1. Introduction
Oral health issues are a matter of global concern. Dentition defect and tooth loss have a profound influence on people’s health and quality of life [1]. Since Professor Brånemark introduced the theory of “osseointegration”, the field of oral implantology has experienced vigorous development [2]. Due to its excellent restorative effects on oral function and aesthetics, dental implantation has become a common and popular treatment for dentition defect [3,4].
The success of implant surgery depends not only on the skill of the dentist during the procedure but also on factors such as post-operative wound healing, osseointegration, and alveolar bone repair and regeneration, all of which significantly affect the final outcome of the implant. This is particularly crucial in cases involving multiple teeth or challenging surgical procedures. After the implantation of the implant into the jawbone, the initial process involves the differentiation, migration, and adhesion of osteoblasts to the surface of the implant [5,6]. Early formation of immature woven bone occurs. Over the following months, bone remodeling is completed, leading to the formation of dense lamellar bone [6]. Implants and the bone that surrounds them are biologically interconnected by osseointegration, a fundamental process that produces a functional and stable connection between the two. However, implant osseointegration can not be successfully completed by everyone, such as patients with diabetes and osteoporosis [7,8,9,10]. While achieving successful osseointegration and subsequent prosthetic rehabilitation is significant, ensuring the long-term health and stability of the implant-supported restoration remains a significant concern for both clinicians and patients in clinical practice [3]. Bone tissue exhibits dynamic remodeling and is subject to resorption [11] under the influence of various intrinsic and extrinsic factors, including patient-specific characteristics and pathological conditions. Loss of bone around the implant is called marginal bone loss [12,13]. Marginal bone loss not only affects implant stability but can also have a substantial impact on the aesthetic outcome for the patient [13]. Among the factors that compromise implant stability and marginal bone loss, peri-implant diseases and associated inflammatory reactions represent the most prevalent and clinically significant challenges. Similar to the impact of periodontal disease on natural teeth, peri-implantitis poses obstacles to the health of dental implants [14]. The prevalence of peri-implantitis is notably high, reaching up to 43% in Europe, South America, and North America [15]. It is an infectious disease primarily induced by various pathogens within the oral cavity. The peri-implant soft and hard tissues are gradually destroyed by this process [15,16]. Ultimately, this can result in implant loosening and failure [16]. According to the latest insights from osteoimmunology, marginal bone loss around dental implants is not solely attributed to the action of pathogenic bacteria of peri-implant diseases [11]. Immune-mediated inflammatory responses can also influence the peri-implant bone [11], which involves the overall systemic condition of the patient. This further emphasizes the significant role of the immune system in implant dentistry. There are still many challenges that dentists need to solve after implantation surgery. It is crucial to recognize that post-implant treatment requires ongoing maintenance and care by both the dentist and the patient.
The oral cavity, as a complex microbial ecosystem, maintains a close relationship between oral microbiota and the human body [17,18]. Whether oral mucosal wound healing or subsequent peri-implant diseases, it has been demonstrated that local microbiota plays a significant role in these processes. However, not all microorganisms are harmful [19]. Certain types of microorganisms naturally occurring in the human body and the environment possess beneficial functions for the body and hold significant importance in health management. These microorganisms are commonly referred to as “probiotics” [19]. As defined by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), probiotics are live microorganisms that, when given in adequate amounts, have a beneficial effect on the body [20,21,22]. Typically, probiotics belong to certain strains of bacterial species, for example, Lacticaseibacillus rhamnosus, Lactobacillus acidophilus, Lactiplantibacillus plantarum, Lacticaseibacillus casei, Limosilactobacillus reuteri, Lacticaseibacillus paracasei, Bifidobacterium longum, Bifidobacterium infantis and Bifidobacterium animalis [22,23]. Bacillus subtilis is an example of a species within the Bacillus genus known for its probiotic properties [23]. In addition, Akkermansia muciniphila is now considered a new generation of probiotics that play beneficial roles in various diseases [24]. In addition to the aforementioned probiotics in the gut, within the oral microbiome, Streptococcus salivarius stands out as a prominent representative and is widely recognized as a probiotic. It plays a significant role in the prevention of dental caries and periodontal disease [25,26].
Throughout history, the regulatory effects of probiotics on gut microbiota have been acknowledged, suggesting their potential in preventing and managing gastrointestinal disorders [27]. However, the impact of probiotics extends beyond the gastrointestinal tract. Emerging evidence highlights the pivotal role of probiotics in modulating systemic tissues and organs in the human body [28,29,30], particularly in the realm of immune regulation. This phenomenon is exemplified by the widely recognized and extensively studied concepts of the gut–brain axis and the gut–bone axis, which elucidate the intricate interplay between the gut and other physiological systems at a molecular and cellular level. Recent abundant high-quality studies have revealed the significant role of probiotics in the prevention and treatment of tumors by modulating the immune system’s functionality and utilizing their own byproducts [31]. Bender et al. discovered that Lacticaseibacillus rhamnosus enhances the production of IFN by CD8+ T cells in the vicinity of melanoma, thus effectively augmenting the immune-mediated anti-tumor response [32]. In the context of the lungs, oral administration of probiotics has been shown to regulate pulmonary immune function, thereby reducing the incidence and severity of respiratory allergic diseases and acute respiratory infections [22,33]. Sun et al. suggest that probiotics have potential as a therapeutic approach in the occurrence and progression of type II diabetes [34,35]. Notably, Nole et al. summarized the connection between gut probiotics and the skin, highlighting the beneficial effects of probiotics in modulating immune system function and maintaining epithelial barrier integrity, thus offering potential benefits for conditions such as atopic dermatitis, acne, and wound healing [36]. Moreover, emerging evidence indicates that probiotics also exert positive influences on cognition, emotions, and other aspects of human behavior [37,38].
The oral cavity, as part of the digestive system, is susceptible to the potential benefits of probiotics. Probiotics are commonly used to influence oral diseases [39]. These probiotics have been found to antagonize pathogens and inhibit their virulence, thus playing a positive role in maintaining oral health and preventing diseases. Numerous studies have applied probiotics in the treatment of oral mucosal diseases, periodontal disease, dental caries, peri-implant diseases, and even oral cancer, with specific administration recommendations provided [39,40,41,42,43,44]. It is crucial to recognize that post-implant care in the field of oral implantology requires not only local effects but also systemic preparations. The subsequent health of dental implants involves aspects such as soft tissue healing, bone tissue regeneration, control of pathogenic bacteria around implants, and modulation of immune system-mediated inflammatory responses. Considering the significant roles that probiotics play systemically and locally, it appears plausible that probiotics may further address some issues encountered after dental implant surgery. Harnessing the potential of microorganisms to promote oral implant health seems to be a viable approach. This article aims to summarize the potential roles of probiotics in dental implant health and provide feasible directions for future research.
2. The Role of Probiotics in Bone Regeneration and Bone Homeostasis
Successful oral implant restoration relies on stable implants, and the prerequisite for implants to function optimally is stable and healthy bone tissue. Once the implants are placed by the oral surgeon, the relationship between bone and implants becomes interdependent, with their success or failure intertwined. The theory of osseointegration, initially proposed by Professor Brånemark, is still widely accepted and used today [45]. The fundamental requirement for implants to achieve proper functionality is good osseointegration, which serves as the theoretical basis and key to implant success [46]. The classic definition of osseointegration is the direct contact and close binding between the implant and bone tissue, without any other intervening tissue components [5,47]. Some researchers currently consider osseointegration as a foreign body reaction, where the interface generates compact bone as a defensive response, and oral implants utilize this kind of reaction [48]. This emphasizes the influence of the immune system on osseointegration. Regardless of the specific definition, osseointegration requires the ability of osteogenic differentiation and bone regeneration [6]. Due to similarities in osteogenic activity, inflammatory response, and angiogenesis, osseointegration is often compared to processes such as fracture healing, although there are some differences [49]. For patients commonly encountered in clinical practice who have insufficient bone volume or bone defects, the current conventional approach in the field of oral implantology is to perform bone augmentation surgeries, such as guided bone regeneration and maxillary sinus floor lifting [50,51]. These procedures involve the use of osteoconductive or bone-inductive materials prior to or during implant surgery, followed by a period of several months to allow for bone tissue regeneration. The success of these procedures greatly depends on the patient’s ability for bone regeneration [52,53].
It is not only the bone-forming ability and bone quality during the early stages of implant surgery that concern oral implantologists. The quality and quantity of the bone surrounding the implant and the bone augmentation area also require attention after the surgery [54]. Marginal bone loss around implants is a common occurrence, especially in elderly patients and those with underlying diseases [7]. Even in the absence of peri-implant diseases or poorly placed restorations, marginal bone loss can still occur around the implants. Any marginal bone loss that occurs after the initial stages of implantation can potentially affect the longevity of the implant [12]. Therefore, post-implantation stability of the bone volume in the surgical area is also crucial. From both the pre-implantation and post-implantation perspectives, the patient’s bone-forming ability and bone homeostasis are essential factors. Enhancing the patient’s bone regeneration ability and reducing bone resorption are directions that ensure the success of implant treatment.
In recent years, the beneficial effects of probiotics on systemic bone tissue have been gradually discovered, even in the absence of direct contact [55,56]. Generally, the influence of probiotics on bone is considered to be remote, as bone tissue is typically considered a completely sterile environment. However, there is a growing awareness of the significant role of gut microbiota, referred to as the gut microbiota–bone axis, in bone health [57,58]. Within this context, probiotics, as a specific group of gut microbiota, play an important role.
The evidence for the beneficial effects of probiotics initially came from studies on fracture healing and osteoporosis treatment, indicating their systemic effects. Numerous animal and human studies have found that the administration of various probiotic strains, such as Lactobacillaceae and Bifidobacterium species, can accelerate fracture healing, promote osteogenic differentiation, and improve bone trabecular parameters [59,60,61,62]. It has been suggested in the literature that supplementation with specific strains of Bifidobacterium, particularly in the elderly population, can expedite fracture repair [62]. In models of osteoporosis induced by various factors, administration of probiotics such as Lactobacillaceae and Bifidobacterium has been shown to increase bone density, attenuate bone loss, and maintain bone homeostasis [56,61,63,64,65,66]. In clinical research, the effects of probiotic supplementation have been comparable to those of vitamin D and calcium supplementation, and it has been suggested as one of the therapeutic approaches for osteoporosis patients [67,68]. In healthy individuals, probiotics such as Lactobacillaceae and Bifidobacterium induce upregulation of bone-related genes and proteins (Runx2, Sp7, Bmp, Osteocalcin, Osteonectin, Osteopontin, Bonesialoprotein, and collagen type I), promote osteogenic differentiation of stem cells, and enhance bone regeneration capacity [69,70,71,72,73,74,75,76]. In the animal model of Zebrafish, probiotics such as Lactococcus lactis and Bacillus subtilis can also promote osteogenic differentiation and bone formation [73]. For the health of the jawbone, the role of probiotics is also prominent [77]. There have also been studies exploring the role of local application of probiotics in promoting implant osseointegration, which has shown promising results. Tan et al. found that locally loaded inactivated Lacticaseibacillus casei biofilm on the implant surface can accelerate osseointegration and enhance its effectiveness by activating TLR signaling pathways in macrophages, secreting osteogenic factors such as oncostatin M, and improving osteogenic differentiation of mesenchymal stem cells [78]. Additionally, researchers have discovered that surface coating of titanium implants with yeast-derived polysaccharides can stimulate bone tissue to secrete osteogenic factors and promote implant osseointegration [79].
Currently, the impact of probiotics on bone tissue primarily originates from the gut. Probiotic strains from gut play a positive role in promoting jawbone health and stability. The mechanisms by which probiotics exert their effects on bone tissue are believed to involve several interconnected aspects (Figure 1).
