1. Introduction
Increasing interest in healthy human food worldwide introduces a rising consumption of natural compounds [1], which are known for their various biological activities and have been applied for a long time in the pharmaceutical area. They are often characterized as multi-component and multi-functional to treat diseases, which might be more effective due to possible synergistic actions [2].
Marine algae have recently been considered a new source of bioactive compounds [3]. Polysaccharides are the most common macromolecules produced by algae [4,5]. Like polynucleotides and proteins, polysaccharides play key roles in life activities such as fertilization, signal recognition, cell communication and adhesion, blood clotting, pathogenesis prevention, and system development [6,7,8]. Recently, pharmacological reports have shown that polysaccharides isolated from algae have various pharmacological activities, including immunomodulation, antitumor, anti-inflammatory, and antioxidant capacities [7,8]. The biological abilities of polysaccharides are probably related to the presence and positions of sulfate groups. The macromolecules have different levels of spatial organization, including primary, secondary, tertiary, and quaternary structures. Their primary forms can affect the various properties of polysaccharides, like their water-solubility, gel viscosity, and biological functions [9]. Functional polysaccharides could prevent the oxidative stress provoked by reactive oxygen species (ROS).
Generally, the endogenous antioxidant system can scavenge ROS amounts generated by cellular metabolism [10]. However, excessive environmental stresses, like ultraviolet irradiation and toxic chemicals, such as pesticides, can cause abnormal ROS production, which leads to oxidative stress.
Tebuconazole (TEB) is an effective fungicide used for the control of mildew and rust on wheat, barley, rice, fruits, and vegetables [11]. In the long term, it was one kind of toxicant for marine organisms, as it might induce an adverse effect on the aquatic environment [12]. Animals were sensitive to the influence of TEB because they could uptake and retain xenobiotics from circumstances via active or passive processes. TEB provokes adrenal gland hypertrophy in chronic dog studies and mouse teratogenic effects [13,14]. Sancho et al. [15] showed that short-term exposure to TEB induced physiological impairment and endocrine reproduction perturbation in male zebrafish. TEB is also deemed to cause hepatic and reproductive damage by inducing lipid peroxidation, decreasing antioxidant enzyme activities, and releasing free radicals [12,15]. To date, no investigation has been carried out on the biological activities of polysaccharides obtained from Alsidium corallinum (A. corallinum). Only a few studies have been realized on phenolic compounds extracted from A. corallinum, a red alga from the Mediterranean Sea, demonstrating their interesting biological activities, including antioxidant and hepatoprotective properties [16].
The present study aims to investigate the protective effect of ACPs against TEB-induced hepatotoxicity in adult rats. Identification of ACPs in vitro is necessary to understand their structural and functional properties and antioxidant potential. Furthermore, the binding affinities and molecular interactions of ACPs with TyrRS from S. aureus (1JIJ), human peroxiredoxin (1HD2), and Acyl-CoA: cholesterol acyltransferase (ACAT, 1WL4) were assessed using computational modeling.
2. Results and Discussion
2.1. Extraction Yield and Physicochemical Analysis of ACPs
The yield and chemical analysis of polysaccharides isolated from the alga Alsidium corallinum are reported in .
The yield percent of ACPs (20.93%) is better than the yields previously reported for polysaccharides from several other algae, including Gelidium crinale (2.6%) [17], Sargassum swartzii (11%) [18], and Chaetomorpha linum (16%) [19]. The yield is similar to polysaccharides isolated from fenugreek [20].
Polysaccharides are polar macromolecules that are easy to dissolve in water because they can replace water–water interactions with water–solute interactions. Polysaccharide extraction yield is influenced by algal species, period of collection, and extraction parameters. Moreover, the pH presented a 6.2 ± 03. Ktari et al. [21] reported that the pH of fenugreek polysaccharides solution at 37 °C is 6.4. ACPs have relatively low levels of moisture (2.96 ± 0.09%) and ash (3.00 ± 0.08%).
