1. Introduction
Gastric cancer (GC) is a significant health issue worldwide [1]. The global prevalence of gastric cancer is increasing significantly, emphasizing the need for improved detection methods. Currently, gastric cancer ranks as the fifth most common malignant cancer and the fourth leading cause of cancer-related mortality worldwide. Despite a decline in incidence rates, the global burden of this malignancy is projected to increase by 62% by 2040 [2]. This escalation is further highlighted by predictions from the International Agency for Research on Cancer (IARC), which forecasts an increase to about 1.8 million new cases and approximately 1.3 million deaths by 2040, representing increases of about 63% and 66%, respectively, compared with 2020 [3].
The five-year survival rate for stage Ia cancer is over 95%, compared to 66.5% and 46.9% for stages II and III, respectively [4]. These survival differences underscore the importance of detecting treatable early-stage neoplastic lesions in the stomach. However, subtle surface changes suggestive of gastric neoplasias can be very challenging to identify endoscopically [5]. An estimated 5–10% of individuals diagnosed with gastric cancer had a negative upper endoscopy within 3 years, suggesting a failure to diagnose early-stage gastric neoplasias [5,6,7]. Procedures performed by non-expert endoscopists have an additional 14% decrease in the absolute gastric neoplasia detection rate compared to those performed by expert endoscopists [6]. Recent advancements in artificial intelligence (AI)-assisted endoscopy offer hope. Computer-aided detection (CADe) and diagnosis (CADx) AI systems aim to improve the real-time detection of gastric neoplasias by detecting subtle neoplastic changes in gastric mucosa with high accuracy [8,9].
Deep learning is a sophisticated branch of artificial intelligence that uses algorithms inspired by the human brain, known as artificial neural networks. These networks consist of layers of nodes that transform input data, enabling the machine to learn from large datasets. The learning process involves adjusting the network’s internal parameters to reduce prediction errors, utilizing methods like backpropagation and gradient descent. Deep learning is distinguished by its use of numerous layers that enable the recognition of complex patterns, making it ideal for applications in image and speech recognition, among others [10].
The proliferation of AI tools can be largely attributed to advances in computational power, algorithmic improvements, and the abundance of data available in the digital age. These tools have found applications across various sectors, including healthcare, automotive, finance, and customer service. The development of accessible frameworks like TensorFlow and PyTorch has democratized AI, allowing a broader range of users to develop AI models. Additionally, the integration of AI into products and services by many companies has fueled further demand for these tools.
Convolutional neural networks (CNNs) are a type of deep learning (DL) model used in computer vision. CNNs are trained using vast quantities of annotated images and videos that are labelled to facilitate particular tasks such as image classification or segmentation. Via the segmentation process, which begins with the collecting and preprocessing of gastric images, followed by expert annotation to mark cancerous tissues, the AI model is trained on these annotated images to learn the distinguishing features of neoplastic tissue. Once trained, the model can segment neoplastic areas in new images, aiding in diagnosis and treatment planning [11].
Once trained, these models can independently analyze new visual data, effectively detecting (CADe) or diagnosing (CADx) gastric neoplasias [12]. The real-world use of CNN-assisted endoscopy has shown encouraging results for identifying upper gastrointestinal neoplasms, including GC [7]. AI-based systems can also estimate GC’s invasion depth, which is crucial for treatment planning [13,14].
In many cases, endoscopic AI systems match or surpass the diagnostic performance of experienced endoscopists [15,16,17,18].
Real-time segmentation models have also successfully distinguished between gastric intestinal metaplasia (GIM) and healthy stomach tissue [17]. AI technology has the potential to revolutionize gastrointestinal endoscopy, improving patient outcomes. Yet, integrating CADe/CADx systems into clinical workflows is not without challenges. Algorithm training bias, the management of human–AI interaction complexities, and dealing with false-positive outcomes all need to be addressed to ensure successful AI implementation [9].
This review aims to probe the current literature on DL’s role in gastric neoplasia detection and diagnosis. We aim to analyze the strengths and weaknesses of CNN-based systems used for endoscopic evaluation of gastric neoplasias, and to discuss the challenges of integrating these systems into clinical practice.
2. Methods
We carried out this systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1. Search Strategy
We conducted a comprehensive search of PubMed/MEDLINE from their inception until May 2023. We used a combination of Medical Subject Headings (MeSH) and free-text terms related to “gastric cancer”, “gastric neoplasm”, “stomach neoplasm”, “stomach cancer”, “gastric carcinoma”, “artificial intelligence”, “deep learning”, “machine learning”, and “endoscopy”. We restricted the search to articles published in English. We manually checked the reference lists of eligible articles for any additional relevant studies.
2.2. Selection Criteria
We included studies that met the following criteria: 1—original research articles, 2—application of DL algorithms for gastric neoplasia detection or diagnosis, 3—use of endoscopic images for analysis, 4—provided quantitative diagnostic performance measures, and 5—compared AI performance with that of human endoscopists where possible.
We excluded studies if they: 1—were reviews, case reports, editorials, letters, or conference abstracts, 2—did not independently assess gastric neoplasias, 3—did not use endoscopic imaging, or 4—lacked sufficient performance measures.
2.3. Data Extraction
Two reviewers independently extracted data using a pre-defined extraction form using Microsoft Excel. The data included variables like the first author’s name, publication year, and study design, among others. Disagreements were resolved through discussion or consultation with a third reviewer.
2.4. Data Synthesis and Analysis
We performed a narrative synthesis to summarize the studies’ findings. Because of the expected heterogeneity in AI algorithms, imaging techniques, and study designs, we did not plan a meta-analysis. We presented the results in a tabular format and discussed the key findings.
3. Results
We identified 42 studies that met the inclusion criteria. The designs, sample sizes, and algorithms used varied across the studies. summarizes all studies included in the review and offers data on the research subject, clinical task, study design, sample size, detection/classification, objective, AI model/algorithm, AI model performance, clinical implication, and subject. The flowchart delineating the selection procedure of the studies included is depicted in Figure 1.
