1. Introduction
Psychological interventions, including cognitive behavioural therapy (CBT), mindfulness-based cognitive therapy, cognitive remediation, etc., aim to promote one’s ability to adapt to a given situation, leading to improved functioning [1]. Despite the increasing clinical use of psychological interventions, their efficacy can be limited. A systematic review of 419 randomized controlled trials (RCTs) from clinical and non-clinical populations found that psychological interventions produced only small-to-moderate effect sizes on mental well-being and/or indicators of illness [2]. Efficacy is often also lower in patients with more severe symptoms and in those who have failed other therapies [3]. The long duration (e.g., 3–12 months) required for a psychological intervention to take effect [4] is a further consideration. For these reasons, the combination of psychological therapies with biological therapies (e.g., pharmacotherapy) is often recommended in clinical practice guidelines [5,6]. While the efficacy of the combination of different psychological interventions with pharmacotherapies has been commonly studied [7,8], combining psychological therapies with non-invasive brain stimulation (NIBS) treatments is a relatively new area of research interest with the potential to improve therapeutic outcomes.
Transcranial magnetic stimulation (TMS) is a form of NIBS that has been extensively utilized for research and clinical applications [9,10]. TMS exerts its effects by delivering magnetic pulses that go through the scalp and skull, which induce electrical currents that can depolarize neurons in the brain [11,12]. Repetitive transcranial magnetic stimulation (rTMS), which delivers multiple repeated magnetic pulses, causes long-lasting effects on cortical excitability beyond the stimulation period [13]. RTMS can also improve cognitive functions in various domains in both healthy [14] and clinical populations [15,16,17]. RTMS parameters can be adjusted to have different effects: high frequency (≥5 Hz) rTMS and intermittent theta-burst stimulation (iTBS) facilitate neural activity, while low frequency (≤1 Hz) rTMS and continuous theta-burst stimulation (cTBS) inhibit it [9,18]. Theta burst stimulation (TBS), a newer form of rTMS, involves delivering patterned stimulation to the brain at specific frequencies (typically at a theta frequency with gamma frequency “bursts”) and has a shorter treatment duration than standard rTMS [19]. Evidence from several meta-analyses has shown that compared to sham stimulation, active rTMS can alleviate symptoms of depression [20] and improve clinical outcomes in other neuropsychiatric illnesses [21,22,23,24,25,26]. Research has shown that, typically, at least 20–30 rTMS sessions administered over consecutive weekdays are necessary for optimal clinical effects [27].
In vivo and in vitro studies have shown that rTMS can affect neurotransmission, influencing adaptive synaptic plasticity and producing long-lasting effects [24]. It can also prolong the time window for the neural interactions subserving behavioural adaptation [28]. Psychological interventions such as CBT and cognitive training (CT) can also facilitate synaptic plasticity [29]. Based on the above, it is possible that rTMS could potentially enhance the effectiveness of psychological interventions by maximizing plasticity. Neuroplasticity is considered to be a critical treatment target for clinical improvement and enhancing cognitive and functional abilities in various neuropsychiatric diseases [30,31]. Existing studies that have paired rTMS with psychological interventions, including exposure therapies [32], CT [33], and CBT [34] have provided preliminary evidence supporting the augmentation effect of rTMS on clinical outcomes across a broad range of neuropsychiatric disorders. While meta-analyses of RCTs of rTMS alone or psychological interventions alone for individual disorders are available, there is currently no systematic examination specifically focusing on the use of rTMS to augment different types of psychological interventions.
In the current study, we aimed to investigate the potential augmentation effects of rTMS on psychological interventions in healthy and clinical populations for different outcomes (i.e., clinical, functional, and cognitive outcomes) to identify common trends and patterns. A secondary aim was to explore which type of psychological interventions may exhibit greater augmentation effects when combined with rTMS. To address these aims, we conducted a systematic review and meta-analysis of RCTs of active rTMS combined with psychological interventions compared to sham rTMS combined with psychological interventions.
2. Materials and Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered on the International Prospective Register of Systematic Reviews, PROSPERO [35]. The PRISMA checklist for this systematic review and meta-analysis is provided in the [36].
2.1. Search Strategy and Study Selection
Keywords (‘repetitive transcranial magnetic stimulation’ OR ’rTMS’, AND ’psychological interventions’ OR ’psychotherapies’, AND ‘randomized controlled trial’ OR ‘RCT’) were searched in four electronic databases including PubMed, EMBASE (Ovid), Cochrane Library, and PsycINFO (Ovid) up to 22 April 2023. The search strategies are shown in . Studies were included if they fulfilled the following criteria: (1) healthy or clinical populations ≥ 18 years; (2) the comparison of rTMS combined with psychological interventions (e.g., CBT, exposure therapy, and CT) to sham rTMS combined with psychological interventions; (3) reporting pre-intervention and post-intervention assessment of clinical outcomes (i.e., symptom severity, functioning, and quality of life) or cognitive outcomes; (4) RCTs published in peer-reviewed English journals. After removing duplicates in the original search results, the titles and abstracts were screened independently against the selection criteria by two authors. The full texts were then reviewed and eligible studies were included in the meta-analysis. Any disagreements were resolved by discussion until a consensus was reached or by consulting a third author.
2.2. Data Extraction and Outcome Measures
All data were extracted by one author and checked by another author. We also contacted the study authors to request data related to the meta-analysis that was not accessible from the original publications. The following data were extracted: author, year of publication, characteristics of the study population (health condition, sample sizes, age, and gender), study design, rTMS parameters (the number of sessions, stimulation sites, frequency, intensity, total pulses, and timing of stimulation), type of psychological interventions, and pre-intervention and post-intervention outcomes. The outcome measures of interest assessed clinical symptoms and cognitive functions, the former including disease symptom severity and functional outcomes measured by standardised questionnaires, and the latter from standardised cognitive tasks or questionnaires . The means, standard deviations (SD), and sample sizes for outcome measures in each group were extracted for the pooled analysis. We also used a Web-based program called WebPlotDigitizer (WebPlotDigitizer, Austin, TX, USA, A. Rohatgi, 2018) to estimate data from figures. If the standard error (SE) was reported, we calculated SD using the equation SD = SE × √N.
