1. Introduction
The developmental origins of health and disease (DOHaD) hypothesis proposes that increased susceptibility to chronic disease is partly shaped by early-life nutritional disorders, which has been supported by evidence from both epidemiological investigations and animal studies [1,2,3]. A maternal high-fat diet (HFD) during pregnancy has been observed to have detrimental effects on various aspects of health in offspring. Studies have revealed that a maternal HFD during pregnancy can impact bone development, mesolimbic function, and social behaviors in offspring [4,5,6]. Furthermore, a large number of studies have highlighted the unfavorable outcomes of maternal HFD on lipid metabolism in offspring. These effects include significantly elevated serum triglyceride (TG) levels and the occurrence of hepatic lipid accumulation in weanling offspring, with persistent effects in adulthood [7,8,9]. Although supplementation with active substances such as lycopene and betaine during pregnancy can mitigate the effects of maternal HFD on lipid metabolism disorder in offspring in animal studies [10,11], these active substances are treated with caution during human pregnancy due to safety concerns.
Folic acid is a water-soluble vitamin, and its supplementation is one of the focuses of periconceptional health care for women in childbearing age owing to its role in preventing fetal neural tube abnormalities [12]. Over the past few years, several population and animal studies have demonstrated that folic acid supplementation during pregnancy remedied lipid metabolism disorder in offspring. This effect is achieved by inducing alterations in the DNA methylation of PPAR-α and PPAR-γ [13,14]. Administering methyl donors, which encompass folic acid, to dams subjected to high-fat sucrose diet during lactation confers protection upon their offspring against the accumulation of fat in liver via DNA methylation, fatty acid oxidation, and insulin resistance [15]. However, DNA methylation is not sufficient to explain the programming effects mediated by folic acid, and other potential mechanisms still need to be explored.
Evidence indicates that folic acid possesses the capability to improve obesity, alcoholic liver injury and hyperuricemia by stabilizing the gut microbiota [16,17,18], which provides new clues to our study. Numerous studies have demonstrated that the maternal diet significantly influences the establishment and development of the offspring’s gut microbiota, subsequently impacting the disease susceptibility. For example, maternal aspartame and stevia exposure altered the microbiota in offspring, which promoted obesity and impaired glucose tolerance [19]. Metformin treatment in HFD dams restored the gut microbiota composition in offspring and improved male offspring’s metabolic phenotype [20]. However, the efficacy of folic acid in mitigating the maternal HFD-induced disorders of the gut microbiota in offspring, thereby alleviating hepatic steatosis via the gut–liver axis, is unclear.
In this study, we gave maternal rats HFD with 5 mg/kg folic acid, focusing on male offspring hepatic steatosis at weaning and exploring the mechanism through the gut–liver axis.
2. Materials and Methods
2.1. Animals
Eight-week-old male and female Sprague Dawley rats weighing 250 ± 10 g were purchased from Vital River (Beijing, China). The animals were kept in the animal center of Qingdao University with standardized environment (21 ± 2 °C, 50% ± 10% relative humidity, and 12 h light/dark cycle). The rats were provided with freely available food and water. All experimental protocols were approved by the Ethics Committee Medical College of Qingdao University, and we adhered to the institution’s guidelines of laboratory animals (approval number: QDU-AEC-2023341).
2.2. Experimental Design
After one week of adaptation, females were randomly divided into two groups. The control group (CON) was given 2 mg folic acid/kg diet. The control with folic acid supplementation group (CS) was given 5 mg folic acid/kg diet. Male rats designated for breeding were provided with an identical diet to that of the CON females before mating. After 2 weeks, the females were mated with males (three females and one male per cage). Pregnancy was confirmed by the detection of sperm in the vaginal smear. On the day when pregnancy was detected, the dams were then further divided randomly into two groups within the original divisions to a control (3850 kcal/kg; 10% kcal from fat, 20% kcal from protein, and 70% kcal from carbohydrate) or high fat (5243 kcal/kg; 60% kcal from fat, 20% kcal from protein, and 20% kcal from carbohydrate) diet. The high-fat group (HF) and a high-fat with folic acid supplementation group (HFS) were established. At birth, within each group, every litter was reduced to 6 male pups to mitigate variations in milk provision. All dams were fed with a CON diet during lactation. The specific dietary compositions are outlined in . The schedule of the experimental and dietary intervention protocol is portrayed in Figure 1.
Figure 1. Schedule of the experimental and dietary intervention protocol.
The body weight of offsprings were measured weekly. At 3 weeks of age, we randomly selected one pup from each litter, and a total of 10 pups were selected for each group. After fasting for 10 h, the pups were anesthetized, followed by blood, liver, colon and colon content sample collection. The collected blood was centrifuged (3000 rpm, 10 min, and 4 °C) after being left for 30 min to clot, and the obtained serum was promptly stored at −80 °C. The liver was promptly excised and weighed for the calculation of the liver index (liver index % = liver weight/body weight × 100%) accurately. A portion of the liver and colon tissues were fixed in formaldehyde (10%) solution for histopathological analysis. Remaining liver and colon tissues were rapidly frozen using liquid nitrogen and subsequently stored at −80 °C.
2.3. Measurement of Metabolites in Serum
The fasting serum levels of glucose (GLU), insulin (INS), TG, total cholesterol (TC), high-density lipoprotein cholesterols (HDL-C), and low-density lipoprotein cholesterols (LDL-C) were measured using an AU5400 automatic biochemical analyzer (Beckman, Los Angeles, CA, USA). The homeostatic model assessment of insulin resistance (HOMA-IR) is calculated as follows: fasting serum glucose (mmol/L) × serum insulin (mIU/L)/22.5. Serum lipopolysaccharide (LPS) concentration was measured using the Endpoint Chromogenic Endotoxin Detection LAL Kit.
2.4. Hepatic Biochemical Analysis
Hepatic TG, TC, interleukin-1β (IL-1β), interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) levels were assessed by ELISA using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
2.5. Oral Glucose Tolerance Test (OGTT)
At 3 weeks of age, male offsprings were given sterile glucose by gavage (50% glucose solution, 2 g/kg body weight) after fasting for 10 h. Blood samples were collected through the tail vein at 0, 15, 30, 60, and 120 min. Glucose concentrations were quantified using the ACCU-CHEK Mobile (Roche, Basel, Switzerland).
2.6. Histopathological Examination
The liver and colon samples were fixed using a 10% paraformaldehyde solution and subsequently embedded in paraffin prior to being sectioned. Hematoxylin and eosin (H&E) staining was then conducted on the liver sections to evaluate hepatic steatosis. Histopathological alterations in the liver and colon sections were observed BX60 light microscope (Olympus, Tokyo, Japan). The analysis of the number of lipid droplets followed previous studies [21,22].
