1. Introduction
Cancer-related fatigue (CRF) is defined by the National Comprehensive Cancer Network (NCCN) as a distressing and persistent subjective feeling of exhaustion or tiredness that is cognitive, emotional, or physical. It is associated with cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning [1]. CRF is a frequently reported symptom, with a prevalence of 45 to 80% among all cancer patients [2,3], with a particular emphasis on those undergoing radiotherapy or chemotherapy [4].
The pathogenesis of CRF is unclear and may be related to inflammation, endocrine system disorders, adenosine triphosphate (ATP) metabolic abnormalities, 5-hydroxy tryptamine (5-HT) system dysfunction, and genetic factors [5,6,7,8,9,10,11]. The most popular and supported hypothesis includes the inflammatory hypothesis, which states that cancer and cancer treatments activate the immune system to release pro-inflammatory factors that affect the central nervous system, resulting in symptoms such as sleep disturbances, fever, and severe fatigue [5,7].
In the state of fatigue, the patient’s immune function is reduced and susceptible to infections, as well as feeling weak and discouraged, seriously affecting their therapeutic effect and quality of life and even increasing the risk of death [4,12,13]. Effective early screening and fatigue assessment are essential for these patients [14]. Although CRF management is strongly recommended in guidelines and the literature, its implementation in clinical practice is often lacking, leading to underestimation and undertreatment [15,16,17]. The primary barrier to implementing CRF management is the lack of accurate knowledge by care providers about fatigue and its treatment options and the effects of fatigue in patients [18]. Furthermore, patients often do not voluntarily report this symptom for fear of interfering with treatment or because they feel that fatigue is unavoidable [14,19].
In personalized treatment and care, precise and effective CRF prediction can impact the status quo and direct treatment decisions for patients and providers. With the rapid development of artificial intelligence, several studies have shown that prediction models based on machine learning algorithms can be a good aid for early screening and assessment of diseases [20] and have promising applications in CRF [21,22]. The selection of predictors, which serve the accuracy and interpretability of the model to a certain extent, is the primary foundation of constructing a prediction model. The selection of easily accessible electronic health records (EHRs) for modeling [23] purposes is a common practice observed in the previous research. Nevertheless, predictive models may need to be improved in their applicability and accuracy due to variations in biology, genetics, and environmental factors among different populations [24]. Thus, a comprehensive and systematic criterion for selecting predictors is required in similar studies in the future.
Sleight et al. proposed the predisposing, precipitating, and perpetuating (3P) factors model to facilitate risk prediction and clinical care of fatigue [25]. Predisposing factors are personal traits contributing to fatigue, such as biological behaviors like age, gender, and genetic variation, and also psychosocial factors like depression and anxiety. In this context, researchers have summarized the potentially significant associations of genetic polymorphisms associated with the neurotransmitter system, the hypothalamic–pituitary–adrenal (HPA) axis, and immune-mediated inflammation with fatigue [26]. Furthermore, Susanne et al. found that baseline levels of fatigue and depression were significant predictors of fatigue in all dimensions [27]. Precipitating factors stimulate the onset or change of fatigue, for instance, inflammatory changes caused by radiotherapy and chemotherapy. Raudonis et al. found that chemotherapy type and serum interleukin-6 (IL-6) were significant predictors of fatigue [8]. Perpetuating factors include poor sleep and chronic nutritional deficiencies, contributing to the exacerbation or gradual onset of fatigue. An increased risk of fatigue related to cancer was associated with a low recent protein intake, as determined via a 24 h recall conducted by Stobäus et al. [28].
In summary, there have been many attempts to predict cancer-related fatigue. However, they were single and one-sided, and studies still need to identify more comprehensive predictive markers of CRF based on the perspective of the occurrence and development of CRF. In this systematic review, we utilized the 3P model as a theoretical foundation to review various possible predictors of cancer-related fatigue other than the genomic domain to provide a broader and more personalized approach to the clinical diagnosis, treatment, and management of cancer-related fatigue.
2. Methods
2.1. Search Strategy
This study was conducted according to The Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) guidelines [29]. The PubMed, Web of Science, Embase, and Scopus databases were thoroughly searched for relevant literature until 16 March 2023. The search strategy was explicitly adapted to the retrieval systems of the different databases, and the search was based on a combination of subject terms and accessible terms. Using search terms such as “cancer-related fatigue, predictor,” the search was limited to English articles and human participants, and the literature search was conducted independently by two researchers (Y.W. and L.T.), resulting in 27 papers. All search strategies were determined after several pre-searches (see for details of the search strategies.) The PRISMA flowchart showed the detailed strategies for paper search and screening. Y.W. and L.T. independently evaluated the articles regarding the inclusion and exclusion criteria (see below), and any disagreement was discussed and negotiated to determine the final study for this review. This study has been registered on the Prospero website (CRD42023408601).
2.2. Inclusion and Exclusion Criteria
The inclusion criteria for this review were as follows:
- (1)
- Study participants were cancer patients with fatigue due to various cancer or cancer treatment (including solid and liquid tumors).
- (2)
- Studies which focused on the association of biomarker or risk factor with cancer-related fatigue.
- (3)
- Studies in which the primary outcome or secondary outcome was cancer-related fatigue.
The exclusion criteria were as follows:
- (1)
- Studies in which the outcome indicator was chronic fatigue syndrome or other disease-related fatigue rather than CRF.
- (2)
- Studies which did not report any correlation between biomarkers or risk factors and CRF.
- (3)
- Biomarker or risk factor of CRF was any gene polymorphism.
- (4)
- Studies that were not published in English or were not available in full text.
- (5)
- Reviews, meta-analyses, protocols, animal experiments, conference reports, medications, case reports, and non-human studies.
- (6)
- Duplication.
2.3. Data Extraction
In this study, data that satisfied the aforementioned criteria were extracted and saved in a Microsoft Excel spreadsheet by two researchers (Y.W. and X.L.). The primary outcome measures were predictors and assessments of CRF. The predictors were classified into predisposing, precipitating, and perpetuating factors according to the 3P model defined by Sleight et al. [25]. The precipitating and perpetuating factors were determined solely based on the above theory. According to the research of Hwang et al. [30], the predisposing factors were categorized into baseline fatigue, demographic characteristics, clinical characteristics, psychosocial traits, and physical symptoms. The following data were also extracted for each included study: author, year of publication, country of origin, study type, data source, sample size, cancer types, and definition of CRF.
2.4. Quality Assessment
The quality of cohort studies was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS) [31], including selection of study cohort populations, comparability between groups, and outcomes/exposures. Total scores ranged from 0 to 9, and poor-quality works were excluded (with a score ≥6 indicating high quality). The critical appraisal checklist from the Joanna Briggs Institute (JBI) was used to evaluate the quality of cross-sectional and longitudinal studies [32]. The questionnaire contains eight questions that were answered with yes, no, or unclear. A score of “yes” for >5 times, 3–4 times, and 0–2 times is considered high methodological quality, moderate methodological quality, and low methodological quality, respectively. The Cochrane Collaboration’s tool for assessing the risk of bias was used to evaluate the quality of randomized controlled trials (RCTs) [33], covering selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. The assessment of study quality was independently conducted by two reviewers (Y.W. and H.Z.), and the results were compared until a consensus was reached. If a study received a low rating in all areas, it would be excluded from the review.
3. Results
3.1. Overview
An initial search of electronic databases identified 22,839 articles for review. After removing 6719 duplicates, 16,120 documents were retained. A total of 8892 articles were excluded based on title, keywords, and abstract, and 188 full-text articles were reviewed for eligibility. Ultimately, 27 articles published between 2002 and 2023 were included in this systematic review based on our inclusion criteria. The PRISMA flowchart is shown in Figure 1.
