1. Introduction
The oral administration route is the most common approach for the local and systemic therapeutic treatments of a wide range of pathologies [1,2,3]. It is the natural physiological pathway to incorporate nutrients into the body and the easiest way to administer drugs. Active compounds can be absorbed in three different sections of the gastrointestinal tract (GIT): the stomach, small intestine, and large intestine. The stomach is a structure specialized in decompounding the ingested food, but its capability for the absorption of drugs is limited. The small intestine is the section specialized in nutrient absorption due to its tremendous surface, where the drugs can easily penetrate by paracellular transport to the systemic circulation. Finally, in recent decades, the colon has been postulated as an area of high interest for drug delivery. The physiological characteristics of the colon and the high residence times in this area facilitate absorption, especially for drugs that are degradable by intestinal enzymes. Furthermore, colonic administration allows the local treatment of certain pathologies such as inflammatory bowel diseases (IBD) or colorectal cancer [4,5,6].
Successful colonic administration requires protection of the drug against different pHs, enzymes, microorganisms, or peristaltic movements through the GIT [6,7]. Currently, capsules or tablets with pH-dependent, pressure-dependent, or time-modified release coatings are used for colonic delivery. Once in the colon, the drug should be released from the delivery system at specific rates and solubilized in the tissues, avoiding toxic effects and getting an optimum pharmacological response [6,8]. Unfortunately, there are great intra-individual and inter-individual variabilities due to the gastric and intestinal transit times, volumes of liquid through the GIT, diet, food–drug interactions, pathologies, gender, or age of patients that compromise the efficacy of the formulations [9].
Polysaccharides have been proposed as the main excipients for colonic formulations, as coating materials, matrices, hydrogel precursors, or prodrug ingredients [6,10,11]. Their physicochemical properties confer on them enzymatic and/or pH resistance. This allows polysaccharides to pass unaltered through the GIT, thus protecting the drug. Then, once in the colonic area, polysaccharides are decomposed by a microbiota composed of a huge number of microorganisms (1011 CFU/g fecal content), allowing the complete release of the drug [10,11]. Polysaccharides also have some industrial advantages, like their abundance and low price, biodegradability, and non-toxicity for the environment [11,12,13].
Hydrogels can be formed from polysaccharides by physical or chemical crosslinking, giving rise to three-dimensional systems of high porosity that can host and protect drugs and then release them in response to different stimuli [14,15,16]. Dry gels are advantageous over hydrogels in terms of drug stability as they are less susceptible to microbiological and chemical degradation [17]. Polysaccharide-based hydrogels can be dried using various methods, and their choice extraordinarily affects the structural properties of the resulting dry gel. Heat drying results in xerogels with significant structural shrinkage, and freeze-drying yields cryogels suitable for certain pharmaceutical applications like oral disintegrating formulations (e.g., ODTs) or scaffolds [18,19]. Supercritical fluid (SCF) drying produces dry gels with a preserved porous nanostructure (the so-called aerogels) and different formats and sizes depending on the chosen preparation technique [20].
Aerogels are dry solids with extremely low weight and bulk density (<0.2 g/m3), high porosity (>90%) predominantly in the mesoporous range (2–50 nm), and high specific surface area (>200 m2/g) [20,21]. They can be prepared from a wide range of inorganic and organic sources, including polysaccharides. The initial mesoporosity of the wet structures is preserved in the aerogels, which gives them a high surface area and excellent drug-loading capacity in the amorphous state [20]. A significant proportion of newly discovered New Molecular Entities (NME) fail to realize their full clinical potential, primarily because of their limited aqueous solubility, stability issues, and, in many instances, inadequate tissue targeting properties. The possibility of loading and stabilizing drugs in aerogels in an amorphous state opens new perspectives for the development of pharmaceutical formulations with poorly soluble NME. Its incorporation into mesoporous structures that remain in a metastable state would improve its dissolution rate, overcoming this problem [22].
A number of aerogel formulations for drug delivery have been recently investigated and proposed for different applications, such as pulmonary drug delivery [23], wound healing [24], or oral administration of drugs [20]. Various polysaccharides, such as alginate, pectin, or starch, among others, processed using various technologies such as emulsification or drip-gelation, have demonstrated their usefulness in producing aerogel particles that can incorporate drugs in their amorphous state. Furthermore, they exhibit modified drug release profiles, offering promising opportunities for enhancing drug absorption and stability [22,25,26].
The enzymatic- and/or pH-resistance of certain polysaccharide aerogels also makes them potential candidates for the development of colonic delivery dosage forms [6]. This approach is attractive for delivering biologics like peptides, given their reduced susceptibility to proteolytic degradation in the colon compared to other sections of the GIT. Additionally, the extended residence times and favorable pH levels in this region contribute to improved bioavailability [27,28]. However, the requirements of colonic formulations are higher than those of conventional oral dosage forms (burst release) [15]. The release of the drug must be specific and controlled in the colonic area. Therefore, the formulation must bypass the aforementioned adverse conditions of the GIT until reaching the colon and then release the drug at the appropriate rate to ensure that no toxic effects or problems of therapeutic inefficacy occur [29].
This review explores recent advances in porous polysaccharide-based formulations for oral drug delivery, providing a critical assessment of aerogels’ potential for colonic drug delivery for the first time. The sections of this article will cover an overview of the GIT characteristics with a special focus on the large intestine, the selection of suitable polysaccharides (alginate, chitosan, pectin, cellulose, starch, and glucomannan, among others) for colonic drug delivery, as well as the analysis of formulation strategies for aerogel design and prior art on aerogels with potential colonic application, including their preparation methods. Finally, alternative technological approaches for producing aerogels with appropriate properties for colonic drug delivery will be presented as future perspectives.
2. Gastrointestinal Conditions: An Overview and Essential Considerations
Digestion through the GIT is a complex process involving enzymes, pH ranges, microorganisms, and movements designed to process food and absorb nutrients. The pH values, microbiomes, and transit times are shown in Figure 1, and the main enzymes implicated in the digestion are disclosed in . Oral dosage forms for colonic drug delivery need to withstand adverse conditions throughout the GIT until reaching the colon and then releasing the drug payload into the tissues before being eliminated [3].
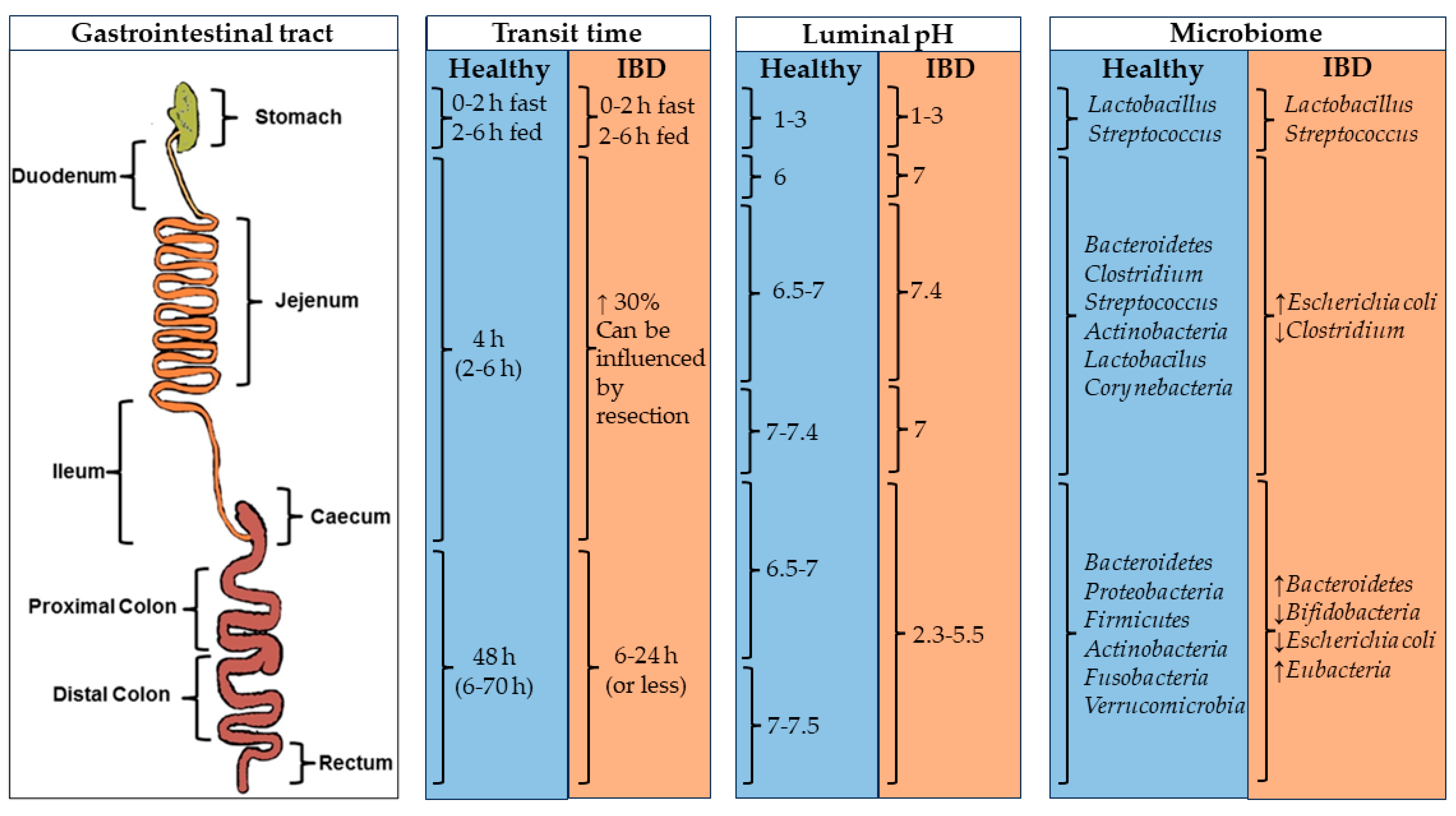
Figure 1. Physiological factors (transit time, luminal pH, and microbiome) in the GIT of healthy and IBD patients influence oral drug delivery. Image adapted from [3].
Each section within the GIT is characterized by a different pH, residence time, ionic force, enzymatic conditions, and gut pressure [3,11]. The upper GIT starts in the mouth, where the food components are chewed and mixed. After that, the food is transported to the stomach through the pharynx and the esophagus. In the stomach, different mixing movements and the low pH (approximately 1–3) allow the degradation of the food content. The residence time of the compounds in the stomach depends on the diet and the gastric state (fast state: 0–2 h; fed state: 2–6 h). After that, the chyme is transported from the stomach to the duodenum, where it is digested by many enzymes at a pH of ca. 6 [3,30,31].
The lower GIT comprises the rest of the small intestine (i.e., jejunum and ileum) and the large intestine [3]. In the small intestine, the efficient absorption of digested nutrients is facilitated by its substantial surface area, which, in healthy individuals, allows for a residence time of 4 h. The pH values in this region are around 7. Finally, in the colon, non-digestible food undergoes water and nutrient processing, with fecal content moving slowly through the colon segments. Bacterial activity in the large intestine, driven by enzymes not produced by the human body, degrades polysaccharides, fatty acids, and active compounds. This process usually takes around 48 h but can last up to 70 h in some cases and is shortened to 6 h or less in pathological conditions. Finally, residues accumulate in the rectum and are expelled as stools [30,32]. pH levels in the cecum and ascending colon range from approximately 6.5 to 7.0. As transit progresses through the large intestine, pH levels increase to 7.5 in the sigmoid colon and rectum. Generally, the pH values tend to be lower in pathological states compared to healthy conditions.
Recently, the microbiota has gained special attention due to its impact on the therapeutic activity of certain treatments [36,37,38] and its potential involvement in the development of chronic diseases, such as IBD [39], Parkinson’s [40], or Alzheimer’s [41]. GIT sections are colonized by different types of microorganisms. From a digestive perspective, the microbiota plays a crucial role in breaking down components that resist digestion in preceding sections of the GIT due to enzymatic systems. Also, certain microbiota metabolites, such as short-chain fatty acids, serve as a source of energy for enterocytes [3,42].
Dietary choice influences the microbiota [42]. While microorganisms inhabit the entire GIT from mouth to anus, the major microbial population grows under anaerobic conditions in the colon. The mouth microbiota has a remarkable diversity of microorganisms (>1000 species), including Bacteroidetes, Proteobacteria, Streptococcus, Escherichia, Clostridium, Firmicutes, Actinobacteria, Spirochaetes, and Fusobacteria, the main phyla. The microbiota variety and population decrease in the stomach due to the enzymatic activity and the low pH. However, the bacterial population gradually increases again until it reaches the colon [43].
In the colon, the bacterial population increases significantly, reaching levels of up to 1 × 1011 CFU/g of wet content. Assuming a colon volume above 400 mL and a wet content density of 1 g/mL, the number of bacteria in the total colon volume would be around 4 × 1014 units [44]. The main species in this area are Bacteroidetes, Proteobacteria, Firmicutes, Actinobacteria, Fusobacteria, and Verrucomicrobia spp. [3,31,42]. Although the bacterial population remains relatively constant throughout the colon within individual patients, there is substantial interpersonal variability in terms of biodiversity. Nevertheless, microbial functions remain remarkably consistent among different patients [42].
Colonic bacteria, in symbiosis with their host, produce numerous enzymes that decompose dietary polysaccharides and peptides into lactate and short-chain fatty acids (SCFAs). Colonocytes use these SCFAs for energy and metabolism [45,46,47]. Key enzyme groups include glycoside hydrolases and the polysaccharide lyases primarily produced by the Bacteroidetes and Firmicutes bacteria phylum [45,46,48]. The Azoreductase family, which specializes in clearing azo groups, is also significant and has potential applications in prodrug development, ensuring drug release specifically in the colonic area [49].
