1. Introduction
One of the major patient safety concerns during hospitalization is the occurrence of healthcare-associated infections (HAIs). This is because HAIs cause an increase in morbidity, mortality, and healthcare-associated cost [1]. There are variations in the rate of HAIs between countries, with 4% in the United States (US) [2], 6.5% in Europe [3], 9.0% in Asia [4], and approximately 16% in developing countries [5]. Africa has a two-fold higher rate of HAIs as compared to the developed countries [6,7]. HAIs are potentially preventable through compliance with infection control and prevention recommendations [1]. Hand hygiene is the mainstay for the prevention of HAIs and this is beneficial in reducing the transmission of multidrug-resistant organisms [8]. Infection control and prevention programs were disrupted during the COVID-19 pandemic, and this has a potential impact on the incidence of HAIs and transmission of multidrug-resistant organisms. The rate of multidrug-resistant Gram-negative and Gram-positive pathogens has increased during the COVID-19 pandemic [9]. Prior to the COVID-19 pandemic, compliance with recommendations from guidelines on hand hygiene was poor among healthcare workers [10]. However, improved hand hygiene and environmental hygiene was reported during the COVID-19 pandemic [11], and this could potentially reduce the rate of HAIs and transmission of multidrug-resistant organisms.
Conversely, hospital resources, including infection prevention and control resources, were diverted to the management of the COVID-19 pandemic, and this could potentially affect the compliance with infection control and prevention recommendations leading to an increase in the rate of HAIs [12]. The diversion of hospital resources may potentially nullify the benefits of improved hand hygiene on the rate of HAIs during the COVID-19 pandemic [13]. In addition, there was a decline in hospital visits and overcrowding due to the enforcement of movement restrictions during the pandemic, and this could potentially benefit infection prevention and control programs [14,15]. Furthermore, the transmission of hospital-acquired respiratory pathogens was reduced due to the increase in the use of face masks by healthcare workers and patients [15]. Currently, the effect of the COVID-19 pandemic on the rate of HAIs is a subject of debate. While some believe that COVID-19 mitigation strategies could potentially reduce the rate of HAIs [13,16], others have argued that the diversion of hospital resources during the pandemic could potentially increase the rate of HAIs [13]. This study aimed to synthesize the effect of the COVID-19 pandemic on the overall risk of HAIs, and determine the effect of the pandemic on the risk of individual types of HAIs, including central line-associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), Clostridium difficile infection (CDI), surgical site infections (SSI), and ventilator-associated pneumonia/hospital-acquired pneumonia (VAP/HAP).
2. Materials and Methods
2.1. Study Design
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statements 2020 was used to conduct and report this systematic review [17]. The study protocol was registered with PROPSPERO (reference ID: CRD42023463262).
2.2. Eligibility Criteria
2.3. Information Sources
PubMed and Scopus databases were searched by two reviewers to find potentially eligible studies. Supplementary search of Google Scholar was conducted to identify eligible studies. The reference list of the selected studies was manually examined to find additional studies.
2.4. Search Strategy
The relevant keywords for HAIs and the COVID-19 pandemic were combined using Boolean indicators (AND/OR). The following keywords were used for the search: impact OR effect OR change AND “hospital-acquired infection*” OR “healthcare-associated infection*” OR “nosocomial infection*” AND “SARS-CoV-2” OR “COVID-19” OR “coronavirus disease 2019” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus infection” OR “coronavirus pandemic” OR “COVID-19 pandemic”.
2.5. Selection Process
The results of the searches from all the databases were combined in one folder and duplicate studies were removed. The titles and abstracts of the studies were initially assessed and irrelevant studies were excluded. The full-text articles of the remaining studies were assessed based on the inclusion and exclusion criteria for selection and data extraction.
2.6. Data Extraction Process
The included studies were reviewed for data extraction using a predefined data collection form. Data extraction was performed by an independent reviewer (UA) and the extracted data were checked by a second reviewer for accuracy. All disagreements were resolved by the reviewers through dialogue.
2.7. Data Items
Data items extracted from the included studies include: name of author and year of publication, study location, study setting, the study design, study period, sample size, hospital units involved, rate of HAIs before and during the COVID-19 pandemic, type of HAIs, and the p-value. In addition, the frequency of HAI, the number of patients, the total patient days and total device days (for urinary catheter and central catheter) for both periods were extracted.
2.8. Study Risk of Bias Assessment
Methodological quality of the included studies was assessed by two independent reviewers (AHY and KA) using the Newcastle–Ottawa scale (NOS) [18]. NOS consists of three sections including: selection, comparability, and outcomes. The reviewers resolved any discrepancies through dialogue.