2.7. Western Blotting
Western blotting was conducted in accordance with previously established protocols [23]. The protein content was quantified using the BCA Protein Assay Kit (Beyotime, Zhenjiang, China). Proteins were separated by 10% polyacrylamide gels and subsequently transferred onto PVDF membranes (Millipore, MA, USA) and then subjected to an overnight incubation at 4 °C with primary antibodies against Notch1, NF-κB, ACC, FAS, Occludin (Cell Signaling Technology, MA, USA), TLR4, IκBα, p-IκBα, PPAR-α, SREBP-1c, ZO-1, Claudin1, β-actin and Lamin B (Santa Cruz, CA, USA). The membranes were washed three times with TBST, then exposed to the corresponding secondary antibody for 2 h at 37 °C. β-Actin and lamin B served as internal control for cytosolic and nuclear proteins.
2.8. DNA Extraction and 16S rRNA Gene Sequencing for Microbiome Analysis
Seven samples from each group were randomly selected for 16S rRNA gene sequencing. Sequencing methods followed previous studies [17,24]. Specifically, DNA was extracted using the DNA Kit from Tiangen Biotech (Beijing, China). Forward primer 338F: 5′-ACTCCTACGGAGGCAGCA-3′ and reverse primer 806R: 50-GGACTACHVGGGTWTCTAAT-3′ was used to amplify the variable region 3–4 (V3–V4). The PCR products were subjected to agarose gel electrophoresis, and DNA quantification was performed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). For the constructed library, sequencing was performed using Illumina NovaSeq6000 platform (San Diego, CA, USA). The raw data were spliced using USEARCH (version 10). To obtain high-quality tag sequences, chimeras were eliminated using UCHIME (version 8.1). Sequences were clustered using USEARCH to filter out OTUs at a 97% similarity threshold. The alpha diversity and beta diversity were analyzed by using QIIME. In addition, the LDA effect size (LEfSe) algorithm was used to analyze the differential abundance of each group of gut microbiota. The logarithmic LDA score was set at 4.0 to determine significant differences.
2.9. Statistical Analysis
Data analysis and graphical design were performed using SPSS 26.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, Boston, MA, USA). All data were presented as mean ± SD. Group differences were estimated using one-way analysis of variance (ANOVA). Tukey’s post hoc test was used to analyze the difference between two groups. The significance in gut microbiota analysis was assessed using the Kruskal–Wallis ranking test. Correlations between gut microbiota and biochemical indices and inflammatory factors were tested using Spearman’s correlation analysis. The correlation heat maps were produced using the R software package (version 3.6.3). A significance level of p < 0.05 was regarded as statistically significant.
3. Results
3.1. Maternal Food Consumption during Pregnancy and Male Pups’ Body Weight, Liver Weight
During pregnancy, there was no significant difference in the maternal average daily food consumption among the four groups. The maternal average daily energy intake in the HF group (83.75 ± 8.27 kcal/d) and the HFS group (84.15 ± 8.32 kcal/d) were both significantly higher compared to the CON group (62.92 ± 6.89 kcal/d). The maternal average daily folic acid intake was significantly higher in the CS group (82.88 ± 9.24 µg/d) and the HFS group (80.25 ± 7.93 µg/d) compared to the CON group (32.69 ± 3.58 µg/d) and the HF group (31.95 ± 3.15 µg/d).
In male offspring, no significant difference was found in body weight from birth to the first two weeks. At 3 weeks old, a significant increase in body weight was found in the HF group compared to the CON group (p < 0.05; Figure 2A), whereas the body weight in the HFS group was not significantly different from that of the CON group. The net weight gain at 3 weeks old exhibited a significant increase in the HF group compared to the CON group (p < 0.05; Figure 2B). There was no significant difference in net weight gain between the HFS group and the CON group. The HF group displayed a significantly higher liver weight and liver index compared to the CON group, whereas these parameters in the HFS group were significantly normalized (p < 0.05; Figure 2C,D).
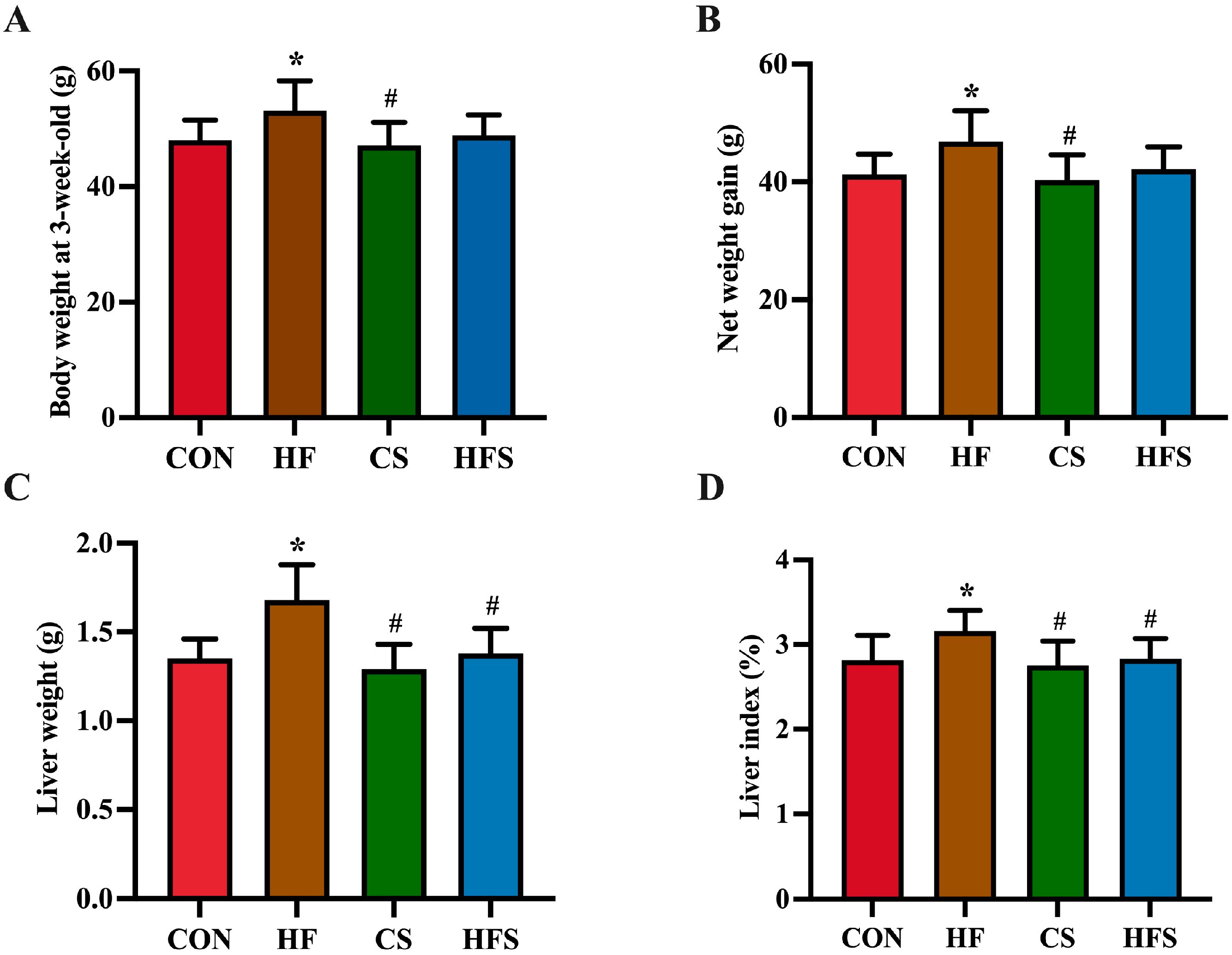
Figure 2. Body weight and liver weight. (A) Body weight at 3-week-old; (B) net weight gain; (C) liver weight; and (D) liver index. Data are presented as mean ± SD (n = 10). * p < 0.05 vs. CON; # p < 0.05 vs. HF.