The quantitative estimation of ACPs showed a significant contribution of carbohydrates and less uronic acid and proteins. Proteins are part of cell wall structure, and are associated closely with polysaccharides. It has been considered a potential contaminant of polysaccharides [22]. After depigmentation and extraction of ACPs, we tried to denature proteins and eliminate the majority of lipids, and that is why we have acquired relatively low levels of proteins (3%) compared to other work done on the other polysaccharides [8], which suggests the efficiency of the extraction method. Amounts of proteins depend mainly on the way of extraction and deproteination processes. Fleury and Lahaye [23] indicated that precipitation of proteins during extraction at 100 °C contributed probably to their indigestibility. The homogeneity of the polysaccharide was confirmed by elemental microanalyses, which showed a protein content of 3% of protein residues. The ACPs protein contents were similar to those from the endodermis of Shaddock [24]. Our results indicate that extracting and isolating ACPs was suitable for yielding a compound free of undesired molecules that could interfere in the subsequent experiments.
Moreover, the results presented in revealed that ACPs had relatively high carbohydrate levels (66.06%).
On the other hand, the uronic acid content of ACPs (11.03%) was similar to those obtained by polysaccharides extracted from Sargassum vulgare (brown alga) [25]. The marine origin, seasonal periods, conditions, and extraction method are determining factors for the variations in all these contents.
2.2. Spectroscopic Analysis of ACPs
2.3. In Vitro Biological Activities of ACPs
Antioxidant activities of ACPs have been determined by various methods such as DPPH radical scavenging, ABTS radical scavenging, ferric-reducing antioxidant power, and nitric oxide scavenging assays.
2.4. Phytotoxicity Essay of ACPs
As shown in Figure 6, ACPs phytotoxicity was determined in different concentrations (1, 0.5, 0.25, 0.125, 0.0625 mg/mL) against cress seeds. Our results have shown that seed germination percent ≥80% with the different concentrations of ACPs, which confirms that polysaccharides from Alsidium corallinum are not phytotoxic [42].
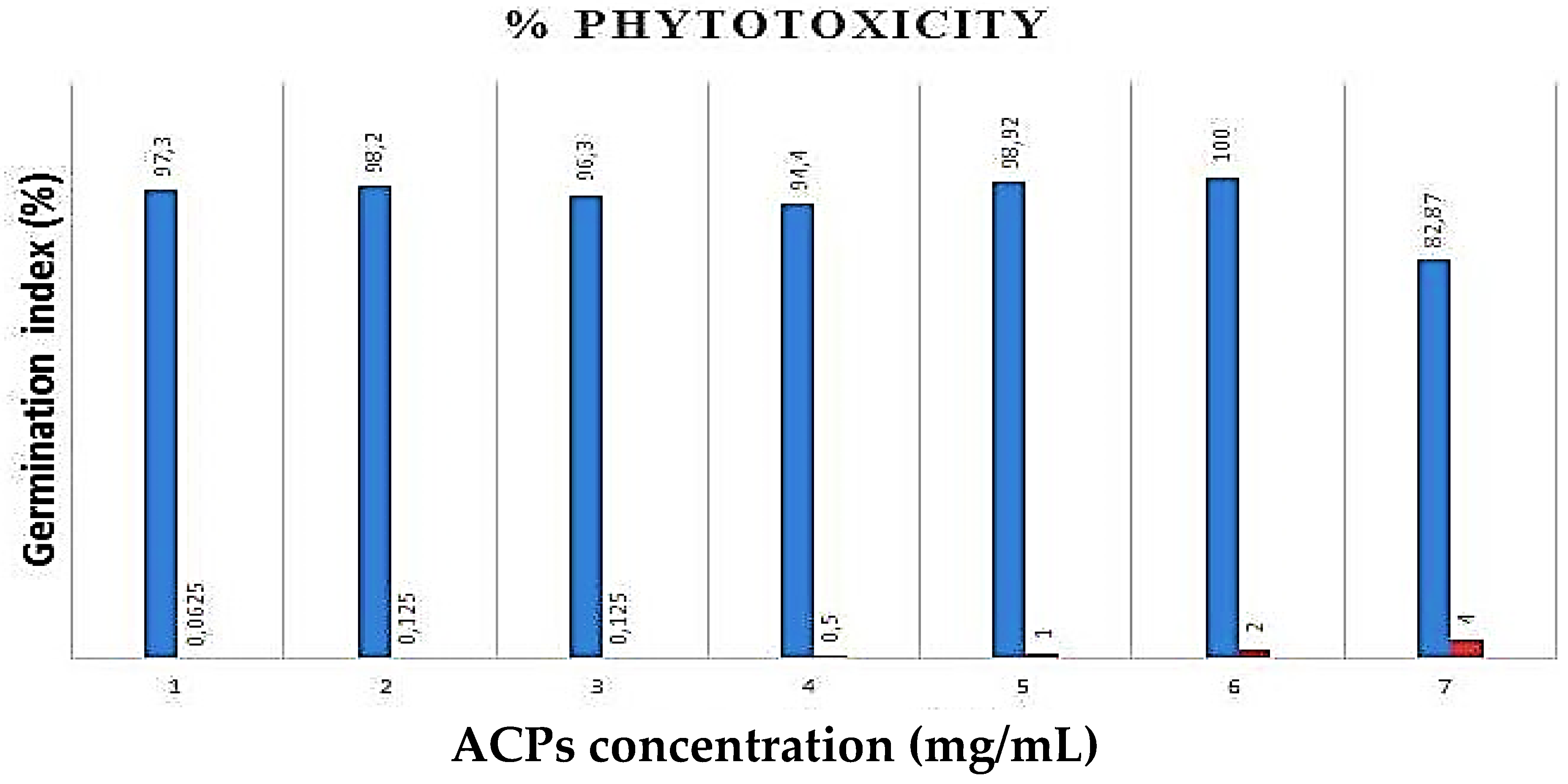
Figure 6. Effects of ACPs on the seed germination (%).