2.9. Outcome Assessment and Effect Measures
The primary outcome was the effect of the COVID-19 pandemic on the overall risk of HAIs, and this was determined by comparing the overall rate of HAIs before versus during the COVID-19 pandemic. The Centers for Disease Control and Prevention (CDC) [19] and the European Centres for Disease Prevention and Control (ECDC) guidelines were used to define HAIs [20]. The secondary outcomes assessed include the risk of CLABSI, CAUTI, CDI, SSI, and VAP/HAP presented as odds ratio with 95% confidence interval. These infections are referred by CDC as types of HAIs.
2.10. Data Synthesis
Both qualitative and quantitative synthesis was used. Review Manager (RevMan) [Computer program], version 5.4. The Cochrane Collaboration, 2020 was used for the quantitative synthesis. The pooled estimate was determined using random-effects meta-analysis, and the findings were presented using forest plots. Higgins I2 statistic was employed to assess the level of heterogeneity using the following criteria; <40% = low heterogeneity, 30–60% = moderate heterogeneity, 50–90% = substantial heterogeneity, and 75–100% considerable heterogeneity [21]. The overall rate of HAIs was evaluated as the number of patients with HAI as a proportion of all hospitalized patients. The overall risk of HAIs was estimated by comparing the overall rate of HAIs before versus the rate during the COVID-19 pandemic. Furthermore, the risk for the different types of HAIs (CLABSI, CAUTI, SSI, CDI, and HAP/VAP) was estimated by comparing the rate of HAIs (number of events divided by the total patient days or total-device days) between the period before and the period during the COVID-19 pandemic. For each type of HAI, data were meta-analyzed when at least two studies reported that particular HAI.
3. Results
3.1. Study Selection
The database searches produced 6133 articles, out of which 88 duplicates were removed. The title and abstract of the de-duplicated articles was screened and 5954 irrelevant articles were excluded. The remaining 91 full-text articles were evaluated for inclusion, and 37 articles that fulfilled the criteria were eventually selected. Figure 1 illustrates the PRISMA flow diagram for the screening and selection process.
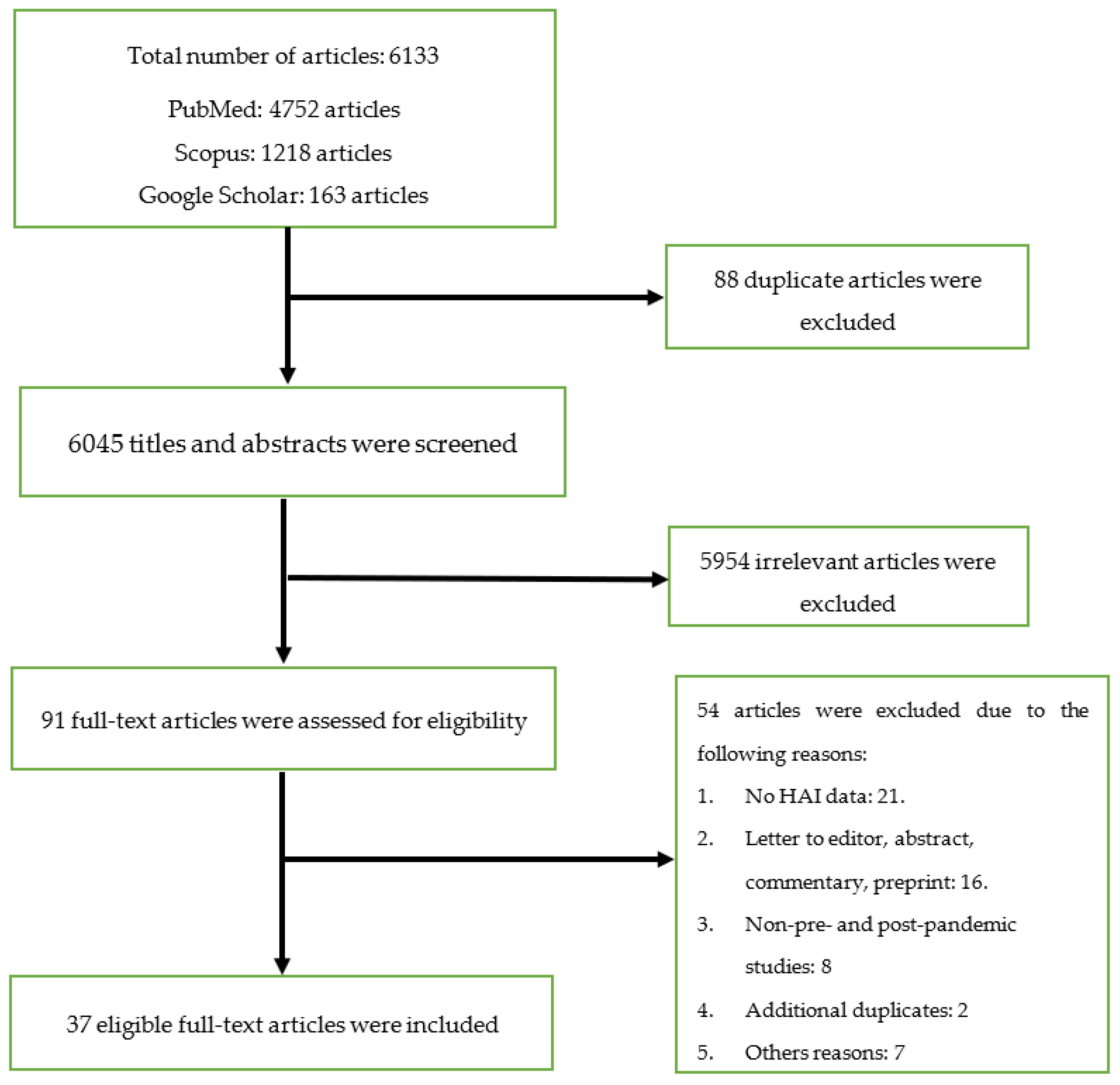
Figure 1. Flow chart for the screening and selection processes.