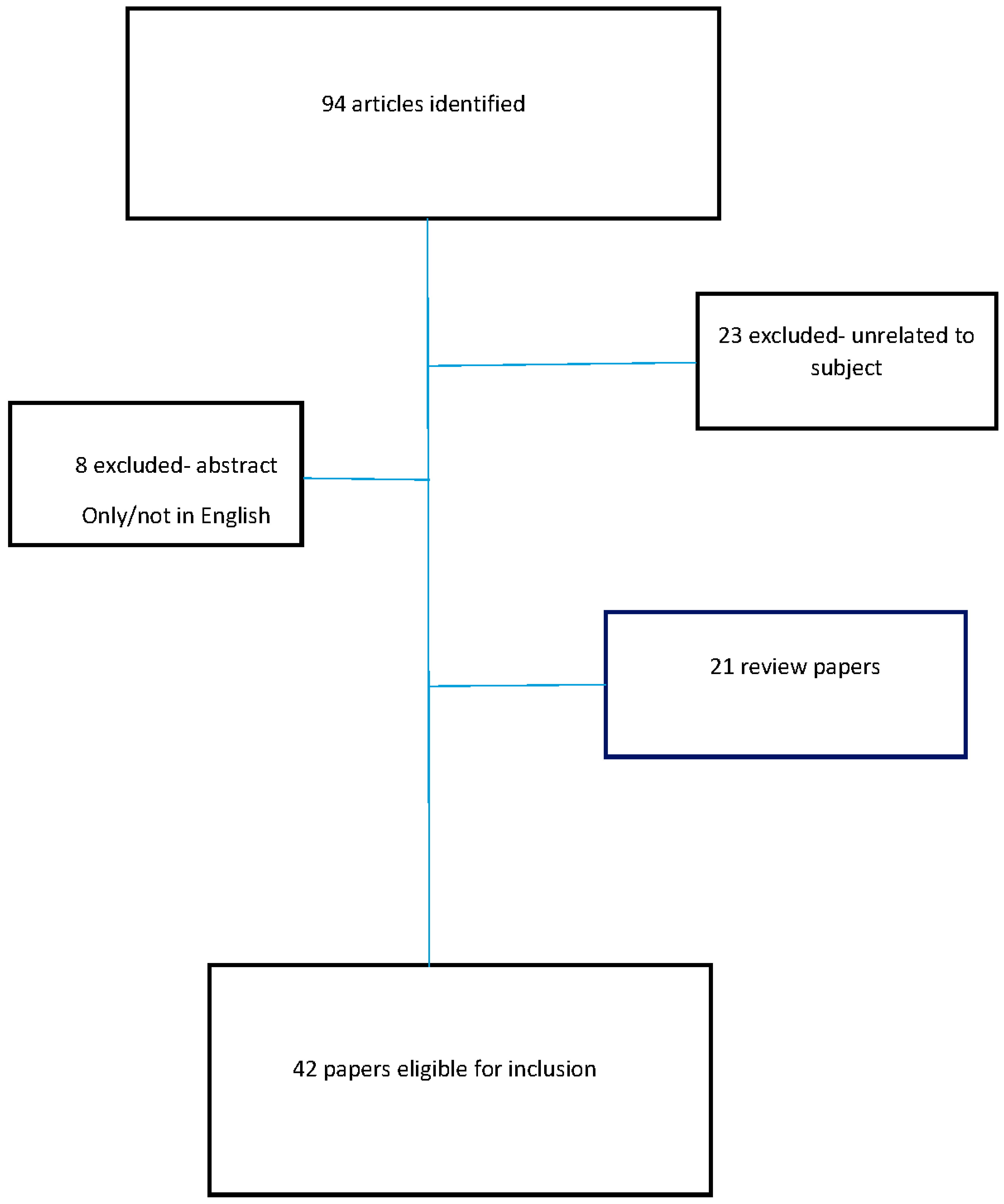
Figure 1. Flowchart delineating the selection procedure of the studies included in this review.
All sensitivity, specificity, and accuracy values represent the AI models’ performance.
3.1. Gastric Neoplasm Classification
Several studies utilized DL to classify gastric endoscopic images. Lee JH et al. [19] used Inception, ResNet, and VGGNet to differentiate between normal, benign ulcer, and cancer images, achieving high areas under the curves (AUCs) of 0.95, 0.97, and 0.85, respectively.
Notably, Inception, ResNet, and VGGNet are influential architectures in the field of deep learning, particularly for image processing tasks:
Inception: Known for its efficiency and depth, the Inception architecture, especially its popular version Inception v3, uses a combination of different-sized convolutional filters within the same layer (called a “network in network” approach). This allows it to capture spatial hierarchies at different scales and reduces computational cost.
ResNet (Residual Network): This architecture introduces residual learning to alleviate the vanishing gradient problem in very deep networks. By using skip connections that bypass one or more layers, ResNet can effectively train networks with many layers, including popular variants like ResNet50 and esNet101.
VGGNet (Visual Geometry Group Network): VGGNet, particularly VGG16 and VGG19, is known for its deep but simple architecture, using multiple convolutional layers with small-sized filters (3 × 3) before pooling layers. Its uniform architecture makes it easy to understand and implement, and it has been highly influential in demonstrating the effectiveness of depth in convolutional neural networks [19].
Cho BJ et al. [20] used Inception-Resnet-v2 for gastric neoplasm classification, reaching 84.6% accuracy. Three studies focused on diagnosing GC.
The ResNet50 model is a variant of the ResNet architecture, which is widely used in deep learning for image recognition and processing tasks. It is 50 layers deep, hence the ‘50’ in its name. One of the key features of ResNet is the use of “residual blocks” that help in combatting the vanishing gradient problem in deep neural networks, enabling the training of much deeper networks.
In a ResNet50 model, these residual blocks consist of layers with skip connections that allow the activation from one layer to be fast-forwarded to a later layer, bypassing one or more layers in between. This design helps in preserving the learning and signal from the initial layers to the deeper layers in the network. ResNet50, specifically, is known for its balance between depth and complexity, making it a popular choice for many image recognition tasks [21].
ResNet50 was applied to Magnifying Endoscopy with Narrow-Band Imaging (ME-NBI) images [21], resulting in 98.7% accuracy. Yusuke Horiuchi et al. [22] achieved 85.3% accuracy in differentiating early gastric cancer (EGC) from gastritis using a 22-layer CNN. Another study [23] achieved 92% sensitivity and 75% specificity in distinguishing malignant from benign gastric ulcers using a CNN.
Liu Y et al. [24] used the CNN Xception model enhanced by a residual attention mechanism to automatically classify benign and malignant gastric ulcer lesions in digestive endoscopy images.
The Xception model is an advanced convolutional neural network (CNN) architecture that builds upon the principles of Inception networks. The key innovation in Xception is the concept of depthwise separable convolutions. This approach separates the convolution process into two parts: a depthwise convolution that applies a single filter per input channel, followed by a pointwise convolution that applies a 1 × 1 convolution to combine the outputs of the depthwise convolution.
This architecture allows Xception to have fewer parameters and computations than a traditional convolutional approach, while maintaining or improving model performance. Xception is particularly effective for tasks in computer vision, demonstrating strong performance in image classification and other related tasks.
The enhanced Xception model achieved an accuracy of 81.4% and F1 score of 81.8% in diagnosing benign and malignant gastric ulcers.
3.2. Gastric Neoplasm Detection
Two studies [25,26] used SSD-GPNet and YOLOv4 for detecting gastric polyps, both achieving high mean average precision (mAP). YOLO, which stands for “You Only Look Once”, is a revolutionary AI algorithm used for real-time object detection in computer vision. It streamlines the traditional two-step object detection process into a single step utilizing a single convolutional neural network. This approach allows YOLO to quickly identify and classify multiple objects within an image by dividing it into a grid and predicting bounding boxes and class probabilities for each grid cell. Renowned for its speed, YOLO also maintains a commendable level of accuracy, although it is slightly less effective with small objects. Its applications range from autonomous vehicles to real-time surveillance. Over time, various iterations of YOLO have emerged, each enhancing its speed, accuracy, and detection capabilities, making it a crucial tool in the field of AI-assisted object detection.
