1. Introduction
Scientific evidence is necessary to support the importance of the bioavailability of nutraceutical/pharmaceutical substances, especially those that are poorly soluble, such as flavonoids. Many flavonoids currently used as dietary supplements or medical devices are poorly soluble and demonstrate poor bioavailability [1]. In 2021, according to the Nutrition Business Journal, U.S. herbal dietary supplement retail sales were worth about USD 12.350 billion. That is a 10% increase in total sales compared to the previous year and is the second highest for these products after 2020’s record increase of 17% from 2019 [2]. Unfortunately, the bioavailability of herbal supplements is often overlooked, which complicates the assessment of their dose-related effects and the comparison of marketed products. At the same time, the pharmaceutical industry and regulatory authorities (European Medicines Agency and U.S. Food and Drug Administration) have already developed approaches to the assessment and classification of poorly soluble drugs (e.g., biopharmaceutical classification system), which could be used and applied to flavonoids with non-medical claims [3,4].
The aim of this investigation is to use a representative list of flavonoids; overview their bioavailability-related physiochemical properties pKa, log P, solubility, solubility parameters, melting temperature, and permeability; and discuss the formulation-related attributes of flavonoids in the context of their bioavailability.
2. Discussion
2.1. Flavonoids—Classification, Health Effects, Applications
Hydroxylated polyphenols, also called flavonoids, are a group of secondary plant metabolites that are abundantly present throughout the whole plant kingdom, including cereals, fruits, vegetables, herbs, seeds, and flowers of numerous plants, which are important dietary sources. Flavonoids are widely distributed in all plant parts, including the roots, leaves, and shoots. In the literature, 8000–10,000 compounds of polyphenols have been identified as flavonoids [5,6]. Numerous studies have revealed that flavonoids have several biological effects and properties [7,8,9,10,11]. The properties of flavonoids include antiviral activities, particularly against various types of human herpesviruses such as tumor herpesviruses. Flavonoids have antiallergenic and anticancer effects, as well as the potential to aid in the development of personalized, preventive, and predictive medicine. Thanks to these health benefits, more people are taking flavonoids not only with food but also as dietary supplements—single purified compounds and/or complex mixtures of compounds [6,12,13,14]. It has been reported that the mean intake of flavonoids from different foods in Europe was 428 ± 49 mg per day. Different food choices by country and, consequently, different preferences for the primary dietary sources may influence flavonoid consumption. Plants that contain flavonoids have also been used medicinally for a long time [15].
Regarding the chemistry of these hydroxylated polyphenols, the word “flavonoid” can be applied to compounds in three cases: (1) compounds with a C15 skeleton (also known as C6-C3-C6 backbone structure) that are derivatives of phenyl-substituted propylbenzene; (2) phenyl-substituted propylbenzene derivatives with a C16 backbone (rotenoids, mainly used as insecticides); (3) compounds with a structure that is based on phenyl-substituted propylbenzene derivatives condensed with C6-C3 precursors, also known as flavonolignans [16]. Flavonoids consisting of 15 carbon atoms are structured into two 6-carbon rings (referred to as the A- and B-rings) joined by a heterocyclic benzopyran C-ring that contains oxygen (Figure 1). Based on the position of the B-ring, as well as saturation and oxidation of the C-ring, flavonoids are classified into eight subclasses: flavones, isoflavones, flavonols, chalcones, anthocyanidins, flavanones, flavanols (flavan-3-ols), and flavanonols. Furthermore, flavonoids in natural products exist not only as aglycones (the parent cyclic structures, free form) but also as β-glycosides (O- and C-glycosylated derivatives) [13,16]. Many studies conducted previously have focused mostly on compounds with a C15 skeleton and have rarely included flavonolignans or did not include compounds from all eight subclasses.
Figure 1. The general skeleton structure of flavonoids.
Flavones, found in the leaves, flowers, and fruits of plants such as mint and chamomile, are a subgroup of compounds with a ketone in position 4 of the C ring and a double bond between positions 2 and 3. The main and more commonly described compounds of this subgroup are apigenin and luteolin (their glycosides, apigetrin and cynaroside) [17]. Additionally, for many years, there has been interest in baicalin and its aglycone, baicalein, a major flavonoid found in the rhizomes of the traditional Chinese medicinal herb Scutellaria baicalensis L. [18,19,20].
Flavonols are ketone-containing flavonoids found in an assortment of vegetables (e.g., onions) and fruits (e.g., apples and berries), as well as in beverages (tea and wine). The most studied compounds in this group are quercetin, myricetin, fisetin, and kaempferol. Quercetin should especially be emphasized in this group, as it is at the top of the list of best-selling dietary supplements [21,22]. Consumption of flavonols has been linked to a number of health advantages, including antioxidant activity and a decreased risk of vascular disease. Additionally, a quercetin glycoside called quercetin 3-O-rutinoside (rutin) is a well-liked flavonoid supplement that is frequently combined with quercetin dosages [23]. Thus, rutin trihydrate (also known as rutoside trihydrate), in addition to trypsin and bromelain, is a medication used to relieve musculoskeletal pain in conditions such as osteoarthritis and rheumatoid arthritis [24,25].
Isoflavones are isomers of flavones that are dominantly found in leguminous plants and are mostly extracted from soybeans. Commonly found isoflavones are the aglycones daidzein and genistein and the glycosides daidzin and genistin. Isoflavones are referred to as phytoestrogens because of their structural and biological similarities to the female hormone estrogen. It has been discovered that phytoestrogens may be used as an alternative therapy for hormone-dependent diseases such as cancer, menopausal syndrome, cardiovascular disorders, and osteoporosis. By boosting bone density, isoflavones also contribute to bone health and bone strength [26].
Furthermore, the flavanones have the C-ring saturated and can also be called dihydroflavones. Unlike flavones, these compounds have a saturated double bond between positions 2 and 3. Flavanones are mostly associated with oranges, lemons, and grapefruits, as they are present in all citrus fruits and are responsible for the bitter taste in juice and peel. The most known compounds of this subclass are hesperetin and naringerin, but even better known is a combination of two compounds: hesperidin (hesperetin 7-O-β-rutinoside) and diosmin (diosmetin 7-O-rutinoside). Although diosmin and its aglycone diosmetin belong to flavones, this combination is widely presented in supplements and is used in authorized medicine (Daflon®). Citrus flavonoids have intriguing pharmacological properties such as antioxidant and anti-inflammatory properties; they can also fulfill the role of blood lipid-lowering and cholesterol-lowering agents [17,27].
Flavanols, also called as “flavan-3-ols” or “catechins”, have a hydroxyl group at position 3 but lack both the ketone group and the saturated bond between positions 2 and 3. Catechins are widely found in fruits and berries as well as herbal plants [13,28]. Flavanonols, also called “dihydroflavonols,” are limitedly distributed in citrus fruits and Glycosmis L. species [29]. Taxifolin, engeletin, and puyanol are the representatives of this class.
Chalcones are characterized by the absence of the so-called “C-ring” of the basic flavonoid structure. This group can also be referred to as open-chain flavonoids. Representatives of this group are phloretin, phlorizin, arbutin, and more. Chalcones occur in vegetables, fruits and berries such as tomatoes, pears, and strawberries [17]. Anthocyanidins are groups of compounds characterized by the lack of the ketone group and by having two double bonds between positions 1 and 2, as well as between 3 and 4 (C-ring). Representatives such as delphinidin, malvidin, and cyanidin are found in flowers and berries [13].
A popular compound among flavonolignans is silibinin. Silibinin is found in sylimarin, which is a common extract from the seeds of milk thistle (Silybum marianum L.). Currently, the main uses of milk thistle are for hepatoprotection, the prevention and therapy of liver disease and hepatic injury [30]. Figure 2 describes the structure and classification of the monomeric flavonoids and glycosides (reviewed in the paper), utilizing the framework for flavonoid structural organization [31].
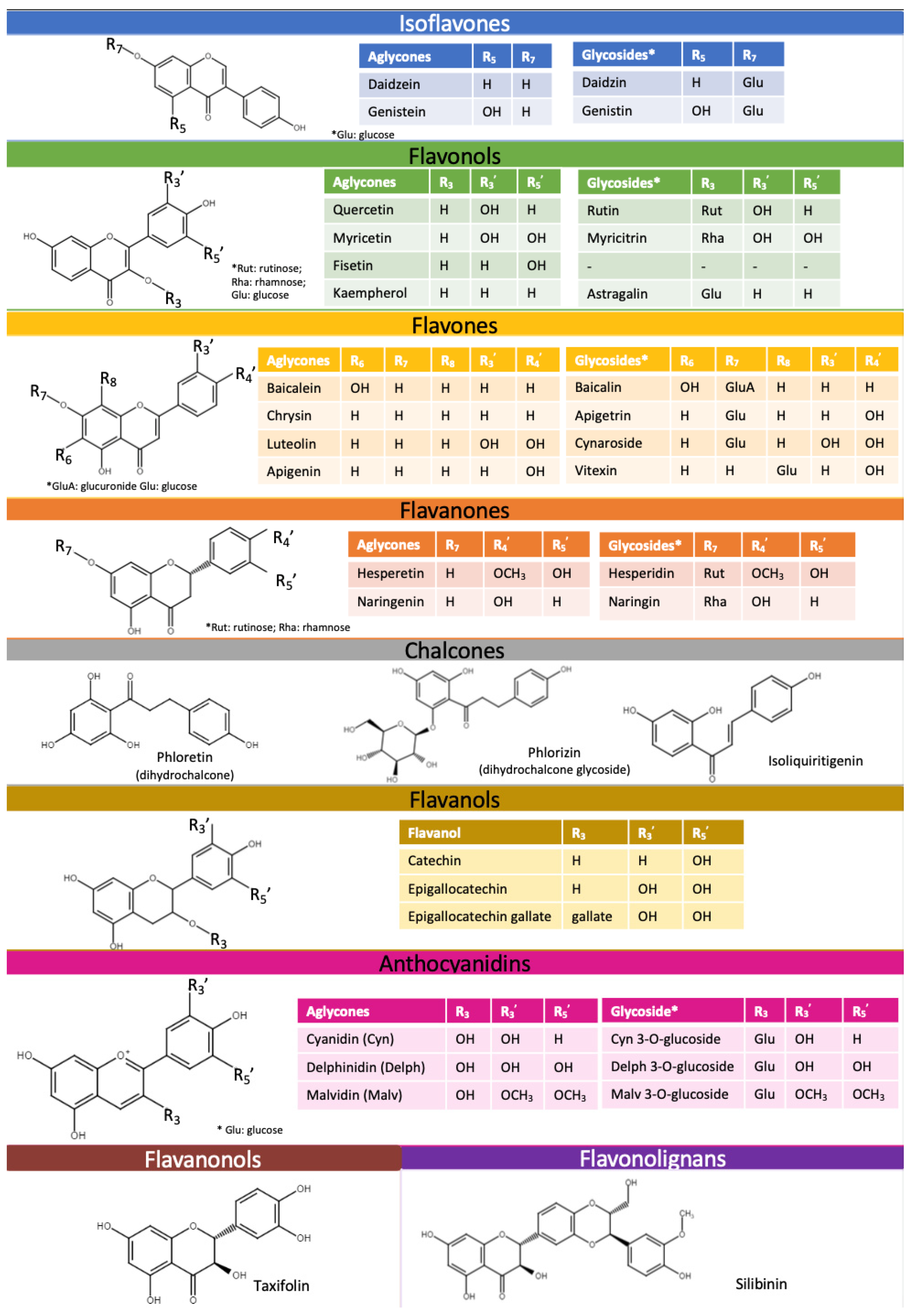
Figure 2. Structures of reviewed flavonoids (aglycones and glycosides) organized by their subclass. This color classification is utilized in all graphs throughout this review.
2.2. Acid-Base Dissociation Constant (pKa)
The pKa of a compound significantly impacts biopharmaceutical attributes such as solubility, lipophilicity, permeability, and protein binding. Understanding the dissociation constant of weak acid and base drugs is crucial for determining their ionic state across various pH levels [32]. Despite recent attention on flavonoids, there is a lack of comprehensive knowledge about their experimental pKa values and other physicochemical properties. The likely reason for this is that they are mostly extracted and used as a sum of compounds but not as highly purified compounds. The molecular complexity of flavonoids also contributes to this issue [33]. Computer-aided calculations in cheminformatics and computational pharmacology are widely used to determine new drug-like molecules, saving time, human effort, and resources, especially when experimental data are unavailable [34]. However, these predicted values rarely match the experimentally determined data due to a variety of factors. Additionally, it is impossible to distinguish the range of acidity of certain -OH groups in polyhydroxylated phenols using spectrophotometric or potentiometric titrations. To the best of our knowledge, only a few studies have actually addressed the location of the main dissociation [35]. Since experimental values are missing for some representatives of aglycones and several representatives of glycosides, this is an area for potential research.