3.2. Study Characteristics
North America (n = 14; 37.8%), Europe (n = 11; 29.7%), and Asia (n = 5; 13.5%) had the highest number of studies. The US had the highest number of studies (n = 13; 35.1%) followed by Italy (n = 4; 10.8%), and Spain (n = 3; 8.1%). Most of the studies (n = 27; 72.9%) included hospital-wide data, while four studies (10.8%) involved data from intensive care units (ICUs) only. Furthermore, the majority of the studies (n = 26; 70.3%) included multiple study centers. Six studies compared the overall prevalence of HAIs between the period before the COVID-19 pandemic and during the pandemic [22,23,24,25,26,27]. CLABSIs (n = 15; 40.5%) [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], CAUTIs (n = 15; 40.5%) [22,28,30,31,32,33,34,35,36,39,40,41,42,43,44], and CDI (n = 14; 37.8%) [28,30,31,32,34,36,40,43,45,46,47,48,49,50] were the most reported HAIs in the selected studies. presents the characteristics of the studies included in this review.
3.3. Quality Assessment of the Studies
Most of the included studies had a truly or somewhat representative target population. In addition, the sample size for most of the studies was satisfactory and justified. The quality score for the included studies ranged from 6 to 7, with 33 studies (89.2%) scoring 7 points. Overall, the methodological quality was good in the majority of the studies (89.2%), although, four studies were found to have a fair methodological quality. illustrates the quality assessment results of the included studies.
3.4. Qualitative Summary of Results
3.5. Quantitative Summary of Results
4. Discussion
This review examined the effect of the COVID-19 pandemic on the rate of HAIs, and included studies from different continents across the world. The majority of the studies were from North America and Europe with a few studies coming from Africa, Asia, South America, and Oceania. There was no difference in the overall risk of HAIs between the two periods. Conversely, patients hospitalized before the COVID-19 pandemic had a lower risk of CLABSI compared to those in the COVID-19 pandemic period. Similarly, there was a significant 20% increase in the risk of CDI during the COVID-19 pandemic. There was no significant increase in the risk of CAUTI and SSI during the pandemic. Therefore, infection prevention and control programs should be strengthened to reduce the burden of HAIs during and after the pandemic. The available evidence has shown that HAIs, particularly those involving multidrug-resistant organisms, have a high mortality rate [59,60]. There were no variations in the overall risk of HAIs between the two periods, and this implies that COVID-19 mitigation strategies did not affect the overall risk of HAIs. The improvements in hand and environmental hygiene during the COVID-19 pandemic was expected to reduce the incidence of HAIs [16]. However, this potential benefit could be counteracted by the disruption of other infection prevention and control programs such as the surveillance of HAIs, contact precaution and isolation of those colonized with multidrug-resistant pathogens in a separate room [12,13,61]. Therefore, the COVID-19 mitigation strategies that improved hand and environmental hygiene should be sustained, while the infection control measures that were disrupted during the pandemic should be resumed to reduce the incidence of HAIs.
The result also revealed that there was an increase in the risk of CLABSI during the pandemic compared to the period before the pandemic. Generally, hospitalized COVID-19 patients, especially those who are critically ill, have a higher risk of bloodstream infections compared to hospitalized non-COVID-19 patients [62]. This was attributed to the frequent use of a central line, use of immunosuppressive therapy, and reduced compliance with hand hygiene due to increased workload [62,63]. Therefore, improved hand hygiene is recommended to reduce the incidence of CLABSIs [64]. Furthermore, COVID-19 was significantly associated with a higher risk of CDI. CDI has been significantly associated with antibiotic use, the number of prescribed antibiotics, and the duration of antibiotic therapy [65,66,67]. There was a high rate of antibiotic prescription among COVID-19 patients [68,69,70]. The excessive use of antibiotics in COVID-19 patients despite a low rate of secondary infections explains the increase in the risk of CDI during the pandemic [71,72]. Therefore, antimicrobial stewardship is recommended to promote the rational use of antibiotics to reduce the risk of CDI. The effectiveness of antimicrobial stewardship programs in reducing the risk of CDI has been established [73]. In addition, infection control and prevention recommendations should be improved to minimize the horizontal transmission of CDI [74].