The SSD-GPNet was shown to significantly increase polyp detection recalls by more than 10% (p < 0.001), particularly in the detection of small polyps [25], and YOLOv4 was shown to have an 88.0% mean average precision [26].
With respect to gastric neoplasm detection, Lianlian Wu et al. [27] showed a decreased miss rate using AI: the incidence of missed gastric neoplasms was significantly reduced in the AI-first group compared to the routine-first group (6.1%, [3 out of 49 patients] versus 27.3%, [12 out of 44 patients]; p = 0.015). Hongliu Du et al. [16] achieved the highest accuracy using a multimodal (white light and weak magnification) model, achieving 93.55% accuracy in prospective validation and outperforming endoscopists in the evaluation of multimodal data (90.0% vs. 76.2%, p = 0.002). Furthermore, when assisted by the multimodal ENDOANGEL-MM model, non-experts experienced a significant improvement in accuracy (85.6% versus 70.8%, p = 0.020), reaching a level not significantly different from that of experts (85.6% versus 89.0%, p = 0.159).
Xu M et al. [28] retrospectively analyzed endoscopic images from five Chinese hospitals to evaluate the efficacy of a computer-aided detection (CADe) system in diagnosing precancerous gastric conditions. The CADe system demonstrated high accuracy, ranging between 86.4% and 90.8%. This was on par with expert endoscopists and notably better than non-experts. A single-shot multibox detector (SSD) was used by one study [29] for gastric cancer detection, achieving 92.2% sensitivity. Another study [30] achieved 58.4% sensitivity and 87.3% specificity in early detection using a CNN.
Leheng Liu et al. [11] developed a computer-aided diagnosis (CAD) system to assist in diagnosing and segmenting Gastric Neoplastic Lesions (GNLs). The study utilized two CNNs: CNN1 for diagnosing GNLs and CNN2 for segmenting them. CNN1 demonstrated excellent diagnostic performance with an accuracy of 90.8%. It also achieved an impressive area under the curve (AUC) of 0.928. The use of CNN1 improved the accuracy rates for all participating endoscopists compared to their individual diagnostic methods. CNN2, focused on segmentation, also performed well, achieving an average intersection over union (IOU) of 0.584 and high values for precision, recall, and the Dice coefficient.
3.3. Assessment of Tumor Invasion Depth
Bang CS et al. [31] utilized AutoDL for classifying the invasion depth of gastric neoplasms with 89.3% accuracy.
VGG-16 is a convolutional neural network model proposed by the Visual Graphics Group (VGG) from the University of Oxford. It is composed of 16 layers (hence the name VGG-16), including 13 convolutional layers and 3 fully connected layers. One of the key characteristics of VGG-16 is its use of a large number of convolutional layers with small-sized filters (3 × 3), which allows it to capture complex patterns in the data while keeping the computational complexity manageable [32].
Hong Jin Yoon et al. [32] used VGG-16 for assessing tumor invasion depth in EGC, achieving highly discriminative AUC values of 0.981 and 0.851 for EGC detection and depth prediction, respectively. Of the potential factors influencing AI performance when predicting tumor depth, only histologic differentiation showed a significant association. Undifferentiated-type histology was associated with lower-accuracy AI predictions.
Kenta Hamada et al. [33] used ResNet152 for and achieved 78.9% accuracy in diagnosing mucosal cancers. Yan Zhu et al. [13] achieved an AUC of 0.94 using ResNet50. When using a threshold value of 0.5, the system demonstrated a sensitivity of 76.47% and a specificity of 95.56%. The overall accuracy of the CNN-CAD system was measured at 89.2%.
Notably, the CNN-CAD system exhibited a significantly higher accuracy (by 17.3%) and specificity (by 32.2%) when compared to human endoscopists.
Dehua Tang et al. [34] differentiated between intramucosal and advanced gastric cancer using a deep convolutional neural network (DCNN). The DCNN model exhibited notable discrimination between intramucosal gastric cancer (GC) and advanced GC, as indicated by an area under the curve (AUC) of 0.942. The model achieved a sensitivity of 90.5% and a specificity of 85.3%. When comparing the diagnostic performance of novice and expert endoscopists, it was observed that their accuracy (84.6% vs. 85.5%), sensitivity (85.7% vs. 87.4%), and specificity (83.3% vs. 83.0%) were all similar when assisted by the DCNN model. Furthermore, the mean pairwise kappa (a measure of agreement) among endoscopists significantly increased with the assistance of the DCNN model, improving from 0.430–0.629 to 0.660–0.861. The utilization of the DCNN model was also associated with a reduction in diagnostic duration, from 4.4 s to 3.0 s. Furthermore, the correlation between the perseverance of effort and diagnostic accuracy among endoscopists diminished with the use of the DCNN model, with the correlation coefficient decreasing from 0.470 to 0.076. This suggests that the DCNN model’s assistance helps mitigate the influence of subjective factors, such as perseverance, on diagnostic accuracy.
In a recent study, Gong EJ et al. [18] used 5017 endoscopic images to train two models: one for lesion detection and another for lesion classification in the stomach. In a randomized pilot study, the lesion detection rate for the computer decision support system (CDSS) was 95.6% in internal tests. Although not statistically significant, CDSS-assisted endoscopy showed a higher lesion detection rate (2.0%) compared to conventional screening (1.3%). In a prospective multicenter external test, the CDSS achieved an 81.5% accuracy rate for four-class lesion classification and an 86.4% accuracy rate for invasion depth prediction.
Goto A et al. [14] aimed at differentiating between intramucosal and submucosal early gastric cancers. The performance of this AI classifier was then compared to a majority vote by endoscopists. The results demonstrated that the AI classifier had better internal evaluation scores, with an accuracy of 77%, sensitivity of 76%, specificity of 78%, and an F1 measure of 0.768. Notably, the F1 score is a statistical measure used to evaluate the accuracy of a test. It considers both the precision (the number of correct positive results divided by the number of all positive results) and the recall (the number of correct positive results divided by the number of positive results that should have been identified). The F1 score is the harmonic mean of precision and recall, providing a balance between them. It is especially useful in situations where the class distribution is imbalanced.
In contrast, the endoscopists had lower performance measures, with 72.6% accuracy, 53.6% sensitivity, 91.6% specificity, and an F1 measure of 0.662. Importantly, the diagnostic accuracy improved when the AI and endoscopists collaborated, achieving 78% accuracy, 76% sensitivity, 80% specificity, and an F1 measure of 0.776 on the test images. The combined approach yielded a higher F1 measure compared to using either the AI or endoscopists individually.
3.4. Gastric Neoplasm Segmentation
The ENDOANGEL system demonstrated commendable performance with both capsule endoscopy (CE) images and white-light endoscopy (WLE) images, achieving accuracy rates of 85.7% and 88.9%, respectively, when compared to the manual markers labeled by experts. Notably, these results were obtained using an overlap ratio threshold of 0.60 [35]. In the case of endoscopic submucosal dissection (ESD) videos, the resection margins predicted by ENDOANGEL effectively encompassed all areas characterized by high-grade intraepithelial neoplasia and cancers. The minimum distance between the predicted margins and the histological cancer boundary was measured at 3.44 ± 1.45 mm, outperforming the resection margin based on ME-NBI.
