1. Introduction
The risk of chronic disease in adulthood is associated with environmental events during perinatal life and early childhood in a period known as the first 1000 days of life, from conception to the age of two years. According to this paradigm, environmental factors and dietary habits early in life are determinants of individual development and subsequent health, particularly for non-communicable diseases. Since Barker’s first observations in the late 1980s, the early postnatal period has been shown to be associated with the risk of long-term cardiovascular diseases [1]. Epidemiological studies have subsequently confirmed Barker’s work and suggested a role for the early environment in the occurrence of neurological, metabolic or cardiovascular disorders later in life [2,3,4,5,6]. The food restrictions during the 1944 famine in the Netherlands led to an increase in chronic pathologies, including further obesity among the generations born at that time [7], with persistent effects for the following generations [8]. Consequently, this work also highlights the fact that maternal malnutrition during gestation impedes the normal development of placentation, with subsequent consequences for the risk of chronic degenerative disorders [9] or inflammatory bowel disease (IBD) [10].
All these prior observations were of growing interest to the scientific community and led to the paradigm of the developmental origin of health and disease (DOHaD) [11,12]. Epigenetics, which modulates the expressions of genes without modifying their sequences, is one of the biological components of the calibration and perpetuation of early environmental events that influence an individual’s health [13,14]. For instance, genetic inheritance and/or epigenetics can partly predict risks of metabolic disorders [14]. The microbes that colonise the neonatal gut immediately following birth and shape the host immunity [15] are able to regulate the chemical phenomena of histone acetylation and DNA methylation via the metabolites it produces, such as short-chain fatty acids (SCFAs) [16]. Breastfeeding by modulating the development of the child’s microbiota could also participate in epigenetic modifications [17,18,19].
Early parent–child interactions, educational factors (sleep, exposure to screens), the parental lifestyle (diet, exposure to psycho-social stressors, physical activity) and exposure to toxic substances are all environmental factors with likelihoods of leaving lasting imprints on a child’s health [5,20,21,22]. Environmental stressors, including exposure to environmental xenobiotics and poor nutritional status, like inadequate fat or carbohydrate intake, can have multiple consequences for placental functions, with consequences for future health [23,24]. Other epidemiological studies in humans have highlighted the many perinatal factors, such as the mode of delivery, type of infant feeding, antibiotic therapy or tobacco exposure during the first months of life, which can have determining influences on the subsequent risk of chronic intestinal diseases, such as celiac disease or IBD, including ulcerative colitis (UC) and Crohn’s disease (CD) [25].
2. Breastfeeding
2.1. General
Exclusive breastfeeding for at least the first 6 months is the benchmark for optimal infant growth [26]. This recommendation is based on evidence that the composition of breastmilk and its energy intake are perfectly suited to the child’s needs [27,28], with beneficial effects depending on the duration of breastfeeding and the age of complementary feedings [29]. The most obvious benefits of breastfeeding include neurodevelopment in preterm infants and the prevention of respiratory and gastrointestinal infections and allergies in children [30,31]. It is also well known that breastfed preterm infants present a lower risk of necrotising enterocolitis (NEC) [32]. As an example, the PROBIT (Promotion of Breastfeeding Intervention Trial) interventional study, previously implemented in Byelorussia, which was specifically aimed at promoting breastfeeding, showed a health benefit by decreasing the risk of gastrointestinal-tract infections and atopic eczema at one year of age, but with no change in the prevalence of respiratory-tract infection [33]. However, while the positive influence of breastfeeding seems to be most evident in low-income countries, a more moderate effect is observed in developed countries where health and social security are better developed [31]. Furthermore, a relationship between breastfeeding and the risk of long-term health outcomes has also been widely emphasised, with sometimes contradictory findings, showing, in particular, a likely effect of breastfeeding on reducing early adiposity rebound, obesity and type 2 diabetes [31,34]. These observations are supported by several works that have suggested that the early disruption of the gut microbiota increases the propensity for later metabolic deregulation [35]. These vulnerabilities manifest as long-lasting endocrine, metabolic and inflammatory effects on the offspring [6]. Breastfeeding has been involved in the protection against various immune-mediated diseases [36], although this is still a matter of debate [31].
2.2. Immune and Gut Microbiota Maturation
The epithelial barrier and microbiota together contribute to immune homeostasis and the acquisition of tolerance to commensal bacteria and dietary antigens during the early postnatal period. Numerous studies indicate that early feeding, and particularly breastmilk, influences the development of the gut barrier and microbiota colonisation and enhances the maturation of the immune system [27,37]. The beneficial influence of breastmilk can be directly attributed to its bioactive components (macronutrients and micronutrients, oligosaccharides, immunoglobulins, cytokines, leukocytes as well as viable microbiota) [37,38]. In particular, lactoferrin is an iron-binding protein that plays an important role in protection against microbial infections. Lactoferrin also exhibits properties that modulate the host immune defence in the intestine [39]. Moreover, the microbiota in human milk may play a defensive role against gastrointestinal infections by participating in the early colonisation of the gut in newborns, contributing to the maturation of the immune system [39]. Interestingly, studies have unravelled the immune development driven by gut microbiota in newborns and its postnatal adaptation to environmental insults [40,41]. In this vein, it has been suggested that the duration of breastfeeding has a greater impact on the intestinal microbial diversity of infants born via caesarean section than that of infants born vaginally [42]. The role of breastfeeding on the immunological status of the child is actually evident in the first months of life [38]: the production of secretory immunoglobulin A (sIgA), detectable in the stool, is increased early in life in breastfed children compared to children receiving infant formula [43,44]. sIgA is involved in intestinal homeostasis by regulating the expressions of genes involved in inflammation, modulating the diversity of the gut microbiota and protecting against infections [45,46,47]. The gut microbiota in early life undergoes a progressive increase in α-diversity and is shaped mainly by the child’s diet, as shown in Figure 1 [48,49,50,51,52,53,54]. In fact, the composition of the gut microbiota differs significantly between breastfed infants and those receiving infant formula (higher proportions of bifidobacteria and lactobacilli, which are overall beneficial for health in breastfed infants) [15,55,56]. Otherwise, in formula-fed infants, the gut microbiome is usually dominated by an increased abundance of Enterococcus or Streptococcus [54,57]. The cessation of breastfeeding, more than the introduction of solid foods, is the main driver in the dynamics of microbiota development during the first year of life [52,58]. The impact of the weaning stage on microbiota development has been poorly investigated but is thought to contribute to gut microbiota alpha diversity [15]. At weaning, increased abundances of the adult-type microorganisms Bacteroides, Prevotella, Clostridium, Ruminococcaceae or Veillonella occur, with decreases in Bifidobacterium, Enterobacteriaceae and Streptococcaeae [37,59]. Surprisingly, other studies indicate that the alpha diversity was even lower in preschoolers than in adults, but the long-term influence of early dietary habits on the transition to a mature microbiota in children remains poorly characterised [60]. A growing body of literature points to changes in the gut microbiota as the source of an early immune imprint that may influence long-term health [40,61].
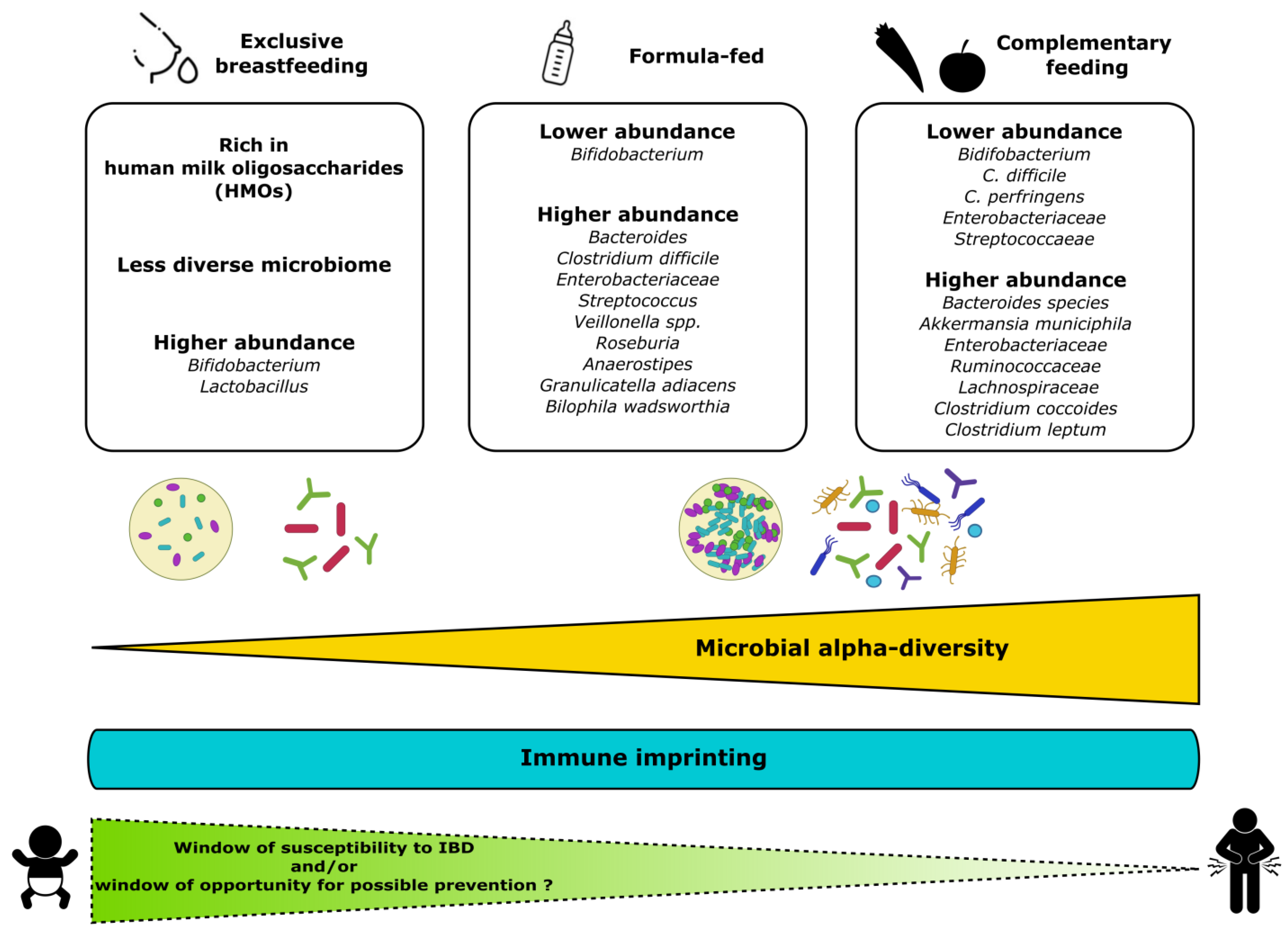
Figure 1. Composition of gut microbiota in early life in relation to child’s diet. Bifidobacterium predominates in exclusively breastfed infants, while, in formulae-fed infants, the composition is less uniform and notably enriched with Bacteroides, Streptococcus or Clostridium. The introduction of solid foods leads to a wider range of microorganisms with greater microbial α-diversity and abundance. The establishment of interactions between the host immunity and the microbiota may result in susceptibility to or protection against the onset of IBD later in life. It is relevant to consider the first months of life as a window of opportunity for preventive dietary intervention to promote early protective effects.