3.2. Effects of Maternal Folic Acid Supplementation on Serum Parameters and Glucose Tolerance in Male Offspring
As demonstrated in , the serum concentrations of TG, LDL-C and LPS were significantly elevated in the HF group compared to the CON group (p < 0.05). The HFS group exhibited significantly reduced levels of TG and LPS compared to the HF group.
Maternal HFD during pregnancy resulted in a significant increase in GLU and HOMA-IR in male offspring. In the HFS group, GLU showed a significant decrease compared to the HF group (p < 0.05). There was no significant difference in HOMA-IR between the HFS group and the CON group. No significant difference was found in serum INS levels among the four groups.
During OGTT, as shown in Figure 3A, the level of blood glucose in the HF group exhibited a significant increase at 0, 15, and 30 min, whereas the CS and HFS group displayed significantly lower blood glucose levels at 0 and 120 min compared to the HF group (p < 0.05). In addition, the area under the curve (AUC) in the HF group showed a significant increase compared to that in the CON group (p < 0.05; Figure 3B), whereas the AUC in the HFS group was not significantly different from that in the CON group.
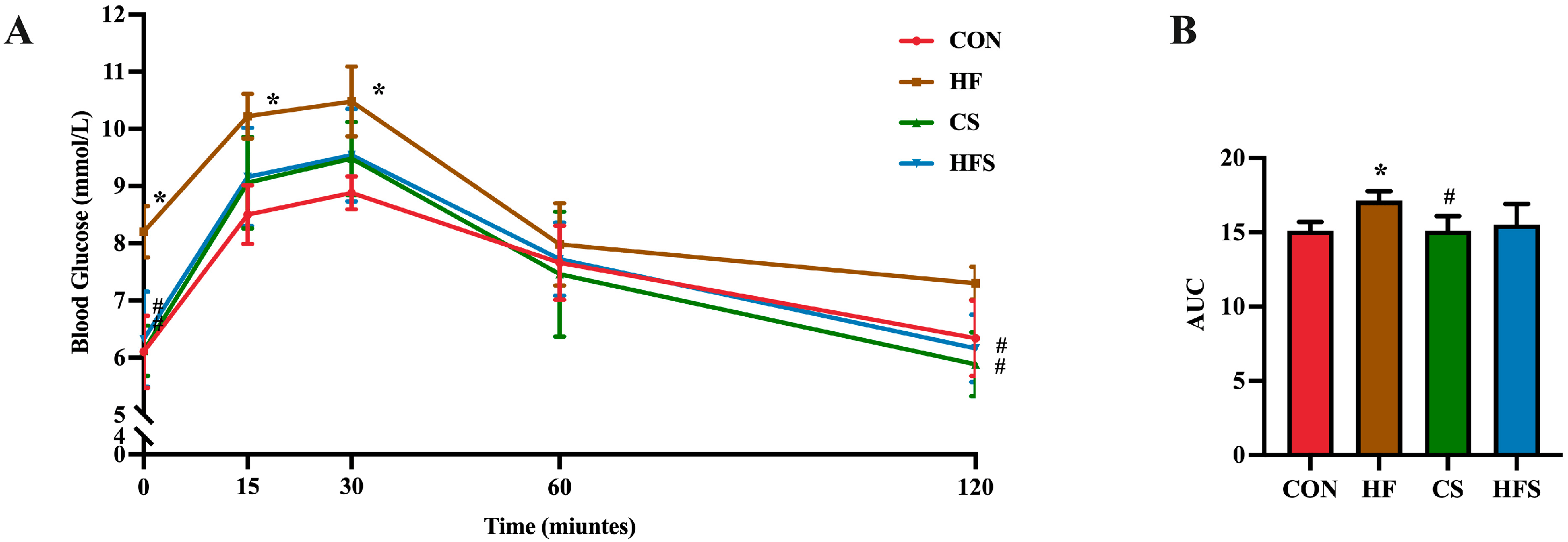
Figure 3. Effects of folic acid on glucose tolerance in male offspring. (A) Blood glucose analysis of glucose tolerance test; (B) AUC of blood glucose. Data are presented as mean ± SD (n = 5). * p < 0.05 vs. CON, # p < 0.05 vs. HF.
3.3. Effects of Maternal Folic Acid Supplementation on Hepatic Histopathology and Parameters in Male Offspring
As shown in Figure 4A, H&E staining revealed that the HF group had disorganized hepatic cords with inflammatory cell infiltration. The number of lipid droplets was significantly higher in the HF group than in the CON group (Figure 4B). Compared with the HF group, the hepatic cords in the HFS group were arranged radially, without obvious inflammatory infiltrate. The number of hepatic lipid droplets was significantly lower in the HFS group than in the HF group. The HF group displayed significantly higher hepatic TG levels compared to the CON group, while the HFS group had significantly more normalized hepatic TG levels (p < 0.05; Figure 4C). In comparison to the CON group, the HF group demonstrated a significant increase in hepatic levels of TNF-α, IL-1β, and IL-6, whereas hepatic TNF-α, IL-1β, and IL-6 levels in the HFS group were significantly lower than those in the HF group (p < 0.05; Figure 4E–G).