The results indicate that there was no significant increase in the risk of CAUTI and SSI during the pandemic. This implies that the infection control recommendations implemented to curb the transmission of COVID-19 did not significantly impact the risk of CAUTI and SSI. In the case of SSI, there are other measures besides infection control recommendations that are used to prevent SSI before, during, and after surgery. Typically, SSIs are preventable through preoperative antimicrobial prophylaxis. Previous studies have shown a low rate of compliance with recommendations for surgical antibiotic prophylaxis before the pandemic [75,76,77]. However, there was an increase in the use of preoperative antimicrobial prophylaxis for genitourinary procedures in the pandemic era compared to the period before the pandemic [78]. In addition to surgical antimicrobial prophylaxis, the duration of surgery, comorbidities such as diabetes and hypertension, tobacco smoking, and the American Society of Anesthesia (ASA) score, are significantly associated with SSIs [79,80,81,82]. These factors could explain the lack of significant improvement in the SSI rate in the pandemic era. Therefore, managing the modifiable risk factors associated with SSI coupled with infection control measures, and surgical antimicrobial prophylaxis is required to reduce the burden of SSI.
The results of this systematic review and meta-analysis should be interpreted with caution in light of some limitations. First, the distribution of the included studies was skewed towards North America and Europe, which accounted for most of the studies and this may affect the generalizability of the findings. However, all the continents were represented in the qualitative and quantitative analyses. Second, there were variations in the definition of HAIs and the classification of HAIs among the included studies, and this is a potential source of assessment and measurement bias. Third, the heterogeneous risk estimates were used by the included studies, where some studies reported the prevalence, while others reported the incidence per 1000 device days or per 1000 patient days. These variations reduced the number of studies included in the meta-analyses, which could potentially affect the findings. However, it is noteworthy that only studies with similar units of measurement were meta-analyzed. In addition, the study period for the included studies was highly variable. While some studies compared the prevalence or incidence in 2019 with 2020, others compared 2019 with 2021. Fourth, the infection prevention and control practices vary from one institution to another and between countries; therefore, the impact of the pandemic on HAIs could be inconsistent. Fifth, most of the studies used a before and after study design, which is associated with a high rate of bias. Sixth, the results for HAP/VAP were not meta-analyzed because the included studies used different units of measurement. Finally, substantial statistical heterogeneity was found in most of the meta-analyses. In spite of the limitations, this study shows evidence of the effect of the COVID-19 pandemic on the risk of HAIs among hospitalized patients.
5. Conclusions
The overall risk of HAI was observed to be unaffected by the COVID-19 pandemic. However, the COVID-19 pandemic was significantly associated with a higher risk of CLABSI and CDI. Therefore, more stringent infection prevention and control measures as well as prudent antimicrobial stewardship programs are warranted across all healthcare facilities to reduce the burden of HAIs during such pandemics. Further studies are required from developing countries, especially those in Africa and Asia.
References
- Storr, J.; Twyman, A.; Zingg, W.; Damani, N.; Kilpatrick, C.; Reilly, J.; Price, L.; Egger, M.; Grayson, M.L.; Kelley, E.; et al. Core components for effective infection prevention and control programmes: New WHO evidence-based recommendations. Antimicrob. Resist. Infect. Control 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care–associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.L.; Apisarnthanarak, A.; Madriaga, G. The burden of healthcare-associated infections in Southeast Asia: A systematic literature review and meta-analysis. Clin. Infect. Dis. 2015, 60, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Amir, O.; Rodríguez-Baño, J. Healthcare-associated infections in Africa: A systematic review and meta-analysis of point prevalence studies. J. Pharm. Policy Pract. 2022, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob. Resist. Infect. Control 2020, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Loftus, M.J.; Guitart, C.; Tartari, E.; Stewardson, A.J.; Amer, F.; Bellissimo-Rodrigues, F.; Lee, Y.F.; Mehtar, S.; Sithole, B.L.; Pittet, D. Hand hygiene in low-and middle-income countries. Int. J. Infect. Dis. 2019, 86, 25–30. [Google Scholar] [CrossRef]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 pandemic on multidrug resistant gram positive and gram negative pathogens: A systematic review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef]
- Ataiyero, Y.; Dyson, J.; Graham, M. Barriers to hand hygiene practices among health care workers in sub-Saharan African countries: A narrative review. Am. J. Infect. Control 2019, 47, 565–573. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Qiao, F.; Feng, B.; Hu, F.; Xi, Z.A.; Wu, W.; Ni, Z.L.; Liu, L.; Yuan, Y. Compared hand hygiene compliance among healthcare providers before and after the COVID-19 pandemic: A rapid review and meta-analysis. Am. J. Infect. Control 2022, 50, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Doll, M.; Pryor, R.; Godbout, E.; Cooper, K.; Bearman, G. Impact of COVID-19 on traditional healthcare-associated infection prevention efforts. Infect. Control Hosp. Epidemiol. 2020, 41, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Rossolini, G.M.; Schultsz, C.; Tacconelli, E.; Murthy, S.; Ohmagari, N.; Holmes, A.; Bachmann, T.; Goossens, H.; Canton, R.; et al. Antimicrobial resistance research in a post-pandemic world: Insights on antimicrobial resistance research in the COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2021, 25, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Subramanya, S.H.; Czyż, D.M.; Acharya, K.P.; Humphreys, H. The potential impact of the COVID-19 pandemic on antimicrobial resistance and antibiotic stewardship. Virus Dis. 2021, 32, 330–337. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.L. The impact of visiting restrictions during the COVID-19 pandemic on pediatric patients. J. Pediatr. Nurs. 2021, 61, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Usman, M.N.; Baba, M.; Sulaiman, A.; Kolo, M.; Adamu, F.; Jaber, A.A. Practices and perception of healthcare workers towards infection control measures during the COVID-19 pandemic: A cross-sectional online survey from Nigeria. J. Infect. Dev. Ctries. 2022, 16, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 3 September 2023).
