1. Introduction
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into a variety of cell types, including bone, cartilage, muscle, and fat cells. They are commonly isolated from bone marrow but can also be found in other tissues, such as adipose tissue and the umbilical cord. MSCs are attractive for medical applications due to their ability to migrate to sites of injury or inflammation and their potential to differentiate into cells that can repair damaged tissue [1]. In addition, MSCs have immunomodulatory properties, making them useful for treating conditions such as autoimmune disorders and graft-versus-host disease. MSCs can be expanded in culture and manipulated ex vivo to promote specific cellular differentiation and are considered a promising tool for regenerative medicine [2]. However, further research is needed to fully understand the mechanisms underlying MSC function and to optimize their use for various clinical applications.
MicroRNAs (miRNAs) are small, non-coding RNA molecules that play a critical role in the regulation of gene expression. miRNAs bind target messenger RNA (mRNA) molecules, leading to their degradation or inhibition, preventing them from being translated into proteins [3]. This allows miRNAs to regulate the expression of multiple genes, making them an important component of gene regulation and cellular function. miRNAs have been shown to play a key role in regulating gene expression, and to be involved in a wide range of biological processes, including development, cell growth and division, and apoptosis [4,5,6,7]. miRNAs have also been implicated in the development and progression of various diseases, including cancer, cardiovascular disease, and neurological disorders [8,9,10]. By regulating the expression of genes involved in disease, miRNAs can act as either oncogenes or tumour suppressors [11,12,13,14].
The involvement of miRNA in a multitude of diseases makes them potential biomarkers for diagnostics as well as therapeutic tools, targeting genes responsible for a specific condition [15,16,17]
Furthermore, miRNAs play a crucial role in regulating MSC differentiation into various cell types, such as bone and cartilage [18,19]. MSCs can secrete miRNAs that promote or inhibit the differentiation of neighbouring cells [20]. The regulation of miRNAs in MSC differentiation is complex, and the role of specific miRNAs in the process is still being elucidated.
miRNAs exert a crucial influence on the intricate regulation of MSCs. Notably, certain miRNAs have been identified as key regulators of the immunosuppressive properties possessed by MSCs, underscoring their significance in unlocking the full therapeutic potential of these cells [21,22]. By introducing specific miRNAs into MSCs, researchers can target and tailor their therapeutic effects for specific diseases or conditions [23,24]. For instance, engineering MSCs to express anti-inflammatory miRNAs holds promise for combating inflammatory diseases, while harnessing miRNAs that promote tissue repair could revolutionize the treatment of tissue injuries [25,26]. This intersection of miRNAs and MSC engineering offers a promising frontier for advancing regenerative medicine and personalized therapeutic interventions.
Long non-coding RNAs (lncRNAs) are RNA molecules that are longer than 200 nucleotides but do not encode proteins [27,28]. Unlike protein-coding mRNA, lncRNA do not have a conserved open reading frame and are not translated into proteins. Despite their lack of coding capacity, lncRNA play critical roles in gene regulation and cellular processes. They have been shown to act as epigenetic regulators, scaffolds for protein complexes, and decoys for miRNA, among other functions [29,30,31,32,33]. lncRNA can also serve as molecular markers for various diseases, including cancer, and can be used for diagnostic and prognostic purposes [34,35,36]. The discovery of lncRNA has expanded our understanding of the diversity and complexity of RNA-mediated gene regulation and has opened up new avenues for the development of therapeutic strategies [37]. However, much remains to be learned about the full extent of lncRNA functions and the mechanisms underlying their effects on gene expression [38,39].
A number of studies have identified lncRNA as playing a key role in regulating MSC differentiation into various cell types [40,41]. For example, the lncRNA HOTAIR has been shown to regulate the differentiation of MSCs into osteoblasts [42]. In addition, the lncRNA MALAT1 has been shown to promote the ability of MSCs to form new blood vessels and promote proliferation [43]. Studies have highlighted the potential application of lncRNAs as innovative biomarkers for diagnosis and as potential targets for therapeutic treatments [44,45,46].
2. Characteristics and Function of MSCs
MSCs are a type of stem cell that have the ability to differentiate into a variety of cell lines, including bone, cartilage, muscle, and fat cells. They are commonly isolated from bone marrow, but they can also be found in other tissues, such as adipose tissue and umbilical cord (Figure 1) [47,48,49]. MSCs exhibit a range of characteristic properties, which enable their identification, as well as facilitate the range of their physiological functions (Figure 1) [50].
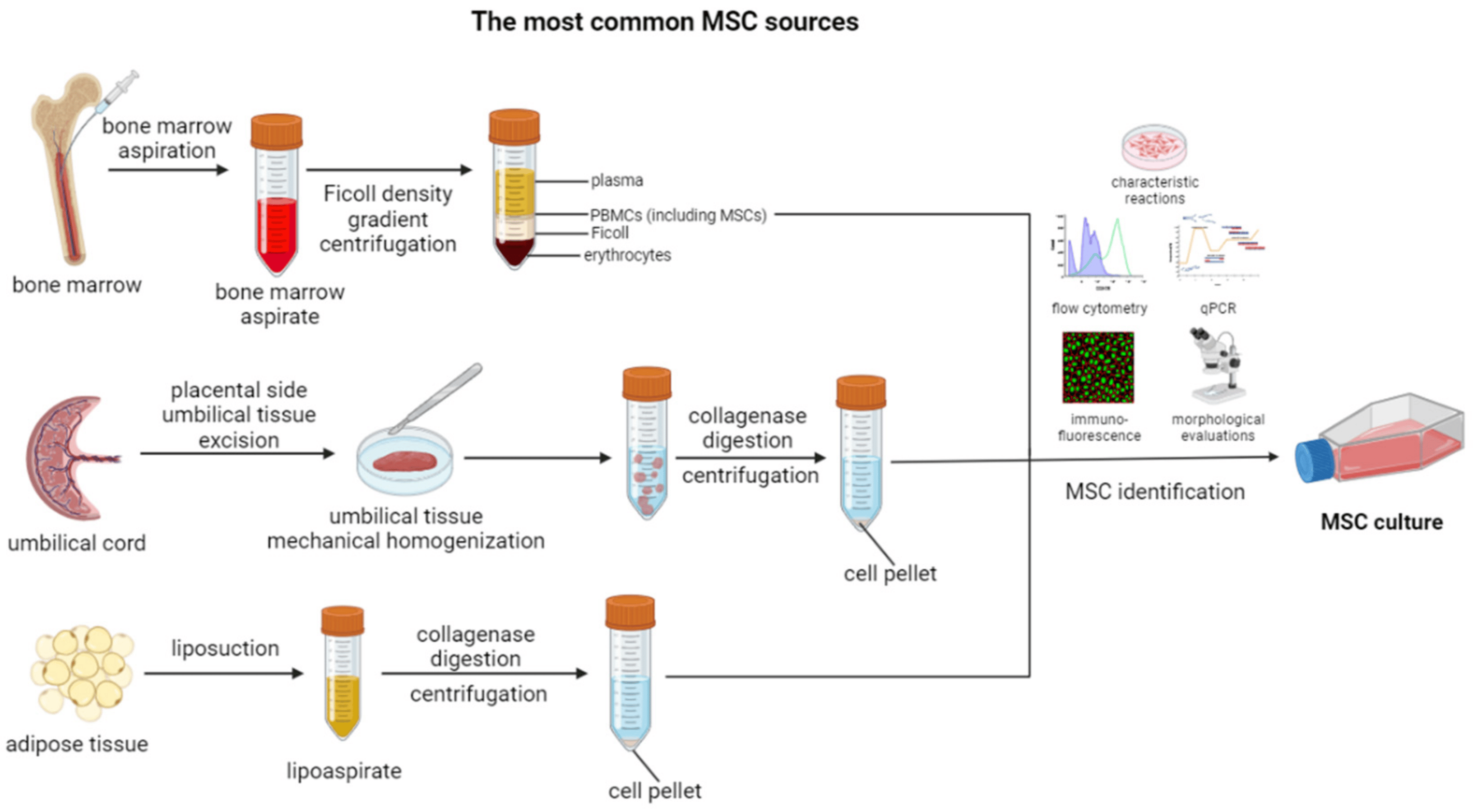
Figure 1. Overview of the most common MSC sources and methods of their isolation. Created with Biorender.com.
MSCs are characterized by specific cell surface markers such as CD73, CD90, and CD105, and lack the expression of hematopoietic cell markers like CD45, CD34, and CD14. These markers are used to identify and isolate MSCs from other cell types [51]. Moreover, there is a number of characteristic properties, that further allow to identify MSCs among other stem cell populations (Figure 2).
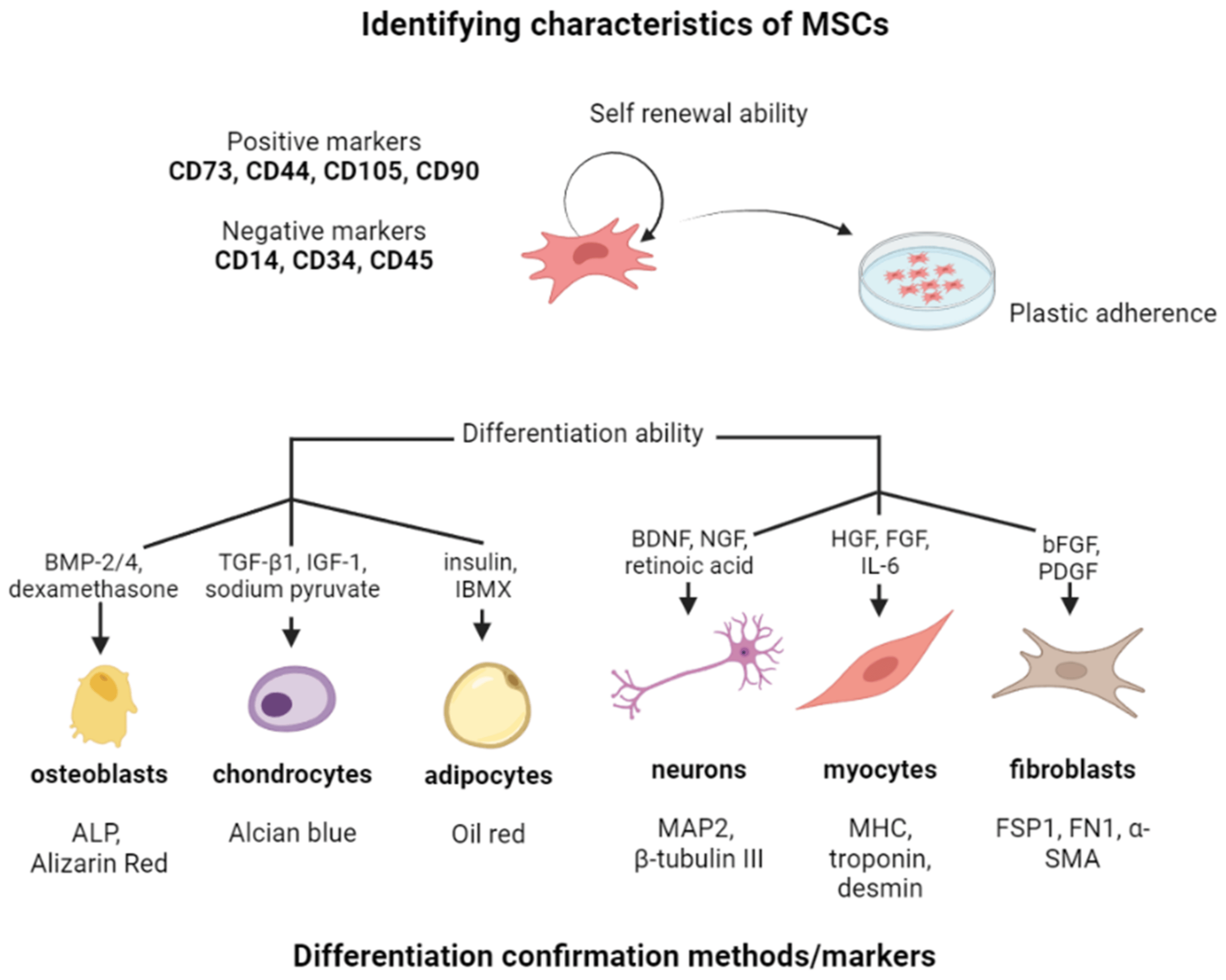
Figure 2. The overview of the identifying characteristics of MSCs. Created with Biorender.com.
MSCs are characterized by their multipotency, which means that they have the ability to differentiate into multiple cell types, including osteocytes, chondrocytes, adipocytes, and myocytes [52,53]. MDC differentiation potential makes them an important tool for regenerative medicine and tissue engineering [2]. The process of MSC differentiation is regulated by a variety of factors, including growth factors, cytokines, and the extracellular matrix. Differentiation involves a series of molecular events that result in changes in gene expression and cell morphology. MSC differentiation can be induced by specific factors, such as dexamethasone, ascorbic acid, and beta-glycerophosphate for osteogenic differentiation, transforming growth factor-beta (TGF-beta) and bone morphogenetic protein-2 (BMP-2) for chondrogenic differentiation, and insulin and dexamethasone for adipogenic differentiation [53,54]. Osteogenic differentiation is the process by which MSCs differentiate into osteoblasts, which are cells responsible for bone formation. During osteogenic differentiation, MSCs undergo changes in gene expression and cell morphology that result in the production of bone matrix proteins, such as collagen and osteocalcin. The resulting osteoblasts then mineralize the bone matrix to form new bone tissue [55,56]. Chondrogenic differentiation is the process where MSCs differentiate into chondrocytes, which are cells responsible for cartilage formation. During chondrogenic differentiation, MSCs undergo changes in gene expression and cell morphology that result in the production of cartilage matrix proteins, such as collagen and aggrecan [57,58]. The resulting chondrocytes then produce a cartilage matrix that can be used for tissue engineering applications [58]. Adipogenic differentiation is the process in which MSCs differentiate into adipocytes, which are cells responsible for fat storage [59]. During adipogenic differentiation, MSCs undergo changes in gene expression and cell morphology that result in the production of lipid droplets. The resulting adipocytes can be used for tissue engineering applications, such as the development of adipose tissue for reconstructive surgery [60,61]. Finally, myogenic differentiation is the process where MSCs differentiate into myocytes, which are cells responsible for muscle formation [62,63]. During myogenic differentiation, MSCs undergo changes in gene expression and cell morphology that result in the production of myogenic proteins, such as MyoD and myogenin. The resulting myocytes can be used for tissue engineering applications, such as the development of muscle tissue for reconstructive surgery [64].
Furthermore, MSCs have the ability to self-renew, which means that they can freely proliferate to create the exact copies of themselves in an almost indefinite manner. This ability is essential for the maintenance of a pool of MSCs in the body that can be used for tissue regeneration and repair when needed. Self-renewal is a complex process that involves several mechanisms. One of the key factors involved in self-renewal is the expression of specific genes that regulate stem cell function. In MSCs, the expression of genes such as Sox2, Oct4, and Nanog has been found to be important for self-renewal [65,66]. The process of self-renewal is strongly influenced by growth factors and cytokines, as they play a crucial role in signaling mesenchymal stem cells (MSCs) to retain their stem cell characteristics and undergo division, resulting in the generation of additional stem cells [67,68,69,70]. For example, fibroblast growth factor-2 (FGF-2) is important for the self-renewal of MSCs [69,71]. The extracellular matrix (ECM) is a complex network of proteins and other molecules that surrounds cells and provides structural support which plays an important role in MSC self-renewal [72]. Interactions with the ECM modulate MSCs’ behaviour, including their self-renewal capacity. Notably, a laminin peptide, an ECM molecule, has been identified as a promoter of MSC self-renewal [73]. Finally, the microenvironment, or niche, where MSCs reside, plays a crucial role in their self-renewal. Within this niche, MSCs receive specific signals that govern their behaviour, including the capacity to self-renew. For instance, The hypoxic microenvironment is crucial for maintaining undifferentiated MSCs by keeping them quiescent and promoting necessary self-renewal. Hypoxia inducible factor (HIF) acts as a molecular regulator within this environment, controlling MSC differentiation and survival [74].