GIT fluids should be particularly taken into account when designing colonic forms. These fluids vary in volume and composition based on multiple factors such as GIT section, diet, or GIT state (fed or fasted) [47,50]. Generally, stomach fluid content is higher in the fed state (686 mL) and decreases during digestion (45 mL). In the small intestine, the fasting state has more water (105 mL) compared to the postprandial state (54 mL). Finally, in the colon, the fasting state has slightly more liquids (13 mL) than the fed state (11 mL) [50,51]. Factors like the number of pockets of liquid in the colon, their water content, and their distribution significantly affect drug administration in the colonic region, as they influence the disintegration and dissolution processes of the dosage forms [50,51].
3. Polysaccharides as Carriers for Colonic Delivery
Several strategies have been explored to achieve colonic drug delivery with oral dosage forms [6,7,32]. An effective approach involves the use of polymers capable of withstanding pH variations across the GIT (pH 1 to 7) to ensure the release of the drug, specifically in the colon. These polymers include a wide range of derivates of acrylic and methacrylic acids (markets like Eudragits®), or derivates of cellulose, like hydroxypropyl methylcellulose phthalate (HPMCP), soluble with pHs above 5.5, or cellulose acetate phthalate, also called cellacefate, soluble with pHs above 6.2 [52,53,54]. Another option is the use of time-release dosage forms, given the extended colon residence time compared to other segments of GIT [7,55]. Extensive research has also been conducted on the use of prodrugs to reach the colon, avoiding absorption in the upper GIT. Prodrugs are drugs covalently bound to a ligand involving various hydrophilic and lipophilic functional groups or specific sites on cellular transporters. After their administration, the active compound is activated through the cleavage of the bond between the ligand and the drug mediated by an enzymatic process that involves hydrolytic enzymes (such as carboxylesterases, azoreductases, or phosphatases), oxidoreductases, transferases, and liases from various colonic bacteria [56,57]. Another suitable technological alternative is the use of resistant polymers against human enzymes to retain the drug content until reaching the colon, where a diverse range of bacterial enzymes can digest the dosage forms [7].
Some polysaccharides are excellent candidates as polymeric excipients for specific colon treatments due to their resistance to gastrointestinal enzymes. The unique glycoside bonds of polysaccharides prevent degradation by gastrointestinal enzymes, allowing the polysaccharide-based dosage forms to reach the large intestine intact. There, the enzymatic system of the colon microbiota breaks the glycoside bonds, facilitating drug release. This property gives them suitable characteristics to produce various forms of colonic administration, including coated tablets, multiparticulate systems, and hydrophilic matrix tablets [4,11,47].
Polysaccharides are readily available from natural resources such as plants, algae, animals, or microorganisms , and they can be easily modified to suit specific needs [58,59]. These biodegradable biopolymers are recognized as GRAS (Generally Recognized As Safe) materials by the FDA (Food and Drug Administration) and offer versatility in formulating drug delivery systems for the colon.
3.1. Alginate (Alg)
Alginate is a linear anionic polysaccharide known for its ability to form gels when crosslinked with different divalent and trivalent cations (affinity order is Pb2+ > Cu2+ > Cd2+ > Ba2+ > Sr2+ > Ca2+ > Fe3+ > Co2+, Ni2+, and Mn2+). Among them, calcium cation is the most used due to its good affinity and low toxicity [17,73]. The crosslinking process relies on coordination bonds between oxygen atoms from the guluronic monomers (G-blocks) and the cation. Particularly, the oxygen atoms of two G-blocks of two different polymer chains form a hydrophilic space where the cation can be introduced, generating a 3D network. The model that describes this interaction is known as the “egg-box” model [17]. The strength of these hydrogels can be influenced by the G/M ratio, as it affects the formation of “egg-box” structures [59,60].
Different alginate gel formulations were developed to target the colonic tissue. Core–shell particles consisting of a core of alginate of varying polymer concentrations, loaded with sodium naproxen, and coated with Eudragit S100 were developed by the emulsification method [74]. The purpose of the Eudragit S100 coating was to safeguard the alginate in the stomach and duodenum, ensuring the release of naproxen in the colonic area. In vitro release tests in three different solution media (pH 1.2, 6.8, and 7.4) revealed variations in drug release rates among formulations (Figure 2). Complete release was achieved in all formulations, although a reduced release rate was observed as the concentration of Eudragit® S-100 increased. Formulations effectively retain drug content within the acidic solution at pH 1.2, exhibiting minimal release as pH gradually rises to 6.8. The majority of drug release occurs only after the pH surpasses 7, requiring a minimum of 2 h of lag time to initiate the drug release.
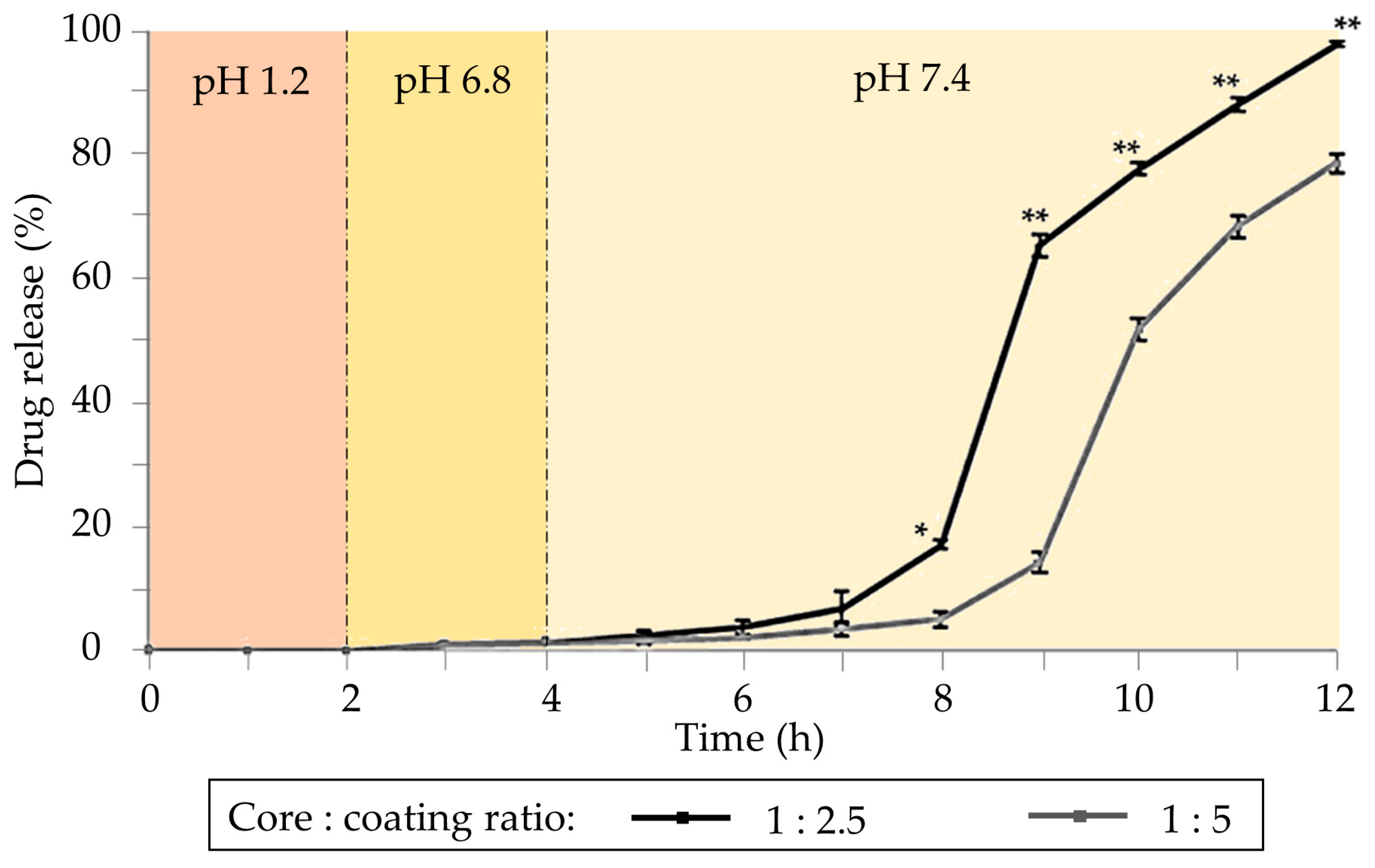
Figure 2. Cumulative drug release from coated formulations. The core is based on drug/alginate (1:6 weight ratio) coated with solutions of Eudragit® S-100 with different concentrations (2.5% and 5% w/v). Notation: * Statistically significant difference at p < 0.001. ** Statistically significant difference at p < 0.0001 from 1:5 sample as determined by Student’s t-test. Image adapted from [74].
Hybrid calcium alginate (CA) and carboxymethyl cellulose (CMC) beads were developed to modulate the release of 5-fluorouracil in the GIT [75]. Dry beads were produced by dropping mixtures of Alg (1.8% w/v) and CMC (0%, 0.5%, or 1% w/v) onto CaCl2 solutions (2% w/v), followed by an aging process involving a mixture of glutaraldehyde, ethanol (EtOH), and hydrochloric acid (HCl). These beads effectively loaded 5-fluorouracil through swelling and exhibited remarkable control of the drug release in an acidic medium (pH 1). At pH 7.4 and 6.8, the formulations experienced a burst release for the first hour, followed by sustained drug release lasting for 100 h. The addition of enzymes to the dissolution media promoted drug release after the first 24 h. Higher CMC concentrations in the formulations resulted in higher drug loadings and slower drug releases. Finally, these formulations released most of their drug content in the simulated colonic fluids.
3.2. Chitosan (CS)
Chitosan is the only semisynthetic cationic polysaccharide [47,59]. There are several varieties of chitosan of different molecular weights (MW) and deacetylation degrees (DD) ranging between 70 and 98%, depending on the specific alkaline treatment applied to the chitin [59,76]. CS has versatile applications in tissue engineering, wound healing, drug products (small molecules, DNA, and peptides), vaccines, cosmetics, and cancer diagnostics [61]. Its mucoadhesive properties make it an ideal candidate for colonic drug delivery, facilitating ionic interaction with the mucus wall (negatively charged) due to its positive charge [77]. In addition, CS acts as a permeation enhancer for transdermal drug delivery [78,79].
Nanoparticles (NPs) based on CS, or thiolate–chitosan, and alginate were prepared by the ionotropic gelation method [80]. These NPs were designed for the treatment of colorectal cancer and loaded with α-mangostin, an active compound with anti-inflammatory and antioxidative properties. Genipin was used as a crosslinking agent. In some formulations, Eudragit® L-100 was added dropwise to the preparation solution to coat the NPs. The formulations crosslinked with genipin reduced the burst release to pH 1.2, whereas the Eudragit coating favored a controlled drug release at pH values over 1.2 and especially at pH 6.8 (50% drug released after 8 h). Additionally, these nanoparticles exhibited promising mucoadhesive properties, making them potential candidates for the treatment of colonic diseases.
3.3. Pectin
Pectin is an anionic heteropolysaccharide, also proposed for preparing colonic drug delivery systems as tablets, beads, pellets, or microparticles. It stands out for its resistance to degradation by intestinal enzymes while being susceptible to breakdown by colonic microflora [14,59]. The gelation properties of pectin and the behavior of pectin gels in gastrointestinal fluids depend on their degrees of amidation (DA) and esterification (DE). Low ester pectins (DE < 50%) can gel in the presence of calcium cations, while high ester pectins (DE > 50%) require acidic conditions and additional sugars to reach gelation. Similarly, the amidated groups within the pectin structure can enhance gelation in conjunction with calcium ions [14,81].
Core–shell polysaccharide microparticles containing betamethasone were prepared by coaxial prilling as a colonic formulation [82]. Amidated low-methoxy pectin was used as the core to preserve the drug, and alginate crosslinked with zinc was selected as the coating material. This type of coating exhibits high resistance against acid environments but degrades in the pH conditions of the small intestine. Once the colon was reached, the formulation could release betamethasone due to the degradation of pectin.
Pectin–silica beads containing mesalazine for IBD treatment were prepared [83]. Various pectin sources (Silene vulgaris callus pectin (SVC), Lemna minor callus pectin (LMC), and commercial apple pectin (AU)), crosslinking times (5 and 60 min) using 0.34 M CaCl2, and silica concentrations (0, 6.4, and 22.2 mg/mL) were used. These beads, of an average size of 1 mm, exhibited a colon-specific release profile of mesalazine, facilitated by a thin silica layer on their surface. Interestingly, this coating is formed spontaneously during the crosslinking process. The best-sustained release profiles were observed for those colonic formulations based on SLV and LMC pectins, in combination with the higher concentration of silica and 60 min of the aging process (Figure 3). This can be attributed to lower methyl-esterified groups in LMC and SVC pectins compared to the AU variety, enhancing their resistance to low pHs.