2.3. Statistical Analysis
Statistical analysis was conducted using R version 4.2.1 (R Core Team, 2022 [37]), RStudio software version 2022.12.0.353 Posit Team, 2022 [38]) and ‘meta’, ‘metafor’ packages. The effect of active or sham rTMS combined with psychological interventions was examined using standardized mean differences (SMD) with 95% confidence intervals (CIs) for each outcome measure as part of a random-effects model. Meta-analyses were conducted when outcomes were available from at least three studies. There were three outcome measures of interest: clinical symptoms, functional outcomes, and cognition. For outcomes where higher scores were associated with poorer performance or more severe disease functional outcomes, these scores were recoded to represent positive effect sizes in favour of the active condition. Cognitive tasks and questionnaires were categorized according to six cognitive domains as defined in the DSM-5, including perceptual-motor function, language, learning and memory, complex attention, social cognition, and executive function [39]. Executive function included tasks which involved updating, shifting, or inhibition [40]. Furthermore, we included two additional cognitive domains: global cognition and working memory. Global cognition was added because a global cognitive score rather than scores within cognitive domains was reported in some of the studies which were included. Similarly, working memory was analysed because it was a commonly assessed cognitive outcome. If multiple cognitive outcomes from the same cognitive task or questionnaire were reported in an individual study, the most commonly used outcome measure as defined by the authors was selected [41] . If the primary outcome measure was not specified for a particular task, we included the most relevant measure based on our predefined cognitive domains. When studies assessed multiple cognitive tasks within the same domain, outcomes from different tasks were averaged to generate domain-specific aggregate effect sizes [14]. Where multiple questionnaires measured the symptom severity for the same disease in each study, the primary outcome measure as defined by the original authors was extracted and analysed [41]. Heterogeneity between studies was assessed using the I2 test. I2 values > 25%, 50%, and 75% indicate low, moderate, and high heterogeneity, respectively [42]. Secondary subgroup analyses were conducted according to different types of psychological interventions (i.e., rTMS + CBT, rTMS + CT, rTMS + exposure therapy, and rTMS + Mindfulness-Based Stress Reduction (MBSR)), study populations (healthy and clinical populations), and cognitive domains where sufficient data was available for meta-analyses. Given that patients exhibit faster improvement or recovery when subjected to a higher number of psychotherapy sessions [43], we additionally performed subgroup analyses on studies involving 10 or more sessions of combined interventions to examine the effects on clinical symptoms, functional outcomes, and cognition. The revised Cochrane risk of bias assessment tool for RCTs (Risk of Bias tool, RoB 2) was used to independently assess the quality of included studies by two authors. All discrepancies were resolved by consulting a third author. The tool was used to assess bias across five domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result [44]. Each study was judged as having a low risk of bias, some concerns, or a high risk of bias. Publication bias was assessed using funnel plots and Egger’s test [45] for the outcomes in which 10 or more available studies were included.
3. Results
3.1. Overview
A total of 3969 articles were identified initially, and 27 studies were ultimately included in the meta-analysis (see Figure 1). The characteristics of the included studies are summarized in .
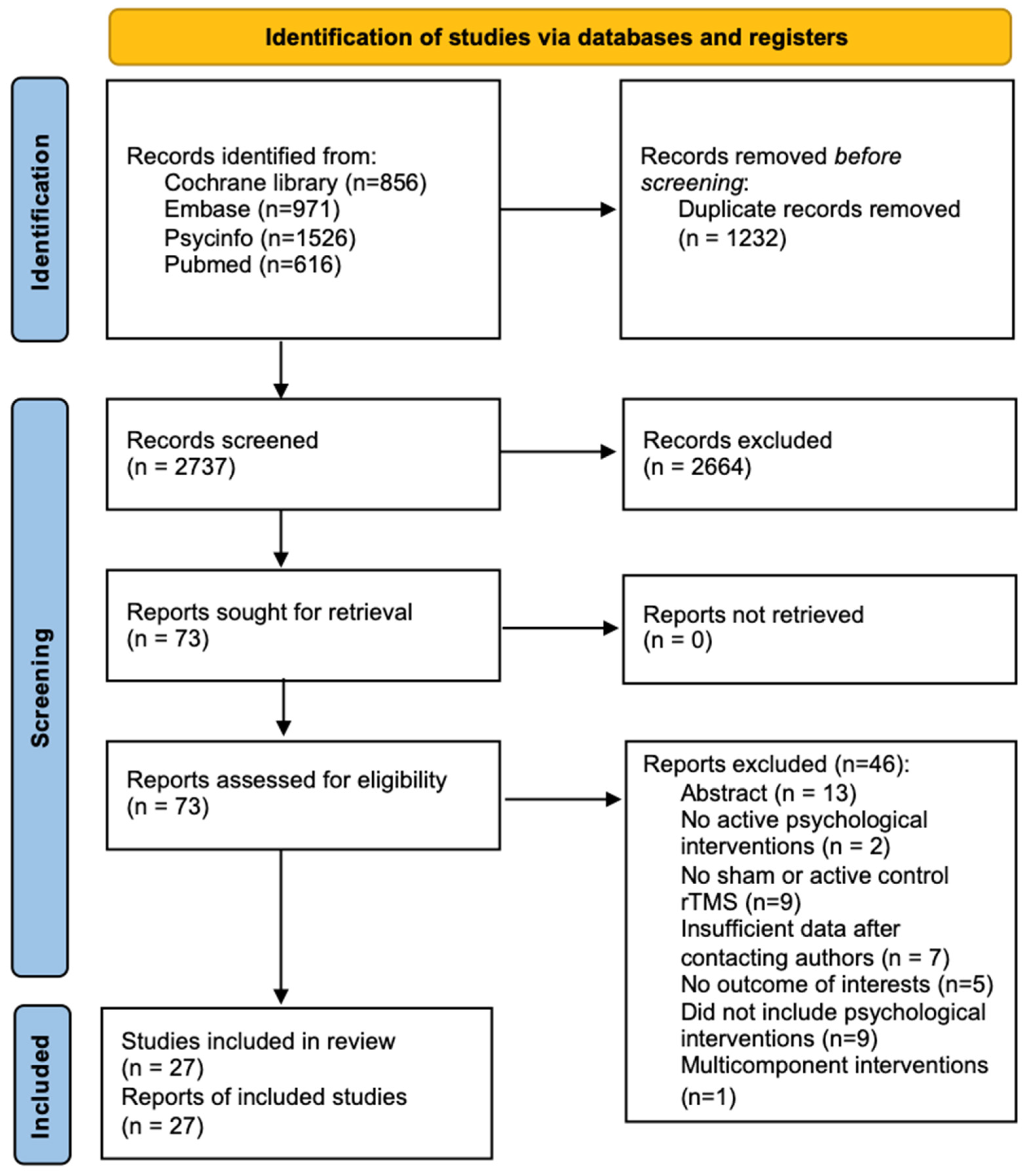
Figure 1. Flowchart of study searching and selection.
All studies utilized a between-subject design, other than studies from Gy et al. [46] and Osuch et al. [47], which used a within-subject design. In the study conducted by Gy et al. [46], participants were randomized to receive 30 sessions of active or sham rTMS + cognitive stimulation in the first phase and then crossed over to receive the opposite type of rTMS with the same cognitive stimulation in the second phase after a 4-week washout period; we only included the pre- and post-data of the first phase prior to crossover. Osuch et al. [47] reported change scores between baseline and endpoint for each condition, which were then used to calculate the SMD. In studies with multiple treatment arms [48,49,50,51,52,53,54,55,56], only the active/sham rTMS + psychological intervention arms were analysed. Of note, 4 studies had two active arms [50,52,57,58]. In these cases, the sample size of the control group was halved to avoid calculating the same control group twice in the meta-analysis [59]. In summary, 31 treatment arms were included. There were 72 healthy participants and 1060 participants from a clinical population included in the meta-analysis. Clinical populations included patients with low cognitive restructuring ability (n = 46), post-traumatic stress disorder (PTSD) (n = 180), anxiety disorder (n = 83), smokers (n = 156), alcohol-dependent patients (n = 119), Alzheimer’s disease (AD) (n = 167), obsessive compulsive disorder (OCD) (n = 30), attention-deficit/hyperactivity disorder (ADHD) (n = 62), cognitive impairment (n = 88), attention dysfunction (n = 58), post-stroke depression (n = 47), and major depressive disorder (MDD) (n = 24). For healthy participants, data were only available to assess the effect on clinical symptoms and cognition in two RCTs. Four treatment arms involved low-frequency (1 Hz) rTMS, three involved iTBS, and twenty-four involved high-frequency (≥5 Hz) rTMS. Most of the studies (77.8%) stimulated one brain region (especially the dorsolateral prefrontal cortex (DLPFC): 59.3% of total studies), and only six studies stimulated multiple sites. In the included studies, only one utilized a single-session intervention, while the majority employed multiple sessions. Notably, 85.2% of the studies involved 10 or more sessions.