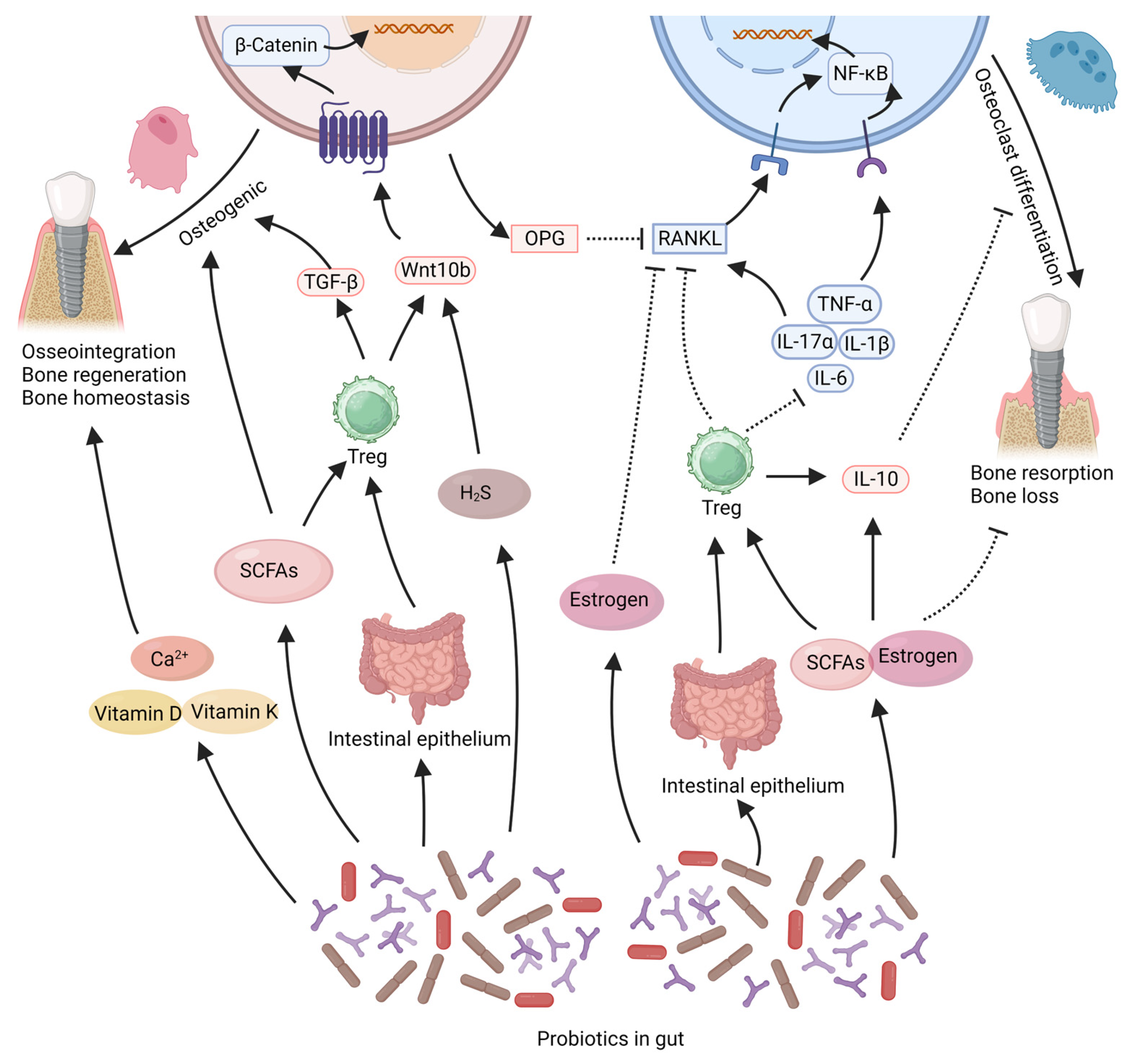
Figure 1. This illustration summarizes the potential beneficial effects of gut probiotics on bone tissue around implants. Probiotics in the intestine can secrete SCFAs, estrogen, which can directly promote osteogenic effects or regulate immunity to maintain bone homeostasis. Probiotics can regulate gut microbiota to enhance the stability of the intestinal epithelium. This can promote the stability of bone tissue mediated by the immune system. In addition probiotics can promote the absorption of nutrients that contribute to bone integration, such as calcium, vitamin D, and vitamin K. The solid arrows in the figure represent “promotion”, while the dashed arrows indicate “inhibition”. SCFAs: short-chain fatty acids, Treg: T regulatory cell. Created with BioRender.com (accessed on 29 June 2023).
2.1. Probiotics Exert Effects by Products
One distinctive way in which probiotics exert their effects is through the production of beneficial bacterial metabolites, such as short-chain fatty acids (SCFAs), estrogen-like compounds, and vitamins. It has been reported that Lactobacillaceae species can produce SCFAs and hydrogen sulfide (H2S) [80], while Bifidobacterium species can secrete SCFAs and estrogen-like compounds [81]. Lactobacillus acidophilus and Some Bacillus such as Bacillus subtilis can also produce vitamin K2, which is considered an important nutrient for bone formation [82]. These factors are widely recognized as crucial contributors to the pro-osteogenic and bone-stabilizing effects of probiotics [69,80,81,83]. In both in vitro and in vivo studies, SCFAs produced by probiotics (such as acetate, propionate, or butyrate) have been shown to increase the production of osteocalcin and osteoprotegerin, stimulate osteogenic differentiation of stem cells, enhance bone formation, and significantly improve trabecular bone parameters [70,84]. SCFAs can also inhibit osteoclast differentiation and suppress bone resorption in mice [85], thus contributing to maintaining bone mass stability. H2S produced by Lactobacillaceae species enhances osteoblast activity and prevents osteoblast apoptosis by activating the Wnt signaling pathway in osteoblasts [86]. Estrogen is well-known for its protective effects on bone tissue, and estrogen deficiency leads to bone loss. Probiotics can produce estrogen-like compounds [87], such as equol produced by Bifidobacterium species [81,87]. These estrogen-like compounds generated by probiotics can inhibit osteoclastogenesis via the suppression of the RANKL pathway, thereby reducing bone resorption [88]. Additionally, the estrogen-like compounds and SCFAs produced by probiotics are believed to influence systemic immunity and inflammation, which will be further discussed in subsequent sections.
2.2. Probiotics Exert Effects by Regulating Inflammation Levels
One important way in which probiotics exert their effects remotely within the gut is by regulating immunity and inflammatory responses. It is increasingly recognized that both implant osseointegration and stability of peri-implant bone tissue are closely associated with immune modulation [12,89]. Immune cells and inflammatory factors greatly influence bone tissue regeneration and stability. Probiotics may regulate systemic and local inflammation levels, thereby promoting bone regeneration and maintaining bone homeostasis [30,62]. The RANKL/RANK pathway, the TNF-α/NF-κB signaling pathway and the Wnt pathway are crucial for regulating osteogenic and osteoclastic activities within bone tissue, and their modulation can be influenced by the inflammatory milieu [90,91,92,93,94]. Reports indicate that Bifidobacterium longum, Lacticaseibacillus paracasei, Limosilactobacillus reuteri, Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, and Lactobacillus acidophilus can reduce the expression of the pro-inflammatory cytokine TNF-α within bone tissue, thereby decreasing the activation of the TNF-α/NF-κB signaling pathway, reducing osteoclast activation, and preventing bone loss [81,83,88,95,96,97]. The reduction in TNF-α within bone tissue also leads to decreased RANKL expression, increased osteoprotegerin (OPG) expression by osteoblasts, ensuring osteoblast activity, and reducing osteoclast differentiation [56,88,96,98,99]. Lacticaseibacillus rhamnosus and Limosilactobacillus reuteri can upregulate the expression of Wnt10b within tissues, activate the Wnt/β-catenin signaling pathway, counteract the inhibitory effects of TNF-α, and enhance osteogenic capacity in mice [70,95,100]. Probiotics such as Bifidobacterium longum, Lacticaseibacillus paracasei, Lacticaseibacillus casei, Limosilactobacillus reuteri, and Lactiplantibacillus plantarum can also reduce the expression of IL-1β, IL-6, IL-17 within bone tissue while increasing IL-10 expression [83,101,102], thereby inhibiting the osteoclastic effects mediated by the RANKL/RANK and TNF-α/NF-κB pathways, and maintaining stability in the peri-implant bone tissue [81,102,103,104,105]. The balance between Treg cells and Th17 cells plays a crucial role in bone homeostasis [104,106]. Probiotics such as Lactobacillus acidophilus, Lacticaseibacillus rhamnosus and Bifidobacterium longum can upregulate the Treg/Th17 ratio within bone tissue, thereby enhancing osteogenic activity and suppressing osteoclast differentiation [97,103,107,108]. An increase in Treg cell within bone tissue also leads to reduced expression of TNF-α and RANKL, as well as increased expression of IL-10 and Wnt10b [70,100,103].
The ability of probiotics to suppress inflammation may be linked to their capacity to enhance the stability of the intestinal epithelium [62]. The stability of the intestinal epithelium has a direct impact on the translocation of intestinal pathogens, which in turn affects the systemic inflammatory response and the stability of bone tissue [69]. Certain probiotic strains, such as Bifidobacterium adolescentis, Bifidobacterium longum, Limosilactobacillus reuteri and Akkermansia muciniphila have shown the ability to upregulate the expression of the Ocln gene in the intestinal epithelium. This leads to the reinforcement of tight intercellular connections among epithelial cells, resulting in a reduction in systemic inflammation [55,58,59,62,68,109]. These effects play a constructive role in promoting bone regeneration and maintaining bone mass stability [55,58,59,62,68,109]. The ability of probiotics to counteract systemic inflammation may also be linked to their production of compounds such as estrogen-like compounds and SCFAs. These substances have the potential to reduce the levels of pro-inflammatory cytokines (TNF-α, IL-1, IL-6) while enhancing the expression of the anti-inflammatory mediator IL-10 [88,110,111]. Furthermore, these short-chain fatty acids have been implicated in stimulating the migration of Tregs from the intestinal lining to the bone marrow, leading to an increase in the expression of Wnt10b in the bone marrow and facilitating the process of bone regeneration [81,100,112].
2.3. Probiotics Exert Effects by Promoting Angiogenesis
In the context of implant osseointegration and bone augmentation surgeries, the effective utilization of osteogenic capacity also relies on the promotion of tissue angiogenesis, which is indispensable [113]. Limited in vivo and in vitro studies have suggested that certain probiotics, whether in live form or in bacterial culture supernatant, can locally stimulate angiogenesis. They have been shown to induce the production of VEGF, thereby promoting endothelial cell growth and migration [114,115,116]. Liu et al. found that in a mouse fracture model, the use of Akkermansia muciniphila in the intestine can also induce type H vessel formation in callus tissue, thereby promoting bone regeneration [109]. However, the current evidence mostly suggests that the angiogenic effects are dependent on the local action of probiotics, and further study is needed regarding their application in the regeneration of peri-implant bone tissue.
2.4. Probiotics Exert Effects by Promoting Nutrient Absorption
In addition, probiotics that promote the digestion and absorption of nutrients can also contribute to the health of the skeletal system [55]. Various probiotics, such as Lactobacillus acidophilus, Lacticaseibacillus casei, Limosilactobacillus reuteri and Bifidobacterium longum have been shown to enhance the absorption of calcium, vitamin D, and vitamin K [64,117,118,119]. These nutrients are considered crucial for implant osseointegration and stability of the surrounding bone tissue [120,121,122,123,124].
Based on current research findings, the use of probiotics appears beneficial for meeting the skeletal demands of implant treatments. They promote osteogenesis, maintain bone homeostasis, and may even facilitate blood vessel formation.
3. The Role of Probiotics in Wound Healing
The speed and quality of wound healing after implant surgery are crucial and easily observable factors. Good wound healing is one of the prerequisites for subsequent osseointegration of the implant and infection control [3,4]. In particular, for implant patients who have undergone bone augmentation procedures, optimal wound healing is a key factor for surgical success [4,113,125]. If the surgical site is extensive, there are higher demands on wound healing [4,113,125]. Some studies suggest that the quality of the mucosa can even affect the occurrence of peri-implantitis [4,126]. Wound healing not only alleviates patient discomfort but also reduces the risk of surgical failure. Furthermore, patients with certain underlying diseases, such as diabetes, experience slower wound healing. Enhancing the speed and quality of wound healing holds significant importance for both patient experience and implant efficacy.
The mechanisms underlying mucosal wound healing are similar to those of the skin; however, due to structural differences, there are subtle variations in the healing process. In contrast to the skin, mucosal wounds exhibit accelerated healing with reduced scar formation [127]. Following the occurrence of an oral mucosal wound, the healing process unfolds through distinct stages: hemostasis, inflammatory response, proliferation, and remodeling. Within minutes of the wound initiation, a cascade of hemostatic reactions is triggered. Subsequently, an inflammatory response ensues over the course of several days [128]. At this stage, neutrophil debridement and macrophage mediated secretion of inflammatory cytokines. Within approximately one week, the inflammatory response subsides, giving way to the proliferation stage, during which fibroblasts proliferate, migrate, and orchestrate collagen deposition and neovascularization, thereby facilitating wound closure. In the subsequent weeks, tissue remodeling occurs, culminating in the further maturation of the dense collagen network [127]. Wound healing is intricately connected with tissue regeneration, infection control, immune modulation, and inflammation regulation. Facilitating epithelialization and modulating excessive inflammatory responses are fundamental strategies to promote optimal quality and expeditious healing of mucosal wounds [127].
The role of probiotics in promoting wound healing has been increasingly recognized [116,129]. Initially, probiotics were found to contribute to the healing of skin wounds. Local or systemic application of probiotics in patients after burns or surgery can accelerate wound healing and reduce wound related complications [130,131,132]. In particular for patients with wound healing disorders such as diabetes, probiotics can have a good effect on wound healing [133]. Numerous studies focusing on oral mucosal wounds have also revealed the significance of probiotics in oral wound healing. Han et al. demonstrated that local application of Limosilactobacillus reuteri lysate significantly enhanced wound healing in a mouse gingival wound model [134]. They also demonstrated the beneficial effects of Limosilactobacillus reuteri on wound healing in mouse palatal wounds [135]. Probiotics have the following effects in promoting wound healing (Figure 2).