Moreover, MSCs have immunomodulatory properties, they can regulate the various elements of the immune system. They can suppress the activity of T-cells and other immune cells, reducing inflammation and preventing immune-mediated tissue damage [75]. MSCs can aid in tissue repair and regeneration by secreting factors that promote the growth and activity of immune cells and anti-inflammatory factors that can reduce inflammation and promote tissue repair [76]. These factors include interleukin-10 (IL-10), transforming growth factor-beta (TGF-β), and prostaglandin E2 (PGE2) [77,78]. MSCs can also secrete factors that promote the growth of new blood vessels, a process known as angiogenesis. This function can play an important role in in repairing damaged tissues that require a new source of blood supply [79,80]. Furthermore, MSCs have been shown to have neuroprotective properties, meaning they can protect neurons from damage and promote their survival [81]. They can secrete factors that promote nerve cell growth and regeneration, making them a potential therapy for neurological disorders [82]. MSCs can also promote wound healing by secreting growth factors that promote the growth of new skin cells and blood vessels [83,84]. Finally, MSCs are able to remodel the extracellular matrix (ECM) of tissues. The ECM is the complex network of proteins and other molecules that provides structural support to tissues. MSCs can produce enzymes that break down and remodel the ECM, which is important for tissue repair and regeneration [85].
In conclusion, MSCs have a wide range of known physiological functions in the body, including tissue repair and regeneration, immune modulation, anti-inflammatory effects, angiogenesis, and neuroprotection. It also needs to be noted that these cells are a subject of continuous research, indicating that there might by a wide array of yet undiscovered functions that could bring additional promise to their application in further fields of science and medicine.
3. Preclinical Studies, Clinical Trials, and Therapies
Preclinical studies, clinical trials, and therapies involving mesenchymal stem cells (MSCs) are aimed at exploring the therapeutic potential of these cells in various diseases and conditions. The development of MSC-based therapies has been driven by their unique characteristics, including the ability to self-renew, differentiate into various cell types, and exert immunosuppressive effects [86]. Preclinical studies are conducted in laboratory settings or in animals, and are used to evaluate the safety and efficacy of MSCs before they can be tested in humans. These studies have demonstrated that MSCs have the potential to regenerate damaged tissues, reduce inflammation, and promote tissue repair [1]. MSCs have been shown to improve outcomes in preclinical models of a range of diseases and conditions, including heart disease, osteoarthritis, liver disease, and spinal cord injury, among [87,88,89]. MSC-based therapies involve the administration of MSCs directly to patients with the aim of treating specific diseases or conditions. MSCs can be delivered to patients either through injections into the affected tissues or intravenously. MSCs are capable of homing to damaged tissues and promoting tissue repair through mechanisms such as secreting growth factors, reducing inflammation, and inducing angiogenesis [53,79]. Clinical trials are conducted in humans to evaluate the safety and efficacy of MSC-based therapies. Clinical trials involving MSCs are currently underway in various stages, ranging from phase I to phase III. Phase I trials are usually small and focus on evaluating the safety of MSC treatments, while phase II and III trials are larger and focus on evaluating the efficacy of MSC treatments. The results of these trials have been promising, with MSCs showing the potential to treat a range of diseases and conditions, including osteoarthritis, Crohn’s disease, heart failure, and spinal cord injury. The currently completed and terminated studies related to MSCs were presented in . Furthermore, according to the ClinicalTrials.gov database, there are 318 ongoing clinical trials related to mesenchymal stem cells, in different completion stages, with no results yet reported.
While the potential for MSCs in regenerative medicine is vast, there are still many challenges that need to be overcome. One of the major challenges is to ensure the safety and efficacy of MSC treatments, which requires rigorous preclinical and clinical testing [90]. Additionally, the high cost of MSC treatments, as well as the limited availability of funding and insurance coverage, continue to be major barriers to their widespread use.
In conclusion, preclinical studies, clinical trials, and MSC-based therapies are contributing to the development of new treatments for a range of diseases and conditions. While the results of these studies have been promising, further research is needed to fully understand the mechanisms of action of MSCs and to determine their safety and efficacy in the treatment of specific diseases and conditions [90]. Nevertheless, MSCs hold great promise as a new class of regenerative therapies, and their continued development and testing is essential to realizing their full therapeutic potential.
4. MSC Differentiation
Based on their ability to differentiate, MSCs support tissue homeostasis by acting as a source of renewable progenitor cells for the repair of damaged tissues and the replacement of cells in routine cellular turnover throughout adult life [91,92,93]. When cultured under specific conditions, they can differentiate into multiple mesenchymal lineage cell types, including osteoblasts, chondrocytes, adipocytes, and myoblasts [94,95,96,97]. The classical method for osteogenic differentiation of human MSCs involves incubation in fetal bovine serum (FBS)-containing medium supplemented with ascorbic acid, β-glycerophosphate, and dexamethasone, resulting in an increase in calcium accumulation and alkaline phosphatase activity [98,99]. Chondrogenic differentiation is accomplished using pelleted micromass cultured in the presence of transforming growth factor (TGF)-β in serum-free medium, which produces cartilage-specific, highly sulfated proteoglycans and type II collagen [98]. Adipogenic differentiation of MSCs is demonstrated through the detection of lipid vacuoles after dexamethasone, insulin, isobutyl methyl xanthine, and indomethacin are added to medium containing FBS [9]. MSCs can also differentiate into myoblasts when treated with 5-azacytidine and amphotericin B, which fuse into rhythmically beating myotubes [100]. Furthermore, MSCs can also give rise to cross-lineage cell types such as endodermal-hepatocytes and β-cells of pancreatic islets and ectodermal-neurons, a process known as trans-differentiation [101,102]. The liver cells were obtained from MSCs in two stages by culturing them in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with HGF, bFGF and nicotinamide, and in the next stage with the addition of oncostatin M, dexamethasone, and ITS+ (insulin, transferring, selenium). Albumin, α-fetoprotein, and hepatocyte nuclear factor 4 (HNF-4) are present in the resulting cells, which are hepatocyte typical markers [103]. Pancreatic islets of β-cells capable of producing insulin were obtained from MSCs by treating them with a mixture of growth factors secreted by regenerating cells of the pancreas and also by using acitin A, sodium butyrate, taurine, and nicotinamide [104,105]. According to Hofstetter and colleagues, neuron-like cells differentiated from MSCs lack voltage-gated ion channels that are required for action potential generation; thus, they may not be considered as true neurons [106]. Additionally, transdifferentiate of MSCs into endothelial cells expressing endothelial nitric oxide synthase have been reported that contribute to endothelial function improvement in vascular injury rat model [107,108]. There has been widespread evidence that miRNAs and lncRNAs play an important role in the differentiation of MSCs, both positively and negatively, as reported herein and .
5. Signalling Pathways Governing MSC Function
Based on the widely accepted definition of ‘tissue engineering’ that was proposed by Robert Nerem in 1988, MSCs can be regarded as an inherent component of the modern regenerative medicine, since they can readily be used for the generation of different cell lineages. The growing success of today’s regenerative medicine stems from the pluripotent nature of MSCs that renders them capable of transforming into other cell types with regards to their microenvironment, which consists of non-coding RNAs, among others [303]. A strikingly high proportion of studies have focused on identification of ncRNAs that facilitate or impair the differentiation of MSCs. These ncRNAs usually constitute an elaborate network or axis of interactions involving lncRNAs, miRNAs, mRNAs and other types of ncRNAs, which can ultimately affect the proliferative and regenerative activity of these cells. Generally, in RNA-based regulatory pathways, lncRNAs bind and sponge miRNAs to indirectly promote the translation of certain mRNAs to their final product. As such, a basic lncRNA/miRNA/mRNA pathway includes an inhibitory pathway accompanied by an indirect de-repressing effect. While a range of other lncRNAs and miRNAs might be involved in this inhibitory process, they usually are the final effector molecule that determines the final cell fate [304]. For instance, if the axis ends in ‘vascular endothelial growth factor’ (VEGF) with a net de-repressing or stimulatory effect, the MSCs occurring in that microenvironment will be compelled to differentiate into endothelial cells, giving rise to vasculature [305]. In addition to microenvironmental properties, the biological origin of MSCs may influence the course of differentiation. Bone marrow, umbilical cord, adipose tissue, peripheral blood and synovium stand among the most frequently preferred sources of MSCs in experimental and clinical applications. Despite being pluripotent, MSCs are still subject to epigenetic regulatory programs associated with the source from which they are derived. In this sense, MSCs extracted from the synovial space are theoretically anticipated to yield better results when used for cartilage regeneration in joint disorders [306]. Still, there are no strict rules regarding the source, as there are reports of successful trials of seemingly contrasting sources for regenerative purposes such as application of adipose-derived MSCs for osteogenic regeneration in patients with osteoarthritis [307], suggesting, once again, that environmental factors and regulatory pathways are as important as the source. Figure 3 illustrates various miRNAs that are critical during MSCs differentiation.
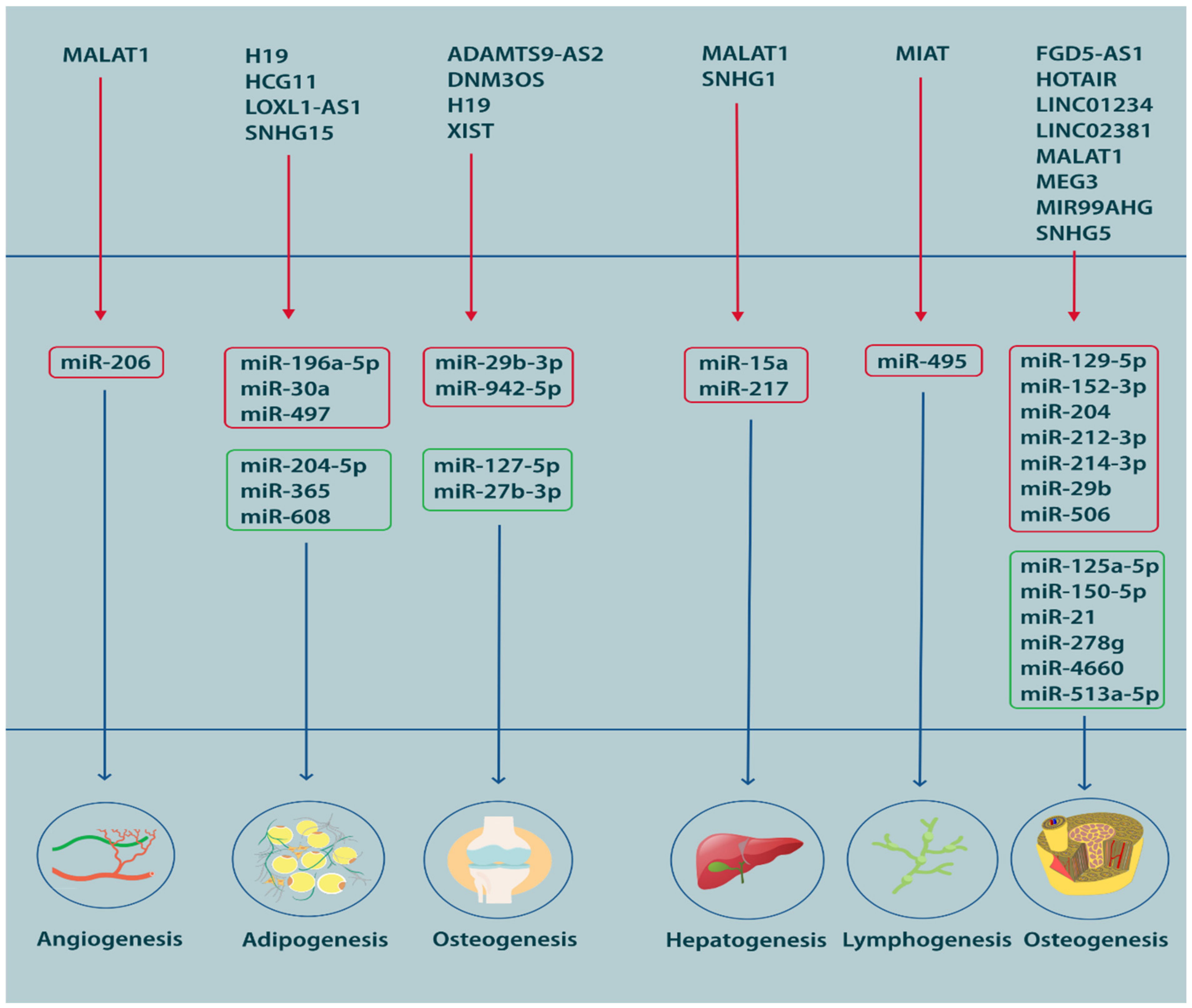
Figure 3. A visual representation of miRNA participation in the process of MSC differentiation.
The lnRNA-miRNA basis of MSC differentiation has primarily been studied in the case of osteogenic, chondrogenic and adipogenic differentiation. More scantly, the role of ncRNAs in hepatogenic, angiogenic and lymphatogenic differentiation has also been explored, albeit, to a much lesser extent. Induction of osteogenic differentiation is of utmost importance in the treatment of degenerative bone diseases. Accordingly, regulatory lncRNAs and miRNAs can be used as therapeutic agents or targets with regards to their stimulatory or inhibitory effects, respectively. One good example is ‘metastasis associated lung adenocarcinoma transcript 1′ (MALAT1), a tumor-associated lncRNA with known osteogenic effects [308,309]. Considering its mechanism of action, MALAT1 can either be used as an exogenous therapeutic agent for induction of osteogenesis or targeted by proxy when it is lowly expressed. Downregulation of MALAT1, as an osteogenic lncRNA, results in de-repression of anti-osteogenic miRNAs, which can be targeted and silenced using specialized short hairpin RNAs (shRNAs) [308,309]. Though, differentiation is not necessarily a desirable outcome, particularly when it comes to malignancies. MALAT1, which is a beneficial factor in the case of hypoproliferative disorders, may assume an adverse role in the context of oncogenesis, where overexpression of MALAT1 stimulates formation of new endothelial cells, hence, promoting angiogenesis in osteosarcoma [310]. However, the regulation of differentiation is important in treating disorders associated with impaired formation or degeneration of vasculature, which may benefit from overexpression of MALAT1 [305]. One reason for this presumable divergent function of MALAT1, or any other lncRNA for that matter, is the difference in miRNAs which are targeted and sponged in each scenario. When it is a beneficial pro-angiogenic factor, MALAT1 targets miR-206 to upregulate VEGFA in the population of endothelial cells that might be overproducing the anti-angiogenic miR-206 [305]. When it is an aggravator of tumor-associated angiogenesis, MALAT1 targets the anti-angiogenic miR-150-5p, when it should not be sponged [310]. In this sense, a good understanding of the lncRNA-miRNA networks governing cell differentiation in health and disease can substantially contribute to the performance of regenerative medicine. The full overview of knowledge regarding the participation of miRNAs and lncRNAs in differentiation of MSCs, as well as the different lncRNA-miRNA axes regulates differentiation into different lineages .
6. Practical Implications and Future Perspective of lncRNA and miRNA in MSCs Treatment
Numerous studies highlight the potential of mesenchymal stem cells (MSCs) in repairing various organs like the lungs, heart, and skin. Exosomes, tiny vesicles produced by MSCs, have gained importance in regenerative medicine [335]. Exosomes, packed with RNA and proteins, are safer and more stable than direct MSC transplants [336]. They play a crucial role in healing by delivering therapeutic substances, especially microRNAs (miRNAs), which regulate gene activity in nearby or distant cells [337].Studies show that MSC-derived exosomes can transport miRNAs, such as miR-132–3p, to endothelial cells, improving their growth and reducing blood-brain barrier dysfunction in a brain injury model [338]. These exosomes boost the expression of essential genes in traumatic brain injury.