Wenju Du et al. [36] validated the use of correlation information among gastroscopic images as a means to enhance the accuracy of EGC segmentation. The authors used the Jaccard similarity index and the Dice similarity coefficient for validation. The Jaccard similarity index is a statistic that measures the similarity and diversity of sample sets. It is defined as the size of the intersection divided by the size of the union of the sample sets. The Jaccard coefficient is widely used in computer science, ecology, genomics, and other sciences where binary or binarized data are used. It is a ratio of Intersection over Union, taking values between 0 and 1, where 0 indicates no similarity and 1 indicates complete similarity.
Similar to the Jaccard index, the Dice similarity coefficient is a measure of the overlap between two samples. For image segmentation, it is calculated as twice the area of overlap between the predicted segmentation and the ground truth divided by the sum of the areas of the predicted segmentation and the ground truth. It is a common metric for evaluating the performance of image segmentation algorithms, particularly in medical imaging.
When applied to an unseen dataset, the EGC segmentation method demonstrated a Jaccard similarity index (JSI) of 84.54%, a threshold Jaccard index (TJI) of 81.73%, a Dice similarity coefficient (DSC) of 91.08%, and a pixel-wise accuracy (PA) of 91.18%. Tingsheng Ling et al. [37] achieved a high accuracy of 83.3% when correctly predicting the differentiation status of EGCs. Notably, in the man–machine contest, the CNN1 model significantly outperformed the five human experts, achieving an accuracy of 86.2% compared to the experts’ accuracy of 69.7%. When delineating EGC margins while utilizing an overlap ratio of 0.80, the model achieved high levels of accuracy for both differentiated EGCs and undifferentiated EGCs, with accuracies of 82.7% and 88.1%, respectively.
For invasive gastric cancer detection and segmentation, Atsushi Teramoto et al. [38] reported 97.0% sensitivity and 99.4% specificity using U-Net. In a case-based evaluation, the approach achieved flawless sensitivity and specificity scores of 100%.
3.5. Pre-Malignant Lesion Detection, Classification, and Segmentation
Several studies used AI to detect and classify gastric lesions associated with an increased risk of progression to cancer. A CNN with TResNet [39] was used to classify atrophic gastritis and gastric intestinal metaplasia (GIM). The AUC for atrophic gastritis classification was determined to be 0.98 CI, indicating high discriminatory power. The sensitivity, specificity, and accuracy rates for atrophic gastritis classification were 96.2%, 96.4%, and 96.4%, respectively. Similarly, the AUC for GIM classification was 0.99 (95% CI 0.98–1.00), indicating excellent discriminatory ability. The sensitivity, specificity, and accuracy rates for GIM diagnosis were 97.9%, 97.5%, and 97.6%, respectively. ENDOANGEL-LD [40] achieved 96.9% sensitivity for pre-malignant lesion detection.
Hirai K et al. [41] investigated the yield of CNN in classifying subepithelial lesions in endoscopic ultrasonography (EUS) images. The results showed that the AI system exhibited a commendable 86.1% accuracy when classifying lesions into five distinct categories: Gastrointestinal Stromal Tumors (GIST), leiomyoma, schwannoma, Neuroendocrine Tumors (NET), and ectopic pancreas. Notably, this accuracy rate surpassed that achieved by all endoscopists by a significant margin. Furthermore, the AI system demonstrated a sensitivity of 98.8%, specificity of 67.6%, and an overall accuracy of 89.3% in distinguishing GISTs from non-GISTs, vastly surpassing the sensitivity and accuracy of all endoscopists in the study at the expense of slightly decreased specificity.
Lastly, Passin Pornvoraphat et al. [17] aimed to create a real-time GIM segmentation system using a BiSeNet-based model. This model was able to process images at a rate of 173 frames per second (FPS). The system achieved sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and mean intersection over union (IoU) values of 91%, 96%, 91%, 91%, 96%, and 55%, respectively.
3.6. Early Gastric Cancer Detection
In recent years, the early detection of GC has gained significant attention [42,43,44,45,46,47,48,49,50,51]. Sakai Y et al. [42] employed transfer learning techniques and achieved a commendable accuracy of 87.6% in detecting EGC. Similarly, Lan Li et al. [43] used Inception-v3 (CNN model), reporting a high sensitivity of 91.2% and a specificity of 90.6%. Tang D et al. [44] evaluated DCNNs and their system demonstrated accuracy rates ranging from 85.1% to 91.2%.
Hu H et al. [12] developed an EGC model based on the VGG-19 architecture. The model was found to be comparable to senior endoscopists in performance but showed significant improvements over junior endoscopists. Wu L et al. [45] conducted a comprehensive study, reporting a sensitivity rate of 87.8% for detecting EGCs, thereby outperforming human endoscopists.
HE X et al. [46] developed ENDOANGEL-ME, which achieved a diagnostic accuracy of 88.4% on internal images and 90.5% on external images. Yao Z et al. [47] introduced a system called EGC-YOLO, achieving accuracy rates of 85.2% and 86.2% in two different test sets. Li J et al. [48] developed another system called ENDOANGEL-LA that significantly outperformed single deep learning models and was on par with expert endoscopists.
Jin J et al. [49] used Mask R-CNN technology and showed high accuracy and sensitivity in both white light images (WLIs) and NBIs. Another noteworthy study [50] utilized the EfficientDet architecture within CNN and achieved a sensitivity of 98.4% and an accuracy of 88.3% in detecting EGC. Finally, Su X et al. [51] assessed three typical Region-based Convolutional Neural Network models, each showing similar accuracy rates, although Cascade RCNN showed a slightly higher specificity of 94.6%.
Figure 2 shows the typical morphological features of malignant (A) and benign (B) ulcers used for AI application.