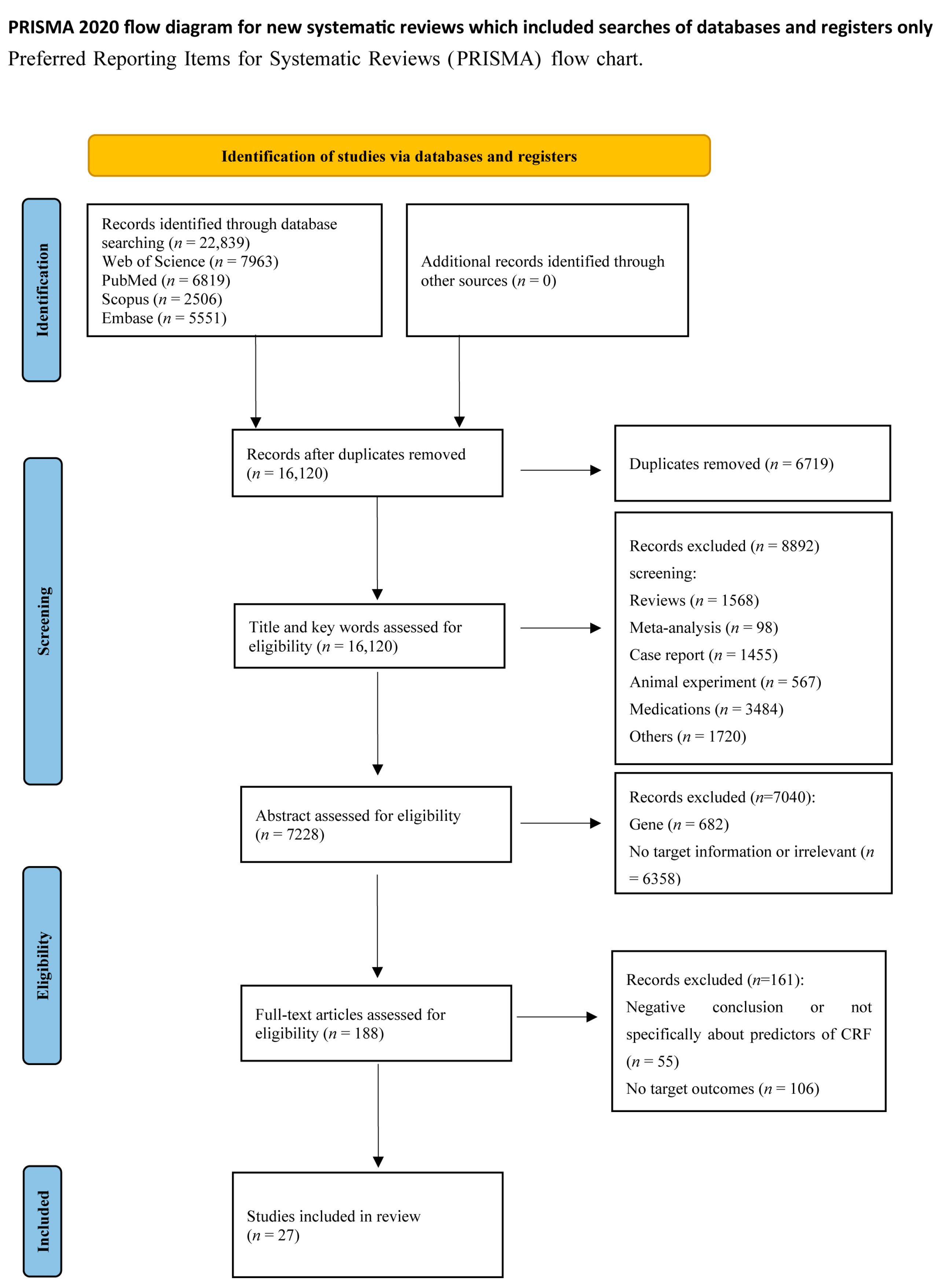
Figure 1. PRISMA diagram for study selection.
The studies were published between 2002 and 2023 (Figure 2B) and included eight cross-sectional studies, fifteen longitudinal studies, three cohort studies, and one RCT (Figure 2A). Studies were conducted primarily in the United States (n = 11), Canada (n = 4), China (n = 3), and Australia (n = 3) (Figure 2C). The number of patients included in the studies ranged from 11 to 3492 with various types of cancer, with breast cancer (n = 9) and mixed tumor populations (n = 8) accounting for approximately two-thirds of the total number of studies (Figure 2D). Data for the study were mainly collected using questionnaires (n = 26), electronic medical records (n = 22), and blood samples (n = 14) (Figure 2E). There was no standardized instrument for evaluating CRF, and most of the studies were assessed using reliability-tested scales, including the Functional Assessment of Cancer Therapy Fatigue (FACT-F) (n = 5), Multidimensional Fatigue Inventory-20 (MFI-20) (n = 5), Brief Fatigue Inventory (BFI) (n = 4), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) (n = 2), Memorial Symptom Assessment Scale (MSAS) (n = 2), Piper Fatigue Scale Revised (PFS-R) (n = 2), Chalder Fatigue Questionnaire (CFQ) (n = 1), Cancer Fatigue Scale (CFS) (n = 1), European Organization for Research and Treatment of Cancer Quality of Life—fatigue assessment 12 item (EORTC QLQ-FA12) (n = 1), The Fatigue Symptom Inventory (FSI) (n = 1), Multidimensional Fatigue Symptom Inventory—Short Form (MFSI-SF) (n = 1), Somatic and Psychological Health Report questionnaire (SPHERE) (n = 1), and Verbal Numerical Rating (VNR) (n = 1) (Figure 2F).
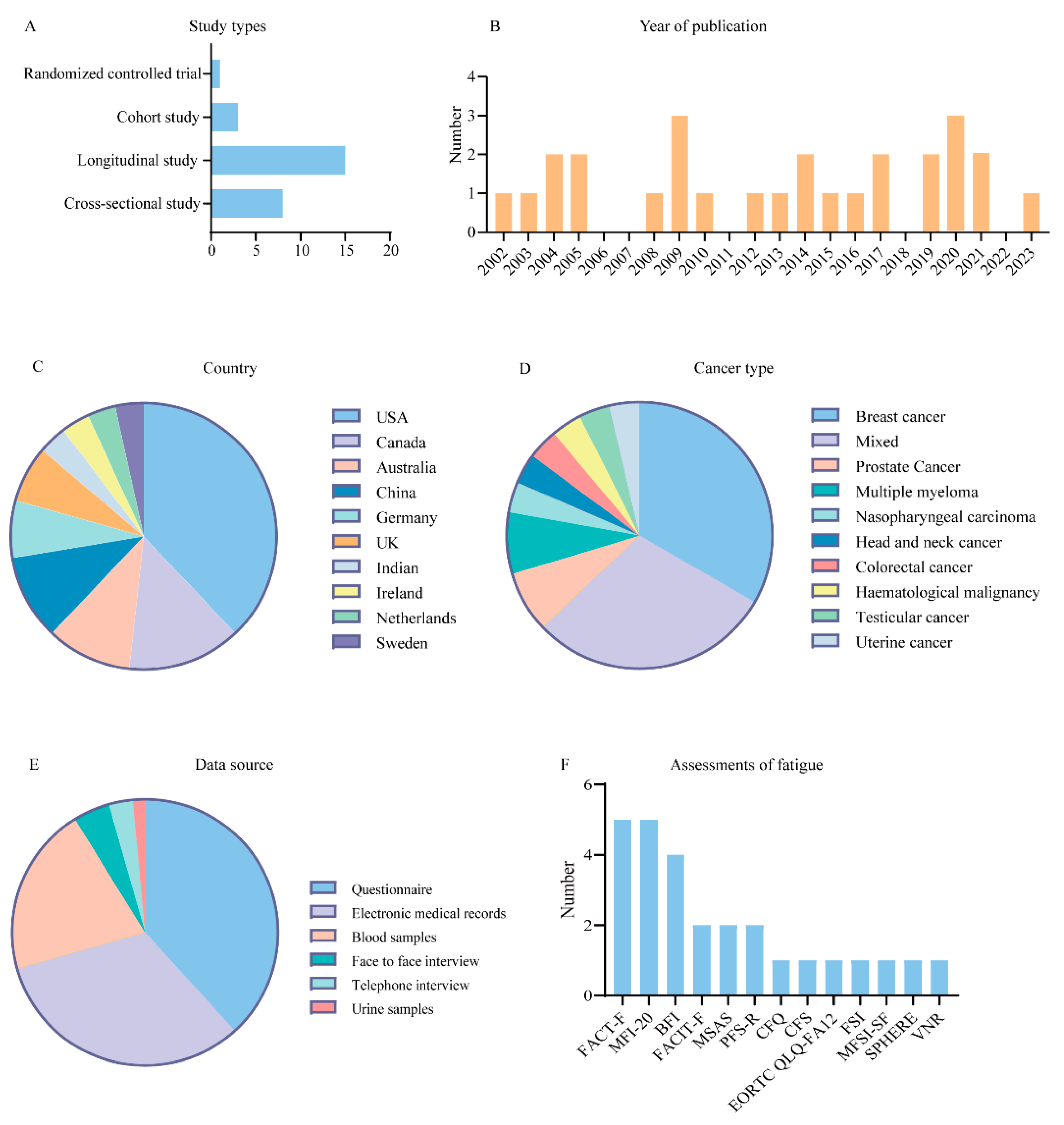
Figure 2. Characteristics of the included studies. (A) Bar chart for types of study of included studies. (B) Bar chart for numbers of publication years of included studies. (C) Pie chart for countries of included studies. (D) Pie chart for types of cancer of included studies. (E) Pie chart for data source of included studies. (F) Bar chart for fatigue assessment scales of included studies.