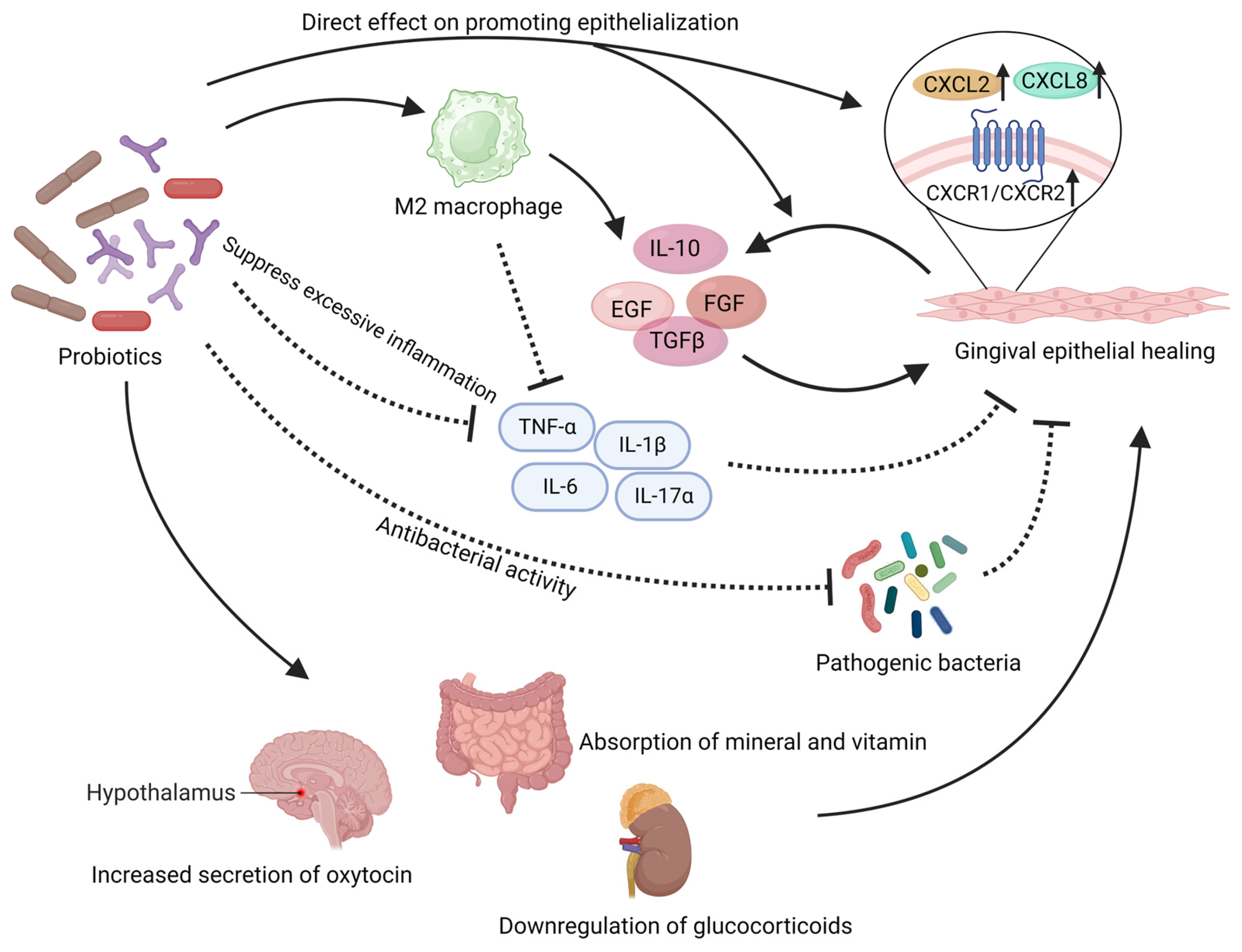
Figure 2. This illustration summarizes the beneficial effects that probiotics may exert in the local mucosal wound. Probiotics have demonstrated their ability to directly promote the proliferation and migration of gingival epithelium both in vivo and in vitro. They can also regulate the level of inflammation in the wound area to accelerate epithelial healing. Probiotics can exert adverse effects on pathogenic microorganisms in the wound area. In addition, probiotics can also regulate the body’s endocrine levels and provide a positive impact on wound healing. The solid arrows in the figure represent “promotion”, while the dashed arrows indicate “inhibition”. EGF: epidermal growth factor, FGF: fibroblast growth factor, TGFβ: transforming growth factor-beta. Created with BioRender.com (accessed on 29 June 2023).
3.1. Probiotics Have Direct Effects on the Gingival Epithelium
The regeneration and migration functions of epithelial cells, fibroblasts, and mesenchymal stem cells are essential for mucosal wound healing and epithelial formation [136]. Certain probiotics have been found to directly enhance cell regeneration and migration capabilities [116]. In in vitro experiments, Limosilactobacillus reuteri lysates was shown to activate the potential of gingival mesenchymal stem cells (GMSCs), enhancing their migration capability and expediting the wound healing process [134,135]. Probiotic mixtures comprising various Lactobacillus and Lactococcus have been shown to enhance the growth and migration abilities of human fibroblasts [137]. Studies have found that the lysates of Lactobacillus salivarius, Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus promote the growth and migration of keratinocytes [138]. Emmanuel et al. demonstrated that Lactobacillaceae and Bifidobacterium directly enhance migration and proliferation of gingival epithelium in co-culture conditions [139]. In vivo experiments have also shown that Lactobacillus bulgaricus and Lactiplantibacillus plantarum can increase the local population of fibroblasts, thereby accelerating wound healing [140]. The direct effects of probiotics on gingival epithelial regeneration can be explained through the expression of CXCL and its receptors, CXCR1/CXCR2. Lacticaseibacillus rhamnosus has been reported to enhance the expression of CXCL2 and CXCR2 in keratinocytes [139]. Under the influence of Lacticaseibacillus casei and Lactobacillus acidophilus, Bifidobacterium pseudolongum, the secretion of CXCL8 by gingival epithelial cells significantly increases, accompanied by a substantial increase in the surface receptors CXCR1/CXCR2 on gingival epithelial cells [141,142,143]. CXCL2 and CXCL8 can activate CXCR1/CXCR2 receptors in a classical manner, stimulating epithelial cell proliferation and migration [139,144].
Probiotics also exert the ability to stimulate the activation of wound repair-associated growth factors [145], thereby expediting the healing process. Yousef Ashoori proposed that probiotic metabolites such as polysaccharides, lactic acid, and acetic acid present in the supernatant of Limosilactobacillus reuteri can effectively trigger the local release of growth factors, including epidermal growth factor (EGF), fibroblast growth factor (FGF), and transforming growth factor-beta (TGFβ) [146]. These growth factors play a pivotal role in the intricate process of wound healing [147,148,149,150,151,152]. Notably, both in vitro and in vivo investigations have demonstrated the profound impact of Limosilactobacillus reuteri in activating the PI3K/AKT/β-catenin pathway within mesenchymal stem cells, ultimately culminating in enhanced proliferation, differentiation, and wound closure facilitated by upregulated TGFβ1 expression [134]. Furthermore, the utilization of probiotics derived from kefir has shown promising potential in augmenting the expression of TGFβ1 and FGF genes [137]. Local application of Lactobacillus bulgaricus and Lactiplantibacillus plantarum has been found to effectively heighten the expression of TGFβ1 in wound tissues [140]. Additionally, the administration of a probiotic mixture consisting of eight strains, including Bifidobacterium and Lactobacillaceae, has demonstrated substantial elevation in mucosal levels of EGF and TGFβ1, significantly enhancing the regenerative capacity and wound healing efficacy [115]. Studies focusing on the intestine have shown that the use of Lacticaseibacillus rhamnosus can enhance the expression of EGF, thereby promoting mucosal protection and regeneration [153,154].
3.2. Probiotics Exert Effects by Reversing the Impact of Oral Pathogens
Bacterial dysbiosis has been shown to impair wound healing [135]. In an imbalanced oral microbiota environment, the functionality of oral gingival and palatal mesenchymal stem cells (MSCs) is compromised, resulting in a significantly slowed healing process of oral soft tissues [155]. However, probiotics can counteract the detrimental effects of pathogenic bacteria or reverse their unfavorable impact on tissue repair [135]. Han et al. discovered that Porphyromonas gingivalis, a periodontal pathogen, slows down wound healing due to the influence of its lipopolysaccharide (LPS). However, when co-administered with Limosilactobacillus reuteri the wound healing speed increased and exceeded that of the control group [135]. Even the ultrasound extract of Limosilactobacillus reuteri can reverse the adverse effects of Porphyromonas gingivalis LPS on the migration of gingival MSCs [135]. Local application of the supernatant derived from Lactobacillus VITSAMJ1, isolated from goat milk, can inhibit the growth of Staphylococcus aureus in the wound area and reduce apoptosis in epithelial cells [145].
3.3. Probiotics Exert Effects by Regulating Local Inflammation Levels
Probiotics exert an additional significant mechanism in promoting wound healing, which involves the regulation of the inflammatory response [116,146,156,157]. Excessive inflammation at the site of the wound can cause delays in epithelialization and impede the healing process [127]. Probiotics possess the capability to downregulate pro-inflammatory cytokines while upregulating anti-inflammatory factors. Research conducted by Emmanuel et al. revealed that a variety of Lactobacillaceae and Bifidobacterium strains (such as Limosilactobacillus reuteri, Lacticaseibacillus rhamnosus, Lactobacillus acidophilus, Lacticaseibacillus casei, Bifidobacterium longum, Bifidobacterium animalis, Bifidobacterium breve, Bifidobacterium pseudolongum, and Bifidobacterium bifidum) can effectively downregulate the expression of IL-1β and TNF-α, which are pro-inflammatory cytokines, in damaged gingival epithelial tissue [142]. In the context of diabetic wound healing, the administration of Lactobacillus bulgaricus and Lactiplantibacillus plantarum has been observed to significantly reduce the levels of IL-1β and TNF-α [140]. Notably, Lacticaseibacillus casei has been reported to modestly inhibit the expression of various pro-inflammatory cytokines, such as IL-1β, IL-2, IL-6, IL-12, IL-17α, IFN-γ, and TNF-α [140], thereby contributing to the facilitation of the wound healing process. Lacticaseibacillus rhamnosus not only regulates the levels of inflammatory factors but also exerts an inhibitory effect on immune cells (Th1, Th2, and Th17) to control inflammation [158,159]. Additionally, probiotics upregulate local anti-inflammatory factors. Lactiplantibacillus plantarum enhances the expression of IL-10 in post-operative mucosal tissues, contributing to a favorable healing environment [160]. Similarly, Lactobacillus bulgaricus can increase the local expression of IL-10 and TGFβ1 in the wound area, effectively reducing inflammation levels and supporting the healing process [140]. The administration of Limosilactobacillus reuteri induces the upregulation of the anti-inflammatory cytokine IL-10, thereby minimizing tissue damage at the wound edge and further downregulating the anti-inflammatory mediator IL-17A [161]. Consequently, following probiotic or probiotic supernatant treatment, there is a significant decrease in local inflammation levels [140]. Numerous studies suggest that the beneficial effects of probiotics on wound healing are closely associated with their ability to modulate inflammation [116,162]. By resolving inflammation, macrophages transition into an M2 phenotype, which in turn promotes the release of regenerative growth factors such as FGF, EGF, and VEGF [127,163]. This elucidates the importance of attenuating inflammation for facilitating mucosal healing.
3.4. Probiotics Provide Indirect Support for Mucosal Healing
In addition to directly influencing local immunity, inflammation, and tissue repair, probiotics can provide indirect support for wound healing. One understandable pathway is that probiotics can improve the absorption of essential nutrients required for wound healing, particularly vitamins, minerals, and cofactors for tissue repair enzymes [116,164]. Research has shown that probiotics can enhance the absorption of inorganic salts, such as calcium ions [165]. Furthermore, Lactobacillus rossiae have been shown to produce vitamin B12 that is beneficial to wound healing [166]. Limosilactobacillus reuteri and Lactobacillus acidophilus can increase the absorption of dietary vitamin D and E [117,167,168,169,170], which is crucial for wound healing.
Certain studies propose that oral administration of probiotics can exert regulatory effects on systemic immunity, inflammation, and tissue repair. Remarkably, the consumption of Limosilactobacillus reuteri lysate alone has been demonstrated to enhance endogenous oxytocin levels [171], a pivotal hormone implicated in promoting wound healing [172,173]. Notably, an augmentation of oxytocin-producing cells within the paraventricular nucleus (PVN) of the hypothalamus adjacent to the third ventricle has been observed in mice treated with probiotic lysate [171]. Clinically, probiotic supplementation has exhibited a substantial elevation in local oxytocin levels within oral mucosal wounds [174]. Moreover, investigations suggest that probiotic intake, including Limosilactobacillus reuteri, Lactobacillus helveticus, and Bifidobacterium longum, can induce a decline in systemic glucocorticoid levels, thus fostering a conducive milieu for wound healing [171,175].
Furthermore, probiotic supplementation may potentially alleviate post-operative pain perception in patients. A clinical study focusing on mucosal wound healing following tooth extraction revealed a significant reduction in swelling sensation among individuals administered oral probiotics [176]. Moreover, patients receiving probiotics reported fewer sleep disturbances, reduced sick leave duration, higher healing score indices, and improved wound healing outcomes [176]. These findings may be associated with factors such as wound healing rate, infection control, among others, warranting further investigation.