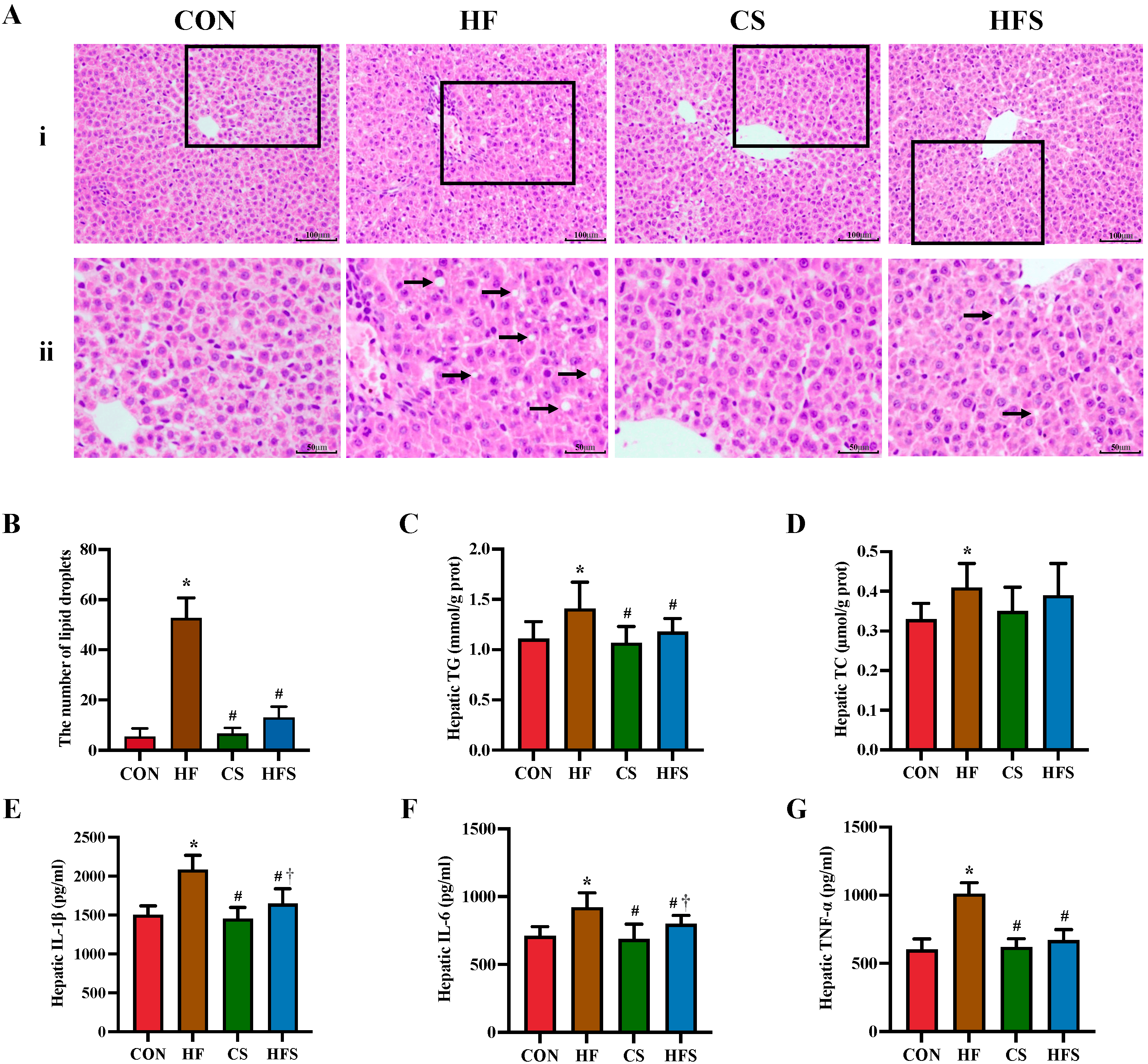
Figure 4. Hepatic pathological changes, lipid profiles, and inflammatory cytokines. (A) (i) row shows H&E staining of hepatic histopathology (×200); representative pathological visual fields were shown in the inset boxes; (ii) row shows an enlarged version of the inset boxes in i row; lipid droplets were indicated by black arrows; (B) the number of lipid droplets in fields (n = 5); (C) hepatic TG; (D) hepatic TC; (E) hepatic IL-1β; (F) hepatic IL-6; and (G) hepatic TNF-α. Data are presented as mean ± SD (n = 10). * p < 0.05 vs. CON, # p < 0.05 vs. HF, † p < 0.05 vs. CS.
3.4. Effects of Maternal Folic Acid Supplementation on Colon Histopathology and the Tight Junction Protein Expression Levels in Male Offspring
In the H&E staining, the colon mucosa mechanical barrier in the HF group was compromised, characterized by damaged mucosal surface integrity and disordered glandular arrangement. In contrast, in the HFS group, the mucosal integrity was restored, and the glandular arrangement was relatively normal in shape (Figure 5A).

Figure 5. Colon histopathology and the tight junction protein expression levels in male offspring. (A) the (i) row shows colon histopathology assessed using H&E staining (100×); representative pathological visual fields were shown in the inset boxes; the (ii) row shows an enlarged version of the inset boxes in the (i) row; damaged mucosal surface or disordered glandular arrangement were denoted as black arrows and black arrowheads, respectively; (B) the expression of Claudin 1, Occludin, and ZO-1 protein in the colon detected using Western blot. Data are presented as mean ± SD (n = 3). * p < 0.05 vs. CON, # p < 0.05 vs. HF.
We detected tight junction proteins in the colon and observed that the expression levels of tight junction proteins (Claudin 1, Occludin, and ZO-1) were significantly reduced in the HF group, while the expression of these proteins significantly recovered in the HFS group (p < 0.05; Figure 5B).
3.5. Maternal Folic Acid Supplementation Regulates Hepatic TLR4/NF-κB Signaling Pathway and Lipid Metabolism in Male Offspring
In comparison to the CON group, the HF group exhibited notably elevated expression levels of TLR4, Notch1, p-IκBα, as well as NF-κB (p < 0.05). A significant reduction in the expression levels of these proteins was observed in the HFS group when compared to the HF group (p < 0.05, Figure 6A).