2.5. In Vivo Biological Activities of ACPs
3. Materials and Methods
3.1. Source of Alga-Derived Polysaccharides
The marine red algae Alsidium corallinum was collected in the month of March from the coastal area of Sidi Mansour, Sfax, Tunisia. The voucher specimen of this species was deposited and identified in the herbarium of the Biology Laboratory at the Faculty of Sciences of Sfax. Alga was rinsed with tap water, followed by deionized water. Next, it was dried and ground before starting the extraction.
3.2. Extraction of Sulfated Polysaccharides (ACPs)
ACPs were extracted according to Chen’s method [65]. Briefly, A. corallinum powder was pre-extracted with ethanol (90%) to remove pigments. The dry residue was extracted twice with deionized water at 70 °C and stirred over six hours. The extract was combined and filtered, and the filtrate evaporated under a vacuum. The concentrated liquid was precipitated with ethanol for 24 h at 4 °C and then centrifuged for 15 min. The obtained residue was re-dissolved in double distilled water. The water phase was dialyzed at 4 °C against distilled water for 48 h. The dialysate was concentrated through rotary evaporation to obtain ACPs. The latter were stored at −20 °C for additional use.
3.3. Extraction Yield and Physicochemical Analysis of ACPs
ACPs extraction yield was measured based on the wet weight of A. corallinum powder. The yield of ACPs was expressed as a percentage (%) of the mass (g) of polysaccharides against the mass (g) of A. corallinum powder.
pH of ACPs (1% solution at 25 °C) was measured using a pH meter with complete immerging of the glass electrode into the solution.
The moisture and ash contents were determined at 105 and 550 ◦C, according to the AOAC standard methods 930.15 and 942.05, respectively [66].
The protein content was estimated by Bradford’s method [67]. Absorbance was measured by a spectrophotometer (595 nm). Protein levels were determined from a standard curve plotted using bovine serum albumin as a reference protein. All tests were carried out in triplicate.
Uronic acid amounts were determined by Blumenkrantz and Asbœ-Hansen’s method [68]. The absorbance was determined with a spectrophotometer (520 nm). The uronic acid amount was determined using a standard curve of glucuronic acid.
Carbohydrate amounts were determined by the method of Masuko [69]. Carbohydrate contents were measured by spectrophotometer at 490 nm. The result was estimated using glucose as a reference sugar from a standard curve.
3.4. Spectroscopic Analysis
3.5. In Vitro Biological Activities of ACPs
3.6. ACPs Phytotoxicity Analysis
The phytotoxicity of the polysaccharides obtained from A. corallinum was evaluated by methods of Zucconi and Monaco [81] using the seed germination technique. Throughout the assay, control included only distilled water sterile. The length of seedling growths was measured as mm. The phytotoxicity of our polysaccharides was evaluated by percentage of germination index (GI %).