During the review, experimental data from different sources as well as calculated values of aglycones (Figure 3) and flavonoid glycosides (Figure 4) were collected. An analysis of the literature on dissociation constants reveals significant variation among published values for most flavonoids. Most often, the experimental pKa values of flavonoids found in the literature are scattered and appear as secondary aspects in broader studies. One of the exceptions is a recently published paper by Fuguet et al. [33]. Due to this reason, multiple experimental values were plotted as ranges of values. Only pKa values that are partially or fully ionized within a physiological pH (approximately up to 7.5) were included in the plots [36,37]. For many of the chosen flavonoids, dissociation constants exceeded the pH range mentioned above; thereby, mainly the pKa1 was included, and for individual compounds, the pKa2 was also included. No experimental values for aglycones, such as diosmetin and isoliquiritigenin, and glycosides, such as myricitrin, cynaroside, phlorizin, and apigetrin, were found in the literature.
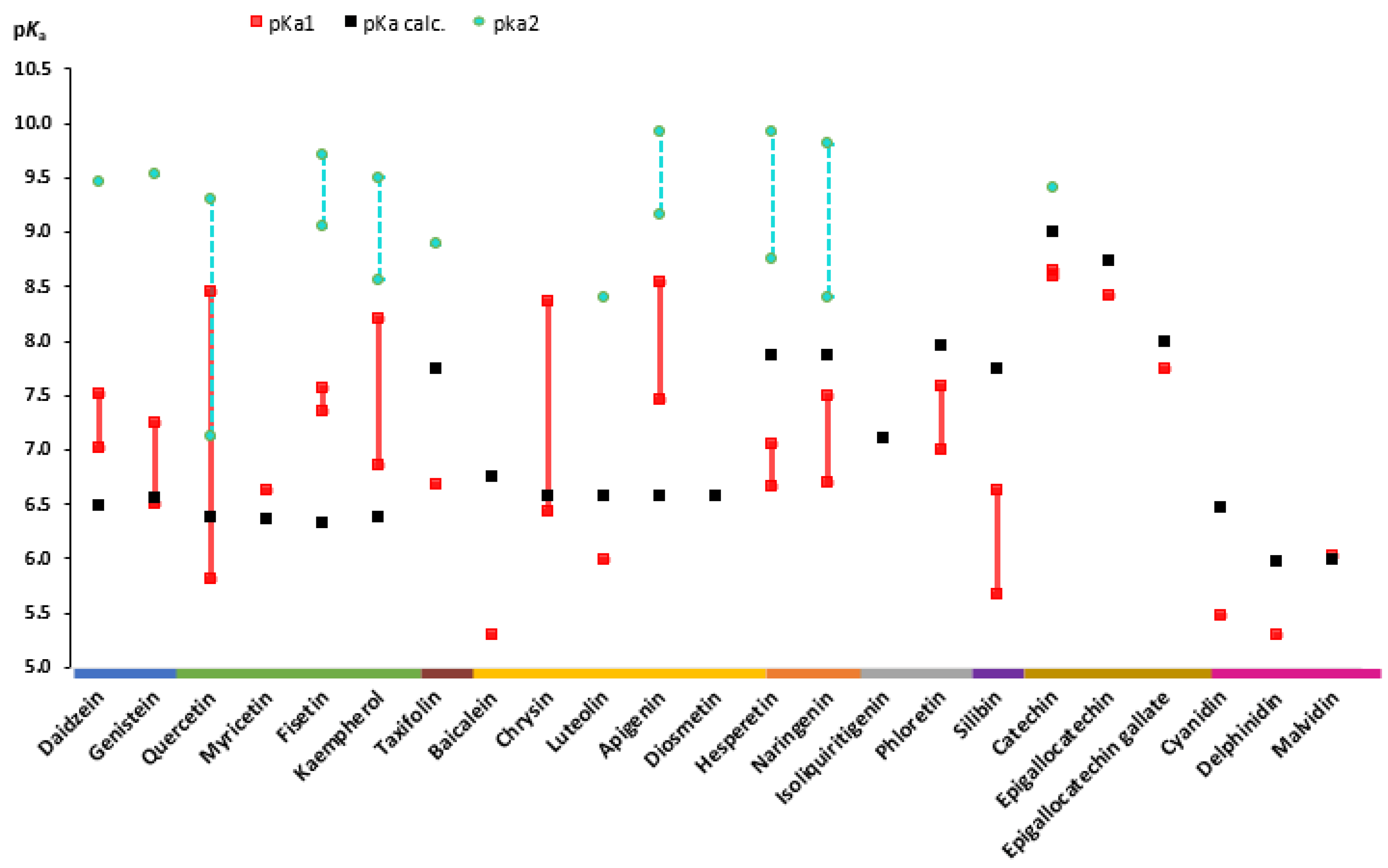
Figure 3. Experimental and theoretically calculated pKa values of aglycones (based on the data from Table A1 [30,33,35,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]).
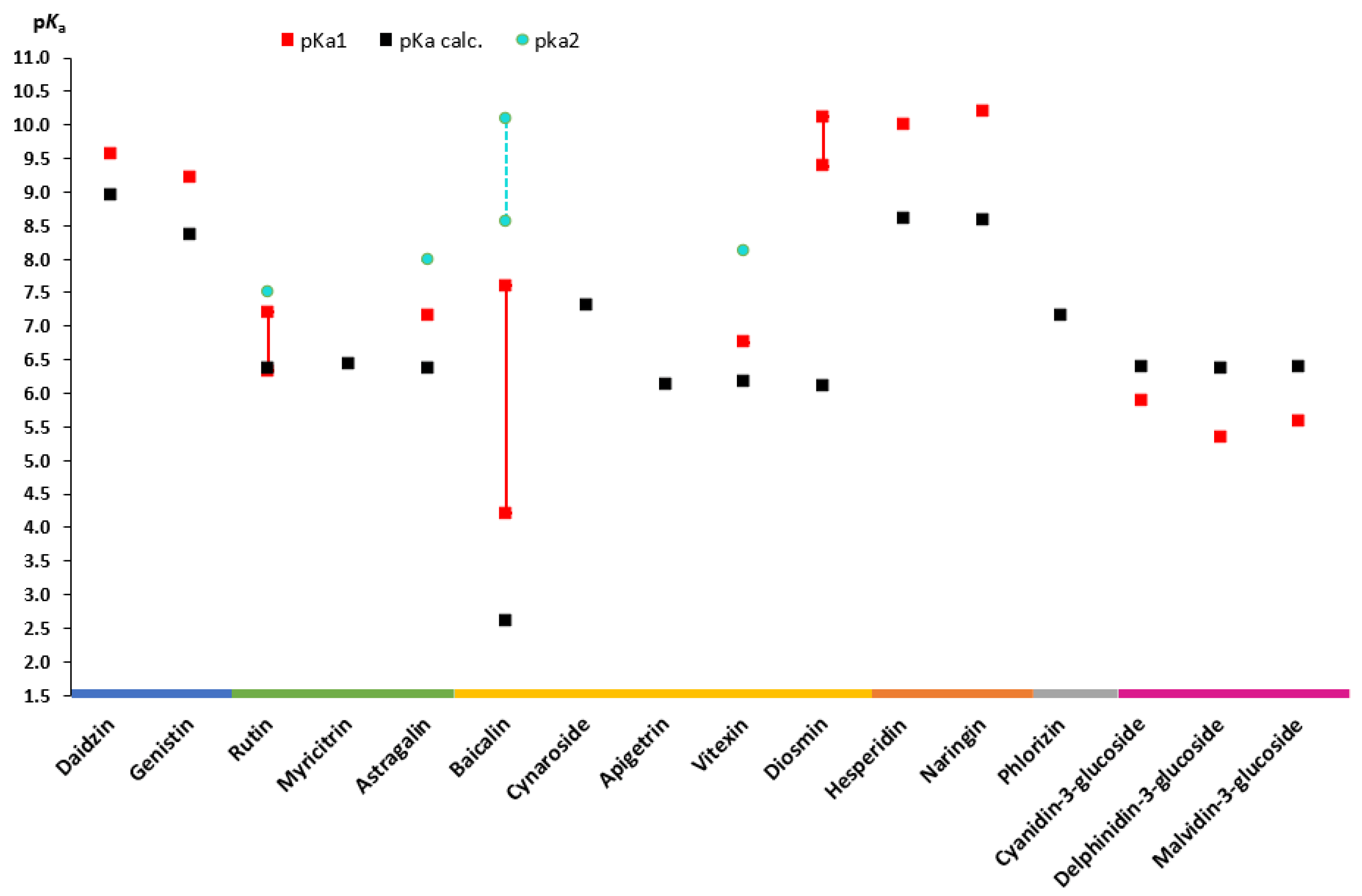
Figure 4. Experimental and theoretically calculated pKa values of glycosides (based on the data from Table A1 [18,20,40,42,50,53,54,58,59,60,61,62,63,64]).
Isoflavonones, flavonols, and flavones are all around the same pKa value (6.5) according to calculated pKa values. However, experimentally determined pKa1 values for the same compounds range from 5.30 (baicalein) to 8.54 (apigenin). The largest range of pKa1 values 5.81–8.45 was observed for quercetin, a flavonol. This is due to the fact that quercetin and its derivatives are among the most common compounds in a large number of plants. Quercetin comprises approximately 60–75% of the total intake of dietary flavonoids, and many biological studies have been carried out in the last few years including physical parameters [65,66]. Another reason for such a wide range can be the variety of determination methods (spectrophotometry, potentiometry, and capillary zone electrophoresis) and method/working conditions (solvents, concentrations, and compound solubility) used [33,67,68].
When comparing flavanols to other flavonoid subclasses, the dissociation constants are higher. The calculated pKa of catechin, epigallocatechin (EGC), and epigallocatechin gallate (EGCG) is 9.00, 8.73, and 7.99, respectively. The minimal experimental pKa1 values of the same compounds are 8.60, 8.41, and 7.68, respectively. A drop in pKa values is caused by the additional OH group that creates the pyrogallol moiety in the EGC structure and the additional galloyl moiety of the catechin molecule in EGCG [43,57].
The plot of aglycone pKa’s presented also remarks that the experimental values of anthocyanidins are in the range from 5.3 (delphinidin) to 6.0 (malvidin). In addition, the values of the glucosides of the same anthocyanidin aglycones are in a similar range (5.30–6.02).
Regarding the glycosides, it is worth mentioning baicalin, as it is one of the well-described glycosides in the literature with experimental constant values. Thus, the pKa values for this compound are also in the largest range with pKa1 in the range from 4.21 to 7.6 and pKa2 in the range from 8.56 to 10.1 [18,20]. The calculated dissociation constant for this compound is 2.62, which means that baicalin can be ionized since entering the stomach environment.
Apart from anthocyanidins, it is clear that the experimentally determined pKa1 values are higher than the calculated values for most glycosides (Figure 4). Only a few glycosides, notably rutin, baicalin, and diosmin, have ranges, unlike aglycones, where most glycosides’ pKa values are individual values that can be seen on the graph.
Based on the findings on dissociation constant data, it can be concluded that flavonoids are weak acidic molecules that are nonionized in the stomach under fasted conditions (pH 1–2). However, a small fraction of a few compounds including baicalein, cyanidin, delphinidin and baicalin can be ionized in postprandial conditions (pH up to 4). Moreover, most of the flavonoids are partially or to a certain extent ionized in the intestines, where the pH is 6.8–7.4.
2.3. Solubility and Permeability
Since 1995, when Amidon et al. introduced a relationship between human jejunal permeability rate measures (Peff) and the fraction of dosage absorbed, the biopharmaceutical classification system (BCS) has become a useful tool in drug development. According to their aqueous solubility and intestinal permeability, compounds are categorized into four classes [69,70]. Whereas compounds of high solubility, such as Class III and Class I, with low and high permeability, are relatively unproblematic, poorly soluble compounds of Class II and IV with high and low permeability are challenging cases to be delivered by oral route. In 2010, Butler and Dressman [71] developed a revised BCS and termed it the developability classification system (DCS), where Class II was further divided into dissolution rate-limited (IIa) and solubility-limited (IIb). For more realistic volumes of fluid available in the gastrointestinal tract and the compensatory nature of permeability on low solubility, the volume of 500 mL (DCS vs. 250 in BCS) was considered for the dose/solubility ratio [71]. To the best of our knowledge, only a few studies have reported flavonoids in the context of BCS, but as a sum of components and not as individual flavonoids [72,73].