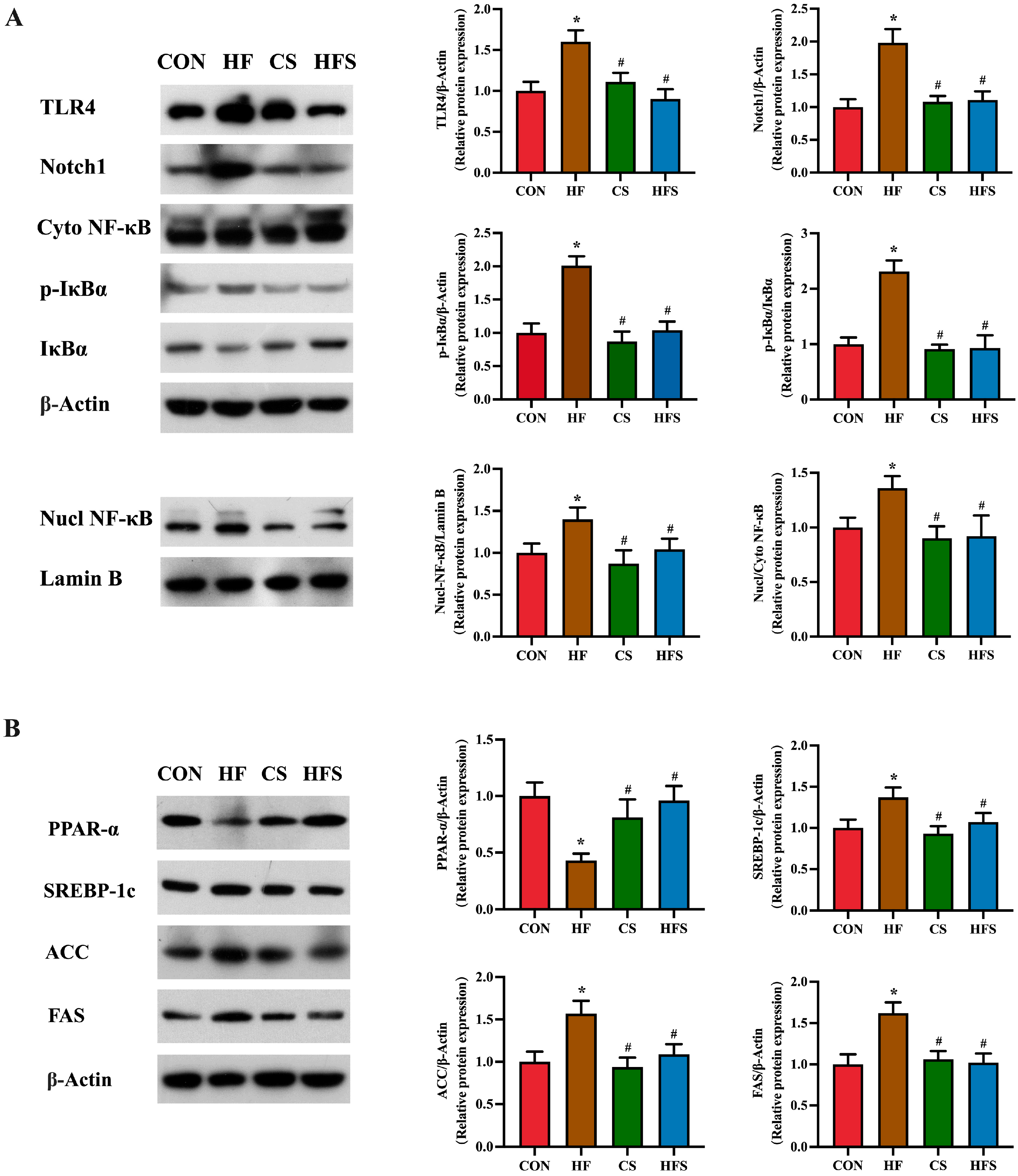
Figure 6. TLR4/NF-κB signaling pathway and lipid metabolism in liver. (A) The expression of TLR4, Notch1, p-IκBα, p-IκBα/IκBα, Nucl NF-κB, and Cyto NF-κB protein levels in liver, detected using Western blot; (B) the expression of PPAR-α, SREBP-1c, ACC, and FAS protein levels in liver, detected using Western blot. Data are presented as mean ± SD (n = 3). * p < 0.05 vs. CON, # p < 0.05 vs. HF.
As shown in Figure 6B, compared with the CON group, PPAR-α expression levels were significantly downregulated, and SREBP-1c, ACC, and FAS expression levels were significantly upregulated in the HF group. Compared with the HF group, the HFS group had significantly increased PPAR-α expression and significantly decreased SREBP-1c, ACC and FAS expressions.
3.6. Effects of Maternal Folic Acid Supplementation on Gut Microbiota in Male Offspring
4. Discussion
Maternal folic acid supplementation during pregnancy demonstrated a protective effect against hepatic steatosis in male offspring of HFD-exposed dams. The alterations detected in the composition and abundance of gut microbiota in male offspring may be relevant to maternal folic acid supplementation during pregnancy, subsequently enhancing gut barrier integrity. The enhancement of intestinal barrier function prevents the influx of endotoxins into the liver with the blood, thereby inhibiting the TLR4/NF-κB pathway and mitigating lipid metabolism disorders.
HFD is a prevalent unfavorable dietary pattern during pregnancy [25]. Maternal HFD has been demonstrated to have adverse effects on lipid metabolism in offspring and promote the onset of hepatic steatosis [20,26,27]. Hepatic steatosis serves as the beginning of non-alcoholic fatty liver disease (NAFLD), often accompanied by dysregulated blood and hepatic lipid levels [28]. Histopathological examination is widely recognized as the gold standard for its diagnosis [29]. In the present study, we administered HFD to dams during the entire pregnancy and observed significantly high serum and hepatic TG levels in their 3-week-old male offspring. Histopathological examination further revealed the presence of hepatic lipid accumulation and inflammatory infiltration in the offspring of HFD dams. The findings above indicate that maternal HFD during pregnancy promotes the development of hepatic steatosis in 3-week-old offspring. Notably, hepatic steatosis during childhood has the potential to advance into end-stage liver disease during adulthood, severely affecting quality of life [30]. Whether active dietary intervention during pregnancy is effective in mitigating the promoting effect of HFD on hepatic steatosis in offspring deserves further investigation.
Folic acid, known as a water-soluble vitamin, is effective in preventing neural tube abnormalities [31]. In recent years, it has been discovered that folic acid supplementation during pregnancy, either as a standalone intervention or in combination with other substances, can mitigate the adverse impacts of an unfavorable intrauterine environment on offspring, including lipid metabolism, nephrogenesis, and neurogenesis [32,33,34]. In these studies, 5 mg/kg folic acid supplementation, based on the upper tolerable intake limit for pregnant women (1000 μg/day), has been widely used in rodents [35]. In this study, the average litter size of dams among the four groups showed no significant difference, which does not impact the initial maternal folic acid stores and requirements. Estrogen levels have been found to affect lipid metabolism, with male subjects showing more pronounced and consistent results in studies related to disorders of lipid metabolism [36,37]. Therefore, only male offspring were researched in this study. Through observations of serum and hepatic TG levels, and histopathological changes in liver, we found that maternal 5 mg/kg folic acid supplementation during pregnancy effectively ameliorated hepatic steatosis induced by a maternal HFD in 3-week-old offspring.
While DNA methylation is a crucial mechanism frequently invoked to elucidate the influence of folic acid supplementation during pregnancy on the health of offspring, it alone does not provide a comprehensive explanation for the programming effects [32,38]. In recent years, the gut microbiota has been established to be intricately associated with the development of lipid metabolic disorders [39]. The gut microbiota could ameliorate intestinal barrier dysfunction and the propagation of inflammation to organs closely associated with the intestine (such as the liver), affecting its function [40]. The microbial communities from different maternal sources, such as vaginal and breast milk, can impact the establishment of an offspring’s gut microbiota, thereby impacting its disease susceptibility [41,42,43,44,45]. At 3 weeks old, rodent offspring are at a pivotal stage for the development of their gut barrier and the colonization of the gut microbiota [46]. It has been shown that a maternal high-sucrose diet significantly modified the gut microbiota of 3-week-old rat pups, and disrupted their intestinal barrier function, exacerbating hepatic steatosis in adulthood [47]. Furthermore, changes in the gut microbiota have been observed in 3-week-old offspring of mice fed a Western-style diet during pregnancy, and it was revealed that the formation of the gut microbiota during the weaning period is essential for the development of NAFLD in offspring [48]. Our previous study demonstrated the capacity of folic acid to regulate the abundance of gut microbiota [18]. Therefore, we suppose that folic acid supplementation during pregnancy may influence the colonization of the gut microbiota in offspring and consequently have effects on the liver metabolism of offspring. The gut microbiota may serve as another significant mechanism elucidating the intergenerational effects of folic acid.