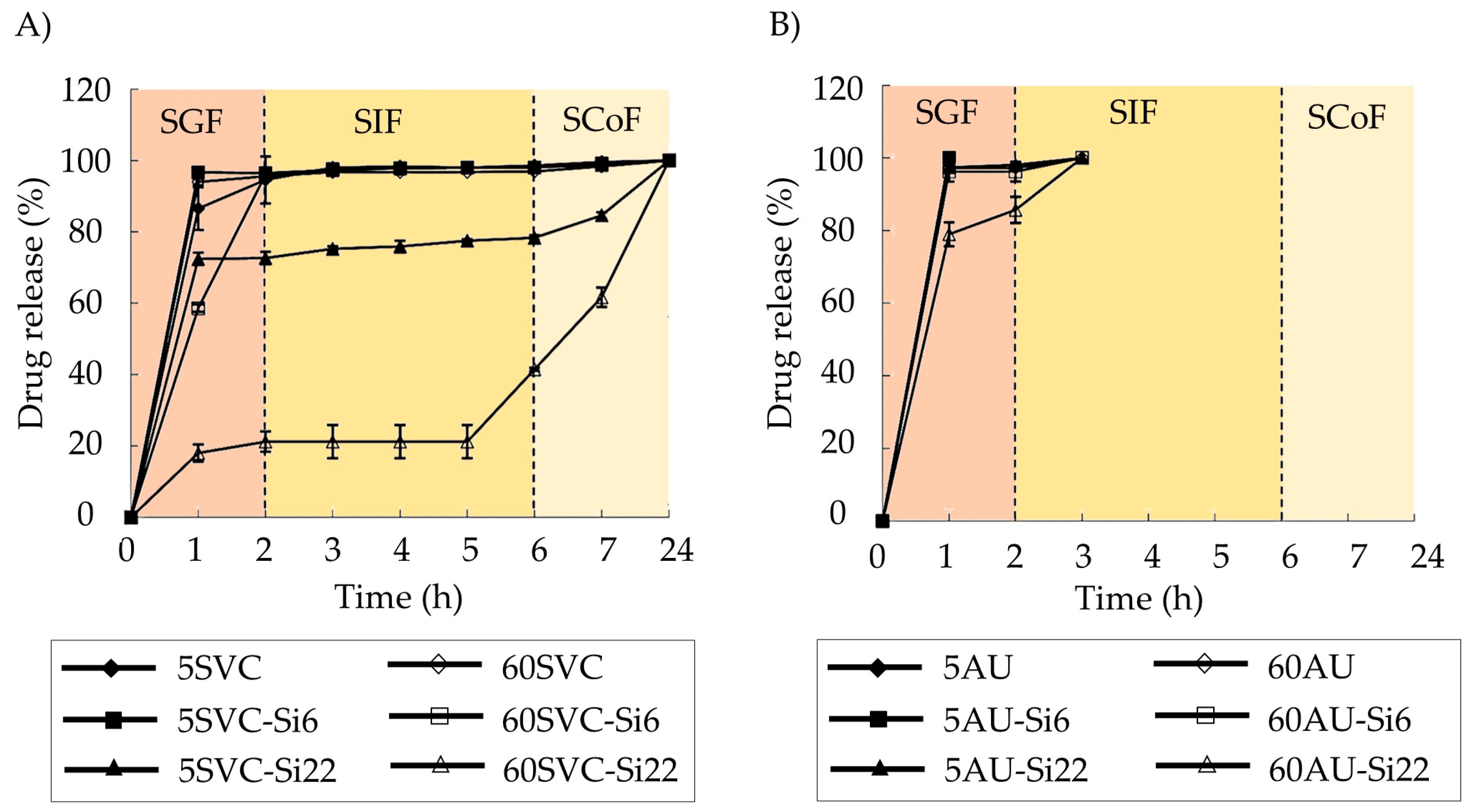
Figure 3. Mesalazine release profiles in the simulated digestive fluids for calcium pectin–silica and calcium pectinate gel beads based on the SVC (A) and AU701 (B) pectins. The formulations with high silica concentrations (22.2 mg/mL, Si22) and extended crosslinking times (60 min, 60) demonstrated the most suitable drug release profiles for colonic drug delivery applications. Image adapted from [83].
3.4. Cellulose
Cellulose is the most abundant homopolysaccharide on earth and has been widely used as an excipient in pharmaceutical formulations. Its modification by substitution of hydroxyl groups of the glucose units to include ether groups (methylcellulose, ethylcellulose, carboxymethylcellulose, hydroxyethylcellulose) or ester groups (acetate, nitrate, or sulfate groups) gives the molecule great variations in solubility, chemical structure, or gelation properties, opening possibilities for new applications in the biomedical field [62].
Coated cellulose-based pellets designed for the treatment of IBD were prepared using extrusion/spheronization [84]. Wet mixtures comprising microcrystalline cellulose (MCC), Carbopol 940 (CP940), high-substitute hydroxypropyl cellulose (H-HPC), sodium chloride (10.68% w/w), and specific amounts of either cyclosporine (2% w/w) or curcumin (4% w/w) were extruded, spheronized, and dried. Subsequently, pellets were coated with Eudragit® S100 using a fluidized bed coater. The most effective formulation, exhibiting superior bioadhesive properties, consisted of 71.82% w/w of MCC, 5.75% w/w of CP940, and 5.75% w/w of H-HPC. Dissolution release profiles revealed that the most suitable pellets for colonic drug delivery were those coated with 20% weight gain relative to the initial formulation. These coated pellets maintained all drug content at pH 1.2 and promoted a constant drug release for 22 h in the intestine.
3.5. Starch
Starch is a polysaccharide widely used in the pharmaceutical industry as a disintegrant or binder in solid dosage forms [64]. Some varieties of resistant starch have been postulated as excipients for oral dosage forms for colonic drug delivery. They are obtained by retrogradation, a type of crystalline reorganization resulting from their heating in solution at high temperatures and subsequent cooling. Retrograded starches have limited hydration properties, making them less accessible to human enzymes and, therefore, less digestible than other starch varieties [47].
OPTICORE™ is a multilayer technology based on resistant starches and Eudragit® S coating, especially designed to target the colonic area [85]. Resistant starches allow drug release triggered by colonic enzymatic activity and Eudragit® S by the pH variation in the medium (Figure 4A). An additional alkaline layer accelerates drug release. OPTICORE™ system (F14) starts the release 1 h after achieving simulated colonic fluids, while in conventional formulations based on Eudragit® S (F1), the drug release starts 2 h after reaching the colon, increasing the possibility of failure of the treatment (Figure 4B). Also, when the formulations are prepared without starch and evaluated in a human fecal slurry (F2 had the outer layer of the OPTICORE™ formulation without the alkaline layer, and F3 was composed of an alkaline layer and an Eudragit® S layer), drug release is achieved only with formulations with resistant starch in their composition (Figure 4C). This was due to this fecal medium having a pH of 6.8, representative of the pH medium of the large intestine, which is insufficient to degrade the Eudragit® S layer. Consequently, the formulation that guarantees a sustained release with a complete release independently of the pH conditions is based on OPTICORE™.
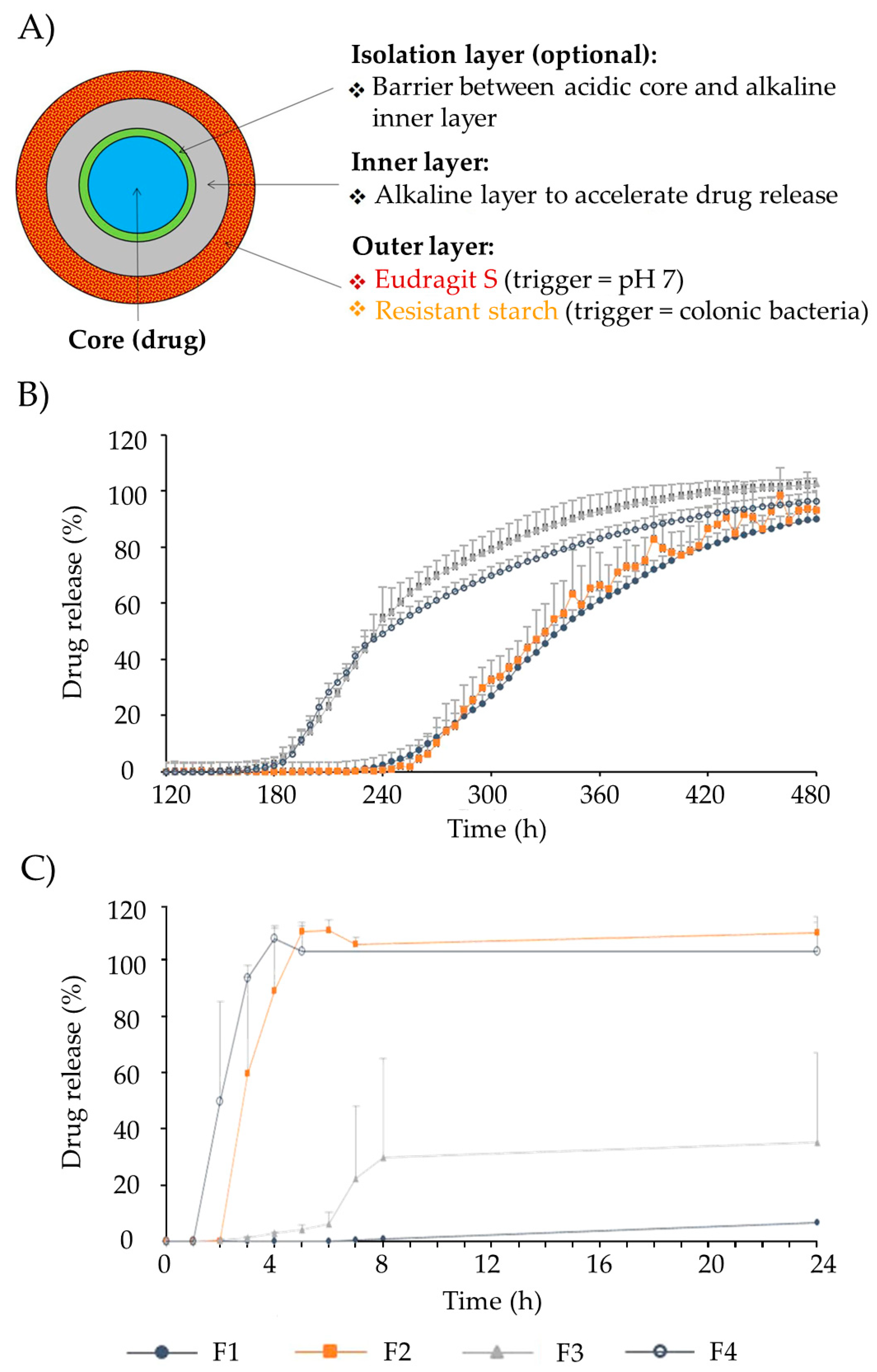
Figure 4. (A) Representation of the different layers that comprise the OPTICORE™ drug tablet system. (B) Effect of the starch-Eudragit outer layer on drug release patterns from several formulations, as follows: (F1) Single layer Eudragit® S coating; (F2) Phloral layer (resistant starch and Eudragit S, without alkaline layer); (F3) Inner layer neutralized Eudragit® S and outer layer Eudragit® S; (F4) Outer layer Phloral™ (OPTICORE™) in Krebs buffer solution (pH 7.4). (C) Effect of the starch-Eudragit outer layer in drug release from formulations (mentioned in 7.B) in fecal human slurry (pH 6.8). Image adapted from [85].
3.6. Konjac Glucomannan (KGM)
Konjac glucomannan is a non-ionic polysaccharide widely used in traditional Asian medicine due to its laxative, anti-obesity, antidiabetic, and anti-inflammatory properties. Upon hydration, KGM has a unique capacity to absorb water and expand significantly, improving peristaltic movements. Furthermore, hydrated KGM gel prevents the absorption of fatty acids and different sugars in the GIT, reducing hyperglycemic peaks [66]. KGM has been used for different biotechnological and chemical applications within the biomedical field, like tissue engineering [86], cosmetics [87], or the encapsulation of bioactive compounds [88].
Hydrophilic matrices based on mixtures of KGM, xanthan gum, and sucrose have shown adequate properties to achieve colonic drug delivery (Figure 5) [89]. These types of matrices controlled the release of highly soluble drugs, such as diltiazem, for long periods of time. KGMs from different origins have shown variations in the control of diltiazem release (Figure 5). Both matrices of Japanese KGM (Figure 5A.i) and American KGM (Figure 5B.i) under certain proportions in the formulation allowed the control drug release. The addition of β-mannanase to the dissolution medium containing J7 or A7 formulations (Figure 5A.ii,B.ii) accelerated the release of the drug, especially for the Japanese KGM (Figure 5A.ii), which has demonstrated the specificity of the formulation for the colonic area.
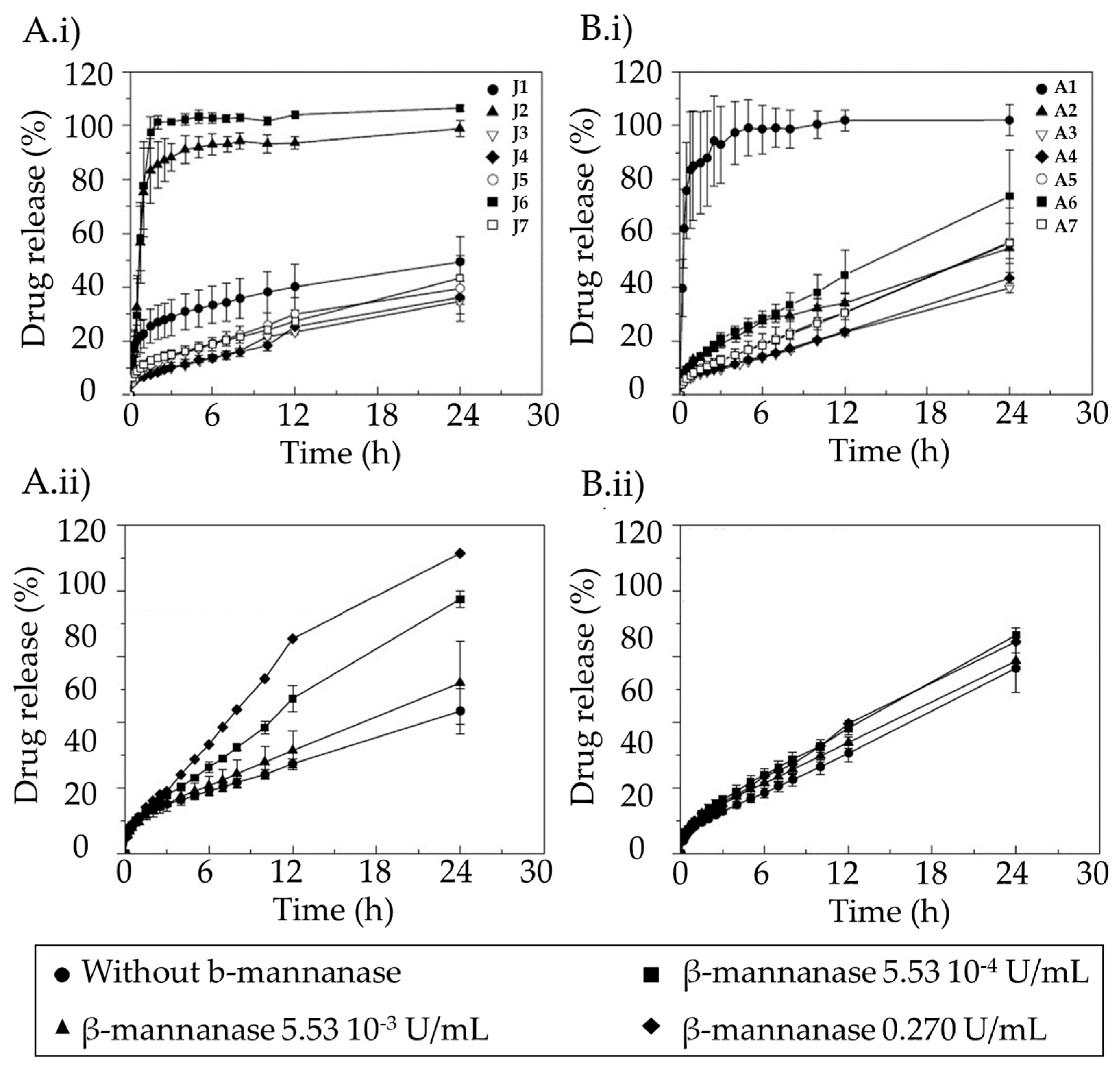
Figure 5. Dissolution profiles of (A.i,B.i) diltiazem into the simulated intestinal fluid (SIF) without β-mannanase and release profiles of the drug from formulations prepared with Japanese KGM (A.i,A.ii) and American KGM (B.i,B.ii), and (A.ii,B.ii) containing various concentrations of β-mannanase for formulations J7 (A.i,A.ii) and A7 (B.i,B.ii). Image extracted from [89].