Most of the studies analyzed fatigue scores as continuous variables. Based on this criterion, some of them transformed into categorical variables considering the minimal clinically important differences (MCIDs) as a cut-off score, differentiating between changes in fatigue as clinically significant or not, or using a boundary value to classify the severity of fatigue. A FACIT-Fatigue score less than 43 was considered fatigue [34], and a score less than 30 was severe fatigue [35]. A FACT-F standardized score of 68 or less was considered fatigue [36], and a change of at least 3 was deemed clinically significant [37,38,39]. A change in CFQ score greater than fourwas considered clinically significant [40]. In different studies, the designation of clinically significant fatigue (CSF) was defined as BFI scores equal to or greater than four [28], similar to VNR scores [41]. Hwang et al. used a threshold score of three to differentiate fatigue from non-fatigue [30].
and Figure 2 depict the main characteristics of the included studies.
Y.W. and Y.T. evaluated the quality of the included studies separately, and found that most of them were of high quality. However, some cross-sectional and longitudinal studies failed to identify or did not address potential confounders; one cohort study had a pre-existing outcome event, fatigue, before the start of the study, and the only RCT did not mention whether it was blinded to the outcome of the study. , and demonstrate the quality assessment results of these studies.
3.2. Predisposing Factors
Predisposing factors in the identified studies focused on four areas: baseline fatigue, demographic characteristics, clinical characteristics, and psychosocial traits. Further details regarding these domains are provided below.
3.3. Precipitating Factors
For precipitating factors, most of the included studies focused on exploring the underlying immune and inflammatory factors for CRF, with the correlation between proinflammatory factors and fatigue severity being the most commonly examined, apart from the effect of radiotherapy and chemotherapy on CRF, which produced mixed results.
3.4. Perpetuating Factors
Poor exercise and dietary habits are persistent factors that influence cancer-related fatigue in patients. Fatigue has been linked to the physical activity levels of patients in three investigations [35,41,51]. Specifically, patients with higher levels of fatigue are less physically active, which may subsequently lead to body dysmorphic disorder, exacerbating the persistence of fatigue [59]. Fatigue at three and six months within the age group of elderly patients can be predicted by their baseline physical activity level [51]. Two studies have shown that survivors with a better nutritional status have relatively lower levels of fatigue [28,43]. Patients with CRF had significantly lower protein and energy intake than those without CRF. Moreover, recent inadequate protein intake (<1 g/kg body weight) and protein intake (g/kg body weight) were significant contributors to cancer-related fatigue [28].
4. Discussion
This systematic review aimed to identify predictors of cancer-related fatigue and categorize them based on the complex biological and psychological processes behind CRF using the 3P model as a framework (Figure 3). We found that predisposing factors (baseline fatigue, demographic characteristics, clinical characteristics, psychosocial traits, physical symptoms), precipitating factors (radiotherapy and chemotherapy, inflammatory factors, laboratory indicators, and metabolites), and perpetuating factors (physical activity levels, nutritional status) predicted the development of CRF to varying degrees.
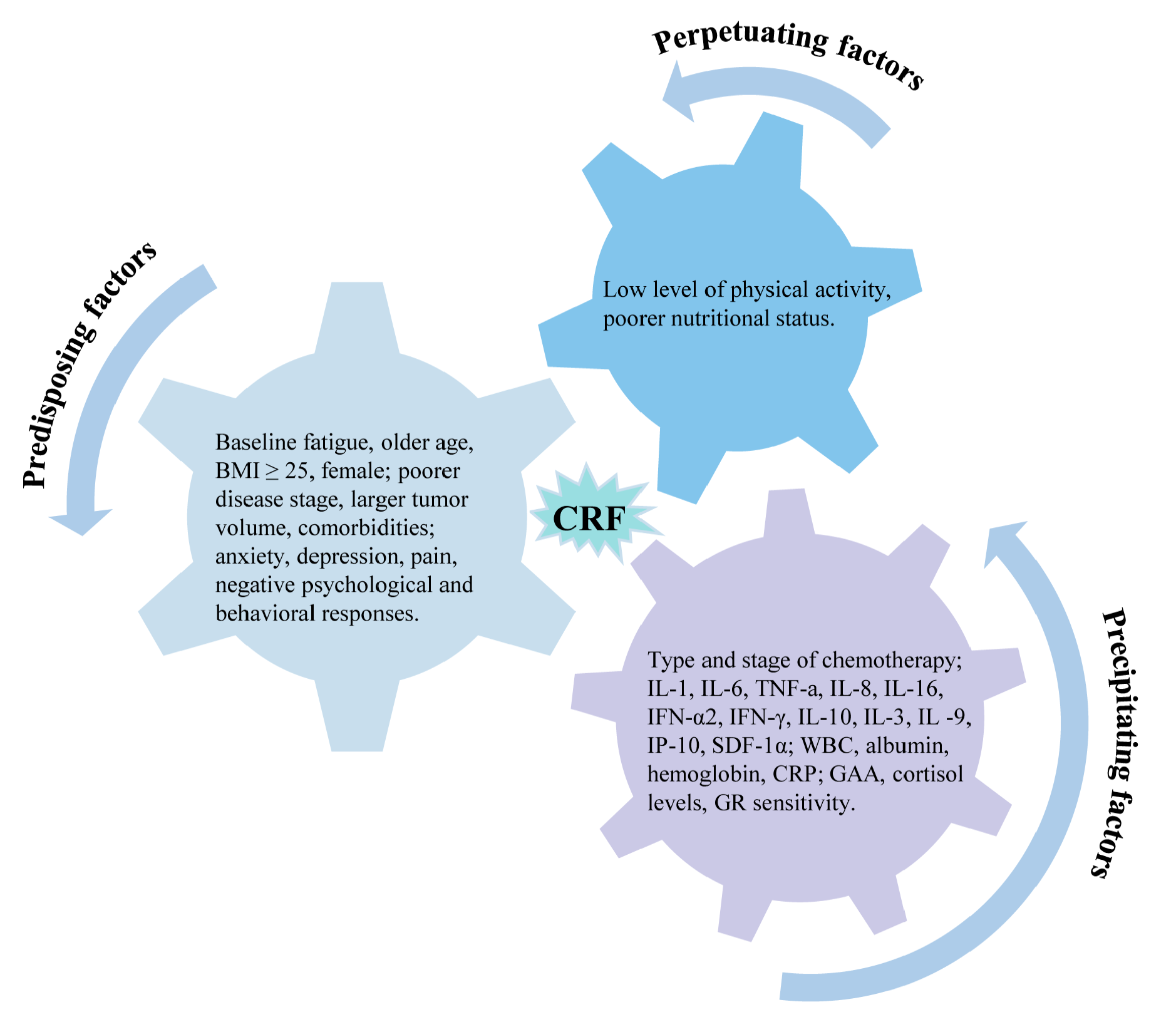
Figure 3. Predictors of cancer-related fatigue based on 3P factor model.
