeation is presented, e.g., hydroxypropyl-β-cyclodextrin (HP-β-CD) at pH 6.2 increases the permeability of albendazole; conversely, the permeation of the drug is reduced, e.g., HP-β-CD at pH 6.2 reduces the permeability of bupivacaine. For drugs with good solubility, cyclodextrins inhibit their permeability in vivo [29]. Current studies on SBE-β-CD have focused on the effect on solubility, with less exploration of permeability and a lack of further studies of the mechanisms, both of which play a pivotal role in drug absorption. It is important to investigate the mechanisms through which SBE-β-CD affects drug permeability in vitro and in vivo based on the fully dissolved state of the drug. To elucidate the mechanism by which SBE-β-CD affects drug permeability, ranitidine was chosen as a model drug to study the changes in permeability under human intestinal pH conditions. As a histamine-like H2 receptor blocker [30], ranitidine is commonly used in the treatment of peptic gastric ulcer and acid reflux; the drug is readily soluble in water, which precludes osmotic effects due to increased solubility.
In this study, the effects of different concentrations of SBE-β-CD on the permeability of ranitidine and its absorption in rats were investigated using parallel artificial membrane permeability assay (PAMPA), a permeability study on Caco-2 cells, and pharmacokinetic studies in rats with oral administration of ranitidine and SBE-β-CD. Based on this finding, a zeta potential study was then designed to investigate the mechanism through which SBE-β-CD reduces drug permeability (Figure 1).

Figure 1. SBE-β-CD and ranitidine form a complex to reduce permeability.
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. In Vitro Analysis of Inhibitory Effect of Ranitidine by SBE-β-CD
3.2. In Vivo Analysis of Inhibitory Effect of Ranitidine by SBE-β-CD
The peak areas of ranitidine obtained from the rat plasma samples were determined and brought into the regression equation to calculate the concentration of ranitidine in plasma samples. shows the pharmacokinetic parameters detected in rats after the administration of ranitidine and SBE-β-CD at a dose of 0.12% and 0.36%. Pharmacokinetic parameters such as elimination half-life (t1/2), time to peak (Tmax), peak concentration (Cmax), area under the drug–time curve (AUCall), apparent volume of distribution (Vz), body clearance (CL), and mean residence time (MRTlast) were calculated using the non-compartment model of Winnonlin software 5.0 after administration of 0.12% SBE-β-CD and 0.36% SBE-β-CD with ranitidine. The peak concentration of ranitidine after SBE-β-CD was significantly lower than that of ranitidine API (0.73 ± 0.18 μg/mL vs. 0.064 ± 0.009 μg/mL, 0.30 ± 0.05 μg/mL, 0.13 ± 0.007), indicating that both the rate and extent of absorption of ranitidine were influenced by SBE-β-CD (Figure 4). The area under the drug–time curve of ranitidine after injection of 0.12% SBE-β-CD and 0.36% SBE-β-CD was also significantly reduced compared to that of ranitidine only (3.6 ± 1.2 h μg/mL vs. 0.3 ± 0.1 h·μg/mL, 0.5 ± 0.1 h·μg/mL). This result may be due to SBE-β-CD affecting the rate and extent of absorption of ranitidine, resulting in a decline in the amount of drug reaching the systemic circulation.