Human milk is composed of diverse non-digestible oligosaccharides (human milk oligosaccharides (HMOs)) that enable the early growth of bifidobacteria, which encode HMO-utilising genes and are predominant during the first months of life [62]. By metabolising HMOs, bifidobacteria promote the release of SCFAs, which improve the epithelial barrier integrity or immune regulatory response by reducing Th2 and Th17 cytokines through interaction with G-protein-coupled receptors, such as GPR43, GPR41 and GPR109A expressed by epithelial and immune cells [40,63]. Beyond this, recent studies using selected HMOs in adult mice have shown that these prebiotics are able to reduce fat mass development, insulin resistance and hepatic steatosis [64,65], suggesting a therapeutic application of HMOs against the metabolic syndrome through the probable involvement of the release of numerous specific microbial metabolites.
Moreover, recent data have demonstrated that microbial metabolites largely mediate the impact of the microbiome on the host physiology [66,67]. Most of the metabolites generated by microbiota metabolism (e.g., SCFAs, such as acetate, propionate and butyrate, or other common metabolites, such as trimethylamine N-oxide (TMAO) or tryptophan derivates) may play a role in the induction of immune tolerance, the intestinal barrier function, signalling or epigenetic modulation that can determine the increased likelihood of developing immune-mediated diseases and systemic effects on health [27,68,69]. For instance, breastfeeding may promote Bifidobacterium species to convert tryptophane into metabolite derivatives, notably kynurenine and indole, which activate the aryl-hydrocarbon receptor (AhR). The AhR is associated with the expansion of type 3 innate lymphocytes (ILC3) and IL-22 production and regulates regulatory T-cell differentiation [70,71,72]. In turn, IL-22 signalling may influence the composition and function of the gut microbiota [72]. It is worth noting that a reduction in AhR ligand production and, consequently, IL-22 activation has been observed in IBD patients [73]. Although research in this field is presently sparse, this converging evidence suggests that microbial-derived metabolites can strongly influence developmental programming in breastfed infants [67]. Moreover, it can also be postulated that these compounds may also have potential impacts on intestinal and metabolic health as new “postbiotic” therapeutics to treat microbiome-related non-communicable diseases (NCDs) in infants and adults.
3. Breastfeeding and Risk of IBD
IBDs are chronic intestinal diseases, and perinatal factors may be partly responsible for their onset, although there is little evidence to suggest this [74]. Given that human milk can shape the gut immune response and microbiota with long-term benefits against immune-related diseases [36], the role of breastfeeding on the subsequent risk of CD and UC has been extensively examined. We propose to review the existence of a link between breastfeeding and IBD from experimental and clinical studies.
3.1. IBD Presentation
CD and UC are the two main clinical forms of IBD. Defined empirically on the basis of clinical, endoscopic and radiological criteria, they are characterised by the chronic and recurrent inflammation of the intestinal wall. Although the exact origin of IBD remains unknown, the current hypothesis is that it is a complex, multifactorial disease, occurring in genetically predisposed individuals and resulting in an abnormal mucosal immune response to intestinal microflora [75]. Over the past 20 years, more than 200 susceptibility genes associated with IBD have been identified [76,77,78]. To date, only smoking and appendectomy are environmental factors recognised as being linked to IBD, even if their mechanisms have not yet been clarified. The impact of current smoking on the IBD course has been studied extensively; smoking is deleterious in CD and beneficial in UC [75,79].
Of note, the incidence and prevalence of IBD, and particularly in paediatric onset, are increasing, with a key role played by environmental risk factors [80,81]. In detail, the epidemiology of IBD is evolving steadily worldwide: the prevalence continues to rise in Western countries (Europe, North America), reaching over 0.3%, while the incidence is increasing rapidly in newly industrialised countries in Africa, Asia and South America [82]. Particular attention needs to be paid to the increase in IBDs in children and adolescents because of the impact that these diseases can have on their quality of life, such as stunted growth, school absenteeism and the psychological effect of a chronic disease on the patient and family [80]. Except for enteral nutrition, there are only limited data regarding the impact of diet on the disease course either considering adults [83,84] or children [85]. It should be noted that there is growing evidence of the role of the Western diet in the increasing prevalence of IBD worldwide [75,82,86].
3.2. Milk Components and Gut Inflammation: What Does an Experimental Model of Colitis Tell Us?
Over the last 30 years, numerous experimental models of colitis have been developed in rodents to decipher the underlying mechanisms of the IBD pathophysiology, identify molecular targets and evaluate new therapeutic strategies [87]. Among these different models of colitis, the most widespread are those induced by chemical compounds such as dextran sulphate sodium (DSS) or 2,4,6-trinitrobenzene sulphonic acid (TNBS), which are reputed to have many similarities with human UC and CD, respectively [88,89]. Genetic models built on the basis of susceptibility genes identified in IBD are also available but are less frequently used [90]. The potentially beneficial effects of breastmilk in these experimental models of gut inflammation have been tested by various teams, with a particular focus on milk-derived oligosaccharides and extracellular vesicles (EVs). In an initial study in 2002, Madsen et al. used interleukin-10-deficient mice, which developed spontaneous colitis, to study the role of breastfeeding on the progression of intestinal inflammation. They observed that breastfeeding had a beneficial effect on reducing the histological inflammation of the colon, as well as the circulating levels of TNF and IFNγ [91]. Subsequently, it was demonstrated that a rodent diet enriched with goat’s milk oligosaccharides (GMOs), administered in a preventive manner seven days before the induction of colitis, was able to reduce the acute intestinal inflammation induced by DSS in rats [92]. In control animals that did not receive DSS, the GMO diet caused a modification of the colonic microbiota with an enrichment in lactobacilli and bifidobacteria. At the same time, the preventive and anti-inflammatory effect of GMOs was also demonstrated in a TNBS rat model [93]. Fuhrer et al. used a different and original approach to investigate the role of the sialylated milk oligosaccharides in mucosal immunity [94]. In order to identify the respective roles of α2,3-sialyllactose (3′-SL) and α2,6-sialyllactose (6′-SL) on gut immunity, these authors used 2,3- and 2,6-sialylltransferase-deficient mice (St3gal4−/− and St6gal1−/− mice, respectively) and applied a cross-breeding protocol in which wild-type and knock-out neonates were exchanged at birth and fed either normal milk or milk deficient in 3′-SL or 6′-SL. At seven weeks of age, the animals were exposed to DSS for five days. Surprisingly, the St3gal4-deficient mice or wild-type mice fed with 3′-SL-deficient milk from St3gal4 knock-out mice were more resistant to DSS-induced colitis than the wild-type mice and St3gal4 knock-out mice fed with normal milk. An analysis of the gut microbiota showed different colonisation profiles depending on the presence or absence of 3′-SL in the milk. The presence of 3′-SL was associated with an enrichment in bacterial species belonging to the Ruminococcaceae family. The reconstitution of germ-free mice with gut microbiota isolated from St3gal4 knock-out mice demonstrated that these reconstituted mice exhibited the same sensitivity to DSS as their microbiota donor animals. Cross-breeding experiments with normal and 6′-SL-deficient milk showed no impact on the susceptibility to DSS-induced acute colitis. This elegant study clearly demonstrates the role of breastmilk oligosaccharides in shaping the intestinal flora and promoting a healthy gut immune system in adulthood. It is particularly interesting because of its experimental design, which respects the temporality and mode of the administration of breastmilk and makes it possible to study the impact of breastfeeding in adult individuals. However, sialylated oligosaccharides are not the major sugars found in human breastmilk, which contains mainly fucosylated oligosaccharides, of which 2′-fucosyl lactose (2′-FL) is the most abundant [95]. 2′-FL is not detected in mouse milk [96]. Interestingly, almost 30 years ago, a transgenic mouse model was constructed with the human gene encoding α1,2-fucosyltransferase and enabling the synthesis of 2′-FL. The expression of this gene in the mouse mammary gland promoted the significant production of 2′-FL in the milk of transgenic animals, up to a level representing 45% of the total oligosaccharides [96]. Unfortunately, to the best of our knowledge, this model has not been used to study the contribution of 2′-FL during breastfeeding on the physiology of the intestinal mucosal immunity in adulthood.