3.2. Clinical Outcomes in Healthy and Clinical Populations
Sixteen studies (19 treatment arms) reported the effect of rTMS combined with psychological interventions on clinical outcomes involving 751 participants: five studies used rTMS + CBT [52,60,61,62,63], three studies used rTMS + CT [55,57,70], one study used rTMS + mindfulness-based stress reduction [51], and seven studies used rTMS + exposure therapy [47,48,50,53,71,72,73]. Of note, data for healthy participants were only available to assess the effect on clinical outcomes in one study using rTMS + CBT [62]. Across the entire sample, significantly greater improvements in clinical symptoms were found after active rTMS + psychological interventions relative to sham rTMS + psychological interventions (SMD = 0.31, CIs 0.08 to 0.54, Z = 2.66, p < 0.01). A subgroup analysis in clinical populations yielded significance indicating greater improvements in clinical outcomes following rTMS + psychological interventions (SMD = 0.32, CIs 0.07 to 0.56, Z = 2.54, p = 0.01, I2 = 94%). Subgroup analyses according to different types of psychological interventions were further explored. The results showed that neither rTMS + CBT (SMD = 0.14, CIs −0.03 to 0.32, Z = 1.58, p = 0.11), rTMS + CT (SMD = 0.59, CIs −0.15 to 1.32, Z = 1.55, p = 0.12), nor rTMS + exposure therapy (SMD = 0.35, CIs −0.06 to 0.75, Z = 1.66, p = 0.10) had a statistically significant greater benefit for improving clinical outcomes (Figure 2). Moreover, data were only available to do subgroup analyses of rTMS + exposure therapy on clinical symptoms in patients with PTSD and smokers. Results showed no significant difference between active rTMS + exposure therapy and sham rTMS + exposure therapy on clinical symptoms in patients with PTSD or smokers . Subgroup analysis in studies with 10 or more sessions of combined interventions yielded a significant improvement in clinical symptoms following rTMS + psychological interventions (SMD = 0.34, CIs 0.07 to 0.61, Z = 2.49, p = 0.01, I2 = 94%). Of note, the results showed that the augmentation effects of rTMS became significant for CBT when pooling studies with 10 or more sessions (SMD = 0.21, CIs 0.05 to 0.36, Z = 2.49, p < 0.01, I2 = 78%) .
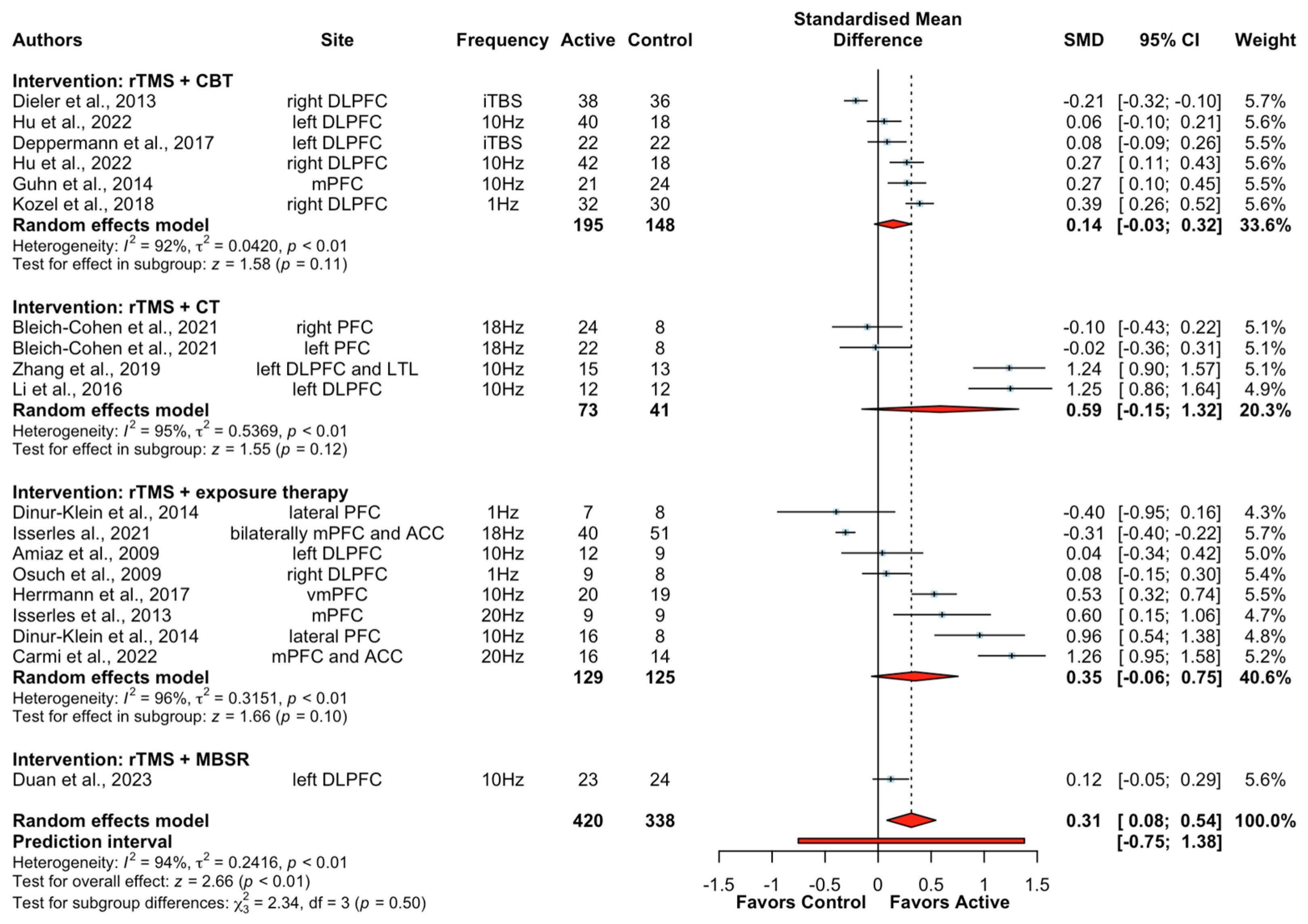
Figure 2. Forest plots of the effect of rTMS + psychological interventions on clinical symptoms [47,48,50,51,52,53,55,57,60,61,62,63,70,71,72,73]. Note: CI: confidence interval; SMD: standardized mean differences.