We conducted sequencing of the colon microbiota in 3-week-old male pups. The diversity analysis revealed that the structure of the gut microbiota in the HFS and CON groups exhibited a higher degree of similarity compared to the differences observed between the HF and CON groups. The administration of folic acid during pregnancy successfully rectified the abundance changes observed in Firmicutes and Bacteroidetes (including Bacteroides) and the Firmicutes/Bacteroidetes (F/B) ratio in offspring caused by maternal HFD. A previous study has confirmed that Firmicutes exhibits superior efficacy over Bacteroidetes in enhancing energy absorption, consequently promoting obesity [49]. Consequently, the higher F/B ratios in rodents fed with HFD have been widely accepted. Several studies have suggested that interventions involving plant polyphenols can modulate the F/B ratio and bring about weight loss [50]. Moreover, we observed a reduction in Desulfobacterota and Desulfovibrio in the offspring of the HFS group. Desulfobacterota, an opportunistic pathogenic bacterium, is associated with the pathogenesis of obesity, inflammation, and metabolic disorders [51]. Desulfobacterota is capable of producing hydrogen sulfide, which can damage the intestinal barrier integrity and promote the activation of inflammation [52]. Desulfovibrio, within the Desulfobacterota phylum, has been recognized as a major factor in the production of LPS [53]. Furthermore, correlation analysis showed that microbiota such as Desulfobacterota and Desulfovibrio were positively correlated with hepatic inflammation-related indices, while microbiota such as Bacteroides, Bacteroidota, and Lactobacillus were negatively correlated with hepatic inflammation-related indices, which are consistent with previous findings [54,55,56]. The results of the gut microbiota suggest that folic acid supplementation during pregnancy ameliorates gut microbiota dysbiosis in male offspring induced by maternal HFD, potentially serving as one of the important factors contributing to the beneficial effects of folic acid on preventing hepatic steatosis in male offspring.
The gut microbiota plays a pivotal role in maintaining the homeostasis of the gut–liver axis [57]. With the disruption of the gut microbiota, bacterial metabolites (such as endotoxins) increase, affecting the integrity of the intestinal barrier [58]. Tight junction proteins, including ZO-1, Claudin 1, and Occludin, are pivotal in ensuring the functionality of the intestinal barrier, thereby serving as indicators of its integrity [59]. Bacterial metabolites are transferred to the liver with the blood when the intestinal barrier is compromised. Within the liver, LPS binding to TLR4 receptor in the liver activates Notch1, causing IκBα to be phosphorylated, resulting in the dissociation of the NF-κB/IκBα complex. The translocation of NF-κB from the cytoplasm to the nucleus facilitates inflammatory factor expression [60,61]. Hepatic inflammation not only contributes to the onset of hepatic steatosis but also exacerbates its progression [62]. In this study, compared to the HF group, the offspring of the HFS group exhibited significant enhancement in colonic mucosal integrity and the upregulation of the expression of tight junction proteins. Maternal folic acid supplementation during pregnancy effectively mitigated the detrimental effects of HFD on the intestinal barrier in the offspring. The improvement in the integrity of the intestinal barrier may lead to a reduction in the translocation of bacterial metabolites into the liver. Therefore, we examined serum levels of LPS in offspring and found that LPS levels were significantly lower in the HFS group. Moreover, we found that folic acid supplementation downregulated the hepatic protein expression levels of TLR4, Notch1, and p-IκBα, consequently inhibiting the nuclear translocation of NF-κB. In short, maternal folic acid supplementation during pregnancy can enhance intestinal barrier integrity in offspring, resulting in LPS leakage reduction and the suppression of liver inflammatory pathway activation.
The inflammatory state of the liver has a substantial impact on lipid metabolism. Numerous studies have confirmed that the overexpression of inflammatory cytokines can inhibit PPAR-α expression and activate SREBP-1c expression [63,64,65]. PPAR-α has been demonstrated to be involved in fatty acid metabolism in various tissues, including the liver, and the activation of PPAR-α leads to significant improvements in hepatic steatosis and inflammation in NAFLD [66]. SREBP-1c can increase the expression levels of lipogenic genes such as FAS and ACC, which enhances the synthesis of fatty acids and accelerates the accumulation of TG [67]. The upregulation of SREBP-1c, along with its downstream genes including FAS and ACC, as well as the downregulation of PPAR-α, are recognized as the interfering factors contributing to the development of hepatic steatosis [68]. In this study, the inhibition of the TLR4/NF-κB inflammatory pathway and reduction in the levels of inflammatory cytokines were followed by the upregulation of PPAR-α and downregulation of SREBP-1c, FAS, and ACC in the livers of 3-week-old male offspring from the HFS group. These findings further explain the observed decrease in serum and liver TG levels, as well as the reduction in hepatic steatosis in the HFS group.
There were some limitations in this study. Firstly, due to the sample limitations, we did not perform Oil Red O (ORO) staining to observe lipid droplets in liver tissues. Although lipid droplet count cannot provide a vivid visualization of lipid droplets, it does enable a quantitative assessment of the extent of hepatic steatosis. Secondly, lard was used as a source of fat, which may produce different results than vegetable fat, because lard exhibits a distinct fatty acid composition and metabolic impact compared to vegetable fats. Thirdly, we gave maternal rats feed containing different doses of folic acid for free consumption, making it difficult to accurately control the intake of folic acid. Fourthly, single nucleotide polymorphisms (SNPs) in the methylenetetrahydrofolate reductase (MTHFR) gene can affect folate metabolism and responsiveness, which may make the results less applicable to individuals with specific genetic characteristics.
5. Conclusions
This is the first time it has been demonstrated that folic acid supplementation during pregnancy could alleviate male offspring hepatic steatosis induced by maternal HFD via gut–liver homeostasis. The changes in the gut microbiota composition observed in the 3-week-old male offspring may be attributed to maternal folic acid supplementation during pregnancy, leading to enhanced intestinal barrier integrity. The improved function of the intestinal barrier prevents the entry of endotoxins into the liver, thus inhibiting the expression of the TLR4/NF-κB pathway, mitigating inflammation and the resulting hepatic steatosis. Whether these programming effects endure into adulthood deserves attention in future study.