4.1. Predisposing Factors
In this review, predisposing factors primarily pertain to the following factors: (1) baseline fatigue; (2) demographic characteristics such as older age, BMI ≥ 25, and female gender; (3) clinical characteristics such as stage of disease, larger tumor volume, increased upper limb volume, and multiple comorbidities; (4) psychosocial traits such as anxiety, depression, pain, and negative psychological and behavioral responses; (5) physical symptoms including pain, lack of appetite, feeling drowsy, dyspnea, and urinary dysfunction.
We found that the demographic characteristics of high age, BMI ≥25, and female are predictors of CRF in patients, consistent with previous findings [50,54,60,61]. However, one study found lower BMI to be a predictor of general fatigue [44], implying that low BMI represents the poorer nutritional status of the patient. Furthermore, one study found that in the breast cancer population, younger rather than older patients reported more severe fatigue and poor quality of life [62], which may be related to their experience of sudden menopause and more severe psychological fallout [63]. Moreover, literacy level and occupation are also associated with fatigue. In this context, Fleer et al. found that testicular cancer patients with higher levels of education reported more symptoms on the dimensions of general and physical fatigue [54]. Chen et al. found that unemployed participants experienced more mental fatigue than those engaged in occupations [44]; however, it could not be used as a significant predictor of CRF. Knowing that patients with such characteristics will experience severe CRF is beneficial because it allows clinicians to focus on such patient populations in advance and provide preventive measures.
Similarly, disease characteristics with a worse prognosis also imply more severe fatigue. Patients with refractory or recurrent disease and those with more extensive tumor volumes are significantly more likely to experience fatigue [35,46], which may be related to increased tumor burden, compromised body systems, and tumor-associated inflammatory responses. Increased upper extremity volume in breast cancer patients predicts CSF [41], which may also be associated with an inflammatory response, where the edema exudate may contain high levels of proinflammatory cytokines [64]. Furthermore, patients with multiple baseline comorbidities have more severe fatigue [50,51], regardless of age. More severe disease conditions increase the physical burden on patients and imply more severe fatigue.
Apart from fatigue, cancer patients frequently experience various long-standing symptoms, such as pain, depression, and anxiety. These symptoms are correlated and can interact [65,66] and synergistically negatively impact the quality of life for cancer survivors [67]. The four studies in this review showed that pain positively correlated with fatigue severity [30,35,39,56]. Moreover, previous clinical investigations have observed that pain and depression are frequently comorbid [68] and often respond to the same treatments and exacerbate each other [69]. Thus, it is not difficult to understand that depression can predict CRF [27,39], which is consistent with the findings of Vardy et al. [36] and Andrykowski et al. [55], whereby increased depressive symptoms were found to correspond to more fatigue. However, using multiple logistic regression, Hwang et al. determined independent predictors of CSF in 180 oncology patients; they found that the Zung Self-Depression Scale (SDS) score, which represents depression, was not an independent predictor [30]. Moreover, anxiety [40,54], feeling sad and feeling irritable [30,46], fatigue catastrophizing [55], stress passive coping [56], mood disorders [53], cancer-related catastrophes, all-or-nothing behaviors, avoidance behaviors, and perceived punishing responses from others [40] can be significant variables in predicting fatigue. Most of the studies mentioned above were conducted on breast cancer patient populations [36,40,53,55,56]. A recent review reaffirmed that CRF is the most prevalent and distressing symptom in breast cancer survivors after treatment [70]. Moreover, another study highlighted that breast cancer-related fatigue (BCRF) is a multidimensional concept that impacts physical, cognitive, and emotional domains [71]. There is growing evidence that the perception and experience of BCRF are related to multiple psychological factors [70,72]; however, a more comprehensive overview has not been established in this area, which necessitates further research.
In summary, patients with these traits are more likely to have fatigue. For all categories of people, baseline fatigue often predicts continued feelings of fatigue in subsequent periods [27,36,54]. A study found that self-reported fatigue represents a poor prognosis for older patients with hematologic malignancies [73], implying that patients with higher levels of fatigue also have a more significant overall symptom burden. Identifying fatigued individuals early in the disease trajectory is required to reduce post-treatment fatigue, which has significant implications for our understanding of fatigue development and management.
4.2. Precipitating Factors
Precipitating factors are states and features that cause or accelerate the onset of cancer-related fatigue. In this review, the following are classified as predictors of CRF precipitating factors: (1) radiotherapy and chemotherapy and related symptoms, (2) inflammatory factors, (3) laboratory indicators and metabolites.
As shown in the introduction, the mechanism of CRF needs to be clarified, related to finely accelerated aging [74,75] (e.g., premature shortening of telomeres and alterations in DNA methylation), inflammation (overproduction of proinflammatory cytokines), and metabolic dysregulation (alterations in metabolic genes and regulatory pathways) due to radiotherapy [25]. Fatigue is exacerbated in patients during radiotherapy and chemotherapy [8,36,44,45,55,57], and the type [8,45] and course [44] of chemotherapy also affect fatigue to varying degrees. Inflammation may be responsible for this phenomenon as a critical biological pathway leading to CRF [76,77]. Fatigue is strongly associated with proinflammatory cytokines, including IL-1 [47,53], IL-6 [8,57], and TNF-α [47], according to several review studies. The result is consistent with a recent finding that biological pathways such as polymorphisms in inflammatory risk genes, alterations in the HPA axis, and alterations in the cellular immune system regulate the production of proinflammatory factors [77]. Thus, many cytokine receptors are located in the hypothalamus, which is richly connected to the brainstem, frontal cortex, and limbic system. These brain regions further influence mood, behavior, and motor flexibility. In essence, the neuroimmune interaction leads to the disease behavior represented by CRF [78].
Furthermore, immune system-related markers such as white blood cells (WBCs) [41], hemoglobin [58], albumin [35], CRP [48,52], and cortisol [53] can be considered independent predictors of CRF. Although lymphocyte count does not predict overall fatigue, it is significantly related to general and mental fatigue and a risk indicator for predicting mental fatigue [44], consistent with previous findings which found that altered lymphocyte subsets and anemia are associated with fatigue during treatment [76,79,80]. As part of the immune system, WBCs usually increase in number when inflammation or infection occurs, and simultaneously, CRP produced by the liver is released into the bloodstream. Maurer et al. suggested that higher levels of CRP are often associated with CRF [81], consistent with the outcomes of the present review. Hemoglobin levels can directly determine the oxygen-carrying capacity of red blood cells, where low levels can lead to anemia and insufficient oxygen supply, fatigue, and is strongly associated with fatigue [80]. Conversely, albumin level is closely related to the nutritional status of the patient, since protein intake is disrupted by cancer and radiotherapy-induced nausea, vomiting, and loss of appetite, which consequently lead to fatigue [82,83]; studies showed that improved nutritional status can change this situation [84]. As for cortisol-predicting CRF in breast cancer patients [53], the HPA axis system controls cortisol release [85], and its secretion usually follows a diurnal pattern. Previous studies suggested that lower cortisol in the morning indicates HPA dysregulation [86], affecting the activity of the immune system and manifests itself in patients as increased fatigue [87].
Furthermore, the questionnaires and clinical data which comprised the majority of information sources for the studies included in this review, as well as a subset of the patient’s blood samples, were collected to examine for conventional markers such as inflammatory factors. However, one study used emerging metabolomic techniques to analyze serum metabolites in patients with multiple myeloma and found that GAA could predict fatigue [42]. Metabolomics which follows in the footsteps of the “big three” (genomics, transcriptomics, and proteomics) is an emerging histology and is presently utilized extensively in disease diagnosis and personalized therapy [88]. Studies have shown that the magnitude of fatigue is related to metabolic patterns [89], and metabolomics-based CRF analysis can help to elucidate the mechanisms underlying CRF [90].
4.3. Perpetuating Factors
Perpetuating factors are characteristics and behaviors that exacerbate or prolong CRF. Low physical activity levels [35,41,51] and poor nutritional status [28,43] were categorized as the factors in the studies included in this review.