More recently, the respective role of HMOs containing fucosyl and sialyl residues on the development of gut inflammation in rodent models has been studied in a more traditional way via the oral supplementation of these oligosaccharides after weaning or in adult animals. Different models of acute or chronic colitis were used (DSS- or IL-10-deficient mice), and different doses of HMOs, alone or mixed, were administered, either preventively or curatively. It is therefore difficult to compare these different data. Nevertheless, all these studies clearly suggest that the administration of specific HMOs (mainly 2′-FL) after weaning can modify the composition of the gut microbiota in order to reduce the acute or chronic inflammation observed in the various mouse models, supporting HMO intervention as a strategy against IBD [97,98,99,100,101].
In addition to HMOs, milk also contains EVs, which are small lipid membrane vesicles that carry bioactive factors such as proteins or RNA. The oral administration of purified EVs from commercial cow’s milk for 6 days after the induction of acute colitis with DSS in C57BL/6 mice attenuated the gut inflammation and restored the gut barrier more rapidly compared to untreated animals [102]. Similar results were obtained in Balb/c mice, with a more pronounced beneficial effect of EVs purified from cow’s milk compared with those from human milk [103]. In order to assess the influence of EVs derived from cow’s milk on the composition of the gut microbiota, Zhou et al. studied two groups of mice: one fed with a diet supplemented with cow’s milk (exosome-/RNA-sufficient diet), and the other one fed with a diet supplemented with ultrasonicated cow’s milk (exosome-/RNA-depleted diet) [104]. Feeding was started at 3 weeks of age, and the intestinal content (cecum) was collected at ages 7, 15 and 47 weeks. At ages 15 and 47 weeks, the gut bacterial communities between both groups of mice turned out to be different and showed characteristics associated with certain pathologies, such as IBD, as evidenced by the decrease in the relative abundance of the Lachnospiraceae family in mice fed the exosome-/RNA-sufficient diet. This alteration in the gut microbiota by bovine-milk-derived EVs has been confirmed by others [105]. The same group has also recently shown and confirmed that bovine-milk-derived EVs, administered preventively, displayed a protective effect on DSS-induced colitis (acute and chronic) by suppressing intestinal inflammation and improving the gut barrier integrity [106,107]. Altogether, these results strongly suggest a beneficial immunomodulatory role for milk-derived EVs during intestinal physiology and mucosal homeostasis. However, no early conclusions should be drawn, as there are still major methodological differences between studies, particularly regarding the purification and analysis of EVs, making it impossible to compare the available data rigorously. In addition, the quantities of EVs administered are regularly supra-physiological and do not allow conclusions to be drawn about the role that they play at the doses found in breastmilk.
3.3. The Role of Breastfeeding in the Development of Human IBDs: Clinical Evidence
We herein propose a review of publications investigating an association between breastfeeding and the risk of developing IBD in humans (summaries of the studies can be found in and . For this review, references published in English were obtained from a search of the PUBMED electronic database until June 2023 using combinations of the English search terms “Early nutrition”, “Early diet”, “Breastfeeding”, “Human milk”, “Inflammatory Bowel diseases”, “Crohn’s disease”, “Ulcerative colitis” and “gut health”. We identified fifty-three publications between 1979 and 2023, the majority of which relied on case–control studies (n = 40). Some of these studies included a broad range of predictor variables, like the environment, parental health, diet, early antibiotic usage, smoking or life-type behaviours, education and mode of delivery, that we will not be discussing in detail in this review. Most of the case–control studies analysed possible association between breastfeeding by using multivariate analysis and the diagnosis of either CD or UC as the outcome (n = 29): seven only had CD as the main outcome, and four only had UC as the main outcome. Five prospective cohort studies [74,108,109,110,111], seven systemic review or meta-analyses [112,113,114,115,116,117] and one recent Mendelian randomisation analysis [118] were also conducted. Among the case–control studies, nine were carried out in Asia/Pacific or Iran [119,120,121,122,123,124,125,126,127], seven in North America [128,129,130,131,132,133,134], one in Brazil [135] and twenty-two in Europe [136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155] and Israel [156,157], while one international study was conducted [158]. Thirteen case–control studies found that breastfeeding could have a marked protective effect on the development of IBD in adults [121,127,140,143,144,149] or paediatric IBD [120,125,130,148,152]. It is worth mentioning that having ever been breastfed has been associated with a differential relationship with CD or UC with a separate preventive effect [119,128,129,136,137,145,154]. Conversely, it is also commonly reported that there is no positive link between being breastfed and the occurrence of IBD [74,110,122,123,124,126,131,132,133,134,135,138,141,142,150,151,153,155,156,157,158]. Of note, it has been suggested that breastfeeding is associated with a higher risk of developing CD [139,147] or UC [144]. Overall, the literature remains inconsistent and does not support a clear association between breastfeeding and IBD. This level of great heterogeneity across studies emerged in systematic reviews [113,115,116,159] and was reported in diverse geographical areas and ethnic groups [115,159]. Concerning the latter points, it has been underlined that the magnitude of protection in individuals who were breastfed during infancy appeared higher in Asian populations compared with Caucasian people [115].
Among the case–control studies and prospective studies, 29 out of 45 analyses did consider the breastfeeding duration. Despite the considerable heterogeneity that remains in the literature regarding the interval of receiving breastfeeding, numerous studies have observed that a prolonged duration of breastfeeding could reduce the odds of having UC or CD [119,121,125,127,130,140,148,149,152,155]. Other findings have reported that a short duration of breastfeeding provides substantial protection against CD or UC [136,137,154]. Therefore, shortly after birth, breastfeeding might reduce the risk, although there is contrasting evidence that suggests that initiating breastfeeding is actually not sufficient to confer a protective effect [133]. There are population-based studies that contrast with these observations, as they did not observe associations between the length of breastfeeding and UC and/or CD diagnosis [110,124,128,129,132,136,141,142]. Few studies apart from Lopez-Serrano and Lindoso [108,145] have shown a link between exclusive breastfeeding and a change in the risk of IBD incidence [134,138,147,151] or severe illness [109]. It is worth pointing out that Lindoso et al., in their prospective study, did not reveal any association between the duration of exclusive breastfeeding and complicated disease at diagnosis [108].
Generally, the meta-analyses tended to conclude that breastfed infants are less susceptible to developing adult and paediatric-onset IBD [116,117], and that longer durations of human milk exposure increase the risk of developing IBD, although the level of evidence is low [112,113]. However, the authors acknowledged that numerous studies were of poor quality and were not strictly designed for analysing breastfeeding effects, with a lack of information on the quality and duration of breastfeeding. Failure in a proper definition of breastfeeding, the absence of a well-documented history of breastfeeding, such as inaccurate reporting of weaning, and the biased recall of whether a child was breastfed or for how long in cohort studies can lead to misinterpretations and preclude a clear conclusion of a direct link between breastfeeding and IBD. Therefore, it is still difficult to state with certainty that well-established breastfeeding prevents the onset of IBD. In fact, a spectrum of risk may cluster with breastmilk to influence early programming, including the timing of introducing different types of foods. Key variants include not only the use of bottle feeding versus exclusively breastfeeding, caesarean delivery, exposure to antibiotics or tobacco and physical activity, but also the type of IBD outcome (incidence or severity), age at diagnosis or community control design [118,159]. In addition, the paradigm that a Western lifestyle and diet [160,161] may play a key role in the development of IBD and the possibility that the strongest effect of breastfeeding on the subsequent risk of IBD was observed in Asian studies [115] fit well with the major role of the exposome in the dependent early-life effect [162]. In this case, a changing diet, socio-economic conditions of life or even improved hygiene and infection outcomes all represent relevant confounders that could underpower studies. Finally, Decker et al. pointed out that children born between 1995 and 2006 were breastfed significantly longer than children born between 1992 and 1994 [148], while Piovani et al. highlighted that the protective influence of being breastfed was higher before 2000 (OR: 0.58; 0.46–0.74) than after 2000 (OR: 0.82; 0.71–0.94) [159]. These observations have raised critical ambiguities in the overall interpretation and comparison between analyses since the 1980s in the sense that, over time, studies can differ according to the quality of the breastfeeding promotion in maternity wards and the overall improvement in the duration of breastfeeding, particularly exclusive breastfeeding.
In conclusion, despite the heterogeneity across studies, there is a trend that suggests that breastfeeding may imprint the risk of IBD. There are actually many biological plausibilities, such as microbiota development and inflammatory priming, that, under the influence of genetic predisposition [75,160], including the genetic predisposition to breastfeeding [118] or environmental exposures, make a complex interplay between breastfeeding and IBD credible.
4. Early Determinants of Microbiota and Colitis Trajectories
4.1. General
It is now well established that the gut microbiota is a major contributor to the pathogeneses of IBDs in adults [163]. However, in addition to the genetic determinants of IBD, the exact environmental causes of microbial dysbiosis and the timeframe of the acquisition of a pre-dysbiotic state early in life to further predispose to IBD is far from elucidated. Whether the pathogens identified in adults are inherited directly from vertical transfer from the mother or secondarily is still unclear. Consequently, the question of the maternal transmission of beneficial bacteria that are likely to colonise the infant’s gut on a long-term basis and prevent the resilience of adult intestinal homeostasis is still being debated [164]. Lastly, the inflammatory context, possibly induced by C-section compared with vaginal delivery [165], and an inappropriate diet(s) or subsequent environmental factors may both favour pathobiont colonisation and the expansion and limit abundance of symbionts.