Exosomes and miRNAs offer promise in treating various diseases, including neurological, cardiovascular, and kidney disorders. Exosomes containing specific miRNAs have beneficial effects on neurological conditions, reducing cell death and inflammation. MiRNAs like miR-126 and miR-184 help brain recovery in stroke models [339]. In autoimmune encephalomyelitis, BM-MSC exosomes deliver miR-367–3p, reducing symptoms [340]. MSC-derived exosomes are also promising in cardiovascular diseases. They target specific genes, reducing inflammation and improving heart function. For example, exosomes containing miR-149 have been used to target genes and modulate the inflammatory response [341]. In kidney repair, they counter calcification and promote recovery. Exosomes containing miR-874-3p have been shown to control necroptosis, decrease renal tubular cell damage, and improve healing in acute kidney injury [342]. For liver issues, exosomes enriched with miR-148a mitigate symptoms, and miR-20a-5p promotes liver repair [343,344]. Lung diseases, arthritis, and osteoarthritis also show potential for exosome therapy. Lung diseases, such as cystic fibrosis, pulmonary fibrosis, and radiation-induced lung injury, have been studied in the perspective of exosomal therapy. MiR-466f-3p and miR-186 have shown therapeutic potential in reducing inflammation, fibrosis, and promoting repair [345,346]. In the case of rheumatoid arthritis, exosomes containing miR-150-5p have been used to downregulate MMP14 and VEGF, reducing inflammation and protecting against cartilage and bone degradation [347]. Exosomes can be used to encourage direct intracellular transfer of miRNAs between cells, thereby promoting anti-inflammatory effects. Osteoarthritis has been studied in the context of BMP2-induced chondrogenesis and the Wnt signaling pathway. Exosomal miR-181c-5p and miR-92a-3p have been implicated in cartilage repair and Wnt inhibition [348,349].
LncRNAs have shown exciting potential in addressing various health conditions and guiding MSCs through various cellular processes. In osteogenic differentiation, LncRNAs like H19, HULC, and MALAT1 exert their influence, promoting bone formation through mechanisms involving miRNAs and key signaling pathways [279,286,287]. Notably, researchers have uncovered a distinctive LncRNA, lncRNA-OG, driving bone growth alongside hnRNPK, which could pave the way for better bone-related treatments [289]. While the immunoregulatory potential of MSCs is significant, only a few studies, like one involving LncRNA-MALAT1, have delved into this arena [43]. Investigating LncRNA-driven immune regulation in MSCs is an area rich in potential. Furthermore, LncRNAs including Lnc-ZNF354A, Lnc-LIN54, Lnc-FRG2C, and Lnc-USP50, were found to be closely associated with pathological bone formation in ankylosing spondylitis [350].Adipogenic differentiation, the process of forming fat cells, is also influenced by LncRNAs such as GAS5 and HOTAIR [299,300]. The balance between osteogenesis and adipogenesis in MSCs is delicately controlled by LncRNAs like H19 and TCONS_00041960, offering a potential therapeutic angle for conditions like osteoporosis [292,296]. Interestingly, LncRNA lnc13728 surfaces, significantly influencing the proliferation of fat cells and modulating genes associated with obesity, presenting opportunities to tackle obesity-related challenges more effectively [298]. In the context of chondrogenic differentiation, LncRNAs like ZBED3-AS1 steer MSCs toward the formation of cartilage tissue, influencing pivotal pathways such as Wnt/β-catenin and offering prospects for therapeutic interventions, particularly in conditions like osteoarthritis [295]. Venturing into the realms of neurogenesis, myogenesis, and endothelial differentiation, LncRNAs like H19, MIAT, MEG3, and HULC actively contribute to the formation of neural, smooth muscle, and endothelial cells [351,352]. Their roles in addressing nerve injuries and cardiovascular therapies beckon for deeper exploration.
Meanwhile, the impact of exosomes from lung cancer on the LncRNA expression profile of MSCs emphasizes the participation of LncRNAs in the intricate interplay between MSCs and tumor cells, ultimately affecting the progression of diseases [353]. This underscores the potential of using LncRNA profiles in circulating MSCs as personalized diagnostic tools for specific medical conditions. Circulating MSCs in peripheral blood hold promise as diagnostic markers for various diseases, offering a novel and precise diagnostic method by identifying specific LncRNAs or patterns within these MSCs. Furthermore, there is an uncharted frontier in enhancing the clinical effectiveness of MSC-based therapies by manipulating LncRNAs that govern MSC behavior. Employing gene editing techniques to fine-tune specific LncRNA expressions has the potential to enhance the immunoregulatory capabilities of MSCs in autoimmune diseases and guide their differentiation into specialized cell types for tissue and regeneration engineering. This dynamic approach opens exciting avenues for refining MSC-based therapies across various diseases.
Overall, LncRNAs are master conductors of MSC behavior, orchestrating a symphony of cellular functions, from differentiation to proliferation and immunoregulation. while, exosomes and miRNAs have opened exciting avenues in regenerative medicine, offering hope for various health conditions. Understanding and harnessing the power of LncRNAs in MSCs offer promising avenues for innovative therapeutics and regenerative medicine.
7. Conclusions
It is important to consider the intricate regulatory roles of miRNAs and lncRNAs in governing the signalling pathways that dictate MSC functioning and differentiation. The findings presented underscore the pivotal significance of these small RNA molecules in the realm of regenerative medicine and hold great promise for future therapeutic applications. The characterization and functional attributes of MSCs have been thoroughly examined, revealing their remarkable potential in tissue repair and immune modulation. As highlighted by an array of preclinical studies, clinical trials, and innovative therapies, MSCs have demonstrated their transformative capability in addressing diverse medical conditions, further emphasizing their significance as a regenerative resource. The emerging understanding of lncRNAs as key modulators of lineage commitment. The intricate interplay between lncRNAs and signalling pathways provides crucial insights into the mechanisms governing MSC fate determination, offering opportunities for targeted interventions and precision therapeutics. Furthermore, the regulatory impact of miRNAs on MSC differentiation has been comprehensively analysed, unravelling the complexity of gene expression network. The interplay between miRNAs and their target genes offers a deep understanding of the regulatory landscape driving MSC differentiation processes, paving the way for potential therapeutic strategies targeting these molecular interactions.
In conclusion, the knowledge amassed serves as a crucial foundation for further advancements in regenerative medicine. Harnessing the regulatory potential of miRNAs and lncRNAs in MSCs presents exciting prospects for developing targeted therapies and personalized treatment approaches, ultimately enhancing the efficacy of regenerative strategies and positively impacting patient outcomes. As research in this field continues to evolve, it is imperative to explore and exploit the vast potential of miRNAs and lncRNAs as therapeutic agents. The findings presented here provide a solid basis for ongoing investigations, fuelling the quest to fully unlock the regenerative potential of MSCs.
References
- Wong, S.P.; Rowley, J.E.; Redpath, A.N.; Tilman, J.D.; Fellous, T.G.; Johnson, J.R. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol. Ther. 2015, 151, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and MiR-16 Induce Apoptosis by Targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Goodfellow, S.J.; White, R.J. Regulation of RNA Polymerase III Transcription During Mammalian Cell Growth. Cell Cycle 2007, 6, 2323–2326. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Calin, G.A.; Lopez-Berestein, G.; Sood, A.K. miRNA Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discov. 2016, 6, 235–246. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNA Dysregulation in Cancer: Diagnostics, Monitoring and Therapeutics. A Comprehensive Review. EMBO Mol. Med. 2012, 4, 143–159. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, M.; Cao, Y. New Insight into microRNA Functions in Cancer: Oncogene–microRNA–Tumor Suppressor Gene Network. Front. Mol. Biosci. 2017, 4, 46. [Google Scholar] [CrossRef]
- O’Bryan, S.; Dong, S.; Mathis, J.M.; Alahari, S.K. The roles of oncogenic miRNAs and their therapeutic importance in breast cancer. Eur. J. Cancer 2017, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, X.; Cobb, G.; Anderson, T. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef]
- Otmani, K.; Lewalle, P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Valihrach, L.; Androvic, P.; Kubista, M. Circulating miRNA analysis for cancer diagnostics and therapy. Mol. Asp. Med. 2019, 72, 100825. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Croce, C.M. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef]
- Hodges, W.M.; O’brien, F.; Fulzele, S.; Hamrick, M.W. Function of microRNAs in the Osteogenic Differentiation and Therapeutic Application of Adipose-Derived Stem Cells (ASCs). Int. J. Mol. Sci. 2017, 18, 2597. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Bononi, I.; Frontini, F.; Mazzoni, E.; Oton-Gonzalez, L.; Rotondo, J.C.; Torreggiani, E.; Tognon, M.; et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 2021, 11, 6573–6591. [Google Scholar] [CrossRef]
- Martin, E.; Qureshi, A.; Dasa, V.; Freitas, M.; Gimble, J.; Davis, T. MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: Perspectives on miRNA biogenesis and cellular transcriptome. Biochimie 2016, 124, 98–111. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.-M.; Maumus, M.; Bony, C.; Jorgensen, C.; Noël, D. Contribution of microRNAs to the immunosuppressive function of mesenchymal stem cells. Biochimie 2018, 155, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Noël, D.; Pers, Y.M.; Apparailly, F.; Jorgensen, C. Deregulation and Therapeutic Potential of MicroRNAs in Arthritic Diseases. Nat. Rev. Rheumatol. 2016, 12, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Yin, G.; Xie, Q. Therapeutic prospects of MicroRNAs carried by mesenchymal stem cells-derived extracellular vesicles in autoimmune diseases. Life Sci. 2021, 277, 119458. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.J. MicroRNA-Based Therapeutics in Central Nervous System Injuries. J. Cereb. Blood Flow. Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Della Porta, G.; Peretti, G.M.; Maffulli, N. Therapeutic potential of microRNA in tendon injuries. Br. Med Bull. 2020, 133, 79–94. [Google Scholar] [CrossRef]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef]
- Zealy, R.W.; Fomin, M.; Davila, S.; Makowsky, D.; Thigpen, H.; McDowell, C.H.; Cummings, J.C.; Lee, E.S.; Kwon, S.-H.; Min, K.-W.; et al. Long noncoding RNA complementarity and target transcripts abundance. Biochim. et Biophys. Acta BBA Gene Regul. Mech. 2018, 1861, 224–234. [Google Scholar] [CrossRef]
- Arora, R.; Lee, Y.; Wischnewski, H.; Brun, C.M.; Schwarz, T.; Azzalin, C.M. RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat. Commun. 2014, 5, 5220. [Google Scholar] [CrossRef]
- Aznaourova, M.; Janga, H.; Sefried, S.; Kaufmann, A.; Dorna, J.; Volkers, S.M.; Georg, P.; Lechner, M.; Hoppe, J.; Dökel, S.; et al. Noncoding RNA MaIL1 is an integral component of the TLR4–TRIF pathway. Proc. Natl. Acad. Sci. USA 2020, 117, 9042–9053. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Postepska-Igielska, A.; Giwojna, A.; Gasri-Plotnitsky, L.; Schmitt, N.; Dold, A.; Ginsberg, D.; Grummt, I. LncRNA Khps1 Regulates Expression of the Proto-oncogene SPHK1 via Triplex-Mediated Changes in Chromatin Structure. Mol. Cell 2015, 60, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, X.; Duan, W.; Wang, R.; Wang, L.; Zheng, G.; Yan, K.; Wang, L.; Li, J.; Zhang, X.; et al. Cell-free microRNA expression signatures in urine serve as novel noninvasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Oncotarget 2017, 8, 40832–40842. [Google Scholar] [CrossRef]
- Li, Q.; Shao, Y.; Zhang, X.; Zheng, T.; Miao, M.; Qin, L.; Wang, B.; Ye, G.; Xiao, B.; Guo, J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumor Biol. 2014, 36, 2007–2012. [Google Scholar] [CrossRef]
- Dong, L.; Lin, W.; Qi, P.; Xu, M.-D.; Wu, X.; Ni, S.; Huang, D.; Weng, W.-W.; Tan, C.; Sheng, W.; et al. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 1158–1166. [Google Scholar] [CrossRef]
- Jiang, M.-C.; Ni, J.-J.; Cui, W.-Y.; Wang, B.-Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366. [Google Scholar]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Sebastian-Delacruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The Role of lncRNAs in Gene Expression Regulation through mRNA Stabilization. Non Coding RNA 2021, 7, 3. [Google Scholar] [CrossRef]
- Barter, M.J.; Gomez, R.; Hyatt, S.; Cheung, K.; Skelton, A.J.; Xu, Y.; Clark, I.M.; Young, D.A. The Long Non-Coding RNA ROCR Contributes to Sox9 Expression and Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Development 2017, 144, 4510–4521. [Google Scholar] [CrossRef]
- Ju, C.; Liu, R.; Zhang, Y.-W.; Zhang, Y.; Zhou, R.; Sun, J.; Lv, X.-B.; Zhang, Z. Mesenchymal stem cell-associated lncRNA in osteogenic differentiation. Biomed. Pharmacother. 2019, 115, 108912. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wei, W.; Zhao, B.; Guo, X.; Liu, S. Long Non-Coding RNA HOTAIR Inhibits MIR-17-5p to Regulate Osteogenic Differentiation and Proliferation in Nontraumatic Osteonecrosis of Femoral Head. PLoS ONE 2017, 12, e0169097. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Liu, F.; Liu, D.; Miao, H.; Ren, J.; Xu, J.; Ding, L.; Hu, Y.; Wang, Z.; et al. Long Non-Coding RNA MALAT1 Promotes Proliferation, Angiogenesis, and Immunosuppressive Properties of Mesenchymal Stem Cells by Inducing VEGF and IDO. J. Cell. Biochem. 2017, 118, 2780–2791. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Gu, P.-C.; Xu, S.-Z.; Lin, X.-J. Long non-coding RNA-DANCR in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. Biosci. Biotechnol. Biochem. 2015, 79, 732–737. [Google Scholar] [CrossRef]