Gerber et al. found that low levels of physical activity in patients with primary breast cancer were correlated with persistent CSF [41], consistent with the findings of another study in this area of 440 older mixed cancer patients [51] which demonstrated that the poorer physical functioning and performance status of the patients was a significant predictor of CRF [35]. Factors related to cancer and treatment may initially contribute to acute fatigue and limit the patient’s daily activities to some extent, and then reduced physical activity may lead to a decline in physical fitness, contributing to the long-term persistence of CRF [35,78]. Further longitudinal studies are required to assess the temporal associations between fatigue, physical inactivity, and susceptibility to fatigue in cancer survivors and examine the existence of a potential causal relationship [91]. Moreover, the nutritional status of the patient has also shown to be associated with fatigue [28,43], consistent with the results of one of the largest fish oil supplementation trials conducted among breast cancer survivors, where supplementation showed more significant improvements in the physical fatigue and vigor dimensions of MFSI. Furthermore, survivors with better nutritional status had a tremendous increase in total serum omega-3 fatty acids from fish oil supplementation, which positively moderated its effects on cancer-related fatigue [43]. A study of colorectal cancer patients also found that laboratory markers of nutritional status were strongly associated with CRF [92]. Existing research indicates that fatigue in cancer populations can be moderated by nutritional status. Furthermore, dietary interventions and improved nutritional status have been linked to numerous health benefits [93]. Therefore, addressing malnutrition and conducting screenings could be an excellent starting point for reducing patient fatigue.
4.4. Limitations and Prospects
This is the first systematic review to categorize cancer-related fatigue predictors in terms of the 3P model, which will facilitate future researchers to comprehend and characterize CRF from the perspective of complex biological and psychological mechanisms behind CRF. However, we could not draw firm conclusions based on the present research due to the prevailing gaps in our knowledge of fatigue and the limitations of the included studies.
We found that the outcomes of the exact predictor were only somewhat consistent across studies; this may be attributable to the heterogeneity of the study populations and the different measurement instruments used. In all the studies included in this systematic review, cancer-related fatigue was measured by utilizing 13 different instruments, with the most commonly used instruments being the FACT-F, MFI-20, and BFI. Unidimensional questionnaires similar to the BFI have fewer questions, which are easier to use. However, the lack of predictors for different dimensions of fatigue has somewhat hindered the exploration of predictors for different dimensions of fatigue using the 3P model; out of the 27 studies included, only 11 defined fatigue, which needed to be more consistent. Most definitions differentiate the presence or absence of fatigue by a specific cut-off value of the questionnaire scores. Agarwal et al. and Raudonis et al. differentiated the severity of fatigue [8,35]. In contrast, Feng et al. compared the value of change in fatigue using the minimum clinically important difference to examine a clinically meaningful difference [37,39]. The lack of uniform definitional criteria to define fatigue may lead to non-comparability and confounding of results. A recent meta-analysis showed that the incidence of cancer-related fatigue measured using the different study instruments varied, which may be associated with inconsistencies in the sensitivity and specificity of the scales [94]. However, the diagnosis and assessment of cancer-related fatigue is currently inconsistent globally, and it is recommended that clinical practitioners establish a benchmark for this evaluation to increase its comparability and dependability.
Furthermore, most of the information obtained from the literature was found in cross-sectional and longitudinal studies without a control group; thus, we could not determine whether the associations between these predictors and fatigue were specific to disease diagnosis and/or treatment. Fatigue is a common symptom in the community, with up to half of the general population reporting fatigue in extensive surveys rather than being specific to cancer patients. Chronic fatigue syndrome (CFS) is a medical condition distinguished by severe and incapacitating fatigue that persists for a minimum of six months and is accompanied by various symptoms like rheumatologic, infectious, and neuropsychiatric [95]. While there may be similarities with the mechanisms underlying CRF, such as inflammation and permanent deconditioning [96], they are not precise. Hence, it could be advantageous to conduct a comparative analysis of CRF results and fatigue in other prolonged conditions using the 3P model as a framework to determine whether significant distinctions exist.
Finally, considering the above limitations, this review has three other areas of improvement. First, our search was limited to four extensively utilized databases, potentially leaving out ongoing unpublished studies and studies from other databases. Second, we should have included works studying the association of genetic polymorphisms with CRF because research involving these aspects already exists [26]; however, we incorporated comprehensive demographic, physical, and psychological indicators to the greatest extent possible. Third, because the approaches and definitions of assessing fatigue were not entirely consistent across studies, we performed only descriptive summaries and did not perform any statistical analyses.
5. Conclusions
This systematic review summarized for the first time the independent predictors of cancer-related fatigue based on the theoretical framework of the 3P model. (1) Predisposing factors—baseline fatigue; demographic characteristics such as older age, BMI ≥ 25, and females; clinical characteristics such as poorer disease stage, larger tumor volume, multiple comorbidities; psychosocial traits such as anxiety, depression, and physical symptoms such as pain and urinary dysfunction. (2) Precipitating factors—type and stage of chemotherapy, IL-1, IL-6, TNF-a, IL-8, IL-16, IFN-α2, IFN-γ, IL-10, IL-3, IL -9, IP-10, and SDF-1α and other inflammatory factors; laboratory markers such as WBC, hemoglobin, albumin, and CRP; metabolic changes such as GAA, cortisol levels, and GR sensitivity. (3) Perpetuating factors—a low level of physical activity and poorer nutritional status. These findings suggest that future management of cancer-related fatigue should focus on the above risk factors, especially the controllable precipitating factors.
Future research is anticipated to incorporate additional emerging technologies, such as genomics and metabolomics, to identify accurate CRF predictors grounded in a unified definition of CRF. This will be complemented by a greater number of large-scale prospective studies that investigate and validate these predictors, thereby establishing a theoretical foundation for clinical practice involving comprehensive and personalized management and treatment of CRF.