3.7. In Vivo Antioxidant Activities of ACPs
3.8. Computational Analysis and Interactions Assay
The four monosaccharides in Alsidium corallinum, which had been identified, were used in the computational study to decipher their molecular interactions and confirm their potential antioxidant, antimicrobial, and hypolipidemic effects. The chemical structure of these monosaccharides was collected from the Pubchem website. The 3D crystal structure of TyrRS from Staphylococcus aureus (1JIJ), human peroxiredoxin (1HD2), and Acyl-CoA: cholesterol acyltransferase (ACAT, 1WL4) were obtained from the RCSB PDB. The studied monosaccharides and three targeted receptors were prepared, processed for depreciation, and then saved in pdbqt format [61,63]. As previously reported, they have been subjected to a CHARMm force field after targeting the grid box by selecting some key residues within the pocket region [57,58,59,60]. The reasons behind choosing these receptors are the massive responsibility of hospital-acquired infections by S. aureus, the key role of reducing hydrogen peroxide and alkyl hydroperoxides using equivalents, and the key involvement in fatty acid metabolism.
3.9. Statistical Analyses
All experiences and statistical analyses were made in triplicate. All results are expressed as the mean standard deviation. Statistical analysis was performed with the SPSS 17.0 statistical package for Windows (SPSS, Inc., Chicago, IL, USA). A two-way ANOVA followed by Tukey’s post hoc test was performed to compare treatment and control groups. Statistical significance was set at p = 0.05.
4. Conclusions
The polysaccharides from A. corallinum exhibited protective effects against TEB-induced toxicity by reducing ROS production. Both finding scores and molecular interactions of A. corallinum polysaccharides may explain the experimental in vitro and in vivo findings, which may result in the antioxidant, antimicrobial, and hypolipidemic activities. Further studies are required to clarify the mechanism of action of algae polysaccharides on liver cells and to probe the clinical availability of these compounds in the form of algal foods, food supplements, and regulated therapeutics.
References
- Thygesen, K.; Alpert, J.S.; White, D.W.; on Behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur. Heart. J. 2007, 28, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, E.G.; Escrig, A.J.; Ruperez, P. Dietary fibre and physicochemical properties of several edible seaweeds from northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Marine Nutraceuticals: Prospects and Perspectives. Eur. J. Neur. 2005, 21, 2649–2659. [Google Scholar] [CrossRef]
- Tehila, T.S.; Margalit, B.; Dorit, M.; Shlomo, G.; Shoshana, A. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, K.N.; Lee, S.H. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS stimulated RAW macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar] [CrossRef]
- Dore, C.M.; Alves, M.G.C.F.; Will, L.S. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Kozlova, L.V.; Mikshina, P.V. Spatial structure of plant cell wall polysaccharides and its functional significance. Biochemistry 2013, 78, 836–853. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef]
- Tully, D.B.; Bao, W.; Goetz, A.K.; Blystone, C.R.; Ren, H.; Schmid, J.E.; Strader, L.F.; Wood, C.R.; Best, D.S.; Narotsky, M.G.; et al. Gene expression profiling in liver and testis of rats to characterize the toxicity of triazole fungicides. Toxicol. Appl. Pharmacol. 2006, 215, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, W.; Liu, D. Stereo selective Degradation of Tebuconazole in Rat Liver Microsomes. Chirality 2012, 24, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Petricca, S.; Flati, V.; Celenza, G.; Di Gregorio, J.; Rita Lizzi, A.; Luzi, C.; Cristiano, L.; Cinque, B.; Rossi, G.; Festuccia, C. Tebuconazole and Econazole Act Synergistically in Mediating Mitochondrial Stress, Energy Imbalance, and Sequential Activation of Autophagy and Apoptosis in Mouse Sertoli TM4 Cells: Possible Role of AMPK/ULK1 Axis. Toxicol. Sci. 2019, 169, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Yargholin, M.; Esfehani Tahere, A.; Sadat Musavi, S.; Mehdi Erfani, A.; Salar Amoli, J. Poisoning of dog by tebuconazole fungicide—A case report. Iran. Vet. Rep. 2016, 11, 114–119. [Google Scholar]