References
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Krikke, G.G.; Grooten, I.J.; Vrijkotte, T.G.; van Eijsden, M.; Roseboom, T.J.; Painter, R.C. Vitamin B12 and folate status in early pregnancy and cardiometabolic risk factors in the offspring at age 5-6 years: Findings from the ABCD multi-ethnic birth cohort. BJOG 2016, 123, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of well-being. Philos. Trans. R. Soc. B 2004, 359, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Ribaroff, G.A.; Wastnedge, E.; Drake, A.J.; Sharpe, R.M.; Chambers, T.J.G. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: A meta-regression analysis. Obes. Rev. 2017, 18, 673–686. [Google Scholar] [CrossRef]
- Buckels, E.J.; Bolam, S.M.; Tay, M.L.; Matthews, B.G. The Impact of Maternal High-Fat Diet on Bone Microarchitecture in Offspring. Front. Nutr. 2021, 8, 730037. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237.e1–237.e13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ye, T.; Liu, C.; Fang, F.; Chen, Y.; Dong, Y. Maternal high-fat diet during pregnancy and lactation affects hepatic lipid metabolism in early life of offspring rat. J. Biosci. 2017, 42, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xu, H.; Wu, J.; Li, J.; Zhou, Y.; Ding, Z.; Siwko, S.K.; Yuan, X.; Schalinske, K.L.; Alpini, G.; et al. Maternal high-fat diet disrupted one-carbon metabolism in offspring, contributing to nonalcoholic fatty liver disease. Liver Int. 2021, 41, 1305–1319. [Google Scholar] [CrossRef]
- Cao, C.; Sun, S.; Li, J.; Song, C.; Meng, Q.; Shi, B.; Shan, A. Lycopene modulates lipid metabolism in rats and their offspring under a high-fat diet. Food Funct. 2021, 12, 8960–8975. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, L.; Zhai, X.; Wang, D.; Xiao, C.; Hui, X.; Sun, T.; Yu, M.; Zhang, Q.; Li, M.; et al. Maternal Dietary Betaine Prevents High-Fat Diet-Induced Metabolic Disorders and Gut Microbiota Alterations in Mouse Dams and Offspring from Young to Adult. Front. Microbiol. 2022, 13, 809642. [Google Scholar] [CrossRef] [PubMed]
- Talaulikar, V.S.; Arulkumaran, S. Folic acid in obstetric practice: A review. Obstet. Gynecol. Surv. 2011, 66, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Ishiguro, L.; Kim, D.; Im, D.; Kim, S.E.; Sohn, K.J.; Croxford, R.; Kim, Y.I. Maternal folic acid supplementation modulates DNA methylation and gene expression in the rat offspring in a gestation period-dependent and organ-specific manner. J. Nutr. Biochem. 2016, 33, 103–110. [Google Scholar] [CrossRef]
- Sie, K.K.; Li, J.; Ly, A.; Sohn, K.J.; Croxford, R.; Kim, Y.I. Effect of maternal and postweaning folic acid supplementation on global and gene-specific DNA methylation in the liver of the rat offspring. Mol. Nutr. Food Res. 2013, 57, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Cordero, P.; Milagro, F.I.; Campion, J.; Martinez, J.A. Supplementation with methyl donors during lactation to high-fat-sucrose-fed dams protects offspring against liver fat accumulation when consuming an obesogenic diet. J. Dev. Orig. Health Dis. 2014, 5, 385–395. [Google Scholar] [CrossRef]
- Chen, S.; Yang, M.; Wang, R.; Fan, X.; Tang, T.; Li, P.; Zhou, X.; Qi, K. Suppression of high-fat-diet-induced obesity in mice by dietary folic acid supplementation is linked to changes in gut microbiota. Eur. J. Nutr. 2022, 61, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Zheng, X.; Gao, J.; Shang, M.; Xu, J.; Liang, H. Folic Acid Protects against Hyperuricemia in C57BL/6J Mice via Ameliorating Gut-Kidney Axis Dysfunction. J. Agric. Food Chem. 2022, 70, 15787–15803. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, Y.; Zhao, H.; Zhao, H.; Wang, Y.; Zhang, X.; Zhang, J.; Wang, P.; Sun, L.; Zhang, H.; et al. Folic acid ameliorates alcohol-induced liver injury via gut-liver axis homeostasis. Front. Nutr. 2022, 9, 989311. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef]
- Song, L.; Cui, J.; Hu, S.; Wang, R.; Li, H.; Sun, B. Maternal Treatment with Metformin Persistently Ameliorates High-Fat Diet-Induced Metabolic Symptoms and Modulates Gut Microbiota in Rat Offspring. Nutrients 2022, 14, 3612. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liu, Z.; Ruan, X.; Wang, H.; Zhang, Q.; Cao, L.; Song, L.; Chen, Y.; Sun, Y. Moderate Treadmill Exercise Alleviates NAFLD by Regulating the Biogenesis and Autophagy of Lipid Droplet. Nutrients 2022, 14, 4910. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, Y.; Zuo, Y.; Zhang, X.; Gao, Y.; Wang, P.; Sun, L.; Zhang, H.; Liang, H. Nicotinamide riboside ameliorates high-fructose-induced lipid metabolism disorder in mice via improving FGF21 resistance in the liver and white adipose tissue. Food Funct. 2022, 13, 12400–12411. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Wang, Y.; Zhao, X.; Zhang, L.; Li, J.; Zhang, Y.; Wang, P.; Liang, H. Dietary Folic Acid Supplementation. Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats. Nutrients 2023, 15, 3263. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, C.; Xie, B.; Wang, T.; Liu, H.; Chen, X.; Huang, Q.; Zhang, C.; Li, T.; Deng, W. Cordyceps guangdongensis lipid-lowering formula alleviates fat and lipid accumulation by modulating gut microbiota and short-chain fatty acids in high-fat diet mice. Front. Nutr. 2022, 9, 1038740. [Google Scholar] [CrossRef] [PubMed]
- Gawlinska, K.; Gawlinski, D.; Filip, M.; Przegalinski, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, H.; Song, Y.; Yu, H.; Zhang, M.; Rao, W.; Wang, Y.; Xiao, X.; Chen, Q.; He, Q. Maternal obesity induces liver lipid accumulation of offspring through the lncRNA Lockd/mTOR autophagy pathway. Mol. Genet. Genom. 2022, 297, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef] [PubMed]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudas, I.; Vereczkey, A.; Banhidy, F. Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Awazu, M.; Hida, M. Folic acid supplementation alleviates reduced ureteric branching, nephrogenesis, and global DNA methylation induced by maternal nutrient restriction in rat embryonic kidney. PLoS ONE 2020, 15, e0230289. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A.; Stachowiak, M.; Gawecki, J.; Pruszynska-Oszmalek, E.; Tubacka, M. Protein and folic acid content in the maternal diet determine lipid metabolism and response to high-fat feeding in rat progeny in an age-dependent manner. Genes Nutr. 2012, 7, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, C.E.; Foster, J.E.; Ramdath, D.D. A maternal high-fat, high-sucrose diet alters insulin sensitivity and expression of insulin signalling and lipid metabolism genes and proteins in male rat offspring: Effect of folic acid supplementation. Br. J. Nutr. 2017, 118, 580–588. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Sun, X.; He, Y.; Li, Y.; Sun, C. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int. J. Mol. Sci. 2014, 15, 6298–6313. [Google Scholar] [CrossRef]