- Seo, J.-S.; Kwak, M.; Hong, S.; Yu, S.-L.; Sim, B.-W.; Kang, J. Parthenogenetic embryonic stem cells with H19 siRNA-mediated knockdown as a potential resource for cell therapy. Int. J. Mol. Med. 2011, 29, 257–262. [Google Scholar] [CrossRef]
- Gloss, B.S.; Dinger, M.E. The specificity of long noncoding RNA expression. Biochim. et Biophys. Acta BBA Gene Regul. Mech. 2016, 1859, 16–22. [Google Scholar] [CrossRef]
- Patel, A.N.; Vargas, V.; Revello, P.; Bull, D.A. Mesenchymal Stem Cell Population Isolated from the Subepithelial Layer of Umbilical Cord Tissue. Cell Transplant. 2013, 22, 513–519. [Google Scholar] [CrossRef]
- Halvorsen, Y.; Wilkison, W.; Gimble, J. Adipose-derived stromal cells—Their utility and potential in bone formation. Int. J. Obes. 2000, 24, S41–S44. [Google Scholar] [CrossRef]
- Surrati, A.; Evseev, S.; Jourdan, F.; Kim, D.-H.; Sottile, V. Osteogenic Response of Human Mesenchymal Stem Cells Analysed Using Combined Intracellular and Extracellular Metabolomic Monitoring. Cell. Physiol. Biochem. 2021, 55, 311–326. [Google Scholar] [CrossRef]
- De Souza Fernandez, T.; de Souza Fernandez, C. Mesenchymal Stem Cells: Biological Characteristics and Potential Clinical Applications for Haematopoietic Stem Cell Transplantation. In Pluripotent Stem Cells—From the Bench to the Clinic; IntechOpen: London, UK, 2016. [Google Scholar]
- Mihaylova, Z. Stem cells and mesenchymal stem cell markers. Int. J. Med. Sci. Clin. Invent. 2019, 6, 4544–4547. [Google Scholar] [CrossRef]
- Guo, X.; Bai, Y.; Zhang, L.; Zhang, B.; Zagidullin, N.; Carvalho, K.; Du, Z.; Cai, B. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Stem Cell Res. Ther. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, M.C.; Malpasso, G.; Musarò, P.; Turco, V.; Gnecchi, M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Methods Mol. Biol. 2016, 1416, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus. Med. Hemother. 2008, 35, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.-T.; Huang, Y.-J.; Wu, H.-H.; Liu, Y.-A.; Liu, Y.-S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef]
- Kärner, E.; Bäckesjö, C.-M.; Cedervall, J.; Sugars, R.V.; Ährlund-Richter, L.; Wendel, M. Dynamics of gene expression during bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Biochim. et Biophys. Acta BBA Gen. Subj. 2009, 1790, 110–118. [Google Scholar] [CrossRef]
- Griffin, M.; Hindocha, S.; Khan, W.S. Chondrogenic Differentiation of Adult MSCs. Curr. Stem Cell Res. Ther. 2012, 7, 260–265. [Google Scholar] [CrossRef]
- Yang, X.; Tian, S.; Fan, L.; Niu, R.; Yan, M.; Chen, S.; Zheng, M.; Zhang, S. Integrated regulation of chondrogenic differentiation in mesenchymal stem cells and differentiation of cancer cells. Cancer Cell Int. 2022, 22, 169. [Google Scholar] [CrossRef]
- Sekiya, I.; Larson, B.L.; Vuoristo, J.T.; Cui, J.-G.; Prockop, D.J. Adipogenic Differentiation of Human Adult Stem Cells from Bone Marrow Stroma (MSCs). J. Bone Miner. Res. 2003, 19, 256–264. [Google Scholar] [CrossRef]
- Stosich, M.S.M.; Mao, J.J.D. Adipose Tissue Engineering from Human Adult Stem Cells: Clinical Implications in Plastic and Reconstructive Surgery. Plast. Reconstr. Surg. 2007, 119, 71–83. [Google Scholar] [CrossRef]
- Beahm, E.K.; Walton, R.L.; Patrick, C.W. Progress in adipose tissue construct development. Clin. Plast. Surg. 2003, 30, 547–558. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Li, X.; Fan, Z.; Liu, Z.; Xie, X.; Guan, J. Regulating myogenic differentiation of mesenchymal stem cells using thermosensitive hydrogels. Acta Biomater. 2015, 26, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.P.; Bitto, F.F.; Lange, C.; Klumpp, D.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Horch, R.E.; Kneser, U. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol. Int. 2011, 35, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Meligy, F.Y.; Shigemura, K.; Behnsawy, H.M.; Fujisawa, M.; Kawabata, M.; Shirakawa, T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. Vitr. Cell. Dev. Biol. Anim. 2012, 48, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Su, P.-F.; Huang, Y.-F.; Yew, T.-L.; Hung, S.-C. Oct4 and Nanog Directly Regulate Dnmt1 to Maintain Self-Renewal and Undifferentiated State in Mesenchymal Stem Cells. Mol. Cell 2012, 47, 169–182. [Google Scholar] [CrossRef]
- Kolf, C.M.; Cho, E.; Tuan, R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007, 9, 204. [Google Scholar] [CrossRef]
- Schwartz, J.; Van De Pavert, S.; Clarke, I.; Rao, A.; Ray, D.; Vrana, K. Paracrine Interactions within the Pituitary Gland. Ann. N. Y. Acad. Sci. 1998, 839, 239–243. [Google Scholar] [CrossRef]
- Kléber, M.; Sommer, L. Wnt signaling and the regulation of stem cell function. Curr. Opin. Cell Biol. 2004, 16, 681–687. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Shimazu, A.; Miyazaki, K.; Pan, H.; Koike, C.; Yoshida, E.; Takagishi, K.; Kato, Y. Retention of Multilineage Differentiation Potential of Mesenchymal Cells during Proliferation in Response to FGF. Biochem. Biophys. Res. Commun. 2001, 288, 413–419. [Google Scholar] [CrossRef]
- Jiang, Y.; Vaessen, B.; Lenvik, T.; Blackstad, M.; Reyes, M.; Verfaillie, C.M. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp. Hematol. 2002, 30, 896–904. [Google Scholar] [CrossRef]
- Zaragosi, L.; Ailhaud, G.; Dani, C. Autocrine Fibroblast Growth Factor 2 Signaling Is Critical for Self-Renewal of Human Multipotent Adipose-Derived Stem Cells. Stem Cells 2006, 24, 2412–2419. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.T.; Sohi, A.N.; Esmaeili, E.; Karami, S.; Soleimanifar, F.; Nasoohi, N. Immobilized Laminin-derived Peptide Can Enhance Expression of Stemness Markers in Mesenchymal Stem Cells. Biotechnol. Bioprocess Eng. 2019, 24, 876–884. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, X.; Xu, Y.; Yu, J. Hypoxia-Inducible Non-coding RNAs in Mesenchymal Stem Cell Fate and Regeneration. Front. Dent. Med. 2021, 2. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Ghannam, S.; Pène, J.; Torcy-Moquet, G.; Jorgensen, C.; Yssel, H. Mesenchymal Stem Cells Inhibit Human Th17 Cell Differentiation and Function and Induce a T Regulatory Cell Phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Y.; Zhang, Y.; Liu, J.; Xu, Z. Exosomes Secreted by Adipose-Derived Stem Cells Contribute to Angiogenesis of Brain Microvascular Endothelial Cells Following Oxygen–Glucose Deprivation In Vitro Through MicroRNA-181b/TRPM7 Axis. J. Mol. Neurosci. 2018, 65, 74–83. [Google Scholar] [CrossRef]
- Han, Y.; Ren, J.; Bai, Y.; Pei, X.; Han, Y. Exosomes from Hypoxia-Treated Human Adipose-Derived Mesenchymal Stem Cells Enhance Angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019, 109, 59–68. [Google Scholar] [CrossRef]
- Uccelli, A.; Benvenuto, F.; Laroni, A.; Giunti, D. Neuroprotective features of mesenchymal stem cells. Best Pr. Res. Clin. Haematol. 2011, 24, 59–64. [Google Scholar] [CrossRef]
- Ohtaki, H.; Ylostalo, J.H.; Foraker, J.E.; Robinson, A.P.; Reger, R.L.; Shioda, S.; Prockop, D.J. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc. Natl. Acad. Sci. USA 2008, 105, 14638–14643. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Zhang, C.; Hwang, Y.; Varghese, S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 97–106. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Potapova, I.A.; Gaudette, G.R.; Brink, P.R.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; Doronin, S.V. Mesenchymal Stem Cells Support Migration, Extracellular Matrix Invasion, Proliferation, and Survival of Endothelial Cells In Vitro. Stem Cells 2007, 25, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Jasim, S.A.; Yumashev, A.V.; Abdelbasset, W.K.; Margiana, R.; Markov, A.; Suksatan, W.; Pineda, B.; Thangavelu, L.; Ahmadi, S.H. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res. Ther. 2022, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Sensebé, L. Mesenchymal stromal cells: Misconceptions and evolving concepts. Cytotherapy 2013, 15, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, S.; Richter, W. Chondrogenesis of mesenchymal stem cells: Role of tissue source and inducing factors. Stem Cell Res. Ther. 2010, 1, 31. [Google Scholar] [CrossRef]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W.; Bakillah, A.; Hussain, M.M.; Song, Y.-S.; Lee, D.H.; Yu, J.-H.; Oh, D.-K.; et al. Current Methods of Adipogenic Differentiation of Mesenchymal Stem Cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Westhrin, M.; Xie, M.; Olderøy, M.; Sikorski, P.; Strand, B.L.; Standal, T. Osteogenic Differentiation of Human Mesenchymal Stem Cells in Mineralized Alginate Matrices. PLoS ONE 2015, 10, e0120374. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, X.; Liu, C.; Li, J.; Liu, F.; Huang, Y. Co-expression of Akt1 and Wnt11 promotes the proliferation and cardiac differentiation of mesenchymal stem cells and attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Biomed. Pharmacother. 2018, 108, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997, 64, 295–312. [Google Scholar] [CrossRef]
- Wakitani, S.; Saito, T.; Caplan, A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995, 18, 1417–1426. [Google Scholar] [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004, 18, 980–982. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Der Lee, K.; Kuo, T.K.C.; Whang-Peng, J.; Chung, Y.F.; Lin, C.T.; Chou, S.H.; Chen, J.R.; Chen, Y.P.; Lee, O.K.S. In Vitro Hepatic Differentiation of Human Mesenchymal Stem Cells. Hepatology 2004, 40, 1275–1284. [Google Scholar] [CrossRef]
- Phadnis, S.M.; Joglekar, M.V.; Dalvi, M.P.; Muthyala, S.; Nair, P.D.; Ghaskadbi, S.M.; Bhonde, R.R.; Hardikar, A.A. Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo. Cytotherapy 2011, 13, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, V.; Ronald, V.; Abdullah, A.; Nathan, K.G.; Aziz, Z.A.; Abdullah, M.; Musa, S.; Abu Kasim, N.; Bhonde, R. Differentiation of Dental Pulp Stem Cells into Islet-like Aggregates. J. Dent. Res. 2011, 90, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, C.P.; Schwarz, E.J.; Hess, D.; Widenfalk, J.; El Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ma, A.; Wang, T.; Han, K.; Liu, Y.; Zhang, Y.; Zhao, X.; Dong, A.; Du, Y.; Huang, X.; et al. Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats. Transpl. Int. 2006, 19, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.-M.; Liu, W.; Bi, Y.-W.; He, X.-P.; Sun, W.-Y.; Pang, X.-Y.; Gu, X.-H.; Wang, X.-P. Mesenchymal Stem Cells Differentiate into an Endothelial Phenotype, Reduce Neointimal Formation, and Enhance Endothelial Function in a Rat Vein Grafting Model. Stem Cells Dev. 2008, 17, 785–794. [Google Scholar] [CrossRef]
- Li, Z.; Hassan, M.Q.; Volinia, S.; van Wijnen, A.J.; Stein, J.L.; Croce, C.M.; Lian, J.B.; Stein, G.S. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc. Natl. Acad. Sci. USA 2008, 105, 13906–13911. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zhang, Y.; Ma, L.; Lin, L.; Meng, J.; Jiang, L.; Wang, L.; Zhou, P.; Zhang, Y. LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed. Pharmacother. 2017, 89, 1178–1186. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Zhao, Y.; Cao, C.; Wu, K.; Zhao, L.; Zhang, Y. Non-viral oligonucleotide antimiR-138 delivery to mesenchymal stem cell sheets and the effect on osteogenesis. Biomaterials 2014, 35, 7734–7749. [Google Scholar] [CrossRef]
- Hu, J.; Liao, H.; Ma, Z.; Chen, H.; Huang, Z.; Zhang, Y.; Yu, M.; Chen, Y.; Xu, J. Focal Adhesion Kinase Signaling Mediated the Enhancement of Osteogenesis of Human Mesenchymal Stem Cells Induced by Extracorporeal Shockwave. Sci. Rep. 2016, 6, 20875. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Yagi, K.; Tokuzawa, Y.; Kanesaki-Yatsuka, Y.; Suda, T.; Katagiri, T.; Fukuda, T.; Maruyama, M.; Okuda, A.; Amemiya, T.; et al. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem. Biophys. Res. Commun. 2008, 368, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Schoolmeesters, A.; Eklund, T.; Leake, D.; Vermeulen, A.; Smith, Q.; Aldred, S.F.; Fedorov, Y. Functional Profiling Reveals Critical Role for miRNA in Differentiation of Human Mesenchymal Stem Cells. PLoS ONE 2009, 4, e5605. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Lu, J.; Yu, X.; Yu, Y. Expression of Sp7 in Satb2-induced osteogenic differentiation of mouse bone marrow stromal cells is regulated by microRNA-27a. Mol. Cell. Biochem. 2016, 417, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, L.; Xing, L.; Di Chen, D. MicroRNA-204 Regulates Runx2 Protein Expression and Mesenchymal Progenitor Cell Differentiation. Stem Cells 2009, 28, 357–364. [Google Scholar] [CrossRef]
- Inose, H.; Ochi, H.; Kimura, A.; Fujita, K.; Xu, R.; Sato, S.; Iwasaki, M.; Sunamura, S.; Takeuchi, Y.; Fukumoto, S.; et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 20794–20799. [Google Scholar] [CrossRef]
- Luzi, E.; Marini, F.; Sala, S.C.; Tognarini, I.; Galli, G.; Brandi, M.L. Osteogenic Differentiation of Human Adipose Tissue-Derived Stem Cells Is Modulated by the miR-26a Targeting of the SMAD1 Transcription Factor. J. Bone Miner. Res. 2007, 23, 287–295. [Google Scholar] [CrossRef]
- Lv, R.; Pan, X.; Song, L.; Sun, Q.; Guo, C.; Zou, S.; Zhou, Q. MicroRNA-200a-3p accelerates the progression of osteoporosis by targeting glutaminase to inhibit osteogenic differentiation of bone marrow mesenchymal stem cells. BioMedicine 2019, 116, 108960. [Google Scholar] [CrossRef]
- Cui, Q.; Xing, J.; Yu, M.; Wang, Y.; Xu, J.; Gu, Y.; Nan, X.; Ma, W.; Liu, H.; Zhao, H. Mmu-miR-185 depletion promotes osteogenic differentiation and suppresses bone loss in osteoporosis through the Bgn-mediated BMP/Smad pathway. Cell Death Dis. 2019, 10, 172. [Google Scholar] [CrossRef]
- Gu, Z.; Long, J.; Li, Y.; Wang, X.; Wang, H. MiR-125a-3p Negatively Regulates Osteoblastic Differentiation of Human Adipose Derived Mesenchymal Stem Cells by Targeting Smad4 and Jak1. Am. J. Transl. Res. 2019, 11, 2603–2615. [Google Scholar]