3.7. Other Polysaccharides
Other polysaccharides of marine (agar), microbial (xanthan gum), and plant (guar gum and locust bean gum) origins have also been studied for colonic administration purposes [90].
Agar has the remarkable ability to create strong gels when an aqueous suspension is prepared and subjected to a heating process between 65 and 100 °C, followed by a cooling step (4–8 °C) to achieve complete and homogeneous gelation [69,91]. This technique has been widely applied in the formulation of various hydrogels designed for delivery systems [92]. These structures show exceptional resistance to disintegration in SIF, and their adequate coating with chitosan allows probiotics to reach the colonic area.
Xanthan gum has a polyanionic composition and is soluble in hot and cold water [59,69]. Its combination with other polysaccharides, such as locust bean gum or agarose, and its processing by autoclaving allow the formation of hydrogels with diverse mechanical properties that have been studied as delivery systems [69,93].
Guar gum is a polysaccharide extracted from the seeds of Cyamopsis tetragonolobus, known for its high stability against gastrointestinal fluids, making it an ideal candidate for use in coatings or as matrix systems [47]. Colonic administration systems employing guar gum can be prepared by compression [94,95] or by employing water-in-oil emulsification with a simultaneous incorporation of acrylic acids [96] or succinic anhydride [97].
New derivatives have also been produced from existing polysaccharides. An example is dextrins, which are produced by partial acidic or enzymatic hydrolysis of starch, resulting in low-molecular-weight amylose or amylopectin chains [98]. They can be chemically modified to create pH-responsive coatings for drug delivery systems, thus allowing controlled release in specific environments [99].
4. Polysaccharide-Based Aerogels as Dry Carriers for Colonic Delivery
There is a paucity of information on polysaccharide-based aerogels for oral drug administration, with even more limited data on their colonic administration applications. However, they offer several advantages, such as their economical and environmentally friendly preparation, which makes them interesting materials for this purpose. Their mesoporous structure allows them to keep the active compounds in the amorphous state, thereby enhancing the drug solubility characteristics beyond what conventional oral pharmaceutical forms can achieve [20]. For colonic administration, polysaccharide-based aerogels can also be designed as matrix systems whose enzyme-resistant properties serve to preserve the drug until it reaches the colon [47]. Alternatively, they can be developed as release-modulated coated systems, with drug release controlled by the disintegration induced by the colonic flora [47]. These benefits enable lower administration doses to achieve the desired therapeutic effects, reducing side effects and treatment costs. Furthermore, the specific drug delivery in the colonic area should allow the local treatment of pathologies such as IBD or colon cancer [100].
The subsequent sections provide an overview of the available information regarding the methodologies for preparing polysaccharide-based aerogels and loading them with drugs of different solubility characteristics. Furthermore, these sections will explore the ongoing advances in the design of dosage systems with aerogels, whether monolithic or multiparticulate. Special mention will be given to the types of drug delivery achieved and their potential adaptation for colonic applications. Finally, the topic of aerogel coating will be addressed as an additional step to successfully reach delivery in the colonic region.
4.1. Polysaccharide-Based Aerogel Preparation
The preparation of aerogels typically follows a stepwise procedure, encompassing sol–gel preparation, gel crosslinking, solvent exchange, and solvent removal (Figure 6A) [101]. Polysaccharide-based sol–gels are prepared by dispersing solid polymers in an aqueous medium. Subsequently, crosslinking can be induced by employing methods such as pH-induced gelation, non-solvent-induced phase separation (NIPS), temperature-induced gelation, covalent crosslinking, or ionotropic gelation (Figure 6B), depending on the functional groups in the polymer molecule structure [17,20,102]. Gelation significantly influences the textural properties of the aerogel, determined by the union between the molecular chains of the polysaccharides [17,18,20,103].
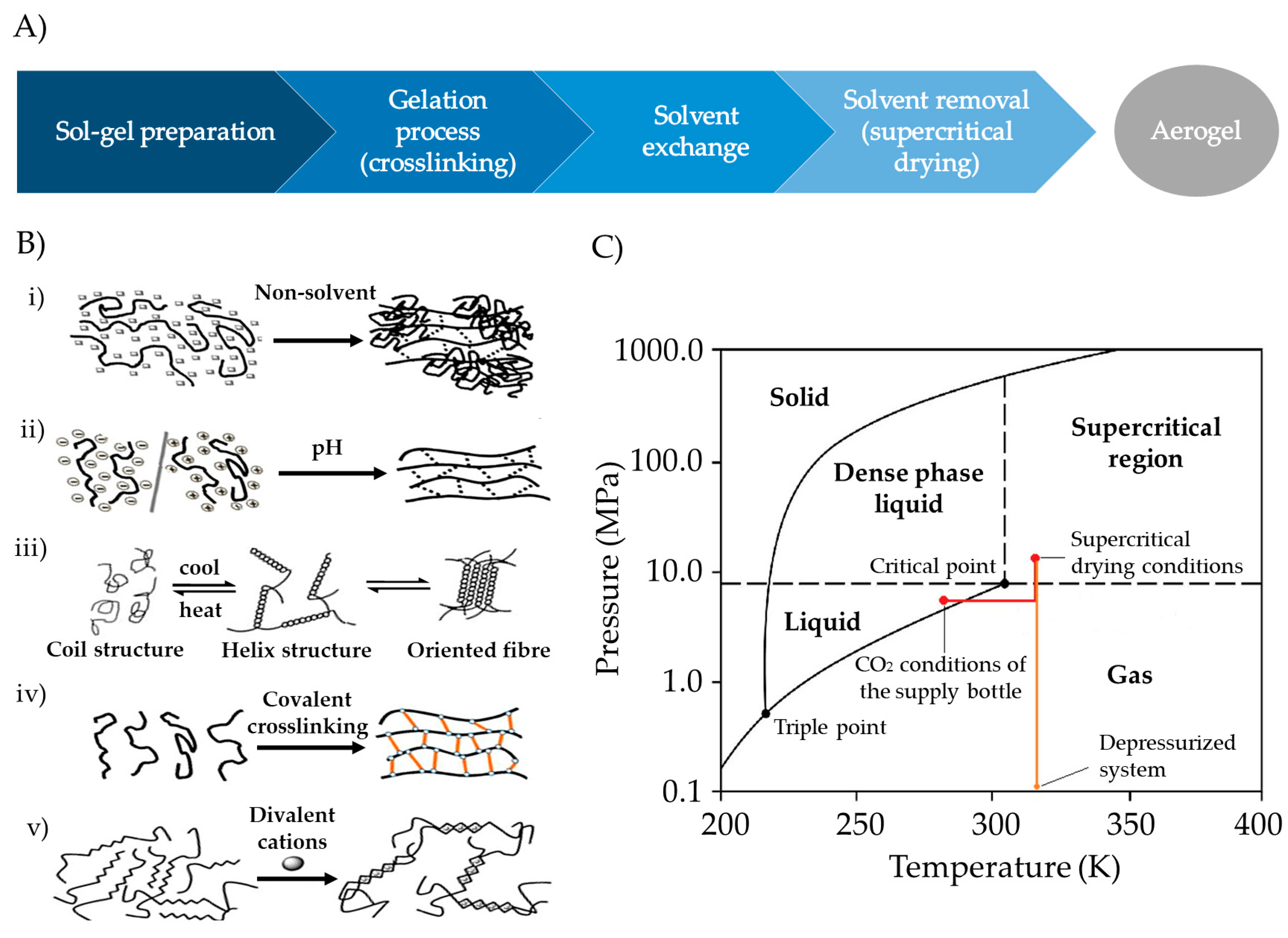
Figure 6. (A) Stepwise procedure for polysaccharide-based aerogel preparation. (B) Crosslinking techniques for gelation: (i) NIPS, (ii) pH-induced gelation, (iii) temperature-induced gelation, (iv) chemical gelation, and (v) ionotropic gelation. Image adapted from [17]. (C) CO2 phase diagram used for the SCF drying process. CO2 is continuously pumped and heated until SCF drying temperature and pressure conditions are achieved. After drying, depressurization must be carried out at a specific temperature and flow rate until the atmospheric pressure is reached. Image adapted from [107].
After preparing a gel, different techniques can be used for drying, but many of them struggle to counteract the capillary forces that may lead to the collapse of the mesoporous structure within the gel. Among them, vacuum drying or oven drying results in materials known as xerogels, while freeze-drying typically yields cryogels [18,104]. Alternatively, aerogels are usually obtained by SCF drying with supercritical CO2 (scCO2). Due to the reduced capacity of CO2 to solubilize water, SCF drying with scCO2 requires an additional step of replacing the water in the hydrogel with alcohol (solvent exchange), resulting in an alcogel [17,20,81]. Next, ethanol is removed from the alcogels in a high-pressure autoclave using scCO2 (Figure 6C) [105,106,107]. scCO2 demonstrates superior permeation within porous structures compared to liquids and shows greater solvation capacity for dissolving substances than gases. Consequently, they can effectively eliminate the solvent from gels, avoiding capillary forces and preserving the mesoporous structures of the original wet material [18,105,108].
4.2. Drug Loading within Aerogels Impregnation Technique
Drug loading into the aerogel structures can be carried out at different steps of the production process: into the sol–gel mixture, during the solvent exchange, or during the SCF drying (Figure 7). The solubility of the active compound is a key parameter in selecting the loading strategy and determining the drug loading efficiency. Hydrophilic drugs are good candidates to be loaded into the gel solution prior to the crosslinking process. Hydrophobic drugs can be loaded during solvent exchange or SCF drying. Moreover, drug loading can be performed after SCF drying by adsorption–precipitation method (supercritical impregnation) [20,109].
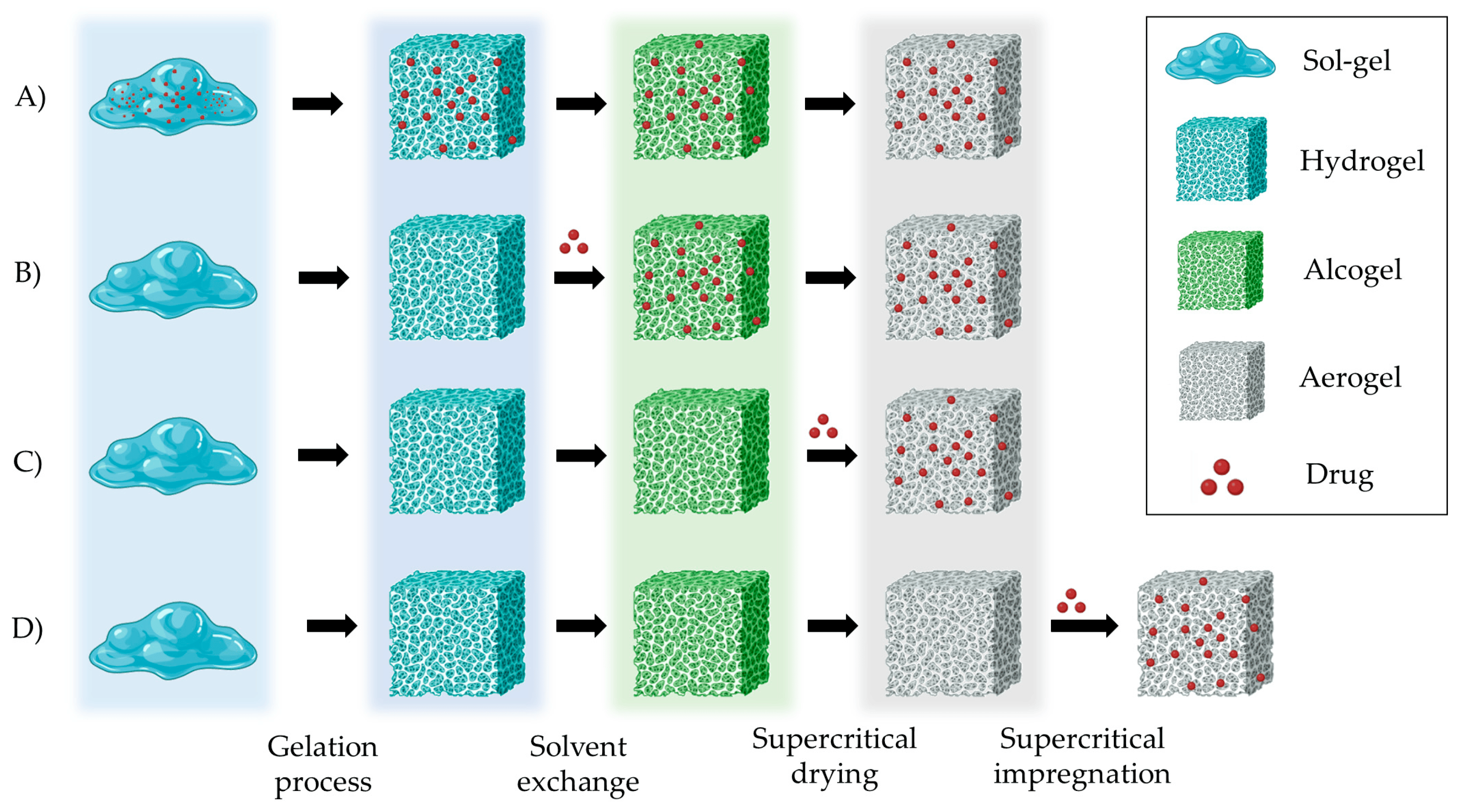
Figure 7. Strategies for preparing drug-loaded aerogels. (A) Incorporating the drug into the gel solution; (B) Adding the drug into the solvent for loading in the gels by solvent diffusion; (C) Adding the drug during the SCF drying process; (D) Loading the drug into the pre-prepared aerogels by impregnation technique. Notation: Red dots represent the drug that is loaded in the aerogel carrier.