4.2. Maternal IBD and Gut Microbiota
While women with IBD maintain an intestinal dysbiosis during pregnancy, characterised by an increase in gamma-proteobacteria and a decrease in bacteroidetes, babies born to these mothers with IBD show reduced diversity and lower counts of bifidobacteria [166]. Of note, the biomarker of gut inflammation, faecal calprotectin, assessed in IBD mothers during pregnancy and babies, was correlated to their respective gut microbiome compositions [167]. In addition, the IBD status of mothers is a predictor of higher calprotectin levels in babies. This suggests the influence of early inflammation and the role of both maternal diseases as well as maternal microbiota on the development of further dysbiotic infant gut microbiota, regardless of genetic factors. However, obviously all babies from IBD mothers will not develop IBD, and the functional redundancy among microbes may compensate for the possible lacks.
4.3. Gut Microbiota and IBD: A Possible Intervention?
Defining the microbial markers of dysbiosis and what constitutes a healthy microbiota in adults is already a challenge, although many bacterial genera and even species have been clearly identified as symbionts or pathobionts. Thus, attributing specific anti-inflammatory roles and functionalities of bacteria in the early-life “unstable” microbiota is quite tricky [168]. The development of the human gut microbiome, along with distinct diets, corresponds to complex and individual dynamics comprising early and late colonisers [15,169,170]. Among these species, dominant and less abundant taxa have shown overall anti-inflammatory potential, such as species from the Bifidobacterium and Bacteroidetes genera, and, to a lesser extent, Lactobacillus spp. In line, other anaerobic bacteria, like Akkermansia and Faecalibacterium prausnitzii, have also demonstrated regulatory functions that contribute to homeostasis and lower inflammation. In contrast, colitogenic properties have been attributed to taxa such as Enterococcus and Clostridium spp. representatives together with an abundance of Gamma-Proteobacteria like E. coli [164]. A higher occurrence of adherent-invasive E. coli (AIEC) has been fully demonstrated in adult IBD patients [171] as well as in paediatric CD patients [172], but, to the best of our knowledge, there is no evidence on an early asymptomatic carriage of AIEC in neonates that could influence the onset of colitis and inflammatory symptoms. The vertical transmission of AIEC was reported in mice [173], but more consistent and reliable clinical studies are actively needed. Lastly, the breastmilk route of such a possible mother-to-infant transmission, as reported for intestinal obligate anaerobic species like Bifidobacteria, Bacteroides and Clostridia, should be addressed in depth [174,175].
Experimental studies have clearly demonstrated that specific dietary habits have an impact on the development of the intestinal barrier and the composition of the neonatal microbiota, with a possible influence on overall health [176] and the long-term susceptibility to chronic diseases, including inflammatory colitis [177,178,179]. During the last decades, preclinical and clinical nutritional interventions have shown great potential to address IBDs by targeting adult microbiota with either prebiotics, probiotics, synbiotics or postbiotics, based on key microbial-derived metabolites [180]. For example, a promising effect of a symbiotic preparation has been shown in reducing symptoms of paediatric IBD with a mean age of 12.6 years old [181]. Only a few trials on children have reported changes in microbiota that normalise or lower some dysbiotic-associated bacterial species [182]. However, clear data in humans are scarce, as no longitudinal clinical studies address the early microbiota composition or nutritional- and microbiota-targeting interventions with further follow-up of the onset and development of IBD.
Recently, Guo and colleagues [183] reviewed the early microbial imprinting of neonates that could define and possibly modulate either resilience against or susceptibility to IBD (see also Figure 1). They finally proposed the design of “tailored interventions” based on prebiotics or probiotics, depending on the distinct mother influence types. Of note, the timing of such interventions has to be clearly defined. Indeed, the introduction of solid foods at 3 months of age, for instance, increased the short-chain fatty acids but appeared detrimental for the gut microbiota [51]. Dosing also has to be taken into account. Barone and colleagues, in attempts to decipher the role of C-section-induced dysbiosis in gut barrier dysfunction and the associated inflammation in mice, found that an excessive exposure to very diverse microbiota too early in life was harmful, sustaining the too much too early principle [165]. In line, the mechanisms involved the “weaning reaction” occurring within a specific time window to prevent susceptibility to inflammatory diseases in the adult and to promote regulatory T-cell-mediated protection [184].
5. Conclusions
Most of the current recommendations for pregnant women and young children do not always consider the long-term health consequences of nutrition. Implementing optimal nutrition programs from the very beginning of life is crucial to improving child development and the well-being of populations for sustainable health. In a context in which the promotion of breastfeeding is a global priority, the focus on the benefits of breastfeeding in modifying the risk of chronic non-communicable diseases is a priority for the development of preventive strategies to promote long-term health. In this review, we summarise the evidence concerning the link between breastfeeding and the reduced risk of IBD. Overall, the data remain uncertain, partly due to the considerable heterogeneity and lack of standardisation between studies. The duration of exclusive breastfeeding is probably decisive for its lasting effect on inflammatory-mediated diseases. The microbial development origin of diseases suggests that the colonisation of the microbiota regulates immune development and may program susceptibility to hyperinflammation later in life [185]. Indeed, even an early transient dysbiosis could determine a health outcome. The composition of breastmilk (i.e., the maternal microbiome or HMOs, for example), the quality of complementary feedings, the use of antibiotics and the place of residence are all variable factors that can promote or disrupt the process of a child’s gut microbiota colonisation and pathological imprinting [184,186,187,188,189]. It is therefore difficult to identify the exact role of breastfeeding and the gut microbiome in the onset of IBD. A more holistic approach is needed to examine the impact of breastfeeding on later life events. A key question is how to translate nutritional factors into biomarkers of interest, with systemic biology as a strategic tool to characterise the molecular/biological alterations leading to IBD. As such, specific improvements in our knowledge could support interventions targeting the gut microbiome, such as prebiotics, probiotics or postbiotics, that could be used to treat or prevent diseases in a precision medicine framework.
References
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Delpierre, C.; Lepeule, J.; Cordier, S.; Slama, R.; Heude, B.; Charles, M.A. DOHaD: Epidemiological researches. Med. Sci. 2016, 32, 21–26. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.D.; Diaz-Castillo, C.; Chamorro-Garcia, R. Multigenerational metabolic disruption: Developmental origins and mechanisms of propagation across generations. Front. Toxicol. 2022, 4, 902201. [Google Scholar] [CrossRef]
- Nicholas, L.M.; Morrison, J.L.; Rattanatray, L.; Zhang, S.; Ozanne, S.E.; McMillen, I.C. The early origins of obesity and insulin resistance: Timing, programming and mechanisms. Int. J. Obes. 2016, 40, 229–238. [Google Scholar] [CrossRef]
- Roseboom, T.; de Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef]
- Painter, R.C.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.I.; Roseboom, T.J. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 2008, 115, 1243–1249. [Google Scholar] [CrossRef]
- De Rooij, S.R.; Bleker, L.S.; Painter, R.C.; Ravelli, A.C.; Roseboom, T.J. Lessons learned from 25 Years of Research into Long term Consequences of Prenatal Exposure to the Dutch famine 1944-45: The Dutch famine Birth Cohort. nt. J. Environ. Health Res. 2022, 32, 1432–1446. [Google Scholar] [CrossRef]
- Klooker, T.K.; Braak, B.; Painter, R.C.; de Rooij, S.R.; van Elburg, R.M.; van den Wijngaard, R.M.; Roseboom, T.J.; Boeckxstaens, G.E. Exposure to severe wartime conditions in early life is associated with an increased risk of irritable bowel syndrome: A population-based cohort study. Am. J. Gastroenterol. 2009, 104, 2250–2256. [Google Scholar] [CrossRef]
- Charles, M.A.; Delpierre, C.; Breant, B. Developmental origin of health and adult diseases (DOHaD): Evolution of a concept over three decades. Med. Sci. 2016, 32, 15–20. [Google Scholar] [CrossRef]
- Barnes, M.D.; Heaton, T.L.; Goates, M.C.; Packer, J.M. Intersystem Implications of the Developmental Origins of Health and Disease: Advancing Health Promotion in the 21st Century. Healthcare 2016, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Junien, C.; Panchenko, P.; Fneich, S.; Pirola, L.; Chriett, S.; Amarger, V.; Kaeffer, B.; Parnet, P.; Torrisani, J.; Bolanos Jimenez, F.; et al. Epigenetics in transgenerational responses to environmental impacts: From facts and gaps. Med. Sci. 2016, 32, 35–44. [Google Scholar] [CrossRef]
- Marousez, L.; Lesage, J.; Eberle, D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients 2019, 11, 2966. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Loret de Mola, C.; Davies, N.M.; Victora, C.G.; Relton, C.L. Breastfeeding effects on DNA methylation in the offspring: A systematic literature review. PLoS ONE 2017, 12, e0173070. [Google Scholar] [CrossRef]
- Indrio, F.; Martini, S.; Francavilla, R.; Corvaglia, L.; Cristofori, F.; Mastrolia, S.A.; Neu, J.; Rautava, S.; Russo Spena, G.; Raimondi, F.; et al. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development. Front. Pediatr. 2017, 5, 178. [Google Scholar] [CrossRef]
- Van Esch, B.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.J.; van Neerven, R.J.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020, 11, 2141. [Google Scholar] [CrossRef]
- Prado, E.L.; Larson, L.M.; Cox, K.; Bettencourt, K.; Kubes, J.N.; Shankar, A.H. Do effects of early life interventions on linear growth correspond to effects on neurobehavioural development? A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e1398–e1413. [Google Scholar] [CrossRef]
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B.; EDEN Mother-Child Cohort Study Group. Breastfeeding, Polyunsaturated Fatty Acid Levels in Colostrum and Child Intelligence Quotient at Age 5–6 Years. J. Pediatr. 2017, 183, 43–50.e43. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Ibanez, C.; Martinez-Samayoa, P.M.; Lomas-Soria, C.; Durand-Carbajal, M.; Rodriguez-Gonzalez, G.L. Maternal Obesity: Lifelong Metabolic Outcomes for Offspring from Poor Developmental Trajectories During the Perinatal Period. Arch. Med. Res. 2016, 47, 1–12. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef] [PubMed]
- Mastorci, F.; Linzalone, N.; Ait-Ali, L.; Pingitore, A. Environment in Children’s Health: A New Challenge for Risk Assessment. Int. J. Environ. Res. Public Health 2021, 18, 10445. [Google Scholar] [CrossRef]
- Ley, D.; Desseyn, J.L.; Mischke, M.; Knol, J.; Turck, D.; Gottrand, F. Early-life origin of intestinal inflammatory disorders. Nutr. Rev. 2017, 75, 175–187. [Google Scholar] [CrossRef]
- Breastfeeding. Available online: https://apps.who.int/nutrition/topics/exclusive_breastfeeding/en/index.html (accessed on 1 June 2021).