- Sangani, R.; Periyasamy-Thandavan, S.; Kolhe, R.; Bhattacharyya, M.H.; Chutkan, N.; Hunter, M.; Isales, C.; Hamrick, M.; Hill, W.D.; Fulzele, S. MicroRNAs-141 and 200a regulate the SVCT2 transporter in bone marrow stromal cells. Mol. Cell. Endocrinol. 2015, 410, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Liu, S.; Zhang, K.; Miao, X.; Li, J.; Shi, Z.; Gao, Y. miR-384-5p Targets Gli2 and Negatively Regulates Age-Related Osteogenic Differentiation of Rat Bone Marrow Mesenchymal Stem Cells. Stem Cells Dev. 2019, 28, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Yuan, S.; Zhang, H.; Liu, J. MicroRNA-23a inhibits osteogenesis of periodontal mesenchymal stem cells by targeting bone morphogenetic protein signaling. Arch. Oral Biol. 2019, 102, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.-M.; Zhou, B.; Yuan, K.-F. Role of p53 mediated miR-23a/CXCL12 pathway in osteogenic differentiation of bone mesenchymal stem cells on nanostructured titanium surfaces. BioMedicine 2019, 112, 108649. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, H.; Wang, Y.; Li, T.; Fan, J.; Xiao, K.; Zhao, R.C.; Weng, X. microRNA-23a inhibits osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by targeting LRP5. Int. J. Biochem. Cell Biol. 2016, 72, 55–62. [Google Scholar] [CrossRef]
- Ren, G.; Sun, J.; Li, M.; Zhang, Y.; Li, R.; Li, Y. MicroRNA-23a-5p regulates osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by targeting mitogen-activated protein kinase-13. Mol. Med. Rep. 2018, 17, 4554–4560. [Google Scholar] [CrossRef]
- Seenprachawong, K.; Nuchnoi, P.; Nantasenamat, C.; Prachayasittikul, V.; Supokawej, A. Computational Identification of MiRNAs That Modulate the Differentiation of Mesenchymal Stem Cells to Osteoblasts. PeerJ 2016, 2016, e1976. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, J.-F.; Shi, L.; Yang, Z.-M.; Wu, T.-Y.; Wang, H.-X.; Lin, W.-P.; Lu, Y.-F.; Lo, J.H.T.; Zhu, D.-H.; et al. MicroRNA-378 Suppressed Osteogenesis of MSCs and Impaired Bone Formation via Inactivating Wnt/β-Catenin Signaling. Mol. Ther. Nucleic Acids 2020, 21, 1017–1028. [Google Scholar] [CrossRef]
- Xiao, J.; Qin, S.; Li, W.; Yao, L.; Huang, P.; Liao, J.; Liu, J.; Li, S. Osteogenic differentiation of rat bone mesenchymal stem cells modulated by MiR-186 via SIRT6. Life Sci. 2020, 253, 117660. [Google Scholar] [CrossRef]
- Ma, W.; Dou, Q.; Ha, X. Let-7a-5p inhibits BMSCs osteogenesis in postmenopausal osteoporosis mice. Biochem. Biophys. Res. Commun. 2019, 510, 53–58. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Wang, X.-B.; Zhao, H.; Zhou, C.-N. MicroRNA-9-5p promotes osteoporosis development through inhibiting osteogenesis and promoting adipogenesis via targeting Wnt3a. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Gao, H.; Liu, F.; Qiu, B. Regulation of Runx2 by microRNA-9 and microRNA-10 modulates the osteogenic differentiation of mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 1046–1052. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, H.; Xiong, Y.; Tang, X.; Yang, Y.; Hu, Z.; Li, C.; Chen, S.; Yu, X. miR-16-2* Interferes with WNT5A to Regulate Osteogenesis of Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2018, 51, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Wang, S.; Wei, J.; Fan, J.; Li, J.; Han, Q.; Liao, L.; Shao, C.; Zhao, R.C. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013, 10, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wu, C.; Dong, Y.; Ma, Y.; Jin, Y.; Ji, Y. MicroRNA-24 Regulates Osteogenic Differentiation via Targeting T-Cell Factor-1. Int. J. Mol. Sci. 2015, 16, 11699–11712. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhou, H.; Hong, Y.; Li, J.; Jiang, X.; Huang, H. miR-30 Family Members Negatively Regulate Osteoblast Differentiation. J. Biol. Chem. 2012, 287, 7503–7511. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wu, S.; Zhou, H.; Bi, X.; Wang, Y.; Hu, Y.; Gu, P.; Fan, X.; Sehic, A.; Tulek, A.; et al. Effects of a miR-31, Runx2, and Satb2 Regulatory Loop on the Osteogenic Differentiation of Bone Mesenchymal Stem Cells. Stem Cells Dev. 2013, 22, 2278–2286. [Google Scholar] [CrossRef]
- Baglìo, S.R.; Devescovi, V.; Granchi, D.; Baldini, N. MicroRNA Expression Profiling of Human Bone Marrow Mesenchymal Stem Cells during Osteogenic Differentiation Reveals Osterix Regulation by MiR-31. Gene 2013, 527, 321–331. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, Z.; Bi, X.; Zhou, H.; Wang, Y.; Gu, P.; Fan, X. Effects of miR-31 on the osteogenesis of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 446, 98–104. [Google Scholar] [CrossRef]
- Fukuda, T.; Ochi, H.; Sunamura, S.; Haiden, A.; Bando, W.; Inose, H.; Okawa, A.; Asou, Y.; Takeda, S. MicroRNA-145 regulates osteoblastic differentiation by targeting the transcription factor Cbfb. FEBS Lett. 2015, 589, 3302–3308. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Q.-S.; Ding, W.-B.; Zhang, L.-L.; Wang, H.-C.; Zhu, Y.-J.; He, W.; Chai, Y.-N.; Liu, Y.-W. Increased microRNA-93-5p inhibits osteogenic differentiation by targeting bone morphogenetic protein-2. PLoS ONE 2017, 12, e0182678. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Q.; Wu, X.-P.; He, H.-B.; Fu, L. MiR-96 regulates bone metabolism by targeting osterix. Clin. Exp. Pharmacol. Physiol. 2017, 45, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J.; Zhu, C.; Lin, L.; Wang, J.; Zhang, H.; Li, J.; Yu, X.; Zhao, Z.; Dong, W.; et al. MicroRNA-98 regulates osteogenic differentiation of human bone mesenchymal stromal cells by targetingBMP2. J. Cell. Mol. Med. 2016, 21, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Qu, X.; Li, H.; Huang, S.; Wang, S.; Xu, Q.; Lin, R.; Han, Q.; Li, J.; Zhao, R.C. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett. 2012, 586, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lin, X.; Zhong, J.; Xu, F.; Wu, F.; Liao, X.; Cui, R.; Li, F.; Yuan, L. miR-124 regulates the osteogenic differentiation of bone marrow-derived mesenchymal stem cells by targeting Sp7. Mol. Med. Rep. 2019, 19, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, Z.; Hou, T.; Li, Z.; Huang, K.; Gong, J.; Zhou, W.; Tang, K.; Xu, J.; Dong, S. MiR-125b Regulates the Osteogenic Differentiation of Human Mesenchymal Stem Cells by Targeting BMPR1b. Cell. Physiol. Biochem. 2017, 41, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.-L.; Meng, Y.-L.; Ge, J.-H. Upregulation of miR-132 attenuates osteoblast differentiation of UC-MSCs. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1580–1587. [Google Scholar] [CrossRef]
- Schaap-Oziemlak, A.M.; Raymakers, R.A.; Bergevoet, S.M.; Gilissen, C.; Jansen, B.J.; Adema, G.J.; Kögler, G.; le Sage, C.; Agami, R.; van der Reijden, B.A.; et al. MicroRNA hsa-miR-135b Regulates Mineralization in Osteogenic Differentiation of Human Unrestricted Somatic Stem Cells. Stem Cells Dev. 2010, 19, 877–885. [Google Scholar] [CrossRef]
- Kong, L.; Zuo, R.; Wang, M.; Wang, W.; Xu, J.; Chai, Y.; Guan, J.; Kang, Q. Silencing MicroRNA-137-3p, which Targets RUNX2 and CXCL12 Prevents Steroid-induced Osteonecrosis of the Femoral Head by Facilitating Osteogenesis and Angiogenesis. Int. J. Biol. Sci. 2020, 16, 655–670. [Google Scholar] [CrossRef]
- Long, H.; Sun, B.; Cheng, L.; Zhao, S.; Zhu, Y.; Zhao, R.; Zhu, J. miR-139-5p Represses BMSC Osteogenesis via Targeting Wnt/β-Catenin Signaling Pathway. DNA Cell Biol. 2017, 36, 715–724. [Google Scholar] [CrossRef]
- Hwang, S.; Park, S.-K.; Lee, H.Y.; Kim, S.W.; Lee, J.S.; Choi, E.K.; You, D.; Kim, C.-S.; Suh, N. miR-140-5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett. 2014, 588, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Geng, J.; Wei, X.; Zhang, R.; Jiang, S. MiR-144-3p regulates osteogenic differentiation and proliferation of murine mesenchymal stem cells by specifically targeting Smad4. FEBS Lett. 2016, 590, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lv, Q.; Lv, C. MicroRNA-153 suppresses the osteogenic differentiation of human mesenchymal stem cells by targeting bone morphogenetic protein receptor type II. Int. J. Mol. Med. 2015, 36, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, C.; Han, L.; Liu, L.; Jing, W.; Tang, W.; Tian, W.; Long, J. MiR-154-5p regulates osteogenic differentiation of adipose-derived mesenchymal stem cells under tensile stress through the Wnt/PCP pathway by targeting Wnt11. Bone 2015, 78, 130–141. [Google Scholar] [CrossRef]
- Davis, C.; Dukes, A.; Drewry, M.; Helwa, I.; Johnson, M.H.; Isales, C.M.; Hill, W.D.; Liu, Y.; Shi, X.; Fulzele, S.; et al. MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng. Part A 2017, 23, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Lin, H.; Fu, H.; Wang, B.; Han, G.; Fan, M. MicroRNA-195-5p Regulates Osteogenic Differentiation of Periodontal Ligament Cells Under Mechanical Loading. J. Cell. Physiol. 2017, 232, 3762–3774. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, L.; Zhou, J.; Chen, Y.; Yang, L.; Deng, F.; Hu, Y. miR-203-3p participates in the suppression of diabetes-associated osteogenesis in the jaw bone through targeting Smad1. Int. J. Mol. Med. 2018, 41, 1595–1607. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Z.; Peng, T.; Wang, G.; Xu, Q.; Li, G. miR-204 inhibits the osteogenic differentiation of mesenchymal stem cells by targeting bone morphogenetic protein 2. Mol. Med. Rep. 2019, 21, 43–50. [Google Scholar] [CrossRef]
- Hu, N.; Feng, C.; Jiang, Y.; Miao, Q.; Liu, H. Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and ERK/MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 10491–10506. [Google Scholar] [CrossRef]
- Wang, C.-G.; Liao, Z.; Xiao, H.; Liu, H.; Hu, Y.-H.; Liao, Q.-D.; Zhong, D. LncRNA KCNQ1OT1 promoted BMP2 expression to regulate osteogenic differentiation by sponging miRNA-214. Exp. Mol. Pathol. 2019, 107, 77–84. [Google Scholar] [CrossRef]
- Qiu, J.; Huang, G.; Na, N.; Chen, L. MicroRNA-214-5p/TGF-β/Smad2 signaling alters adipogenic differentiation of bone marrow stem cells in postmenopausal osteoporosis. Mol. Med. Rep. 2018, 17, 6301–6310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-L.; Wang, S.; Ding, D.-G.; Xu, L.; Zhu, H.-T. miR-217 inhibits osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells by binding to Runx2. Mol. Med. Rep. 2017, 15, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Deng, R.; Lai, S.; Wen, Q.; Zeng, Y.; Gao, L.; Liu, Y.; Kong, P.; Zhong, J.; Su, Y.; et al. Inhibition of microRNA-221-5p induces osteogenic differentiation by directly targeting smad3 in myeloma bone disease mesenchymal stem cells. Oncol. Lett. 2019, 18, 6536–6544. [Google Scholar] [CrossRef]
- Yan, J.; Guo, D.; Yang, S.; Sun, H.; Wu, B.; Zhou, D. Inhibition of miR-222-3p activity promoted osteogenic differentiation of hBMSCs by regulating Smad5-RUNX2 signal axis. Biochem. Biophys. Res. Commun. 2016, 470, 498–503. [Google Scholar] [CrossRef]
- Tome, M.E.; López-Romero, P.; Albo, C.; Sepulveda, J.C.; Fernandez-Gutierrez, B.; Dopazo, A.; Bernad, A.; Gonzalez, M.A. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2010, 18, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Q.; Wan, C.; Li, L.; Zhang, L.; Chen, Z. MicroRNA-338-3p Regulates Osteogenic Differentiation of Mouse Bone Marrow Stromal Stem Cells by Targeting Runx2 and Fgfr2. J. Cell. Physiol. 2014, 229, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhu, Y.; Lin, Z.; Wan, J.; Cheng, L.; Zeng, M.; Tang, Y.; Zhao, R. miR-381 modulates human bone mesenchymal stromal cells (BMSCs) osteogenesis via suppressing Wnt signaling pathway during atrophic nonunion development. Cell Death Dis. 2019, 10, 470. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Z.; Jin, X.; Shi, H. miR-383 negatively regulates osteoblastic differentiation of bone marrow mesenchymal stem cells in rats by targeting Satb2. Bone 2018, 114, 137–143. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kang, I.-H.; Lee, J.W.; Jang, W.-G.; Koh, J.-T. MiR-433 mediates ERRγ-suppressed osteoblast differentiation via direct targeting to Runx2 mRNA in C3H10T1/2 cells. Life Sci. 2013, 92, 562–568. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.H.; Lee, S.Y.; Shin, K.K.; Cho, H.H.; Bae, Y.C.; Jung, J.S.; Li, J.; Ohliger, J.; Pei, M.; et al. miR-486-5p Induces Replicative Senescence of Human Adipose Tissue-Derived Mesenchymal Stem Cells and Its Expression Is Controlled by High Glucose. Stem Cells Dev. 2012, 21, 1749–1760. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.; Li, R.; Liu, H.; Du, L.; Chen, H.; Zhang, Y.; Zhang, S.; Liu, D. MicroRNA-503-5p inhibits stretch-induced osteogenic differentiation and bone formation. Cell Biol. Int. 2016, 41, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Yang, S.; Xu, W.; Shen, J.K.; Ye, S.; Liu, X.; Dong, Z.; Xiao, B.; Feng, Y. MiR-708 promotes steroid-induced osteonecrosis of femoral head, suppresses osteogenic differentiation by targeting SMAD3. Sci. Rep. 2016, 6, 22599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.-H.; Meng, Y. microRNA-1297 promotes the progression of osteoporosis through regulation of osteogenesis of bone marrow mesenchymal stem cells by targeting WNT5A. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4541–4550. [Google Scholar] [CrossRef]
- Camp, E.; Pribadi, C.M.P.; Anderson, P.J.; Zannettino, A.C.W.; Gronthos, S. miRNA-376c-3p Mediates TWIST-1 Inhibition of Bone Marrow-Derived Stromal Cell Osteogenesis and Can Reduce Aberrant Bone Formation of TWIST-1 Haploinsufficient Calvarial Cells. Stem Cells Dev. 2018, 27, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, H. Restoration of miR-1305 relieves the inhibitory effect of nicotine on periodontal ligament-derived stem cell proliferation, migration, and osteogenic differentiation. J. Oral Pathol. Med. 2016, 46, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wei, W.; Ruan, J.; Ding, Y.; Zhuang, A.; Bi, X.; Sun, H.; Gu, P.; Wang, Z.; Fan, X. Effects of miR-146a on the osteogenesis of adipose-derived mesenchymal stem cells and bone regeneration. Sci. Rep. 2017, 7, 42840. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Fu, W.-M.; He, M.-L.; Wang, H.; Wang, W.-M.; Yu, S.-C.; Bian, X.-W.; Zhou, J.; Lin, M.C.M.; Lu, G.; et al. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol. Biol. Cell 2011, 22, 3955–3961. [Google Scholar] [CrossRef]
- Lian, W.-S.; Wu, R.-W.; Lee, M.S.; Chen, Y.-S.; Sun, Y.-C.; Wu, S.-L.; Ke, H.-J.; Ko, J.-Y.; Wang, F.-S. Subchondral mesenchymal stem cells from osteoarthritic knees display high osteogenic differentiation capacity through microRNA-29a regulation of HDAC4. J. Mol. Med. 2017, 95, 1327–1340. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bae, S.W.; Yu, S.S.; Bae, Y.C.; Jung, J.S. miR-196a Regulates Proliferation and Osteogenic Differentiation in Mesenchymal Stem Cells Derived from Human Adipose Tissue. J. Bone Miner. Res. 2009, 24, 816–825. [Google Scholar] [CrossRef]
