1. Introduction
Probiotics are living microorganisms that, when administered in adequate amounts, confer a health benefit on the host [1] They encompass various taxa, including yeasts (Saccharomyces spp.…) and bacteria (Lactobacillus spp., Bifidobacterium spp. …), which can be used in various clinical situations such as the prevention or treatment of antibiotic-associated diarrhea, gastroenteritis and functional bowel disorders [2]. Probiotic products come in a wide range of formulations and dosages, containing a single or multiple strains. Most probiotics are available as food supplements, while some are recognized as medications with marketing authorization from the European Medicines Agency (EMA).
Probiotics play a crucial role in regulating the digestive microbiota, using a combination of mechanisms that are still not fully understood. Well-established mechanisms include their immunomodulatory effects, such as the recruitment of lymphocytes to the intestinal mucosa, enhanced antigen transport, facilitating a more rapid immune response and simulation of the intestinal tight-junction proteins. Probiotics also exert direct antibacterial action and outcompete intestinal pathogens, thus aiding the colonization of beneficial commensal bacteria [3].
In healthy full-term infants, the colonization of the digestive tract by micro-organisms occurs gradually after birth. This process is influenced by various factors, such as the mode of delivery and feeding method [4]. But other elements are also involved: breastfeeding and absence of post-natal antibiotic therapy further contribute to a greater diversity of microbiota [5].
In premature infants, gut colonization occurs when gastrointestinal function and immune system are still immature. This immaturity of the immune system leads to a reduced level of immune factors (antibodies, intestinal mucus, antimicrobial peptides…), and increased pro-inflammatory factors such as Toll-like receptors [6]. As a result of this physiological immaturity and environmental factors associated with hospitalization, colonization occurs more slowly and with less diversity in preterm infants compared to full-term infants [7]. Fecal samples from preterm infants with necrotizing enterocolitis (NEC) often exhibit a higher abundance of Proteobacteria spp. than those from healthy preterm infants. This bacterial phylum is associated with intestinal dysbiosis, and the dysbiosis associated with its overabundance is known to precede the onset of NEC [8,9]. Furthermore, the composition of stool microbiota differs between healthy preterm infants and those with NEC, with the latter displaying even lower microbial diversity, and a prevalence of a single bacterial species [10].
NEC remains a poorly understood disease, with a relatively stable incidence over the last years, ranging from 2 to 7% among preterm infants under 32 gestational weeks (GW), and a mortality rate of 15 to 30% [11]. Regarding this dysbiosis in preterm infants as possible cause for prematurity complications such as NEC, the administration of probiotics in this vulnerable population holds promise for restoring microbiota balance.
Since the 1990s, several placebo-controlled studies have investigated the efficacy of probiotics to prevent NEC in preterm infants, yielding promising initial results. Large-scale trials have shown a decrease in the incidence of NEC and NEC-related mortality [12]. Subsequently, numerous randomized controlled trials have been conducted among preterm infants, continuing to provide valuable insights into the use of probiotics. The most recent systematic review, which included over 10,000 premature infants, demonstrated a significative reduction in NEC risk (RR 0.54, 95% CI 0.45–0.65) with probiotic supplementation [13].
These trials have also revealed additional benefits of probiotics in preterm infants, including a decrease in the incidence of late-onset sepsis (LOS) and overall mortality. The administration of probiotics resulted in a 12–14% reduction in LOS incidence [14,15]. Furthermore, there is evidence suggesting a 24% decrease in mortality rates, although the certainty of this evidence is currently low [13].
Probiotics have also shown positive effects on other outcomes, such as reduction in the time required to achieve full enteral feeding by 1.5 days [16], and a shorter length of hospital stay by 3.8 days (5400 newborns) [17]. Importantly, the administration of probiotics does not lead to an increased risk of adverse effects on intraventricular hemorrhage, neurodevelopment, bronchopulmonary dysplasia, periventricular leukomalacia or retinopathy of prematurity, thus suggesting an excellent safety profile [17,18,19,20].
In addition to these benefits, a pilot study revealed a decrease in the diversity of expressed antibiotic resistance genes in the gut microbiome of preterm infants who received probiotics. This reduction persisted up to 5 months of age and suggests a potential role for probiotics in limiting the development of antibiotic resistance [21].
To date, over 5000 preterm infants have received probiotics during randomized control trials [13], with an additional 21,000 infants included in cohort studies [22], not to mention the numerous preterm infants who have received probiotics outside clinical trials. Among all those infants, the incidence of adverse events has been extremely low. As previously mentioned, probiotics do not increase the risk of neurological impairment, neurosensorial complications or respiratory issues.
Despite the global reduction in LOS incidence, few cases have been reported of sepsis caused by probiotic strains or infection caused by contamination of the probiotic product. Saccharomyces spp. has been implicated in most sepsis cases [23,24], which highlights its unsuitability for use in preterm infants [25]. Three cases of Bifidobacterium longum bacteriemia were reported in Switzerland in 2015 among newborns receiving this probiotic strain. Two of them were asymptomatic and did not require treatment, while the third had necrotizing enterocolitis [26]. Another concern regarding probiotic use is the potential severity of sepsis resulting from contamination of probiotic product with pathogenic microorganisms [27]. This emphasizes the need for stringent quality control measures for probiotic products used in preterm infants.
Despite the substantial evidence supporting the administration of probiotics and the low occurrence of adverse events, there has been no definitive recommendation for their routine use until recently. The primary concern lies in the selection of probiotic strains due to the heterogeneity observed in the products tested in both randomized controlled trials and cohort studies. While safety appears to be recognized across all tested strains, variations in efficacy have been observed depending on the specific strains and combinations utilized [13].