The supercritical impregnation process is typically carried out by conditioning the aerogel and the active substance in permeable cartridges in a high-pressure autoclave. Injection of supercritical CO2 at a suitable pressure and temperature for a certain time (typically several hours) will achieve equilibrium between the drug-saturated scCO2 and the internal porous matrix of the aerogels. Controlled depressurization is necessary to return to atmospheric conditions and eliminate CO2 [20,109]. A successful impregnation process requires a careful definition of various factors. Initially, it is crucial to ensure that the drug is soluble in scCO2. Consequently, potential alterations in temperature and pressure values or the addition of cosolvents must be considered, as they can significantly influence the solubility of the drug in the supercritical medium [109]. The depressurization rate can directly influence the amount of drug retained in the matrix structure and its crystalline state. The aerogel composition and its microstructure influence the amount of drug adsorbed on the porous surface [20,109,110]. The type and density of functional groups in the matrix porous structure determine the affinity of the drug molecule for the skeletal structure of the aerogel and, therefore, the impregnation process [110]. The functional groups of the primary monomers in biopolymer aerogels, which directly influence their impregnation capability, are illustrated in Figure 8 [110].
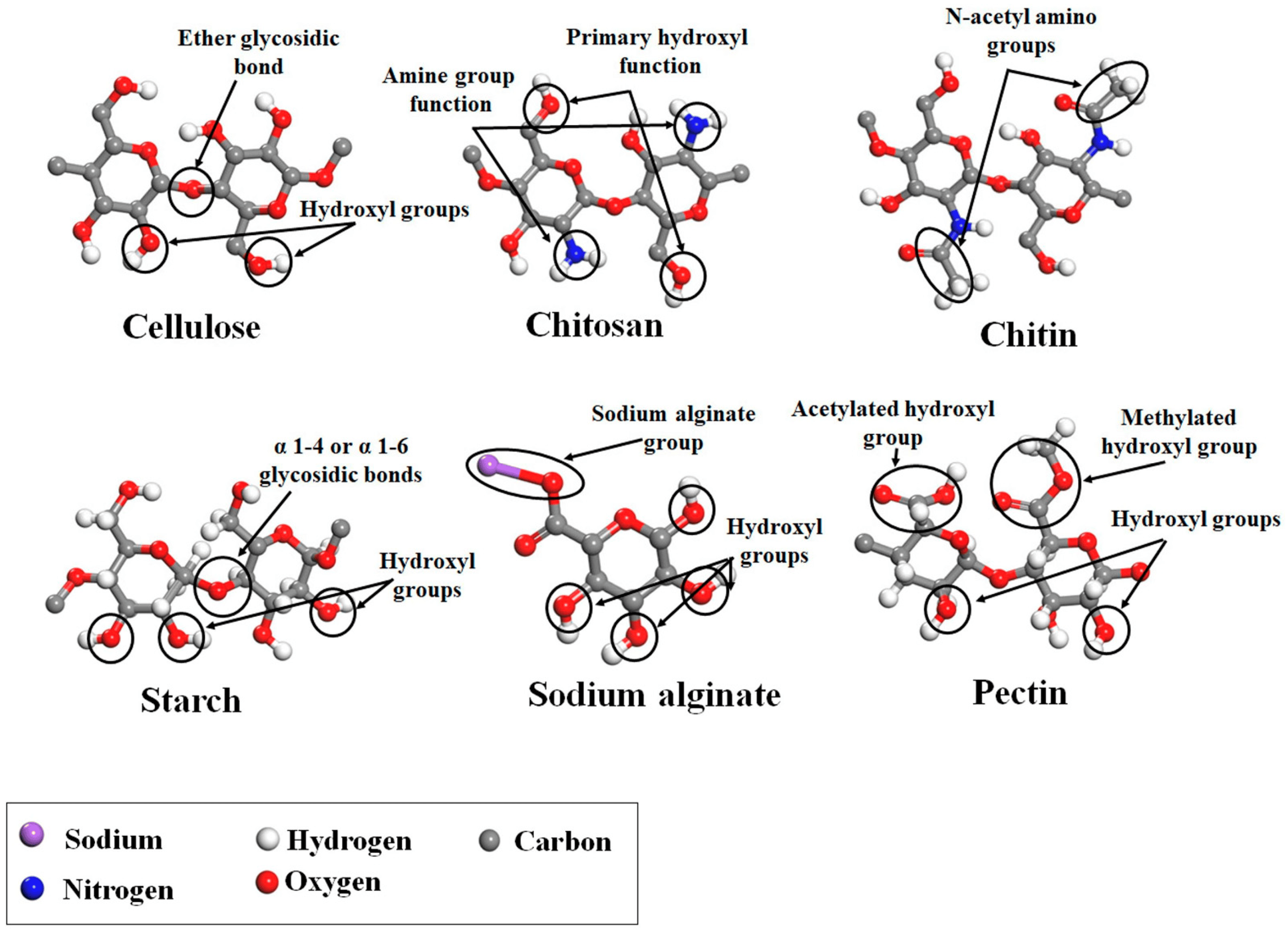
Figure 8. Main monomer functional groups of polysaccharide aerogels. Image adapted from [110].
The effect of the abovementioned factors is exemplified in the results from polysaccharide aerogels of pectin, alginate, and starch produced through the emulsion–gelation method and SCF drying [103]. The loading of these aerogels was carried out by impregnation at 180 bar (40 °C for ketoprofen and 55 °C for benzoic acid) for 24 h. Aerogels with a greater surface area exhibited more efficient drug loading. The drug loading efficiency was also influenced by the type and density of functional groups (hydroxyl, carboxyl, and amino) in the skeletal structure of the aerogels, in alignment with the molecular structure of benzoic acid and ketoprofen. Specifically, the molecular structure of the starch-based aerogels promoted higher ketoprofen loading. This can be attributed to starch’s higher concentration of hydroxyl groups in comparison to other polymers and its lack of acid groups. Lastly, the molecular weight of the drugs can also dictate the loading efficiency, with smaller molecules being more favorable for impregnation.
4.3. Polysaccharide-Based Aerogel Dosage Systems: Production and Release Properties
The combination of various aerogel production techniques with different gelation and drug loading methods yields aerogel dosage systems of varying shapes and distinct behaviors [103]. The shape, together with the aerogel composition and the drug loading technique, have a strong influence on the drug release profile of the formulations .
Depending on the location of the crosslinking agent (Figure 9A), gelation can be classified as external, internal, or inverse [17]. External gelation involves introducing the polymer solution into a bath containing the gelation promoter (e.g., salts and organic solvents) [125]. Alternatively, a water-in-oil (W/O) emulsion of the polymer can be prepared, followed by the dropwise addition of a crosslinking agent into the emulsion to induce polymer gelation [126]. Internal gelation involves adding the crosslinking agent in an insoluble state into the polymer solution and then dropping this mixture in a bath capable of solubilizing the crosslinking agent, thereby promoting the gelation of the polymer. This procedure is especially convenient for the gelation of Alg. It involves adding calcium carbonate (CaCO3) to an Alg solution and then dropping the Alg in an acidic bath. This promotes the dissolution of calcium and the gelation of Alg. Alternatively, it is also feasible to introduce an acid solution dropwise into an emulsion containing Alg and CaCO3 dispersed within Alg [127]. Finally, in the process of inverse gelation, the crosslinking agent (e.g., in a water solution) is dropped into a polymer solution, promoting the gelation from the innermost droplet and progressing outward [128].
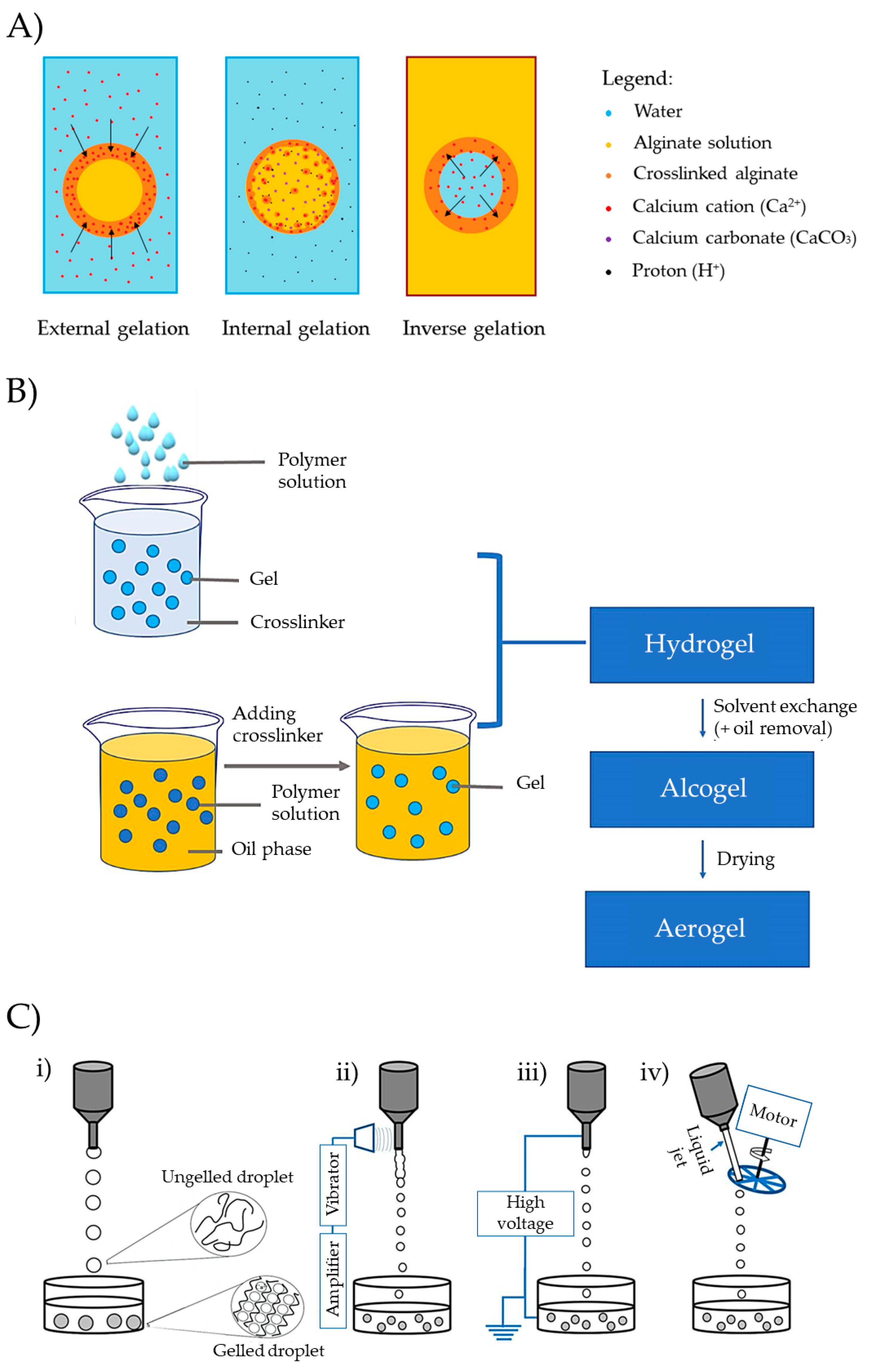
Figure 9. (A) Representation of external, internal, or inverse gelation. This figure illustrates the gelation process of alginate with Ca2+ as an example, but the underlying concept is applicable to various polymers and crosslinking agents. The arrows indicate the direction of the crosslinking front. (B) Preparation of aerogel microparticles by internal gelation and external gelation. Image extracted from [100]. (C) Dripping gelation modalities: (i) conventional, (ii) vibrating, (iii) electrostatic, and (iv) jet-cutting dripping methods. Image extracted from [17].
Molds of various shapes, such as cylinders or spheres, can be used to prepare monolithic forms. Examples of monolithic shapes prepared from starch (Entry 1) [111], pectin (Entry 2) [112], or kappa-carrageenan (Entries 7 and 10) [117,121] are presented in . Cylinders can also be produced through extrusion, and other shapes can be achieved using 3D printers [103]. Various technologies, such as emulsification, drip-gelation, or prilling, allow the development of multiparticle aerogel systems that can load drugs in an amorphous state, enabling controlled release. These technologies offer promising prospects for improving absorption and stability [20]. The choice of technique significantly influences the size and morphology of the gel particles, with emulsion–gelation and dripping being among the most commonly employed methods (Figure 9B) [23,103]. Emulsion gelation techniques create solid particulate aerogels by forming a W/O emulsion with polysaccharides in the aqueous phase and liquid paraffin in the oil phase. Emulsion droplets ranging from 1 to 3000 µm in size are crosslinked, washed, and dried to successfully produce solid multiparticulate aerogels. This method has been widely explored for polysaccharide-based aerogels of alginate, starch, and pectin (Entry 13) [22] and MCC (Entry 3) [113].