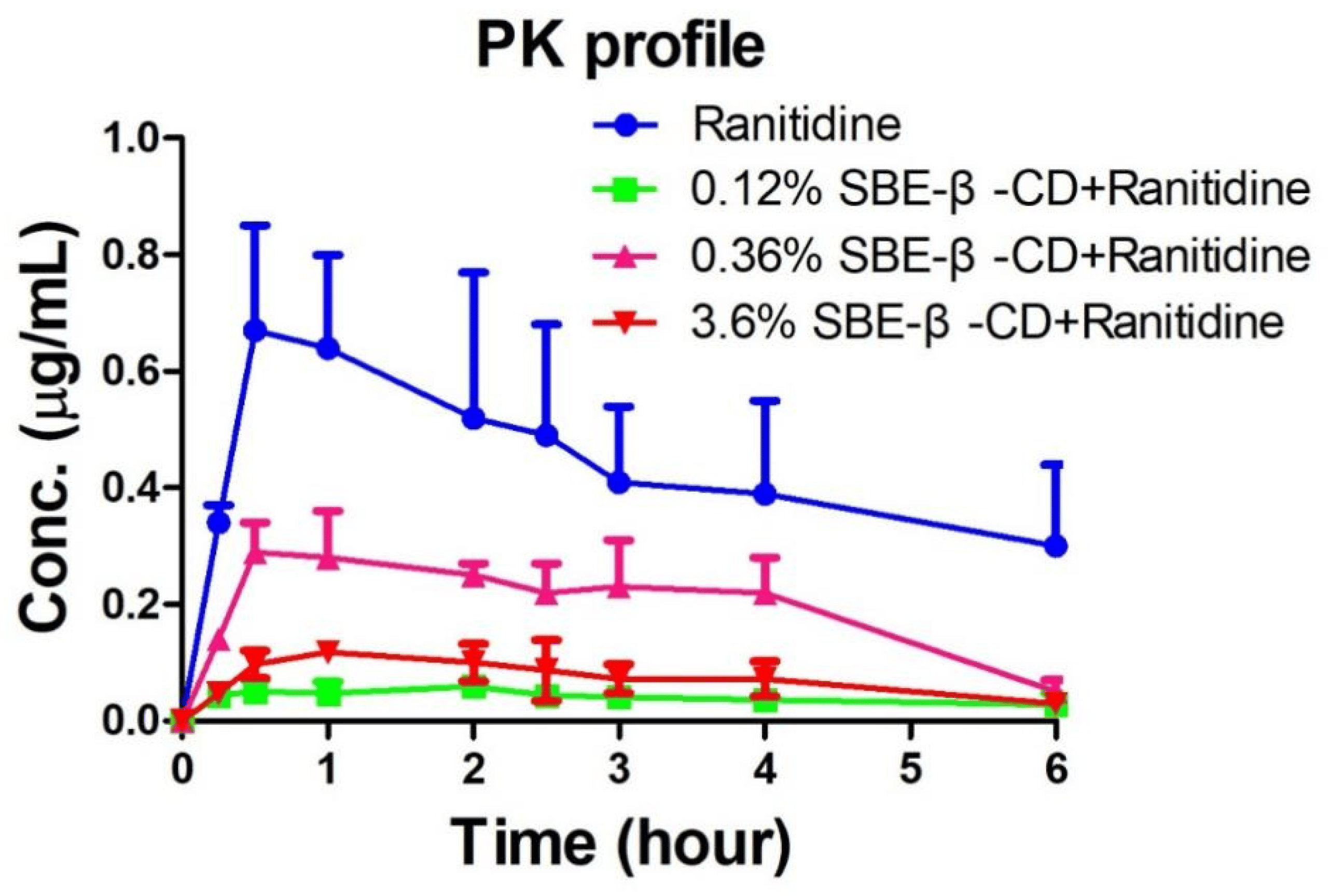
Figure 4. Ranitidine plasma concentration–time curve in rats.
3.3. Mechanism of Inhibition of Ranitidine Permeability In Vivo by SBE-β-CD
To investigate the mechanism through which SBE-β-CD affects the permeability of ranitidine, zeta potential measurements were performed on ranitidine hydrochloride solution, 0.06%, 0.12%, 0.24%, 0.36%, 0.72%, 1.44% and 3.6% of SBE-β-CD solution, and the corresponding concentrations of the mixture of SBE-β-CD and ranitidine. The experimental results (see and Figure 5) showed that the zeta potential of the mixed solution of SBE-β-CD with ranitidine increased with increasing concentrations of SBE-β-CD. The zeta potential of the 0.06% SBE-β-CD and ranitidine mixture was 16.60 mV, which was significantly lower than the 0.06% SBE-β-CD solution (−5.09 mV). The zeta potential of the 0.12% SBE-β-CD and ranitidine mixture was −17.90 mV, which was significantly lower than that of the 0.12% SBE- β-CD solution (−3.66 mV). The zeta potential of 0.24% SBE-β-CD and ranitidine mixture was −16.6 mV, which was lower and significantly different from that of 0.24% SBE-β-CD solution (−0.52 mV). Continuing to increase the concentration of SBE-β-CD in ranitidine solution concentration, the zeta potential gradually approached that of the SBE-β-CD solution, probably due to the excess of SBE-β-CD after increasing the concentration, consequential in the dominance of the potential of SBE-β-CD in the solution. Using Pearson correlation analysis, the Zeta potential was positively and strongly correlated with the concentration of SBE-β-CD in the ranitidine mixture (r = 0.7282, p = 0.0405).