- Chen, B.; Meng, J.; Zeng, Y.-T.; Du, Y.-X.; Zhang, J.; Si, Y.-M.; Yuan, X. MicroRNA-7-5p regulates osteogenic differentiation of hMSCs via targeting CMKLR1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7826–7831. [Google Scholar] [CrossRef]
- Cai, Q.; Zheng, P.; Ma, F.; Zhang, H.; Li, Z.; Fu, Q.; Han, C.; Sun, Y. MicroRNA-224 enhances the osteoblastic differentiation of hMSCs via Rac1. Cell Biochem. Funct. 2019, 37, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Tokuzawa, Y.; Ninomiya, Y.; Yagi, K.; Yatsuka-Kanesaki, Y.; Suda, T.; Fukuda, T.; Katagiri, T.; Kondoh, Y.; Amemiya, T.; et al. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett. 2009, 583, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, H.; Liu, W.; Hu, R.; Huang, B.; Tan, Y.F.; Liao, E.Y.; Xu, K.; Sheng, Z.F.; Zhou, H.D.; et al. A Novel MicroRNA Targeting HDAC5 Regulates Osteoblast Differentiation in Mice and Contributes to Primary Osteoporosis in Humans. J. Clin. Investig. 2009, 119, 3666–3677. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Jin, Y.; Zheng, J.; Liu, K.; Zhao, J.; Zhang, S.; Wu, F.; Sun, Z. MiR-217 promotes cell proliferation and osteogenic differentiation of BMSCs by targeting DKK1 in steroid-associated osteonecrosis. Biomed. Pharmacother. 2018, 109, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.A.; Boucher, S.; Ricupero, C.L.; Fenstermacher, S.; Swerdel, M.; Chase, L.G.; Adams, C.C.; Chesnut, J.; Lakshmipathy, U.; Hart, R.P. Differentiating human multipotent mesenchymal stromal cells regulate microRNAs: Prediction of microRNA regulation by PDGF during osteogenesis. Exp. Hematol. 2008, 36, 1354–1369.e2. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Gu, R.; Zhang, W.; Shao, L.; Li, F.; Wu, C.; Sun, Y. MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/β-Catenin signaling pathway via downregulating Myd88. J. Cell. Physiol. 2019, 234, 22675–22686. [Google Scholar] [CrossRef]
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J.; et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res. Ther. 2019, 10, 65. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Kou, J.; Wang, Q.; Zheng, X.; Yu, T. MiR-9 promotes osteoblast differentiation of mesenchymal stem cells by inhibiting DKK1 gene expression. Mol. Biol. Rep. 2016, 43, 939–946. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Fan, L.; Li, T.; Yang, Y.; Xu, H.; Deng, L.; Li, J.; Li, T.; Weng, X.; et al. MiRNA-10b Reciprocally Stimulates Osteogenesis and Inhibits Adipogenesis Partly through the TGF-β/Smad2 Signaling Pathway. Aging Dis. 2018, 9, 1058–1073. [Google Scholar] [CrossRef]
- Jia, J.; Feng, X.; Xu, W.; Yang, S.; Zhang, Q.; Liu, X.; Dai, Y.F.Z. MiR-17-5p Modulates Osteoblastic Differentiation and Cell Proliferation by Targeting SMAD7 in Non-Traumatic Osteonecrosis. Exp. Mol. Med. 2014, 46, e107–e108. [Google Scholar] [CrossRef]
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Carbonare, L.D.; Mottes, M. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis. Cells 2019, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, C.; Zhang, P.; Liu, Y.; Jiang, Y.; Wu, E.; Xue, H.; Liu, C.; Li, Z. miR-26b modulates OA induced BMSC osteogenesis through regulating GSK3β/β-catenin pathway. Exp. Mol. Pathol. 2019, 107, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wan, M.; Xu, X.; Gao, B.; Zhou, Y.; Sun, J.; Cheng, L.; Klein, O.; Zhou, X.; Zheng, L. Crosstalk between miR-34a and Notch Signaling Promotes Differentiation in Apical Papilla Stem Cells (SCAPs). J. Dent. Res. 2014, 93, 589–595. [Google Scholar] [CrossRef]
- Hupkes, M.; Sotoca, A.M.; Hendriks, J.M.; van Zoelen, E.J.; Dechering, K.J. MicroRNA miR-378 promotes BMP2-induced osteogenic differentiation of mesenchymal progenitor cells. BMC Mol. Biol. 2014, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cai, J.; Cai, X.-H.; Chen, L. miR-346 Regulates Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells by Targeting the Wnt/β-Catenin Pathway. PLoS ONE 2013, 8, e72266. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Zhang, Z.; Zhang, D.; You, X.; Zhong, Y.; Chen, M.; Liu, S. miR-10a restores human mesenchymal stem cell differentiation by repressing KLF4. J. Cell. Physiol. 2013, 228, 2324–2336. [Google Scholar] [CrossRef]
- Gámez, B.; Rodríguez-Carballo, E.; Bartrons, R.; Rosa, J.L.; Ventura, F. MicroRNA-322 (miR-322) and Its Target Protein Tob2 Modulate Osterix (Osx) mRNA Stability. J. Biol. Chem. 2013, 288, 14264–14275. [Google Scholar] [CrossRef]
- Yang, N.; Wang, G.; Hu, C.; Shi, Y.; Liao, L.; Shi, S.; Cai, Y.; Cheng, S.; Wang, X.; Liu, Y.; et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J. Bone Miner. Res. 2012, 28, 559–573. [Google Scholar] [CrossRef]
- Laine, S.K.; Alm, J.J.; Virtanen, S.P.; Aro, H.T.; Laitala-Leinonen, T.K. MicroRNAs miR-96, miR-124, and miR-199a regulate gene expression in human bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 2687–2695. [Google Scholar] [CrossRef]
- Huang, S.; Wang, S.; Bian, C.; Yang, Z.; Zhou, H.; Zeng, Y.; Li, H.; Han, Q.; Zhao, R.C.; Li, B.; et al. Upregulation of miR-22 Promotes Osteogenic Differentiation and Inhibits Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells by Repressing HDAC6 Protein Expression. Stem Cells Dev. 2012, 21, 2531–2540. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Zhong, W.-J.; Wang, L. A signal-amplification circuit between miR-218 and Wnt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation. Bone 2014, 58, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, Y.; Lin, Z.; Wan, J.; Xu, C.; Zeng, Y.; Zhu, Y. miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3β/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2016, 477, 749–754. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, Q.; Bonewald, L.F.; He, X.; Stein, G.; Lian, J.; Chen, J. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J. Bone Miner. Res. 2011, 26, 1953–1963. [Google Scholar] [CrossRef]
- Tang, X.; Lin, J.; Wang, G.; Lu, J. MicroRNA-433-3p Promotes Osteoblast Differentiation through Targeting DKK1 Expression. PLoS ONE 2017, 12, e0179860. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, W.; Zhou, L. MiR-590-3p regulates osteogenic differentiation of human mesenchymal stem cells by regulating APC gene. Biochem. Biophys. Res. Commun. 2016, 478, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Xu, Y.; Zhang, S.; Guan, H.; Song, S.; Wang, X.; Wang, Y.; Li, Y.; Zhao, G. miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARγ and GREM1. Sci. Rep. 2016, 6, 38491. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chang, H.; Hou, Y.; Wang, Y.; Zhou, Z.; Wang, M.; Huang, Z.; Yu, B. Lentivirus-mediated microRNA-26a overexpression in bone mesenchymal stem cells facilitates bone regeneration in bone defects of calvaria in mice. Mol. Med. Rep. 2018, 18, 5317–5326. [Google Scholar] [CrossRef]
- Su, X.; Liao, L.; Shuai, Y.; Jing, H.; Liu, S.; Zhou, H.; Liu, Y.; Jin, Y. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis. 2015, 6, e1851. [Google Scholar] [CrossRef]
- Liu, H.; Su, H.; Wang, X.; Hao, W. MiR-148a regulates bone marrow mesenchymal stem cells-mediated fracture healing by targeting insulin-like growth factor 1. J. Cell. Biochem. 2018, 120, 1350–1361. [Google Scholar] [CrossRef]
- Fan, X.; Teng, Y.; Ye, Z.; Zhou, Y.; Tan, W.-S. Gap junction-mediated MiR-200b on osteogenesis and angiogenesis in coculture between MSCs and HUVECs. J. Cell Sci. 2018, 131, jcs.216135. [Google Scholar] [CrossRef]
- Yan, X.; Wang, H.; Li, Y.; Jiang, Y.; Shao, Q.; Xu, W. MicroRNA-92a overexpression promotes the osteogenic differentiation of bone mesenchymal stem cells by impeding Smad6-mediated runt-related transcription factor 2 degradation. Mol. Med. Rep. 2018, 17, 7821–7826. [Google Scholar] [CrossRef] [PubMed]
- Vishal, M.; Vimalraj, S.; Ajeetha, R.; Gokulnath, M.; Keerthana, R.; He, Z.; Partridge, N.; Selvamurugan, N. MicroRNA-590-5p Stabilizes Runx2 by Targeting Smad7 During Osteoblast Differentiation. J. Cell. Physiol. 2016, 232, 371–380. [Google Scholar] [CrossRef]
- Yang, S.; Guo, S.; Tong, S.; Sun, X. Exosomal miR-130a-3p regulates osteogenic differentiation of Human Adipose-Derived stem cells through mediating SIRT7/Wnt/β-catenin axis. Cell Prolif. 2020, 53, e12890. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Song, M.; Du, J.; Yu, J.; Zheng, W.; Zhang, C.; Wang, Y. MiR-497-5p Regulates Osteo/Odontogenic Differentiation of Stem Cells from Apical Papilla via the Smad Signaling Pathway by Targeting Smurf2. Front. Genet. 2020, 11, 582366. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.A.; Kong, L.; Bai, X.-H.; Luan, Y.; Liu, C.-J. miR-199a*, a Bone Morphogenic Protein 2-responsive MicroRNA, Regulates Chondrogenesis via Direct Targeting to Smad1. J. Biol. Chem. 2009, 284, 11326–11335. [Google Scholar] [CrossRef] [PubMed]
- Guérit, D.; Brondello, J.-M.; Chuchana, P.; Philipot, D.; Toupet, K.; Bony, C.; Jorgensen, C.; Noël, D. FOXO3A Regulation by miRNA-29a Controls Chondrogenic Differentiation of Mesenchymal Stem Cells and Cartilage Formation. Stem Cells Dev. 2014, 23, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Liang, T.; Jin, S.; Dai, X.; Zhou, Z.; Gao, M.; Huang, S.; Luo, J.; Zou, L.; Zou, X. Methylation-mediated silencing of miR-124 facilitates chondrogenesis by targeting NFATc1 under hypoxic conditions. Am. J. Transl. Res. 2017, 9, 4111–4124. [Google Scholar]
- Bai, M.; Yin, H.; Zhao, J.; Li, Y.; Wu, Y. miR-182-5p overexpression inhibits chondrogenesis by down-regulating PTHLH. Cell Biol. Int. 2019, 43, 222–232. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Yang, G.; Yu, H.; Zhou, Z.; Tang, M. MicroRNA-30a regulates chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells through targeting Sox9. Exp. Ther. Med. 2019, 18, 4689–4697. [Google Scholar] [CrossRef]
- Wa, Q.; He, P.; Huang, S.; Zuo, J.; Li, X.; Zhu, J.; Hong, S.; Lv, G.; Cai, D.; Xu, D.; et al. miR-30b regulates chondrogenic differentiation of mouse embryo-derived stem cells by targeting SOX9. Exp. Ther. Med. 2017, 14, 6131–6137. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, D.S.; Paik, S.; Lee, K.-M.; Jang, Y.; Lee, J.W. microRNA-495 Inhibits Chondrogenic Differentiation in Human Mesenchymal Stem Cells by Targeting Sox9. Stem Cells Dev. 2014, 23, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guo, H.; Zhang, Y.; Chen, L.; Ying, D.; Dong, S. MicroRNA-145 Regulates Chondrogenic Differentiation of Mesenchymal Stem Cells by Targeting Sox9. PLoS ONE 2011, 6, e21679. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Jung, H.S.; Lee, S.; Yoon, D.S.; Park, M.S.; Lee, J.W. miR-449a Regulates the Chondrogenesis of Human Mesenchymal Stem Cells Through Direct Targeting of Lymphoid Enhancer-Binding Factor-1. Stem Cells Dev. 2012, 21, 3298–3308. [Google Scholar] [CrossRef] [PubMed]
- Guérit, D.; Philipot, D.; Chuchana, P.; Toupet, K.; Brondello, J.-M.; Mathieu, M.; Jorgensen, C.; Noël, D. Sox9-Regulated miRNA-574-3p Inhibits Chondrogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2013, 8, e62582. [Google Scholar] [CrossRef] [PubMed]
- Lolli, A.; Narcisi, R.; Lambertini, E.; Penolazzi, L.; Angelozzi, M.; Kops, N.; Gasparini, S.; van Osch, G.J.; Piva, R. Silencing of Antichondrogenic MicroRNA-221 in Human Mesenchymal Stem Cells Promotes Cartilage Repair In Vivo. Stem Cells 2016, 34, 1801–1811. [Google Scholar] [CrossRef]
- Anderson, B.A.; McAlinden, A. miR-483 targets SMAD4 to suppress chondrogenic differentiation of human mesenchymal stem cells. J. Orthop. Res. 2017, 35, 2369–2377. [Google Scholar] [CrossRef]
- Tian, J.; Rui, Y.-J.; Xu, Y.-J.; Zhang, S.-A. MiR-143-3p regulates early cartilage differentiation of BMSCs and promotes cartilage damage repair through targeting BMPR2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8814–8821. [Google Scholar]
- Xiao, Y.; Yan, X.; Yang, Y.; Ma, X. Downregulation of long noncoding RNA HOTAIRM1 variant 1 contributes to osteoarthritis via regulating miR-125b/BMPR2 axis and activating JNK/MAPK/ERK pathway. Biomed. Pharmacother. 2018, 109, 1569–1577. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, Y.; Wang, J.; Cao, Y.; Hang, D.H. MiR-26b Regulates Cartilage Differentiation of Bone Marrow Mesenchymal Stem Cells in Rats through the Wnt/β-Catenin Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5084–5092. [Google Scholar]
- Shen, P.F.; Wang, B.; Qu, Y.X.; Zheng, C.; Xu, J.D.; Xie, Z.K.; Ma, Y. MicroRNA-23c Inhibits Articular Cartilage Damage Recovery by Regulating MSCs Differentiation to Chondrocytes via Reducing FGF2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 932–940. [Google Scholar] [CrossRef]
- Zhao, C.; Miao, Y.; Cao, Z.; Shi, J.; Li, J.; Kang, F.; Dou, C.; Xie, Z.; Xiang, Q.; Dong, S. MicroRNA-29b regulates hypertrophy of murine mesenchymal stem cells induced toward chondrogenesis. J. Cell. Biochem. 2019, 120, 8742–8753. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kang, Y.; Liao, W.-M.; Yu, L. MiR-194 Regulates Chondrogenic Differentiation of Human Adipose-Derived Stem Cells by Targeting Sox5. PLoS ONE 2012, 7, e31861. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yang, Z.; Kang, Y.; Zhang, Z.; Fu, M.; He, A.; Zhang, Z.; Liao, W. MiR-193b regulates early chondrogenesis by inhibiting the TGF-beta2 signaling pathway. FEBS Lett. 2015, 589, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, S.; Nakasa, T.; Otsuki, S.; Grogan, S.P.; Higashiyama, R.; Inoue, A.; Kato, Y.; Sato, T.; Lotz, M.K.; Asahara, H. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009, 60, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef]
- Barter, M.J.; Tselepi, M.; Gómez, R.; Woods, S.; Hui, W.; Smith, G.R.; Shanley, D.P.; Clark, I.M.; Young, D.A. Genome-Wide MicroRNA and Gene Analysis of Mesenchymal Stem Cell Chondrogenesis Identifies an Essential Role and Multiple Targets for miR-140-5p. Stem Cells 2015, 33, 3266–3280. [Google Scholar] [CrossRef]
- Karlsen, T.A.; Jakobsen, R.B.; Mikkelsen, T.S.; Brinchmann, J.E. microRNA-140 Targets RALA and Regulates Chondrogenic Differentiation of Human Mesenchymal Stem Cells by Translational Enhancement of SOX9 and ACAN. Stem Cells Dev. 2014, 23, 290–304. [Google Scholar] [CrossRef]