2. Materials and Methods
2.1. Study Design
This study employed an observational, multicentric, transversal design to investigate medical practices related to probiotic use. The analysis focused on three categories: routine use, occasional use and absence of use.
2.2. Settings
The primary objective of the study was to determine the proportion of Neonatal Intensive Care Units (NICUs) in French-speaking European countries that routinely use probiotics. The secondary objectives included comparing administration protocols among NICUs and exploring the reasons for non-use.
The data were collected through an online questionnaire administered between October 2020 and June 2021, spanning a duration of 9 months.
The questionnaire was conducted using the Limesurvey website, and hosted on the server of the University of Caen Normandy. Responses were also stored through the Limesurvey website, ensuring anonymization of the physicians but enabling the identification of each response according to the represented NICU, to avoid duplication and facilitate data categorization by country.
Participants received clear information prior to their involvement in the study, and data were securely stored on the server of the University of Caen Normandy.
Approval for this study was obtained from the Local Health Research Ethics Committee of the Caen Normandy University Hospital under ID 2074.
2.3. Participants
The study involved European NICUs. For France, Switzerland and Belgium, we listed all the NICUs (67 in France, 9 in Switzerland, and 19 in Belgium). We obtained e-mail addresses through private contact lists, or hospital websites, and sent the questionnaire via email to at least one physician per unit. For the remaining European countries, the questionnaire was sent by e-mail to the respective national neonatal societies (or pediatric societies in the absence of a neonatal society), with a request for distribution to physicians working in NICUs.
The questionnaire link was sent via email to targeted physicians between October 2020 and June 2021, with a maximum of 5 reminders.
2.4. Variables
The primary endpoint of the study was to describe the use of probiotics at the NICU level, based on the answer to the second question: routine use, occasional use or absence of use. Secondary endpoints included comparing administration protocols, exploring reasons for non-use, and examining any correlation between probiotic use and the size of the NICU.
2.5. Data Sources/Measurements
The questionnaire was provided in French for France and French-speaking areas from Belgium and Switzerland, while other regions received the English version.
The first question of the survey collected information about the hospital’s name, city and country, to facilitate the tracking of responses and prevent duplication. The second question inquired about the type of probiotic use: routinely, as part of a research protocol, occasionally or never. Subsequent questions varied depending on the type of probiotic use, including inquiries about administration protocol or reasons for non-use, or both in the case of occasional use.
The second part of the questionnaire consisted of 12 common questions for all participants, covering aspects such as NICU size, typical patient profiles, and feeding protocols for preterm infants.
The questionnaire comprised open-ended, single-response and multiple-response questions. Only the first two questions were mandatory, the others were optional. Physicians had the opportunity to upload their administration protocol on the Limesurvey website.
The number of deliveries in 2019 at the hospital’s maternity ward was recorded for each French NICU using the scopesante.fr website, ensuring accurate data for comparing the NICUs.
Only one response per NICU was included, and if multiple responses were received from the same department, the most complete response was chosen based on the order of receipt.
2.6. Quantitative Variables
Data collected on the Limesurvey website were exported to Excel in spreadsheet format. Subgroup analysis were conducted for countries where more than 50% of answers were received.
2.7. Statistical Methods
For these countries, the frequency of each type of probiotic use was estimated using the Wald 95% confidence interval.
Subsequently, the responses were analyzed separately based on the type of probiotic use, with descriptive statistics, like percentages. For routine use, administration protocols were compared in terms of indications, contraindications, initiation time, and treatment duration. Indications and contraindications were compared for occasional use. For both types of use, the specific probiotic strains, pharmaceutical products, doses, and administration schedules were described.
Additionally, obstacles for routine use in the presence of occasional or no use were explored.
The pvalue.io (accessed on 20 April 2023) software was used to conduct univariate analysis using Chi2 and Fisher’s tests, aiming to identify a potential relationship between department characteristics and the type of probiotic use. This analysis was initially performed with three groups: routine use, occasional use, and absence of use. Subsequently, the occasional use and absence of use groups were combined into one category for further analysis.
3. Results
3.1. Participating Centers
All 67 NICUs in France, 9 NICUs in Switzerland, and 20 NICUs in Belgium were contacted through at least one of their physicians. Additionally, 31 European neonatal or pediatric societies were contacted, 5 societies agreed to forward the questionnaire, 2 refused, and the others did not respond. A total of 109 responses were received, of which 20 were excluded (Figure 1). Ultimately, 89 responses were retained, representing 11 different European countries (Figure 2).