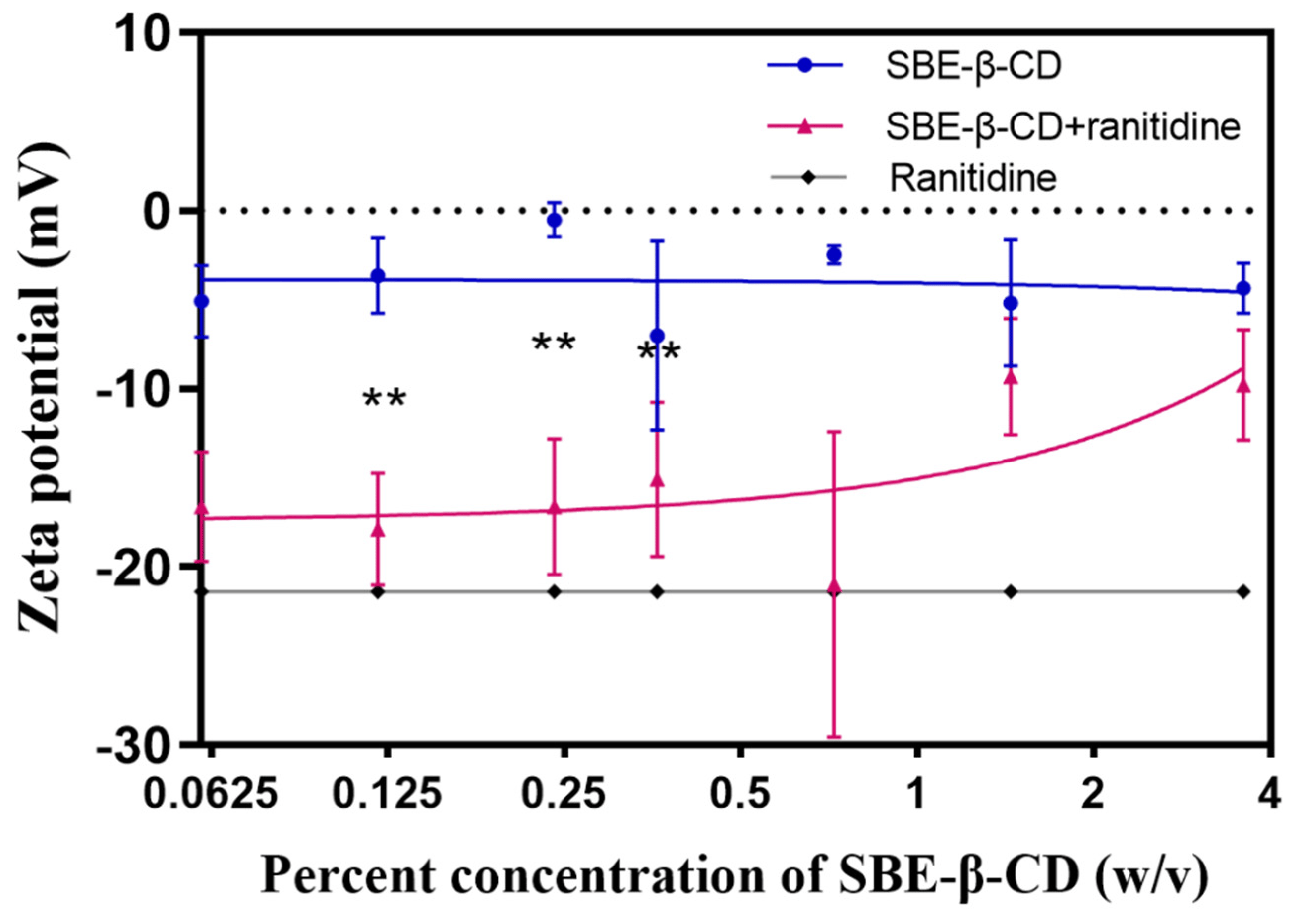
Figure 5. The zeta potential curves of different concentrations of SBE-β-CD solutions and mixed solutions of SBE-β-CD and ranitidine. ** p < 0.01.