4. The Role of Probiotics against Peri-Implant Diseases
The most common biological complication in implantology is peri-implantitis, which refers to the inflammation of the tissues surrounding the implant, including both the soft and hard tissues. These tissues provide essential support and protection for the implant [177]. If peri-implant diseases occur, they can significantly affect the functionality and aesthetics of the implant. Peri-implant diseases are classified into “peri-implant mucositis” (involving only soft tissues) and “peri-implantitis” (involving both soft and hard tissues) [178]. Generally, peri-implant mucositis, if left untreated, can progress to peri-implantitis. Peri-implant diseases can cause damage to the peri-implant soft and hard tissues and directly lead to implant failure [179].
Overall, the incidence of peri-implantitis ranges from 1.1% to 85.0% [8]. A survey conducted on patients with implants for over one year found a prevalence of 10.7% for peri-implantitis at the implant level [180]. Within three years after implant placement, the incidence rates of peri-implant mucositis and peri-implantitis were reported as 29.48% and 9.25%, respectively, based on the number of implants [181]. The most recent epidemiological study conducted in China revealed that after 2.5 years of implantation, the incidence rates of peri-implant mucositis and peri-implantitis were 36.69% and 7.66% at the implant level [182]. Peri-implant diseases pose a significant challenge to the long-term functionality of dental implants and have become a serious public health issue [14].
The primary etiology of peri-implant diseases is microbial infection of the peri-implant tissues [183,184]. Peri-implantitis is characterized by a dysbiosis of the subgingival microbial ecosystem [185]. There are significant similarities between peri-implantitis and periodontitis in terms of etiology and pathological changes. Some studies believe that there is no difference between the pathogens of diseases around implants and Periodontal disease [186]. However, more recent research indicates that the pathogenic bacterial populations in peri-implantitis may have slight differences [185,187,188,189], and there is considerable variation in the microbial profiles among individuals [187]. In addition to the bacterial complexes commonly found in periodontitis, such as Red-Complex Bacteria and other bacteria (including Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, Tannerella forsythia, and Treponema denticola), peri-implant diseases also frequently involve bacteria such as Staphylococcus aureus, Prevotella nigrescens, and Campylobacter species [177,190]. However, it should be noted that the traditional periodontal pathogens, including Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, Tannerella forsythia and Treponema denticola, are highly prevalent and play an important role in peri-implant diseases [177,187,188,190,191]. In particular, Porphyromonas gingivalis, Fusobacterium nucleatum, and Tannerella forsythia exhibit high abundance in peri-implant diseases [188]. Under the attack of pathogenic bacteria, the host’s excessive inflammatory response leads to the destruction of peri-implant soft and hard tissues, ultimately resulting in the failure of implant treatment.
Probiotics have long been proven to be a treatment for infections, including dysbiosis and infectious diseases within the oral cavity [192]. They also appear to have targeted benefits for peri-implant diseases. Probiotics play a significant role in regulating the dysbiosis of microbial populations around dental implants. They can effectively inhibit the colonization of pathogenic bacteria in peri-implant tissues, balance the oral microbiota, and thus exert an anti-infective effect [135]. Microscopically, probiotics mainly exert their effects through the following ways (Figure 3).
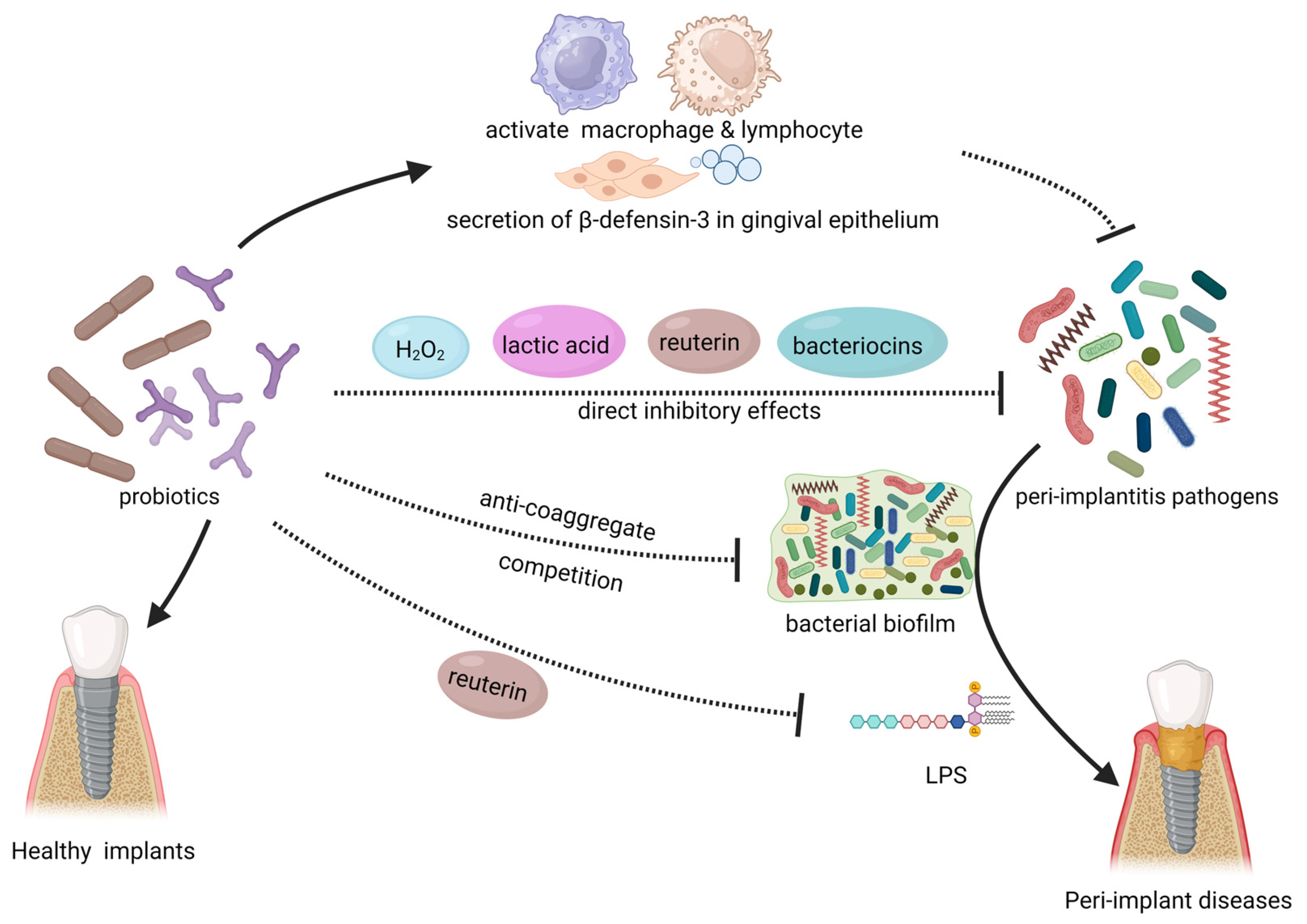
Figure 3. This illustration summarizes the local mechanism of probiotics in combating peri-implant diseases. Probiotics have an inhibitory effect on peri-implantitis pathogens and biofilm in the oral cavity directly. Probiotics can also enhance the body’s immune system and enhance the epithelial barrier. The use of probiotics can also resist the toxic effects of pathogenic bacteria. The solid arrows in the figure represent “promotion”, while the dashed arrows indicate “inhibition”. LPS: lipopolysaccharide. Created with BioRender.com (accessed on 29 June 2023).
4.1. Direct Inhibitory Effect on Pathogens
Probiotics directly hinder the growth and proliferation of pathogenic microorganisms in the human body. Probiotic strains such as Weissella cibaria, Limosilactobacillus reuteri, and Lactobacillus salivarius have been shown to significantly inhibit the growth of peri-implantitis pathogens such as Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, Tannerella forsythia, and Staphylococcus aureus in vitro [193,194,195]. They also affect the colonization of these bacteria on titanium surfaces [195]. Vacca et al. demonstrated that Streptococcus salivarius, a member of the oral micro-biome, exhibits the ability to inhibit the formation of pathogenic biofilms on implant materials in vitro. These findings suggest that Streptococcus salivarius could be utilized for the prevention or treatment of peri-implant diseases [196]. Bifidobacterium species exhibit inhibitory effects against various peri-implant disease pathogens, including Porphyromonas gingivalis and Fusobacterium nucleatum, with particularly strong inhibition against P. gingivalis [197,198,199]. The inhibitory effects of probiotics on pathogenic microorganisms may arise from the production of specific substances. Various Lactobacillaceae and Bifidobacterium, including Limosilactobacillus fermentum and Lactobacillus acidophilus, exert direct inhibitory effects on peri-implant disease pathogens through producing hydrogen peroxide, bacteriocins and organic acids [200,201,202]. Reuterin (3-hydroxypropionaldehyde) is an antimicrobial metabolite produced by Lactobacillus species. Limosilactobacillus reuteri, in previous studies, was found to exert antibacterial effects by utilizing reuterin to oxidize thiol groups in peri-implantitis pathogens [116]. Radaic et al. discovered that Lactococcus lactis producing nisin (a bacteriocin) exhibited inhibitory effects against various peri-implantitis pathogens such as Fusobacterium nucleatum, Porphyromonas gingivalis, and Treponema denticola [203]. This particular Lactococcus lactis can disrupt the biofilm formation of pathogenic bacteria on the surface of implant materials [203]. As one of the bacteria capable of colonizing the oral cavity, probiotics compete with other oral microbiota, particularly for nutrients and binding sites. Due to its own competitive advantage in terms of nutrients and growth factors, Bifidobacterium can competitively inhibit peri-implant disease pathogens such as Porphyromonas gingivalis [198,204]. Bifidobacterium may have potential implications for bacterial aggregation and biofilm formation of pathogens, as it can compete for binding sites and consequently affect pathogen coaggregation [205,206,207].
4.2. Probiotics Prevent Tissue Damage Mediated by Pathogenic Bacteria
Furthermore, certain probiotic strains have the ability to neutralize toxic substances produced by pathogenic bacteria or alleviate the detrimental effects mediated by these pathogens. Han et al. discovered that reuterin, a unique and effective compound secreted by Limosilactobacillus reuteri, can mitigate the toxic effects of Porphyromonas gingivalis lipopolysaccharide (LPS) on the host [135]. Probiotics may play a role in modulating inflammation locally in the periodontal tissues, thereby alleviating tissue damage and disease progression caused by pathogen-induced inflammation. Flichy-Fernández et al. found that after treatment with Limosilactobacillus reuteri in patients with peri-implant mucositis, there was a significant decrease in inflammatory mediators such as IL-1β and TNF-α in peri-implant gingival crevicular fluid [105]. The authors suggested that the local inhibition of excessive inflammatory responses by probiotics is an important factor in relieving clinical symptoms, preventing further tissue destruction, and promoting tissue repair. As mentioned earlier, probiotics have been shown to modulate systemic inflammation in the gut, and this may also have significant implications for mitigating inflammation in the peri-implant region.
4.3. Probiotics Enhance Immune Function
Some researchers have found that the direct intervention of probiotics on pathogenic bacteria cannot fully explain their significance in peri-implant diseases [208]. Probiotics can also enhance the host oral mucosa’s resistance to pathogenic bacteria in the peri-implant region [206]. The local upregulation of β-defensins, which are antimicrobial peptides, by probiotics has been widely reported and can help maintain the body’s barriers [116,209]. Invernic et al. found that treatment with Bifidobacterium animalis subsp lactis HN019 led to increased secretion of β-defensin-3 in gingival epithelium, thereby enhancing the resistance against pathogenic bacteria such as Porphyromonas gingivalis [197]. Moreover, local expression of Toll-like receptor 4 (TLR4) and CD-4, CD-57-positive immune cells was upregulated. These cells play a role in pathogen recognition and activation of innate immunity. The local use of probiotics significantly improves the body’s own defense capabilities [197]. It has also been reported that probiotics possess immunostimulatory extracellular polysaccharides that can activate macrophages and lymphocytes, thereby enhancing immune function [210].
4.4. Clinical Studies on the Treatment of Peri-Implant Diseases with Probiotics
Introducing probiotics into the unique environment of the oral cavity holds promising prospects for combating peri-implant diseases. In recent years, researchers have proposed the use of probiotics for the treatment or adjunctive treatment of peri-implantitis. Their main basis is the significant changes observed in clinical indicators of peri-implant diseases in clinical trials or animal studies. Clinical studies have compared the effects of mechanical plaque removal alone or in combination with local application of Limosilactobacillus reuteri in the treatment of peri-implant diseases. The results showed that patients who received probiotic supplementation showed better improvements in clinical parameters such as probing bleeding and probing depth, regardless of the extent of the disease [208,211]. Butera et al. suggested that the use of Limosilactobacillus reuteri, Lactobacillus brevis, or Lactiplantibacillus plantarum in the treatment of peri-implant diseases led to varying degrees of improvement in clinical indices such as bleeding on probing, attachment loss, and plaque index [212]. The latest systematic review indicates that probiotics, as an adjunctive treatment for peri-implant mucositis, have good clinical efficacy, surpassing the use of antibiotics [213,214]. The authors suggest that probiotics should be used as an adjunct to mechanical plaque removal. There is also a systematic review that suggests that probiotics have positive significance in improving clinical parameters such as probe depth in peri-implantitis [215]. However, there are also some studies doubt that the use of probiotics can cure patients who have already developed peri-implant inflammation, which remains to be discussed [216,217,218].