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- European Center for Disease Prevention and Control. Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals—Protocol Version 5.3; ECDC: Stockholm, Sweden, 2016.
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Mazzalai, E.; Alessandri, F.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 87. [Google Scholar] [CrossRef]
- Jabarpour, M.; Dehghan, M.; Afsharipour, G.; Hajipour Abaee, E.; Mangolian Shahrbabaki, P.; Ahmadinejad, M.; Maazallahi, M. The impact of COVID-19 outbreak on nosocomial infection rate: A case of Iran. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6650920. [Google Scholar] [CrossRef] [PubMed]
- Ghali, H.; Ben Cheikh, A.; Bhiri, S.; Khefacha, S.; Latiri, H.S.; Ben Rejeb, M. Trends of Healthcare-associated Infections in a Tuinisian University Hospital and Impact of COVID-19 Pandemic. INQUIRY J. Health Care Organ. Provis. Financ. 2021, 58, 00469580211067930. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, P.; Zhang, Y.; Liu, B. Effect of the “Normalized Epidemic Prevention and Control Requirements” on hospital-acquired and community-acquired infections in China. BMC Infect. Dis. 2021, 21, 1178. [Google Scholar] [CrossRef] [PubMed]
- Irelli, E.C.; Orlando, B.; Cocchi, E.; Morano, A.; Fattapposta, F.; Di Piero, V.; Toni, D.; Ciardi, M.R.; Giallonardo, A.T.; Fabbrini, G.; et al. The potential impact of enhanced hygienic measures during the COVID-19 outbreak on hospital-acquired infections: A pragmatic study in neurological units. J. Neurol. Sci. 2020, 418, 117111. [Google Scholar] [CrossRef] [PubMed]
- Tham, N.; Fazio, T.; Johnson, D.; Skandarajah, A.; Hayes, I.P. Hospital Acquired Infections in Surgical Patients: Impact of COVID-19-Related Infection Prevention Measures. World J. Surg. 2022, 46, 1249–1258. [Google Scholar] [CrossRef]
- Alsuhaibani, M.; Kobayashi, T.; McPherson, C.; Holley, S.; Marra, A.R.; Trannel, A.; Dains, A.; Abosi, O.J.; Jenn, K.E.; Meacham, H.; et al. Impact of COVID-19 on an infection prevention and control program, Iowa 2020–2021. Am. J. Infect. Control 2022, 50, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Granda, M.J.; Carrillo, C.S.; Rabadán, P.M.; Valerio, M.; Olmedo, M.; Muñoz, P.; Bouza, E. Increase in the frequency of catheter-related bloodstream infections during the COVID-19 pandemic: A plea for Control. J. Hosp. Infect. 2022, 119, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Conceicao, E.P.; Tan, J.Y.; Magesparan, K.D.; Amin, I.B.; Ismail, B.B.; Toh, H.X.; Jin, P.; Zhang, J.; Wee, E.G.; et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 469–477. [Google Scholar] [CrossRef]
- Halverson, T.; Mikolajczak, A.; Mora, N.; Silkaitis, C.; Stout, S. Impact of COVID-19 on hospital acquired infections. Am. J. Infect. Control 2022, 50, 831–833. [Google Scholar] [CrossRef]
- Advani, S.D.; Sickbert-Bennett, E.; Moehring, R.; Cromer, A.; Lokhnygina, Y.; Dodds-Ashley, E.; Kalu, I.C.; DiBiase, L.; Weber, D.J.; Anderson, D.J.; et al. The Disproportionate Impact of COVID-19 Pandemic on Healthcare-Associated Infections in Community Hospitals: Need for Expanding the Infectious Disease Workforce. Clin. Infect. Dis. 2022, 76, e34–e41. [Google Scholar] [CrossRef]
- Fakih, M.G.; Bufalino, A.; Sturm, L.; Huang, R.H.; Ottenbacher, A.; Saake, K.; Winegar, A.; Fogel, R.; Cacchione, J. Coronavirus disease 2019 (COVID-19) pandemic, central-line–associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts. Infect. Control Hosp. Epidemiol. 2022, 43, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, L.J.; Satyanarayana, G.; Baum, L.V.; Nebhan, C.A.; Kassim, A.A.; Gatwood, K.S. Evaluation of healthcare-associated infection rates in patients with hematologic malignancies and stem cell transplantation during the coronavirus disease 2019 (COVID-19) pandemic. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e11. [Google Scholar] [CrossRef] [PubMed]