There have been multiple instances of published measurements for the Peff of the human intestinal barrier, focusing on various pharmaceuticals. These measurements were attained by employing a single-pass perfusion method in the proximal jejunum. Due to the complex nature and significant expenses associated with in situ experiments, ongoing endeavors are directed toward simulating the in vivo system through in vitro apparent permeability (Papp) measurements. For instance, Caco-2 cultured cell lines (monolayers) are utilized to model drug absorption in humans [74,75,76]. In this review, we used Papp (apical-basolateral) data obtained experimentally (Figure 5 and Figure 6), as they were available for the majority of flavonoids of interest, in contrast to Peff data, which were frequently lacking. It should be noted that Papp data from Caco-2 cell monolayer models have been used in numerous studies [74,75,76] to predict oral drug absorption in humans (Peff). Although in vitro Papp values show a good correlation with in situ Peff values, they cannot be directly equated due to the list of factors.
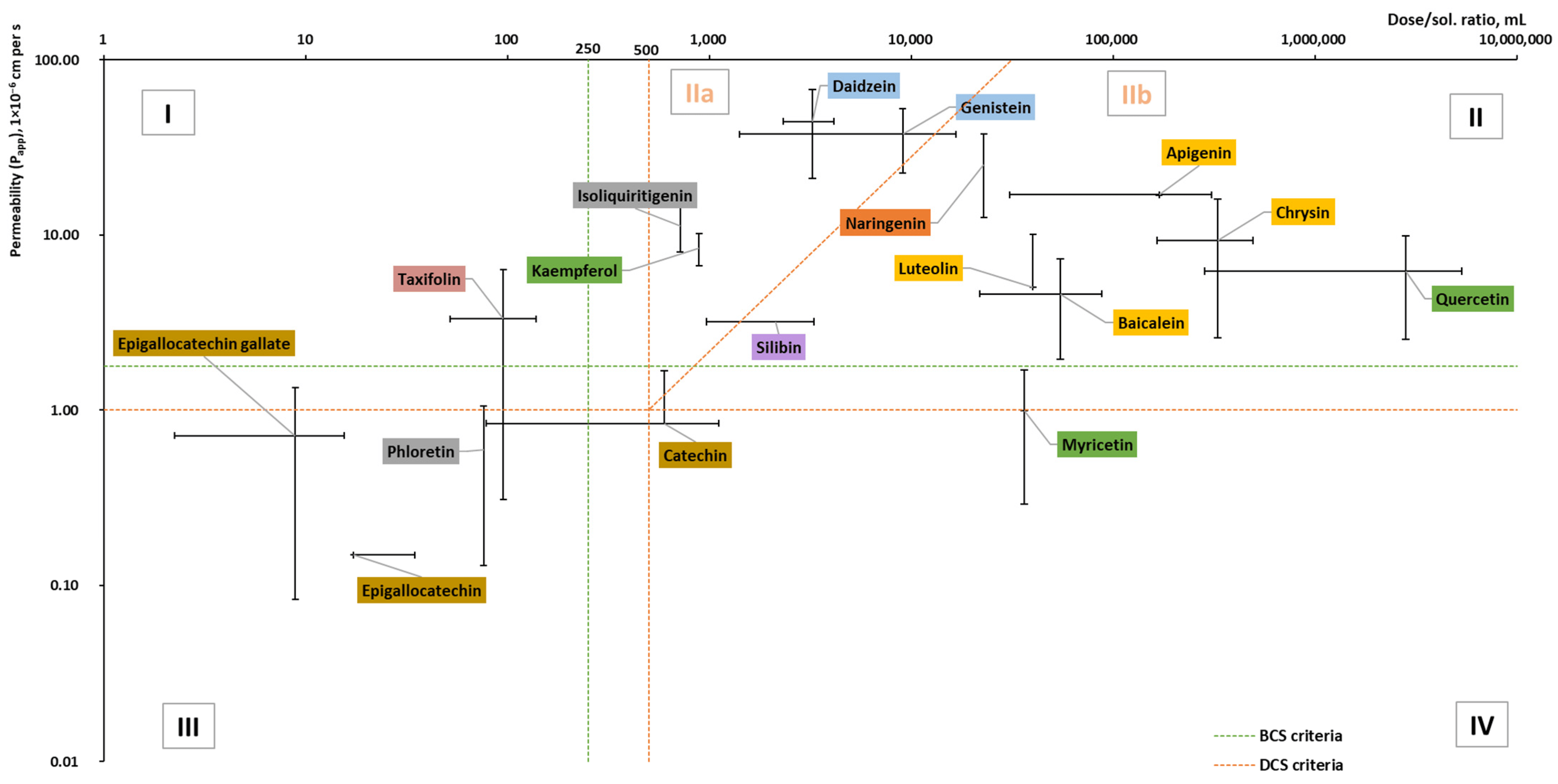
Figure 5. Dose/solubility ratio and permeability (Papp) values of aglycones (based on the data from Table A2 [30,38,42,44,54,72,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124]). BCS and DCS criteria based on [69,71,125].
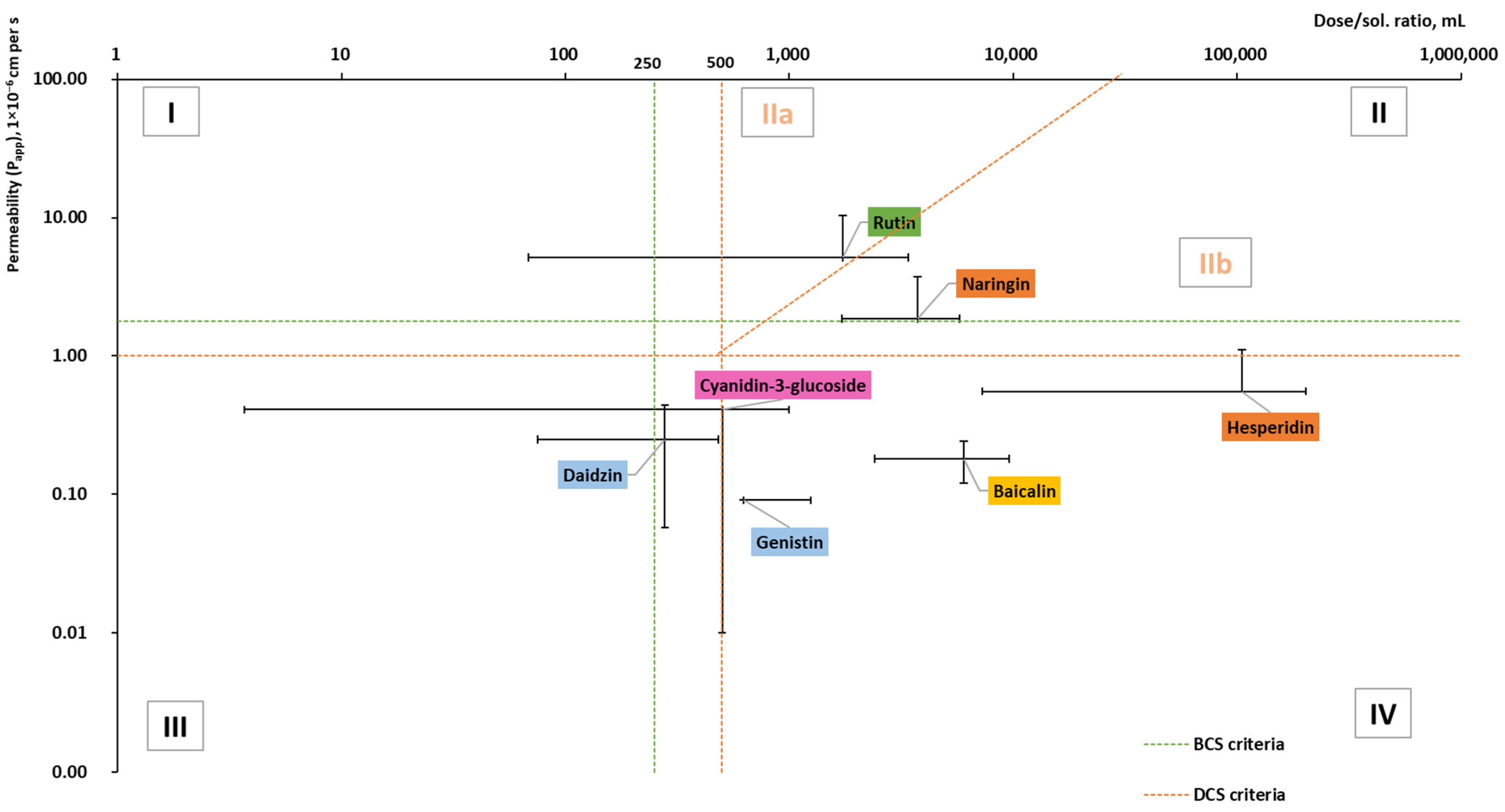
Figure 6. Dose/solubility ratio and permeability (Papp) values of glycosides (based on the data from Table A2 [42,54,62,77,78,79,81,82,86,103,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147]).
Based on the collected data (Figure 5), the reviewed aglycones belong to Classes II and III with the exception of taxifolin and myricetin. Taxifolin is in Class I but is approaching Classes II and III, whereas myricetin belongs to Class IV but is on the borderline of Class II. In addition, all reviewed flavanols belong to Class III, whereas all flavones belong to Class II. For certain aglycones such as hesperetin and diosmetin, the permeability has been determined; however, due to missing doses (there is no information on dietary supplements (DS), as in DS and medicines, the glycosides of these compounds are used), they were not included in the graph.
Glycosides are mostly in classes II and IV, but only half of all reviewed glycosides were included in the graph (Figure 6). Myricitrin, astragalin, cynaroside, apigetrin, delphinidin 3-glucoside, and malvidin 3-glucoside lacked permeability and dose data. No permeability studies were performed for vitexin or phlorizin.
2.4. Solubility Parameters
Based on the Hansen solubility parameters, calculated with the Fedors and Van Krevelen/Hoftyzer group contribution method, Breitkreutz [148] proposed a Bagley plot (δH vs. δV; please see the Section 3). In accordance with the findings, drugs in a circular region with a center of 1.3 and 20.3, and a radius of 3, had absorption times >10 h and were absorbed within the whole gastrointestinal tract. Outside this circular region, at higher δH and/or δV but below δH of 17, the absorption times were 4–9 h. Drugs above δH of 17 were absorbed in the upper part of the gastrointestinal tract with an absorption time ≤3 h. In accordance with the abovementioned findings, none of the flavonoids fell into the circular region, and almost all flavonoids fell into region with δH > 17 (above the dotted line; Figure 7 and Figure 8), corresponding to the absorption in the upper parts of the small intestine and shorter absorption times. It can be observed that aglycones and glycosides are arranged in two areas that do not overlap.