Zeta potential is a measure of the stability of the dispersion system, and changes in this indicator suggest the formation of complexes in the dispersion system. The interaction between SBE-β-CD and ranitidine consists of commonly attractive charge interactions. Additionally, the sulphury element in SBE-β-CD and the nitrogen element in ranitidine readily form hydrogen bonds, causing them to bind and form complexes. In the human intestine there is an unstirred aqueous layer consisting of in vivo mucus with a thickness of 30–100 μm [25]. Once formed, the complexes need to pass successively through the hydrated layer and the lipid barrier before they can enter the body. The change in size and shape causes the complex to encounter greater spatial site resistance when passing through the unstirred aqueous layer and biofilm, resulting in slower absorption and reduced permeability of ranitidine.
In summary, the charge interaction between SBE-β-CD and ranitidine occasioned the formation of a complex, which in turn altered the rate of absorption and permeability of ranitidine. This interaction may have influenced the behavior of the drug as it passed through the hydrated layer and biofilm by increasing the spatial site resistance. This has important implications for understanding the mechanisms through which SBE-β-CD affects drug permeability and provides a useful reference for drug design and optimizing drug delivery systems.
4. Discussion
Drug permeability is one of great factors for drug development and therapeutic efficacy, as it directly affects the bioavailability, pharmacodynamic properties and therapeutic efficacy of the drug. Drug permeability is influenced by many factors, such as particle size [31]. SBE-β-CD is often used as a co-solvent and excipient in pharmaceutical formulations, and the mechanisms through which this excipient affects drug permeability are less well considered. Therefore, in this study, two in vitro permeation systems, PAMPA assay and Caco-2 cell assay, were applied to investigate the effect of SBE-β-CD on the permeability of ranitidine. After that, pharmacokinetic experiments in rats were carried out to further confirm that SBE-β-CD could inhibit the permeability of ranitidine in vivo. The mechanism is related to the formation of the complex, with changes in size and shape leading to increased spatial site resistance to passage through the intestinal barrier, which in turn leads to slower absorption and reduced permeability.
For drugs with poor oral permeability, the correct choice of drug excipient is crucial to improve the bioavailability and efficacy of the drug. This study investigates the mechanism of SBE-β-CD inhibition of drug permeability and fills a gap in this research field. Our results provide an important reference for the prescription design of drugs with poor oral permeability and are of great significance. For drugs with poor oral permeability, changes in permeability can affect the acquisition of biological immunity [32], so the dose of SBE-β-CD should be carefully selected. Further studies could explore the mechanism of the effect of other cyclodextrin polymers on drug permeability. Cyclodextrin polymers [33] are a class of substances based on the modification of their substituents by the cyclodextrin structure, which have similar cavity structures and molecular recognition capabilities, but may differ in their chemical structure and properties. By studying the interaction of different types of cyclodextrin polymers with drugs, a more comprehensive understanding of the effects of these excipients on drug permeability can be obtained and can provide a theoretical basis for the development of novel cyclodextrin excipients. In addition, the effect of the application of SBE-β-CD in combination with other excipients on drug permeability can be further investigated. The selection and combination of excipients may have a synergistic effect on drug permeability, which helps to improve the bioavailability and efficacy of the drug. By studying the combination and ratio of different excipients, the permeability and therapeutic effect of the drug can be optimized and the absorption and bioavailability of the orally administered drug can be improved.
5. Conclusions
In this study, ranitidine was selected as a model drug, and its permeability was investigated in vitro and in vivo with SBE-β-CD at human intestinal pH, and the mechanism through which SBE-β-CD affects drug permeability in vivo was investigated based on the complete dissolution of the drug. SBE-β-CD was found to inhibit the in vivo permeability of ranitidine under human intestinal pH conditions with a mechanism related to the formation of complexes, where the charge of SBE-β-CD interacts with ranitidine to form complexes that are subject to greater resistance to crossing the physiological barrier, resulting in changes in drug permeability. Consequently, the dose of SBE-β-CD should be carefully selected when applying SBE-β-CD for the oral administration of drugs with poor permeability.