- Lin, X.; Wu, L.; Zhang, Z.; Yang, R.; Guan, Q.; Hou, X.; Wu, Q. MiR-335-5p Promotes Chondrogenesis in Mouse Mesenchymal Stem Cells and Is Regulated Through Two Positive Feedback Loops. J. Bone Miner. Res. 2013, 29, 1575–1585. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, R.; Shi, B.; Chen, L.; Yang, L.; Fu, Q. MicroRNA-30a promotes chondrogenic differentiation of mesenchymal stem cells through inhibiting Delta-like 4 expression. Life Sci. 2016, 148, 220–228. [Google Scholar] [CrossRef]
- Mao, G.; Hu, S.; Zhang, Z.; Wu, P.; Zhao, X.; Lin, R.; Liao, W.; Kang, Y. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J. Cell. Mol. Med. 2018, 22, 5354–5366. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Zhang, Z.; Yang, Z.; Kang, Y.; Zhao, X.; Long, D.; Hu, S.; Gu, M.; He, S.; et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018, 8, 2862–2883. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huang, Z.; Wu, P.; Chang, Z.; Liao, W.; Zhang, Z. CDK6 and miR-320c Co-Regulate Chondrocyte Catabolism Through NF-κB Signaling Pathways. Cell. Physiol. Biochem. 2018, 51, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qiu, X.; Gao, B.; Lian, C.; Peng, Y.; Liang, A.; Xu, C.; Gao, W.; Zhang, L.; Su, P.; et al. Melatonin-mediated miR-526b-3p and miR-590-5p upregulation promotes chondrogenic differentiation of human mesenchymal stem cells. J. Pineal Res. 2018, 65, e12483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Luo, D.; Sun, H.; Qi, Y.; Xu, W.; Jin, X.; Li, C.; Lin, Z.; Li, G. MiR-132-3p regulates ADAMTS-5 expression and promotes chondrogenic differentiation of rat mesenchymal stem cells. J. Cell. Biochem. 2017, 119, 2579–2587. [Google Scholar] [CrossRef]
- Çelik, E.; Bayram, C.; Denkbaş, E.B. Chondrogenesis of human mesenchymal stem cells by microRNA loaded triple polysaccharide nanoparticle system. Mater. Sci. Eng. C 2019, 102, 756–763. [Google Scholar] [CrossRef]
- Lee, J.M.; Ko, J.-Y.; Kim, H.Y.; Park, J.-W.; Guilak, F.; Im, G.-I. miR-892b Inhibits Hypertrophy by Targeting KLF10 in the Chondrogenesis of Mesenchymal Stem Cells. Mol. Ther. Nucleic Acids 2019, 17, 310–322. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Z.; Sun, B.; Han, B.; Fu, Q.; Han, Y.; Yuan, W.; Xu, Z.; Chen, A. MiR-520d-5p modulates chondrogenesis and chondrocyte metabolism through targeting HDAC1. Aging 2020, 12, 18545–18560. [Google Scholar] [CrossRef]
- Xue, Z.; Meng, Y.; Ge, J. miR-127-5p promotes chondrogenic differentiation in rat bone marrow mesenchymal stem cells. Exp. Ther. Med. 2017, 14, 1481–1486. [Google Scholar] [CrossRef]
- Lakshmipathy, U.; Hart, R.P. Concise Review: MicroRNA Expression in Multipotent Mesenchymal Stromal Cells. Stem Cells 2007, 26, 356–363. [Google Scholar] [CrossRef]
- Yang, Z.; Bian, C.; Zhou, H.; Huang, S.; Wang, S.; Liao, L.; Zhao, R.C.; Nardelli, C.; Granata, I.; Iaffaldano, L.; et al. MicroRNA hsa-miR-138 Inhibits Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells Through Adenovirus EID-1. Stem Cells Dev. 2011, 20, 259–267. [Google Scholar] [CrossRef]
- Sun, F.; Wang, J.; Pan, Q.; Yu, Y.; Zhang, Y.; Wan, Y.; Wang, J.; Li, X.; Hong, A. Characterization of function and regulation of miR-24-1 and miR-31. Biochem. Biophys. Res. Commun. 2009, 380, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, J.; Hou, J.; Long, J.; Li, C.; Liu, L. A Novel Negative Regulator of Adipogenesis: MicroRNA-363. Stem Cells 2014, 32, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Zhang, S.; Ye, L.; Cui, J.; Sun, Q.; Li, K.; Wu, H.; Liu, L. MiR-540 as a Novel Adipogenic Inhibitor Impairs Adipogenesis Via Suppression of PPARγ. J. Cell. Biochem. 2015, 116, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Chen, M.; Ren, S.; Cheng, P.; Zhang, H. miR-301b~miR-130b—PPARγ axis underlies the adipogenic capacity of mesenchymal stem cells with different tissue origins. Sci. Rep. 2017, 7, 1160. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, K.; Liu, A.; Yang, Z.; Hu, C.; Chen, D.; Wang, H. miR-330-5p inhibits H2O2-induced adipogenic differentiation of MSCs by regulating RXRγ. Int. J. Mol. Med. 2018, 42, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, J.; Hu, X.; Bao, P.; Pan, J.; Chen, Z.; Xian, J. Expression of Concern: MiR-27b Impairs Adipocyte Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells by Targeting LPL. Cell. Physiol. Biochem. 2018, 47, 545–555. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Yan, R.; Xu, X.; Gao, L.; Mei, J.; Liu, J.; Wang, X.; Zhang, J.; Wu, P.; et al. miR-377-3p regulates adipogenic differentiation of human bone marrow mesenchymal stem cells by regulating LIFR. Mol. Cell. Biochem. 2018, 449, 295–303. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; He, X.; Zhang, S.; Wang, K.; Wu, H.; Chen, L. LncRNA TINCR/miR-31-5p/C/EBP-α feedback loop modulates the adipogenic differentiation process in human adipose tissue-derived mesenchymal stem cells. Stem Cell Res. 2018, 32, 35–42. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Liu, X.; Hong, T.; Wang, T.; Dong, A.; Li, J.; Xu, X.; Cao, L. MiR-431 Inhibits Adipogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells via Targeting Insulin Receptor Substance 2. Stem Cell Res. Ther. 2018, 9, 231. [Google Scholar] [CrossRef]
- Karbiener, M.; Fischer, C.; Nowitsch, S.; Opriessnig, P.; Papak, C.; Ailhaud, G.; Dani, C.; Amri, E.-Z.; Scheideler, M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARγ. Biochem. Biophys. Res. Commun. 2009, 390, 247–251. [Google Scholar] [CrossRef]
- Skårn, M.; Namløs, H.M.; Noordhuis, P.; Wang, M.-Y.; Meza-Zepeda, L.A.; Myklebost, O. Adipocyte Differentiation of Human Bone Marrow-Derived Stromal Cells Is Modulated by MicroRNA-155, MicroRNA-221, and MicroRNA-222. Stem Cells Dev. 2012, 21, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hou, J.; Ye, L.; Chen, Y.; Cui, J.; Tian, W.; Li, C.; Liu, L. MicroRNA-143 Regulates Adipogenesis by Modulating the MAP2K5–ERK5 Signaling. Sci. Rep. 2014, 4, 3819. [Google Scholar] [CrossRef] [PubMed]
- Trohatou, O.; Zagoura, D.; Orfanos, N.K.; Pappa, K.I.; Marinos, E.; Anagnou, N.P.; Roubelakis, M.G. miR-26a Mediates Adipogenesis of Amniotic Fluid Mesenchymal Stem/Stromal Cells via PTEN, Cyclin E1, and CDK6. Stem Cells Dev. 2017, 26, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, Y.; Yang, H.; Hong, P.; Fang, X.; Hu, Y. H19/miR-30a/C8orf4 axis modulates the adipogenic differentiation process in human adipose tissue-derived mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 20925–20934. [Google Scholar] [CrossRef]
- Shuai, Y.; Yang, R.; Mu, R.; Yu, Y.; Rong, L.; Jin, L. MiR-199a-3p mediates the adipogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating KDM6A/WNT signaling. Life Sci. 2019, 220, 84–91. [Google Scholar] [CrossRef]
- Hamam, D.; Ali, D.; Vishnubalaji, R.; Hamam, R.; Al-Nbaheen, M.; Chen, L.; Kassem, M.; Aldahmash, A.; Alajez, N.M. microRNA-320/RUNX2 axis regulates adipocytic differentiation of human mesenchymal (skeletal) stem cells. Cell Death Dis. 2014, 5, e1499. [Google Scholar] [CrossRef]
- Oskowitz, A.Z.; Lu, J.; Penfornis, P.; Ylostalo, J.; McBride, J.; Flemington, E.K.; Prockop, D.J.; Pochampally, R. Human multipotent stromal cells from bone marrow and microRNA: Regulation of differentiation and leukemia inhibitory factor expression. Proc. Natl. Acad. Sci. USA 2008, 105, 18372–18377. [Google Scholar] [CrossRef]
- Zaragosi, L.-E.; Wdziekonski, B.; Brigand, K.; Villageois, P.; Mari, B.; Waldmann, R.; Dani, C.; Barbry, P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011, 12, R64. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.J.; Bae, Y.C.; Jung, J.S. MiR-21 Regulates Adipogenic Differentiation through the Modulation of TGF-β Signaling in Mesenchymal Stem Cells Derived from Human Adipose Tissue. Stem Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef]
- Karbiener, M.; Pisani, D.F.; Frontini, A.; Oberreiter, L.M.; Lang, E.; Vegiopoulos, A.; Mössenböck, K.; Bernhardt, G.A.; Mayr, T.; Hildner, F.; et al. MicroRNA-26 Family Is Required for Human Adipogenesis and Drives Characteristics of Brown Adipocytes. Stem Cells 2013, 32, 1578–1590. [Google Scholar] [CrossRef]
- Karbiener, M.; Neuhold, C.; Opriessnig, P.; Prokesch, A.; Bogner-Strauss, J.G.; Scheideler, M. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol. 2011, 8, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.S.; Woo, K.M.; Ryoo, H.-M.; Yi, T.; Song, S.U.; Baek, J.-H. MiR-124 Inhibits Myogenic Differentiation of Mesenchymal Stem Cells Via Targeting Dlx5. J. Cell. Biochem. 2014, 115, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Lin, M.; Lv, X.; Wang, J.; Wei, Q.; Zhang, Z.; Li, L. MicroRNA-124-3p affects myogenic differentiation of adipose-derived stem cells by targeting Caveolin-1 during pelvic floor dysfunction in Sprague Dawley rats. Ann. Transl. Med. 2021, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Sun, B.; Xu, P.; Zhu, Y.; Meng, X.-H.; Teng, G.-J.; Xiao, Z.-D. MiR-218 Induces Neuronal Differentiation of ASCs in a Temporally Sequential Manner with Fibroblast Growth Factor by Regulation of the Wnt Signaling Pathway. Sci. Rep. 2017, 7, 39427. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.-F.; Wu, H.; Wang, W. miR-142-5p Improves Neural Differentiation and Proliferation of Adipose-Derived Stem Cells. Cell. Physiol. Biochem. 2018, 50, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Patel, S.; Gregory, L.; Rameshwar, P. Neurogenesis: Role for microRNAs and mesenchymal stem cells in pathological states. Curr. Med. Chem. 2010, 17, 2159–2167. [Google Scholar] [CrossRef]
- Liang, W.-C.; Fu, W.-M.; Wang, Y.-B.; Sun, Y.-X.; Xu, L.-L.; Wong, C.-W.; Chan, K.-M.; Li, G.; Waye, M.M.-Y.; Zhang, J.-F. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci. Rep. 2016, 6, 20121. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Jia, L.; Li, W. Long Noncoding RNA H19 Promotes Osteoblast Differentiation Via TGF-β1/Smad3/HDAC Signaling Pathway by Deriving miR-675. Stem Cells 2015, 33, 3481–3492. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, J.; Sun, L.; Pan, Y.; Wang, H.; Zhang, W.-B. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone 2018, 108, 62–70. [Google Scholar] [CrossRef]
- Zhuang, W.; Ge, X.; Yang, S.; Huang, M.; Zhuang, W.; Chen, P.; Zhang, X.; Fu, J.; Qu, J.; Li, B. Upregulation of lncRNA MEG3 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells From Multiple Myeloma Patients By Targeting BMP4 Transcription. Stem Cells 2015, 33, 1985–1997. [Google Scholar] [CrossRef]
- Deng, L.; Hong, H.; Zhang, X.; Chen, D.; Chen, Z.; Ling, J.; Wu, L. Down-regulated lncRNA MEG3 promotes osteogenic differentiation of human dental follicle stem cells by epigenetically regulating Wnt pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jin, C.; Chen, S.; Zheng, Y.; Huang, Y.; Jia, L.; Ge, W.; Zhou, Y. Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Mol. Cell. Biochem. 2017, 433, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tao, Z.; Wang, Y. Long non-coding RNA DANCR regulates the proliferation and osteogenic differentiation of human bone-derived marrow mesenchymal stem cells via the p38�MAPK pathway. Int. J. Mol. Med. 2017, 41, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Lei, P.; Wen, T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging 2019, 11, 8777–8791. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, F.; Wang, C.; Wang, C.; Cui, P.; Zhang, X.; Chen, X. Long noncoding RNA MALAT1 promotes osterix expression to regulate osteogenic differentiation by targeting miRNA-143 in human bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2018, 119, 6986–6996. [Google Scholar] [CrossRef]
- Jiang, X.-R.; Guo, N.; Li, X.-Q.; Yang, H.-Y.; Wang, K.; Zhang, C.-L.; Li, G.-S. Long non-coding RNA HULC promotes proliferation and osteogenic differentiation of bone mesenchymal stem cells via down-regulation of miR-195. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2954–2965. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, X.; Feng, X.; Zhu, E.; Zhou, J.; Wang, G.; Tian, L.; Wang, B. A novel long noncoding RNA PGC1β-OT1 regulates adipocyte and osteoblast differentiation through antagonizing miR-148a-3p. Cell Death Differ. 2019, 26, 2029–2045. [Google Scholar] [CrossRef]
- Tang, S.; Xie, Z.; Wang, P.; Li, J.; Wang, S.; Liu, W.; Li, M.; Wu, X.; Su, H.; Cen, S.; et al. LncRNA-OG Promotes the Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Under the Regulation of hnRNPK. Stem Cells 2018, 37, 270–283. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Chen, Z.; Zhang, D. Osteogenic growth peptide promotes osteogenic differentiation of mesenchymal stem cells mediated by LncRNA AK141205-induced upregulation of CXCL13. Biochem. Biophys. Res. Commun. 2015, 466, 82–88. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Tan, H.; Li, J.; Zhu, M.; Xu, Y. Silencing of Long Non-Coding RNA NONHSAT009968 Ameliorates the Staphylococcal Protein A-Inhibited Osteogenic Differentiation in Human Bone Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2016, 39, 1347–1359. [Google Scholar] [CrossRef]
- Shang, G.; Wang, Y.; Xu, Y.; Zhang, S.; Sun, X.; Guan, H.; Zhao, X.; Wang, Y.; Li, Y.; Zhao, G. Long non-coding RNA TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of rat bone marrow mesenchymal stem cell by targeting miR-204-5p and miR-125a-3p. J. Cell. Physiol. 2018, 233, 6041–6051. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Liu, N.; Wang, W. High glucose prevents osteogenic differentiation of mesenchymal stem cells via lncRNA AK028326/CXCL13 pathway. BioMedicine 2016, 84, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X.; Chen, S.; Yang, C.; Shi, B.; Zhou, L.; Zhao, J. Long noncoding RNA DANCR regulates miR-1305-Smad 4 axis to promote chondrogenic differentiation of human synovium-derived mesenchymal stem cells. Biosci. Rep. 2017, 37, BSR20170347. [Google Scholar] [CrossRef] [PubMed]
- Ou, F.; Su, K.; Sun, J.; Liao, W.; Yao, Y.; Zheng, Y.; Zhang, Z. The LncRNA ZBED3-AS1 induces chondrogenesis of human synovial fluid mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 487, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, Y.; Jin, C.; Li, X.; Jia, L.; Li, W. Long Non-coding RNA H19 Inhibits Adipocyte Differentiation of Bone Marrow Mesenchymal Stem Cells through Epigenetic Modulation of Histone Deacetylases. Sci. Rep. 2016, 6, 28897. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, Z.; Cen, S.; Li, M.; Liu, W.; Tang, S.; Ye, G.; Li, J.; Zheng, G.; Li, Z.; et al. Long noncoding RNA repressor of adipogenesis negatively regulates the adipogenic differentiation of mesenchymal stem cells through the hnRNP A1-PTX3-ERK axis. Clin. Transl. Med. 2020, 10, e227. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.; Fan, L.; Deng, L.; Fan, J.; Li, D.; Li, H.; Zhao, R.C. Lnc13728 facilitates human mesenchymal stem cell adipogenic differentiation via positive regulation of ZBED3 and downregulation of the WNT/β-catenin pathway. Stem Cell Res. Ther. 2021, 12, 176. [Google Scholar] [CrossRef]