Peri-implantitis is currently challenging to eradicate, and its treatment principle involves the removal of biofilm. Current treatment options include mechanical therapy, the use of antibiotics [15,126,219], and even surgical procedures. Once peri-implant diseases progress to the stage of peri-implantitis, the difficulty of treatment increases significantly. Based on existing research, if left untreated in the early stages, peri-implant mucositis tends to rapidly progress [220]. Considering the cost and difficulty of treating peri-implantitis, the best approach is to intervene before the onset of peri-implant diseases or in the early stages of the disease to prevent further progression [126,184]. Although probiotics are controversial for the cure of peri-implantitis, more than one study emphasizes the clinical significance of probiotics in controlling and preventing peri-implant diseases [105,208,213,221,222]. Increasingly, research suggests that probiotics have a beneficial effect on the early stages of peri-implant diseases, especially peri-implant mucositis, which serves as a precursor to peri-implantitis [213]. Probiotics have shown promising therapeutic effects in this regard, indicating their potential for prevention and control of peri-implant diseases. Tan et al. found that the surface loading of inactivated Lactobacillus biofilm on implants exhibited strong resistance against (MRSA) and could be used to prevent the occurrence and progression of peri-implantitis [78]. Zhu et al. demonstrated that when Bifidobacteria first colonize the environment, they can strongly inhibit the colonization and biofilm formation of peri-implant pathogens such as Porphyromonas gingivalis, Fusobacterium nucleatum, and Prevotella intermedia [200]. Bifidobacterium are beneficial for the growth of oral microbiota (Actinomyces naeslundii and Streptococcus mitis) associated with gingival health while inhibiting the proportion of peri-implant pathogens [199,223]. In studies related to periodontitis, it has been found that early intervention with probiotics in a healthy state significantly reduces the severity of subsequent periodontal disease models [224]. Therefore, these findings suggest that the early use of probiotics may have a more significant preventive effect on peri-implantitis.
Probiotics can play a crucial role in preventing peri-implant diseases by inhibiting the growth of pathogens and influencing the formation of biofilm structures. However, Clinical studies investigating the prevention of peri-implant diseases with probiotics are currently lacking on a large scale. According to existing literature, probiotics such as Lactobacillus and Bifidobacterium have shown potential in the prevention and control of peri-implant diseases in the oral cavity. Their single or combined use may become important tools in preventing peri-implant diseases.
5. Conclusions and Outlook
Probiotics exert extensive and remarkable effects in the human body. They have the ability to directly influence the prognosis of implants by impacting local hard and soft tissues. Probiotics can also indirectly support post-operative healing and osseointegration of implants by affecting systemic conditions. From a localized perspective, probiotics can promote the regeneration of local soft and hard tissues, exhibit antimicrobial properties against pathogenic bacteria, and reduce local inflammatory reactions. From a systemic standpoint, the ingestion of probiotics can regulate systemic immune-inflammatory responses and enhance the absorption of nutrients. Probiotics also possess the capability to remotely assist in bone regeneration and homeostasis, making them particularly valuable for post-implantation health care.
The utilization of probiotics as an intervention measure holds intrinsic value due to its benefits, including a relatively low safety risk and non-invasive mode of intervention. Probiotics are worthy of widespread utilization [22,225,226,227,228]. However, several considerations need to be addressed before their formal implementation in clinical practice.
Probiotics confer benefits to human health, and the utilization of probiotics has a long-standing history. In general, probiotics are associated with minimal adverse effects on the human body [81,192,229]. While adverse effects of probiotics are really rare in clinical settings, as living microorganisms, it may be prudent to exercise caution when employing probiotics in clinical applications [229]. Probiotics have been associated with several potential and theoretical risks [230]. There is a potential risk of probiotics causing systemic infections [231,232]. Actually, the likelihood of probiotics causing systemic infections is generally low in patients without specific diseases or specific interventions [233,234]. Based on current clinical research, there may be a risk of systemic infection associated with the use of probiotics in patients with short bowel syndrome (SBS), heart valve disease, and those requiring central venous catheters [230,233,235]. Probiotics, due to the secretion of substances such as D-lactic, have been reported in some studies to potentially cause gastrointestinal adverse reactions in a subset of patients undergoing intestinal surgery, such as abdominal cramping, nausea, soft stools, flatulence, and taste disturbance [229,236]. However, compared to patients not receiving probiotics, those who received probiotics generally experienced fewer gastrointestinal reactions [235]. Due to the immunomodulatory effects of probiotics, they may also potentially trigger an exaggerated immune response in the body. Some reports have indicated an increased risk of allergic rhinitis, asthma and atopic allergies in certain individuals after the use of probiotics [233]. However, overall, the occurrence of such events is very low [229]. Probiotics, as bacteria, may potentially undergo antibiotic resistance transfer with pathogenic bacteria. Currently, it is widely believed that the probability of antibiotic resistance transfer in probiotics is very low [231,233,236]. However, as required by the European Food Safety Authority, the genetic nature of clinically relevant antibiotic resistance genes must be explained for probiotics [236]. The gene transfer of probiotics is currently being regulated and monitored. In a study published in Cell in 2018, it was reported that probiotics may have long-term negative effects on the recovery of the gut microbiota following antibiotic treatment [237]. Although there is no clinical evidence to suggest that this phenomenon may have adverse health impacts [238], the safety concerns associated with probiotics deserve ongoing attention during their usage. Furthermore, additional clinical trial results and experimental models are needed to further refine the guidelines for probiotic use. It is important to use probiotics within a defined range and regulate their use diligently to ensure their optimal clinical benefits can be realized.
The clinical effects of different probiotic strains vary significantly. Even within the same species of traditional probiotics such as Lactobacillaceae and Bifidobacterium, distinct strains exhibit diverse properties. For instance, it has been reported that Limosilactobacillus fermentum has been shown to reduce the vitality of keratinocytes and suppress re-epithelialization [116,139]. Furthermore, not all probiotic strains exert equal effects on processes such as osteogenesis, wound healing, and combating peri-implantitis pathogens. In addition, the acceptance of different probiotic strains may vary among individuals, which can also influence the effectiveness of probiotics across different individuals [239]. Therefore, the selection of probiotics should prioritize strain specificity. Further research is needed to enhance the evidence concerning different strains in various aspects of dental implants. This can aid in identifying the optimal combination of probiotics and even explore the possibility of developing engineered strains through biotechnology, merging the advantages of different probiotic strains.
Based on current findings, further basic and clinical research is needed for the clinical application of probiotics in implantology. While there is substantial evidence supporting their role in combating peri-implant diseases, promoting oral mucosal wound healing, and enhancing jawbone health and stability, studies specifically focused on implant surgery are still lacking. In our opinion, conducting research on oral implantation in conjunction with probiotics is crucial based on the existing evidence. Moreover, research on probiotics may be more important not only for healthy patients (animals), but also for patients with certain underlying diseases and elderly individuals. Current evidence suggests that probiotics exhibit significant potential in mitigating the impact of underlying diseases, particularly in the context of diabetes and osteoporosis [64,67,133]. In the field of oral implantology, conditions such as diabetes and osteoporosis are generally recognized as high risks for implant health [7,9,10]. However, some researchers now argue that underlying diseases may not be absolute contraindications for implant surgery [7]. Therefore, employing probiotic intervention in implant patients with underlying diseases seems to be even more meaningful, as these patients may derive greater benefits compared to healthy individuals.
Probiotics are significant microorganisms in the human microbiota. Due to their safety and efficacy, probiotics can be applied to various diseases. We have summarized the benefits that probiotics can provide in several aspects of post-implantation. Based on this review, we believe that probiotics hold potential for application in promoting soft and hard tissue healing, maintaining bone homeostasis, and combating peri-implant diseases in oral implantation. We call for further research to explore their potential value.
References
- Kimble, R.; Papacosta, A.O.; Lennon, L.T.; Whincup, P.H.; Weyant, R.J.; Mathers, J.C.; Wannamethee, S.G.; Ramsay, S.E. The Relationship of Oral Health with Progression of Physical Frailty among Older Adults: A Longitudinal Study Composed of Two Cohorts of Older Adults from the United Kingdom and United States. J. Am. Med. Dir. Assoc. 2023, 24, 468–474.e463. [Google Scholar] [CrossRef] [PubMed]
- Renouard, F.; Renouard, E.; Rendón, A.; Pinsky, H.M. Increasing the margin of patient safety for periodontal and implant treatments: The role of human factors. Periodontol. 2000 2023. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Chopra, D.; Kocak-Oztug, N.A.; Verron, E. Fit and forget: The future of dental implant therapy via nanotechnology. Adv. Drug Deliv. Rev. 2023, 199, 114900. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Roccuzzo, A.; Marruganti, C.; Fickl, S. The importance of soft tissue condition in bone regenerative procedures to ensure long-term peri-implant health. Periodontol. 2000 2023. [Google Scholar] [CrossRef]
- Marco, F.; Milena, F.; Gianluca, G.; Vittoria, O. Peri-implant osteogenesis in health and osteoporosis. Micron 2005, 36, 630–644. [Google Scholar] [CrossRef]
- Lee, J.W.Y.; Bance, M.L. Physiology of Osseointegration. Otolaryngol. Clin. N. Am. 2019, 52, 231–242. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; de Oliveira, A.S.; Faé, D.S.; Oliveira, H.; Del Rei Daltro Rosa, C.D.; Bento, V.A.A.; Verri, F.R.; Pellizzer, E.P. Do dental implants placed in patients with osteoporosis have higher risks of failure and marginal bone loss compared to those in healthy patients? A systematic review with meta-analysis. Clin. Oral Investig. 2023, 27, 2483–2493. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal. Res. 2018, 53, 657–681. [Google Scholar] [CrossRef]
- Almehmadi, A.H. Awareness of population regarding the effects of diabetes on dental implant treatment in Jeddah, Saudi Arabia. Heliyon 2019, 5, e02407. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Sun, H. Impact of diabetes mellitus on the poor prognosis in patients with osseointegrated dental implants: A meta-analysis of observational studies. Biotechnol. Genet. Eng. Rev. 2023, 39, 1–19. [Google Scholar] [CrossRef]
- Albrektsson, T.; Tengvall, P.; Amengual, L.; Coli, P.; Kotsakis, G.A.; Cochran, D. Osteoimmune regulation underlies oral implant osseointegration and its perturbation. Front. Immunol. 2022, 13, 1056914. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Tengvall, P.; Amengual-Peñafiel, L.; Coli, P.; Kotsakis, G.; Cochran, D.L. Implications of considering peri-implant bone loss a disease, a narrative review. Clin. Implant. Dent. Relat. Res. 2022, 24, 532–543. [Google Scholar] [CrossRef]