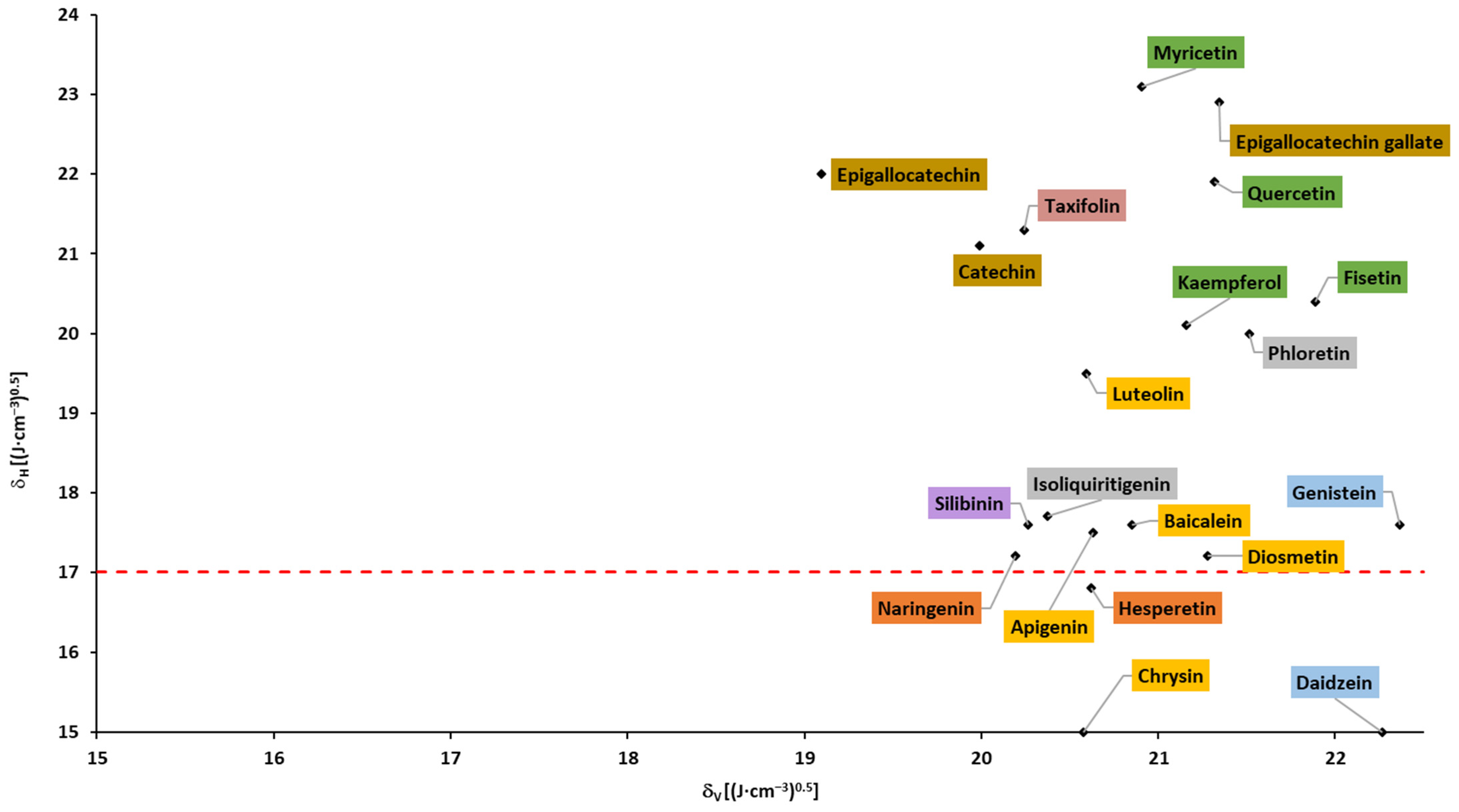
Figure 7. Bagley plot for aglycones (based on the data from Table A3).
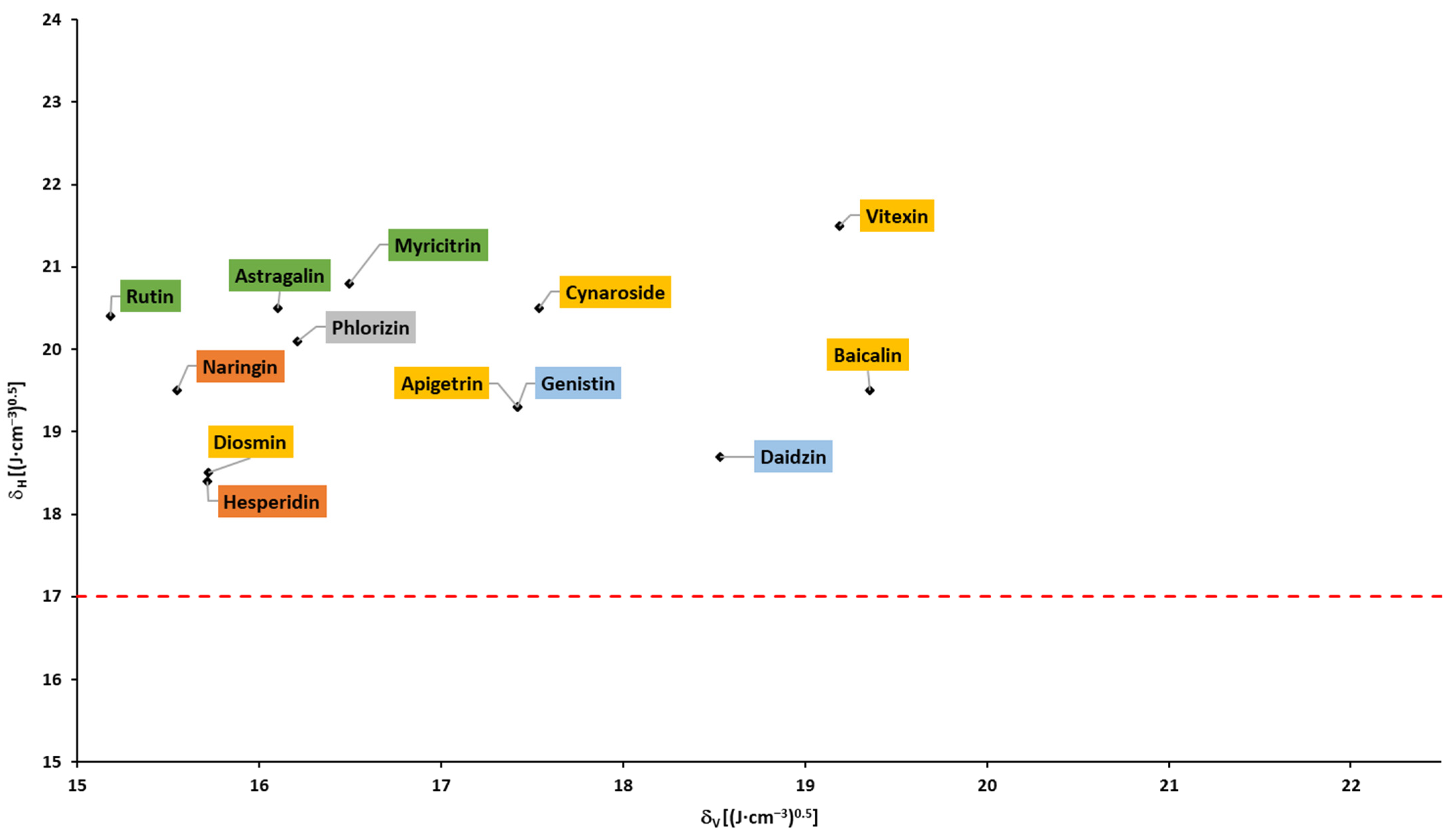
Figure 8. Bagley plot for glycosides (based on the data from Table A3).
Based on the Breitkreutz findings and presented results, an absorption window in the upper part of the small intestine and a limited (up to 3 h) absorption time of all flavonoids can be expected.
2.5. The Octanol/Water Partition Coefficient (KO/W) and Melting Point (Tm)
The KO/W and Tm are used for solubility calculation in accordance with the General Solubility Equation proposed by Jain and Yalkowsky [149]:
KO/W, or its logarithmic expression (log P), is one of the key parameters in drug discovery, design, and development [150]. Whereas water media can represent aqueous physiological media, the trivially employed amphiphilic n-octanol is considered to mimic the characteristics of the phospholipid membrane [151].
To differentiate formulation strategies based on the properties of the poorly soluble compounds such as log P and Tm, the “grease ball/brick dust” classification was proposed and is currently widely used. The log P and Tm are used to characterize compounds’ lipophilicity and crystal lattice strength, respectively. A relatively low lipophilicity (log P < 3) and a Tm higher than 100 and 200 °C are used to classify the compound as “brick dust”. Another group, so-called “grease balls”, is characterized by relatively high lipophilicity (log P > 3) and substantially lower melting temperatures [152,153,154,155]. For example, while “grease balls” are more likely to be included in the lipid-based formulation, the “brick dust” candidates can be considered for polymer-based amorphous solid dispersions [156].
The same as with pKa values, the experimentally determined distribution coefficients vary between publications, and log P values for flavonoids are quite scattered as it is one of the parameters that are typically determined as a secondary or additional parameter [157,158]. In addition, most publications provide log P values for flavonoids, which have been calculated using one or various of the calculation tools, for example, SwissADME and ChemBioDraw Ultra 11.0 [34,159].
Plotting the collected log P against the melting point data (Table A1; Figure 9), it can be concluded that most studied flavonoid aglycones belong to the “brick dust” category, and only a few compounds (chalcones, phloretin, and isoliquiritigenin; flavone diosmetin) are moving toward the “grease balls” category. The most hydrophilic compounds (with relatively low log P) of the aglycones are the representatives of flavanols: catechin, epigallocatechin, epigallocatechin gallate, and flavonolignan silibinin. The representatives of each subclass are spotted in relative proximity to each other and can be circled as areas. For the compounds isoliquiritigenin, phloretin, and epigallocatechin, the calculated log P values were used since the experimental values were not available. Phloretin has the widest range regarding the partition coefficient value. It can be characterized by different calculation parameters. Additionally, the way the experiment was set up, the substance used, etc., can help explain why the ranges of values for other compounds, such as kaempferol, apigenin, and luteolin, whose values are determined experimentally, are so large. The log P values of the reviewed aglycones are within the range of 0.4–3.2. Regarding the Tm of the aglycones, the values differ from 141 °C to 358 °C, and it can be said that each subclass of flavonoids is characterized by a certain melting temperature range. Flavanols have a lower range of Tm (141–220 °C), while flavanones have a Tm ranging from 227 °C to 251 °C. The melting point for isoflavones, flavonols, and flavones is over 250 °C.
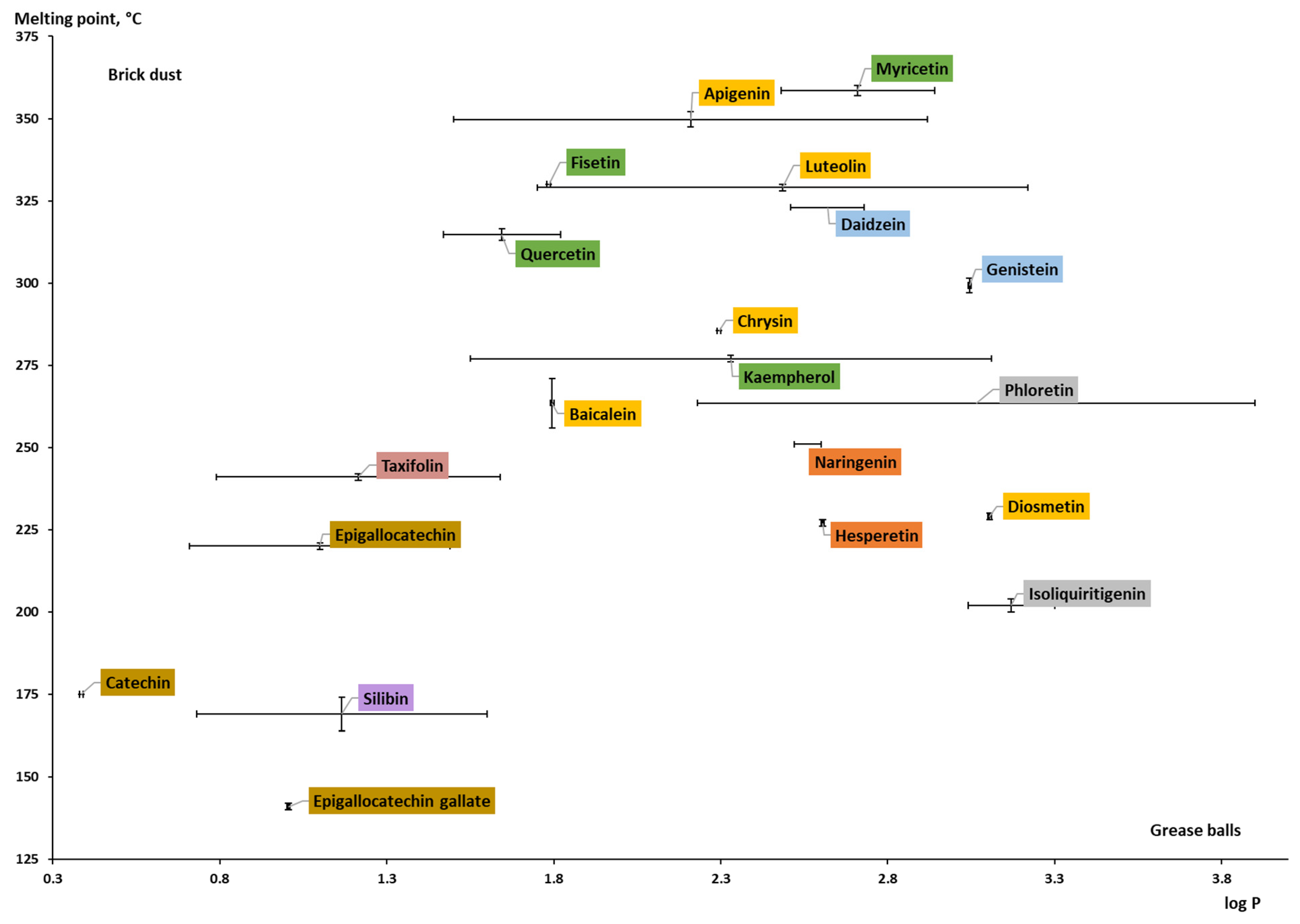
Figure 9. Experimental and theoretically calculated log P values and melting point of aglycones (based on the data from Table A1 [30,38,42,43,44,48,157,161,162,163,164,165,166,167,168,169,170,171,172,173]).