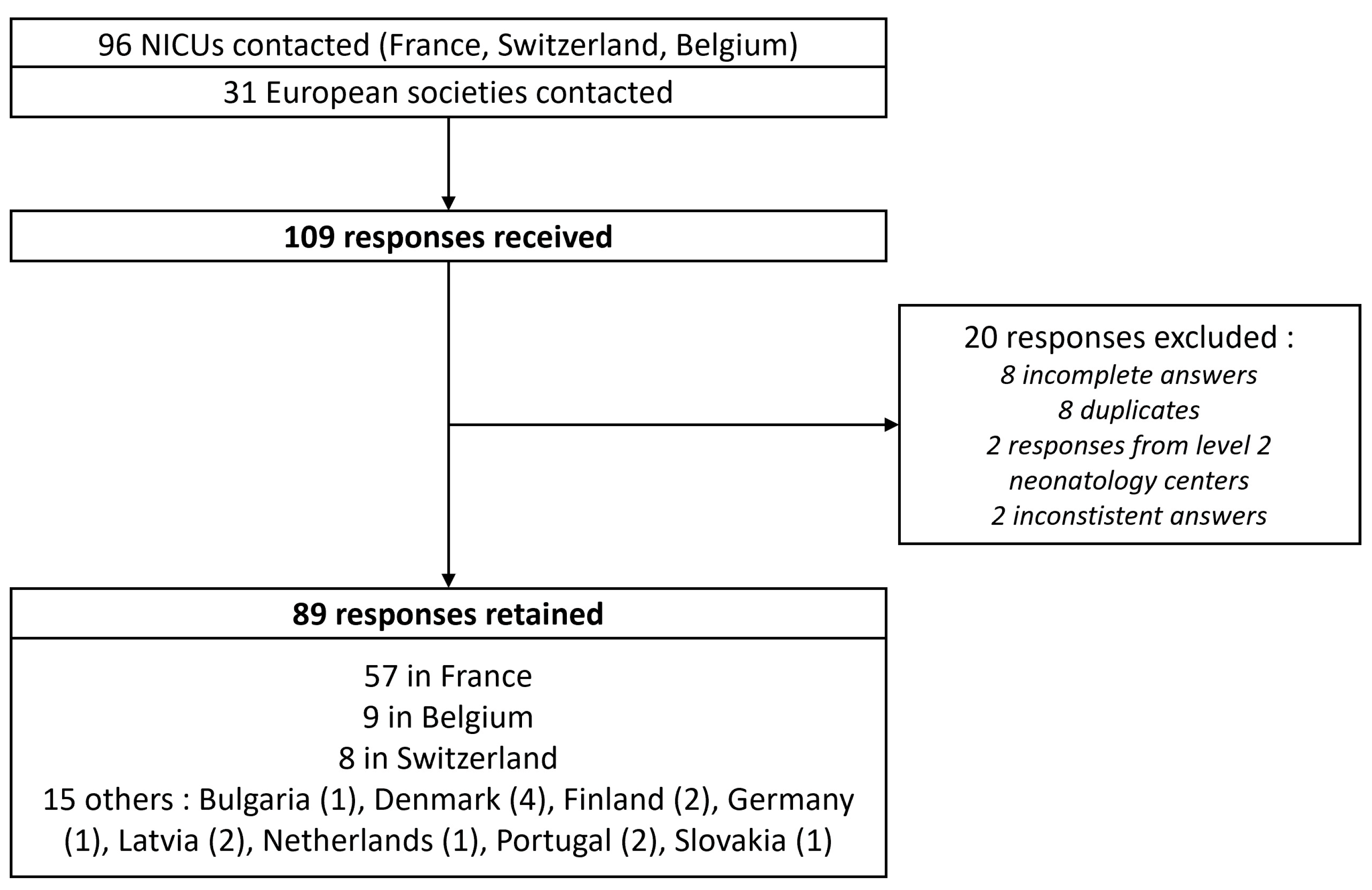
Figure 1. Flow chart of the study. NICU: Neonatal Intensive Care Unit.
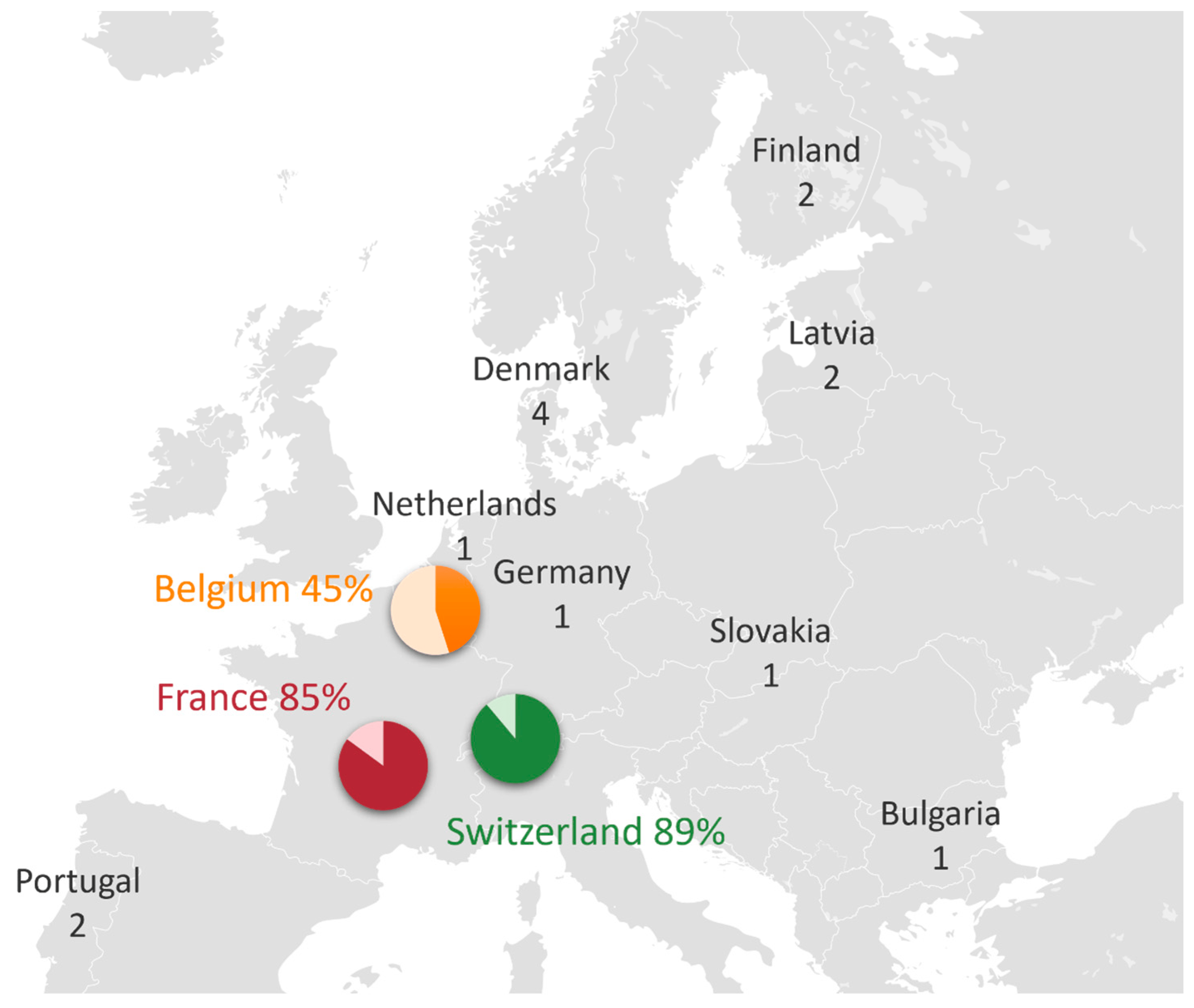
Figure 2. Geographical distribution of questionnaire responses among countries: each diagram represents the proportion of NICUs that responded to the questionnaire out of the total number of NICUs in each country. For other countries that were contacted through their societies, the number of responses is reported.