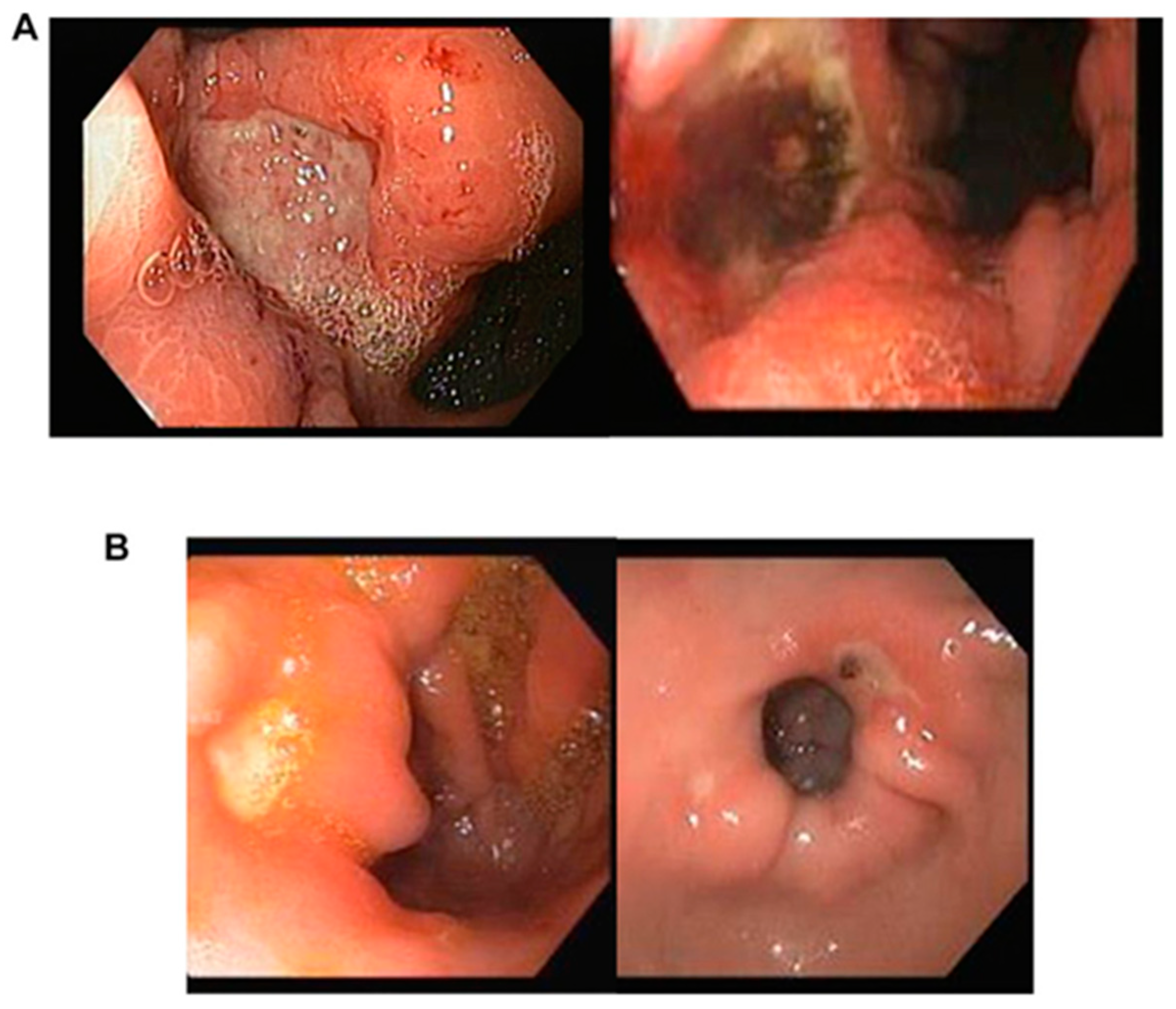
Figure 2. Morphological attributes of malignant (A) and benign (B) ulcers are as follows: (A) Malignant gastric ulcers exhibit typically larger dimensions, measuring over 1 cm, and possess irregular borders. These borders display elevation in comparison to the ulcer base, and discoloration of the base is frequently observed. (B) Conversely, benign gastric ulcers tend to be smaller in size, measuring less than 1 cm, and feature a clean unblemished base with consistently flat and regular borders.
4. Discussion
AI-assisted endoscopy promises to improve the detection rates of pre-malignant and early gastric neoplasias. The addition of computationally efficient DL computer vision models allows endoscopists to identify more potentially treatable gastric lesions during real-time upper endoscopy [15,16,17,18]. AI also shows promise in diagnosing and managing GC, as supported by two recent systematic reviews [8,9]. Our review delves into the use of deep learning (DL) models to detect gastric pre-malignant, early-stage, and neoplastic lesions. This review explores the potential benefits and implications of employing advanced AI techniques in this context.
DL models like Inception, ResNet, and VGGNet demonstrated high accuracy in identifying gastric pathologies [52,53]. However, their real-world performance depends on the quality and diversity of the training data. Training these models on diverse datasets is essential to account for patient demographic factors and subtle differences in disease presentation. AI systems like SSD-GPNet and YOLOv4 are good at detecting gastric pathologies, particularly polyps [24,25,54,55]. However, their real-world effectiveness remains uncertain, as variables like endoscopy lighting conditions, image quality, and inter-operator variability can impact performance.
As a group, the studies reviewed in this analysis have consistently demonstrated promising results in utilizing AI models for GC detection and segmentation. The accuracy achieved by the addition of these AI systems to standard endoscopy has shown significant improvements over un-assisted endoscopy, even outperforming human experts in some cases. The use of CNNs and other DL architectures has proven to be particularly effective in handling the complexity and variability of GC images [19,56,57,58].
In terms of GC detection, the AI models have exhibited high sensitivity and specificity, with values ranging between 80 and 92% in different studies [26,27,28,29,30]. These results suggest that AI has the potential to serve as a reliable tool for the initial screening and detection of GC.
Furthermore, AI-based real-time segmentation systems have shown great promise in segmenting the boundaries of gastric tumors. The segmentation accuracy achieved by these models has consistently surpassed that of traditional endoscopist-based approaches, providing reproducibly and accurately delineated tumor margins [18,36]. This capability is crucial for resection planning, treatment evaluation, and follow-up assessments. The high performance of AI models in differentiating between differentiated and undifferentiated GCs further highlights their potential in guiding treatment decisions and predicting prognoses [49,50,51,56].
Notably, the use of AI-assisted endoscopy significantly improved diagnostic performance and reduced diagnostic variability of endoscopic examinations of the stomach. This was particularly true for less-experienced practitioners. The AI-assisted diagnostic process not only enhanced the sensitivity and specificity of gastric lesion diagnosis, but also reduced the diagnostic duration and enhanced interobserver agreement among endoscopists.
Future ventures for algorithms can be to process and understand a wide range of medical data, from patient records and medical images to genetic data and biomarker profiles. This vast data set can provide AI models a deep understanding of GC patterns. This may allow the models to identify subtle signs and predictive markers that may be missed by human analysis [8,9,10].
AI has shown potential for early GC detection, as shown in multiple studies [42,43,44,45,46,47,48,49,50,51]. Improved endoscopic detection rates of pre-malignant gastric lesions and early GC could make large-scale screening more effective. Using a screening-based approach, earlier diagnosis of premalignant gastric lesions and gastric neoplasias would allow a greater proportion of patients to be treated with endoscopic interventions that have high cure rates and low complication rates [8,51]. Further studies are necessary to assess the feasibility and structure of such screening programs across different populations.
Despite these promising results, there are still challenges that need to be addressed before widespread clinical implementation of AI-assisted endoscopy for pre-malignant and early-stage GC detection and segmentation. One such challenge is the need for larger and more diverse datasets to ensure robust model performance across different populations and imaging conditions. Additionally, there is a requirement for rigorous validation and standardization of AI models to ensure their reliability and generalizability.