- Geffers, C.; Schwab, F.; Behnke, M.; Gastmeier, P. No increase of device associated infections in German intensive care units during the start of the COVID-19 pandemic in 2020. Antimicrob. Resist. Infect. Control 2022, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Botero Suarez, C.S.; Rahamatalli, B.; Shankweiler, J.; Karasik, O. Hand Hygiene and Hospital-Acquired Infections During COVID-19 Increased Vigilance: One Hospital’s Experience. HCA Healthc. J. Med. 2021, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.P.; Borges, I.C.; Buss, L.; Machado, A.; Bassetti, B.R.; Cocentino, B.; Bicalho, C.S.; Carrilho, C.M.; Rodrigues, C.; Neto, E.A.; et al. Healthcare-associated infections on the intensive care unit in 21 Brazilian hospitals during the early months of the coronavirus disease 2019 (COVID-19) pandemic: An ecological study. Infect. Control Hosp. Epidemiol. 2022, 44, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Weiner-Lastinger, L.M.; Dudeck, M.A.; Fike, L.V.; Kuhar, D.T.; Edwards, J.R.; Pollock, D.; Benin, A. Impact of COVID-19 pandemic on central-line–associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Myatra, S.N.; Divatia, J.V.; Biswas, S.; Shrivastava, A.; Al-Ruzzieh, M.A.; Ayaad, O.; Bat-Erdene, A.; Bat-Erdene, I.; Narankhuu, B.; et al. The impact of COVID-19 on health care–associated infections in intensive care units in low-and middle-income countries: International Nosocomial Infection Control Consortium (INICC) findings. Int. J. Infect. Dis. 2022, 118, 83–88. [Google Scholar] [CrossRef]
- Lastinger, L.M.; Alvarez, C.R.; Kofman, A.; Konnor, R.Y.; Kuhar, D.T.; Nkwata, A.; Patel, P.R.; Pattabiraman, V.; Xu, S.Y.; Dudeck, M.A. Continued Increases in HAI Incidence During the Second Year of the COVID-19 Pandemic. Infect. Control Hosp. Epidemiol. 2023, 44, 997–1001. [Google Scholar] [CrossRef]
- Samaroo-Campbell, J.; Qiu, W.; Asrat, H.; Abdallah, M.; Fornek, M.; Episcopia, B.; Quale, J. The initial and lingering impact of coronavirus disease 2019 (COVID-19) on catheter-associated infections in a large healthcare system in New York City. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e77. [Google Scholar] [CrossRef]
- AlAhdal, A.M.; Alsada, S.A.; Alrashed, H.A.; Al Bazroun, L.I.; Alshoaibi, A. Impact of the COVID-19 Pandemic on Levels of Device-Associated Infections and Hand Hygiene Compliance. Cureus 2022, 14, e24254. [Google Scholar] [CrossRef]
- Ochoa-Hein, E.; González-Lara, M.F.; Huertas-Jiménez, M.A.; Chávez-Ríos, A.R.; de-Paz-García, R.; Haro-Osnaya, A.; González-González, R.; Cruz-Juárez, B.S.; Hernández-Gilsoul, T.; Rivero-Sigarroa, E.; et al. Surge in Ventilator-Associated Pneumonias and Bloodstream Infections in An Academic Referral Center Converted to Treat COVID-19 Patients. Rev. Investig. Clínica 2021, 73, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, A.; Colgrove, G.; Scheutzow, M.; Ramic, M.; Monaco, K.; Hill, J.L., Jr. Decreasing Catheter-Associated Urinary Tract Infection (CAUTI) at a community academic medical center using a multidisciplinary team employing a multi-pronged approach during the COVID-19 pandemic. Am. J. Infect. Control 2023, 51, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, E.; Alessio, G.; Spuntarelli, V.; Luciani, M.; Santino, I.; Simmaco, M.; Martelletti, P. Impact of COVID-19 prevention measures on risk of health care-associated Clostridium difficile infection. Am. J. Infect. Control 2021, 49, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.B.; Du, T.; Silva, A.; Golding, G.R.; Pelude, L.; Mitchell, R.; Rudnick, W.; Hizon, R.; Al-Rawahi, G.N.; Chow, B.; et al. Trends in Clostridioides difficile infection rates in Canadian hospitals during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2022, 44, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Hein, E.; Rajme-López, S.; Rodríguez-Aldama, J.C.; Huertas-Jiménez, M.A.; Chávez-Ríos, A.R.; de Paz-García, R.; Haro-Osnaya, A.; González-Colín, K.K.; González-González, R.; González-Lara, M.F.; et al. Substantial reduction of healthcare facility-onset Clostridioides difficile infection (HO-CDI) rates after conversion of a hospital for exclusive treatment of COVID-19 patients. Am. J. Infect. Control 2021, 49, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Manea, E.; Jipa, R.; Milea, A.; Roman, A.; Neagu, G.; Hristea, A. Healthcare-associated infection during the COVID-19 pandemic in a tertiary care hospital in Romania. Rom. J. Intern. Med. 2021, 59, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Alonso, M.; De La Fuente, J.S.; Rincón-Carlavilla, A.; Moreno-Nunez, P.; Martínez-García, L.; Escudero-Sánchez, R.; Pintor, R.; García-Fernández, S.; Cobo, J. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect. Control Hosp. Epidemiol. 2021, 42, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Sipos, S.; Vlad, C.; Prejbeanu, R.; Haragus, H.; Vlad, D.; Cristian, H.; Dumitrascu, C.; Popescu, R.; Dumitrascu, V.; Predescu, V. Impact of COVID-19 prevention measures on Clostridioides difficile infections in a regional acute care hospital. Exp. Ther. Med. 2021, 22, 1215. [Google Scholar] [CrossRef]
- Sturm, L.K.; Saake, K.; Roberts, P.B.; Masoudi, F.A.; Fakih, M.G. Impact of COVID-19 pandemic on hospital onset bloodstream infections (HOBSI) at a large health system. Am. J. Infect. Control 2022, 50, 245–249. [Google Scholar] [CrossRef]
- Polly, M.; de Almeida, B.L.; Lennon, R.P.; Cortês, M.F.; Costa, S.F.; Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am. J. Infect. Control 2022, 50, 32–38. [Google Scholar] [CrossRef]
- Kitt, E.; Brennan, L.; Harrison, C.; Hei, H.; Paul, E.; Satchell, L.; Wilson, K.B.; Smathers, S.; Handy, L.; Coffin, S.E. Dodging the bundle—Persistent healthcare-associated rhinovirus infection throughout the pandemic. Am. J. Infect. Control 2022, 50, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.L.; Cabral, J.; Marques-Pinto, A.; Vila, F.; Lindoro, J.; Fraga, A. How the COVID-19 pandemic changed postoperative infections in urology wards: A retrospective cohort study from two urology departments. Can. Urol. Assoc. J. 2022, 16, E267. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Khatami, F.; Azimbeik, Z.; Khajavi, A.; Aloosh, M.; Aghamir, S.M. Hospital-acquired infections in a tertiary hospital in Iran before and during the COVID-19 pandemic. Wien. Med. Wochenschr. 2022, 172, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, P.; Paiano, L.; Samardzic, N.; Germani, P.; Bernardi, L.; Borelli, M.; Pozzetto, B.; de Manzini, N.; Bortul, M. Impact of lockdown for SARS-CoV-2 (COVID-19) on surgical site infection rates: A monocentric observational cohort study. Updates Surg. 2020, 72, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Ereth, M.H.; Fine, J.; Stamatatos, F.; Mathew, B.; Hess, D.; Simpser, E. Healthcare-associated infection impact with bioaerosol treatment and COVID-19 mitigation measures. J. Hosp. Infect. 2021, 116, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martínez, A.; Fernández-Cruz, A.; Domínguez, F.; Forteza, A.; Cobo, M.; Sánchez-Romero, I.; Asensio, A. Hospital-acquired infective endocarditis during Covid-19 pandemic. Infect. Prev. Pract. 2020, 2, 100080. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Tangiisuran, B.; Elnaem, M.H.; Sulaiman, S.A.; Khan, F.U. Mortality and its predictors among hospitalized patients with infections due to extended spectrum beta-lactamase (ESBL) Enterobacteriaceae in Malaysia: A retrospective observational study. Future J. Pharm. Sci. 2022, 8, 17. [Google Scholar] [CrossRef]
- Abubakar, U.; Zulkarnain, A.I.; Rodríguez-Baño, J.; Kamarudin, N.; Elrggal, M.E.; Elnaem, M.H.; Harun, S.N. Treatments and Predictors of Mortality for Carbapenem-Resistant Gram-Negative Bacilli Infections in Malaysia: A Retrospective Cohort Study. Trop. Med. Infect. Dis. 2022, 7, 415. [Google Scholar] [CrossRef]
- Elliott, T.M.; Hurst, C.; Doidge, M.; Hurst, T.; Harris, P.N.; Gordon, L.G. Unexpected benefit of COVID-19 hospital restrictions: Reduction in patients isolating with multidrug resistant organisms after restrictions were lifted. Infect. Dis. Health 2022, 27, 10–14. [Google Scholar] [CrossRef]
- Ippolito, M.; Simone, B.; Filisina, C.; Catalanotto, F.R.; Catalisano, G.; Marino, C.; Misseri, G.; Giarratano, A.; Cortegiani, A. Bloodstream infections in hospitalized patients with COVID-19: A systematic review and meta-analysis. Microorganisms 2021, 9, 2016. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef]