Because the Tm of anthocyanidins (both aglycones and glycosides) could not be found or were questionable, they were not included in the graphs. A few sources reported values that were used as beginning points or comparisons rather than as precise values. For instance, it was said that cyanidin had a Tm of >300 °C (see Table A1). Thus, this subgroup was not graphically described.
When the log P values of aglycones and glycosides are compared (Figure 9 and Figure 10), glycosides have a lower partition coefficient (maximum of 1.3 for baicalin), which means that aglycones are more lipophilic. The reason behind this is that log P for a sugar moiety is much lower than for the -OH group. For example, the log P for a glucose substituent is reported as roughly −2.4 [160], while for the hydroxyl group, it was calculated as −0.27 using the Molinspiration online interactive model. It is worth noting that the presence of a sugar moiety has a significant impact on the overall structure [157]. The log P values for naringin, hesperidin, and rutin are all negative. Furthermore, it is evident that in contrast to aglycones of flavanones, glycosides of the same subclass are distributed around the plot. Experimental data for glycosides have not been found; therefore, only calculated log P values were given for several conjugated flavonoids, including myricitrin, astragalin, cynaroside, apigetrin, vitexin, and naringin.
To be permeated and consequently absorbed, the poorly soluble flavonoids should be dissolved and made available in the small intestine where the permeation takes place. Oral formulation improvement strategies for flavonoids can be handled in numerous ways, such as structural transformation and chemical modification (glycosylation, metabolic conjugation, prenylation, “prodrugs”), absorption enhancers, as well as formulation and technological processing (e.g., cocrystals, nanotechnology) [1,182,183]. From the pharmaceutical technology side, the BCS/DCS and “brick dust/grease balls” classifications can help to choose the formulation approach. Most flavonoids were classified as “brick dust” (relatively high Tm and low log P), while a few were classified as “grease balls.” Class I and III “brick dust” compounds are relatively unproblematic and can be formulated as immediate- or fast-release dosage forms. For Class IIa “brick dust” flavonoids whose dissolution rates are limited, such as daidzein, genistein, kaempferol, and rutin, the simplest way to increase the bioavailability is to increase the dissolution rate via particle size reduction. Whereas for solubility-limited Class IIb “brick dust” flavonoids, such as naringenin, apigenin, quercetin, and glycosides, the formulation should consider the increased apparent solubility and can include the solid-state modification (including solid dispersions and amorphous solid dispersions) [184]. These solid dispersions can be prepared by hot-melt extrusion, spray-drying, or electrospinning, as well as by the loading of flavonoids onto mesoporous carriers as can be exemplified by silybin (or Silymarin) [185]. For poorly soluble “grease balls” (relatively low Tm and high log P), bioavailability can be enabled using different techniques. The simplest bioavailability-improving approach can be considered the co-consumption of fat-containing foods. The other approach is lipid-based formulations, including self-emulsifying (or self-micro-emulsifying) drug delivery systems (SEDDS/SMEDDS), micro-/nano-emulsions, and liposomes. Regarding Class IV compounds flavonoids (such as myricetin and the glycosides genistein, baicalin, and hesperidin), the same formulation approaches as for Class IIb “brick dust” and “grease balls” may be considered; however, cellular efflux issues should also be brought to attention [186]. For example, while the bioavailability of “brick dust” flavonoid glycoside hesperidin was improved by micronization [187], at the same time, in accordance with DCS, it is related to Class IV (on the border between IIb and IV; Figure 6). This means that its bioavailability could be further improved by enabling formulations.
3. Materials and Methods
The selection of flavonoids for this investigation was performed based on the expert opinion of the authors to representatively cover the diversity of flavonoids (different subclasses of aglycones as well as their glycosides). Based on the selected list of flavonoids, a targeted search of specific properties (such as pKa, log P, melting point, solubility, permeability, minimal and maximal dose) was performed. The collected data were systematized in the form of tables, including the respective references , which were converted into graphics and discussed. Wherever it was possible, experimental values (pKa, log P, Tm, Papp, and solubility) were used; otherwise, calculated values were taken (Table A1 and Table A2). Google Scholar was used for data collection using the following combination of keywords: “name of the flavonoid and name of the property”, e.g., “apigenin” and “pKa”. Abstracts were screened for inclusion criteria. Full-text articles were used to extract experimental and calculated values.
If the maximum and/or minimum daily dose for flavonoids as dietary supplements was not reported in scientific literature, the U.S. and/or EU currently marketed dietary supplements were sources for this purpose. The maximum and/or minimum daily dose and the recommended daily intake were then used to calculate the dose-to-solubility ratio by dividing the doses by the minimum solubility.
Hansen solubility parameters for flavonoids were calculated using the Van Krevelen group contribution method. For this purpose, the HSPiP v5.1.03 software [188] was used. The molecular volume for each flavonoid was calculated using the Y-MB engine within HSPiP. The symmetry planes of molecules were calculated using the Webmo.net online tool. Flavonoid solubility parameters δD, δP, and δH were calculated by counting molecular groups in molecules (Table A3). For the construction of a Bagley plot, δH was plotted against δV, where the δV values were calculated using the following formula:
4. Conclusions
Based on the dose-to-solubility ratio and Papp values, most flavonoids are classified as poorly soluble compounds with relatively high and low permeability (Class IIa and IIb followed by Class IV respectively). Based on their pKa and the fact that they are weakly acidic, the flavonoids reviewed should be unionized in the stomach under fasted conditions, while in fed-state (pH up to 4), only a few of them can be partially ionized. The ionization of flavonoids is expected to occur at intestinal conditions starting from the duodenum (pH approx. 5.5), following the jejunum and ileum, where the permeation should happen. In accordance with the Bagley plot, the absorption window is in the upper part of the small intestine, and a limited (up to 3 h) absorption time of all flavonoids can be expected.
Considering the mentioned absorption window and limited absorption time, formulations with Class I and III flavonoids are expected to provide immediate or fast release. In general, a lot of dietary supplements with powdered flavonoids and simple formulations (except Class I and III flavonoids) can be justifiably criticized. Albeit, based on the pKa and log P of some of them, administration before/after/with a meal or with a fat meal can be used to increase their bioavailability.
Nevertheless, to decrease inter- and intra-variability, as well as food and food content effects on bioavailability to achieve more reproducible health effects, enabling formulations should be considered for Class IIa/IIb and IV flavonoids. For the Class IV flavonoids, the same approach can be used as for Class IIb; however, cellular efflux issues and first-pass metabolism should also be brought to attention.
The current review of selected flavonoids allowed us to describe their diversity, compare their properties, and assess the potential effect of these properties on biopharmaceutical consequences as well as the applicability of possible formulation strategies. A list of gaps in the flavonoid’s investigation was actualized (pKa, Papp, log P, solubility, and recommended maximum daily dose), and an enormous potential to improve the health benefits of flavonoids through improved or enabled formulations was concluded.
References
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Resetar, H.; Morton, C. US sales of herbal supplements increase by 9.7% in 2021. HerbalGram 2022, 136, 42–69. [Google Scholar]
- U.S. Department of Health and Human Services; Food and Drug, Administration. M9 Biopharmaceutics Classification System Based Biowaivers. Available online: https://www.fda.gov/media/148472/download (accessed on 25 September 2023).
- European Medicines Agency. Guideline on the Investigation of Bioequivalence. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 25 September 2023).
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids—Food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 68, 79–85. [Google Scholar]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, K. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar] [CrossRef]
- Šudomová, M.; Berchová-Bímová, K.; Mazurakova, A.; Šamec, D.; Kubatka, P.; Hassan, S.T.S. Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights. Viruses 2022, 14, 592. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Šudomová, M. Molecular Mechanisms of Flavonoids against Tumor Gamma-Herpesviruses and Their Correlated Cancers—A Focus on EBV and KSHV Life Cycles and Carcinogenesis. Int. J. Mol. Sci. 2023, 24, 247. [Google Scholar] [CrossRef]
- Pinto, C.; Cidade, H.; Pinto, M.; Tiritan, M. Chiral Flavonoids as Antitumor Agents. Pharmaceuticals 2021, 14, 1267. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Ahmed, A. Flavonoids and cardiovascular risk factors: A review. Pharmadvances 2021, 3, 521–547. [Google Scholar] [CrossRef]
- Egert, S.; Rimbach, G. Which sources of flavonoids: Complex diets or dietary supplements? Adv. Nutr. 2011, 2, 8–14. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.N.; Spencer, J.P.; Schroeter, H.; Khaw, K.T.; Kuhnle, G.G. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Rauter, A.P.; Ennis, M.; Hellwich, K.-H.; Herold, B.J.; Horton, D.; Moss, G.P.; Schomburg, I. Nomenclature of flavonoids (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 1429–1486. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Jakab, G.; Bogdán, D.; Mazák, K.; Deme, R.; Mucsi, Z.; Mándity, I.M.; Noszál, B.; Kállai-Szabó, N.; Antal, I. Physicochemical Profiling of Baicalin Along with the Development and Characterization of Cyclodextrin Inclusion Complexes. AAPS PharmSciTech 2019, 20, 314. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, A.; Pang, H.; Xue, W.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Li, K.; Xiao, W.; et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J. Ethnopharmacol. 2014, 156, 210–215. [Google Scholar] [CrossRef]
- Liang, R.; Han, R.-M.; Fu, L.-M.; Ai, X.-C.; Zhang, J.-P.; Skibsted, L. Baicalin in Radical Scavenging and Its Synergistic Effect with β-Carotene in Antilipoxidation. J. Agric. Food Chem. 2009, 57, 7118–7124. [Google Scholar] [CrossRef]
- Amazon.com. Amazon Best Sellers: Best Sellers in Flavonoid Vitamin Supplements. Available online: https://www.amazon.com/Best-Sellers-Flavonoid-Vitamin-Supplements/zgbs/hpc/3773561 (accessed on 10 August 2023).
- Amazon.co.uk. Amazon Best Sellers: Best Sellers in Flavonoid Antioxidant Supplements (UK). Available online: https://www.amazon.co.uk/Best-Sellers-Flavonoid-Antioxidant-Supplements/zgbs/drugstore/5977712031 (accessed on 10 August 2023).
- Amazon.co.uk. Quercetin 500 mg with Bromelain & Vitamin C—120 Vegan Capsules—For Immune Support—Blended with Rosehip, Bioflavonoids, Acerola and Rutin—Made in The UK by Nutravita. Available online: https://www.amazon.co.uk/Quercetin-Complex-Vitamin-Contributes-Bioflavonoids/dp/B083F34SVQ/ref=zg_bs_g_5977712031_sccl_1/257-5385247-4093119?psc=1 (accessed on 10 August 2023).
- Trypsin+Bromelain+Rutoside Trihydrate. Available online: https://www.apollopharmacy.in/salt/trypsin%2bbromelain%2brutoside%20trihydrate (accessed on 11 August 2023).