Another challenge is the explainability of DL models that plays a pivotal role in clinical acceptance and decision-making. These DL models, trained on vast datasets of endoscopic images, have demonstrated high proficiency in identifying early signs of gastric cancer. However, the challenge lies in deciphering how these models arrive at their conclusions. For gastroenterologists, understanding the rationale behind a model’s prediction is crucial for diagnosis and treatment planning. Explainability in this context involves the model highlighting specific features in endoscopic images, such as subtle changes in tissue texture, color, or vascular patterns, that signify pre-malignant or malignant changes. Techniques like Gradient-weighted Class Activation Mapping (Grad-CAM) [58] can be employed to visualize these decisive image regions, providing clinicians with a visual explanation of the model’s diagnostic process. This level of transparency is essential, as it not only builds trust in the AI system but also aids in the educational aspect, helping clinicians to identify and understand subtle early signs of gastric cancer. Thus, enhancing the explainability of DL models in AI-assisted endoscopy is a crucial step towards integrating these advanced tools into routine clinical practice for more effective and early detection of gastric cancer.
The AI models explored in this review echo the promise of a time when early cancer detection is common, not exceptional. Thus, AI may be a transformative force in the prevention and treatment of early-stage GC in the near future.
In AI-assisted endoscopy for gastric neoplasia, researchers could focus on developing algorithms for enhanced detection and classification of early-stage lesions, offering real-time procedural assistance to endoscopists, and automating pathology correlation to potentially reduce biopsy needs. Further exploration could involve patient-specific risk assessments incorporating personal and genetic data, developing AI-driven post-endoscopic surveillance recommendations, and creating training tools for endoscopists. Additionally, the integration of endoscopic data with other imaging modalities like CT or MRI could provide a more comprehensive diagnostic approach to gastric neoplasia.
We acknowledge the limitations of this review. Most of the included studies were single-center, potentially limiting their scope of patient demographics and disease presentations. Additionally, AI model training and validation often used undisclosed datasets, raising generalizability concerns. The retrospective training and validation of AI algorithms may not fully reflect prospective clinical performance. Furthermore, the lack of disclosure of models’ technical details can make study reproduction difficult [58,59,60,61]. Lastly, we selected PubMed/MEDLINE for its relevance in biomedical research, recognizing that this choice narrows our review’s scope. This might exclude studies from other databases, possibly limiting diverse insights. This limitation was considered to balance focus and comprehensiveness in our review.
In conclusion, AI’s potential in diagnosing and managing pre-malignant early-stage gastric neoplasia is significant due to several reasons. Firstly, AI algorithms, powered by machine learning, can analyze complex medical data at unprecedented speeds and with high accuracy. This ability enables the early detection of gastric neoplasia, which is crucial for successful treatment outcomes.
Secondly, AI can assist in differentiating between benign and malignant gastric lesions, which is often a challenging task in medical practice. By doing so, AI can help reduce unnecessary biopsies and surgeries, leading to better patient outcomes and reduced healthcare costs.
Thirdly, AI can continuously learn from new data, enhancing its diagnostic capabilities over time. This aspect is particularly important in the field of oncology, where early and accurate diagnosis can significantly impact patient survival rates.
However, further research, larger-scale studies, and prospective clinical trials are necessary to confirm these findings. Future research should also focus on transparency, providing detailed reports on models’ technical details and the datasets used for training and validation.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Thrift, A.P.; Wenker, T.N.; El-Serag, H.B. Global burden of gastric cancer: Epidemiological trends, risk factors, screening and prevention. Nat. Rev. Clin. Oncol. 2023, 20, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Katai, H.; Ishikawa, T.; Akazawa, K.; Isobe, Y.; Miyashiro, I.; Oda, I.; Tsujitani, S.; Ono, H.; Tanabe, S.; Fukagawa, T.; et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer 2018, 21, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Januszewicz, W.; Witczak, K.; Wieszczy, P.; Socha, M.; Turkot, M.H.; Wojciechowska, U.; Didkowska, J.; Kaminski, M.F.; Regula, J. Prevalence and risk factors of upper gastrointestinal cancers missed during endoscopy: A nationwide registry-based study. Endoscopy 2022, 54, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, O.; Hattori, M.; Douden, K.; Hayashi, H.; Ohta, K.; Kaizaki, Y. Difference in accuracy between gastroscopy and colonoscopy for detection of cancer. Hepatogastroenterology 2007, 54, 442–444. [Google Scholar] [PubMed]
- Menon, S.; Trudgill, N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc. Int. Open 2014, 2, E46–E50. [Google Scholar] [CrossRef]
- Ochiai, K.; Ozawa, T.; Shibata, J.; Ishihara, S.; Tada, T. Current Status of Artificial Intelligence-Based Computer-Assisted Diagnosis Systems for Gastric Cancer in Endoscopy. Diagnostics 2022, 12, 3153. [Google Scholar] [CrossRef]
- Sharma, P.; Hassan, C. Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia. Gastroenterology 2022, 162, 1056–1066. [Google Scholar] [CrossRef]