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing early life nutritional sources and human milk feeding practices: Personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef]
- Chong, H.Y.; Tan, L.T.; Law, J.W.; Hong, K.W.; Ratnasingam, V.; Ab Mutalib, N.S.; Lee, L.H.; Letchumanan, V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef]
- Le Huerou-Luron, I.; Blat, S.; Boudry, G. Breast-v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef]
- Roze, J.C.; Darmaun, D.; Boquien, C.Y.; Flamant, C.; Picaud, J.C.; Savagner, C.; Claris, O.; Lapillonne, A.; Mitanchez, D.; Branger, B.; et al. The apparent breastfeeding paradox in very preterm infants: Relationship between breast feeding, early weight gain and neurodevelopment based on results from two cohorts, EPIPAGE and LIFT. BMJ Open 2012, 2, e000834. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Nolan, L.S.; Parks, O.B.; Good, M. A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis. Nutrients 2019, 12, 14. [Google Scholar] [CrossRef]
- Kramer, M.S.; Chalmers, B.; Hodnett, E.D.; Sevkovskaya, Z.; Dzikovich, I.; Shapiro, S.; Collet, J.P.; Vanilovich, I.; Mezen, I.; Ducruet, T.; et al. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA 2001, 285, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J. The adiposity rebound in the 21st century children: Meaning for what? Korean J. Pediatr. 2018, 61, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Carpen, N.; Helve, O.; de Vos, W.M.; Korpela, K.; Salonen, A. Early-life gut microbiota and its connection to metabolic health in children: Perspective on ecological drivers and need for quantitative approach. EBioMedicine 2021, 69, 103475. [Google Scholar] [CrossRef]
- Alotiby, A.A. The role of breastfeeding as a protective factor against the development of the immune-mediated diseases: A systematic review. Front. Pediatr. 2023, 11, 1086999. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Gao, Y.; de Groot, N.; Vonk, M.M.; Ulfman, L.; van Neerven, R.J.J. Babies, Bugs, and Barriers: Dietary Modulation of Intestinal Barrier Function in Early Life. Annu. Rev. Nutr. 2022, 42, 165–200. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Morales, A.; Caba, M.; Garcia-Juarez, M.; Caba-Flores, M.D.; Viveros-Contreras, R.; Martinez-Valenzuela, C. Breastfeeding Contributes to Physiological Immune Programming in the Newborn. Front. Pediatr. 2021, 9, 744104. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e3811. [Google Scholar] [CrossRef]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic Immune System Development in Newborn Children. Cell 2018, 174, 1277–1292.e1214. [Google Scholar] [CrossRef]
- Coker, M.O.; Laue, H.E.; Hoen, A.G.; Hilliard, M.; Dade, E.; Li, Z.; Palys, T.; Morrison, H.G.; Baker, E.; Karagas, M.R.; et al. Infant Feeding Alters the Longitudinal Impact of Birth Mode on the Development of the Gut Microbiota in the First Year of Life. Front. Microbiol. 2021, 12, 642197. [Google Scholar] [CrossRef] [PubMed]
- Bridgman, S.L.; Konya, T.; Azad, M.B.; Sears, M.R.; Becker, A.B.; Turvey, S.E.; Mandhane, P.J.; Subbarao, P.; Scott, J.A.; Field, C.J.; et al. Infant gut immunity: A preliminary study of IgA associations with breastfeeding. J. Dev. Orig. Health Dis. 2016, 7, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Hida, M.; Kohgo, T.; Fukunaga, Y. Changes in salivary and fecal secretory IgA in infants under different feeding regimens. Pediatr. Int. 2009, 51, 342–345. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory IgA: Designed for Anti-Microbial Defense. Front. Immunol. 2013, 4, 222. [Google Scholar] [CrossRef]
- Guo, J.; Ren, C.; Han, X.; Huang, W.; You, Y.; Zhan, J. Role of IgA in the early-life establishment of the gut microbiota and immunity: Implications for constructing a healthy start. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Baldridge, M.T.; Wallace, M.A.; Burnham, C.-A.D.; Virgin, H.W.; Stappenbeck, T.S. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 2015, 521, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, E.; Magenes, V.C.; Fiore, G.; Agostinelli, M.; La Mendola, A.; Acunzo, M.; Francavilla, R.; Indrio, F.; Bosetti, A.; D’Auria, E.; et al. Special Diets in Infants and Children and Impact on Gut Microbioma. Nutrients 2022, 14, 3198. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Ostbye, T.; Mueller, N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Chichlowski, M.; van Diepen, J.A.; Prodan, A.; Olga, L.; Ong, K.K.; Kortman, G.A.M.; Dunger, D.B.; Gross, G. Early development of infant gut microbiota in relation to breastfeeding and human milk oligosaccharides. Front. Nutr. 2023, 10, 1003032. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatter, C.; Fernandez Trigo, N.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front. Immunol. 2021, 12, 683022. [Google Scholar] [CrossRef] [PubMed]
- Lif Holgerson, P.; Esberg, A.; West, C.E.; Johansson, I. The breast milk and childhood gastrointestinal microbiotas and disease outcomes: A longitudinal study. Pediatr. Res. 2023, 93, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Azad, M.B.; Vehling, L.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Field, C.J.; Lefebvre, D.; Sears, M.R.; Becker, A.B.; et al. Association of Exposure to Formula in the Hospital and Subsequent Infant Feeding Practices with Gut Microbiota and Risk of Overweight in the First Year of Life. JAMA Pediatr. 2018, 172, e181161. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Eberl, G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 2020, 13, 183–189. [Google Scholar] [CrossRef]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef]
- Van den Elsen, L.W.J.; Rekima, A.; Verhasselt, V. Early-Life Nutrition and Gut Immune Development. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Chleilat, F.; Klancic, T.; Ma, K.; Schick, A.; Nettleton, J.E.; Reimer, R.A. Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats. Nutrients 2020, 12, 1532. [Google Scholar] [CrossRef] [PubMed]
- Gart, E.; Salic, K.; Morrison, M.C.; Giera, M.; Attema, J.; de Ruiter, C.; Caspers, M.; Schuren, F.; Bobeldijk-Pastorova, I.; Heer, M.; et al. The Human Milk Oligosaccharide 2’-Fucosyllactose Alleviates Liver Steatosis, ER Stress and Insulin Resistance by Reducing Hepatic Diacylglycerols and Improved Gut Permeability in Obese Ldlr-/-.Leiden Mice. Front. Nutr. 2022, 9, 904740. [Google Scholar] [CrossRef]
- Spivak, I.; Fluhr, L.; Elinav, E. Local and systemic effects of microbiome-derived metabolites. EMBO Rep. 2022, 23, e55664. [Google Scholar] [CrossRef]
- Stinson, L.F.; Geddes, D.T. Microbial metabolites: The next frontier in human milk. Trends Microbiol. 2022, 30, 408–410. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The role of the gut microbiota in health and cardiovascular diseases. Mol. Biomed. 2022, 3, 30. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e1128. [Google Scholar] [CrossRef]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Morbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Molgaard, C.; et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Parks, O.B.; Pociask, D.A.; Hodzic, Z.; Kolls, J.K.; Good, M. Interleukin-22 Signaling in the Regulation of Intestinal Health and Disease. Front. Cell Dev. Biol. 2015, 3, 85. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Roberts, S.E.; Wotton, C.J.; Williams, J.G.; Griffith, M.; Goldacre, M.J. Perinatal and early life risk factors for inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 743–749. [Google Scholar] [CrossRef]
- Noble, A.J.; Nowak, J.K.; Adams, A.T.; Uhlig, H.H.; Satsangi, J. Defining Interactions Between the Genome, Epigenome, and the Environment in Inflammatory Bowel Disease: Progress and Prospects. Gastroenterology 2023, 165, 44–60.e42. [Google Scholar] [CrossRef]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; Brand, S.; et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet 2016, 387, 156–167. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Zielinska, M.; Sokal, A.; Filip, R. Genetic and Epigenetic Etiology of Inflammatory Bowel Disease: An Update. Genes 2022, 13, 2388. [Google Scholar] [CrossRef]
- Gaya, D.R.; Russell, R.K.; Nimmo, E.R.; Satsangi, J. New genes in inflammatory bowel disease: Lessons for complex diseases? Lancet 2006, 367, 1271–1284. [Google Scholar] [CrossRef]