References
- Cella, D.; Davis, K.; Breitbart, W.; Curt, G. Fatigue Coalition Cancer-Related Fatigue: Prevalence of Proposed Diagnostic Criteria in a United States Sample of Cancer Survivors. J. Clin. Oncol. 2001, 19, 3385–3391. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Loiselle, S.-E.; Agnihotram, R.V.; Ferri, L.E.; Carli, F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018, 153, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Zhao, F.; Fisch, M.J.; O’Mara, A.M.; Cella, D.; Mendoza, T.R.; Cleeland, C.S. Prevalence and Characteristics of Moderate to Severe Fatigue: A Multicenter Study in Cancer Patients and Survivors. Cancer 2014, 120, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Al Maqbali, M.; Al Sinani, M.; Al Naamani, Z.; Al Badi, K.; Tanash, M.I. Prevalence of Fatigue in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Pain. Symptom Manag. 2021, 61, 167–189.e14. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; Hong, S.; Natarajan, L.; Mills, P.J.; Dimsdale, J.E. The Association between Fatigue and Inflammatory Marker Levels in Cancer Patients: A Quantitative Review. Brain Behav. Immun. 2007, 21, 413–427. [Google Scholar] [CrossRef]
- Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019, 8, 738. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The Central Role of Hypothalamic Inflammation in the Acute Illness Response and Cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Raudonis, B.M.; Kelley, I.H.; Rowe, N.; Ellis, J. A Pilot Study of Proinflammatory Cytokines and Fatigue in Women with Breast Cancer During Chemotherapy. Cancer Nurs. 2017, 40, 323–331. [Google Scholar] [CrossRef]
- Bower, J.E. The Role of Neuro-immune Interactions in Cancer-related Fatigue: Biobehavioral Risk Factors and Mechanisms. Cancer 2019, 125, 353–364. [Google Scholar] [CrossRef]
- Dhruva, A.; Aouizerat, B.E.; Cooper, B.; Paul, S.M.; Dodd, M.; West, C.; Wara, W.; Lee, K.; Dunn, L.B.; Langford, D.J.; et al. Cytokine Gene Associations with Self-Report Ratings of Morning and Evening Fatigue in Oncology Patients and Their Family Caregivers. Biol. Res. Nurs. 2015, 17, 175–184. [Google Scholar] [CrossRef]
- Tian, T.; Qin, W.; Liu, B.; Wang, D.; Wang, J.; Jiang, T.; Yu, C. Catechol-O-Methyltransferase Val158Met Polymorphism Modulates Gray Matter Volume and Functional Connectivity of the Default Mode Network. PLoS ONE 2013, 8, e78697. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.M.; Bruner, D.W.; Mitchell, S.A.; Minasian, L.M.; Basch, E.; Dueck, A.C.; Cella, D.; Reeve, B.B. A Literature Synthesis of Symptom Prevalence and Severity in Persons Receiving Active Cancer Treatment. Support. Care Cancer 2013, 21, 1525–1550. [Google Scholar] [CrossRef]
- Rutherford, C.; Campbell, R.; White, K.; King, M. Patient-Reported Outcomes as Predictors of Survival in Patients with Bowel Cancer: A Systematic Review. Qual. Life Res. 2019, 28, 2871–2887. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mitchell, S.A.; Jacobsen, P.B.; Pirl, W.F. Screening, Evaluation, and Management of Cancer-Related Fatigue: Ready for Implementation to Practice? CA A Cancer J. Clin. 2015, 65, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef]
- Hilarius, D.L.; Kloeg, P.H.; van der Wall, E.; Komen, M.; Gundy, C.M.; Aaronson, N.K. Cancer-Related Fatigue: Clinical Practice versus Practice Guidelines. Support. Care Cancer 2011, 19, 531–538. [Google Scholar] [CrossRef]
- Tolotti, A.; Bonetti, L.; Pedrazzani, C.; Bianchi, M.; Moser, L.; Pagnucci, N.; Sari, D.; Valcarenghi, D. Nursing Management of Fatigue in Cancer Patients and Suggestions for Clinical Practice: A Mixed Methods Study. BMC Nurs. 2021, 20, 182. [Google Scholar] [CrossRef]
- Piper, B.F.; Borneman, T.; Sun, V.C.-Y.; Koczywas, M.; Uman, G.; Ferrell, B.; James, R.L. Cancer-Related Fatigue: Role of Oncology Nurses in Translating National Comprehensive Cancer Network Assessment Guidelines into Practice. Clin. J. Oncol. Nurs. 2008, 12, 37–47. [Google Scholar] [CrossRef]
- Scott, J.A.; Lasch, K.E.; Barsevick, A.M.; Piault-Louis, E. Patients’ Experiences with Cancer-Related Fatigue: A Review and Synthesis of Qualitative Research. Oncol. Nurs. Forum 2011, 38, E191–E203. [Google Scholar] [CrossRef]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A Guide to Machine Learning for Biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Satheeshkumar, P.S.; Pili, R.; Epstein, J.B.; Thazhe, S.B.K.; Sukumar, R.; Mohan, M.P. Characteristics and Predictors Associated with Cancer-Related Fatigue among Solid and Liquid Tumors. J. Cancer Res. Clin. Oncol. 2023, 149, 13875–13888. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Du, J.; Yang, M.; Xu, Q.; Huang, J.; Tan, W.; Xu, T.; Wang, L.; Nie, W.; Zhao, L. Development and External Validation of a Machine Learning-Based Prediction Model for the Cancer-Related Fatigue Diagnostic Screening in Adult Cancer Patients: A Cross-Sectional Study in China. Support. Care Cancer 2023, 31, 106. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kors, J.A.; Ioannou, S.; John, L.H.; Markus, A.F.; Rekkas, A.; de Ridder, M.A.J.; Seinen, T.M.; Williams, R.D.; Rijnbeek, P.R. Trends in the Conduct and Reporting of Clinical Prediction Model Development and Validation: A Systematic Review. J. Am. Med. Inform. Assoc. 2022, 29, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.A.; Navar, A.M.; Pencina, M.J.; Ioannidis, J.P.A. Opportunities and Challenges in Developing Risk Prediction Models with Electronic Health Records Data: A Systematic Review. J. Am. Med. Inform. Assoc. 2017, 24, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Sleight, A.G.; Crowder, S.L.; Skarbinski, J.; Coen, P.; Parker, N.H.; Hoogland, A.I.; Gonzalez, B.D.; Playdon, M.C.; Cole, S.; Ose, J.; et al. A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care. Cancers 2022, 14, 1982. [Google Scholar] [CrossRef]
- Wang, T.; Yin, J.; Miller, A.H.; Xiao, C. A Systematic Review of the Association between Fatigue and Genetic Polymorphisms. Brain Behav. Immun. 2017, 62, 230–244. [Google Scholar] [CrossRef]
- Susanne, K.; Michael, F.; Thomas, S.; Peter, E.; Andreas, H. Predictors of Fatigue in Cancer Patients: A Longitudinal Study. Support. Care Cancer 2019, 27, 3463–3471. [Google Scholar] [CrossRef]
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.-D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chang, V.T.; Rue, M.; Kasimis, B. Multidimensional Independent Predictors of Cancer-Related Fatigue. J. Pain Symptom Manag. 2003, 26, 604–614. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; McInerney, P.; Soares, C.B.; Khalil, H.; Parker, D. Methodology for Jbi Scoping Reviews. In The Joanna Briggs Institute Reviewers Manual 2015; Joanna Briggs Institute: Adelaide, Australia, 2015; pp. 3–24. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.R.; Juneau, P.; Regan, J.M.; Liwang, J.; Alshawi, S.; Wang, A.; Saligan, L.N. Brain-Derived Neurotrophic Factor Polymorphism Val66Met Protects against Cancer-Related Fatigue. Transl. Psychiatry 2020, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Agarwal, S.; Minhas, V.; Bhatnagar, S.; Mishra, S.; Kumar, V.; Bharati, S.; Gupta, N.; Khan, M. To Assess the Prevalence and Predictors of Cancer-Related Fatigue and Its Impact on Quality of Life in Advanced Cancer Patients Receiving Palliative Care in a Tertiary Care Hospital: A Cross-Sectional Descriptive Study. Indian. J. Palliat. Care 2020, 26, 523. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Renton, C.; Dodd, A.; Zhang, H.; Clarke, S.J.; Tannock, I.F. Fatigue in People with Localized Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Longitudinal Prospective Study. Ann. Oncol. 2016, 27, 1761–1767. [Google Scholar] [CrossRef]