- Sarker, I.H. Deep Learning: A Comprehensive Overview on Techniques, Taxonomy, Applications and Research Directions. SN Comput. Sci. 2021, 2, 420. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Z.; Cheng, J.; Bu, X.; Qiu, K.; Yang, C.; Wang, J.; Niu, W.; Wu, X.; Xu, J.; et al. Diagnosis and segmentation effect of the ME-NBI-based deep learning model on gastric neoplasms in patients with suspected superficial lesions—A multicenter study. Front. Oncol. 2023, 12, 1075578. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gong, L.; Dong, D.; Zhu, L.; Wang, M.; He, J.; Shu, L.; Cai, Y.; Cai, S.; Su, W.; et al. Identifying early gastric cancer under magnifying narrow-band images with deep learning: A multicenter study. Gastrointest. Endosc. 2021, 93, 1333–1341.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Q.C.; Xu, M.D.; Zhang, Z.; Cheng, J.; Zhong, Y.S.; Zhang, Y.Q.; Chen, W.F.; Yao, L.Q.; Zhou, P.H.; et al. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest. Endosc. 2019, 89, 806–815.e1. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Kubota, N.; Nishikawa, J.; Ogawa, R.; Hamabe, K.; Hashimoto, S.; Ogihara, H.; Hamamoto, Y.; Yanai, H.; Miura, O.; et al. Cooperation between artificial intelligence and endoscopists for diagnosing invasion depth of early gastric cancer. Gastric Cancer 2023, 26, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Lin, M.C.; Li, Y.J. Artificial Intelligence in Gastric Cancer: Identifying Gastric Cancer Using Endoscopic Images with Convolutional Neural Network. Cancers 2021, 13, 5253. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Dong, Z.; Wu, L.; Li, Y.; Liu, J.; Luo, C.; Zeng, X.; Deng, Y.; Cheng, D.; Diao, W.; et al. A deep-learning based system using multi-modal data for diagnosing gastric neoplasms in real-time (with video). Gastric Cancer 2023, 26, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Pornvoraphat, P.; Tiankanon, K.; Pittayanon, R.; Sunthornwetchapong, P.; Vateekul, P.; Rerknimitr, R. Real-time gastric intestinal metaplasia diagnosis tailored for bias and noisy-labeled data with multiple endoscopic imaging. Comput. Biol. Med. 2023, 154, 106582. [Google Scholar] [CrossRef]
- Gong, E.J.; Bang, C.S.; Lee, J.J.; Baik, G.H.; Lim, H.; Jeong, J.H.; Choi, S.W.; Cho, J.; Kim, D.Y.; Lee, K.B.; et al. Deep learning-based clinical decision support system for gastric neoplasms in real-time endoscopy: Development and validation study. Endoscopy 2023, 55, 701–708. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.J.; Kim, Y.W.; Park, S.; Choi, Y.I.; Kim, Y.J.; Park, D.K.; Kim, K.G.; Chung, J.W. Spotting malignancies from gastric endoscopic images using deep learning. Surg. Endosc. 2019, 33, 3790–3797. [Google Scholar] [CrossRef]
- Cho, B.J.; Bang, C.S.; Park, S.W.; Yang, Y.J.; Seo, S.I.; Lim, H.; Shin, W.G.; Hong, J.T.; Yoo, Y.T.; Hong, S.H. Automated classification of gastric neoplasms in endoscopic images using a convolutional neural network. Endoscopy 2019, 51, 1121–1129. [Google Scholar] [CrossRef]
- Ueyama, H.; Kato, Y.; Akazawa, Y.; Yatagai, N.; Komori, H.; Takeda, T.; Matsumoto, K.; Ueda, K.; Matsumoto, K.; Hojo, M.; et al. Application of artificial intelligence using a convolutional neural network for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging. J. Gastroenterol. Hepatol. 2021, 36, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Aoyama, K.; Tokai, Y.; Hirasawa, T.; Yoshimizu, S.; Ishiyama, A.; Yoshio, T.; Tsuchida, T.; Fujisaki, J.; Tada, T. Convolutional Neural Network for Differentiating Gastric Cancer from Gastritis Using Magnified Endoscopy with Narrow Band Imaging. Dig. Dis. Sci. 2020, 65, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Klang, E.; Barash, Y.; Levartovsky, A.; Barkin Lederer, N.; Lahat, A. Differentiation Between Malignant and Benign Endoscopic Images of Gastric Ulcers Using Deep Learning. Clin. Exp. Gastroenterol. 2021, 14, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Hao, Z. An xception model based on residual attention mechanism for the classification of benign and malignant gastric ulcers. Sci. Rep. 2022, 12, 15365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, F.; Yu, T.; An, J.; Huang, Z.; Liu, J.; Hu, W.; Wang, L.; Duan, H.; Si, J. Real-time gastric polyp detection using convolutional neural networks. PLoS ONE 2019, 14, e0214133. [Google Scholar] [CrossRef] [PubMed]
- Durak, S.; Bayram, B.; Bakırman, T.; Erkut, M.; Doğan, M.; Gürtürk, M.; Akpınar, B. Deep neural network approaches for detecting gastric polyps in endoscopic images. Med. Biol. Eng. Comput. 2021, 59, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shang, R.; Sharma, P.; Zhou, W.; Liu, J.; Yao, L.; Dong, Z.; Yuan, J.; Zeng, Z.; Yu, Y.; et al. Effect of a deep learning-based system on the miss rate of gastric neoplasms during upper gastrointestinal endoscopy: A single-centre, tandem, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2021, 6, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, W.; Wu, L.; Zhang, J.; Wang, J.; Mu, G.; Huang, X.; Li, Y.; Yuan, J.; Zeng, Z.; et al. Artificial intelligence in the diagnosis of gastric precancerous conditions by image-enhanced endoscopy: A multicenter, diagnostic study (with video). Gastrointest. Endosc. 2021, 94, 540–548.e4. [Google Scholar] [CrossRef]
- Hirasawa, T.; Aoyama, K.; Tanimoto, T.; Ishihara, S.; Shichijo, S.; Ozawa, T.; Ohnishi, T.; Fujishiro, M.; Matsuo, K.; Fujisaki, J.; et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer 2018, 21, 653–660. [Google Scholar] [CrossRef]
- Ikenoyama, Y.; Hirasawa, T.; Ishioka, M.; Namikawa, K.; Yoshimizu, S.; Horiuchi, Y.; Ishiyama, A.; Yoshio, T.; Tsuchida, T.; Takeuchi, Y.; et al. Detecting early gastric cancer: Comparison between the diagnostic ability of convolutional neural networks and endoscopists. Dig. Endosc. 2021, 33, 141–150. [Google Scholar] [CrossRef]
- Bang, C.S.; Lim, H.; Jeong, H.M.; Hwang, S.H. Use of Endoscopic Images in the Prediction of Submucosal Invasion of Gastric Neoplasms: Automated Deep Learning Model Development and Usability Study. J. Med. Internet Res. 2021, 23, e25167. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, S.; Kim, J.H.; Keum, J.S.; Oh, S.I.; Jo, J.; Chun, J.; Youn, Y.H.; Park, H.; Kwon, I.G.; et al. A Lesion-Based Convolutional Neural Network Improves Endoscopic Detection and Depth Prediction of Early Gastric Cancer. J. Clin. Med. 2019, 8, 1310. [Google Scholar] [CrossRef]
- Hamada, K.; Kawahara, Y.; Tanimoto, T.; Ohto, A.; Toda, A.; Aida, T.; Yamasaki, Y.; Gotoda, T.; Ogawa, T.; Abe, M.; et al. Application of convolutional neural networks for evaluating the depth of invasion of early gastric cancer based on endoscopic images. J. Gastroenterol. Hepatol. 2022, 37, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhou, J.; Wang, L.; Ni, M.; Chen, M.; Hassan, S.; Luo, R.; Chen, X.; He, X.; Zhang, L.; et al. A Novel Model Based on Deep Convolutional Neural Network Improves Diagnostic Accuracy of Intramucosal Gastric Cancer (With Video). Front. Oncol. 2021, 11, 622827. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Yang, D.; Wang, J.; Wu, L.; Zhou, J.; Zeng, Z.; Huang, X.; Xiao, Y.; Hu, S.; Chen, Y.; et al. A deep learning method for delineating early gastric cancer resection margin under chromoendoscopy and white light endoscopy. Gastric Cancer 2020, 23, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Rao, N.; Yong, J.; Adjei, P.E.; Hu, X.; Wang, X.; Gan, T.; Zhu, L.; Zeng, B.; Liu, M.; et al. Early gastric cancer segmentation in gastroscopic images using a co-spatial attention and channel attention based triple-branch ResUnet. Comput. Methods Programs Biomed. 2023, 231, 107397. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Wu, L.; Fu, Y.; Xu, Q.; An, P.; Zhang, J.; Hu, S.; Chen, Y.; He, X.; Wang, J.; et al. A deep learning-based system for identifying differentiation status and delineating the margins of early gastric cancer in magnifying narrow-band imaging endoscopy. Endoscopy 2021, 53, 469–477. [Google Scholar] [CrossRef]