- Jayachandran, S.; Khobre, P. Efficacy of Bromelain along with Trypsin, Rutoside Trihydrate Enzymes and Diclofenac Sodium Combination Therapy for the treatment of TMJ Osteoarthritis—A Randomised Clinical Trial. J. Clin. Diagn. Res. 2017, 11, zc09–zc11. [Google Scholar] [CrossRef]
- Kim, I.S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Lukaseder, B.; Vajrodaya, S.; Hehenberger, T.; Seger, C.; Nagl, M.; Lutz-Kutschera, G.; Robien, W.; Greger, H.; Hofer, O. Prenylated flavanones and flavanonols as chemical markers in Glycosmis species (Rutaceae). Phytochemistry 2009, 70, 1030–1037. [Google Scholar] [CrossRef]
- Mohylyuk, V.; Pauly, T.; Dobrovolnyi, O.; Scott, N.; Jones, D.S.; Andrews, G.P. Effect of carrier type and Tween® 80 concentration on the release of silymarin from amorphous solid dispersions. J. Drug Deliv. Sci. Technol. 2021, 63, 102416. [Google Scholar] [CrossRef]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Manallack, D.T. The pKa Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Mañé, M.; Ruiz, R.; Bosch, E. Acidity constants of hydroxyl groups placed in several flavonoids: Two flavanones, two flavones and five flavonols. Talanta 2023, 253, 124096. [Google Scholar] [CrossRef]
- Bitew, M.; Desalegn, T.; Demissie, T.B.; Belayneh, A.; Endale, M.; Eswaramoorthy, R. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: Molecular docking and DFT study. PLoS ONE 2021, 16, e0260853. [Google Scholar] [CrossRef]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of Hydroxyl Groups: An Overlooked Influence on Antiradical Properties of Flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]
- O’ Donovan, D.H.; De Fusco, C.; Kuhnke, L.; Reichel, A. Trends in Molecular Properties, Bioavailability, and Permeability across the Bayer Compound Collection. J. Med. Chem. 2023, 66, 2347–2360. [Google Scholar] [CrossRef]
- Fraczkiewicz, R.; Lobell, M.; Göller, A.H.; Krenz, U.; Schoenneis, R.; Clark, R.D.; Hillisch, A. Best of Both Worlds: Combining Pharma Data and State of the Art Modeling Technology To Improve in Silico pKa Prediction. J. Chem. Inf. Model. 2015, 55, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci. Biotechnol. Biochem. 2013, 77, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Nan, G.; Shi, J.; Huang, Y.; Sun, J.; Lv, J.; Yang, G.; Li, Y. Dissociation Constants and Solubilities of Daidzein and Genistein in Different Solvents. J. Chem. Eng. Data 2014, 59, 1304–1311. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between Structure and Antioxidant Capacity and Activity of Glycosylated Flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef]
- Lemanska, K.; Szymusiak, H.; Tyrakowska, B.; Zielinski, R.; Soffers, A.; Rietjens, I. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Rastogi, H.; Jana, S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 33–43. [Google Scholar] [CrossRef]
- Tungjai, M.; Poompimon, W.; Loetchutinat, C.; Kothan, S.; Dechsupa, N.; Mankhetkorn, S. Spectrophotometric Characterization of Behavior and the Predominant Species of Flavonoids in Physiological Buffer: Determination of Solubility, Lipophilicity and Anticancer Efficacy. Open Drug Deliv. J. 2008, 2, 10–19. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, G.; Xie, Y.; Ma, P.; Li, G.; Meng, Q.; Wu, T. Preformulation studies of myricetin: A natural antioxidant flavonoid. Pharmazie 2014, 69, 19–26. [Google Scholar] [CrossRef]
- Kumar, R.M.; Kumar, H.; Bhatt, T.; Jain, R.; Panchal, K.; Chaurasiya, A.; Jain, V. Fisetin in Cancer: Attributes, Developmental Aspects, and Nanotherapeutics. Pharmaceuticals 2023, 16, 196. [Google Scholar] [CrossRef]
- Álvarez-Diduk, R.; Ramírez-Silva, M.T.; Galano, A.; Merkoçi, A. Deprotonation Mechanism and Acidity Constants in Aqueous Solution of Flavonols: A Combined Experimental and Theoretical Study. J. Phys. Chem. B 2013, 117, 12347–12359. [Google Scholar] [CrossRef]
- Herrero-Martínez, J.M.; Sanmartin, M.; Rosés, M.; Bosch, E.; Ràfols, C. Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis 2005, 26, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Siquet, C.; Alves, C.; Boal, I.; Marques, M.P.; Borges, F.; Lima, J.L.F.C.; Reis, S. Structure–property studies on the antioxidant activity of flavonoids present in diet. Free Radic. Biol. Med. 2005, 39, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Yoshizuka, K.; Ohta, H.; Inoue, K.; Kitazaki, H.; Ishimaru, M. Selective separation of flavonoids with a polyvinyl alcohol membrane. J. Membr. Sci. 1996, 118, 41–48. [Google Scholar] [CrossRef]
- Memon, A.F.; Solangi, A.R.; Memon, S.Q.; Mallah, A.; Memon, N. Quantitative separation of hesperidin, chrysin, epicatechin, epigallocatechin gallate, and morin using ionic liquid as a buffer additive in capillary electrophoresis. Electrophoresis 2018, 39, 1606–1612. [Google Scholar] [CrossRef]
- Thompson, M.; Williams, C.R.; Elliot, G.E.P. Stability of flavonoid complexes of copper(II) and flavonoid antioxidant activity. Anal. Chim. Acta 1976, 85, 375–381. [Google Scholar] [CrossRef]
- Ramešová, S.; Sokolová, R.; Degano, I.; Bulíčková, J.; Zabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef]
- Kron, I.; Pudychová-Chovanová, Z.; Velika, B.; Guzy, J.; Perjesi, P. (E)-2-Benzylidenebenzocyclanones, part VIII: Spectrophotometric determination of pK a values of some natural and synthetic chalcones and their cyclic analogues. Monatshefte Chem. Chem. Mon. 2011, 143, 13–17. [Google Scholar] [CrossRef]
- Serra, H.; Mendes, T.; Bronze, M.R.; Simplício, A.L. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg. Med. Chem. 2008, 16, 4009–4018. [Google Scholar] [CrossRef]
- Valenta, C.; Cladera, J.; O’Shea, P.; Hadgraft, J. Effect of phloretin on the percutaneous absorption of lignocaine across human skin. J. Pharm. Sci. 2001, 90, 485–492. [Google Scholar] [CrossRef]
- Bai, T.-C.; Zhu, J.-J.; Hu, J.; Zhang, H.-L.; Huang, C.-G. Solubility of silybin in aqueous hydrochloric acid solution. Fluid Phase Equilibria 2007, 254, 204–210. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Gliszczyńska-Świgło, A.; Szymusiak, H.; Tyrakowska, B. The influence of stereochemistry on the antioxidant properties of catechin epimers. Eur. Food Res. Technol. 2012, 235, 1001–1009. [Google Scholar] [CrossRef]
- Borkowski, T.; Szymusiak, H.; Gliszczyńska-Świgło, A.; Rietjens, I.M.C.M.; Tyrakowska, B. Radical Scavenging Capacity of Wine Anthocyanins Is Strongly pH-Dependent. J. Agric. Food Chem. 2005, 53, 5526–5534. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kong, X.; Zhang, C.; Chen, Y.; Hua, Y. Adsorption of soy isoflavones by activated carbon: Kinetics, thermodynamics and influence of soy oligosaccharides. Chem. Eng. J. 2013, 215–216, 113–121. [Google Scholar] [CrossRef]
- Voirin, B.; Sportouch, M.; Raymond, O.; Jay, M.; Bayet, C.; Dangles, O.; El Hajji, H. Separation of flavone C-glycosides and qualitative analysis of Passiflora incarnata L. by capillary zone electrophoresis. Phytochem. Anal. 2000, 11, 90–98. [Google Scholar] [CrossRef]
- Freag, M.S.; Elnaggar, Y.S.R.; Abdallah, O.Y. Development of novel polymer-stabilized diosmin nanosuspensions: In vitro appraisal and ex vivo permeation. Int. J. Pharm. 2013, 454, 462–471. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: A natural bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef]
- Oulianova, N.; Falk, S.; Berteloot, A. Two-Step Mechanism of Phlorizin Binding to the SGLT1 Protein in the Kidney. J. Membr. Biol. 2001, 179, 223–242. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Iland, P.G.; Tate, M.E.; Jones, G.P. Charge equilibria and pKa of malvidin-3-glucoside by electrophoresis. Anal. Biochem. 2003, 318, 291–299. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef]
- Sampson, L.; Rimm, E.; Hollman, P.C.H.; de Vries, J.H.M.; Katan, M.B. Flavonol and Flavone Intakes in US Health Professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Reijenga, J.; Van Hoof, A.; Van Loon, A.; Teunissen, B. Development of Methods for the Determination of pKa Values. Anal. Chem. Insights 2013, 8, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Horvat, A.J.M.; Mutavdžić Pavlović, D.; Kaštelan-Macan, M. Determination of pKa values of active pharmaceutical ingredients. TrAC Trends Anal. Chem. 2007, 26, 1043–1061. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of In Vitro Drug Product Dissolution and In Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Larregieu, C.A.; Benet, L.Z. Distinguishing between the permeability relationships with absorption and metabolism to improve BCS and BDDCS predictions in early drug discovery. Mol. Pharm. 2014, 11, 1335–1344. [Google Scholar] [CrossRef]
- Butler, J.M.; Dressman, J.B. The developability classification system: Application of biopharmaceutics concepts to formulation development. J. Pharm. Sci. 2010, 99, 4940–4954. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Fong, S.Y.K.; Liu, M.; Wei, H.; Löbenberg, R.; Kanfer, I.; Lee, V.H.L.; Amidon, G.L.; Zuo, Z. Establishing the Pharmaceutical Quality of Chinese Herbal Medicine: A Provisional BCS Classification. Mol. Pharm. 2013, 10, 1623–1643. [Google Scholar] [CrossRef]
- Grès, M.C.; Julian, B.; Bourrié, M.; Meunier, V.; Roques, C.; Berger, M.; Boulenc, X.; Berger, Y.; Fabre, G. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: Comparison with the parental Caco-2 cell line. Pharm. Res. 1998, 15, 726–733. [Google Scholar] [CrossRef]
- Takenaka, T.; Harada, N.; Kuze, J.; Chiba, M.; Iwao, T.; Matsunaga, T. Application of a Human Intestinal Epithelial Cell Monolayer to the Prediction of Oral Drug Absorption in Humans as a Superior Alternative to the Caco-2 Cell Monolayer. J. Pharm. Sci. 2016, 105, 915–924. [Google Scholar] [CrossRef]
- Avdeef, A.; Tam, K.Y. How well can the Caco-2/Madin-Darby canine kidney models predict effective human jejunal permeability? J. Med. Chem. 2010, 53, 3566–3584. [Google Scholar] [CrossRef]
- Tian, X.J.; Yang, X.W.; Yang, X.; Wang, K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009, 367, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, T.; Ito, M.; Ohno, K.; Ota, N.; Kobayashi, K.; Sato, H.; Iwao, T.; Matsunaga, T.; Tanaka, M.; Hisaka, A. In vitro-based prediction of human plasma concentrations of food-related compounds. ALTEX—Altern. Anim. Exp. 2023. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Study of Structure and Permeability Relationship of Flavonoids in Caco-2 Cells. Nutrients 2017, 9, 1301. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, H.; Hu, M. Metabolism of Flavonoids via Enteric Recycling: Role of Intestinal Disposition. J. Pharmacol. Exp. Ther. 2003, 304, 1228–1235. [Google Scholar] [CrossRef]
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.P.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199. [Google Scholar] [CrossRef]
- Dai, J.-y.; Yang, J.-l.; Li, C. Transport and metabolism of flavonoids from Chinese herbal remedy Xiaochaihu-tang across human intestinal Caco-2 cell monolayers. Acta Pharmacol. Sin. 2008, 29, 1086–1093. [Google Scholar] [CrossRef]
- Zhao, Y.y.; Fan, Y.; Wang, M.; Wang, J.; Cheng, J.x.; Zou, J.b.; Zhang, X.f.; Shi, Y.j.; Guo, D.y. Studies on pharmacokinetic properties and absorption mechanism of phloretin: In vivo and in vitro. Biomed. Pharmacother. 2020, 132, 110809. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Cuyàs, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Verdura, S.; González-Álvarez, I.; Bermejo, M.; Joven, J.; Micol, V.; Bosch-Barrera, J.; et al. Intestinal Permeability Study of Clinically Relevant Formulations of Silibinin in Caco-2 Cell Monolayers. Int. J. Mol. Sci. 2019, 20, 1606. [Google Scholar] [CrossRef]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced uptake and transport of (+)-catechin and (-)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int. J. Nanomed. 2014, 9, 2157–2165. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Chow, M.; Zuo, Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int. J. Pharm. 2005, 287, 1–12. [Google Scholar] [CrossRef]
- Granja, A.; Neves, A.R.; Sousa, C.T.; Pinheiro, M.; Reis, S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon 2019, 5, e02020. [Google Scholar] [CrossRef] [PubMed]
- Amazon.co.uk. SOYA Isoflavones 3000 mg, 6000 mg Daily Dose. 120 Vegan Capsules, 2 Months Supply. Available online: https://www.amazon.co.uk/Isoflavones-400mg-Capsules-Months-Supply/dp/B06Y395PW7 (accessed on 2 August 2023).
- Amazon.com. Twinlab Maxlf Mega Soy, 60 CAP. Available online: https://www.amazon.com/-/es/Twinlab-maxlf-Mega-soja-60-Cap/dp/B0057UZCVA (accessed on 2 August 2023).
- Minghetti, P.; Cilurzo, F.; Casiraghi, A.; Montanari, L. Evaluation of ex vivo human skin permeation of genistein and daidzein. Drug Deliv. 2006, 13, 411–415. [Google Scholar] [CrossRef]
- Vitalnutrients.co. Genistein 125 mg. Available online: https://www.vitalnutrients.co/products/genistein-125mg (accessed on 2 August 2023).