- Li, M.; Xie, Z.; Wang, P.; Li, J.; Liu, W.; Tang, S.; Liu, Z.; Wu, X.; Wu, Y.; Shen, H. The long noncoding RNA GAS5 negatively regulates the adipogenic differentiation of MSCs by modulating the miR-18a/CTGF axis as a ceRNA. Cell Death Dis. 2018, 9, 554. [Google Scholar] [CrossRef]
- Kalwa, M.; Hänzelmann, S.; Otto, S.; Kuo, C.-C.; Franzen, J.; Joussen, S.; Fernandez-Rebollo, E.; Rath, B.; Koch, C.; Hofmann, A.; et al. The lncRNA HOTAIR impacts on mesenchymal stem cells via triple helix formation. Nucleic Acids Res. 2016, 44, 10631–10643. [Google Scholar] [CrossRef]
- Li, Y.; Shan, Z.; Yang, B.; Yang, D.; Men, C.; Cui, Y.; Wu, J. LncRNA HULC promotes epithelial and smooth-muscle-like differentiation of adipose-derived stem cells by upregulation of BMP9. Pharmazie 2018, 73, 49–55. [Google Scholar] [CrossRef]
- Farzi-Molan, A.; Babashah, S.; Bakhshinejad, B.; Atashi, A.; Taha, M.F. Down-regulation of the non-coding RNA H19 and its derived miR-675 is concomitant with up-regulation of insulin-like growth factor receptor type 1 during neural-like differentiation of human bone marrow mesenchymal stem cells. Cell Biol. Int. 2018, 42, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Gugjoo, M.B.; Pal, A. Mesenchymal Stem Cell Differentiation Properties and Available Microenvironment. In Mesenchymal Stem Cell in Veterinary Sciences; Gugjoo, M.B., Pal, A., Eds.; Springer: Singapore, 2020; pp. 67–87. ISBN 9789811560378. [Google Scholar]
- Karagkouni, D.; Karavangeli, A.; Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Characterizing MiRNA–LncRNA Interplay. In Methods in Molecular Biology; Zhang, L., Hu, X., Eds.; Humana: New York, NY, USA, 2021; Volume 2372, pp. 243–262. [Google Scholar]
- Sun, X.; Luo, L.; Li, J. LncRNA MALAT1 facilitates BM-MSCs differentiation into endothelial cells via targeting miR-206/VEGFA axis. Cell Cycle 2020, 19, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Carp, D.M.; Liang, Y. Universal or Personalized Mesenchymal Stem Cell Therapies: Impact of Age, Sex, and Biological Source. Cells 2022, 11, 2077. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.-Y.; Yang, X.-L.; Li, W.-J.; Liu, G.-L. Long Noncoding RNA Metastasis-Associated Lung Adenocarcinoma Transcript 1 Promotes the Osteoblast Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells by Targeting the microRNA-96/Osterix Axis. J. Craniofacial Surg. 2021, 33, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zheng, Z.; Zeng, X.; Zhao, Y.; Ai, Z.; Yu, M.; Wu, Y.; Jiang, J.; Li, J.; Li, S. LncRNA MALAT1 Mediates Osteogenic Differentiation of Bone Mesenchymal Stem Cells by Sponging MiR-129-5p. PeerJ 2022, 10, e13355. [Google Scholar] [CrossRef]
- Vimalraj, S.; Subramanian, R.; Dhanasekaran, A. LncRNA MALAT1 Promotes Tumor Angiogenesis by Regulating MicroRNA-150-5p/VEGFA Signaling in Osteosarcoma: In-Vitro and In-Vivo Analyses. Front. Oncol. 2021, 11, 742789. [Google Scholar] [CrossRef]
- Min, Z.H.; Wang, S.; Zhou, Y.; Yu, Z.W.; Chen, P.Q.; Wang, H.D.; Gu, R. Effect of Long-Chain Non-Coding RNA Small Nucleo Ar RNA Host Gene 15 on Osteogenic/Adipogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in High Glucose Environment via Targeting Mir-497. J. Biomater. Tissue Eng. 2020, 10, 1655–1661. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, H.; Li, S. LncRNA LOXL1-AS1 controls osteogenic and adipocytic differentiation of bone marrow mesenchymal stem cells in postmenopausal osteoporosis through regulating the miR-196a-5p/Hmga2 axis. J. Bone Miner. Metab. 2020, 38, 794–805. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Gao, W.; Han, J.; Yuan, R.; Zhang, M.; Ge, Z. LncRNA HCG11 Inhibits Adipocyte Differentiation in Human Adipose-Derived Mesenchymal Stem Cells by Sponging miR-204-5p to Upregulate SIRT1. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef]
- Wang, H.; Wei, P.; Zhang, Y.; Li, Y.; Yin, L. LncRNA TCONS_00023297 Regulates the Balance of Osteogenic and Adipogenic Differentiation in Bone Marrow Mesenchymal Stem Cells and the Coupling Process of Osteogenesis and Angiogenesis. Front. Cell Dev. Biol. 2021, 9, 697858. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liang, D. Effect of Long-Chain Non-Coding RNA GAS5 on Osteogenic/Adipogenic Differentiation of Bone Marrow Mesenchymal Stem Cells Under Oxidative Stress Through Targeting MiR-365. J. Biomater. Tissue Eng. 2019, 9, 1751–1757. [Google Scholar] [CrossRef]
- Huang, M.-J.; Zhao, J.-Y.; Xu, J.-J.; Li, J.; Zhuang, Y.-F.; Zhang, X.-L. lncRNA ADAMTS9-AS2 Controls Human Mesenchymal Stem Cell Chondrogenic Differentiation and Functions as a ceRNA. Mol. Ther. Nucleic Acids 2019, 18, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Dai, X. Platelet lysate induces chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells by regulating the lncRNA H19/miR-29b-3p/SOX9 axis. FEBS Open Bio 2020, 10, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, W.; Wang, Y.; Zhang, H.; Zhang, L.; Li, C.; Yao, S.; Huang, Z.; Huang, L.; Luo, D. LncRNA DNM3OS regulates GREM2 via miR-127-5p to suppress early chondrogenic differentiation of rat mesenchymal stem cells under hypoxic conditions. Cell. Mol. Biol. Lett. 2021, 26, 22. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, R.; Wen, L.-M. Long non-coding RNA XIST regulates chondrogenic differentiation of synovium-derived mesenchymal stem cells from temporomandibular joint via miR-27b-3p/ADAMTS-5 axis. Cytokine 2020, 137, 155352. [Google Scholar] [CrossRef]
- Tan, Y.-F.; Tang, L.; OuYang, W.-X.; Jiang, T.; Zhang, H.; Li, S.-J. β-catenin-coordinated lncRNA MALAT1 up-regulation of ZEB-1 could enhance the telomerase activity in HGF-mediated differentiation of bone marrow mesenchymal stem cells into hepatocytes. Pathol. Res. Pr. 2019, 215, 546–554. [Google Scholar] [CrossRef]
- Sun, J.; Sun, X.; Hu, S.; Wang, M.; Ma, N.; Chen, J.; Duan, F. Long noncoding RNA SNHG1 silencing accelerates hepatocyte-like cell differentiation of bone marrow-derived mesenchymal stem cells to alleviate cirrhosis via the microRNA-15a/SMURF1/UVRAG axis. Cell Death Discov. 2022, 8, 77. [Google Scholar] [CrossRef]
- Dai, X.-W.; Luo, W.; Lv, C.-L. lncRNA-MIAT facilitates the differentiation of adipose-derived mesenchymal stem cells into lymphatic endothelial cells via the miR-495/Prox1 axis. Mol. Med. Rep. 2021, 23, 323. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Lei, P. LncRNA HOTAIRM1 promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by targeting miR-152-3p/ETS1 axis. Mol. Biol. Rep. 2023, 50, 5597–5608. [Google Scholar] [CrossRef]
- Ouyang, X.; Ding, Y.; Yu, L.; Xin, F.; Yang, X. LncRNA TUG Regulates Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells via MiRNA-204/SIRT 1. J. Musculoskelet. Neuronal Interact. 2022, 22, 401–410. [Google Scholar] [PubMed]
- Han, Y.; Yang, Q.; Huang, Y.; Jia, L.; Zheng, Y.; Li, W. Long non-coding RNA SNHG5 promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the miR-212-3p/GDF5/SMAD pathway. Stem Cell Res. Ther. 2022, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Wei, S.; Huang, M. Long non-coding RNA KCNQ1OT1 overexpression promotes osteogenic differentiation of staphylococcus aureus-infected human bone mesenchymal stem cells by sponging microRNA miR-29b-3p. Bioengineered 2022, 13, 5855–5867. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, X.; Shi, Y.; Zhao, H. FGD5-AS1 facilitates the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells via targeting the miR-506-3p/BMP7 axis. J. Orthop. Surg. Res. 2021, 16, 665. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, H.; Wang, Z.; Cai, J.; Ma, X.; Chen, W.; Jiang, X.; Zhao, D.; Hou, W.; Tao, Y. lncRNAs MALAT1 and LINC00657 upstream to miR-214-3p/BMP2 regulate osteogenic differentiation of human mesenchymal stem cells. Mol. Biol. Rep. 2022, 49, 6847–6857. [Google Scholar] [CrossRef]
- Liu, C.; Liang, T.; Zhang, Z.; Chen, J.; Xue, J.; Zhan, X.; Ren, L. MEG3 alleviates ankylosing spondylitis by suppressing osteogenic differentiation of mesenchymal stem cells through regulating microRNA-125a-5p-mediated TNFAIP3. Apoptosis 2022, 28, 498–513. [Google Scholar] [CrossRef]
- Yan, Z.; He, Q. LINC01234 Sponging of the miR-513a-5p/AOX1 Axis is Upregulated in Osteoporosis and Regulates Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Mol. Biotechnol. 2023, 1–11. [Google Scholar] [CrossRef]
- Wang, F.; Deng, H.; Chen, J.; Wang, Z.; Yin, R. LncRNA MIAT can regulate the proliferation, apoptosis, and osteogenic differentiation of bone marrow-derived mesenchymal stem cells by targeting miR-150-5p. Bioengineered 2022, 13, 6343–6352. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Zhou, X.; Ding, H.; Sun, C.; Wang, Y.; Zhang, F.; Zhao, J. METTL3-mediated long non-coding RNA MIR99AHG methylation targets miR-4660 to promote bone marrow mesenchymal stem cell osteogenic differentiation. Cell Cycle 2022, 22, 476–493. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Feng, S. Knockdown of long non-coding RNA HOTAIR promotes bone marrow mesenchymal stem cell differentiation by sponging microRNA miR-378g that inhibits nicotinamide N-methyltransferase. Bioengineered 2021, 12, 12482–12497. [Google Scholar] [CrossRef]
- Zhao, G.; Luo, W.D.; Yuan, Y.; Lin, F.; Guo, L.M.; Ma, J.J.; Chen, H.B.; Tang, H.; Shu, J. LINC02381, a Sponge of MiR-21, Weakens Osteogenic Differentiation of HUC-MSCs through KLF12-Mediated Wnt4 Transcriptional Repression. J. Bone Miner. Metab. 2022, 40, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Cervio, E.; Barile, L.; Moccetti, T.; Vassalli, G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015, 2015, 482171. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Masood, M.; Adlat, S.; Gang, D.; Zhu, S.; Li, G.; Li, N.; Chen, J.; Zhu, P. Mesenchymal stem cell-derived exosome microRNA as therapy for cardiac ischemic injury. Biomed. Pharmacother. 2021, 143, 112118. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Kuang, X.; Cai, S.; Wang, X.; Du, D.; Wang, J.; Wang, Y.; Chen, Y.; Bihl, J.; Chen, Y.; et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res. Ther. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Fujimiya, M. Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders. Neural Regen. Res. 2021, 16, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Han, Y.; Sun, H.; Sun, S.; Wang, Y.; Guo, R.; Guo, J.; Tian, X.; Wang, J.; Wang, J. Mesenchymal stem cell-derived exosomal microRNA-367–3p alleviates experimental autoimmune encephalomyelitis via inhibition of microglial ferroptosis by targeting EZH2. BioMedicine 2023, 162, 114593. [Google Scholar] [CrossRef]
- Zou, L.; Ma, X.; Wu, B.; Chen, Y.; Xie, D.; Peng, C. Protective effect of bone marrow mesenchymal stem cell-derived exosomes on cardiomyoblast hypoxia-reperfusion injury through the miR-149/let-7c/Faslg axis. Free. Radic. Res. 2020, 54, 722–731. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, M.; Guo, Q.; Shen, L.; Liu, X.; Pan, J.; Zhang, Y.; Xu, T.; Zhang, D.; Wei, G. Human umbilical cord mesenchymal stem cell exosome-derived miR-874-3p targeting RIPK1/PGAM5 attenuates kidney tubular epithelial cell damage. Cell. Mol. Biol. Lett. 2023, 28, 12. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, X.; Zhang, M.; Cui, L.; Li, B.; Liu, Y.; Su, R.; Sun, K.; Hu, Y.; Yang, F.; et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res. Ther. 2022, 13, 330. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Li, X.; Lin, D.; Li, Z.; Wang, J.; Chen, J.; Gao, Z.; Lin, B. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote liver regeneration via miR-20a-5p/PTEN. Front. Pharmacol. 2023, 14, 1168545. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.-S.; Kline, H.L.; Lee, C.-H.; Wilkes, D.S.; Zhang, C. Regulation of Collagen V Expression and Epithelial-Mesenchymal Transition by miR-185 and miR-186 during Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2016, 186, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Z.; Jiang, X.; Wang, Y.; Yang, Z.; Mao, Y.; Wu, Z.; Li, G.; Chen, H. Mouse mesenchymal stem cell-derived exosomal miR-466f-3p reverses EMT process through inhibiting AKT/GSK3β pathway via c-MET in radiation-induced lung injury. J. Exp. Clin. Cancer Res. 2022, 41, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Xia, Y.; Yan, F.; Lu, Y. Therapeutic Potential of Mesenchymal Cell–Derived miRNA-150-5p–Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J. Immunol. 2018, 201, 2472–2482. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Q.; Cao, L.; Cao, L.; Zou, S.; Zou, S.; Feng, Y.; Feng, Y.; Miao, X.; Miao, X.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MicroRNA-181c-5p Promote BMP2-Induced Repair of Cartilage Injury through Inhibition of SMAD7 Expression. Stem Cells Int. 2022, 2022, 1157498. [Google Scholar] [CrossRef]
- Mao, G.; Kang, Y.; Zhang, Z.; Liao, W. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and prevent the development of osteoarthritis. Osteoarthr. Cartil. 2018, 26, S103. [Google Scholar] [CrossRef]
- Xie, Z.; Li, J.; Wang, P.; Li, Y.; Wu, X.; Wang, S.; Su, H.; Deng, W.; Liu, Z.; Cen, S.; et al. Differential Expression Profiles of Long Noncoding RNA and mRNA of Osteogenically Differentiated Mesenchymal Stem Cells in Ankylosing Spondylitis. J. Rheumatol. 2016, 43, 1523–1531. [Google Scholar] [CrossRef]
- Sun, C.; Huang, L.; Li, Z.; Leng, K.; Xu, Y.; Jiang, X.; Cui, Y. Long non-coding RNA MIAT in development and disease: A new player in an old game. J. Biomed. Sci. 2018, 25, 23. [Google Scholar] [CrossRef]
- Tye, C.E.; Gordon, J.A.R.; Martin-Buley, L.A.; Stein, J.L.; Lian, J.B.; Stein, G.S. Could LncRNAs Be the Missing Links in Control of Mesenchymal Stem Cell Differentiation? J. Cell Physiol. 2015, 230, 526–534. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhu, R.; Han, Q.; Zhao, R.C. Lung cancer exosomes initiate global long non-coding RNA changes in mesenchymal stem cells. Int. J. Oncol. 2015, 48, 681–689. [Google Scholar] [CrossRef]