A subgroup analysis was additionally conducted to examine the effects of rTMS combined with psychological interventions on depressive symptoms. Ten studies (twelve treatment arms) reported depressive symptom severity outcomes in patients with MDD (n = 24), ADHD (n = 62), PTSD (n = 180), AD (n = 50), post-stroke depression (n = 47), alcohol-dependent patients (n = 119), and mild cognitive impairment (MCI) (n = 22) were included. The meta-analysis showed that active rTMS combined with psychological interventions did not produce a significantly greater improvement in depressive symptoms compared to sham rTMS + psychological intervention in the analysed clinical populations (SMD = 0.06, CIs − 0.16 to 0.29, Z = 0.55, p = 0.58, I2 = 86%; Figure 3). A further subgroup analysis in patients with PTSD showed no difference in depressive symptoms between active or sham rTMS when combined with exposure therapy .
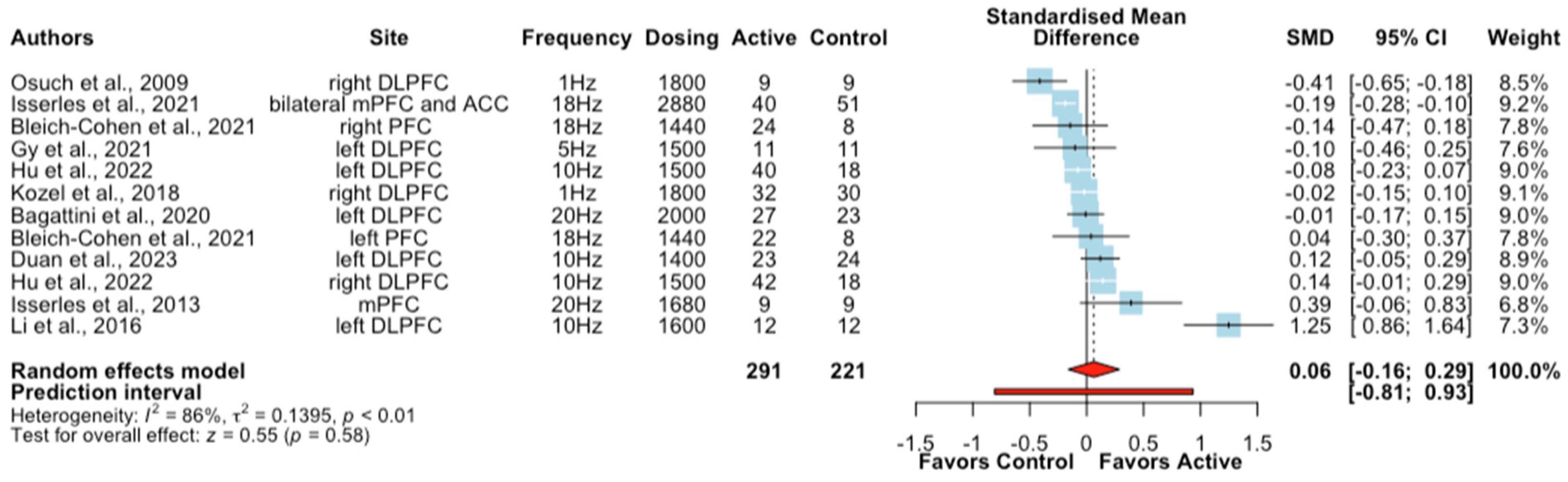
Figure 3. Forest plots of the effect of rTMS + psychological interventions on depressive symptoms [46,47,51,52,53,55,57,60,67,71].
3.3. Functional Outcomes in Clinical Populations
Eight studies (ten treatment arms) examined the effect of rTMS + psychological interventions on functional outcomes in clinical populations [46,51,57,58,60,64,69,70]. Meta-analysis revealed that active rTMS + psychological interventions did not significantly improve functional outcomes relative to sham rTMS + psychological interventions (SMD = 0.10, CIs −0.12 to 0.32, Z = 0.87, p = 0.38; ). In the subgroup analysis of interventions involving 10 or more sessions, no significant outcomes were observed either .
3.4. Cognitive Outcomes in Healthy and Clinical Populations
We investigated the effect of active/sham rTMS + CT on cognitive function from 13 studies (14 treatment arms) for 741 participants across 7 cognitive domains: perceptual-motor function, language, executive function, learning and memory, complex attention, working memory, and global cognition. Data for the effect on cognition were only available in one study in healthy participants (n = 27). When data from these ten studies were pooled, there was an overall significantly greater effect with active rTMS + CT compared to sham rTMS + CT (SMD = 0.28, CIs 0.15 to 0.42, Z = 4.03, p < 0.01). However, the results were highly heterogeneous (I2 = 88%). Due to insufficient data, subgroup analyses were limited to the cognitive domains of complex attention, executive function, learning and memory, and global cognition. Results showed that the combination of rTMS + CT had a small effect on global cognition (SMD = 0.45, CIs 0.21 to 0.68, Z = 3.75, p < 0.01), with significant heterogeneity (I2 = 89%). When subgroup analysis in clinical populations was conducted, the results remained significant on overall cognition when all domains were collapsed (SMD = 0.33, CIs 0.18 to 0.49, Z = 4.22, p < 0.01, I2 = 88%) and for global cognition (SMD = 0.47, CIs 0.22 to 0.72, Z = 3.70, p < 0.01, I2 = 89%). Furthermore, the results of subgroup analysis in patients with AD also remained significant for overall cognition (SMD = 0.43, CIs 0.20 to 0.66, Z = 3.6, p < 0.01; ) and for global cognition (SMD = 0.41, CIs 0.08 to 0.75, Z = 2.41, p = 0.02; ). There was no significant difference between conditions for the complex attention, learning and memory, and executive function domains (Figure 4).

Figure 4. Forest plots of the effect of rTMS combined with cognitive training for different cognitive domains [46,49,54,55,56,57,64,65,66,67,68,69,70].
3.5. Publication Bias
The funnel plots for the effect sizes of clinical symptoms, depressive symptoms, functional outcomes, overall cognitive effects, and global cognition showed symmetry, indicating no evidence of publication bias . This was supported by the results of the Egger’s tests (p > 0.05).
3.6. Risk of Bias Assessment and Sensitivity Analyses
Two studies (7.4%) were assessed as having a high risk of bias. Herrmann et al. [73] had a high risk of bias arising from the randomization process and Osuch et al. [47] had a high risk of bias due to deviations from the intended intervention . Results from the ROB2 tool are shown in . After removing these two studies, the effect of active rTMS combined with psychological interventions was still significantly greater for clinical outcomes compared to sham rTMS combined with psychological interventions (SMD = 0.32, CIs 0.06 to 0.57, Z = 2.41, p = 0.02, I2 = 95%). When pooling studies with 10 or more sessions of combined interventions, subgroup analysis showed rTMS had augmentation effects on psychological interventions for overall clinical symptoms with a small-sized effect (SMD = 0.36, CIs 0.07 to 0.64, Z = 2.47, p < 0.01, I2 = 95%) after excluding one study with a high risk of bias [47]. Functional and cognitive outcomes were not affected by the removal of these two studies as they only examined clinical outcomes.
4. Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to investigate whether rTMS can potentially augment the efficacy of psychological interventions. In summary, the results provided preliminary evidence that: (1) rTMS could potentially augment the effects of psychological interventions on overall clinical symptoms with a small-sized effect across a broad range of health conditions; and (2) active rTMS could potentially produce small-sized cognitive-enhancing benefits on CT for overall cognition in studies involving healthy and clinical populations.
While the combined effects of rTMS and psychological interventions are increasingly being investigated, it remains unclear whether rTMS can augment clinical outcomes. Overall, the results showed that there may be a small-sized beneficial effect of rTMS on psychological interventions for clinical outcomes. More intervention sessions seemed to slightly increase effect size when conducted subgroup analysis including studies with 10 or more sessions of combined interventions. Heterogeneity between studies, however, was high, and subgroup analyses according to different treatment strategies (CBT, CT, and exposure therapy) failed to reach statistical significance, likely due to reduced statistical power available in subgroup analyses. Interestingly, rTMS showed a potential augmentation effect on CBT in studies which included 10 or more sessions, suggesting rTMS may facilitate more rapid clinical effects of CBT with a higher number of sessions. In the subgroup analyses of patients with PTSD or smokers, no beneficial effect was observed following rTMS combined with exposure therapy; however, there were insufficient studies to do subgroup analyses on other health conditions. Of note, only three studies were available for the subgroup analyses of patients with PTSD and smokers, respectively, and the rTMS protocol utilized in each study was unique, which limited statistical power to detect a beneficial effect and so suggests the need for further research. Nevertheless, the current findings provide preliminary and promising evidence suggesting a potential augmentation effect of rTMS on psychological interventions for clinical outcome measures in a broad context. Further research, particularly with larger sample sizes within specific disorders and rTMS protocols with sufficient therapeutic parameters, is warranted to clarify and validate these preliminary findings.
A recent review provided preliminary evidence that greater improvement in depressive symptoms could be achieved by rTMS combined with psychological interventions, such as CBT and cognitive-emotional reactivation, but no meta-analysis was conducted [3]. Active rTMS combined with psychological interventions was not significantly more beneficial for improving depressive symptoms in clinical populations in this analysis. The result may be due to the large variability in the types of psychological interventions used in this analysis: three studies used rTMS + exposure therapy, four studies used rTMS + CT, two studies used rTMS + CBT, whereas only one study used rTMS + MBSR to investigate the effect on depressive symptoms. A subgroup analysis revealed that patients with PTSD who received rTMS + exposure therapy did not significantly improve their depressive symptoms, which might be partially attributed to no improvement in the clinical symptoms mentioned above. There is a parallel association between improvement in PTSD symptoms and improvements in depression [74]. Depressive symptom outcomes were obtained from different clinical populations, which may also have contributed to the non-significant results. It is also important to note that there were only two studies investigating the effect on depressive symptoms in patients with depression, which limits any conclusions about the use of rTMS to boost the effects of psychological interventions in this population. The majority of patients in the included studies were treated with a minimum of 10 sessions of combined interventions, with either high frequency rTMS applied to the left PFC or low frequency rTMS applied to the right PFC. A large naturalistic study found that rTMS combined with psychotherapy resulted in a 66% response and a 56% remission rate after treatment, with no difference found between high-frequency and low-frequency rTMS [75]. No standard dosage of rTMS was applied, which may also explain the lack of augmentation effects of rTMS on psychological interventions for depressive symptoms. Except for one study, that utilized 2880 pulses per session, the TMS dose ranged from 1400–2000 pulses per session, which was much lower than the FDA-approved rTMS protocol of 3000 pulses per session [75]. Future randomized trials with a larger number of sessions of rTMS and psychological interventions in patients with MDD or comorbid depression are needed to determine the efficacy of this combined intervention for improving depressive symptoms.
We did not find an additional benefit of rTMS on psychological interventions for functional outcomes in clinical populations or in sub-samples treated with 10 or more sessions. However, it is important to note that only a small number of studies (N = 8) reported functional outcomes, meaning that a lack of effect cannot be concluded from this preliminary analysis, which included diverse functional outcomes measures (e.g., quality of life, vocational and social functioning, and activities of daily living). Deficits in social and vocational functioning, as well as compromised quality of life, are critical and shared features across psychiatric disorders [76,77]. Given the significance of these functional outcomes for patients, it is critical that future research investigates the augmentation effects of rTMS on psychological interventions to assess these outcomes.
Previous meta-analyses have found greater improvement in cognition with active rTMS combined with CT compared to rTMS alone [78,79]. In the present study, we extended these past findings by examining whether active rTMS combined with CT was more beneficial compared to sham rTMS combined with CT in a sample of studies involving healthy and clinical populations. The results showed that overall cognitive functions were potentially improved following active rTMS combined with CT, albeit with a small effect size and significant heterogeneity. Additionally, we also observed a greater improvement in overall cognition following active rTMS combined with CT compared to sham rTMS combined with CT in a subgroup analysis of patients with AD. In addition, high-frequency rTMS applied to the prefrontal cortex, particularly the DLPFC, was used in most of the included studies assessing the effects of rTMS + CT. Existing evidence has demonstrated that high-frequency rTMS targeted over the prefrontal cortex has beneficial effects on cognitive functioning [17,78]. In this study, we provide further evidence that multiple sessions of high-frequency rTMS sessions administered to this region could have additional cognitive-enhancing effects on CT.
Interestingly, following a subgroup analysis of different cognitive domains, rTMS + CT was only significantly beneficial for improving global cognition with a small effect size. Global cognition measures a wide range of cognitive skills, including orientation, attention, memory, and visuospatial and constructional abilities [80]. A recent systematic review and meta-analysis of neuropsychiatric symptoms across various diagnoses also found rTMS to the left DLPFC alone had a small-sized effect not only for global cognition, but also for declarative memory, working memory, and cognitive control [80]. The current study extends this finding by showing that rTMS might augment the effects of CT in improving global cognition. In this subgroup analysis, nine out of eleven studies examined the effect on global cognition in older populations, who were more likely to be cognitively impaired at baseline. Moreover, a subgroup analysis showed the augmentation effect on global cognition remained significant in patients with AD, who were characterized by significant cognitive impairment and probably benefited most from these combined interventions [81]. The failure to find cognitive-enhancing effects for the executive function, learning and memory, and complex attention domains was likely due to the small number of studies included for these domains. Therefore, we cannot rule out that there are no additional beneficial effects of rTMS on CT for these outcomes at this stage.