- Montbarbon, M.; Pichavant, M.; Langlois, A.; Erdual, E.; Maggiotto, F.; Neut, C.; Mallevaey, T.; Dharancy, S.; Dubuquoy, L.; Trottein, F.; et al. Colonic inflammation in mice is improved by cigarette smoke through iNKT cells recruitment. PLoS ONE 2013, 8, e62208. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e1144. [Google Scholar] [CrossRef] [PubMed]
- Sykora, J.; Pomahacova, R.; Kreslova, M.; Cvalinova, D.; Stych, P.; Schwarz, J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 2741–2763. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Gubatan, J.; Kulkarni, C.V.; Talamantes, S.M.; Temby, M.; Fardeen, T.; Sinha, S.R. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients 2023, 15, 579. [Google Scholar] [CrossRef]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef]
- Albenberg, L. The Role of Diet in Pediatric Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2023, 52, 565–577. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Impact of Diet on Risk of IBD. Crohns Colitis 360 2020, 2, otz054. [Google Scholar] [CrossRef]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory Bowel Disease: A Review of Pre-Clinical Murine Models of Human Disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Fedorak, R.N.; Tavernini, M.M.; Doyle, J.S. Normal Breast Milk Limits the Development of Colitis in IL-10-Deficient Mice. Inflamm. Bowel Dis. 2002, 8, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Lara-Villoslada, F.; Debras, E.; Nieto, A.; Concha, A.; Galvez, J.; Lopez-Huertas, E.; Boza, J.; Obled, C.; Xaus, J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin. Nutr. 2006, 25, 477–488. [Google Scholar] [CrossRef]
- Daddaoua, A.; Puerta, V.; Requena, P.; Martinez-Ferez, A.; Guadix, E.; de Medina, F.S.; Zarzuelo, A.; Suarez, M.D.; Boza, J.J.; Martinez-Augustin, O. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J. Nutr. 2006, 136, 672–676. [Google Scholar] [CrossRef]
- Fuhrer, A.; Sprenger, N.; Kurakevich, E.; Borsig, L.; Chassard, C.; Hennet, T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J. Exp. Med. 2010, 207, 2843–2854. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Prieto, P.A.; Mukerji, P.; Kelder, B.; Erney, R.; Gonzalez, D.; Yun, J.S.; Smith, D.F.; Moremen, K.W.; Nardelli, C.; Pierce, M.; et al. Remodeling of mouse milk glycoconjugates by transgenic expression of a human glycosyltransferase. J. Biol. Chem. 1995, 270, 29515–29519. [Google Scholar] [CrossRef]
- Grabinger, T.; Glaus Garzon, J.F.; Hausmann, M.; Geirnaert, A.; Lacroix, C.; Hennet, T. Alleviation of Intestinal Inflammation by Oral Supplementation with 2-Fucosyllactose in Mice. Front. Microbiol. 2019, 10, 1385. [Google Scholar] [CrossRef]
- Li, A.-L.; Ni, W.-W.; Li, Y.; Zhang, X.; Yang, J.-J.; Ma, X.-Y.; Jia, X.-D.; Li, C.; Liu, L.-B. Effect of 2′-fucosyllactose supplementation on intestinal flora in mice with intestinal inflammatory diseases. Int. Dairy J. 2020, 110, 104797. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Yin, J.; Zhang, B.; Wang, J.; Wang, S. Differential responses on gut microbiota and microbial metabolome of 2’-fucosyllactose and galactooligosaccharide against DSS-induced colitis. Food Res. Int. 2022, 162, 112072. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, L.; Zheng, N.; Blecker, C.; Delcenserie, V.; Li, H.; Wang, J. 2’-Fucosyllactose Ameliorates Inflammatory Bowel Disease by Modulating Gut Microbiota and Promoting MUC2 Expression. Front. Nutr. 2022, 9, 822020. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.H.; Shin, C.S.; Yoon, J.W.; Jeon, S.M.; Song, Y.H.; Kim, K.Y.; Kim, K. 2’-Fucosyllactose and 3-Fucosyllactose Alleviates Interleukin-6-Induced Barrier Dysfunction and Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Modulating the Intestinal Microbiome. Nutrients 2023, 15, 1845. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Diallo, I.; Salem, M.; Michel, S.; Gilbert, C.; Sevigny, J.; Provost, P. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental colitis. Sci. Rep. 2019, 9, 14661. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Elbaum-Shiff, Y.; Koroukhov, N.; Shilo, I.; Musseri, M.; Golan-Gerstl, R. Cow and Human Milk-Derived Exosomes Ameliorate Colitis in DSS Murine Model. Nutrients 2020, 12, 2589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Paz, H.A.; Sadri, M.; Cui, J.; Kachman, S.D.; Fernando, S.C.; Zempleni, J. Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G618–G624. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, e1901251. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 2023, 9, eade5041. [Google Scholar] [CrossRef]
- Lindoso, L.; Mondal, K.; Venkateswaran, S.; Somineni, H.K.; Ballengee, C.; Walters, T.D.; Griffiths, A.; Noe, J.D.; Crandall, W.; Snapper, S.; et al. The Effect of Early-Life Environmental Exposures on Disease Phenotype and Clinical Course of Crohn’s Disease in Children. Am. J. Gastroenterol. 2018, 113, 1524–1529. [Google Scholar] [CrossRef]
- Guo, A.Y.; Stevens, B.W.; Wilson, R.G.; Russell, C.N.; Cohen, M.A.; Sturgeon, H.C.; Thornton, A.; Giallourakis, C.; Khalili, H.; Nguyen, D.D.; et al. Early life environment and natural history of inflammatory bowel diseases. BMC Gastroenterol. 2014, 14, 216. [Google Scholar] [CrossRef]
- Khalili, H.; Ananthakrishnan, A.N.; Higuchi, L.M.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Early life factors and risk of inflammatory bowel disease in adulthood. Inflamm. Bowel Dis. 2013, 19, 542–547. [Google Scholar] [CrossRef]
- Niewiadomski, O.; Studd, C.; Wilson, J.; Williams, J.; Hair, C.; Knight, R.; Prewett, E.; Dabkowski, P.; Alexander, S.; Allen, B.; et al. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern. Med. J. 2016, 46, 669–676. [Google Scholar] [CrossRef]
- Agrawal, M.; Sabino, J.; Frias-Gomes, C.; Hillenbrand, C.M.; Soudant, C.; Axelrad, J.E.; Shah, S.C.; Ribeiro-Mourao, F.; Lambin, T.; Peter, I.; et al. Early life exposures and the risk of inflammatory bowel disease: Systematic review and meta-analyses. EClinicalMedicine 2021, 36, 100884. [Google Scholar] [CrossRef]
- Gungor, D.; Nadaud, P.; Dreibelbis, C.; LaPergola, C.C.; Wong, Y.P.; Terry, N.; Abrams, S.A.; Beker, L.; Jacobovits, T.; Jarvinen, K.M.; et al. Infant milk-feeding practices and diagnosed celiac disease and inflammatory bowel disease in offspring: A systematic review. Am. J. Clin. Nutr. 2019, 109, 838S–851S. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Yuan, A. A Systematic Review of Epidemiology and Risk Factors Associated with Chinese Inflammatory Bowel Disease. Front. Med. 2018, 5, 183. [Google Scholar] [CrossRef]
- Xu, L.; Lochhead, P.; Ko, Y.; Claggett, B.; Leong, R.W.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.R.; Russell, R.K.; Wilson, M.L.; Gilmour, W.H.; Satsangi, J.; Wilson, D.C. Systematic review: The role of breastfeeding in the development of pediatric inflammatory bowel disease. J. Pediatr. 2009, 155, 421–426. [Google Scholar] [CrossRef]
- Klement, E.; Cohen, R.V.; Boxman, J.; Joseph, A.; Reif, S. Breastfeeding and risk of inflammatory bowel disease: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2004, 80, 1342–1352. [Google Scholar] [CrossRef]
- Saadh, M.J.; Pal, R.S.; Arias-Gonzales, J.L.; Orosco Gavilan, J.C.; Jc, D.; Mohany, M.; Al-Rejaie, S.S.; Bahrami, A.; Kadham, M.J.; Amin, A.H.; et al. A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors. Nutrients 2023, 15, 1202. [Google Scholar] [CrossRef]
- Lee, W.S.; Song, Z.L.; Wong, S.Y.; Gan, C.W.; Koay, Z.L.; Em, J.M.; Chong, S.Y.; Lim, C.B.; Wong, S.Y.; Chew, K.S.; et al. Environmental risk factors for inflammatory bowel disease: A case control study in Southeast Asian children. J. Paediatr. Child. Health 2022, 58, 782–790. [Google Scholar] [CrossRef]
- Urashima, H.; Ohmori, I.; Shiraki, K. Epidemiological Survey on Chronic Inflammatory Bowel Disease Developed during Childhood in Japan, and a Case-Control Study on Nutrition during Infancy. Yonago Acta Medica 1999, 42, 95–102. [Google Scholar]
- Gearry, R.B.; Richardson, A.K.; Frampton, C.M.; Dodgshun, A.J.; Barclay, M.L. Population-based cases control study of inflammatory bowel disease risk factors. J. Gastroenterol. Hepatol. 2010, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xia, B.; Li, J.; Ye, M.; Deng, C.; Ding, Y.; Luo, H.; Ren, H.; Hou, X.; Liu, H.; et al. Risk factors for ulcerative colitis in a Chinese population: An age-matched and sex-matched case-control study. J. Clin. Gastroenterol. 2007, 41, 280–284. [Google Scholar] [CrossRef]