- Amazon.co.uk. Quercetin 85 mg + Quercetin Phospholipid Complex with Vitamins C & D3. Available online: https://www.amazon.co.uk/Quercetin-Phospholipid-Bioavailable-Formulation-Vegetarian/dp/B08KJGWX4K/ref=zg_bs_5977712031_sccl_13/257-5385247-4093119?psc=1 (accessed on 2 August 2023).
- Amazon.co.uk. Liposomal Quercetin Phytosome 1600 mg Softgels with Bromelain & Vitamin C, Immunity Booster Supplement to Improve Respiratory Health & Immune Defense, Supports Internal Circulation Health. Available online: https://www.amazon.co.uk/Liposomal-Antioxidant-Supplement-Respiratory-Circulation/dp/B09YYKHG6F/ref=zg_bs_5977712031_sccl_38/257-5385247-4093119?th=1 (accessed on 2 August 2023).
- Walmart.com. Source Naturals Myricetin 100 mg, Antioxidant Flavonoid, 30 Tablets. Available online: https://www.walmart.com/ip/Source-Naturals-Myricetin-100mg-Antioxidant-Flavonoid-30-Tablets/136753540 (accessed on 2 August 2023).
- Grynkiewicz, G.; Demchuk, O.M. New Perspectives for Fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Amazon.co.uk. Fisetin Pro Liposomal 150 mg. Available online: https://www.amazon.co.uk/MCS-Formulas-Fisetin-Pro-Liposomal/dp/B0BG9RZ9H3/ref=sr_1_15?crid=T2MDICFOZTJO&keywords=fisetin+tablets&qid=1689070552&s=drugstore&sprefix=fisetin+tablets%2Cdrugstore%2C97&sr=1-15 (accessed on 2 August 2023).
- Amazon.co.uk. Fisetin with Quercetin 1200 mg | Liposomal Encapsuled for Increased Nutrient Utilization | Powerful Anti Aging & Rejuvenating Supplement | Non-GMO & Gluten Free. Available online: https://www.amazon.co.uk/Quercetin-Encapsuled-Bioavailability-Rejuvenating-Supplement/dp/B0B6NGGKFV/ref=zg_bs_5977712031_sccl_7/257-5385247-4093119?th=1 (accessed on 2 August 2023).
- Deng, S.P.; Yang, Y.L.; Cheng, X.X.; Li, W.R.; Cai, J.Y. Synthesis, Spectroscopic Study and Radical Scavenging Activity of Kaempferol Derivatives: Enhanced Water Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 975. [Google Scholar] [CrossRef]
- Amazon.com. Kaempferol 100 mg 60 Count Bottle, Capsule, Kaempferia Galangal, Rice Flour, Magnesium Sterate, Gelatin Capsule. Available online: https://www.amazon.com/Kaempferol-100mg-60-Count-Bottle/dp/B07H9FSMYS (accessed on 2 August 2023).
- Amazon.com. Supersmart—Taxifolin 60 mg per Day (Dihydroquercetin). Available online: https://www.amazon.com/Supersmart-Taxifolin-Dihydroquercetin-Protection-Antioxidant/dp/B00LPJP8OI (accessed on 2 August 2023).
- Divari.lt. Taxifolin. Available online: https://divari.lt/en/vitamir-dihydroquercetin-50-tablets (accessed on 2 August 2023).
- Liu, Y.; Sun, J.; Zhong, L.; Li, Y.; Er, A.N.; Li, T.; Yang, L.; Dong, L. Combination of a biopharmaceutic classification system and physiologically based pharmacokinetic models to predict absorption properties of baicalein in vitro and in vivo. J. Tradit. Chin. Med. Sci. 2021, 8, 238–247. [Google Scholar] [CrossRef]
- Amazon.com. Nootropics Depot Baikal Skullcap Extract Tablets | 250mg | 120 Count | >15% Apigenin, 20% Baicalein, 50% Baicalin. Available online: https://www.amazon.com/Skullcap-Extract-Apigenin-Baicalein-Baicalin/dp/B09MZQ3M4F?th=1 (accessed on 2 August 2023).
- Sunday de Baicalein. Available online: https://www.sunday.de/en/baicalein-capsules-200mg.html (accessed on 2 August 2023).
- Lee, S.; Lee, Y.-S.; Song, J.; Han, H.-K. Improved in vivo effect of chrysin as an absorption enhancer via the preparation of solid dispersion with Brij®L4 and aminoclay. Curr. Drug Deliv. 2018, 15, 86–92. [Google Scholar] [CrossRef]
- Amazon.com. Chrysin 500 mg | 60 Capsules | Passion Flower Extract | Non-GMO, Gluten Free Supplement | By Horbaach. Available online: https://www.amazon.com/Horbaach-Capsules-Promotes-Supports-Testosterone/dp/B07GRFPDDK (accessed on 2 August 2023).
- Rajhard, S.; Hladnik, L.; Vicente, F.A.; Srčič, S.; Grilc, M.; Likozar, B. Solubility of Luteolin and Other Polyphenolic Compounds in Water, Nonpolar, Polar Aprotic and Protic Solvents by Applying FTIR/HPLC. Processes 2021, 9, 1952. [Google Scholar] [CrossRef]
- Amazon.com. Supersmart—Luteolin and Super Quercetin Bundle (Immunity Booster)—Bioflavonoids Supplements. Available online: https://www.amazon.com/Supersmart-Luteolin-Quercetin-Bioflavonoids-Supplements/dp/B0C7HJ4FM8/ref=sr_1_3?keywords=luteolin&qid=1689077126&sr=8-3 (accessed on 2 August 2023).
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Amazon.co.uk. Apigenin—50mg × 120 Capsules—98%+ Purity—Optimal Dose for Sleep & Relaxation Support. Available online: https://www.amazon.co.uk/New-Relaxation-Naturally-Grapefruit-Standards/dp/B0C3J54NZJ/ref=zg_bs_5977712031_sccl_50/257-5385247-4093119?psc=1 (accessed on 2 August 2023).
- Amazon.co.uk. Liposomal Apigenin 500 mg Softgels—Optimal Apigenin Supplement with Fisetin, Quercetin and Theaflavins—2 Month Supply. Available online: https://www.amazon.co.uk/Liposomal-apigenin-500mg-Softgels-Theaflavins/dp/B09QS6XGVC/ref=sr_1_7?crid=3KH4H62K06QNN&keywords=apigenin&qid=1684933802&sprefix=apigenin%2Caps%2C102&sr=8-7 (accessed on 2 August 2023).
- Wen, J.; Liu, B.; Yuan, E.; Ma, Y.; Zhu, Y. Preparation and physicochemical properties of the complex of naringenin with hydroxypropyl-beta-cyclodextrin. Molecules 2010, 15, 4401–4407. [Google Scholar] [CrossRef]
- Amazon.com. 100 mg Naringenin 99% Powder Flavone, Flavonoid, Grapfruit Extract. Available online: https://www.amazon.com/NARINGENIN-FLAVONE-FLAVONOID-GRAPFRUIT-Extract/dp/B07GQXXVFJ/ref=sr_1_1?keywords=naringenin&qid=1689077888&sr=8-1 (accessed on 2 August 2023).
- Wu, X.; Li, J.; Hu, C.; Zheng, Y.; Zhang, Y.; Li, J.; Li, M.; Xiao, D.; Lu, L.; Huang, Y.; et al. Inclusion Complex of Isoliquiritigenin with Sulfobutyl Ether-β-Cyclodextrin: Preparation, Characterization, Inclusion Mode, Solubilization, and Stability. Front. Chem. 2022, 10, 930297. [Google Scholar] [CrossRef]
- Nootropicsdepot.com. Isoliquiritigenin Controlled Dissolve Tablets | 25mg | Licorice Extract | Glycyrrhiza Glabra | Stress, Neuroprotection, & Inflammation Support. Available online: https://nootropicsdepot.com/isoliquiritigenin-controlled-dissolve-tablets/ (accessed on 2 August 2023).
- Herbadiet.in. Phloretin 98% Extract Capsules. Available online: https://herbadiet.in/products/phloretin-pure-green-apple-extract-veg-capsules-immune-support-skin-health?variant=42274888581370 (accessed on 2 August 2023).
- Generica.world. Silybin Active Complex, 60 Capsules. Available online: https://generica.world/produkt/silybin-active-complex-60-capsules/ (accessed on 2 August 2023).
- Amazon.com. Life Extention Advanced Milk Thistle—Milk Thistle Supplement for Liver Function Support, Kidney Health & Detox—With Silymarin, Silibinins, Isosilybin A,B—Gluten-Free, Non-GMO—60 Softgels. Available online: https://www.amazon.com/Life-Extension-European-Thistle-Advanced-Phospholipid/dp/B019ZFQFB8 (accessed on 2 August 2023).
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6–331.2K: Fundamental data to design polyphenol extraction processes. Fluid Phase Equilibria 2014, 382, 279–285. [Google Scholar] [CrossRef]
- Aor.us. Active Green Tea. Available online: https://aor.us/products/active-green-tea/ (accessed on 2 August 2023).
- Amazon.com. Epicatechin—500 mgs Per Serving—60 Servings—Max Strength—May Reduce Myostatin—May Increase Lean Muscle and Strength—Helps in Protein Synthesis—For Men and Women—By Genetic Enhancement. Available online: https://www.amazon.com/EPICATECHIN-Servings-Myostatin-Synthesis-ENHANCEMENT/dp/B082BGLTX7 (accessed on 2 August 2023).
- Hu, C.; Wang, Q.; Zhao, G.; Yao, W.; Xia, Q. Improved oral absorption of (−)-epigallocatechin-3-gallate via self-double-emulsifying solid formulation. Eur. J. Lipid Sci. Technol. 2016, 118, 1115–1124. [Google Scholar] [CrossRef]
- Walmart.com. Green Tea Plus with EGCG, Extra Strength, Premium Formula, Appetite Suppressant for Weight Loss and Fat Loss, Natural Weight Loss Supplement, Detox Metabolism Booster to Burn Belly Fat, Made in USA. Available online: https://www.walmart.com/ip/Green-Tea-Plus-EGCG-Extra-Strength-Premium-Formula-Appetite-suppressant-Weight-Loss-Fat-Loss-Natural-Supplement-Detox-Metabolism-Booster-Burn-Belly-F/545797741 (accessed on 2 August 2023).
- Amazon.com. EGCG Green Tea Extract Pills | 180 Capsules | Max Potency | Non-GMO & Gluten Free Supplement | By Horbaach. Available online: https://www.amazon.com/Extract-Capsules-Potency-Supplement-Horbaach/dp/B07Z9P7PX4 (accessed on 2 August 2023).
- Waldmann, S.; Almukainzi, M.; Bou-Chacra, N.A.; Amidon, G.L.; Lee, B.-J.; Feng, J.; Kanfer, I.; Zuo, J.Z.; Wei, H.; Bolger, M.B.; et al. Provisional Biopharmaceutical Classification of Some Common Herbs Used in Western Medicine. Mol. Pharm. 2012, 9, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, Y.H.; Yu, H.-J.; Cho, N.-S.; Kim, T.-H.; Kim, D.-C.; Chung, C.-B.; Hwang, Y.-I.; Kim, K.H. Enhanced Bioavailability of Soy Isoflavones by Complexation with β-Cyclodextrin in Rats. Biosci. Biotechnol. Biochem. 2007, 71, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Vitalabo.com. Solaray Kudzu Extract. Available online: https://www.vitalabo.com/solaray/kudzu-extract (accessed on 2 August 2023).
- Amazon.com. NaturesPlus Advanced Therapeutics Isoflavone Rx Phytoestrogen—125 mg, 30 Vegetarian Tablets—Soy Supplement. Available online: https://www.amazon.com/Natures-Plus-Isoflavone-RxPhytoestrogen/dp/B00H57X9D6 (accessed on 2 August 2023).
- Amazon.com. Solgar Quercetin Complex with Ester-C Plus, 100 Vegetable Capsules—50 Servings—Supports Immune Health Vitamin C 1000 mg, 250 Vegetable Capsules—Overall Health. Available online: https://www.amazon.com/Solgar-Quercetin-Complex-Vegetable-Capsules/dp/B09R7R781K/ref=sr_1_9?keywords=rutin&qid=1685443031&sr=8-9 (accessed on 2 August 2023).