A response rate of 85% was obtained for France, and 89% for Switzerland. Five responses initially classified as routine use were reclassified to occasional use because the administration was according to specific indications.
3.2. Frequency of Probiotic Use
From all the responses, 33% reported routine use, 22% reported occasional use and 45% reported an absence of probiotic use.
Among French NICUs, 53% reported never using probiotics (n = 30, IC95% 0.46–0.60), 21% reported routine use (n = 12, IC95% 0.16–0.26), and 26% reported occasional use (n = 15, IC95% 0.20–0.32). All eight responding Swiss NICUs reported routine probiotic use. Due to a low response rate from other countries, we were unable to evaluate the frequency of probiotic use.
Upon conducting univariate analysis, no significant differences were observed in type of probiotic use according to breastfeeding rates, characteristics of hospitalized newborns, or the annual number of births.
3.3. Comparison of Routine Use Protocols
3.4. Comparison of Occasional Use Protocols
Indications for occasional use of probiotics include newborn colics in 55% of cases, antibiotic therapy in 40% of cases, and diarrhea or abdominal bloat in 35% of cases each. Other indications such as gastroeosophageal reflux, constipation, diaper rash or multiresistant bacterial colonization were reported in less than 10% of cases.
Contraindications for occasional probiotic use are not clearly defined, due to the lack of a specific protocol. However, many physicians rather like not to give probiotics to the most immature infants, with varying thresholds. Some physicians set a threshold of 1 kg of body weight, while others wait until corrected term. Additionally, two physicians reported the central veinous line as a contraindication.
3.5. Different Probiotic Strains Used
A total of 63% of Europeans NICUs use only a single probiotic strain, 20% use an association of two strains, and 10% use an association of three strains.
In France, probiotics used consist of a single strain of Lactobacillus spp., while in Switzerland, all NICUs use an association of Bifidobacterium spp. and Lactobacillus spp.
Among all the responses received, 63% of NICUs use only probiotics from the genus Lactobacillus, 29% use an association of strains from Lactobacillus and Bifidobacterium, and 2% use an association of Bifidobacterium and Streptococcus. A total of 15 different probiotic products have been reported, including 16 different bacterial strains .
3.6. Rhythm of Administration
The rhythm of administration varies between one to four times a day, with differences observed between countries. In France, probiotics are mainly administered once a day (70% of NICUs), while in Switzerland, they are administered twice or four times a day (37.5% of NICUs each).
3.7. Daily Dose
Daily doses are usually between 0.1 to 1 billion CFU (Colony Forming Units) per day, for both routine and occasional use. However, higher doses have also been reported, reaching up to 30 billion CFU per day for occasional use (Figure 3).