- Han, D.Y.; Fraser, A.G.; Dryland, P.; Ferguson, L.R. Environmental factors in the development of chronic inflammation: A case-control study on risk factors for Crohn’s disease within New Zealand. Mutat. Res. 2010, 690, 116–122. [Google Scholar] [CrossRef]
- Vahedi, H.; Chaharmahali, M.; Momtahen, S.; Kolahdoozan, S.; Khademi, H.; Olfati, G.; Tabrizian, T.; Rashtak, S.; Khaleghnejad, R.; Naserimoghadam, S.; et al. A Case-Control study on the risk factors of IBD in 258 Iranian patients. Govaresh 2011, 16, 61–67. [Google Scholar]
- Ng, S.C.; Tang, W.; Leong, R.W.; Chen, M.; Ko, Y.; Studd, C.; Niewiadomski, O.; Bell, S.; Kamm, M.A.; de Silva, H.J.; et al. Environmental risk factors in inflammatory bowel disease: A population-based case-control study in Asia-Pacific. Gut 2015, 64, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Ou-Yang, Q.; Xia, B.; Liu, L.N.; Gu, F.; Zhou, K.F.; Mei, Q.; Shi, R.H.; Ran, Z.H.; Wang, X.D.; et al. Multicenter case-control study of the risk factors for ulcerative colitis in China. World J. Gastroenterol. 2013, 19, 1827–1833. [Google Scholar] [CrossRef]
- Ko, Y.; Kariyawasam, V.; Karnib, M.; Butcher, R.; Samuel, D.; Alrubaie, A.; Rahme, N.; McDonald, C.; Cowlishaw, J.; Katelaris, P.; et al. Inflammatory Bowel Disease Environmental Risk Factors: A Population-Based Case-Control Study of Middle Eastern Migration to Australia. Clin. Gastroenterol. Hepatol. 2015, 13, 1453–1463.e1451. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Griffiths, A.; Corey, M.; Smith, C.; Sherman, P. Infant feeding practices and ulcerative colitis in childhood. BMJ 1991, 302, 1580–1581. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Sherman, P.; Corey, M.; Griffiths, A.; Smith, C. Role of infant feeding practices in development of Crohn’s disease in childhood. BMJ 1989, 298, 1617–1618. [Google Scholar] [CrossRef]
- Rigas, A.; Rigas, B.; Glassman, M.; Yen, Y.Y.; Lan, S.J.; Petridou, E.; Hsieh, C.C.; Trichopoulos, D. Breast-feeding and maternal smoking in the etiology of Crohn’s disease and ulcerative colitis in childhood. Ann. Epidemiol. 1993, 3, 387–392. [Google Scholar] [CrossRef]
- Gruber, M.; Marshall, J.R.; Zielezny, M.; Lance, P. A case-control study to examine the influence of maternal perinatal behaviors on the incidence of Crohn’s disease. Gastroenterol. Nurs. 1996, 19, 53–59. [Google Scholar] [CrossRef]
- Amre, D.K.; Lambrette, P.; Law, L.; Krupoves, A.; Chotard, V.; Costea, F.; Grimard, G.; Israel, D.; Mack, D.; Seidman, E.G. Investigating the hygiene hypothesis as a risk factor in pediatric onset Crohn’s disease: A case-control study. Am. J. Gastroenterol. 2006, 101, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Banerjee, A.; Targownik, L.E.; Singh, H.; Ghia, J.E.; Burchill, C.; Chateau, D.; Roos, L.L. Cesarean Section Delivery Is Not a Risk Factor for Development of Inflammatory Bowel Disease: A Population-based Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hutfless, S.; Li, D.K.; Heyman, M.B.; Bayless, T.M.; Abramson, O.; Herrinton, L.J. Prenatal and perinatal characteristics associated with pediatric-onset inflammatory bowel disease. Dig. Dis. Sci. 2012, 57, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V.C.L.; Luiz, R.R.; Boechat, N.; Schorr, B.C.; Leao, I.S.; Nunes, T.; Zaltman, C. Crohn’s disease environmental factors in the developing world: A case-control study in a statewide catchment area in Brazil. World J. Gastroenterol. 2017, 23, 5549–5556. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, S.A.; Fournier, N.; Biedermann, L.; Pittet, V.; Schreiner, P.; Misselwitz, B.; Scharl, M.; Rogler, G.; Siebenhuner, A.R. The Influence of Breastfeeding, Cesarean Section, Pet Animals, and Urbanization on the Development of Inflammatory Bowel Disease: Data from the Swiss IBD Cohort Study. Inflamm. Intest. Dis. 2020, 5, 170–179. [Google Scholar] [CrossRef]
- Van der Sloot, K.W.J.; Weersma, R.K.; Alizadeh, B.Z.; Dijkstra, G. Identification of Environmental Risk Factors Associated with the Development of Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 1662–1671. [Google Scholar] [CrossRef]
- Ekbom, A.; Adami, H.O.; Helmick, C.G.; Jonzon, A.; Zack, M.M. Perinatal risk factors for inflammatory bowel disease: A case-control study. Am. J. Epidemiol. 1990, 132, 1111–1119. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Giugliano, F.; Martinelli, M.; Cenni, S.; Greco, L.; Staiano, A.; Miele, E. Impact of Environmental and Familial Factors in a Cohort of Pediatric Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 569–574. [Google Scholar] [CrossRef]
- Bergstrand, O.; Hellers, G. Breast-feeding during infancy in patients who later develop Crohn’s disease. Scand. J. Gastroenterol. 1983, 18, 903–906. [Google Scholar] [CrossRef]
- Samuelsson, S.M.; Ekbom, A.; Zack, M.; Helmick, C.G.; Adami, H.O. Risk factors for extensive ulcerative colitis and ulcerative proctitis: A population based case-control study. Gut 1991, 32, 1526–1530. [Google Scholar] [CrossRef]
- Persson, P.G.; Leijonmarck, C.E.; Bernell, O.; Hellers, G.; Ahlbom, A. Risk indicators for inflammatory bowel disease. Int. J. Epidemiol. 1993, 22, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Tragnone, A.; Caprilli, R.; Trallori, G.; Papi, C.; Andreoli, A.; Di Paolo, M.; Riegler, G.; Rigo, G.P.; Ferrau, O.; et al. Risk of inflammatory bowel disease attributable to smoking, oral contraception and breastfeeding in Italy: A nationwide case-control study. Cooperative Investigators of the Italian Group for the Study of the Colon and the Rectum (GISC). Int. J. Epidemiol. 1998, 27, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.P.; Montgomery, S.M.; Wadsworth, M.E.; Pounder, R.E.; Wakefield, A.J. Early determinants of inflammatory bowel disease: Use of two national longitudinal birth cohorts. Eur. J. Gastroenterol. Hepatol. 2000, 12, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Serrano, P.; Perez-Calle, J.L.; Perez-Fernandez, M.T.; Fernandez-Font, J.M.; Boixeda de Miguel, D.; Fernandez-Rodriguez, C.M. Environmental risk factors in inflammatory bowel diseases. Investigating the hygiene hypothesis: A Spanish case-control study. Scand. J. Gastroenterol. 2010, 45, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Russel, M.G.; Engels, L.G.; Muris, J.W.; Limonard, C.B.; Volovics, A.; Brummer, R.J.; Stockbrugger, R.W. Modern life’ in the epidemiology of inflammatory bowel disease: A case-control study with special emphasis on nutritional factors. Eur. J. Gastroenterol. Hepatol. 1998, 10, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Turck, D.; Leplat, C.; Merle, V.; Gower-Rousseau, C.; Marti, R.; Yzet, T.; Lerebours, E.; Dupas, J.L.; Debeugny, S.; et al. Environmental risk factors in paediatric inflammatory bowel diseases: A population based case control study. Gut 2005, 54, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.; Engelmann, G.; Findeisen, A.; Gerner, P.; Laass, M.; Ney, D.; Posovszky, C.; Hoy, L.; Hornef, M.W. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 2010, 125, e1433–e1440. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.S.; Jess, T.; Vind, I.; Elkjaer, M.; Nielsen, M.F.; Gamborg, M.; Munkholm, P. Environmental factors in inflammatory bowel disease: A case-control study based on a Danish inception cohort. J. Crohns Colitis 2011, 5, 577–584. [Google Scholar] [CrossRef]
- Sonntag, B.; Stolze, B.; Heinecke, A.; Luegering, A.; Heidemann, J.; Lebiedz, P.; Rijcken, E.; Kiesel, L.; Domschke, W.; Kucharzik, T.; et al. Preterm birth but not mode of delivery is associated with an increased risk of developing inflammatory bowel disease later in life. Inflamm. Bowel Dis. 2007, 13, 1385–1390. [Google Scholar] [CrossRef]
- Radon, K.; Windstetter, D.; Poluda, A.L.; Mueller, B.; von Mutius, E.; Koletzko, S.; Chronische Autoimmunerkrankungen und Kontakt zu Tieren Study, G. Contact with farm animals in early life and juvenile inflammatory bowel disease: A case-control study. Pediatrics 2007, 120, 354–361. [Google Scholar] [CrossRef]