- Strauss, F.J.; Siegenthaler, M.; Hämmerle, C.H.F.; Sailer, I.; Jung, R.E.; Thoma, D.S. Restorative angle of zirconia restorations cemented on non-original titanium bases influences the initial marginal bone loss: 5-year results of a prospective cohort study. Clin. Oral Implant. Res. 2022, 33, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M. Prevention and treatment of peri-implant diseases-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H.; Wang, H.L. Breaking the wave of peri-implantitis. Periodontol. 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral microbiome and peri-implant diseases: Where are we now? Ther. Clin. Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Tuganbaev, T.; Yoshida, K.; Honda, K. The effects of oral microbiota on health. Science 2022, 376, 934–936. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and Potentially Probiotic Yeasts-Characteristics and Food Application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; Ter Haar Née Younes, J.A.; Heschl, A.; Mičetić Turk, D.; Rogelj, I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. Biomed. Res. Int. 2019, 2019, 7585486. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6, 1128. [Google Scholar] [CrossRef]
- Van Holm, W.; Carvalho, R.; Delanghe, L.; Eilers, T.; Zayed, N.; Mermans, F.; Bernaerts, K.; Boon, N.; Claes, I.; Lebeer, S.; et al. Antimicrobial potential of known and novel probiotics on in vitro periodontitis biofilms. NPJ Biofilms Microbiomes 2023, 9, 3. [Google Scholar] [CrossRef]
- Fang, J.; Yang, Y.; Xie, W. Chinese expert consensus on the application of live combined Bifidobacterium, Lactobacillus, and Enterococcus powder/capsule in digestive system diseases (2021). J. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Louis, P. The impact of nutrition on intestinal bacterial communities. Curr. Opin. Microbiol. 2017, 38, 59–65. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kołat, D.; Yang, K.; Hu, J.K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.J.; McPherson, A.C.; Phelps, C.M.; Pandey, S.P.; Laughlin, C.R.; Shapira, J.H.; Medina Sanchez, L.; Rana, M.; Richie, T.G.; Mims, T.S.; et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 2023, 186, 1846–1862.e1826. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Wang, X.; Jin, Z.; Zhang, P.; Su, H.; Sun, X. Effect of Probiotics on Respiratory Tract Allergic Disease and Gut Microbiota. Front. Nutr. 2022, 9, 821900. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, X.; Li, J.; Li, Z.; Hu, Q.; Li, L.; Hao, X.; Song, M.; Li, C. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2020, 60, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef]
- Baquerizo Nole, K.L.; Yim, E.; Keri, J.E. Probiotics and prebiotics in dermatology. J. Am. Acad. Dermatol. 2014, 71, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Accettulli, A.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Campaniello, D.; Racioppo, A.; Altieri, C.; Bevilacqua, A. Psycho-Microbiology, a New Frontier for Probiotics: An Exploratory Overview. Microorganisms 2022, 10, 2141. [Google Scholar] [CrossRef]
- Cenzato, N.; Khijmatgar, S.; Carloni, P.; Dongiovanni, P.; Meroni, M.; Del Fabbro, M.; Tartaglia, G.M. What is the use of nutraceuticals in dentistry? A scoping review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4899–4913. [Google Scholar] [CrossRef]
- Hoare, A.; Marsh, P.D.; Diaz, P.I. Ecological Therapeutic Opportunities for Oral Diseases. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Seminario-Amez, M.; López-López, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jané-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e282–e288. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, V.H.; Bandara, H.M.; Mayer, M.P.; Samaranayake, L.P. Probiotics as Antifungals in Mucosal Candidiasis. Clin. Infect. Dis. 2016, 62, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Brody, H.; Lin, G.H.; Rangé, H.; Kuraji, R.; Ye, C.; Kamarajan, P.; Radaic, A.; Gao, L.; Kapila, Y. Probiotics, including nisin-based probiotics, improve clinical and microbial outcomes relevant to oral and systemic diseases. Periodontol. 2000 2020, 82, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontol. 2000 2019, 79, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. 1), 4–7. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef]
- Misch, C.M. The future of bone augmentation. Int. J. Oral Implantol. 2022, 15, 103–104. [Google Scholar]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Fu, X.; Zeng, W.; Chen, A.; Luo, Z.; Li, Y.; Zhou, Z.; Li, J. Chopped fibers and nano-hydroxyapatite enhanced silk fibroin porous hybrid scaffolds for bone augmentation. J. Mater. Chem. B 2023, 11, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; dos Santos, D.M.; Santiago, J.F., Jr.; Moreno, A.; Pellizzer, E.P. Longevity of dental implants in type IV bone: A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Saini, C.; Garg, B.; Gupta, R.; Verma, B.; Mishra, P.K.; Srivastava, R.K. Long-term implications of COVID-19 on bone health: Pathophysiology and therapeutics. Inflamm. Res. 2022, 71, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, K.; Wu, C.; Chen, J.; Pan, H.; Liu, Y.; Wu, P.; Yuan, J.; Huang, F.; Lang, J.; et al. An emerging role of Prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice. Am. J. Clin. Nutr. 2021, 114, 1304–1313. [Google Scholar] [CrossRef]
- Villa, C.R.; Ward, W.E.; Comelli, E.M. Gut microbiota-bone axis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1664–1672. [Google Scholar] [CrossRef]
- Roberts, J.L.; Liu, G.; Darby, T.M.; Fernandes, L.M.; Diaz-Hernandez, M.E.; Jones, R.M.; Drissi, H. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed. Pharmacother. 2020, 132, 110831. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, S.; Wang, Y.; Yu, D.; Hua, L.; Guo, C.; Wang, D.; Lei, M. Oral administration of Lactobacillus casei Shirota improves recovery of hand functions after distal radius fracture among elder patients: A placebo-controlled, double-blind, and randomized trial. J. Orthop. Surg. Res. 2019, 14, 257. [Google Scholar] [CrossRef]
- Guo, X.; Zhong, K.; Zou, L.; Xue, H.; Zheng, S.; Guo, J.; Lv, H.; Duan, K.; Huang, D.; Tan, M. Effect of Lactobacillus casei fermented milk on fracture healing in osteoporotic mice. Front. Endocrinol. 2022, 13, 1041647. [Google Scholar] [CrossRef]
- Roberts, J.L.; Golloshi, M.; Harding, D.B.; Conduah, M.; Liu, G.; Drissi, H. Bifidobacterium longum supplementation improves age-related delays in fracture repair. Aging Cell 2023, 22, e13786. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Han, Y.; Li, F.; Gu, X.; Su, D.; Yu, W.; Li, Z.; Xiao, J. Neuropeptide Y1 Receptor Antagonist Alters Gut Microbiota and Alleviates the Ovariectomy-Induced Osteoporosis in Rats. Calcif. Tissue Int. 2020, 106, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Dabbaghmanesh, M.H.; Ghasemi, Y.; Koohpeyma, F.; Talezadeh, P.; Montazeri-Najafabady, N. The ameliorative role of specific probiotic combinations on bone loss in the ovariectomized rat model. BMC Complement. Med. Ther. 2022, 22, 241. [Google Scholar] [CrossRef] [PubMed]
- Montazeri-Najafabady, N.; Ghasemi, Y.; Dabbaghmanesh, M.H.; Ashoori, Y.; Talezadeh, P.; Koohpeyma, F.; Abootalebi, S.N.; Gholami, A. Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Complement. Med. Ther. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ai, J.; Mao, H.; Gao, X. Effects of Eclipta prostrata on gut microbiota of SAMP6 mice with osteoporosis. J. Med. Microbiol. 2019, 68, 402–416. [Google Scholar] [CrossRef]
- Biver, E.; Herrou, J.; Larid, G.; Legrand, M.A.; Gonnelli, S.; Annweiler, C.; Chapurlat, R.; Coxam, V.; Fardellone, P.; Thomas, T.; et al. Dietary recommendations in the prevention and treatment of osteoporosis. Jt. Bone Spine 2023, 90, 105521. [Google Scholar] [CrossRef]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol. Spectr. 2017, 5, 213–233. [Google Scholar] [CrossRef]
- Rizzoli, R. Nutritional influence on bone: Role of gut microbiota. Aging Clin. Exp. Res. 2019, 31, 743–751. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131.e7. [Google Scholar] [CrossRef]
- McCabe, L.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; Castro, A.S.; Rodrigues, V.C.; Fernandes, S.A.; Fontes, E.A.; de Oliveira, T.T.; Martino, H.S.; de Luces Fortes Ferreira, C.L. Yacon flour and Bifidobacterium longum modulate bone health in rats. J. Med. Food. 2012, 15, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Sojan, J.M.; Raman, R.; Muller, M.; Carnevali, O.; Renn, J. Probiotics Enhance Bone Growth and Rescue BMP Inhibition: New Transgenic Zebrafish Lines to Study Bone Health. Int. J. Mol. Sci. 2022, 23, 4748. [Google Scholar] [CrossRef] [PubMed]
- Sojan, J.M.; Gioacchini, G.; Giorgini, E.; Orlando, P.; Tiano, L.; Maradonna, F.; Carnevali, O. Zebrafish caudal fin as a model to investigate the role of probiotics in bone regeneration. Sci. Rep. 2022, 12, 8057. [Google Scholar] [CrossRef]
- Maradonna, F.; Gioacchini, G.; Falcinelli, S.; Bertotto, D.; Radaelli, G.; Olivotto, I.; Carnevali, O. Probiotic supplementation promotes calcification in Danio rerio larvae: A molecular study. PLoS ONE 2013, 8, e83155. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R.; Karimi, G.; Hwei, A.N.; Abdul-Majeed, S.; Ahmad, Z.; Ibrahim, Z.; Jamaluddin, R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. Biomed. Res. Int. 2015, 2015, 897639. [Google Scholar] [CrossRef]
- Eaimworawuthikul, S.; Tunapong, W.; Chunchai, T.; Suntornsaratoon, P.; Charoenphandhu, N.; Thiennimitr, P.; Chattipakorn, N.; Chattipakorn, S.C. Altered gut microbiota ameliorates bone pathology in the mandible of obese-insulin-resistant rats. Eur. J. Nutr. 2020, 59, 1453–1462. [Google Scholar] [CrossRef]
- Tan, L.; Fu, J.; Feng, F.; Liu, X.; Cui, Z.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, Z.; et al. Engineered probiotics biofilm enhances osseointegration via immunoregulation and anti-infection. Sci. Adv. 2020, 6, eaba5723. [Google Scholar] [CrossRef]
- Shi, Y.C.; Wang, L.T.; Niu, Y.M.; Yu, N.; Xing, P.F.; Dong, L.; Wang, C.M. Fungal Component Coating Enhances Titanium Implant-Bone Integration. Adv. Funct. Mater. 2018, 28, 14. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, L.; Chen, Y.; Han, X.; Sun, L.; Chen, H.; Chen, Q. Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front. Endocrinol. 2023, 14, 1109296. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yamada, Y.; Ohtani, Y.; Mitsui, N.; Murasawa, H.; Araki, S. Production of menaquinone (vitamin K2)-7 by Bacillus subtilis. J. Biosci. Bioeng. 2001, 91, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ho, W.T.P.; Liu, C.; Chow, S.K.; Ip, M.; Yu, J.; Wong, H.S.; Cheung, W.H.; Sung, J.J.Y.; Wong, R.M.Y. The role of gut microbiota in bone homeostasis. Bone Jt. Res. 2021, 10, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Katono, T.; Kawato, T.; Tanabe, N.; Suzuki, N.; Iida, T.; Morozumi, A.; Ochiai, K.; Maeno, M. Sodium butyrate stimulates mineralized nodule formation and osteoprotegerin expression by human osteoblasts. Arch. Oral Biol. 2008, 53, 903–909. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Grassi, F.; Tyagi, A.M.; Calvert, J.W.; Gambari, L.; Walker, L.D.; Yu, M.; Robinson, J.; Li, J.Y.; Lisignoli, G.; Vaccaro, C.; et al. Hydrogen Sulfide Is a Novel Regulator of Bone Formation Implicated in the Bone Loss Induced by Estrogen Deficiency. J. Bone Miner. Res. 2016, 31, 949–963. [Google Scholar] [CrossRef]
- Xu, X.; Jia, X.; Mo, L.; Liu, C.; Zheng, L.; Yuan, Q.; Zhou, X. Intestinal microbiota: A potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017, 5, 17046. [Google Scholar] [CrossRef]
- Rizzoli, R. Dairy products and bone health. Aging Clin. Exp. Res. 2022, 34, 9–24. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Abu-Amer, Y. NF-κB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef]
- Takegahara, N.; Kim, H.; Choi, Y. RANKL biology. Bone 2022, 159, 116353. [Google Scholar] [CrossRef] [PubMed]
- Hooshiar, S.H.; Tobeiha, M.; Jafarnejad, S. Soy Isoflavones and Bone Health: Focus on the RANKL/RANK/OPG Pathway. Biomed. Res. Int. 2022, 2022, 8862278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, M.; Lu, C.; Han, J.; Tang, S.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. Tuna Bone Powder Alleviates Glucocorticoid-Induced Osteoporosis via Coregulation of the NF-κB and Wnt/β-Catenin Signaling Pathways and Modulation of Gut Microbiota Composition and Metabolism. Mol. Nutr. Food Res. 2020, 64, e1900861. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Zhang, J.; Motyl, K.J.; Irwin, R.; MacDougald, O.A.; Britton, R.A.; McCabe, L.R. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology 2015, 156, 3169–3182. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.K.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef]
- Tousen, Y.; Matsumoto, Y.; Nagahata, Y.; Kobayashi, I.; Inoue, M.; Ishimi, Y. Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation. Nutrients 2019, 11, 297. [Google Scholar] [CrossRef]
- Amin, N.; Boccardi, V.; Taghizadeh, M.; Jafarnejad, S. Probiotics and bone disorders: The role of RANKL/RANK/OPG pathway. Aging Clin. Exp. Res. 2020, 32, 363–371. [Google Scholar] [CrossRef]