- Teramoto, A.; Shibata, T.; Yamada, H.; Hirooka, Y.; Saito, K.; Fujita, H. Detection and Characterization of Gastric Cancer Using Cascade Deep Learning Model in Endoscopic Images. Diagnostics 2022, 12, 1996. [Google Scholar] [CrossRef]
- Lin, N.; Yu, T.; Zheng, W.; Hu, H.; Xiang, L.; Ye, G.; Zhong, X.; Ye, B.; Wang, R.; Deng, W.; et al. Simultaneous Recognition of Atrophic Gastritis and Intestinal Metaplasia on White Light Endoscopic Images Based on Convolutional Neural Networks: A Multicenter Study. Clin. Transl. Gastroenterol. 2021, 12, e00385. [Google Scholar] [CrossRef]
- Wu, L.; Xu, M.; Jiang, X.; He, X.; Zhang, H.; Ai, Y.; Tong, Q.; Lv, P.; Lu, B.; Guo, M.; et al. Real-time artificial intelligence for detecting focal lesions and diagnosing neoplasms of the stomach by white-light endoscopy (with videos). Gastrointest. Endosc. 2022, 95, 269–280.e6. [Google Scholar] [CrossRef]
- Hirai, K.; Kuwahara, T.; Furukawa, K.; Kakushima, N.; Furune, S.; Yamamoto, H.; Marukawa, T.; Asai, H.; Matsui, K.; Sasaki, Y.; et al. Artificial intelligence-based diagnosis of upper gastrointestinal subepithelial lesions on endoscopic ultrasonography images. Gastric Cancer 2022, 25, 382–391. [Google Scholar] [CrossRef]
- Sakai, Y.; Takemoto, S.; Hori, K.; Nishimura, M.; Ikematsu, H.; Yano, T.; Yokota, H. Automatic detection of early gastric cancer in endoscopic images using a transferring convolutional neural network. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 4138–4141. [Google Scholar] [PubMed]
- Li, L.; Chen, Y.; Shen, Z.; Zhang, X.; Sang, J.; Ding, Y.; Yang, X.; Li, J.; Chen, M.; Jin, C.; et al. Convolutional neural network for the diagnosis of early gastric cancer based on magnifying narrow band imaging. Gastric Cancer. 2020, 23, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, L.; Ling, T.; Lv, Y.; Ni, M.; Zhan, Q.; Fu, Y.; Zhuang, D.; Guo, H.; Dou, X.; et al. Development and validation of a real-time artificial intelligence-assisted system for detecting early gastric cancer: A multicentre retrospective diagnostic study. eBioMedicine 2020, 62, 103146. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, J.; He, X.; Zhu, Y.; Jiang, X.; Chen, Y.; Wang, Y.; Huang, L.; Shang, R.; Dong, Z.; et al. Deep learning system compared with expert endoscopists in predicting early gastric cancer and its invasion depth and differentiation status (with videos). Gastrointest. Endosc. 2022, 95, 92–104.e3. [Google Scholar] [CrossRef]
- He, X.; Wu, L.; Dong, Z.; Gong, D.; Jiang, X.; Zhang, H.; Ai, Y.; Tong, Q.; Lv, P.; Lu, B.; et al. Real-time use of artificial intelligence for diagnosing early gastric cancer by magnifying image-enhanced endoscopy: A multicenter diagnostic study (with videos). Gastrointest. Endosc. 2022, 95, 671–678.e4. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Jin, T.; Mao, B.; Lu, B.; Zhang, Y.; Li, S.; Chen, W. Construction and Multicenter Diagnostic Verification of Intelligent Recognition System for Endoscopic Images from Early Gastric Cancer Based on YOLO-V3 Algorithm. Front. Oncol. 2022, 12, 815951. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Y.; Dong, Z.; He, X.; Xu, M.; Liu, J.; Zhang, M.; Tao, X.; Du, H.; Chen, D.; et al. Development and validation of a feature extraction-based logical anthropomorphic diagnostic system for early gastric cancer: A case-control study. eClinicalMedicine 2022, 46, 101366. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, Q.; Dong, B.; Ma, T.; Mei, X.; Wang, X.; Song, S.; Peng, J.; Wu, A.; Dong, L.; et al. Automatic detection of early gastric cancer in endoscopy based on Mask region-based convolutional neural networks (Mask R-CNN) (with video). Front. Oncol. 2022, 12, 927868. [Google Scholar] [CrossRef]
- Zhou, B.; Rao, X.; Xing, H.; Ma, Y.; Wang, F.; Rong, L. A convolutional neural network-based system for detecting early gastric cancer in white-light endoscopy. Scand. J. Gastroenterol. 2023, 58, 157–162. [Google Scholar] [CrossRef]
- Su, X.; Liu, Q.; Gao, X.; Ma, L. Evaluation of deep learning methods for early gastric cancer detection using gastroscopic images. Technol. Health Care 2023, 31 (Suppl. S1), 313–322. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, D.; Li, F.; Li, Z.; Bao, Z. Application of Artificial Intelligence in Early Gastric Cancer Diagnosis. Digestion 2022, 103, 69–75. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.J.; Park, S.C. Usefulness of artificial intelligence in gastric neoplasms. World J. Gastroenterol. 2021, 27, 3543–3555. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.J.; Wen, Y.C.; Lai, W.Y.; Lin, Y.Y.; Yang, Y.P.; Chien, Y.; Yarmishyn, A.A.; Hwang, D.K.; Lin, T.C.; Chang, Y.C.; et al. Application of artificial intelligence-driven endoscopic screening and diagnosis of gastric cancer. World J. Gastroenterol. 2021, 27, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yoshitaka, T.; Yoshio, T.; Tada, T. Artificial intelligence for cancer detection of the upper gastrointestinal tract. Dig. Endosc. 2021, 33, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, H.; Ishihara, R.; Aoyama, K.; Kono, M.; Fukuda, H.; Shimamoto, Y.; Nakagawa, K.; Ohmori, M.; Iwatsubo, T.; Iwagami, H.; et al. Stratification of gastric cancer risk using a deep neural network. JGH Open 2019, 4, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, Y.; Wei, K.; Li, Z.; Li, D.; Song, R.; Ren, J.; Gao, X.; Yang, X. Deep learning predicts resistance to neoadjuvant chemotherapy for locally advanced gastric cancer: A multicenter study. Gastric Cancer 2022, 25, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, S.; Sasaki, Y.; Mikami, T.; Sakuraba, H.; Fukuda, S. Anatomical classification of upper gastrointestinal organs under various image capture conditions using AlexNet. Comput. Biol. Med. 2020, 124, 103950. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K.; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Nat Med. 2020, 26, 1364–1374. [Google Scholar] [CrossRef]
- Cruz Rivera, S.; Liu, X.; Chan, A.W.; Denniston, A.K.; Calvert, M.J.; SPIRIT-AI and CONSORT-AI Working Group; SPIRIT-AI and CONSORT-AI Steering Group; SPIRIT-AI and CONSORT-AI Consensus Group. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI extension. Nat Med. 2020, 26, 1351–1363. [Google Scholar] [CrossRef]
- Parasa, S.; Repici, A.; Berzin, T.; Leggett, C.; Gross, S.A.; Sharma, P. Framework and metrics for the clinical use and implementation of artificial intelligence algorithms into endoscopy practice: Recommendations from the American Society for Gastrointestinal Endoscopy Artificial Intelligence Task Force. Gastrointest. Endosc. 2023, 97, 815–824.e1. [Google Scholar] [CrossRef] [PubMed]