- Jakobsen, C.; Paerregaard, A.; Munkholm, P.; Wewer, V. Environmental factors and risk of developing paediatric inflammatory bowel disease—A population based study 2007–2009. J. Crohns Colitis 2013, 7, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Diaferia, M.; Morace, F.; Labianca, O.; Meucci, C.; Cuomo, A.; Panarese, A.; Romano, M.; Sorrentini, I.; D’Onofrio, C.; et al. Risk factors for inflammatory bowel diseases according to the “hygiene hypothesis”: A case-control, multi-centre, prospective study in Southern Italy. J. Crohns Colitis 2012, 6, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Whorwell, P.J.; Holdstock, G.; Whorwell, G.M.; Wright, R. Bottle feeding, early gastroenteritis, and inflammatory bowel disease. Br. Med. J. 1979, 1, 382. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, T.; Toth, J.; Koller, T.; Krajcovicova, A.; Oravcova, S.; Zelinkova, Z.; Huorka, M. Smoking, breastfeeding, physical inactivity, contact with animals, and size of the family influence the risk of inflammatory bowel disease: A Slovak case-control study. United Eur. Gastroenterol. J. 2013, 1, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Reif, S.; Farbstein, H.; Halak, A.; Gilat, T. Preillness non dietary factors and habits in inflammatory bowel disease. Ital. J. Gastroenterol. Hepatol. 1998, 30, 247–251. [Google Scholar]
- Velosa, M.; Hochner, H.; Yerushalmi, B.; Harel, S.; Friss, C.; Calderon-Margalit, R.; Paltiel, O.; Manor, O.; Balicer, R.D.; Greenfeld, S.; et al. Pre- and Perinatal Factors Predicting Inflammatory Bowel Disease: A Population-Based Study with Fifty Years of Follow-Up. J. Crohns Colitis 2022, 16, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Gilat, T.; Hacohen, D.; Lilos, P.; Langman, M.J. Childhood factors in ulcerative colitis and Crohn’s disease. An international cooperative study. Scand. J. Gastroenterol. 1987, 22, 1009–1024. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e644. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Adolph, T.E.; Zhang, J. Diet fuelling inflammatory bowel diseases: Preclinical and clinical concepts. Gut 2022, 71, 2574–2586. [Google Scholar] [CrossRef]
- Van der Sloot, K.W.J.; Amini, M.; Peters, V.; Dijkstra, G.; Alizadeh, B.Z. Inflammatory Bowel Diseases: Review of Known Environmental Protective and Risk Factors Involved. Inflamm. Bowel Dis. 2017, 23, 1499–1509. [Google Scholar] [CrossRef]
- Raygoza Garay, J.A.; Turpin, W.; Lee, S.H.; Smith, M.I.; Goethel, A.; Griffiths, A.M.; Moayyedi, P.; Espin-Garcia, O.; Abreu, M.; Aumais, G.L.; et al. Gut Microbiome Composition Is Associated with Future Onset of Crohn’s Disease in Healthy First-Degree Relatives. Gastroenterology 2023, 165, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, A.; Chan, J.; De Wolfe, T.J.; Yang, H.; Vallance, B.A. Pathobionts in Inflammatory Bowel Disease: Origins, Underlying Mechanisms, and Implications for Clinical Care. Gastroenterology 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Ramayo-Caldas, Y.; Estelle, J.; Tambosco, K.; Chadi, S.; Maillard, F.; Gallopin, M.; Planchais, J.; Chain, F.; Kropp, C.; et al. Gut barrier-microbiota imbalances in early life lead to higher sensitivity to inflammation in a murine model of C-section delivery. Microbiome 2023, 11, 140. [Google Scholar] [CrossRef]
- Torres, J.; Hu, J.; Seki, A.; Eisele, C.; Nair, N.; Huang, R.; Tarassishin, L.; Jharap, B.; Cote-Daigneault, J.; Mao, Q.; et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut 2020, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Tarassishin, L.; Eisele, C.; Barre, A.; Nair, N.; Rendon, A.; Hawkins, K.; Debebe, A.; White, S.; Thjomoe, A.; et al. Longitudinal Changes in Fecal Calprotectin Levels among Pregnant Women with and without Inflammatory Bowel Disease and Their Babies. Gastroenterology 2021, 160, 1118–1130.e1113. [Google Scholar] [CrossRef]
- Quin, C.; Gibson, D.L. Human behavior, not race or geography, is the strongest predictor of microbial succession in the gut bacteriome of infants. Gut Microbes 2020, 11, 1143–1171. [Google Scholar] [CrossRef]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494. [Google Scholar] [CrossRef]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.C.; Kiilerich, P.; Akrami, R.; Kramer, M.; Uhlen, M.; et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 2021, 29, 765–776.e763. [Google Scholar] [CrossRef]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef]
- Conte, M.P.; Longhi, C.; Marazzato, M.; Conte, A.L.; Aleandri, M.; Lepanto, M.S.; Zagaglia, C.; Nicoletti, M.; Aloi, M.; Totino, V.; et al. Adherent-invasive Escherichia coli (AIEC) in pediatric Crohn’s disease patients: Phenotypic and genetic pathogenic features. BMC Res. Notes 2014, 7, 748. [Google Scholar] [CrossRef] [PubMed]
- Wymore Brand, M.; Proctor, A.L.; Hostetter, J.M.; Zhou, N.; Friedberg, I.; Jergens, A.E.; Phillips, G.J.; Wannemuehler, M.J. Vertical transmission of attaching and invasive E. coli from the dam to neonatal mice predisposes to more severe colitis following exposure to a colitic insult later in life. PLoS ONE 2022, 17, e0266005. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Martin, R.; Ishikawa, E.; Gawad, A.; Kubota, H.; Sakai, T.; Oishi, K.; Tanaka, R.; Ben-Amor, K.; Knol, J.; et al. Multilocus sequence typing of bifidobacterial strains from infant’s faeces and human milk: Are bifidobacteria being sustainably shared during breastfeeding? Benef. Microbes 2015, 6, 563–572. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Zhang, L.; Liu, N.; Li, Z.; Zhang, F.; Liu, X.; Ma, X. Dietary Inulin Regulated Gut Microbiota and Improved Neonatal Health in a Pregnant Sow Model. Front. Nutr. 2021, 8, 716723. [Google Scholar] [CrossRef]
- Chatelais, L.; Jamin, A.; Gras-Le Guen, C.; Lalles, J.P.; Le Huerou-Luron, I.; Boudry, G. The level of protein in milk formula modifies ileal sensitivity to LPS later in life in a piglet model. PLoS ONE 2011, 6, e19594. [Google Scholar] [CrossRef] [PubMed]
- Boudry, G.; Jamin, A.; Chatelais, L.; Gras-Le Guen, C.; Michel, C.; Le Huerou-Luron, I. Dietary protein excess during neonatal life alters colonic microbiota and mucosal response to inflammatory mediators later in life in female pigs. J. Nutr. 2013, 143, 1225–1232. [Google Scholar] [CrossRef]
- Reddy, K.V.; Naidu, K.A. Maternal and neonatal dietary intake of balanced n-6/n-3 fatty acids modulates experimental colitis in young adult rats. Eur. J. Nutr. 2016, 55, 1875–1890. [Google Scholar] [CrossRef]
- Le, A.; Mantel, M.; Marchix, J.; Bodinier, M.; Jan, G.; Rolli-Derkinderen, M. Inflammatory bowel disease therapeutic strategies by modulation of the microbiota: How and when to introduce pre-, pro-, syn-, or postbiotics? Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G523–G553. [Google Scholar] [CrossRef]
- Haskey, N.; Dahl, W.J. Synbiotic therapy: A promising new adjunctive therapy for ulcerative colitis. Nutr. Rev. 2006, 64, 132–138. [Google Scholar] [CrossRef]
- Savino, F.; Cordisco, L.; Tarasco, V.; Palumeri, E.; Calabrese, R.; Oggero, R.; Roos, S.; Matteuzzi, D. Lactobacillus reuteri DSM 17938 in infantile colic: A randomized, double-blind, placebo-controlled trial. Pediatrics 2010, 126, e526–e533. [Google Scholar] [CrossRef]
- Guo, F.; Cai, D.; Li, Y.; Gu, H.; Qu, H.; Zong, Q.; Bao, W.; Chen, A.; Liu, H.Y. How Early-Life Gut Microbiota Alteration Sets Trajectories for Health and Inflammatory Bowel Disease? Front. Nutr. 2021, 8, 690073. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Dejardin, F.; Sparwasser, T.; Berard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288.e1275. [Google Scholar] [CrossRef] [PubMed]
- Stiemsma, L.T.; Michels, K.B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 2018, 141, e20172437. [Google Scholar] [CrossRef] [PubMed]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef] [PubMed]
- Theochari, N.A.; Stefanopoulos, A.; Mylonas, K.S.; Economopoulos, K.P. Antibiotics exposure and risk of inflammatory bowel disease: A systematic review. Scand. J. Gastroenterol. 2018, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Kaplan, G.G.; Otley, A.R.; Nguyen, G.C.; Underwood, F.E.; Guttmann, A.; Jones, J.L.; Potter, B.K.; Catley, C.A.; Nugent, Z.J.; et al. Rural and Urban Residence During Early Life is Associated with Risk of Inflammatory Bowel Disease: A Population-Based Inception and Birth Cohort Study. Am. J. Gastroenterol. 2017, 112, 1412–1422. [Google Scholar] [CrossRef]
- Meng, X.; Dunsmore, G.; Koleva, P.; Elloumi, Y.; Wu, R.Y.; Sutton, R.T.; Ambrosio, L.; Hotte, N.; Nguyen, V.; Madsen, K.L.; et al. The Profile of Human Milk Metabolome, Cytokines, and Antibodies in Inflammatory Bowel Diseases Versus Healthy Mothers, and Potential Impact on the Newborn. J. Crohns Colitis 2019, 13, 431–441. [Google Scholar] [CrossRef]