- McHugh, J. Wnt signalling in the gut microbiota-bone axis. Nat. Rev. Rheumatol. 2019, 15, 4. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Shokeen, N.; Porwal, K.; Saini, C.; Bhardwaj, A.; Mathew, M.; Mishra, P.K.; Chattopadhyay, N.; Dar, H.Y.; Verma, B.; et al. Bifidobacterium longum Ameliorates Ovariectomy-Induced Bone Loss via Enhancing Anti-Osteoclastogenic and Immunomodulatory Potential of Regulatory B Cells (Bregs). Front. Immunol. 2022, 13, 875788. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef]
- Flichy-Fernández, A.J.; Ata-Ali, J.; Alegre-Domingo, T.; Candel-Martí, E.; Ata-Ali, F.; Palacio, J.R.; Peñarrocha-Diago, M. The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: A double-blind randomized controlled trial. J. Periodontal. Res. 2015, 50, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of Osteoporosis-Role of T Cells. Front. Immunol. 2018, 9, 657. [Google Scholar] [CrossRef]
- Sapra, L.; Dar, H.Y.; Bhardwaj, A.; Pandey, A.; Kumari, S.; Azam, Z.; Upmanyu, V.; Anwar, A.; Shukla, P.; Mishra, P.K.; et al. Lactobacillus rhamnosus attenuates bone loss and maintains bone health by skewing Treg-Th17 cell balance in Ovx mice. Sci. Rep. 2021, 11, 1807. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509–27515. [Google Scholar] [CrossRef]
- Liu, J.H.; Yue, T.; Luo, Z.W.; Cao, J.; Yan, Z.Q.; Jin, L.; Wan, T.F.; Shuai, C.J.; Wang, Z.G.; Zhou, Y.; et al. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis. Model. Mech. 2020, 13, dmm043620. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Takegahara, N.; Kim, H.; Choi, Y. Updating osteoimmunology: Regulation of bone cells by innate and adaptive immunity. Nat. Rev. Rheumatol. 2018, 14, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Malik Tyagi, A.; Li, J.Y.; Adams, J.; Denning, T.L.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. PTH induces bone loss via microbial-dependent expansion of intestinal TNF(+) T cells and Th17 cells. Nat. Commun. 2020, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Im, E.; Choi, Y.J.; Kim, C.H.; Fiocchi, C.; Pothoulakis, C.; Rhee, S.H. The angiogenic effect of probiotic Bacillus polyfermenticus on human intestinal microvascular endothelial cells is mediated by IL-8. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G999–G1008. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, P.; De Simone, C.; Chadee, K. The probiotic mixture VSL#3 accelerates gastric ulcer healing by stimulating vascular endothelial growth factor. PLoS ONE 2013, 8, e58671. [Google Scholar] [CrossRef]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: A post hoc analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Montazeri-Najafabady, N.; Ghasemi, Y.; Dabbaghmanesh, M.H.; Talezadeh, P.; Koohpeyma, F.; Gholami, A. Supportive Role of Probiotic Strains in Protecting Rats from Ovariectomy-Induced Cortical Bone Loss. Probiotics Antimicrob. Proteins 2019, 11, 1145–1154. [Google Scholar] [CrossRef]
- Werny, J.G.; Sagheb, K.; Diaz, L.; Kämmerer, P.W.; Al-Nawas, B.; Schiegnitz, E. Does vitamin D have an effect on osseointegration of dental implants? A systematic review. Int. J. Implant. Dent. 2022, 8, 16. [Google Scholar] [CrossRef]
- Javed, F.; Al Amri, M.D.; Kellesarian, S.V.; Al-Kheraif, A.A.; Vohra, F.; Calvo-Guirado, J.L.; Malmstrom, H.; Romanos, G.E. Efficacy of parathyroid hormone supplementation on the osseointegration of implants: A systematic review. Clin. Oral Investig. 2016, 20, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Muresan, G.C.; Hedesiu, M.; Lucaciu, O.; Boca, S.; Petrescu, N. Effect of Vitamin D on Bone Regeneration: A Review. Medicina 2022, 58, 1337. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Welldon, K.J.; Wijenayaka, A.R.; Bonewald, L.F.; Findlay, D.M. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am. J. Physiol. Physiol. 2009, 297, C1358–C1367. [Google Scholar] [CrossRef]
- Bazal-Bonelli, S.; Sánchez-Labrador, L.; Cortés-Bretón Brinkmann, J.; Cobo-Vázquez, C.; Martínez-Rodríguez, N.; Beca-Campoy, T.; Santos-Marino, J.; Rodríguez-Fernández, E.; Alvarado-Lorenzo, M. Influence of Serum Vitamin D Levels on Survival Rate and Marginal Bone Loss in Dental Implants: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10120. [Google Scholar] [CrossRef] [PubMed]
- 1Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Molina, A.; Sanz, M. Complications in bone-grafting procedures: Classification and management. Periodontol. 2000 2022, 88, 86–102. [Google Scholar] [CrossRef]
- Schwarz, F.; Ramanauskaite, A. It is all about peri-implant tissue health. Periodontol. 2000 2022, 88, 9–12. [Google Scholar] [CrossRef]
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef]
- desJardins-Park, H.E.; Mascharak, S.; Chinta, M.S.; Wan, D.C.; Longaker, M.T. The Spectrum of Scarring in Craniofacial Wound Repair. Front. Physiol. 2019, 10, 322. [Google Scholar] [CrossRef]
- Siddharthan, R.; Chapek, M.; Warren, M.; Martindale, R. Probiotics in Prevention of Surgical Site Infections. Surg. Infect. 2018, 19, 781–784. [Google Scholar] [CrossRef]
- El-Ghazely, M.H.; Mahmoud, W.H.; Atia, M.A.; Eldip, E.M. Effect of probiotic administration in the therapy of pediatric thermal burn. Ann. Burn. Fire Disasters 2016, 29, 268–272. [Google Scholar]
- Esposito, C.; Roberti, A.; Turrà, F.; Cerulo, M.; Severino, G.; Settimi, A.; Escolino, M. Frequency of Antibiotic-Associated Diarrhea and Related Complications in Pediatric Patients Who Underwent Hypospadias Repair: A Comparative Study Using Probiotics vs Placebo. Probiotics Antimicrob. Proteins 2018, 10, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Togo, C.; Zidorio, A.P.; Gonçalves, V.; Botelho, P.; de Carvalho, K.; Dutra, E. Does Probiotic Consumption Enhance Wound Healing? A Systematic Review. Nutrients 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Daher, G.S.; Choi, K.Y.; Wells, J.W.; Goyal, N. A Systematic Review of Oral Nutritional Supplement and Wound Healing. Ann. Otol. Rhinol. Laryngol. 2022, 131, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Lactobacillus reuteri extracts promoted wound healing via PI3K/AKT/β-catenin/TGFβ1 pathway. Stem Cell Res. Ther. 2019, 10, 243. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Guo, L.; Su, Y.; Luo, Z.; Du, J.; Mei, S.; Liu, Y. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res. Ther. 2020, 11, 61. [Google Scholar] [CrossRef]
- Liu, P.; Choi, J.W.; Lee, M.K.; Choi, Y.H.; Nam, T.J. Wound Healing Potential of Spirulina Protein on CCD-986sk Cells. Mar Drugs 2019, 17, 130. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Eskandari, M.H. Kefir Accelerates Burn Wound Healing Through Inducing Fibroblast Cell Migration In Vitro and Modulating the Expression of IL-1ß, TGF-ß1, and bFGF Genes In Vivo. Probiotics Antimicrob. Proteins 2019, 11, 874–886. [Google Scholar] [CrossRef]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Ishikawa, K.H.; Amado, P.P.; Nicoli, J.R.; Holzhausen, M.; Mayer, M.P.A. Probiotics improve re-epithelialization of scratches infected by Porphyromonas gingivalis through up-regulating CXCL8-CXCR1/CXCR2 axis. Anaerobe 2021, 72, 102458. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque-Souza, E.; Balzarini, D.; Ando-Suguimoto, E.S.; Ishikawa, K.H.; Simionato, M.R.L.; Holzhausen, M.; Mayer, M.P.A. Probiotics alter the immune response of gingival epithelial cells challenged by Porphyromonas gingivalis. J. Periodontal. Res. 2019, 54, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Mendi, A.; Köse, S.; Uçkan, D.; Akca, G.; Yilmaz, D.; Aral, L.; Gültekin, S.E.; Eroğlu, T.; Kiliç, E.; Uçkan, S. Lactobacillus rhamnosus could inhibit Porphyromonas gingivalis derived CXCL8 attenuation. J. Appl. Oral Sci. 2016, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Baumgart, D.C.; d’Heureuse, J.H.; Hotz, A.; Wiedenmann, B.; Dignass, A.U. CXCL8 modulates human intestinal epithelial cells through a CXCR1 dependent pathway. Cytokine 2005, 29, 42–48. [Google Scholar] [CrossRef]
- Sinha, A.; Sagar, S.; Madhumathy, M.; Osborne, W.J. Probiotic Bacteria in Wound Healing; An In-Vivo Study. Iran. J. Biotechnol. 2019, 17, e2188. [Google Scholar] [CrossRef]
- Ashoori, Y.; Mohkam, M.; Heidari, R.; Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Golkar, N.; Gholami, A. Development and In Vivo Characterization of Probiotic Lysate-Treated Chitosan Nanogel as a Novel Biocompatible Formulation for Wound Healing. Biomed. Res. Int. 2020, 2020, 8868618. [Google Scholar] [CrossRef]
- Azuma, N.; Katada, Y.; Sano, H. Deterioration in saliva quality in patients with Sjögren’s syndrome: Impact of decrease in salivary epidermal growth factor on the severity of intraoral manifestations. Inflamm. Regen. 2018, 38, 6. [Google Scholar] [CrossRef]
- Oxford, G.E.; Tayari, L.; Barfoot, M.D.; Peck, A.B.; Tanaka, Y.; Humphreys-Beher, M.G. Salivary EGF levels reduced in diabetic patients. J. Diabetes Complicat. 2000, 14, 140–145. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.H. The regulation of TGFbeta signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Riedwyl, D.; Aizawa, R.; Raabe, C.; Couso-Queiruga, E.; Chappuis, V. Local Concentrations of TGF-β1 and IGF-1 Appear Determinant in Regulating Bone Regeneration in Human Postextraction Tooth Sockets. Int. J. Mol. Sci. 2023, 24, 8239. [Google Scholar] [CrossRef]
- Leoni, G.; Neumann, P.A.; Sumagin, R.; Denning, T.L.; Nusrat, A. Wound repair: Role of immune-epithelial interactions. Mucosal. Immunol. 2015, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Ajima, R.; Luo, C.T.; Yamaguchi, T.P.; Stappenbeck, T.S. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 2012, 338, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Fordjour, L.; D’Souza, A.; Cai, C.; Ahmad, A.; Valencia, G.; Kumar, D.; Aranda, J.V.; Beharry, K.D. Comparative effects of probiotics, prebiotics, and synbiotics on growth factors in the large bowel in a rat model of formula-induced bowel inflammation. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 507–513. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes 2012, 3, 25–28. [Google Scholar] [CrossRef]
- Su, Y.; Chen, C.; Guo, L.; Du, J.; Li, X.; Liu, Y. Ecological Balance of Oral Microbiota Is Required to Maintain Oral Mesenchymal Stem Cell Homeostasis. Stem. Cells 2018, 36, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2020, 182, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Patel, K.H.; Huang, R.Y.; Lee, C.N.; Moochhala, S.M. The Gut-Skin Microbiota Axis and Its Role in Diabetic Wound Healing-A Review Based on Current Literature. Int. J. Mol. Sci. 2022, 23, 2375. [Google Scholar] [CrossRef]
- Gouriet, F.; Million, M.; Henri, M.; Fournier, P.E.; Raoult, D. Lactobacillus rhamnosus bacteremia: An emerging clinical entity. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- De Groote, M.A.; Frank, D.N.; Dowell, E.; Glode, M.P.; Pace, N.R. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr. Infect. Dis. J. 2005, 24, 278–280. [Google Scholar] [CrossRef]
- Liu, P.C.; Yan, Y.K.; Ma, Y.J.; Wang, X.W.; Geng, J.; Wang, M.C.; Wei, F.X.; Zhang, Y.W.; Xu, X.D.; Zhang, Y.C. Probiotics Reduce Postoperative Infections in Patients Undergoing Colorectal Surgery: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2017, 2017, 6029075. [Google Scholar] [CrossRef]
- Erdman, S.E.; Poutahidis, T. Probiotic ‘glow of health’: It’s more than skin deep. Benef. Microbes 2014, 5, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef] [PubMed]
- Askelson, T.E.; Campasino, A.; Lee, J.T.; Duong, T. Evaluation of phytate-degrading Lactobacillus culture administration to broiler chickens. Appl. Environ. Microbiol. 2014, 80, 943–950. [Google Scholar] [CrossRef]
- Rooj, A.K.; Kimura, Y.; Buddington, R.K. Metabolites produced by probiotic Lactobacilli rapidly increase glucose uptake by Caco-2 cells. BMC Microbiol. 2010, 10, 16. [Google Scholar] [CrossRef]
- De Angelis, M.; Bottacini, F.; Fosso, B.; Kelleher, P.; Calasso, M.; Di Cagno, R.; Ventura, M.; Picardi, E.; van Sinderen, D.; Gobbetti, M. Lactobacillus rossiae, a vitamin B12 producer, represents a metabolically versatile species within the Genus Lactobacillus. PLoS ONE 2014, 9, e107232. [Google Scholar] [CrossRef]
- Degirolamo, C.; Rainaldi, S.; Bovenga, F.; Murzilli, S.; Moschetta, A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014, 7, 12–18. [Google Scholar] [CrossRef]
- Roager, H.M.; Sulek, K.; Skov, K.; Frandsen, H.L.; Smedsgaard, J.; Wilcks, A.; Skov, T.H.; Villas-Boas, S.G.; Licht, T.R. Lactobacillus acidophilus NCFM affects vitamin E acetate metabolism and intestinal bile acid signature in monocolonized mice. Gut Microbes 2014, 5, 296–303. [Google Scholar] [CrossRef]
- Bikle, D.D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Investig. 2023, 46, 205–212. [Google Scholar] [CrossRef]
- Oda, Y.; Tu, C.L.; Menendez, A.; Nguyen, T.; Bikle, D.D. Vitamin D and calcium regulation of epidermal wound healing. J. Steroid. Biochem. Mol. Biol. 2016, 164, 379–385. [Google Scholar] [CrossRef]