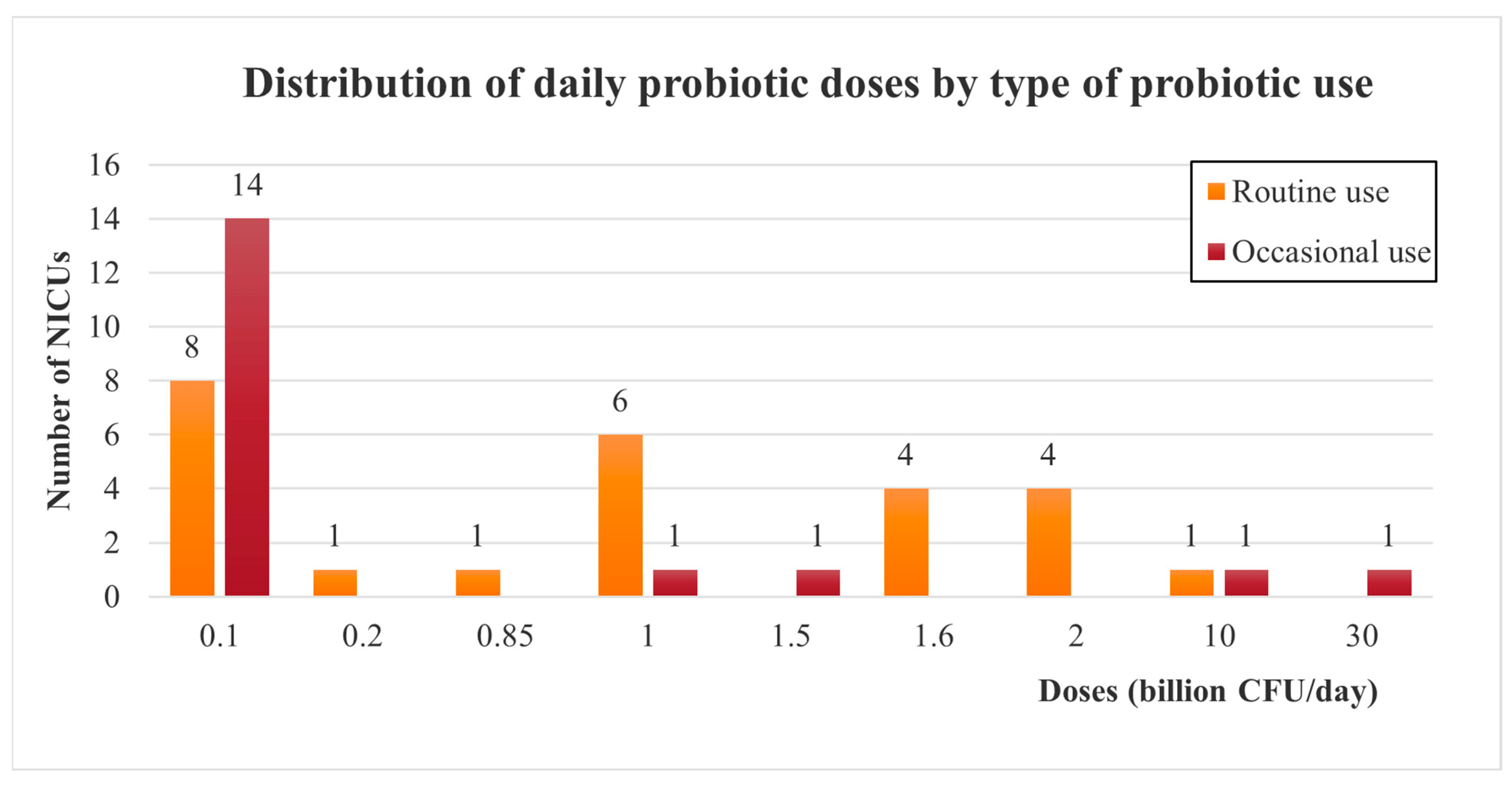
Figure 3. Doses of probiotics administered among European NICUs, depending on the type of probiotic use (routinely or occasionally). CFU: Colony Forming Unit.
3.8. Barriers to Routine Use of Probiotics
Among European NICUs that did not use probiotics routinely (occasional or absence of use), the most commonly reported obstacles are a lack of recommendations from endorsed societies (67%), lack of scientific evidence (38%), difficulty in obtaining probiotic products (21%), and fear of adverse effects (20%, Figure 4).
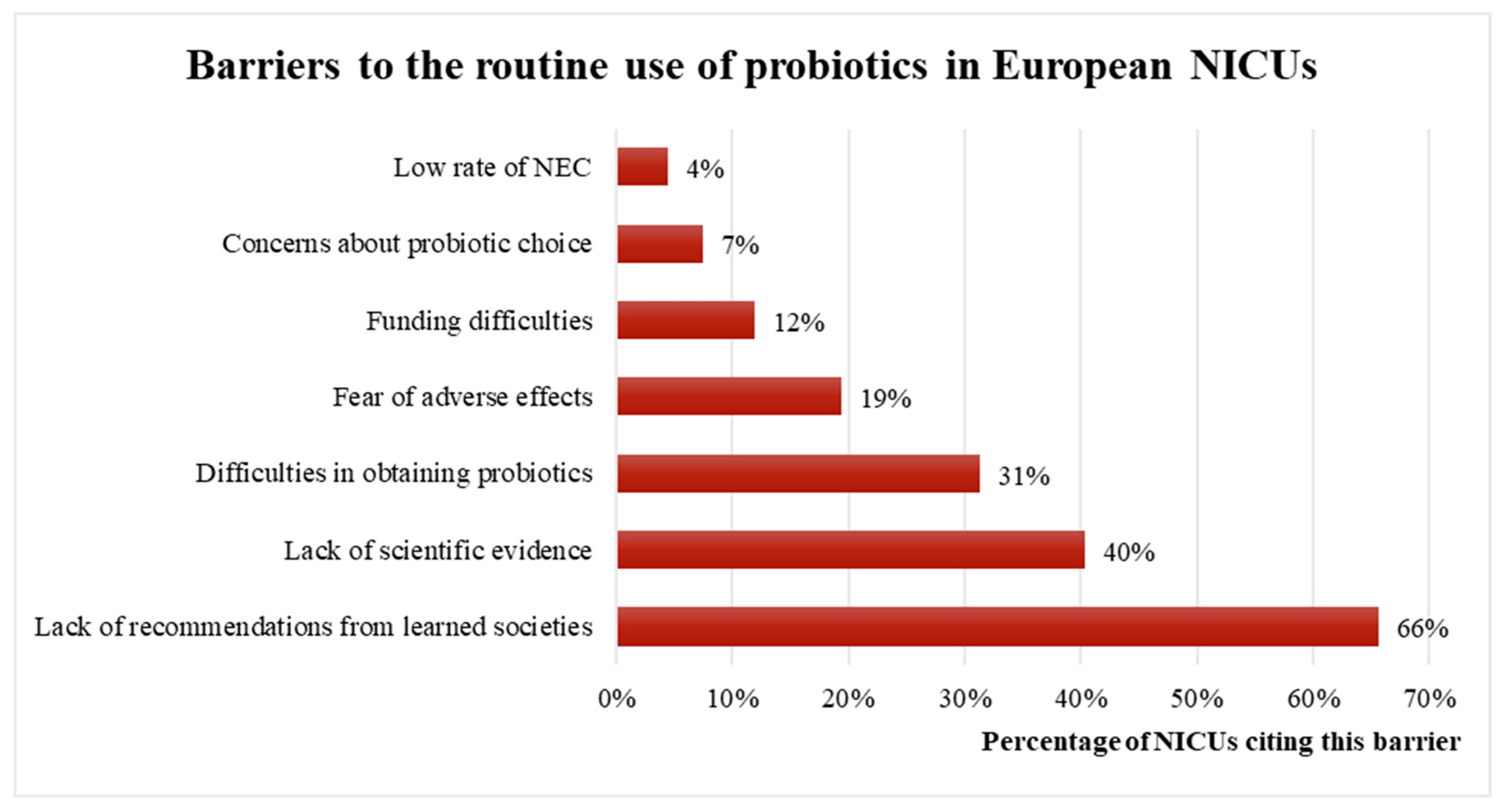
Figure 4. Barriers to the routine use of probiotics among European NICUs. NEC: necrotizing enterocolitis.