- Kirsch, R.; Clarkson, V.; Shephard, E.G.; Marais, D.A.; Jaffer, M.A.; Woodburne, V.E.; Kirsch, R.E.; Hall Pde, L. Rodent nutritional model of non-alcoholic steatohepatitis: Species, strain and sex difference studies. J. Gastroenterol. Hepatol. 2003, 18, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.E.; Wu, Q.; Wang, X.; Deng, L.; Caudill, M.A.; Rozen, R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J. Nutr. 2010, 140, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Lillycrop, K.A.; Phillips, E.S.; Jackson, A.A.; Hanson, M.A.; Burdge, G.C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J. Nutr. 2005, 135, 1382–1386. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef]
- Bolte, E.E.; Moorshead, D.; Aagaard, K.M. Maternal and early life exposures and their potential to influence development of the microbiome. Genome Med. 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Mjaaseth, U.N.; Norris, J.C.; Aardema, N.D.J.; Bunnell, M.L.; Ward, R.E.; Hintze, K.J.; Cho, C.E. Excess Vitamins or Imbalance of Folic Acid and Choline in the Gestational Diet Alter the Gut Microbiota and Obesogenic Effects in Wistar Rat Offspring. Nutrients 2021, 13, 4510. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 423. [Google Scholar] [CrossRef]
- Murgas Torrazza, R.; Neu, J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J. Perinatol. 2011, 31 (Suppl. S1), S29–S34. [Google Scholar] [CrossRef]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef]
- Dai, X.; Guo, Z.; Chen, D.; Li, L.; Song, X.; Liu, T.; Jin, G.; Li, Y.; Liu, Y.; Ajiguli, A.; et al. Maternal sucralose intake alters gut microbiota of offspring and exacerbates hepatic steatosis in adulthood. Gut Microbes 2020, 11, 1043–1063. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.M.; Friedman, J.E. Maternal modifiers of the infant gut microbiota: Metabolic consequences. J. Endocrinol. 2017, 235, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Xue, B.; Xie, J.; Huang, J.; Chen, L.; Gao, L.; Ou, S.; Wang, Y.; Peng, X. Plant polyphenols alter a pathway of energy metabolism by inhibiting fecal Bacteroidetes and Firmicutes in vitro. Food Function 2016, 7, 1501–1507. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, J.; Huo, J.; Yan, Y. Sesamolin Attenuates Kidney Injury, Intestinal Barrier Dysfunction, and Gut Microbiota Imbalance in High-Fat and High-Fructose Diet-Fed Mice. J. Agric. Food Chem. 2023, 71, 1562–1576. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xu, J.; Yang, P.; Liang, X.; Zeng, Z.; Luo, H.; Tang, X.; Wu, X.; Xiao, X. The effects of a set amount of regular maternal exercise during pregnancy on gut microbiota are diet-dependent in mice and do not cause significant diversity changes. PeerJ 2022, 10, e14459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. The gut microbiota and obesity: From correlation to causality. Nat. Rev. Microbiol. 2013, 11, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Su, J.; Yu, J.J.; Yan, M.Q.; Shi, M.L.; Huang, Q.D.; Li, B.; Wu, W.Y.; Xia, R.S.; Li, S.F.; et al. Buddleoside-Rich Chrysanthemum indicum L. Extract has a Beneficial Effect on Metabolic Hypertensive Rats by Inhibiting the Enteric-Origin LPS/TLR4 Pathway. Front. Pharmacol. 2021, 12, 755140. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Guo, X.; Song, G.; Pang, S.; Fang, W.; Peng, Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients 2022, 14, 2760. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Wang, F.; Li, Y.; Zheng, X. Black Current Anthocyanins Improve Lipid Metabolism and Modulate Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2021, 65, e2001090. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Solis-Urra, P.; Rodriguez-Rodriguez, F.; Olivares-Arancibia, J.; Navarro-Oliveros, M.; Abadia-Molina, F.; Alvarez-Mercado, A.I. The Gut Barrier, Intestinal Microbiota, and Liver Disease: Molecular Mechanisms and Strategies to Manage. Int. J. Mol. Sci. 2020, 21, 8351. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol. Med. Rep. 2014, 9, 2352–2356. [Google Scholar] [CrossRef]
- Xu, J.; Chi, F.; Tsukamoto, H. Notch signaling and M1 macrophage activation in obesity-alcohol synergism. Clin. Res. Hepatol. Gastroenterol. 2015, 39 (Suppl. S1), S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Minter, L.M.; Cho, O.H.; Gottipati, S.; Fauq, A.H.; Golde, T.E.; Sonenshein, G.E.; Osborne, B.A. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 2006, 25, 129–138. [Google Scholar] [CrossRef]
- Poeta, M.; Pierri, L.; Vajro, P. Gut-Liver Axis Derangement in Non-Alcoholic Fatty Liver Disease. Children 2017, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Bonda, T.A.; Szynaka, B.; Sokolowska, M.; Dziemidowicz, M.; Waszkiewicz, E.; Winnicka, M.M.; Bernaczyk, P.; Wawrusiewicz-Kurylonek, N.; Kaminski, K.A. Interleukin 6 modulates PPARalpha and PGC-1alpha and is involved in high-fat diet induced cardiac lipotoxicity in mouse. Int. J. Cardiol. 2016, 219, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yang, Y.Z.; Zheng, Y.J.; Wang, S.C.; Gu, H.M.; Pan, Y.; Wang, S.J.; Xu, H.J.; Kong, L.D. Magnesium isoglycyrrhizinate blocks fructose-induced hepatic NF-kappaB/NLRP3 inflammasome activation and lipid metabolism disorder. Eur. J. Pharmacol. 2017, 809, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Han, S.; Li, C.; Meng, T.; Huo, Y.; Liu, X.; Huang, Y.; Yang, L. Rhinacanthin C Ameliorates Insulin Resistance and Lipid Accumulation in NAFLD Mice via the AMPK/SIRT1 and SREBP-1c/FAS/ACC Signaling Pathways. Evid. Based Complement. Alternat. Med. 2023, 2023, 6603522. [Google Scholar] [CrossRef]
- Zhong, M.; Yan, Y.; Yuan, H.; Rong, A.; Xu, G.; Cai, F.; Yang, Y.; Wang, Y.; Zhang, W. Astragalus mongholicus polysaccharides ameliorate hepatic lipid accumulation and inflammation as well as modulate gut microbiota in NAFLD rats. Food Funct. 2022, 13, 7287–7301. [Google Scholar] [CrossRef] [PubMed]