This study has several limitations. First, when a reasonable lower limit of 5 studies is recommended for subgroup analyses [82], the limited number of studies in some subgroups hampers our comprehensive assessment of the effects of certain treatment strategies (e.g., rTMS + MBSR), outcome measures, or in certain disorders (e.g., panic disorders, ADHD). This limited statistical power to detect potential beneficial effects of rTMS on psychological interventions, suggesting that more studies across diverse categories are warranted to validate and further extend upon these preliminary findings. Secondly, the included studies exhibited substantial clinical and methodological heterogeneity. We attempted to conduct subgroup and sensitivity analyses to identify potential sources of heterogeneity. However, the heterogeneity remained high. The current study examined the effects of four different treatment strategies (various combinations of rTMS and psychological intervention parameters) in participants with twelve health conditions on three outcome measures grouped into different domains. Thus, aggregating effects from different conditions likely contributed to heterogeneity. The high heterogeneity made it challenging to draw meaningful and generalizable conclusions from our current meta-analysis. Future research would benefit from including larger sample sizes in different clinical populations and further examination of the specific efficacy of rTMS with a standardized protocol (e.g., sufficient number of sessions) on psychological interventions in improving clinical, functional, and cognitive outcomes. Third, the combination of rTMS with psychological interventions is a relatively new and emerging area of research, so the number of included studies in the respective analyses was limited, which limited the statistical power to detect bias. Fourth, a high proportion of included studies were assessed as having some concern of risk of bias, while two studies exhibited high risk. Nevertheless, sensitivity analyses showed that the results were not changed on overall cognition after the removal of these two studies. Fifth, we only included studies comparing active or sham rTMS combined with active psychological interventions. Future research involving sham psychological interventions is needed to determine whether improved clinical outcomes with rTMS combined with psychological interventions are due to synergistic or additive mechanisms.
5. Conclusions
In conclusion, this study provides preliminary evidence that active rTMS may augment clinical outcomes when combined with psychological interventions. Further, rTMS might produce a small-sized cognitive-enhancing effect on CT, warranting further research into this combined intervention. These preliminary results, however, must be interpreted with caution due to high heterogeneity and, therefore, require confirmation and replication in future research. In particular, studies with large sample sizes are needed to better understand and optimize the combined effect of rTMS and psychological interventions for specific clinical conditions.
References
- Ricou, M.; Marina, S.; Vieira, P.M.; Duarte, I.; Sampaio, I.; Regalado, J.; Canário, C. Psychological intervention at a primary health care center: Predictors of success. BMC Fam. Pract. 2019, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- van Agteren, J.; Iasiello, M.; Lo, L.; Bartholomaeus, J.; Kopsaftis, Z.; Carey, M.; Kyrios, M. A systematic review and meta-analysis of psychological interventions to improve mental wellbeing. Nat. Hum. Behav. 2021, 5, 631–652. [Google Scholar] [CrossRef] [PubMed]
- Tatti, E.; Phillips, A.L.; Paciorek, R.; Romanella, S.M.; Dettore, D.; Di Lorenzo, G.; Ruffini, G.; Rossi, S.; Santarnecchi, E. Boosting psychological change: Combining non-invasive brain stimulation with psychotherapy. Neurosci. Biobehav. Rev. 2022, 142, 104867. [Google Scholar] [CrossRef] [PubMed]
- Miklowitz, D.J.; Efthimiou, O.; Furukawa, T.A.; Scott, J.; McLaren, R.; Geddes, J.R.; Cipriani, A. Adjunctive Psychotherapy for Bipolar Disorder: A Systematic Review and Component Network Meta-analysis. JAMA Psychiatry 2021, 78, 141–150. [Google Scholar] [CrossRef]
- Gelenberg, A.J.; Freeman, M.; Markowitz, J.; Rosenbaum, J.; Thase, M.; Trivedi, M.; Van Rhoads, R. American Psychiatric Association practice guidelines for the treatment of patients with major depressive disorder. Am. J. Psychiatry 2010, 167, 9–118. [Google Scholar]
- Malhi, G.S.; Bell, E.; Bassett, D.; Boyce, P.; Bryant, R.; Hazell, P.; Hopwood, M.; Lyndon, B.; Mulder, R.; Porter, R.; et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust. N. Z. J. Psychiatry 2021, 55, 7–117. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.A.; Meredith, L.R.; Kiluk, B.D.; Walthers, J.; Carroll, K.M.; Magill, M. Combined Pharmacotherapy and Cognitive Behavioral Therapy for Adults with Alcohol or Substance Use Disorders: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e208279. [Google Scholar] [CrossRef]
- Guidi, J.; Fava, G.A. Sequential Combination of Pharmacotherapy and Psychotherapy in Major Depressive Disorder: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 261–269. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Martin, D.M.; Moffa, A.; Nikolin, S.; Bennabi, D.; Brunoni, A.R.; Flannery, W.; Haffen, E.; McClintock, S.M.; Moreno, M.L.; Padberg, F.; et al. Cognitive effects of transcranial direct current stimulation treatment in patients with major depressive disorder: An individual patient data meta-analysis of randomised, sham-controlled trials. Neurosci. Biobehav. Rev. 2018, 90, 137–145. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial magnetic stimulation and the human brain. Nature 2000, 406, 147–150. [Google Scholar] [CrossRef]
- Martin, D.M.; McClintock, S.M.; Forster, J.; Loo, C.K. Does Therapeutic Repetitive Transcranial Magnetic Stimulation Cause Cognitive Enhancing Effects in Patients with Neuropsychiatric Conditions? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Neuropsychol. Rev. 2016, 26, 295–309. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Xu, M.; Nikolin, S.; Samaratunga, N.; Chow, E.J.H.; Loo, C.K.; Martin, D.M. Cognitive Effects Following Offline High-Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) in Healthy Populations: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2023, 1–27. [Google Scholar] [CrossRef]
- Chou, Y.H.; Ton That, V.; Sundman, M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2020, 86, 1–10. [Google Scholar] [CrossRef]
- Iimori, T.; Nakajima, S.; Miyazaki, T.; Tarumi, R.; Ogyu, K.; Wada, M.; Tsugawa, S.; Masuda, F.; Daskalakis, Z.J.; Blumberger, D.M.; et al. Effectiveness of the prefrontal repetitive transcranial magnetic stimulation on cognitive profiles in depression, schizophrenia, and Alzheimer’s disease: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 31–40. [Google Scholar] [CrossRef]
- Martin, D.M.; McClintock, S.M.; Forster, J.J.; Lo, T.Y.; Loo, C.K. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress. Anxiety 2017, 34, 1029–1039. [Google Scholar] [CrossRef]