- Balla, K.C.; Rao, S.P.; Arul, C.; Shashidhar, A.; Prashantha, Y.N.; Nagaraj, S.; Suresh, G. Decreasing central line-associated bloodstream infections through quality improvement initiative. Indian Pediatr. 2018, 55, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Eze, P.; Balsells, E.; Kyaw, M.H.; Nair, H. Risk factors for Clostridium difficile infections–an overview of the evidence base and challenges in data synthesis. J. Glob. Health 2017, 7, 010417. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Akram, A.R.; Singanayagam, A.; Wilcox, M.H.; Hill, A.T. Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J. Infect. 2016, 73, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Loo, V.G.; Bourgault, A.M.; Poirier, L.; Lamothe, F.; Michaud, S.; Turgeon, N.; Toye, B.; Beaudoin, A.; Frost, E.H.; Gilca, R.; et al. Host and pathogen factors for Clostridium difficile infection and colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Dieringer, T.D.; Furukawa, D.; Graber, C.J.; Stevens, V.W.; Jones, M.M.; Rubin, M.A.; Goetz, M.B. Inpatient antibiotic utilization in the Veterans’ Health Administration during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2021, 42, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Elligsen, M.; Wan, M.; Lam, P.W.; Lo, J.; Taggart, L.R.; Chan, A.J.; Downing, M.; Gough, K.; Seah, J.; Leung, E. Trends in hospital antibiotic utilization during the coronavirus disease 2019 (COVID-19) pandemic: A multicenter interrupted time-series analysis. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e128. [Google Scholar] [CrossRef] [PubMed]
- Castro-Lopes, A.; Correia, S.; Leal, C.; Resende, I.; Soares, P.; Azevedo, A.; Paiva, J.A. Increase of antimicrobial consumption in a tertiary care hospital during the first phase of the COVID-19 pandemic. Antibiotics 2021, 10, 778. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Patton, A.; Davey, P.; Harbarth, S.; Nathwani, D.; Sneddon, J.; Marwick, C.A. Impact of antimicrobial stewardship interventions on Clostridium difficile infection and clinical outcomes: Segmented regression analyses. J. Antimicrob. Chemother. 2018, 73, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Clostridium difficile infection: Epidemiology, risk factors and management. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U. Antibiotic use among hospitalized patients in northern Nigeria: A multicenter point-prevalence survey. BMC Infect. Dis. 2020, 20, 86. [Google Scholar] [CrossRef]
- Abubakar, U.; Syed Sulaiman, S.A.; Adesiyun, A.G. Utilization of surgical antibiotic prophylaxis for obstetrics and gynaecology surgeries in Northern Nigeria. Int. J. Clin. Pharm. 2018, 40, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Syed Sulaiman, S.A.; Adesiyun, A.G. Impact of pharmacist-led antibiotic stewardship interventions on compliance with surgical antibiotic prophylaxis in obstetric and gynecologic surgeries in Nigeria. PLoS ONE 2019, 14, e0213395. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Nomi, M.; Kozawa, S.; Yanagiuchi, A.; Shigemura, K.; Sengoku, A. The impact of the coronavirus disease 2019 pandemic on changes in antimicrobial prophylaxis and development of genito-urinary tract infections after urodynamic study: A retrospective comparative study of a single rehabilitation hospital in Japan. Neurourol. Urodyn. 2022, 41, 1440–1450. [Google Scholar] [CrossRef]
- AlGamdi, S.S.; Alawi, M.; Bokhari, R.; Bajunaid, K.; Mukhtar, A.; Baeesa, S.S. Risk factors for surgical site infection following spinal surgery in Saudi Arabia: A retrospective case–control study. Medicine 2021, 100, e25567. [Google Scholar] [CrossRef]
- Patel, S.; Thompson, D.; Innocent, S.; Narbad, V.; Selway, R.; Barkas, K. Risk factors for surgical site infections in neurosurgery. Ann. R. Coll. Surg. Engl. 2019, 101, 220–225. [Google Scholar] [CrossRef]
- Xue, D.Q.; Qian, C.; Yang, L.; Wang, X.F. Risk factors for surgical site infections after breast surgery: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 375–381. [Google Scholar] [CrossRef]
- Jain, R.K.; Shukla, R.; Singh, P.; Kumar, R. Epidemiology and risk factors for surgical site infections in patients requiring orthopedic surgery. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 251–254. [Google Scholar] [CrossRef]