Dripping a polymer solution into a gelation bath is also a versatile technique to obtain multiparticulate systems. The bath composition dictates the gelation process (ionic crosslinking, coagulation), while the dripping method (conventional, vibrating, electrostatic, or mechanical cutting) influences particle characteristics. The conventional method relies on factors such as solution viscosity, surface tension, nozzle diameter, and gravity forces to form spherical structures in the air with specific dimensions, followed by gelation in the bath. Alternative modalities employ different physical principles to break the preformed drops within the nozzle, enhancing efficiency or reducing particle size or shape (Figure 9C) [17,103,129]. The scale-up of the dripping gelation technique can be carried out by using multiple nozzles to drop the formulations simultaneously into the bath. This method consists of installing a rack of nozzles of the same geometry over a crosslinking bath and keeping the same distance between the bath and the tip of the nozzles [130].
Multiparticulate aerogels have been produced with pectin by mechanical-cutting or jet-cutting dripping technique (Entry 5) [115], Alg by vibrating dripping technique (Entry 15) [124], or pectin or alginate aerogels by conventional dripping technique (Entries 4 [114] and 14 [123]) .
Polysaccharide-based aerogel systems can be used to improve the solubilization characteristics of poorly soluble drugs. For example, starch aerogel monoliths were prepared by dissolving the starch powder in water at 14.1% w/v, heating at 117 °C, and cooling to promote the retrogradation process (Entry 1, . Afterward, a solvent exchange and subsequent SCF drying were carried out. They were loaded with celecoxib during the solvent exchange. They have shown faster drug release compared to the raw material as supplied in both SIF (pH 7.4) and SGF (pH 1.2) at any of the paddle speeds studied (50 and 100 rpm) (Figure 10) [111]. This is undoubtedly related to the large dissolution surface area of the porous structures of the aerogel. The drug release profiles fit the Korsmeyer–Peppas model, demonstrating the diffusion–erosion mechanism. The Fickian diffusion of celecoxib is complemented by the degradation of the aerogel matrix structure.
Monolithic aerogels with high methoxyl pectin content have also been proposed to improve the oral bioavailability of nifedipine (Entry 2, [112]. Aqueous pectin solutions (1, 2, and 4% w/v) were molded, gelled in 10% v/v EtOH, solvent exchanged (absolute EtOH), and scCO2 dried. Nifedipine loading occurred during solvent exchange (3 h in a drug-saturated solution) or impregnation after drying (200 bar and 60 °C for 24 h). Incorporating nifedipine into pectin aerogels has not significantly enhanced its release in SGF; in fact, it notably delays the release, particularly for aerogels loaded during the solvent exchange (Figure 11A). However, the formulation of nifedipine in pectin aerogels expedites drug release in neutral-basic media (Figure 11B), especially when loaded through SCF impregnation. In this case, the profiles fit the Korsmeyer–Peppas model, indicating a mechanism of erosion-diffusion for those aerogels in PBS medium.
Polysaccharide-based aerogels can also be engineered to control drug release within the GIT. For instance, cellulose aerogels loaded with acetaminophen were prepared using the emulsion–gelation method to address this goal (Entry 3, [113]. Cellulose was first pretreated with dimethylformamide at 100 °C, dissolved in 1-butyl-3-methylimidazolium chloride (an ionic liquid), and emulsified in a cyclohexane solution with Hypermer 1599™ and Tween® 80 as additives. The resulting emulsion was scCO2-dried. The acetaminophen loading was carried out during the solvent exchange step (absolute EtOH). Cellulose aerogels (3% w/v) effectively controlled acetaminophen release in PBS (pH 7.4) in comparison with acetaminophen crystals. Release profiles followed first-order kinetics, suggesting diffusion in water as the main release mechanism, with no observable carrier degradation except for initial shrinkage in the first few minutes. These systems have the potential for targeting colon drug delivery, where microorganisms can degrade cellulose structures and promote drug release.
Controlled diclofenac release has also been achieved from Alg, low-methoxy pectin, and their mixtures (1:1) aerogels prepared through the dripping method (Entry 4, [114]. Solutions of polysaccharide (2% w/v) containing diclofenac (1% w/v) were dropped, crosslinked with Ca2+, Zn2+, or Sr2+ (2% w/v), cured for 24 h, and supercritically dried (100 bar and 40 °C) to produce the aerogels. All aerogels were stable in SGF over a period of 7 h and started to swell when in contact with PBS. Figure 12 presents the diclofenac release profiles in a PBS medium (pH 6.8), showing a strong dependence on the polysaccharide composition and the crosslinking agent. Calcium-crosslinked aerogels provided the highest surface areas, and zinc crosslinked the lowest. As a result, pectin aerogels crosslinked with zinc exhibit slower drug release, making them more promising as controlled delivery systems.
4.4. Coating of Polysaccharide-Based Aerogels for Colonic Applications
Coated polysaccharide-based aerogels are regarded as a valuable tool to develop formulations for colonic administration. This approach is expected to reduce drug losses in the upper regions of the GIT while boosting the local drug dose in the colon. Despite existing examples of aerogel coating in the literature , none of them have demonstrated the creation of a colonic delivery system. Nevertheless, the conducted research can serve as the basis for designing effective colonic formulations, as coating has the potential to impact mechanical properties, slow water uptake, and modify the drug release mechanism.
A method used for coating polysaccharide-based aerogels is the fluidized bed technique that has been used to modify drug release profiles [131]. For instance, Alg aerogels were produced through dripping, ionic crosslinking (Ca2+), and SCF drying. These aerogels were loaded with ibuprofen through impregnation (at 200 bar, 40 °C, 3 h), and a Wurster fluidized bed was used to apply a coating with an aqueous dispersion of methacrylic acid-ethyl acrylate (“Aquarius™ Control ENA”, ENA) at 60 °C. Under these conditions, the coating process did not alter the porous structure of the aerogel or modify the release of the drug. Under acidic conditions, the particles exhibited a well-preserved coating without drug release due to their enteric coat. Under basic conditions (PBS, pH 7.4), the coated particles initially retained the ibuprofen. The formation of microscopic pores in the coating after 15 min facilitated the controlled release of ibuprofen (Figure 13).
In another example, coated cellulose aerogels were prepared using spouted bed technology and evaluated for colonic purposes [132]. The particles were produced by jet-cutting dripping of cellulose dispersions into an alkaline aqueous medium, followed by drying and supercritical impregnation with vanillin (125 bar, 60 °C, 16 h). The particles were coated with ethanolic solutions of shellac (a natural resin) at different concentrations. The uncoated aerogels released 50% of the vanillin content during the first 20 min, while the coating with a volume/mass ratio of approximately 1 mL/g was able to retain 50% of the vanillin content for up to 90 min.
The coating can also modify the water uptake and mechanical properties of the aerogel. Hybrid Alg–starch aerogels with a cylindrical tablet shape were prepared in a fluidizer bed coater [133]. CaCO3 was dispersed into the polymer solution to promote the gelation process. Afterward, a solvent exchange to EtOH, followed by an SCF drying process, was carried out. For the coating of the aerogels, a fluidized bed coater was used to spray an aqueous Eudragit® 30 D-55 solution at 30% w/v. Water uptake data for the coated aerogels exhibited minimal values at low pH (pH 1.2) and increased from pH 5.5 as the coating became permeable. At pH 7.4, water uptake was unconstrained.
Aerogel coatings can be produced through alternative methods such as cold plasma processes or immersion. When employing the cold plasma coating technique with perfluoro-acrylates (e.g., 1H,1H,2H,2H-perfluorooctyl acrylate), it becomes possible to effectively modulate the surface wettability of polysaccharide-based aerogels like Alg or cellulose, making them superhydrophobic. Furthermore, this deposition method offers flexibility and quick processing times [134].
The multi-step sol-gel process is a straightforward technique that has enabled the preparation of multilayer hydrogels and, therefore, aerogels. Alg beads at a concentration of 1.5% w/v were coated by immersing them in successive layers of alginate solution at 0.75% w/v. The wetted beads were then collected and subjected to gelation in a 0.2 M CaCl2 solution. This procedure allowed the incorporation of a water-soluble drug (nicotinic acid) either within the initial bead cores or within distinct layers. The subsequent solvent exchange and SCF drying steps enable the production of loaded aerogels, whose controlled drug release depends on the number of layers incorporated [135].
Coaxial prilling is another method that enables the production of coated aerogels with remarkable versatility. This innovative technique allows for the one-step preparation of core–shell hydrogels, which, upon SCF drying, results in the formation of core–shell aerogels [136]. As an example, spherical core–shell particles (mean diameter: 3.25 mm) were achieved by pumping an inner solution of 3.5% w/v pectin containing doxycycline (3.5% w/w) through a 0.4 mm internal nozzle while simultaneously employing an annular solution of 1.5–1.75% w/v aqueous Alg through a 0.6 mm outer nozzle. The crosslinking process occurred in a gelling bath (0.5 M CaCl2 dissolved in 96° EtOH). A vibration frequency of 350 Hz was maintained with the coaxial nozzle positioned 25 cm away from the crosslinking bath. After the core–shell beads were prepared, they were dried using scCO2 technology. The obtained particles had an encapsulation efficiency of up to 87%, and the sustained drug release was maintained for 48 h.
The preceding examples clearly demonstrate the considerable potential of polysaccharide aerogels as effective oral drug delivery systems, particularly for colonic administration. Nevertheless, the existing body of research on aerogels tailored for colonic applications remains quite limited. Despite the extensive research dedicated to developing polysaccharide-based aerogels with Alg, cellulose, starch, or CS, there remains a critical need to address the gaps in understanding the ideal composition characteristics and operational conditions necessary for these systems to effectively reach the colonic area and optimize drug release for efficient treatments [20]. While certain strategies, such as pH-dependent polymer coatings, offer potential improvements in release profiles, none of the existing studies have conclusively demonstrated their specificity at the colonic level.
5. Future Trends in Research on Aerogels as Colonic Drug Delivery Systems
Undoubtedly, the rise in colonic pathologies, including colorectal cancer, Crohn’s disease, ulcerative colitis, diverticulitis, and irritable bowel syndrome, can be attributed to a combination of factors, such as diet, lifestyle, and various other determinants that the world, especially the more industrialized one, is currently experiencing. For example, the number of new cases of colorectal cancer in 2020 was higher than 1.9 million worldwide, according to the World Health Organization [137]. Also, the prevalence of IBD has increased significantly. Particularly in Western countries, Europe, and the United States, the prevalence is already between 0.5 and 0.75% [138,139]. Those diseases greatly impact patients’ quality of life, hinder their productivity [5,140,141], and pose an extraordinary burden on treatment costs for national health systems [142].
In this scenario, innovation in colonic drug delivery systems designed to target local treatment of these pathologies, facilitate the administration of NME (poor-soluble drugs, peptides, or proteins), or improve the effectiveness of existing drugs offers promising prospects.
According to the precedent sections, the possibility of engineered polysaccharide-based aerogels that can respond to pH, enzymes within the colon microbiota, and even specific molecules as reactive oxygen species (ROS) produced by the inflammatory cells involved in these pathologies is a reality, but also a path that remains relatively unexplored [20,143]. The selection of polysaccharides first determines their release, which can be any of those mentioned or even a combination of two of them, giving the aerogels great selectivity to target the colon.
The literature review has shown that the most successful strategy for polysaccharide aerogels to reach the colon while avoiding the early release of the drug is the formation of monolithic or multiparticulate systems coated with pH or enzymatic-resistant materials. Consequently, the development of core–shell formulations of aerogels presents the best prospects.
Work involving the development of coatings with synthetic polymers like Eudragit® typically employs fluid bed coaters or spouted bed technology, which have shown excellent results in terms of maintenance of the aerogel structures and release profiles. These techniques are widely recognized and easily scalable. Nonetheless, there is potential for innovation in producing core gel particles with polysaccharide shells, offering interesting possibilities for combining various functionalities, such as mucoadhesion and responsiveness to pH or both pH and enzymatic responses.
Coated aerogels can also be prepared by immersing aerogel cores in diverse polysaccharide shells using coaxial nozzles and SCF drying. This method enables the formation of multiparticle aerogel systems with variable sizes and offers a range of release mechanisms, allowing for customized release rates [136]. Its versatility extends to the use of various polysaccharide combinations, high production rates, and straightforward scalability [144,145]. Despite the considerable potential of this technique to advance the development of colonic aerogels, a notable gap exists in effectively incorporating poorly soluble drugs or other types of NME at appropriate doses and demonstrating the influence of the system properties on their bioavailability.
The powder coating method for aerogels is an unexplored approach that entails mixing the final aerogel formulation with low-melting atomized materials. Upon heating, fusion and coalescence occur, creating a lasting coating on the particle surfaces. This coating will persist after cooling [146]. This technique is environmentally friendly, as it eliminates the use of organic solvents in coating solutions.
Treatments for cancer or IBD frequently include antitumorals, anti-inflammatories, corticosteroids, immunomodulators, antibiotics, and monoclonal antibodies, some of which have poor bioavailability due to their low solubility properties [5]. The inclusion of these drugs in the amorphous state in stable and solid aerogel formulations should help overcome solubilization problems and enhance the efficacy of treatments [20]. Some of these active compounds have already been formulated into coated aerogels with the aim of colonic administration .
There is a paucity of knowledge concerning the drug crystalline state within aerogels and its evolution upon storage, particularly in terms of maintaining stability in the amorphous state and solubility characteristics [20,109]. There is also no information in the literature about the mucoadhesive properties of the polysaccharide-based aerogel particles formulated for colonic delivery purposes. Additionally, drug release profiles are not fully explored. The impact of the microbiota on the drug-release process from these aerogels remains undocumented, and there is a lack of in vivo studies in the existing literature.
These areas represent critical research frontiers that warrant further exploration and investigation within the field of polysaccharide-based aerogels as colonic delivery systems.