- Mix, A.; Benali, A.; Eysel, U.T.; Funke, K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur. J. Neurosci. 2010, 32, 1575–1586. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Mulsant, B.H.; Thorpe, K.E.; McClintock, S.M.; Konstantinou, G.N.; Lee, H.H.; Nestor, S.M.; Noda, Y.; Rajji, T.K.; Trevizol, A.P.; et al. Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs. Bilateral Theta Burst Stimulation in Older Adults with Depression: The FOUR-D Randomized Noninferiority Clinical Trial. JAMA Psychiatry 2022, 79, 1065–1073. [Google Scholar] [CrossRef]
- De Risio, L.; Borgi, M.; Pettorruso, M.; Miuli, A.; Ottomana, A.M.; Sociali, A.; Martinotti, G.; Nicolò, G.; Macrì, S.; di Giannantonio, M.; et al. Recovering from depression with repetitive transcranial magnetic stimulation (rTMS): A systematic review and meta-analysis of preclinical studies. Transl. Psychiatry 2020, 10, 393. [Google Scholar] [CrossRef]
- Xiang, H.; Sun, J.; Tang, X.; Zeng, K.; Wu, X. The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2019, 33, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Dong, Z.; Pan, L.; Liu, Y.; Ye, Z.; Qin, L.; Liu, Q.; Qin, C. Effects of repetitive transcranial magnetic stimulation on gait disorders and cognitive dysfunction in Parkinson’s disease: A systematic review with meta-analysis. Brain Behav. 2022, 12, e2697. [Google Scholar] [CrossRef]
- Teselink, J.; Bawa, K.K.; Koo, G.K.; Sankhe, K.; Liu, C.S.; Rapoport, M.; Oh, P.; Marzolini, S.; Gallagher, D.; Swardfager, W.; et al. Efficacy of non-invasive brain stimulation on global cognition and neuropsychiatric symptoms in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res. Rev. 2021, 72, 101499. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Perera, M.P.N.; Mallawaarachchi, S.; Miljevic, A.; Bailey, N.W.; Herring, S.E.; Fitzgerald, P.B. Repetitive Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Meta-analysis of Randomized, Sham-Controlled Trials. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 947–960. [Google Scholar] [CrossRef]
- Song, S.; Zilverstand, A.; Gui, W.; Li, H.J.; Zhou, X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimul. 2019, 12, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Schulze, L.; Feffer, K.; Lozano, C.; Giacobbe, P.; Daskalakis, Z.J.; Blumberger, D.M.; Downar, J. Number of pulses or number of sessions? An open-label study of trajectories of improvement for once-vs. twice-daily dorsomedial prefrontal rTMS in major depression. Brain Stimul. 2018, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorff, J.; Göke, K.; Kiebs, M.; Kasten, F.H.; Herrmann, C.S.; Witt, K.; Hurlemann, R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sci. 2022, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef]
- Tzioras, M.; McGeachan, R.I.; Durrant, C.S.; Spires-Jones, T.L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 19–38. [Google Scholar] [CrossRef]
- van Spronsen, M.; Hoogenraad, C.C. Synapse pathology in psychiatric and neurologic disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.; Zinbarg, R.E.; Mittal, V.A. Efficacy and mechanisms of non-invasive brain stimulation to enhance exposure therapy: A review. Clin. Psychol. Rev. 2019, 70, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Sathappan, A.V.; Luber, B.M.; Lisanby, S.H. The Dynamic Duo: Combining noninvasive brain stimulation with cognitive interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Conelea, C.A.; Jacob, S.; Redish, A.D.; Ramsay, I.S. Considerations for Pairing Cognitive Behavioral Therapies and Non-invasive Brain Stimulation: Ignore at Your Own Risk. Front. Psychiatry 2021, 12, 660180. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The, P.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 26 October 2023).
- Posit Team. RStudio: Integrated Development for R; Posit Software, PBC: Boston, MA, USA, 2022; Available online: http://www.posit.co/ (accessed on 26 October 2023).
- Sachdev, P.; Blacker, D.; Blazer, D.; Ganguli, M.; Jeste, D.; Paulsen, J.; Petersen, R. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Tiemens, B.; Kloos, M.; Spijker, J.; Ingenhoven, T.; Kampman, M.; Hendriks, G.-J. Lower versus higher frequency of sessions in starting outpatient mental health care and the risk of a chronic course; a naturalistic cohort study. BMC Psychiatry 2019, 19, 228. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Gy, R.R.; Reyes-López, J.V.; Garcell, R.; Ar, C.M.; Na, A.G. Effect of transcranial magnetic stimulation as an enhancer of cognitive stimulation sessions on mild cognitive impairment: Preliminary results. Psychiatry Res. 2021, 304, 114151. [Google Scholar] [CrossRef]
- Osuch, E.A.; Benson, B.E.; Luckenbaugh, D.A.; Geraci, M.; Post, R.M.; McCann, U. Repetitive TMS combined with exposure therapy for PTSD: A preliminary study. J. Anxiety Disord. 2009, 23, 54–59. [Google Scholar] [CrossRef]
- Amiaz, R.; Levy, D.; Vainiger, D.; Grunhaus, L.; Zangen, A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009, 104, 653–660. [Google Scholar] [CrossRef]
- Brem, A.K.; Di Iorio, R.; Fried, P.J.; Oliveira-Maia, A.J.; Marra, C.; Profice, P.; Quaranta, D.; Schilberg, L.; Atkinson, N.J.; Seligson, E.E.; et al. Corticomotor Plasticity Predicts Clinical Efficacy of Combined Neuromodulation and Cognitive Training in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 200. [Google Scholar] [CrossRef]
- Dinur-Klein, L.; Dannon, P.; Hadar, A.; Rosenberg, O.; Roth, Y.; Kotler, M.; Zangen, A. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: A prospective, randomized controlled trial. Biol. Psychiatry 2014, 76, 742–749. [Google Scholar] [CrossRef]
- Duan, H.; Yan, X.; Meng, S.; Qiu, L.; Zhang, J.; Yang, C.; Liu, S. Effectiveness Evaluation of Repetitive Transcranial Magnetic Stimulation Therapy Combined with Mindfulness-Based Stress Reduction for People with Post-Stroke Depression: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 930. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, T.; Ma, H.; Zhou, X.; Wang, H.; Wang, X.; Cheng, C.; Li, Y.; Duan, R.; Zhang, B.; et al. Repetitive transcranial magnetic stimulation combined with cognitive behavioral therapy treatment in alcohol-dependent patients: A randomized, double-blind sham-controlled multicenter clinical trial. Front. Psychiatry 2022, 13, 935491. [Google Scholar] [CrossRef]
- Isserles, M.; Shalev, A.Y.; Roth, Y.; Peri, T.; Kutz, I.; Zlotnick, E.; Zangen, A. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder—A pilot study. Brain Stimul. 2013, 6, 377–383. [Google Scholar] [CrossRef]
- Lechner, W.V.; Philip, N.S.; Kahler, C.W.; Houben, K.; Tirrell, E.; Carpenter, L.L. Combined Working Memory Training and Transcranial Magnetic Stimulation Demonstrates Low Feasibility and Potentially Worse Outcomes on Delay to Smoking and Cognitive Tasks: A Randomized 2 × 2 Factorial Design Pilot and Feasibility Study. Nicotine Tob. Res. 2022, 24, 1871–1880. [Google Scholar] [CrossRef]