- Sancho, E.; Villarroel, M.J.; Andreu, E.; Ferrando, M.D. Disturbances in energy metabolism of Daphnia magna after exposure to tebuconazole. Chemosphere 2009, 74, 1171–1178. [Google Scholar] [CrossRef]
- Ben Saad, H.; Kharrat, N.; Krayem, N. Biological properties of Alsidium corallinum and its potential protective effects against damage caused by potassium bromate in the mouse liver. Environ. Sci. Pollut. Res. 2015, 23, 3809–3823. [Google Scholar] [CrossRef]
- Pereira, M.S.; Vilela-Silva, A.-C.E.S.; Valente, A.-P.; Mourao, P.A.S. A 2-sulfated, 3- linked α-l-galactan is an anticoagulant polysaccharide. Carbohydr. Res. 2002, 337, 2231–2238. [Google Scholar] [CrossRef]
- Kurup, G.M.; Jose, M.G. In Vitro Antioxidant Properties of Edible Marine Algae. Pharm. Bioprocess. 2016, 4, 100–108. [Google Scholar]
- Hamzaoui, A.; Ghariani, M.; Sallem, I. Extraction characterization and biological properties of polysaccharide derived from green seaweed “Chaetomorpha linum” and its potential application in Tunisian beef sausages. Int. J. Biol. Macromol. 2020, 148, 1156–1168. [Google Scholar] [CrossRef]
- Ktari, N.; Feki, A.; Trabelsi, I.; Triki, M. Structure, functional and antioxidant properties in Tunisian beef sausage of a novel polysaccharide from Trigonella foenum-graecum seeds. Int. J. Biol. Macromol. 2017, 98, 169–181. [Google Scholar] [CrossRef]
- Ktari, N.; Trabelsi, I.; Bardaa, S. Effects in rat cutaneous wound healing of a novel polysaccharide from fenugreek (Trigonella foenum- graecum) seeds. Int. J. Biol. Macromol. 2017, 95, 625–634. [Google Scholar] [CrossRef]
- Hao, H.; Han, Y.; Yang, L.; Huang, R. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int. J. Biol. Macrom. 2019, 134, 891–900. [Google Scholar] [CrossRef]
- Fleury, N.; Lahaye, M. Chemical and physico-chemical characterisation of fibres from Laminaria digitata (kombu breton): A physiological approach. J. Sci. Food Agric. 1991, 55, 389–400. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.; Chen, L. Chemical composition and bioactivities of a water-soluble polysaccharide from the endodermis of shaddock. Int. J. Biol. Macrom. 2012, 51, 763–766. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.A.; Kim, K.N. Antioxidative and antimicrobial activities of Sargassum muticum extracts. J. Korean Soc. Food. Sci. Nutr. 2007, 36, 663–669. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef]
- Hammed, A.; Irwandi, J.; Senay, S.; Azura, A.; Zahangir, A. Chemical structure of sulfated polysaccharides from brown seaweed (Turbinaria turbinata). Int. J. Food. Prop. 2017, 20, 1457–1469. [Google Scholar]
- Robic, A.; Gaillard, C.D.; Sassi, J.F.O.; Lerat, Y.; Lahaye, M. Ultrastructureof ulvan: A polysaccharide from green seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef]
- Phyo, P.; Wang, T.; Xiao, C.; Anderson, C.T.; Hong, M. Effects of pectin molecular weight changes on the structure, dynamics, and polysaccharide interactions of primary cell walls of Arabidopsis thaliana: Insights from solid-state NMR. Biomacromolecules 2017, 18, 2937–2950. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Krichen, F.; Karoud, W.; Sila, A. Extraction, characterization and antimicrobial activity of sulfated polysaccharides from fish skins. Int. J. Biol. Macrom. 2015, 75, 283–289. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Ox. Med. Cell. Long. 2016, 2016, 5692852. [Google Scholar]
- Leong, L.P.; Shui, G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Khaskheli, M.; Arain, M.A.; Chaudhry, S.; Soomro, A.H.; Qureshi, T.A. PhysicoChemical Quality of Camel Milk. J. Agric. Soc. Sci. 2005, 1, 164–166. [Google Scholar]
- Ruperez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucusve siculosus. J. Agric. Food. Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Huimin, Q.; Tingting, Z.; Quanbin, Z.; Zhien, L.; Zengqin, Z.; Ronge, X. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A. High molecular weight plant phenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macrom. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Mayer, A.; Hamann, M. Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. J. Comp. Biochem. Physiol. 2005, 140, 265–286. [Google Scholar] [CrossRef]
- Kaewsrithong, J.; Intarak, K.; Longpol, T.; Chairgulprasert, V.; Prasertsongsakun, S.; Chotimakorn, C.; Ohshima, T. Antibacterial activity and bioactive compounds of some brown algae from Thailand. In JSPS-NRCT International Symposium Joint Seminar; Kasetsart University: Bangkok, Thailand, 2007; pp. 608–613. [Google Scholar]