- Feng, L.R.; Wolff, B.S.; Lukkahatai, N.; Espina, A.; Saligan, L.N. Exploratory Investigation of Early Biomarkers for Chronic Fatigue in Prostate Cancer Patients Following Radiation Therapy. Cancer Nurs. 2017, 40, 184–193. [Google Scholar] [CrossRef]
- Feng, L.R.; Fernández-Martínez, J.L.; Zaal, K.J.M.; deAndrés-Galiana, E.J.; Wolff, B.S.; Saligan, L.N. mGluR5 Mediates Post-Radiotherapy Fatigue Development in Cancer Patients. Transl. Psychiatry 2018, 8, 110. [Google Scholar] [CrossRef]
- Feng, L.R.; Fuss, T.; Dickinson, K.; Ross, A.; Saligan, L.N. Co-Occurring Symptoms Contribute to Persistent Fatigue in Prostate Cancer. Oncology 2019, 96, 183–191. [Google Scholar] [CrossRef]
- Hughes, A.; Suleman, S.; Rimes, K.A.; Marsden, J.; Chalder, T. Cancer-Related Fatigue and Functional Impairment–Towards an Understanding of Cognitive and Behavioural Factors. J. Psychosom. Res. 2020, 134, 110127. [Google Scholar] [CrossRef]
- Gerber, L.H.; Stout, N.; McGarvey, C.; Soballe, P.; Shieh, C.; Diao, G.; Springer, B.A.; Pfalzer, L.A. Factors Predicting Clinically Significant Fatigue in Women Following Treatment for Primary Breast Cancer. Support. Care Cancer 2011, 19, 1581–1591. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, H.; Xiao, H.; Zhang, Z.; Huang, C.; Huang, M. Serum Metabolomics Reveals the Effects of Accompanying Treatment on Fatigue in Patients with Multiple Myeloma. Support. Care Cancer 2023, 31, 43. [Google Scholar] [CrossRef]
- Kleckner, A.S.; Culakova, E.; Kleckner, I.R.; Belcher, E.K.; Demark-Wahnefried, W.; Parker, E.A.; Padula, G.D.A.; Ontko, M.; Janelsins, M.C.; Mustian, K.M.; et al. Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study. Nutrients 2021, 14, 184. [Google Scholar] [CrossRef]
- Chen, L.-M.; Yang, Q.-L.; Duan, Y.-Y.; Huan, X.-Z.; He, Y.; Wang, C.; Fan, Y.-Y.; Cai, Y.-C.; Li, J.-M.; Chen, L.-P.; et al. Multidimensional Fatigue in Patients with Nasopharyngeal Carcinoma Receiving Concurrent Chemoradiotherapy: Incidence, Severity, and Risk Factors. Support. Care Cancer 2021, 29, 5009–5019. [Google Scholar] [CrossRef]
- Xiao, C.; Eldridge, R.C.; Beitler, J.J.; Higgins, K.A.; Chico, C.E.; Felger, J.C.; Wommack, E.C.; Knobf, T.; Saba, N.F.; Shin, D.M.; et al. Association Among Glucocorticoid Receptor Sensitivity, Fatigue, and Inflammation in Patients with Head and Neck Cancer. Psychosom. Med. 2020, 82, 508–516. [Google Scholar] [CrossRef]
- Zordan, R.; Manitta, V.; Nandurkar, H.; Cole-Sinclair, M.; Philip, J.; Saligan, L.N.; Fernández-Martínez, J.L.; deAndrés-Galiana, E.J.; Sonis, S. Prevalence and Predictors of Fatigue in Haemo-Oncological Patients. Cancer Inform. 2014, 44, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, D.; Peng, Y.; Yang, Y.; Zhuang, X.; Li, Z.; Wang, M.; Chen, L.; Zhang, H. Cancer-Related Fatigue and Chemotherapy-Associated Adverse Effects: Correlation with TNF-α, IL-1 and 17-Hydroxycorticosteroids. Future Oncol. 2014, 10, 1619–1626. [Google Scholar] [CrossRef]
- Pertl, M.M.; Hevey, D.; Boyle, N.T.; Hughes, M.M.; Collier, S.; O’Dwyer, A.M.; Harkin, A.; Kennedy, M.J.; Connor, T.J. C-Reactive Protein Predicts Fatigue Independently of Depression in Breast Cancer Patients Prior to Chemotherapy. Brain Behav. Immun. 2013, 34, 108–119. [Google Scholar] [CrossRef]
- Goldstein, D.; Bennett, B.K.; Webber, K.; Boyle, F.; de Souza, P.L.; Wilcken, N.R.C.; Scott, E.M.; Toppler, R.; Murie, P.; O’Malley, L.; et al. Cancer-Related Fatigue in Women with Breast Cancer: Outcomes of a 5-Year Prospective Cohort Study. J. Clin. Oncol. 2012, 30, 1805–1812. [Google Scholar] [CrossRef]
- Hoffman, A.J.; von Eye, A.; Gift, A.G.; Given, B.A.; Given, C.W.; Rothert, M. Testing a Theoretical Model of Perceived Self-Efficacy for Cancer-Related Fatigue Self-Management and Optimal Physical Functional Status. Nurs. Res. 2009, 58, 32–41. [Google Scholar] [CrossRef]
- Luctkar-Flude, M.; Groll, D.; Woodend, K.; Tranmer, J. Fatigue and Physical Activity in Older Patients with Cancer: A Six-Month Follow-Up Study. Oncol. Nurs. Forum 2009, 36, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Booker, R.; Olson, K.; Pilarski, L.M.; Noon, J.P.; Bahlis, N.J. The Relationships Among Physiologic Variables, Quality of Life, and Fatigue in Patients with Multiple Myeloma. Oncol. Nurs. Forum 2009, 36, 209–216. [Google Scholar] [CrossRef]
- Von Ah, D.M.; Kang, D.-H.; Carpenter, J.S. Predictors of Cancer-Related Fatigue in Women with Breast Cancer Before, during, and after Adjuvant Therapy. Cancer Nurs. 2008, 31, 134–144. [Google Scholar] [CrossRef]
- Fleer, J.; Sleijfer, D.T.; Hoekstra, H.J.; Tuinman, M.A. Prevalence, Changes in and Correlates of Fatigue in the First Year after Diagnosis of Testicular Cancer. Anticancer Res. 2005, 25, 4647–4653. [Google Scholar]
- Andrykowski, M.A.; Schmidt, J.E.; Salsman, J.M.; Beacham, A.O.; Jacobsen, P.B.; Dupont, A.; Bower, J.E.; Stanton, A.L.; Ganz, P.A. Use of a Case Definition Approach to Identify Cancer-Related Fatigue in Women Undergoing Adjuvant Therapy for Breast Cancer. J. Clin. Oncol. 2005, 23, 6613–6622. [Google Scholar] [CrossRef]
- Gélinas, C.; Fillion, L. Factors Related to Persistent Fatigue Following Completion of Breast Cancer Treatment. Oncol. Nurs. Forum 2004, 31, 269–278. [Google Scholar] [CrossRef]
- Ahlberg, K.; Ekman, T.; Gaston-Johansson, F. Levels of Fatigue Compared to Levels of Cytokines and Hemoglobin during Pelvic Radiotherapy: A Pilot Study. Biol. Res. Nurs. 2004, 5, 203–210. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.; Chang, C.-H.; Peterman, A.; Slavin, M. Fatigue in Cancer Patients Compared with Fatigue in the General United States Population. Cancer 2002, 94, 528–538. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Bennett, J.A.; Nail, L.; Schwartz, A. Strength, Physical Activity, and Age Predict Fatigue in Older Breast Cancer Survivors. Oncol. Nurs. Forum 2008, 35, 815–821. [Google Scholar] [CrossRef]
- Álvarez-Bustos, A.; de Pedro, C.G.; Romero-Elías, M.; Ramos, J.; Osorio, P.; Cantos, B.; Maximiano, C.; Méndez, M.; Fiuza-Luces, C.; Méndez-Otero, M.; et al. Prevalence and Correlates of Cancer-Related Fatigue in Breast Cancer Survivors. Support. Care Cancer 2021, 29, 6523–6534. [Google Scholar] [CrossRef] [PubMed]
- van Baar, H.; Bours, M.J.L.; Beijer, S.; van Zutphen, M.; van Duijnhoven, F.J.B.; Kok, D.E.; Wesselink, E.; de Wilt, J.H.W.; Kampman, E.; Winkels, R.M. Body Composition and Its Association with Fatigue in the First 2 Years after Colorectal Cancer Diagnosis. J. Cancer Surviv. 2021, 15, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Champion, V.L.; Wagner, L.I.; Monahan, P.O.; Daggy, J.; Smith, L.; Cohee, A.; Ziner, K.W.; Haase, J.E.; Miller, K.; Pradhan, K.; et al. Comparison of Younger and Older Breast Cancer Survivors and Age-Matched Controls on Specific and Overall QoL Domains. Cancer 2014, 120, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Carreira, H.; Williams, R.; Dempsey, H.; Stanway, S.; Smeeth, L.; Bhaskaran, K. Quality of Life and Mental Health in Breast Cancer Survivors Compared with Non-Cancer Controls: A Study of Patient-Reported Outcomes in the United Kingdom. J. Cancer Surviv. 2021, 15, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Yeo, W.; Mo, K.F.; Tse, K.Y.; Lee, Y.M.; Tse, S.M.; Ho, F.P.; Kwan, W.H. A Case Control Study on Risk Factors of Lymphedema after Axillary Lymph Node Dissection for Breast Cancer in Hong Kong. J. Clin. Oncol. 2007, 25, 9048. [Google Scholar] [CrossRef]