- Varian, B.J.; Poutahidis, T.; DiBenedictis, B.T.; Levkovich, T.; Ibrahim, Y.; Didyk, E.; Shikhman, L.; Cheung, H.K.; Hardas, A.; Ricciardi, C.E.; et al. Microbial lysate upregulates host oxytocin. Brain. Behav. Immun. 2017, 61, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Gouin, J.P.; Carter, C.S.; Pournajafi-Nazarloo, H.; Glaser, R.; Malarkey, W.B.; Loving, T.J.; Stowell, J.; Kiecolt-Glaser, J.K. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology 2010, 35, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Vitalo, A.; Fricchione, J.; Casali, M.; Berdichevsky, Y.; Hoge, E.A.; Rauch, S.L.; Berthiaume, F.; Yarmush, M.L.; Benson, H.; Fricchione, G.L.; et al. Nest making and oxytocin comparably promote wound healing in isolation reared rats. PLoS ONE 2009, 4, e5523. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Keller, M.K.; Lee, L.; Yucel-Lindberg, T.; Pedersen, A.M.L. Effect of probiotic lozenges containing Lactobacillus reuteri on oral wound healing: A pilot study. Benef. Microbes 2018, 9, 691–696. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Wälivaara, D.; Sjögren, I.; Gerasimcik, N.; Yucel-Lindberg, T.; Twetman, S.; Abrahamsson, P. Effects of Lactobacillus reuteri-containing lozenges on healing after surgical removal of mandibular third molars: A randomised controlled trial. Benef. Microbes 2019, 10, 653–659. [Google Scholar] [CrossRef]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Serra, P.; Francesco, I.; Di Carmine, M.; Tari, S.R.; Leo, L.; Lorusso, F. Current Status of Peri-Implant Diseases: A Clinical Review for Evidence-Based Decision Making. J. Funct. Biomater. 2023, 14, 210. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Monje, A.; Nart, J. Management and sequelae of dental implant removal. Periodontol. 2000 2022, 88, 182–200. [Google Scholar] [CrossRef]
- Tsaousoglou, P.; Chatzopoulos, G.S.; Tsalikis, L.; Lazaridou, T.; Mikrogeorgis, G.; Vouros, I. Prevalence and risk indicators of peri-implantitis: A university based cross-sectional study. Quintessence Int. 2023, 54, 558–568. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, W.; Huang, J.; Fang, M.; Dong, Y.; Chen, J.; Ji, Z.; Tian, M. Prevalence and Risk Factors of Peri-Implant Disease: A Retrospective Case-Control Study in Western China. Int. J. Environ. Res. Public Health 2022, 19, 12667. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, A.; Imber, J.C.; Salvi, G.E.; Roccuzzo, M. Peri-implantitis as the consequence of errors in implant therapy. Periodontol. 2000 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Chen, C.J.; Gallucci, G.O. Prevention and management of peri-implant disease. Clin. Implant. Dent. Relat. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.; Santagati, M.; Guni, A.; Torrisi, P.; Spitale, A.; Stefani, S.; Ferlito, S.; Nibali, L. Microbiome differences in periodontal, peri-implant, and healthy sites: A cross-sectional pilot study. Clin. Oral Investig. 2022, 26, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Retamal-Valdes, B.; Formiga, M.C.; Almeida, M.L.; Fritoli, A.; Figueiredo, K.A.; Westphal, M.; Gomes, P.; Feres, M. Does subgingival bacterial colonization differ between implants and teeth? A systematic review. Braz. Oral Res. 2019, 33, e064. [Google Scholar] [CrossRef]
- Gazil, V.; Bandiaky, O.N.; Renard, E.; Idiri, K.; Struillou, X.; Soueidan, A. Current Data on Oral Peri-Implant and Periodontal Microbiota and Its Pathological Changes: A Systematic Review. Microorganisms 2022, 10, 2466. [Google Scholar] [CrossRef]
- Shi, Y.; Tong, Z.; Zhang, Y.; Si, M.; He, F. Microbial profiles of peri-implant mucositis and peri-implantitis: Submucosal microbial dysbiosis correlates with disease severity. Clin. Oral Implant. Res. 2022, 33, 172–183. [Google Scholar] [CrossRef]
- Kensara, A.; Saito, H.; Mongodin, E.F.; Masri, R. Microbiological profile of peri-implantitis: Analyses of microbiome within dental implants. J. Prosthodont. 2023. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Di Spirito, F.; D’Ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms 2022, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.; Hegde, S.; Koshy, A.; Mulla, M. Effect of Probiotic Lactobacillus salivarius on Peri-Implantitis Pathogenic Bacteria: An In Vitro Study. Cureus 2021, 13, e20808. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.; Mulla, M.; Hegde, S.; Koshy, A.V. In vitro assessment of the effect of probiotic Lactobacillus reuteri on peri-implantitis microflora. BMC Oral Health 2021, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Park, G.Y. In Vitro Evaluation of the Effect of Oral Probiotic Weissella cibaria on the Formation of Multi-Species Oral Biofilms on Dental Implant Surfaces. Microorganisms 2021, 9, 2482. [Google Scholar] [CrossRef]
- Vacca, C.; Contu, M.P.; Rossi, C.; Ferrando, M.L.; Blus, C.; Szmukler-Moncler, S.; Scano, A.; Orrù, G. In vitro Interactions between Streptococcus intermedius and Streptococcus salivarius K12 on a Titanium Cylindrical Surface. Pathogens 2020, 9, 1096. [Google Scholar] [CrossRef]
- Invernici, M.M.; Furlaneto, F.A.C.; Salvador, S.L.; Ouwehand, A.C.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.I.; Silva, P.H.F.; et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS ONE 2020, 15, e0238425. [Google Scholar] [CrossRef]
- Hojo, K.; Nagaoka, S.; Murata, S.; Taketomo, N.; Ohshima, T.; Maeda, N. Reduction of vitamin K concentration by salivary Bifidobacterium strains and their possible nutritional competition with Porphyromonas gingivalis. J. Appl. Microbiol. 2007, 103, 1969–1974. [Google Scholar] [CrossRef]
- Argandoña Valdez, R.M.; Ximenez-Fyvie, L.A.; Caiaffa, K.S.; Rodrigues Dos Santos, V.; Gonzales Cervantes, R.M.; Almaguer-Flores, A.; Duque, C. Antagonist effect of probiotic bifidobacteria on biofilms of pathogens associated with periodontal disease. Microb. Pathog. 2021, 150, 104657. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, L.; Shen, D.; Hao, Y. Competition between yogurt probiotics and periodontal pathogens in vitro. Acta Odontol. Scand. 2010, 68, 261–268. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Salvador, S.L.; Silva, P.H.; Furlaneto, F.A.; Figueiredo, L.; Casarin, R.; Ervolino, E.; Palioto, D.B.; Souza, S.L.; Taba, M., Jr.; et al. Benefits of Bifidobacterium animalis subsp. lactis Probiotic in Experimental Periodontitis. J. Periodontol. 2017, 88, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Brody, H.; Contreras, F.; Hajfathalian, M.; Lucido, L.; Kamarajan, P.; Kapila, Y.L. Nisin and Nisin Probiotic Disrupt Oral Pathogenic Biofilms and Restore Their Microbiome Composition towards Healthy Control Levels in a Peri-Implantitis Setting. Microorganisms 2022, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.J.; Chang, D.; Adams, G.G.; Butler, C.A.; Reynolds, E.C.; Darby, I.B.; Dashper, S.G. Microbiome profiles of non-responding and responding paired periodontitis sites within the same participants following non-surgical treatment. J. Oral Microbiol. 2022, 14, 2043595. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Hojo, K.; Murata, S.; Mori, T.; Ohshima, T.; Maeda, N. Interactions between salivary Bifidobacterium adolescentis and other oral bacteria: In vitro coaggregation and coadhesion assays. FEMS Microbiol. Lett. 2008, 281, 183–189. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Fakhruddin, K.S.; Ngo, H.; Samaranayake, L.P. Probiotic Bifidobacteria in Managing Periodontal Disease: A Systematic Review. Int. Dent. J. 2023, 73, 11–20. [Google Scholar] [CrossRef]
- Santos, C.M.A.; Pires, M.C.V.; Leão, T.L.; Hernández, Z.P.; Rodriguez, M.L.; Martins, A.K.S.; Miranda, L.S.; Martins, F.S.; Nicoli, J.R. Selection of Lactobacillus strains as potential probiotics for vaginitis treatment. Microbiology 2016, 162, 1195–1207. [Google Scholar] [CrossRef]
- Galofré, M.; Palao, D.; Vicario, M.; Nart, J.; Violant, D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J. Periodontal. Res. 2018, 53, 378–390. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef]
- Alqahtani, F.; Alshaikh, M.; Mehmood, A.; Alqhtani, N.; Alkhtani, F.; Alenazi, A. Role of Probiotics for the Treatment of Peri-Implant Mucositis in Patients with and without Type 2 Diabetes Mellitus. J. Oral Implantol. 2022, 48, 37–42. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Gennai, S.; Bollain, J.; Ambrosio, N.; Marruganti, C.; Graziani, F.; Figuero, E. Efficacy of adjunctive measures in peri-implant mucositis. A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.; Alshaikh, M.; Mehmood, A.; Alqhtani, N.; Alkhtani, F.; Alenazi, A. Efficacy of Antibiotic Versus Probiotics As Adjuncts to Mechanical Debridement for the Treatment of Peri-Implant Mucositis. J. Oral Implantol. 2022, 48, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Arbildo-Vega, H.I.; Panda, S.; Bal, A.; Mohanty, R.; Rendón-Alvarado, A.; Das, A.C.; Cruzado-Oliva, F.H.; Infantes-Ruíz, E.D.; Manfredi, B.; Vásquez-Rodrigo, H.; et al. Clinical effectiveness of Lactobacillus reuteri in the treatment of peri-implant diseases: A systematic review and meta-analysis. J. Biol. Regul. Homeost. Agents 2021, 35, 79–88. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, H.; Wang, Y.; Lai, W.; Jian, F. Efficacy of Probiotics as Adjunctive Therapy to Nonsurgical Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 541752. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.; Barallat, L.; Vilarrasa, J.; Vicario, M.; Violant, D.; Nart, J. Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: A triple-blind randomized clinical trial. Clin. Oral Investig. 2019, 23, 1673–1683. [Google Scholar] [CrossRef]
- Sayardoust, S.; Johansson, A.; Jönsson, D. Do Probiotics Cause a Shift in the Microbiota of Dental Implants-A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 823985. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I. Treatment of pathologic peri-implant pockets. Periodontol. 2000 2018, 76, 180–190. [Google Scholar] [CrossRef]
- Schwarz, F.; Jepsen, S.; Obreja, K.; Galarraga-Vinueza, M.E.; Ramanauskaite, A. Surgical therapy of peri-implantitis. Periodontol. 2000 2022, 88, 145–181. [Google Scholar] [CrossRef]
- Lauritano, D.; Carinci, F.; Palmieri, A.; Cura, F.; Caruso, S.; Candotto, V. Reuterinos® as adjuvant for peri-implant treatment: A pilot study. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419827745. [Google Scholar] [CrossRef]
- Alqahtani, F.; Alqahtani, M.; Shafqat, S.S.; Akram, Z.; Al-Kheraif, A.A.; Javed, F. Efficacy of mechanical debridement with adjunctive probiotic therapy in the treatment of peri-implant mucositis in cigarette-smokers and never-smokers. Clin. Implant. Dent. Relat. Res. 2019, 21, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.P.; de Almeida Silva Levi, Y.L.; Henrique Félix Silva, P.; Carla Wons, L.; Pizzo Pitelli, L.; Goulart de Castro, J.; da Silva Dólens, E.; Gregorio, D.; Gouveia Straioto, F.; Dos Santos Santinoni, C.; et al. Impact of Bifidobacterium animalis subsp. lactis HN019 probiotic in the prevention of periodontitis associated with immunosuppression. J. Periodontol. 2023, 94, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.; Brigida, M.; Migneco, A.; Gunawardena, G.; Zanza, C.; Candelli, M.; Franceschi, F.; Ojetti, V. Lactobacillus Reuteri DSM 17938 (Limosilactobacillus reuteri) in Diarrhea and Constipation: Two Sides of the Same Coin? Medicina 2021, 57, 643. [Google Scholar] [CrossRef] [PubMed]
- van den Nieuwboer, M.; Brummer, R.J.; Guarner, F.; Morelli, L.; Cabana, M.; Claassen, E. Safety of probiotics and synbiotics in children under 18 years of age. Benef. Microbes 2015, 6, 615–630. [Google Scholar] [CrossRef]
- Sheyholislami, H.; Connor, K.L. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2382. [Google Scholar] [CrossRef]
- Lundelin, K.; Poussa, T.; Salminen, S.; Isolauri, E. Long-term safety and efficacy of perinatal probiotic intervention: Evidence from a follow-up study of four randomized, double-blind, placebo-controlled trials. Pediatr. Allergy Immunol. 2017, 28, 170–175. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S129–S134. [Google Scholar] [CrossRef]
- Snydman, D.R. The safety of probiotics. Clin. Infect. Dis. 2008, 46 (Suppl. S2), S104–S111, discussion S144–S151. [Google Scholar] [CrossRef]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Tyrrell, K.L.; Citron, D.M. Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S98–S107. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.; Hörmannsperger, G.; Huys, G.; Levy, D.D.; Lutgendorff, F.; Mack, D.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]
- van den Nieuwboer, M.; Claassen, E. Dealing with the remaining controversies of probiotic safety. Benef. Microbes 2019, 10, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.L.; Boyte, M.E.; Elkins, C.A.; Goldman, V.S.; Heimbach, J.; Madden, E.; Oketch-Rabah, H.; Sanders, M.E.; Sirois, J.; Smith, A. Considerations for determining safety of probiotics: A USP perspective. Regul. Toxicol. Pharmacol. 2022, 136, 105266. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef]
- Langella, P.; Chatel, J.M. Risk assessment of probiotics use requires clinical parameters. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 202–204. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef]