- Belhaj, D.; Frikha, D.; Athmouni, K. Box-Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): Partial characterization, in vitro antioxidant and antimicrobial activities. Int. J. Biol. Macromol. 2017, 105, 1501–1510. [Google Scholar] [CrossRef]
- Belhaj, D.; Elloumi, N.; Jerbi, B. Effects of sewage sludge fertilizer on heavy metal accumulation and consequent responses of sunflower (Helianthus annuus). Environ. Sci. Pollut. Res. 2016, 23, 20168–20177. [Google Scholar] [CrossRef]
- Bissell, M. Chronic liver injury, TGF-β, and cancer. Experim. Mol. Med. 2001, 33, 179–190. [Google Scholar] [CrossRef]
- Sanchez, V.; Valle, C.; Chavez-Tapia, N.; Uribe, M.; Mendez-Sanchez, N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Kamel, J.; Mellouli, L.; Nasri, M.; Ben Amara, I. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- Locckie, L.Z.; Meerman, J.H.N.; Commander, J.N.M.; Vermeulen, N.P.E. Biomarkers of free radical damage: Application in experimental animals and in human. Free Rad. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Ben Saad, H.; Driss, D.; Ellouz-Chaabouni, S. Vanillin mitigates potassium bromate-induced molecular, biochemical and histopathological changes in the kidney of adult mice. Chem. Biol. Inter. 2016, 252, 102–113. [Google Scholar] [CrossRef]
- Knebe, C.; Neeb, J.; Zahn, E.F. Propiconazole, Tebuconazole, and Their Mixture on the Receptors CAR and PXR in Human Liver Cells. Toxicol. Sci. 2018, 163, 170–181. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Zhou, G.; Lu, X.; Xu, Z.; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol. Res. 2003, 48, 151–155. [Google Scholar] [CrossRef]
- Pu, X.; Ma, X.; Liu, L. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr. Polym. 2016, 137, 154–164. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Hu, R.; Zhang, K.; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg. Med. Chem. Lett. 2006, 16, 2441–2445. [Google Scholar] [CrossRef]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Fan, Y.; Yan, P.; Li, S.; Zhou, X. The effect of boletus polysaccharides on diabetic hepatopathy in rats. Chem. Biol. Inter. 2019, 308, 61–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Hu, T. Metabonomic profiling in study hepatoprotective effect of polysaccharides from Flammulina velutipes on carbon tetrachloride-induced acute liver injury rats using GC–MS. Int. J. Biol. Macromol. 2017, 12, 149. [Google Scholar] [CrossRef]
- Lekshmi, V.S.; Arun, A.R.; Muraleedhara, K.G. Sulfated polysaccharides from the edible marine algae Padina tetrastromatica attenuates isoproterenol-induced oxidative damage via activation of PI3K/ Akt/Nrf2 signaling pathway—An in vitro and in vivo approach. Chem. Biol. Inter. 2019, 308, 258–268. [Google Scholar] [CrossRef]
- Guo, T.; Xu, H.; Zhang, L. In vivo protective effect of Porphyra yezoensis polysaccharide against carbon tetrachloride induced hepatotoxicity in mice. Regul. Toxicol. Pharmacol. 2007, 49, 101–106. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Badraoui, R.; Saoudi, M.; Hamadou, W.S.; Elkahoui, S.; Siddiqui, A.J.; Alam, J.A. Antiviral effects of Artemisinin and its derivatives against SARS-CoV-2 main protease: Computational evidences and interactions with ACE2 allelic variants. Pharmaceuticals 2022, 15, 129. [Google Scholar] [CrossRef]
- Akacha, A.; Badraoui, R.; Rebai, T.; Zourgui, L. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: A biochemical, docking and histological study. J. Biomol. Struct. Dynam. 2022, 40, 4341–4351. [Google Scholar] [CrossRef]
- Rahmouni, F.; Badraoui, R.; Ben-Nasr, H.; Bardakci, F.; Elkahoui, S. Pharmacokinetics and therapeutic potential of Teucrium polium against liver damage associated hepatotoxicity and oxidative injury in rats: Computational, biochemical and histological studies. Life 2022, 12, 1092. [Google Scholar] [CrossRef]
- Mhadhbi, N.; Issaoui, N.; Hamadou, W.S.; Alam, J.M.; Elhadi, A.S.; Adnan, M. Physico-Chemical Properties, Pharmacokinetics, Molecular Docking and In-Vitro Pharmacological Study of a Cobalt (II) Complex Based on 2-Aminopyridine. ChemSelect 2022, 7, e202103592. [Google Scholar] [CrossRef]