4. Discussion
This study reveals that the use of probiotics in European NICUs varies significantly. Among the countries included, Switzerland has a 100% rate of routine probiotic use, while France has a rate of 21%. The administration protocols and choice of probiotic strains differ widely among the NICUs. The main reasons cited for not using probiotics are the lack of recommendations and scientific evidence.
Comparisons with previous studies reveal significant variations in the rates of routine probiotic use across different regions. Probiotics are used by 8.8% of NICUs in United States [28], 17% in England [29], 19% in Germany [30], and 100% in New-Zealand [31]. In Canada in 2014–2015, 21% of preterm infants under 29 GW received prophylactic probiotics [32]. Viswanathan et al. in 2016 reported also a heterogeneous use of probiotics in United States, with an introduction mainly occurring at the initiation of enteral feeding, and a duration of treatment ranging from a few days until discharge home. A total of 16 different pharmaceutical products were used, only 4 of which have been studied for VLBW infants in randomized controlled trials [28]. Among European studies conducted between 2008 and 2018, most of them used Lactobacillus rhamnosus, with an association of at least two different strains in 53% of trials. In contrast, in this study, only 30% of European NICUs used an association of different strains, and none in France, and the genus Lactobacillus is the most frequently used in France. Administration duration and doses were found to be highly diverse across studies, showing a similar order of magnitude as observed in this study. However, none of the administration protocols have been able to demonstrate superiority over others in terms of efficacy [33].
In terms of strain selection, several meta-analyses have compared the administration of a single strain of Lactobacillus or Bifidobacterium with the use of multiple strains involving a combination of at least two genres. These studies have shown the superiority of the association of Bifidobacterium spp. and Lactobacillus spp. in reducing NEC and death [34,35]. Based on these findings, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) has issued a position paper recommending specific strains for probiotic use. The paper recommends the use of either Lactobacillus rhamnosus GG ATCC 53103 or the combination of Bifidobacterium infantis Bb-02, Bifidobacterium lactis Bb-12 and Streptococcus thermophilus TH-4 [25,36,37].
Indeed, while the recommendations from the ESPGHAN position paper provide guidance on specific strains that have shown promise in preterm infants, they do not offer detailed instructions on the administration protocol for probiotics. Important considerations such as the initiation and duration of treatment are not fully addressed in these recommendations, leaving room for variations in clinical practice [25]. To note, the questionnaire was administered prior to the publication of the EPSGHAN position paper, which may have contributed to an increased response rate due to the absence of official recommendations at the time.
In 2020, the American Gastroenterological Association published recommendations about probiotic use in different gastrointestinal disorders, and recommended the use of an association of Lactobacillus spp. and Bifidobacterium spp. for preterm infants for the prevention of NEC [35,38]. However, a few months later, the American Academy of Pediatrics issued recommendations that did not support the use of probiotics in preterm infants. This decision was based on the lack of FDA-regulated pharmaceutical-grade probiotic products available in the United States [39].
The safety aspect of probiotic use in preterm infants is indeed a critical consideration. Most probiotic products worldwide are marketed as dietary supplements rather than medicines, and current legislation governing dietary supplements is less stringent. Scientific societies raised concerns regarding the quality of probiotic products, the quality control process and potential discrepancies between the label and actual content [40]. This lower level of regulation increases the risk of non-compliance with product standards. A composition analysis of 16 probiotic products revealed that only one of them matched its label claims perfectly, while the others exhibited both pill-to-pill and lot-to-lot variations [41]. Contamination of probiotic products can result in ingestion of unexpected pathogens, potentially leading to severe infections, like the one case of fatal gastrointestinal mucormycosis associated with a contaminated dietary supplement [27]. This highlights the need for careful monitoring of probiotic production, as is required for medicines.
Recent recommendations emphasize the importance of good quality control of the probiotic product chosen for administration to preterm infants, and parental information about the physician’s choice regarding probiotic administration to their infant. Another important point underlined by these recommendations is the ability of the microbiology laboratory to identify probiotic bacteria in blood cultures, in case of sepsis due to probiotic species [25].