References
- Donovan, D.H.O.; De Fusco, C.; Kuhnke, L.; Reichel, A. Trends in Molecular Properties, Bioavailability, and Permeability across the Bayer Compound Collection. J. Med. Chem. 2023, 66, 2347–2360. [Google Scholar] [CrossRef]
- Garg, A.; Garg, R. A Comprehensive Review on Recent Advances and Considerations for the Selection of Cell-based In-vitro Techniques for the Assessment of Permeability of Drug Molecules. Curr. Drug Deliv. 2023, 20, 526–544. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Visentin, S.; Butnarasu, C.; Stein, P.C.; di Cagno, M.P. Commercially Available Cell-Free Permeability Tests for Industrial Drug Development: Increased Sustainability through Reduction of In Vivo Studies. Pharmaceutics 2023, 15, 592. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, Z.; Tu, Y.; Hu, W.; Xie, C.; Ye, L. Experimental and computational models to investigate intestinal drug permeability and metabolism. Xenobiotica 2023, 53, 25–45. [Google Scholar] [CrossRef]
- Ruiz-Picazo, A.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I.; Bermejo, M. Effect of Common Excipients on Intestinal Drug Absorption in Wistar Rats. Mol. Pharm. 2020, 17, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Picazo, A.; Lozoya-Agullo, I.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Effect of excipients on oral absorption process according to the different gastrointestinal segments. Expert Opin. Drug Deliv. 2020, 18, 1005–1024. [Google Scholar] [CrossRef]
- Takizawa, Y.; Goto, N.; Furuya, T.; Hayashi, M. Influene of Pharmaceutical Excipients on the Membrane Transport of a P-glycoprotein Substrate in the Rat Small Intestine. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Deepanjan, D.; Panchal, D.S.; Venuganti, V.V.K. Transdermal Delivery of Vancomycin Hydrochloride: Influence of Chemical and Physical Permeation Enhancers. Int. J. Pharm. 2021, 609, 120663. [Google Scholar]
- Sadashivaiah, R.; Satheesha Babu, B.K.; Rohith, G. A Comparative Evaluation of Permeation Enhancers for Ropinirole Hydrochloride a Bcs Class Iii Drug to Be Formulated as Transdermal Drug Delivery System. Int. J. Pharm. Sci. Res. 2020, 11, 6149–6156. [Google Scholar]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef]
- Li, S.K.; Chantasart, D. Skin Permeation Enhancement in Aqueous Solution: Correlation with Equilibrium Enhancer Concentration and Octanol/Water Partition Coefficient. J. Pharm. Sci. 2018, 108, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Pawar, B.M.; Rahman, S.N.R.; Pawde, D.M.; Goswami, A.; Shunmugaperumal, T. Orally Administered Drug Solubility-Enhancing Formulations: Lesson Learnt from Optimum Solubility-Permeability Balance. Aaps. Pharmscitech. 2021, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Feitosa, R.; Souza Ribeiro Costa, J.; van Vliet Lima, M.; Sawa Akioka Ishikawa, E.; Cogo Müller, K.; Bonin Okasaki, F.; Sabadini, E.; Garnero, C.; Longhi, M.R.; Lavayen, V.; et al. Supramolecular Arrangement of Doxycycline with Sulfobutylether-β-Cyclodextrin: Impact on Nanostructuration with Chitosan, Drug Degradation and Antimicrobial Potency. Pharmaceutics 2023, 15, 1285. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.E.M.; Almaiman, A.A.; Alshehade, S.A.; Alsalemi, W.; Kamran, S.; Suliman, F.O.; Alshawsh, M.A. Characterization of Thymoquinone-Sulfobutylether-Beta-Cyclodextrin Inclusion Complex for Anticancer Applications. Molecules 2023, 28, 4096. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Zagami, R.; De Plano, L.M.; Burduja, N.; Guglielmino, S.P.P.; Scolaro, L.M.; Mazzaglia, A. Antimicrobial and Antibiofilm Photodynamic Action of Photosensitizing Nanoassemblies Based on Sulfobutylether-β-Cyclodextrin. Molecules 2023, 28, 2493. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cui, H.; Guo, X.; Dong, Q.; You, X.; Guo, X.; Qin, S.; Jia, L. Enantioseparation by Zeolitic Imidazolate Framework-8-Silica Hybrid Monolithic Column with Sulfobutylether-Beta-Cyclodextrin as a Chiral Additive in Capillary Electrochromatography. Microchimica Acta 2023, 190, 315. [Google Scholar] [CrossRef]