- Harris, C.S.; Kober, K.M.; Cooper, B.; Conley, Y.P.; Dhruva, A.A.; Hammer, M.J.; Paul, S.; Levine, J.D.; Miaskowski, C.A. Symptom Clusters in Outpatients with Cancer Using Different Dimensions of the Symptom Experience. Support. Care Cancer 2022, 30, 6889–6899. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, C.; Dodd, M.; Lee, K. Symptom Clusters: The New Frontier in Symptom Management Research. J. Natl. Cancer Inst. Monogr. 2004, 2004, 17–21. [Google Scholar] [CrossRef]
- Kroenke, K.; Theobald, D.; Wu, J.; Loza, J.K.; Carpenter, J.S.; Tu, W. The Association of Depression and Pain with Health-Related Quality of Life, Disability, and Health Care Use in Cancer Patients. J. Pain Symptom Manag. 2010, 40, 327–341. [Google Scholar] [CrossRef]
- Reyes, C.C.; Anderson, K.O.; Gonzalez, C.E.; Ochs, H.C.; Wattana, M.; Acharya, G.; Todd, K.H. Depression and Survival Outcomes after Emergency Department Cancer Pain Visits. BMJ Support. Palliat. Care 2019, 9, e36. [Google Scholar] [CrossRef]
- Li, J.-X. Pain and Depression Comorbidity: A Preclinical Perspective. Behav. Brain Res. 2015, 276, 92–98. [Google Scholar] [CrossRef]
- Ruiz-Casado, A.; Álvarez-Bustos, A.; de Pedro, C.G.; Méndez-Otero, M.; Romero-Elías, M. Cancer-Related Fatigue in Breast Cancer Survivors: A Review. Clin. Breast Cancer 2021, 21, 10–25. [Google Scholar] [CrossRef]
- Fox, R.S.; Ancoli-Israel, S.; Roesch, S.C.; Merz, E.L.; Mills, S.D.; Wells, K.J.; Sadler, G.R.; Malcarne, V.L. Sleep Disturbance and Cancer-Related Fatigue Symptom Cluster in Breast Cancer Patients Undergoing Chemotherapy. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 845–855. [Google Scholar] [CrossRef]
- Wang, S.-H.; He, G.-P.; Jiang, P.-L.; Tang, L.-L.; Feng, X.-M.; Zeng, C.; Wang, G.-F. Relationship between Cancer-Related Fatigue and Personality in Patients with Breast Cancer after Chemotherapy. Psychooncology 2013, 22, 2386–2390. [Google Scholar] [CrossRef] [PubMed]
- Hofer, F.; Koinig, K.A.; Nagl, L.; Borjan, B.; Stauder, R. Fatigue at Baseline Is Associated with Geriatric Impairments and Represents an Adverse Prognostic Factor in Older Patients with a Hematological Malignancy. Ann. Hematol. 2018, 97, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Port, J.D.; Kaufman, K.R.; Jatoi, A.; Hart, C.R.; Gries, K.J.; Lanza, I.R.; Kumar, R. Skeletal Muscle Mitochondrial Dysfunction and Muscle and Whole Body Functional Deficits in Cancer Patients with Weight Loss. J. Appl. Physiol. 2022, 132, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Sanoff, H.K.; Deal, A.M.; Krishnamurthy, J.; Torrice, C.; Dillon, P.; Sorrentino, J.; Ibrahim, J.G.; Jolly, T.A.; Williams, G.; Carey, L.A.; et al. Effect of Cytotoxic Chemotherapy on Markers of Molecular Age in Patients with Breast Cancer. J. Natl. Cancer Inst. 2014, 106, dju057. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-Related Fatigue--Mechanisms, Risk Factors, and Treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Lamkin, D.M. Inflammation and Cancer-Related Fatigue: Mechanisms, Contributing Factors, and Treatment Implications. Brain Behav. Immun. 2013, 30, S48–S57. [Google Scholar] [CrossRef]
- Brownstein, C.G.; Twomey, R.; Temesi, J.; Wrightson, J.G.; Martin, T.; Medysky, M.E.; Culos-Reed, S.N.; Millet, G.Y. Physiological and Psychosocial Correlates of Cancer-Related Fatigue. J. Cancer Surviv. 2022, 16, 1339–1354. [Google Scholar] [CrossRef]
- Koornstra, R.H.T.; Peters, M.; Donofrio, S.; van den Borne, B.; de Jong, F.A. Management of Fatigue in Patients with Cancer—A Practical Overview. Cancer Treat. Rev. 2014, 40, 791–799. [Google Scholar] [CrossRef]
- Aapro, M.; Scotte, F.; Bouillet, T.; Currow, D.; Vigano, A. A Practical Approach to Fatigue Management in Colorectal Cancer. Clin. Colorectal Cancer 2017, 16, 275–285. [Google Scholar] [CrossRef]
- Maurer, T.; Jaskulski, S.; Behrens, S.; Jung, A.Y.; Obi, N.; Johnson, T.; Becher, H.; Chang-Claude, J. Tired of Feeling Tired–The Role of Circulating Inflammatory Biomarkers and Long-Term Cancer Related Fatigue in Breast Cancer Survivors. Breast 2021, 56, 103–109. [Google Scholar] [CrossRef]
- Yennu, S.; Urbauer, D.L.; Bruera, E. Factors Associated with the Severity and Improvement of Fatigue in Patients with Advanced Cancer Presenting to an Outpatient Palliative Care Clinic. BMC Palliat. Care 2012, 11, 16. [Google Scholar] [CrossRef]
- Wang, X.S.; Giralt, S.A.; Mendoza, T.R.; Engstrom, M.C.; Johnson, B.A.; Peterson, N.; Broemeling, L.D.; Cleeland, C.S. Clinical Factors Associated with Cancer-Related Fatigue in Patients Being Treated for Leukemia and Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 1319–1328. [Google Scholar] [CrossRef]
- Ghoshal, A.; Damani, A.; Muckaden, M. Association of Cancer-Related Fatigue with Other Symptoms and Impact on Quality of Life of Palliative Care Patients in a Tertiary Cancer Institute: A Prospective Observational Study (S751). J. Pain Symptom Manag. 2016, 51, 435. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Semik, J.; Habermann, N.; Wiskemann, J.; Ulrich, C.M.; Steindorf, K. Cancer-Related Fatigue Shows a Stable Association with Diurnal Cortisol Dysregulation in Breast Cancer Patients. Brain Behav. Immun. 2016, 52, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Luecken, L.J.; Dausch, B.; Gulla, V.; Hong, R.; Compas, B.E. Alterations in Morning Cortisol Associated with PTSD in Women with Breast Cancer. J. Psychosom. Res. 2004, 56, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.K. Altered Circadian Rhythms and Cancer-Related Fatigue Outcomes. Integr. Cancer Ther. 2011, 10, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in Cancer Research and Emerging Applications in Clinical Oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic Features of Chronic Fatigue Syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E3749. [Google Scholar] [CrossRef]
- Surowiec, I.; Gjesdal, C.G.; Jonsson, G.; Norheim, K.B.; Lundstedt, T.; Trygg, J.; Omdal, R. Metabolomics Study of Fatigue in Patients with Rheumatoid Arthritis Naïve to Biological Treatment. Rheumatol. Int. 2016, 36, 703–711. [Google Scholar] [CrossRef]
- Twomey, R.; Martin, T.; Temesi, J.; Culos-Reed, S.N.; Millet, G.Y. Tailored Exercise Interventions to Reduce Fatigue in Cancer Survivors: Study Protocol of a Randomized Controlled Trial. BMC Cancer 2018, 18, 757. [Google Scholar] [CrossRef]
- Sharour, L.A. Cancer-Related Fatigue, Laboratory Markers as Indicators for Nutritional Status among Patients with Colorectal Cancer. Nutr. Cancer 2020, 72, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Skinner, T.L.; Wright, O.R.L. Nutrition Therapy for the Management of Cancer-Related Fatigue and Quality of Life: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2019, 122, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, B.; Jiang, M.; Yang, Y.; Wang, C.; Huang, C.; Han, L. Prevalence and Risk Factors of Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2020, 111, 103707. [Google Scholar] [CrossRef] [PubMed]
- Afari, N.; Buchwald, D. Chronic Fatigue Syndrome: A Review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef]
- Servaes, P.; Prins, J.; Verhagen, S.; Bleijenberg, G. Fatigue after Breast Cancer and in Chronic Fatigue Syndrome: Similarities and Differences. J. Psychosom. Res. 2002, 52, 453–459. [Google Scholar] [CrossRef]