Data about long-term outcomes are also limited to guide assessment of long-term efficacy. In a randomized trial of very low-birth-weight infants with follow-up of 249 infants at 18–24 months’ corrected age, the use of Lactobacillus reuteri did not increase or decrease the risk of adverse neurocognitive outcomes, assessed using the Bayley Scales of Infant and Toddler Development II [42].
According to recent studies, there is a consensus that future probiotic trials should focus on comparing different probiotic products against each other and comparing different administration protocols, rather than comparing probiotic administration to a placebo [13,43]. Simultaneous analysis of stool samples could provide valuable insights into the effects of probiotic administration on gut colonization and the persistence of probiotics in stool after discontinuation of treatment. This approach could help determine the optimal duration of treatment.
Study Limitations
The strength of this study lies in the high response rates in France and Switzerland, which provide a reliable representation of probiotic use in these two countries. However, the study does have some limitations. Firstly, there is a potential selection bias due to the use of two different methods of contact. The method involving neonatal societies resulted in a low response rate for other European countries, which hindered the accurate estimation of probiotic use in those regions. Furthermore, the data collected in this study are based on self-reporting, which introduces the possibility of recall bias and potential misclassifications. It is important to acknowledge that participants may not accurately recall or report their probiotic usage, leading to potential inaccuracies in the data. Finally, it is also possible that physicians who actively use probiotics were more inclined to complete the questionnaire, introducing a non-response bias and potentially leading to an overestimation of probiotic use.
5. Conclusions
Despite the demonstrated benefits of probiotics in reducing NEC, sepsis and mortality in preterm infants, the rate of routine probiotic use remains low in European NICUs. Comparison of administration protocols reveals a great heterogeneity, reflecting the lack of comparability between studies on this subject. Considering that probiotics are intended for preventive use, ensuring their safety is of paramount importance for routine implementation. This study highlights the importance of rigorous microbiological quality control measures for probiotic product production to ensure their safety and efficacy in NICUs. Additional studies are needed to guide clinicians in choosing the most appropriate probiotic product to decrease NEC. Studies should focus on comparing different probiotic products and administration protocols, as well as exploring the impact of probiotic administration on gut colonization and the persistence of probiotics in the stool. These efforts will contribute to enhance the evidence-based practice of probiotic use in the care of preterm infants, ultimately improving their outcomes and reducing the risk of neonatal complications.
References
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO Working Group: Rome, Italy, 2002; Volume 21, pp. 1–11. [Google Scholar]
- Marteau, P.R. Probiotics in clinical conditions. Clin. Rev. Allergy Immunol. 2002, 22, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Cruchet, S.; Furnes, R.; Maruy, A.; Hebel, E.; Palacios, J.; Medina, F.; Ramirez, N.; Orsi, M.; Rondon, L.; Sdepanian, V.; et al. The Use of Probiotics in Pediatric Gastroenterology: A Review of the Literature and Recommendations by Latin-American Experts. Pediatr. Drugs 2015, 17, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Gewolb, I.H.; Schwalbe, R.S.; Taciak, V.L.; Harrison, T.S.; Panigrahi, P. Stool microflora in extremely low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 80, F167. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Gate-keeper function of the intestinal epithelium. Benef. Microbes 2013, 4, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Gruhl, B.; Löbnitz, M.; Michel, P.; Radke, M.; Blaut, M. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr. Res. 2003, 54, 393–399. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Simon Kroll, J.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Hoenig, J.D.; Malin, K.J.; Qamar, S.; Petrof, E.O.; Sun, J.; Antonopoulos, D.A.; Chang, E.B.; Claud, E.C. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009, 3, 944–954. [Google Scholar] [CrossRef]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of neonatal necrotising enterocolitis in high-income countries: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, A.B. Reduced Incidence of Necrotizing Enterocolitis Associated with Enteral Administ-Ation of Lactobacillus acidopbilus and Bifidobacterium infantis to Neonates in an Intensive Care Unit. 1999, Volume 3. Available online: https://www.ijidonline.com/article/S1201-9712(99)90024-3/pdf (accessed on 2 July 2019).