- Alreshidi, M.; Badraoui, R.; Adnan, M.; Patel, M.; Alotaibi, A.; Saeed, M. Phytochemical profiling, antibacterial, and antibiofilm activities of Sargassum sp. (brown algae) from the Red Sea: ADMET prediction and molecular docking analysis. Algal Res. 2023, 69, 102912. [Google Scholar] [CrossRef]
- Noumi, E.; Ahmad, I.; Bouali, N.; Patel, H.; Ghannay, S.; ALrashidi, A.A. Thymus musilii Velen. Methanolic Extract: In Vitro and In Silico Screening of Its Antimicrobial, Antioxidant, Anti-Quorum Sensing, Antibiofilm, and Anticancer Activities. Life 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jin, C.; Tong, Z. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016, 136, 187–197. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockyville, MD, USA, 2005. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 29, 21770–21776. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wu, F.J.; Du, L.; Li, G.Y.; Takahashi, K.; Xue, Y. Effects of polysaccharides from abalone (Haliotis discus hannai Ino) on HepG2 cell proliferation. Int. J. Biol. Macromol. 2014, 66, 354–361. [Google Scholar] [CrossRef]
- Van, V.F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends. Food Sci. Technol. 2002, 13, 73–92. [Google Scholar]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Huang, S.S.; Huang, G.J.; Lin, Y.H. Antioxidant and antiproliferative activities of the four Hydrocotyle species from Taiwan. Bot. Stud. 2008, 49, 311–322. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J. A new method for measuring antioxidant activity. Biochem. Soc. Trans. 1993, 21, 95S. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L.; Theron, K.I. Chemical and phytochemical properties and antioxidant activities of three pomegranate cultivars grown in South Africa. Food Bioprocess Technol. 2012, 5, 2934–2940. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Nilsson-Ehle, P.; Carlstrom, S.; Belfrage, P. Rapid effects on lipoprotein lipase activity in adipose tissue of humans after carbohydrate and lipid intake: Time course and relation to plasma glycerol, triglyceride, and insulin levels. Scand. J. Clin. Lab. Invest. 1975, 35, 373–378. [Google Scholar] [CrossRef]
- Bassole, I.; Ouattara, A.; Nebie, R. Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry 2003, 62, 209–212. [Google Scholar] [CrossRef]
- Zucconi, F.A.; Monaco, M. Phytotoxins during the stabilization of organic matter. In Composting of Agricultural and Other Wastes; Gasser, J.K.R., Ed.; Elsevier Applied Science Publication: New York, NY, USA, 1985; pp. 73–86. [Google Scholar]
- Chen, X.; Zhu, Q.; Li, X.; Huang, T.; Wang, S.; Wang, Y.; Chen, X.; Lin, Z.; Ge, R. Pubertal exposure to tebuconazole increases testosterone production via inhibiting testicular aromatase activity in rats. Chemosphere 2019, 230, 519–526. [Google Scholar] [CrossRef]
- Kammoun, I.; Bkhairia, I.; Ben Abdallah, F.; Jaballi, I.; Ktari, N.; Boudawara, O.; Nasri, M.; Gharsallah, N.; Hakim, A.; Ben Amara, I. Potential protective effects of polysaccharide extracted from Ulva lactuca against male reprotoxicity Induced by thiacloprid. Arch. Physiol. Biochem. 2017, 123, 334–343. [Google Scholar] [CrossRef]
- Council of European Communities Council Directive 86/609/EEC of 24 November, on the Approximation of Laws, Regulations and Administrative Provisions of the Member States regarding the Protection of Animals Used for Experimental and Other Scientific Purposes; Food and Agriculture Organization of the United Nations: Rome, Italy, 1986; Volume 358, pp. 1–18.
- Lowry, O.H.; Rosebrough, N.J.; Farr, L.A.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Chem. Biol. 1951, 193, 65–275. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods. Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Wolff, S.P. A discontinuous method for catalase determination at near physiological concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J. Biochem. Biophys. Methods 1996, 31, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Witko, V.; Nguyen, A.T.; Descamps-Latscha, B. Microtiter plate assay for phagocyte-derived taurine chloramines. J. Clin. Lab. Anal. 1992, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improve assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillete, J.R. Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4 bromobenzeneoxide as the hepatotoxic intermediate. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Gabe, M. Techniques Histologiques; Masson: Paris, France, 1968; pp. 838–879. [Google Scholar]