- Li, C.-T.; Hsieh, J.-C.; Huang, H.-H.; Chen, M.-H.; Juan, C.-H.; Tu, P.-C.; Lee, Y.-C.; Wang, S.-J.; Cheng, C.-M.; Su, T.-P. Cognition-modulated frontal activity in prediction and augmentation of antidepressant efficacy: A randomized controlled pilot study. Cereb. Cortex 2016, 26, 202–210. [Google Scholar] [CrossRef]
- Vecchio, F.; Quaranta, D.; Miraglia, F.; Pappalettera, C.; Di Iorio, R.; L’Abbate, F.; Cotelli, M.; Marra, C.; Rossini, P.M. Neuronavigated Magnetic Stimulation combined with cognitive training for Alzheimer’s patients: An EEG graph study. Geroscience 2022, 44, 159–172. [Google Scholar] [CrossRef]
- Bleich-Cohen, M.; Gurevitch, G.; Carmi, N.; Medvedovsky, M.; Bregman, N.; Nevler, N.; Elman, K.; Ginou, A.; Zangen, A.; Ash, E.L. A functional magnetic resonance imaging investigation of prefrontal cortex deep transcranial magnetic stimulation efficacy in adults with attention deficit/hyperactive disorder: A double blind, randomized clinical trial. Neuroimage Clin. 2021, 30, 102670. [Google Scholar] [CrossRef]
- Neacsiu, A.D.; Beynel, L.; Powers, J.P.; Szabo, S.T.; Appelbaum, L.G.; Lisanby, S.H.; LaBar, K.S. Enhancing Cognitive Restructuring with Concurrent Repetitive Transcranial Magnetic Stimulation: A Transdiagnostic Randomized Controlled Trial. Psychother. Psychosom. 2022, 91, 94–106. [Google Scholar] [CrossRef]
- Wang, J.; Luo, H.; Schülke, R.; Geng, X.; Sahakian, B.J.; Wang, S. Is transcranial direct current stimulation, alone or in combination with antidepressant medications or psychotherapies, effective in treating major depressive disorder? A systematic review and meta-analysis. BMC Med. 2021, 19, 319. [Google Scholar] [CrossRef]
- Kozel, F.A.; Motes, M.A.; Didehbani, N.; DeLaRosa, B.; Bass, C.; Schraufnagel, C.D.; Jones, P.; Morgan, C.R.; Spence, J.S.; Kraut, M.A.; et al. Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial. J. Affect. Disord. 2018, 229, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Deppermann, S.; Vennewald, N.; Diemer, J.; Sickinger, S.; Haeussinger, F.B.; Dresler, T.; Notzon, S.; Laeger, I.; Arolt, V.; Ehlis, A.C.; et al. Neurobiological and clinical effects of fNIRS-controlled rTMS in patients with panic disorder/agoraphobia during cognitive-behavioural therapy. Neuroimage Clin. 2017, 16, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Guhn, A.; Dresler, T.; Andreatta, M.; Muller, L.D.; Hahn, T.; Tupak, S.V.; Polak, T.; Deckert, J.; Herrmann, M.J. Medial prefrontal cortex stimulation modulates the processing of conditioned fear. Front. Behav. Neurosci. 2014, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Dieler, A.C.; Dresler, T.; Joachim, K.; Deckert, J.; Herrmann, M.J.; Fallgatter, A.J. Can intermittent theta burst stimulation as add-on to psychotherapy improve nicotine abstinence? Results from a pilot study. Eur. Addict. Res. 2014, 20, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Ba, L.; Zhang, F.; Jian, S.; Zhang, M.; Zhu, W. Cerebral blood flow changes induced by high-frequency repetitive transcranial magnetic stimulation combined with cognitive training in Alzheimer’s disease. Front. Neurol. 2023, 14, 1037864. [Google Scholar] [CrossRef] [PubMed]
- Yingli, B.; Zunke, G.; Wei, C.; Shiyan, W. Cerebral activity manipulation of low-frequency repetitive transcranial magnetic stimulation in post-stroke patients with cognitive impairment. Front. Neurol. 2022, 13, 951209. [Google Scholar] [CrossRef] [PubMed]
- Palaus, M.; Viejo-Sobera, R.; Redolar-Ripoll, D.; Marrón, E.M. Cognitive Enhancement via Neuromodulation and Video Games: Synergistic Effects? Front. Hum. Neurosci. 2020, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Bagattini, C.; Zanni, M.; Barocco, F.; Caffarra, P.; Brignani, D.; Miniussi, C.; Defanti, C.A. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul. 2020, 13, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, H.; Yu, Q.; Yin, L.; Li, K.; Li, Y.; Fu, J. Cerebral Functional Manipulation of Repetitive Transcranial Magnetic Stimulation in Cognitive Impairment Patients after Stroke: An fMRI Study. Front. Neurol. 2020, 11, 977. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, M.; Luo, J.; Huang, L.; Zhang, S.; Pan, C.; Hu, X. Effects of transcranial magnetic stimulation on the performance of the activities of daily living and attention function after stroke: A randomized controlled trial. Clin. Rehabil. 2020, 34, 1465–1473. [Google Scholar] [CrossRef]
- Zhang, F.; Qin, Y.; Xie, L.; Zheng, C.; Huang, X.; Zhang, M. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J. Neural. Transm. 2019, 126, 1081–1094. [Google Scholar] [CrossRef]
- Isserles, M.; Tendler, A.; Roth, Y.; Bystritsky, A.; Blumberger, D.M.; Ward, H.; Feifel, D.; Viner, L.; Duffy, W.; Zohar, J.; et al. Deep Transcranial Magnetic Stimulation Combined with Brief Exposure for Posttraumatic Stress Disorder: A Prospective Multisite Randomized Trial. Biol. Psychiatry 2021, 90, 721–728. [Google Scholar] [CrossRef]
- Carmi, L.; Alyagon, U.; Barnea-Ygael, N.; Zohar, J.; Dar, R.; Zangen, A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018, 11, 158–165. [Google Scholar] [CrossRef]
- Herrmann, M.J.; Katzorke, A.; Busch, Y.; Gromer, D.; Polak, T.; Pauli, P.; Deckert, J. Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimul. 2017, 10, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, J.F.; Salas, J.; Norman, S.B.; Schnurr, P.P.; Chard, K.M.; Tuerk, P.; Schneider, F.D.; van den Berk-Clark, C.; Cohen, B.E.; Friedman, M.J.; et al. Association Between Clinically Meaningful Posttraumatic Stress Disorder Improvement and Risk of Type 2 Diabetes. JAMA Psychiatry 2019, 76, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Donse, L.; Padberg, F.; Sack, A.T.; Rush, A.J.; Arns, M. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018, 11, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.; Bellack, A.S.; Gold, J.M. Social/communication skills, cognition, and vocational functioning in schizophrenia. Schizophr. Bull. 2007, 33, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Lydon, S.; Healy, O. Employment and Vocational Skills among Individuals with Autism Spectrum Disorder: Predictors, Impact, and Interventions. Rev. J. Autism Dev. Disord. 2014, 1, 266–275. [Google Scholar] [CrossRef]
- Cheng, C.P.W.; Wong, C.S.M.; Lee, K.K.; Chan, A.P.K.; Yeung, J.W.F.; Chan, W.C. Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2018, 33, e1–e13. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Ling, Z.; Yu, X. Repetitive transcranial magnetic stimulation for cognitive impairment in Alzheimer’s disease: A meta-analysis of randomized controlled trials. J. Neurol. 2020, 267, 791–801. [Google Scholar] [CrossRef]
- Kan, R.L.D.; Padberg, F.; Giron, C.G.; Lin, T.T.Z.; Zhang, B.B.B.; Brunoni, A.R.; Kranz, G.S. Effects of repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex on symptom domains in neuropsychiatric disorders: A systematic review and cross-diagnostic meta-analysis. Lancet Psychiatry 2023, 10, 252–259. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]