- Kovacs, T.; Kurtan, K.; Varga, Z.; Nagy, P.; Panyi, G.; Zakany, F. Veklury® (Remdesivir) Formulations Inhibit Initial Membrane-Coupled Events of Sars-Cov-2 Infection Due to Their Sulfobutylether-Beta-Cyclodextrin Content. Br. J. Pharmacol. 2023, 180, 2064–2084. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Liang, H.; Huang, X.; Meng, N.; Zhou, N. Silver Nanoparticles Based on Sulfobutylether-Beta-Cyclodextrin Functionalized Graphene Oxide Nanocomposite: Synthesized, Characterization, and Antibacterial Activity. Colloids Surf. B-Biointerfaces 2023, 221, 113009. [Google Scholar] [CrossRef]
- Das, S.K.; Kahali, N.; Bose, A.; Khanam, J. Physicochemical Characterization and in Vitro Dissolution Performance of Ibuprofen-Captisol® (Sulfobutylether Sodium Salt of Beta-Cd) Inclusion Complexes. J. Mol. Liq. 2018, 261, 239–249. [Google Scholar] [CrossRef]
- Khurana, R.; Kakatkar, A.S.; Chatterjee, S.; Barooah, N.; Kunwar, A.; Bhasikuttan, A.C.; Mohanty, J. Supramolecular Nanorods of (N-Methylpyridyl) Porphyrin With Captisol: Effective Photosensitizer for Anti-bacterial and Anti-tumor Activities. Front. Chem. 2019, 7, 452. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced Solubility, Stability, Permeation and Anti-Cancer Efficacy of Celastrol-Β-Cyclodextrin Inclusion Complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, L.; Wang, H.; Dai, J.; Bao, Y.; Hou, B.; Yin, Q. Enhancing the Water Solubility of 9-Fluorenone Using Cyclodextrin Inclusions: A Green Approach for the Environmental Remediation of OPAHs. Crystals 2023, 13, 775. [Google Scholar] [CrossRef]
- Petitprez, J.; Legrand, F.-X.; Tams, C.; Pipkin, J.D.; Antle, V.; Kfoury, M.; Fourmentin, S. Huge Solubility Increase of Poorly Water-Soluble Pharmaceuticals by Sulfobutylether-Beta-Cyclodextrin Complexation in a Low-Melting Mixture. Environ. Chem. Lett. 2022, 20, 1561–1568. [Google Scholar] [CrossRef]
- Szabó, Z.-I.; Gál, R.; Gáll, Z.; Vancea, S.; Rédai, E.; Fülöp, I.; Sipos, E.; Donáth-Nagy, G.; Noszál, B.; Tóth, G. Cyclodextrin complexation improves aqueous solubility of the antiepileptic drug, rufinamide: Solution and solid state characterization of compound-cyclodextrin binary systems. J. Incl. Phenom. Macrocycl. Chem. 2017, 88, 43–52. [Google Scholar] [CrossRef]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef]
- Fine-Shamir, N.; Beig, A.; Dahan, A. Adequate Formulation Approach for Oral Chemotherapy: Etoposide Solubility, Permeability, and Overall Bioavailability from Cosolvent- Vs. Vitamin E Tpgs-Based Delivery Systems. Int. J. Pharm. 2021, 597, 120295. [Google Scholar] [CrossRef]
- Porat, D.; Dahan, A. Active intestinal drug absorption and the solubility-permeability interplay. Int. J. Pharm. 2018, 537, 84–93. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Michalak, D.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. Antioxidant and Anti-Glycation Potential of H2 Receptor Antagonists—In Vitro Studies and a Systematic Literature Review. Pharmaceuticals 2023, 16, 1273. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Q.; Zhang, Y.; Ding, J.; Yang, M.; Yang, L.; Wang, W.; Cui, P.; Dai, Z.; Ma, L. Preparation and Evaluation of Liposomes Containing Ethanol and Propylene Glycol as Carriers for Nicotine. Curr. Drug Deliv. 2024, 21, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Metry, M.; Polli, J.E. Evaluation of Excipient Risk in BCS Class I and III Biowaivers. AAPS J. 2022, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, V.R.; Dahiya, L.; Singh, G.; Sarwal, A. Impact of cyclodextrin derivatives on systemic release of duloxetine HCl via buccal route. Drug Dev. Ind. Pharm. 2020, 46, 931–945. [Google Scholar] [CrossRef] [PubMed]