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Sathish, D.; Himabindu, S.; Shravan Kumar, Y.; Shayeda; Madhusudan Rao, Y. Floating Drug Delivery Systems for Prolonging Gastric Residence Time: A Review. Curr. Drug Deliv. 2011, 8, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent Advances in Colon Drug Delivery Systems. J. Control. Release 2020, 327, 703–724. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.W. Advances in Colonic Drug Delivery. Drugs 2005, 65, 1991–2007. [Google Scholar] [CrossRef]
- Dening, T.J.; Amidon, G.E.; He, X.; Hageman, M.J. Physicochemical Characterization and Oral Dosage Form Design and Selection. In Burger’s Medicinal Chemistry and Drug Discovery; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1–49. ISBN 978-0-471-26694-5. [Google Scholar]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of Gastrointestinal Tract Variability on Oral Drug Absorption and Pharmacokinetics: An UNGAP Review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Sensoy, I. A Review on the Food Digestion in the Digestive Tract and the Used In Vitro Models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Lenourry, A.; Charrueau, C.; Chaumeil, J.-C. The Use of Polysaccharides to Target Drugs to the Colon. Carbohydr. Polym. 2002, 48, 219–231. [Google Scholar] [CrossRef]
- Shah, N.; Shah, T.; Amin, A. Polysaccharides: A Targeting Strategy for Colonic Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Kumria, R. Polysaccharides in Colon-Specific Drug Delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Colombo, G.; Sonvico, F. Pectin Matrix as Oral Drug Delivery Vehicle for Colon Cancer Treatment. AAPS PharmSciTech 2010, 12, 201–214. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Paukkonen, H.; Kunnari, M.; Laurén, P.; Hakkarainen, T.; Auvinen, V.-V.; Oksanen, T.; Koivuniemi, R.; Yliperttula, M.; Laaksonen, T. Nanofibrillar Cellulose Hydrogels and Reconstructed Hydrogels as Matrices for Controlled Drug Release. Int. J. Pharm. 2017, 532, 269–280. [Google Scholar] [CrossRef]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and Formulation Design of Polysaccharide-Based Hydrogels for Drug Delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Costa, J.S.R.; de Oliveira Cruvinel, K.; Oliveira-Nascimento, L. A Mini-Review on Drug Delivery through Wafer Technology: Formulation and Manufacturing of Buccal and Oral Lyophilizates. J. Adv. Res. 2019, 20, 33–41. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in Drug Delivery: From Design to Application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-Based Aerogel Microspheres for Oral Drug Delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef]
- Duong, T.; López-Iglesias, C.; Szewczyk, P.K.; Stachewicz, U.; Barros, J.; Alvarez-Lorenzo, C.; Alnaief, M.; García-González, C.A. A Pathway From Porous Particle Technology Toward Tailoring Aerogels for Pulmonary Drug Administration. Front. Bioeng. Biotechnol. 2021, 9, 671381. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-Loaded Chitosan Aerogel Particles for Chronic Wound Applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and Biomedical Applications of Aerogels: Possibilities and Challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Feng, S.; Zhang, Z.; Wu, C.; Zhang, X.; Kang, F. Nano-Porous Silica Aerogels as Promising Biomaterials for Oral Drug Delivery of Paclitaxel. J. Biomed. Nanotechnol. 2019, 15, 1532–1545. [Google Scholar] [CrossRef]
- Wang, J.; Yadav, V.; Smart, A.L.; Tajiri, S.; Basit, A.W. Stability of Peptide Drugs in the Colon. Eur. J. Pharm. Sci. 2015, 78, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yadav, V.; Smart, A.L.; Tajiri, S.; Basit, A.W. Toward Oral Delivery of Biopharmaceuticals: An Assessment of the Gastrointestinal Stability of 17 Peptide Drugs. Mol. Pharm. 2015, 12, 966–973. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, V.; Arduino, I.; Vacca, M.; Iacobazzi, R.M.; Altamura, D.; Lopalco, A.; Rizzi, R.; Cutrignelli, A.; Laquintana, V.; Massimo, F.; et al. Colonic Budesonide Delivery by Multistimuli Alginate/Eudragit® FS 30D/Inulin-Based Microspheres as a Paediatric Formulation. Carbohydr. Polym. 2023, 302, 120422. [Google Scholar] [CrossRef] [PubMed]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological Parameters for Oral Delivery and In Vitro Testing. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in Oral Nano-Delivery Systems for Colon Targeted Drug Delivery in Inflammatory Bowel Disease: Selective Targeting to Diseased versus Healthy Tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Anil, K.; Philip, B.P. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 19–87. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Colonic Drug Delivery: Prodrug Approach. Pharm. Res. 2001, 18, 557–564. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Klünemann, M.; Andrejev, S.; Blasche, S.; Mateus, A.; Phapale, P.; Devendran, S.; Vappiani, J.; Simon, B.; Scott, T.A.; Kafkia, E.; et al. Bioaccumulation of Therapeutic Drugs by Human Gut Bacteria. Nature 2021, 597, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Lebwohl, B.; Abrams, J.A. The Impact of Proton Pump Inhibitors on the Human Gastrointestinal Microbiome. Hum. Microbiome 2014, 34, 771–785. [Google Scholar] [CrossRef]
- Hold, G.L.; Hansen, R. Impact of the Gastrointestinal Microbiome in Health and Disease: Co-Evolution with the Host Immune System. In Molecular Mechanisms of Inflammation: Induction, Resolution and Escape by Helicobacter pylori; Backert, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 303–318. ISBN 978-3-030-15138-6. [Google Scholar]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- Lubomski, M.; Tan, A.H.; Lim, S.-Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s Disease and the Gastrointestinal Microbiome. J. Neurol. 2020, 267, 2507–2523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Qin, H.-Y.; Li, T.-T. Advances in the Study of the Relationship between Alzheimer’s Disease and the Gastrointestinal Microbiome. Ibrain 2022, 8, 465–475. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function after a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Compare, D. The Human Gastric Microbiota: Is It Time to Rethink the Pathogenesis of Stomach Diseases? United Eur. Gastroenterol. J. 2015, 3, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W. Effects of Short Chain Fatty Acids on Gut Morphology and Function. Gut 1994, 35, 35–38. [Google Scholar] [CrossRef]
- Rasmussen, H.S.; Holtug, K.; Mortensen, P.B. Degradation of Amino Acids to Short-Chain Fatty Acids in Humans: An In Vitro Study. Scand. J. Gastroenterol. 1988, 23, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Madla, C.M.; McCoubrey, L.E.; Ferraro, F.; Gavins, F.K.H.; Buanz, A.; Gaisford, S.; Orlu, M.; Siepmann, F.; Siepmann, J.; et al. Clinical Translation of Advanced Colonic Drug Delivery Technologies. Adv. Drug Deliv. Rev. 2022, 181, 114076. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. (JMB) 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ha, W.; Gao, K.; Shi, Y. Precisely Traceable Drug Delivery of Azoreductase-Responsive Prodrug for Colon Targeting via Multimodal Imaging. Anal. Chem. 2020, 92, 9039–9047. [Google Scholar] [CrossRef] [PubMed]
- Schiller, C.; Fröglich, C.P.; Giessmann, T.; Siegmund, W.; Mönnikes, H.; Hosten, N.; Weitschies, W. Intestinal Fluid Volumes and Transit of Dosage Forms as Assessed by Magnetic Resonance Imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marciani, L. Quantification of Gastrointestinal Liquid Volumes and Distribution Following a 240 mL Dose of Water in the Fasted State. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A Technology Evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Fülöpová, N.; Pavloková, S.; DeBono, I.; Vetchý, D.; Franc, A. Development and Comparison of Various Coated Hard Capsules Suitable for Enteric Administration to Small Patient Cohorts. Pharmaceutics 2022, 14, 1577. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Q.; Xu, Y.; Wu, D.; Sun, Y. pH-Controlled Drug Release from Mesoporous Silica Tablets Coated with Hydroxypropyl Methylcellulose Phthalate. Mater. Res. Bull. 2009, 44, 606–612. [Google Scholar] [CrossRef]
- Kumar, S.; Jeet, K.; Baldi, A. Recent Technological Advancements in Multiparticulate Formulations: The Smart Drug Delivery Systems. Asian J. Pharm. 2015, 9, S13–S25. [Google Scholar]
- Yang, Y.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L. Enzyme-Mediated Hydrolytic Activation of Prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Dahan, A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12, 1031. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Garcia-Triñanes, P.; Echezarreta López, M.M.; Santoveña, A.; Landin, M. Mineralized Alginate Hydrogels Using Marine Carbonates for Bone Tissue Engineering Applications. Carbohydr. Polym. 2018, 195, 235–242. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-Conventional Starch Sources. Curr. Opin. Food Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A Review of Starch, a Unique Biopolymer—Structure, Metabolism and in Planta Modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Channab, B.-E.; Ayoub, E.I.; Zahouily, M.; Motamedi, E. A Comprehensive Review on Starch: Structure, Modification, and Applications in Slow/Controlled-Release Fertilizers in Agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-Promoting Effects of Konjac Glucomannan and Its Practical Applications: A Critical Review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Manceñido, F.; Braeckmans, K.; De Smedt, S.C.; Demeester, J.; Landin, M.; Martínez-Pacheco, R. Characterization of Diffusion of Macromolecules in Konjac Glucomannan Solutions and Gels by Fluorescence Recovery after Photobleaching Technique. Int. J. Pharm. 2006, 316, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; He, P.; Lin, X. The Mechanism of Sodium Hydroxide Solution Promoting the Gelation of Konjac Glucomannan (KGM). Food Hydrocoll. 2013, 30, 92–99. [Google Scholar] [CrossRef]
- Khoobbakht, F.; Khorshidi, S.; Bahmanyar, F.; Hosseini, S.M.; Aminikhah, N.; Farhoodi, M.; Mirmoghtadaie, L. Modification of Mechanical, Rheological and Structural Properties of Agar Hydrogel Using Xanthan and Locust Bean Gum. Food Hydrocoll. 2024, 147, 109411. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A Decade of Change in the Seaweed Hydrocolloids Industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Mandal, S.; Hwang, S.; Shi, S.Q. Guar Gum, a Low-Cost Sustainable Biopolymer, for Wastewater Treatment: A Review. Int. J. Biol. Macromol. 2023, 226, 368–382. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust Bean Gum: Processing, Properties and Food Applications—A Review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Raghav, S.; Jain, P.; Kumar, D. Alginates: Properties and Applications. In Polysaccharides; Scrivener Publishing: Beverly, MA, USA, 2021; pp. 399–422. ISBN 978-1-119-71141-4. [Google Scholar]
- Chawla, A.; Sharma, P.; Pawar, P. Eudragit S-100 Coated Sodium Alginate Microspheres of Naproxen Sodium: Formulation, Optimization and In Vitro Evaluation. Acta Pharm. 2013, 62, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayana, S.N.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium Alginate-Carboxymethyl Cellulose Beads for Colon-Targeted Drug Delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Agarose, Alginate and Chitosan Nanostructured Aerogels for Pharmaceutical Applications: A Short Review. Front. Bioeng. Biotechnol. 2021, 9, 688477. [Google Scholar] [CrossRef] [PubMed]
- Gulbake, A.; Jain, S.K. Chitosan: A Potential Polymer for Colon-Specific Drug Delivery System. Expert Opin. Drug Deliv. 2012, 9, 713–729. [Google Scholar] [CrossRef]
- Schipper, N.G.M.; Vårum, K.M.; Stenberg, P.; Ocklind, G.; Lennernäs, H.; Artursson, P. Chitosans as Absorption Enhancers of Poorly Absorbable Drugs: 3: Influence of Mucus on Absorption Enhancement. Eur. J. Pharm. Sci. 1999, 8, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of Chitosan on the Permeability of Monolayers of Intestinal Epithelial Cells (Caco-2). Pharm. Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Mucoadhesive Chitosan and Thiolated Chitosan Nanoparticles Containing Alpha Mangostin for Possible Colon-Targeted Delivery. Pharm. Dev. Technol. 2021, 26, 362–372. [Google Scholar] [CrossRef]
- Veronovski, A.; Tkalec, G.; Knez, Ž.; Novak, Z. Characterisation of Biodegradable Pectin Aerogels and Their Potential Use as Drug Carriers. Carbohydr. Polym. 2014, 113, 272–278. [Google Scholar] [CrossRef]
- Auriemma, G.; Cerciello, A.; Aquino, R.P.; Del Gaudio, P.; Fusco, B.M.; Russo, P. Pectin and Zinc Alginate: The Right Inner/Outer Polymer Combination for Core-Shell Drug Delivery Systems. Pharmaceutics 2020, 12, 87. [Google Scholar] [CrossRef]
- Günter, E.A.; Markov, P.A.; Melekhin, A.K.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Preparation and Release Characteristics of Mesalazine Loaded Calcium Pectin-Silica Gel Beads Based on Callus Cultures Pectins for Colon-Targeted Drug Delivery. Int. J. Biol. Macromol. 2018, 120, 2225–2233. [Google Scholar] [CrossRef]