- Amazon.com. Amazing Formulas Rutin 500 mg 100 Tablets Supplement | Non-GMO | Gluten Free | Made in USA. Available online: https://www.amazon.com/Amazing-Formulas-Rutin-Antioxidant-Properties/dp/B071CTNYTY (accessed on 2 August 2023).
- Wu, H.; Long, X.; Yuan, F.; Chen, L.; Pan, S.; Liu, Y.; Stowell, Y.; Li, X. Combined use of phospholipid complexes and self-emulsifying microemulsions for improving the oral absorption of a BCS class IV compound, baicalin. Acta Pharm. Sin. B 2014, 4, 217–226. [Google Scholar] [CrossRef]
- Amazon.com. Supersmart—90% Baicalin 500 mg per Day (Scutellaria baicalensis). Available online: https://www.amazon.com/Supersmart-Scutellaria-Baicalensis-Alternatives-Vegetarian/dp/B01G5MZJWK (accessed on 2 August 2023).
- Costa, E.C.; Menezes, P.M.N.; de Almeida, R.L.; Silva, F.S.; de Araújo Ribeiro, L.A.; de Silva, J.A.; de Oliveira, A.P.; da Cruz Araújo, E.C.; Rolim, L.A.; Nunes, X.P. Inclusion of vitexin in β-cyclodextrin: Preparation, characterization and expectorant/antitussive activities. Heliyon 2020, 6, e05461. [Google Scholar] [CrossRef] [PubMed]
- Osavi.com. Passiflora 250 mg. Available online: https://osavi.com/en/passiflora-250-mg.html (accessed on 2 August 2023).
- Amazon.com. NOW Supplements, Passion Flower (Passiflora incarnata) 350 mg, Natural Stress Relief*, 90 Veg Capsules. Available online: https://www.amazon.com/NOW-Passion-Flower-350-Capsules/dp/B002J0KCQ6 (accessed on 2 August 2023).
- Europa, E. Diosmin—Water Solubility (Registration dossier). Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/21071/4/9# (accessed on 2 August 2023).
- Amazon.com. Dulàc Hemorrhoid and Leg Vein Supplement Omniven 500-40 Tablets Diosmin, Horse Chestnut Extract, Butchers Broom, Hesperidin for Restless Legs Syndrome Relief, Varicose and Spider Veins. Available online: https://www.amazon.com/Hemorrhoid-Leg-Vein-Supplement-Omniven/dp/B01IF089E0 (accessed on 2 August 2023).
- Amazon.com.au. Diosmin and Hesperidin | 1200 mg | 180 Capsules | Non-GMO, Gluten Free | By Horbaach. Available online: https://www.amazon.com.au/Diosmin-Hesperidin-Capsules-Non-GMO-Horbaach/dp/B07QG6LT1D (accessed on 2 August 2023).
- Ostrovit.com. OstroVit Vitamin C + Hesperidin + Rutin 60 caps. Available online: https://ostrovit.com/en/products/ostrovit-vitamin-c-hesperidin-rutin-60-caps-25893.html (accessed on 2 August 2023).
- Amazon.com. Hesperidin 500 mg—100% Pure Ingredient no Mixes or Additives for Blood Circulation, Leg Veins Health, Purity Guarantee 90 Capsules. Available online: https://www.amazon.com/Hesperidin-500mg-Ingredient-Additives-Circulation/dp/B07P7J3F45 (accessed on 2 August 2023).
- Teja, A.; Musmade, P.B.; Khade, A.B.; Dengale, S.J. Simultaneous improvement of solubility and permeability by fabricating binary glassy materials of Talinolol with Naringin: Solid state characterization, in-vivo in-situ evaluation. Eur. J. Pharm. Sci. 2015, 78, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Greenorganicsupplements.com. Hesperidin, Diosmin, Naringin, Bio-Flavonoids. Available online: https://greenorganicsupplements.com/products/hesperidin-diosmin (accessed on 2 August 2023).
- Amazon.com. Swanson Naringin 500 Milligrams 60 Capsules. Available online: https://www.amazon.com/Swanson-Naringin-500-Milligrams-Capsules/dp/B0017OFS0K (accessed on 2 August 2023).
- Herrera-González, A.; Núñez-López, G.; Núñez-Dallos, N.; Amaya-Delgado, L.; Sandoval, G.; Remaud-Simeon, M.; Morel, S.; Arrizon, J.; Hernández, L. Enzymatic synthesis of phlorizin fructosides. Enzym. Microb. Technol. 2021, 147, 109783. [Google Scholar] [CrossRef]
- Mass-zone.eu. Life Extension Tri Sugar Shield 60 Vegetarian Capsules. Available online: https://mass-zone.eu/en/life-extension-tri-sugar-shield-60-vegetarian-capsules-p-5852.html (accessed on 2 August 2023).
- Yang, M.; Lu, X.; Xu, J.; Liu, X.; Zhang, W.; Guan, R.; Zhong, H. Cellular uptake, transport mechanism and anti-inflammatory effect of cyanidin-3-glucoside nanoliposomes in Caco-2/RAW 264.7 co-culture model. Front. Nutr. 2022, 9, 995391. [Google Scholar] [CrossRef]
- Spectrumsupplements.ca. Macuguard Ocular Support—60 Softgels. Available online: https://www.spectrumsupplements.ca/supplement/macuguard-ocular-support-60-softgels/ (accessed on 2 August 2023).
- Breitkreutz, J. Prediction of Intestinal Drug Absorption Properties by Three-Dimensional Solubility Parameters. Pharm. Res. 1998, 15, 1370–1375. [Google Scholar] [CrossRef]
- Jain, N.; Yalkowsky, S.H. Estimation of the aqueous solubility I: Application to organic nonelectrolytes. J. Pharm. Sci. 2001, 90, 234–252. [Google Scholar] [CrossRef]
- Hill, A.P.; Young, R.J. Getting physical in drug discovery: A contemporary perspective on solubility and hydrophobicity. Drug Discov. Today 2010, 15, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Bergström, C.A.S.; Larsson, P. Computational prediction of drug solubility in water-based systems: Qualitative and quantitative approaches used in the current drug discovery and development setting. Int. J. Pharm. 2018, 540, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Holm, R.; Müllertz, A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Charman, W.N.; Porter, C.J.H. Computational prediction of formulation strategies for beyond-rule-of-5 compounds. Adv. Drug Deliv. Rev. 2016, 101, 6–21. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Avdeef, A. Perspectives in solubility measurement and interpretation. ADMET DMPK 2019, 7, 88–105. [Google Scholar] [CrossRef]
- Williams, H.; Trevaskis, N.; Charman, S.; Shanker, R.; Charman, W.; Pouton, C.; Porter, C. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental Determination of Octanol−Water Partition Coefficients of Quercetin and Related Flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Murota, K.; Shimizu, S.; Chujo, H.; Moon, J.-H.; Terao, J. Efficiency of Absorption and Metabolic Conversion of Quercetin and Its Glucosides in Human Intestinal Cell Line Caco-2. Arch. Biochem. Biophys. 2000, 384, 391–397. [Google Scholar] [CrossRef]
- Tsopelas, F.; Tsagkrasouli, M.; Poursanidis, P.; Pitsaki, M.; Vasios, G.; Danias, P.; Panderi, I.; Tsantili-Kakoulidou, A.; Giaginis, C. Retention behavior of flavonoids on immobilized artificial membrane chromatography and correlation with cell-based permeability. Biomed. Chromatogr. 2018, 32, e4108. [Google Scholar] [CrossRef]
- Yang, B.; Kotani, A.; Arai, K.; Kusu, F. Estimation of the Antioxidant Activities of Flavonoids from Their Oxidation Potentials. Anal. Sci. 2001, 17, 599–604. [Google Scholar] [CrossRef] [PubMed]
- PubChem Substance and Compound Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 30 July 2023).
- FooDB Database Version 1.0. Available online: www.foodb.ca (accessed on 31 July 2023).
- Drugbank Online Database. Available online: https://go.drugbank.com/ (accessed on 31 July 2023).
- Royal Society of Chemistry. ChemSpider Database. Available online: http://www.chemspider.com/ (accessed on 30 July 2023).
- Singha Roy, A.; Utreja, J.; Badhei, S. Characterization of the binding of fisetin and morin with chicken egg lysozyme using spectroscopic and molecular docking methods. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 385–394. [Google Scholar] [CrossRef]
- Shubina, V.S.; Shatalin, Y.V. Antioxidant and iron-chelating properties of taxifolin and its condensation product with glyoxylic acid. J. Food Sci. Technol. 2017, 54, 1467–1475. [Google Scholar] [CrossRef]
- Shatalin, Y.V.; Shubina, V.S. Partitioning of taxifolin-iron ions complexes in octanol-water system. Biophysics 2014, 59, 351–356. [Google Scholar] [CrossRef]
- Molecule of the Week Archive Chrysin. Available online: https://www.acs.org/ (accessed on 29 July 2023).
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Perrissoud, D.; Testa, B. Inhibiting or potentiating effects of flavonoids on carbon tetrachloride-induced toxicity in isolated rat hepatocytes. Arzneimittelforschung 1986, 36, 1249–1253. [Google Scholar]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- ChemBK Online Database. Available online: https://www.chembk.com/en (accessed on 2 August 2023).
- Fernandes, G.; Pusuluri, S.L.A.; Nikam, A.N.; Birangal, S.; Shenoy, G.G.; Mutalik, S. Solvent Free Twin Screw Processed Silybin Nanophytophospholipid: In Silico, In Vitro and In Vivo Insights. Pharmaceutics 2022, 14, 2729. [Google Scholar] [CrossRef] [PubMed]
- NIST (National Institute of Standards and Technology) Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/ (accessed on 31 July 2023).
- Chemicalbook.com. Vitexin. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3119208.htm (accessed on 31 July 2023).
- Rastija, V.; Nikolić, S.; Masand, V.H. Quantitative relationships between structure and lipophilicity of naturally occurring polyphenols. Acta Chim. Slov. 2013, 60, 781–789. [Google Scholar]
- Bino, A.; Vicentini, C.B.; Vertuani, S.; Lampronti, I.; Gambari, R.; Durini, E.; Manfredini, S.; Baldisserotto, A. Novel Lipidized Derivatives of the Bioflavonoid Hesperidin: Dermatological, Cosmetic and Chemopreventive Applications. Cosmetics 2018, 5, 72. [Google Scholar] [CrossRef]
- AK Scientific Inc. Cyanidin 3-O-Glucoside Product. Available online: https://aksci.com/item_detail.php?cat=X1117 (accessed on 31 July 2023).
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.; Manfredini, S.; Vertuani, S. Synthesis, Antioxidant and Antimicrobial Activity of a New Phloridzin Derivative for Dermo-Cosmetic Applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, H.; Tu, L.; Jin, Y.; Zhang, Z.; Wang, M.; Liu, S.; Wang, Y.; He, S. Kinetics of Enzymatic Synthesis of Cyanidin-3-Glucoside Lauryl Ester and Its Physicochemical Property and Proliferative Effect on Intestinal Probiotics. Biology 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Guimarães, M.; Araújo, P.; Évora, A.; de Freitas, V.; Mateus, N. Malvidin 3-Glucoside–Fatty Acid Conjugates: From Hydrophilic toward Novel Lipophilic Derivatives. J. Agric. Food Chem. 2017, 65, 6513–6518. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Zhao, X.; Liu, Q.; Song, S.-J. A comprehensive review: Biological activity, modification and synthetic methodologies of prenylated flavonoids. Phytochemistry 2021, 191, 112895. [Google Scholar] [CrossRef] [PubMed]
- Beekmann, K.; Actis-Goretta, L.; van Bladeren, P.J.; Dionisi, F.; Destaillats, F.; Rietjens, I.M.C.M. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012, 3, 1008–1018. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef]
- Elder, D. Effective formulation development strategies for poorly soluble Active Pharmaceutical Ingredients (APIs). Pharm. Outsourcing 2011, 12, 56–61. [Google Scholar]
- Lyseng-Williamson, K.A.; Perry, C.M. Micronised purified flavonoid fraction: A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003, 63, 71–100. [Google Scholar] [CrossRef]
- Abbott, S. Solubility Science: Principles and Practice; University of Leeds: Leeds, UK, 2017; pp. 109–110. [Google Scholar]
- Franklin, S.J.; Myrdal, P.B. Solid-State and Solution Characterization of Myricetin. AAPS PharmSciTech 2015, 16, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Rosita, N.; Meitasari, V.; Rianti, M.; Hariyadi, D.; Miatmoko, A. Enhancing skin penetration of epigallocatechin gallate by modifying partition coefficient using reverse micelle method. Ther. Deliv. 2019, 10, 409–417. [Google Scholar] [CrossRef] [PubMed]