- Sharif, S.; Meader, N.; Oddie, S.J.; Rojas-Reyes, M.X.; McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2020, 2020, CD005496. [Google Scholar] [CrossRef]
- Dermyshi, E.; Wang, Y.; Yan, C.; Hong, W.; Qiu, G.; Gong, X.; Zhang, T. The “golden Age” of Probiotics: A Systematic Review and Meta-Analysis of Randomized and Observational Studies in Preterm Infants. Neonatology 2017, 112, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Rev. Artic. Pediatr. 2016, 137, e20153684. [Google Scholar] [CrossRef] [PubMed]
- Athalye-Jape, G.; Deshpande, G.; Rao, S.; Patole, S. Benefits of probiotics on enteral nutrition in preterm neonates: A systematic review. Am. J. Clin. Nutr. 2014, 100, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Marwah, G.; Westgarth, M.; Buys, N.; Ellwood, D.; Gray, P.H. Effects of Probiotics on Necrotizing Enterocolitis, Sepsis, Intraventricular Hemorrhage, Mortality, Length of Hospital Stay, and Weight Gain in Very Preterm Infants: A Meta-Analysis. Adv. Nutr. Int. Rev. J. 2017, 8, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Villamor-Martínez, E.; Filippi, L.; Mosca, F.; Villamor, E. Probiotic supplementation in preterm infants does not affect the risk of retinopathy of prematurity: A meta-analysis of randomized controlled trials. Sci. Rep. 2017, 7, 3014. [Google Scholar] [CrossRef]
- Upadhyay, R.P.; Taneja, S.; Chowdhury, R.; Strand, T.A.; Bhandari, N. Effect of prebiotic and probiotic supplementation on neurodevelopment in preterm very low birth weight infants: Findings from a meta-analysis. Pediatr. Res. 2020, 87, 811–822. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.; Villamor, E. Probiotic supplementation in preterm infants does not affect the risk of bronchopulmonary dysplasia: A meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1197. [Google Scholar] [CrossRef]
- Guitor, A.K.; Yousuf, E.I.; Raphenya, A.R.; Hutton, E.K.; Morrison, K.M.; McArthur, A.G.; Wright, G.D.; Stearns, J.C. Capturing the antibiotic resistome of preterm infants reveals new benefits of probiotic supplementation. Microbiome 2022, 10, 136. [Google Scholar] [CrossRef]
- Deshmukh, M.; Patole, S. Prophylactic Probiotic Supplementation for Preterm Neonates—A Systematic Review and Meta-Analysis of Nonrandomized Studies. Adv. Nutr. 2021, 12, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Belet, N.; Dalgiç, N.; Öncel, S.; Ciftçi, E.; Ince, E.; Güriz, H.; Barlas, M.; Doǧru, Ü. Catheter-related fungemia caused by Saccharomyces cerevisiae in a newborn. Pediatr. Infect. Dis. J. 2005, 24, 1125. [Google Scholar] [CrossRef] [PubMed]
- Chioukh, F.Z.; Ben Hmida, H.; Ben Ameur, K.; Toumi, A.; Monastiri, K. Septicémie à Saccharomyces cerevisiae chez un prématuré traité par Ultra-Levure®. Med. Mal. Infect. 2013, 43, 359–360. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, C.H.P.; van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Berni Canani, R.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, A.; Zbinden, R.; Berger, C.; Arlettaz, R. Case series of Bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology 2015, 107, 56–59. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Walker, T.A.; Lockhart, S.R.; Ng, D.; Chiller, T.; Melchreit, R.; Brand, M.E.; Smith, R.M. Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. Morb. Mortal. Wkly. Rep. 2015, 64, 155–156. [Google Scholar]
- Viswanathan, S.; Lau, C.; Akbari, H.; Hoyen, C.; Walsh, M.C. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. J. Perinatol. 2016, 36, 1106–1111. [Google Scholar] [CrossRef]
- Duffield, S.D.; Clarke, P. Current use of probiotics to prevent necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F228. [Google Scholar] [CrossRef]
- Denkel, L.A.; Schwab, F.; Garten, L.; Geffers, C.; Gastmeier, P.; Piening, B. Protective Effect of Dual-Strain Probiotics in Preterm Infants: A Multi-Center Time Series Analysis. PLoS ONE 2016, 11, e0158136. [Google Scholar] [CrossRef]
- Meyer, M.P.; Chow, S.S.W.; Alsweiler, J.; Bourchier, D.; Broadbent, R.; Knight, D.; Lynn, A.M.; Patel, H. Probiotics for Prevention of Severe Necrotizing Enterocolitis: Experience of New Zealand Neonatal Intensive Care Units. Front. Pediatr. 2020, 8, 119. [Google Scholar] [CrossRef]
- Singh, B.; Shah, P.S.; Afifi, J.; Simpson, C.D.; Mitra, S.; Dow, K.; El-Naggar, W. Probiotics for preterm infants: A National Retrospective Cohort Study. J. Perinatol. 2019, 39, 533–539. [Google Scholar] [CrossRef]
- Kutylowksi, J.; Yahia, N. Types, Frequency, Duration, and Dosage of Probiotics to Prevent Necrotizing Enterocolitis in Preterm Infants among Countries. Adv. Neonatal Care 2019, 19, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A. Probiotics and the prevention of necrotizing enterocolitis. J. Pediatr. Surg. 2019, 54, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B.; Chang, Y.; Florez, I.D.; Foroutan, F.; Shahid, S.; Zeraatkar, D. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgia, N.; et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial. Early Hum. Dev. 2014, 90, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.; Cummings, J.; Hand, I.; Adams-Chapman, I.; Aucott, S.W.; Puopolo, K.M.; Goldsmith, J.P.; Kaufman, D.; Martin, C.; Mowitz, M. Use of probiotics in preterm infants. Pediatrics 2021, 147, e2021051485. [Google Scholar] [CrossRef]
- Kolaček, S.; Hojsak, I.; Berni Canani, R.; Guarino, A.; Indrio, F.; Orel, R.; Pot, B.; Shamir, R.; Szajewska, H.; Vandenplas, Y.; et al. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 117–124. [Google Scholar] [CrossRef]
- Lewis, Z.T.; Shani, G.; Masarweh, C.F.; Popovic, M.; Frese, S.A.; Sela, D.A.; Underwood, M.A.; Mills, D.A. Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatr. Res. 2016, 79, 445–452. [Google Scholar] [CrossRef]
- Akar, M.; Eras, Z.; Oncel, M.Y.; Arayici, S.; Guzoglu, N.; Canpolat, F.E.; Uras, N.; Oguz, S.S. Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. J. Matern. Fetal. Neonatal Med. 2017, 30, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Umberger, E.; Patel, R.M. Safety and Efficacy of Probiotic Administration to Preterm Infants: Ten Common Questions. Pediatr. Res. 2020, 88, 48. [Google Scholar] [CrossRef] [PubMed]