- Desai, N.; Momin, M. Colon Targeted Bioadhesive Pellets of Curcumin and Cyclosporine for Improved Management of Inflammatory Bowel Disease. Drug Deliv. Transl. Res. 2020, 10, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.; Freire, A.C.; Bravo, R.; Basit, A.W. OPTICORE™, an Innovative and Accurate Colonic Targeting Technology. Int. J. Pharm. 2020, 583, 119372. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Pang, Y.; Lu, C.; Yang, Y.; Gao, M.; Zheng, L.; Zhao, J. Photo-Crosslinkable Methacrylated Konjac Glucomannan (KGMMA) Hydrogels as a Promising Bioink for 3D Bioprinting. Biomater. Sci. 2022, 10, 6549–6557. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Tester, R.F. Effect of Konjac Glucomannan Hydrolysates and Probiotics on the Growth of the Skin Bacterium Propionibacterium Acnes In Vitro. Int. J. Cosmet. Sci. 2010, 32, 139–142. [Google Scholar] [CrossRef]
- Wattanaprasert, S.; Borompichaichartkul, C.; Vaithanomsat, P.; Srzednicki, G. Konjac Glucomannan Hydrolysate: A Potential Natural Coating Material for Bioactive Compounds in Spray Drying Encapsulation. Eng. Life Sci. 2017, 17, 145–152. [Google Scholar] [CrossRef]
- Alvarez-Manceñido, F.; Landin, M.; Martínez-Pacheco, R. Konjac Glucomannan/Xanthan Gum Enzyme Sensitive Binary Mixtures for Colonic Drug Delivery. Eur. J. Pharm. Biopharm. 2008, 69, 573–581. [Google Scholar] [CrossRef]
- Putro, J.N.; Soetaredjo, F.E.; Lunardi, V.B.; Irawaty, W.; Yuliana, M.; Santoso, S.P.; Puspitasari, N.; Wenten, I.G.; Ismadji, S. Polysaccharides Gums in Drug Delivery Systems: A Review. Int. J. Biol. Macromol. 2023, 253, 127020. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar Gel Strength: A Correlation Study between Chemical Composition and Rheological Properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Albadran, H.A.; Monteagudo-Mera, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Development of Chitosan-Coated Agar-Gelatin Particles for Probiotic Delivery and Targeted Release in the Gastrointestinal Tract. Appl. Microbiol. Biotechnol. 2020, 104, 5749–5757. [Google Scholar] [CrossRef]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Dinesh Kumar, B.; Karthikeyan, R.S.; Bhaskar, P. In Vivo Evaluation of Guargum-Based Colon-Targeted Oral Drug Delivery Systems of Celecoxib in Human Volunteers. Eur. J. Drug Metab. Pharmacokinet. 2002, 27, 273–280. [Google Scholar] [CrossRef]
- Kenyon, C.J.; Nardi, R.V.; Wong, D.; Hooper, G.; Wilding, I.R.; Friend, D.R. Colonic Delivery of Dexamethasone: A Pharmacoscintigraphic Evaluation. Aliment. Pharmacol. Ther. 1997, 11, 205–213. [Google Scholar] [CrossRef]
- Shahid, M.; Bukhari, S.A.; Gul, Y.; Munir, H.; Anjum, F.; Zuber, M.; Jamil, T.; Zia, K.M. Graft Polymerization of Guar Gum with Acryl Amide Irradiated by Microwaves for Colonic Drug Delivery. Int. J. Biol. Macromol. 2013, 62, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Seeli, D.S.; Prabaharan, M. Guar Gum Succinate as a Carrier for Colon-Specific Drug Delivery. Int. J. Biol. Macromol. 2016, 84, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Nunes, C.; Pereira, I.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A.; Gama, F.M. Structural Analysis of Dextrins and Characterization of Dextrin-Based Biomedical Hydrogels. Carbohydr. Polym. 2014, 114, 458–466. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, D.; Liu, J.; Kuang, Y.; Li, Q.; Zhang, M.; Ye, H.; Qin, H.; Xu, Y.; Li, C.; et al. pH-Sensitive Drug Delivery System Based on Modified Dextrin Coated Mesoporous Silica Nanoparticles. Int. J. Biol. Macromol. 2016, 85, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Teruel, A.H.; Gonzalez-Alvarez, I.; Bermejo, M.; Merino, V.; Marcos, M.D.; Sancenon, F.; Gonzalez-Alvarez, M.; Martinez-Mañez, R. New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy. Int. J. Mol. Sci. 2020, 21, 6502. [Google Scholar] [CrossRef]
- Payanda Konuk, O.; Alsuhile, A.A.A.M.; Yousefzadeh, H.; Ulker, Z.; Bozbag, S.E.; García-González, C.A.; Smirnova, I.; Erkey, C. The Effect of Synthesis Conditions and Process Parameters on Aerogel Properties. Front. Chem. 2023, 11, 1294520. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-Based Aerogels—Promising Biodegradable Carriers for Drug Delivery Systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Patel, S.M.; Doen, T.; Pikal, M.J. Determination of End Point of Primary Drying in Freeze-Drying Process Control. AAPS PharmSciTech 2010, 11, 73–84. [Google Scholar] [CrossRef]
- Kankala, R.K.; Xu, P.-Y.; Chen, B.-Q.; Wang, S.-B.; Chen, A.-Z. Supercritical Fluid (SCF)-Assisted Fabrication of Carrier-Free Drugs: An Eco-Friendly Welcome to Active Pharmaceutical Ingredients (APIs). Adv. Drug Deliv. Rev. 2021, 176, 113846. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical Drying of Aerogels Using CO2: Effect of Extraction Time on the End Material Textural Properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Witkowski, A.; Majkut, M.; Rulik, S. Analysis of Pipeline Transportation Systems for Carbon Dioxide Sequestration. Arch. Thermodyn. 2014, 35, 117–140. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M.; Knez, Ž.; Knez Marevci, M. Supercritical Fluid Technologies for the Incorporation of Synthetic and Natural Active Compounds into Materials for Drug Formulation and Delivery. Pharmaceutics 2022, 14, 1670. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Amorphization of Drugs by Adsorptive Precipitation from Supercritical Solutions: A Review. J. Supercrit. Fluids 2018, 132, 105–125. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bashir Yahya, E.; Jummaat, F.; Adnan, A.S.; Olaiya, N.G.; Rizal, S.; Abdullah, C.K.; Pasquini, D.; Thomas, S. Biopolymers Based Aerogels: A Review on Revolutionary Solutions for Smart Therapeutics Delivery. Prog. Mater. Sci. 2023, 131, 101014. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moghaddas, J. Mesoporous Tablet-Shaped Potato Starch Aerogels for Loading and Release of the Poorly Water-Soluble Drug Celecoxib. Chin. J. Chem. Eng. 2020, 28, 1778–1787. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Fast Production of High-Methoxyl Pectin Aerogels for Enhancing the Bioavailability of Low-Soluble Drugs. J. Supercrit. Fluids 2015, 106, 16–22. [Google Scholar] [CrossRef]
- Lin, W.-H.; Jana, S.C. Analysis of Porous Structures of Cellulose Aerogel Monoliths and Microparticles. Microporous Mesoporous Mater. 2021, 310, 110625. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. PH Sensitive Mesoporous Materials for Immediate or Controlled Release of NSAID. Microporous Mesoporous Mater. 2016, 224, 190–200. [Google Scholar] [CrossRef]
- Méndez, D.A.; Schroeter, B.; Martínez-Abad, A.; Fabra, M.J.; Gurikov, P.; López-Rubio, A. Pectin-Based Aerogel Particles for Drug Delivery: Effect of Pectin Composition on Aerogel Structure and Release Properties. Carbohydr. Polym. 2023, 306, 120604. [Google Scholar] [CrossRef] [PubMed]
- Groult, S.; Buwalda, S.; Budtova, T. Tuning Bio-Aerogel Properties for Controlling Drug Delivery. Part 2: Cellulose-Pectin Composite Aerogels. Biomater. Adv. 2022, 135, 212732. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, Z.; Namazi, H. Nontoxic Double-Network Polymeric Hybrid Aerogel Functionalized with Reduced Graphene Oxide: Preparation, Characterization, and Evaluation as Drug Delivery Agent. J. Polym. Res. 2022, 29, 37. [Google Scholar] [CrossRef]
- Li, Y.; Fan, R.; Xing, H.; Fei, Y.; Cheng, J.; Lu, L. Study on Swelling and Drug Releasing Behaviors of Ibuprofen-Loaded Bimetallic Alginate Aerogel Beads with pH-Responsive Performance. Colloids Surf. B Biointerfaces 2021, 205, 111895. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Tuning Bio-Aerogel Properties for Controlling Theophylline Delivery. Part 1: Pectin Aerogels. Mater. Sci. Eng. C 2021, 126, 112148. [Google Scholar] [CrossRef] [PubMed]
- Pantić, M.; Kravanja, K.A.; Knez, Ž.; Novak, Z. Influence of the Impregnation Technique on the Release of Esomeprazole from Various Bioaerogels. Polymers 2021, 13, 1882. [Google Scholar] [CrossRef]
- Agostinho, D.A.S.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.M.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of κ-Carrageenan Aerogels Prepared by Using Different Dissolution Media and Its Application as Drug Delivery Systems. Mater. Chem. Phys. 2020, 253, 123290. [Google Scholar] [CrossRef]
- Veres, P.; Sebők, D.; Dékány, I.; Gurikov, P.; Smirnova, I.; Fábián, I.; Kalmár, J. A Redox Strategy to Tailor the Release Properties of Fe(III)-Alginate Aerogels for Oral Drug Delivery. Carbohydr. Polym. 2018, 188, 159–167. [Google Scholar] [CrossRef]
- Veronovski, A.; Knez, Ž.; Novak, Z. Comparison of Ionic and Non-Ionic Drug Release from Multi-Membrane Spherical Aerogels. Int. J. Pharm. 2013, 454, 58–66. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of Alginate-Based Aerogel for Nonsteroidal Anti-Inflammatory Drugs Controlled Delivery Systems Using Prilling and Supercritical-Assisted Drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the Printer to the Lungs: Inkjet-Printed Aerogel Particles for Pulmonary Delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Alnaief, M.; Alzaitoun, M.A.; García-González, C.A.; Smirnova, I. Preparation of Biodegradable Nanoporous Microspherical Aerogel Based on Alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.M.; Smirnova, I. Alginate-Based Hybrid Aerogel Microparticles for Mucosal Drug Delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Zacco, R.; Rekkas, D.M.; Politis, S.; Garofalo, E.; Del Gaudio, P.; Aquino, R.P. Application of Experimental Design for the Development of Soft-Capsules through a Prilling, Inverse Gelation Process. J. Drug Deliv. Sci. Technol. 2019, 49, 577–585. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, H.; Widmer, F. A New Multinozzle Encapsulation/Immobilisation System to Produce Uniform Beads of Alginate. J. Biotechnol. 1998, 63, 73–80. [Google Scholar] [CrossRef]
- Akgün, I.S.; Ulker, Z.; Demir, E.; Işık, M.; Ekmekçiyan, N.; Darvishi, S.; Karaz, S.; Şenses, E.; Erkey, C. Enteric Coating of Drug Loaded Aerogel Particles in a Wurster Fluidized Bed and Its Effect on Release Behaviour. J. Drug Deliv. Sci. Technol. 2023, 82, 104279. [Google Scholar] [CrossRef]
- Schroeter, B.; Yonkova, V.P.; Goslinska, M.; Orth, M.; Pietsch, S.; Gurikov, P.; Smirnova, I.; Heinrich, S. Spray Coating of Cellulose Aerogel Particles in a Miniaturized Spouted Bed. Cellulose 2021, 28, 7795–7812. [Google Scholar] [CrossRef]
- Antonyuk, S.; Heinrich, S.; Gurikov, P.; Raman, S.; Smirnova, I. Influence of Coating and Wetting on the Mechanical Behaviour of Highly Porous Cylindrical Aerogel Particles. Powder Technol. 2015, 285, 34–43. [Google Scholar] [CrossRef]
- Schroeter, B.; Jung, I.; Bauer, K.; Gurikov, P.; Smirnova, I. Hydrophobic Modification of Biopolymer Aerogels by Cold Plasma Coating. Polymers 2021, 13, 3000. [Google Scholar] [CrossRef] [PubMed]
- Veronovski, A.; Knez, Ž.; Novak, Z. Preparation of Multi-Membrane Alginate Aerogels Used for Drug Delivery. J. Supercrit. Fluids 2013, 79, 209–215. [Google Scholar] [CrossRef]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and Supercritical Drying: A Successful Duo to Produce Core-Shell Polysaccharide Aerogel Beads for Wound Healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- Colorectal Cancer. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 7 November 2023).
- Jones, G.R.; Lyons, M.; Plevris, N.; Jenkinson, P.W.; Bisset, C.; Burgess, C.; Din, S.; Fulforth, J.; Henderson, P.; Ho, G.-T.; et al. IBD Prevalence in Lothian, Scotland, Derived by Capture–Recapture Methodology. Gut 2019, 68, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Coward, S.; Clement, F.; Benchimol, E.I.; Bernstein, C.N.; Avina-Zubieta, J.A.; Bitton, A.; Carroll, M.W.; Hazlewood, G.; Jacobson, K.; Jelinski, S.; et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019, 156, 1345–1353. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Seyedian, S.; Nokhostin, F.; Dargahi, M. A Review of the Diagnosis, Prevention, and Treatment Methods of Inflammatory Bowel Disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Burisch, J.; Vardi, H.; Schwartz, D.; Friger, M.; Kiudelis, G.; Kupčinskas, J.; Fumery, M.; Gower-Rousseau, C.; Lakatos, L.; Lakatos, P.L.; et al. Health-Care Costs of Inflammatory Bowel Disease in a Pan-European, Community-Based, Inception Cohort during 5 Years of Follow-Up: A Population-Based Study. Lancet Gastroenterol. Hepatol. 2020, 5, 454–464. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Wang, P.; Wang, C.; Niu, J.; Liu, Y.; Chen, Y. Research Progress of Colon-Targeted Oral Hydrogel System Based on Natural Polysaccharides. Int. J. Pharm. 2023, 643, 123222. [Google Scholar] [CrossRef]
- Sellitto, M.R.; Amante, C.; Aquino, R.P.; Russo, P.; Rodríguez-Dorado, R.; Neagu, M.; García-González, C.A.; Adami, R.; Del Gaudio, P. Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds. Gels 2023, 9, 492. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Amante, C.; Civale, R.; Bizzarro, V.; Petrella, A.; Pepe, G.; Campiglia, P.; Russo, P.; Aquino, R.P. In Situ Gelling Alginate-Pectin Blend Particles Loaded with Ac2-26: A New Weapon to Improve Wound Care Armamentarium. Carbohydr. Polym. 2020, 227, 115305. [Google Scholar] [CrossRef] [PubMed]
- Sauer, D.; Cerea, M.; DiNunzio, J.; McGinity, J. Dry Powder Coating of Pharmaceuticals: A Review. Prog. Film Coat. 2013, 457, 488–502. [Google Scholar] [CrossRef] [PubMed]

